Enabling stable aqueous Zn metal anodes using scandium acetate electrolyte additives†
Chun
Chen
 abc,
Liansheng
Li
abc,
Liansheng
Li
 ab,
Zuxin
Long
ab,
Zuxin
Long
 ab,
Edison Huixiang
Ang
ab,
Edison Huixiang
Ang
 *d and
Qinghua
Liang
*d and
Qinghua
Liang
 *abc
*abc
aKey Laboratory of Rare Earth, Chinese Academy of Sciences, Ganzhou, Jiangxi 341000, China. E-mail: qhliang@gia.cas.cn
bGanjiang Innovation Academy, Chinese Academy of Sciences, Ganzhou, Jiangxi 341000, China
cSchool of Rare Earth, University of Science and Technology of China, Hefei, Anhui 230026, China
dNatural Sciences and Science Education, National Institute of Education, Nanyang Technological University, Singapore 637616, Singapore. E-mail: edison.ang@nie.edu.sg
First published on 11th June 2024
Abstract
The potential of zinc metal anodes (ZMAs) for use in emerging aqueous electrochemical devices like rechargeable zinc-ion batteries and hybrid capacitors is substantial, owing to their high theoretical capacity, low redox potential, non-toxicity, abundant availability, and cost-effectiveness. However, the practical application of ZMAs faces limitations due to issues such as uncontrolled zinc dendrite growth and side reactions. In this study, we demonstrate that simultaneously incorporating scandium ions (Sc3+) and acetate anions (Ac−) as electrolyte additives into a common ZnSO4 solution significantly enhances the cycling stability and reversibility of ZMAs. Our findings reveal that the Ac− acts as a pH regulator, dynamically buffering the electrolyte pH to be around 4.3, effectively suppressing water-induced side reactions. Additionally, the synergistic effect of Sc3+ and Ac− (Sc3+/Ac−) facilitates the desolation process of Zn2+ and lowers the energy barrier for electrochemical Zn plating, resulting in uniform Zn plating without noticeable zinc dendrite growth. Consequently, Zn‖Zn symmetric cells utilizing the Sc3+/Ac− electrolyte additive exhibit an ultra-long lifespan exceeding 1000 hours at 2.0 mA cm−2 and 1.0 mA h cm−2. Moreover, the Zn‖Cu cell demonstrates a high average coulombic efficiency of 99.4% after 400 plating/stripping cycles at 1.0 mA cm−2 and 1.0 mA h cm−2. Notably, when paired with an activated carbon (AC) cathode, Zn‖AC hybrid capacitors maintain a high-specific capacity of 62 mA h g−1 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1.0 A g−1. The research outcomes indicate that Sc3+ in combination with Ac− are promising electrolyte additives for achieving highly stable aqueous ZMAs.
000 cycles at 1.0 A g−1. The research outcomes indicate that Sc3+ in combination with Ac− are promising electrolyte additives for achieving highly stable aqueous ZMAs.
1. Introduction
Zinc metal anodes (ZMAs) are poised to revolutionize various emerging aqueous electrochemical applications, spanning rechargeable batteries,1 hybrid capacitors,2 desalination devices,3 sensors,4 and electrochromic devices.5 Their allure stems from a multitude of attributes: a high theoretical capacity (820 mA h g−1 and 5855 mA h cm−3), low redox potential (−0.76 V vs. standard hydrogen electrode), non-toxicity, widespread availability, and cost-effectiveness.6 Despite these advantages, the practical implementation of ZMAs in aqueous electrolytes encounters significant challenges such as the hydrogen evolution reaction (HER), corrosion, by-product accumulation, and zinc dendrite formation, substantially affecting the reversibility of zinc plating and stripping, as well as cycle stability.7,8 Therefore, it is imperative to develop efficient and pragmatic strategies to tackle these issues associated with ZMAs.A range of effective strategies have been devised to tackle the above-mentioned interfacial challenges and bolster the stability and reversibility of ZMAs. These strategies encompass interface engineering,9–11 ZMA structure design,12,13 electrolyte optimization,14,15 and separator modification.16,17 Notably, electrolyte optimization, particularly through the incorporation of electrolyte additives, has emerged as a straightforward and efficient approach. This strategy offers distinct advantages, including simplicity in manufacturing, adaptability, and cost-effectiveness. A diverse array of electrolyte additives have been explored to optimize ZMA performance, spanning polymers, organic molecules, and ion compounds. Generally, electrolyte additives enhance ZMA reversibility in aqueous electrolytes by modulating the electric double layer (EDL),18,19 facilitating the formation of a solid electrolyte interface (SEI),20 or altering the solvation structure of Zn2+.21 Some organic electrolyte additives, such as trimethyl phosphate,22 gamma-butyrolactone,23 sulfolane,24 benzyl trimethylammonium,25etc., functioned by displacing water in the Zn2+ solvation sheath, restricting free water activity, and reshaping the hydrogen-bonding network, thereby mitigating the water-involved HER and diminishing zinc dendrite formation.26 Some organic electrolyte additives facilitate the in situ construction of polymeric-rich SEI layers with abundant functional groups and exceptional hydrophilicity, enabling dendrite-free zinc deposition by modulating Zn nucleation.27 Some ion compounds electrolyte additives, including thioacetamide,28 tetraethyl ammonium chloride,29 monosodium glutamate,30 glucose,31 trifluoro borate,32etc., have also been extensively investigated for their ability to modulate the solvation structure of Zn2+. In addition, certain ionic electrolyte additives, such as alkylammonium salt,33 methylammonium acetate,34 Zn(H2PO4)2,35 and glycine,36 can promote uniform Zn plating by facilitating the in situ formation of SEI layers. Despite notable advancements in electrolyte additive strategies, the cycle stability and reversibility of ZMAs remain heavily reliant on the specific electrolyte additive chosen. Moreover, considering the cost and toxicity issues associated with organic additives, there is a critical need to explore the development of economically feasible, environmentally sustainable, and efficacious additives to further enhance the electrochemical performance of ZMAs in aqueous electrolytes. Our recent study has revealed that the inclusion of a trace amount of Sc3+ additive (1.0 mol%) enhances the coulombic efficiency (CE) and cycle stability of ZMAs in aqueous ZnSO4 electrolyte by reducing nucleation overpotential and improving kinetics for Zn plating/stripping.37 However, the presence of even 1.0 mol% Sc3+ leads to a more acidic environment (pH = 2.3), accelerating unwanted HER side reactions and contributing to the persistently inadequate stability of ZMAs.
