Identification of features associated with plant cell wall recalcitrance to pretreatment by alkaline hydrogen peroxide in diverse bioenergy feedstocks using glycome profiling†
Muyang
Li
ab,
Sivakumar
Pattathil
cd,
Michael G.
Hahn
cde and
David B.
Hodge
*abfg
aDepartment of Biosystems and Agriculture Engineering, Michigan State University, East Lansing, MI 48824, USA. E-mail: hodgeda@egr.msu.edu
bGreat Lakes Bioenergy Research Center (GLBRC), Michigan State University, USA
cComplex Carbohydrate Research Center, The University of Georgia, 315 Riverbend Rd., Athens, Georgia 30602, USA
dBioEnergy Science Center (BESC), Oak Ridge National Laboratory, Oak Ridge, TN 37831, USA
eDepartment of Plant Biology, University of Georgia, Athens, GA 30602, USA
fDepartment of Chemical Engineering and Materials Science, Michigan State University, East Lansing, MI 48824, USA
gDivision of Sustainable Process Engineering, Luleå University of Technology, Luleå, Sweden
First published on 26th March 2014
Abstract
A woody dicot (hybrid poplar), an herbaceous dicot (goldenrod), and a graminaceous monocot (corn stover) were subjected to alkaline hydrogen peroxide (AHP) pretreatment and subsequent enzymatic hydrolysis in order to assess how taxonomically and structurally diverse biomass feedstocks respond to a mild alkaline oxidative pretreatment and how differing features of the cell wall matrix contribute to its recalcitrance. Using glycome profiling, we determined changes in the extractability of non-cellulosic glucans following pretreatment by screening extracts of the pretreated walls with a panel of 155 cell wall glycan-directed monoclonal antibodies to determine differences in the abundance and distribution of non-cellulosic glycan epitopes in these extracts and assess pretreatment-induced changes in the structural integrity of the cell wall. Two taxonomically-dependent outcomes of pretreatment were identified that both improved the subsequent enzymatic hydrolysis yields but differed in their impacts on cell wall structural integrity. Specifically, it was revealed that goldenrod walls exhibited decreases in all classes of alkali-extractable glycans indicating their solubilization during pretreatment, which was accompanied by an improvement in the subsequent extractability of the remaining cell wall glycans. The corn stover walls did not show the same decreases in glycan abundance in extracts following pretreatment, but rather mild increases in all classes of cell wall glycans, indicating overall weaker associations between cell wall polymers and improved extractability. The hybrid poplar walls were relatively unaffected by pretreatment in terms of composition, enzymatic hydrolysis, and the extractability of cell wall glycans due presumably to their higher lignin content and denser vascular structure.
Introduction
The lignified cell walls of vascular plants are the result of nearly half a billion years of evolution to resist biological degradation while serving the structural and physiological needs of the plant.1–4 It is believed that the majority of terrestrial carbon in the biosphere is sequestered within plant cell walls,5 yet this vast resource of reduced carbon is used primarily by humans for its existing structural value (as fiber/paper and as a structural/building material), as a fuel for combustion, or as ruminant forage rather than for the value contained in its existing chemical constituents. This is due to the recalcitrance of the cell wall to deconstruction by chemical and biological treatments and is set by features that are both structural and chemical that cut across the length scales at the molecular, macromolecular, and cellular levels.6,7The plant cell wall matrix is a complex network of cellulose and other matrix polysaccharides including hemicelluloses and pectin, lignin, and structural proteins and presents an obvious barrier to water and cellulolytic enzyme penetration, in particular due to lignin's capacity to set cell wall hydrophobicity and porosity. However, underpinning this lignin barrier is a network of non-cellulosic, cell wall matrix polysaccharides providing structure and organization. The major classes of matrix polysaccharides include the xylans (GAXs and GXs), glucomannans (GMs), xyloglucans (XyGs), mixed-linkage glucans (MLGs), pectins, and cell wall protein glycosylations.8–10 The abundance, composition, and substitution patterns of these glycans vary temporally during plant growth and cell wall expansion, spatially within cell walls and between plant tissues, and taxonomically across diverse plants. In dicots, XyGs and GAXs are the predominant non-cellulosic glycans in the primary cell walls with GXs the predominant hemicellulose in the secondary cell walls.11 In grasses, GAXs have been found to occupy a significant fraction of the interstitial space between cellulose microfibrils in the primary cell walls in addition to mixed-linkage glucans (MLGs) and grass-specific XyGs and GMs.2,12 Pectic polysaccharides are complex and can comprise up to 30% of the primary cell walls of dicots, but significantly less in grasses,13 where some of their functions are thought to be performed by other glycans including MLGs and GAXs.2 Structural proteins may comprise up to 10% of the cell wall in some plant tissues and can be significantly glycosylated, for example with arabinogalactans (AGs).8
Matrix polysaccharide content, diversity, interactions, and distribution play a role in recalcitrance by setting the accessibility of cellulose to cellulolytic enzymes and defining the porosity of the cell wall.13–15 This network of macromolecules is built up from a combination of physical entanglement of structural polymers as well as non-covalent and covalent cross-links between macromolecules.16 These non-covalent interactions are important and form the principle mechanism of association between cell wall glycans.15 Taken together, this complex composite structure presents a challenge for characterization and a number of approaches have been developed recently with a focus on relating structural features of the cell wall to its recalcitrance as reviewed recently by Foston et al.17
Immunological methods using glycan-directed mAbs are widely used tools to investigate plant cell wall structure.18,19 Besides the cell wall composition and structure, mAbs can be used for qualitative and quantitative detection of carbohydrate epitopes in sequential extracts of plant cell walls. This has been performed in order to characterize pretreatments using one approach that blotted polysaccharides from three increasingly severe cell wall extracts (CDTA, NaOH, cadoxen) onto a microarray, which was then probed with mAbs and CBMs20 in order to identify changes in content and extractability of xylans, XyGs, and MLGs epitopes in hydrothermal pretreated wheat straw.21 Recently, Pattathil et al. assembled a collection of glycan-directed mAbs22 shown in ESI Table S1† and an ELISA-based screen was used to categorize these mAbs with respect to binding affinity for structurally diverse plant cell wall glycans.22,23 They described a method, glycome profiling, that uses these mAbs to screen sequential extracts of cell walls in order to evaluate the structural, accessibility, and extractability changes of cell wall glycans in hybrid poplar during dilute acid pretreatments24 and to compare differences in the structural features underpinning cellulose digestibility in switchgrass and hybrid poplar.25
