A hybrid approach to oil structuring – combining wax oleogels and capillary suspensions†
Selvyn
Simoes
and
Dérick
Rousseau
 *
*
Food and Soft Materials Research Group, Department of Chemistry and Biology, Toronto Metropolitan University, 350 Victoria St, Toronto, Canada. E-mail: rousseau@torontomu.ca; Fax: + 1-416-979-5044; Tel: + 1-416-979-5000x552155
First published on 14th May 2024
Abstract
There is continuing interest in finding new approaches to gel liquid oil for processed food applications. Here, we combined oleogels and capillary suspensions to generate model oil-continuous networks consisting of a wax oleogel and a water-bridged, glass particle network. The composition map tested comprised 30 vol% polar or non-polar glass beads dispersed in a 70 vol% non-particle phase consisting of water (≤9 vol%) as well as 2 wt% hexatriacontane as oleogelator in canola oil. While the hexatriacontane wax alone gelled the oil, presence of the glass beads (but no water) prevented oleogelation. Self-supporting capillary networks formed with polar particles and 1 vol% water or non-polar glass beads and 3 vol% water in canola oil. The capillary suspensions demonstrated significant differences in rheological behaviour as the polar particles yielded much higher elastic moduli than their non-polar particle counterparts. Polar hybrids were weakened by inclusion of the wax whereas the non-polar particle hybrid network displayed elastic moduli greater than the respective contributions of both capillary and wax gel networks. This hybrid method of oleogelation can be applied to virtually any food particles and uses minimal water and wax.
1. Introduction
The solid-like properties of fat are integral to the functionality of many food products.1 For instance, the crystallinity of cocoa butter will determine the melting behaviour of chocolate. In dough, fat crystals act as a barrier to flour hydration, yielding tenderness. As well, the plasticity of solid fat allows for its lamination in dough resulting in puffed croissant layers.2,3 While critically important for product performance, awareness surrounding the negative environmental and health impacts of saturated fat has also resulted in its continued removal from processed foods and increased interest in alternatives that confer a fat-like texture and mouthfeel.Oleogelation is emerging as a strategy to replace solid fat in some food formulations, one that hinges on the immobilization of liquid oil via formation of a percolated network to yield a viscoelastic solid.4 Many materials have been tested for their ability to gel oil, including low-molecular weight species (waxes, emulsifiers, etc.), polymers (e.g., ethylcellulose) and particles (e.g., fumed silica).5–7 Of relevance, plant or animal waxes (candelilla, rice bran, carnauba, bee, etc.) have been extensively investigated as they are natural by-products of agriculture and their upcycling reduces waste. They typically consist of wax esters and alcohols, n-alkanes, free fatty acids, fatty alcohols as well as other minor components.8 They can confer solid-like properties to liquid oils when added at concentrations below 10 wt% through formation of a network that immobilizes the oil.9
Progress from concept to commercialization of oleogel-containing foods has remained slow for several reasons. For example, wax oleogels lack plasticity and may leak oil at low gelator concentrations. When added at higher concentrations, they confer a waxy mouthfeel due to their high melting temperature.9 In most instances, simply swapping fat with gelled oil is not suitable, e.g., when used to replace saturated fat in ice cream and shortbread, they lack the crystallinity to reproduce the microstructure and associated texture generated via a fat crystal network.10,11
A further challenge limiting oleogelation uptake in processed foods is the presence of dispersed particles (e.g., in chocolates, cookie fillings and nut spreads). Little is known of the effects of dispersed food particles (e.g., protein particles, crystalline sweeteners, or starch granules), specifically whether they hinder oleogelator crystal network formation by their mere presence, alter wax crystallization kinetics and crystal morphology or interact with the oleogelator to alter a food's sensory attributes and shelf stability.12
Food particles dispersed in oil usually cannot produce self-supporting networks unless coaxed to do so. Such a challenge may be overcome via addition of sufficient water to form a percolated capillary network in the oil phase that gives the material a yield stress.13–15 The resulting gels will differ in morphology and strength based on several parameters, including dispersed particle size and mass/volume fraction, oil–water interfacial tension, three-phase contact angle and extent of particle interactions.16 Understanding the interplay between these parameters offers possibilities to tune the texture and rheology of oil-continuous, self-supporting networks using particle types commonly found in food formulations.
Here, we investigated the microstructure and rheology of model hybrid gels comprising glass particles dispersed within a hexatriacontane wax oleogel at its critical gelling concentration. The tuning parameters investigated were glass particle polarity and water content. The introduction of attractive particle interactions through the addition of water may enhance overall gel strength, allowing for firmer textures with reduced wax content, depending on water concentration and particle properties. To our knowledge, this first targeted report on combined oleogels and capillary networks presents a new approach to gel edible oils, that of hybrid oleogelation.
