Efficient doped and non-doped light-emitting diodes based on a TADF-emitting Cu4Br4 cluster†
Xin
Lu
abc,
Shao-Jie
Wu
abc,
Ya-Shu
Wang
bc,
Shan-Yue
Wei
cd,
Lingyi
Meng
 abc,
Xi-He
Huang
abc,
Xi-He
Huang
 *a,
Xu-Lin
Chen
*abc and
Can-Zhong
Lu
*a,
Xu-Lin
Chen
*abc and
Can-Zhong
Lu
 *abc
*abc
aCollege of Chemistry, Fuzhou University, Fuzhou, Fujian 350108, China. E-mail: xlchem@fjirsm.ac.cn; xhhuang@fzu.edu.cn; czlu@fjirsm.ac.cn
bState Key Laboratory of Structural Chemistry, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China
cXiamen Key Laboratory of Rare Earth Photoelectric Functional Materials, Xiamen Institute of Rare-earth Materials, Haixi Institutes, Chinese Academy of Sciences, Xiamen, Fujian 361021, China
dEngineering Research Center of Environment-Friendly Function Materials, Ministry of Education, Institute of Materials Physical Chemistry, Huaqiao University, Xiamen 361021, P. R. China
First published on 8th April 2024
Abstract
Copper-halide clusters emerge as a promising category of photoluminescent materials, attributed to their rich structural and photophysical properties in solid states. However, their environment-dependent emission quenching and challenges in film formation pose obstacles to their application in electroluminescent (EL) devices. Herein, we present Cu4Br4(AcNP)2, a sublimable copper-halide cluster chelated by a bidentate N^P ligand with a donor–acceptor configuration. The steric and electronic modification effects of the dimethylacridine group not only induce predominant (metal + halide)-to-ligand charge transfer and intra-ligand charge transfer characters in the excited states of the cluster, ensuring efficient and short-lived thermally activated delayed fluorescence, but also effectively prevent concentration quenching in films by avoiding intermolecular π–π stacking. As a consequence, 40 wt% doped and non-doped devices achieve maximum external quantum efficiencies of 12.8% and 10.2%, respectively. We anticipate that the current study offers insights into the design of copper-halide clusters for fabricating highly efficient EL devices, especially for non-doped EL devices.
Introduction
Organic light-emitting diodes (OLEDs) have been leading innovation in the field of solid-state displays due to their ability to deliver high-quality and flexible products.1–3 In order to attain high electroluminescence (EL) efficiency, it is imperative to utilize highly efficient emitters capable of utilizing both singlet (25%) and triplet (75%) excitons during device fabrication. Phosphorescence and thermally activated delayed fluorescence (TADF) emitters have emerged as the two most promising candidates for achieving highly efficient OLEDs. Phosphorescent transition-metal complexes with d6 and d8 electron configurations have the potential to achieve a theoretical internal quantum efficiency of 100% thanks to the robust spin–orbit coupling (SOC) interactions between the triplet state (T1) and the singlet states (S1 and S0).4 TADF materials can efficiently harvest triplet excitons for fluorescence through reverse intersystem crossing (RISC) from the T1 state to the energetically proximate S1 state.5,6 Many phosphorescence and TADF emitters typically exhibit relatively long exciton lifetimes, spanning from microseconds to milliseconds, and often suffer from severe aggregation-caused quenching (ACQ) in neat films.7–9 To mitigate the concentration quenching and exciton annihilation in the emitting layer of OLEDs, these emitters must be employed in co-doping systems with precise control of the doping concentration. In comparison with doped OLEDs, non-doped OLEDs offer advantages, including process simplicity, reduced fabrication costs, and the avoidance of phase separation. The development of emitters for efficient non-doped OLEDs has gained significant attention recently. However, the performances of non-doped OLEDs still fall significantly short of those achieved in doped OLEDs.10–12Copper(I) complexes have shown promise as cost-effective EL emitters because of their diverse structural and photophysical properties, along with their capacity to efficiently harness both singlet and triplet excitons for emission. The versatile coordination chemistry of copper(I) ions allows them to create remarkable structural diversity in combination with both organic and inorganic ligands. Among them, copper(I)–halide clusters are well known for their rich photophysical properties. Owing to the low oxidation potentials of copper(I) and halide ions, copper(I)–halide clusters often exhibit (metal + halide)-to-ligand charge transfer (M + X)LCT character in their lowest excited states, endowing them with typical TADF properties.13–21 Moreover, the heavy atom effect (HAE) exhibited by these clusters can lead to significant SOCs between the lowest triplet and singlet states, thereby facilitating intersystem crossing (ISC) and phosphorescence processes, and the high rigidity of the cluster structure effectively inhibits the photothermal decomposition of Cu(I) and the quenching of the excited state caused by the Jahn–Teller distortion.22–25 These attributes of copper(I)–halide clusters make them potential high-efficiency EL emitters. Nonetheless, copper(I)–halide clusters often come with significant drawbacks when it comes to the fabrication of efficient EL devices. First, copper(I)–halide clusters frequently exhibit an intricate composition in their excited states, involving both organic ligands and inorganic cluster cores. The presence of non-radiative, low-lying cluster-centered triplet states (3CC) can potentially quench the emission.26,27 In addition, a substantial number of copper(I)–halide clusters are insoluble in conventional solvents and exhibit instability during sublimation, which makes them unsuitable for fabricating cluster light-emitting diodes (CLEDs) via solution-based processes and vacuum deposition. Despite some progress,10,28–35 the rational design of copper(I)–halide clusters for highly efficient CLEDs and the comprehension of structure–property relationships continue to pose challenges.
