DOI:
10.1039/C5RA17921A
(Review Article)
RSC Adv., 2015,
5, 92799-92811
Selenium nanostructures: microbial synthesis and applications
Received
3rd September 2015
, Accepted 9th October 2015
First published on 9th October 2015
Abstract
Due to considerable impact on day-to-day life through the superior functions in energy, medicine, electronics, sensors and space industries, young researchers are being attracted towards the fields of nanotechnology and biotechnology. Selenium (Se) acts as an essential element in most organisms and is a beneficial nutrient in higher plants. Se nanostructures (NSs), i.e. nanospheres or nanoparticles (NPs) and nanowires (NWs), have unusual properties compared to their bulk counterparts. They have seven-fold lower toxicity than their other biologically utilizable materials. Several physical & chemical methods have been applied for the synthesis of Se NSs, but the development of eco-friendly routes is of considerable importance towards biological applications. Recently, microorganisms have been reported for the synthesis of Se NSs via the reduction of oxyions in anaerobic and aerobic environments. This review briefly proposes the recent developments in the microbial synthesis, characterization, and synthesis mechanism of Se NSs, along with their non-biological (solar, sensor, photocatalysis, etc.) and biological (anticancer, antioxidant, antiprotozoal, etc.) applications. Advantages of the Se NSs synthesized using green approaches, over the commonly used, are explored.
 Shubhangi Shirsat | Ms. Shubhangi Shirsat gained her bachelor’s and master’s degrees from S.G.B. Amravati University, Amravati, India in 2004 and 2006, respectively, and since 2012 has been involved in research activities, as part of a doctoral degree, in Biotechnology at Swami Ramanand Teerth Marathwada (S.R.T.M.), University, Nanded, India. She passed CSIR-UGC NET with an all-India ranking of 57, as well as GATE, GRE, TOEFL, etc. Since the beginning of the decade she has been working as an Asst. Prof. at a graduate and undergraduate level. |
 Ambadas Kadam | Dr Ambadas Kadam received MPhil and PhD degrees from Amravati University, Amravati, India and S.R.T.M., University, Nanded, India in 1999 and 2003, respectively. His major interest is tissue culture. Since 1994 he has been the Head of the Botany Department at the DSM’S ACS College, Jintur, Dist. Parbhani, India. To his credit, about 40 journal articles are available. He holds responsibilities as a member of the Management Council, Senate, Board of Studies in Botany, Faculty of Science, Standing Committee, Purchase Committee, etc. Due to his social and scientific contributions he was awarded with a Best Teacher Award. |
 Mu. Naushad | Dr Mu. Naushad is currently working as an Assoc. Prof. in the Department of Chemistry, College of Science, King Saud University (KSU), Saudi Arabia and received his PhD Degree in Analytical Chemistry from A.M.U. Aligarh, India in 2007. He has received scholarships from University Grants Commission in India, California, Washington DC and Michigan. He is the author of more than 130 research articles and several book chapters of international repute. He is also the Editor of several books. His research area is wastewater treatment, adsorption and nanocomposite materials. He is the life member of the National Environmental Science Academy, Delhi, India. |
 Rajaram S. Mane | Prof. Rajaram S. Mane received his PhD in Physics from the Shivaji University, Kolhapur, India in 2000 and worked as a postdoctoral fellow followed by a position as an Asst. Prof. during 2004–2006 and 2007–2008, respectively, in the Chemistry Department at Hanyang University, Korea. In 2009, he was at the research faculty of Oxford University, UK. Since 2010, he has been a regular professor and a visiting professor at S.R.T.M., University, Nanded, India and at King Saud University, Saudi Arabia, respectively. With more than 200 research articles and 4000 citations, he is actively engaged in synthesis, characterization, solar cells, electrochemical supercapacitors, chemical sensors and bioactive applications of various metal chalcogenide/oxide nanostructures/thin films. |
1. Introduction
Nanotechnology, an interdisciplinary field, includes the understanding of biological, material, physical and chemical sciences for the development of a variety of technologies. Nanostructures (NSs), including nanoparticles or nanospheres (NPs) and nanowires (NWs), are found to be a structural and functional part of nanotechnology. These are materials of one or more dimensions in the order of 100 nm or less.1 Nanostructured materials have attracted considerable attention in recent years as they exhibit useful and unusual properties compared to their polycrystalline counterparts. The physical and chemical properties of metal NPs like colour, melting temperature, conductivity and reactivity are mainly depend upon their sizes, shapes, compositions, crystallinities and structures etc.2 NPs display a high surface/volume ratio and are being used to build long-lasting, cleaner and safer products for a variety of applications in medicine, transportation and agriculture. Nanotechnology not only offers quality products but also improves the manufacturing processes.3 Based on their chemical composition, there are two types of NPs, i.e. carbon-based (fullerenes, carbon nanotubes, etc.) and inorganic. Inorganic NPs can further be categorized as metal oxides (zinc oxide, iron oxide, titanium dioxide, etc.), metals (gold, silver, iron, etc.) and quantum dots (cadmium sulphide, cadmium selenide, etc.).4 NPs can also be categorized as engineered NPs (ENPs) and non-engineered NPs (NENPs). ENPs are, usually, produced intentionally by considering a specific application. Their origin can be biological, like lipids, phospholipids, lactic acid, etc., or chemical, such as carbon molecules, silica, various metals, etc. On the other hand, NENPs are obtained in undesired ways such as particles emitted as byproducts; for example, during the combustion of fuels.5 There are four types of NSs based on their dimensions: 0D-nanoclusters, 1D-multilayers, 2D-nanograin layers, and 3D-bulk solids.6 Se is one of the chalcogenides naturally occurring as selenate (SeO42−) and selenite oxyions (SeO32−, Se2−) which can be reduced to the elemental form, Se0, in the presence of an appropriate reducing agent. The reduction of soluble Se4+ and Se6+ by microbes to insoluble non-toxic elemental Se is an effective way to remove it from the environment.7
A variety of physical, chemical, biological, and hybrid methods are being used to synthesize different types of NPs.8,9 Although physical and chemical methods are popular in the synthesis of NPs, toxic chemicals are required in both methods, greatly reducing their biomedical applications. Physical and chemical methods produce large quantities of NPs with finite sizes and shapes in a short time. These methods are complicated, costly, and result in hazardous toxic waste as byproducts, which are harmful not only to ecosystems but also to human health. Therefore, the development of safer and eco-friendly methods for the synthesis of Se NPs is of utmost importance to expand their biomedical applications. One of the choices to achieve this goal is to use microbial methods. NPs produced by biogenic enzymatic processes are far superior, in several ways, to those produced by chemical methods. With an enzymatic process, which is also known as a green synthesis, the use of expensive chemicals and high temperature can be eliminated. The “biogenic” approach is further supported by the fact that the majority of bacteria inhabit ambient conditions of temperature, pH, pressure, etc. The NPs generated through green processes revealed a higher catalytic reactivity and a greater specific surface area.10 Biogenic synthesis of Se NPs is frequently achieved through the reduction of selenate/selenite in the presence of bacterial proteins or plant extracts containing phenolic groups, flavonoids, alcohols, proteins, aldehydes, etc. However, a simple and reproducible bio-inspired method for the preparation of stable Se NPs on a large scale with potential applications is still missing. This review provides a brief overview of the current research activities that center on the microbial synthesis and the characterization of Se NPs, followed by discussion of the available mechanisms and plausible applications.
