Site preference and defect engineering of a highly efficient blue-emitting phosphor Sr2SiO4:Ce3+/K+ toward thermally enhanced luminescence†
Kai
Zhao‡
a,
Zhihong
Ma‡
a,
Li
Yin
 a,
Bin
Hui
a,
Han
Si
a,
Xinlin
Tong
b,
Huidong
Tang
c,
Peng
Cao
a,
Bin
Hui
a,
Han
Si
a,
Xinlin
Tong
b,
Huidong
Tang
c,
Peng
Cao
 d and
Saifang
Huang
d and
Saifang
Huang
 *a
*a
aSchool of Materials Science and Engineering, Jiangsu University of Science and Technology, Zhenjiang 212003, P. R. China. E-mail: s.huang@just.edu.cn
bSchool of Materials Science and Engineering, Changzhou University, Changzhou 213164, P. R. China
cDepartment of Chemistry and Materials Engineering, Changzhou Vocational Institute of Engineering, Changzhou 213164, P. R. China
dDepartment of Chemical and Materials Engineering, The University of Auckland, Private Bag 92019, Auckland 1142, New Zealand
First published on 10th March 2023
Abstract
The blue-emitting phosphor is one of the trichromatic phosphors essential for the development of near-ultraviolet (nUV) pumped phosphor-converted white light-emitting diodes (pc-WLEDs). Efficiency and thermal stability are two critical factors for the potential application of phosphors in solid-state lighting. In this work, we report a highly efficient blue-emitting phosphor, α-Sr2SiO4:0.02Ce3+/0.02K+ (emission peak at 425 nm) with superior thermally enhanced photoluminescence properties. The Rietveld refinement results suggest that Ce3+ preferentially occupies the nine-fold coordinated Sr2 site only whereas K+ occupies both Sr1 and Sr2 sites in the host structure. Its emission intensity gradually increases with increasing temperature and retains 120% of its room-temperature peak intensity at 250 °C. Besides, its luminescence spectral peak does not shift at elevated temperatures. Under nUV irradiation, the phosphor is highly luminous with an excellent internal quantum efficiency (IQE) of 91.7%. The abnormal thermal quenching phenomenon can be ascribed to the defect energy levels introduced by defect engineering using an aliovalent doping strategy of Ce3+ and K+ in the α-Sr2SiO4 host structure. The as-fabricated WLED prototypes demonstrated the great potential of the highly efficient blue-emitting phosphor with superior thermal stability for solid-state lighting applications.
1 Introduction
Phosphor-converted white light-emitting diodes (pc-WLEDs) have attracted extensive attention due to their numerous advantages such as small size, long life span, fast response, high luminous efficacy, and low energy consumption.1,2 The traditional approach for making white LEDs is to combine a blue-emitting chip with a yellow garnet phosphor (YAG:Ce3+).3,4 However, this approach lacks a red emission component, thus leading to a poor color rendering index (CRI, Ra < 80) and a relatively high correlated color temperature (CCT > 4500 K).5 An alternative strategy has been attracting significant attention in recent years, namely combining near ultraviolet (nUV) pumped red/green/blue (RGB) emitting phosphors.6 This new approach enables the full visible spectrum, thereby leading to the feasibility of a high CRI (Ra ≥ 95), a tunable CCT and a wide color gamut of white LED devices for different application scenarios. To meet the new development trend, highly efficient blue phosphors with superior thermal stability are of particular interest and importance.Recent efforts have been devoted to the exploration of new blue-emitting phosphors with better photoluminescence properties.7,8 The properties of phosphors largely determine the performance of solid-state lighting devices.1,2 Among them, the thermal quenching effect plays a vital role in the luminescence stability of phosphors at increased temperatures, further affecting the photoelectric properties of the pc-WLED devices whose working temperature can reach ca. 150 °C.9 The luminescence thermal stability of phosphors is particularly crucial for high-power lighting applications in which the working temperature can be even higher (over 200 °C).10 To date, several blue phosphors have been reported as showing excellent quantum efficiencies and thermal stability.7 Most of them are Eu2+-activated phosphors, such as Ca2PO4Cl:Eu2+,11 RbBaPO4:Eu2+,12 Na3Sc2(PO4)3:Eu2+,13 BaLi2[Be4O6]:Eu2+,14 BaAl12O19:Eu2+,15 (Sr0.69Ba0.3)2P2O7:Eu2+16 and the commercially available BAM phosphor (BaMgAl11O17:Eu2+).11 These phosphors have an external quantum efficiency (EQE) of 40–70% and a thermal stability of 83–110%@200 °C (the percentage of its integrated emission intensity at 200 °C relative to that at room temperature).7 Our recent work developed a blue phosphor, K2SrxBa2−x(PO4)2:Eu2+, with a thermal stability of 93%@200 °C, an IQE of 96.4% and an EQE of 76.4%.7,8 Nevertheless, blue-emitting phosphors activated by Ce3+ are less studied. Ce3+-activated boride phosphors such as Ba3Y2B6O15:Ce3+ (ref. 17) and NaSrBO3:Ce3+ (ref. 18) have poor thermal stability, retaining only 20%@200 °C and 58%@200 °C respectively. Some other phosphors exhibit relatively better thermal stability at the level of 70–80%@200 °C, such as Ca3ZrSi2O9:Ce3+ (ref. 19) and SrLu2O4:Ce3+.20 Therefore, it is of great interest to explore Ce3+-activated blue-emitting phosphors with excellent thermal stability and quantum efficiencies.