Chemical pretreatments are one route to overcoming cell wall recalcitrance26 and can be coupled to an enzymatic depolymerization of cell wall polysaccharides whereby the sugar monomers generated can be subsequently biologically converted to fuels and chemicals, providing a path forward for developing a bio-based fuels and chemicals industry that is renewable and petroleum-displacing. Ultimately this recalcitrance to cell wall deconstruction lies in the challenge for cellulolytic enzyme infiltration into the cell wall which can be seen as a combination of cell wall porosity, hydrophobicity (or water penetration/swelling), and glycan accessibility. Alkaline hydrogen peroxide (AHP) pretreatment is comparable to alkaline hydrogen peroxide pulp bleaching stages in the paper industry with the difference that higher pH and higher hydrogen peroxide loadings are employed to affect a mild delignification rather than brightening as the outcome.27,28 AHP pretreatment has several potential advantages compared to other pretreatment processes including minimal loss of polysaccharides,29 operation at low temperature and pressure, minimal formation of inhibitors for fermentation,30 and potentially high enzymatic digestibilities in grasses. In particular, we have recently been able to achieve ethanol titers greater than 50 g L−1 from undetoxified hydrolysates of 12.5% (w/w) H2O2 loading AHP-pretreated corn stover and switchgrass including complete glucose and xylose utilization using Saccharomyces cerevisiae strains metabolically engineered and evolved for xylose fermentation.30
It has been relatively well-established that hydrothermal pretreatments such as dilute acid31 or liquid hot water32 overcome recalcitrance through thermal effects by melting and redistributing lignin and catalyzing xylan hydrolysis and solubilization. This effect on xylan removal has been validated using similar characterization approaches for quantifying glycan extractability and epitope abundance.21,24 As AHP pretreatment is mechanistically different from acidic hydrothermal pretreatments and targets lignin solubilization at low temperature while preserving carbohydrates considerably, glycome profiling should be able to provide new insight into matrix polysaccharide-specific contributions to cell wall recalcitrance.
Specifically, in this study we investigate the response of cell wall glycans of diverse plants including hybrid poplar (woody dicot), goldenrod (herbaceous dicot), and corn stover (monocot grass) to AHP pretreatment with increasing H2O2 loadings. The cell wall response to pretreatment was characterized by compositional changes and overall mass loss of the cell wall, glucan and xylan enzymatic yields using a commercial enzyme cocktail, and glycome profiling of the sequential glycan extracts of the untreated and AHP-pretreated cell walls at 12.5% (w/w) H2O2 loading on biomass. Using this information, we draw conclusions about the structural changes associated with AHP pretreatment and additionally are able to gain insights into the role that differences in plant cell wall architecture have on cell wall recalcitrance.
Experimental
1. Pretreatment
Biomass consisted of a commercial hybrid corn stover (Pioneer Hi-Bred 36H56) provided through the Great Lakes Bioenergy Research Center (GLBRC), debarked hybrid poplar (Populus nigra var. charkoviensis x caudina cv. NE-19) grown at the University of Wisconsin Arlington Agricultural Research Station and provided through the GLBRC, and goldenrod (Solidago canadensis) collected locally in East Lansing, MI and obtained from Dr Jonathan Walton (MSU, Plant Biology). Biomass was initially milled with a Wiley MiniMill (Thomas Scientific) to pass a 2 mm screen and air-dried to ∼5% moisture. The milled biomass was subjected to alkaline hydrogen peroxide (AHP) pretreatment using H2O2 loadings of either 12.5%, 25%, 50% (g H2O2 per g biomass). These were performed in shake flasks with a 100 g total mass and 2% (w/v) solids concentration for 24 h at 30 °C with orbital shaking at 170 rpm and periodic pH adjustment to 11.5. After pretreatments, the liquid in the samples were removed by vacuum filtration (#113 Whatman filter paper) and the slurry was washed several times with deionized water to remove solubles and air-dried at room temperature. The mass yield during pretreatment was quantified as the ratio between the mass (dry basis) after pretreatment and the initial mass before pretreatment.2. Composition analysis, enzymatic hydrolysis and digestibility determination
The pretreated biomass was subjected to a two-stage acid hydrolysis according to NREL composition analysis33 to determine neutral polysaccharide content, Klason lignin, and ash with the difference that Aminex HPX-87H (Bio-Rad, Hercules, CA) column was used to quantify the glucan, xylan + galactan + mannan, arabinan, as well as acetate content. The total uronic acids were assayed enzymatically (K-Uronic, Megazyme, Wicklow, Ireland). The extractives content was determined by a sequential 3-step extraction including 70% ethanol, followed by 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) methanol and chloroform mixture, then acetone. Three extraction cycles for each solvent were performed and followed by centrifugation at 10
1 (v/v) methanol and chloroform mixture, then acetone. Three extraction cycles for each solvent were performed and followed by centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes for each cycle. Before the enzymatic hydrolysis, the pretreated biomass was ball-milled for 3 cycles using a QIAGEN TissueLyser II equipped with 25 mL Teflon jars and 20 mm diameter Teflon balls at 30 Hz for 2 minutes with liquid nitrogen cooling. The ball-milled samples were incubated with Cellic CTec2 (Novozymes, Bagsværd, Denmark) at a loading of 30 mg protein per g glucan at 50 °C, 10% (w/v) solid loading and 5 mL total volume in 0.05 M Na–citrate buffer pH 4.8, for 24 hours or 72 hours. The glucan and xylan yields (based on only glucan and xylan remaining after pretreatment) were determined by the HPLC analyzable glucose and xylose concentrations after hydrolysis divided by the original glucan and xylan contents in the pretreated samples.
