A highly effective “naked eye” colorimetric and fluorimetric curcumin-based fluorescent sensor for specific and sensitive detection of H2O2in vivo and in vitro†
Wenhao
Du
,
Zheyu
Shen
,
Yueying
Liang
,
Shuai
Gong
,
Zhiyuan
Meng
,
Mingxing
Li
,
Zhonglong
Wang
* and
Shifa
Wang
 *
*
Co-Innovation Center of Efficient Processing and Utilization of Forest Resources, International Innovation Center for Forest Chemicals and Materials, College of Chemical Engineering, Nanjing Forestry, University, Nanjing, 210037, China. E-mail: wang_zhonglong@njfu.edu.cn; wangshifa65@163.com; Fax: +86-25-85428369; Tel: +86-25-85428369
First published on 13th March 2023
Abstract
Hydrogen peroxide (H2O2) is involved in many important tasks in normal cell metabolism and signaling. However, abnormal levels of H2O2 are associated with the occurrence of several diseases. Therefore, it is important to develop a new method for the detection of H2O2in vivo and in vitro. A turn-off sensor, 2,2-difluoro-4,6-bis(3-methoxy-4-((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)oxy)styryl)-2H-1,3,2-dioxaborine (DFCB), based on curcumin was developed for the detection of H2O2. The DFCB, an orange-emitting sensor, was constructed by employing 2,2-difluoro-4,6-bis(4-hydroxy-3-methoxystyryl)-2H-1,3,2-dioxaborine (DFC) as the main carrier, and 2-(4-bromomethylphenyl)-4,4,5,5-tetramethyl-1,3,2-doxaborolane as the recognition site. The recognition group on the DFCB sensor could be completely cleaved by H2O2 to generate the intermediate DFC, which would lead to a colorimetric change from bright orange to light blue accompanying by a significantly quenched fluorescence, which could be seen by the naked eye. This sensor exhibited a highly specific fluorescence response to H2O2, in preference to other relevant species, with an excellent anti-interference performance. The sensor DFCB also possessed some advantages including a wide pH response range (6–11), a broad linear range (0–300 μM), and a low detection limit (1.31 μM). The sensing mechanism of the DFCB sensor for H2O2 was verified by HRMS analysis, 1H-NMR titration and DFT calculations. In addition, the use of the DFCB sensor was compatible with the fluorescence imaging of H2O2 in living cells and zebrafish.
Introduction
Hydrogen peroxide (H2O2) is one of the most important reactive oxygen species (ROS), and one of its key tasks is to activate immune cells and maintain the normal metabolism.1–4 However, abnormal H2O2 generation will cause irreversible damage to the organism, and may attack cellular biomolecules such as protein and DNA,5–10 affect cell metabolism and proliferation, and cause cancer, abnormal amino acid modification, Alzheimer's disease and other diseases.11–13 Due to the complex pathologies that arise in organisms, H2O2 can cause oxidative stress and eventually lead to cell damage and necrosis.14–16 Therefore, to develop efficient methods for detecting H2O2 is of great importance in disease prevention and medical diagnosis.Existing quantitative detection methods, such as titrimetry, electrochemistry, spectrophotometry and so on, are relatively mature techniques.17–19 Fluorescent sensor analysis has the advantages of fast response, strong selectivity, adaptability and microscopic properties, and it has been widely used for detecting some analytes in biological and environmental systems.20–23 At present, there are many reported sensors for H2O2 which use aryl borate or phenyl boric acid reactions to generate phenol, phenol oxidation to give quinone, oxonium or diketone to give acid, carbon–carbon double bond breaking, and so on.24–26 In recent years, a limited number of fluorescent sensors have been widely used for monitoring H2O2 in living organisms and cells.27–30 However, some reported sensors for H2O2 have suffered from some drawbacks, such as unsatisfactory sensitivity, narrow pH usage range, and so on. Therefore, it is necessary to design pH-compatible fluorescent sensors for detecting H2O2 in biological systems.
Curcumin, a natural product with a unique fluorescence nature, is found mainly in the rhizomes of the plant families Zingiberaceae and Araceae.31,32 Curcumin has received much attention due to its wide range of health care functions and biological properties, such as antibacterial, anti-inflammatory, anti-infective, anti-cancer and liver protective activities. We propose that the curcumin-based fluorescent sensor may have low cytotoxicity.33–35 Moreover, curcumin and its derivatives have a large conjugate structure, which could enhance the π electron delocalization. The curcumin and its derivatives are better to be emitted to generate outstanding long-wavelength fluorescence. The fluorescence sensor with a long-wavelength emission can effectively avoid interference with self-emission fluorescence, and is an ideal tool for in vivo imaging. In this case, some excellent curcumin-based fluorescent sensors have constructed.36–38 Therefore, it is promising for the development of a new curcumin-based fluorescent sensor for H2O2 detection.