2. Materials and methods
2.1 Materials
Hollow glass beads (9–13 μm, 1.1 g mL−1) (Sigma Aldrich, St. Louis, Missouri, USA) were dried at 80 °C overnight in a vacuum oven (Acros international, Livingston, NJ, USA) before use. Hexatriacontane (C36H74) (98% purity) was used as oleogelator (Sigma Aldrich, St. Louis, MO, USA). Canola oil was purchased from a local supermarket (Mazola brand, Metro Inc. Toronto, ON, Canada). To remove partial glycerides and free fatty acids, 20 wt% silica (60 Å, 70–230 mesh, 63–200 μm, Sigma Aldrich, Oakville, ON, Canada) was added to the oil at room temperature (RT, 21 °C) and stirred for 24 hr. The oil–silica blend was allowed to sediment and the supernatant oil was filtered and stored in air-tight, amber, screw-cap bottles until use.2.2 Glass bead surface modification
In a screw-cap bottle, the dried hollow glass beads were suspended in n-heptane (Sigma Aldrich, St. Louis, MO, USA). Chlorotrimethylsilane (Sigma Aldrich, St. Louis, MO, USA) was added as the hydrophobizing agent (10 wt% relative to glass beads). The mixture was periodically inverted by hand and allowed to react for 24 hr. The particles were collected via vacuum filtration, washed with ethanol and subsequently with dichloromethane. The beads were dried overnight at 80 °C in a vacuum oven and stored in an air-tight, screw-cap bottle till use.2.3 Gel preparation
The hybrid gels were a combination of the wax oleogel and capillary suspension. All samples were prepared at a total volume of 30 mL. The hybrid composition map tested consisted of 30 vol% polar or non-polar glass beads dispersed in a 70 vol% non-particle phase consisting of ≤9 vol% DI water and 91 to 99 vol% canola oil, with 2 wt% hexatriacontane dissolved in the oil (Fig. 1). The corresponding capillary suspensions were prepared by replacing the wax with canola oil. Oleogel networks were prepared at 2 wt% wax in oil (corresponding to its critical gelling concentration).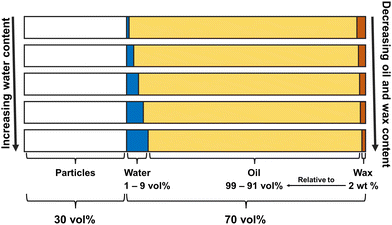 | ||
| Fig. 1 Visual representation of the experimental hybrid and capillary suspension compositions. Wax content was kept constant at 2 wt% of the oil phase. | ||
The wax oleogel was produced by combining 2 wt% hexatriacontane and purified canola oil, heating and degassing at 80 °C for 1 h, mixing in a centrifugal planetary mixer (2000 rpm, 30 s) (Kakuhunter sk-300sii, Clifton, NJ, USA) and storage in the air-tight mixing container for 24 h at RT before measurement. Particle effects on wax gelation were determined by mixing 30 vol% particles (1760 rpm, 1 min) into the wax mixture before heating, degassing and storage. Capillary suspensions were prepared by mixing particles and purified canola oil in the planetary mixer (1760 rpm, 1 min), followed by heating and degassing at 80 °C and −1 atm for 1 h. Water preheated to 80 °C was then added using a micropipette and the mixture was mixed again in the centrifugal planetary mixer (2000 rpm, 30 s). The hybrid gels were prepared as above but also included the wax in the non-volatile phase (i.e., wax, oil, and particles).
2.4 Microstructure
Gel microstructure was assessed using an LSM800 confocal laser scanning microscope (CLSM) (Zeiss Inc., Toronto, ON, Canada). Samples were prepared using 0.01 wt% Rhodamine B in the aqueous phase and 0.025 wt% Fluorol yellow 088 in the oil phase as fluorescent labels. A small amount of sample was deposited onto a microscope slide and covered with a cover slip. Samples were imaged at a magnification of 200× at RT.Samples were also characterized using a cryo-scanning electron microscope (SEM) (Thermo Fisher Quattro S environmental SEM, Eindhoven, The Netherlands) equipped with a Quorum PP3010T sputter coating and cryo-transfer system (Quorum Technologies, East Sussex, UK). Samples were adhered to copper stubs using colloidal carbon adhesive and freeze-fractured at −196 °C and then sublimated at −90 °C for 5 min. They were then sputter-coated using a platinum target with a current of 5 mA for 60 s. Imaging was performed under high vacuum at 3 to 5 kV and magnifications of 1000×.
Polarized light images of the wax crystals and glass particles in oil were obtained at RT using a Carl Zeiss Axiovert 200 M inverted light microscope (Zeiss Inc., Toronto, ON, Canada). A small amount of aged sample was deposited onto a microscope slide and gently covered with a cover slip. Images were acquired at a magnification of 630× using a Q-Imaging CCD camera and processed using the Q-Capture Pro software v.7.0 (Q-Imaging Inc., Surrey, BC, Canada).
