Open Access Article
Open Access ArticleAdsorptive molecular sieving of aromatic hydrocarbons over cyclic aliphatic hydrocarbons via an intrinsic/extrinsic approach†
Gengwu
Zhang‡
a,
Xuanfu
Zhu‡
a,
Lukman O.
Alimi
a,
Xin
Liu
a,
Aiping
Chen
b,
Basem M.
Moosa
 a and
Niveen M.
Khashab
a and
Niveen M.
Khashab
 *a
*a
aSmart Hybrid Materials Laboratory (SHMs), Advanced Membranes and Porous Materials Center (AMPMC), King Abdullah University of Science and Technology (KAUST), Thuwal, 23955-6900, Saudi Arabia. E-mail: niveen.khashab@kaust.edu.sa
bClean Combustion Research Center (CCRC), King Abdullah University of Science and Technology (KAUST), Thuwal 23955-6900, Saudi Arabia
First published on 9th January 2024
Abstract
Trianglimine (TI) and trianglamine (TA) macrocycles offer an efficient adsorptive molecular sieving strategy to separate toluene (Tol) from an equimolar mixture of Tol and methylcyclohexane (MCH) with 98.3% and 96.4% selectivity, respectively. Selective separation is achieved through the intrinsic cavity or extrinsic channel within the crystalline molecular absorbents, driven by C–H⋯π and π⋯π interactions. Moreover, TI and TA exhibit exceptional recyclability, highlighting their potential for sustainable chemical separation processes.
Cyclic aliphatics, such as cycloalkanes and alkyl-cycloalkanes, are often used as high-performance jet fuels and endothermic fuels to overcome the severe “thermal barrier” issue for hypersonic vehicles.1,2 Among them, methylcyclohexane (MCH), a typical hexatomic ring alkyl-cycloalkane with the simplest branched structure, is frequently chosen as a jet-fuel surrogate for many practical fuels.3–5 In chemical industry production, MCH is predominantly obtained through the hydrogenation of the corresponding aromatic toluene (Tol), resulting in the presence of unreacted Tol.6 The separation of residual Tol from MCH is crucial for producing high grade MCH. However, conventional extraction and distillation methods face significant challenges in the separation of cyclic aliphatic and aromatic hydrocarbons due to their similar physical properties, such as close boiling points (384 K for Tol and 374 K for MCH) and the formation of azeotropes.7 Consequently, developing sustainable and efficient technology for separating cyclic aliphatic and aromatic hydrocarbons becomes imperative in addressing these challenges.
Porous materials-based adsorptive separation technology has been recognized as an alternative strategy for the separation of cyclic aliphatic and aromatic hydrocarbons, owing to its high efficiency and low energy consumption.8–23 In recent years, various adsorbents such as metal–organic frameworks (MOFs),24 porous organic molecular frameworks (POMFs),25 organic macrocycles,26–28 and molecular cages29 have been reported for the separation of MCH and Tol. Typically, these examples showed that MCH and Tol could be selectively adsorbed into the extrinsic void of assembled materials or the intrinsic cavity of macrocycles, depending on the differences in their molecular sizes, conformational structures, and electrical charge distributions. However, different separation mechanisms between the adsorption in the intrinsic cavity and extrinsic void are still unclear at the molecular level. Therefore, understanding the different host–guest complexation modes during separation is essential for developing novel adsorbents and exploring new separation strategies.
Herein, we present that the simple trianglimine (TI) and trianglamine (TA) macrocycles can be used for the separation of MCH and Tol by different adsorption behaviors (Fig. 1). TI exhibits a rigid molecular structure that enables the adsorption of the Tol molecule into its intrinsic cavity, while TA, with a flexible conformation, forms extrinsic channels in the crystalline assembly structure to capture the Tol molecule. Notably, to the best of our knowledge, this is the first example of conformational transitions being introduced to modulate adsorption occurring in the intrinsic cavity and extrinsic void.