Silicate phosphors are an important family that has been extensively investigated due to its advantages of chemical and structural diversity, abundance of raw materials, and simple and cost-effective synthesis methods.21–25 Alkaline earth metal orthosilicates, M2SiO4 (M = Ca, Sr, and Ba), have shown their suitability for application as a host in commercial phosphors such as (Sr,Ba)2SiO4:Eu2+. These phosphors are tunable for green/yellow emission, but their thermal stability remains poor as they retain merely 50% at 200 °C.26 Although Sr2SiO4-based phosphors have recently been rekindling research interest, those activated by Ce3+ are rarely studied. In addition, when divalent alkaline metal ions are substituted by trivalent ions such as Eu3+, charge compensation with monovalent ions is essential for the bright emission of phosphors.27,28
Herein, we report the preparation and characterization of a blue-emitting phosphor, Sr2SiO4:Ce3+/K+, featuring an abnormal thermal quenching effect and excellent quantum efficiency, where Ce3+ acts as the activator and K+ as the charge compensator. The effect of the doping concentration on the phase structure and luminescence properties has been investigated. The quantum efficiencies and thermal stability of phosphors as well as the mechanism involved have been characterized and discussed. To demonstrate their potential application, white LEDs have been prototyped and their photoelectric properties have been studied.
2 Experimental section
2.1 Material synthesis
Sr2SiO4:xCe3+/xK+ phosphor powders were synthesized via a high-temperature solid-state reaction. The raw chemicals, including K2CO3, SrCO3, SiO2, and CeO2, were weighed in stoichiometric amounts. K+ ions were introduced to compensate for the excess charge of Ce3+ at the Sr2+ site. Then the mixtures were thoroughly ground in an agate mortar for half an hour, and transferred to alumina crucibles for calcination in air at 700 °C for 8 h. After that, the calcined samples were re-ground and transferred into alumina crucibles with lids for synthesis at 1250 °C for 4 h under a reducing atmosphere of 95% Ar/5% H2 forming gas. After synthesis, the samples were ground gently into powders for further analysis.2.2 Characterization methods
The phase structure of the Sr2SiO4-based phosphor powders was characterized by X-ray diffraction (XRD) using a diffractometer (D2 Phaser, AXS Bruker, Germany) with Cu Kα radiation (λ = 1.5418 Å) under operating conditions of 30 kV and 10 mA. The structure refinement upon X-ray diffraction was conducted using the Fullprof Suite program.29 The morphology and elemental distribution of phosphors were examined with a scanning electron microscope (SEM, JEOL JSM-6480, Japan), operating at an acceleration voltage of 20 kV. Transmission electron microscopy (TEM, Tecnai G2 F30 S-TWIN) was performed to investigate the morphology, lattice structure and elemental distribution of the prepared phosphor samples.Photoluminescence excitation (PLE) and emission (PL) spectra were acquired using a spectrofluorometer (Fluoromax PLUS, Horiba, Japan) equipped with a 150 W xenon lamp at room temperature (20 °C) and elevated temperatures (25–250 °C). Each temperature-dependent spectrum was collected after the target temperature had been maintained for no less than 5 min so that the set temperature could be attained. Quantum efficiencies (QE) were measured with an integral sphere and BaSO4 as the reference material. The spectral bands used for QE calculation are 380–500 nm (for integrating the emission intensity of the phosphor) and 335–365 nm (for integrating the excitation light source). The internal quantum efficiency (IQE) refers to the ratio of the number of photons emitted to that absorbed, which is calculated using eqn (1):
 | (1) |
The thermoluminescence (TL) curves were measured on an SL08 system (Guangzhou RuiDi Aisheng Technology Co. Ltd, China) in a range of 20–500 °C with a ramp rate of 1 K s−1. Each sample was irradiated under 254 nm UV light for 5 min before TL testing.
2.3 Fabrication of white LED prototypes
To demonstrate its potential application in solid-state lighting, we used the as-prepared phosphor Sr2SiO4:0.02Ce3+/0.02K+ to fabricate prototype white LED devices with a near-ultraviolet (n-UV) InGaN LED chip (λ = 365 nm) and commercial green phosphor (Sr,Ba)2SiO4:Eu2+ and red phosphor (Sr,Ba)AlSiN3:Eu2+. The phosphors were mixed with epoxy resin, and the obtained mixture was dropped on top of the LED chips. The photoelectric properties, including the electroluminescence (EL) spectrum, correlated color temperature (CCT), color rendering index (Ra), CIE chromaticity coordinates and luminous efficacy of the WLED prototypes, were collected using an OHSP-350M spectrophotometer system (Hangzhou Hopoo Light & Color Co. Ltd, China) equipped with a 0.5 m integrating sphere.3 Results and discussion
3.1 Phase and structure analysis
Alkaline earth metal orthosilicates, M2SiO4 (M = Ca, Sr, and Ba), have two polymorphs, namely the orthorhombic α′ phase (isostructural to β-K2SO4 type structure, space group Pmnb) and the monoclinic β phase (space group P21/n). The α′ phase of pure Sr2SiO4 transforms to the β phase at 358 K (85 °C) while cooling during the synthesis process.30 Studies show that elemental doping can stabilize the α′ phase structure of Sr2SiO4 at room temperature.31The XRD patterns of the Sr2SiO4:xCe3+/xK+ phosphor samples synthesized at 1250 °C for 4 h are shown in Fig. 1. As clearly shown in Fig. 1b, a characteristic peak at 2θ of ∼32.40° indicates the existence of monoclinic β-Sr2SiO4 in the samples with an x value of 0.02 or smaller. Thus, biphasic powders were received if the x value was 0.02 or lower whereas the orthorhombic α′-Sr2SiO4 was the main phase and the monoclinic β-Sr2SiO4 was diminishing gradually with the increase in Ce3+ concentration (x). Pure α′-Sr2SiO4 powders were obtained for samples with x ≥ 0.3.
