Temperature-dependent self-trapped models regulating energy transfer in rare earth double perovskites via 5s2 electron doping†
Chujun
Tan
,
Shuai
Zhang
,
Haiyan
Wang
,
Jiandong
Yao
,
Hongbang
Liu
*,
Bingsuo
Zou
 * and
Ruosheng
Zeng
* and
Ruosheng
Zeng
 *
*
State Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures, School of Physical Science and Technology, Guangxi University, Nanning 530004, China. E-mail: liuhb@gxu.edu.cn; zoubs@gxu.edu.cn; zengrsh@guet.edu.cn
First published on 8th May 2024
Abstract
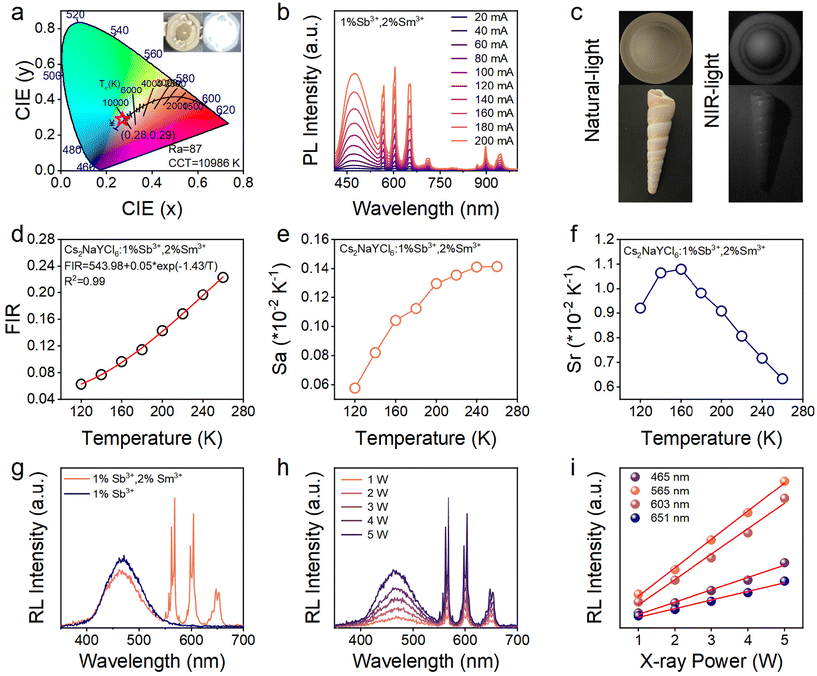
Due to their environmental friendliness, structural plasticity, and tunable emission, lead-free halide double perovskites offer a broad spectrum of applications in light-emitting diode (LED), photodetectors, infrared imaging, and temperature sensing. Herein, we synthesized rare earth-based Cs2NaYCl6 double perovskites using a solvothermal method, and Sb3+/Sm3+ co-doping can effectively modulate the luminescence by adjusting the band gap structure and channels of energy transfer. With the Sm3+-feeding concentration increasing, the emission could be adjusted from blue to white, attributed to an effective energy transfer from the self-trapped state to Sm3+. Temperature-dependent photoluminescence spectra indicate that the double self-trapped exciton emission at low temperatures originated from two minima in the excited state of 3P1. The relative sensitivity of the optical temperature sensor reached 1.08% K−1, which was better than that of other rare earth perovskites. The LED device based on Sb3+/Sm3+ co-doped Cs2NaYCl6@polymethylmethacrylate displays a chromaticity coordinate of (0.29, 0.28), a color rendering index of 87, and the correlated color temperature of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 986 K. Our work explores an in-depth understanding of energy transfer in double self-trapped states and provides new material for advanced applications.
986 K. Our work explores an in-depth understanding of energy transfer in double self-trapped states and provides new material for advanced applications.
1. Introduction
Lead-based halide perovskites (A = MA+/FA+/Cs+ and X = Cl−/Br−/I−), typically characterized by APbX3, have garnered significant attention due to their excellent photovoltaic properties, including high light absorption coefficients, low defect densities, and large electron sizes.1–3 Based on these advantages, lead-based perovskites have been found to have broad applications in many fields, such as light emitting diodes (LEDs) and4,5 X-ray detectors.6 The disadvantages of a low stability and environmental unfriendliness are also accompanied by lead-based perovskites.7 However, environmentally friendly cation substitution is an effective strategy to overcome the toxicity of Pb2+ ions. Replacing two Pb2+ cations as B+ and B3+ sites in halide double perovskites with the formula A2B+B3+X6 (e.g. Cs2NaInCl6)8 is a universal strategy.9–15 However, indirect bandgap or parity prohibited transition cause a weak photoluminescence (PL) in pristine lead-free perovskites. Doping with metal ions or alloying can alter the bandgap or disrupt the symmetry of double perovskites, breaking forbidden transitions and thereby enhancing their optical properties. By introducing Na+ and Bi3+ into the Cs2AgInCl6 host, Tang's group broke the inversion-symmetry-induced parity-forbidden transition and reduced the electronic dimensionality of the semiconductor, thus achieving an effective white light emission.16 By introducing Sb3+ into the Cs2NaInCl6 host, our group effectively regulated the density of states (DOS) and significantly enhanced the PL quantum yield (QY) of the self-trapped exciton (STE) (up to 75.89%).8 However, aforementioned studies only achieved a single luminescence mode. It is widely recognized that doping with rare earth elements is an effective method for tuning luminescent color.17–22 Rare earth ions typically exhibit a wide emission range from ultraviolet (UV) to infrared due to their rich (4fn) electronic configurations, along with long PL lifetimes and excellent optical stability.23–26 Xia's group reported on Bi3+:Cs2Ag(In1−xTbx)Cl6 halide perovskites,27 which modulated luminescence from green to orange. However, the tuning range of luminescence did not extend to cover the entire spectrum. Tang et al. confirmed the existence of an energy transfer from a STE to a rare earth in Ho3+:Cs2(Na, Ag)InCl6 and fabricated white LED.28 Zhu et al. obtained white light emission