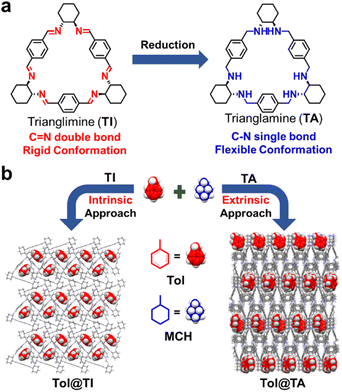 | ||
| Fig. 1 (a) Chemical structures of TI and TA and their transformation. (b) Schematic illustration of the intrinsic and extrinsic capture approaches of Tol from the mixture of Tol/MCH by TI and TA. | ||
TI and TA macrocycles were synthesized using the published methods,30,31 respectively, and the purity of products was confirmed through 1H NMR and 13C NMR (Fig. S1–S4†). The crystalline TI and TA materials were obtained through recrystallization of their ethyl acetate solutions, followed by activation at 90 °C under vacuum for 12 hours to remove residual solvent molecules. Thermogravimetric analysis (TGA) and powder X-ray diffraction (PXRD) analysis indicated that both activated TI and TA displayed excellent thermal stability (Fig. S5 and S6†) and persistent crystallinity (Fig. S7 and S8†) after the activation. Furthermore, the N2 sorption isotherm at 77 K demonstrated that the activated TI and TA crystals were not porous to N2 at 77 K, exhibiting a Brunauer–Emmett–Teller (BET) surface area of 1.68 m2 g−1 for TI (Fig. S9†) and 6.99 m2 g−1 for TA (Fig. S10†), respectively.
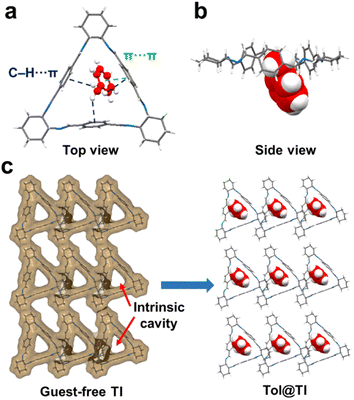
To investigate the host–guest binding behaviors of guest molecules in TI and TA, efforts were made to grow single crystals of the macrocycles with Tol and MCH, followed by single crystal X-ray diffraction (SCXRD) analysis to gain a better understanding of the host–guest interactions. Specifically, by dissolving TI in a pure Tol solution and allowing it to cool/evaporate at room temperature for several days, colorless cubic crystals of Tol@TI were formed. The SCXRD analysis revealed that Tol@TI crystallizes in a monoclinic system with a P21 space group (Table S1†). Each asymmetric unit of Tol@TI consisted of one TI host macrocycle and one Tol guest molecule (Fig. 2a and b). The host TI molecule exhibited a regular triangular structure with an intrinsic cavity. The guest Tol molecule was found to be partially located within the cavity of the TI macrocycle, with the methyl group positioned appropriately inside the cavity. Notably, the methyl group of Tol formed multiple C–H⋯π interactions (3.025 Å, 3.128 Å and 3.245 Å, Fig. S11†) individually with the three benzene rings in the TI cavity, and the benzene ring of Tol exhibited a π⋯π stacking interaction (3.257 Å) with one benzene group in the TI macrocycle (Fig. S12†). Moreover, the packing arrangement of Tol@TI along the crystallographic a axis reveals an inclined 1D packing structure (Fig. S13 and S14†). Additionally, from the guest-free single layer 2D packing structure, it clearly shows the guest-accessible voids within the intrinsic cavity of TI and their capacity to adsorb guest Tol molecules (Fig. 2c).
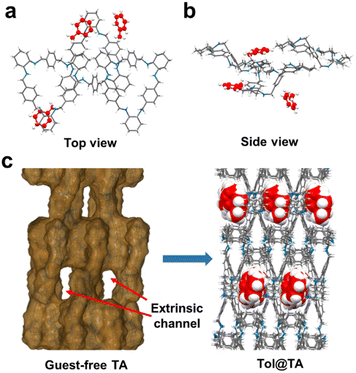
A single crystal of Tol@TA was obtained by the same method. SCXRD analysis revealed that Tol@TA crystallizes in an orthorhombic system with a P212121 space group (Table S1†), which consists of three TA macrocycles and three Tol guest molecules within the asymmetric unit (Fig. 3a and b). In contrast to TI, TA with a highly flexible conformation exhibited a distorted zigzag structure (Fig. S15†), which restricted the volume of guest-accessible voids within its intrinsic cavity and consequently limited its ability to capture guest molecules (Fig. S16†). However, in the crystal packing structure, six TA macrocycles assembled to form a 1D extrinsic channel along the c axis through host–host C–H⋯π interactions, accommodating all Tol guest molecules within these voids (Fig. 3c, S17 and S18†). Two of the Tol guest molecules (TolI and TolII) were situated between two adjacent TA host macrocycles (TA1 and TA2) and stabilized by C–H⋯π interactions between the methyl group of TA and benzene of Tol (Fig. S19 and S20†). The third Tol guest molecule TolIII was linked by one TA macrocycle, as well as connected with the Tol guest molecule TolI (Fig. S21†). These host–guest and guest–guest interactions also resulted in the formation of Tol channels along the crystallographic c-axis, which is consistent with the extrinsic channels formed by TA (Fig. S22†).
