Assessing the properties of supercritical water in terms of structural dynamics and electronic polarization effects†
Abstract
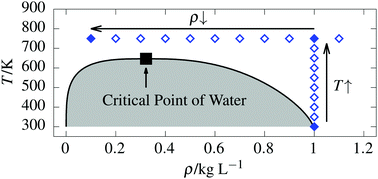
Supercritical water features fascinating physical properties which are fundamentally different compared to ambient liquid water. Importantly, it can gradually be compressed from gas-like to liquid-like densities while avoiding any thermodynamic phase transition. Although the interest in supercritical water has recently increased, many microscopic characteristics still remain unknown. Based on extensive ab initio molecular dynamics simulations using the RPBE-D3 density functional along a supercritical isotherm and the isochore from the ambient liquid into the supercritical phase, we provide a comprehensive picture of supercritical water regarding its structural, dynamical and electronic properties depending on the chosen thermodynamic state point. Our results do not only show that the effective molecular dipole moment of water can be gradually tuned as a function of density along an isotherm, but also that it correlates linearly with the number of H-bond neighbors all the way from liquid-like to gas-like densities which is shown to be caused by many-body electronic polarization and charge transfer effects. Remarkably, these polarization and charge transfer effects are still present even at rather low gas-like densities. Regarding the dynamics, the H-bond lifetime is largely decreased in supercritical water and follows an Arrhenius-type behavior as a function of temperature, while it is essentially unaffected by the extreme density changes along the supercritical isotherm. In contrast, the self-diffusion coefficient dramatically varies as a function of density along the isotherm, while it scales essentially linearly as a function of temperature along the isochore.
- This article is part of the themed collections: Frontiers in Molecular Simulation of Solvated Ions, Molecules and Interfaces and 2019 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...