by combining CsPbBr3 NCs and Cs2Ag1−xKxIn0.125Bi0.875Cl6 double perovskites.29 The stability of double perovskites is significantly improved through K+ ion alloying. However, the lower PLQY of 15.96% limited broader application. Overall, abovementioned works only focus on LED applications and do not realize the multi-functional application of phosphors. Furthermore, the luminescence of all these works originates from the single self-trapped state and how to regulate the energy transfer process is not understood in depth.In this work, Sb3+/Sm3+ co-doped Cs2NaYCl6 were synthesized by a solvent-thermal method, resulting in the emission color transitioning steadily from blue to warm white with increasing Sm3+ feeding concentration. When a 2% Sm3+ precursor is the feed, the PLQY is as high as 68%. We propose a model to explain physical processes associated with energy transfer from the STE to the Sm3+ energy level. The single STE emission was observed at a higher temperature (up to 260 K), but the excited state 3P1 was split into two minima and resulted in a double STE emission at a lower temperature (below 260 K). 5s2 electron doping (Sb3+-doping) facilitates the energy transfer efficiency. Furthermore, we attempted multifunctional applications of the phosphors. At room temperature, the LED device exhibits chromaticity coordinates (CIE) of (0.28, 0.29), a color rendering index (CRI) of 87 and the correlated color temperature (CCT) of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 986 K, and the efficient infrared emission (400–1250 nm) is suitable for advanced infrared imaging. Further, the non-contact optical thermometry of Sb3+/Sm3+ co-doped Cs2NaYCl6 has demonstrated that absolute and relative sensitivities can reach 0.14% K−1 and 1.08% K−1, respectively. Our work provides new physical insights into regulating energy transfer and references for the rational design of functional materials.
986 K, and the efficient infrared emission (400–1250 nm) is suitable for advanced infrared imaging. Further, the non-contact optical thermometry of Sb3+/Sm3+ co-doped Cs2NaYCl6 has demonstrated that absolute and relative sensitivities can reach 0.14% K−1 and 1.08% K−1, respectively. Our work provides new physical insights into regulating energy transfer and references for the rational design of functional materials.
2. Results and discussion
Pristine and Sb3+/Sm3+ co-doped Cs2NaYCl6 are of a highly symmetric three-dimensional cubic structure belonging to the Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. Cs2NaYCl6 consists of alternately arranged [NaCl6]5− and [YCl6]3− octahedra (Fig. 1a). The powder X-ray diffraction (PXRD) analysis reveals that the introduction of Sb3+ ions causes a shift in diffraction peaks towards higher angles, as seen in Fig. 1b. This shift is due to the smaller ionic radius of Sb3+ (76.0 pm) compared to that of Y3+ (90.0 pm), which results in the contraction of the lattice. Conversely, an increase in the Sm3+-feeding concentration leads to the diffraction peaks shifting towards lower angles. This shift originates from the substitution of Sb3+/Sm3+ ions for Y3+ ions due to the larger ionic radius of Sm3+ (95.8 pm) compared to that of Y3+ (90.0 pm), and lattice expansion dominates a further shift. Due to the low doping content, no large change in lattice parameters was observed. The impurity peaks at 31.7° and 45.6° were from NaCl; however, a slight excess of NaCl is necessary for synthesizing samples with high yields. The X-ray photoelectron spectra (XPS) of pristine and doped perovskites show that the peaks at 160.4 and 158.5 eV are attributed to the Y 3d orbital (Fig. 1c) with no significant deviations. Fig. 1d shows the peaks of the Sb 3d orbital of Sb3+/Sm3+ co-doped Cs2NaYCl6, which are located at 535.9 and 531.9 eV. Moreover, Fig. 1e shows the peak of the Sb 3d orbital, which is located at 1088.4 eV. The XPS spectra of Cs, Na and Cl of pristine and Sb3+/Sm3+ co-doped Cs2NaYCl6 samples in Fig. S1† indicate that no striking deviations in binding energies are observed between pristine and Sb3+/Sm3+ co-doped Cs2NaYCl6 perovskites, which is attributed to the strong electronegativity of the rare earth ions. As shown in Fig. 1f, a scanning electron microscopy (SEM) image shows that the Sb3+/Sm3+ co-doped Cs2NaYCl6 perovskites are of micrometer scale. Furthermore, the energy-dispersive X-ray spectroscopy (EDS) mapping indicated Cs, Na, Y, Cl, Sb and Sm elements were uniformly distributed in the crystal.
