Enabling efficient near-infrared emission in lead-free double perovskite via a codoping strategy†
Xiangyan
Yun
ab,
Hanlin
Hu
 c,
Haizhe
Zhong
b,
Jingheng
Nie
*d,
Henan
Li
e and
Yumeng
Shi
c,
Haizhe
Zhong
b,
Jingheng
Nie
*d,
Henan
Li
e and
Yumeng
Shi
 *ae
*ae
aKey Laboratory of Luminescence and Optical Information Ministry of Education Institute of Optoelectronic Technology, Beijing Jiaotong University, Beijing 100044, P. R. China. E-mail: ymshi@bjtu.edu.cn
bInternational Collaborative Laboratory of 2D Materials for Optoelectronics Science and Technology of Ministry of Education, Institute of Microscale Optoelectronics, Shenzhen University, Shenzhen 518060, P. R. China
cHoffman Institute of Advanced Materials, Shenzhen Polytechnic, Shenzhen 518060, P. R. China
dGuangdong Rare Earth Photofunctional Materials Engineering Technology Research Center, School of Chemistry and Environment, Jiaying University, Meizhou, 514015, P. R. China. E-mail: niejh@jyu.edu.cn
eSchool of Electronics and Information Engineering, Shenzhen University, Shenzhen 518060, P. R. China
First published on 9th August 2024
Abstract
Metal halides show great promise as a new generation of near-infrared (NIR) light-emitting materials. Compared with other light-emitting materials, double perovskites possess structures with different dimensionalities, which can support multiple emission centers, leading to varied photoluminescence. Among various doping centers, ytterbium(III) (Yb3+) has attracted attention because of its unique two-energy-level structure (2F5/2 and 2F7/2). However, the NIR emission of Yb3+ remains unsatisfactory because of poor resonance energy transfer between Yb3+ and sensitizers. Here, effective NIR-emitting lead-free perovskites are developed by co-doping antimony(III) (Sb3+) and lanthanide(III) ions into Cs2NaInCl6. Under excitation at 318 nm, Cs2NaInCl6:Sb3+/Yb3+ showed a broadband NIR emission peak at 1001 nm, whereas Cs2NaInCl6:Sb3+/Nd3+ exhibited three NIR emission peaks at 896, 1077, and 1358 nm. The exciton dynamics of the materials were investigated. Experiments and density functional theory calculations revealed that the NIR emission of Yb3+ originated from a charge-transfer state (CTS) and energy transfer, whereas that of Nd3+ arose from resonance energy transfer. Profiting from the high-energy self-trapped exciton (STE) emission and CTS with Yb3+, a high photoluminescence quantum yield of 48.95% was realized. The excellent NIR luminescence performance combined with high environmental stability demonstrates the potential of these metal halides for use in night-vision technologies.
1. Introduction
All-inorganic metal halide perovskites are promising materials for light-emitting technologies because of their excellent photophysical properties such as high photoluminescence quantum yield (PLQY), tunable light emission wavelength, and cost-effective synthesis.1–4 Presently, the development and preparation techniques for visible light-emitting perovskites are relatively mature, adequately meeting demands in areas such as illumination and displays.5–7 However, the evolving needs of medical imaging, optical communications, optical storage, military reconnaissance, security monitoring, and sensing generate demand for lead-free near-infrared (NIR)-emitting metal halide perovskites.8–11 This demand arises from the advantageous properties of NIR light sources, such as strong penetration, minimal biological damage, and high resolution.12–14 NIR light-emitting diodes (LEDs) that can be used for full-spectrum (400–1100 nm) illumination, infrared security anti-counterfeiting, and infrared imaging have emerged as a burgeoning area of research.15 The most commonly used method for achieving NIR luminescence from perovskites involves doping with lanthanide or transition metal ions.16–18 However, the traditional ABX3 perovskite structure cannot meet the requirements of trivalent-ion dopants.19 Consequently, a strategy of heterovalent double-cation co-substitution (e.g., B+ + B′3+ → Pb2+ + Pb2+) has been adopted to fabricate stable inorganic double-perovskite systems (A2BB′X6).20–22 The challenges of low PLQY and restricted emission range have emerged as technological bottlenecks in the development of NIR-emitting perovskites.23 Thus, the pursuit of an effective method to achieve high-efficiency, long-wavelength NIR emission holds paramount importance for expanding the applications of NIR technology.Currently, lanthanide ions (Ln3+; e.g., Er3+, Ho3+, Tm3+, Sm3+, Dy3+, Yb3+, Nd3+) and transition metal ions (e.g., Mn4+, Cr3+, Cr4+) are popular activators for NIR luminescence.24–26 However, achieving high PLQYs in halide matrices with these activator ions through single ion-doping strategies is rarely possible. Energy transfer (ET) is an effective approach to improve NIR PLQY and extend the emission wavelength range of metal halides.27,28 For example, NIR luminescence was achieved by co-doping Te4+ and Nd3+ into a Cs2ZrCl6 host; ET from yellow-emitting Te4+ to NIR-emitting Nd3+ was clearly observed.29 The simple energy-level structure (2F5/2 and 2F7/2) of Yb3+, which helps to suppress non-radiative energy losses, has led to it becoming a popular activator ion in the field of NIR luminescence.30–32 However, the NIR PLQYs and ET efficiency of Yb3+-doped materials remain low because of the reliance of the traditional resonant ET mechanism on the degree of overlap between the emission band of the photoreceptor and absorption band of the activator. For example, while Bi3+/Yb3+-co-doped Cs2Na0.6Ag0.4InCl6 double perovskites showed enhanced NIR emission because of ET from self-trapped excitons (STEs) to Yb3+, the ET efficiency was not ideal.33,34 Therefore, suitable transfer strategies need to be identified to facilitate ET and obtain high-efficiency NIR emission.