In this work, we report a new copper(I)–bromine cluster, Cu4Br4(AcNP)2, supported by a bidentate N^P ligand featuring an electron donor (D)–acceptor (A) configuration. Cu4Br4(AcNP)2 emits efficient EL in films benefiting from the nearly degenerate S1 and T1 states, both originating from (M + X)LCT and intra-ligand charge transfer (ILCT) transitions. Moreover, the highly twisted and bulky D–A configuration effectively prevents concentration quenching in films by effectively separating the emission centers of adjacent molecules and preventing intermolecular π–π stacking.36–38 Cu4Br4(AcNP)2 in 40 wt%-doped and neat films show PLQYs of 47% and 38% and decay times of 2.7 and 1.9 μs, respectively. The doped OLEDs employing Cu4Br4(AcNP)2 as the dopant achieved peak EQEs reaching 12.8% with a small efficiency roll-off. Remarkably, the non-doped OLED utilizing Cu4Br4(AcNP)2 maintains high performance with a maximum EQE of 10.2%, which is excellent among those attained by non-doped OLEDs based on Cu(I) complexes.
Results and discussion
Synthesis and characterization
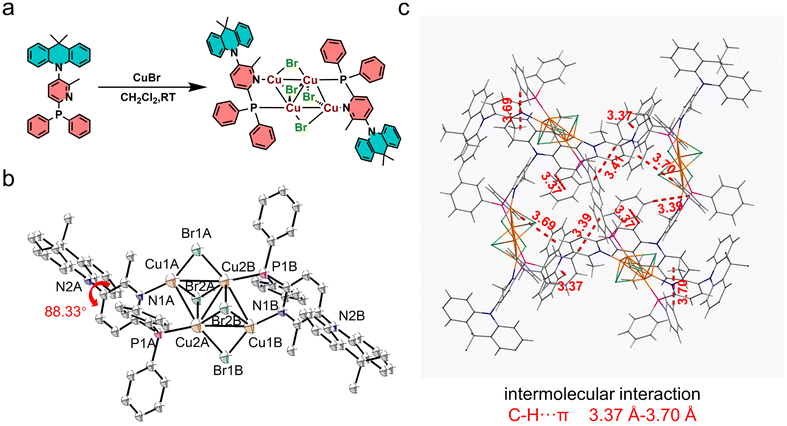
The D–A type bidentate N^P ligand (AcNP) was synthesized in three steps, resulting in an overall yield of 38% (refer to the ESI† for detailed synthesis procedures). The copper(I)–bromine cluster, Cu4Br4(AcNP)2, was prepared from the reaction of one equivalent of the AcNP ligand and two equivalents of CuBr in dichloromethane (as illustrated in Fig. 1a). After purification through recrystallization, accomplished by the slow diffusion of ether into its dichloromethane solution, the cluster Cu4Br4(AcNP)2 was obtained as yellow single crystals with a moderate yield of 55%. The obtained cluster was further purified by vacuum sublimation before conducting characterization studies and device fabrications. The molecular structure of Cu4Br4(AcNP)2 was determined with 1H-NMR,13C-NMR, and single-crystal X-ray diffraction analysis. The CCDC number of Cu4Br4(AcNP)2 is 2323855.†Single-crystal structure analysis
The single crystal information in the X-ray structure analysis and the selected bond lengths and bond angles are listed in Tables S1 and S2,† respectively. As depicted in Fig. 1b, Cu4Br4(AcNP)2 exhibits a Ci symmetric structure in the crystal. The four coordinated copper atoms form a parallelogram structure with an acute angle of 60.66°, and adjacent sides (Cu–Cu distances) of 2.81 and 2.91 Å. In addition, the two copper atoms positioned at the vertices of the obtuse angles of the parallelogram form a Cu–Cu bond at a distance of 2.89 Å. Each set of three copper atoms forming an acute angle is μ3-capped with a bromine atom, respectively. The other two bromine atoms form μ2-bonds at the two long sides of the parallelogram, each featuring a Cu–Br–Cu angle of 74.76°. Two AcNP ligands coordinate with copper atoms on the two shorter sides of the parallelogram in a C2-symmetric arrangement. The ligand AcNP exhibits a D–A dihedral angle of 88.33° between the acridine unit and the pyridine ring. The highly distorted D–A structure of the ligand is expected to hinder the formation of intermolecular π–π stackings that typically lead to emission quenching, especially at high doping concentrations. As shown in Fig. 1c, only weak intermolecular C–H⋯π interactions (3.37–3.70 Å) were observed, and no π–π interactions were found in the crystal lattice of Cu4Br4(AcNP)2. Moreover, the peripheral non-planar dimethylacridine units effectively separate the emission centers of adjacent molecules (Fig. 1c), thereby inhibiting short-distance Dexter energy transfer (DET), which is primarily responsible for concentration quenching.38Thermal and electrochemical properties
Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) were used to evaluate the thermal stability of the powder sample of Cu4Br4(AcNP)2. As shown in Fig. S1,† the decomposition temperature (Td, 5% weight loss) was detected to be 365 °C, and no obvious signs of glass transition were observed in the temperature range of 50–360 °C. The good thermal stability of Cu4Br4(AcNP)2 could ensure its ability to endure high thermal stress during the thermal evaporation process and Joule heating during the operation of OLEDs. Moreover, to ascertain whether high-temperature evaporation causes decomposition and validate the purity of the evaporated cluster film, we recorded the 1H-NMR spectrum of a Cu4Br4(AcNP)2 sample deposited through evaporation (Fig. S15†), and compared it with those of the ligand AcNP and a Cu4Br4(AcNP)2 sample without evaporation (Fig. S16†). The results indicate the absence of an uncoordinated ligand or other impurities in the evaporated Cu4Br4(AcNP)2 sample, confirming that thermal evaporation does not cause the decomposition of the cluster.The electrochemical properties of both the ligand AcNP and the Cu4Br4(AcNP)2 cluster were investigated by cyclic voltammetry (CV). The cyclic voltammograms for the oxidations of AcNP and Cu4Br4(AcNP)2 in dichloromethane are shown in Fig. S2.† Using the Eonset values of the initial oxidative peaks, the HOMO energy levels of AcNP and Cu4Br4(AcNP)2 were calculated to be −5.28 and −5.22 eV, respectively. In conjunction with the optical band gaps, which were determined as 3.17 and 2.86 eV from the UV-Vis absorption onset values (Fig. 3a and Fig. S3†), the LUMO levels of AcNP and Cu4Br4(AcNP)2 were calculated to be −2.11 and −2.36 eV, respectively (Table S6†).
