Unveiling the mechanism of improved capacity retention in Pmn21 Li2FeSiO4 cathode by cobalt substitution†
Abstract
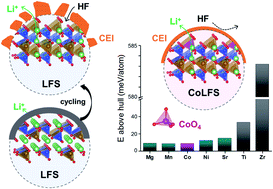
Li2FeSiO4 (LFS) is a sustainable Li-ion cathode material composed of earth-abundant elements with potentially high energy density but suffers from limited reversible storage capacity (less than one Li), low intrinsic conductivities, and cycling instability. In search of deeper understanding of the structural chemistry of LFS towards overcoming some of these challenges, the viability of Fe-site doping by Co and other dopants in Pmn21 LFS is investigated using both first-principles calculations and experimental testing. Computational results suggest that the formation of Li2Fe1−xCoxSiO4 is energetically favorable, predictions confirmed by successful hydrothermal synthesis. Substitution of Co in LFS is revealed to have a dual effect; firstly catalyzing faster electrochemically induced phase transformation to the stable inverse-Pmn21 phase; and secondly enabling the formation of a low-resistant, uniform and stable fluorine-rich cathode-electrolyte interphase (CEI) layer that inhibits detrimental reactions between the cathode and electrolyte. As a result, Co-substituted LFS displays enhanced reversibility with 95% capacity retention after 50 cycles as compared to 80% of the undoped LFS.


 Please wait while we load your content...
Please wait while we load your content...