It has been reported that β-dicarbonyl compounds which form a rigid structure with boron trifluoride (BF3), could enhance the fluorescence emission wavelength.39,40 Based on this research, BF3 was purposefully added to curcumin to amplify its conjugation effect and sequentially modulate its fluorescence properties. In the research reported in this paper, a novel curcumin-based fluorescent sensor based on DFCB was developed. The quenching mechanism of DFCB on H2O2 was confirmed by high resolution mass spectrometry (HRMS) analysis, 1H-NMR titration and DFT calculations. The bilateral ether bond of the DFCB sensor would be broken in the presence of H2O2, which leads to a significant change of the color which can be seen by the “naked eye”, and the spectral properties of the sensor. The DFCB sensor emitted orange fluorescence at 601 nm and exhibited a significantly quenched fluorescence response to H2O2. The DFCB sensor showed a high sensitivity and favorable selectivity for H2O2 in PBS solution (containing 50% CH3CN, pH = 7.4). More importantly, the DFCB sensor could detect H2O2 in HeLa cells as well as in living zebrafish, which confirmed that the DFCB sensor could be used to effectively monitor exogenous and endogenous H2O2 in a biological system.
Results and discussion
Design and synthesis of the DFCB sensor
In this research, a new curcumin-based fluorescent sensor was constructed for H2O2 detection. In this sensor, the curcumin unit is used logically as a highly effective fluorescent framework due to its lipophilic long-chain conjugate. After that, boron trifluoride diethyl etherate (BF3·(C2H5)2O) was introduced into the curcumin framework to form the DFC compound, which can produce a strong electron push–pull effect. Next, the 2-(4-bromomethylphenyl)-4,4,5,5-tetramethyl-1,3,2-doxaborolane group was introduced into the molecule and used as a recognition group for H2O2. As expected, the sensor exhibited a significant fluorescence emission in the PBS solution (containing 50% CH3CN, pH = 7.4). After reaction with H2O2, the 2-(4-bromomethylphenyl)-4,4,5,5-tetramethyl-1,3,2-doxaborolane group of the DFCB sensor was selectively broken, allowing for the formation of the intermediate DFC, which would lead to a significantly quenched fluorescence emission. The chemical structure of the DFCB sensor was confirmed using 13C-NMR, 1H-NMR and HRMS analyses (see the ESI†).Spectral properties of DFCB in response to H2O2
The optical properties of the DFCB sensor in the absence and presence of H2O2 were determined using UV-vis absorption and fluorescence spectroscopy. As shown in Fig. 1a, the DFCB sensor had a sharp and strong absorption band at 503 nm. After adding 300 μM H2O2 into the solution of the DFCB sensor, the absorbance at 503 nm decreased significantly, whereas a new absorption band appeared at 601 nm. At the same time, the color of the solution changed from bright orange to light blue. The previous data indicated that the DFCB sensor can serve as a colorimetric sensor for detecting H2O2. As shown in Fig. 1b, the fluorescence spectra of DFCB sensor had a strong fluorescence emission at 601 nm. However, after addition of 300 μM H2O2, the fluorescence emission of the DFCB sensor decreased significantly, and the color of the fluorescence changed from bright orange to colorless. The fluorescence spectral results indicated that the DFCB sensor could be used as a turn off fluorescence sensor for H2O2 detection.Sensitivity of the DFCB sensor to H2O2
In order to explore the sensitivity of the DFCB sensor for detecting H2O2, the UV-vis absorption and fluorescence spectra of the DFCB sensor in response to different concentrations of H2O2 were studied. As shown in Fig. 2a, with gradual addition of H2O2 (0–300 μM), the absorption peak of sensor DFCB at 503 nm weakened gradually, whereas a new UV absorption peak appeared at 601 nm and was gradually enhanced. The absorption intensity (A601 nm/A503 nm) of the DFCB sensor showed a good linear relationship with the H2O2 concentration (0–300 μM), and the linear regression equation was y = 0.00469 + 0.00117x (R2 = 0.99152). These experimental data indicated that the DFCB sensor could be employed for the colorimetric quantitative detection of H2O2.The fluorescence spectra of the DFCB sensor for detecting H2O2 were measured. As shown in Fig. 2c, with the gradual addition of 0–300 μM H2O2, the fluorescence emission peak of the DFCB sensor at 601 nm gradually weakened, and the fluorescence intensity of the DFCB sensor showed a linear relationship with the concentration of H2O2 (0–300 μM). The linear regression equation was: y = 829.10482 − 3.08365x (R2 = 0.99001). According to the literature, the formula for the limit of detection was: LOD = 3σ/κ, and the detection limit was calculated to be 1.31 μM,11 which was comparable to that of a previously reported sensor. Based on the UV-vis absorption and fluorescence spectroscopy, the DFCB sensor had high sensitivity and a low detection limit for H2O2.
