Geometric isomers of asymmetric rigid four-membered chelating ring based deep-red-emitting iridium complexes featuring three charged (0, −1, −2) ligands†
Ruoqi
Zeng
a,
Nengquan
Li
 b,
Feiyang
Li
b,
Feiyang
Li
 a,
Chao
Shi
a,
Chao
Shi
 *a,
Zhen
Jiang
a,
Fuzheng
Zhang
a,
Qiuxia
Li
*a,
Kaishun
Ye
a,
Aihua
Yuan
*a,
Zhen
Jiang
a,
Fuzheng
Zhang
a,
Qiuxia
Li
*a,
Kaishun
Ye
a,
Aihua
Yuan
 *a and
Chuluo
Yang
*a and
Chuluo
Yang
 *b
*b
aSchool of Environmental and Chemical Engineering, Jiangsu University of Science and Technology, Zhenjiang 212003, P. R. China. E-mail: shichao@just.edu.cn; liqiuxia2019@just.edu.cn; aihua.yuan@just.edu.cn
bCollege of Materials Science and Engineering, Shenzhen University, Shenzhen 518060, P. R. China. E-mail: clyang@szu.edu.cn
First published on 19th April 2023
Abstract
Geometric isomers are very important and interesting in the field of optical materials. In this work, we have designed and synthesized a new series of geometric isomers of iridium complexes featuring three charged (0, −1, −2) ligands, which contain a rigid asymmetric four-membered Ir–N–C–S chelating ring. The reaction of iridium complex precursors (2a and 2b) with an equal amount of the four-membered ring S^N ligand at a low temperature produces the kinetic isomers Ir1K and Ir2K, while a higher temperature leads to the formation of their corresponding thermodynamic isomers Ir1T and Ir2T. The X-ray diffraction analysis shows that the kinetic isomers exhibit a trans-S^N configuration, which is in contrast with the trans-N^N configuration of the thermodynamic isomers, and their coordination bond lengths, bond angles and packing patterns are also quite different. More importantly, all isomers showed efficient deep-red emission (619–676 nm), and the thermodynamic isomers have shorter emission wavelengths, longer excited state lifetimes and higher luminescent efficiencies than their corresponding kinetic isomers. Theoretical calculations show that the four-membered ring S^N ligand in the thermodynamic isomers is more involved in the excited state than that in the kinetic isomers, and the 3MLCT effects are more pronounced in the thermodynamic isomers. Notably, OLED devices incorporating both thermodynamic and kinetic isomers (Ir2T and Ir2K) as emitting layers can achieve good maximum external quantum efficiencies (EQEs) (5.0% and 4.6%) peaking at 642 nm and 643 nm of the deep-red region with CIE coordinates (0.675, 0.323) and (0.668, 0.329), respectively, accompanied by a low turn-on voltage (3.0 V). This study provides an important strategy for the design of deep-red emitting geometric isomers of iridium complexes and their photoelectric applications.
Introduction
As a kind of important optical material, phosphorescent iridium complexes with octahedral structure are widely used in various optical fields, including organic light-emitting diodes (OLEDs),1–5 solar cells,6–8 chemical sensing,9,10 biological imaging,11–13 photocatalysts,14etc. Notably, geometric isomers are very important and interesting in this class of octahedral coordination structures and can often lead to a great difference in the photophysical, electrochemical and thermodynamic properties of the complexes. For example, in the classical phosphorescent iridium complex system with three monoanionic (−1, −1, −1) ligands (Scheme 1a), the facial isomer fac-Ir(ppy)3 (ppy is 2-phenylpyridine) is thermodynamically stable and exhibits strong green emission, while the meridional isomer mer-Ir(ppy)3 is kinetically stable and exhibits a relatively weak yellow emission (Scheme 1a).15–17 However, most of the geometric isomers reported to date exist in iridium complexes with three monoanionic (−1, −1, −1) ligands,15–22 while none have been reported in three charged (0, −1, −2) ligand based iridium complexes (Fig. 1a),23–27 which have been developed in recent years and tend to emit red or near-infrared light more easily. Therefore, it is of great significance to study the synthesis, structures and optical properties of geometric isomers in such new three-charged (0, −1, −2) ligand systems.On the other hand, compared with five or six-membered chelating ring based iridium complexes, four-membered chelating ring based iridium complexes often have smaller coordination angles, which leads to greater coordination strain energy.28,29 In addition, an iridium complex with a four-membered ring structure can also shorten the distance between the ligand and the iridium atom, which can further regulate and improve the optical properties of the complex. Nevertheless, the four-membered ring ligands reported so far are mainly the symmetric ligands30–35 (Scheme 1b), which mainly contain two coordinated nitrogen or sulfur atoms and eventually form the Ir–N–C–N or Ir–S–C–S backbone (Scheme 1b), while asymmetric ligands with the Ir–N–C–S backbone are rarely reported,36 and these coordination atoms are not in a rigid structural framework, which is not beneficial for the improvement of the luminescence efficiency. Notably, benzo[d]thiazole-2-thiol is a cheap, low cost industrial raw material with a rigid coordination skeleton (Scheme 1b), which is an ideal asymmetric four-membered ring S^N ligand.