Theoretical calculations
Density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations were performed at the B3LYP/6-31G* level to investigate the frontier molecular orbitals (FMOs) and excited states of Cu4Br4(AcNP)2. As shown in Fig. 2 and Table S3,† the HOMO of this cluster is primarily situated on the Cu4Br4 cluster and extends partially to the arylphosphine unit of the ligand, whereas the LUMO is predominantly distributed over the ligand. The effective separation of FMOs is expected to result in a small singlet–triplet splitting (ΔEST), a crucial requirement for facilitating TADF emission.39,40 Although a D–A type ligand was employed, DFT calculations revealed that the dimethylacridine unit on the ligand does not contribute to the HOMO of Cu4Br4(AcNP)2. This could be attributed to the lower oxidation potential of the Cu4Br4 cluster compared to the dimethylacridine unit, which is confirmed from the CV measurement. TD-DFT calculations unveiled that the S1 and T1 states of Cu4Br4(AcNP)2 are close in energy (ΔEST = 0.15 eV) and both featured with predominant intramolecular charge transfer (ICT) transitions (Table S4† and Fig. 2). According to orbital transition analysis (Table S5†), both the S1 and T1 states of Cu4Br4(AcNP)2 comprise (M + X)LCT plus ILCT transitions.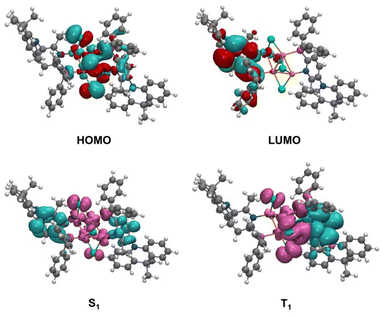 | ||
| Fig. 2 HOMO and LUMO orbital distributions and redistribution of electron density of the lowest excited states (S1 and T1) of Cu4Br4(AcNP)2. | ||
Photophysical properties
Photophysical properties of Cu4Br4(AcNP)2 were investigated in dichloromethane, doped and neat films. Fig. 3a shows the ultraviolet-visible (UV-Vis) absorption and photoluminescence (PL) spectra of the Cu4Br4(AcNP)2 cluster and the ligand AcNP in dichloromethane (c = 2 × 10−5 M) at room temperature. Cu4Br4(AcNP)2 and AcNP exhibit similar absorption profiles, each consisting of a strong band below 330 nm and a weak shoulder band above 330 nm. The analogous high-energy absorption bands of both the cluster and the ligand can be assigned to the spin-allowed π–π* transitions localized at the NP ligand. The weak low-energy shoulder band of AcNP could be ascribed to the ILCT transition from the dimethylacridine donor unit to the pyridine acceptor unit. In contrast, the low-energy shoulder band of Cu4Br4(AcNP)2 should stem from different CT transitions, as evidenced by its absorption onset at 445 nm, which represents a significant redshift compared to that of the ligand at 390 nm. According to theoretical calculations (vide supra, Table S4†), the S0 → S1 absorption bands of Cu4Br4(AcNP)2 mainly involve the transitions from the Cu4Br4 cluster and P atoms to the ligand. Consequently, the low-energy CT absorption band of Cu4Br4(AcNP)2 originates from a combination of (M + X)LCT and ILCT transitions. In degassed dichloromethane solution, the ligand AcNP emits a greenish-blue light with a featureless emission spectrum, peaking at 496 nm. In comparison, the Cu4Br4(AcNP)2 cluster exhibits a notably weaker emission, significantly redshifted and broadened, with a peak at 707 nm. The weak emission of this cluster in solution is attributed to the flattening distortion in the MLCT excited state, exacerbating nonradiative decay.41,42 Such an excited-state distortion is common in copper(I) complexes and can be effectively suppressed in a rigid environment.43,44 Despite numerous reports of high-efficiency copper(I) complexes in the powder state,45,46 addressing the excited-state distortion in solution and film states through molecular design remains a formidable challenge.As shown in Table 1, in doped (40 wt%-doped in BCPO host, BCPO = Bis-(4-(N-carbazolyl) phenyl)-phenylphosphine oxide) and neat films (deposited via thermal evaporation), Cu4Br4(AcNP)2 exhibits efficient greenish-yellow emission with a peak maxima of 552 nm and 567 nm, photoluminescence quantum yields (PLQYs) of 47% and 38%, and short emission lifetimes of 2.7 and 1.9 μs, respectively. Aggregation-caused quenching and emission redshift with increasing doping concentration are not significant. This phenomenon may be attributed to the highly twisted D–A structure of the ligand, which impedes the formation of intermolecular π–π stackings. The radiative (kr) and non-radiative rate constant (knr) are calculated to be 1.7 × 105 and 2.0 × 105 for the doped film, and 2.0 × 105 and 3.3 × 105 for the neat film, respectively. Evidently, the comparatively reduced PLQY of Cu4Br4(AcNP)2 in the neat film, as opposed to the doped film, can be ascribed to more pronounced nonradiative decay in the neat film. To gain a deep understanding of the emissive mechanism, we examined the temperature dependence of both emission spectra and decay times. As depicted in Fig. 3c and d, Fig. S4 and S5,† Cu4Br4(AcNP)2 in both doped and neat films exhibit slight red-shifted emission spectra and considerably prolonged lifetimes on decreasing the temperature from 300 to 77 K. This suggests that the emission of this cluster may stem from two thermally equilibrated excited states, namely S1 and T1. Assuming a rapid thermal equilibrium between the S1 and T1 states, the temperature dependence of the emission decay time (τ) is expressed by the following Boltzmann equation:14,47–49