The SEM image of the Sr2SiO4:0.02Ce3+/0.02K+ phosphor powders calcined at 1250 °C is shown in Fig. 1c. The phosphor powders are spherical or round in shape with a relatively homogeneous grain size of 2–5 μm, and some grains are clustered together as a result of solid-state synthesis. We also used TEM to investigate the microstructure of the prepared phosphor powder. Fig. 1d shows a typical high-resolution TEM image (HRTEM) of a single grain, and EDS mapping was conducted to examine the elemental distribution in the grain (Fig. 1f). As seen from the HRTEM image, the grain reveals a well-ordered lattice infringed structure with a lattice spacing of 0.282 Å, which corresponds to the (200) lattice plane of the orthorhombic α′-Sr2SiO4 phase. The mapping data further demonstrate the homogeneous elemental distribution of Ce, K, Sr, Si and O.
In order to understand the crystallographic sites of Ce3+ and K+ in Sr2SiO4, Rietveld refinements were performed for the Sr2SiO4:0.05Ce3+/0.05K+ compound upon its X-ray powder diffraction patterns using the FullProf Suite. The refined diffraction pattern is plotted in Fig. 2a. Details about the crystallographic data, refinement parameters, atomic positions and site occupancies are given in Tables 1 and 2. Further details of the crystal structure investigation may be obtained from an online deposition service of the joint CCDC/FIZ Karlsruhe: https://www.ccdc.cam.ac.uk/structures/ by quoting the deposition number CCDC 2225570.†
 | ||
| Fig. 2 (a) The refined XRD pattern and (b) crystal structure of Ce3+/K+ co-doped α′-Sr2SiO4 and the coordination of Sr sites (Sr1O10 and Sr2O9). | ||
| Composition from the refinement | Ce0.0225K0.1275Sr1.85SiO4 |
|---|---|
| Radiation source | X-ray (Cu Kα) |
| Source wavelength (Å) | 1.5418 |
| Crystal system | Orthorhombic |
| Space group | Pmnb (no. 62) |
| No. of refined parameters | 38 |
| R exp | 6.2956 |
| R wp | 12.0198 |
| Bragg R-factor | 5.7439 |
| χ 2 | 3.6452 |
| Z | 4 |
| a (Å) | 5.6624(1) |
| b (Å) | 7.0790(1) |
| c (Å) | 9.7392(2) |
| α, β, γ (°) | 90 |
| V (Å3) | 390.39(1) |
| 2θ range (°) | 10–110 |
| Atom | x | y | z | B iso | Occ. | Wyckoff pos. |
|---|---|---|---|---|---|---|
| Si1 | 0.25 | 0.7765(7) | 0.5837(8) | 0.0430(19) | 1 | 4c |
| Sr1 | 0.2697(13) | 0.3400(2) | 0.5795(2) | 0.0336(7) | 0.452(7) | 8d |
| K1 | 0.2697(13) | 0.3400(2) | 0.5795(2) | 0.0336(7) | 0.048(7) | 8d |
| Sr2 | 0.2734(9) | −0.0013(2) | 0.3025(2) | 0.0336(7) | 0.473(7) | 8d |
| Ce2 | 0.2734(9) | −0.0013(2) | 0.3025(2) | 0.0336(7) | 0.011(7) | 8d |
| K2 | 0.2734(9) | −0.0013(2) | 0.3025(2) | 0.0336(7) | 0.0157 | 8d |
| O1 | 0.312(2) | 1.0050(12) | 0.5697(10) | 0.021(2) | 0.5 | 8d |
| O2 | 0.302(2) | 0.6661(17) | 0.4261(10) | 0.021(2) | 0.5 | 8d |
| O3 | 0.030(2) | 0.7312(15) | 0.6863(17) | 0.021(2) | 0.5 | 8d |
| O4 | 0.0108 | 0.6882 | 0.6471 | 0.021(2) | 0.5 | 8d |
The phosphor crystallized in the structure of α′-Sr2SiO4 (space group Pnma).32 It is interesting to find out that any attempt at putting Ce at the Sr1 site led to negative values for its occupancy, whereas the refinement of Ce at Sr2 went nicely. This suggests that the activator Ce3+ ions prefer not to reside at the Sr1 site but only at the Sr2 site. The refinements demonstrated that the actual amount of Ce3+ was ∼0.0225 per formula unit, which was less than the nominal amount of 0.05. The remaining Ce3+ forms as CeO2 impurity in a trace amount. On the other hand, K+ ions tend to reside at the Sr1 (dominantly) and Sr2 sites in the structure of the host α′-Sr2SiO4. The content of K+ ions largely increased to compensate for the unbalanced charge in the system due to the aliovalent doping of Ce3+. As per the refinement, the chemical formula of the phosphor is Ce0.0225K0.1275Sr1.85SiO4, and its crystal structure is shown in Fig. 2b.