Based on the above considerations, we have constructed a new series of geometric isomers (Ir1K, Ir2K, Ir1T and Ir2T) of three charged (0, −1, −2) ligand based iridium complexes, in which benzo[d]thiazole-2-thiol is used as an asymmetric rigid four-membered ring monoanionic (−1) ligand, biphenyl as a dianionic (−2) ligand, and 2,2′-bipyridyl or 1,10-phenanthroline as a neutral (0) ligand (Fig. 1a and b). Notably, the reaction of the iridium complex precursors (2a and 2b) with an equal amount of the benzo[d]thiazole-2-thiol ligand at a low temperature produces the kinetic isomers Ir1K and Ir2K, respectively, while a higher temperature leads to the formation of their corresponding thermodynamic isomers (Ir1T and Ir2T). The X-ray diffraction analysis shows that the kinetic isomers exhibit a trans-S^N configuration, which is in contrast with the trans-N^N configuration of the thermodynamic isomers, and their coordination bond lengths, bond angles and packing patterns are also quite different. More importantly, all isomers showed efficient deep-red emission (619–676 nm), and the thermodynamic isomers have shorter emission wavelengths, longer excited state lifetimes and higher luminescent efficiencies than their corresponding kinetic isomers. Theoretical calculations show that the four-membered ring S^N ligand in the thermodynamic isomers is more involved in the excited state than that in the kinetic isomers, and the 3MLCT effects are more pronounced in the thermodynamic isomers. In addition, OLED devices incorporating these isomers as emitting layers can also achieve efficient deep-red emission. Notably, both thermodynamic and kinetic isomers (Ir2T and Ir2K) can achieve good maximum external quantum efficiencies (EQEs) (5.0% and 4.6%) peaking at 642 nm and 643 nm with CIE coordinates (0.675, 0.323) and (0.668, 0.329), respectively, accompanied by a low turn-on voltage (3.0 V).
Results and discussion
Synthesis and X-ray single structures
All of the geometric isomers of the iridium(III) complex can be prepared using a three-step process (Fig. 1b and Fig. S1†). First, the iridium complex precursors (2a and 2b) can be obtained from biphenylene and the iridium(I) complex [Ir(COD)Cl]2 using the previously reported two-step method.23,24 Then the reaction of the iridium complex precursors (2a and 2b) with an equal amount of the four-membered ring S^N ligand at a low temperature (90 °C) produces the kinetic isomers Ir1K and Ir2K, while a higher temperature (130 °C) leads to the formation of their corresponding thermodynamic isomers (Ir1T and Ir2T). (Fig. S1, see the ESI for details†). Next, the structures of the new geometric isomers of the iridium(III) complex were fully characterized and confirmed by 1H NMR, 13C NMR, X-ray single structure, mass spectrometry and elemental analysis. Notably, the small structural changes between the thermodynamic isomers and kinetic isomers can be well reflected in the 1H NMR spectra. For example, the thermodynamic isomer Ir2T and the kinetic isomer Ir2K show significant differences in the 1H NMR aromatic ring region (Fig. 1c). Additionally, according to the calculated sum of electronic and zero-point energies (E0) and the sum of electronic and thermal free energies (G) of all complexes (Fig. 1d), the values of Ir1T (E0 = −2182.1380 Hartree, G = −2182.1974 Hartree) and Ir2T (E0 = −2258.3575 Hartree, G = −2258.3173 Hartree) are smaller than those of their corresponding isomers Ir1K (E0 = −2182.1339 Hartree, G = −2182.1939 Hartree) and Ir2K (E0 = −2258.3537 Hartree, G = −2258.4140 Hartree), which further indicates that both Ir1T and Ir2T are thermodynamically stable products, while Ir1K and Ir2K are kinetically stable products.The single-crystals of Ir1K, Ir1T and Ir2T were all gained by slow diffusion of ethanol into their CH2Cl2 solutions, respectively. The single-crystal of Ir2K was obtained by slow diffusion of ethanol into its CHCl3 solution. The X-ray diffraction analysis shows that all iridium complexes possess a four-membered ring Ir–N–C–S skeleton (Fig. 2a and Fig. S2–S5, Table S1†), in which the Ir–S bond lengths in the thermodynamic isomers (Ir1T: Ir1–S1: 2.565 Å, Ir2T: Ir1–S1: 2.586 Å) are significantly longer than those in the corresponding kinetic isomers (Ir1K: Ir1–S1: 2.379 Å, Ir2K: Ir1–S1: 2.377 Å), while the Ir–N bond lengths in the thermodynamic isomers (Ir1T: Ir1–N3: 2.058 Å, Ir2T: Ir1–N3: 2.057 Å) are shorter than those in the corresponding kinetic isomers (Ir1K: Ir1–N3: 2.216 Å, Ir2K: Ir1–N3: 2.196 Å) (Fig. 2a). Furthermore, the coordination bond angles in the thermodynamic isomers (Ir1T: N3–Ir1–S1: 66.22°, Ir2T: N3–Ir1–S1: 65.70°) are smaller than those in the corresponding kinetic isomers (Ir1K: N3–Ir1–S1: 67.37°, Ir2K: N3–Ir1–S1: 67.92°) (Fig. 2a and Fig. S2–S5†). Notably, the kinetic isomers (Ir1K and Ir2K) exhibit a trans-S^N configuration between the monoanionic (−1) ligand and the neutral (0) ligand, which is in contrast with the trans-N^N configuration of the thermodynamic isomers (Ir1T and Ir2T). Another interesting structural feature is the intermolecular interactions of the complexes Ir2K and Ir2T, which exhibit two distinct π–π stacking modes, respectively (Fig. 2b and c). For the kinetic isomer Ir2K, the first intermolecular π–π stacking mode is derived from two four-membered ring monoanionic (−1) ligands, benzo[d]thiazole-2-thiol, and the interaction distance is 3.32 Å (mode 1, Fig. 2b). The second intermolecular π–π stacking mode is derived from two neutral (0) ligands, 1,10-phenanthroline, and the interaction distance is 3.42 Å (mode 2, Fig. 2b). For the thermodynamic isomer Ir2T, both stacking modes are derived from the neutral (0) ligand 1,10-phenanthroline (Fig. 2c), and the interaction distances are 3.48 Å (mode 1) and 3.52 Å (mode 2), respectively.