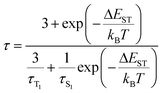 | (1) |
| Cu4Br4(AcNP)2 | λ max /nm | Φ PL /% | τ /μs | k r /s−1 | k nr /s−1 | ΔESTf/eV |
|---|---|---|---|---|---|---|
| a Emission maximum. b Photoluminescence quantum yield. c Emission decay time. d Radiative decay rate. e Nonradiative decay rate. f Energy gap between the S1 and T1 states determined from the emission spectra at 77 and 300 K. | ||||||
| 40% | 552 | 47 | 2.7 | 1.7 × 105 | 2.0 × 105 | 0.059 |
| 100% | 567 | 38 | 1.9 | 2.0 × 105 | 3.3 × 105 | 0.050 |
PL spectra and decay curves were also measured for a spin-coated neat film of Cu4Br4(AcNP)2 and a thermal-deposited neat film of AcNP, and then compared with those obtained from the thermal-deposited Cu4Br4(AcNP)2 neat film. As shown in Fig. S7,† both the PL spectrum and decay curve of the spin-coated cluster film closely resemble those of the thermal-deposited cluster film, both exhibiting an emission spectrum peak at 567 nm and an emission lifetime of around 1.9 μs. In contrast, the ligand AcNP in the neat film exhibits sky-blue emission with a spectrum peaking at 469 nm and an emission lifetime of 12.3 ns, which can be attributed to be prompt fluorescence. The absence of emission from the ligand further confirms the purity of the thermal-deposited cluster film.
Electroluminescence (EL) properties
To evaluate the EL properties of Cu4Br4(AcNP)2, vacuum deposition OLEDs were fabricated with a structure of ITO/HATCN (10 nm)/TAPC (40 nm)/mCP (5 nm)/emitting layer (30 nm)/DPEPO (5 nm)/TmPyPB (30 nm)/Liq (1 nm)/Al (100 nm) (Fig. 4a). Here, Cu4Br4(AcNP)2 was used as the emitter; BCPO served as the host material to disperse the emitter; HATCN, TAPC, mCP, DPEPO, TmPyPB and Liq act as the hole-injection, hole-transporting, electron-blocking, hole-blocking, electron-transporting, and electron-injection layers, respectively. The device energy-level diagram and molecular structures of the used organic materials are depicted in Fig. 4a and Fig. S9,† respectively.We explored the impact of the doping concentration on the EL performance, varying the emitter doping concentrations from 20 wt% to 100 wt% (non-doped). The EL performances are presented in Fig. 4b–d and Table 2. A progressive spectral redshift in EL was observed as the doping concentration increased, with spectral peaks red-shifting from 557 to 572 nm and the corresponding Commission Internationale de L'Eclairage (CIE) color coordinates from (0.39, 0.46) to (0.42, 0.49), when going from 20 wt% to 100 wt%. The doping concentration-dependent EL spectra of D–A emitters are commonly associated with the solid solvation effect.50–52 Specifically, at elevated concentration levels, the emissive CT states of the emitters are more likely stabilized by the polar neighboring molecules. In comparison to the majority of reported D–A emitters, the redshift observed in the EL spectra of Cu4Br4(AcNP)2 with increasing doping concentration is not substantial. This may be attributed to the presence of large steric ligands, which effectively separate the neighboring chromophores. All these OLEDs exhibited remarkably low turn-on voltages, ranging from 3.3 to 3.6 V, indicating efficient carrier injection and transport within the devices. The maximum EQEs of the OLEDs exhibited a gradual increase with the rise in the doping concentration from 20 wt% to 40 wt%, reaching a highest value of 12.8% in the devices doped at 40 wt%. As the doping concentration continues to increase, the maximum EQE values exhibit only a slight decrease, going from 12.8% in the 40 wt%-doped device to 10.8% in the non-doped device. The dependence of efficiency roll-off on the doping concentration can be explained as follows: At low doping concentrations (20 wt% and 30 wt%), the presence of a shoulder band in the high-energy region of the EL spectrum suggests insufficient host–guest energy transfer which causes low efficiencies and reduced luminance in the device. In these cases, under high current density, the high-concentration excitons of host molecules may undergo bimolecular quenching before diffusing into the guest molecules for radiative transition, leading to severe efficiency roll-offs.53–55 This efficiency roll-off behavior could be alleviated by optimizing the doping-concentration for ensuring efficient host–guest energy transfer. The OLED doped at 40 wt% exhibits a significantly smaller efficiency roll-off compared to the devices doped at 20 wt% and 30 wt%. However, when the dopant concentration is excessively high (e.g., 100 wt%), the accumulation of dopant excitons under high current density inevitably intensifies bimolecular quenching, thereby exacerbating efficiency roll-off. In summary, 40 wt%-doped and non-doped OLEDs achieved the maximum EQE of 12.8% and 10.8%, a peak power efficiency (PE) of 32.0 and 25.3 lm W−1, a maximum current efficiency (CE) of 34.0 and 27.0 cd A−1, and a maximum luminance of 5904 and 1045 cd m−2, respectively. It is uncommon to observe such minimal dependence of the EL performance on the doping concentration for copper(I)-based emitters.10,28,32,56–58 Notably, as summarized in Table S7,† the device performances of the non-doped OLED can be comparable to that of the state-of-the-art non-doped OLEDs based on copper(I) emitters.