1.1 Role of Se in mammals
Dietary Se metabolites are taken up into the cell, where, together with the existing intracellular pool, they become metabolized by different pathways. As a result of the metabolic pathway, selenide forms, which serves as the Se source for selenocysteine (SeCys) biosynthesis. Fig. 1a gives a simplified view of Se metabolism in mammals. Most metals interact with proteins in the form of a cofactor; likewise, Se can be incorporated into the polypeptide chain as the SeCys amino acid. Proteins that contain SeCys as an integral part of their polypeptide chain are known as selenoproteins. Selenoproteins are present in all living entities and thirty different types of selenoproteins have been characterized so far.11 Seventeen selenoprotein families together form a human selenoproteome. It consists of glutathione peroxidases (GPx), thioredoxin reductases (TrxR), iodothyronine deiodinases, selenophosphate synthetases 2 (SPS2), Sep15, SelH, SelI, SelK, SelM, SelN, SelO, SelP/SepP, SelR, SelS, SelT, SelV, SelW, etc. These selenoproteins have antioxidant properties and they, in addition, help in cellular processes like in the biosynthesis of dNTPs, removing signalling peroxides, reducing oxidized proteins and membranes, regulating redox signalling, thyroid hormone metabolism, protein folding and selenium transportation. A summary of human selenoproteins and their functions is presented in Fig. 1b.11 Se deficiency is known to be associated with over forty human diseases.12
 |
| | Fig. 1 (a) Selenium metabolism in mammalian organisms (Se – selenium, GSSeSG – selenodiglutathione, CH3SeH – methylselenol, H2Se – selenide, SeMet – selenomethionine, SeCys – selenocysteine, GSH – glutathione, TrxR – thioredoxin reductase, and Trx – thioredoxin). (b) Human selenoproteome and its functions, and (c) a schematic overview of Se metabolism in plants. APSe – adenosine phospho selenate, SAT – serine acetyl transferase, OAS – O-acetylserine, OPH – O-phosphohomoserine, SeCys – selenocysteine, SeMet – selenomethionine, DMSeP – dimethylselenoproprionate, DMSe – dimethylselenide, and DMDSe – dimethyldiselenide. Numbers denote known enzymes. (1) ATP sulfurylase, (2) adenosine phosphosulfate reductase, (3) sulfite reductase (or glutathione), (4) OAS thiol lyase, (5) SeCys methyltransferase, (6) SeCys lyase, (7) cystathionine-g-synthase, (8) cystathionine-b-lyase, (9) methionine synthase, (10) methionine methyltransferase, (11) DMSP lyase, and (12) g-glutamylcysteine synthetase (reprinted with the permission of ref. 13). | |
1.2 Role of Se in plants
Se acts as a beneficial nutrient in higher plants, not as an essential element. A plant homolog of selenoproteins, like glutathione peroxidase (GPx), does not contain SeCys, but contains Cys in its active sites.13 Under low dosages, it can stimulate the growth of the plant, while at high dosages, it also can cause harm to plants.14 Members of the genus Brassica, e.g. mustards and cabbages, normally known as accumulators, accumulate 0.1% Se of their total dry weights. Plants belonging to families Fabaceae and Asteraceae are only found on seleniferous soils and typically accumulate Se to hundred-fold higher levels than the nearby vegetation in the field and are called as hyperaccumulators.15 Higher plants take up inorganic Se from the surrounding environment and convert it into organic compounds using selenium-assimilating enzymes. Firstly, a SeCys amino acid is formed, which non-specifically incorporates into proteins and results in toxicity development. An alternative to SeCys is selenomethionine (SeMet), which is also going to incorporate into proteins but shows less harmful effects. SeMet is the major selenocompound in cereal grains, legumes, and soybeans, and can be transformed to volatile dimethylselenide (DMSe) so that the plant can release excess Se. SeCys can also be transformed in plants to elemental Se and alanine.16 Fig. 1c represents the possible Se metabolism pathways in higher plants.
2. Existing methods
Methods employed for the synthesis of NPs are broadly classified under two processes; “top-down” and “bottom-up” processes. In the top-down approach, bulk material is broken down into NPs at the nanoscale level with various lithographic techniques like grinding and milling. While in the bottom-up approach, atoms self-assemble into new nuclei grown into a nanoscale particle.9 Currently, a number of chemical and physical methods are being used for the synthesis of Se NPs.
The summary (with ref. 17–31) in Table 1 encompasses the existing physical and chemical methods used in synthesis of Se NPs with their respective importance.
Table 1 Physical and chemical methods for the synthesis of Se NSs
| Method of synthesis |
Nanostructure material synthesized |
Disadvantages |
References |
| Wet chemical reduction |
Amorphous Se NPs |
Capital intensive, low production rate, difficult to scale up |
17–22 |
| Hydrothermal routes |
Amorphous Se NPs, Se NWs, t-Se NWs and nanotubes |
Difficult to control process reproducibility |
23–26 |
| Solvothermal and aging routes |
Se NPs |
High cost |
22 |
| Sonochemical approaches |
Se NPs |
Inability to control particle size |
27–29 |
| Photothermal assisted synthesis methods |
Se NPs |
Low rate of production, high energy consumption, highly economical |
22 |
| Photocatalytic reduction |
Se NPs |
High energy consumption |
30 and 31 |
3. Biosynthesis
Biological entities and inorganic materials have been in constant contact with each other ever since the start of life on the earth. Because of the constant interaction of cells with metals, living cells have well-organized mineral deposits and these metals have important functions in these cells. Recently, scientists have been paying attention for finding the interactions between inorganic molecules and biological species.32 A variety of microorganisms, enzymes, fungi, and plant extracts have been used to synthesize Se NPs of different sizes and morphologies. The summary in Table 2 presents ways preferred so far for developing various NPs from different microorganisms.33–60 Microorganisms reduce the toxic selenate and selenite oxyions into non-toxic elemental Se, which is insoluble in water. It is a simple process to detoxify selenites/selenates to Se NPs as the reverse reaction is too slow to produce Se compounds.61 There are two modes of oxyion reduction as presented below.
Table 2 Biosynthesis of metal NPs by different microorganisms
| Microorganisms |
Nanoparticle synthesized |
Size (nm) |
Shape |
Site of synthesis |
References |
| Rhodococcus sp |
Au |
5–15 |
Spherical |
Intracellular |
33 |
| Plectonema boryanum |
Au |
<10–25 |
Cubic |
Intracellular |
34 |
| Plectonema boryanum UTEX 485 |
Au |
10 nm to 6 μm |
Octahedral |
Extracellular |
34 |
| Escherichia coli |
Au |
20–30 |
Triangles, hexagons |
Extracellular |
35 |
| Yarrowia lipolytica |
Au |
15 |
Triangles |
Extracellular |
33 |
| Pseudomonas aeruginosa |
Au |
15–30 |
Not available |
Extracellular |
36 |
| Rhodopseudomonas capsulate |
Au |
10–20 |
Spherical |
Extracellular |
37 |
| Shewanella algae |
Au |
10–20 |
Not available |
Intracellular |
38 |
| Brevibacterium casei |
Au, Ag |
10–50 |
Spherical |
Intracellular |
39 |
| Trichoderma viride |
Ag |
5–40 |
Spherical |
Extracellular |
40 |
| Phanerochaete chrysosporium |
Ag |
50–200 |
Pyramidal |
Extracellular |
41 |
| Corynebacterium glutamicum |
Ag |
5–50 |
Irregular |
Extracellular |
42 |
| Bacillus cereus |
Ag |
4–5 |
Spherical |
Intracellular |
43 |
| Aspergillus flavus |
Ag |
8.92 ± 1.61 |
Spherical |
Extracellular |
41 |
| Aspergillus fumigatus |
Ag |
5–25 |
Spherical |
Extracellular |
44 |
| Verticillium sp |
Ag |
25 ± 8 |
Spherical |
Extracellular |
45 |
| Fusarium oxysporum |
Ag |
5–50 |
Spherical |
Extracellular |
45 |
| Neurospora crassa |
Au, Au/Ag |
32, 20–50 |
Spherical |
Intracellular, extracellular |
46 |
| Shewanella algae |
Pt |
5 |
Not available |
Intracellular |
47 |
| Enterobacter sp |
Hg |
2–5 |
Spherical |
Intracellular |
48 |
| Escherichia coli |
CdTe |
2.0–3.2 |
Spherical |
Extracellular |
49 |
| Yeast |
Au/Ag |
9–25 |
Irregular polygonal |
Extracellular |
50 |
| Desulfovibrio desulfuricans |
Pd |
50 |
Spherical |
Extracellular |
51 |
| Shewanella oneidensis |
Fe3O4 |
40–50 |
Rectangular, rhombic, hexagonal |
Extracellular |
52 |
| Saccharomyces cerevisiae |
Sb2O3 |
2–10 |
Spherical |
Intracellular |
53 |
| Lactobacillus sp |
BaTiO3 |
20–80 |
Tetragonal |
Extracellular |
54 |
| Fusarium oxysporum |
TiO2 |
6–13 |
Spherical |
Extracellular |
55 |
| Fusarium oxysporum |
BaTiO3 |
4–5 |
Spherical |
Extracellular |
56 |
| Fusarium oxysporum |
ZrO2 |
3–11 |
Spherical |
Extracellular |
57 |
| Rhodopseudomonas palustris |
CdS |
8 |
Cubic |
Intracellular |
58 |
| Rhodobacter sphaeroides |
ZnS |
10.5 ± 0.15 |
Spherical |
Extracellular |
59 |
| Sulfate-reducing bacteria |
FeS |
2 |
Spherical |
Extracellular |
60 |
3.1 Anaerobic reduction of oxyions by microorganisms
