Structural peculiarities of keto-carotenoids in water-soluble proteins revealed by simulation of linear absorption†
Abstract
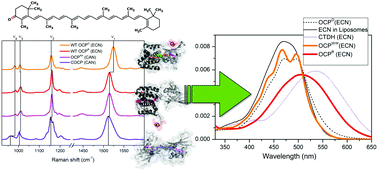
To prevent irreversible damage caused by an excess of incident light, the photosynthetic machinery of many cyanobacteria uniquely utilizes the water-soluble orange carotenoid protein (OCP) containing a single keto-carotenoid molecule. This molecule is non-covalently embedded into the two OCP domains which are interconnected by a flexible linker. The phenomenon of OCP photoactivation, causing significant changes in carotenoid absorption in the orange and red form of OCP, is currently being thoroughly studied. Numerous additional spectral forms of natural and synthetic OCP-like proteins have been unearthed. The optical properties of carotenoids are strongly determined by the interaction of their electronic states with vibrational modes, the surrounding protein matrix, and the solvent. In this work, the effects of the pigment–protein interaction and vibrational relaxation in OCP were studied by computational simulation of linear absorption. Taking into account Raman spectroscopy data and applying the multimode Brownian oscillator model as well as the cumulant expansion technique, we have calculated a set of characteristic microparameters sufficient to demarcate different carotenoid states in OCP forms, using the model carotenoids spheroidene and spheroidenone in methanol/acetone solution as benchmarks. The most crucial microparameters, which determine the effect of solvent and protein environment, are the Huang–Rhys factors and the frequencies of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C–C stretching modes, the low-frequency mode and the FWHM due to inhomogeneous line broadening. Considering the difference of linear absorption between spheroidene and spheroidenone, which remarkably resembles the photoinduced changes of OCP absorption, and applying quantum chemical calculations, we discuss structural and functional determinants of carotenoid binding proteins.
C and C–C stretching modes, the low-frequency mode and the FWHM due to inhomogeneous line broadening. Considering the difference of linear absorption between spheroidene and spheroidenone, which remarkably resembles the photoinduced changes of OCP absorption, and applying quantum chemical calculations, we discuss structural and functional determinants of carotenoid binding proteins.


 Please wait while we load your content...
Please wait while we load your content...