Retracted Article: Synthesis and characterization of Co/Ti layered double hydroxide and its application as a photocatalyst for degradation of aqueous Congo Red†
Priyadarshi Roy Chowdhury and
Krishna G. Bhattacharyya*
and
Krishna G. Bhattacharyya*
Department of Chemistry, Gauhati University, Guwahati-781014, Assam, India. E-mail: kgbhattacharyya@gmail.com; Fax: +91 3612570599; Tel: +91 9864031987
First published on 20th October 2015
Abstract
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti layered double hydroxide (LDH) was synthesized hydrothermally using commercially available Co(NO3)2·6H2O and TiCl4, on a urea template. The high surface area material (∼180 m2 g−1) had a narrow band gap (2.67 eV) and shallow and deep trap defect sites. The layered nanomaterial exhibited remarkable semiconductor properties and demonstrated excellent visible light decolourisation efficiency for the anionic dye Congo Red in aqueous medium. The photocatalytic efficiency of the LDH was better than common commercial materials in use such as ZnO, ZnS, NiO, CoO, TiO2 and Degussa P25. The presence of different surface states of defect sites in the LDH was confirmed by PL, EIS and XPS measurements. XRD, DRS, FT-IR, AFM, TEM, SEM/EDX and TG/DTG analyses yielded information about the structural, morphological properties and thermal stability of the LDH. BET N2 adsorption–desorption measurements at 77 K gave surface area and porosity data for the LDH. The surface charge characteristics of the LDH were evaluated with ξ-potential measurements over a wide pH-range in aqueous medium. The photocatalytic behaviour towards decolourisation of the dye was evaluated depending on the reaction variables of pH, LDH amount, initial dye concentration and effects of quenchers, and variation of molar ratios of Co/Ti LDH. The pseudo-first order model satisfactorily described the degradation kinetics of the anionic dye. The photocatalytic mechanistic pathways of the LDH were explained on the basis of an electron–hole (e−–h+) hopping conduction model and also photosensitization of the dye. The maximum catalytic efficiency was observed with 15.0 mg of LDH at pH 4 for the anionic Congo Red dye at a concentration 1 × 10−5 M. The LDH was stable even after the fifth catalytic cycle, indicating its remarkable efficiency in potential decolourisation treatments. The dye degradation products were analysed with GC-MS and a reaction mechanism was proposed for the breakdown of the dye to simple and less toxic components.
1 Co/Ti layered double hydroxide (LDH) was synthesized hydrothermally using commercially available Co(NO3)2·6H2O and TiCl4, on a urea template. The high surface area material (∼180 m2 g−1) had a narrow band gap (2.67 eV) and shallow and deep trap defect sites. The layered nanomaterial exhibited remarkable semiconductor properties and demonstrated excellent visible light decolourisation efficiency for the anionic dye Congo Red in aqueous medium. The photocatalytic efficiency of the LDH was better than common commercial materials in use such as ZnO, ZnS, NiO, CoO, TiO2 and Degussa P25. The presence of different surface states of defect sites in the LDH was confirmed by PL, EIS and XPS measurements. XRD, DRS, FT-IR, AFM, TEM, SEM/EDX and TG/DTG analyses yielded information about the structural, morphological properties and thermal stability of the LDH. BET N2 adsorption–desorption measurements at 77 K gave surface area and porosity data for the LDH. The surface charge characteristics of the LDH were evaluated with ξ-potential measurements over a wide pH-range in aqueous medium. The photocatalytic behaviour towards decolourisation of the dye was evaluated depending on the reaction variables of pH, LDH amount, initial dye concentration and effects of quenchers, and variation of molar ratios of Co/Ti LDH. The pseudo-first order model satisfactorily described the degradation kinetics of the anionic dye. The photocatalytic mechanistic pathways of the LDH were explained on the basis of an electron–hole (e−–h+) hopping conduction model and also photosensitization of the dye. The maximum catalytic efficiency was observed with 15.0 mg of LDH at pH 4 for the anionic Congo Red dye at a concentration 1 × 10−5 M. The LDH was stable even after the fifth catalytic cycle, indicating its remarkable efficiency in potential decolourisation treatments. The dye degradation products were analysed with GC-MS and a reaction mechanism was proposed for the breakdown of the dye to simple and less toxic components.
1. Introduction
The search for suitable semiconductor photocatalysts for wastewater remediation is one of the most important aspects of environmental science. Light assisted degradation of carcinogenic and neurotoxic dyes present in industrial effluents by photocatalysts is a complicated task, since it occurs through several photon-initiated redox reactions. Conventional photocatalytic systems employing TiO2, have been extensively investigated, but the considerably large band gap of TiO2 (>3 eV) limits its use under UV irradiation only. The introduction of suitable dopants has been shown to be very effective towards extending the photocatalytic activity of TiO2 into the visible light region. However, as the dopants act as recombination centres of charge carriers, the photocatalytic efficiency decreases. In order to overcome this limitation, recent approaches are centred on the creation of Ti3+ centres by narrowing the band gap.Layered double hydroxides (LDHs) constitute a large family of anionic clay materials possessing 2D inorganic layered matrices and have the general formula, [MII1−xMIIIx(OH)2](An−)x/n·yH2O, where MII, MIII represent divalent, trivalent cations respectively and An− is the charge balancing anion present in the interlayer. The LDH layers are brucite-like containing divalent metal cations (Mg2+, Fe2+, Co2+, Ni2+, Zn2+, etc.) in octahedral positions coordinated to the hydroxyl groups. The divalent cations can be substituted isomorphously with trivalent cations (Al3+, Cr3+, Ga3+, In3+, Mn3+, Ti3+, Fe3+, etc.) creating positively charged layers. Water molecules and inorganic or organic charge-compensating exchangeable anions occupy the interlayer galleries. The hydroxyl groups are oriented towards the interlayer region or may be hydrogen bonded to the interlayer anions as well as to water molecules. LDH materials possess weak interlayer bonding due to which they exhibit excellent properties of expansion. The LDHs have found use as polymer fillers1–3 and as novel materials with unique magnetic,4–6 catalytic and photochemical functions7 due to their structural and compositional variability. The LDH could be converted to the corresponding mixed metal oxide, MIIM2IIIO4 by heating.8 The LDH decomposition is extensively employed in designing oxide and oxide-supported catalysts.
In recent times, attention has been increasingly focused towards the development of novel and efficient photocatalysts for application in the field of environmental management. Important considerations in the different approaches have been low cost, stability and large scale applicability in redox processes. The LDH-based composite photocatalysts have been extensively tested in a number of redox reactions, including photocatalytic degradation of organic pollutants and selective organic transformations.9
In the present work, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH was synthesized on urea template, characterized with a number of physical, chemical and spectroscopic techniques and was tried as a photocatalyst for the degradation of the anionic dye, Congo Red in water. Congo Red is extensively used in printing, textile and photographic industries8–10 and is a component of the coloured effluent discharged by such industries. This work tries to destroy the dye through photochemical reaction over 2
1 Co/Ti LDH was synthesized on urea template, characterized with a number of physical, chemical and spectroscopic techniques and was tried as a photocatalyst for the degradation of the anionic dye, Congo Red in water. Congo Red is extensively used in printing, textile and photographic industries8–10 and is a component of the coloured effluent discharged by such industries. This work tries to destroy the dye through photochemical reaction over 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH in aqueous medium using visible light as the energy source.
1 Co/Ti LDH in aqueous medium using visible light as the energy source.
2. Experimental section
2.1. Materials
All analytical grade chemicals were purchased from Merck Chemicals, USA and were used without further purification. De-ionized and decarbonated water was extensively used throughout the experiments.2.2. LDH synthesis
Co(NO3)2·6H2O, TiCl4 and urea were used for synthesizing 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti-LDH using co-precipitation method from a homogenous solution. In the typical synthesis, 11.78 g of Co(NO3)2·6H2O, 1.1 ml TiCl4 and 3.0 g of urea, were dissolved in 100 ml decarbonated water at room temperature (∼30 °C) and the contents were vigorously stirred for 3 h. The mixture was allowed to age in Teflon lined autoclave at 140 °C for 36 h and the dark pink crystalline product was extracted by centrifugation, washed several times with de-ionized water and dried at 100 °C for 4 h. The product was designated as 2
1 Co/Ti-LDH using co-precipitation method from a homogenous solution. In the typical synthesis, 11.78 g of Co(NO3)2·6H2O, 1.1 ml TiCl4 and 3.0 g of urea, were dissolved in 100 ml decarbonated water at room temperature (∼30 °C) and the contents were vigorously stirred for 3 h. The mixture was allowed to age in Teflon lined autoclave at 140 °C for 36 h and the dark pink crystalline product was extracted by centrifugation, washed several times with de-ionized water and dried at 100 °C for 4 h. The product was designated as 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH.
1 Co/Ti LDH.
2.3. Preparation of Congo Red solution
The stock solution of the anionic Congo Red (CR) (Fig. 1) of 1.0 × 10−3 M [=619.66 mg L−1 of CR] was prepared by dissolving its accurate amount in de-ionized water. The stock solution was diluted further to obtain the experimental solutions of 1.0 × 10−4 and 1.0 × 10−5 M respectively to be used for degradation reactions.2.4. Characterization of the LDH
X-ray diffraction (XRD) pattern was recorded using a PANalytical X'Pert PRO diffractometer that allowed reflection as well as transmission mode measurements. Reflection mode experiments were performed with a conventional Bragg–Brentano geometry. The incident CuKα X-ray beam (40 kV; 30 mA) was passed through a 0.02 rad Soller slit, a 1/8 divergence slit, a 15 mm fixed mask, having a 1/4 anti-scatter slit. The diffracted beam was detected with a PIXcel linear sensitive detector possessing β filter, a 0.02 rad Soller slit and a 1/8 anti-scatter slit. The diffraction patterns were recorded in 2θ range of 5–80° with step size of 0.013° and acquisition time of 15 s per step. The transmission-mode data was recorded using an elliptic focusing mirror, 0.5° divergence slit, 0.5° anti-scatter slit and 0.02 rad Soller slit in the primary beam.FT-IR measurements were performed with a Shimadzu FT-IR 3000 spectrometer (resolution 4 cm−1); the sample was mixed with KBr in the weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and was pressed into a disc.
100 and was pressed into a disc.