| Cu4Br4(AcNP)2 | λ EL /nm | V on /V | L max /cd m−2 | EQEd/% | CEe/cd A−1 | PEf/lm W−1 | CIEg (x, y) |
|---|---|---|---|---|---|---|---|
| a The wavelength at EL maximum (recorded at 9 V). b Turn-on voltage at 1 cd m−2. c Maximum luminance. d EQE maximum value, value at 100 cd m−2. e Maximum current efficiency. f Maximum power efficiency. g Commission Internatinale de L'Eclairage coordinates measured at 9 V. | |||||||
| 20 wt% | 557 | 3.5 | 544 | 7.4/3.8 | 19.7 | 18.8 | (0.39, 0.46) |
| 30 wt% | 558 | 3.6 | 1655 | 9.1/7.3 | 23.6 | 21.0 | (0.40, 0.48) |
| 40 wt% | 561 | 3.3 | 5904 | 12.8/11.9 | 34.0 | 32.0 | (0.41, 0.50) |
| 50 wt% | 562 | 3.4 | 3689 | 11.1/9.9 | 29.2 | 27.7 | (0.41, 0.50) |
| 60 wt% | 562 | 3.3 | 4570 | 11.0/10.3 | 29.1 | 27.3 | (0.41, 0.50) |
| 100 wt% | 572 | 3.5 | 1045 | 10.2/8.2 | 27.0 | 25.3 | (0.42, 0.49) |
Conclusions
In summary, a novel yellow-emissive Cu4Br4 cluster, namely Cu4Br4(AcNP)2, was synthesized utilizing a D–A type bidentate N^P ligand. At room temperature, Cu4Br4(AcNP)2 exhibited efficient TADF with PLQYs of 47% and 38%, along with short decay times of 2.7 and 1.9 μs in 40 wt%-doped and neat films, respectively. OLEDs utilizing Cu4Br4(AcNP)2 as the emitter achieved high EL performance. The 40 wt%-doped device and non-doped device achieved maximum EQEs of 12.8% and 10.2%, respectively. The high efficiencies and well-suppressed concentration quenching observed in both PL and EL for this cluster are outstanding among copper(I)-based emitters and can be attributed to the distinctive D–A ligand configuration. This study offers insights into the design and structure–property relationships of copper(I)-based emitters with the aim of achieving highly efficient non-doped OLEDs.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 52073286 and 21805281), the Natural Science Foundation of Fujian Province (Grant No. 2006L2005), and the Fujian Science and Technology Innovation Laboratory for Optoelectronic Information of China (Grant No. 2021ZR132 and 2021ZZ115).References
- S. Reineke, F. Lindner, G. Schwartz, N. Seidler, K. Walzer, B. Lussem and K. Leo, White organic light-emitting diodes with fluorescent tube efficiency, Nature, 2009, 459, 234–238 CrossRef CAS PubMed.
- L. Xiao, Z. Chen, B. Qu, J. Luo, S. Kong, Q. Gong and J. Kido, Recent progresses on materials for electrophosphorescent organic light-emitting devices, Adv. Mater., 2011, 23, 926–952 CrossRef CAS PubMed.
- H. Sasabe and J. Kido, Development of high performance OLEDs for general lighting, J. Mater. Chem. C, 2013, 1, 1699–1707 RSC.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Very high-efficiency green organic light-emitting devices based on electrophosphorescence, Appl. Phys. Lett., 1999, 75, 4–6 CrossRef CAS.
- A. Endo, K. Sato, K. Yoshimura, T. Kai, A. Kawada, H. Miyazaki and C. Adachi, Efficient up-conversion of triplet excitons into a singlet state and its application for organic light emitting diodes, Appl. Phys. Lett., 2011, 98, 083302 CrossRef.
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 CrossRef CAS PubMed.
- J. Guo, X. L. Li, H. Nie, W. Luo, S. Gan, S. Hu, R. Hu, A. Qin, Z. Zhao, S. J. Su and B. Z. Tang, Achieving High–Performance Nondoped OLEDs with Extremely Small Efficiency Roll–Off by Combining Aggregation–Induced Emission and Thermally Activated Delayed Fluorescence, Adv. Funct. Mater., 2017, 27, 1060458 Search PubMed.
- R. Furue, T. Nishimoto, I. S. Park, J. Lee and T. Yasuda, Aggregation–Induced Delayed Fluorescence Based on Donor/Acceptor–Tethered Janus Carborane Triads: Unique Photophysical Properties of Nondoped OLEDs, Angew. Chem., Int. Ed., 2016, 55, 7171–7175 CrossRef CAS PubMed.