Selectivity of the DFCB sensor to H2O2
The selectivity and anti-interference properties of the DFCB sensor for the detection of H2O2 were measured. As shown in Fig. 3a, the fluorescence spectra of the DFCB sensor after the addition of different analytes (AcO−, Ag+, Ba2+, Br−, Ca2+, Cl−, ClO−, ClO4−, Co2+, CrO72−, Cs2+, Cu2+, H2O2, H2PO4−, Hg2+, HSO3−, HSO4−, I−, K+, meta-chloroperoxybenzoic acid (MCPBA), Mg2+, Mn2+, Na+, Ni2+, NO2−, NO3−, ˙OH, ONOO−, PAA, SO42−, TBHP, Zn2+). After adding these analytes, the fluorescence intensity of the DFCB sensor was sharply quenched after the reaction with H2O2. In contrast, after the addition of the other analytes, the fluorescence intensity of the DFCB sensor exhibited no significant changes. These data demonstrated the superior selectivity of the DFCB sensor to H2O2. The anti-interference properties of the DFCB sensor for detecting H2O2 were investigated. As shown in Fig. 3b, the fluorescence intensity of the DFCB sensor with H2O2 was not affected by the presence of other analytes. Previous results had indicated that the DFCB sensor had excellent selectivity and anti-interference properties for H2O2.Effects of pH and response time
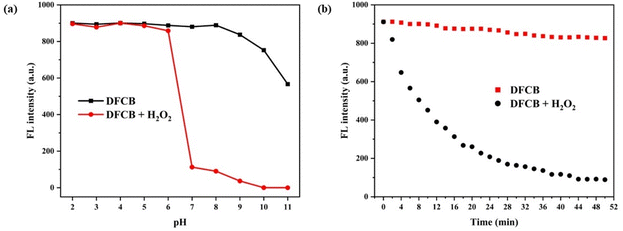
The pH detection range determines whether the sensor can be applied in a biological environment. As shown in Fig. 4a, the DFCB sensor exhibited a high fluorescence intensity at 601 nm, which indicated its good stability. When the pH was located in the range of 2–6, the DFCB sensor showed no remarkable fluorescence change because the H2O2 can react with acid to produce H2O and O2 in an acidic environment. However, when the pH value increased to above 7, the fluorescence emission of the DFCB sensor was sharply quenched, which suggested that the DFCB sensor had an excellent detection ability over a wide pH range, and could be applied to living organisms. The response time of the DFCB sensor to H2O2 was evaluated. As shown in Fig. 4b, after the addition of H2O2, the fluorescence intensity of the DFCB sensor dramatically decreased and reached saturation after 44 min, which suggested that the DFCB sensor can be employed for real-time monitoring of H2O2 (Table 1).| Sensors | Response time | Detection limit (μM) | Linear range | pH range | Application |
|---|---|---|---|---|---|
|
120 min | 4.0 | 0–1 μM | Not mentioned | Living mice |
| Ref. 41 | |||||

|
70 min | 20 | 0–10 μM | 48 | Living mice |
| Ref. 42 | |||||

|
180 min | 6.0 | 0–100 μM | 5–8 | Living cells |
| Ref. 43 | |||||

|
60 min | 4.6 | 0–1 mM | 4–11 | Living cells |
| Ref. 44 | |||||

|
60 min | 1.35 | 0–200 μM | 4–10 | Living cells and living zebrafish |
| Ref. 45 | |||||

|
44 min | 1.31 | 0–300 μM | 6–11 | Living cells and living zebrafish |
| This work |
Detection mechanism
In order to demonstrate the reaction mechanism of DFCB to H2O2, HRMS analysis of the reaction product of the DFCB sensor with H2O2 was performed. As shown in Fig. 5(a), it was observed that the main molecular ion peaks were recorded at an m/z value of 439.1144, which was consistent with the stable DFC ([M + Na]+ = m/z 439.1135). It was hypothesized that the boronate ester group of the DFCB sensor was oxidized and then dissociated fully to form the intermediate ([M + K]+ = m/z 665.1566) in the presence of H2O2, followed by quinoid dissociation to form stable DFC. In addition, the results of the HRMS analysis confirmed that the probable peak was recorded at an m/z value of 665.1585 when about 2 eq. H2O2 was added. The HRMS analysis confirmed the logic of our preliminary analytical reaction mechanism.To demonstrate the reaction mechanism between the DFCB sensor and H2O2, the 1H-NMR spectra of the DFCB sensor after reaction with different amounts of H2O2 were recorded. As shown in Fig. 6, after adding H2O2 into the DMSO-d6 solution of the DFCB sensor, the proton signal peaks (Ha) of the phenylborate groups at 1.29 ppm disappeared with the increase of the H2O2 equivalent. At the same time, the proton signal peaks (Hb) of the methylene units at 5.5 gradually disappeared, which indicated that the boronate ester group of the DFCB sensor can react with H2O2 and was then removed. Based on HRMS analysis and 1H-NMR titration, the sensing mechanism of DFCB on H2O2 was selective boron oxidation which formed the intermediate, and the elimination reactions to release DFC, which might break the original structure of the sensor and would result in a significant weakening of the fluorescence emission at 601 nm.