Photophysical and electrochemical properties
The photophysical properties of all geometric isomers of iridium complexes were studied by spectroscopy (Fig. 3 and Fig. S6–S9, Table 1†). From the UV/visible absorption spectra, the intense absorption peaks below 360 nm can be assigned to the π → π* and the n → π* of all ligands (Fig. 3a and Table 1). The weaker low-energy absorption bands are mainly located in the 400–700 nm range, which can be attributed to the metal-to-ligand charge transfer (MLCT) and the ligand-to-ligand charge transfer (LLCT) transitions. Interestingly, the lowest absorption energy bands of the thermodynamic isomers (Ir1T and Ir2T) are slightly blue shifted compared to those of their corresponding kinetic isomers (Ir1K and Ir2K) (Fig. 3a), which is consistent with the calculated UV spectra (Fig. S13†). This result indicates that the excited state energy of the thermodynamic isomers is higher than that of the kinetic isomers. The photoluminescence spectra of the geometric isomers in dichloromethane solution also showed the same trend (Fig. 3b and Table 1). The kinetic isomers (Ir1K: 676 nm, Ir2K: 672 nm) show distinct deep-red emission and exhibit significant red shift relative to their corresponding thermodynamic isomers (Ir1T: 660 nm, Ir2T: 646 nm). Furthermore, the PS film (1 wt%) of the kinetic isomers (Ir1K: 660 nm, Ir2K: 643 nm) also showed obvious redshift emission compared to the thermodynamic isomers (Ir1T: 624 nm, Ir2T: 619 nm) (Fig. 3c and Table 1).| Complex |
λ
abs (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) ε)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a (nm) a (nm) |
PL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a,b (nm) a,b (nm) |
E Ox1/2 (eV) |
E
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (eV) c (eV) |
HOMO/LUMOc (eV) |
Φ
PL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a,b (%) a,b (%) |
τ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (ns) b (ns) |
|---|---|---|---|---|---|---|---|
| a Recorded in CH2Cl2 (2 × 10−5 M) at 298 K with an excitation wavelength of 370 nm. Φp is referred to absolute quantum yields of phosphorescence determined by employing an integrating sphere. b Recorded in PS films (1 wt%) (excitation wavelength 370 nm). c The HOMO (eV) = −(Eonsetox + 4.8) eV, Eg = 1240/λ, λ is the absorption wavelength threshold. LUMO (eV) = Eg + HOMO. | |||||||
| Ir1K | 229(4.78); 295(4.45); 329(4.24); 361(4.00); 441(3.46); 568(3.03); 612(2.36) | 676/660 | 0.25 | 1.83 | −5.05/−3.22 | 3/6 | 557 |
| Ir1T | 228(4.75); 288(4.47); 325(4.18); 361(3.97); 440(3.49); 559(3.20); 606(3.06) | 660/624 | 0.29/0.44/0.69 | 1.97 | −5.09/−3.12 | 6/8 | 1198 |
| Ir2K | 229(4.87); 264(4.69); 324(4.21); 361(3.91); 432(3.60); 565(3.07); 609(1.93) | 672/643 | 0.24 | 1.89 | −5.04/−3.15 | 4/10 | 1167 |
| Ir2T | 227(4.76); 263(4.59); 324(4.03); 361(4.05); 435(3.46); 552(3.11); 603(2.86) | 646/619 | 0.27/0.45/0.72 | 2.00 | −5.07/−3.07 | 8/12 | 2385 |
Interestingly, the excited state lifetimes of the thermodynamic isomers (τ = 1198 ns for Ir1T, τ = 2385 ns for Ir2T) are significantly longer than those of their corresponding kinetic isomers (τ = 557 ns for Ir1K, τ = 1167 ns for Ir2K) (Fig. 3d and Table 1), which indicates that stereoisomerism has a strong effect on the excited state lifetime of the iridium complex. Notably, the thermodynamic isomers exhibit a higher phosphorescence efficiency than their corresponding kinetic isomers in both solution and solid films (Table 1). Additionally, the photoluminescence and absorption spectra of all complexes in different solvents are recorded (Fig. S6–S9†). It is found that all complexes exhibit some solvation effect, especially the kinetic isomers Ir1K and Ir2K, whose