Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A sustainable aviation fuel pathway from biomass: life cycle environmental and cost evaluation for dimethylcyclooctane jet fuel†
Rahamim
Batten
 a,
Mukund
Karanjikar
b and
Sabrina
Spatari
a,
Mukund
Karanjikar
b and
Sabrina
Spatari
 *ac
*ac
aFaculty of Civil and Environmental Engineering, Technion – Israel Institute of Technology, Haifa 3200003, Israel. E-mail: ssabrina@technion.ac.il
bTechnology Holding, Salt Lake City, UT 84119, USA
cGrand Technion Energy Program, Technion – Israel Institute of Technology, Haifa 3200003, Israel
First published on 20th March 2024
Abstract
Biomass is a promising renewable feedstock for conversion to sustainable aviation fuel (SAF) to mitigate near-term greenhouse gas (GHG) emissions. Through metabolic engineering, sugars derived from pretreated and hydrolyzed cellulose and hemicellulose can be directly fermented to isoprene and catalytically upgraded to 1-4-dimethylcyclooctane (DMCO), an environmentally beneficial and high performance alternative to petroleum-based jet fuel. Cellulosic sugars may allow for greater GHG emission reduction compared to first generation sugars and meet scaling needs to reduce dependence on petroleum-based kerosene. Here, we assess the environmental impact and economic feasibility of utilizing direct isoprene fermentation from residual biomass sugars as an intermediate step in the production of DMCO via life cycle assessment (LCA) and techno-economic analysis (TEA). We use chemical process modeling to simulate the conversion of sugars from biomass to isoprene, dimerization to dimethylcyclooctadiene (DMCOD) and catalytic hydrotreatment to DMCO. The bottom-up process model serves as the basis for constructing the life cycle inventory to assess environmental impacts and to predict economic feasibility. Results show a GHG intensity of 7.2 gCO2e MJ−1 that is significantly lower than that of current petroleum jet (89 gCO2e MJ−1) when using Zea mays L. residue (corn stover) as feedstock. The TEA indicated that the target costs have the potential to be competitive with a minimum fuel selling price of DMCO between $1.01 and $1.32 per L. Direct fermentation of isoprene could improve the overall process efficiency and reduce energy consumption, while also enhancing the environmental sustainability of the process.
1 Introduction
The expected growth in the global demand for air travel in the coming decade along with the nearly complete dependence on petroleum-based fuels with limited alternative options has raised the need to develop low carbon fuels to mitigate climate change. Drop-in jet fuel derived from lignocellulose (biomass) is projected to be an important resource for producing sustainable aviation fuel (SAF) to mitigate greenhouse gas (GHG) emissions from air travel in the near future. Most aviation fuel is conventionally supplied from kerosene produced from crude oil with an annual global consumption surpassing 330 Mt (2019).1 The rapid growth in the aviation industry suggests that rising global demand for aviation fuel is expected in the next few decades and it has been projected to more than double by 2050.2 The challenges of crude oil prices, national security, and meeting sustainability goals in aviation make it necessary to have short and medium-term solutions to decarbonize the sector. Developing SAF blendstocks enables a path to decarbonizing aviation while using existing infrastructure and aircraft fleet.Lignocellulose is an abundant, renewable and sustainable resource that does not compete with the food chain and includes agricultural waste, forest residues, and woody biomass.3–6 Many countries around the world have adopted low carbon and renewable fuel standards that target biofuels as part of their energy policies to mitigate GHG emissions from their transportation sectors.7 Those policies use life cycle assessment (LCA) to benchmark candidate fuels having an average fuel carbon intensity (AFCI), which measures GHG emissions in gCO2e MJ−1, below that of a petroleum-based fuel. As an example of such a policy, the U.S. Environmental Protection Agency, through its Renewable Fuel Standard,8 aims to achieve at least a 60% reduction in the AFCI of cellulosic fuels, measured in carbon dioxide equivalents (CO2-eq.) relative to a baseline