Thermogravimetric analysis (TG) from 32 to 750 °C was done in a Mettler Toledo Thermal Analyser in an inert atmosphere with a heating rate of 10 °C min−1.
Brunauer–Emmett–Teller (BET) N2-adsorption method (Micromeritics TriStar 3000 V6.08 analyzer) along with Barrett–Joyner–Halenda (BJH) computation procedure was used to obtain specific surface area, pore volume and pore size. Co/Ti-LDH sample was degassed at 120 °C for 3 h before analysis.
The electrochemical impedance spectroscopy (EIS) analysis of the Co/Ti LDH was conducted with CHI 760E Chenhua Instruments (Shanghai) electrochemical workstation in a conventional three-electrode cell having Pt wire as the counter electrode, saturated calomel electrode as the reference electrode and LDH deposited on ITO glass as the working electrode. 0.5 M Na2SO4 solution was taken as the electrolyte.
The X-ray photoelectron spectroscopy (XPS) surface survey of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti-LDH was taken with PHI Quantum 5800 ESCA instrument, equipped with micro-focussing, monochromatic Al-Kα as the X-ray source. UV-vis diffuse reflectance spectra (DRS) of LDH were obtained with Hitachi U4100 spectrometer on BaSO4 background. The morphology of 2
1 Co/Ti-LDH was taken with PHI Quantum 5800 ESCA instrument, equipped with micro-focussing, monochromatic Al-Kα as the X-ray source. UV-vis diffuse reflectance spectra (DRS) of LDH were obtained with Hitachi U4100 spectrometer on BaSO4 background. The morphology of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH was examined with Zeiss Supra 55 scanning electron microscope (SEM) with an accelerating voltage of 20 kV. The layered structure was investigated using a Philips TECNAI-20 and JEOL JEM-2010 high resolution transmission electron microscope with accelerating voltage of 200 kV. The elemental composition was determined with Oxford INCA EDX 300 microanalysis attachment associated with the SEM instrument. The morphology and the LDH layers were investigated with atomic force microscopy (AFM) in the tapping mode (Cypher S atomic force microscope, Asylum Research, Oxford Instruments). The sample was prepared on 4 × 4 cm2 mica plate. A Si tip with 10 nm radius (Mikromasch) was used to record high resolution micrographs. Height-mode images were recorded simultaneously with 512 × 512 pixels at the scan rate of 1–2 Hz.
1 Co/Ti LDH was examined with Zeiss Supra 55 scanning electron microscope (SEM) with an accelerating voltage of 20 kV. The layered structure was investigated using a Philips TECNAI-20 and JEOL JEM-2010 high resolution transmission electron microscope with accelerating voltage of 200 kV. The elemental composition was determined with Oxford INCA EDX 300 microanalysis attachment associated with the SEM instrument. The morphology and the LDH layers were investigated with atomic force microscopy (AFM) in the tapping mode (Cypher S atomic force microscope, Asylum Research, Oxford Instruments). The sample was prepared on 4 × 4 cm2 mica plate. A Si tip with 10 nm radius (Mikromasch) was used to record high resolution micrographs. Height-mode images were recorded simultaneously with 512 × 512 pixels at the scan rate of 1–2 Hz.
The electrical properties of the LDH surface in aqueous medium were determined with a Malvern Zetasizer Nano ZS instrument in the pH range of 4–11.
Photoluminescence (PL) spectra were obtained at different excitation wavelengths in a fluorescence spectrometer (Hitachi F-2500 FL spectrophotometer) with a Xe-lamp as the excitation source. The absorption spectra of the dyes were monitored with Shimadzu 1800 UV-vis spectrometer. The decolorized end products of photodegradation were identified by GC/MS (Varian 3900GC-Saturn 2100T) with column temperature programmed as 40 °C (1 min), 40–200 °C (7 °C min−1, hold time: 5 min).
2.5. Photocatalytic reactions
The photo-assisted dye degradation experiments were carried out in a visible light photocatalytic reactor (Fig. 2) comprising of a stainless steel chamber fitted with a 300 W tungsten lamp (Philips 38941-1; PS25, Frost-6100) as the visible-light source and a wavelength pass filter (λ > 400 nm) beneath the chamber. Prior to visible light irradiation, the reactants were stirred vigorously for 30 min in dark to establish adsorption/desorption equilibrium between the catalyst and the dye. The degradation of the test dye, Congo Red was investigated in aqueous solution at 30 °C (room temperature) with continuous circulation of running water through the outer jacket of the reactor ensuring constant temperature. At each 15 min interval, 10 ml aliquots were taken out, centrifuged to remove solid LDH particles and the centrifugate was analyzed for unconverted CR dye using Shimadzu UV-1800 spectrophotometer at λmax value of 497 nm for aqueous CR.Blank (without catalyst) and dark reactions were carried out following the same procedure. The effects of pH on photo-assisted degradation were investigated from pH 4.0 to 11.0 by addition of either HCl or NaOH (0.1 M). The effects of LDH amount were studied with loadings of 5.0, 10.0, 15.0 and 20.0 mg in 200 ml dye solution (1.0 × 10−5 M) at pH 4.0 (optimum pH). In another set of experiments, initial dye concentrations of 1.0 × 10−3, 1.0 × 10−4 and 1.0 × 10−5 M were taken with fixed LDH loading of 15.0 mg in 200 ml of dye solution. The photocatalytic effects were also investigated by varying the molar concentration of Co and Ti in the LDH under optimum conditions. For comparison of the performance of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH, CR photodegradation was carried out with commercial ZnO, ZnS, NiO, CoO, TiO2 and Degussa P25 photocatalysts (15.0 mg of catalyst in 200 ml of 1.0 × 10−5 M Congo Red solution).
1 Co/Ti LDH, CR photodegradation was carried out with commercial ZnO, ZnS, NiO, CoO, TiO2 and Degussa P25 photocatalysts (15.0 mg of catalyst in 200 ml of 1.0 × 10−5 M Congo Red solution).
The degradation kinetics was evaluated under optimum conditions (dye concentration = 1.0 × 10−5 M, pH = 4, catalyst 15.0 mg/200 ml, temperature 30 °C). To study the role of quenchers, Na-EDTA (h+ scavenger), n-butanol (˙OH scavenger), and benzoquinone (O2˙− scavenger) were added to the dye solution at the beginning of the degradation experiments. The photostability of the LDH was evaluated by comparative FT-IR analysis of the pure LDH and the LDH recovered after a fifth catalytic cycle.
3. Results and discussion
3.1. X-ray diffraction analysis
The X-ray diffraction pattern of the synthesized LDH (Fig. 3) shows characteristic Bragg reflections from the hexagonal LDH phase with interlayer CO32− ions, similar to the previously reported X-ray diffraction data for LDHs.The diffractogram showed the existence of well-defined series of (00l) Bragg reflections indicating the presence of parallel layered house-of cards type stacking of Co/Ti LDH crystallites. The presence of (003), (006) and (009) reflections at 2θ values of 11.49°, 22.98° and 34.47° respectively indicates intercalation of CO32− ions and H2O molecules within the LDH lattice,2,6 while the (100) XRD peak was observed at 32.31° (2θ). The d-spacing corresponding to the (003) peak was 0.763 nm (2θ ≈ 11.49°) and that of (110) was 0.146 nm (2θ ≈ 28.35°). These are in good agreement with previously reported data for Ti-LDHs with interlayer CO32− anions.8,11–15 Since the basal spacing of the synthesized 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH is similar to that of the normal LDHs, it is most likely that the planar orientation of the interlayer CO32− and H2O molecules has retained a similar pattern. Moreover (110) and (101) peaks at 2θ values of 28.35° and 37.44° indicate the existence of the anatase phase of TiO2 in the layered nanomaterial. The diffraction peaks at (018), (01
1 Co/Ti LDH is similar to that of the normal LDHs, it is most likely that the planar orientation of the interlayer CO32− and H2O molecules has retained a similar pattern. Moreover (110) and (101) peaks at 2θ values of 28.35° and 37.44° indicate the existence of the anatase phase of TiO2 in the layered nanomaterial. The diffraction peaks at (018), (01![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif) ), (113) and (10
), (113) and (10![[1 with combining low line]](https://www.rsc.org/images/entities/char_0031_0332.gif)
![[3 with combining low line]](https://www.rsc.org/images/entities/char_0033_0332.gif) ) at 2θ values of 47.12°, 53.35°, 67.61° and 76.58° respectively could also be indexed to typical LDHs. The presence of narrow and sharp diffraction peaks represents good crystallinity.2,3 The XRD lattice parameters are illustrated in Tables S1 and S2; (ESI†).12,16–18
) at 2θ values of 47.12°, 53.35°, 67.61° and 76.58° respectively could also be indexed to typical LDHs. The presence of narrow and sharp diffraction peaks represents good crystallinity.2,3 The XRD lattice parameters are illustrated in Tables S1 and S2; (ESI†).12,16–18
3.2. X-ray photoelectron spectroscopy analysis
X-ray photoelectron spectroscopy (XPS) is employed to have a well-established surface survey of the LDH and the results are presented in Fig. 4(A–G). The chemical states of the elements were identified by comparing the photoelectron binding energies (BE) of the ones reported in the literature. The XPS survey spectrum identifies the presence of elements in their respective oxidation states on the surface of the Co/Ti-LDH which is accompanied by oxygen vacancies. Fig. 4(A) shows the photoelectron lines at binding energies of 198, 289, 403, 455.4, 529 and ∼780–805 eV which could be attributed to Cl2p, C1s, N1s, Ti2p, O1s and Co2p respectively. The high resolution Ti2p XPS spectrum of Co/Ti-LDH [Fig. 4(B)] indicates two asymmetric peaks at binding energies of 455.4 eV and 462.7 eV corresponding to Ti2p3/2 and Ti2p1/2 spin states. Thus, the LDH surface shows the presence of two titanium species. Apart from the contributions from Ti4+ species, shoulders associated with Ti2p3/2 and Ti2p1/2 peaks at binding energies of 457.6 eV and 464.7 eV respectively could be attributed to Ti3+, as observed in previous reports.19–24 The surface reduction of Ti indicates the existence of negatively charged oxygen vacancies on the surface, the negative charge being shared by Ti cations adjacent to the defect sites. | ||
| Fig. 4 (A) XPS full surface survey (B) XPS Ti2p line (C) XPS Co2p line (D) XPS Cl2p line (E) XPS C1s line (F) XPS N1s line (G) XPS O1s line. | ||
Co2p XPS spectra yielded four peaks [Fig. 4(C)]. Two sharp peaks were observed at 780.4 eV and 795.9 eV corresponding to the spin states, Co2p3/2 and Co2p1/2 respectively. The peak splitting between the two states was ΔE = 15.5 eV, representing Co2+ and Co3+ in their respective oxide phases. The weak satellite peaks at 785.3 eV and 805.2 eV also support the existence of Co in two oxidation states on the LDH surface.