maximum PL spectrum bathochromic shifts are 58 nm and 55 nm, respectively, which can be attributed to the CT excited state properties of the complexes. The electrochemical properties of all geometric isomers of iridium complexes in dichloromethane solution were evaluated by cyclic voltammetry (Fig. S10† and Table 1). Both the kinetic isomers exhibit only one irreversible oxidation peak (0.25 eV for Ir1K and 0.24 eV for Ir2K), which can be attributed to the dianionic (−2) biphenyl ligand. In contrast, the thermodynamic isomers not only showed the oxidation peak (0.29 eV for Ir1T and 0.27 eV for Ir2T) of the biphenyl ligand, but also showed the reversible oxidation peak (0.69 eV for Ir1T and 0.72 eV for Ir2T) of the metal iridium and the irreversible oxidation peak (0.44 eV for Ir1T and 0.45 eV for Ir2T) of the four-membered ring ligand. This result demonstrates that stereoisomerism can greatly affect the redox properties of iridium complexes. Furthermore, the HOMO values of the thermodynamic isomers (−5.09 eV for Ir1T and −5.07 eV for Ir2T) are lower than those of their corresponding kinetic isomers (−5.05 eV for Ir1K and −5.04 eV for Ir2K), suggesting that the spatial configuration of the thermodynamic isomers is conducive to increasing the HOMO level of complexes. In addition, the thermogravimetric (TG) experiment showed that the thermodynamic isomers (Ir1T: 260 °C and Ir2T: 335 °C) have a higher decomposition temperature than kinetic isomers (Ir1K: 146 °C and Ir2K: 229 °C) and thus exhibit better thermal stability (Fig. S11†).
Theoretical calculations
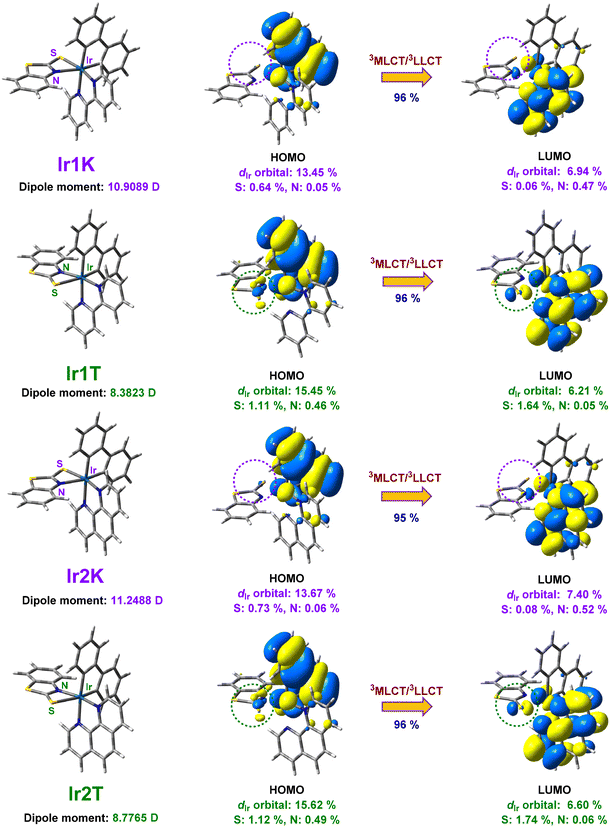
In order to elucidate the excited state properties of the novel complexes described above and to account for the large optical differences between the thermodynamic isomers (Ir1T and Ir2T) and the kinetic isomers (Ir1K and Ir2K), TD-DFT calculations were carried out at the B3LYP/6-31G(d) level (Fig. 4 and Fig. S12–S14, Table S2†). The lowest triplet excited state (T1) of all geometric isomers is characterized mainly by the HOMO → LUMO transitions (Fig. 4 and Table S2†). The HOMOs of these isomers are mainly concentrated on the metal iridium center (dIr orbital: 13.45% for Ir1K, 15.45% for Ir1T, 13.67% for Ir2K, 15.62% for Ir2T) and the dianionic (−2) biphenyl ligand, while their LUMOs are mainly distributed on the entire neutral (0) ligand (2,2′-bipyridyl or 1,10-phenanthroline) and part of the metal iridium center (dIr orbital: 6.94% for Ir1K, 6.21% for Ir1T, 7.40% for Ir2K, 6.60% for Ir2T). As a result, the T1 of all geometric isomers shows significant charge transfer from both the metal-to-ligand charge-transfer (MLCT) and the dianionic (−2) ligand to the neutral (0) ligand (LLCT). Notably, the 3MLCT effects on the thermodynamic isomers (Ir1T: 9.35%, Ir2T: 9.16%) are more pronounced than those on the kinetic isomers (Ir1K: 7.06%, Ir2K: 6.87%), which can explain the relatively high phosphorescence efficiency of the thermodynamic isomers compared to that of the kinetic isomers.Interestingly, the HOMO and LUMO distributions of S and N coordination atoms from the four-membered ring ligand in the kinetic isomers (HOMOs: S: 0.64% and N: 0.05% for Ir1K, S: 0.73% and N: 0.06% for Ir2K, LUMOs: S: 0.06% and N: 0.47% for Ir1K, S: 0.08% and N: 0.52% for Ir2K) are significantly lower than those of N and S coordination atoms from the four-membered ring ligand in the thermodynamic isomers (HOMOs: S: 1.11% and N: 0.46% for Ir1T, S: 1.12% and N: 0.49% for Ir2T, LUMOs: S: 1.64% and N: 0.05% for Ir1T, S: 1.74% and N: 0.06% for Ir2T) (Fig. 4). This result suggests that the four-membered ring S^N ligand in the thermodynamic isomers is more involved in the excited state than that in