Herein, high-efficiency NIR emission is achieved by co-doping Sb3+ and Ln3+ in Cs2NaInCl6. The Jahn–Teller deformation of the [SbCl6]3− octahedron in the STE process leads to efficient formation of ET channels, enabling efficient NIR luminescence from Ln3+ in Cs2NaInCl6:Sb3+. With the help of a charge-transfer state (CTS) of Yb3+, efficient NIR emission at 1001 nm with a high PLQY of 48.95% is obtained from Cs2NaInCl6:Sb3+/Yb3+ under 318 nm excitation. In addition, three NIR emission peaks at 896, 1077, and 1358 nm with a high PLQY of 11.29% are exhibited by Cs2NaInCl6:Sb3+/Nd3+ under 318 nm excitation. Experimental and density functional theory (DFT) calculation results reveal that the NIR emission of Yb3+ could be derived from a CTS and ET, whereas that of Nd3+ could originate from resonance ET.
2. Results and discussion
Cs2NaInCl6 crystallizes into a cubic structure (space group: Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m) consisting of alternating [InCl6]3− and [NaCl6]5− octahedra with large Cs+ occupying the center of the cuboctahedral cavities (Fig. 1a).35 High-quality Cs2NaInCl6:Sb3+/Ln3+ (Ln3+ = Nd3+ or Yb3+) single crystals were prepared via a facile hydrothermal synthesis method (Fig. S1†). Sb3+ and Ln3+ tend to occupy the In3+ sites because of their similar ionic radii and valence.36 Powder X-ray diffraction (PXRD) measurements showed that all diffraction peaks matched well with the standard pattern of Cs2NaInCl6 (ICSD#132718). With increasing Nd3+ or Yb3+ content, the diffraction peaks shifted to lower angles because the ionic radii of Nd3+ (98 pm) and Yb3+ (87 pm) are larger than that of In3+ (80 pm), as shown in Fig. S2 and S3.†37 Scanning electron microscopy (SEM) images showed that the samples were polyhedral with mean sizes of about 5 μm. Energy-dispersive X-ray spectroscopy (EDS) elemental mapping revealed that the dopants were homogeneously distributed in the samples (Fig. 1c and S4–S6†). These results suggest the successful incorporation of Sb3+ and Ln3+ in the double perovskite crystal lattice. The dopant concentrations were estimated by inductively coupled plasma mass spectrometry (ICP-MS); the results are listed in Tables S1 and S2.† The ICP-MS results are similar to the EDS element content results, and the accuracy of the measurement is verified again (Table S3†). The actual content of Ln3+ in the samples is shown in Fig. 1d. There was a considerable difference between the actual doping concentration of the Cs2NaInCl6:Sb3+/xLn3+ samples and the feed ratio of Yb3+ and Nd3+. Similar trends have been reported for doped halides such as Cs2KInCl6:Sb3+/Ho3+, Cs2AgInCl6:Cr3+/Er3+, and Cs2ZrCl6:Sb3+/Nd3+.38–40 The doping ratio stated in this study refers to the feed ratio of Ln3+ rather than the actual doping concentration.
m) consisting of alternating [InCl6]3− and [NaCl6]5− octahedra with large Cs+ occupying the center of the cuboctahedral cavities (Fig. 1a).35 High-quality Cs2NaInCl6:Sb3+/Ln3+ (Ln3+ = Nd3+ or Yb3+) single crystals were prepared via a facile hydrothermal synthesis method (Fig. S1†). Sb3+ and Ln3+ tend to occupy the In3+ sites because of their similar ionic radii and valence.36 Powder X-ray diffraction (PXRD) measurements showed that all diffraction peaks matched well with the standard pattern of Cs2NaInCl6 (ICSD#132718). With increasing Nd3+ or Yb3+ content, the diffraction peaks shifted to lower angles because the ionic radii of Nd3+ (98 pm) and Yb3+ (87 pm) are larger than that of In3+ (80 pm), as shown in Fig. S2 and S3.†37 Scanning electron microscopy (SEM) images showed that the samples were polyhedral with mean sizes of about 5 μm. Energy-dispersive X-ray spectroscopy (EDS) elemental mapping revealed that the dopants were homogeneously distributed in the samples (Fig. 1c and S4–S6†). These results suggest the successful incorporation of Sb3+ and Ln3+ in the double perovskite crystal lattice. The dopant concentrations were estimated by inductively coupled plasma mass spectrometry (ICP-MS); the results are listed in Tables S1 and S2.† The ICP-MS results are similar to the EDS element content results, and the accuracy of the measurement is verified again (Table S3†). The actual content of Ln3+ in the samples is shown in Fig. 1d. There was a considerable difference between the actual doping concentration of the Cs2NaInCl6:Sb3+/xLn3+ samples and the feed ratio of Yb3+ and Nd3+. Similar trends have been reported for doped halides such as Cs2KInCl6:Sb3+/Ho3+, Cs2AgInCl6:Cr3+/Er3+, and Cs2ZrCl6:Sb3+/Nd3+.38–40 The doping ratio stated in this study refers to the feed ratio of Ln3+ rather than the actual doping concentration.
