Accretion product formation in the self-reaction of ethene-derived hydroxy peroxy radicals†
Abstract
In this study we revisit one of the simplest 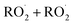 reactions: the self-reaction of the ethene-derived hydroxyperoxy radical formed via sequential addition of ˙OH and O2 to ethene. Previous studies of this reaction suggested that the branching to ‘accretion products’, compounds containing the carbon backbone of both reactants, was minimal. Here, CF3O− GC-CIMS is used to quantify the yields of ethylene glycol, glycolaldehyde, a hydroxy hydroperoxide produced from
reactions: the self-reaction of the ethene-derived hydroxyperoxy radical formed via sequential addition of ˙OH and O2 to ethene. Previous studies of this reaction suggested that the branching to ‘accretion products’, compounds containing the carbon backbone of both reactants, was minimal. Here, CF3O− GC-CIMS is used to quantify the yields of ethylene glycol, glycolaldehyde, a hydroxy hydroperoxide produced from 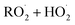 , and a C4O4H10 accretion product. These experiments were performed in an environmental chamber at 993 hPa and 294 K. We provide evidence that the accretion product is likely dihydroxy diethyl peroxide (HOC2H4OOC2H4OH
, and a C4O4H10 accretion product. These experiments were performed in an environmental chamber at 993 hPa and 294 K. We provide evidence that the accretion product is likely dihydroxy diethyl peroxide (HOC2H4OOC2H4OH ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ROOR) and forms in the gas-phase with a branching fraction of 23 ± 5%. We suggest a new channel in the
ROOR) and forms in the gas-phase with a branching fraction of 23 ± 5%. We suggest a new channel in the 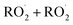 chemistry leading directly to the formation of
chemistry leading directly to the formation of 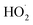 (together with glycolaldehyde and an alkoxy radical). Finally, by varying the ratio of the formation rate of
(together with glycolaldehyde and an alkoxy radical). Finally, by varying the ratio of the formation rate of 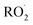 and
and 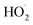 in our chamber, we constrain the ratio of the rate coefficient for the reaction of
in our chamber, we constrain the ratio of the rate coefficient for the reaction of 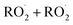 to that of
to that of 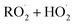 and find that this ratio is 0.22 ± 0.07, consistent with previous flash photolysis studies.
and find that this ratio is 0.22 ± 0.07, consistent with previous flash photolysis studies.

- This article is part of the themed collections: Outstanding Papers 2023 – Environmental Science: Atmospheres and Ab initio reaction mechanisms


 Please wait while we load your content...
Please wait while we load your content...