New structural topologies in 4f-metal cluster chemistry from vertex-sharing butterfly units: {LnIII7} complexes exhibiting slow magnetization relaxation and ligand-centred emissions†
Eleni C. Mazarakiotia,
Luís Cunha-Silvab,
Vlasoula Bekiaric,
Albert Escuerd and
Theocharis C. Stamatatos*a
aDepartment of Chemistry, Brock University, 500 Glenridge Ave, L2S 3A1 St. Catharines, Ontario, Canada. E-mail: tstamatatos@brocku.ca
bREQUIMTE/LAQV & Department of Chemistry and Biochemistry, Faculty of Sciences, University of Porto, 4169-007 Porto, Portugal
cDepartment of Aquaculture and Fisheries Management, Technological Educational Institute of Western Greece, 30 200 Messolonghi, Greece
dDepartament de Quimica Inorganica, Institut de Nanociencia i Nanotecnologia (IN2UB), Universitat de Barcelona, Diagonal 645, 08028 Barcelona, Spain
First published on 22nd October 2015
Abstract
The employment of N-salicylidene-o-aminophenol in 4f-metal cluster chemistry has led to a family of {Ln7} clusters with a new core topology that comprises two {Ln4} butterflies sharing a common metal vertex; the {Dy7} derivative exhibits slow magnetization relaxation whereas all heptanuclear compounds show ligand-centred blue-green emissions.
One of the current most attractive directions in modern inorganic and coordination chemistry is towards the synthesis of complex molecular materials with appealing structures and multiple physical properties. The goal is undoubtedly to construct multifunctional (or hybrid) materials, where two or more properties will cooperate synergistically in the presence of an external stimulus, such as light, electric current or magnetic field.1 In order to achieve the synthesis of such molecular, zero-dimensional (0-D) species, the nature of metals and bridging ligands is of vital importance.
In terms of metal ions, lanthanides have shown a remarkable ability to yield beautiful structures with interesting magnetic and optical properties.2 This is due to the preference of 4f-metal ions to bind to O- and/or N-based ligands, the nature of the f-electrons and orbitals, and the unique electronic structures they possess (ground state: 2S+1LJ). The large number of unpaired electrons, in conjunction with the appreciable magnetoanisotropy originating from the spin–orbit coupling and ligand field effects, of some 4f-metal ions (i.e., DyIII and TbIII) make them suitable candidates for single-molecule magnetism behaviors.3 Single-molecule magnets (SMMs) derive their properties from the combination of a large magnetic moment in the ground state with a large magnetic anisotropy, as reflected in a large and negative zero-field splitting parameter (D).4 As a result, SMMs often possess an appreciable barrier to magnetization relaxation at low temperatures, and they display out-of-phase ac magnetic susceptibility signals and magnetization hysteresis loops.5 In addition, 4f-metal complexes have also shown intense, sharp and long-lived emissions;6 they can thus be used for a variety of optical and medical applications such as display devices, luminescent sensors, and probes for clinical use.7 This particularly applies to EuIII and TbIII complexes with red and green luminescence due to 5D0 → 7FJ and 5D4 → 7FJ transitions, respectively, and occasionally to DyIII compounds with characteristic blue or yellow-centred 4F9/2 → 6HJ transitions.8
Thus, it becomes apparent that polynuclear 4f-metal clusters can satisfy all the above requirements and yield both structurally interesting molecular species and emissive SMMs.9 The nature of the organic bridging ligand becomes of great importance because it will not only foster the formation of a high-nuclearity species but also dictate the nature of the intramolecular magnetic exchange interactions between the metal ions and the efficiency of the energy transfer from its triplet state to the metals' accessible emissive states (‘antenna’ effect). For these reasons, we have decided to follow up with our previous success in employing Schiff base bridging ligands in 3d-metal cluster chemistry,10 but this time to explore their use in lanthanide chemistry as a means of obtaining novel compounds with unprecedented topologies and magneto-optical behaviors. We herein show that the use of the tridentate chelating/bridging ligand N-salicylidene-o-aminophenol (saphH2) in 4f-metal chemistry can lead to a new family of heptanuclear clusters with an unforeseen metal vertex-sharing double-butterfly topology, and SMM and emission behaviors. We have also managed to show that although saphH2 is not a new ligand in lanthanide chemistry,11 the hydrolysis of the metal ions via the use of a different combination of solvent and starting materials can lead to higher nuclearity products.