000 rpm for 10 minutes for each cycle. Before the enzymatic hydrolysis, the pretreated biomass was ball-milled for 3 cycles using a QIAGEN TissueLyser II equipped with 25 mL Teflon jars and 20 mm diameter Teflon balls at 30 Hz for 2 minutes with liquid nitrogen cooling. The ball-milled samples were incubated with Cellic CTec2 (Novozymes, Bagsværd, Denmark) at a loading of 30 mg protein per g glucan at 50 °C, 10% (w/v) solid loading and 5 mL total volume in 0.05 M Na–citrate buffer pH 4.8, for 24 hours or 72 hours. The glucan and xylan yields (based on only glucan and xylan remaining after pretreatment) were determined by the HPLC analyzable glucose and xylose concentrations after hydrolysis divided by the original glucan and xylan contents in the pretreated samples.
3. Sequential extraction, glycome profiling
Sequential cell wall extractions and glycome profiling were carried out as described previously.22,24,34 The six extractions included (in order of extraction) oxalate to remove “loose” pectins, carbonate to remove “more tightly” bound pectins, 1 M KOH to remove “loose” hemicelluloses along with “tightly” bound pectins, 4 M KOH to remove “tightly” bound hemicelluloses along with “tightly” bound pectins, acid chlorite to oxidize and solubilize lignin and release lignin-embedded hemicelluloses, and a 4 M KOH post-chlorite treatment to remove additional lignin-bound polysaccharides. Plant glycan-directed mAbs were from laboratory stocks (CCRC, JIM and MAC series) at the Complex Carbohydrate Research Center (available through CarboSource Services; http://www.carbosource.net) or were obtained from BioSupplies (Australia) (BG1, LAMP). A description of the mAbs used in this study can be found in the ESI, Table S1,† which includes links to a web database, WallMabDB (http://www.wallmabdb.net) that provides detailed information about each antibody.Results and discussion
1. Changes in composition and mass loss
Three types of biomass representing three classes of plants that may offer promise as feedstocks for cellulosic biofuels were tested in this study. Corn stover represents the agricultural residue with the highest production and availability for bioenergy applications in the U.S.,35 while short-rotation hybrid poplar has agronomic, logistical, and environmental advantages as a feedstock.36 “Low-input high-diversity” bioenergy landscapes have many attractive sustainability attributes37 and comprise mixed communities of plants on marginal or degraded lands, and in this study we use goldenrod (Solidago canadensis) as a representative herbaceous dicot that may be present in these landscapes. The composition of the untreated biomass is presented in Table 1. Notable differences include the low content of pectic polysaccharides (as uronic acids) in the corn stover, which is 5-fold lower than the goldenrod and 2-fold lower than the hybrid poplar. The goldenrod has a substantially higher extractives content (23.5%) relative to the other two biomass types. Additionally, lignin content of the corn stover is nearly half that of the goldenrod and poplar.| Hybrid poplar (w/w%) | Goldenrod (w/w%) | Corn stover (w/w%) | |
|---|---|---|---|
| Glc | 48.3 ± 2.0 | 27.1 ± 0.8 | 38.4 ± 0.3 |
| Xyl + Man + Gal | 16.9 ± 0.6 | 13.6 ± 0.8 | 25.2 ± 0.2 |
| Ara | 1.33 ± 0.07 | 2.96 ± 0.03 | 3.92 ± 0.02 |
| Acetyl | 4.14 ± 0.09 | 2.59 ± 0.06 | 3.20 ± 0.10 |
| Total uronic acids | 1.88 ± 0.10 | 4.75 ± 0.03 | 0.88 ± 0.02 |
| Lignin (Klason) | 20.85 ± 1.1 | 19.92 ± 0.7 | 12.57 ± 1.2 |
| Extractives | 5.79 ± 0.22 | 23.5 ± 0.23 | 12.0 ± 0.74 |
| Ash | 1.95 ± 0.48 | 6.08 ± 1.02 | 3.03 ± 0.29 |
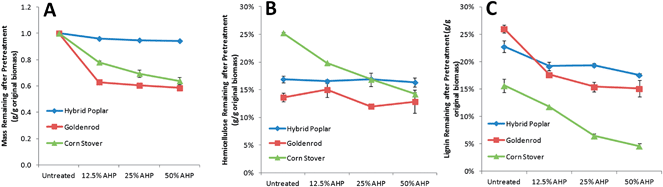
AHP pretreatment was performed at increasing H2O2 loadings (12.5, 25, and 50% w/w on biomass) which would be significantly higher than would be economically practicable industrially. The reason for these high loadings was to compare and analyze how the cell walls from phylogenetically diverse plants differ in their susceptibility to low temperature mild oxidative delignification and hemicellulose extraction and as a screen for overall differences in enzymatic hydrolyzability. The total cell wall mass loss (excluding extractives), xylan loss, and lignin loss for the three biomass types following pretreatment with increasing H2O2 loadings show distinct responses between the biomass types (Fig. 1). For poplar, minimal material was solubilized with pretreatment (<1% by mass), while up to 20% and 25% of the mass the cell walls of the goldenrod and corn stover, respectively, was solubilized by pretreatment at the higher H2O2 loadings. The mass of corn stover decreased continuously with increasing H2O2 loading, while the sample mass of poplar and goldenrod decreased abruptly with the mildest treatment (12.5% H2O2). For individual cell wall components, AHP pretreatments resulted in minimal changes in glucan for all biomass types representing preservation of cellulose (data not shown) which is consistent with our previous findings.27,29 while the xylan content decreased only for the corn stover (Fig. 1B). For goldenrod, the Klason lignin content was only slightly reduced by AHP pretreatment and did not change significantly by increasing H2O2 loading. The simultaneous removal of xylan and Klason lignin in corn stover was increased by increasing H2O2 loading. This high extractability of lignin is a well-known property of grass cell walls38 and alkali-only extraction of lignin and xylans in grasses is known to be significantly higher in grasses than in woody dicots.25,39 Besides alkali solubility, cleavage of ester and ether cross-links between xylan and lignin or lignin and lignin mediated by ferulate12,40 in grasses are thought to be an important target of AHP pretreatment29 and likely contribute to these outcomes.