The distinct host–guest complexation modes of TI and TA with Tol can be attributed to the structural differences between the two macrocycles. Specifically, TI with C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds exhibits a rigid molecular skeleton that enables it to maintain intrinsic cavities in the solid packing structure, thereby capturing guest molecules. However, upon reduction to TA, the rigid C
N double bonds exhibits a rigid molecular skeleton that enables it to maintain intrinsic cavities in the solid packing structure, thereby capturing guest molecules. However, upon reduction to TA, the rigid C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bonds are transformed into C–H single bonds, allowing for flexible rotation. As a result, TA adopts a more flexible conformation that makes it difficult to maintain guest accessible voids within its intrinsic cavities in the solid packing structure (Fig. S16†). Nevertheless, TA is more capable of forming extrinsic voids or channels to absorb guest molecules.
N double bonds are transformed into C–H single bonds, allowing for flexible rotation. As a result, TA adopts a more flexible conformation that makes it difficult to maintain guest accessible voids within its intrinsic cavities in the solid packing structure (Fig. S16†). Nevertheless, TA is more capable of forming extrinsic voids or channels to absorb guest molecules.
Subsequently, attempts were made to crystallize TA and TI with MCH. However, despite using various solvents and methods, all attempts were unsuccessful (Table S2†). This outcome may be attributed to MCH's low electronegativity profile resulting from its non-aromatic structure, which limits its capacity to establish strong host–guest interactions with the host macrocycles (Fig. S39†). Specifically, Tol contains a CH3 methyl group and an electron-rich benzene ring, which can not only form guest-to-host C–H⋯π interactions with the benzene rings on TI and TA but also can form additional π⋯π interactions and host-to-guest C–H⋯π interactions. In contrast, MCH solely comprises aliphatic hydrocarbon atoms, which can only form relatively weak guest-to-host C–H⋯π interactions with the benzene rings on TI or TA.
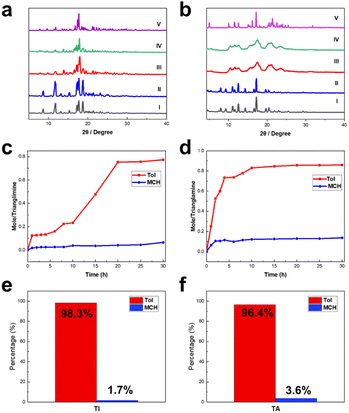
To further evaluate the potential of TI and TA as candidates for the adsorption of Tol and MCH, solid–vapor sorption experiments were performed on the activated TI and TA upon exposure to the single component of Tol and MCH, as well as their equimolar mixture, respectively. As revealed by 1H NMR spectroscopy, each TA or TI macrocycle can capture approximately 0.9 molecules of Tol on average after reaching adsorption saturation (Fig. S23–S26†). In contrast, both TI and TA exhibited negligible uptake after being exposed to MCH vapors (Fig. S27 and S28†). The low adsorption capacity of TI and TA towards MCH explains the challenge in obtaining host–guest single crystals between TA/TI and MCH. Furthermore, PXRD experiments were conducted to investigate the structural changes of TI and TA after the uptake of Tol and MCH. The absorption of Tol molecules with activated TI and TA led to significant guest-induced structural changes, which were consistent with the simulated PXRD patterns of Tol@TI and Tol@TA (Fig. 3a and b). However, there were almost no changes in PXRD patterns for TI and TA after exposure to MCH vapor. Moreover, the different adsorption results were also verified through TGA analysis by comparing the relative weight loss of activated host macrocycles after absorbing Tol and MCH guest molecules, respectively (Fig. S29–S32†). These observations highlight the exceptional selectivity of TI and TA towards Tol over MCH.