the kinetic isomers. Additionally, we also calculate the T1 level and band gap (Eg) of all geometric isomers (Fig. S14 and Table S2†), which are in good agreement with their corresponding experimental results, so it can also explain the difference in the luminescent wavelength of these isomers. In addition, the calculated dipole moments of all isomers at the T1 state show that the polarity of the thermodynamic isomers (Ir1T: 8.3823 D, Ir2T: 8.7765 D) is significantly lower than that of the kinetic isomers (Ir1K: 10.9089 D, Ir2K: 11.2488 D) (Fig. 4), which is in accordance with that in the S0 state (Fig. S12†).
OLED device application
To evaluate their electroluminescence (EL) performance, all geometric isomers of iridium complexes were tested as emitters in typical OLED devices with the same architecture as follows: ITO/HAT-CN (5 nm)/TAPC (30 nm)/TCTA (15 nm)/mCBP (10 nm)/DMIC-TRZ (85%): Ir dopant (15%) (45 nm)/PO-T2T (35 nm)/ANT-BIZ (30 nm)/Liq (2 nm)/Al (100 nm) (Fig. 5a), in which HAT-CN is hexaazatriphenylenehexacarbonitrile, TAPC is 4,4′-cyclohexylidenebis[N,N-bis(p-tolyl)aniline], TCTA is 4,4′,4′′-tris(carbazol-9-yl)-triphenylamine, mCBP is 3,3′-di(9H-carbazol-9-yl)biphenyl, DMIC-TRZ is 5-(3-(4,6-diphenyl-1,3,5-triazin-2-yl)phenyl)-7,7-dimethyl-5,7-dihydroindeno[2,1-b]carbazole, PO-T2T is 2,4,6-tris[3-(diphenylphosphinyl)phenyl]-1,3,5-triazine, ANT-BIZ is 1-(4-(10-([1,1′-biphenyl]-4-yl)anthracen-9-yl)-phenyl)-2-ethyl-1H-benzo[d]imidazole, and Liq is (8-quinolinolato)lithium. DMIC-TRZ is used as the host material, PO-T2T and ANT-BIZ as electron transport materials, TAPC and TCTA as hole transport materials, and mCBP as the exciton blocking material. The fabricated devices with geometric isomers of iridium complexes (Ir1K: 650 nm, Ir1T: 639 nm, Ir2K: 643 nm and Ir2T: 642 nm) all exhibit efficient deep-red emission with CIEx,y coordinates (Ir1K: (0.657, 0.338), Ir1T: (0.663, 0.337), Ir2K: (0.668, 0.329) and Ir2T: (0.675, 0.323)) originating only from themselves (Fig. 5b, c and Table 2), suggesting the effective energy transfer from the host material to the dopant.| Complex |
V
on![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [V] a [V] |
EQEmax/CEmax/PEmax![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b [%/cd A−1/lm W−1] b [%/cd A−1/lm W−1] |
λ
ems![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c [nm] c [nm] |
CIE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (x, y) d (x, y) |
|---|---|---|---|---|
| a Voltage at the luminance of 1 cd m−2. b Maximum external quantum efficiency (EQEmax), maximum current efficiency (CEmax), and maximum power efficiency (PEmax). c Maximum emission wavelength of the EL spectra. d The Commission Internationale de I′Eclairage (CIE) coordinates. | ||||
| Ir1K | 3.6 | 2.3/1.5/1.5 | 650 | (0.657, 0.338) |
| Ir1T | 3.5 | 2.6/1.9/1.8 | 639 | (0.663, 0.337) |
| Ir2K | 3.0 | 4.6/2.9/2.7 | 643 | (0.668, 0.329) |
| Ir2T | 3.0 | 5.0/3.1/3.0 | 642 | (0.675, 0.323) |
Notably, the device external quantum efficiencies (EQEs) of the complexes Ir2T and Ir2K are 5.0% and 4.6%, respectively (Fig. 5e and Table 2), which are significantly higher than those of the complexes Ir1T (2.6%) and Ir1K (2.3%), resulting from the fact that the 1,10-phenanthroline ligand is more rigid than the 2,2′-bipyridyl ligand. Furthermore, the thermodynamic isomers (Ir1T and Ir2T) show a higher EQE than their corresponding kinetic isomers (Ir1K and Ir2K). In addition, the devices with complexes Ir2T and Ir2K exhibit a lower turn-on voltage (3.0 V) than those with complexes Ir1T (3.5 V) and Ir1K (3.6 V) (Fig. 5d and Table 2), which may be due to the presence of the 1,10-phenanthroline ligand leading to better carrier transport of Ir2K and Ir2T.