2.5 Glass bead properties
The particle size of the glass beads dispersed in oil was determined by image analysis. In triplicate, 10 wt% particles were dispersed in canola oil and imaged at 200×. Five images per replicate were selected and the particle size distribution was analyzed using ImageJ (v.1.53, NIH, Bethesda, MD, US). The three-phase contact angle of the glass beads at an oil–water interface was determined as per Bossler and Koos.17 Particles were first dispersed in both the oil and water. Using a linear microchannel with a 200 μm depth and 1 mm width, a 0.025% dispersion of particles in canola oil was used to fill the microchannel, with a 0.025% dispersion of particles in DI water used to backfill the microchannel and create an oil–water interface. After resting for 45 min, micrographs were taken using an inverted light microscope (Zeiss Axiovert 200M, Zeiss Inc, Toronto, Canada). Micrographs of particles situated at the interface were taken and, through geometric fitting using ImageJ, the contact angle was determined. The zeta potential of the glass beads was determined using a LiteSizer 500 (Anton Paar Canada Inc, Saint-Laurent, QC, Canada). Suspensions of either bead type with concentrations of 0.05% in water were prepared. Samples were measured in triplicates at RT.2.6 Rheology
Rheological measurements were performed with an Anton Paar MCR302 rheometer (Anton Paar Canada Inc, Saint-Laurent, QC, Canada). A 25 mm sand-blasted, parallel plate geometry (PP25) was used with a gap set of 1 mm. Amplitude sweep measurements took place after a 15 min equilibration from 0.001 to 100% at a frequency of 1 Hz and at RT. The elastic (G′) and viscous (G′′) moduli in the linear viscoelastic regime (LVR), as well as the critical strain, defined as a 5% deviation from the plateau elastic modulus in the LVR, crossover strain and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ were extracted from the amplitude sweeps. Frequency sweeps were performed in the LVR from 0.01 to 100 to Hz. Samples were prepared and measured in triplicate at RT, following 24 h of storage. All measurements were recorded at 20 °C.
δ were extracted from the amplitude sweeps. Frequency sweeps were performed in the LVR from 0.01 to 100 to Hz. Samples were prepared and measured in triplicate at RT, following 24 h of storage. All measurements were recorded at 20 °C.
3. Results
3.1 General observations
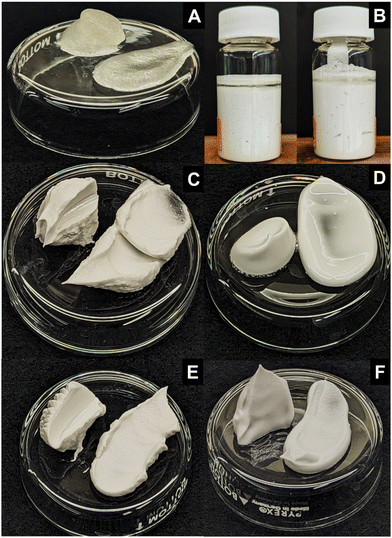
The visual appearance of the hybrid and component gels is shown in Fig. 2. The 2 wt% hexatriacontane wax gel yielded a translucent, delicate, soft solid (Fig. 2A). Scooping it from its container caused oil exudation and some loss of structure. Addition of either polar or non-polar glass beads to the hexatriacontane gel was ineffective in forming self-supporting gels (Fig. 2B). The polar capillary suspensions were spreadable soft solids with an opaque white appearance due to the presence of the micron-sized glass particles (Fig. 2C). The non-polar capillary suspensions were similarly white and opaque (Fig. 2D). Unlike the capillary suspensions which appeared glossy, the hybrid gels had a matte appearance (Fig. 2E and F). The polar hybrid gel was similar in texture to its capillary suspension counterpart, while the non-polar hybrid was visibly firmer and resembled the polar hybrid. The resulting spreadable gels were opaque and showed brittleness not observed in either the oleogels or capillary suspensions.3.2 Glass bead characteristics
The average diameter for both the native and surface-modified glass beads was ∼12 μm. Surface modification changed the three-phase (oil–water-particle) contact angle measured through aqueous phase from 57 ± 6° to 122 ± 9°, demonstrating that surface modification yielded hydrophobized (non-polar) particles compared to the hydrophilic (polar) unmodified glass beads. The zeta potential of the polar particles was −31.8 ± 1.0 mV vs. −0.2 ± 0.1 mV for the non-polar particles, further confirming glass bead surface hydrophobization. Fig. S1 (ESI†) depicts polar and non-polar particles dispersed in oil. Polar particles were clustered whereas non-polar particles were more evenly dispersed.3.3 Gel microstructure
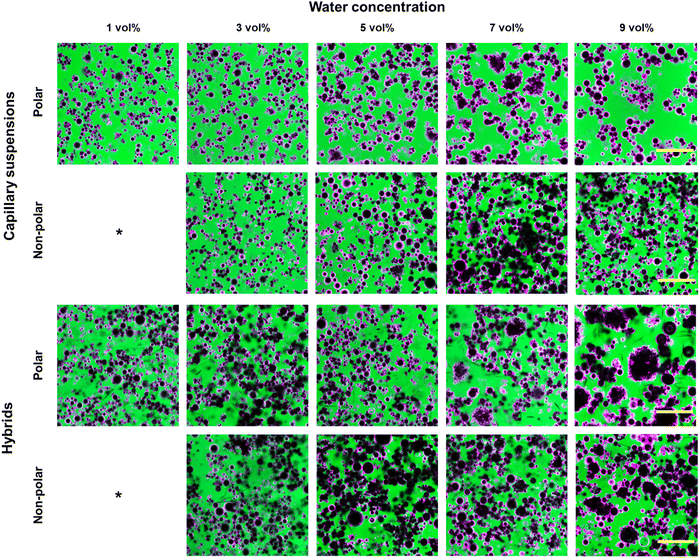
Fig. 3 shows the effect of wax presence, particle surface polarity and water content on the microstructure of the capillary networks. Irrespective of surface polarity, glass bead dispersions alone in oil did not form self-supporting networks. Addition of at least 1 vol% water to a dispersion of polar particles produced sample-spanning networks resistant to phase separation. Increasing the water content from 1 vol% to 9 vol% resulted in the increased presence of spherical particle aggregates. By comparison, the non-polar particles required the addition of at least 3 vol% water to produce capillary suspensions. At equivalent water loads to the polar particle dispersions, the non-polar particles did not form spherical aggregates; instead, clusters of particles around a central droplet of water were apparent.The presence of hexatriacontane in the capillary suspensions led to wax crystal platelet formation in the continuous oil phase for both particle types (Fig. 3 hybrids). Wax crystal presence resulted in more extensive particle agglomeration resulting in what appeared to be tighter aggregates.