m space group. Cs2NaYCl6 consists of alternately arranged [NaCl6]5− and [YCl6]3− octahedra (Fig. 1a). The powder X-ray diffraction (PXRD) analysis reveals that the introduction of Sb3+ ions causes a shift in diffraction peaks towards higher angles, as seen in Fig. 1b. This shift is due to the smaller ionic radius of Sb3+ (76.0 pm) compared to that of Y3+ (90.0 pm), which results in the contraction of the lattice. Conversely, an increase in the Sm3+-feeding concentration leads to the diffraction peaks shifting towards lower angles. This shift originates from the substitution of Sb3+/Sm3+ ions for Y3+ ions due to the larger ionic radius of Sm3+ (95.8 pm) compared to that of Y3+ (90.0 pm), and lattice expansion dominates a further shift. Due to the low doping content, no large change in lattice parameters was observed. The impurity peaks at 31.7° and 45.6° were from NaCl; however, a slight excess of NaCl is necessary for synthesizing samples with high yields. The X-ray photoelectron spectra (XPS) of pristine and doped perovskites show that the peaks at 160.4 and 158.5 eV are attributed to the Y 3d orbital (Fig. 1c) with no significant deviations. Fig. 1d shows the peaks of the Sb 3d orbital of Sb3+/Sm3+ co-doped Cs2NaYCl6, which are located at 535.9 and 531.9 eV. Moreover, Fig. 1e shows the peak of the Sb 3d orbital, which is located at 1088.4 eV. The XPS spectra of Cs, Na and Cl of pristine and Sb3+/Sm3+ co-doped Cs2NaYCl6 samples in Fig. S1† indicate that no striking deviations in binding energies are observed between pristine and Sb3+/Sm3+ co-doped Cs2NaYCl6 perovskites, which is attributed to the strong electronegativity of the rare earth ions. As shown in Fig. 1f, a scanning electron microscopy (SEM) image shows that the Sb3+/Sm3+ co-doped Cs2NaYCl6 perovskites are of micrometer scale. Furthermore, the energy-dispersive X-ray spectroscopy (EDS) mapping indicated Cs, Na, Y, Cl, Sb and Sm elements were uniformly distributed in the crystal.
To further investigate the optical properties of Sb3+/Sm3+ co-doped Cs2NaYCl6, PL, PL excitation (PLE), time-resolved PL (TRPL), and absorption spectroscopy have been performed. As shown in Fig. S2,† the absorption spectrum of pristine Cs2NaYCl6 shows a strong absorption at 310 nm and two weak absorption bands at 355 and 402 nm. After Sb3+ doping, the whole absorption band is enhanced, and a new absorption band appears at 260 and 300 nm originating from the 1S0 → 3P1 of Sb3+. The absorption spectra of Sm3+-doped samples are basically the same as those of undoped samples, which indicate that the introduction of Sm3+ does not contribute to the absorption, or absorption peaks overlap. As shown in Fig. S3a,† under 268 nm excitation, the double perovskites exhibit a broadband blue emission centered at 465 nm with a Stokes shift of 1.96 eV. After Sb3+ doping, the PL intensity of Sb3+:Cs2NaYCl6 is more than 10 times that of the pristine sample, with a Stokes shift of 0.93 eV. A large Stokes shift is a distinctive feature of STE emission. Simultaneously, the PL intensity of Sb3+/Sm3+ co-doped Cs2NaYCl6 is significantly enhanced by Sb3+ doping, achieving more than 30 times that of Sm3+:Cs2NaYCl6, as illustrated in Fig. S3b.† As a bridge of energy transfer, 5s2 dopants can more effectively transfer excited electrons from the excited state to the energy level of the rare earth ions. This is because STE emission possesses a wide excitation region and large absorption strength, which can overcome the weak absorption of the f–f transition through the efficient energy transfer. These indicate that the doping of Sb3+ greatly improves the luminescence efficiency of the samples. At room temperature, under 345 nm excitation, the Sb3+/Sm3+ co-doped Cs2NaYCl6 double perovskites exhibits STE emission centered at 465 nm (Fig. 2a), where the visible (VIS) emission at 565, 603, 651 and 712 nm is from the 4G5/2 → 6HJ=(5/2, 7/2, 9/2, 11/2) energy level transitions of Sm3+, and the near-infrared (NIR) emission at 941, 951, 1026 and 1170 nm is from the 4G5/2 → 6FK=(9/2, 7/2, 5/2, 3/2) energy level transitions of Sm3+.30 By normalizing the intensity of STE emission at 465 nm, the intensity of the rare earth emission from Sm3+ is gradually enhanced with increasing Sm3+-feeding concentration. Three excitation peaks at 285, 320, and 345 nm of the Sb3+/Sm3+ co-doped Cs2NaYCl6 double perovskites at different emissions can be observed in Fig. 2b. The peak at 285 nm is attributed to the transition from the singlet 1S0 to 1P1 of Sb3+, while excitation peaks at 320 and 345 nm correspond to the transitions from the ground state 1S0 to triplet 3P1. The excitation peak at 410 nm originates from the 6H5/2 → 4F7/2 transition of the Sm3+ ion.31 The profiles of the PLE in NIR emission in Fig. 2c is similar to that in Fig. 2b, indicating that almost all NIR emissions are from the energy transfer from Sb3+ to Sm3+, and only a very small portion comes from 6H5/2 → 4F7/2 of the Sm3+ ions. Fig. 2d shows PL spectra normalized at 465 nm under different excitations. As the excitation wavelength increases, the emission intensity of Sm3+ emission relative to STE emission gradually increases, which indicates that Sb3+/Sm3+ co-doped Cs2NaYCl6 has an excitation wavelength dependence.32 The integral area ratio S550–750/S400–550 of STE emission to Sm3+ emission is shown in Fig. 2e, which is positively correlated with the Sm3+-feeding concentration, indicating the promoted energy transfer from 3P1 of Sb3+ to 4D5/2 of Sm3+. CIE coordinates that correspond to the PL spectra at varying Sm3+-feeding concentrations are depicted in Fig. S4.† As the Sm3+-feeding concentration increases, the luminescence gradually shifts from blue to white. The initial CIE coordinate is (0.19, 0.21), and the final CIE coordinate is (0.36, 0.32). The PLQY reaches a maximum of 68% at a 2% Sm3+-feeding concentration and further gradually decreases with further increasing the Sm3+-feeding concentration (Fig. S5†). Broad-band STE emission is usually accompanied by a large Stokes shift, with barely any self-absorption in the PL process. This is also the reason why the sample has a higher PLQY. Fig. 2f demonstrates that the integral area ratio of Sm3+ emission to STE emission increases with increasing excitation wavelength. The PL decay spectra of Sb3+/Sm3+ co-doped Cs2NaYCl6 at 465 nm under a 345 nm excitation are shown in Fig. 2g, and specific values are expressed in Table S1.† The PL lifetime at 465 nm decreases from 1.21 to 0.79 μs with increasing Sm3+-feeding concentration. All the emissions exhibit microsecond lifetimes corresponding to the 1S0 → 3P1 transition of Sb3+. This changing trend indicates an efficient energy transfer from the STE to Sm3+ exists (Fig. 2h). The energy transfer efficiency of the STE to Sm3+ can be expressed by the following eqn (1):27,33