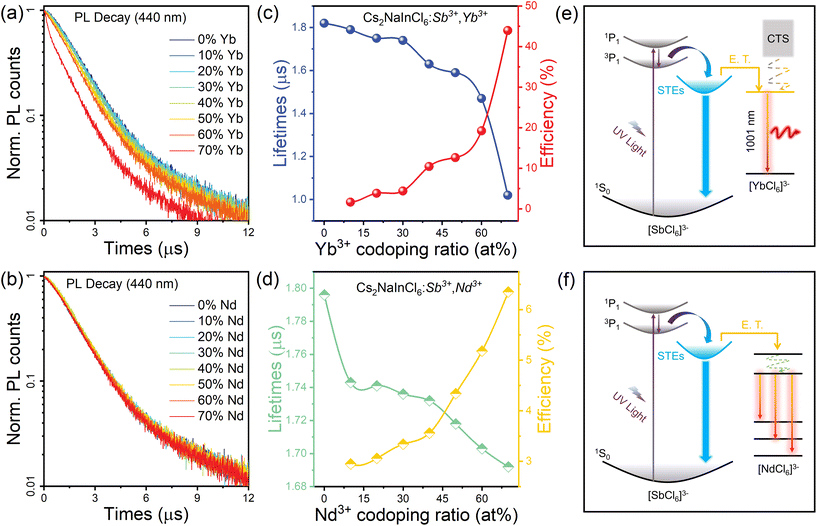
To explore the photophysical process of Cs2NaInCl6:Sb3+/Ln3+ in detail, photoluminescence spectra in the visible and NIR regions were recorded. The PLQY of the Cs2NaInCl6 matrix at RT was less than 1%. The Sb3+-doped sample showed a high-energy blue emission peak at 446 nm with a full width at half maximum (FWHM) of 128 nm (Fig. S7†), which was attributed to the STE emission in the [SbCl6]3− octahedra caused by strong Jahn–Teller lattice distortion.41 Under 318 nm excitation, in addition to the blue emission, an intense NIR emission at 1001 nm was observed in Sb3+/Yb3+-co-doped Cs2NaInCl6, which was assigned to the 2F5/2 → 2F7/2 transitions of Yb3+ (Fig. 2a and b).31 As shown in Fig. 2c, the STE emission intensity decreased and the NIR emission intensity greatly increased as the Yb3+ doping content increased to 60%. Higher Yb3+ doping concentration than 60% led to a decrease in NIR efficiency because of concentration quenching. The Sb3+/Nd3+-co-doped Cs2NaInCl6 samples displayed a blue emission peak at 446 nm and three sharp NIR emission bands at 896, 1077, and 1358 nm, which were assigned to the 4F3/2 → 4I9/2, 4F3/2 → 4I11/2, and 4F3/2 → 4I13/2 transitions of Nd3+, respectively (Fig. 2d and e).42 The NIR emission signals exhibited millisecond-scale lifetimes of 3.29 ms for that originating from Yb3+ and 4.08 ms for that derived from Nd3+ (Fig. S8 and S9†), which were characteristic of Laporte forbidden f–f transitions.32,40 The NIR emission intensity greatly increased and STE emission intensity decreased with increasing Nd3+ content, as depicted in Fig. 2e. Both Sb3+/Yb3+ and Sb3+/Nd3+ co-doped samples showed excellent NIR luminescence properties. The highest NIR emission intensity of Sb3+/Yb3+-co-doped samples with a PLQY of 48.95% was obtained when the Yb3+ feed ratio was 60% (Fig. S10†). It has shown good performance in the recently reported NIR PLQY of metal halides (Table S4†). The highest NIR PLQY of Sb3+/Nd3+-co-doped Cs2NaInCl6 of 11.29% was obtained when the Nd3+ feed ratio was 70% (Fig. S11†). The sensitization induced by Sb3+ caused the NIR emission intensity of Sb3+/Yb3+ and Sb3+/Nd3+ co-doped samples to increase considerably compared to that of Yb3+ or Nd3+ singly doped samples (Fig. S12 and S13†).
To reveal the luminescence mechanism of the samples, absorption and photoluminescence excitation (PLE) spectra of the co-doped samples were collected. As shown in Fig. 3a, Yb3+ singly doped Cs2NaInCl6 exhibited an additional absorption peak at about 270 nm compared to the absorption spectra of undoped and Nd3+ singly doped Cs2NaInCl6, matching closely with the PLE spectra (Fig. 3b). The excitation band at 266 nm corresponded to the Yb3+ absorption of the Cl− → Yb3+ CTS.29,43,44 Sb3+/Yb3+ and Sb3+/Nd3+ co-doped Cs2NaInCl6 samples exhibited an intense absorption peak at 320 nm, which was attributed to Sb3+. As the feed ratio of Ln3+ rose, the actual doping concentration of Sb3+ increased (Tables S1 and S2†), and thus the corresponding absorption peak of Sb3+ increased in intensity at high Ln3+ doping concentration, as shown in Fig. S14 and S15.† The PLE spectrum monitored at 446 nm displayed characteristic excitation bands at 318 and 333 nm, which were assigned to the 1S0–3P1 transition of Sb3+ (PLE spectra are shown in Fig. S16 and S17†). The PLE spectrum of Sb3+/Nd3+ co-doped Cs2NaInCl6 monitored at 1077 nm contained two weak narrow excitation peaks at 534 and 588 nm, consistent with that of Nd3+ singly doped Cs2NaInCl6, which were attributed to the direct excitation of Nd3+.42 The PLE spectra of visible (446 nm) and NIR (1077 nm) emissions were similar (Fig. 3c), indicating that these emissions have the same excited-state origin. Notably, the emission of Ln3+ originating from the f–f transition is usually sensitive to the excitation wavelength, while the emission spectra were almost constant with the variation of the excitation wavelength (Fig. 3e), suggesting that the NIR luminescence of Nd3+ mainly depends on the sensitization of Sb3+. In addition, the excitation intensities at 534 and 588 nm were much lower than that at 318 nm, indicating that Nd3+ excitation through ET was more efficient than direct excitation. Because of the wide excitation range and high absorption strength of STE emission in the Sb3+/Nd3+ co-doped Cs2NaInCl6 system, the weak absorption of the f–f transition can be overcome by the efficient ET, thus realizing efficient NIR luminescence. Notably, however, the NIR efficiency is still relatively low, which may be related to the small overlap between the emission region of Sb3+ and absorption region of Nd3+ limiting the ET efficiency.