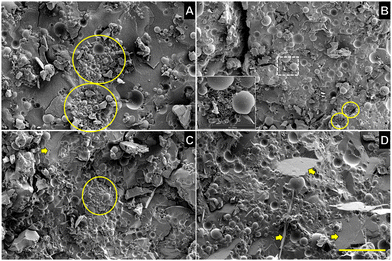
The SEM images in Fig. 4 show the effects of wax and particle polarity on the morphology of the capillary suspensions consisting of 9 vol% water whereas Fig. S2 (ESI†) shows the effects at 3 vol% water. As per the CLSM results, increased presence of water led to polar particle agglomerates as large as 100 μm in diameter (Fig. 4A circles). Expectedly, at the same water concentration, the non-polar particles instead of forming aggregates encapsulated free water in a manner akin to a coarse Pickering emulsion (Fig. 4B circles and inset).13
Both polar and non-polar particles caused a decrease in the size of wax crystals to different extents. The wax platelets in the hybrid gels (Fig. 4C and D) were smaller compared to the control oleogel, which consisted of wax crystals 100 to 300 μm in length arranged in a “house-of-cards” structure of interconnected platelets (Fig. S3, ESI†).18 Incorporating polar glass beads led to wax crystals ∼50 μm in length whereas with non-polar particles, they grew to ∼100 μm in length - still smaller than in the control wax oleogel.
3.4 Comparison of hybrid and constituent gel rheology
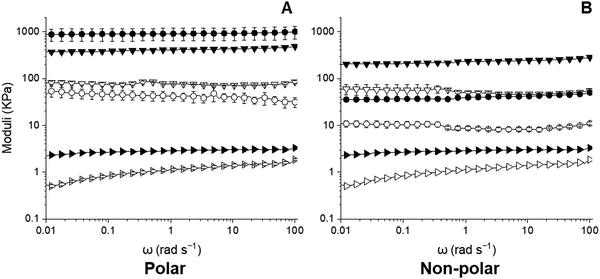
Fig. 5 contrasts the strain sweeps of the hybrid gels containing 30 vol% polar or non-polar particles, 3 vol% water, and 2% hexatriacontane vs. their capillary suspension counterparts, and the 2% wax oleogel with and without particles. The hybrid and component gels containing the other water loads are shown in Fig. S4 (ESI†). All materials were characterized by a G′ plateau at small strain amplitudes, followed by a decrease in G′ at higher strains. The 2 wt% hexatriacontane oleogels exhibited a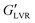 of ∼3.5 kPa, similar to plant wax oleogels in triglyceride oil at their respective critical gelling concentration.19 Both particle types precluded wax oleogel formation and resulted in supernatant oil within 24 hours. When present at 30 vol%, particle inclusion reduced wax oleogel
of ∼3.5 kPa, similar to plant wax oleogels in triglyceride oil at their respective critical gelling concentration.19 Both particle types precluded wax oleogel formation and resulted in supernatant oil within 24 hours. When present at 30 vol%, particle inclusion reduced wax oleogel 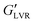 to ∼0.10 kPa and decreased the gap between G′ and G′′ indicating a loss of structure with the reduced ability to resist deformation. The polar capillary suspension containing 3 vol% water yielded a
to ∼0.10 kPa and decreased the gap between G′ and G′′ indicating a loss of structure with the reduced ability to resist deformation. The polar capillary suspension containing 3 vol% water yielded a 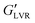 of 1800 kPa while its non-polar counterpart demonstrated a lower
of 1800 kPa while its non-polar counterpart demonstrated a lower 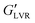 at 60 kPa – a 30-fold difference. This is consistent with early findings by Koos and Willenbacher who noted that non-polar particles in an oily (hydrophobic and viscous) medium, with water as added secondary fluid, required more water to reach the capillary state and resulted in a weaker network.20 The
at 60 kPa – a 30-fold difference. This is consistent with early findings by Koos and Willenbacher who noted that non-polar particles in an oily (hydrophobic and viscous) medium, with water as added secondary fluid, required more water to reach the capillary state and resulted in a weaker network.20 The 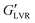 of the hybrid gel containing polar particles was ∼800 kPa vs. ∼400 kPa for the hybrid gels with non-polar particles. This indicated that at 3 vol% water, wax oleogel presence was deleterious to polar hybrid gel strength, yet beneficial to non-polar hybrid gel strength.