petroleum fuel. For aviation, international standards developed through the Carbon Offsetting and Reduction Scheme for International Aviation (CORSIA) define criteria for SAF-eligible drop-in fuels as requiring at least a 10% reduction in life cycle GHG emissions compared to emissions from a baseline petroleum jet-fuel set at 89 gCO2e MJ−1.9 CORSIA has certified many biofuel pathways originating from first generation feedstocks made from edible crops and second generation (lignocellulosic) feedstocks that all meet SAF eligibility criteria. The lignocellulosic feedstocks have potential to achieve the lowest AFCI among certified SAFs. This is owing to them having low GHG emissions during feedstock cultivation and harvesting stages and no or minimal land use change (LUC) GHG emissions.10 According to a review by Jeswani et al.,11 the absence of LUC GHG emissions and reduced soil GHG emissions make agricultural residues and dedicated energy crops suitable for producing low-AFCI fuels. Moreover, other studies have noted potential ecosystem service benefits if these feedstocks are grown on marginal lands.12
The vast majority of prior LCA studies on second generation biofuels (lignocellulosic feedstocks and technologies) via the bioconversion platform have focused on ethanol.13–19 Those studies demonstrated a significant reduction in life cycle GHG emissions relative to a petroleum-based gasoline baseline of 93 gCO2e MJ−1. The AFCI range for lignocellulosic ethanol depends on feedstock source and management practice, biofuel pre-treatment and conversion technology, and how co-products are treated. Previous studies have mostly concluded that lignocellulosic ethanol pathways sourced from agricultural residues (−25 to 40 gCO2e MJ−1)16,20 or dedicated energy crops like switchgrass (2 to 15 gCO2e MJ−1)19,21 and miscanthus (5 to 22 gCO2e MJ−1)22 grown on non-arable agricultural land or annual crops like biomass sorghum (25 to 60 gCO2e MJ−1)23,24 would meet policy objectives in the U.S. under select conditions. Studies have also found the potential for deep decarbonization as low as −179 gCO2e MJ−1 if coupling lignocellulosic ethanol production with bioenergy carbon capture and storage (BECCS)23,25 and advanced ethanol conversion involving consolidated bioprocessing.26,27 However, ethanol has blend wall restrictions that limit its market expansion. In contrast, drop-in fuel for long-haul biofuel markets like aviation will continue to rise with the need to decarbonize the sector. Thus recent studies have examined both the cost and GHG emissions through LCA of promising emerging thermochemical and biochemical production to meet the SAF demand from biofuels. Reported GHG emission results for SAF production from thermochemical routes (e.g. FT synthesis and fast pyrolysis) range between −1.6 and 70 gCO2e MJ−1 jet fuel28–32 as compared to biochemical production routes (e.g. alcohol-to-jet and sugar-to-jet) of 12 to 90 gCO2e MJ−1 jet fuel.33–38Fig. 1 details a GHG comparison between recent SAF pathways for jet fuel production and includes the 80.1 gCO2e MJ−1 threshold for SAF eligible fuels according to CORSIA.10
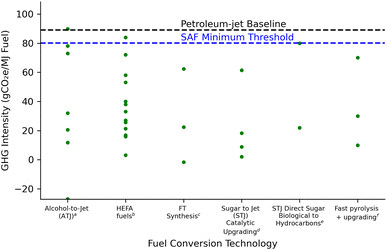 | ||
| Fig. 1 GHG intensity SAF pathway comparison to fuel conversion technology pathways: ATJ, HEFA fuels, FT synthesis, and STJ catalytic. a Han (2017),37 Gollakota (2021),39 Staples (2014),33 Budsberg (2016),34 Tanzil (2022).40b Cox (2014),38 Stratton (2011),41 de Jong (2017),42 Bailis (2010),15 Seber (2014).43c Shonnard (2010),44 de Jong (2017),42 Suresh (2018).29dRiazi (2018),35 Baral (2021).36e Han (2017),37 Cox (2014).38fSorunmu (2017),30 Elkasabi (2020),45 Fitriasari (2023).31 | ||
Chemical process simulations have been used for cost estimation of many early-stage industrial processes, including the conversion of biomass to fuels and value-added co-products.46 They help estimate the effect of variations in raw materials, changes in operational conditions, process configurations or scaling and integration of early-stage technology.47 These simulations provide the material and energy basis for LCA and technological and financial basis for TEA to evaluate production alternatives, including for the production of bio-jet fuel blendstock produced from biochemical35,36,48–50 and thermochemical31,32 conversion processes.