3.2 Luminescence properties and the mechanism involved
Our preliminary studies showed that charge compensation (adding K+ ions) can improve the emission intensity of the phosphor, as demonstrated in Fig. S2.† Thus, we further investigated the concentration quenching effect of Sr2SiO4:xCe3+/xK+. Their photoluminescence (PL) spectra are shown in Fig. 3a. The peak wavelength and full width at half maximum (FWHM) of the emission spectra are fixed at around 425 nm and 65.5 nm respectively while varying the doping concentration of Ce3+/K+. The optimal Ce3+/K+ concentration was around 0.02, beyond which the phosphors demonstrated a concentration quenching effect. The concentration corresponding to its critical distance is the optimal doping concentration. Thus, the critical distance between the activator Ce3+ can be calculated using the Blasse equation:33 | (2) |
Through calculation, the critical distance for concentration quenching of the Sr2SiO4:xCe3+/xK+ phosphor was found to be 16.75 Å. Since the calculated value is greater than 5 Å, the energy transfer between Ce3+ ions is achieved by multipole interaction. According to the literature, there are three types of multipole interactions: electric dipole/electric dipole interaction, electric dipole/electric quadrupole interaction, and electric quadrupole/electric quadrupole interaction.34 The type of multipole interaction can be determined according to Dexter's energy transfer theory using formula (3):35
 | (3) |
According to this formula, log(I/x) can be used as the ordinate and log (x) as the abscissa to fit data and derive the slope 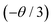 and θ values. The fitting data are shown in Fig. S1,† and the slope is −1.005. Thus θ equals 3.015, indicating that the energy transfer in the Sr2SiO4:Ce3+ phosphor is mainly attributed to the electric dipole interaction.
and θ values. The fitting data are shown in Fig. S1,† and the slope is −1.005. Thus θ equals 3.015, indicating that the energy transfer in the Sr2SiO4:Ce3+ phosphor is mainly attributed to the electric dipole interaction.
Fig. 3b plots the PL and PLE spectra of the Sr2SiO4:0.01Ce3+ phosphor. This phosphor shows a blue emission band peaking at 425 nm with a FWHM of 65.8 nm at room temperature, which can be ascribed to the typical 5d → 4f transition. When monitored at 425 nm, the excitation spectrum demonstrates a band from 300 to 390 nm, peaking at around 342 nm. By applying Gaussian deconvolution to the PL spectrum, two peaks at 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 368 cm−1 (428 nm) and 25
368 cm−1 (428 nm) and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 037 cm−1 (399 nm) can be obtained (Fig. 3c). The energy difference between the two as-fitted Gaussian peaks is 1669 cm−1. Such a difference is close to the energy difference of 2000 cm−1 between the 2F5/2 and 2F7/2 transitions of Ce3+.36 We further measured the emission spectra of Sr2SiO4:0.02Ce3+/0.02K+ at different excitation wavelengths, and the normalized spectra are shown in Fig. S3.† When the excitation wavelength gradually increases from 282 to 365 nm, a subtle change can be observed in the emission spectrum. This confirms the single emission center in the phosphor.
037 cm−1 (399 nm) can be obtained (Fig. 3c). The energy difference between the two as-fitted Gaussian peaks is 1669 cm−1. Such a difference is close to the energy difference of 2000 cm−1 between the 2F5/2 and 2F7/2 transitions of Ce3+.36 We further measured the emission spectra of Sr2SiO4:0.02Ce3+/0.02K+ at different excitation wavelengths, and the normalized spectra are shown in Fig. S3.† When the excitation wavelength gradually increases from 282 to 365 nm, a subtle change can be observed in the emission spectrum. This confirms the single emission center in the phosphor.
Fig. 3d shows the fluorescence decay plots of the Sr2SiO4:xCe3+/xK+ samples monitored at 425 nm under a 365 nm excitation. All decay curves are fitted using the following formula:37
 | (4) |
When the doping concentration of Ce3+ is 0.03 or smaller, the fluorescence lifetime gradually increases from 27.84 ns to 28.83 ns, and when it is higher than 0.03, the fluorescence lifetime gradually decreases. The phenomenon of increasing fluorescence lifetime for 0.005 ≤ x ≤ 0.03 can be ascribed to the biphasic composition. According to the analysis of XRD, when the Ce3+ doping concentration is lower than 0.03, the phosphors consist of α′ and β phases where β-Sr2SiO4 is gradually diminished. Accordingly, the fluorescence lifetime of Ce3+ in the β phase is likely shorter than that in the α′ phase structure. As the Ce3+ doping concentration reaches 0.03 or higher (x ≥ 0.03), the phosphor is composed of a single α′ phase. The fluorescence lifetime decreases because of the concentration quenching effect.
3.3 Luminescence thermal stability and the associated mechanism
The temperature-dependent emission spectra of Sr2SiO4:xCe3+/xK+ (x = 0.01 and 0.02) were collected from 25 to 250 °C under the excitation at 342 nm and are shown in Fig. 4a and c. The as-synthesized Sr2SiO4:Ce3+/K+ phosphor samples exhibit excellent thermal quenching resistance. Fig. 4b and d show the normalized intensities versus temperature for samples with x = 0.01 and 0.02, respectively. The thermal stabilities of the two samples are compared in Fig. S4† with respect to their peak intensity and integrated intensity.When the Ce3+ doping concentration is 0.01, the peak intensity and integrated intensity of the sample Sr2SiO4:0.01Ce3+/0.01K+ increase gradually, then they decrease when the temperature is higher than 150 °C. Specifically, the sample with x = 0.02 (Sr2SiO4:0.02Ce3+/0.02K+) has a superior thermal enhancement effect such that the luminescence intensities (peak and integrated) continuously increase with the rise in temperature. At 250 °C, the peak intensity and integrated intensity can retain 119.7% and 122.9% of their initial intensity (at 25 °C), respectively. The temperature-dependent PL spectra do not reveal a shift in the peak position but only show subtle changes in the spectral shape (Fig. S5†). This result suggests that there is no color drift while the working temperature changes. The sample with x = 0.01 shows zero thermal quenching up to 200 °C. Therefore, the phosphor with an optimal doping concentration, namely Sr2SiO4:0.02Ce3+/0.02K+, has both the brightest emission and superior thermal enhancement features, which are very promising for high-power nUV-pumped white LED devices.