of the hybrid gel containing polar particles was ∼800 kPa vs. ∼400 kPa for the hybrid gels with non-polar particles. This indicated that at 3 vol% water, wax oleogel presence was deleterious to polar hybrid gel strength, yet beneficial to non-polar hybrid gel strength.
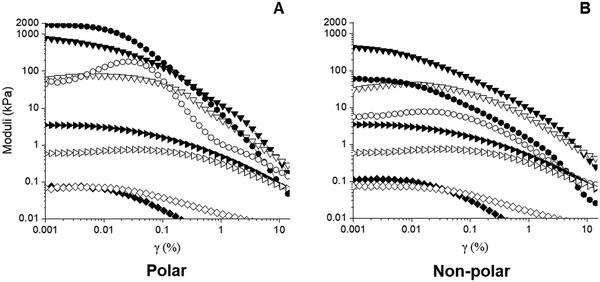 | ||
| Fig. 5 Strain sweeps of capillary suspensions and associated hybrid gels containing 3 vol% water with (A) polar or (B) non-polar glass beads. (●;○) capillary suspensions; (▼;▽) hybrid gels; (▶;▷) wax oleogel; (◆;◇) wax + particle “gel”. Filled symbols – G′ and open symbols – G′′. Strain sweeps at 1, 5, 7 and 9 vol% water are shown in Fig. S4 (ESI†). | ||
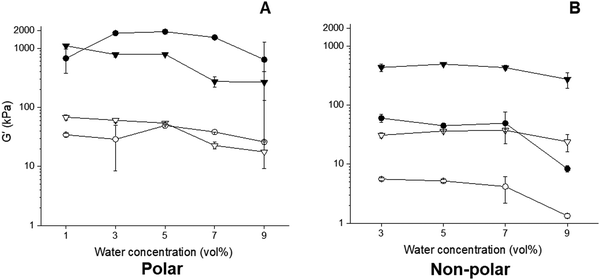
The viscoelastic moduli of the hybrid gels and associated capillary suspensions were affected by water content, though this depended on particle polarity (Fig. 6). The polar capillary suspensions showed much higher 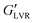 values than their non-polar particle counterparts (Fig. 6A and B). At 1 vol% water, the polar hybrid gel showed the highest
values than their non-polar particle counterparts (Fig. 6A and B). At 1 vol% water, the polar hybrid gel showed the highest 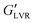 (1100 kPa), which was 1.6 × greater than the corresponding capillary suspension without wax, suggesting that polar hybrid produced a stronger network. However, it was the polar capillary suspension at 3–7 vol% showed the highest
(1100 kPa), which was 1.6 × greater than the corresponding capillary suspension without wax, suggesting that polar hybrid produced a stronger network. However, it was the polar capillary suspension at 3–7 vol% showed the highest 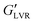 values of all formulation (∼2000 kPa).
values of all formulation (∼2000 kPa).
The increase in gel strength with increasing water concentration is typical for capillary suspensions as they transition from the pendular state to the funicular state.21 The pendular state is categorized by secondary fluid menisci connecting two particles, and the transition occurs when >3 particles become connected by a continuous meniscus. This transition requires more than the minimum amount of secondary fluid to achieve percolation and is associated with an increase in gel strength.22 By contrast, increasing water load weakened the polar hybrid gels, based on the decrease in 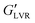 to ∼800 kPa at 3 vol% and further to ∼300 kPa at 9 vol% water. The water content-dependent evolution in G′′ was similar for both the capillary suspensions and associated hybrid gels containing polar particles.
to ∼800 kPa at 3 vol% and further to ∼300 kPa at 9 vol% water. The water content-dependent evolution in G′′ was similar for both the capillary suspensions and associated hybrid gels containing polar particles.
The weakest gels were the non-polar capillary suspensions whose 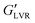 steadily diminished from 60 to 10 kPa with an increase in water load from 3 to 9 vol%, which corresponded to the transition to a coarse Pickering emulsion (Fig. 4B). The
steadily diminished from 60 to 10 kPa with an increase in water load from 3 to 9 vol%, which corresponded to the transition to a coarse Pickering emulsion (Fig. 4B). The 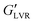 of the non-polar hybrids at 3 to 7 vol% water similar (∼400 kPa), with a small decrease at 9 vol% water (∼280 kPa). As opposed to the systems with polar particles, the
of the non-polar hybrids at 3 to 7 vol% water similar (∼400 kPa), with a small decrease at 9 vol% water (∼280 kPa). As opposed to the systems with polar particles, the 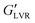 of the non-polar hybrids was greater than the corresponding capillary suspensions across the tested water concentrations.
of the non-polar hybrids was greater than the corresponding capillary suspensions across the tested water concentrations.
