A novel extra-broadband visible-emitting garnet phosphor for efficient single-component pc-WLEDs†
Qianyi
Chen‡
a,
Zhenjie
Lun‡
a,
Dongdan
Chen
 *a,
Yongsheng
Sun
a,
Puxian
Xiong
*a,
Yongsheng
Sun
a,
Puxian
Xiong
 ac,
Siyun
Li
a,
Shanhui
Xu
*a and
Zhongmin
Yang
ab
ac,
Siyun
Li
a,
Shanhui
Xu
*a and
Zhongmin
Yang
ab
aSchool of Physics and Optoelectronics, State Key Laboratory of Luminescent Materials and Devices, School of Materials Science and Engineering, Guangdong Engineering Technology Research Center of Special Optical Fiber Materials and Devices, Guangdong Provincial Key Laboratory of Fiber Laser Materials and Applied Techniques, Institute of Optical Communication Materials, South China University of Technology, Guangzhou 510641, China. E-mail: ddchen@scut.edu.cn; flxshy@scut.edu.cn
bSouth China Normal University, Guangzhou 510641, China
cDepartment of Electrical and Electronic Engineering, The University of Hong Kong, Hong Kong, China
First published on 1st November 2024
Abstract
The development of extra-broadband visible emission phosphors is crucial to achieve next-generation illumination with better color experience. Herein, a defect engineering strategy mediated by the structural cationic substitution is proposed and experimentally demonstrated for specific ultra-broadband emission in a garnet phosphor. The induced oxygen vacancies and interstitial cation through lattice distortion break the periodic potential field of the crystal and provide electronic levels in the band gap. As a result, excited by blue-light-emitting diodes, the novel Y3Sc2Al3O12:B3+ shows an ultra-broad emission with a full width at half maximum (FWHM) of ∼170 nm. Compared to general defect-emitting phosphors, the unique Y3Sc2Al3O12:B3+ exhibits excellent thermal quenching resistance and superior internal quantum efficiency of up to 95%. These findings not only show great promise of Y3Sc2Al3O12:B3+ as an extra-broadband emitter but also provide a new design strategy to achieve a full-visible-spectrum phosphor in a single-component material for white-light applications.
1. Introduction
Broadband visible lighting underpins a plethora of applications in optics and photonics, including optical communication, visible spectroscopy1,2 and, particularly, white-light-emitting diodes (WLED).3,4 The typical commercial phosphor-converted WLEDs (pc-WLEDs) were achieved by the combination of a blue InGaN chip with a yellow-emitting Y3Al5O12:Ce3+ (YAG:Ce) phosphor.5 However, such WLEDs often suffer from deficiency of the red-light component and “cyan cavity”, leading to the severe problem of poor colour rendition. After the discovery of red-emitting phosphors6–8 (e.g. CaAlSiN3:Eu2+, BaAl4Sb2O12:Eu2+ or SrLiAl3N4:Eu2+) and cyan phosphors9–12 (e.g. NaMgBO3:Ce3+, Ca2LaHf2Al3O12:Ce3+, Ba3.92Ca0.9La0.1(PO4)3Cl:Eu2+ and CaLuGaO4:Eu2+), an alternative approach of combining tricolour phosphors with a near-ultraviolet (n-UV) LED chip has achieved a high colour rendering index (CRI) and low correlated colour temperature (CCT). Unfortunately, it has been observed that such pc-WLEDs based on a multi-phase phosphor system inevitably faces the problems of reabsorption energy loss and dissonance deterioration rates during long-term operation.13 Thus, it is essential to develop an ultra-broad emission phosphor with full-spectrum emission in a single-component material.One option is to co-dope several rare-earth ions into a single system to introduce multiple luminescence,14–16 but this also results in reabsorption and poor luminescence efficiency. Recently, defect engineering is effectively applied for design and modulation of novel luminescent materials.17 In principle, controlling the intrinsic defects in the lattice can affect the energy level distribution of the electrons, which could act as unusual activators. A feasible route to investigate broadband emissions is to modulate the oxygen vacancy (VO) and interstitial atoms to introduce defect levels that can trap electrons from the conduction band. In this respect, it is preferred to choose wide bandgap oxides (energy gap > 3.5 eV) because their range of native defects provides an electronic level in the bandgap that is capable of generating an emission band in the visible range (Fig. 1). Guided by this design strategy, a few binary oxides, such as ZnO18 and TiO2,19 with full-visible spectrum have been explored. However, these materials are very sensitive to the surroundings and possess relatively poor thermal quenching owing to the low activation energy of the thermally activated defects,20,21 which may hinder the practical applications of pc-WLED devices. Therefore, it is essential to develop a defect-stabilized luminescent phosphor that broadens the choices for broadband visible emission.