Herein, we observed that the addition of acetate anions (Ac−) synergistically enhances the positive impact of Sc3+ additives, leading to long-term and highly reversible zinc plating/stripping of ZMAs. The hydrolysis reaction of Ac− ions within the standard ZnSO4 electrolyte dynamically regulates the concentration of H+, maintaining an optimal pH range (∼4.3). This adjustment effectively reduces the occurrence of the HER, corrosion and side reactions. Additionally, the synergistic effect of Sc3+ and Ac− lowers the energy barrier for Zn plating, thus modulating the plating behaviour of Zn2+ and achieving uniform and dense zinc plating. Consequently, the HER, side reactions and zinc dendrite growth are notably suppressed. As a result, after introducing a small amount of Sc3+ and Ac− additives into 2.0 M ZnSO4 (ZSO) electrolyte, the lifespan of Zn‖Zn symmetric cells demonstrates more than a ten-fold increase in cycle life compared to those employing ZSO electrolyte alone at 2.0 mA cm−2 and 1.0 mA h cm−2. Moreover, the Zn‖Cu asymmetric cell achieves a high average CE of 98.45% within 400 plating/stripping cycles. Notably, the Zn‖activated carbon (AC) hybrid capacitor exhibits stable cycling performance at a current density of 1.0 A g−1, maintaining a specific capacity of 62 mA h g−1 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
2. Results and discussion
2.1 Evaluation of electrolytes
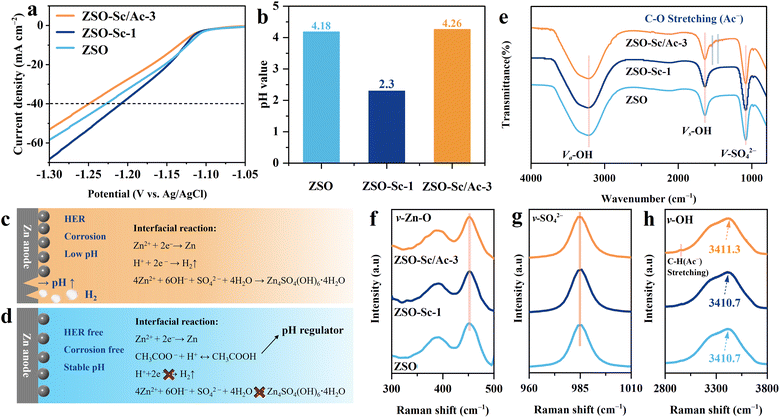
The commonly encountered challenge in the use of electrolyte additives lies in finding the balance between insufficient inhibition of Zn dendrite growth and interfacial side reactions with a small amount of additive, and the retardation of electrochemical reaction rates with an excessive amount. Our previous studies have established that the optimal concentration of Sc3+ additive in ZSO is 1.0 mol%.37 In this study, electrolytes containing ZSO with 1.0 mol% Sc3+ ions and varying concentrations of Ac− ions (1.0 mol%, 3.0 mol%, 5.0 mol%, and 10.0 mol%, labelled ZSO–Sc/Ac-1, ZSO–Sc/Ac-3, ZSO–Sc/Ac-5, and ZSO–Sc/Ac-10, respectively), were prepared to ascertain the optimal amount of Ac− ions. All the prepared electrolytes remain clear and transparent for over two months, indicating that they are highly stable (Fig. S1†).To assess the corrosion rate of ZMAs in various electrolytes, Tafel plots were initially obtained. The corrosion current densities were determined for Zn plates in the ZSO, ZSO–Sc/Ac-1, ZSO–Sc/Ac-3, ZSO–Sc/Ac-5, and ZSO–Sc/Ac-10 electrolytes (Fig. S2†), yielding values of 4.58, 4.15, 1.29, 1.81, and 2.37 mA cm−2, respectively. Notably, the optimal amount of Ac− additive exhibits a mitigating effect on the corrosion rate, whereas excessive Ac− shows a reduced inhibitory effect on ZMA corrosion.38 Particularly in the ZSO–Sc/Ac-3 electrolyte, the lowest corrosion current density was observed, indicating enhanced corrosion resistance of ZMAs. Furthermore, Zn‖Cu batteries were employed to assess the reversibility of Zn plating/stripping across different electrolytes. As the concentration of Ac− in the electrolyte rises from 1.0 mol% to 5.0 mol%, the CE of the corresponding Zn‖Cu cell increases from 95.07% to 97.73% (Fig. S3†), indicating an improved reversibility of Zn plating/stripping within the concentration range of 1.0 mol% to 5.0 mol%. However, with excessive Ac− concentration (≥10 mol%), the polarization voltage of the Zn‖Cu cell notably elevates during operation. This phenomenon may be attributed to the accumulation of “dead Zn” or by-products on the ZMA surface, leading to increased diffusion and reduction resistance of Zn2+.35 We subsequently conducted a comparison of the cycling stability of ZMAs in ZSO–Sc/Ac-3 and ZSO–Sc/Ac-5 electrolytes. The Zn‖Zn symmetric cell utilizing the ZSO–Sc/Ac-3 electrolyte exhibits an extended lifespan of up to 1000 hours at 2.0 mA cm−2 under 1.0 mA h cm−2, while the Zn‖Zn symmetric cell employing the ZSO–Sc/Ac-5 electrolyte only cycles for 200 hours (Fig. S4†). Likewise, the Zn‖Zn symmetric cell utilizing the ZSO–Sc/Ac-3 electrolyte also demonstrates a prolonged lifespan of up to 500 hours, whereas the Zn‖Zn battery with the ZSO–Sc/Ac-5 electrolyte cycles for only 100 hours at 5.0 mA cm−2 under 2.0 mA h cm−2. These experimental findings underscore that the ZMAs in ZSO electrolytes with 1.0 mol% Sc3+ and 3.0 mol% Ac− ions exhibit superior corrosion resistance, cycling stability, and reversibility. We also studied the effect of only 1.0 mol% Sc3+ or 3.0 mol% Ac− in ZSO electrolyte on the performances of ZMAs. The lifespan of Zn‖Zn cells assembled with ZSO–Sc-1 electrolyte or ZSO–Ac-3 electrolyte is 358 hours and 306 hours, respectively (Fig. S5†). The cycle life of Zn‖Zn cells with both ZSO–Sc-1 electrolyte and ZSO–Ac-3 electrolyte is much shorter than that of the Zn‖Zn cell with ZSO–Sc/Ac-3 electrolyte, indicating the synergistic effect of Sc3+ and Ac− additives.