Cl2p XPS line [Fig. 4(D)] indicates the presence of a strong photoelectron peak at 198.3 eV representing 2p3/2 spin state of Cl and a satellite at 199.5 eV. Their presence indicates that Cl− ions are physisorbed on the LDH surface. The occurrence of Cl− is due to the use of TiCl4 as a precursor of Ti in synthesizing Co/Ti-LDH.
The positions of the carbon components could be correlated with the high resolution C1s spectrum of the synthesized LDH [Fig. 4(E)] and it is established that the peak corresponding to the binding energy of 289.3 eV is due to the presence of CO32− species in the interlayer gallery of Co/Ti-LDH. C and N peaks are observed in the XPS spectra due to the use of urea and Co-nitrate salts in synthesizing Co/Ti LDH.
N1s line [Fig. 4(F)] shows two XPS peaks at binding energies of 403.2 eV and 405 eV respectively. The binding energy associated with the N1s line is very sensitive to the chemical environment and varies from 396 eV to 408 eV. The X-ray photoelectron peak at 403.2 eV is associated with the terminally bonded well screened molecular nitrogen (γ-N2) and that at 405 eV could be attributed to the terminally bonded poorly screened molecular nitrogen (γ-N2) species. Thus the spectroscopic features of N1s XPS line showed striking similarity with the chemisorbed states of N2 as illustrated in the previous reports.26 Meanwhile, the peak at 408 eV arises due to the presence of NO3− as an impurity on the surface of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti-LDH, which originated as a result of utilizing hydrated cobalt nitrate as a precursor of cobalt in synthesizing the LDH.24,25
1 Co/Ti-LDH, which originated as a result of utilizing hydrated cobalt nitrate as a precursor of cobalt in synthesizing the LDH.24,25
O1s line reveals two peaks – OI and OII representing the two different types of oxygen species on the LDH surface [Fig. 4(G)]. This is found to be in accordance with the previous reports that OI with a lower BE of 529.4 eV is most likely to be the lattice oxygen bound to metal cations in the LDH, whereas OII having higher BE of 530.9 eV could most likely belong to surface oxygen species, including mainly the oxygen species of hydroxyl groups.19
3.3. Electrochemical impedance spectroscopy analysis
The electrochemical impedance spectroscopy (EIS) analysis of the Co/Ti LDH was carried out to investigate the charge transfer resistance and the separation efficiency between the photo-generated electrons and holes. The impedance analysis was done using 2.5 mM K3[Fe(CN)6]/K4[Fe(CN)6] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture in 0.5 M Na2SO4 aqueous solution as the redox probe. The arc radius of the impedance spectrum reflects the interface layer resistance arising from the LDH electrode surface. Smaller arc radius infers higher charge transfer efficiency of the material. In case of the synthesized LDH, a small arc radius was observed in the electrochemical Nyquist plot [Fig. 7(A)], suggesting that interfacial charge-transfer could occur easily and rapid transport of charge carriers could be achieved with an effective charge separation facilitating the semiconduction mechanism. Thus, the overall electron transporting properties of 2
1) mixture in 0.5 M Na2SO4 aqueous solution as the redox probe. The arc radius of the impedance spectrum reflects the interface layer resistance arising from the LDH electrode surface. Smaller arc radius infers higher charge transfer efficiency of the material. In case of the synthesized LDH, a small arc radius was observed in the electrochemical Nyquist plot [Fig. 7(A)], suggesting that interfacial charge-transfer could occur easily and rapid transport of charge carriers could be achieved with an effective charge separation facilitating the semiconduction mechanism. Thus, the overall electron transporting properties of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH could be associated with the suppression of e−–h+ pair recombination, which is supported by photoluminescence results, leading to the highly efficient photoassisted degradation of dyestuffs.9
1 Co/Ti LDH could be associated with the suppression of e−–h+ pair recombination, which is supported by photoluminescence results, leading to the highly efficient photoassisted degradation of dyestuffs.9
In order to investigate the electronic properties associated with the synthesized Co/Ti LDH, Mott–Schottky (MS) measurements were performed using the impedance technique and the results are shown in [Fig. 7(B)]. The consistency in the reverse sigmoid nature of the MS plot of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH electrode signifies its n-type semiconductor properties. The flat band potential (Vfb) could be obtained from the x-intercept of the linear region of the MS plot. The negative Vfb (−0.79 V vs. SCE; equivalent to −0.54 vs. NHE) suggests the presence of different surface states of metals which could lead to considerable changes in the band positions. The phenomenon of the presence of negative flat band and the presence of different surface states is illustrated on the basis of Fermi-level pinning. Surface states occurring at the semiconductor–liquid interface, play an important role in the performance of the photoelectrochemical devices. In the absence of a redox couple, the flat band potential (V0fb) corresponds close to the energy of the conduction band edge (Ec) in an n-type semiconductor (Fig. 5).
1 Co/Ti LDH electrode signifies its n-type semiconductor properties. The flat band potential (Vfb) could be obtained from the x-intercept of the linear region of the MS plot. The negative Vfb (−0.79 V vs. SCE; equivalent to −0.54 vs. NHE) suggests the presence of different surface states of metals which could lead to considerable changes in the band positions. The phenomenon of the presence of negative flat band and the presence of different surface states is illustrated on the basis of Fermi-level pinning. Surface states occurring at the semiconductor–liquid interface, play an important role in the performance of the photoelectrochemical devices. In the absence of a redox couple, the flat band potential (V0fb) corresponds close to the energy of the conduction band edge (Ec) in an n-type semiconductor (Fig. 5).
At Vfb, in absence of specific adsorption, the potential drops across the Helmholtz layer, ΔφH, which corresponds to the potential attributable to the dipoles oriented at the interface, Vd, while the potential at the space charge region of the semiconductor, ΔφSC, is zero. In the impedance measurements, a reasonably concentrated electrolyte has been used so that the potential drop across the diffuse double layer could be neglected. If the potential difference between the semiconductor LDH surface and the solution is varied by application of an external potential (located at Eredox = eVredox), ΔφH remains unchanged, while ΔφSC, remains equal to the applied potential (assuming no specific adsorption of redox couple and no change in Vd). This forms the basis of the usual model in which the band energies are said to have remained fixed [i.e. Δ(ΔφH) ≈ 0] and under these conditions, the open circuit photopotential (ΔV) is represented by the equation
| ΔV = |V0fb − Vredox| | (1) |
For couples with energies outside the band gap, no photoresponse is expected. Eqn (1) forms the basis of selection of redox couples with potentials corresponding to energies near the valence band edge (for n-type semiconductor) in order to maximize the output photovoltage of a photoelectrochemical cell. The flat band potential, however, shifts by specific adsorption of ions, since the potential drop across the Helmholtz layer in presence of the surface charge, qs, is given by eqn (2)
 | (2) |
 | ||
Fig. 7 (A) Electrochemical impedance Nyquist plot and (B) Mott–Schottky plot of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH electrode. 1 Co/Ti LDH electrode. | ||
The existence of different oxidation states in Co/Ti-LDH is in accordance with the XPS results. Moreover, Co has excellent electrochemical properties owing to its electronic configuration. Therefore, the rapid transport of charge carriers with an effective charge separation could be easily achieved. The overall transport properties of the LDH could be attributed to the suppression of charge recombination, which contributes to its high rate of photocatalytic performance.
On the basis of the impedance results, the enhanced photocatalytic behaviour of the Co/Ti LDH could be attributed to the separation of charge carriers, extended photo responding range, the negative flat-band potential accompanied by higher migration efficiency of photo induced electrons. The conduction band potential (ECB) of n-type semiconductor ranges from 0 to −0.2 V; very close to the Vfb obtained for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti-LDH, and is dependent on the effective electronic mass and carrier concentration. In this measurement, the voltage difference between the conduction band and the flat band potential is fixed at 0.1 V. Thus the negative value of Fermi level in the Co/Ti LDH electrode causes ECB to lie at −0.54 V vs. NHE. The conduction band potential, so obtained for the LDH indicates a stronger reductive power, which may be effectively involved in the oxygen reduction reaction. As the standard redox potential of O2/O2˙− (−0.28 V vs. NHE) is more positive than ECB measured for the LDH, it suggests that the photo-generated electrons could theoretically react readily with the adsorbed O2 to form O2˙− on Co/Ti LDH surface. Thus, Co/Ti-LDH could efficiently transfer photo-generated electrons from the surface leaving more h+-type charge carriers that assist in the photo-degradation of dyestuffs in aqueous medium.
1 Co/Ti-LDH, and is dependent on the effective electronic mass and carrier concentration. In this measurement, the voltage difference between the conduction band and the flat band potential is fixed at 0.1 V. Thus the negative value of Fermi level in the Co/Ti LDH electrode causes ECB to lie at −0.54 V vs. NHE. The conduction band potential, so obtained for the LDH indicates a stronger reductive power, which may be effectively involved in the oxygen reduction reaction. As the standard redox potential of O2/O2˙− (−0.28 V vs. NHE) is more positive than ECB measured for the LDH, it suggests that the photo-generated electrons could theoretically react readily with the adsorbed O2 to form O2˙− on Co/Ti LDH surface. Thus, Co/Ti-LDH could efficiently transfer photo-generated electrons from the surface leaving more h+-type charge carriers that assist in the photo-degradation of dyestuffs in aqueous medium.