2. Enzymatic hydrolysis yields of poplar, goldenrod, and corn stover
Fig. 2 shows the enzymatic hydrolysis yields of glucose for poplar, goldenrod, and corn stover subjected to increasing H2O2 loadings for 24 and 72 h hydrolysis times using a commercial cellulase (Cellic CTec2) with no xylanase supplementation. These results represent screening only and were not focused on optimizing enzyme cocktail or loading. As such, improved glucose (and xylose) yields at lower enzyme loadings would be observed if xylanase and pectinase were used. These results show that the glucose yields were increased with increasing H2O2 loading for the three types of biomass. Similar to the results for compositional changes (Fig. 1), the yield changes with pretreatment are significantly different between the three classes of plants tested. For poplar, the glucose yields were significantly lower than goldenrod and corn stover with the highest glucose yields approaching 40%. For goldenrod, the glucose yields “saturate” at approximately 70% at the mildest pretreatment condition. For corn stover, the glucose yield approaches 100% with increased H2O2 loading and it is known that corn stover as well as many other cereal stovers are often considerably more digestible than many undomesticated grasses.29,40 Gould41 determined that diverse graminaceous monocots responded considerably better to AHP pretreatment at 100% (w/w) H2O2 loadings than herbaceous dicots/forbs (including goldenrod) and that the 7 grasses tested had on average more than double the improvement in digestibility of the 11 forbs tested following AHP pretreatment, although the goldenrod showed the largest improvement in digestibility of the dicots tested and was among the highest in terms of absolute glucose release per gram of biomass.3. Glycome profiling of poplar, goldenrod, and corn stover
Glycome profiling (GP) provides quantitative information on both how pretreatment impacts the strength of association between cell wall glycans and other cell wall matrix polymers and how pretreatment impacts cell wall glycan composition. For GP, the samples were subjected to increasingly severe extractions to sequentially remove cell wall polymer, followed by quantification of recovered materials in each extraction step, and probing of the binding strength for the diverse array of mAbs covering a range of non-cellulosic cell wall polysaccharide epitopes.22,24,34 It should be noted that the GP approach does not yield information on small glycan molecules (e.g. oligomeric glycans and monosaccharides) as only larger cell wall glycans are able to effectively adsorb to the ELISA plates.22 For the sequential extractions, the strength of association between individual glycans and other cell wall matrix polymers may be hypothesized to be mechanistically due to differences in: (1) the strength of non-covalent association or physical entanglement between polymers or “encrustation” within lignin, (2) location within the cell wall (surface vs. interior), and (3) tissue type (e.g. lignified parenchyma and sclerenchyma versus low lignin pith tissues). The role of lignin in setting the alkali-extractability of cell wall glycans was recently demonstrated by Pattathil et al.,42 whereby it was identified that alfalfa lines with disrupted monolignol synthesis resulting in a low-lignin phenotype contained considerably more alkali-extractable glycans than the control line where much more of the cell wall glycans were extractable only after chlorite delignification.The GP results from this work are presented in Fig. 3 for the three biomass types for either no pretreatment or AHP pretreatment at 12.5% (w/w) H2O2 loading on the biomass. Substantial differences can be observed between biomass types and for biomass subjected to pretreatment as quantified for both mass partitioning of extracted glycans (top panel of Fig. 3) and differences in the abundance of glycan epitopes in these extracts (heat map in the lower part of Fig. 3). For the extract mass partitioning of glycans, it is clear that the two dicots have similar profiles for the four most severe extracts, i.e. the 1 M KOH, 4 M KOH, chlorite, and 4 M KOH PC. Goldenrod biomass shows a very high content of the oxalate- and carbonate-extractable polysaccharides relative to the other two types of biomass. This may be a consequence of the goldenrod having a higher proportion of pectic polysaccharide-rich leaves compared to the poplar which consists of only stem heartwood, and the corn stover, which as a graminaceous monocot is known to have low pectic polysaccharide content.43
 | ||
| Fig. 3 Glycome profiling of hybrid poplar, goldenrod and corn stover biomass samples before and after AHP pretreatment (12.5% H2O2 loading). Sequential cell wall extracts were made from untreated and pretreated biomass samples using increasingly harsh reagents as explained in materials and methods. Various extracts obtained were ELISA screened using 155 mAbs directed against most major plant cell wall glycans (see ESI Table S1†). The resulting binding response data are represented as heatmaps with yellow-red-black scale indicating the strength of the ELISA signal (yellow, red and dark-blue colors depict strong, medium, and no binding, respectively). The mAbs are grouped based on the cell wall glycans they recognize as depicted in the panel at right hand side of the figure. The actual amounts of materials extracted out at each extraction condition are depicted as bar graphs at the top of the heatmaps with color codes for the reagents used in the preparation of the extracts. | ||
A number of noteworthy differences are apparent in comparing the glycan epitope abundances within the six extracts for the three types of untreated biomass. One difference is that the XyG epitopes show significantly different partitioning between the three biomass types and the goldenrod is the only biomass exhibiting abundant XyG epitopes in the 1 M KOH extract. The xylan epitopes are more abundant in the corn stover extracts and more abundantly distributed into the two most severe extracts (chlorite and 4 M KOH PC) that might correspond to “lignin-bound” xylan. Pectic polysaccharides and AG domains show different partitioning behavior between the biomass types as well, with the cell wall extracts from goldenrod exhibiting the most abundant content of these classes of epitopes. Two intense MLG epitopes are present in all the corn stover cell wall extracts corresponding to antibodies LAMP2H12H7 and BG1 and the abundance of these epitopes increase in extracts (particularly in the 2 mildest and the chlorite delignification) following AHP pretreatment. Weak epitope binding for both of these antibodies is present in some of the poplar extracts and goldenrod extracts. These observations are consistent with the primary cell wall models proposed by Carpita,12 where for grasses (Type II cell wall), the MLGs and GXs have a more important structural role and may act in the capacity that XyGs and pectic polysaccharides act in dicots (Type I cell wall).
