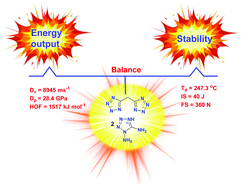
To develop environmentally friendly green energetic materials with excellent insensitivity, thermal stability, and detonation performance, a series of nitrogen-rich salts were synthesized from di(1H-tetrazol-5-yl)methane and N,N′-(methylenebis(1H-1,2,4-triazole-3,5-diyl))dinitramide using a simple and straightforward high-yield approach. The molecules were characterized using NMR, IR, elemental analysis, and differential scanning calorimetry. Single-crystal X-ray diffraction studies confirm the molecular structures of salts 4 and 15. Their key energetic parameters, viz., energy content, density, detonation performance, melting and decomposition temperatures, and impact/friction sensitivities, were assessed. All of these salts showed excellent thermal stability over 210 °C as measured using DSC. The crystal structures of compounds 4 and 15 show a strong intramolecular network of hydrogen bonding that is expected to stabilize the salts and induce a higher insensitivity to mechanical stimuli. All the newly synthesized derivatives exhibit a superior energetic performance over TNT, close to RDX. The salts 5, 6, 10–14, and 16 display properties better than TATB and are expected to serve as potential energetic materials.