Subsequently, equimolar mixtures of Tol and MCH were used to evaluate the separation performance of TI and TA. The results showed that the uptake of Tol in TI and TA increased over time and reached saturation within 20 h and 10 h, respectively (Fig. 4c, d, S33 and S34†). In contrast, MCH absorption was almost negligible throughout the entire experimental period for both TI and TA. Additionally, the PXRD patterns of TI and TA upon adsorption of Tol/MCH equimolar mixture vapor were in complete agreement with the patterns of TI and TA upon capturing Tol vapor and those simulated from Tol@TI and Tol@TA (Fig. 4a and b), respectively, implying the phase transformation from TI and TA into Tol@TI and Tol@TA. Furthermore, gas chromatography confirmed the high selectivity for Tol over MCH, with the proportion of Tol adsorbed in TI and TA reaching 98.3% and 96.4%, respectively (Fig. S35 and S36†).
In practical applications, the recyclability of absorbents is a crucial aspect that influences their potential use. For TI and TA, regeneration was achieved by heating the saturated samples at 90 °C under vacuum for 12 hours. Remarkably, the restored TI and TA samples maintained their selectivity towards Tol without any performance loss even after undergoing five cycles (Fig. S37†).
Conclusions
In conclusion, we have investigated the potential of TI and TA for the separation of aromatic Tol from aliphatic cyclic MCH. Both TI and TA exhibited high selectivity towards Tol, with adsorption capacities of 98.3% and 96.4%, respectively. Crystal structure analysis revealed that TI, with its rigid structure, could capture Tol through its intrinsic cavities, while the flexible conformation of the reduced product TA was able to assemble into extrinsic channels for absorbing guest molecules. This study demonstrates that altering the structure of a macrocycle can induce conformational changes to regulate intrinsic and extrinsic voids for separation. We expect that this research will not only provide a new strategy for designing novel absorbents but also inspire the application of supramolecular macrocycles in energy-intensive separation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank King Abdullah University of Science and Technology (KAUST) and the AMPM center for supporting this work.References
- Z. Wang, G. Liu and X. Zhang, Fuel, 2023, 331, 125732 CrossRef CAS.
- B. W. Weber, W. J. Pitz, M. Mhel, E. J. Silke, A. C. Davis and C. J. Sung, Combust. Flame, 2014, 161, 1972–1983 CrossRef CAS.
- B. Liu, Z. Wang, Q. Zhu, X. Li and J. Wang, Fuel, 2017, 200, 387–394 CrossRef CAS.
- D. Hu, S. Zhang, C. Yang, G. Yao, Q. Zhu and Y. Chen, J. Anal. Appl. Pyrolysis, 2022, 168, 105730 CrossRef CAS.
- F. Zhang, Z. Wang, Z. Wang, L. Zhang, Y. Li and F. Qi, Energy Fuels, 2013, 27, 1679–1687 CrossRef CAS.
- L. Foppa and J. Dupont, Chem. Soc. Rev., 2015, 44, 1886–1897 RSC.
- J. G. Villaluenga and A. T. Mohammadi, J. Membr. Sci., 2000, 169, 159–174 CrossRef.
- M. Yamad, R. Yoshizaki, F. Uemura, H. Katagiri, S. Kato, K. Akimoto and F. Hamada, Chem. Commun., 2023, 59, 2604–2607 RSC.
- P. F. Cui, X. R. Liu, Y. J. Liu, Z. H. Li and G.-X. Jin, J. Am. Chem. Soc., 2022, 144, 6558–6565 CrossRef CAS PubMed.
- J. Zhou, G. Yu, Q. Li, M. Wang and F. Huang, J. Am. Chem. Soc., 2020, 142, 2228–2232 CrossRef CAS PubMed.
- S. Mukjerjee, D. Sensharma, O. T. Qazvini, S. Dutta, L. K. Macreadie, S. K. Ghosh and R. Babarao, Coord. Chem. Rev., 2021, 15, 213852 CrossRef.
- Y. Ding, L. O. Alimi, B. M. Moosa, C. Maaliki, J. Jacquemin, F. Huang and N. M. Khashab, Chem. Sci., 2021, 12, 5315–5318 RSC.
- A. A. Sapianiki, K. A. Kovalenko, D. G. Samsonenko, M. O. Barsukova, D. N. Dybtsev and V. P. Fedin, Chem. Commun., 2020, 56, 8241–8244 RSC.
