Mechanisms of Mg carbonates precipitation and implications for CO2 capture and utilization/storage
Abstract
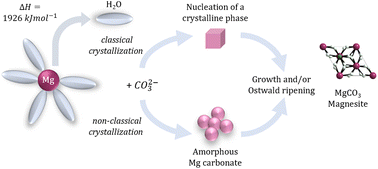
The mechanisms involved in the natural formations of dolomite (CaMg(CO3)2) and magnesite (MgCO3) have endured as challenging research questions over centuries, being yet a matter under investigation in multiple fields. From a geochemical perspective, it is still unknown why there are recent natural formations of dolomite and magnesite at ambient conditions, and yet most available synthetic routes for precipitating these minerals require high temperatures and/or pressures. The core scientific gap is that even though dolomite and magnesite are the most thermodynamically stable phases among the respective polymorphs/intermediates, their formation is controlled by slow kinetics and their syntheses at ambient conditions remain a challenge. Research findings lead to possible explanations based on the chemical and thermodynamical properties of the system: (i) the high energy barrier for dehydrating the Mg2+·6H2O cations hinders the carbonation of Mg precursors, inducing a preferential formation of the hydrated magnesium carbonates polymorphs, (ii) the intrinsic structural/spatial barrier of the CO32− groups in the rhombohedral arrangement of dolomite and magnesite shifts the system towards the formation of the respective polymorphs. However, further studies are still needed to enable a clearer understanding of the phenomenon. Recently, the research question at hand gained broader significance due to the relevance of Mg carbonates for routes of carbon capture and utilization/storage, which has been seen as one of the most promising solutions for such processes. The main socio-economic motivations behind such interest on these carbon mineralization methods are the high availability of Mg precursors (from natural sources to industrial waste-streams), the long-term geological storage of CO2 as magnesite, the possibility of utilizing the carbonate products in construction materials applications, and the relevance of the routes for climate mitigation actions. Therefore, understanding the mechanisms and kinetics of Mg carbonates precipitation is of fundamental importance for many fields, ranging from geology to necessary environmental actions. This review focuses on gathering the main information concerning the geochemical and chemical advances on the dynamics and mechanisms of Mg carbonates precipitation. It aims at providing a comprehensive summary of the developments from the fundamental sciences to the applications of Mg carbonates.
- This article is part of the themed collections: 2023 Inorganic Chemistry Frontiers Review-type Articles and Recent Open Access Articles in Frontiers Journals


 Please wait while we load your content...
Please wait while we load your content...