Interestingly, we successfully adopted defect engineering in the Y3Sc2Al3O12 (YSAG) phosphor without a lanthanide dopant, which presents a highly reproducible and intense broadband emission that spans the cyan to orange-red region. YSAG is an outstanding matrix with high structural rigidity, which has been widely applied in the field of solid-state lasers, displays, and illumination. However, it is still difficult for YSAG phosphors based on rare-earth ion or transition metal ion doping to meet the requirements of high-performance single-component WLEDs due to their narrow emission bandwidth.22–24 Herein, we demonstrate novel emitting through the consecutive substitution of matrix cations in this garnet material. The crystal local field and electronic microstructures are purposefully modulated, thereby controlling the broadband performance through variation in the defect concentration. The finding provides new insight into the garnet structure design and oxygen vacancy tailoring for luminescent applications.
2. Results and discussion
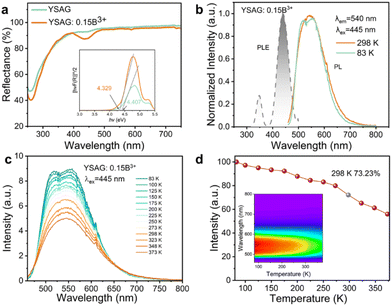
To experimentally elaborate the defect engineering strategy, we synthesized a series of YSAG powders doped with varying B3+ concentrations using the traditional high-temperature solid-state reaction. The diffuse reflectance (DR) spectra of the novel YSAG:xB3+ (x = 0.00 and 0.15) are exhibited in Fig. 2a. For the garnet phosphor, the solid optical absorption is observed in the UV and visible region from 250 to 500 nm. Notably, the Y3Sc2Al3O12 phosphor shows one prominent band, which is centered at around 258 nm. After B3+-doping, there is also a prominent band in the region of 400–500 nm. According to the Kubelka–Munk function, the direct optical bandgap value can be obtained from a fit to the linear part of the DRS spectrum as follows:25| [hνF(R)]2 = A(hν − Eg) | (1) |
| F(R) = (1 − R)2/2R | (2) |
To explore the origin of the unique cyan-red emission of the B3+ ions in YSAG, the crystal structure configuration and composition of YSAG:B3+ were investigated. YSAG is a typical garnet with a highly rigid framework structure composed of a [YO8] dodecahedron, [ScO6] octahedrons and [AlO4] tetrahedrons. In YSAG:B3+, because B3+ and Al3+ have more similar ionic radii than Sc3+ or Y3+, this smaller size allows B3+ to fit more comfortably into the crystal lattice where Al3+ is present, facilitating substitution. Moreover, B3+ typically prefers a lower coordination number and a tetrahedral geometry, and might better match the electronegativity of the surrounding anions when replacing Al3+. Therefore, when B3+ enters the lattice, it prefers to replace Al3+ sites rather than Sc3+ or Y3+.