- W. Li, B. Li, X. Cai, L. Gan, Z. Xu, W. Li, K. Liu, D. Chen and S. J. Su, Tri–Spiral Donor for High Efficiency and Versatile Blue Thermally Activated Delayed Fluorescence Materials, Angew. Chem., Int. Ed., 2019, 58, 11301–11305 CrossRef CAS PubMed.
- N. Zhang, Y. Li, S. Han, Y. Wei, H. Hu, R. Huo, C. Duan, J. Zhang, C. Han, G. Xie and H. Xu, Cluster Light–Emitting Diodes Containing Copper Iodine Cube with 100% Exciton Utilization Using Host–Cluster Synergy, Angew. Chem., Int. Ed., 2023, 62, e202305018 CrossRef CAS PubMed.
- H. Wang, J. X. Chen, Y. Z. Shi, X. Zhang, L. Zhou, X. Y. Hao, J. Yu, K. Wang and X. H. Zhang, An A–D–A–Type Thermally Activated Delayed Fluorescence Emitter with Intrinsic Yellow Emission Realizing Record–High Red/NIR OLEDs upon Modulating Intermolecular Aggregations, Adv. Mater., 2023, 35, 2302984 CrossRef PubMed.
- T. Chatterjee and K. T. Wong, Perspective on Host Materials for Thermally Activated Delayed Fluorescence Organic Light Emitting Diodes, Adv. Opt. Mater., 2018, 7, 1800565 CrossRef.
- X. L. Chen, R. Yu, Q. K. Zhang, L. J. Zhou, X. Y. Wu, Q. Zhang and C. Z. Lu, Rational Design of Strongly Blue-Emitting Cuprous Complexes with Thermally Activated Delayed Fluorescence and Application in Solution-Processed OLEDs, Chem. Mater., 2013, 25, 3910–3920 CrossRef CAS.
- H. Yersin, A. F. Rausch, R. Czerwieniec, T. Hofbeck and T. Fischer, The triplet state of organo-transition metal compounds. Triplet harvesting and singlet harvesting for efficient OLEDs, Coord. Chem. Rev., 2011, 255, 2622–2652 CrossRef CAS.
- R. Czerwieniec, J. Yu and H. Yersin, Blue-Light Emission of Cu(I) Complexes and Singlet Harvesting, Inorg. Chem., 2011, 17, 8293–8301 CrossRef PubMed.
- T. Hofbeck, U. Monkowius and H. Yersin, Highly efficient luminescence of Cu(I) compounds TADF combined with short-lived phosphorescence, J. Am. Chem. Soc., 2014, 50, 8293–8301 Search PubMed.
- M. J. Leitl, V. A. Krylova, P. I. Djurovich, M. E. Thompson and H. Yersin, Phosphorescence versus thermally activated delayed fluorescence. Controlling singlet-triplet splitting in brightly emitting and sublimable Cu(I) compounds, J. Am. Chem. Soc., 2014, 136, 16032–16038 CrossRef CAS PubMed.
- X. L. Chen, C. S. Lin, X. Y. Wu, R. Yu, T. Teng, Q. K. Zhang, Q. Zhang, W. B. Yang and C. Z. Lu, Highly efficient cuprous complexes with thermally activated delayed fluorescence and simplified solution process OLEDs using the ligand as host, J. Mater. Chem. C, 2015, 3, 1187–1195 RSC.
- M. J. Leitl, D. M. Zink, A. Schinabeck, T. Baumann, D. Volz and H. Yersin, Copper(I) Complexes for Thermally Activated Delayed Fluorescence: From Photophysical to Device Properties, Top. Curr. Chem., 2016, 374, 1–34 CrossRef CAS PubMed.
- X. Zhang and H. Xu, Electroluminescent Clusters, Angew. Chem., Int. Ed., 2024, 63, e202317597 CrossRef CAS PubMed.
- N. Zhang, L. Qu, S. Dai, G. Xie, C. Han, J. Zhang, R. Huo, H. Hu, Q. Chen, W. Huang and H. Xu, Intramolecular charge transfer enables highly-efficient X-ray luminescence in cluster scintillators, Nat. Commun., 2023, 14, 2901 CrossRef CAS PubMed.
- D. F. Schrempp, S. Leingang, M. Schnurr, E. Kaifer, H. Wadepohl and H.-J. Himmel, Inter- and Intramolecular Electron Transfer in Copper Complexes: Electronic Entatic State with Redox-Active Guanidine Ligands, Chem. – Eur. J., 2017, 23, 13607–13611 CrossRef CAS PubMed.
- A. P. Borges, A. P. S. Gaspari, C. G. Oliveira, S. F. de Sousa, R. S. daSilva, V. M. Deflon, A. E. H. Machado, A. O. T. Patrocínio and P. I. S. Maia, Photophysical and DFT Studies of Cationic Ag(I) Complexes with Thiosemicarbazides Derived from p–Toluenesulfohydrazide, ChemistrySelect, 2018, 3, 2108–2114 CrossRef CAS.
- H. Yersin, R. Czerwieniec, M. Z. Shafikov and A. F. Suleymanova, TADF material design - Photophysical background and case studies focusing on Cu(I) and Ag(I) complexes, ChemPhysChem, 2017, 18, 3508–3535 CrossRef CAS PubMed.
- R. Czerwieniec, M. J. Leitl, H. H. H. Homeier and H. Yersin, Cu(I) complexes - Thermally activated delayed fluorescence. Photophysical approach and material design, Coord. Chem. Rev., 2016, 325, 2–28 CrossRef CAS.