3.4. Photoluminescence analysis
Photoluminescence technique (PL) is employed to understand the fate of e−–h+ pairs in the LDH as well as to examine the mechanism of trapping, migration and transfer of charge carriers [Fig. 8(A)]. Photoluminescence emissions that usually give a better understanding of the optical properties, surface states and defect sites, are broadly divided into two categories: the band–band PL emissions due to electronic transitions from the conduction band (CB) bottom to the valence band (VB) top and excitonic PL emissions resulting from the surface oxygen vacancies and other defects present in the semiconductors. For band–band PL emissions, the lower the intensity, higher is the separation rate of photo-induced electron–hole [(eCB−)–(hVB+)] pair and higher is the photocatalytic activity.9,27 In case of excitonic emissions, the relationship between the luminescence intensity and photocatalytic activity is quite complicated and depends on the electronic properties of semiconducting materials. | ||
Fig. 8 (A) Photoluminescence spectra at different excitation wavelengths (B) mechanism of photoluminescence emission of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH. 1 Co/Ti LDH. | ||
The ultraviolet emission analysis of Co/Ti LDH was carried out under excitation wavelengths of 390 nm, 400 nm and 410 nm respectively. It is seen that the intensity decreased on increasing the excitation wavelength, which is attributed to higher separation of the charge carriers. The higher separation of charge carriers reflects the consistency with the results of electrochemical impedance measurements. Studies have suggested that oxygen vacancies along with surface defects bind the photo-induced electrons forming excitons, so that the PL signal can easily occur. Moreover, Ti doped photocatalytic systems consist of two kinds of trap sites for holes, the deep hole and the shallow hole. The deep trapped holes exhibit lower oxidizing potential, prefer to undergo reaction with physisorbed substances.
The synthesized LDH showed several excitonic PL emissions at 432 nm (2.87 eV), 463 nm (2.67 eV), 472 nm (2.63 eV), 511 nm (2.43 eV), 534 nm (2.32 eV), 563 nm (2.2 eV), 573 nm (2.16 eV), accompanied by a valley extending up to 633 nm, resulting from the oxygen vacancies associated with the surface and defects within the LDH, which has led to a significant enhancement in its optical properties. The emission peak at 463 nm (2.67 eV) is attributed to the exciton from conduction band (eCB−) to valence band (hVB+), equal to the band gap energy of the synthesized Co/Ti LDH nanomaterial (determined from Tauc plot analysis). Apart from this peak, the other emission peaks are mostly associated with excitons as well as oxygen defect related shallow and deep trap centers. The emission at 432 nm is observed due to self-trapped exciton (STE), generated on the LDH lattice due to hole capture by a trapped electron. The STE is of two types, direct and indirect. The direct STE emission is caused by the direct recombination of the charge carriers, while indirect STE emission is caused by recombination via an oxygen vacancy. Since Ti doping generates oxygen vacancy, the STE emission at 432 nm is assumed to be formed via oxygen vacancies and is of indirect nature.36 The emission peaks at 472, 534, 563 and 573 nm are due to F or F2+ and F+ color centers respectively. An F-center is the oxygen vacancy with two trapped electrons; F+ is with a singly trapped electron whereas F2+ is the oxygen vacancy with no trapped electrons. The oxygen defects associated with these emissions occur due to oxygen vacancies in the shallow and deep level in the band gap of LDH. The absorption peaks associated with the oxygen defects are in the range of 450–630 nm, therefore the emission peaks in the luminescence spectrum of the LDH are certainly associated with different types of charged (F, F+ or F2+) oxygen vacancy states and are of deep trap emissions in nature. On the other hand, the emission peaks below 450 nm are shallow trap emissions. The oxygen defects associated with the grain boundary are distributed randomly, are charged (F, F+ or F2+) and act as shallow trap centers. In addition, lattice site oxygen defects, forming deep trap centers, are also charged. The free electrons, not trapped in these defects migrate throughout the lattice and recombine with the holes inside or on the surface of the LDH. But the migrating electrons are strongly repelled by the charged oxygen vacancy sites and are not easily allowed to recombine with the holes. Since the existence of defect states originate due to doping, the mobility of carriers is slowed down, which delays the recombination process.
The dopants present on the LDH surface act as an obstacle to efficient overlapping of carriers on the surface. The defect sites act as active trap centers and separate the charge carriers. Doping also shifts the band edges and the conduction band electrons have to traverse an indirect path for undergoing recombination with the holes. This delays the recombination and quenches the emission intensity. Since the emission intensity is reduced with an increase in excitation wavelength, the quenching effect is expected to play an important role. In conformity with this, doping increases the interaction among dopant and defects and form quenching centers. The electrons are first captured by shallow trap levels, and then they jump to Co or Ti d-states, which further jump from one d-state to another and thus, form some quenching centers. The electrons, after passing through a channel of dopant and defects, ultimately find a recombination center and emit light. But, this process increases the time period for the charge carriers to undergo recombination and therefore a reduction in the emission intensity is observed. Thus, the presence of defects within the microstructures of the synthesized LDH is expected to play a major role in its highly efficient photocatalytic performance.
3.5. Zeta potential measurements
The variation of the surface charge of aqueous Co/Ti-LDH with pH was monitored with zeta potential measurements from pH 4–11 (Fig. S1; ESI†). The pH was adjusted by adding drops of 0.1 M HCl or NaOH to the aqueous LDH solution. It was observed that the surface charge of the LDH decreased monotonically with the increase in pH, attaining zero point charge (zpc) at pH 7.44, the iso-electric point of the LDH.28,29 Thus, Co/Ti-LDH surface attains positive charge at pH < pHzpc, attracting negatively charged Congo Red anions in aqueous solution, enabling a higher photodegradation efficiency. The maximum photocatalytic degradation was observed at the pH 4, when the LDH surface is sufficiently positive.3.6. BET/BJH-N2 sorption analysis
The low temperature N2-sorption analysis in an inert atmosphere showed a type IV isotherm with a considerably large H3-type of hysteresis loop (p/p0 > 0.4–0.8) for the Co/Ti LDH [Fig. S2(A); ESI†]. The large hysteresis loop indicates the condensation of N2 inside the pores of the LDH. In the desorption phase, the gas is released through a different path with reduction in the pressure, a property shown by the mesoporous materials.8,11 Thus, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH consists of mesopores formed by the accumulation of particles. The comparatively broad average pore width of 3.91 nm in 2
1 Co/Ti LDH consists of mesopores formed by the accumulation of particles. The comparatively broad average pore width of 3.91 nm in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH is most likely to have originated due to use of urea as the template during the hydrothermal synthesis [Fig. S2(B); ESI†]. The high BET specific surface area of 180 m2 g−1 and the BJH adsorption and desorption cumulative pore volumes of 0.246 and 0.218 cm3 g−1 respectively for Co/Ti-LDH indicate the suitability of the material as a catalyst. The LDH is dominated by micro- and mesopores of 1 to 6 nm diameter. The mesoporous character of the material with appropriate spatial arrangement of the pores is likely to assist in e−–h+ transfer within the LDH framework resulting in high photocatalytic performance.8
1 Co/Ti LDH is most likely to have originated due to use of urea as the template during the hydrothermal synthesis [Fig. S2(B); ESI†]. The high BET specific surface area of 180 m2 g−1 and the BJH adsorption and desorption cumulative pore volumes of 0.246 and 0.218 cm3 g−1 respectively for Co/Ti-LDH indicate the suitability of the material as a catalyst. The LDH is dominated by micro- and mesopores of 1 to 6 nm diameter. The mesoporous character of the material with appropriate spatial arrangement of the pores is likely to assist in e−–h+ transfer within the LDH framework resulting in high photocatalytic performance.8
3.7. FT-IR analysis
The FT-IR spectrum of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH (Fig. S3; ESI†) showed the characteristic frequencies associated with the layered structure having interlayer CO32− ions and H2O molecules. The intense and strong absorption band centered on 3435 cm−1 could be attributed to stretching vibrations of surface and interlayer water molecules and hydroxyl groups. The lower frequency in Co/Ti LDH compared to normal OHstr vibration of free water at 3600 cm−1, indicates the presence of H2O molecules in the interlayer gallery of the LDH. This could also be due to the formation of hydrogen bonds associated with interlayer water with guest anions and hydroxide groups of the intrinsic layers.8 The band at 3173 cm−1 accompanied by a hump near 2932 cm−1 could also be attributed to hydrogen bonding between the interlayer H2O and CO32− ions. The band at 1635 cm−1 is assigned to bending vibrations of H2O molecules. In most hydrotalcites, three IR active absorption bands arising from CO32− species are observed at 1408 cm−1 (ν3), 1125 cm−1 (ν1) and 714 cm−1 (ν2). The band at 1408 cm−1 is attributed to ν3 mode of interlayer CO32− species of the LDH.8 The band at 862 cm−1 is observed due to metal–hydroxyl (M–OH) groups present in the lattice of the LDH.17,18 The 622 cm−1 sharp band is associated with in-plane quadrant bending and that of 714 cm−1 could be attributed to ν2 mode of CO32− species. The occurrence of the three characteristic bands in the LDH synthesized in the present work, along with the XRD data, confirms successful incorporation of CO32− and H2O molecules in the interlayer gallery of the Co/Ti LDH.
1 Co/Ti LDH (Fig. S3; ESI†) showed the characteristic frequencies associated with the layered structure having interlayer CO32− ions and H2O molecules. The intense and strong absorption band centered on 3435 cm−1 could be attributed to stretching vibrations of surface and interlayer water molecules and hydroxyl groups. The lower frequency in Co/Ti LDH compared to normal OHstr vibration of free water at 3600 cm−1, indicates the presence of H2O molecules in the interlayer gallery of the LDH. This could also be due to the formation of hydrogen bonds associated with interlayer water with guest anions and hydroxide groups of the intrinsic layers.8 The band at 3173 cm−1 accompanied by a hump near 2932 cm−1 could also be attributed to hydrogen bonding between the interlayer H2O and CO32− ions. The band at 1635 cm−1 is assigned to bending vibrations of H2O molecules. In most hydrotalcites, three IR active absorption bands arising from CO32− species are observed at 1408 cm−1 (ν3), 1125 cm−1 (ν1) and 714 cm−1 (ν2). The band at 1408 cm−1 is attributed to ν3 mode of interlayer CO32− species of the LDH.8 The band at 862 cm−1 is observed due to metal–hydroxyl (M–OH) groups present in the lattice of the LDH.17,18 The 622 cm−1 sharp band is associated with in-plane quadrant bending and that of 714 cm−1 could be attributed to ν2 mode of CO32− species. The occurrence of the three characteristic bands in the LDH synthesized in the present work, along with the XRD data, confirms successful incorporation of CO32− and H2O molecules in the interlayer gallery of the Co/Ti LDH.