The limit of the LVR for all samples was between 0.01 to 0.04% strain, the sole exception being the polar hybrid gel at 9 vol% (γ = 0.06%) (Fig. S5A, ESI†). The hybrid gels across the tested water concentrations did not exhibit a clear LVR and deviation from the ordinate value occurred at low strains. Due to the hybrid network being more brittle, it is possible that the structure was deformed during transfer to the rheometer or during gap setting. The crossover strain describes deformation required for a material to transition from elastic-dominant to viscous-dominant behaviour (G′ = G′′). It tended towards a general value of 0.08% in all gels as water content increased (Fig. S5B, ESI†). The crossover strain of the hybrid gels was greater than their corresponding capillary suspensions. The tangent delta (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ), which is the ratio of G′′ to G′ with values approaching 0 indicating an increasing solid-like (elastic) behaviour was 0.02 to 0.1, the sole exception being the non-polar capillary at tan
δ), which is the ratio of G′′ to G′ with values approaching 0 indicating an increasing solid-like (elastic) behaviour was 0.02 to 0.1, the sole exception being the non-polar capillary at tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ = 0.16 (Fig. S5C, ESI†). The polar capillary suspensions at 3, 5 and 7 wt% water presented the lowest tan
δ = 0.16 (Fig. S5C, ESI†). The polar capillary suspensions at 3, 5 and 7 wt% water presented the lowest tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ, signifying that they were the most solid-like of all gels.
δ, signifying that they were the most solid-like of all gels.
Frequency sweeps of the capillary and hybrid suspensions made with 3 vol% water are shown in Fig. 7. All materials were elastic-dominant (G′ > G′′) and exhibited largely frequency-independent G′ values. Similar trends were observed at all water loads (Fig. S6, ESI†). There was no crossover between G′ and G′′ within the frequency range examined of any material. As per the strain sweeps, the G′ of the 2% wax oleogel was lowest and remained generally frequency-independent. Its G′′ was frequency-dependent, indicating weak gel-like behaviour.19
4. Discussion
This study considered two modes of gelation, namely a hexatriacontane wax crystal network in a house-of-cards-like structure at its critical gelling concentration and capillary suspensions consisting of spherical particles connected by bridges of water which aggregates the particles into a network. These two modes of oleogelation are generally regarded as independent of one another in the literature. The former has been extensively studied in the context of different natural waxes for saturated fat replacement and arises from attractive interactions between sintered crystals.23 As shown here and elsewhere, interactions in capillary suspensions are more complex as they depend on the type of particle present, water load, and the interfacial forces between the majority, particle, and secondary phases.24,25 With non-polar particles, van der Waals interactions will dominate whereas hydrogen bonding will predominate with polar particles. In the presence of water added above its solubility limit, capillary forces will promote particle association into a percolated network, with hydrophobic interactions also playing a role if the particles are non-polar.Glass beads were surface-modified to compare the microstructures afforded by wetting (polar) and nonwetting (non-polar) particles with water capillary bridges. Water content was varied between (i) the minimum amount to produce a capillary suspension that resisted oil separation for 24 hours (1 and 3 vol% polar and non-polar particles, respectively), and (ii) the amount of water required to transition to an aggregated state with network weakening (9 vol%, both particle types). Particle type and quantity of water determined the type of interaction and, by extension, the resulting microstructure and gel rheology in each gel type.
The impetus in hybridizing these gelation methods stemmed from the use of a crystalline wax to kinetically trap the capillary network, and exploitation of capillary forces to provide structure to an otherwise delicate oleogel.
Wax and particle polarity significantly affected the viscoelastic profiles of the oleogels and particle suspensions. If we consider the base oleogels as our control 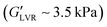 (Fig. 5), inclusion of polar and non-polar particles precluded gel formation
(Fig. 5), inclusion of polar and non-polar particles precluded gel formation 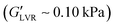 . As above, this was the result of the wax crystals' inability to effectively enmesh into a percolated network. There was no evidence of particle surface polarity effects on wax nucleation as both the polar and non-polar particles appeared to promote nucleation of wax crystal growth (Fig. S7, ESI†). Sintering of wax crystals was impeded by the particles acting as a physical barrier thereby reducing their size and ability to form a space-spanning network, irrespective of particle polarity. Ultimately, the wax crystals and particles underwent segregation that dictated their microstructure and rheological behaviour.
. As above, this was the result of the wax crystals' inability to effectively enmesh into a percolated network. There was no evidence of particle surface polarity effects on wax nucleation as both the polar and non-polar particles appeared to promote nucleation of wax crystal growth (Fig. S7, ESI†). Sintering of wax crystals was impeded by the particles acting as a physical barrier thereby reducing their size and ability to form a space-spanning network, irrespective of particle polarity. Ultimately, the wax crystals and particles underwent segregation that dictated their microstructure and rheological behaviour.