Pretreatment can conceivably alter the binding of mAbs to their glycan epitopes in the same extracts from different biomass samples in three ways by: (1) altering the cell wall structural integrity to shift the glycan epitope into a more easily (or more difficult) extractable category, (2) solubilizing the glycan epitope during pretreatment, and (3) structurally altering the glycan epitope, for example, through alkali-induced deacetylation or demethylesterification. These three modes of action are used to interpret the changes associated with pretreatment. To better visualize the effects of pretreatment on glycan extractability, the GP data are replotted in Fig. 4 and 5 after normalizing to epitope abundance per mass of original cell wall. In this representation, glycan epitopes that are increased in their abundance in individual extracts after pretreatment will appear to the left of the x–y line, while epitopes that are decreased will appear to the right. It can be observed that for the poplar, pretreatment has very little effect on the total extractable glycans in most of the six fractions. The apparent increase in the xylan epitopes in oxalate and carbonate extracts of poplar biomass suggest that the extractability of xylan by mild solvents may be enhanced by pretreatment (Fig. 4, subplot B). However, considering that the total content of carbohydrates in these extracts are unchanged and that the xylan-specific antibodies were developed for deacetylated, alkali-extracted xylans, this result likely indicates that easily extractable xylans were deacetylated by pretreatment and that the abundance of deacetylated xylan epitopes increase as a consequence. Another possibility is that a small fraction of the total xylan becomes more easily extractable following pretreatment. The slight differences in other epitopes in the 4 harshest extracts (Fig. 5, subplots A–C) suggests that AHP pretreatment of poplar biomass results in only minor alterations in the extractability of other major cell wall glycans indicating minimal impact on the structural and compositional organization of the cell wall in agreement with the results in Fig. 1 and 2. An exception is the xyloglucan epitopes in the 1 M KOH extract (Fig. 5A), which are slightly improved in their extractability by pretreatment.
 | ||
| Fig. 4 Comparison of the changes in the relative abundance of three glycan epitope categories due to AHP pretreatment from the oxalate (blue dots) and carbonate (red dots) extracts in glycome profiling, for poplar (A, B and C, for xyloglucans, xylans and pectic polysaccharides), goldenrod (D–F for xyloglucans, xylans and pectic polysaccharides) and corn stover (G–I for xyloglucans, xylans and pectic polysaccharides). Data are replotted from Fig. 3, but are normalized to represent mAb binding strength per mass of original AIR to make absolute values comparable between conditions and samples. The red dash line represents x = y and denotes the case where the abundance of these glycan epitopes was unchanged after AHP pretreatment in these extracts. Data points above and below the dash lines represent increased or decreased abundance of the glycan epitopes appearing in each extract, respectively. | ||
 | ||
| Fig. 5 Comparison of the changes in the relative abundance of three glycan epitope categories due to AHP pretreatment from each of the four harshest extracts including 1 M KOH (green triangles), 4 M KOH (blue diamonds), chlorite (red squares), and 4 M KOH PC (purple crosses) in glycome profiling. Data are normalized to represent relative abundance per mass original AIR. See Fig. 4 for additional details. | ||
For goldenrod, the GP results show that xylan epitopes and XyG epitopes in the oxalate extract increased considerably (Fig. 4, subplots D–F); potentially as a consequence of pretreatment-induced deacetylation or by pretreatment increasing the extractability in agreement with the increase in total glycan mass in the oxalate extract with pretreatment (Fig. 3). Unlike the poplar, the epitopes for HG backbones, RG-I/AG, and AG are decreased in both the oxalate and carbonate extracts, indicating that these pectic polysaccharides in goldenrod are likely solubilized during AHP pretreatment. In the four most severe extracts, a number of important trends are apparent (Fig. 5, subplots D–F). The first is that virtually all epitopes are decreased in the 1 M KOH extract (corresponding to the alkali-soluble glycans not closely associated with lignin) as a consequence of pretreatment, while slight increases in the abundance of epitopes in the 4 M KOH, chlorite, and 4 M KOH post-chlorite extracts (corresponding to lignin-embedded glycans) are observed after pretreatment. These results indicate that the likely target of AHP pretreatment for improving enzymatic hydrolysis in goldenrod is glycan (XyG and xylan) solubilization to improve cell wall accessibility to glycolytic enzymes and minor delignification which slightly improves the extractability of lignin-embedded polysaccharides.