3.8. UV-visible DRS analysis
The UV-vis diffuse reflectance spectroscopy analysis is performed to determine the electronic properties of the Co/Ti LDH [Fig. 9(A)]. The DRS investigated the nature and coordination state of Co and Ti within the layered material. The hump at ∼260 nm is attributed to d–d transitions. The strong absorption band around 320 nm could be assigned to typical Co(II) coordinated to the CO32− anions present in the interlayer galleries.1,8,21 The broad absorption band from ∼410–700 nm is observed with a maximum at ∼548 nm indicating the presence of Ti4+ in the brucite like sheets. It also signifies guest–guest or guest–host supramolecular interactions. In comparison to the commercial catalysts, Co/Ti LDH showed an overall band broadening accompanied by a red shift in the UV-visible absorption spectrum, which is most likely to be due to the formation of aggregates.8 | ||
Fig. 9 (A) UV-vis DRS of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH along with those of the commercial catalysts. (B) Tauc plot of 2 1 Co/Ti LDH along with those of the commercial catalysts. (B) Tauc plot of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH with commercial catalysts. 1 Co/Ti LDH with commercial catalysts. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti-LDH with a few important commercial catalysts like ZnO, ZnS, CoO, TiO2, NiO and Degussa P25 [Fig. 9(A)] showed strong absorbance at ∼260–450 nm for all the catalysts, with a minor shift in λmax position for the synthesized Co/Ti LDH. Moreover, Co/Ti-LDH also exhibited broad absorption between 410–700 nm, which was not observed in any of the other commercial catalysts. The strong absorbance in the visible region makes the LDH, a highly efficient photocatalyst compared to commercial ZnO, ZnS, CoO, TiO2, NiO and Degussa P25. UV-vis absorption analysis is considered to be very convenient and effective for explaining the band structure of semiconductor materials. The band gap of LDH along with that of the commercial catalysts was calculated from solid UV-visible diffuse reflectance study [Fig. 9(B)] using the Tauc equation21
1 Co/Ti-LDH with a few important commercial catalysts like ZnO, ZnS, CoO, TiO2, NiO and Degussa P25 [Fig. 9(A)] showed strong absorbance at ∼260–450 nm for all the catalysts, with a minor shift in λmax position for the synthesized Co/Ti LDH. Moreover, Co/Ti-LDH also exhibited broad absorption between 410–700 nm, which was not observed in any of the other commercial catalysts. The strong absorbance in the visible region makes the LDH, a highly efficient photocatalyst compared to commercial ZnO, ZnS, CoO, TiO2, NiO and Degussa P25. UV-vis absorption analysis is considered to be very convenient and effective for explaining the band structure of semiconductor materials. The band gap of LDH along with that of the commercial catalysts was calculated from solid UV-visible diffuse reflectance study [Fig. 9(B)] using the Tauc equation21| εhν = C(hν − Eg)n | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH, the best fit of (εhν)2 vs. hν was obtained for n = 1/2, suggesting a direct allowed electronic transition across the energy band gap. The extrapolation of hν at ε = 0 gives absorption edge energy corresponding to Eg = 2.67 eV for 2
1 Co/Ti LDH, the best fit of (εhν)2 vs. hν was obtained for n = 1/2, suggesting a direct allowed electronic transition across the energy band gap. The extrapolation of hν at ε = 0 gives absorption edge energy corresponding to Eg = 2.67 eV for 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH, which is found to be much smaller than that of pure NiO (∼2.94 eV), ZnO (∼3.23 eV), ZnS (∼3.54 eV), CoO (∼2.68 eV), TiO2 (∼3.25 eV) and Degussa P25 (∼3.18 eV).
1 Co/Ti LDH, which is found to be much smaller than that of pure NiO (∼2.94 eV), ZnO (∼3.23 eV), ZnS (∼3.54 eV), CoO (∼2.68 eV), TiO2 (∼3.25 eV) and Degussa P25 (∼3.18 eV).The narrow band gap imparts better semiconducting properties to Co/Ti LDH in comparison to the commercial ZnO, ZnS, CoO, TiO2, NiO and Degussa P25. Moreover, a red shift is observed in the UV-visible spectrum of the LDH, which could be attributed to the narrow band gap, suggesting that photo-generated e−–h+ pairs could be generated by the LDH through irradiation with longer wavelength of visible light.30 The presence of narrow band gap in the LDH could be attributed to localized gap states induced by Ti3+ accompanied by oxygen vacancies within the layered framework. This is also confirmed by PL and XPS observations. The narrow band gap imparts excellent photocatalytic properties to the LDH for degradation of dye-stuffs than that of other commercial photocatalysts.
3.9. Thermogravimetric analysis
The TG measurements showed three degradation steps for Co/Ti LDH (Fig. S4; ESI†), which indicated that the thermal stability of the synthesized LDH was very much comparable with that of other LDHs.3,8 Weight loss between 83–130 °C with a peak at ∼89 °C, indicated by the DTG curve, could be assigned to the loss of physisorbed and interlayer water constituting ∼13% by weight of the material. The second loss, observed from ∼240 to 293 °C with a sharp peak at 262 °C, accounted for 17% weight loss, and was obviously due to dehydroxylation of the brucite like layers. The third weight loss of ∼22% from 294 to 430 °C, associated with a broad peak at 335 °C, is likely to have occurred due to the decomposition of the interlayer CO32− ions.17,18 No further weight loss was observed above 450 °C, which indicated the presence of very negligible amount of carbonaceous residues in the LDH. The thermogravimetric data have been found to be in agreement with XRD and FT-IR results, indicating the presence of H2O and CO32− ions in the interlayer galleries of the layered nanomaterial.3.10. Morphology and particle size distribution
The morphology of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH was investigated using HR-TEM, SEM and AFM measurements. The HR-TEM micrograph [Fig. 10(A)] shows ultra-fine hexagonal shaped particles with stacking of one layer over the other, in good agreement with the SEM results [Fig. 10(E)]. The bright spots in the SAED pattern reveal crystalline nature of the LDH [Fig. 10(B)] with a well ordered house-of cards type stacking. The interlayer distance of the periodic lattice fringes of (003) plane of 2
1 Co/Ti LDH was investigated using HR-TEM, SEM and AFM measurements. The HR-TEM micrograph [Fig. 10(A)] shows ultra-fine hexagonal shaped particles with stacking of one layer over the other, in good agreement with the SEM results [Fig. 10(E)]. The bright spots in the SAED pattern reveal crystalline nature of the LDH [Fig. 10(B)] with a well ordered house-of cards type stacking. The interlayer distance of the periodic lattice fringes of (003) plane of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti-LDH could be measured as ∼0.763 nm from HR-TEM analysis [Fig. 10(C)], which is in accordance with that obtained from X-ray diffraction studies of Co/Ti LDH (Table S2, ESI†). The average particle size was calculated to be around 104 nm; evident from the histogram plot [Fig. 10(D)]. The cross-sectional TEM analysis (Fig. S5; ESI†) yielded the distance between two consecutive brucite sheets to be 0.39 nm and the thickness of the brucite like metal hydroxide layer to be 0.42 nm. The sum of the interline distances (brucite like sheet thickness + interlayer thickness) is 0.81 nm, which is in good agreement with the c′ lattice parameter of the LDH determined from XRD data (Table S2, ESI†).
1 Co/Ti-LDH could be measured as ∼0.763 nm from HR-TEM analysis [Fig. 10(C)], which is in accordance with that obtained from X-ray diffraction studies of Co/Ti LDH (Table S2, ESI†). The average particle size was calculated to be around 104 nm; evident from the histogram plot [Fig. 10(D)]. The cross-sectional TEM analysis (Fig. S5; ESI†) yielded the distance between two consecutive brucite sheets to be 0.39 nm and the thickness of the brucite like metal hydroxide layer to be 0.42 nm. The sum of the interline distances (brucite like sheet thickness + interlayer thickness) is 0.81 nm, which is in good agreement with the c′ lattice parameter of the LDH determined from XRD data (Table S2, ESI†).
The SEM micrograph [Fig. 10(E)] showed regular hexagonal platelets, consistent with the TEM results. The presence of a large number of platelets could be an evidence of subsequent crystallization of layered double hydroxide incorporated with CO32− and H2O molecules in the interlayer.19–24 The presence of CO32− in the interlayer galleries of the LDH has produced a change in the superficial interaction between the particles influencing the stacking pattern and resulting in the formation of a 3D architecture accompanied by pores of different sizes, as determined from the surface area measurements.
The tapping mode AFM micrograph [Fig. 10(G)] also confirms the presence of hexagonal platelets, thereby supporting the HR-TEM and SEM results. The LDH particles possess regular hexagonal shapes with 120° internal angles, reminiscent of the hexagonal morphology of the Co/Ti LDH. The parallel layer-by-layer house-of-cards packing of brucite like layers was further confirmed by AFM observations. The formation of flat crystallite terrace in the height profile of crystallites [Fig. 10(H and J)], provides strong evidence for a parallel multilayered structure, which is also consistent with the cross-sectional TEM results. Fig. 10(I) represents the 3D AFM micrograph where the darker zones (between the hexagonal plates) represent the voids in the hexagonal LDH platelets. The number of parallel LDH layers could be calculated though the total thickness along c-axis and was found to be ∼31 nm from the height profile AFM of LDH crystallites. Dividing the total thickness of Co/Ti LDH with the lattice parameter c′ obtained from XRD results, it is confirmed that Co/Ti LDH comprises of 38 to 39 parallel house-of cards type brucite like layers.
The elemental composition of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH was investigated using energy-dispersive X-ray spectroscopy (EDX) [Fig. 10(F)]. The EDX analysis indicates the presence of Co, Ti, O, Cl, C and N in the LDH having Co/Ti atomic ratio of 2.03
1 Co/Ti LDH was investigated using energy-dispersive X-ray spectroscopy (EDX) [Fig. 10(F)]. The EDX analysis indicates the presence of Co, Ti, O, Cl, C and N in the LDH having Co/Ti atomic ratio of 2.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in good agreement with the Co/Ti nominal ratio (2
1, in good agreement with the Co/Ti nominal ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) taken for the synthesis. The EDX data are listed in Table 1. The fractionally higher atomic ratio of Co/Ti in the synthesized LDH, might have resulted due to the presence of CoOx impurity in the hydrated cobalt nitrate salt used as a precursor of Co during LDH synthesis.