The XRD pattern of YSAG:xB3+ (x = 0.00–0.17) could be indexed with the standard Y3Sc2Al3O12 (PDF No. 79-1846), thereby demonstrating the formation of the targeted phase (see Fig. S2†). Fig. 3a, Fig. S3 and Table S2† display the Rietveld refinement XRD results of YSAG:xB3+. All of the as-prepared samples crystalized in a cubic structure with a space group of Ia3d. In terms of defect formation, the difference of the ion radii between the doped ions and host cations can be evaluated by the parameter Dr, which is obtained from the empirical formula:30
 | (3) |
The Raman spectra are shown in Fig. 3f, reflecting the evolution of the microscopic structure with and without the B3+ dopant. The bands around 251, 375 and 766 cm−1 are induced by the characteristic vibrations of the YSAG garnet.22 Additionally, the bands of YSAG:0.15B3+ shift toward lower frequency, suggesting that the local lattice coordination environment in the matrix slightly changes with B3+ incorporation. EPR measurements were performed to analyze the valence state in the YSAG:0.15B3+ samples. The valence state via the g-factor value can be calculated by the following formula:31
| g = hv/βH | (4) |
To reveal the crystal local field-related defects in YSAG, the effect of oxygen deficiency and the local electron structure variation around the B3+ ions on the luminescence behavior of YSAG:B3+ was firstly explored by density functional theory (DFT) calculation. The calculated energy band structure and electron densities of state (DOS) in the YSAG matrix and YSAG:B3+ are shown in Fig. S6 and S7.† The calculated bandgap (Eg) value (4.39 eV) is close to the experimental value (4.407 eV). DFT calculations in Fig. 4a reveal that no electron transition energy levels ions are observed in the band gap for the dopant of B3+ in the absence of VO, which implies that this model cannot provide effective energy levels for electron transitions. To actualize defective engineering, we purposefully introduced Vo randomly around the B ion. We calculated the electron structures of YSAG:B3+ with three types of Vo defects, which are exhibited in Fig. 4b. The whole electron distribution shifts in the low-energy direction. DFT results show that the electron transition energy levels of the B 2p orbital appear between the conduction band and the valence band of the YSAG matrix.
To further investigate the influence of oxygen vacancies on the B3+ luminescence behavior in the YSAG matrix, the electron localization function (ELF) maps around the B atoms (Fig. 4c) without and with oxygen vacancies are analyzed. Thus, when B3+ ions occupy Al3+ ions without generating oxygen vacancies, the B 2s energy level is embedded in the valence band, while the 2p energy level contributes to the valence band and the conduction band. The separation energy between the 2p energy levels is almost equal to the band gap value. There are no electron transitions on B3+ ions for generating luminescence. In the presence of VO, the B 2p excited-state energy level appears between the conduction band and the valence band, and the 2s energy level mainly embeds the maximum of the valence band. In addition, DFT calculations show that B3+ substitution of Al3+ results in the formation of interstitial aluminum Ali with an energy level (2.30 eV) in the bandgap, as depicted in Fig. 4d and e. The proposed electron transition, which is based on DFT calculations, is summarized schematically in Fig. 4f. Our experimental results agree well with this theoretical calculation. The Gaussian fitting of the broadband PL spectra (Fig. S8†) supports the existence of two luminescence centers with peak positions at 2.30 eV (535 nm) and 2.26 eV (548 nm).
To utilize the advantages of this newly designed phosphor, we firstly fabricated two prototype pc-WLED devices, where the YSAG:0.15B3+ and commercial YAG:Ce3+ phosphors acting as light converters are each pumped by a blue LED chip (445 nm). Fig. 5a and b demonstrate the electroluminescence (EL) spectra of the LED devices in the 400–800 nm region at a driving current of 30 mA. It exhibits a broad emission peak around 460 nm–700 nm with a surprising FWHM of approximately 170 nm. The FWHM of other visible broadband emission phosphors include YSAG:Ce3+ with 80–90 nm,34 GdYAG:Ce3+ with 100–110 nm,35 and SrAl12O19:Eu2+ with 80–90 nm,36 which highlight the application potential of YSAG:B3+ in advanced lighting. In addition, compared to commercial YAG:Ce3+, the YSAG:0.15B3+ phosphor exhibits stronger luminescence in the cyan-blue region. Notably, the blue peak of YSAG:0.15B3+ located at ∼460 nm is ascribed to the electronic transition of VO2, which agrees well with the DFT calculations. The emission intensity increases with increasing currents, while demonstrating stable output efficiency (Fig. 5c). With 3 V and 30 mA driving current, the lumens efficiency of the as-prepared pc-WLED is 42.1 lm W−1. As shown in Fig. 5d, the Commission Internationale de L'Eclairage (CIE) chromaticity coordinate of the WLED with a correlated colour temperature (CCT) of 7245 K is (0.296, 0.337). These results demonstrate that the developed Y3Sc2Al3O12:0.15B3+ is a promising broadband emitter for application in blue-based pc-WLED lighting.