 | ||
| Fig. 6 The 1H-NMR spectra of the DFCB sensor before and after the reaction with different amounts of H2O2. | ||
The DFT calculation was carried out using the Gaussian 09 program to further reveal the optical changes of the DFCB sensor before and after the reaction with H2O2. Fig. 7 shows the spatial distribution and energy orbitals of the lowest unoccupied molecular orbital (LUMO) and the highest occupied molecular orbital (HOMO) of the DFCB sensor and the DFC compound. The LUMO and HOMO of the DFCB sensor were mainly distributed on the fluorine-boron core, the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, and the benzene ring, indicating the weak charge transfer effect of the DFCB sensor. However, the LUMO of the DFCB sensor was distributed on the fluorine-boron core and the C
C bond, and the benzene ring, indicating the weak charge transfer effect of the DFCB sensor. However, the LUMO of the DFCB sensor was distributed on the fluorine-boron core and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, whereas its HOMO was distributed on the phenol group, which would activate the charge transfer effect of the DFCB sensor after reaction with H2O2, and then it led to a red-shifted absorption spectra.
C bond, whereas its HOMO was distributed on the phenol group, which would activate the charge transfer effect of the DFCB sensor after reaction with H2O2, and then it led to a red-shifted absorption spectra.
 | ||
| Fig. 7 Optimized geometric configurations and frontier molecular orbitals of the DFCB sensor and the DFC intermediate. | ||
Imaging of DFCB in HeLa cells
The use of the DFCB sensor for imaging H2O2 was investigated in living HeLa cells. Prior to the cell fluorescence imaging experiment, the cytotoxicity of the DFCB sensor was measured. As shown in Fig. S7 (ESI†), the DFCB sensor (0–4 μM) had no significant effect on the survival rate of the HeLa cells, and the cell survival rate was over 90%. This indicated that the DFCB sensor was less cytotoxic to cells and that it could be applied in the cell imaging experiment.Furthermore, the DFCB sensor was used for fluorescence imaging in living cells. As shown in Fig. 8, the control cells showed no observable fluorescence emission. With the addition of the DFCB sensor, the HeLa cells emitted an orange fluorescence, which demonstrated that the sensor had good cell permeability and biocompatibility. After further incubation with increasing concentrations of H2O2 (6 and 12 μM), the fluorescence intensity of the cells gradually decreased and was completely quenched. The 3D surface plot and the relative fluorescence intensity were calculated to support the fluorescence quenching effect in living cells, which demonstrated that the DFCB sensor could be utilized for imaging exogenous H2O2in vitro.
The fluorescence labeling, with endogenous H2O2, was carried out in HeLa cells using the DFCB sensor. As shown in Fig. 9, in the presence of the free DFCB sensor (4 μM), there was significant fluorescence emission in the HeLa cells. Since phorbol myristate acetate (PMA, a ROS stimulant) could promote endogenous H2O2 production, the HeLa cells displayed quenching which was supplemented with PMA (1 μg ml−1), and subsequently the cells were cultured for 30 min. If the cells were cultured with PMA for 60 min, the fluorescence intensity decreased further, which indicated that the DFCB sensor could detect endogenous H2O2. In addition, to verify that the endogenous H2O2 production could cause the fluorescence quenching in the cells, the PMA-stimulated cells were further supplemented with N-acetyl-L-cysteine (NAC, a ROS scavenger, 1 mM) and then stained with the DFCB sensor. From Fig. 9(b) it was concluded that the average fluorescence intensity was enhanced after the removal of the endogenous H2O2 from the HeLa cells. The previously described experimental imaging data confirmed the excellent imaging ability of the DFCB sensor for generating endogenous H2O2. In addition, the untreated HeLa cells were selected as the control group. Significant fluorescence emission could not be observed in the cells due to the absence of the DFCB sensor, and these experimental data confirmed that the DFCB sensor could be used to monitor endogenous H2O2 production in living cells. All these cellular experiments confirmed that the DFCB sensor could image endogenous H2O2 in living cells.