Conclusion
A new series of asymmetric four-membered Ir–N–C–S chelating ring based geometric isomers (kinetic and thermodynamic) of iridium complexes featuring three charged (0, −1, −2) ligands have been designed and synthesized. Notably, the kinetic isomers exhibit a trans-S^N configuration, which is in contrast with the trans-N^N configuration of the thermodynamic isomers, and their coordination bond lengths, bond angles and packing patterns are also quite different. More importantly, all isomers showed effective deep-red emission (619–676 nm), and the thermodynamic isomers have shorter emission wavelengths, longer excited state lifetimes and higher luminescent efficiencies than their corresponding kinetic isomers. Theoretical calculations show that the four-membered ring ligand in the thermodynamic isomers is more involved in excited states than those in the kinetic isomers. In addition, OLED devices incorporating these isomers as emitting layers can also achieve efficient deep-red emission. Notably, both thermodynamic and kinetic complexes (Ir2T and Ir2K) with a 1,10-phenanthroline ligand can achieve good maximum external quantum efficiencies (EQEs) (5.0% and 4.6%) peaking at 642 nm and 643 nm with CIE coordinates (0.675, 0.323) and (0.668, 0.329), accompanied by a low turn-on voltage (3.0 V). This research provides an important strategy for the design and photoelectric applications of deep-red emitting geometric isomers of iridium complexes.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (22171109 and 22001097), the Natural Science Foundation of Jiangsu Province of China (BK20201003), the Postdoctoral Research Foundation of China (2021M701657) and the Shenzhen Science and Technology Program (KQTD20170330110107046).References
- M. A. Baldo, M. E. Thompson and S. R. Forrest, High-efficiency fluorescent organic light-emitting devices using a phosphorescent sensitizer, Nature, 2000, 403, 750–753 CrossRef CAS PubMed.
- G. Lu, Z. G. Wu, R. Wu, X. Cao, L. Zhou, Y.-X. Zheng and C. Yang, Semitransparent Circularly Polarized Phosphorescent Organic Light-Emitting Diodes with External Quantum Efficiency over 30% and Dissymmetry Factor Close to 10–2, Adv. Funct. Mater., 2021, 31, 2102898–2102905 CrossRef CAS.
- Q. Li, C. Shi, M. Huang, C. Wu, H. Wang, H. Wu, Y. Zheng, C. Yang and A. Yuan, Three Types of Charged Ligands Based Carboxyl-Containing Iridium(III) Complexes: Structures, Photophysics, and Solution Processed OLED Application, Inorg. Chem., 2021, 60, 17699–17704 CrossRef CAS PubMed.
- M. Penconi, A. B. Kajjam, M.-C. Jung, M. Cazzaniga, C. Baldoli, D. Ceresoli, M. E. Thompson and A. Bossi, Advancing Near-Infrared Phosphorescence with Heteroleptic Iridium Complexes Bearing a Single Emitting Ligand: Properties and Organic Light-Emitting Diode Applications, Chem. Mater., 2022, 34, 574–583 CrossRef CAS.
- M. Wu, N. Li, C. Shi, J. Song, R. Zeng, F. Li, Q. Li, A. Yuan and C. Yang, A Narrowband Red-Emitting Asymmetric Iridium(III) Complex Featuring B- and N-Embedded π-Conjugation Units: Structure,Photophysics and OLED Application, Inorg. Chem. Front., 2023, 10, 1262–1269 RSC.
- M. Qian, R. Zhang, J. Hao, W. Zhang, Q. Zhang, J. Wang, Y. Tao, S. Chen, J. Fang and W. Huang, Dramatic Enhancement of Power Conversion Efficiency in Polymer Solar Cells by Conjugating Very Low Ratio of Triplet Iridium Complexes to PTB7, Adv. Mater., 2015, 27, 3546–3552 CrossRef CAS PubMed.
- Y. Jin, Y. Zhang, Y. Liu, J. Xue, W. Li, J. Qiao and F. Zhang, Limitations and Perspectives on Triplet-Material-Based Organic Photovoltaic Devices, Adv. Mater., 2019, 31, 1900690 CrossRef PubMed.
- M. Qian, R. Zhang, J. Hao, W. Zhang, Q. Zhang, J. Wang, Y. Tao, S. Chen, J. Fang and W. Huang, Metallated terpolymer donors with strongly absorbing iridium complex enables polymer solar cells with 16.71% efficiency, Chem. Eng. J., 2022, 430, 132832 CrossRef.