Thauera selenatis,61 Enterobacter cloacae,62 Shewanella sp. HN-41,63 Sulfurospirillum barnesii, Bacillus selenitireducens, Selenihalanaerobacter shriftii,64 Clostridium pasteurianum,65 etc. strains have been found and studied to reduce selenium oxyions from selenate/selenite oxyions. These strains grow microaerophilically but reduce selenium oxyions to elemental selenium only in anaerobic conditions with the formation of stable, uniform NPs of Se.64 Se nanowires (NWs) and stellated polyhedral structures have been successfully synthesized by Shewanella putrefaciens. Mercury (Hg) has been directly reacted with Se NWs or the stellated polyhedral structures in water, and a core–shell structure of Hg–Se can be obtained after 12 h of incubation at an ambient temperature.66 It has been reported that membrane-associated proteins play an important role in the reduction of the Se oxyions. The reduction occurs at the cell surface and the precipitate is rapidly expelled through the membrane-associated efflux pumps.67 But, the exact biochemistry and enzymology behind the reduction of the Se oxyions are still unprecedented.68
3.2 Aerobic reduction of oxyions by microorganisms
Large scale biomanufacturing processes for the synthesis of Se NPs through the anaerobic mode are very tedious and challenging. Researchers are actively engaged in finding aerobic microorganisms which tolerate the high concentration of Se oxyions and reduce them to elemental Se within the NP range. Different types of Se NPs were synthesized using proteins, small peptides and other reducing agents.69 It has been reported that the particle size is decreased in the presence of O2 because oxygen promotes the oxidation of Se (backward reaction). As a consequence, the redox step becomes slower by producing smaller Se NPs.70 Smaller particle size can be obtained through aerobic respiration in contrast to the anaerobic reduction approach of Se NP synthesis. Microbes like Aspergillus terreus,70 Klebsiella pneumonia,71 Pseudomonas sp.,72–74 Bacillus sp.,69,75 Duganella sp.,76 Agrobacterium sp.,76 Bacillus selenitireducens strain MLS10,77 Rhodospirillum rubrum,78 Stenotrophomonas maltophilia,79 Wollinella succinogenes,80 etc., have been majorly reported to reduce selenium oxyions in aerobic conditions with the formation of Se NPs with different sizes. Bacillus subtilis reduces the Se oxyions initially so as to form spherical Se NPs. Once these unstable Se NPs dissolve into the solution, Se atoms develop. Se atoms precipitated out as nanocrystalline (trigonal) t-Se via a self-aggregation process turned out to be nanorods.
4. Characterization of Se NPs
4.1 Structure
After incubating bacterial cells with selenite/selenate oxyions, a gradual colour change with time is observed from colorless to red, followed by intense red. The distinguishing red colour of Se NPs is due to the excitation of the surface plasmon vibration of monoclinic Se. The UV-visible absorption spectrum of the selenium NPs recovered from the culture broth produces a characteristic peak at 590 nm (Fig. 2a). Se NPs produced by anaerobic bacteria Sulfurospirillum barnesii, Bacillus selenitireducens, and Selenihalanaerobacter shriftii present large variations in the UV-visible and Raman shift measurements. Se-reducing bacteria produce Se NPs with different atomic structures because of the diversity of the enzymes that catalyze the reduction of Se oxyanions.69
 |
| | Fig. 2 (a) Absorption spectrum and (b) zeta potential measurements of the selenium NPs isolated from the Bacillus cereus strain CM100B (taken from an open access source, ref. 69). | |
4.2 Stability
Se NSs stored for prolonged time periods confirm agglomeration property by changing their appearance to black. The zeta potential measured for Se NPs produced by different microorganisms has demonstrated a relatively higher negative charge (Fig. 2b). If all the particles in the suspension have a large negative or positive zeta potential then they have little tendency of agglomeration and greater stability. Fourier transform infrared analysis spectra of Se NPs produced by P. alcaliphila with and without Se NPs confirm that the intensity of the spectral peaks containing Se NPs is drastically diminished, suggesting a strong interaction between the Se atoms and the protein molecules present in P. alcaliphila. Se NPs and proteins link via electrostatic interactions because the intensity of the sample containing Se atoms decreased with an increase of wave number from 3421 to 3435 cm−1. Se NPs produced by microorganisms can tightly bind to proteins produced by cells and protect Se NPs from further transformation to the black form, indicating a greater stability and prolonged life.81
4.3 Size and shape
The size and shape of NPs produce changes in properties such as conductivity, color, mechanical strength, magnetic behaviour, melting point, etc. Se NPs produced by all the reported microorganisms were spherical and in some cases transformed from spherical particles to nanowires. The size of the Se NPs depends on the production time and the type of microorganism reducing the Se oxyanions. All microorganisms studied, so far, have produced polydisperse NPs with sizes ranging from 50 nm to 500 nm. The average size is above 100 nm.64,69 Temperature, oxygen concentration and production time have a strong influence on the size and shape of the Se NPs. The average size of the NPs is higher at elevated (4, 15 and 30 °C) temperatures.82 When the O2 concentration in the medium was increased, the average size of the Se NPs decreased and the shape of the particles became more irregular (Fig. 3). In Shewanella sp. HN-41 the production time was increased from 2 h to 12 h and the Se NP size also increased from 35–40 nm to 120 nm.63 The spherical (50–400 nm) monoclinic Se NP structure generated by Bacillus subtilis was changed into an anisotropic, one dimensional (1D) trigonal structure in 24 h when kept at an ambient temperature in aqueous solution.82 The color of the solution changed from red to black, recognizing the formation of trigonal Se NWs (Fig. 4).
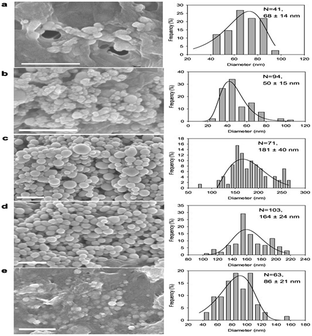 |
| | Fig. 3 The SEM images and the size distributions of the Se(0) NPs produced from N2-purged incubations at 4 °C (a), 15 °C (b), and 30 °C (c), from N2–O2-purged incubations (d) and from O2-purged incubations (e). The number of particles counted, and the average and standard deviations of the diameters of the Se(0) nanoparticles are shown in the side-diagrams. The scale bars given represent 500 nm. Solid lines depict the estimation using the log normal function (reprinted with the permission of ref. 82). | |
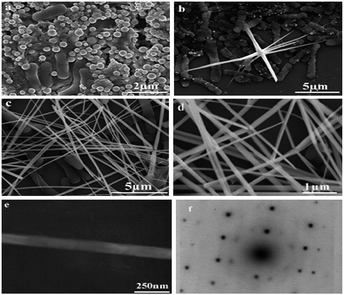 |
| | Fig. 4 Spherical Se NPs change their crystal structure from monoclinic to trigonal selenium over time. This transformation was observed on Se NPs produced by Bacillus subtilis. Field emission scanning electron microscopy and transmission electron microscopy (TEM) images of selenium nanoparticles. (a) 0 h, (b) 12 h, (c) 24 h and (d) the high magnification image of (c). TEM image (e) and the electron diffraction pattern (f) of an individual Se NW (reprinted with the permission of ref. 85). | |
5. Probable mechanism
Microbial transformations of Se oxyions (selenite/selenate) to insoluble forms such as elemental Se0 may not be the only end product in the transformation process.68,69 It can also generate assimilable organic volatile forms like dimethyl selenide (DMSe, CH3SeCH3), dimethyl diselenide (DMDSe, CH3SeSeCH3), and dimethyl selenenyl sulfide (DMSeS, CH3SeSCH3) in the headspace of culture vessels.83 The majority of metal transformations in anaerobic and aerobic environments are the result of the direct enzymatic activity of bacteria.77
In some bacterial species, selenite reduction may serve functions such as detoxification and upholding the redox component of the electron transport system via cytoplasmic reductase enzymes.73 Selenite/selenate reduction activity is observed mainly in the membrane fraction when it is incubated in the presence of selenite/selenate as a substrate.69 In a few bacterial strains, growth took place in the presence of selenite, suggesting that these reductases are probably respiratory enzymes.67 Studies have indicated that NADPH/NADH-dependent selenate reductase enzymes are responsible for the reduction of Se (selenite/selenate) oxyions.74,75 The reduction seems to be initiated by electron transfer from NADPH/NADH by a NADPH/NADH-dependent reductase as the electron carrier (Fig. 5).69 Recently it has been reported that E. coli can also produce specific forms of proteins (AdhP, Idh, OmpC, and AceA) which are associated with the synthesis of Se NPs. These proteins play an important role in generating nearly identical Se NPs. It has been found that AdhP proteins have a strong affinity for Se NPs.84
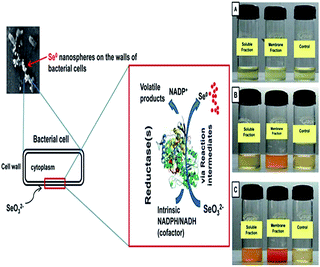 |
| | Fig. 5 Schematic representation of the proposed mechanism of biogenesis of selenium (Se0) nanospheres (taken from an open access source, ref. 69). | |
6. Applications
6.1 Non-biological
Se has a low melting point (∼217 °C), a high photoconductivity (∼0.8 × 105 S cm−1), catalytic activity towards hydration and corrosion reactions, and high piezoelectric, thermoelectric, and nonlinear optical responses. Se can react with a wealth of chemicals that can be used to convert Se into other functional materials such as Bi2Se3, ZnSe, CdSe, etc.31 Due to these properties, Se finds non-biological applications.