Bioimaging in zebrafish
The DFCB sensor was further used to detect H2O2in vivo. As shown in Fig. 10, zebrafish treated with the DFCB sensor (4 μM) exhibited an intense orange fluorescence, suggesting that the DFCB sensor possessed excellent cell permeability. However, the fluorescence intensity of the DFCB sensor gradually decreased until it was completely quenched by the increase of H2O2 concentration (6 and 12 μM). Furthermore, the fluorescence imaging phenomenon in living zebrafish was supported by the 3D surface and the relative fluorescence intensity plots. The previous experimental results showed that the DFCB sensor can detect exogenous H2O2 in organisms.As shown in Fig. 11, the zebrafish incubated with the DFCB sensor (4 μM) emitted an orange fluorescence unlike the control group, which confirmed that zebrafish cannot emit fluorescence by themselves. With the addition of PMA (1 μg ml−1) for different times, the emission fluorescence of the DFCB sensor in zebrafish was gradually quenched, which indicated that the PMA supplement could stimulate the production of endogenous H2O2 in zebrafish. Compared with the zebrafish with the DFCB sensor, the fluorescence intensity was stronger in the zebrafish which was treated with NAC (1 mM) and then cultured with the DFCB sensor. These experimental results confirmed that the DFCB sensor had good potential for use in biological imaging and in the detection of endogenous H2O2. In addition, the relative fluorescence intensity chart was used to support the biological imaging experiments. These experimental data indicated that the DFCB sensor can detect exogenous and endogenous levels of H2O2 in biological systems.
Experiments
Instruments and materials
The chemical reagents used in the experiments are normally available commercially and were used directly. Curcumin was purchased from the Shanghai Titan Technology Company. The BF3·(C2H5)2O was obtained from the Shanghai Lingfeng Chemical Reagent Company. The 2-(4-bromomethylphenyl)-4,4,5,5-tetramethyl-1,3,2-doxaborolane was obtained from the Shanghai Haohong Biomedical Technology Company. The 1H-NMR and 13C-NMR spectra were recorded on a AV-600 NMR spectrometer (Bruker). The HRMS was carried out on a JMS-800D mass spectrometer (Jeol). The UV-vis absorption spectra were recorded on a UV-2450 spectrometer (Shimadzu). The fluorescence emission spectra were recorded using a LS55 fluorescence spectrometer (PerkinElmer).Synthesis of compound DFC
Curcumin (1 mmol) was dissolved in 30 mL of CH2Cl2 at ambient temperature, and BF3·(C2H5)2O (2.4 mmol) was added dropwise. The reaction was continued for 5 h, and monitored with thin layer chromatography. After concentrating and removing half of the solvent, the reaction mixture was placed in a refrigerator for crystallization to take place. The red solid product DFC was obtained by filtering, washing with CH2Cl2, and drying, with a yield of 98%. 1H-NMR (600 MHz, DMSO-d6) δ: 10.09 (s, 2H), 7.92 (d, J = 15.5 Hz, 2H), 7.47 (s, 2H), 7.34 (d, J = 10.3 Hz, 2H), 7.01 (d, J = 15.6 Hz, 2H), 6.87 (s, 2H), 6.45 (s, 1H), 3.85 (s, 6H). 13C-NMR (151 MHz, DMSO-d6) δ: 178.89, 151.52, 148.35, 147.14, 126.16, 125.44, 118.03, 116.13, 112.56, 101.29, 55.94. HRMS (m/z): [M + H]+ calcd for C21H19BO6F2Na + H+, 439.1140; found, 439.1141.Synthesis of the DFCB sensor
The DFC (1 mmol) and 2-(4-bromomethylphenyl)-4,4,5,5-tetramethyl-1,3,2-doxaborolane (2.4 mmol), and K2CO3 (2.4 mmol) were dissolved in 8 mL of CH3CN, and the reaction mixture was then refluxed for 6 h. The reaction mixture was kept in cryogenic refrigerator overnight, and the brown precipitate was filtered to obtain the crude DFCB. The crude DFCB was dissolved in ethyl acetate, and washed with deionized water until the pH became neutral. After drying with MgSO4, followed by filtration, and distillation of the solvent, the DFCB was finally obtained, with a yield of 53% (Scheme 1). 1H-NMR (600 MHz, DMSO-d6) δ: 7.96 (d, J = 15.6 Hz, 2H), 7.70 (d, J = 7.6 Hz, 4H), 7.53 (s, 2H), 7.44 (dd, J = 17.0, 8.1 Hz, 6H), 7.17–7.07 (m, 4H), 6.52 (s, 1H), 5.23 (s, 4H), 3.86 (s, 6H), 1.29 (s, 24H). 13C-NMR (151 MHz, DMSO-d6) δ: 179.43, 158.87, 158.65, 158.42, 158.20, 151.59, 149.70, 147.12, 144.36, 140.22, 134.91, 127.77, 127.32, 125.34, 124.51, 119.42, 117.70, 115.75, 113.67, 111.96, 84.00, 70.03, 56.12, 24.95. HRMS (m/z): [M + H]+ calcd for C47H53B3O10F2Na + H+, 871.3784; found, 871.3794.Spectral measurements