Interestingly, the PLE spectra of the Sb3+/Yb3+-co-doped sample for the emissions at 446 and 1001 nm showed different patterns. Sb3+/Yb3+-co-doped Cs2NaInCl6 showed a broadband excitation above 300 nm, which was consistent with the excitation of Sb3+-doped Cs2NaInCl6. This result implies that STE and NIR emissions could originate from the same excited state, and an ET process occurs from Sb3+ to Yb3+.45 The PLE spectrum of Sb3+/Yb3+-co-doped Cs2NaInCl6 displayed an additional excitation peak at 273 nm, which may originate from the Cl− → Yb3+ CTS. As shown in Fig. 3d, the photoluminescence and PLE pseudo-color maps further confirmed that the NIR emission can be realized by ET and CTS. The characteristic excitation peaks of Yb3+ are different to those of other Ln3+, as shown in Fig. S18.† Yb3+ has only two energy levels, 2F5/2 and 2F7/2, whereas other Ln3+ (e.g., Er3+, Ho3+, Nd3+) typically possess abundant 4f electronic states (Fig. S19†). Because Yb3+ has no energy levels that match the 3P1 state of Sb3+, Förster resonance ET from Sb3+ to Yb3+ was difficult. The spectral characteristics of the Sb3+/Yb3+-co-doped Cs2NaInCl6 system exhibit certain similarities to those reported for Cs2ZrCl6:Te4+/Yb3+ halides.29
To further determine the ET process in the co-doped samples, time-resolved photoluminescence measurements of STE emissions were recorded. As shown in Fig. 4a and b, the excitation lifetime monitored at 446 nm shortened as the Ln3+ content increased (Tables S5 and S6†), implying that the improved ET from the STEs to Ln3+ promoted decay of the STE emission.46 The ET efficiency (ηT) from STE to Ln3+ can be calculated using the following equation:
 | (1) |
To further unveil the photophysics of the Sb3+–Ln3+ pair, DFT calculations were performed to reveal the electronic structure of Cs2NaInCl6:Sb3+/Ln3+. In the pure Cs2NaInCl6 system, the conduction-band minimum (CBM) is mainly composed of Cl p states and In s states and the valence-band maximum (VBM) mainly consists of Cl p orbitals, as shown in Fig. S20.† For the Sb3+/Yb3+- and Sb3+/Nd3+-co-doped systems, a new band consisting of the Cl p states and Sb lone-pair s states appears above the host valence band, while the SbCl6 CBM appears in a gap deep in the host conduction band, as illustrated in Fig. 5. The highest occupied molecular orbitals are composed of Sb s and Cl p states and the lowest unoccupied molecular orbitals are composed of Sb p and Cl p states. Meanwhile, the 4f bands of Yb3+ or Nd3+ are located in the gap between the CBM and VBM of SbCl6, which provides the possibility to realize ET.51–53 These calculation results demonstrate that the introduced Sb3+ can modulate the host electronic structure and the introduced Ln3+ are located in the gap between the CBM and VBM of SbCl6, confirming the possibility of ET.
To illustrate a potential application of Cs2NaInCl6:Sb3+/Ln3+, NIR LEDs were fabricated. The prepared Cs2NaInCl6:Sb3+/Yb3+ was coated on a commercially available 365 nm UV-LED chip. The as-fabricated LEDs exhibited strong luminescence, as shown in Fig. 6a. The NIR emission intensity increased with the drive current, showing excellent NIR emission performance (Fig. 6b). Fig. 6c shows NIR applications of Cs2NaInCl6:Sb3+/Yb3+ using different cameras (visible and NIR) and light sources (natural and NIR). To demonstrate NIR anti-counterfeiting, a picture with the acronyms “SZU”, “DLB”, “NIR”, and “LED” in different colors exhibited distinct patterns when illuminated by natural and NIR light. The principle behind this behavior is that the black “SZU” letters contained carbon black, resulting in strong absorption of NIR light. For infrared detection applications, the area of a picture covered by the long-wavelength filter was not detected by the visible camera under natural light. No image was captured by the NIR camera when the NIR LEDs were switched off. In contrast, when the NIR LEDs were switched on, a clear and complete image was detected by the NIR camera. Similar detection applications can also be found in biology, where yellow-green leaf veins can be clearly captured using the NIR LEDs. To demonstrate night-vision applications, black-and-white photographs of fruit were captured upon illumination with the NIR LEDs. The image clarity was relatively high, with the images clearly displaying the contours of the photographed objects. These results reveal the great potential of the prepared Cs2NaInCl6:Sb3+/Yb3+ samples for use in NIR anti-counterfeiting, infrared detection, and night-vision technologies. The environmental stability of the Cs2NaInCl6:Sb3+/Yb3+ and Cs2NaInCl6:Sb3+/Nd3+ samples was evaluated. As shown in Fig. S21,† the PXRD peaks of the Cs2NaInCl6:Sb3+/Yb3+ and Cs2NaInCl6:Sb3+/Nd3+ samples remained unchanged after continuous illumination under 365 nm UV light. As shown in Fig. S22,† the PL performance of Cs2NaInCl6:Sb3+/Yb3+ and Cs2NaInCl6:Sb3+/Nd3+ samples were almost unchanged even upon heating at 420 K for 6.5 h. These experimental results indicate that Cs2NaInCl6:Sb3+/Ln3+ perovskites are promising for NIR LED applications in advanced night-vision detection and anti-counterfeiting.