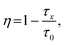 | (1) |
To gain more understanding about the electron-optical phonon coupling of 1% Sb3+, 2% Sm3+ co-doped Cs2NaYCl6, temperature-dependent PL spectroscopy was utilized to explore the photophysical process. The PL spectra at 140 and 300 K are shown in Fig. 3a. The PL spectra shows a double STE emission at 140 K and only a single STE emission at 300 K. The emission centered at 465 nm is defined as STE1, and the emission centered at 550 nm is defined as STE2. Comparing Sb3+/Sm3+ co-doped Cs2NaYCl6 with similar Sb-doped systems,34 there are two possible explanations for the double STE emission at low temperatures. One is due to the bimodality that arises from the dynamical Jahn–Teller coupling of the orbital triplet state 3P1 to two minima.35 The excited state shows two minima, which result in two different emission bands. Another reason is that the single state 1P1 → 1S0 transition and triple state 3P1 → 1S0 transition co-exist together. To study the source of the double STE emission at low temperatures, the lifetimes of STE1 and STE2 emissions were monitored separately at 140 K (Fig. 3b). The PL lifetimes of 0.52 and 1.22 μs for high-energy and low-energy emissions are obtained (Fig. 3b), respectively. The PL lifetimes of both STE emissions are in the microsecond scale. Sb3+ is characterized by a typical electron configuration of 5s2,36 exhibiting STE emission with nanosecond lifetimes resulting from the transition of 1P1 to 1S0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 37 and microsecond lifetimes arising from the transition of 3P1 to 1S0. Therefore, the STE1 and STE2 emissions of Sb3+/Sm3+ co-doped Cs2NaYCl6 belong to the triplet state emission. Temperature-dependent PL spectra and pseudo-color maps are shown in Fig. 3c and S8,† and double STE emission can be clearly detected from 80 to 260 K and Sm3+ emission positions are largely independent of temperature. This is because the f–f transition of rare earths is not easily affected by temperature, and the two minima of the 3P1 orbital of Sb3+ result in a double STE emission at low temperatures. Fig. 3d shows the variation of PL lifetime and intensity at 540 nm from 140 to 260 K, and both show a decreasing trend with increasing temperatures due to thermal quenching. As shown in Fig. S9,† the PL intensity at 465 nm increases gradually with temperature. This is attributed to the electrons gaining more energy and so tend to a higher energy radiative transition with increasing temperature. Fig. 3e shows the variation of PL lifetime and intensity at 651 nm from 120 to 400 K. PL intensity and lifetime increase from 120 to 280 K, which is attributed to the weakening of phonon scattering and reduction of defects more favorable to radiative recombination at low temperature. While PL intensity and lifetime show a decreasing trend from 280 to 400 K, which is attributed to thermal quenching at the high temperature. As shown in Fig. 3f and i, STE1 and STE2 were fitted using eqn (2), respectively.38,39
37 and microsecond lifetimes arising from the transition of 3P1 to 1S0. Therefore, the STE1 and STE2 emissions of Sb3+/Sm3+ co-doped Cs2NaYCl6 belong to the triplet state emission. Temperature-dependent PL spectra and pseudo-color maps are shown in Fig. 3c and S8,† and double STE emission can be clearly detected from 80 to 260 K and Sm3+ emission positions are largely independent of temperature. This is because the f–f transition of rare earths is not easily affected by temperature, and the two minima of the 3P1 orbital of Sb3+ result in a double STE emission at low temperatures. Fig. 3d shows the variation of PL lifetime and intensity at 540 nm from 140 to 260 K, and both show a decreasing trend with increasing temperatures due to thermal quenching. As shown in Fig. S9,† the PL intensity at 465 nm increases gradually with temperature. This is attributed to the electrons gaining more energy and so tend to a higher energy radiative transition with increasing temperature. Fig. 3e shows the variation of PL lifetime and intensity at 651 nm from 120 to 400 K. PL intensity and lifetime increase from 120 to 280 K, which is attributed to the weakening of phonon scattering and reduction of defects more favorable to radiative recombination at low temperature. While PL intensity and lifetime show a decreasing trend from 280 to 400 K, which is attributed to thermal quenching at the high temperature. As shown in Fig. 3f and i, STE1 and STE2 were fitted using eqn (2), respectively.38,39
| I(t) = I0/(1 + Ae−Ea/TKB), | (2) |
Huang Rhys factor S is obtained by fitting the full width at half-maximum (FWHM) and temperature (T) using the following eqn (3):16,40
 | (3) |
The electron-optical phonon coupling energy Γop was fitted using the following eqn (4):43
| Γ(T) = Γ0 + Γop/(eħωop/KBT − 1), | (4) |
To get a better knowledge of the electrical structure and luminous characteristics of Cs2NaYCl6 and Sb3+/Sm3+ co-doped Cs2NaYCl6, we performed density functional theory (DFT) calculations on pristine and Sb3+/Sm3+ co-doped Cs2NaYCl6 structures. Because of the covalent states of Sb3+/Sm3+ and Y3+, a [SbCl6]3− and [SmCl6]3− octahedron is used to replace the [YCl6]3− octahedron. It is found that pristine Cs2NaYCl6 exhibits a flat energy band structure with a direct band gap of 5.01 eV (Fig. 4a). After Sb3+ doping, the emergence of a new energy level results in a narrower bandgap of 3.75 eV (Fig. 4b). Furthermore, both structures have very flat energy bands with limited dispersion, indicating a large effective mass and confined properties for electrons and holes, which promotes the creation of highly localized electron distributions. The calculated DOS reveals that the valence band maximum (VBM) of Cs2NaYCl6 primarily comprises the Cl 3p state, while the conduction band minimum (CBM) is composed of Y 4d and Cl 3p states, as illustrated in Fig. 4c. The VBM of Sb3+/Sm3+ co-doped Cs2NaYCl6 consisted of Sb 5s and Cl 3p states, and the CBM consisted of the Sb 5p state (Fig. 4d). Dopant s-electrons mainly contribute near the band edges of the host. So, the s-electron dopants (Sb3+) act as sensitizers and emitters. The charge density distribution of pristine Cs2NaYCl6 reveals that the charge of the VBM is primarily concentrated around [YCl6]3− octahedra, as depicted in Fig. 4e. Conversely, the charge of the CBM is concentrated around Cl (Fig. 4f). The charge density distribution of Sb3+/Sm3+ co-doped Cs2NaYCl6 reveals that the charge of the VBM is primarily concentrated around the [YCl6]3− octahedra (Fig. 4g). The charge of the CBM is concentrated around [SbCl6]3− octahedra (Fig. 4h).