The aggregation behaviour of the particles in oil was highly dependent on their surface polarity and resulted in different capillary suspension morphologies. Unmodified glass beads contain surface alcohols capable of hydrogen bonding. Within oil-continuous media, the dipoles of these surface groups cannot be satisfied through interactions with the oil, yielding a higher surface energy on the particle surface. This results in the particles minimizing their contact area with the oil phase by aggregating into clusters. The particles surface-modified to be non-polar had their surface alcohols capped with trimethylsilyl groups limiting their ability to hydrogen bond. Given their reduced surface polarity, the non-polar particles readily dispersed in oil given their lower surface energy (Fig. S1, ESI†).
The particles in the capillary networks were distinct in their organization based on particle polarity and amount of added water. For particles of the same size distribution and volume fraction, the polar particles required less water to form a sample-spanning network than their non-polar counterpart. Stable particle clusters arising from pairwise menisci yielded the pendular regime whereas non-polar clusters required greater amounts of particles and water to form the capillary regime.26 Particle surface modification changed the wettability of the particles by limiting their ability to hydrogen bond. This altered the bridge shape from concave (wetting) to convex (non-wetting) and the capillary force was accordingly weakened by a repulsive capillary pressure.27
These contributing factors also influenced capillary suspension and hybrid morphology and rheology as water content was increased. The polar capillary suspensions transitioned to the funicular regime around 5 vol% water as there was sufficient water to cluster multiple particles around a single attractive meniscus. As the water concentration increased further, multiple particles were pulled into spherical aggregate clusters which grew in size (Fig. 4A). As more particles were pulled into these aggregates, the available surface area of the capillary network decreased along with the corresponding network strength and oil binding capacity.
For non-polar capillary suspensions, as water content increased, the system transitioned to a coarse Pickering emulsion where water droplets were decorated by interfacially bound particles (Fig. 4B).28 We attributed this behaviour to the system tending towards spherical agglomeration which was detrimental to network strength, as the particles and water were sequestered into agglomerates limiting participation in capillary bridges thereby reducing network strength and ability to trap oil.
The presence of wax generally reduced the stiffness of the polar capillary suspensions, with addition of more water gradually decreasing stiffness from ∼106 kPa to 300 kPa by increasing water load from 1 vol% to 9 vol%. Such a decline suggested an antagonistic effect between wax and water on the stiffness of the polar hybrid gels. By contrast, the presence of wax increased the stiffness of the non-polar capillary suspensions. While no capillary or hybrid gel formed at 1 vol% water, addition of ≥3 vol% saw not only the formation of a self-supporting capillary suspension, but also a hybrid gel with greater stiffness than the capillary suspension alone 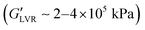 . This increase suggested a synergistic effect of the wax and water on the stiffness of the non-polar hybrid gels, and that water concentrations precluding capillary suspension formation did not lead to hybrid gel formation.
. This increase suggested a synergistic effect of the wax and water on the stiffness of the non-polar hybrid gels, and that water concentrations precluding capillary suspension formation did not lead to hybrid gel formation.
Increasing the water content also decreased the LVR and crossover strain of the polar capillary suspensions. The LVR of the hybrid gels decreased compared to the capillary suspensions, implying that less strain was required to begin the irreversible deformation of the network, hinting at greater brittleness. The crossover strain of the hybrids increased relative to the capillary suspensions, likely due to the increased solids content by virtue of the wax addition. Comparing the wax network to the capillary suspensions, a greater strain was required to reach the cross-over strain in the wax gel.
All capillary suspensions demonstrated a G′′ overshoot with increasing strain corresponding to the reorganization within the capillary network. This phenomenon was reduced in the non-polar capillary suspension counterpart due to the water phase being sequestered by particles. The hybrid suspensions also saw this G′′ overshoot suppressed, though we attributed this to wax crystals preventing particle bridge fragments from encountering other particle network bridges.
5. Conclusion
We have presented the first instance of a hybrid gel combining a capillary suspension and a wax-based oleogel. At the critical gelling concentration of the wax network, both polar and non-polar particle types prevented the wax oleogel from forming, which caused a notable decrease in G′ and enhanced phase separation compared to the oleogel alone. We attributed this behaviour to the reduction in wax platelet size and the lack of a homogeneous particle distribution that prevented connectivity between wax platelets into a network. The use of water to sequester particles in the hybrid network allowed the wax oleogel to form in the oil phase with both polar and non-polar particles yielding a unique microstructure and rheology for the polar and non-polar particles. The hybrid gels made with polar particles were weakened by increasing water fraction whereas the non-polar hybrids were not as sensitive to water load and possessed a G′ greater than the contributions of the component gels. We demonstrated that a wide range of textures were accessible through formation of the hybrids (300 to 1800 kPa) yielding a tuneable soft solid with potential for solid fat replacement. Broadly speaking, as the hybrids formed with both particle types, the present approach could presumably be applied to oleogels containing polar and/or non-polar particles. These results confirmed our hypothesis as both water addition and oleogelation contributed to structure formation in these gels. Future efforts will continue to expand on the combined roles of oleogelation and capillary suspensions on hybrid material structure and rheology.Author contributions
Selvyn Simoes: conceptualization, investigation, methodology, experimentation, data analysis, writing – original draft, review & editing, writing – revised draft, review & editing. Dérick Rousseau: supervision, funding acquisition, conceptualization, investigation, writing – writing – original draft, review & editing, writing – revised draft, review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The Natural Sciences and Engineering Research Council of Canada (NSERC) and AAK are acknowledged for their support. We would like to thank Jeppe Lindegaard Hjorth for his support and insights throughout this work.References
- L. Bayés-García, A. R. Patel, K. Dewettinck, D. Rousseau, K. Sato and S. Ueno, Curr. Opin. Food Sci., 2015, 4, 32–38 CrossRef
.