We particularly select ZSO–Sc/Ac-3 electrolytes for further electrochemical testing. To assess the HER in various electrolytes, linear sweep voltammetry (LSV) was conducted. As depicted in Fig. 1a, to deliver a current density of 40 mA cm−2, the potential of the HER in the electrolyte with Sc3+ (−1.21 V vs. Ag/AgCl) is close to that of the ZSO electrolyte (−1.23 V vs. Ag/AgCl). This indicates a negligible inhibition effect on the HER owing to an increased H+ concentration caused by the hydrolysis of Sc3+ in the electrolyte. However, the potential increases from −1.21 to −1.25 V (vs. Ag/AgCl) at 40 mA cm−2 upon the further introduction of the Ac− additive, indicating the inhibited HER in the presence of Sc3+ and Ac−. All the results mentioned above are associated with the regulatory effect of Ac− on the concentration of H+ in the electrolyte. This can be illustrated by the changes in the pH values of various electrolytes (Fig. S6† and 1b). As mentioned earlier, corrosion and the HER intensely occur in the ZSO–Sc-1 electrolyte during AZB cycling due to a significantly decreased pH value (Fig. 1c). As hydrogen gas continues to evolve, the concentration of OH− at the ZMA interface increases. OH− reacts with Zn2+ and SO42− to form pits and the inactive side product Zn4SO4(OH)6·4H2O.39 The formation of side products increases the ZMA surface roughness and accelerates dendrite growth, consequently weakening the inhibitory effect of the Sc3+ additive on Zn dendrite growth. In sharp contrast, with the introduction of the Ac− additive, the intrinsic hydrolysis reaction of Ac− (Ac− + H+ ↔ HAc) dynamically regulates the pH of the bulk electrolyte to a stable value of 4.3, thereby significantly mitigating the HER, corrosion, and side reactions on ZMAs, while effectively amplifying the inhibitory effect of the Sc3+ additive on Zn dendrites (Fig. 1d).
To investigate the influence of Sc3+/Ac− additives on the solvation structure of Zn2+, attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR) and Raman spectroscopy were conducted. As depicted in Fig. 1e, the peaks originating from ν-Zn–O, ν-SO42–, and ν-O–H can be observed for ZSO, ZSO–Sc-1, and ZSO–Sc/Ac-3 electrolytes. Notably, there is no obvious difference among the ATR-FTIR spectra, suggesting that the Sc3+/Ac− additive does not significantly affect the Zn2+ solvation structure.40 Moreover, the Sc3+/Ac− additive does not induce any significant change in the Raman spectra of the ν-Zn–O, ν-SO42–, and ν-O–H peaks at 350–550, 926–998, and 3000–3800 cm−1, respectively, further indicating that trace Sc3+/Ac− additive does not significantly alter the hydration structure of Zn2+ or the water structure in the electrolyte (Fig. 1f–h).41,42 Furthermore, the contact angle was measured to assess the wettability of the electrolyte on Zn foil (Fig. S7†). With the Sc3+/Ac− additive, the contact angle on the Zn foil slightly decreases from 100.3° in the ZSO electrolyte to 98.4° in the ZSO–Sc/Ac-3 electrolyte. The negligible change indicates that the Sc3+/Ac− additive has a minor effect on electrolyte wettability.43
2.2 Electrochemical test and characterization of Zn plating/stripping processes
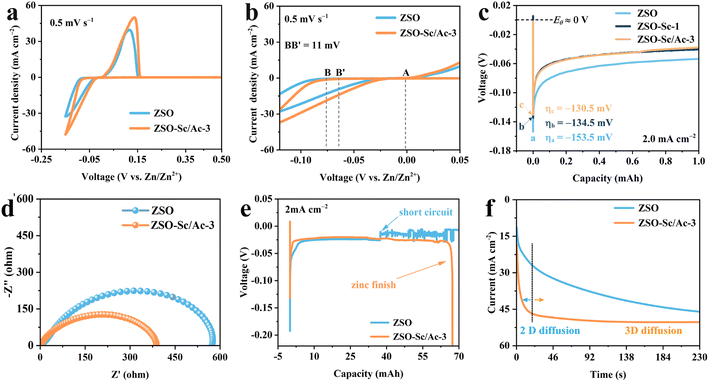
The influence of the Sc3+/Ac− additive on Zn plating and stripping processes was investigated through cyclic voltammetry (CV) tests. As depicted in Fig. 2a and b, the CV of the Zn‖Cu cell with ZSO–Sc/Ac-3 electrolyte displays higher peak current density and lower Zn nucleation overpotential compared to those with ZSO electrolyte, indicating enhanced Zn plating kinetics and a reduced nucleation barrier. Additionally, at a current density of 2.0 mA cm−2, Zn nucleation on Zn foil in ZSO–Sc/Ac-3 electrolyte exhibits a nucleation overpotential of 130.5 mV, lower than that in ZSO–Sc-1 (134.5 mV) and ZSO electrolytes (153.5 mV) (Fig. 2c). A similar decrease in Zn nucleation overpotential is observed