To explore the potential applications of the Sb3+/Sm3+ co-doped Cs2NaYCl6 double perovskites, the stability of the samples in the environment was initially investigated. Fig. S12† presents a comparison of the XRD and PL spectra of the sample after one month with those of the fresh sample, demonstrating a good environmental stability at room temperature and a high humidity of 70%. Our samples were placed in a relatively high humidity environment (70%) for one month. From the XRD pattern, a NaCl impurity was observed, and the main diffraction peaks of the samples did not obviously change, which indicated that the samples were of high humidity stability and good humidity resistance. Fig. 5a demonstrates that the LED device, emitting cool white light, that was fabricated by integrating the Sb3+/Sm3+ co-doped Cs2NaYCl6 double perovskites with polymethylmethacrylate (PMMA) on a 365 nm UV chip, achieving a CRI of up to 87. The LED device exhibits a CIE of (0.28, 0.29) and a CCT of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 986 K. Furthermore, the prepared LED lamp exhibited an excellent spectral stability across various currents ranging from 20 to 200 mA, as illustrated in Fig. 5b. Different imaging photographs of a conch and coffee cap under natural light irradiation and NIR light irradiation are depicted in Fig. 5c. Furthermore, the observation of different energy level transitions of Sm3+ with varying temperature sensitivities prompted us to explore the utilization of Sb3+/Sm3+ co-doped Cs2NaYCl6 for temperature sensing. As shown in Fig. S13,† we observe a decrease in emission at 603 nm with increasing temperature within the range of 120–260 K, while the emission at 951 nm exhibits a positive correlation within the same temperature range. This phenomenon suggests the potential use of the fluorescence intensity ratio (FIR) technique for precise temperature measurements. The relationship between temperature and FIR can be described by the following eqn (5):45
986 K. Furthermore, the prepared LED lamp exhibited an excellent spectral stability across various currents ranging from 20 to 200 mA, as illustrated in Fig. 5b. Different imaging photographs of a conch and coffee cap under natural light irradiation and NIR light irradiation are depicted in Fig. 5c. Furthermore, the observation of different energy level transitions of Sm3+ with varying temperature sensitivities prompted us to explore the utilization of Sb3+/Sm3+ co-doped Cs2NaYCl6 for temperature sensing. As shown in Fig. S13,† we observe a decrease in emission at 603 nm with increasing temperature within the range of 120–260 K, while the emission at 951 nm exhibits a positive correlation within the same temperature range. This phenomenon suggests the potential use of the fluorescence intensity ratio (FIR) technique for precise temperature measurements. The relationship between temperature and FIR can be described by the following eqn (5):45
 | (5) |
 | (6) |
 | (7) |
As shown in Fig. 5e and f, for the samples, the maximum values of Sa and Sr are 0.14 and 1.08% K−1, respectively. In addition to the high sensitivity, the repeatability and stability of optical temperature measurements are also critical. Among the same type of rare-earth doped perovskites, the Sa and Sr values of optical temperature measurements in this work are among leading values (Table S2†). Temperature resolution (δT) is another important parameter to evaluate the performance of optical thermometry, which can be expressed as follows:
 | (8) |
In this case, ΔFIR/FIR stands for the relative error in the measurement process, and its value is approximately 0.5%.47,48 Hence, it is calculated that the minimum temperature resolution is 0.46 at 160 K. At the same time, the non-contact optical thermometry of Sb3+/Sm3+ co-doped Cs2NaYCl6 has a good repeatability. The above results indicate that Sb3+/Sm3+ co-doped Cs2NaYCl6 is a potential material for non-contact optical thermometers. Additionally, Sb3+:Cs2NaYCl6 and the Sb3+/Sm3+ co-doped Cs2NaYCl6 double perovskites exhibit light emission under excitation by high-energy X-rays, as depicted in Fig. 5g. We conducted experiments at various X-ray power intensities to establish the relationship between emission and X-ray intensity. Notably, the radioluminescence (RL) intensity of Sb3+/Sm3+ co-doped Cs2NaYCl6 displays a linear correlation with the power of X-rays, as illustrated in Fig. 5h and i.
