Complementary and selective oxidation of hydrocarbon derivatives by two cytochrome P450 enzymes of the same family†
Abstract
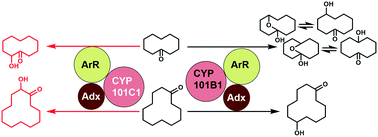
The cytochrome P450 enzymes CYP101B1 and CYP101C1, which are from the bacterium Novosphingobium aromaticivorans DSM12444, can hydroxylate norisoprenoids with high activity and selectivity. With the goal of expanding and establishing their substrate range with a view to developing applications, the oxidation of a selection of cyclic alkanes, ketones and alcohols was investigated. Cycloalkanes were oxidised, but both enzymes displayed moderate binding affinity and low levels of productive activity. We improved the binding and activity of these substrates with CYP101B1 by making the active site more hydrophobic by switching a histidine residue to a phenylalanine (H85F). The presence of a ketone moiety in the cycloalkane skeleton significantly improved the oxidation activity with both enzymes. CYP101C1 preferably catalysed the oxidation of cycloalkanones at the C-2 position whereas CYP101B1 oxidised these substrates with higher productivity and at positions remote from the carbonyl group. This demonstrates that the binding orientation of the cyclic ketones in the active site of each enzyme must be different. Linear ketones were also oxidised by both enzymes but with lower activity and selectivity. Cyclic substrates with an ester directing group were more efficiently oxidised by CYP101B1 than CYP101C1. Both enzymes catalysed oxidation of these esters with high regioselectively on the ring system remote from the ester directing group. CYP101C1 selectively oxidised certain terpenoid ester substrates, such as α-terpinyl and citronellyl acetate more effectively than CYP101B1. Overall, we establish that the high selectivity and activity of these enzymes could provide new biocatalytic routes to important fine chemicals.


 Please wait while we load your content...
Please wait while we load your content...