3. Conclusions
In summary, Cs2NaInCl6:Sb3+/Yb3+ and Cs2NaInCl6:Sb3+/Nd3+ single crystals that displayed efficient NIR emission were synthesized. Under excitation at 318 nm, Cs2NaInCl6:Sb3+/Yb3+ showed a broadband NIR emission peak at 1001 nm with a high PLQY of 48.95%, whereas Cs2NaInCl6:Sb3+/Nd3+ exhibited three NIR emission peaks at 896, 1077, and 1358 nm with a PLQY of 11.29%. The results of experiments and DFT calculations revealed that the NIR emission of Yb3+ was derived from a CTS and ET, whereas the NIR emission of Nd3+ originated from resonance ET. Profiting from the high-energy STE emission and CTS of Yb3+, the high NIR PLQY was realized by efficient ET from STEs to Yb3+. The excellent NIR luminescence performance and high environmental stability of Cs2NaInCl6:Sb3+/Yb3+ endow it with good application prospects in NIR anti-counterfeiting and night-vision devices.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the National Natural Science Foundation of China (Grant No. 61874074), Science and Technology Project of Shenzhen (Grant No. JCYJ20220531100815034), and Guangdong Basic and Applied Basic Research Foundation (General Program, Grant No. 2022A1515012055).References
- Z. Yuan, C. K. Zhou, Y. Tian, Y. Shu, J. Messier, J. C. Wang, L. J. Burgt, K. Kountouriotis, Y. Xin, E. Holt, K. Schanze, R. Clark, T. Siegrist and B. W. Ma, One-dimensional organic lead halide perovskites with efficient bluish white-light emission, Nat. Commun., 2017, 8, 14051 CrossRef PubMed
.
- H. Yin, Q. K. Kong, R. L. Zhang, D. Y. Zheng, B. Yang and K. L. Han, Lead-free rare-earth double perovskite Cs2AgIn1−y−xBixLayCl6 nanocrystals with highly efficient warm-white emission, Sci. China Mater., 2021, 64, 2667–2674 CrossRef CAS
.
- Y. Liu, F. D. Stasio, C. H. Bi, J. B. Zhang, Z. G. Xia, Z. F. Shi and L. Manna, Near-Infrared Light Emitting Metal Halides: Materials, Mechanisms, and Applications, Adv. Mater., 2024, 2312482 CrossRef CAS
.
- R. X. Wu, P. G. Han, D. Y. Zheng, J. F. Zhang, S. Q. Yang, Y. Zhao, X. Y. Miao and K. L. Han, All-Inorganic Rare-Earth-Based Double Perovskite Nanocrystals with Near-Infrared Emission, Laser Photonics Rev., 2021, 15, 2100218 CrossRef CAS
.
- G. D. Zhang, P. P. Dang, H. Z. Lian, K. Li, L. Tian, W. Yang, Z. Y. Cheng and J. Lin, Assembling Two Self-Trapped Exciton Emissions in 0D Metal Halides with Near-Unity Quantum Yield and Zero Thermal-Quenching Photoluminescence, Laser Photonics Rev., 2023, 18, 2300599 CrossRef
.
- Y. Y. Jing, Y. Liu, J. Zhao and Z. G. Xia, Sb3+ Doping-Induced Triplet Self-Trapped Excitons Emission in Lead-Free Cs2SnCl6 Nanocrystals, J. Phys. Chem. Lett., 2019, 10, 7439–7444 CrossRef CAS PubMed
.
- P. G. Han, C. Luo, S. Q. Yang, Y. Yang, W. Q. Deng and K. L. Han, All-Inorganic Lead-Free 0D Perovskites by a Doping Strategy to Achieve a PLQY Boost from <2% to 90%, Angew. Chem., Int. Ed., 2020, 59, 12709–12713 CrossRef PubMed
.
- X. Chen, Y. X. Pan, Y. H. Ding, H. Z. Lian, J. Lin and L. Y. Li, Enhanced Efficiency, Broadened Excitation, and Tailored Er3+ Luminescence Triggered by Te4+ Codoping in Cs2NaYbCl6 Crystals for Multifunctional Applications, Inorg. Chem., 2024, 63, 3525–3534 CrossRef
.
- Z. X. Zeng, G. D. Zhang, Y. S. Wang, M. Zhang, Z. Y. Cheng, H. Z. Lian and J. Lin, Mn2+/Er3+-Codoped Cs2AgInCl6 Double Perovskites with Dual Emission and Photochromism Properties for Anti-Counterfeiting Application, Laser Photonics Rev., 2024, 2300983 CrossRef
.
- R. R. Sun, M. C. Jia, X. Chen, F. Zhang, Z. Z. Ma, Y. Liu, J. B. Zhang, L. Y. Lian, Y. B. Han, M. Y. Li, D. W. Yang, X. J. Li, Y. Zhang, C. X. Shan and Z. F. Shi, Constructing Efficient and Thermostable Red-NIR Emitter via Cross Relaxation and Crystal-Field Engineering of Holmium-Based Perovskite-Type Half Metal, Laser Photonics Rev., 2023, 2301028 Search PubMed
.
- F. Y. Zhao, Z. Song, J. Zhao and Q. L. Liu, Double perovskite Cs2AgInCl6:Cr3+: broadband and near-infrared luminescent materials, Inorg. Chem. Front., 2019, 6, 3621–3628 RSC
.
- S. L. Jin, H. Yuan, T. Pang, M. J. Zhang, Y. W. He, B. Zhuang, T. M. Wu, Y. H. Zheng and D. Q. Chen, Boosting STE and Nd3+ NIR Luminescence in Cs2AgInCl6 Double Perovskite via Na+/Bi3+-Induced Local Structure Engineering, Adv. Funct. Mater., 2023, 33, 2304577 CrossRef CAS
.