- L. S. K. Dassanayake, D. R. Kodali and S. Ueno, Curr. Opin. Colloid Interface Sci., 2011, 16, 432–439 CrossRef CAS
.
-
K. Sato in Crystallization of Lipids: Fundamentals and Applications in Food Cosmetics and Pharmaceuticals, ed. K. Sato, Wiley Blackwell, Hoboken, NJ, 1st edn, 2018, vol. 1, pp. 6–7 Search PubMed
.
- P. Terech and R. G. Weiss, Chem. Rev., 1997, 97, 3133–3159 CrossRef CAS PubMed
.
- A. Puscas, V. Muresan, C. Socaciu and S. Muste, Foods, 2020, 9, 1–27 CrossRef PubMed
.
- P. L. Fuhrmann, J. Powell and D. Rousseau, Food Hydrocolloids, 2023, 139, 108503 CrossRef CAS
.
- C. P. Whitby, Front Sustainable Food Syst., 2020, 4, 1–8 CrossRef
.
- M. Scharfe, J. Niksch and E. Flöter, Eur. J. Lipid Sci. Technol., 2022, 124, 1–19 CrossRef
.
- C. D. Doan, I. Tavernier, P. K. Okuro and K. Dewettinck, Innovative Food Sci. Emerging Technol., 2018, 45, 42–52 CrossRef CAS
.
- B. Mert and I. Demirkesen, Food Chem., 2016, 199, 809–816 CrossRef CAS PubMed
.
- D. C. Zulim Botega, A. G. Marangoni, A. K. Smith and H. D. Goff, J. Food Sci., 2013, 78, 1334–1339 Search PubMed
.
- M. R. Elkatory, E. A. Soliman, A. El Nemr, M. A. Hassaan, S. Ragab, M. A. El-Nemr and A. Pantaleo, Polymers, 2022, 14(16), 3231 CrossRef CAS PubMed
.
- E. Koos, Curr. Opin. Colloid Interface Sci., 2014, 19, 575–584 CrossRef CAS PubMed
.
- S. Hoffmann, E. Koos and N. Willenbacher, Food Hydrocolloids, 2014, 40, 44–52 CrossRef CAS
.
- G. S. Wang, H. Y. Chen, L. J. Wang, Y. Zou, Z. L. Wan and X. Q. Yang, Food Hydrocolloids, 2022, 133, 107912 CrossRef CAS
.
- J. Yang, N. Heinichen and S. S. Velankar, Colloids Surf., A, 2018, 558, 164–170 CrossRef CAS
.
- F. Bossler and E. Koos, Langmuir, 2016, 32, 1489–1501 CrossRef CAS PubMed
.
- D. J. Abdallah, S. A. Sirchio and R. G. Weiss, Langmuir, 2000, 16, 7558–7561 CrossRef CAS
.
- A. R. Patel, M. Babaahmadi, A. Lesaffer and K. Dewettinck, J. Agric. Food Chem., 2015, 63, 4862–4869 CrossRef CAS PubMed
.
- E. Koos and N. Willenbacher, Soft Matter, 2012, 8, 3988–3994 RSC
.
- S. S. Velankar, Soft Matter, 2015, 11, 8393–8403 RSC
.
- S. Bindgen, F. Bossler, J. Allard and E. Koos, Soft Matter, 2020, 16, 8380–8393 RSC
.
- C. D. Doan, C. M. To, M. De Vrieze, F. Lynen, S. Danthine, A. Brown, K. Dewettinck and A. R. Patel, Food Chem., 2017, 214, 717–725 CrossRef CAS PubMed
.
- E. Koos and N. Willenbacher, Science, 2011, 331, 897–900 CrossRef CAS PubMed
.
- F. Bossler and E. Koos, Langmuir, 2016, 32, 1489–1501 CrossRef CAS PubMed
.
- S. Bindgen, J. Allard and E. Koos, Curr. Opin. Colloid Interface Sci., 2022, 58, 101557 CrossRef CAS
.
- K. D. Danov, M. T. Georgiev, P. A. Kralchevsky, G. M. Radulova, T. D. Gurkov, S. D. Stoyanov and E. G. Pelan, Adv. Colloid Interface Sci., 2018, 251, 80–96 CrossRef CAS PubMed
.
- E. Koos, Curr. Opin. Colloid Interface Sci., 2014, 19, 575–584 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sm01619f |
| This journal is © The Royal Society of Chemistry 2024 |