3. Experimental
3.1 Computational methods
First-principles density functional theory (DFT) calculations were performed with the Vienna ab initio simulation package (VASP) code.37,38 The electron-ion interaction was treated with the projector augmented wave (PAW) method. Al (3s23p1), B (2s22p1), O (2s22p4), Sc (3d14s2), and Y (4d15s2) electrons were treated as the valence electrons. The generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) functional was employed as the exchange–correlation potential. A plane-wave cutoff energy of 450 eV was applied in our calculations, and 3 × 3 × 3 Monkhorst–Pack k grids were used during the optimization. The crystal lattice was fully relaxed until the atomic force was less than 0.03 eV Å−1. The energy convergence criterion for self-consistent electronic calculation was set to 10−5 eV per atom.3.2 Materials preparation
A series of Y3Sc2Al3O12:xB3+ (x = 0.00–0.17) samples were produced via conventional high-temperature solid-state reaction. The raw materials, including Y2O3 (99.99% purity, Aladdin), Sc2O3 (99.99%, Aladdin), Al2O3 (99.9%, Macklin), and H3BO3 (A.R. Aladdin), were weighed based on their stoichiometric proportions and ground together for 0.5 h in agate mortars. The mixture was placed in alumina crucibles and sintered in a muffle furnace at 1550 °C for 6 h in air. After being naturally cooled to room temperature, the synthesized samples were ground again for further characterization.3.3 Materials characterizations
Powder X-ray diffraction (XRD) was collected on an X'Pert PRO X-ray diffractometer (PANalytical, Netherland) using Cu Kα (λ = 1.5418 Å) radiation. Rietveld refinements were performed with General Structure Analysis System (GSAS) software based on XRD data. The morphology and elemental mapping were studied via TEM (JEOL-JEM 2100 F) operated at 200 kV and equipped with an energy dispersive X-ray spectrometer (EDS). The DR spectra were measured by a UV-Vis-NIR spectrophotometer (UH4150, Hitachi) over the spectral range of 200–800 nm. The PLE and PL spectra (the excitation light is blocked off by the 450 nm long-pass filter) and lifetime measurements of YSAG:xB3+ were obtained by an Edinburgh FLS-920 spectrometer. The Raman spectra were characterized by a Micro-Raman spectrometer (Renishaw inVia) under the excitation of a 532 nm laser. To analyse the value state of the defect, X-ray photoelectron spectroscopy (XPS, Axis Supra+) measurements were carried out. Accurate elemental quantification of the samples was performed by an X-ray fluorescence spectrometer (Zetium, Malvern Panalytical). The internal quantum efficiency (IQE) of the broadband visible emission phosphor was measured by a fluorescence spectrophotometer (FLS 1000, Edinburgh Instruments) with an additional integrating sphere. All of the above measurements were performed at room temperature. Temperature-dependent PL spectra (83 K–373 K) were recorded on a fluorescence spectrophotometer (FLSP-920, Edinburgh Instruments) with a temperature controller.3.4 LED fabrication and characterizations
A pc-WLED device was fabricated using the as-prepared YSAG:0.15B3+ phosphor and a 445 nm LED chip. Particularly, the YSAG:0.15B3+ phosphor was evenly blended with ultraviolet photosensitive resins (Shenzhen Looking Long Technology Co., LTD, A/B epoxy resin mixed in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio) and the resulting mixture was coated on the LED chip. The epoxy-encapsulated LED chips were cured in an oven at 110 °C for 4 h. The white light emission spectra and performance parameters of the home-made WLED were analyzed by a UV-vis-NIR spectrophotometer with an integrating sphere (PMS-50 PLUS, Everfine).
4 ratio) and the resulting mixture was coated on the LED chip. The epoxy-encapsulated LED chips were cured in an oven at 110 °C for 4 h. The white light emission spectra and performance parameters of the home-made WLED were analyzed by a UV-vis-NIR spectrophotometer with an integrating sphere (PMS-50 PLUS, Everfine).