- K. Li and R. V. Deun, Novel Intense Emission-Tunable Li1.5La1.5WO6:Mn4+,Nd3+,Yb3+ Material with Good Luminescence Thermal Stability for Potential Applications in c-Si Solar Cells
and Plant-Cultivation Far-Red-NIR LEDs, ACS Sustainable Chem. Eng., 2019, 7, 16284–16294 CrossRef CAS
.
- K. Li and R. V. Deun, Effectively realizing broadband spectral conversion of UV/visible to near-infrared emission in (Na,K)Mg(La,Gd)TeO6:Mn4+,Nd3+,Yb3+ materials for c-Si solar cells via efficient energy transfer, J. Mater. Chem. C, 2018, 6, 7302–7310 RSC
.
- B. M. Liu, X. X. Guo, L. Y. Cao, L. Huang, R. Zou, Z. Zhou and J. Wang, A High-efficiency blue-LED-excitable NIR-II-emitting MgO:Cr3+,Ni2+ phosphor for future broadband light source toward multifunctional NIR spectroscopy applications, Chem. Eng. J., 2023, 452, 139313 CrossRef CAS
.
- H. Arfin, A. S. Kshirsagar, J. Kaur, B. Mondal, Z. G. Xia, S. Chakraborty and A. Nag, ns2 Electron (Bi3+ and Sb3+) Doping in Lead-Free Metal Halide Perovskite Derivatives, Chem. Mater., 2020, 32, 10255–10267 CrossRef CAS
.
- Z. C. Zeng, B. L. Huang, X. Wang, L. Lu, Q. Y. Lu, M. Z. Sun, T. Wu, T. F. Ma, J. Xu, Y. S. Xu, S. A. Wang, Y. P. Du and C. H. Yan, Multimodal Luminescent Yb3+/Er3+/Bi3+-Doped Perovskite Single Crystals for X-ray Detection and Anti-Counterfeiting, Adv. Mater., 2020, 32, 2004506 CrossRef CAS PubMed
.
- H. Arfin, J. Kaur, T. Sheikh, S. Chakraborty and A. Nag, Bi3+-Er3+ and Bi3+-Yb3+ Codoped Cs2AgInCl6 Double Perovskite Near-Infrared Emitters, Angew. Chem., Int. Ed., 2020, 59, 11307–11311 CrossRef CAS
.
- Z. J. Shi, J. Guo, Y. H. Chen, Q. Li, Y. F. Pan, H. J. Zhang, Y. D. Xia and W. Huang, Lead-Free Organic–Inorganic Hybrid Perovskites for Photovoltaic Applications: Recent Advances and Perspectives, Adv. Mater., 2017, 29, 1605005 CrossRef
.
- M. B. Gray, S. Hariyani, T. A. Strom, J. D. Majher, J. Brgoch and P. M. Woodward, High-efficiency blue photoluminescence in the Cs2NaInCl6:Sb3+ double perovskite phosphor, J. Mater. Chem. C, 2020, 8, 6797–6803 RSC
.
- A. Noculak, V. Morad, K. M. McCall, S. Yakunin, Y. Shynkarenko, M. Wörle and M. V. Kovalenko, Bright Blue and Green Luminescence of Sb(III) in Double Perovskite Cs2MInCl6 (M = Na, K) Matrices, Chem. Mater., 2020, 32, 5118–5124 CrossRef PubMed
.
- L. Y. Cao, X. F. Jia, W. J. Gan, C. G. Ma, J. Y. Zhang, B. B. Lou and J. Wang, Strong Self-Trapped Exciton Emission and Highly Efficient Near-Infrared luminescence in Sb3+-Yb3+ Co-doped Cs2AgInCl6 Double Perovskite, Adv. Funct. Mater., 2023, 33, 2212135 CrossRef
.
- G. H. Wei, P. L. Li, R. Li, Y. Wang, S. X. He, J. L. Li, Y. W. Shi, H. Suo, Y. B. Yang and Z. J. Wang, How to Achieve Excellent Luminescence Properties of Cr Ion-Doped Near-Infrared Phosphors, Adv. Opt. Mater., 2023, 11, 2301794 CrossRef
.
- F. Q. He, E. H. Song, C. Zhang, H. Chang, G. P. Dong, Z. G. Xia, C. W. Wang and Q. Y. Zhang, Cr3+↔Fe3+ Energy Transfer Offset Enabling Anti-Thermal Quenching Near-Infrared Emission for Coded Wireless-Communication Applications, Laser Photonics Rev., 2023, 18, 2300668 CrossRef
.
- J. M. Xiang, X. Zhou, X. Q. Zhao, Z. Y. Wu, C. H. Chen, X. J. Zhou and C. F. Guo, Ab Initio Site-Selective Occupancy and Luminescence Enhancement in Broadband NIR Emitting Phosphor Mg7Ga2GeO12:Cr3+, Laser Photonics Rev., 2023, 17, 2200965 CrossRef
.
- Y. F. Pei, D. T. Tu, C. L. Li, S. Y. Han, Z. Xie, F. Wen, L. P. Wang and X. Y. Chen, Boosting Near-Infrared Luminescence of Lanthanide in Cs2AgBiCl6 Double Perovskites via Breakdown of the Local Site Symmetry, Angew. Chem., Int. Ed., 2022, 61, e202205276 CrossRef PubMed
.
- D. W. Chen, X. G. Zhang, J. W. Wei, L. Y. Zhou, P. C. Chen, Q. Pang and J. Z. Zhang, Simultaneous enhancement of near infrared luminescence and stability of Cs2AgInCl6:Cr3+ double perovskite single crystals enabled by a Yb3+ dopant, Inorg. Chem. Front., 2022, 9, 4695–4704 RSC
.