(i) Photocatalysis. Polluting substances such as antibiotics, personal care products, plasticizers, surfactants, herbicides, and other persistent organic compounds generate significant environmental problems around the world. Nanotechnology is providing novel and cost-effective ways for the removal of these contaminants through catalytic destruction and adsorption.A number of studies have been carried out on the use of semiconductor photocatalysts for environmental remediation. Se is one of the most important semiconductor photocatalysts as it shows a photomemory effect i.e. photoactivity of Se NPs under light illumination and in dark are same. Se NPs synthesized using tungstosilicate acid (TSA) ions show a very good catalytic activity in the degradation of Congo red. The TSA ion acts as a reducing agent in the synthesis of Se NPs and it also prevents the aggregation of the Se particles and offers stability. For the photodegradation process, aqueous solutions of Congo red were employed along with a Se–TSA solution for UV irradiation. The progress of dye decolorization was observed through the decrease in absorbance of Congo red at 488 nm. The UV light leads to the degradation of the dye structure and the overall reaction follows first-order kinetics. Se NPs without the TSA ion degrade Congo red, but a fast degradation occurred with Se–TSA. The decolorization of the dye increases with an increase in the concentration of the catalyst particles used. The rate of decolorization of Congo red increases with a decrease in the size of the Se NPs.21 The photodegradation of methyl orange can be achieved with metal selenides, such as ultrathin ZnSe nanorods, upon illumination with UV light.31
(ii) Rectifiers. A rectifier is an electrical device that converts alternating current, which periodically reverses direction, to direct current, which flows in only one direction. Currently, various semiconductor materials like Zn and Hg are used for the fabrication of rectifiers. Se NPs are also been used in this rectification process. The rectification in a junction between the NPs and an organic molecule is due to the parity between the free carriers in the former component and the type of carrier-accepting nature in the latter one. Rectifiers fabricated from Se NPs are cheaper and simpler to specify and installation than other rectifiers. These rectifiers are used in the detection of amplitude modulated radio signals, welding, etc.85
(iii) Sensors. Hydrogen peroxide (H2O2) is harmful to biological systems and it is the cause of neuropathology in central nervous system diseases. Se NPs synthesized from B. subtilis are utilized for the detection of H2O2. With a Se sensor, one can detect very low concentration of H2O2 in food, pharmaceutical, clinical, industrial, and environmental samples.85 Melamine is illegally added to milk, baby food, and animal feed to increase nitrogen content and it has serious health hazards. Se NPs conjugated with melamine antibody strips have been developed, which are the simplest method used for the detection of melamine even at very low concentration.86 Dinitrobutylphenol is very detrimental to human and aquatic animals as it is carcinogenic. Dinitrobutylphenol is released into wastewater, causing environmental pollution, and a Se NP-based chemiluminescence system is, so far, the best for its detection.87
(iv) Solar cells. Several research groups, including ours, are searching for efficient materials that can be used in solar cells which efficiently convert solar energy into electrical energy.88–90 Due to its unusual properties, Se can be used in solar cells. Selenium generates an electric current proportional to the amount of light falling on its surface and an inexpensive solar cell can be produced using Se NPs. Se NPs synthesized via a microwave method using SeCl4 as a precursor have been used to fabricate inexpensive solar cells by considering FTO/TiO2/Se/Pt-FTO and FTO/Se/CdS/Pt-FTO (where abbreviations have their usual meanings) configurations with remarkable solar-to-power conversion efficiency values.91
(v) Wastewater treatment. High doses of Se oxyanions (selenate and selenite) are toxic to humans, animals, and aquatic organisms, so they need to be removed from wastewaters prior to their release into the environment. Aerobic reduction of Se oxyanions to either volatilized Se compounds followed by gas trapping or Se NP entrapment in microbial biomass would be a one-step process for the treatment of selenite-containing wastewater. Activated sludge is more suitable for treating Se-rich wastewater compared to pure cultures as it is easy to handle and cheap. The presence of Se NPs in activated sludge flocs improves their settleability. Solid–liquid separation on the basis of gravity settling is a cost-effective method for the industry. Se NP-activated sludge was used as a fertilizer for selenium deficient soils and for the decontamination of heavy metals as Se NPs absorb heavy metals, especially in mercury polluted soils.92
6.2 Biological
Nanomedicine is a burgeoning field of research with tremendous prospects for the improvement of the diagnosis and treatment of human diseases. NPs are being used for various medical applications due to their smaller sizes and higher surface-to-volume ratios that allow more active sites for interacting with biological molecules, such as microorganisms and other bioactive entities.93 There is a fine line between the optimum limit/deficiency and an excess of Se in a living system causes toxicity. Se NPs have a seven-fold lower acute toxicity than sodium selenite and the subchronic toxicity was lower than that of selenite.94 Also, Se NPs demonstrated a higher efficiency than other organic Se forms in upregulating selenoenzymes.95 When Se is reduced to NP range, its biological activity remains the same as that found for the previously used forms.96 Due to this unique property of Se NPs, they are enormously considered in the various fields of medicine and pharmacy.
(i) Anticancer. Most of the time Se deficiency is found to be associated with cancer. As Se compounds have a low therapeutic index, Se compounds have fewer applications in cancer treatment. Apoptosis can be induced in a breast cancer cell line (MCF-7) treated with Se NP-functionalized folic acid (FA). FA-Se NPs can enter into mitochondria and increase the reactive oxygen species (ROS) production capacity, finally causing damage to the mitochondria and inducing MCF-7 cell cycle arrest.97 Researchers have also found that the viability of the cells of a prostate cancer cell line (PC3) can be decreased when treated with Se NPs in comparison with selenomethionine, the major dietary supplement form of Se. After the treatment with Se NPs, PC3 cells lost their typical morphology and shrunken. The cells were detached from the surface of the plate and the adhesion properties decreased with an increase in the Se NP concentration. The Se NPs were also able to induce cytotoxicity in PC3 cells through a caspase-independent necrotic pathway without affecting the non-cancerous hPBMC cell line after a ApoTox-Glo triplex assay.98
(ii) Antioxidant. Free radical molecules can lose one or more electrons and are responsible for biological oxidation. Free radicals steal electrons from proteins, DNA and other cell structures. UVB radiation generates oxidative stress indirectly through ROS. ROS induce DNA single-strand breaks where DNA–protein cross-linking takes place with the formation of oxidized base derivatives, such as 7,8-dihydro-8-oxoguanine (8-oxoG). This genotoxic effect of UV radiation can be reduced by bio-functionalized Se NPs. When lymphocyte cells are exposed to UVB radiation, DNA damage is seen using a comet assay, but upon the addition of Se NPs DNA damage can be prevented greatly.99 Human umbilical vein endothelial cells were exposed to a higher level of D-glucose, where an increased fluorescence of the fluorescent probe 2′,7′-dichlorodihydroflurorescein diacetate acetyl ester was confirmed.100 A higher level of D-glucose and stress conditions resulted in the formation of ROS. When the cells were pre-incubated with cysteine-functionalized Se NPs, the fluorescence level was decreased to a value still lower than in the untreated cells.