4. Conclusions
In summary, based on the DFT structure calculation and defect design, we have discovered a new strategy to achieve ultra-broadband emission by systematically tailoring vacancy defects and cationic defect in the Y3Sc2Al3O12:xB3+ (x = 0.10–0.17) phosphor. At 445 nm excitation, the emission covers the region of 450 nm to 800 nm with a satisfactory FWHM of ∼170 nm and has a high internal quantum efficiency of up to 95%. Importantly, in comparison with most other defective light-emitting phosphors, YSAG:B3+ exhibits promising thermal quenching performance, retaining 73.23% of the original intensity after the test temperature was increased by ∼230 °C. These results reveal the mechanism of defect formation in garnet, and pave the way toward the design of efficient broadband lighting emitters.Author contributions
Qianyi Chen: conceptualization, data curation, formal analysis, investigation, methodology, and writing – original draft; Zhenjie Lun: data curation and software; Dongdan Chen: conceptualization, funding acquisition, project administration, resources, supervision, and writing – review & editing; Yongsheng Sun: data curation; Puxian Xiong: data curation; Siyun Li: data curation; Shanhui Xu: investigation; Zhongmin Yang: investigation.Data availability
The data supporting this article have been included as part of the ESI.† More raw data are available from the corresponding author on reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Natural Science Foundation of China (No. 62235014) and the Fundamental Research Funds for the Central Universities (No. 2023ZYGXZR002).References
- Z. Liu, Z. Li, T. Seto and Y. Wang, An ultra–broadband yellow–emitting phosphor KRbCa0.8Mg0.2PO4F:Eu2+ for white light–emitting diodes with high color rendering index, Adv. Opt. Mater., 2023, 2300845 CrossRef CAS
.
- N. K. Mishra, A. Kumar and K. Kumar, Development of ultra-broad band light emitting single phase garnet phosphor based on energy transfer mechanism and its applications in optical temperature sensing and WLED, J. Alloys Compd., 2023, 947, 169440 CrossRef CAS
.
- Y. H. Kim, P. Arunkumar, B. Y. Kim, S. Unithrattil, E. Kim, S. H. Moon, J. Y. Hyun, K. H. Kim, D. Lee, J. S. Lee and W. B. Im, A zero-thermal-quenching phosphor, Nat. Mater., 2017, 16, 543–550 CrossRef CAS PubMed
.
- J. Qiao, J. Zhao, Q. Liu and Z. Xia, Recent advances in solid-state LED phosphors with thermally stable luminescence, J. Rare Earths, 2019, 37, 565–572 CrossRef CAS
.
- J. Cho, J. H. Park, J. K. Kim and E. F. Schubert, White light-emitting diodes: History, progress, and future, Laser Photonics Rev., 2017, 11, 1600147 CrossRef
.
- H. Wang, Y. Liu, X. Zhu, L. Wei, X. Jiang, Y. Chen and L. Li, Preparation of CaAlSiN3:Eu2+ red-emitting phosphor by a two-step method for solid-state lighting applications, Ceram. Int., 2020, 46, 23035–23040 CrossRef CAS
.
- J. Wang, L. Dong, Z. Lyu, D. Sun, T. Tan and H. You, Facile synthesis of highly efficient and thermally stable BaAl4Sb2O12:Eu2+ phosphor in air, Adv. Funct. Mater., 2023, 33, 2214611 CrossRef CAS
.
- P. Pust, V. Weiler, C. Hecht, A. Tücks, A. S. Wochnik, A.-K. Henß, D. Wiechert, C. Scheu, P. J. Schmidt and W. Schnick, Narrow-band red-emitting Sr[LiAl3N4]:Eu2+ as a next-generation LED-phosphor material, Nat. Mater., 2014, 13, 891–896 CrossRef CAS PubMed
.
- J. Zhong, Y. Zhuo, S. Hariyani, W. Zhao, J. Wen and J. Brgoch, Closing the cyan gap toward full-spectrum LED lighting with NaMgBO3:Ce3+, Chem. Mater., 2019, 32, 882–888 CrossRef
.