- S. Saikia, A. Joshi, H. Arfin, S. Badola, S. Saha and A. Nag, Sb3+-Er3+-Codoped Cs2NaInCl6 for Emitting Blue and Short-Wave Infrared Radiation, Angew. Chem., Int. Ed., 2022, 61, e202201628 CrossRef PubMed
.
- J. Y. Sun, W. Zheng, P. Huang, M. R. Zhang, W. Zhang, Z. H. Deng, S. H. Yu, M. Y. Jin and X. Y. Chen, Efficient Near-Infrared Luminescence in Lanthanide-Doped Vacancy-Ordered Double Perovskite Cs2ZrCl6 Phosphors via Te4+ Sensitization, Angew. Chem., Int. Ed., 2022, 61, e202201993 CrossRef PubMed
.
- R. Q. Shi, S. H. Miao, X. L. Lv, D. X. Chen, Y. Zhang and Y. J. Liang, High-Efficiency Short-Wave Infrared Emitter Enabled by Cr3+–Yb3+ Co-Doped Phosphor, Adv. Opt. Mater., 2024, 2303221 CrossRef
.
- Y. N. Wang, M. M. Shang, S. Huang, Y. X. Sun, Y. Y. Zhu, X. L. Xing, P. P. Dang and J. Lin, Continuous Ultra-Broadband Near-Infrared Sc2O3-Based Nanophosphor Realized by Spectral Bridge of Cr3+-Yb3+-Cr4+ for Multiple Optical Applications, Adv. Opt. Mater., 2023, 11, 2300517 CrossRef
.
- S. Y. Han, D. T. Tu, Z. Xie, Y. Q. Zhang, J. Y. Li, Y. F. Pei, J. Xu, Z. L. Gong and X. Y. Chen, Unveiling Local Electronic Structure of Lanthanide-Doped Cs2NaInCl6 Double Perovskites for Realizing Efficient Near-Infrared Luminescence, Adv. Sci., 2022, 9, 2203735 CrossRef PubMed
.
- G. D. Zhang, Y. Wei, P. P. Dang, H. Xiao, D. J. Liu, X. K. Li, Z. Y. Cheng and J. Lin, Facile solution synthesis of Bi3+/Yb3+ ions co-doped Cs2Na0.6Ag0.4InCl6 double perovskites with near-infrared emission, Dalton Trans., 2020, 49, 15231–15237 RSC
.
- S. Yang, S. F. Gong, Z. L. Zhou, L. F. Wu, M. M. Zhang, L. L. Jiang and W. Z. Wu, Bi and Yb Codoped Cs2Ag0.6Na0.4InCl6 Microcrystals: Visible to Near-Infrared Fluorescence for Thermometry, J. Phys. Chem. C, 2021, 125, 10431–10440 CrossRef
.
- B. Zhou, Z. X. Liu, S. F. Fang, H. Z. Zhong, B. B. Tian, Y. Wang, H. N. Li, H. L. Hu and Y. M. Shi, Efficient White Photoluminescence from Self-Trapped Excitons in Sb3+/Bi3+-Codoped Cs2NaInCl6 Double Perovskites with Tunable Dual-Emission, ACS Energy Lett., 2021, 6, 3343–3351 CrossRef CAS
.
- B. Zhou, A. X. Du, D. Ding, Z. X. Liu, Y. Wang, H. Z. Zhong, H. N. Li, H. L. Hu and Y. M. Shi, Achieving Tunable Cold/Warm White-Light Emission in a Single Perovskite Material with Near-Unity Photoluminescence Quantum Yield, Nano-Micro Lett., 2023, 15, 207 CrossRef CAS
.
- F. Zhang, X. Chen, X. F. Qi, W. Q. Liang, M. Wang, Z. Z. Ma, X. Z. Ji, D. W. Yang, M. C. Jia, D. Wu, X. J. Li, Y. Zhang, Z. F. Shi and C. X. Shan, Regulating the Singlet and Triplet Emission of Sb3+ Ions to Achieve Single-Component White-Light Emitter with Record High Color-Rendering Index and Stability, Nano Lett., 2022, 22, 5046–5054 CrossRef CAS
.
- W. G. Huang, H. Peng, Q. L. Wei, J. J. Xia, X. F. He, B. Ke, Y. Tian and B. S. Zou, Tunable Efficient White Emission in Holmium Doped Double Perovskites Cs2KInCl6 via Antimony Sensitization, Adv. Opt. Mater., 2023, 11, 2203103 CrossRef CAS
.
- W. J. Gan, L. Y. Cao, S. M. Gu, H. W. Lian, Z. G. Xia and J. Wang, Broad-Band Sensitization in Cr3+−Er3+ Co-Doped Cs2AgInCl6 Double Perovskites with 1.5 μm Near-Infrared Emission, Chem. Mater., 2023, 35, 5291–5299 CrossRef CAS
.
- H. W. Li, K. Han, Z. Y. Li, H. X. Yue, X. Y. Fu, X. Y. Wang, Z. G. Xia, S. Y. Song, J. Feng and H. J. Zhang, Multiple Energy Transfer Channels in Rare Earth Doped Multi-Exciton Emissive Perovskites, Adv. Sci., 2023, 11, 2307354 CrossRef PubMed
.
- R. S. Zeng, L. L. Zhang, Y. Xue, B. Ke, Z. Zhao, D. Huang, Q. L. Wei, W. C. Zhou and B. S. Zou, Highly Efficient Blue Emission from Self-Trapped Excitons in Stable Sb3+-Doped Cs2NaInCl6 Double Perovskites, J. Phys. Chem. Lett., 2020, 11, 2053–2061 CrossRef CAS
.