- J. Zhou, J. Li, K. Y. Zhang, S. Liu and Q. Zhao, Phosphorescent iridium(III) complexes as lifetime-based biological sensors for photoluminescence lifetime imaging microscopy, Coord. Chem. Rev., 2022, 453, 214334 CrossRef CAS.
- Q. Li, C. Shi, M. Huang, X. Wei, H. Yan, C. Yang and A. Yuan, B- and N-embedded color-tunable phosphorescent iridium complexes and B–N Lewis adducts with intriguing structural and optical changes, Chem. Sci., 2019, 10, 3257–3263 RSC.
- K. Y. Zhang, Q. Yu, H. Wei, S. Liu, Q. Zhao and W. Huang, Long-Lived Emissive Probes for Time-Resolved Photoluminescence Bioimaging and Biosensing, Chem. Rev., 2018, 118, 1770–1839 CrossRef CAS PubMed.
- J. Shen, T. W. Rees, L. Ji and H. Chao, Recent advances in ruthenium(II) and iridium(III) complexes containing nanosystems for cancer treatment and bioimaging, Coord. Chem. Rev., 2021, 443, 214016–214038 CrossRef CAS.
- T. Yoshihara, N. Matsumura, T. Tamura, S. Shiozaki and S. Tobita, Intracellular and Intravascular Oxygen Sensing of Pancreatic Tissues Based on Phosphorescence Lifetime Imaging Microscopy UsingLipophilic and Hydrophilic Iridium(III) Complexes, ACS Sens., 2022, 7, 545–554 CrossRef CAS PubMed.
- D. N. Tritton, F.-K. Tang, G. B. Bodedla, F. W. Lee, C.-S. Kwan, K. C.-F. Leung, X. Zhu and W.-Y. Wong, Development and advancement of iridium(III)-based complexes for photocatalytic hydrogen evolution, Coord. Chem. Rev., 2022, 459, 214390 CrossRef CAS.
- A. B. Tamayo, B. D. Alleyne, P. I. Djurovich, S. Lamansky, I. Tsyba, N. N. Ho, R. Bau and M. E. Thompson, Synthesis and Characterization of Facial and Meridional Tris-cyclometalated Iridium(III) Complexes, J. Am. Chem. Soc., 2003, 125, 7377–7387 CrossRef CAS PubMed.
- C. Shi, H. Sun, X. Tang, W. Lv, H. Yan, Q. Zhao, J. Wang and W. Huang, Variable Photophysical Properties of Phosphorescent Iridium(III) Complexes Triggered by closo- and nido-Carborane Substitution, Angew. Chem., Int. Ed., 2013, 52, 13434–13438 CrossRef CAS PubMed.
- A. Y. Gitlina, F. Fadaei-Tirani, A. Ruggi, C. Plaice and K. Severin, Acid-base-induced fac→mer isomerization of luminescent iridium(III) complexes, Chem. Sci., 2022, 13, 10370–10374 RSC.
- J. Lee, H.-F. Chen, T. Batagoda, C. Coburn, P. I. Djurovich, M. E. Thompson and S. R. Forrest, Deep blue phosphorescent organic light-emitting diodes with very high brightness and efficiency, Nat. Mater., 2016, 15, 92–98 CrossRef CAS PubMed.
- S. Arroliga-Rocha and D. Escudero, Facial and Meridional Isomers of Tris(bidentate) Ir(III) Complexes: Unravelling Their Different Excited State Reactivity, Inorg. Chem., 2018, 57, 12106–12112 CrossRef CAS PubMed.
- W. Li, J. Liu, B. Wang, S. Hou, X. Lü, G. Fu and W.-Y. Wong, Geometrically isomeric [Ir(iqbt)(ppy)(hpa)] complexes with differential molecule orientations for efficient near-infrared (NIR) polymer light-emitting diodes (PLEDs), J. Mater. Chem. C, 2021, 9, 12068–12072 RSC.
- J. C. Deaton, R. H. Young, J. R. Lenhard, M. Rajeswaran and S. Huo, Photophysical Properties of the Series fac- and mer,-(1-Phenylisoquinolinato-N^C2′)x(2-phenylpyridinato-N^C2′)3-xIridium(III) (x = 1-3), Inorg. Chem., 2010, 49, 9151–9161 CrossRef CAS PubMed.
- E. Baranoff, S. Suàrez, P. Bugnon, C. Barolo, R. Buscaino, R. Scopelliti, L. Zuppiroli, M. Graetzel and M. K. Nazeeruddin, Sublimation Not an Innocent Technique: A Case of Bis-Cyclometalated Iridium Emitter for OLED, Inorg. Chem., 2008, 47, 6575–6577 CrossRef CAS PubMed.