1) taken for the synthesis. The EDX data are listed in Table 1. The fractionally higher atomic ratio of Co/Ti in the synthesized LDH, might have resulted due to the presence of CoOx impurity in the hydrated cobalt nitrate salt used as a precursor of Co during LDH synthesis.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH
1 Co/Ti LDH
| Elements | Weight% | Atomic% | Co/Ti ratio |
|---|---|---|---|
| C K | 1.63 | 1.28 | 2.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| N K | 4.35 | 3.15 | |
| O K | 12.14 | 10.21 | |
| Cl K | 3.73 | 1.27 | |
| Co K | 52.36 | 56.34 | |
| Ti K | 25.79 | 27.75 | |
| Total | 100.00 | 100.00 |
3.11. Photocatalytic reactions
The catalytic activity of the semiconductor Co/Ti LDH depends on the surface area, narrow band gap, light harvesting capacity and separation of e−–h+ pairs by absorption of incident photons with energies larger than its band gap energy (Eg). The ability of e−–h+ pair separation in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH is evident from the luminescence spectra and EIS analyses. The high specific surface area, narrow band gap and presence of different surface states accompanied by oxygen vacancies, as evident from BET, DRS and XPS analyses, indicate the potential of the LDH to generate e−–h+ pairs easily under visible light, resulting in its highly efficient photocatalytic activity.
1 Co/Ti LDH is evident from the luminescence spectra and EIS analyses. The high specific surface area, narrow band gap and presence of different surface states accompanied by oxygen vacancies, as evident from BET, DRS and XPS analyses, indicate the potential of the LDH to generate e−–h+ pairs easily under visible light, resulting in its highly efficient photocatalytic activity.
The photocatalytic reactions of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH were carried out with aqueous Congo Red (CR), with the LDH dispersed in 200 ml aqueous solution of the test dye, followed by vigorous stirring of the reaction mixture for 30 min in the dark for establishing adsorption equilibrium between the LDH and the dye (S7, ESI†). This was followed by irradiation of the experimental mixture with visible light. It has been observed that the synthesized LDH exhibited better photocatalytic degradation of CR (at pH = 4) than commercial ZnO, ZnS, CoO, TiO2, NiO and Degussa P25. The photocatalytic experiments were done with variation of pH, catalyst amount, variation of molar ratios of Co/Ti in the LDH and initial dye concentration. The photocatalytic effects were also studied separately with respect to influence of different quenchers like 2Na-EDTA (h+ scavenger), n-butanol (˙OH scavenger), benzoquinone (O2˙− scavenger) by adding the same at the beginning of the dye degradation experiment in order to indirectly establish the role played by ˙OH, h+ and O2˙− species in the photodegradation process. The reaction was monitored by following the decrease in the absorption intensity of the dye at λmax = 497 nm in 15 min intervals with a Shimadzu 1800 UV-vis spectrophotometer. The extent of decolorization was obtained from the ratio C/C0, where C and C0 were the absorbance values at a particular time interval and at t = 0 respectively. The strong absorption bands of CR decreased gradually on increasing irradiation time and the absorbance of the dye decreased nearly to zero after 75 min of visible light irradiation with the synthesized LDH [Fig. 11(A)]. The solution colour changed to nearly transparent marking the end of the decolorization [Fig. 11(B)]. The highly efficient photocatalytic performance of the synthesized Co/Ti-LDH compared to ZnO, ZnS, TiO2, CoO, NiO and Degussa P25 could be attributed to the presence of high surface area, defects, oxygen vacancies and Ti3+ centers within the layered brucite like structure.8,15,23 Control experiments like blank and dark reactions revealed negligible adsorption, clearly indicating that visible light plays a significant role in the photodegradation process.
1 Co/Ti LDH were carried out with aqueous Congo Red (CR), with the LDH dispersed in 200 ml aqueous solution of the test dye, followed by vigorous stirring of the reaction mixture for 30 min in the dark for establishing adsorption equilibrium between the LDH and the dye (S7, ESI†). This was followed by irradiation of the experimental mixture with visible light. It has been observed that the synthesized LDH exhibited better photocatalytic degradation of CR (at pH = 4) than commercial ZnO, ZnS, CoO, TiO2, NiO and Degussa P25. The photocatalytic experiments were done with variation of pH, catalyst amount, variation of molar ratios of Co/Ti in the LDH and initial dye concentration. The photocatalytic effects were also studied separately with respect to influence of different quenchers like 2Na-EDTA (h+ scavenger), n-butanol (˙OH scavenger), benzoquinone (O2˙− scavenger) by adding the same at the beginning of the dye degradation experiment in order to indirectly establish the role played by ˙OH, h+ and O2˙− species in the photodegradation process. The reaction was monitored by following the decrease in the absorption intensity of the dye at λmax = 497 nm in 15 min intervals with a Shimadzu 1800 UV-vis spectrophotometer. The extent of decolorization was obtained from the ratio C/C0, where C and C0 were the absorbance values at a particular time interval and at t = 0 respectively. The strong absorption bands of CR decreased gradually on increasing irradiation time and the absorbance of the dye decreased nearly to zero after 75 min of visible light irradiation with the synthesized LDH [Fig. 11(A)]. The solution colour changed to nearly transparent marking the end of the decolorization [Fig. 11(B)]. The highly efficient photocatalytic performance of the synthesized Co/Ti-LDH compared to ZnO, ZnS, TiO2, CoO, NiO and Degussa P25 could be attributed to the presence of high surface area, defects, oxygen vacancies and Ti3+ centers within the layered brucite like structure.8,15,23 Control experiments like blank and dark reactions revealed negligible adsorption, clearly indicating that visible light plays a significant role in the photodegradation process.
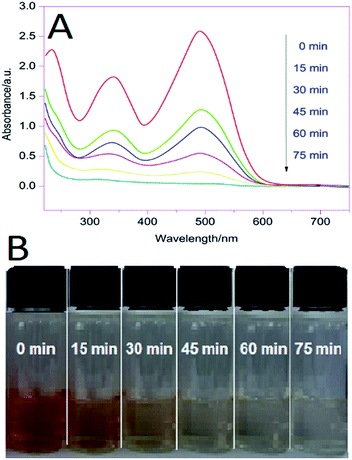 | ||
Fig. 11 (A) UV absorption spectra of CR degradation with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH [pH 4.0; LDH 15.0 mg; aqueous Cr 1 × 10−5 M] (B) snapshot of aliquots of CR at different time intervals. 1 Co/Ti LDH [pH 4.0; LDH 15.0 mg; aqueous Cr 1 × 10−5 M] (B) snapshot of aliquots of CR at different time intervals. | ||
 | ||
Fig. 13 (A) Effects of initial CR concentration on degradation, (B) effects of variation of Co/Ti molar ratio in the LDH on degradation (C) number of recycles of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH. 1 Co/Ti LDH. | ||
The reason for the observed changes could be linked to more and more dye molecules getting adsorbed on the LDH surface when the initial dye concentration was increased. The large amount of adsorbed dye molecules could have caused an inhibitive effect on the photocatalytic degradation due to the lack of direct contact among the photogenerated holes, hydroxyl radicals or superoxide radicals. Moreover, high dye concentration may act as inner filter which shunts the photons away from the LDH surface. Most of the dye molecules after getting adsorbed on the LDH surface might also block the active surface sites for reaction decreasing the photodegradation efficiency.8
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDHs were also synthesized (S8; ESI†) and were used for comparing the photodegradation performances with that of 2
1 Co/Ti LDHs were also synthesized (S8; ESI†) and were used for comparing the photodegradation performances with that of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH. The comparative photodegradation analysis was done at pH 4 with 15.0 mg of LDH and 1 × 10−5 M CR solution. Fig. 13(B) shows that 2
1 Co/Ti LDH. The comparative photodegradation analysis was done at pH 4 with 15.0 mg of LDH and 1 × 10−5 M CR solution. Fig. 13(B) shows that 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH exhibited much higher activity than that of either of the other Co/Ti LDHs. These results suggest a synergistic effect between Co and Ti in the LDH for exhibiting maximum photocatalytic performance. It is most likely that 2
1 Co/Ti LDH exhibited much higher activity than that of either of the other Co/Ti LDHs. These results suggest a synergistic effect between Co and Ti in the LDH for exhibiting maximum photocatalytic performance. It is most likely that 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH provides an optimum contact surface for Ti-species and the visible light photons, giving maximum photocatalytic performance.31
1 Co/Ti LDH provides an optimum contact surface for Ti-species and the visible light photons, giving maximum photocatalytic performance.31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 Co/Ti LDH. After each degradation cycle, the LDH was separated from the reaction system, was regenerated by washing it three times with de-ionized water followed by drying, and was reused as a photocatalyst. The degree of CR degradation for the first cycle was ∼99.7% [Fig. 13(C)]. Although the extent of degradation of the LDH declined in the second, third, fourth and fifth cycles, the rate of photodegradation was still 92% after the fifth cycle indicating that the synthesized LDH is a potential recyclable candidate for photodegradation purposes.