The stock solution of DFCB (1 mM) was prepared in CH3CN. The stock solutions of different metal ions (10 mM) (Ag+, Ba2+, Ca2+, Co2+, Cs2+, Cu2+, Hg2+, K+, Mg2+, Mn2+, Na+, Ni2+, Zn2+), anions (AcO−, Br−, Cl−, ClO−, ClO4−, CrO7−, H2PO4−, HSO3−, HSO4−, I−, NO3−, NO2−, SO42−) and ROS (MCPBA, ˙OH, ONOO−, PAA, TBHP) were prepared in deionized water. The UV-vis absorption and fluorescence spectra of the samples were recorded in PBS solution (50% CH3CN, pH = 7.4) at room temperature. For the fluorescence spectroscopy measurements, the excitation wavelength was λex = 425 nm, and the slit width was λex/λem = 5 nm/5.5 nm.HeLa cell and zebrafish imaging
The HeLa cells were cultured in DMEM containing 10% fetal bovine serum (FBS) with 5% CO2 at 37 °C. Next, the cells were divided into four groups. Except for group 1, the other cells from groups 2 to 4 were incubated in 4 μM DFCB solution for 1 h at 37 °C, and then incubated with different concentrations of H2O2 (0, 6, 12 μM) for another 30 min. Fluorescence images of the HeLa cells were recorded using a laser confocal fluorescence microscope in the orange channel (λex = 405 nm, λem = 450–650 nm).Four-day-old zebrafish were randomly divided into three groups. Each group was incubated with 4 μM of the DFCB sensor for 30 min, and then incubated with different concentrations of H2O2 (0 μM, 6 μM, and 12 μM) for 44 min. After washing the zebrafish three times with the PBS solution, the fluorescence images of the zebrafish were obtained using a laser confocal fluorescence microscope in the orange channel (λex = 405 nm, λem = 450–650 nm).
Conclusions
In summary, a new curcumin-based fluorescent DFCB sensor was designed and synthesized for use in H2O2 detection. The DFCB sensor emitted an intense orange fluorescence and exhibited a significant quenched fluorescence response to H2O2. The color of the DFCB sensor solution changed from orange to blue in the presence of H2O2, when the solution was viewed with the naked eye. The DFCB sensor could selectively detect H2O2 and showed a good anti-interference performance. Moreover, the DFCB sensor exhibited the advantages of a low detection limit (1.31 μM), wide linear range (0–300 μM), and broad pH detection range (6–11). Furthermore, the DFCB sensor with low cytotoxicity and good biocompatibility was successfully used for imaging endogenous and exogenous H2O2 in living HeLa cells, as well as in living zebrafish. We predict that the results of this research will have the potential to be used in future for monitoring the physiological and pathological effects of H2O2in vivo.Author contributions
Wenhao Du: investigation, methodology and writing – original draft. Zheyu Shen: formal analysis. Yueying Liang: software. Shuai Gong: investigation. Zhiyuan Meng: software. Mingxing Li: data curation. Zhonglong Wang: writing – review and editing. Shifa Wang: project administration.Ethics declarations
Ethics approval
All the animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Nanjing University and approved by the Animal Ethics Committee of China.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The authors wish to thank the National Natural Science Foundation of China (Grant No. 32071707 and 32101466) and the Natural Science Foundation of Jiangsu Province (Grant No. BK20210624) for their financial support for this research.References
- X. Li, N. Gao, C. Liu, M. Yu, X. Rong, Y. Zhang, M. Su, X. Wang, H. Zhu, K. Wang, Y. Liu, W. Sheng and B. Zhu, Sens. Actuators, B, 2022, 353, 131051 CrossRef CAS.
- X. Zhang, L. Zhang, X. Y. Cheng, S. D. Liu, S. J. Fang, L. W. Zhang, X. Y. Wang and L. X. Chen, Sens. Actuators, B, 2022, 366, 131982 CrossRef CAS.
- Y. Huang, L. Yu, P. P. Lu, Y. H. Wei, L. L. Fu, J. J. Hou, Y. Q. Wang, X. Y. Wang and L. X. Chen, J. Hazard. Mater., 2022, 424, 127425 CrossRef CAS PubMed.