Recent studies suggest that zero (or abnormal) thermal quenching may be related to the interaction between activator ions and crystal defects acting as electron trapping centers.38,39 In order to investigate the mechanism involved, we further investigated the thermoluminescence (TL) performance of Sr2SiO4:xCe3+/xK+ samples with x values of 0.01 and 0.02. The TL curves in the temperature range of 20–500 °C are shown in Fig. 5a. Three individual peaks are evidently observed at 122, 282 and 415 °C for the sample with x = 0.02, associated with corresponding electron traps due to the existence of defect energy levels. Using the relation ET = T/500 eV where T is the absolute temperature in K,40 the characteristic trap depths (ET) are estimated to be 0.79, 1.11 and 1.38 eV, respectively.
 | ||
| Fig. 5 (a) TL curves of Sr2SiO4:xCe3+/xK+ with x = 0.01 and x = 0.02, and (b) simplified model for the mechanism of the thermally activated emission of Sr2SiO4:0.02Ce3+/0.02K+. | ||
In comparison with x = 0.02, the sample with x = 0.01 has one trap less (trap C), and the peak intensity associated with traps A and B is much weaker, indicating that the population of defects is much smaller. From both the temperature-dependent PL spectra and the TL data of the two samples, the thermal stability of Sr2SiO4:0.01Ce3+/0.01K+ is thermally enhanced in a certain temperature range (namely 25–150 °C) before quenching again, attributed to the thermally activated emission of the electron traps A and B, whereas that of Sr2SiO4:0.02Ce3+/0.02K+ is activated for the additional trap C.
Thus, the abnormal thermal quenching effect of Sr2SiO4:0.02Ce3+/0.02K+ can be explained by the simplified model schematically shown in Fig. 5b. For steady-state luminescence at a given temperature, some 5d electrons of Ce3+ are thermally ionized into the conduction band of the ground state with a trapping process at the defect levels (processes 3 and 4). Then these electrons are released into the conduction band and transferred to the 4f level by a radiative transition (procedures 5, 6 and 2).39 The relationship between emission intensity (I) and temperature (T) is derived by modeling the process using a probability equation:39
 | (5) |
3.4 Quantum efficiency
Quantum efficiency is an important parameter of phosphors for solid-state lighting applications. Therefore, the quantum efficiencies of Sr2SiO4:0.02Ce3+/0.02K+ were measured (Fig. S7†). It is seen that the phosphor has an excellent internal quantum efficiency (IQE) of 91.7% when excited at 350 nm. The absorption rate (Abs) is 39.5%, and the external quantum efficiency (EQE) is 36.2%. The EQE of the phosphor could be further boosted by optimizing the synthesis process of phosphors.Table 3 compares the internal quantum efficiencies and thermal stability of blue-emitting phosphors reported in recent years. As the number of reports of Ce3+-doped blue-emitting phosphors is limited, some Eu2+-doped blue-emitting phosphors are also compared. The IQE of Sr2SiO4:Ce3+/K+ phosphors is higher than that of most blue-emitting Ce3+-doped phosphors. Moreover, the luminescence thermal stability of Sr2SiO4:Ce3+/K+ is superior to most of the blue-emitting phosphors. In view of its excellent PL properties, Sr2SiO4:Ce3+/K+ is very promising for solid-state lighting applications.
| Phosphor | λ Ex (nm) | λ Em (nm) | TQ | IQE (%) | EQE (%) | Ref. |
|---|---|---|---|---|---|---|
| a Percentage estimated from the published plot (relative to the room temperature intensity). | ||||||
| Sr2SiO4:0.02Ce3+/0.02K+ | 342 | 425 | 120%@250 °C | 91.7 | 36.2 | This work |
| Ca6BaP4O17:Ce3+,Si4+ | 400 | 480 | 80%@150 °C | 70 | — | 41 |
| Ba9Y2Si6O24:0.03Ce3+ | 394 | 480 | 85%@200 °C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
57 | — | 42 |
| NaSrBO3:Ce3+ | 365 | 422 | 58%@200 °C | 74.7 | 61.6 | 18 |
| BAM:Eu2+ | 365 | 462 | 70%@250 °C | 89 | — | 43 |
| K2SrxBa2−x(PO4)2:Eu2+ | 330 | 425 | 93%@200 °C | 96.4 | 76.4 | 7 and 8 |
| Sr5(PO4)3Cl:Eu2+ | 395 | 444 | ∼78%@200 °C | 80.5 | — | 44 |
3.5 Fabrication and performance of WLED prototypes
In order to evaluate the potential applications of Sr2SiO4:Ce3+/K+ blue phosphors in improving the color rendering index of white LEDs and the coordinating color temperature, we combined near-ultraviolet InGaN chips (λ = 365 nm) with Sr2SiO4:0.02Ce3+/0.02K+ and commercial (Sr,Ba)2SiO4:Eu2+ (green emitting) and (Sr,Ba)AlSiN3:Eu2+ (red emitting) to fabricate four white LED prototypes. Fig. 6 shows the EL emission spectra of the WLED prototypes at 20 mA. Four main emission peaks (365 nm, 425 nm, 525 nm, 610 nm) can be clearly seen in the emission spectra of these devices, corresponding to the emission peaks of the chip and blue, green and red phosphors, respectively.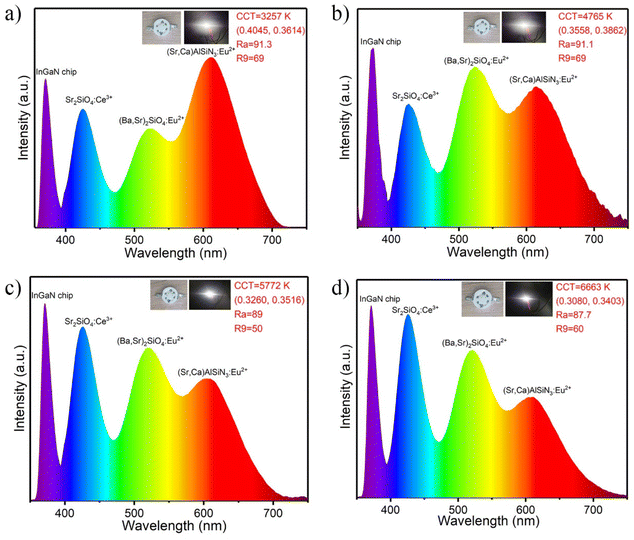 | ||
| Fig. 6 (a–d) The EL spectra and key performances of the assembled white LED devices using Sr2SiO4:Ce3+/K+ with commercial green and red phosphors pumped by an nUV chip. | ||
Table 4 shows the relevant properties measured by the device, such as CRI, including Ra and R9, CCT, CIE coordinates and luminous efficacy. Prototype WLED devices with adjustable CRI (87–92) and CCT (3200–6700 K) are achieved by controlling the addition of phosphors. Explained in detail, the CCT changed from 4765 to 6663 K when we fixed the ratio of green and yellow phosphors and varied the content of blue phosphor (Fig. 6b–d), which further decreased to 3257 K if an additional amount of red phosphor was added (Fig. 6a). These results demonstrate that the Sr2SiO4:Ce3+/K+ phosphors have great potential for high-power solid-state lighting applications.