(iii) Antiprotozoal. Leishmaniasis is one of the most important public health issues in tropical and sub-tropical countries. It is endemic in ninety-eight countries and territories, affecting twelve million people and threatens approximately three hundred and fifty million, worldwide. Antileishmanial activity of biogenic Se NPs alone and grouped with Meglumine antimoniate have been tested against the sensitive and glucantime-resistant L. tropica in vitro. The cell viability MTT assay showed that Se NPs are toxic to the promastigote stage of L. tropica.101
(iv) Antibacterial. With the prevalence and increase of microorganisms resistant to multiple antibiotics it is necessary to find new drugs with greater activity. When the antimicrobial activity of Se NPs was evaluated it was found that Se NPs inhibit the growth of S. aureus within 3–4 h at a very low concentration, indicating that Se NPs are influential against bacterial infections in normal tissues.102 Se NPs also show antimicrobial activity against C. albicans103 and pathogenic Escherichia coli.104
(v) Immune-regulation. Prolonged viral infections and alcohol abuse cause hepatocellular carcinoma through chronic hepatitis, fibrosis, and cirrhosis. Melatonin–Se NPs (MT–Se NPs) were given to mice with immunological liver injuries caused by bacillus Calmette-Guérin.105 After treatment it was observed that the MT–Se NPs had an immunoregulatory effect by inhibiting proinflammatory cytokines and activating lymphocytes. Radiotherapy and chemotherapy cause a considerable deterioration of the immune system by paralyzing the bone marrow. This leads to a decrease of white blood cells, red blood cells, and platelets in patients undergoing chemotherapy or radiotherapy. A decrease in host immunity and ultimately an increase in the risk of infectious diseases is caused by opportunistic microorganisms. Oral supplementation of Se NPs for 30 days to X-ray irradiated mice recovering from BM suppression has provided information that many types of important white blood cells are significantly increased, especially lymphocyte and neutrophil counts.106
(vi) Dietary supplement. The consumption per capita of broiler chicken meat has increased from 0.8 to 2.8 kg during the period from 2000 to 2012. To meet the growing demand, broiler chickens have to gain market weight by the 40th day of their growth. Rapid weight gain can be achieved with high metabolic activity, low disease susceptibility, less physical stress, and adequate micronutrients and trace minerals. During the rearing of broiler chicken, stressors are responsible for causing immunosuppression. Incompetency of the immune system increases susceptibility to various infections, and malignant auto-immune and inflammatory disorders, consequentially decreasing the productive performance. Dietary supplementation with Se NPs improved the growth performance, feed conversion efficiency and antioxidant enzyme activity of the broiler chickens.107
(vii) Inhibitor of protein glycation. The reduction of sugar molecules reacted with an amino acid of a protein stimulates a series of chemical changes that leads to the formation of advanced glycation end products (AGEs). AGEs cause a tricky situation in diabetic conditions such as diabetic nephropathy, neuropathy, and retinopathy, as well as Alzheimer’s disease and aging. Bovine serum albumin (BSA) and glucose incubated with and without Se NPs at 55 °C for 40 h were employed to evaluate the antiglycation effect of Se NPs. After the incubation, an amount of glucose was covalently bound onto BSA and the formation of fructosamine and fluorescent products was measured. It was found that the addition of Se NPs together with glucose to a BSA solution significantly decreased the development of protein glycation.108 As Se NPs have a large surface to volume ratio, BSA can bind to the surfaces of the Se NPs, thus decreasing the reactivity of glucose molecules towards the amino acids of BSA and consequently inhibiting protein glycation. Se NPs inhibit protein glycation in a dose-dependent but time-independent manner under the selected reaction conditions. Se NPs show the greatest inhibitory effect in the early stage, rather than in the advanced stage.108
7. Conclusion
The biosynthesis of nanoparticles using microbial methods is clean, safe, and environmentally acceptable according to “Green Chemistry” measures. Microorganisms are a prospective source for the synthesis of Se NSs which have applications in non-biological fields such as in photocatalysis, rectifiers, sensors, and solar cells, and in biological fields like anticancer, antimicrobial, antiprotozoal, dietary supplementation, etc. Although microbial synthesis is the best approach for Se NP synthesis, considerable research is needed to improve the quality of the products. Microbiologically synthesised Se NSs follow the principle of Ostwald ripening and thus the size of the formed Se NPs is a function of time. Se NPs produced from the reported microorganisms are generally in a polydisperse form with an average diameter greater than 100 nm. Efforts should be made to control the size and polydispersivity. Also, methods should ensure a high yield of Se NPs. Extracellular producers are the best for the synthesis of Se NPs where a purification process from the cells is considered. Recent progress and the ongoing efforts to improve particle synthesis efficiency and explore their non-biological and biological applications would definitely benefit society. There is no doubt that the implementation of these approaches on a commercial scale will improve living standard and life duration in the coming years.
Acknowledgements
Authors, R. S. Mane and M. Naushad, extend their gratitude to the Visiting Professor (VP) Unit of King Saud University (KSU) for the financial support.
Notes and references
- H. D. Synthesis, Properties and applications of iron Nanoparticles, Small, 2005, 1, 482–501 CrossRef PubMed.
- L. Addadi and S. Weiner, Control and design principles in biological mineralization, Angew. Chem., Int. Ed., 1992, 31, 153–169 CrossRef.
- D. A. Bazylinski, R. B. Frankel and K. O. Konhauser, Modes of biomineralization of magnetite by microbes, Geomicrobiol. J., 2007, 24, 465–475 CrossRef CAS.
- Y. J. Nam and J. R. Lead, Manufactured nanoparticles: An overview of their chemistry, interactions and potential environmental implications, Sci. Total Environ., 2008, 400, 396–414 CrossRef PubMed.
- K. Radad, M. Al-Shraim, R. Moldzio and W. D. Rausch, Recent advances in benefits and hazards of engineered nanoparticles, Environ. Toxicol. Pharmacol., 2012, 34, 661–672 CrossRef CAS PubMed.
- M. A. Meyers, A. Mishra and D. J. Benson, Mechanical properties of nanocrystalline materials, Prog. Mater. Sci., 2006, 51, 427–556 CrossRef CAS.
- R. S. Dungan Jr and T. Frankenberger, Microbial transformations of selenium and the bioremediation of seleniferous environments, Biorem. J., 1999, 3, 171–188 CrossRef.
- J. Liu, S. Z. Qiao, H. Q. Hu and G. Q. Lu, Magnetic nanocomposites with mesoporous structures: synthesis and applications, Small, 2011, 7, 425–443 CrossRef CAS PubMed.
- J. Saxena, M. M. Sharma, S. Gupta and A. Singh, Emerging role of fungi in nanoparticles synthesis and their applications, World J. Pharm. Sci., 2014, 3, 1586–1613 Search PubMed.
- R. Bhattacharya and P. Mukherjeem, Biological properties of “naked” metal nanoparticles, Adv. Drug Delivery Rev., 2008, 60, 1289–1306 CrossRef CAS PubMed.
- D. N. Cox and K. Bastiaans, Understanding Australian consumers’ perceptions of selenium and motivations to consume selenium enriched foods, Food Quality and Preference, 2007, 18, 66–76 CrossRef.
- H. Tapiero, D. M. Townsend and K. D. Tew, The antioxidant role of selenium and seleno-compounds, Biomed. Pharmacother., 2003, 57, 134–144 CrossRef CAS.
- S. V. Novoselov, M. Rao, N. V. Onoshko, H. Zhi, G. V. Kryukov, Y. Xiang, D. P. Weeks, D. L. Hatfield and V. N. Gladyshev, Selenoproteins and selenocysteine insertion system in the model plant system, Chlamydomonas reinhardtii, EMBO J., 2002, 21, 3681–3693 CrossRef CAS PubMed.
- G. H. Lyons, Y. Genc, K. Soole, J. C. R. Stangoulis, F. Liu and R. D. Graham, Selenium increases seed production in Brassica, Plant Soil, 2009, 318, 73–80 CrossRef CAS.
- O. A. Beath, C. S. Gilbert and H. F. Eppson, The use of indicator plants in locating seleniferous areas in Western United States. I. General, Am. J. Bot., 1939, 26, 257–269 CrossRef CAS.
- B. G. Lewis, C. M. Johnson and C. C. Delwiche, Release of volatile selenium compounds by plants: collection procedures and preliminary observations, J. Agric. Food Chem., 1966, 14, 638–640 CrossRef CAS.
- F. Gao, Q. Lu, X. Meng and S. Komarneni, Synthesis of nanorods and nanowires using biomolecules under conventional- and microwave-hydrothermal conditions, J. Mater. Sci., 2008, 43, 2377–2386 CrossRef CAS.
- Z. H. Lin and C. R. C. Wang, The size-dependent absorption spectral evolution of selenium nanoparticles, Mater. Chem. Phys., 2005, 92, 591–594 CrossRef CAS.
- J. Ma, X. Liu, Y. Wu, P. Peng and W. Zheng, Controlled synthesis of selenium of different morphologies at room temperature, Cryst. Res. Technol., 2008, 43, 1052–1056 CrossRef CAS.
- Y. Ren, M. Niu, W. Gu and Y. Fang, Facile synthesis of trigonal selenium nanotubes in ethanol at low temperature, Mater. Lett., 2012, 82, 148–151 CrossRef CAS.
- L. B. Yang, Y. H. Shen, A. J. Xie, J. J. Liang and B. C. Zhang, Synthesis of Se nanoparticles by using TSA ion and its photocatalytic application for decolorization of cango red under UV irradiation, Mater. Res. Bull., 2008, 43, 572–582 CrossRef CAS.
- S. Y. Zhang, J. Zhang, H. Y. Wang and H. Y. Chen, Synthesis of selenium nanoparticles in the presence of polysaccharides, Mater. Lett., 2004, 58, 2590–2594 CrossRef CAS.
- C. H. An, K. B. Tang, X. M. Liu and Y. T. Qian, Large-scale synthesis of high quality trigonal selenium nanowires, Eur. J. Inorg. Chem., 2003, 3, 3250–3255 CrossRef.
- Y. T. Chen, W. Zhang, Y. Q. Fan, X. Q. Xu and Z. X. Zhang, Hydrothermal preparation of selenium nanorods, Mater. Chem. Phys., 2006, 98, 191–194 CrossRef CAS.
- X. Y. Gao, T. Gao and L. D. Zhang, Solution–solid growth of alpha-monoclinic selenium nanowires at room temperature, J. Mater. Chem., 2003, 13, 6–8 RSC.
- Y. Shin, J. M. Blackwood, I. T. Bae, B. W. Arey and G. J. Exarhos, Synthesis and stabilization of selenium nanoparticles on cellulose nanocrystal, Mater. Lett., 2007, 61, 4297–4300 CrossRef CAS.
- B. Gates, Y. D. Yin and Y. N. Xia, A solution-phase approach to the synthesis of uniform nanowires of crystalline selenium with lateral dimensions in the range of 10–30 nm, J. Am. Chem. Soc., 2000, 122, 12582–12583 CrossRef CAS.
- B. Gates, B. Mayers, B. Cattle and Y. N. Xia, Synthesis and characterization of uniform nanowires of trigonal selenium, Adv. Funct. Mater., 2002, 12, 219–227 CrossRef CAS.
- B. Gates, B. Mayers, A. Grossman and Y. N. Xia, A sonochemical approach to the synthesis of crystalline selenium nanowires in solutions and on solid supports, Adv. Mater., 2002, 14, 1749–1752 CrossRef CAS.
- T. Triantis, A. Troupis, E. Gkika, G. Alexakos, N. Boukos, E. Papaconstantinou and A. Hiskia, Photocatalytic synthesis of Se nanoparticles using polyoxometalates, Catal. Today, 2009, 144, 2–6 CrossRef CAS.
- N. A. Luechinger, R. N. Grass, E. A. Athanassiou and W. J. Stark, Bottom-up fabrication of metal/metal nanocomposites from nanoparticles of immiscible metals, Chem. Mater., 2010, 22, 155–160 CrossRef CAS.
- D. K. Tiwari, J. Behari and P. Sen, Time and dose-dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach, Curr. Sci., 2008, 95, 647–655 CAS.
- A. Ahmad, S. Senapati and M. I. Khan, Intracellular synthesis of gold nanoparticles by a novel alkalotolerant Actinomycete, Rhodococcus species, Nanotechnology, 2003, 14, 824–828 CrossRef CAS.
- M. F. Lengke, B. Ravel, M. E. Fleet, G. Wanger, R. A. Gordon and G. Southam, Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold(III)-chloride complex, Environ. Sci. Technol., 2006, 40, 6304–6309 CrossRef CAS PubMed.
- L. Du, H. Jiang, X. Liu and E. Wang, Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of haemoglobin, Electrochem. Commun., 2007, 9, 1165–1170 CrossRef CAS.
- M. I. Husseiny, M. A. El-Aziz, Y. Badr and M. A. Mahmoud, Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa, Spectrochim. Acta, Part A, 2007, 67, 1003–1006 CrossRef CAS PubMed.
- S. He, Z. Guo, Y. Zhang, S. Zhang, J. Wang and N. Gu, Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulate, Mater. Lett., 2007, 61, 3984–3987 CrossRef CAS.
- Y. Konishi, T. Tsukiyama, T. Tachimi, N. Saitoh, T. Nomura and S. Nagamine, Microbial deposition of gold nanoparticles by the metal-reducing bacterium Shewanella algae, Electrochim. Acta, 2007, 53, 186–192 CrossRef CAS.
- K. Kalishwaralal, V. Deepak, S. Pandian and R. Kumar, et al. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei, Colloids Surf., B, 2010, 77, 257–262 CrossRef CAS PubMed.
- A. M. Fayaz, K. Balaji, M. Girilal, R. Yadav, P. T. Kalaichelvan and R. Venketesan, Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against Gram-positive and Gram-negative bacteria, Nanomedicine: Nanotechnology, Biology, and Medicine, 2010, 26, 103–109 CrossRef PubMed.
- N. Vigneshwaran, A. A. Kathe, P. V. Varadarajan, R. P. Nachane and R. H. Balasubramanya, Biomimetics of silver nanoparticles by white rot fungus, Phanerochaete chrysosporium, Colloids Surf., B, 2006, 53, 55–59 CrossRef CAS PubMed.
- K. Sneha, M. Sathishkumar, J. Mao, I. S. Kwak and Y. S. Yun, Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes, Chem. Eng. J., 2010, 162, 989–996 CrossRef CAS.
- M. M. G. Babu and P. Gunasekaran, Production and structural characterization of crystalline silver nanoparticles from Bacillus cereus isolate, Colloids Surf., B, 2009, 74, 191–195 CrossRef CAS PubMed.
- K. C. Bhainsa and S. F. D’Souza, Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigates, Colloids Surf., B, 2006, 47, 160–164 CrossRef CAS PubMed.
- S. Senapati, D. Mandal and A. Ahmad, Fungus mediated synthesis of silver nanoparticles: a novel biological approach, Indian J. Phys., A, 2004, 78, 101–105 Search PubMed.
- E. Castro-Longoria, A. R. Vilchis-Nestor and M. Avalos- Borja, Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa, Colloids Surf., B, 2011, 83, 42–48 CrossRef CAS PubMed.
- Y. Konishi, K. Ohno and N. Saitoh, Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae, J. Biotechnol., 2007, 128, 648–653 CrossRef CAS PubMed.
- A. Sinha and S. K. Khare, Mercury bioaccumulation and simultaneous nanoparticle synthesis by Enterobacter sp. cells, Bioresour. Technol., 2011, 102, 4281–4284 CrossRef CAS PubMed.
- H. Bao, Z. Lu and X. Cui, Extracellular microbial synthesis of biocompatible CdTe quantum dots, Acta Biomater., 2010, 6, 3534–3541 CrossRef CAS PubMed.
- D. Zheng, C. Hu, T. Gan, X. Dang and S. Hu, Preparation and application of a novel vanillin sensor based on biosynthesis of Au–Ag alloy nanoparticles, Sens. Actuators, B, 2010, 148, 247–252 CrossRef CAS.
- J. R. Lloyd, P. Yong and L. E. Macaskie, Enzymatic recovery of elemental palladium by using sulfate-reducing bacteria, Appl. Environ. Microbiol., 1998, 64, 4607–4609 CAS.
- T. Perez-Gonzalez, C. Jimenez-Lopez and A. L. Neal, Magnetite biomineralization induced by Shewanella oneidensis, Geochim. Cosmochim. Acta, 2010, 74, 967–979 CrossRef CAS.
- A. K. Jha, K. Prasad and K. Prasad, A green low-cost biosynthesis of Sb2O3 nanoparticles, Biochem. Eng. J., 2009, 43, 303–306 CrossRef CAS.
- A. K. Jha and K. Prasad, Ferroelectric BaTiO3 nanoparticles: biosynthesis and characterization, Colloids Surf., B, 2010, 75, 330–334 CrossRef CAS PubMed.
- V. Bansal, P. Poddar, A. Ahmad and M. Sastry, Room temperature biosynthesis of ferroelectric barium titanate nanoparticles, J. Am. Chem. Soc., 2006, 128, 11958–11963 CrossRef CAS PubMed.
- V. Bansal, D. Rautaray and A. Bharde, Fungus-mediated biosynthesis of silica and titania particles, J. Mater. Chem., 2005, 15, 2583–2589 RSC.
- V. Bansal, D. Rautaray, A. Ahmad and M. Sastry, Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum, J. Mater. Chem., 2004, 14, 3303–3305 RSC.
- H. J. Bai, Z. M. Zhang, Y. Guo and G. E. Yang, Biosynthesis of cadmium sulfide nanoparticles by photosynthetic bacteria Rhodopseudomonas palustris”, Colloids Surf., B, 2009, 70, 142–146 CrossRef CAS PubMed.
- H. J. Bai and Z. M. Zhang, Microbial synthesis of semiconductor lead sulfide nanoparticles using immobilized Rhodobacter sphaeroides, Mater. Lett., 2009, 63, 764–766 CrossRef CAS.
- J. H. P. Watson, D. C. Ellwood, A. K. Soper and J. Charnock, Nanosized strongly-magnetic bacterially-produced iron sulfide materials, J. Magn. Magn. Mater., 1999, 203, 69–72 CrossRef CAS.
- H. DeMoll-Decker and J. M. Macy, The periplasmic nitrite reductase of Thauera selenatis may catalyze the reduction of selenite to elemental selenium, Arch. Microbiol., 1993, 160, 241–247 CAS.
- M. Losi and W. T. Frankenberger Jr, Reduction of selenium by Enterobacter cloacae SLD1a-1: isolation and growth of bacteria and its expulsion of selenium particles, Appl. Environ. Microbiol., 1997, 63, 3079–3084 CAS.
- K. Tam, C. T. Ho, J. H. Lee, M. Lai, C. H. Chang, Y. Rheem, W. Chen and H. G. Hur, Growth mechanism of amorphous selenium nanoparticles synthesized by Shewanella sp. HN-41, Biosci., Biotechnol., Biochem., 2010, 74, 696–700 CrossRef CAS PubMed.
- R. S. Oremland, J. S. Blum, C. W. Culbertson, P. T. Visscher, L. G. Miller, P. Dowdle and F. E. Strohmaier, Isolation, growth and metabolism of an obligately anaerobic, selenate-respiring bacterium, strain SES-3, Appl. Environ. Microbiol., 1994, 60, 3011–3019 CAS.
- L. J. Yanke, R. D. Bryant and E. J. Laishley, Hydrogenase (I) of Clostridium pasteurianum functions a novel selenite reductase, Anaerobe, 1995, 1, 61–67 CrossRef CAS PubMed.
- C. T. Ho, A.-T. Nguyen, T.-T. Duong, T.-P.-Q. Le, D.-K. Dang, T.-C. Tang and H.-G. Hur, Biologically based method for the synthesis of Hg–Se nanostructures by Shewanella spp., RSC Adv., 2015, 20764–20768 RSC.
- M. Sabaty, C. Avazeri, D. Pignol and A. Vermeglio, Characterization of the reduction of selenate and tellurite by nitrate reductases, Appl. Environ. Microbiol., 2001, 67, 5122–5126 CrossRef CAS PubMed.
- L. Ranjard, C. Prigent-Combaret, S. Nazaret and B. Cournoyer, Methylation of inorganic and organic selenium by the bacterial thiopurine methyltransferase, J. Bacteriol., 2002, 184, 3146–3149 CrossRef CAS PubMed.
- S. Dhanjal and S. Cameotra, Aerobic biogenesis of selenium nanospheres by Bacillus cereus isolated from coalmine soil, Microb. Cell Fact., 2010, 9, 1–11 CrossRef PubMed.
- B. Zare, S. Babaie, N. Setayesh and A. R. Shahverdi, Isolation and characterization of a fungus for extracellular synthesis of small selenium nanoparticles, Nanomed. J., 2013, 1, 13–19 Search PubMed.
- P. J. Fesharaki, P. Nazari, M. Shakibaie, S. Rezaie, M. Banoee, M. Abdollahi and A. R. Shahverdi, Biosynthesis of selenium nanoparticles using Klebsiella pneumoniae and their recovery by a simple sterilization process, Braz. J. Microbiol., 2010, 41, 461–466 CrossRef CAS PubMed.
- L. Lortie, W. D. Gould, S. Rajan, R. G. L. Meeready and K. J. Cheng, Reduction of elemental selenium by a Pseudomonas stutzeri isolate, Appl. Environ. Microbiol., 1992, 58, 4042–4044 CAS.
- N. Belzile, G. J. Wu, Y. W. Chen and V. D. Appanna, Detoxification of selenite and mercury by reduction and mutual protection in the assimilation of both elements by Pseudomonas fluorescens, Sci. Total Environ., 2006, 367, 704–714 CrossRef CAS PubMed.
- W. J. Hunter and D. K. Manter, Reduction of selenite to elemental red selenium by Pseudomonas sp. strain CA5, Curr. Microbiol., 2009, 58, 493–498 CrossRef CAS PubMed.
- S. M. Etezad, K. Khajeh, M. Soudi, P. T. M. Ghazvini and B. Dabirmanesh, Evidence on the presence of two distinct enzymes responsible for the reduction of selenate and tellurite in Bacillus sp. STG-83, Enzyme Microb. Technol., 2009, 45, 1–6 CrossRef CAS.
- M. Bajaj, S. Schmidt and J. Winter, Formation of Se(0) nanoparticles by Duganella sp. and Agrobacterium sp. isolated from Se-laden soil of north-east Punjab, India, Microb. Cell Fact., 2012, 11, 64 CrossRef CAS PubMed.
- V. V. Yurkov and J. T. Beatty, Aerobic anoxygenic phototrophic bacteria, Microbiol. Mol. Biol. Rev., 1998, 62, 695–724 CAS.
- J. Kessi, M. Ramuz, E. Wehrli, M. Spycher and R. Bachofen, Reduction of selenite and detoxification of elemental selenium by the phototrophic bacterium Rhodospirillum rubrum, Appl. Environ. Microbiol., 1999, 65, 4734–4740 CAS.
- A. Yamada, M. Miyashita, K. Inoue and T. Matsunaga, Extracellular reduction of selenite by a novel marine photosynthetic bacterium, Appl. Microbiol. Biotechnol., 1997, 48, 367–372 CrossRef CAS PubMed.
- F. A. Tomei, L. L. Barton, C. L. Lemanski and T. G. Zocco, Reduction of selenate and selenite to elemental selenium by Wolinella succinogenes, Can. J. Microbiol., 1992, 38, 1328–1333 CrossRef CAS.
- W. Zhang, Z. Chen, H. Liu, L. Zhang, P. Gaoa and D. Li, Biosynthesis and structural characteristics of selenium nanoparticles by Pseudomonas alcaliphila, Colloids Surf., B, 2011, 88, 196–201 CrossRef CAS PubMed.
