Controlled α-mono- and α,α-di-halogenation of alkyl sulfones using reagent–solvent halogen bonding†
Abstract
The direct and selective α-mono-bromination of alkyl sulfones was achieved through base-mediated electrophilic halogenation. The appropriate combination of solvent and electrophilic bromine source was found to be critical to control the nature of the products formed, where reagent–solvent halogen bonding is proposed to control the selectivity via alteration of the effective size of the electrophilic bromine source. Conversely, the α,α-di-brominated sulfones were selectively obtained in good yields following polyhalogenation followed by selective de-halogenation during workup. Both procedures can be applied on gram scale, and the mono-halogenation was successfully extended to the fully selective α-chlorination, α-iodination and α-fluorination of alkyl sulfones.
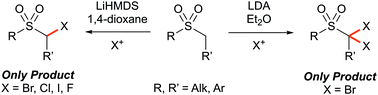
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions


 Please wait while we load your content...
Please wait while we load your content...