| No. | CCT (K) | Ra | R9 | CIE (x, y) | Luminous efficacy (lm W−1) |
|---|---|---|---|---|---|
| 1 | 3257 | 91.3 | 69 | (0.4045, 0.3614) | 24.3 |
| 2 | 4765 | 91.1 | 69 | (0.3558, 0.3865) | 26.5 |
| 3 | 5772 | 89.0 | 50 | (0.3260, 0.3516) | 22.9 |
| 4 | 6663 | 87.7 | 60 | (0.3080, 0.3403) | 27.8 |
4 Conclusions
In summary, a highly efficient blue-emitting phosphor α-Sr2SiO4:0.02Ce3+/0.02K+ with an abnormal thermal quenching effect was successfully developed in this work by a defect engineering strategy. It exhibits superior luminescence thermal stability, retaining 120% of its room-temperature peak intensity at 250 °C. The site preference of aliovalent dopants Ce3+ and K+ in the structure of α-Sr2SiO4 was investigated by structural refinement via the Rietveld method, which suggests that Ce3+ preferentially occupies the nine-fold coordinated Sr2 site only, while K+ occupies both Sr1 and Sr2 sites. Under nUV irradiation (350 nm), the phosphor shows a blue light emission peak at 425 nm and has an excellent internal quantum efficiency (IQE) of 91.7%. The mechanism for the abnormal thermal quenching phenomenon was investigated by thermoluminescence measurements. Several defect energy levels are formed by defect engineering using an aliovalent doping strategy of Ce3+ and K+ in the α-Sr2SiO4 host structure. The defect energy levels serve as electron traps that can be thermally activated, which continuously boost the emission intensity of the phosphor while being heated up to 250 °C. The as-fabricated WLED prototypes demonstrated the great potential of the high-performance blue-emitting phosphor Sr2SiO4:Ce3+/K+ for solid-state lighting applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the Jiangsu Specially-Appointed Professorship Foundation (Grant No. 1064902003) and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (Grant No. SJCX21_1772).References
- Z. Xia and Q. Liu, Progress in discovery and structural design of color conversion phosphors for LEDs, Prog. Mater. Sci., 2016, 84, 59 CrossRef CAS.
- S. Ye, F. Xiao, Y. X. Pan, Y. Y. Ma and Q. Y. Zhang, Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties, Mater. Sci. Eng., R, 2010, 71, 1 CrossRef.
- L. Wang, X. Kong, P. Li, W. Ran, X. Lan, Q. Chen and J. Shi, Narrow-band green emission of Eu2+ in a rigid tunnel structure: site occupations, barycenter energy calculations and luminescence properties, Inorg. Chem. Front., 2019, 6, 3604 RSC.
- M. Zhao, Y. Zhou, M. S. Molokeev, Q. Zhang, Q. Liu and Z. Xia, Discovery of new narrow–band phosphors with the UCr4C4-related type structure by alkali cation effect, Adv. Opt. Mater., 2019, 7, 1801631 CrossRef.
- O. P. Golim, S. Huang, L. Yin, T. Yang, H. Zhou, W. Gao, T. Söhnel and P. Cao, Synthesis, neutron diffraction and photoluminescence properties of a whitlockite structured Ca9MgLi(PO4)7:Pr3+ phosphor, Ceram. Int., 2020, 46, 27476 CrossRef CAS.
- N. C. George, K. A. Denault and R. Seshadri, Phosphors for solid-state white lighting, Annu. Rev. Mater. Sci., 2013, 43, 481 CrossRef CAS.
- T. Yang, T. Zhang, S. Huang, T. D. Christopher, Q. Gu, Y. Sui and P. Cao, Structure tailoring and defect engineering of LED phosphors with enhanced thermal stability and superior quantum efficiency, Chem. Eng. J., 2022, 435, 133873 CrossRef CAS.
- T. Yang, Z. Ma, S. Huang, T. Zhang, K. Zhao, L. Yin, Q. Gu and P. Cao, Ultra-narrow-band blue-emitting K2SrBa(PO4)2:Eu2+ phosphor with superior efficiency and thermal stability, J. Alloys Compd., 2022, 892, 162066 CrossRef CAS.