In this paper, we evaluate the cost and environmental tradeoffs of producing a bio-jet fuel capable of replacing current petroleum derived Jet-A fuel: 1,4-dimethylcyclooctane (DMCO). Although without regulatory approval, DMCO, as a prospective jet fuel blendstock, has demonstrated enhanced gravimetric and volumetric net heats of combustion, surpassing conventional jet fuel by 2.4% and 9.2%, respectively.51 This improvement, which can be attributed to its cyclic structure and inherent ring strain, allows DMCO to be blended in high concentrations of jet fuel.52 Recent work by Walkling et al.53 tested a DMCO and conventional Jet A blend with a ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 and found that the blend satisfies all ASTM fuel standards for industry implementation. DMCO can be catalytically produced from an intermediate product of biomass, isoprene, and then converted to DMCO in two steps: dimerization and subsequent hydrogenation (Fig. 2). There are numerous routes to produce the intermediate product, isoprene. Industrially, isoprene is produced from petroleum sources as a by-product in naphtha cracking for the production of ethylene and is a monomer of natural rubber. Alternatively, it can be produced from sugars in Escherichia coli through a dual-pathway of the mevalonic acid (MVA) or methylerythritol phosphate (MEP) processes as shown by Yang et al.,54 who measured yields of 26.7 w/w%, the highest experimental yield to date, which equates to 26.7 g of isoprene synthesized per 100 g glucose.
90 and found that the blend satisfies all ASTM fuel standards for industry implementation. DMCO can be catalytically produced from an intermediate product of biomass, isoprene, and then converted to DMCO in two steps: dimerization and subsequent hydrogenation (Fig. 2). There are numerous routes to produce the intermediate product, isoprene. Industrially, isoprene is produced from petroleum sources as a by-product in naphtha cracking for the production of ethylene and is a monomer of natural rubber. Alternatively, it can be produced from sugars in Escherichia coli through a dual-pathway of the mevalonic acid (MVA) or methylerythritol phosphate (MEP) processes as shown by Yang et al.,54 who measured yields of 26.7 w/w%, the highest experimental yield to date, which equates to 26.7 g of isoprene synthesized per 100 g glucose.
Synthesizing fully infrastructure compatible (oxygen-free) fuel via metabolic pathways is challenging due to the oxygen present in the carbohydrate substrate. Thus, fermenting an oxygen-free molecule from five- and six-carbon sugars while retaining most carbon atoms from the sugar could be a key step in the synthesis route. Isoprene is a promising molecule for attaining this objective.55 Rosenkoetter et al.51 investigated the selective dimerization of isoprene over an Fe(II) catalyst to produce DMCOD, which can be further hydrogenated to produce DMCO, a high-performance bio-based jet fuel that could meet SAF criteria. Previous research35,36,48 has examined the production of isoprene through indirect fermentation of methyl-butyl-ether fermented from cellulose and hemicellulose sugars with isoprene yields from dehydration of isoprenol of 10.2 to 21.5 g per 100 g of glucose, lower than the direct fermentation yield of 26.7 g isoprene. Yang et al.54 demonstrated the direct fermentation to isoprene from sugars using metabolically engineered E. coli. If scaled, the technology can yield oxygen-free reaction intermediates (isoprene) that can be upgraded to produce infrastructure compatible bio-jet while bypassing the alcohol conversion step. Batten et al.56 evaluated the life cycle global warming differences between DMCO produced from direct fermentation of sugars derived from corn and concluded that land use change could limit deep decarbonization goals if not combined with carbon capture and sequestration processes. That limitation may be overcome if using lignocellulose as feedstock.
Our objective is to investigate the potential for reducing GHG emissions and evaluate costs in the aviation fuel industry by using corn stover feedstock, a lignocellulosic agricultural residue, in biorefineries to convert sugars to renewable jet fuel (DMCO). Riazi et al.35 and Baral et al.36 previously studied the indirect pathways to producing DMCO via dehydration of isoprenol using agricultural residues and purpose grown crops, respectively. Here, we examine the direct fermentation of isoprene from sugars derived from abundant agricultural residues (corn stover) in the U.S. Midwest and subsequent dimerization of isoprene to DMCOD and its conversion to high energy density DMCO through hydrogenation.