- Y. Q. Shi, Q. C. Wu, W. T. Li, L. Lin, F. F. Qu, C. J. Shen, Y. Z. Wei, P. C. Nie, Y. He and X. P. Feng, J. Hazard. Mater., 2022, 432, 128605 CrossRef CAS PubMed.
- Q. Xu, Y. Y. Tang, P. D. Zhu, W. Y. Zhang, Y. Q. Zhang, O. S. Solis, T. S. Hu and J. C. Wang, Nanoscale, 2022, 14, 13771–13778 RSC.
- Y. Wu, Z. Y. Li and Y. M. Shen, ACS Omega, 2019, 4, 16242–16246 CrossRef CAS PubMed.
- Z. L. Wang, Y. Zhang, J. Song, M. X. Li, Y. Q. Yang, X. Xu, H. J. Xu and S. F. Wang, Sens. Actuators, B, 2019, 284, 148–158 CrossRef CAS.
- J. Q. Xu, J. S. Guo, K. F. Xie, M. J. Gao, R. Wei, Z. H. Xin and Y. F. Kang, Dyes Pigm., 2022, 204, 110437 CrossRef CAS.
- X. L. Liu, M. D. Yan, Z. G. Chen, B. X. Zhang, N. C. Yao, S. Zhao, X. X. Zhao, T. Zhang and G. F. Hai, Spectrochim. Acta, Part A, 2023, 286, 121955 CrossRef CAS PubMed.
- M. Chen, Z. H. Liang, G. L. Zeng, Y. Wang, Z. H. Mai, X. Y. Chen, G. Wu and T. S. Chen, Dyes Pigm., 2022, 198, 109995 CrossRef CAS.
- L. Q. Li, M. H. Zheng, X. Y. Yan, H. Huang, S. X. Cao, K. M. Liu and J. B. Liu, J. Photochem. Photobiol., A, 2022, 432, 114069 CrossRef CAS.
- T. Ma, K. Fu, Z. C. Li, C. C. Yuan and W. B. Ma, Spectrochim. Acta, Part A, 2022, 276, 121218 CrossRef CAS PubMed.
- M. X. Wang, F. Zhang, C. Q. Wang, N. Yin, Y. T. Wang, G. X. Qin, Q. L. Xu, J. H. Gong, H. Z. Liu and X. R. Duan, Anal. Chem., 2022, 94, 5962–5969 CrossRef CAS PubMed.
- Y. Q. Li, Y. Zhou, J. N. Lei, Q. J. Lu, X. Qin, Q. Xu, Y. Q. Wang, C. Y. Wu, Z. Yang and B. S. He, J. Mol. Struct., 2023, 1271, 134042 CrossRef CAS.
- Y. Fang, J. Wang, H. Yu, Q. Zhang, S. J. Chen, K. P. Wang and Z. Q. Hu, Sens. Actuators, B, 2022, 371, 132514 CrossRef CAS.
- Y. Tian, S. Y. Liu, W. W. Cao, P. Wu, Z. M. Chen and H. Xiong, Anal. Chem., 2022, 94, 11321–11328 CrossRef CAS PubMed.
- M. Chen, Z. H. Liang, X. H. Fan, R. M. Qu, H. H. Wang and T. S. Chen, Spectrochim. Acta, Part A, 2022, 276, 121163 CrossRef CAS PubMed.
- L. Guo, S. Chen, Y. L. Yu and J. H. Wang, Anal. Chem., 2021, 93, 16240–16247 CrossRef CAS PubMed.
- M. R. Li, B. W. Wang, J. Y. Liu, Z. Z. Zhang, L. G. Chen, Y. Li and X. L. Yan, Anal. Chem., 2022, 94, 9732–9739 CrossRef CAS PubMed.
- K. Yin, F. B. Yu, D. Y. Liu, Z. H. Xie and L. X. Chen, Sens. Actuators, B, 2016, 223, 799–805 CrossRef CAS.
- Z. Y. Zhang, Z. P. Chen, F. B. Cheng, Y. W. Zhang and L. X. Chen, Biosens. Bioelectron., 2017, 89, 932–936 CrossRef CAS PubMed.
- X. Wang, Q. Ding, Y. Tian, W. Wu, F. D. Che, P. Li, W. Zhang, W. Zhang and B. Tang, Chem. Commun., 2022, 58, 6320–6323 RSC.
- J. K. Liang, H. Li, J. R. Wang, H. L. Yu and Y. He, Anal. Chem., 2020, 92, 6548–6554 CrossRef CAS PubMed.