- H. Yao, Y.-M. Wang, M. Quan, M. U. Farooq, L.-P. Yang and W. Jiang, Angew. Chem., Int. Ed., 2020, 59, 19945–19950 CrossRef CAS PubMed.
- C. G. Galán, A. L. Triguero, J. M. V. Luna, A. P. Zaderenko, A. Sławek, R. Sánchez-de-Armas and S. Calero, Chem. Eng. J., 2020, 398, 125678 CrossRef.
- M. J. Emparan-Legaspi, J. Gonzalez, G. Gonzalez-Carrillo, S. G. Ceballos-Magañ, J. Canales-Vazquez, I. A. Aguayo-Villarreal and R. Muñiz-Valencia, Microporous Mesoporous Mater., 2020, 294, 109942 CrossRef CAS.
- L. K. Macreadie, O. T. Qazvini and R. Babarao, ACS Appl. Mater. Interfaces, 2021, 13, 30885–30890 CrossRef CAS PubMed.
- H. L. Tan, Q. B. Chen, T. T. Chen and H. L. Liu, ACS Appl. Mater. Interfaces, 2018, 10, 32717–32725 CrossRef CAS PubMed.
- Y. Han, Y. Chen, Y. Ma, J. Bailey, Z. Wang, D. Lee, A. M. Sheveleva, F. Tuna, E. J. L. Mclinnes, M. D. Frogley, S. J. Day, S. P. Thompson, B. F. Spencer, M. Nikiel, P. Manuel, D. Crawshaw, M. Schröder and S. Yang, Chem, 2023, 9, 739–754 CAS.
- A. Natraj, W. Ji, J. Xin, I. Castano, D. W. Burke, A. M. Evans, M. J. Strauss, M. Ateia, L. S. Hamachi, N. C. Gianneschi, Z. A. Alothman, J. Sun, K. Yusuf and W. R. Dichtel, J. Am. Chem. Soc., 2022, 144, 19813–19824 CrossRef CAS PubMed.
- M. Liang, S. Hu, N. Zhou, Z. Liu, Q. Chen, X. Chen, X. Liu, C. Li, J. Hao and P. Xue, Small, 2023, 2304340 CrossRef CAS PubMed.
- S. Mukjerjee, B. Manna, A. V. Desai, Y. Yin, R. Krishna, R. Babarao and S. K. Ghosh, Chem. Commun., 2016, 52, 8215–8218 RSC.
- W. Yang, K. Samanta, X. Wang, T. U. Thikekar, Y. Chao, S. Li, K. Du, J. Xu, Y. Gao, H. Zuilhof and A. C.-H. Sue, Angew. Chem., Int. Ed., 2020, 59, 3994–3999 CrossRef CAS PubMed.
- L. K. Macreadie, R. Babarao, C. J. Setter, S. J. Lee, O. T. Qazvini, A. J. Seeber, J. Tsanaktsidis, S. G. Telfer, S. R. Batten and M. R. Hill, Angew. Chem., Int. Ed., 2020, 59, 6090–6098 CrossRef CAS PubMed.
- C. Chen, H. Guan, H. Li, Y. Zhou, Y. Huang, W. Wei, M. Hong and M. A. Wu, Angew. Chem., Int. Ed., 2022, e202201646 CAS.
- K. Jie, Y. Zhou, E. Li, R. Zhao and F. Huang, Angew. Chem., Int. Ed., 2018, 57, 12845–12849 CrossRef CAS PubMed.
- W. Wang, Z. Li, C. Song, J. Yang and Y. Yang, Molecules, 2022, 27, 8554 CrossRef CAS PubMed.
- F. Zeng, X. S. Xiao, S. F. Gong, L. Yuan and L. L. Tang, Org. Chem. Front., 2022, 9, 4829–4833 RSC.
- X. Zhao, Y. Liu, Z. Y. Zhang, Y. Wang, X. Jia and C. Li, Angew. Chem., Int. Ed., 2021, 60, 17904–17909 CrossRef CAS PubMed.
- M. Chadim, M. Budesinsky, J. Hodacova, J. Zavada and P. C. Junk, Tetrahedron: Asymmetry, 2001, 12, 127–133 CrossRef CAS.
- J. Gawronski, K. Gawronska, J. Grajewski, M. Kwit, A. Plutecka and U. Rychlewska, Chem.–Eur. J., 2006, 12, 1807–1817 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2287895 and 2287896. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3ta06602a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |