Highly selective and sensitive recognition of histidine based on the oxidase-like activity of Cu2+ ions†
Yan Xua,
Xiao-Qiong Wua,
Jiang-Shan Shen*ab and
Hong-Wu Zhang*a
aKey Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen, 361021, China. E-mail: jsshen@hqu.edu.cn; hwzhang@iue.ac.cn
bCollege of Materials Science and Engineering, Huaqiao University, Xiamen, 361021, China
First published on 23rd October 2015
Abstract
Developing simple, highly sensitive and selective sensing systems for histidine (His) is important due to its biological significance. In this report, Cu2+ ions serving as the oxidase mimics towards O-phenylenediamine (OPD) were investigated in detail. Experimental results revealed that the oxidase-like activity of Cu2+ ions is substantially higher than that of Cu/CuO nanoparticles. On the basis of these findings, a simple, highly sensitive and selective PL sensing platform for His could be developed, with a limit of detection (LOD) as low as 0.33 μM. Furthermore, experiments of His recovery in human urine samples were successfully conducted by employing the established sensing system with satisfactory results.
Introduction
Histidine (His) is a natural α-amino acid, which acts as an important constituent of proteins. His containing an imidazole moiety can function as a neurotransmitter and a regulator of metal transmission in the biological systems.1 Deficiency of His results in an impaired nutritional state in patients with chronic kidney disease.2 Therefore, developing highly selective and sensitive methods for detecting His is of importance due to its biological significance. Until now, a number of approaches have been developed for the detection of His, including high-performance liquid chromatography, electrochemistry, UV-vis absorption spectra, photoluminescence (PL) spectra.3–14 In particular, constructing PL based sensors have recently attracted considerable attentions due to the high sensitivity of PL spectrometry. Most of these reported investigations of probing His focused on metal-complexes,15–17 particularly Cu2+-complexes,18–22 as chemosensing ensembles, which could promise a high selectivity towards His. However, the procedures of preparing metal-complexes in general are tedious, time-consuming and troublesome, and employed organic solvents are environmentally unfriendly. Therefore, it is of importance to develop a novel, simple, highly sensitive and selective PL sensor for His.Artificial enzyme mimics have recently become a very important and exciting branch of biomimetic chemistry,23,24 since that, (1) they can serve as highly stable and low-cost alternatives to natural enzymes; (2) quite a number of artificial enzyme mimics have been found and prepared. Therefore, a wide range of applications of artificial enzyme mimics have been explored.25–29 In particular, numerous nanomaterials have been developed for serving as artificial enzyme mimics with oxidase-, peroxidase- and catalase-like activity,30–36 which were widely employed in the field of chemo-/biosensing by means of PL or UV-vis absorption spectra for the detection of a series of targets including metal ions, anions, toxic substance and biomolecules. Recently, we found that Fe3+ ions possess much higher peroxidase-like activity than that of Fe3O4 magnetic nanoparticles.37 Therefore, as the counterpart of Fe3+ ions, are Cu2+ ions having similar peroxidase-like or oxidase-like activity? However, to the best of our knowledge, such investigation is lack at present. Furthermore, it should be noted that, although several Cu2+-complexes have been employed as PL sensors for His based on the displacement mechanism, developing the sensing system for His, based on the catalytic reaction of artificial enzyme mimics, has not been reported so far.
Herein, we employed Cu2+ ions as the oxidase mimic for catalysing the oxidation of O-phenylenediamine (OPD) to form a photoluminescent compound. The oxidase-like activity of Cu2+ ions was found to be higher than that of Cu/CuO nanoparticles. Introducing a certain amount of His could significantly suppress the oxidation reaction of OPD catalysed by Cu2+ ions, due to the specific strong interaction between His and Cu2+ ions (Scheme 1). Good linear relationship between PL intensity and His concentration could be established, and a limit of detection (LOD) was calculated to be sub μM level. High selectivity has also been revealed by performing control experiments of other 19 natural α-amino acids, although cysteine showed somewhat interference due to it bearing one thiol group with strong binding capacity towards most of transition heavy metal ions. The experiments of His recovery in human urine samples were further performed by employing the established sensing system, and satisfactory results could be obtained.
Experimental section
Materials and instruments
Procedures
Results and discussion
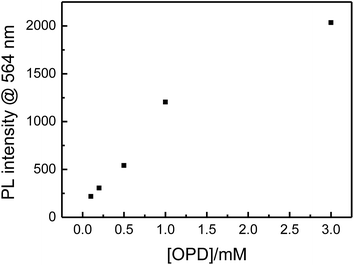
The oxidase-like activity of Cu2+ ions was firstly investigated by employing OPD, TMB and p-acetoamidophenol as the substrates, respectively. It was found that, when 0.5 μM Cu2+ ions was introduced into 50 mM Tris–HCl buffer solution of pH 7.4 containing 15% acetonitrile (by volume) and 0.5 mM OPD under 65 °C of temperature, the original colourless solution gradually turned into light yellow, and the resulting solution could change to emit yellow light from its original nonfluorescent under a 365 nm UV lamp (Fig. 1b and c). This observation revealed that Cu2+ ions can possess the extraordinary oxidase-like activity, similar to that of metal or metal oxides/sulfides nanomaterials.35,38–40 This catalytic reaction would be greatly suppressed if Cu2+ ions or oxygen in the measured solution were absent (the results of the control experiments of free dissolved oxygen were shown in Fig. S2†). However, for TMB employed as the substrate, neither obvious colour substances, nor fluorescent substances, could be observed, under similar experimental conditions. For p-acetoamidophenol catalysed by Cu2+ ions, only very weak PL emission could be observed. Furthermore, PL/UV-vis absorption spectra supported these observations (Fig. 1a). For OPD as the substrate, maximum wavelength of PL emission spectrum could be obtained at 564 nm (Fig. 1a, black line). The reaction time-dependent PL intensity at 564 nm of the OPD–Cu2+ system was showed in Fig. 2.The catalytic activity of Cu2+ ions towards OPD was found to be dependent on pH, temperature, OPD and Cu2+ ions concentrations, too (Fig. 3–6). pH effect on the catalytic activity of Cu2+ ions showed a change tendency, in which the catalytic activity first enhanced and then decreased, when pH was increased from 4.7 to 8.5 (Fig. 3), indicated by the change of PL intensity at 564 nm with varying pH. Taken together pH of the biological condition, 50 mM Tris–HCl buffer solution of pH 7.4 was thus employed in further experiments. When the temperature was set from 25 °C to 80 °C, the catalytic activity demonstrated an enhancement (Fig. 4). Furthermore, the effect of OPD concentration was investigated, and the results were showed in Fig. 5. Experimental results revealed that, when OPD concentration was changed from 0.1 mM to 3.0 mM, PL intensity at 564 nm gradually enhanced with increasing OPD concentration. The effect of Cu2+ ions concentration also indicated that, with increasing Cu2+ ions concentration from 0.05 μM to 5 μM, not only accelerating reaction, but also enhancement of PL intensity, could be observed (Fig. 6). Importantly, even if Cu2+ ions concentration was lowered to 0.1 μM which was 3 orders of magnitude lower than that of OPD fixed at 0.5 mM, a significant reaction could still occur, indicative of the extremely high oxidase-like activity of Cu2+ ions towards OPD. It should be noted that, we recently reported that Fe3+ ions possess much higher peroxidase-like activity than that of Fe3O4 nanoparticles.37
Similarly, in the absence of Cu2+ ions, the time, temperature and pH effect on the PL intensity of only OPD system were investigated, too. It was revealed that, the changes were similar to those of cases in the presence of Cu2+ ions (Fig. S3–S5†), except that PL intensity at 564 nm was significantly lowered.
We suggested that the reversible conversion between Cu2+ and Cu+ ions was responsible for the observable catalytic activity of Cu2+ ions. Since in traditional inorganic chemistry it is well known that, Cu+ ions can be stabilized by the weak coordination of acetonitrile,41,42 introducing acetonitrile into this catalytic system was expected to be helpful for stabilizing Cu+ ions. This suggestion was supported by the experiment of the effect of volume ratio of acetonitrile to buffer aqueous (v/v, %) on the catalytic activity of Cu2+ ions. Experimental results exhibited that, the catalytic activity of Cu2+ ions was substantially enhanced when the volume ratio was changed from 0 to 15%, however, further increasing the volume ratio could lead to the decrease of the catalytic activity (Fig. 7). The decrease of the catalytic activity of Cu2+ ions caused by the addition of excessive acetonitrile should originate from that the redox potential of the catalyst generally decreased when polarity of the solvent was lowered. Therefore, 15% acetonitrile content (by volume) was chosen to obtain preferable catalytic activity of Cu2+ ions in the related experiments.
It is known that, the stability of Cu nanoparticles is usually poor, and Cu nanoparticles are easy to be converted into CuO nanoparticles when Cu nanoparticles aqueous solution contains oxygen. Therefore, it was wondered that whether CuO nanoparticles have similar catalytic performance towards OPD compared with that of Cu2+ ions? Experimental results revealed that, OPD could be catalysed by CuO nanoparticles (the results of XRD and TEM characterization were shown in Fig. S6 and S7†)35 to form an oxidation production, too, similar to that of the case of Cu2+ ions. This result has also been observed by other groups.33 However, when Cu2+ ions concentration in copper salt and Cu content of CuO nanoparticles solution were set to be exactly same, the catalytic activity of CuO nanoparticles was found to be significantly poorer than that of Cu2+ ions (Fig. 8). Furthermore, the catalytic activity of Cu2+ ions obviously decreased when a certain amount of ascorbic acid was added to the OPD–Cu2+ catalytic system (Fig. 9), because ascorbic acid could fast reduce Cu2+ ions to in situ form Cu/CuO nanoparticles (Fig. S8†). This observation further supported that the catalytic activity of Cu2+ ions is superior to that of CuO nanoparticles. It should be noted that, likely only the superficial copper (unbinding or binding Cu2+ ions) in the surface of nanoparticles could act as catalysts for catalysing the oxidation reaction of OPD, similar to our recent observations.37
Considering the strong complexation capability of His towards Cu2+ ions, the catalytic reaction of the OPD–Cu2+ system should be inhibited by adding His. Indeed, it was found that, introducing a certain amount of His, the oxidation reaction of OPD catalysed by Cu2+ ions was His concentration-dependent. As shown in Fig. 10, the catalytic oxidation reaction was gradually suppressed with increasing His concentration, and good linear relationship was found between [His] and the PL intensity at 564 nm wavelength. The linear correlation of PL = 414.7–16.8 [His] (R2 = 0.991) was obtained over the tested concentration range of His from 0.5 to 30 μM when 0.5 μM Cu2+ ions was employed. The limit of detection (LOD) for His was estimated to be 0.47 μM (3σ/k, n = 11). Decreasing Cu2+ ions concentration to 0.1 μM, LOD of this sensing system for His was lowered to be 0.33 μM (Fig. S9†). These LODs were much lower than those of most of reported cases.43–45 The inhibition of the catalytic reaction should be ascribed to forming Cu2+–2His compounds due to high stability constant of Cu2+–His, which could result in reducing the amount of unbound Cu2+ ions.46,47 Other 19 natural α-amino acids were employed to test the selectivity of this sensing system. The results showed that, under similar experimental conditions, employing other amino acids did not suppress the catalytic reaction, besides that, cysteine bearing a thiol group showed somewhat interference, since it has strong binding capacity towards most of transition heavy metal ions (Fig. 11). Furthermore, other structurally similar biomolecules such as adenine and cytosine were employed for conducting control experiments, too, and the results revealed that PL spectral interferences were hardly observed (Fig. S10†). This result indicated that this established sensing system has good selectivity. A comparison of our present sensing system and other reported references were summarized, as shown in Table 1. Compared with tedious and troublesome synthesis of receptors or expensive instruments in the reported references, our present sensing system shows some merits of simplicity, facile operation, and sensitivity.
To evaluate the applicability, the experiments of His recovery in human urine samples were performed by employing our present sensing system. Two human urine samples were collected, and detailed experimental operation was referred to the Experimental section. As shown in Table 2, the quantitative recoveries (96.3% to 102.5%) of spiked His have revealed that this sensing system can have good practical sensing application. Meanwhile, affording His concentration (402 to 610.5 μM) in the human urine samples are consistent with its normal levels (normal level, 130–2100 μM in urine)48 and that by other developed sensors.43,48,49
| Sample | Spiked/μM | Measured/μM | Recovery/% | Found concentration/μM |
|---|---|---|---|---|
| a Note that, the urine sample A and sample B were directly diluted to 150 times by 50 mM Tris–HCl buffer solution of pH 7.4. | ||||
| Sample A | 0 | 2.68 ± 0.07 | 402.0 ± 9.8 | |
| 4 | 4.10 ± 0.18 | 102.5 ± 4.5 | ||
| 8 | 7.70 ± 0.05 | 96.3 ± 0.7 | ||
| Sample B | 0 | 4.07 ± 0.16 | 610.5 ± 23.7 | |
| 4 | 3.88 ± 0.11 | 97.0 ± 2.8 | ||
| 8 | 8.13 ± 0.11 | 101.6 ± 1.4 | ||
Conclusion
Cu2+ ions could be employed as the oxidase mimic to catalyse the oxidation reaction of OPD to “turn on” its PL. The catalytic reaction was investigated in detail for obtaining optimal experimental conditions. It was found that, when OPD was employed as the substrate, the oxidase-like activity of Cu2+ ions was substantially better than that of Cu/CuO nanoparticles. Due to the strong binding of His towards Cu2+ ions, a simple, highly selective and sensitive sensing platform was thus established for His. Furthermore, this sensing system was successfully employed for detecting His in the human urine samples with satisfactory His recovery. All in all, the PL sensing system for His established in this work has several intriguing enlightenments: (i) Cu2+ ions serving as the oxidase mimic is much more simple and stable, compared with its nanomaterials counterparts such as Cu/CuO nanoparticles, because the preparation procedures of nanomaterials are in general troublesome and most of nanomaterials are intrinsically instable during storage and preparation; (ii) our experimental results can afford much more useful insights for the nature of the observed oxidase/peroxidase-like activity of Cu/CuO nanoparticles.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants no. 21377124), and by the project of new faculty of Huaqiao University (Grant no. 15BS202).Notes and references
- Y. Kusakari, S. Nishikawa, S.-i. Ishiguro and M. Tamai, Curr. Eye Res., 1997, 16, 600–604 CrossRef CAS.
- M. Watanabe, M. E. Suliman, A. R. Qureshi, E. Garcia-Lopez, P. Bárány, O. Heimbürger, P. Stenvinkel and B. Lindholm, Am. J. Clin. Nutr., 2008, 87, 1860–1866 CAS.
- N. Tateda, K. Matsuhisa, K. Hasebe, N. Kitajima and T. Miura, J. Chromatogr. B: Biomed. Sci. Appl., 1998, 718, 235–241 CrossRef CAS.
- R. P. Deo, N. S. Lawrence and J. Wang, Analyst, 2004, 129, 1076–1081 RSC.
- F. Darain, C. Ban and Y.-B. Shim, Biosens. Bioelectron., 2004, 20, 857–863 CrossRef CAS PubMed.
- Z. Huang, J. Du, J. Zhang, X.-Q. Yu and L. Pu, Chem. Commun., 2012, 48, 3412–3414 RSC.
- P. G. Sutariya, A. Pandya, A. Lodha and S. K. Menon, Analyst, 2014, 139, 4794–4798 RSC.
- D. L. Ma, W. L. Wong, W. H. Chung, F. Y. Chan, P. K. So, T. S. Lai, Z. Y. Zhou, Y. C. Leung and K. Y. Wong, Angew. Chem., 2008, 120, 3795–3799 CrossRef PubMed.
- A. Buryak and K. Severin, Angew. Chem., 2004, 116, 4875–4878 CrossRef PubMed.
- Y. Zhou and J. Yoon, Chem. Soc. Rev., 2012, 41, 52–67 RSC.
- E. Oliveira, C. Santos, P. Poeta, J. L. Capelo and C. Lodeiro, Analyst, 2013, 138, 3642–3645 RSC.
- R. M. Kong, X. B. Zhang, Z. Chen, H. M. Meng, Z. L. Song, W. Tan, G. L. Shen and R. Q. Yu, Anal. Chem., 2011, 83, 7603–7607 CrossRef CAS PubMed.
- X. Lou, L. Zhang, J. Qin and Z. Li, Langmuir, 2010, 26, 1566–1569 CrossRef CAS PubMed.
- H. Li and Y. Bian, Nanotechnology, 2009, 20, 145502 CrossRef PubMed.
- J. Du, Z. Huang, X.-Q. Yu and L. Pu, Chem. Commun., 2013, 49, 5399–5401 RSC.
- Q.-H. You, A. W.-M. Lee, W.-H. Chan, X.-M. Zhu and K. C.-F. Leung, Chem. Commun., 2014, 50, 6207–6210 RSC.
- Y. He, X. Wang, J. Zhu, S. Zhong and G. Song, Analyst, 2012, 137, 4005–4009 RSC.
- H. Agarwalla, N. Taye, S. Ghorai, S. Chattopadhyay and A. Das, Chem. Commun., 2014, 50, 9899–9902 RSC.
- B. Wang, Y. Gao, H.-W. Li, Z.-P. Hu and Y. Wu, Org. Biomol. Chem., 2011, 9, 4032–4034 CAS.
- Y. Zhou, T. Zhou, M. Zhang and G. Shi, Analyst, 2014, 139, 3122–3126 RSC.
- J.-T. Hou, K. Li, K.-K. Yu, M.-Y. Wu and X.-Q. Yu, Org. Biomol. Chem., 2013, 11, 717–720 CAS.
- J. Sun, F. Yang, D. Zhao, C. Chen and X. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 6860–6866 CAS.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- Y. Lin, J. Ren and X. Qu, Acc. Chem. Res., 2014, 47, 1097–1105 CrossRef CAS PubMed.
- A. Singh, S. Patra, J.-A. Lee, K. H. Park and H. Yang, Biosens. Bioelectron., 2011, 26, 4798–4803 CrossRef CAS PubMed.
- K. S. McKeating, S. Sloan-Dennison, D. Graham and K. Faulds, Analyst, 2013, 138, 6347–6353 RSC.
- W. Shi, Q. Wang, Y. Long, Z. Cheng, S. Chen, H. Zheng and Y. Huang, Chem. Commun., 2011, 47, 6695–6697 RSC.
- G.-L. Wang, X.-F. Xu, L. Qiu, Y.-M. Dong, Z.-J. Li and C. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 6434–6442 CAS.
- Y. Song, K. Qu, C. Zhao, J. Ren and X. Qu, Adv. Mater., 2010, 22, 2206–2210 CrossRef CAS PubMed.
- A. Asati, S. Santra, C. Kaittanis, S. Nath and J. M. Perez, Angew. Chem., Int. Ed., 2009, 48, 2308–2312 CrossRef CAS PubMed.
- A. Asati, C. Kaittanis, S. Santra and J. M. Perez, Anal. Chem., 2011, 83, 2547–2553 CrossRef CAS PubMed.
- M. Liang, K. Fan, Y. Pan, H. Jiang, F. Wang, D. Yang, D. Lu, J. Feng, J. Zhao and L. Yang, Anal. Chem., 2012, 85, 308–312 CrossRef PubMed.
- X. Yang and E. Wang, Anal. Chem., 2011, 83, 5005–5011 CrossRef CAS PubMed.
- D. Zhao, Y. Zheng, M. Li, S. A. Baig, D. Wu and X. Xu, Ultrason. Sonochem., 2014, 21, 1714–1721 CrossRef CAS PubMed.
- W. Chen, J. Chen, Y.-B. Feng, L. Hong, Q.-Y. Chen, L.-F. Wu, X.-H. Lin and X.-H. Xia, Analyst, 2012, 137, 1706–1712 RSC.
- Z. Wang, X. Yang, J. Yang, Y. Jiang and N. He, Anal. Chim. Acta, 2015, 862, 53–63 CrossRef CAS PubMed.
- X.-Q. Wu, Y. Xu, Y.-L. Chen, H. Zhao, H.-J. Cui, J.-S. Shen and H.-W. Zhang, RSC Adv., 2014, 4, 64438–64442 RSC.
- W. Chen, L. Hong, A.-L. Liu, J.-Q. Liu, X.-H. Lin and X.-H. Xia, Talanta, 2012, 99, 643–648 CrossRef CAS PubMed.
- W. He, H. Jia, X. Li, Y. Lei, J. Li, H. Zhao, L. Mi, L. Zhang and Z. Zheng, Nanoscale, 2012, 4, 3501–3506 RSC.
- W. Chen, J. Chen, A. L. Liu, L. M. Wang, G. W. Li and X. H. Lin, ChemCatChem, 2011, 3, 1151–1154 CrossRef CAS PubMed.
- M. Meyer, A.-M. Albrecht-Gary, C. O. Dietrich-Buchecker and J.-P. Sauvage, Inorg. Chem., 1999, 38, 2279–2287 CrossRef CAS.
- J. Eersels, J. Mertens and J. Herscheid, J. Radioanal. Nucl. Chem., 2011, 288, 291–296 CrossRef CAS.
- W. Bian, F. Wang, Y. Wei, L. Wang, Q. Liu, W. Dong, S. Shuang and M. M. Choi, Anal. Chim. Acta, 2015, 856, 82–89 CrossRef CAS PubMed.
- X. Wang, Q. Miao, T. Song, Q. Yuan, J. Gao and G. Liang, Analyst, 2014, 139, 3360–3364 RSC.
- X. Huang, Y. Lin, J. Chen, Y. Chen, Y. Li and W. Gao, New J. Chem., 2015 10.1039/c5nj01819f.
- T. P. Kruck and B. Sarkar, Can. J. Chem., 1973, 51, 3563–3571 CrossRef CAS.
- R. Leberman and B. Rabin, Trans. Faraday Soc., 1959, 55, 1660–1670 RSC.
- P. M. Kovach and M. Meyerhoff, Anal. Chem., 1982, 54, 217–220 CrossRef CAS.
- P. Wu and X.-P. Yan, Biosens. Bioelectron., 2010, 26, 485–490 CrossRef CAS PubMed.
- S. Wadud, M. M. Or-Rashid and R. Onodera, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2002, 767, 369–374 CrossRef CAS.
- J. Meng, W. Zhang, C.-X. Cao, L.-Y. Fan, J. Wu and Q.-L. Wang, Analyst, 2010, 135, 1592–1599 RSC.
- M. K. Amini, S. Shahrokhian and S. Tangestaninejad, Anal. Chem., 1999, 71, 2502–2505 CrossRef CAS PubMed.
- M. Hosseini, M. R. Ganjali, Z. Vaezi, B. Arabsorkhi, M. Dadmehr, F. Faridbod and P. Norouzi, Sens. Actuators, B, 2015, 210, 349–354 CrossRef CAS PubMed.
- Y. He, X. Wang, J. Zhu, S. Zhong and G. Song, Analyst, 2012, 137, 4005–4009 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S10. See DOI: 10.1039/c5ra17900a |
| This journal is © The Royal Society of Chemistry 2015 |