when current densities change from 0.5 to 5.0 mA cm−2 (Fig. S8†), further underscoring the reduced nucleation barrier in ZSO–Sc/Ac-3 electrolyte. Electrochemical impedance spectroscopy (EIS) of Zn‖Zn symmetric cells reveals that the addition of the Sc3+/Ac− additive significantly reduces Zn2+ charge transfer resistance in the ZSO–Sc/Ac-3 electrolyte (Fig. 2d). Thus, the enhanced kinetics of Zn nucleation and reduced nucleation barrier in the ZSO–Sc/Ac-3 electrolyte are primarily attributed to the accelerated charge transfer process of Zn2+, leading to uniform Zn plating.44To further examine the Zn plating process, a constant current discharge test was conducted on Zn‖Zn cells using ZSO and ZSO–Sc/Ac-3 electrolytes at an applied current density of 2.0 mA cm−2. As illustrated in Fig. 2e, the Zn‖Zn symmetric cell assembled with ZSO electrolyte experiences a short circuit when the plating capacity reaches 32.4 mA h, attributed to Zn dendrite growth. Conversely, the Zn‖Zn symmetric cell with ZSO–Sc/Ac-3 electrolyte does not experience a short circuit until the counter Zn metal anode is entirely consumed, indicating that the Sc3+/Ac− additive promotes uniform Zn2+ distribution and smooth Zn plating. Similar outcomes were also obtained when the applied current density was 0.5 or 1.0 mA cm−2 (Fig. S9†). Furthermore, the regulated Zn2+ distribution and plating behaviour in ZSO–Sc/Ac-3 electrolyte are confirmed through chronoamperometry (CA) measurements (Fig. 2f). Following a brief 25 s test, the Zn‖Zn symmetric cell with ZSO–Sc/Ac-3 electrolyte exhibits stable current density for up to 230 s after applying an overpotential of −150 mV, indicating significant restriction of rampant 2D diffusion of Zn2+ responsible for dendrite formation.45
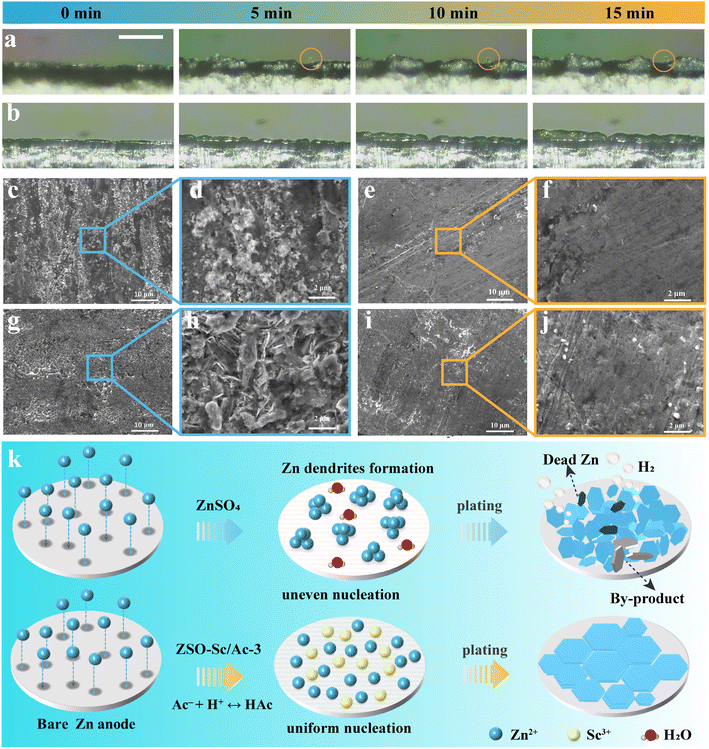
To delve deeper into the influence of the Sc3+/Ac− additive on Zn plating behaviour, we employed in situ optical microscopy to scrutinize the morphological changes during Zn plating in both ZSO and ZSO–Sc/Ac-3 electrolytes on Zn foil (Fig. 3a and b). In ZSO electrolytes, the tendency of Zn2+ to aggregate at the Zn protrusions, known as the “tip effect”, leads to localized plating at specific sites. Within 5 minutes of electroplating, noticeable Zn protrusions emerge on the surface, gradually aggregating into large dendritic crystals over the subsequent 10 minutes. This phenomenon is known to cause internal short circuits, decreased CE, and compromised battery performance over time. In contrast, in the ZSO–Sc/Ac-3 electrolyte, the Zn electrode displays a consistently dense and uniform plating morphology throughout the 15-minute electroplating process. This can be attributed to the enhanced nucleation kinetics, reduced nucleation barrier, and an electrostatic shielding effect of adsorbed Sc3+ provided by the ZSO–Sc/Ac-3 electrolyte, leading to a more uniform Zn plating process. Scanning electron microscopy (SEM) images of the plated Zn confirm these observations. At different current densities, the Zn electrode cycled in the ZSO electrolyte exhibits disordered dendrites and irregular plate-like by-products, disrupting electric/ion field distribution and accelerating battery degradation (Fig. 3c, d, g and h). Conversely, in the ZSO–Sc/Ac-3 electrolyte, the Zn electrode maintains a smooth, dendrite-free surface, indicating homogeneous ion distribution throughout plating (Fig. 3e, f, i and j). Furthermore, X-ray diffraction (XRD) analysis reveals unique diffraction peaks corresponding to the by-product Zn4SO4(OH)6·4H2O on the ZMA electrode in the ZSO electrolyte, confirming the accumulation of by-products (Fig. S10†). Conversely, the inclusion of the Sc3+/Ac− additive allows a more even distribution of Zn2+ across the ZMA surface, leading to a near elimination of the by-product peaks. This underscores the notable suppression of side reactions enabled by the ZSO–Sc/Ac-3 electrolyte.