3. Conclusions
In summary, the Sb3+/Sm3+ co-doped Cs2NaYCl6 rare-earth double perovskites with a tunable emission and excellent stability were synthesized via a solvothermal method. It was found that the transition of 3P1 → 1S0 could be split into two transitions due to the Jahn–Teller effect, resulting in a double STE emission within a temperature range of 80 to 260 K. However, as the temperature increases, the excited electrons tend to move toward a higher energy STE emission, leading to the disappearance of the low-energy STE emission within the same temperature range (80–260 K). By adjusting the Sm3+ feeding concentration, we were able to adjust the energy transfer efficiency between STE and Sm3+ emissions. Furthermore, the Sb3+/Sm3+ co-doped Cs2NaYCl6 double perovskites exhibited multifunctional applications in high-energy X-rays and night vision. These double perovskites possess excellent optical properties and stability, rendering them promising candidates for applications in white-light illumination and temperature sensing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 52162021 and 22175043), Open Foundation of State Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures (Grant No. MMCS2023OF05), and Guangxi Science and Technology Plan Project (Guike AA23073018). The calculation was supported by the high-performance computing platform of Guangxi University.References
- Y. Bao, H. Wang, M. An, H. Tang, J. Li, J. Li, C. Tan, Y. Luo, J. Xu and Y. Yang, Defect-modulated synthesis and optoelectronic properties in chemical vapor deposited CsPbBr3 microplates, Nano Res., 2024, 17, 4610–4615 CrossRef CAS
.
- Y. Jiang, C. Sun, J. Xu, S. Li, M. Cui, X. Fu, Y. Liu, Y. Liu, H. Wan, K. Wei, T. Zhou, W. Zhang, Y. Yang, J. Yang, C. Qin, S. Gao, J. Pan, Y. Liu, S. Hoogland, E. H. Sargent, J. Chen and M. Yuan, Synthesis-on-substrate of quantum dot solids, Nature, 2022, 612, 679–684 CrossRef CAS PubMed
.
- M. Cui, C. Qin, Z. Zhou, Y. Jiang, S. Zhang, Z. Yuan, M. Yuan, K. Yu, Y. Jiang and Y. Liu, Tuning coherent phonon dynamics in two-dimensional phenylethylammonium lead bromide perovskites, Nano Res., 2023, 16, 3408–3414 CrossRef CAS
.
- C. Zhao, C. Zhu, Y. Yu, W. Xue, X. Liu, F. Yuan, J. Dai, S. Wang, B. Jiao and Z. Wu, Multifunctional short-chain 2-thiophenealkylammonium bromide ligand-assisted perovskite quantum dots for efficient light-emitting diodes, ACS Appl. Mater. Interfaces, 2023, 15, 40080–40087 CrossRef CAS PubMed
.
- X. Hu, Y. Xu, J. Wang, J. Ma, L. Wang and W. Jiang, Ligand-modified synthesis of shape-controllable and highly luminescent CsPbBr3 perovskite nanocrystals under ambient conditions, Inorg. Chem. Front., 2022, 9, 6080–6090 RSC
.
- Q. Lin, S. Bernardi, B. Shabbir, Q. Ou, M. Wang, W. Yin, S. Liu, A. S. R. Chesman, S. O. Fürer, G. Si, N. Medhekar, J. Jasieniak, A. Widmer-Cooper, W. Mao and U. Bach, Phase-control of single-crystalline inorganic halide perovskites via molecular coordination engineering, Adv. Funct. Mater., 2022, 32, 2109442 CrossRef CAS
.
- Y. Xie, A. Zhou, X. Zhang, Q. Ou and S. Zhang, Metal cation substitution of halide perovskite nanocrystals, Nano Res., 2022, 15, 6522–6550 CrossRef CAS
.
- R. Zeng, L. Zhang, Y. Xue, B. Ke, Z. Zhao, D. Huang, Q. Wei, W. Zhou and B. Zou, Highly efficient blue emission from self-trapped excitons in stable Sb3+-doped Cs2NaInCl6 double perovskites, J. Phys. Chem. Lett., 2020, 11, 2053–2061 CrossRef CAS PubMed
.
- Z. Xiao, K. Du, W. Meng, J. Wang, D. B. Mitzi and Y. Yan, Intrinsic instability of Cs2In(I)M(III)X6 (M = Bi, Sb; X = Halogen) double perovskites: a combined density functional theory and experimental study, J. Am. Chem. Soc., 2017, 139, 6054–6057 CrossRef CAS PubMed
.
- D. Li, J. Song, Y. Cheng, X. Wu, Y. Wang, C. Sun, C. Yue and X. Lei, Ultra-sensitive, selective and repeatable fluorescence sensor for methanol based on a highly emissive 0D hybrid lead-free perovskite, Angew. Chem., Int. Ed., 2022, 61, e202206437 CrossRef CAS PubMed
.
- Z. Chen, F. Zhang, D. Yang, H. Ji, X. Chen, D. Wu, X. Li, Y. Zhang and Z. Shi, Doping suppresses lattice distortion of vacant quadruple perovskites to activate self-trapped excitons emission, Nano Res., 2024, 17, 3068–3078 CrossRef CAS
.
- S. Wu and Y. Liu, Recent advancements and manipulation strategies of colloidal Cs2BIBIIIX6 lead-free halide double perovskite nanocrystals, Nano Res., 2023, 16, 5572–5591 CrossRef
.
- H. Yang, X. Chen, Y. Chu, C. Sun, H. Lu, M. Yuan, Y. Zhang, G. Long, L. Zhang and X. Li, A universal hydrochloric acid-assistant powder-to-powder strategy for quick and mass preparation of lead-free perovskite microcrystals, Light: Sci. Appl., 2023, 12, 75 CrossRef CAS PubMed
.