- J. Liang, L. Sun, S. Wang, Q. Sun, B. Devakumar and X. Huang, Filling the cyan gap toward full-visible-spectrum LED lighting with Ca2LaHf2Al3O12:Ce3+ broadband green phosphor, J. Alloys Compd., 2020, 836, 155469 CrossRef CAS
.
- Y. Xu, S. Yin, X. Wu, C. Zhong, X. Zhang, L. Zhou and H. You, Improved thermal stability and quantum efficiency of novel cyan phosphors toward full-visible-spectrum lighting, Ceram. Int., 2024, 50, 1159–1165 CrossRef CAS
.
- Z. Jiang, T. Hu, F. Men, H. Yang, W. Lv, D. Wen, Q. Zeng and Y. Gao, An efficient and thermally stable red emitting Eu2+ doped oxide for rechargeable portable flashlight, Chem. Eng. J., 2024, 482, 149086 CrossRef CAS
.
- M. Zhao, Z. Yang, L. Ning and Z. Xia, Tailoring of white luminescence in a NaLi3SiO4:Eu2+ phosphor containing broad-band defect-induced charge-transfer emission, Adv. Mater., 2021, 33, 2101428 CrossRef CAS PubMed
.
- N. Baig, N. S. Dhoble, K. Park, N. S. Kokode and S. J. Dhoble, Enhanced luminescence and white light emission from Eu3+–co–doped K3Ca2(SO4)3Cl:Dy3+ phosphor with near visible ultraviolet excitation for white LEDs, Luminescence, 2014, 30, 479–484 CrossRef PubMed
.
- W. Zhou, G. Wang, X. Zheng, L. Yu, J. Zhang, Z. Qiu and S. Lian, Tunable
colors and applications of Dy3+/Eu3+ co–doped CaO–B2O3−SiO2 glasses, J. Am. Ceram. Soc., 2019, 102, 5890–5898 CrossRef CAS
.
- Y. Zhou, C. Zhu and X. Yang, Near white light emission of Ce3+–, Ho3+–, and Sm3+–doped oxyfluoride silicate glasses, J. Am. Ceram. Soc., 2017, 100, 2059–2068 CrossRef CAS
.
- T. Yang, T. Zhang, S. Huang, T. D. Christopher, Q. Gu, Y. Sui and P. Cao, Structure tailoring and defect engineering of LED phosphors with enhanced thermal stability and superior quantum efficiency, Chem. Eng. J., 2022, 435, 133873 CrossRef CAS
.
- S. Das, U. K. Ghorai, R. Dey, C. K. Ghosh and M. Pal, White light phosphorescence from ZnO nanoparticles for white LED applications, New J. Chem., 2022, 46, 17585–17595 RSC
.
- M. Gallart, T. Cottineau, B. Hönerlage, V. Keller, N. Keller and P. Gilliot, Temperature dependent photoluminescence of anatase and rutile TiO2 single crystals: Polaron and self-trapped exciton formation, J. Appl. Phys., 2018, 124, 133104 CrossRef
.
-
S. Shionoya, Luminescence of Solids, Plenum Press, New York, 1998 Search PubMed
.
- M. A. Reshchikov, Temperature dependence of defect-related photoluminescence in III-V and II-VI semiconductors, J. Appl. Phys., 2014, 115, 012010 CrossRef
.
- S. Ding, H. Wang, J. Luo, W. Liu, Y. Ma and Q. Zhang, 1 micrometer high-efficient radiation resistant laser crystal: Nd:YSAG, J. Lumin., 2019, 214, 116596 CrossRef CAS
.
- H. Li, J. Jiao, X. Xiang, J. Wu, W. Hu, J. Xie, S. Huang, H. Zhang and J. Zhu, Directly identifying multiple Cr3+ emitting centers for broad near–infrared emission in an efficient and near–zero thermal quenching garnet–type phosphor, Adv. Opt. Mater., 2024, 12, 2302391 CrossRef CAS
.
- V. A. Tarala, V. E. Suprunchuk, A. A. Kravtsov, F. F. Malyavin, D. S. Vakalov, L. V. Tarala, V. A. Lapin, O. M. Chapura, E. V. Medyanik, D. T. Dziov and S. V. Kuznetsov, Fabrication and optical properties of YSAG:Cr optical ceramics, Ceram. Int., 2023, 49, 32127–32135 CrossRef CAS
.