Building upon the preceding analysis, a proposed operational mechanism illustrating the role of the Sc3+/Ac− additive in guiding the Zn plating process at the Zn metal anode/electrolyte interface is presented in Fig. 3k. In the ZSO electrolyte, the Zn metal anode experiences severe HER and corrosion, which are primarily induced by the presence of a water-rich interface and the high desolvation energy barrier of Zn(H2O)62+.46 Continuous hydrogen evolution leads to an elevated concentration of OH− at the interface, resulting in the formation of Zn4SO4(OH)6·xH2O by-products. The increased surface roughness due to the scattered corrosion areas disrupts the uniform distribution of the electrical field on the ZMA surface, culminating in an irregular dispersion of Zn2+ at the interface and subsequent dendrite formation. In contrast, in the ZSO–Sc/Ac-3 electrolyte, the Sc3+/Ac− additive can reduce the energy barrier for Zn plating and enhance Zn plating kinetics, promoting uniform Zn2+ flux and deposition. Simultaneously, Sc3+ tends to be absorbed on the Zn anode surface, forming an electrostatic shielding layer.47,48 This shielding layer helps mitigate the “tip effect”, facilitating a uniform Zn-plated layer. Moreover, the hydrolysis reaction of Ac− dynamically regulates the concentration of H+ at the Zn anode/electrolyte interface, maintaining the pH of the electrolyte at approximately 4.3, thereby inhibiting the HER and related side reactions. Consequently, the synergistic effect of Sc3+ and Ac− enables highly stable and reversible ZMAs.
2.3 Evaluation of Zn anode stability and reversibility using cell performances
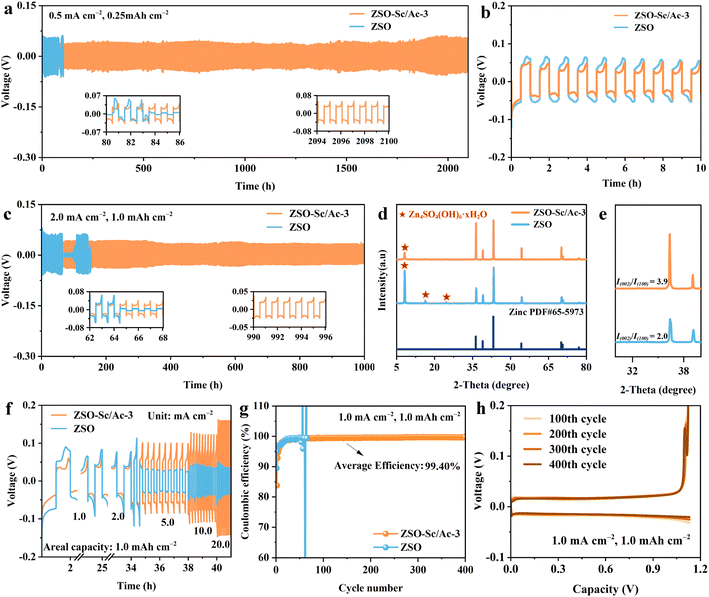
The electrochemical performance test was conducted to illustrate the improved stability and reversibility of the ZMAs facilitated by the inclusion of the Sc3+/Ac− additive. Initially, the cycling stability of the Zn metal anode was evaluated at 0.5 mA cm−2 and 0.25 mA h cm−2. As depicted in Fig. 4a, the Zn‖Zn symmetric cells utilizing ZSO–Sc/Ac-3 electrolyte demonstrate an extended lifespan exceeding 2000 hours, accompanied by reduced overpotential and voltage hysteresis of 74.3 mV. In contrast, the Zn‖Zn symmetric cell employing ZSO electrolyte exhibits a shorter lifetime of 103 hours and a higher voltage hysteresis of 115.9 mV (Fig. 4b). Similarly, at a current density of 2.0 mA cm−2 and a capacity of 1.0 mA h cm−2, the Zn‖Zn symmetric cell assembled with ZSO–Sc/Ac-3 electrolyte also achieves a prolonged cycling life of over 1000 hours, while the Zn‖Zn symmetric cell using ZSO electrolyte shows a shorter lifespan due to uneven Zn plating and side reactions (Fig. 4c). Moreover, even under higher current density and increased plating capacity conditions of 4.0 mA cm−2 and 2.0 mA h cm−2, the lifespan of the Zn‖Zn symmetric cell employing ZSO–Sc/Ac-3 electrolyte (700 hours) exceeds that of the Zn‖Zn cell using ZSO electrolyte (100 hours) by a significant factor of 7 (Fig. S11†). The SEM images and XRD patterns of the ZMAs after stripping/plating cycles in different electrolytes further validate the reasons for the failure of the symmetric cell and the beneficial role of the Sc3+/Ac− additive. In the ZSO electrolyte, the Zn metal anode exhibits severe corrosion with flaky by-products and rough cracking on its surface after 30 cycles, contrasting with the relatively smooth surface observed after 30 cycles in the ZSO–Sc/Ac-3 electrolyte (Fig. S12†). Furthermore, unlike the strong characteristic peaks of by-products observed on the surface of the Zn metal anode after 30 cycles in ZSO electrolyte, the intensity of the characteristic peaks of accumulated by-products on the Zn surface after 30 cycles in ZSO–Sc/Ac-3 electrolyte was comparatively lower, underscoring the inhibitory effect of the Sc3+/Ac− additive on by-product formation (Fig. 4d). Notably, the ratio of the intensity of the (002) crystal plane to the (100) crystal plane of electroplated Zn increased from 2.0 to 3.9 when using ZSO–Sc/Ac-3 electrolyte (Fig. 4e). This observed increase in I(002)/I(100) suggests that the Sc3+/Ac− additive promotes preferential growth along the (002) plane.49 Since Zn metal has a hexagonal close-packed structure, the (002) crystal plane is the densest stacking crystal plane.50 The lowest surface energy and the highest hydrogen evolution barrier of the (002) plane can effectively suppress side reactions at the electrode interface and Zn dendrite growth.51 Therefore, preferential deposition along the (002) plane helps produce a uniform and compact Zn plating layer. Based on the positive effect of the Sc3+/Ac− additive on the formation of Zn (002) crystal place, the Zn‖Zn symmetric cell utilizing ZSO–Sc/Ac-3 electrolyte demonstrates remarkable rate capability across various current densities with a fixed areal capacity of 1.0 mA h cm−2 throughout multiple cycling processes (Fig. 4f).To delve deeper into the impact of the Sc3+/Ac− additive on the reversibility of Zn plating/stripping on ZMAs, Zn‖Cu cells were assembled and evaluated. As depicted in Fig. 4g, the Zn‖Cu cell using the ZSO electrolyte exhibits significant fluctuations in CE after 66 cycles at a current density of 1.0 mA cm−2 and a capacity of 1.0 mA h cm−2. These fluctuations imply insufficient reversibility, likely due to water-induced side reactions and dendrite formation. The Zn‖Cu cell with ZSO–Sc-1.0 exhibits an average CE of 99.11% and a lifespan of 265 cycles. The Zn‖Cu cell assembled with ZSO–Ac-3 shows an average CE of 98.56% and a short life of 160 cycles (Fig. S13†). In contrast, the Zn‖Cu cell employing ZSO–Sc/Ac-3 electrolyte can endure 400 charge/discharge cycles with an average CE of 99.40%, indicating that the Sc3+/Ac− additive contributed to enhancing the reversibility of Zn plating/stripping (Fig. 4h). Notably, at 5.0 mA cm−2 and 2.0 mA h cm−2, Zn‖Cu cells with the Sc3+/Ac− additive also exhibit remarkable stability and reversibility, with an average CE of 99.44% in 200 cycles. In contrast, the Zn‖Cu cell without additives displays noticeable CE fluctuations, which are relatively lower at 99.19% (Fig. S14†). These findings further suggest that the Sc3+/Ac− additive plays a significant role in enhancing the stability and reversibility of Zn plating/stripping.