- K. Zhao, L. Yin, Z. Ma, T. Yang, H. Tang, P. Cao and S. Huang, Investigation of the solid-solution limit, crystal structure, and thermal quenching mitigation of Sr-substituted Rb2CaP2O7:Eu2+ phosphors for white LED applications, Inorg. Chem., 2022, 61, 1627 CrossRef CAS PubMed.
- Y. H. Kim, N. S. M. Viswanath, S. Unithrattil, H. J. Kim and W. B. Im, Review-phosphor plates for high-power LED applications: Challenges and opportunities toward perfect lighting, ECS J. Solid State Sci. Technol., 2018, 7, R3134 CrossRef CAS.
- Y.-C. Chiu, W.-R. Liu, C.-K. Chang, C.-C. Liao, Y.-T. Yeh, S.-M. Jang and T.-M. Chen, Ca2PO4Cl:Eu2+ : An intense near-ultraviolet converting blue phosphor for white light-emitting diodes, J. Mater. Chem., 2010, 20, 1755 RSC.
- H. J. Song, D. K. Yim, H.-S. Roh, I. S. Cho, S.-J. Kim, Y.-H. Jin, H.-W. Shim, D.-W. Kim and K. S. Hong, RbBaPO4:Eu2+: A new alternative blue-emitting phosphor for UV-based white light-emitting diodes, J. Mater. Chem. C, 2013, 1, 500 RSC.
- Y. H. Kim, P. Arunkumar, B. Y. Kim, S. Unithrattil, E. Kim, S.-H. Moon, J. Y. Hyun, K. H. Kim, D. Lee, J.-S. Lee and W. B. Im, A zero-thermal-quenching phosphor, Nat. Mater., 2017, 16, 543 CrossRef CAS PubMed.
- P. Strobel, C. Maak, V. Weiler, P. J. Schmidt and W. Schnick, Ultra-narrow-band blue-emitting oxoberyllates AELi2 [Be4O6]:Eu2+ (AE = Sr, Ba) paving the way to efficient RGB pc–LEDs, Angew. Chem., Int. Ed., 2018, 57, 8739 CrossRef CAS PubMed.
- Y. Wei, L. Cao, L. Lv, G. Li, J. Hao, J. Gao, C. Su, C. C. Lin, H. S. Jang and P. Dang, Highly efficient blue emission and superior thermal stability of BaAl12O19:Eu2+ phosphors based on highly symmetric crystal structure, Chem. Mater., 2018, 30, 2389 CrossRef CAS.
- Y. Zhong, M. Xia, Z. Chen, P. Gao, H. B. Hintzen, W.-Y. Wong, J. Wang and Z. Zhou, Pyrophosphate phosphor solid solution with high quantum efficiency and thermal stability for efficient LED lighting, iScience, 2020, 23, 100892 CrossRef CAS PubMed.
- A. C. Duke, S. Hariyani and J. Brgoch, Ba3Y2B6O15:Ce3+ a high symmetry, narrow-emitting blue phosphor for wide-gamut white lighting, Chem. Mater., 2018, 30, 2668 CrossRef CAS.
- W.-R. Liu, C.-H. Huang, C.-P. Wu, Y.-C. Chiu, Y.-T. Yeh and T.-M. Chen, High efficiency and high color purity blue-emitting NaSrBO3:Ce3+ phosphor for near-UV light-emitting diodes, J. Mater. Chem., 2011, 21, 6869 RSC.
- Z. Zhu, Z. Sun, Z. Guo and X. Zhang, Luminescence of Ca3ZrSi2O9:Ce3+ blue phosphor with good thermal stability, J. Lumin., 2019, 207, 430 CrossRef CAS.
- S. Zhang, Z. Hao, L. Zhang, G.-H. Pan, H. Wu, X. Zhang, Y. Luo, L. Zhang, H. Zhao and J. Zhang, Efficient blue-emitting phosphor SrLu2O4:Ce3+ with high thermal stability for near ultraviolet (∼400 nm) LED-chip based white LEDs, Sci. Rep., 2018, 8, 10463 CrossRef PubMed.
- C. Zhao, Y.-H. Wu, D.-H. Wang, S.-X. Cao, L.-L. Peng and D.-C. Zhu, A near-ultraviolet (NUV) converting blue-violet Mg2SiO4:Ce3+ phosphor for white light-emitting-diodes (w-LEDs), J. Lumin., 2019, 207, 241 CrossRef CAS.
- Y. Yang, Y. Lin, Y. Han, Z. Qiu, W. Zhou, J. Zhang, C. Li, L. Yu and S. Lian, Fine controllable blue emission and its mechanism in Ce3+-doped orthosilicate solid solution phosphors for different plant growths, J. Rare Earths, 2018, 36, 1150 CrossRef CAS.
- Z. Tang, D. Wang, W. U. Khan, S. Du, X. Wang and Y. Wang, Novel zirconium silicate phosphor K2ZrSi2O7:Eu2+ for white light-emitting diodes and field emission displays, J. Mater. Chem. C, 2016, 4, 5307 RSC.
- T. Aitasalo, J. Hassinen, J. Hölsä, M. Lastusaari, M. Malkamäki, J. Niittykoski and P. Novák, Synchrotron radiation investigations of the Sr2MgSi2O7:Eu2+, R3+ persistent luminescence materials, J. Rare Earths, 2009, 27, 529 CrossRef.
- Y. Yonesaki, T. Takei, N. Kumada and N. Kinomura, Crystal structure of Eu2+ -doped M3MgSi2O8 (M: Ba, Sr, Ca) compounds and their emission properties, J. Solid State Chem., 2009, 182, 547 CrossRef CAS.
- L. Z. He, Z. Song, Q. C. Xiang, Z. G. Xia and Q. L. Liu, Relationship between thermal quenching of Eu2+ luminescence and cation ordering in (Ba1−xSrx)2SiO4:Eu phosphors, J. Lumin., 2016, 180, 163 CrossRef CAS.