- C. Shi, H. Huang, Q. Li, J. Yao, C. Wu, Y. Cao, F. Sun, D. Ma, H. Yan, C. Yang and A. Yuan, Three Types of Charged-Ligand-Based Blue–Green to Near-Infrared Emitting Iridium Complexes: Synthesis, Structures, and Organic Light-Emitting Diode Application, Adv. Opt. Mater., 2021, 9, 2002060 CrossRef CAS.
- G. Li, N. Li, Y. Cao, C. Shi, X. Liu, R. Zeng, M. Wu, Q. Li, C. Yang and A. Yuan, Deep-Red/Near-Infrared to Blue-Green Phosphorescent Iridium(III) Complexes Featuring Three Differently Charged (0, −1, and −2) Ligands: Structures, Photophysics, and Organic Light-Emitting Diode Application, Inorg. Chem., 2022, 61, 10548–10556 CrossRef CAS PubMed.
- Y. Zhang, H.-C. Kao, C. Shi, C. Wu, M. Zhu, K. Li, C.-C. Wu and C. Yang, Iridium(III) Complexes with [-2, -1, 0] Charged Ligand Realized Deep-Red/Near-Infrared Phosphorescent Emission, Chem. – Eur. J., 2022, 28, e202103543 CAS.
- Q. Li, C. Shi, M. Huang, C. Wu, H. Wang, H. Wu, Y. Zheng, C. Yang and A. Yuan, Three Types of Charged Ligands Based Carboxyl-Containing Iridium(III) Complexes: Structures, Photophysics, and Solution Processed OLED Application, Inorg. Chem., 2021, 60, 17699–17704 CrossRef CAS PubMed.
- Q. Li, C. Shi, M. Huang, X. Zhang, F. Sun, Y. Zheng, H. Yan, C. Yang and A. Yuan, Three types of charged ligand-based neutral phosphorescent iridium(III) complexes featuring nido-carborane: synthesis, structures, and solution processed organic light-emitting diode applications, Dalton Trans., 2021, 50, 16304–16310 RSC.
- Q. Zhang, X. Wang, X. Wang, L. Wang and J. Zhang, Computational studies of electronic structures and photophysical properties of luminescent iridium(III) complexes based on amidinate/bis(pyridylphenyl) ligands, Org. Electron., 2016, 33, 281–289 CrossRef.
- N. Su and Y.-X. Zheng, Four membered red iridium(III) complexes with Ir-S-P-S structures: rapidly room temperature synthesis and application in OLEDs, Dalton Trans., 2019, 48, 7583–7588 RSC.
- Y. Liu, K. Ye, Y. Fan, W. Song, Y. Wang and Z. Hou, Amidinate-ligated iridium(III) bis(2-pyridyl)phenyl complex as an excellent phosphorescent material for electroluminescence devices, Chem. Commun., 2009, 3699–3701 RSC.
- G.-Z. Lu, N. Su, H.-Q. Yang, Q. Zhu, W.-W. Zhang, Y.-X. Zheng, L. Zhou, J.-L. Zuo, Z.-X. Chen and H.-J. Zhang, Rapid room temperature synthesis of red iridium(III) complexes containing four-membered Ir-S-C-S chelating ring for highly efficient OLEDs with EQE over 30%, Chem. Sci., 2019, 10, 3535–3542 RSC.
- G. Li, D. Zhu, T. Peng, Y. Liu, Y. Wang and M. R. Bryce, Very High Efficiency Orange-Red Light-Emitting Devices with Low Roll-Off at High Luminance Based on an Ideal Host–Guest System Consisting of Two Novel Phosphorescent Iridium Complexes with Bipolar Transport, Adv. Funct. Mater., 2014, 24, 7420–7426 CrossRef CAS.
- B. Liang, Z. Yu, X. Zhuang, J. Wang, J. Wei, K. Ye, Z. Zhang, Y. Liu and Y. Wang, Achieving High-Performance Pure-Red Electrophosphorescent Iridium(III) Complexes based on Optimizing Ancillary Ligand, Chem. – Eur. J., 2020, 26, 4410–4418 CrossRef CAS PubMed.
- G.-Z. Lu, X. Li, L. Liu, L. Zhou, Y.-X. Zheng, W.-W. Zhang, J.-L. Zuo and H. Zhang, Efficient phosphorescent red iridium(III) complexes containing a four-membered Ir–S–C–S ring backbone and large hindered spacers for high-performance OLEDs, J. Mater. Chem. C, 2019, 7, 3862–3868 RSC.
- N. Su, F.-L. Li and Y.-X. Zheng, Four-membered red iridium(III) complexes with Ir-S-C-S structures for efficient organic light-emitting diodes, J. Mater. Chem. C, 2020, 8, 7411–7416 RSC.
- G.-Z. Lu, J. Yao, Z. Chen, D. Ma and C. Yang, Saturated red iridium(III) complexes containing a unique four-membered Ir–S–C–N backbone: mild synthesis and application in OLEDs, J. Mater. Chem. C, 2020, 8, 1391–1397 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, structural characterization and DFT calculations. CCDC 2234483 for Ir1K, 2234484 for Ir1T, 2234485 for Ir2K and 2234486 for Ir2T. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi00224a |
| This journal is © the Partner Organisations 2023 |