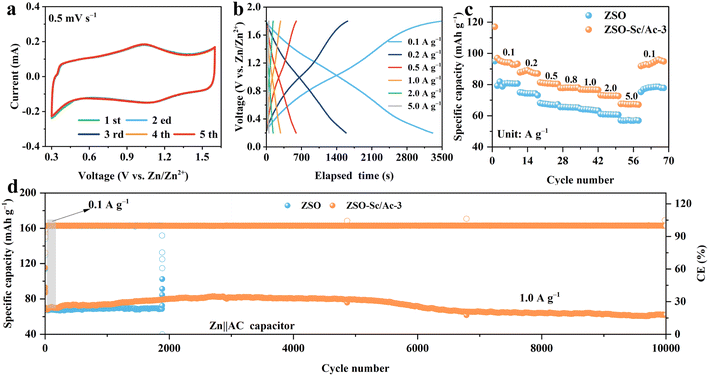
To further assess the efficacy of Sc3+/Ac− additives in a full battery, Zn ion hybrid capacitors (ZHCs) were constructed utilizing commercially available activated carbon (AC), followed by the evaluation of their electrochemical performance. As illustrated in Fig. 5a, the cyclic voltammetry (CV) curve of ZHCs remains stable under a scan rate of 0.5 mV s−1, exhibiting reversible oxidation/reduction peaks associated with the reversible ion adsorption/desorption on the AC cathode and Zn2+ plating/stripping on the ZMA.52Fig. 5b illustrates the constant current charge–discharge profiles of ZHCs across various current densities, ranging from 0.1 to 5.0 A g−1. The charge–discharge behaviour of ZHCs demonstrates a nearly symmetric pattern, characterized by a linear voltage change over time, indicative of a typical capacitive storage mechanism consistent with the CV curve. Moreover, as the current density escalates from 0.1 to 5.0 A g−1, the ZHC utilizing ZSO–Sc/Ac-3 electrolyte exhibits a heightened specific capacity compared to that with ZSO electrolyte (Fig. 5c). Despite a fifty-fold increase in current density, the former displays superior rate capability, retaining 71.4% of its capacitance (from 97 to 69 mA h g−1), while the ZHC with ZSO electrolytes shows diminished specific capacity and capacity retention (69.5%, from 81 to 56 mA h g−1). Furthermore, the ZHC employing ZSO electrolyte demonstrates capacity fluctuations and a noticeable decrease in CE after 1880 cycles at a current density of 1.0 A g−1, likely attributed to potential side reactions at the ZMA interface and dendrite formation (Fig. 5d). In contrast, the ZHC incorporating ZSO–Sc/Ac-3 electrolytes exhibits the capability to undergo continuous 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles while retaining a specific capacity of 62 mA h g−1, highlighting the promising practical applications of ZSO–Sc/Ac-3 electrolyte. Notably, a significant increase in capacity was observed in the ZHC using the ZSO–Sc/Ac-3 electrolyte. This improvement can be attributed to the ongoing activation of the porous carbon electrode during cycling, the inhibition of side reactions, and the enhanced stability and reversibility of the electrode due to the presence of the Sc3+/Ac− additive.53
000 cycles while retaining a specific capacity of 62 mA h g−1, highlighting the promising practical applications of ZSO–Sc/Ac-3 electrolyte. Notably, a significant increase in capacity was observed in the ZHC using the ZSO–Sc/Ac-3 electrolyte. This improvement can be attributed to the ongoing activation of the porous carbon electrode during cycling, the inhibition of side reactions, and the enhanced stability and reversibility of the electrode due to the presence of the Sc3+/Ac− additive.53
3. Conclusions
In conclusion, we have introduced the Sc3+/Ac− electrolyte additive into an aqueous ZnSO4 solution, significantly enhancing the stability and reversibility of ZMAs. Throughout the Zn plating process, Ac− ions act as pH regulators, maintaining the electrolyte pH at an optimal level ∼ 4.3, effectively suppressing the HER and associated side reactions. Moreover, the cooperative influence of Sc3+ and Ac− ions facilitates the desolation process of Zn2+ and lowers the energy barrier for zinc plating, promoting uniform deposition and leading to a dendrite-free ZMA. Consequently, the Zn‖Zn symmetric battery constructed with ZSO–Sc/Ac-3 electrolyte demonstrates remarkable endurance, operating for over 1000 hours at 1.0 mA cm−2 and 1.0 mA h cm−2. Additionally, the Zn‖AC hybrid capacitor assembled with ZSO–Sc/Ac-3 electrolyte exhibits exceptional cycling stability, retaining a specific capacity of 62 mA h g−1 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 1.0 A g−1. These findings provide valuable insights into the rational design of electrolytes for aqueous ZMA systems.