The reaction of Ln(NO3)3·6H2O, saphH2, and NEt3 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 molar ratio in Me2CO gave yellow solutions that upon slow evaporation at room temperature afforded yellow, very thin needle-shaped crystals of (NHEt3)[Ln7(OH)2(saph)10(Me2CO)2] (Ln = Gd (1); Tb (2); Dy (3)) in 45–55% isolated yields.† The chemical and structural identities of the complexes were confirmed by single-crystal X-ray crystallography (complete data set for 1 and unit cell determination for 2 and 3), elemental analyses, IR spectral comparison (Fig. S1†), and powder-XRD studies (Fig. S7†).
6 molar ratio in Me2CO gave yellow solutions that upon slow evaporation at room temperature afforded yellow, very thin needle-shaped crystals of (NHEt3)[Ln7(OH)2(saph)10(Me2CO)2] (Ln = Gd (1); Tb (2); Dy (3)) in 45–55% isolated yields.† The chemical and structural identities of the complexes were confirmed by single-crystal X-ray crystallography (complete data set for 1 and unit cell determination for 2 and 3), elemental analyses, IR spectral comparison (Fig. S1†), and powder-XRD studies (Fig. S7†).
The formula of 1 is based on metric parameters, charge balance considerations and O BVS calculations;12 the latter confirmed the assignment of the μ3-bridging groups as OH− ions (BVS 1.11–1.12). The molecular structure of the anion of 1 (Fig. 1, top) reveals the presence of seven GdIII atoms bridged together by two μ3-OH− ions (O8, O11) and the phenoxido arms of two η2:η1:η2:μ3, four η1:η1:η2:μ and four η1:η1:η3:μ3 saph2− ligands (Scheme 1). The complete [Gd7(μ3-OH)2(μ3-OR)4(μ-OR)8]7+ core of 1 (Fig. 1, bottom) can be conveniently described as two [Gd4(μ3-OH)(μ3-OR)2(μ-OR)4]5+ butterflies that share a common metal vertex (Gd4) at the wing-tip position. The μ3-OH− and μ3-OR− bridging groups are all displaced by 1.04–1.40 Å away from the Gd4 best-mean-planes, implying a significant distortion of the butterflies and a subsequent asymmetrization of the overall structure. Peripheral ligation about the core is provided by the chelating part of the saph2− ligands and two terminally bound Me2CO molecules on Gd2 and Gd5. Two GdIII atoms (Gd1, Gd6) are 7-coordinate with capped octahedron geometries, while the remaining metal ions are 8-coordinate with different coordination geometries (triangular dodecahedral for Gd2 and Gd5; biaugmented trigonal prismatic for Gd3 and Gd7; square antiprismatic for Gd4). All coordination features of the individual GdIII ions have been derived from the program SHAPE (Fig. S2†).13 Finally, the {Gd7} anions are well-isolated in the crystal (Fig. S8†), with the shortest Gd⋯Gd intermolecular separation being 12.2 Å. Although heptanuclear metal complexes are of precedence, with the majority of them comprising two vertex-sharing dicubanes,14 there is no previous report on a topology similar to that of 1, other than a mixed-valence {MnII/III7} with diethylenetriamine chelate ligand.15
Variable-temperature direct current (dc) magnetic susceptibility studies were carried out on freshly prepared, crystalline samples of complexes 1–3 in the temperature range 2.0–300 K under an applied field of 0.3 T. The low-temperature (30–2 K) susceptibility data were re-collected at a very small applied dc field of 0.02 T to avoid saturation effects; these data were identical with the ones collected under 0.3 T. The obtained data for all studied compounds are shown as χMT vs. T plots in Fig. 2. The experimental χMT values at room temperature are in good agreement with the theoretical ones (55.13 cm3 K mol−1 for 1; 82.74 cm3 K mol−1 for 2; 99.19 cm3 K mol−1 for 3) for seven non-interacting GdIII (8S7/2, S = 7/2, L = 0, g = 2), TbIII (7F6, S = 3, L = 3, g = 3/2) and DyIII (6H15/2, S = 5/2, L = 5, g = 4/3) ions. For the isotropic Gd7 (1), the χMT product remains almost constant at a value of ∼55 cm3 K mol−1 from 300 K to ∼40 K and then steadily decreases to a minimum value of 22.21 cm3 K mol−1 at 2.0 K. The identical response under the two measured fields precludes the presence of any anisotropy, and is indicative of the presence of weak intramolecular antiferromagnetic exchange interactions between the seven GdIII centers. For the anisotropic Tb7 (2) and Dy7 (3) complexes, the thermal evolution of the magnetic susceptibility is similar, in which the χMT product remains essentially constant at a value of ∼78 and ∼98 cm3 K mol−1 from 300 K to ∼130 K and then steadily decreases to a minimum value of 44.07 and 63.60 cm3 K mol−1 at 2.0 K, respectively. Such a low temperature decrease of the χMT product is mainly due to depopulation of the excited Stark sublevels of the TbIII and DyIII ions and some weak antiferromagnetic interactions between the metal centers, which cannot be quantified due to the strong orbital momentum.16