- Y. Xiao, P. Xiong, S. Zhang, K. Chen, S. Tian, Y. Sun, P. Shao, K. Qin, M. G. Brik, S. Ye, D. Chen and Z. Yang, Deep-red to NIR mechanoluminescence in centrosymmetric perovskite MgGeO3: Mn2+ for potential dynamic signature anti-counterfeiting, Chem. Eng. J., 2023, 453, 139671 CrossRef CAS
.
- Q. Zong, D. Zhao, R. Zhang, L. Jia and S. Zhu, Defect engineering towards anti-temperature quenching luminescence for application in light emitting diodes, Ceram. Int., 2023, 49, 24794–24801 CrossRef CAS
.
- M. A. Reshchikov, Mechanisms of thermal quenching of defect–related luminescence in semiconductors, Phys. Status Solidi A, 2020, 218, 2000101 CrossRef
.
- J. Dolado, J. García-Fernández, P. Hidalgo, J. González-Calbet, J. Ramírez-Castellanos and B. Méndez, Intense cold-white emission due to native defects in Zn2GeO4 nanocrystals, J. Alloys Compd., 2022, 898, 162993 CrossRef CAS
.
- H. He, Y. Wang, J. Wang and Z. Ye, Extraction of the surface trap level from photoluminescence: a case study of ZnO nanostructures, Phys. Chem. Chem. Phys., 2011, 13, 14902 RSC
.
- Y. Wei, Z. Gao, S. Liu, S. Chen, G. Xing, W. Wang, P. Dang, A. A. Al Kheraif, G. Li and J. Lin, Highly efficient green-to–yellowish–orange emitting Eu2+–doped pyrophosphate phosphors with superior thermal quenching resistance for w–LEDs, Adv. Opt. Mater., 2020, 8, 1901859 CrossRef CAS
.
- A. A. Prokhorov, L. F. Chernush, R. Minikayev, J. Lančok and A. D. Prokhorov, EPR study of chromium ions doped gallium borate, Acta Phys. Pol., A, 2019, 136, 947–951 CrossRef CAS
.
- M. Łapiński, L. Piekara-Sady, R. Kozioł, W. Sadowski and B. Kościelska, Two kinds of oxygen vacancies in lithium titaniate doped with copper as detected by EPR, Solid State Sci., 2020, 106, 106337 CrossRef
.
- N. Zhang, B. Tian, Z. Wang, A. T. Smith, Z. Ma, Z. Xue and L. Sun, Intense mechanoluminescence in undoped LiGa5O8 with persistent and recoverable behaviors, Adv. Opt. Mater., 2021, 9, 2100137 CrossRef CAS
.
- Y. Ma, L. Zhang, T. Zhou, B. Sun, Q. Yao, P. Gao, J. Huang, J. Kang, F. A. Selim, C. Wong, H. Chen and Y. Wang, Weak thermal quenching and tunable luminescence in Ce:Y3(Al,Sc)5O12 transparent ceramics for high power white LEDs/LDs, Chem. Eng. J., 2020, 398, 125486 CrossRef CAS
.
- X. Liu, H. Zhou, Z. Hu, X. Chen, Y. Shi, J. Zou and J. Li, Transparent Ce:GdYAG ceramic color converters for high-brightness white LEDs and LDs, Opt. Mater., 2019, 88, 97–102 CrossRef CAS
.
- B. Ma, Q. Guo, M. S. Molokeev, Z. Lv, J. Yao, L. Mei and Z. Huang, Crystal structure and luminescence properties of green-emitting Sr1−xAl12O19:xEu2+ phosphors, Ceram. Int., 2016, 42, 5995–5999 CrossRef CAS
.
- G. Kresse and J. Furthmüller, Efficiency of ab initio total energy calculations for metals and semiconductors using a plane-wave basis set, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS
.
- G. Kresse and J. Furthmüller, Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi01824a |
| ‡ Co-first author. |
| This journal is © the Partner Organisations 2024 |