- J. H. Lee, J. Han, H. Choi and H. G. Hur, Effects of temperature and dissolved oxygen on Se(IV) removal and Se(0) precipitation by Shewanella sp. HN-41, Chemosphere, 2007, 68, 1898–1905 CrossRef CAS PubMed.
- J. W. Swearingen Jr, D. E. Fuentes, M. A. Araya, M. F. Plishker, C. P. Saavedra, T. G. Chasteen and C. C. Vásquez, Expression of the ubiE gene of Geobacillus stearothermophilus V in Escherichia coli K-12 mediates the evolution of selenium compounds into the headspace of selenite- and selenate- smended cultures, Appl. Environ. Microbiol., 2006, 72, 963–967 CrossRef CAS PubMed.
- J. Dobias, E. I. Suvorova and R. Bernier-Latmani, Role of proteins in controlling selenium nanoparticle size, Nanotechnology, 2011, 22, 195605 CrossRef CAS PubMed.
- T. Wang, Y. Liangbao, Z. Buchang and L. Jinhuai, Extracellular biosynthesis and transformation of selenium nanoparticles and application in H2O2 biosensor, Colloids Surf., B, 2010, 80, 94–102 CrossRef CAS PubMed.
- Z. Wang, D. Zhi, Y. Zhao, H. Zhang, X. Wang, Y. Ru and H. Li, Lateral flow test strip based on colloidal selenium immunoassay for rapid detection of melamine in milk, milk powder, and animal feed, Int. J. Nanomed., 2014, 9, 1699–1707 CrossRef PubMed.
- M. Iranifam, M. Fathinia, T. SadeghiRad, Y. Hanifehpour, A. Khataee and S. Joo, A novel selenium nanoparticles-enhanced chemiluminescence system for determination of dinitrobutylphenol, Talanta, 2012, 107, 263–269 CrossRef PubMed.
- T. Ganesh, H.-M. Nguyen, R. S. Mane, N. Kim, D. V. Shinde, S. S. Bhande, M. Naushad, K. N. Hui and S. H. Han, Promising ZnO-based DSSC performance using HMP molecular d yes of high extinction coefficients, Dalton Trans., 2014, 43, 11305–11308 RSC.
- S. S. Bhande, D. V. Shinde, S. F. Shaikh, S. B. Ambade, R. B. Ambade, R. S. Mane, Inamuddin, M. Naushad and S.-H. Han, Low-temperature solution-processed Zn-doped SnO2 photoanodes: enhancements in charge collection efficiency and mobility, RSC Adv., 2014, 4, 20527–20530 RSC.
- S. S. Bhande, D. V. Shinde, K. K. Tehare, S. A. Patil, R. S. Mane, M. Naushad, Z. A. Alothman, K. N. Hui and S. H. Han, DSSCs synergic effect in thin metal oxide layer-functionalized SnO2 photoanodes, J. Photochem. Photobiol., A, 2014, 295, 64–69 CrossRef CAS.
- P. K. Mokhtar, S. N. Masoud and H. M. Mostafa, Facile microwave synthesis, characterization, and solar cell application of selenium nanoparticles, J. Alloys Compd., 2014, 617, 627–632 CrossRef.
- R. Jain, M. Seder-Colomina, N. Jordan, P. Dessi, J. Cosmidis, E. D. van Hullebusch, S. Weiss, F. Farges and P. N. L. Lens, Entrapped elemental selenium nanoparticles affect physicochemical properties of selenium fed activated sludge, J. Hazard. Mater., 2015, 295, 193–200 CrossRef CAS PubMed.
- J. S. Zhang, X. Y. Gao, L. D. Zhang and Y. P. Bao, Biological effects of a nano red elemental selenium, BioFactors, 2001, 15, 27–38 CrossRef CAS PubMed.
- J. S. Zhang, H. L. Wang, X. X. Yan and L. D. Zhang, Comparison of short term toxicity between Nano-Se and selenite in mice, Life Sci., 2005, 76, 1099–1109 CrossRef CAS PubMed.
- H. Wang, J. Zhang and H. Yu, Elemental selenium at nano size possesses lower toxicity without compromising the fundamental effect on seleno enzymes: comparison with selenomethionine in mice, Free Radical Biol. Med., 2007, 42, 524–533 Search PubMed.
- J. Zhang, X. Wang and T. Xu, Elemental selenium at Nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with Se-methylselenocysteine in mice, Toxicol. Sci., 2008, 101, 22–31 CrossRef CAS PubMed.
- J. Pi, H. Jin, R. Liu, B. Song, Q. Wu and L. Liu, et al. Pathway of cytotoxicity induced by folic acid modified selenium nanoparticles in MCF-7 cells, Appl. Microbiol. Biotechnol., 2013, 97(3), 1051–1062 CrossRef CAS PubMed.
- P. Sonkusre, R. Nanduri, P. Gupta and S. Singh, Improved extraction of intracellular biogenic selenium nanoparticles and their specificity for cancer chemoprevention, J. Nanomed. Nanotechnol., 2014, 5(2), 1000194 Search PubMed.
- K. S. Prasad, H. Patel, T. Patel, K. Patel and K. Selvaraj, Biosynthesis of Se nanoparticles and its effect on UV-induced DNA damage, Colloids Surf., B, 2013, 103, 261–266 CrossRef CAS PubMed.
- S. K. Torrens, V. L. Campos, C. G. Leon, S. M. Rodriguez Llamazares, S. M. Rojas, M. Gozalez, C. Smith and M. A. Mondaca, Biosynthesis of selenium nanoparticles by Pantoea agglomerans and their antioxidant activity, J. Nanopart. Res., 2012, 14, 1236 CrossRef.
- H. Mahmoudvand, M. Shaklbale, R. Tavakoli, S. Jahanbakhsh and I. Sharifi, In vitro study of leishmanicidal activity of biogenic selenium nanoparticles against iranian isolate of sensitive and glucantime-resistant Leishmania tropica, Iran. J. Parasitol., 2014, 9, 452–460 Search PubMed.
- P. A. Tran and T. J. Webster, Selenium nanoparticles inhibit Staphylococcus aureus growth, Int. J. Nanomed., 2011, 6, 1553–1558 CAS.
- E. Kheradmand, F. Rafii, M. H. Yazdi, A. A. Sepahi, A. R. Shahverdi and M. R. Oveisi, The antimicrobial effects of selenium nanoparticle-enriched probiotics and their fermented broth against Candida albicans, Daru, J. Pharm. Sci., 2014, 1, 22–48 Search PubMed.
- J. Yang, K. Huang, S. Qin, X. Wu, Z. Zhao and F. Chen, Antibacterial action of selenium-enriched probiotics against pathogenic Escherichia coli, Dig. Dis. Sci., 2009, 54(2), 246–254 CrossRef CAS PubMed.
- H. Wang, W. Wei, S.-Y. Zhang, Y.-X. Shen, N.-P. Wang, L. Yue and S.-Y. Xu, Melatonin–selenium nanoparticles protectsliver against immunological injury induced by bacillus Calmette-Guérin and lipopolysaccharide, Acta Pharmacol. Sin., 2005, 26(6), 745–752 CrossRef CAS PubMed.
- M. H. Yazdi, M. Masoudifar, B. Varastehmoradi, E. Mohammadi, E. Kheradmand, S. Homayouni and A. R. Shahverdi, Effect of oral supplementation of biogenic selenium nanoparticles on white blood cell profile of BALB/c mice and mice exposed to X-ray Radiation, Avicenna J. Med. Biotechnol., 2013, 5(3), 158–167 CAS.
- X. Zhou and Y. Wang, Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality and glutathione peroxidase activity in Guangxi yellow chicken, Poult. Sci., 2011, 90, 680–686 CrossRef CAS PubMed.
- S. Yu, W. Zhang, W. Liu, W. Zhu, R. Guo, Y. Wang, D. Zhang and J. Wang, The inhibitory effect of selenium nanoparticles on protein glycation in vitro, Nanotechnology, 2015, 26, 145703 CrossRef PubMed.
|
| This journal is © The Royal Society of Chemistry 2015 |
Click here to see how this site uses Cookies. View our privacy policy here. 