The field dependence of magnetization (M) at low temperatures show all the expected characteristics for polynuclear, weakly coupled Ln(III) clusters. Briefly, the lack of saturation in magnetization for complexes 2 and 3 (Fig. S3†) indicates the presence of magnetic anisotropy and/or population of low-lying excited states.17 In the case of 1, the magnetization reaches a quasi saturated value of 47.7 μB at the highest fields (Fig. S4†), which is in excellent agreement with the expected value of 49 μB for seven non-coupled GdIII ions. The deviation of M vs. H for 1 at low fields (below the shape and values expected for a simply Brillouin behavior for 7 GdIII) confirms the weak antiferromagnetic interactions.
Alternating current (ac) magnetic susceptibility studies have been also performed in order to investigate the magnetization dynamics of the anisotropic Tb7 and Dy7 clusters under a zero dc magnetic field. Complex 2 does not show any out-of-phase signals (Fig. S5†); this is somehow usual in polynuclear TbIII complexes because TbIII is a non-Kramers ion and so its complexes will have a bistable ground state only if the ligand-field has an axial symmetry.18 This is particularly difficult and rare when the aggregation of TbIII ions results in a polymetallic cluster compound where many metal ions are present in different coordination environments. In contrast, complex 3 shows frequency-dependent out-of-phase χ′′M tails of signals at temperatures below ∼7 K (Fig. 3), indicative of the slow magnetization relaxation of an SMM with a small energy barrier for magnetization reversal. This is most likely due to the fast tunneling which is frequently observed in high-nuclearity DyIII SMMs, and mainly originates from single-ion anisotropy effects of the individual DyIII Kramers ions.19 In an attempt to quantify the energy barrier and relaxation time for 3, and given the absense of χ′′ peak maxima, we decided to apply the below equation developed by Bartolomé et al.20
| ln(χ′′/χ′) = ln(ωτ0) + Ea/kBT |
Considering a single relaxation process, the least-squares fit of the experimental data (inset of Fig. 3) gave an energy barrier of ∼3.0(1) cm−1 (∼4.3(1) K) and a τ0 = 8.3(2) × 10−6 s which is consistent with the expected τ0 values for a fast relaxing SMM.
In order to gain any possible access into additional physical properties for this family of Ln7 complexes, we decided to perform photoluminescence studies in the solid-state and at room temperature. 4f-metal complexes usually exhibit metal-centred emission bands, which are sharp, intense, and narrow. These bands arise from an efficient energy transfer mechanism which includes the ‘sensitization’ of the metal's excited levels from the triplet (or occasionally singlet) state of the coordinated organic ligand(s).21 In contrast, quenching of Ln emission is relatively rare, but when occurs it is associated with either negligible emission or red-shifted ligand-centred emissions, which are both broad and weak. Reasons for such quenching vary but they are mainly related with structural parameters, such as the coordination of solvate ligands and the presence of lattice solvents and counterions in the crystal, the temperature, as well as the energy of the lowest triplet state of the ligand.22
The free ligand saphH2, and its dianionic form (saph2−),23 appear to have very similar photophysical behaviors. Upon maximum excitation at 490 nm, the ligand shows two strong and broad emission bands at 510 and 540 nm (Fig. 4, left), which are located at the blue-green range of the visible spectrum. Although saphH2 seemed to be a promising ‘sensitizer’ for 4f-metal luminescence, the photophysical properties of all three reported Ln7 compounds are identical and consistent with a ligand-centred emission. In detail, upon maximum excitation of 1–3 at 400 nm, a green emission at 540 nm has been detected, supplemented with a weaker intensity band at 485 nm (Fig. 4, right, and Fig. S6†). The recorded emissions of 1–3 are obviously red-shifted with respect to the metal-free ligand, implying a significant effect of the metal–ligand interactions on the overall optical response. Such a Ln-independent emission can be ascribed to an efficient Ln-to-saph2− back energy transfer process.24 There is no doubt that quenching from the coordinated Me2CO molecules, the presence of Et3NH+ countercations and lattice solvents in the crystals of 1–3, might also contribute to the diminishing of the Ln emission.