The corn stover results show considerable differences in both their glycan extraction plots and antibody binding profiles relative to the hybrid poplar and goldenrod (Fig. 3). From the glycan mass extraction profile at the top of the panel, it can be observed that, unlike goldenrod, the amounts of extractable glycans in the three most severe extracts were significantly altered by pretreatment. One noticeable alteration is that the glycan epitopes in the 4 M KOH post-chlorite extract were shifted into the chlorite extracts after AHP pretreatment. This consequence of the pretreatment changing the glycan extractability profile was not shown for dilute acid pretreated hardwood.24 This result supports the identified changes in composition shown in Fig. 1, and together with the data in Fig. 2, make clear that alteration of the non-cellulosic glycan extractability directly impacts the glucose hydrolysis yield in the subsequent enzymatic treatment. In the two mildest extracts from corn stover, the XyG and xylan epitopes were increased, which could be a consequence of improving extractability or likely due to deacetylation of these glycans by pretreatment (Fig. 4, subplots G and H). Unlike goldenrod and poplar, the epitopes for pectic polysaccharides were increased in the two mildest extracts, possibly indicating differences in the structural roles of pectic polysaccharides between monocots and dicots.12 Additionally, the results for changes in epitope abundance as a result of pretreatment for the four harshest extracts were considerably different for the corn stover than for the goldenrod. Compared to goldenrod, where all glycan epitopes were decreased by pretreatment in the 1 M KOH extract but slightly increased in the lignin-associated extracts (Fig. 5, subplots D–F), the corn stover glycan epitopes showed minimal change or slight increases across all four extracts except in the 4 M KOH post-chlorite extract, in which the lignin-embedded glycan epitopes are decreased by pretreatment (Fig. 5, subplots G–I). These reductions of corn stover glycans in the harshest extracts are likely a consequence of these epitopes being already removed by the earlier less harsh extractions. Increases in the 2 MLG epitopes were observed with pretreatment for corn stover (Fig. 3) for all extracts except the 4 M KOH post chlorite treatment.
These differing responses to AHP pretreatment between monocots and dicots have important implications for structural features of the cell wall contributing to recalcitrance as well as the mechanism or target of pretreatment. The composition and structure of the cell wall are obviously important and many properties of the cell wall impacting recalcitrance have been described in the literature including cell wall hydrophobicity,44 porosity,45,46 xylan content,47 lignin content, cross-linking, and higher order structure.48 Jung et al.1 noted that lignified secondary cell walls were the primary obstacle hindering ruminant digestibility in dicots with stem secondary xylem (i.e. woody biomass) being the most recalcitrant, while increasing lignification in grasses hinders, but does not completely inhibit digestion. The current work identified that AHP pretreatment has a relatively minor impact on the hybrid poplar composition, hydrolysis yields, and glycan extractability profiles.
Substantial work has been devoted to understanding the cell wall properties contributing to ruminant digestibility of grasses and properties including ferulate content, total lignin content, syringyl–guaicyl ratio of lignin monomers, and degree of arabinosylation of xylans have all been linked to differences in hydrolysis yields.1,48–50 Pretreatments may impact any of these afore-mentioned cell wall properties to improve cell wall digestibility. DeMartini et al.25 found that treatment of switchgrass with alkali alone to solubilize xylan (and lignin) from the cell wall was sufficient to result in glucan enzymatic hydrolysis yields approaching the theoretical maximum, while for hybrid poplar, chlorite delignification was necessary to improve enzymatic hydrolysis significantly past alkali-only pretreatment. This is consistent with models for grass cell walls that include alkali-labile ferulate ester cross-links between cell wall polymers as an important structural feature controlling lignin integration into cell walls.40 Our previous work identified that lignin and ferulate removal by AHP pretreatment are important predictors of digestibility in diverse grasses.29 We have previously shown that AHP pretreatment results in the destruction of β–O–4 bonds in grasses29 and, for example, the content of free phenolics in grass lignins may enable improved alkali solubilization or potentially participate in the initiation of β–O–4 scission reactions.
Re-engineering plant cell walls for improved bioconversion outcomes is currently the subject of substantial research interest,51 and the findings of this work and the literature suggest strategies for tailoring bioenergy feedstock phenotypes to an alkaline-oxidative pretreatment process. Specifically, low initial lignin content and/or the capacity of the pretreatment to effectively remove lignin are recognized as important contributors to high enzymatic hydrolysis yields. As such, engineered plant phenotypes that would optimally couple to an alkaline-oxidative pretreatment might include decreased lignin content (without impacting plant fitness) and increasing alkali-labile bonds in lignin for example through the introduction of ester cross-links52 or increasing the β–O–4 content through increasing the S/G ratio.53
Conclusions
Untreated and AHP-pretreated biomass from phylogenetically diverse plants were compared to understand fundamental features impacting cell wall recalcitrance. We found that enzymatic hydrolysis yields, cell wall biopolymer and total mass solubilization, cell wall glycan extractabilities, and glycan epitope abundances in these extracts differed significantly in their response to AHP pretreatment for a woody dicot (hybrid poplar), an herbaceous dicot (goldenrod), and a graminaceous monocot. Using glycome profiling, we identified different mechanisms for how AHP pretreatment overcomes cell wall recalcitrance in goldenrod versus corn stover, while it was relatively ineffective on poplar. For corn stover, mild alkaline-oxidative pretreatment resulted in slight delignification and presumably disruption of cell wall polymer cross-linking. This had the consequence of disrupting the structural integrity of the cell wall which was manifested through improved extractability of important structural glycans including xylans, MLGs, and XyGs and presumably allowed for improved accessibility for glycolytic enzymes into the cell wall during hydrolysis. Goldenrod was found to respond differently in the extractability profiles where all classes of glycan epitopes exhibited considerable decreases in the 1 M KOH extracts following pretreatment rather than an increase as was observed for corn stover. Besides these differences, it was revealed that the pectic polysaccharides (HG, RG-I, and AG) were not only significantly more abundant in goldenrod than in corn stover, but were solubilized by pretreatment as indicated by their decrease following pretreatment in the three mildest extracts for goldenrod. For corn stover, the pectic polysaccharides as well as the significantly more abundant MLGs showed mild increases in extractability following pretreatment indicating “loosening” from the cell wall rather than solubilization. These results call attention to the important role that differences in cell wall structure (e.g. MLGs in graminaceous monocots and pectic polysaccharides in herbaceous dicots) and organization (e.g. ester cross-linking) play in establishing cell wall recalcitrance to deconstruction by pretreatment and enzymatic hydrolysis. Thus, pretreatment conditions that are feedstock-specific are likely to be more effective than general approaches, and future work in breeding and engineering plants with cell walls designed for specific deconstruction approaches should make use of positive synergistic interactions between specific pretreatments and particular cell wall features.Abbreviations
| AG | Arabinogalactan |
| AHP | Alkaline hydrogen peroxide |
| AIR | Alcohol insoluble residue |
| AX | Arabinoxylan |
| CBM | Carbohydrate binding module |
| CCRC | Complex Carbohydrate Research Center |
| CDTA | (1,2-Cyclohexylenedinitrilo)tetraacetic acid |
| CS | Corn stover |
| ELISA | Enzyme-linked immunosorbent assay |
| GAX | Glucuronoarabinoxylan |
| GM | Glucomannan |
| GP | Glycome profiling |
| GX | Glucuronoxylan |
| HGA | Homogalacturonic acid |
| HPLC | High pressure liquid chromatography |
| mAb | Monoclonal antibody |
| MLG | Mixed-linkage glucan |
| RG-I | Rhamnoglucuronan-I |
| SG | Switchgrass |
| XyG | Xyloglucan. |
Acknowledgements
This work was funded by the DOE Great Lakes Bioenergy Research Center (DOE BER Office of Science DE-FC02-07ER64494) and the BioEnergy Science Center administered by Oak Ridge National Laboratory, and funded by a grant (DOE BER Office of Science DE-AC05-00OR22725). The generation of the CCRC series of plant cell wall glycan-directed monoclonal antibodies used in this work was supported by the NSF Plant Genome Program (DBI-0421683 and IOS-0923992). Muyang Li was supported in part by a grant from NSF (NSF CBET 1336622).References
- H.-J. G. Jung, D. A. Samac and G. Sarath, Plant Sci., 2012, 185–186, 65–77 CrossRef CAS PubMed.