1 Co/Ti LDH. After each degradation cycle, the LDH was separated from the reaction system, was regenerated by washing it three times with de-ionized water followed by drying, and was reused as a photocatalyst. The degree of CR degradation for the first cycle was ∼99.7% [Fig. 13(C)]. Although the extent of degradation of the LDH declined in the second, third, fourth and fifth cycles, the rate of photodegradation was still 92% after the fifth cycle indicating that the synthesized LDH is a potential recyclable candidate for photodegradation purposes.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH after the fifth cycle of CR degradation, the FT-IR analysis of the LDH and that recovered at the end of the fifth cycle of CR degradation was carried out (Fig. 14). The comparative analysis reveals that the LDH showed remarkable photostability even after fifth cycle of use with all the characteristic IR-bands present in their respective positions, accompanied by some minor changes in the fingerprint region.32–34
1 Co/Ti LDH after the fifth cycle of CR degradation, the FT-IR analysis of the LDH and that recovered at the end of the fifth cycle of CR degradation was carried out (Fig. 14). The comparative analysis reveals that the LDH showed remarkable photostability even after fifth cycle of use with all the characteristic IR-bands present in their respective positions, accompanied by some minor changes in the fingerprint region.32–34
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH. The observed linearity in the plot of ln(C0/C) vs. irradiation time [Fig. 15(B)] signifies that the degradation of the test dye follows pseudo first-order kinetics, represented by the equation
1 Co/Ti LDH. The observed linearity in the plot of ln(C0/C) vs. irradiation time [Fig. 15(B)] signifies that the degradation of the test dye follows pseudo first-order kinetics, represented by the equation
 | (4) |
 | (5) |
Thus, the photodegradation of CR is primarily dependent on the light intensity and absorption efficiency of the Co/Ti LDH.2,8,16
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 Co/Ti LDH with commercial catalysts. A comparative photodegradation analysis was performed to validate the efficiency of Co/Ti LDH over commercial ZnO, ZnS, CoO, TiO2, NiO and Degussa P25 for degradation of the anionic CR dye. As maximum efficiency of Co/Ti LDH was observed at pH 4 with 15.0 mg LDH in 200 ml dye solution of concentration 1 × 10−5 M at room temperature (30 °C), the comparative studies were done under these conditions with all the catalysts. 2
1 Co/Ti LDH with commercial catalysts. A comparative photodegradation analysis was performed to validate the efficiency of Co/Ti LDH over commercial ZnO, ZnS, CoO, TiO2, NiO and Degussa P25 for degradation of the anionic CR dye. As maximum efficiency of Co/Ti LDH was observed at pH 4 with 15.0 mg LDH in 200 ml dye solution of concentration 1 × 10−5 M at room temperature (30 °C), the comparative studies were done under these conditions with all the catalysts. 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti-LDH showed enhanced degradation of the dye (∼99.7%) in 75 min in comparison to ZnO (∼66%), CoO (∼82%), TiO2 (∼49%), ZnS (∼59%), NiO (∼83%) and Degussa P25 (∼76%) as observed from C/C0 vs. irradiation time plots [Fig. 16(A)]. Thus, the dye solution became colourless in 75 min with Co/Ti LDH indicating a higher efficiency for CR degradation than the other commercial catalysts.8 Blank reaction (without the catalyst) under the same experimental conditions showed ∼18% adsorption, attributed to the effects of the visible light on adsorption of the anionic dye while the dark showed ∼25% adsorption of CR from aqueous solution.
1 Co/Ti-LDH showed enhanced degradation of the dye (∼99.7%) in 75 min in comparison to ZnO (∼66%), CoO (∼82%), TiO2 (∼49%), ZnS (∼59%), NiO (∼83%) and Degussa P25 (∼76%) as observed from C/C0 vs. irradiation time plots [Fig. 16(A)]. Thus, the dye solution became colourless in 75 min with Co/Ti LDH indicating a higher efficiency for CR degradation than the other commercial catalysts.8 Blank reaction (without the catalyst) under the same experimental conditions showed ∼18% adsorption, attributed to the effects of the visible light on adsorption of the anionic dye while the dark showed ∼25% adsorption of CR from aqueous solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH) was undertaken along with that of pure CR [Fig. 16(B)]. It is seen that all the characteristic bands were present at their respective positions for both blank and dark reactions with some peaks becoming broad particularly in the fingerprint region, clearly indicating the occurrence of adsorption in the control experiments. However, the FT-IR spectrum of aqueous CR solution after 75 min (at the end of the decolorization process) indicates the absence of most of the characteristic bands of the anionic dye with some band shifts being observed to newer positions, indicating that 2
1 Co/Ti LDH) was undertaken along with that of pure CR [Fig. 16(B)]. It is seen that all the characteristic bands were present at their respective positions for both blank and dark reactions with some peaks becoming broad particularly in the fingerprint region, clearly indicating the occurrence of adsorption in the control experiments. However, the FT-IR spectrum of aqueous CR solution after 75 min (at the end of the decolorization process) indicates the absence of most of the characteristic bands of the anionic dye with some band shifts being observed to newer positions, indicating that 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH has remarkable ability to degrade the anionic CR dye to simple molecules under visible light irradiation.
1 Co/Ti LDH has remarkable ability to degrade the anionic CR dye to simple molecules under visible light irradiation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
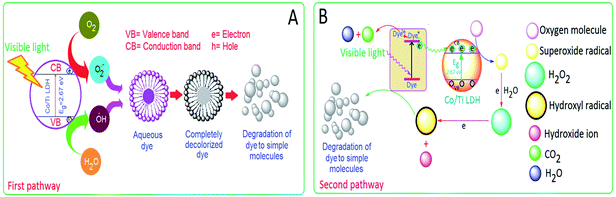
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH is proposed on the basis of band theory by taking O1s orbital as the valence band, Co2p and Ti2p orbitals as the conduction bands. The photocatalytic degradation is supposed to take place in two different pathways by the generation of reactive species like ˙OH and O2˙− in presence of visible light.
1 Co/Ti LDH is proposed on the basis of band theory by taking O1s orbital as the valence band, Co2p and Ti2p orbitals as the conduction bands. The photocatalytic degradation is supposed to take place in two different pathways by the generation of reactive species like ˙OH and O2˙− in presence of visible light.
First pathway. It is proposed that the series of reactions take place in the following sequential pattern (eqn (6)–(17)) upon visible light exposure at 30 °C. The electrons are first excited from valence band to the conduction band, thereby leaving holes in the valence band. The absorption of photons initiates electronic excitation with energy equal to or greater than the band gap energy of the semiconductor, Co/Ti LDH. Thus, 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti-LDH generates e−–h+ pairs (eqn (6)) upon visible light exposure, thereby reducing Co3+ and Ti4+ species on the LDH surface (eqn (7)–(9)). This generates a strong redox reaction (eqn (9)) being initiated by the photo-generated electrons with intense lattice vibrations on the LDH surface. The hopping h+ move to the LDH surface and react with H2O and OH− ions yielding OH˙ radicals (eqn (10) and (11)). The e− are likely to react with adsorbed oxygen present on the surface to produce superoxide (O2˙−) (eqn (12)). Superoxide (O2˙−) further reacts with adsorbed H+ present on the surface to produce peroxide radicals (˙OOH) (eqn (13)), further yielding H2O2 (eqn (14)) and hydroxyl radicals (˙OH) (eqn (15)). Hydroxyl (˙OH) and superoxide (O2˙−) radicals are highly active species that mineralize the dye to simple molecules (eqn (16)). The photo-generated, h+ and e−, may also recombine and dissipate the energy as light or heat (eqn (17)) which reduces the quantum efficiency.8 The first pathway proceeds through the following set of sequential reactions
1 Co/Ti-LDH generates e−–h+ pairs (eqn (6)) upon visible light exposure, thereby reducing Co3+ and Ti4+ species on the LDH surface (eqn (7)–(9)). This generates a strong redox reaction (eqn (9)) being initiated by the photo-generated electrons with intense lattice vibrations on the LDH surface. The hopping h+ move to the LDH surface and react with H2O and OH− ions yielding OH˙ radicals (eqn (10) and (11)). The e− are likely to react with adsorbed oxygen present on the surface to produce superoxide (O2˙−) (eqn (12)). Superoxide (O2˙−) further reacts with adsorbed H+ present on the surface to produce peroxide radicals (˙OOH) (eqn (13)), further yielding H2O2 (eqn (14)) and hydroxyl radicals (˙OH) (eqn (15)). Hydroxyl (˙OH) and superoxide (O2˙−) radicals are highly active species that mineralize the dye to simple molecules (eqn (16)). The photo-generated, h+ and e−, may also recombine and dissipate the energy as light or heat (eqn (17)) which reduces the quantum efficiency.8 The first pathway proceeds through the following set of sequential reactions| Co/Ti LDH + hν → Co/Ti LDH (e− + h+) | (6) |
| Co3+ + e− → Co2+ (electron trap) | (7) |
| Ti4+ + e− → Ti3+ (electron trap) | (8) |
| Co3+ + Ti4+ + hν ↔ Co2+ + Ti3+ (migration) | (9) |
| h+ + H2O → ˙OH + H+ | (10) |
| h+ + OH− → ˙OH | (11) |
| O2 + e− → O2˙− | (12) |
| O2˙− + H+ → HOO˙ | (13) |
| HOO˙ + HOO˙ → H2O2 + O2 | (14) |
| H2O2 + O2˙− → ˙OH + OH− + O2 | (15) |
| CR dye + O2˙− + ˙OH → degradation products | (16) |
| h+ + e− → energy | (17) |
Second pathway. The second pathway proceeds through the photosensitization of the CR dye in the presence of visible light.35–37 In this process, the dye molecules act as sensitizer by absorption of visible light to yield an excited state of the sensitizer (eqn (18)), which further transforms to dye radicals with the release of electrons (eqn (19)). The dye radicals thus generated form intermediate products like CO2 and H2O (eqn (20)). The electrons are injected into the conduction band of the Co/Ti LDH photocatalyst (eqn (21)) where they are scavenged by O2 to form O2˙− species as shown (eqn (22)), yielding H2O2 (eqn (23)). The electron transfer from the excited dye molecule to the conduction band of LDH is very fast (in the range of tens of femtoseconds). H2O2 subsequently generates ˙OH radicals (eqn (24)) that degrade the complex dye to simple molecules (eqn (25)).
| Dye + hν → dye* | (18) |
| Dye* → dye˙+ + e− | (19) |
| Dye˙+ → CO2 + H2O | (20) |
| Co/Ti LDH + e− → Co/Ti LDH˙+ | (21) |
| Co/Ti LDH˙+ + O2 → Co/Ti LDH + O2˙− | (22) |
| O2˙− + 2H2O + e− → 2H2O2 | (23) |
| H2O2 + e− → ˙OH + OH− | (24) |
| ˙OH + CR dye → degradation products | (25) |
These observations clearly demonstrate the involvement of holes (h+), hydroxyl (˙OH) and superoxide (O2˙−) species as highly reactive agents in the Co/Ti LDH mediated photocatalytic degradation of the anionic Congo Red in aqueous medium. The XPS and the PL measurements suggest that both Co and Ti are present in two oxidation states, accompanied by oxygen vacancies in the LDH. The presence of defects and O2/O2˙− transformation in the LDH has also been confirmed by EIS and PL analyses. DRS also indicate the presence of Ti4+ in brucite sheets. The broad nature of absorption at ∼548 nm could be ascribed to supramolecular guest–guest or guest–host interactions. On the basis of these data, the mechanistic pathways has been presented in the form of e−–h+ hopping conduction model and through photosensitization of the dye. The schematic illustrations of the mechanistic pathways of photodegradation are represented in Fig. 17(A and B).