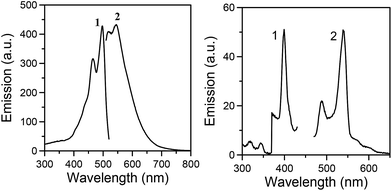 | ||
| Fig. 4 Excitation (1) and emission (2) spectra of solid saphH2 (left) and Dy7 (right) at room temperature. | ||
Conclusions
In conclusion, we have shown that a new family of heptanuclear Ln(III) complexes, bearing the known Schiff base ligand N-salicylidene-o-aminophenol (saphH2), has now joined the growing family of 4f-metal clusters with large nuclearities, unprecedented topologies and interesting physicochemical properties, such as single-molecule magnetism and optics. The reported complexes possess an unusual topology of two {Ln4} butterflies sharing a common, central metal ion at the wing-tip position. This results from the co-presence of μ3-OH−, and μ3- and μ-OR− groups from the double-deprotonated form of saphH2 under the prevailing basic conditions.Acknowledgements
This work was supported by Brock University, NSERC-DG and ERA (to Th. C. S), the Ontario Trillium Foundation (graduate scholarship to E. M), the FEDER (Fundo Europeu de Desenvolvimento Regional) through PT2020 and FCT (Fundação para a Ciência e a Tecnologia) for financial support to the research centre REQUIMTE/LAQV (UID/QUI/50006/2013) (L. C.-S.), and the CICYT (project CTQ2012-30662, to A. E). V. B acknowledges financial support by the European Union (European Social Fund – ESF) and Greek national funds through the Operational Program “Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) – Research Funding Program: Archimedes III.Notes and references
- For example, see: (a) R. Ababei, C. Pichon, O. Roubeau, Y.-G. Li, N. Bréfuel, L. Buisson, P. Guionneau, C. Mathonière and R. Clérac, J. Am. Chem. Soc., 2013, 135, 14840 CrossRef CAS PubMed; (b) J. Long, J. Rouquette, J. M. Thibaud, R. A. S. Ferreira, L. D. Carlos, B. Donnadieu, V. Vieru, L. F. Chibotaru, L. Konczewicz, J. Haines, Y. Guari and J. Larionova, Angew. Chem., Int. Ed., 2015, 54, 2236 CrossRef CAS PubMed; (c) E. Coronado, J. R. Galán-Mascarós, A. Murcia-Martínez, F. M. Romero and A. Tarazón, Organic Conductors, Superconductors and Magnets: From Synthesis to Molecular Electronics, NATO ASI Series, ed. L. Ouahab and E. Yagubskii, Kluwer Academic Publishers, 2004, vol. 139, pp. 127–142 Search PubMed.
- (a) J.-B. Peng, X.-J. Kong, Q.-C. Zhang, M. Orendáč, J. Prokleška, Y.-P. Ren, L.-S. Long, Z. Zheng and L.-S. Zheng, J. Am. Chem. Soc., 2014, 136, 17938 CrossRef CAS PubMed; (b) X. Gu and D. Xue, Inorg. Chem., 2007, 46, 3212 CrossRef CAS PubMed.