- M. C. McCann and N. C. Carpita, Curr. Opin. Plant Biol., 2008, 11, 314–320 CrossRef CAS PubMed.
- P. Sarkar, E. Bosneaga and M. Auer, J. Exp. Bot., 2009, 60, 3615–3635 CrossRef CAS PubMed.
- Z. A. Popper, G. Michel, C. Hervé, D. S. Domozych, W. G. T. Willats, M. G. Tuohy, B. Kloareg and D. B. Stengel, Annu. Rev. Plant Biol., 2011, 62, 567–590 CrossRef CAS PubMed.
- H. J. Gilbert, Plant Physiol., 2010, 153, 444–455 CrossRef CAS PubMed.
- T. Jeoh, C. I. Ishizawa, M. F. Davis, M. E. Himmel, W. S. Adney and D. K. Johnson, Biotechnol. Bioeng., 2007, 98, 112–122 CrossRef CAS PubMed.
- S.-Y. Ding, Y.-S. Liu, Y. Zeng, M. E. Himmel, J. O. Baker and E. A. Bayer, Science, 2012, 338, 1055–1060 CrossRef CAS PubMed.
- M. Josè-Estanyol and P. Puigdomènech, Plant Physiol. Biochem., 2000, 38, 97–108 CrossRef.
- A. Voragen, G.-J. Coenen, R. Verhoef and H. Schols, Struct. Chem., 2009, 20, 263–275 CrossRef CAS.
- A. Ebringerová, Macromol. Symp., 2005, 232, 1–12 CrossRef.
- P. J. Harris and B. A. Stone, in Biomass Recalcitrance, Blackwell Publishing Ltd., 2009, pp. 61–93 Search PubMed.
- N. C. Carpita, Annu. Rev. Plant Physiol. Plant Mol. Biol., 1996, 47, 445–476 CrossRef CAS PubMed.
- W. G. T. Willats, L. McCartney, W. Mackie and J. P. Knox, Plant Mol. Biol., 2001, 47, 9–27 CrossRef CAS.
- C. Somerville, S. Bauer, G. Brininstool, M. Facette, T. Hamann, J. Milne, E. Osborne, A. Paredez, S. Persson, T. Raab, S. Vorwerk and H. Youngs, Science, 2004, 306, 2206–2211 CrossRef CAS PubMed.
- D. J. Cosgrove, Nat. Rev. Mol. Cell Biol., 2005, 6, 850–861 CrossRef CAS PubMed.
- D. J. Cosgrove, Plant Physiol. Biochem., 2000, 38, 109–124 CrossRef CAS.
- M. Foston and A. Ragauskas, Ind. Biotechnol., 2012, 8, 191–208 CrossRef CAS.
- W. G. T. Willats, C. G. Steele-King, L. McCartney, C. Orfila, S. E. Marcus and J. P. Knox, Plant Physiol. Biochem., 2000, 38, 27–36 CrossRef CAS.
- J. P. Knox, in International Review of Cytology, ed. W. J. Kwang, Academic Press, 1997, vol. 171, pp. 79–120 Search PubMed.
- I. Moller, I. Sørensen, A. J. Bernal, C. Blaukopf, K. Lee, J. Øbro, F. Pettolino, A. Roberts, J. D. Mikkelsen, J. P. Knox, A. Bacic and W. G. T. Willats, Plant J., 2007, 50, 1118–1128 CrossRef CAS PubMed.
- A. Alonso-Simón, J. B. Kristensen, J. Øbro, C. Felby, W. G. T. Willats and H. Jørgensen, Biotechnol. Bioeng., 2010, 105, 509–514 CrossRef PubMed.
- S. Pattathil, U. Avci, D. Baldwin, A. G. Swennes, J. A. McGill, Z. Popper, T. Bootten, A. Albert, R. H. Davis, C. Chennareddy, R. Dong, B. O'Shea, R. Rossi, C. Leoff, G. Freshour, R. Narra, M. O'Neil, W. S. York and M. G. Hahn, Plant Physiol., 2010, 153, 514–525 CrossRef CAS PubMed.
- S. Pattathil, U. Avci, J. S. Miller and M. G. Hahn, in Biomass Conversion, 2012, vol. 908, pp. 61–72 Search PubMed.
- J. D. DeMartini, S. Pattathil, U. Avci, K. Szekalski, K. Mazumder, M. G. Hahn and C. E. Wyman, Energy Environ. Sci., 2011, 4, 4332–4339 CAS.