The mass spectrum of the CR degradation products showed nine products. Congo Red is most likely to undergo asymmetric cleavage (Fig. 19) producing sodium(4-amino-3-diazenylnaphthalene)-1-sulfonate (2 molecules) and phenylbenzene (one molecule). Sodium(4-amino-3-diazenylnaphthalene)-1-sulfonate undergoes further dissociation forming sodium(4-amino-3-diazenyl-2-hydroxy)-1-naphthoxide (I) (m/z = 224), which transforms to form the compound (II) as well as 2-(1-amino-2-diazenyl-2-formylvinyl)benzoic acid (III) having m/z of 205 and 217 respectively. Sodium(4-amino-3-diazenylnaphthalene)-1-sulfonate is likely to undergo oxidation yielding 3-diazonium naphthalen-1-ol (IV) with a mass peak of 177, as intermediate product, which also undergoes transformation yielding 2-diazoniumnaphthalen-1,4-diol (V) with m/z of 192. Phenyl benzene is likely to undergo transformation to sodium phenyl phenoxide (VI) (m/z 196). It further reacts to form (VII) and (VIII) having m/z of 200 and 201 respectively, finally yielding 4-(4-oxocyclohexa-1,5-dienyl)cyclohex-4-ene-1,2-dione (IX) having m/z of 202. Apart from these peaks, the remaining region of the mass spectrum reveals some other peaks of negligible intensity, clearly indicating that most of the anionic CR dye molecules have been mineralized. The identification of the intermediates in the mass spectrum of Congo Red degradation products supports the asymmetric cleavage, leading to the formation of simple, less toxic molecules.
4. Conclusions
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti-LDH displayed remarkable photocatalytic properties for degradation of aqueous Congo Red. These properties of the LDH are attributed to the narrow band gap, high surface area, porous structure with surface defects and oxygen vacancies. The photocatalytic performance of the LDH is found to be much higher than that of commercial ZnO, ZnS, TiO2, NiO and Degussa P25. It is observed that adsorption dominated the control experiments carried out in the dark as well as the blank reactions (without catalyst), whereas degradation marked the end of the photochemical reaction with the LDH. The catalytic activity of the LDH is retained even after the fifth cycle and the FT-IR analysis of the LDH before and at the end of the fifth cycle showed excellent photostability. The pseudo-first-order model satisfactorily describes the degradation kinetics. The strategy of applying urea solution as a basic precipitant as well as template in synthesizing Co/Ti LDH has been found to enhance its semiconducting catalytic properties. The mass spectrum analysis supported the asymmetric cleavage of the dye forming simple, less toxic and environment friendly molecules in comparison to the carcinogenic and genotoxic parent dye. The encouraging results of this work show that 2
1 Co/Ti-LDH displayed remarkable photocatalytic properties for degradation of aqueous Congo Red. These properties of the LDH are attributed to the narrow band gap, high surface area, porous structure with surface defects and oxygen vacancies. The photocatalytic performance of the LDH is found to be much higher than that of commercial ZnO, ZnS, TiO2, NiO and Degussa P25. It is observed that adsorption dominated the control experiments carried out in the dark as well as the blank reactions (without catalyst), whereas degradation marked the end of the photochemical reaction with the LDH. The catalytic activity of the LDH is retained even after the fifth cycle and the FT-IR analysis of the LDH before and at the end of the fifth cycle showed excellent photostability. The pseudo-first-order model satisfactorily describes the degradation kinetics. The strategy of applying urea solution as a basic precipitant as well as template in synthesizing Co/Ti LDH has been found to enhance its semiconducting catalytic properties. The mass spectrum analysis supported the asymmetric cleavage of the dye forming simple, less toxic and environment friendly molecules in comparison to the carcinogenic and genotoxic parent dye. The encouraging results of this work show that 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH could be potentially used as a very effective and recyclable photocatalyst for large scale wastewater treatment.
1 Co/Ti LDH could be potentially used as a very effective and recyclable photocatalyst for large scale wastewater treatment.
Acknowledgements
The authors sincerely thank Sanjeev K. Srivastava, Professor of Physics and Meteorology, IIT Kharagpur, India for helping with the XPS analysis of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Co/Ti LDH. The authors are also very grateful to the referees for suggesting certain important modifications to the manuscript.
1 Co/Ti LDH. The authors are also very grateful to the referees for suggesting certain important modifications to the manuscript.
References
- K. M. Parida and L. Mohapatra, Chem. Eng. J., 2012, 179, 131 CrossRef CAS.
- C. G. Silva, Y. Bouizi, V. Fornes and H. Garcia, J. Am. Chem. Soc., 2009, 131, 13833 CrossRef PubMed.
- D. Chen, Y. Li, J. Zhang, J. Z. Zhou, Y. Guo and H. Liu, Chem. Eng. J., 2012, 185–186, 120 CrossRef CAS.
- S. Xu, J. Shen, S. Chen, M. Zhang and T. Shen, J. Photochem. Photobiol., A, 2002, 67, 64 CrossRef CAS.
- L. W. Zhang, H. B. Fu and Y. F. Zhu, Adv. Funct. Mater., 2008, 18, 2180 CrossRef CAS.
- G. Chen, S. Qian, X. Tu, X. Wei, J. Zou, L. Leng and S. Luo, Appl. Surf. Sci., 2014, 293, 345 CrossRef CAS.
- Z. P. Xu, J. Zhang, M. O. Adebajo, H. Zhang and C. Zhou, Appl. Clay Sci., 2011, 53, 139 CrossRef CAS.
- P. Roy Chowdhury and K. G. Bhattacharyya, Dalton Trans., 2015, 44, 6809 RSC.
- N. Zhang, M. Q. Yang, S. Liu, Y. Sun and Y. J. Xu, Chem. Rev., 2015, 115, 10307 CrossRef CAS PubMed.
- Y. Zhao, B. Li, Q. Wang, W. Gao, C. J. Wang, M. Wei, D. G. Evans, X. Duan and D. O'Hare, Chem. Sci., 2014, 5, 951 RSC.
- J. Senthilnathan and L. Philip, Chem. Eng. J., 2010, 161, 83 CrossRef CAS.
- R. Comparelli, E. Fanizza, M. L. Curri, P. D. Cozzoli, G. Mascolo and A. Agostiano, Appl. Catal., B, 2005, 60, 1 CrossRef CAS.
- C. Zhang, L. Gu, Y. Lin, Y. Wang, D. Fu and Z. Gu, J. Photochem. Photobiol., A, 2009, 207, 66 CrossRef CAS.
- Y. F. Zhao, M. Wei, J. Lu, Z. L. Wang and X. Duan, ACS Nano, 2009, 3, 4009 CrossRef CAS PubMed.
- J. Zhang, D. Fu, H. Gao and L. Deng, Appl. Surf. Sci., 2011, 258, 1294 CrossRef CAS.
- Z. Huang, P. Wu, B. Gong, Y. Fange and N. Zhu, J. Mater. Chem. A, 2014, 2, 5534 CAS.
- K. Li, X. Luo, X. Lin, F. Qi and P. Wu, J. Mol. Catal. A: Chem., 2014, 383–384, 1 CAS.
- A. A. A. Ahmed, Z. A. Talib and M. Z. B. Hussein, Appl. Clay Sci., 2012, 56, 68 CrossRef CAS.
- N. Tabet, M. Faiz and A. Al-Oteibi, J. Electron Spectrosc. Relat. Phenom., 2008, 163, 15 CrossRef CAS.
- J. Baltrusaitis, P. M. Jayaweera and V. H. Grassian, Phys. Chem. Chem. Phys., 2009, 11, 8295 RSC.
- S. K. Mehta, S. Kumar, S. Chaudhary and K. K. Bhasin, Nanoscale, 2010, 2, 145 RSC.
- H. Xu and L. Zhang, J. Phys. Chem. C, 2010, 114, 940 CAS.
- L. Zou, X. Xiang, M. Wei, F. Li and D. G. Evans, Inorg. Chem., 2008, 47, 1361 CrossRef CAS PubMed.
- M. M. Khan, S. A. Ansari, D. Pradhan, M. O. Ansari, D. H. Hang, J. Lee and M. H. Cho, J. Mater. Chem. A, 2014, 2, 637 CAS.
- A. Galtayries and J. Grimblot, J. Electron Spectrosc. Relat. Phenom., 1999, 98–99, 267 CrossRef CAS.
- A. J. Bard, F. R. F. Fan, A. S. Gioda, G. Nagasubramanian and H. S. White, Faraday Discuss. Chem. Soc., 1980, 70, 19 RSC.
- B. Choudhury, M. Dey and A. Choudhury, Appl. Nanosci., 2014, 4, 449 CrossRef.
- H. Wang, X. Xiang, F. Li, D. G. Evans and X. Duan, Appl. Surf. Sci., 2009, 255, 6945 CrossRef CAS.
- L. Zou, X. Xiang, J. Fan and F. Li, Chem. Mater., 2007, 19, 6518 CrossRef CAS.
- M. Q. Yang, N. Zhang, M. Pagliaro and Y. J. Xu, Chem. Soc. Rev., 2014, 43, 8240 RSC.
- Y. Zhang, Z. R. Tang, X. Fu and Y. J. Xu, ACS Nano, 2010, 4, 7303 CrossRef CAS PubMed.
- S. Li, Z. Bai and D. Zhao, Appl. Surf. Sci., 2013, 284, 7 CrossRef CAS.
- Y. Zhu, Y. Zhou, T. Zhang, M. He, Y. Wang, X. Yang and Y. Yang, Appl. Surf. Sci., 2012, 263, 132 CrossRef CAS.
- G. J. Cui, X. Y. Xu, T. J. Lin, D. G. Evans and D. Q. Li, Ind. Eng. Chem. Res., 2010, 49, 448 CrossRef CAS.
- R. Yoshihara, A. Katoh, Y. Furube, M. Tamaki, K. Murai, S. Hara, H. Murata, M. Arakawa and M. Tachiya, J. Phys. Chem. B, 2004, 108, 3817 CrossRef.
- S. Liu, Z. R. Tang, Y. Sun, J. C. Colmenares and Y. J. Xu, Chem. Soc. Rev., 2015, 44, 5053 RSC.
- N. Zhang, Y. Zhang and Y. J. Xu, Nanoscale, 2012, 4, 5792 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Characterization and photoassisted degradation results associated with this work. See DOI: 10.1039/c5ra19106h |
| This journal is © The Royal Society of Chemistry 2015 |