- C. Wang, J. Xiao, Z. Yan, X. Niu, T. Lin, Y. Zhou, J. Li and X. Han, Colloidal synthesis and phase transformation of all-inorganic bismuth halide perovskite nanoplates, Nano Res., 2023, 16, 1703–1711 CrossRef CAS
.
- A. Huang, M. Liu, C. Duan, K. Wong and P. A. Tanner, Understanding the ultraviolet, green, red, near infrared and infrared emission properties of bismuth halide double perovskite, Inorg. Chem. Front., 2022, 9, 6379–6390 RSC
.
- J. Luo, X. Wang, S. Li, J. Liu, Y. Guo, G. Niu, L. Yao, Y. Fu, L. Gao, Q. Dong, C. Zhao, M. Leng, F. Ma, W. Liang, L. Wang, S. Jin, J. Han, L. Zhang, J. Etheridge, J. Wang, Y. Yan, E. H. Sargent and J. Tang, Efficient and stable emission of warm-white light from lead-free halide double perovskites, Nature, 2018, 563, 541–545 CrossRef CAS PubMed
.
- S. Bao, H. Yu, G. Gao, H. Zhu, D. Wang, P. Zhu and G. Wang, Rare-earth single atom based luminescent composite nanomaterials: tunable full-color single phosphor and applications in WLEDs, Nano Res., 2022, 15, 3594–3605 CrossRef CAS
.
- Y. Chen, R. Zeng, Q. Wei, S. Zhang, B. Luo, C. Chen, X. Zhu, S. Cao, B. Zou and J. Z. Zhang, Competing energy transfer-modulated dual emission in Mn2+-doped Cs2NaTbCl6 rare-earth double perovskites, J. Phys. Chem. Lett., 2022, 13, 8529–8536 CrossRef CAS PubMed
.
- H. Wang, J. Yao, Q. Wei, J. Zhao, B. Zou and R. Zeng, Simultaneously achieving multicolor emission of down-shifting and up-conversion in Yb3+, Er3+-codoped Cs2NaGdCl6 double perovskites, Adv. Opt. Mater., 2023, 11, 2300694 CrossRef CAS
.
- Y. Liu, F. Di Stasio, C. Bi, J. Zhang, Z. Xia, Z. Shi and L. Manna, Near-infrared light emitting metal halides: materials, mechanisms, and applications, Adv. Mater., 2024, 2312482 CrossRef PubMed
.
- Y. Huang, Y. Pan, C. Peng, Y. Ding, H. Lian, L. Li and J. Lin, Orange/cyan emissive sensors of Sb3+ for probing water via reversible phase transformation in rare-earth-based perovskite crystals, Inorg. Chem. Front., 2023, 10, 991–1000 RSC
.
- Y. Hua, T. Wang, J. S. Yu, W. Ran and L. Li, Modulating A site compositions of europium(III)-doped double-perovskite niobate phosphors, Inorg. Chem. Front., 2022, 9, 6211–6224 RSC
.
- L. Zhang and M. Yuan, Lanthanide doped lead-free double perovskites as the promising next generation ultra-broadband light sources, Light: Sci. Appl., 2022, 11, 99 CrossRef CAS PubMed
.
- Y. Wang, P. Dang, L. Qiu, G. Zhang, D. Liu, Y. Wei, H. Lian, G. Li, Z. Cheng and J. Lin, Multimode luminescence tailoring and improvement of Cs2NaHoCl6 cryolite crystals via Sb3+/Yb3+ alloying for versatile photoelectric applications, Angew. Chem., Int. Ed., 2023, 62, e202311699 CrossRef CAS PubMed
.
- Z. Rao, M. Cao, Z. Chen, X. Zhao and X. Gong, Understanding and effective tuning of red-to-green upconversion emission in Ho-based halide double perovskite microcrystals, Adv. Funct. Mater., 2024, 34, 2311568 CrossRef CAS
.
- D. Chen, X. Zhang, J. Wei, L. Zhou, P. Chen, Q. Pang and J. Z. Zhang, Simultaneous enhancement of near infrared luminescence and stability of Cs2AgInCl6:Cr3+ double perovskite single crystals enabled by a Yb3+ dopant, Inorg. Chem. Front., 2022, 9, 4695–4704 RSC
.
- Y. Liu, X. Rong, M. Li, M. S. Molokeev, J. Zhao and Z. Xia, Incorporating rare-earth Terbium(III) ions into Cs2AgInCl6:Bi nanocrystals toward tunable photoluminescence, Angew. Chem., Int. Ed., 2020, 59, 11634–11640 CrossRef CAS PubMed
.
- S. Li, Q. Hu, J. Luo, T. Jin, J. Liu, J. Li, Z. Tan, Y. Han, Z. Zheng, T. Zhai, H. Song, L. Gao, G. Niu and J. Tang, Self-Trapped Exciton to Dopant Energy Transfer in Rare Earth Doped Lead-Free Double Perovskite, Adv. Opt. Mater., 2019, 7, 1901098 CrossRef CAS
.
- P. Zhu, S. Thapa, H. Zhu, S. Wheat, Y. Yue and D. Venugopal, Composition engineering of lead-free double perovskites towards efficient warm white light emission for health and well-being, J. Alloys Compd., 2023, 960, 170836 CrossRef CAS
.
- X. Li, X. Shen, M. Lu, J. Wu, Y. Zhong, Z. Wu, W. W. Yu, Y. Gao, J. Hu, J. Zhu, Y. Zhang and X. Bai, Wide-coverage and efficient NIR emission from single-component nanophosphors through shaping multiple metal-halide packages, Angew. Chem., Int. Ed., 2023, 62, e202217832 CrossRef CAS PubMed
.
- R. Tang, Y. Yang, Y. Yang, X. Ouyang, J. Che, S. Chen, X. Yao, B. Deng and R. Yu, A novel reddish-orange emitting NaSrBiTeO6:Sm3+ phosphor with high moisture resistance and thermostability for horticultural light emitting diode applications, J. Am. Ceram. Soc., 2024, 107, 3012–3027 CrossRef CAS
.