- H. Nagabhushana, D. V. Sunitha, S. C. Sharma, B. Daruka Prasad, B. M. Nagabhushana and R. P. S. Chakradhar, Enhanced luminescence by monovalent alkali metal ions in Sr2SiO4:Eu3+ nanophosphor prepared by low temperature solution combustion method, J. Alloys Compd., 2014, 595, 192 CrossRef CAS.
- H. Liu, Y. Hao, H. Wang, J. Zhao, P. Huang and B. Xu, Luminescent properties of R+ doped Sr2SiO4:Eu3+ (R+ =Li+, Na+ and K+) red-emitting phosphors for white LEDs, J. Lumin., 2011, 131, 2422 CrossRef CAS.
- J. Rodriguez-Carvajal , Fullprof: A program for Rietveld refinement and pattern matching analysis, Abstracts of the Satellite Meeting on Powder Diffraction of the XV Congress of the IUCr, 1990, 192, 55.
- M. Catti, G. Gazzoni, G. Ivaldi and G. Zanini, The β↔α′ phase transition of Sr2SiO4. I. Order–disorder in the structure of the α′ form at 383 K, Acta Crystallogr., Sect. B: Struct. Sci., 1983, 39, 674 CrossRef.
- S. S. Binti Nasir, K. Yakura, N. Horiuchi, M. Tsuta and A. Kato, Effect of Eu doping on room temperature phase and phase transition of Sr2SiO4:Eu2+ phosphor synthesized by polymerized complex method, J. Phys. Chem. Solids, 2019, 133, 135 CrossRef CAS.
- Y. Li, J. Wang, X.-M. Wang, F. Pan, T. Zhou and R.-J. Xiea, Colour tuning via crystalline site-selected energy transfer in a Sr2SiO4:Eu2+,Pr3+ phosphor, J. Mater. Chem. C, 2017, 5, 1022 RSC.
- C. Yan, Z. Liu, W. Zhuang, R. Liu, X. Xing, Y. Liu, G. Chen, Y. Li and X. Ma, YScSi4N6C:Ce3+ a broad cyan-emitting phosphor to weaken the cyan cavity in full-spectrum white light-emitting diodes, Inorg. Chem., 2017, 56, 11087 CrossRef CAS PubMed.
- J. Zhong, W. Zhuang, X. Xing, R. Liu, Y. Li, Y. Zheng, Y. Hu and H. Xu, Synthesis, structure and luminescence properties of new blue-green-emitting garnet-type Ca3Zr2SiGa2O12:Ce3+ phosphor for near-UV pumped white-LEDs, RSC Adv., 2016, 6, 2155 RSC.
- D. L. Dexter and J. H. Schulman, Theory of concentration quenching in inorganic phosphors, J. Chem. Phys., 1954, 22, 1063 CrossRef CAS.
- M. Liao, Z. Mu, Q. Wang, X. Zhang, H. Dong, M. Wen and F. Wu, Understanding the cyan-emitting phosphor RbNa(Li3SiO4)2:Eu2+ by providing Rb ion vacancies, J. Alloys Compd., 2020, 837, 155084 CrossRef CAS.
- J. Qiao, G. Zhou, Y. Zhou, Q. Zhang and Z. Xia, Divalent europium-doped near-infrared-emitting phosphor for light-emitting diodes, Nat. Commun., 2019, 10, 1 CrossRef CAS PubMed.
- R. Shi, L. Ning, Z. Wang, J. Chen, T. K. Sham, Y. Huang, Z. Qi, C. Li, Q. Tang and H. Liang, Zero-thermal quenching of Mn2+ red luminescence via efficient energy transfer from Eu2+ in BaMgP2O7, Adv. Opt. Mater., 2019, 7, 1901187 CrossRef CAS.
- J. Qiao, L. Ning, M. S. Molokeev, Y.-C. Chuang, Q. Liu and Z. Xia, Eu2+ site preferences in the mixed cation K2BaCa(PO4)2 and thermally stable luminescence, J. Am. Chem. Soc., 2018, 140, 9730 CrossRef CAS PubMed.
- K. V. d. Eeckhout, P. F. Smet and D. Poelman, Persistent luminescence in Eu2+-doped compounds: a review, Materials, 2010, 3, 2536 CrossRef.
- N. Komuro, M. Mikami, Y. Shimomura, E. G. Bithellc and A. K. Cheethamd, Synthesis, structure and optical properties of cerium-doped calcium barium phosphate—a novel blue-green phosphor for solid-state lighting, J. Mater. Chem. C, 2015, 3, 204 RSC.
- J. Brgoch, C. K. H. Borg, K. A. Denault, A. Mikhailovsky, S. P. DenBaars and R. Seshadri, An efficient, thermally stable cerium-based silicate phosphor for solid state white lighting, Inorg. Chem., 2013, 52, 8010 CrossRef CAS PubMed.
- J. Chen, Y. Liu, L. Mei, H. Liu, M. Fang and Z. Huang, Crystal structure and temperature-dependent luminescence characteristics of KMg4(PO4)3:Eu2+ phosphor for white light-emitting diodes, Sci. Rep., 2015, 5, 9673 CrossRef CAS PubMed.
- J. Zheng, Q. Cheng, S. Wu, Z. Guo, Y. Zhuang, Y. Lu, Y. Li and C. Chen, An efficient blue-emitting Sr5(PO4)3Cl:Eu2+ phosphor for application in near-UV white light-emitting diodes, J. Mater. Chem. C, 2015, 3, 11219 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2225570. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3qi00180f |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2023 |