- P. Huang, W. Zheng, D. T. Tu, X. Y. Shang, M. R. Zhang, R. F. Li, J. Xu, Y. Liu and X. Y. Chen, Unraveling the Electronic Structures of Neodymium in LiLuF4 Nanocrystals for Ratiometric Temperature Sensing, Adv. Sci., 2019, 6, 1802282 CrossRef PubMed
.
- Y. Li, J. Wang, W. L. Zhou, G. G. Zhang, Y. Chen and Q. Su, Ultraviolet–Visible–Near-Infrared Luminescence Properties and Energy Transfer Mechanism of a Novel 5d Broadband Sensitized Sr3SiO5:Ce3+,Yb3+ Suitable for Solar Spectral Converter, Appl. Phys. Express, 2013, 6, 082301 CrossRef
.
- M. N. Tran, I. J. Cleveland, G. A. Pustorino and E. S. Aydil, Efficient near-infrared emission from lead-free ytterbium-doped cesium bismuth halide perovskites, J. Mater. Chem. A, 2021, 9, 13026–13035 RSC
.
- Y. H. Wang, S. C. Bai, J. Sun, H. Liang, C. Li, T. Y. Tan, G. Yang and J. W. Wang, Highly efficient visible and near-infrared luminescence of Sb3+, Tm3+ co-doped Cs2NaYCl6 lead-free double perovskite and light emitting diodes, J. Alloys Compd., 2023, 947, 169602 CrossRef CAS
.
- Z. C. Liu, Y. X. Pan, C. D. Peng, Y. H. Ding, H. Z. Lian, J. Lin and L. Y. Li, Enhancing Luminescence in Hue-Tunable White-Light Emitting K4CdCl6:Sb3+,Mn2+ All-Inorganic Halide Perovskites: Insights from Defect Engineering and Energy Transfer, ACS Appl. Mater. Interfaces, 2023, 15, 51462–51473 CrossRef CAS PubMed
.
- D. C. Yu, F. T. Rabouw, W. Q. Boon, T. Kieboom, S. Ye, Q. Y. Zhang and A. Meijerink, Insights into the energy transfer mechanism in Ce3+-Yb3+ codoped YAG phosphors, Phys. Rev. B: Condens. Matter Mater. Phys., 2014, 90, 165126 CrossRef
.
- A. D. Sontakke, J. Ueda, Y. Katayama, P. Dorenbos and S. Tanabe, Experimental insights on the electron transfer and energy transfer processes between Ce3+-Yb3+ and Ce3+-Tb3+ in borate glass, Appl. Phys. Lett., 2015, 106, 131906 CrossRef
.
- Z. Barandiarán, A. Meijerinkc and L. Seijo, Configuration coordinate energy level diagrams of intervalence and metal-to-metal charge transfer states of dopant pairs in solids, Phys. Chem. Chem. Phys., 2015, 17, 19874–19884 RSC
.
- B. Chen, Y. Guo, Y. Wang, Z. Liu, Q. Wei, S. X. Wang, A. L. Rogach, G. C. Xing, P. Shi and F. Wang, Multiexcitonic Emission in Zero-Dimensional Cs2ZrCl6:Sb3+ Perovskite Crystals, J. Am. Chem. Soc., 2021, 143, 17599–17606 CrossRef CAS PubMed
.
- C. Wang, Y. Liu, X. Feng, C. Y. Zhou, Y. L. Liu, X. Yu and G. J. Zhao, Phase Regulation Strategy of Perovskite Nanocrystals from 1D Orthomorphic NH4PbI3 to 3D Cubic (NH4)0.5Cs0.5Pb(I0.5Br0.5)3 Phase Enhances Photoluminescence, Angew. Chem., Int. Ed., 2019, 58, 11642–11646 CrossRef CAS PubMed
.
- C. Wang, N. J. Zhao, H. Y. Zhang, X. F. Zhang, X. H. Lin, H. Liu, F. Dang, W. Zhang, J. Sun, P. Chen, H. L. Chen, P. G. Han and P. Li, Ultrawide UV to NIR Emission in Double Perovskite Nanocrystals via the Self-Trapping State Engineering Strategy, ACS Sustainable Chem. Eng., 2023, 11, 14659–14666 CrossRef
.
- Y. R. Guo, H. W. Guan, P. Li, C. Wang, Y. Wang, J. R. Zhang and G. J. Zhao, Non-adiabatic conformation distortion charge transfer enables dual emission of thermally activated delayed fluorescence and room temperature phosphorescence, Spectrochim. Acta, Part A, 2024, 311, 124032 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, PLQYs testing and calculation, photographs of Cs2NaInCl6:Sb3+/Ln3+ (Ln3+ = Yb3+, Nd3+) single crystals, PXRD patterns, SEM image and EDX elemental mappings, PLE spectra, NIR PL decay, PLQYs, Ultraviolet–visible absorption spectra of Cs2NaInCl6:Sb3+/Ln3+ (Ln3+ = Yb3+, Nd3+) single crystals, PL and PLE mapping of Cs2NaInCl6:Sb3+/Ln3+ (Ln3+ = Yb3+, Nd3+) single crystals, PLE spectra of Cs2NaInCl6:Sb3+/Ln3+ (Ln3+ = Er3+, Ho3+, Nd3+, Yb3+), energy-level diagrams, PDOS, ICP elemental analysis of Cs2NaInCl6:Sb3+/Ln3+ (Ln3+ = Yb3+, Nd3+) single crystals, NIR PLQYs of metal halides (PDF). See DOI: https://doi.org/10.1039/d4qi01518e |
| This journal is © the Partner Organisations 2024 |