000 cycles at 1.0 A g−1. These findings provide valuable insights into the rational design of electrolytes for aqueous ZMA systems.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support from the Natural Science Foundation of Jiangxi Province (No. 20232ACB214001), the Research Projects of Ganjiang Innovation Academy (No. E355F003) of the Chinese Academy of Sciences, and the Research fund from Jiangxi Province Human Resources and Social Security Department and the Chinese Academy of Sciences.Notes and references
- J. Li, Z. Liu, S. Han, P. Zhou, B. Lu, J. Zhou, Z. Zeng, Z. Chen and J. Zhou, Nano-Micro Lett., 2023, 15, 237 CrossRef CAS PubMed.
- Y. Sun, B. Liu, L. Liu, J. Lang and J. Qiu, Small Struct., 2023, 4, 2200345 CrossRef CAS.
- Z. Cao, S. Hu, Q. Yang, J. Yu, Y. Pan, J. Zuo, H. Song, Z. Ye and S. Zhang, Chem. Eng. J., 2022, 450, 138126 CrossRef CAS.
- K. Jiang, X. Wen, Y. Deng, Z. Zhou and Q. Weng, SusMat, 2022, 2, 368–378 CrossRef CAS.
- W. Zhang, H. Li and A. Y. Elezzabi, Adv. Funct. Mater., 2023, 33, 2300155 CrossRef CAS.
- Y. Zhu, G. Liang, X. Cui, X. Liu, H. Zhong, C. Zhi and Y. Yang, Energy Environ. Sci., 2024, 17, 369–385 RSC.
- Y. Kim, Y. Park, K. J. Kim and J. W. Choi, Nat. Commun., 2022, 13, 2371 CrossRef CAS PubMed.
- C. Xie, S. Liu, Z. Yang, Y. Tang, X. Ji, Q. Zhang and H. Wang, Angew. Chem., Int. Ed., 2023, 62, e202218612 CrossRef CAS PubMed.
- A. Chen, C. Zhao, J. Gao, Z. Guo, X. Lu, J. Zhang, Z. Liu, M. Wang, N. Liu, L. Fan, Y. Zhang and N. Zhang, Energy Environ. Sci., 2023, 16, 275–284 RSC.
- J. Yang, X. Xu, Y. Gao, Y. Wang, Q. Cao, J. Pu, F. Bu, T. Meng and C. Guan, Adv. Energy Mater., 2023, 13, 2301997 CrossRef CAS.
- T. Xiong, D. Zhang, J. Y. Yeo, Y. Zhan, Y. K. Ong, C. M. A. Limpo, L. Shi, Y. Rao, Y. Pu, W. Lai, J. Lee, W. S. V. Lee and B. Özyilmaz, J. Mater. Chem. A, 2024, 12, 5499–5507 RSC.
- Q. Cao, Y. Gao, J. Pu, X. Zhao, Y. Wang, J. Chen and C. Guan, Nat. Commun., 2023, 14, 641 CrossRef CAS PubMed.
- G. Nagaraju, S. Tagliaferri, A. Panagiotopoulos, M. Och, R. Quintin-Baxendale and C. Mattevi, J. Mater. Chem. A, 2022, 10, 15665–15676 RSC.
- N. Hu, W. Lv, H. Tang, H. Qin, Y. Zhou, L. Yi, D. Huang, Z. Wu, J. Liu, Z. Chen, J. Xu and H. He, J. Mater. Chem. A, 2023, 11, 14921–14932 RSC.
- H. Li, R. Zhao, W. Zhou, L. Wang, W. Li, D. Zhao and D. Chao, JACS Au, 2023, 3, 2107–2116 CrossRef CAS PubMed.
- Y. Li, X. Peng, L. Dong and F. Kang, Adv. Mater., 2023, 35, 2300019 CrossRef CAS PubMed.
- F. Wu, F. Du, P. Ruan, G. Cai, Y. Chen, X. Yin, L. Ma, R. Yin, W. Shi, W. Liu, J. Zhou and X. Cao, J. Mater. Chem. A, 2023, 11, 11254–11263 RSC.
- L. Jiang, D. Li, X. Xie, D. Ji, L. Li, L. Li, Z. He, B. Lu, S. Liang and J. Zhou, Energy Storage Mater., 2023, 62, 102932 CrossRef.
- X. Feng, M. Li, J. Yin, T. Cui, F. Li, Y. Cheng, S. Ding, X. Xu and J. Wang, ACS Appl. Mater. Interfaces, 2024, 16, 12544–12553 CrossRef CAS PubMed.
- K. Wu, J. Yi, X. Liu, Y. Sun, J. Cui, Y. Xie, Y. Liu, Y. Xia and J. Zhang, Nano-Micro Lett., 2021, 13, 79 CrossRef PubMed.
- L. Zheng, H. Li, X. Wang, Z. Chen, C. Hu, K. Wang, G. Guo, S. Passerini and H. Zhang, ACS Energy Lett., 2023, 8, 2086–2096 CrossRef CAS.
- C. Wei, Y. Zhou, X. Wang, H. Qi, X. Li, T. Zou, W. Wang, Z. Yang and Y. Yu, Mater. Today Phys., 2024, 41, 101356 CrossRef CAS.
- H. Huang, D. Xie, J. Zhao, P. Rao, W. M. Choi, K. Davey and J. Mao, Adv. Energy Mater., 2022, 12, 2202419 CrossRef CAS.
- C. Huang, X. Zhao, Y. Hao, Y. Yang, Y. Qian, G. Chang, Y. Zhang, Q. Tang, A. Hu and X. Chen, Energy Environ. Sci., 2023, 16, 1721–1731 RSC.
- K. Guan, L. Tao, R. Yang, H. Zhang, N. Wang, H. Wan, J. Cui, J. Zhang, H. Wang and H. Wang, Adv. Energy Mater., 2022, 12, 2103557 CrossRef CAS.