- M. Xie, C. Han, J. Zhang, G. Xie and H. Xu, White Electroluminescent Phosphine-Chelated Copper Iodide Nanoclusters, Chem. Mater., 2017, 29, 6606–6610 CrossRef CAS.
- K. R. Kyle and P. C. Ford, Dynamic Quenching of the Metal-to-Ligand Charge-Transfer Excited State of CuJ^pyridine)^ Exciplex Formation and Self-Quenching, J. Am. Chem. Soc., 1989, 111, 5005–5006 CrossRef CAS.
- M. Xie, C. Han, Q. Liang, J. Zhang, G. Xie and H. Xu, Highly efficient sky blue electroluminescence from ligand-activated copper iodide clusters: Overcoming the limitations of cluster light-emitting diodes, Sci. Adv., 2019, 5, eaav9857 CrossRef CAS PubMed.
- Y. Fang, W. Liu, S. J. Teat, G. Dey, Z. Shen, L. An, D. Yu, L. Wang, D. M. O'Carroll and J. Li, A Systematic Approach to Achieving High Performance Hybrid Lighting Phosphors with Excellent Thermal– and Photostability, Adv. Funct. Mater., 2016, 27, 1603444 CrossRef.
- Y. Li, S. Xu, X. Zhang, Y. Man, J. Zhang, G. Zhang, S. Chen, C. Duan, C. Han and H. Xu, Bulk Passivation Enables Hundredfold–Enhanced Electroluminescence of Monophosphine Cu4I4 Cubes, Angew. Chem., Int. Ed., 2023, 62, e202308410 CrossRef CAS PubMed.
- Y. Li, X. Zhang, Y. Man, S. Xu, J. Zhang, G. Zhang, S. Chen, C. Duan, C. Han and H. Xu, Interfacial Passivation Enormously Enhances Electroluminescence of Triphenylphosphine Cu4I4 Cube, Adv. Mater., 2023, 35, 2302984 CrossRef CAS PubMed.
- N. Zhang, H. Hu, L. Qu, R. Huo, J. Zhang, C. Duan, Y. Meng, C. Han and H. Xu, Overcoming Efficiency Limitation of Cluster Light-Emitting Diodes with Asymmetrically Functionalized Biphosphine Cu4I4 Cubes, J. Am. Chem. Soc., 2022, 144, 6551–6557 CrossRef CAS PubMed.
- M. Olaru, E. Rychagova, S. Ketkov, Y. Shynkarenko, S. Yakunin, M. V. Kovalenko, A. Yablonskiy, B. Andreev, F. Kleemiss, J. Beckmann and M. Vogt, A Small Cationic Organo–Copper Cluster as Thermally Robust Highly Photo- and Electroluminescent Material, J. Am. Chem. Soc., 2019, 142, 373–381 CrossRef PubMed.
- N. Zhang, L. Qu, H. Hu, R. Huo, Y. Meng, C. Duan, J. Zhang, C. Han, G. Xie and H. Xu, Sky Blue and Yellow Cluster Light-Emitting Diodes Based on Asymmetric Cu4I4 Nanocubes, Research, 2022, 0005 CrossRef CAS.
- P. Roesch, J. Nitsch, M. Lutz, J. Wiecko, A. Steffen and C. Müller, Synthesis and Photoluminescence Properties of an Unprecedented Phosphinine–Cu4Br4 Cluster, Inorg. Chem., 2014, 53, 9855–9859 CrossRef CAS PubMed.
- J. Huang, H. Nie, J. Zeng, Z. Zhuang, S. Gan, Y. Cai, J. Guo, S.-J. Su, Z. Zhao and B. Z. Tang, Highly Efficient Nondoped Oleds with Negligible Efficiency Roll-Off Fabricated from Aggregation-Induced Delayed Fluorescence Luminogens, Angew. Chem., Int. Ed., 2017, 56, 12971–12976 CrossRef CAS PubMed.
- J. Guo, X.-L. Li, H. Nie, W. Luo, S. Gan, S. Hu, R. Hu, A. Qin, Z. Zhao, S.-J. Su and B. Z. Tang, Achieving High-Performance Nondoped Oleds with Extremely Small Efficiency Roll-Off by Combining Aggregation-Induced Emission and Thermally Activated Delayed Fluorescence, Adv. Funct. Mater., 2017, 27, 1606458 CrossRef.
- J. Lee, N. Aizawa, M. Numata, C. Adachi and T. Yasuda, Versatile Molecular Functionalization for Inhibiting Concentration Quenching of Thermally Activated Delayed Fluorescence, Adv. Mater., 2017, 29, 1604856 CrossRef PubMed.
- F. B. Dias, J. Santos, D. R. Graves, P. Data, R. S. Nobuyasu, M. A. Fox, A. S. Batsanov, T. Palmeira, M. N. Berberan-Santos, M. R. Bryce and A. P. Monkman, The Role of Local Triplet Excited States and D–A Relative Orientation in Thermally Activated Delayed Fluorescence: Photophysics and Devices, Adv. Sci., 2016, 3, 1600080 CrossRef PubMed.
- Y. L. Zhang, Q. Ran, Q. Wang, Y. Liu, C. Hänisch, S. Reineke, J. Fan and L. S. Liao, High–Efficiency Red Organic Light–Emitting Diodes with External Quantum Efficiency Close to 30% Based on a Novel Thermally Activated Delayed Fluorescence Emitter, Adv. Mater., 2019, 31, 1902368 CrossRef CAS PubMed.
- E. W. C. Shirota Jr., G. J. Meyer and L. X. Chen, Ultrafast Structural Rearrangements in the MLCT Excited State for Copper(I) bis-Phenanthrolines in Solution, J. Am. Chem. Soc., 2007, 129, 2147–2160 CrossRef PubMed.