- S. Wang, Y. Zhang, T. R. Wang, Y. J. Liu, S. L. Shen and X. Q. Cao, Spectrochim. Acta, Part A, 2022, 266, 120435 CrossRef CAS PubMed.
- Y. Hua, Y. J. Shang, M. J. Gao, J. Li and Y. F. Kang, Spectrochim. Acta, Part A, 2022, 265, 120320 CrossRef CAS PubMed.
- X. D. Zeng, C. Jiang, Q. Zhang, D. K. Chai, M. S. Ma, J. Chen and Z. G. Liu, J. Lumin., 2021, 240, 118422 CrossRef CAS.
- J. Su, S. P. Zhang, C. R. Wang, M. Li, J. J. Wang, F. Su and Z. J. Wang, ACS Omega, 2021, 6, 14819–14823 CrossRef CAS PubMed.
- L. L. Xu, Y. Zhang, L. H. Zhao, H. Han, S. Q. Zhang, Y. B. Huang, X. H. Wang, D. Q. Song, P. Y. Ma, P. Ren and Y. Sun, Talanta, 2021, 233, 122578 CrossRef CAS PubMed.
- G. Q. Yang, T. Zhu, D. Wang, Z. J. Liu, R. L. Zhang, G. M. Han, X. H. Tian, B. H. Liu, M. Y. Han and Z. P. Zhang, Chem. Commun., 2021, 57, 6628–6631 RSC.
- R. R. Zhou, Q. Y. Peng, D. Wan, C. Yu, Y. Zhang, Y. Hou, Q. Luo, X. Li, S. H. Zhang, L. Xie, P. H. Ou and Y. B. Peng, RSC Adv., 2021, 11, 24032–24037 RSC.
- Y. M. Hao, H. P. Wang, Z. H. Wang, W. J. Dong, Q. Hu, S. M. Shuang, C. Dong and X. J. Gong, Mikrochim. Acta, 2021, 188, 16 CrossRef CAS PubMed.
- X. H. Pan, Y. H. Zhao, T. T. Cheng, A. S. Zheng, A. B. Ge, L. X. Zang, K. H. Xu and B. Tang, Chem. Sci., 2019, 10, 8179–8186 RSC.
- Z. W. Gan, T. Zhang, X. X. An, Q. Tan, S. J. Zhen, Y. M. Hu and X. L. Hu, Microchem. J., 2022, 182, 107939 CrossRef CAS.
- H. Yang, C. Yu, Z. Yin, P. Guan, S. Jin, Y. Wang and X. Feng, J. Sci. Food Agric., 2022, 103, 1550–1560 CrossRef PubMed.
- H. L. Dong, P. Wang, Z. Y. Yang, R. Li, X. L. Xu and J. Shen, Ultrason. Sonochem., 2022, 90, 106188 CrossRef CAS PubMed.
- H. Li, T. Wang, J. Q. Su and P. V. Meeren, Food Hydrocolloids, 2022, 133, 108020 CrossRef CAS.
- J. P. Hu, Y. L. Wang, T. Y. Shao, G. C. Lian, K. B. Hu, Y. Liu, M. Zhou, X. P. Wang, L. Z. Huang, X. L. Meng and G. F. Jin, Arabian J. Chem., 2022, 15, 104087 CrossRef CAS.
- S. Kang, B. Y. Park, S. Lee, N. Lee and M. S. Han, Analyst, 2021, 146, 463–470 RSC.
- Q. Jiang, Z. L. Wang, M. X. Li, J. Song, Y. Q. Yang, X. Xu, H. J. Xu and S. F. Wang, Tetrahedron Lett., 2020, 61, 152103 CrossRef CAS.
- Y. Gao, M. X. Li, X. C. Tian, S. Gong, Y. Zhang, Y. Q. Yang, Z. L. Wang and S. F. Wang, Microchem. J., 2022, 169, 106631 CrossRef.
- C. S. Lim, M. Y. Cho, M. Y. Park and H. M. Kim, ChemistryOpen, 2017, 7, 53–56 CrossRef PubMed.
- R. F. Xu, Y. Wang, H. Y. You, L. W. Zhang, Y. Q. Wang and L. X. Chen, Analyst, 2019, 144, 2556–2564 RSC.
- Y. Liu, J. Nie, J. Niu, F. F. Meng and W. Y. Lin, Sci. Rep., 2017, 7, 7293 CrossRef PubMed.
- G. Masanta, C. H. Heo, C. S. Lim, S. K. Bae, B. R. Cho and H. M. Kim, Chem. Commun., 2012, 48, 3518–3520 RSC.
- K. Xu, L. He, X. Yang, Y. Yang and W. Lin, Analyst, 2018, 43, 3555–3559 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3an00340j |
| This journal is © The Royal Society of Chemistry 2023 |