- S. K. Cushing, M. Li, F. Huang and N. Wu, Origin of strong excitation wavelength dependent fluorescence of graphene oxide, ACS Nano, 2014, 8, 1002–1013 CrossRef CAS PubMed
.
- J. Nie, H. Li, S. Fang, B. Zhou, Z. Liu, F. Chen, Y. Wang and Y. Shi, Efficient red photoluminescence in holmium-doped Cs2NaInCl6 double perovskite, Cell Rep. Phys. Sci., 2022, 3, 100820 CrossRef CAS
.
- J. Li, Z. Tan, M. Hu, C. Chen, J. Luo, S. Li, L. Gao, Z. Xiao, G. Niu and J. Tang, Antimony doped Cs2SnCl6 with bright and stable emission, Front. Optoelectron., 2019, 12, 352–364 CrossRef
.
- Y. Jing, Y. Liu, M. Li and Z. Xia, Photoluminescence of singlet/triplet self-trapped excitons in Sb3+-based metal halides, Adv. Opt. Mater., 2021, 9, 2002213 CrossRef CAS
.
- H. Arfin, A. S. Kshirsagar, J. Kaur, B. Mondal, Z. Xia, S. Chakraborty and A. Nag, ns2 electron (Bi3+ and Sb3+) doping in lead-free metal halide perovskite derivatives, Chem. Mater., 2020, 32, 10255–10267 CrossRef CAS
.
- Y. Jing, Y. Liu, X. Jiang, M. S. Molokeev, Z. Lin and Z. Xia, Sb3+ dopant and halogen substitution triggered highly efficient and tunable emission in lead-free metal halide single crystals, Chem. Mater., 2020, 32, 5327–5334 CrossRef CAS
.
- A. Shinde, R. Gahlaut and S. Mahamuni, Low-temperature photoluminescence studies of CsPbBr3 quantum dots, J. Phys. Chem. C, 2017, 121, 14872–14878 CrossRef CAS
.
- J. Ghosh, L. P. L. Mawlong, M. G. B, A. J. Pattison, W. Theis, S. Chakraborty and P. K. Giri, Solid-state synthesis of stable and color tunable cesium lead halide perovskite nanocrystals and the mechanism of high-performance photodetection in a monolayer MoS2/CsPbBr3 vertical heterojunction, J. Mater. Chem. C, 2020, 8, 8917–8934 RSC
.
- Z. Tan, Y. Chu, J. Chen, J. Li, G. Ji, G. Niu, L. Gao, Z. Xiao and J. Tang, Lead-free perovskite variant solid solutions Cs2Sn1−xTexCl6: bright luminescence and high anti-water stability, Adv. Mater., 2020, 32, 2002443 CrossRef CAS PubMed
.
- D. Zhu, M. L. Zaffalon, J. Zito, F. Cova, F. Meinardi, L. De Trizio, I. Infante, S. Brovelli and L. Manna, Sb-doped metal halide nanocrystals: a 0D versus 3D comparison, ACS Energy Lett., 2021, 6, 2283–2292 CrossRef CAS PubMed
.
- B. Zhou, Z. Liu, S. Fang, H. Zhong, B. Tian, Y. Wang, H. Li, H. Hu and Y. Shi, Efficient white photoluminescence from self-trapped excitons in Sb3+/Bi3+-codoped Cs2NaInCl6 double perovskites with tunable dual-emission, ACS Energy Lett., 2021, 6, 3343–3351 CrossRef CAS
.
- W. Zhang, J. Wei, Z. Gong, P. Huang, J. Xu, R. Li, S. Yu, X. Cheng, W. Zheng and X. Chen, Unveiling the excited-state dynamics of Mn2+ in 0D Cs4PbCl6 perovskite nanocrystals, Adv. Sci., 2020, 7, 2002210 CrossRef CAS PubMed
.
- T. Chang, H. Wang, Y. Gao, S. Cao, J. Zhao, B. Zou and R. Zeng, Component engineering to tailor the structure and optical properties of Sb-doped Indium-based halides, Inorg. Chem., 2022, 61, 1486–1494 CrossRef CAS PubMed
.
- J. Xue, M. Song, H. M. Noh, S. H. Park, B. R. Lee, J. H. Kim and J. H. Jeong, Near-ultraviolet light induced red emission in Sm3+-activated NaSrLa(MoO4)O3 phosphors for solid-state illumination, J. Alloys Compd., 2020, 817, 152705 CrossRef CAS
.
- W. Li, S. Wu, H. Zhang, X. Zhang, J. Zhuang, C. Hu, Y. Liu, B. Lei, L. Ma and X. Wang, Enhanced biological photosynthetic efficiency using light-harvesting engineering with dual-emissive carbon dots, Adv. Funct. Mater., 2018, 28, 1804004 CrossRef
.
- X. Li, D. Wang, Y. Zhong, F. Jiang, D. Zhao, S. Sun, P. Lu, M. Lu, Z. Wang, Z. Wu, Y. Gao, Y. Zhang, W. W. Yu and X. Bai, Halide double perovskite nanocrystals doped with rare-earth ions for multifunctional applications, Adv. Sci., 2023, 10, 2207571 CrossRef CAS PubMed
.
- A. Zhang, Z. Sun, M. Jia, Z. Fu, B. C. Choi, J. H. Jeong and S. H. Park, Sm3+-doped niobate orange-red phosphors with a double-perovskite structure for plant cultivation and temperature sensing, J. Alloys Compd., 2021, 889, 161671 CrossRef
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4qi00649f |
| This journal is © the Partner Organisations 2024 |