- (a) D. N. Woodruff, R. E. P. Winpenny and R. A. Layfield, Chem. Rev., 2013, 113, 5110 CrossRef CAS PubMed; (b) J. D. Rinehart and J. R. Long, Chem. Sci., 2011, 2, 2078 RSC; (c) R. J. Blagg, L. Ungur, F. Tuna, J. Speak, P. Comar, D. N. Collison, W. Wernsdorfer, E. J. L. McInnes, L. Chibotaru and R. E. P. Winpenny, Nat. Chem., 2013, 5, 673 CrossRef CAS PubMed.
- (a) D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS PubMed; (b) L. Sorace, C. Benelli and D. Gatteschi, Chem. Soc. Rev., 2011, 40, 3092 RSC; (c) L. Sorace and D. Gatteschi, in Lanthanides and Actinides in Molecular Magnetism, ed. R. A. Layfield and M. Murugesu, Wiley-VCH, 1st edn, 2015 Search PubMed.
- (a) G. Christou, D. Gatteschi, D. N. Hendrickson and R. Sessoli, MRS Bull., 2000, 25, 66 CrossRef CAS; (b) R. Bagai and G. Christou, Chem. Soc. Rev., 2009, 38, 1011 RSC.
- (a) S. V. Eliseeva and J.-C. Bünzli, New J. Chem., 2011, 35, 1165 RSC; (b) M. D. Ward, Coord. Chem. Rev., 2010, 254, 2634 CrossRef CAS; (c) T. Förster, Chem. Phys. Lett., 1971, 12, 422 CrossRef.
- J.-C. G. Bünzli and S. V. Eliseeva, Chem. Sci., 2013, 4, 1939 RSC.
- (a) S. Cotton, Lanthanide and Actinide Chemistry, John Willey & Sons, West Sussex, 2006 Search PubMed; (b) Y. Bi, X.-T. Wang, W. Liao, X. Wang, R. Deng, H. Zhang and S. Gao, Inorg. Chem., 2009, 48, 11743 CrossRef CAS PubMed; (c) C. E. Burrow, T. J. Burchell, P.-H. Lin, F. Habib, W. Wernsdorfer, R. Clérac and M. Murugesu, Inorg. Chem., 2009, 48, 8051 CrossRef CAS PubMed; (d) C.-J. Li, M.-X. Peng, J.-D. Leng, M.-M. Yang, Z. Li and M.-L. Tong, CrystEngComm, 2008, 10, 1645 RSC.
- (a) D. I. Alexandropoulos, S. Mukherjee, C. Papatriantafyllopoulou, C. P. Raptopoulou, V. Psycharis, V. Bekiari, G. Christou and Th. C. Stamatatos, Inorg. Chem., 2011, 50, 11276 CrossRef CAS PubMed; (b) M. Menelaou, F. Ouharrou, L. Rodríguez, O. Roubeau, S. J. Teat and N. Aliaga-Alcalde, Chem.–Eur. J., 2012, 18, 11545 CrossRef CAS PubMed; (c) E. C. Mazarakioti, K. M. Poole, L. Cunha-Silva, G. Christou and Th. C. Stamatatos, Dalton Trans., 2014, 43, 11456 RSC; (d) D. I. Alexandropoulos, L. Cunha-Silva, L. Pham, V. Bekiari, G. Christou and Th. C. Stamatatos, Inorg. Chem., 2014, 53, 3220 CrossRef CAS PubMed; (e) A. B. Canaj, D. I. Tzimopoulos, A. Philippidis, G. E. Kostakis and C. J. Milios, Inorg. Chem., 2012, 51, 7451 CrossRef CAS PubMed.
- (a) A. A. Athanasopoulou, C. P. Raptopoulou, A. Escuer and Th. C. Stamatatos, RSC Adv., 2014, 4, 12680 RSC; (b) A. A. Athanasopoulou, M. Pilkington, C. P. Raptopoulou, A. Escuer and Th. C. Stamatatos, Chem. Commun., 2014, 50, 14942 RSC.
- For previously reported dinuclear and hexanuclear lanthanide complexes bearing saph2− ligands, see: (a) N. C. Anastasiadis, D. A. Kalofolias, A. Philippidis, S. Tzani, C. P. Raptopoulou, V. Psycharis, C. J. Milios, A. Escuer and S. P. Perlepes, Dalton Trans., 2015, 44, 10200 RSC; (b) S. Mukherjee, A. K. Chaudhari, S. Xue, J. Tang and S. K. Ghosh, Inorg. Chem. Commun., 2013, 35, 144 CrossRef CAS.