- M. Iwamura, S. Takeuchi and T. Tahara, Ultrafast Excited-State Dynamics of Copper(I) Complexes, Acc. Chem. Res., 2015, 48, 782–791 CrossRef CAS PubMed.
- S. Garakyaraghi, C. E. McCusker, S. Khan, P. Koutnik, A. T. Bui and F. N. Castellano, Enhancing the Visible-Light Absorption and Excited-State Properties of Cu(I) MLCT Excited States, Inorg. Chem., 2018, 57, 2296–2307 CrossRef CAS PubMed.
- N. A. Gothard, M. W. Mara, J. Huang, J. M. Szarko, B. Rolczynski, J. V. Lockard and L. X. Chen, Strong Steric Hindrance Effect on Excited State Structural Dynamics of Cu(I) Diimine Complexes, J. Phys. Chem. A, 2012, 116, 1984–1992 CrossRef CAS PubMed.
- X. L. Chen, C. S. Lin, X. Y. Wu, R. Yu, T. Teng, Q. K. Zhang, Q. Zhang, W. B. Yang and C. Z. Lu, Highly efficient cuprous complexes with thermally activated delayed fluorescence and simplified solution process OLEDs using the ligand as host, J. Mater. Chem. C, 2015, 3, 1187–1195 RSC.
- Y. Watanabe, B. M. Washer, M. Zeller, S. Savikhin, L. V. Slipchenko and A. Wei, Copper(I)-Pyrazolate Complexes as Solid-State Phosphors: Deep-Blue Emission through a Remote Steric Effect, J. Am. Chem. Soc., 2022, 144, 10186–10192 CrossRef CAS PubMed.
- B. P. Rand, H. Yersin, C. Adachi, R. Czerwieniec, A. Hupfer and V. van Elsbergen, The Triplet State of fac-Ir(ppy)3, Org. Photonics V, 2012, 8435, 843508 Search PubMed.
- T. Hofbeck and H. Yersin, Singlet harvesting with brightly emitting Cu(I) and metal-free organic compounds, Inorg. Chem., 2010, 49, 9290–9299 CrossRef CAS PubMed.
- H. Yersin, A. F. Rausch and R. Czerwieniec, Organometallic Emitters for OLEDs: Triplet Harvesting, Singlet Harvesting, Case Structures, and Trends, Phys. Org. Semicond., 2012, 371–424 CAS.
- V. Bulovic, R. Deshpande, M. E. Thompson and S. R. Forrest, Tuning the color emission of thin film molecular organic light emitting devices by the solid state solvation effect, Chem. Phys. Lett., 1999, 308, 317–322 CrossRef CAS.
- T. Northey, J. Stacey and T. J. Penfold, The role of solid state solvation on the charge transfer state of a thermally activated delayed fluorescence emitter, J. Mater. Chem. C, 2017, 5, 11001–11009 RSC.
- B. L. Cotts, D. G. McCarthy, R. Noriega, S. B. Penwell, M. Delor, D. D. Devore, S. Mukhopadhyay, T. S. De Vries and N. S. Ginsberg, Tuning Thermally Activated Delayed Fluorescence Emitter Photophysics through Solvation in the Solid State, ACS Energy Lett., 2017, 2, 1526–1533 CrossRef CAS.
- H. Liu, J. Zeng, J. Guo, H. Nie, Z. Zhao and B. Z. Tang, Highly Efficient Nondoped OLEDs with Negligible Efficiency Roll-Off Fabricated from Aggregation-Induced Delayed Fluorescence Luminogens, Angew. Chem., Int. Ed., 2018, 57, 9290–9294 CrossRef CAS PubMed.
- R. J. Holmes, S. R. Forrest, Y. J. Tung, R. C. Kwong, J. J. Brown, S. Garon and M. E. Thompson, Blue organic electrophosphorescence using exothermic host–guest energy transfer, Appl. Phys. Lett., 2003, 82, 2422–2424 CrossRef CAS.
- X. Gong, J. C. Ostrowski, D. Moses, G. C. Bazan and A. J. Heeger, Electrophosphorescence from a Polymer Guest-Host System with an Iridium Complex as Guest: Förster Energy Transfer and Charge Trapping, Adv. Funct. Mater., 2003, 13, 439–444 CrossRef CAS.
- M. Klein, N. Rau, M. Wende, J. Sundermeyer, G. Cheng, C. M. Che, A. Schinabeck and H. Yersin, Cu(I) and Ag(I) Complexes with a New Type of Rigid Tridentate N,P,P-Ligand for Thermally Activated Delayed Fluorescence and OLEDs with High External Quantum Efficiency, Chem. Mater., 2020, 32, 10365–10382 CrossRef CAS.
- D. Liang, X. L. Chen, J. Z. Liao, J. Y. Hu, J. H. Jia and C. Z. Lu, Highly Efficient Cuprous Complexes with Thermally Activated Delayed Fluorescence for Solution-Processed Organic Light-Emitting Devices, Inorg. Chem., 2016, 55, 7467–7475 CrossRef CAS PubMed.
- X. Liu, T. Zhang, T. Ni, N. Jiang, Z. Liu, Z. Bian, Z. Lu and C. Huang, Co-deposited Cu(I) Complex for Tri-layered Yellow and White Organic Light-Emitting Diodes, Adv. Funct. Mater., 2014, 24, 5385–5392 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2323855. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4qi00210e |
| This journal is © the Partner Organisations 2024 |