- J. DeMartini, S. Pattathil, J. S. Miller, H. Li, M. G. Hahn and C. E. Wyman, Energy Environ. Sci., 2013, 6, 898–909 CAS.
- N. Mosier, R. Hendrickson, N. Ho, M. Sedlak and M. R. Ladisch, Bioresour. Technol., 2005, 96, 1986–1993 CrossRef CAS PubMed.
- G. Banerjee, S. Car, T. Liu, D. L. Williams, S. L. Meza, J. D. Walton and D. B. Hodge, Biotechnol. Bioeng., 2012, 109, 922–931 CrossRef CAS PubMed.
- G. Banerjee, S. Car, J. Scott-Craig, D. Hodge and J. Walton, Biotechnol. Biofuels, 2011, 4, 16 CrossRef CAS PubMed.
- M. Li, C. Foster, S. Kelkar, Y. Pu, D. Holmes, A. Ragauskas, C. Saffron and D. Hodge, Biotechnol. Biofuels, 2012, 5, 38 CrossRef CAS PubMed.
- T. K. Sato, T. Liu, L. S. Parreiras, D. L. Williams, D. J. Wohlbach, B. D. Bice, I. M. Ong, R. J. Breuer, L. Qin, D. Busalacchi, S. Deshpande, C. Daum, A. P. Gasch and D. B. Hodge, Appl. Environ. Microbiol., 2014, 80, 540–554 CrossRef PubMed.
- B. S. Donohoe, S. R. Decker, M. P. Tucker, M. E. Himmel and T. B. Vinzant, Biotechnol. Bioeng., 2008, 101, 913–925 CrossRef CAS PubMed.
- M. A. T. Hansen, J. B. Kristensen, C. Felby and H. Jørgensen, Bioresour. Technol., 2011, 102, 2804–2811 CrossRef CAS PubMed.
- A. Sluiter, B. Hames, R. Ruiz, C. Scarlata, J. Sluiter and D. Templeton, NREL Analytical Procedure, National Renewable Energy Laboratory, Golden, CO, 2004 Search PubMed.
- S. Pattathil, U. Avci, J. S. Miller and M. G. Hahn, in Biomass Conversion: Methods and Protocols, ed. M. E. Himmel, Humana Press, 2012, vol. 908, pp. 61–72 Search PubMed.
- U.S. Department of Energy, U.S. Billion-Ton Update: Biomass Supply for a Bioenergy and Bioproducts Industry, 2011; R. D. Perlack and B. J. Stokes (Leads), ORNL/TM-2011/224, Oak Ridge National Laboratory, Oak Ridge, TN, p. 227.
- V. H. Dale, K. L. Kline, L. L. Wright, R. D. Perlack, M. Downing and R. L. Graham, Ecol. Appl., 2011, 21, 1039–1054 CrossRef PubMed.
- D. Tilman, J. Hill and C. Lehman, Science, 2006, 314, 1598–1600 CrossRef CAS PubMed.
- C. Lapierre, D. Jouin and B. Monties, Phytochemistry, 1989, 28, 1401–1403 CrossRef CAS.
- R. J. Stoklosa and D. B. Hodge, Ind. Eng. Chem. Res., 2012, 51, 11045–11053 CrossRef CAS.
- J. Ralph, S. Guillaumie, J. H. Grabber, C. Lapierre and Y. Barrière, C. R. Biol., 2004, 327, 467–479 CrossRef CAS PubMed.
- J. M. Gould, Biotechnol. Bioeng., 1985, 27, 893–896 CrossRef CAS PubMed.
- S. Pattathil, T. Saffold, L. Gallego-Giraldo, M. O'Neill, W. S. York, R. A. Dixon and M. G. Hahn, Ind. Biotechnol., 2012, 8, 217–221 CrossRef CAS.
- M. C. Jarvis, W. Forsyth and H. J. Duncan, Plant Physiol., 1988, 88, 309–314 CrossRef CAS PubMed.
- S. Nakagame, R. P. Chandra, J. F. Kadla and J. N. Saddler, Biotechnol. Bioeng., 2011, 108, 538–548 CrossRef CAS PubMed.
- W. R. Grous, A. O. Converse and H. E. Grethlein, Enzyme Microb. Technol., 1986, 8, 274–280 CrossRef CAS.
- D. N. Thompson, H.-C. Chen and H. E. Grethlein, Bioresour. Technol., 1992, 39, 155–163 CrossRef CAS.
- J. D. McMillan, in Enzymatic Conversion of Biomass for Fuels Production, American Chemical Society, 1994, vol. 566, ch. 15, pp. 292–324 Search PubMed.
- Y. Zhang, T. Culhaoglu, B. Pollet, C. Melin, D. Denoue, Y. Barrière, S. p. Baumberger and V. R. Méchin, J. Agric. Food Chem., 2011, 59, 10129–10135 CrossRef CAS PubMed.
- M. A. Bernard Vailhé, G. J. Provan, L. Scobbie, A. Chesson, M. P. Maillot, A. Cornu and J. M. Besle, J. Agric. Food Chem., 2000, 48, 618–623 CrossRef PubMed.
- A. Mohagheghi, K. Evans, Y.-C. Chou and M. Zhang, Appl. Biochem. Biotechnol., 2002, 98–100, 885–898 CrossRef CAS.
- R. G. Ong, S. P. Chundawat, D. B. Hodge, S. Keskar, and B. E. Dale, in Plants and BioEnergy, Springer, 2014, ch. 14, pp. 231–253 Search PubMed.
- J. H. Grabber, J. Ralph and R. D. Hatfield, J. Agric. Food Chem., 1998, 46, 2609–2614 CrossRef CAS.
- J. J. Stewart, T. Akiyama, C. Chapple, J. Ralph and S. D. Mansfield, Plant Physiol., 2009, 150, 621–635 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra00824c |
| This journal is © The Royal Society of Chemistry 2014 |