Solvent- and catalyst-free mechanochemical synthesis of alkali metal monohydrides†
Abstract
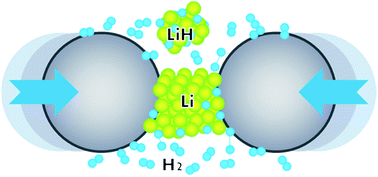
Alkali metal monohydrides, AH (A = Li–Cs) have been synthesized in quantitative yields at room temperature by reactive milling of alkali metals in the presence of hydrogen gas at 200 bar or less. The mechanochemical approach reported here eliminates problems associated with the malleability of alkali metals — especially Li, Na, and K — and promotes effective solid–gas reactions, ensuring their completion. This is achieved by incorporating a certain volume fraction of the corresponding hydride powder as a process control agent, which allows continuous and efficient milling primarily by coating the surface of metal particles, effectively blocking cold welding. Formation of high-purity crystalline monohydrides has been confirmed by powder X-ray diffraction, solid-state NMR spectroscopy, and volumetric analyses of reactively desorbed H2 from as-milled samples. The proposed synthesis method is scalable and particularly effective for extremely air-sensitive materials, such as alkali and alkaline earth metal hydrides. The technique may also be favorable for production in continuous reactors operating at room temperature, thereby reducing the total processing time, energy consumption and, hence, the cost of production of these hydrides or their derivatives and composites.

 Please wait while we load your content...
Please wait while we load your content...