- W. Deng, Z. Xu and X. Wang, Energy Storage Mater., 2022, 52, 52–60 CrossRef.
- M. Wang, Y. Cheng, H. Zhao, J. Gao, J. Li, Y. Wang, J. Qiu, H. Zhang, X. Chen and Y. Wei, Small, 2023, 19, 2302105 CrossRef CAS PubMed.
- K. Ren, M. Li, Q. Wang, B. Liu, C. Sun, B. Yuan, C. Lai, L. Jiao and C. Wang, Nano-Micro Lett., 2024, 16, 117 CrossRef CAS PubMed.
- T. Fang, Q. Liu, A. Hu, J. Meng, Y. Fu and Z. Shi, J. Power Sources, 2023, 581, 233521 CrossRef CAS.
- Y. Zhong, Z. Cheng, H. Zhang, J. Li, D. Liu, Y. Liao, J. Meng, Y. Shen and Y. Huang, Nano Energy, 2022, 98, 107220 CrossRef CAS.
- Z. Zha, T. Sun, D. Li, T. Ma, W. Zhang and Z. Tao, Energy Storage Mater., 2024, 64, 103059 CrossRef.
- C. Lin, L. He, P. Xiong, H. Lin, W. Lai, X. Yang, F. Xiao, X.-L. Sun, Q. Qian, S. Liu, Q. Chen, S. Kaskel and L. Zeng, ACS Nano, 2023, 17, 23181–23193 CrossRef CAS PubMed.
- L. Cao, D. Li, T. Pollard, T. Deng, B. Zhang, C. Yang, L. Chen, J. Vatamanu, E. Hu, M. J. Hourwitz, L. Ma, M. Ding, Q. Li, S. Hou, K. Gaskell, J. T. Fourkas, X.-Q. Yang, K. Xu, O. Borodin and C. Wang, Nat. Nanotechnol., 2021, 16, 902–910 CrossRef CAS PubMed.
- L. Zheng, H. Li, X. Wang, Z. Chen, C. Hu, K. Wang, G. Gup, S. Passerini and H. Zhang, ACS Energy Lett., 2023, 8, 2086–2096 CrossRef CAS.
- X. Zeng, J. Mao, J. Hao, J. Liu, S. Liu, Z. Wang, Y. Wang, S. Zhang, T. Zheng, J. Liu, P. Rao and Z. Guo, Adv. Mater., 2021, 33, 2007416 CrossRef CAS PubMed.
- X. Liang, X. Chen, Z. Zhai, R. Huang, T. Yu and S. Yin, Chem. Eng. J., 2024, 480, 148040 CrossRef CAS.
- C. Chen, L. Li, Z. Long and Q. Liang, Chem. Commun., 2023, 59, 13363–13366 RSC.
- H. Cheng, S. Zhang, W. Guo, Q. Wu, Z. Shen, L. Wang, W. Zhong, D. Li, B. Zhang, C. Liu, Y. Wang and Y. Lu, Adv. Sci., 2024, 11, 2307052 CrossRef CAS PubMed.
- A. Bayaguud, Y. Fu and C. Zhu, J. Energy Chem., 2022, 64, 246–262 CrossRef CAS.
- H. Qin, W. Kuang, N. Hu, X. Zhong, D. Huang, F. Shen, Z. Wei, Y. Huang, J. Xu and H. He, Adv. Funct. Mater., 2022, 32, 2206695 CrossRef CAS.
- M. Xia, H. Fu, K. Lin, A. M. Rao, L. Cha, H. Liu, J. Zhou, C. Wang and B. Lu, Energy Environ. Sci., 2024, 17, 1255–1265 RSC.
- X. Yi, H. Fu, A. M. Rao, Y. Zhang, J. Zhou, C. Wang and B. Lu, Nat. Sustain., 2024, 7, 326–337 CrossRef.
- P. Xiao, Y. Wu, J. Fu, J. Liang, Y. Zhao, Y. Ma, T. Zhai and H. Li, ACS Energy Lett., 2023, 8, 31–39 CrossRef CAS.
- S. Chen, D. Ji, Q. Chen, J. Ma, S. Hou and J. Zhang, Nat. Commun., 2023, 14, 3526 CrossRef CAS PubMed.
- Y. Liu, J. Wang, J. Sun, F. Xiong, Q. Liu, Y. An, L. Shen, J. Wang, Q. An and L. Mai, J. Mater. Chem. A, 2022, 10, 25029–25038 RSC.
- M. Li, Z. Li, X. Wang, J. Meng, X. Liu, B. Wu, C. Han and L. Mai, Energy Environ. Sci., 2021, 14, 3796–3839 RSC.
- Y. Chen, S. Zhou, J. Li, J. Kang, S. Lin, C. Han, H. Duan, S. Liang and A. Pan, Adv. Energy Mater., 2024, 2400398 CrossRef.
- M. K. Kim, S.-J. Shin, J. M. Lee, Y. B. Park, Y. M. Kim, H. J. Kim and J. W. Choi, Angew. Chem., Int. Ed., 2022, 61, e202211589 CrossRef CAS PubMed.
- T.-T. Su, K. Wang, B.-Y. Chi, W.-F. Ren and R.-C. Sun, EcoMat, 2022, 4, e12219 CrossRef CAS.
- Z. Zhang, B. Xi, X. Ma, W. Chen, J. Feng and S. Xiong, SusMat, 2022, 2, 114–141 CrossRef CAS.
- X. Li, Z. Chen, P. Ruan, X. Hu, B. Lu, X. Yuan, S. Tian and J. Zhou, Nanoscale, 2024, 16, 2923–2930 RSC.
- H. Yu, Q. Li, W. Liu, H. Wang, X. Ni, Q. Zhao, W. Wei, X. Ji, Y. Chen and L. Chen, J. Energy Chem., 2022, 73, 565–574 CrossRef CAS.
- P. Wang, X. Xie, Z. Xing, X. Chen, G. Fang, B. Lu, J. Zhou, S. Liang and H. J. Fan, Adv. Energy Mater., 2021, 11, 2101158 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta02133a |
| This journal is © The Royal Society of Chemistry 2024 |