- I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 244 CrossRef CAS . An O BVS in the ∼1.7–2.0, ∼1.0–1.2, and ∼0.2–0.4 ranges is indicative of non-, single- and double-protonation, respectively.
- S. Alvarez, P. Alemany, D. Casanova, J. Cirera, M. Llunell and D. Avnir, Coord. Chem. Rev., 2005, 249, 1693 CrossRef CAS.
- (a) X. Liu, J. A. McAllister, M. P. de Miranda, E. J. L. McInnes, C. A. Kilner and M. A. Halcrow, Chem.–Eur. J., 2004, 10, 1827 CrossRef CAS PubMed; (b) G. P. Guedes, S. Soriano, N. M. Comerlato, N. L. Speziali, M. A. Novak and M. G. F. Vaz, Inorg. Chem. Commun., 2013, 37, 101 CrossRef CAS; (c) S. Petit, P. Neugebauer, G. Pilet, G. Chastanet, A.-L. Barra, A. B. Antunes, W. Wernsdorfer and D. Luneau, Inorg. Chem., 2012, 51, 6645 CrossRef CAS PubMed.
- R. Bhula and D. C. Weatherburn, Angew. Chem., Int. Ed., 1991, 30, 688 CrossRef.
- P. Zhang, Y.-N. Guo and J. Tang, Coord. Chem. Rev., 2013, 257, 1728 CrossRef CAS.
- K. C. Mondal, G. E. Kostakis, Y. Lan and A. K. Powell, Polyhedron, 2013, 66, 268 CrossRef CAS.
- (a) N. Ishikawa, M. Sugita, T. Ishikawa, S.-Y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694 CrossRef CAS PubMed; (b) N. Ishikawa, M. Sugita, T. Ishikawa, S.-y. Koshihara and Y. Kaizu, J. Phys. Chem. B, 2004, 108, 11265 CrossRef CAS; (c) N. Ishikawa, Y. Mizuno, S. Takamatsu, T. Ishikawa and S.-Y. Koshihara, Inorg. Chem., 2008, 47, 10217 CrossRef CAS PubMed.
- (a) P.-H. Lin, W.-B. Sun, M.-F. Yu, G.-M. Li, P.-F. Yan and M. Murugesu, Chem. Commun., 2011, 47, 10993 RSC; (b) P.-H. Lin, W.-B. Sun, Y.-M. Tian, P.-F. Yan, L. Ungur, L. F. Chibotaru and M. Murugesu, Dalton Trans., 2012, 41, 12349 RSC; (c) L. G. Westin, M. Kritikos and A. Caneschi, Chem. Commun., 2003, 1012 RSC.
- J. Bartolomé, G. Filoti, V. Kuncser, G. Schinteie, V. Mereacre, C. E. Anson, A. K. Powell, D. Prodius and C. Turta, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 014430 CrossRef.
- (a) N. Sabbatini, M. Guardigli and J.-M. Lehn, Coord. Chem. Rev., 1993, 123, 201 CrossRef CAS; (b) J.-M. Lehn, Angew. Chem., Int. Ed., 1990, 29, 1304 CrossRef.
- (a) K. Binnemans, Chem. Rev., 2009, 109, 4283 CrossRef CAS PubMed; (b) V. Bekiari and P. Lianos, Adv. Mater., 2000, 12, 1603 CrossRef CAS.
- For the photophysical properties of saph2− in solution, see: A. Kagkelari, V. Bekiari, E. Stathatos, G. S. Papaefstathiou, C. P. Raptopoulou, Th. F. Zafiropoulos and P. Lianos, J. Lumin., 2009, 129, 578 CrossRef CAS.
- D. I. Alexandropoulos, A. Fournet, L. Cunha-Silva, A. M. Mowson, V. Bekiari, G. Christou and Th. C. Stamatatos, Inorg. Chem., 2014, 53, 5420 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic and structural details, and various magnetism and photophysical figures for 1–3. CCDC 1429027. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5ra20454b |
| This journal is © The Royal Society of Chemistry 2015 |