2 Methods
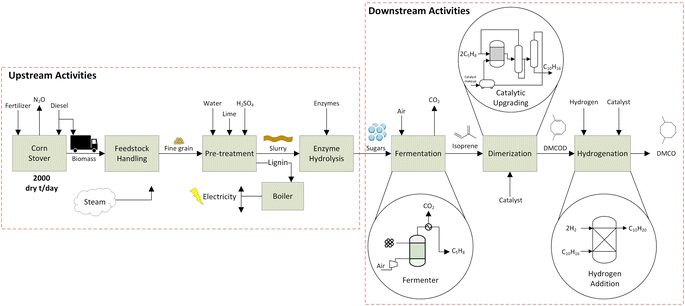
This study applies the LCA framework according to the International Organization for Standardization (ISO 14040/44)57 and TEA to evaluate the environmental and economic impacts associated with DMCO jet-fuel blendstock from corn (Zea mays L.) stover. Material and energy balances for the life cycle inventory (LCI) that track biomass conversion were estimated from chemical process simulations formulated with experimental data. We follow methods set by ISO 2006 (ref. 58) to construct a LCI model from the cradle-to-grave mass and energy balances and use background data from ecoinvent v3.6 (ref. 59) within SimaPro 9.3 software.60 The functional unit is defined as 1 MJ of DMCO jet-fuel.2.1 Biomass feedstock acquisition
Renewable DMCO jet-fuel is modeled as a well-to-wake process that includes feedstock production, harvest (where soil N2O emissions and change in soil organic carbon (SOC) are considered), transport of the feedstock, pretreatment, hydrolysis, fermentation, catalytic isoprene dimerization and hydrogenation to DMCO (Fig. 2). Data from previous literature16,21 are used for the cradle-to-gate LCI inputs related to feedstock acquisition (diesel for farm operations, including for corn stover harvesting) and N–P–K nutrient addition to replace quantities in the biomass removed (Table S2†). To meet the biorefinery's feedstock demand, Iowa, located in the corn belt of the USA, was chosen for corn stover supply following the work of Pourhashem et al.16 and Adler et al.61 Both studies used the DayCent biogeochemical model for Boone county, Iowa to estimate soil N2O and soil organic carbon (SOC) change emissions related to corn stover removal. The feedstock supply model assumes that 50% of corn stover is removed from agricultural fields and transported to the biorefinery by diesel powered trucks. Thus, GHG emissions from feedstock acquisition include cradle-to-gate nutrient replacement inputs, soil N2O and soil organic carbon (SOC) change emissions, which for corn stover removal imparts a depletion of SOC stock, and feedstock transportation to the biorefinery. Table S2 in the ESI† summarizes all assumed inputs into the LCI for feedstock collection and delivery to the biorefinery. The biorefinery is modeled to match the National Renewable Energy Laboratory's (NREL) biorefinery design62 of 2500 metric tons (20% moisture content) of biomass per day (MTPD). The supply of corn stover as the feedstock is assumed to be sourced from within an average 80 km (approx. 50 mile) radius of the biorefinery.2.2 Sugar fermentation to isoprene
Specific areas of the biorefinery were modelled using experimental data to provide heat duty unit operations simulated using UniSim chemical process engineering software. The process flow diagrams for specific unit operations described herein are outlined in the ESI (Fig. S2 and S3†).63 The main inputs for the LCI are provided by the mass and energy balances that are evaluated from the UniSim Process Simulation for the direct fermentation and catalytic upgrading steps. Several unit operations for feedstock handling, pretreatment, and hydrolysis were taken from the literature16,21,35 to build mass balances to estimate the IPCC AR5 impact that included loading of chemicals, enzymes and nutrients.After the corn stover is brought to the biorefinery, pre-treatment and hydrolysis are carried out. Previous studies16,35 were used for these steps assuming dilute acid pretreatment and enzymatic hydrolysis.62 The lignin portion of the feedstock is assumed to be fractionated after pretreatment and combusted to generate electricity and steam onsite providing the energy required for the biorefinery similar to prior LCA models13,14 and chemical process simulations.62 The electricity generated is surplus to the operational needs of the biorefinery, providing an electricity credit that is assumed sold to the electricity grid, reducing the environmental and economic impact of the process.21,64 The Midwest Reliability Organization (MRO) electricity grid, which supplies electricity to Iowa, is used for all LCA calculations.
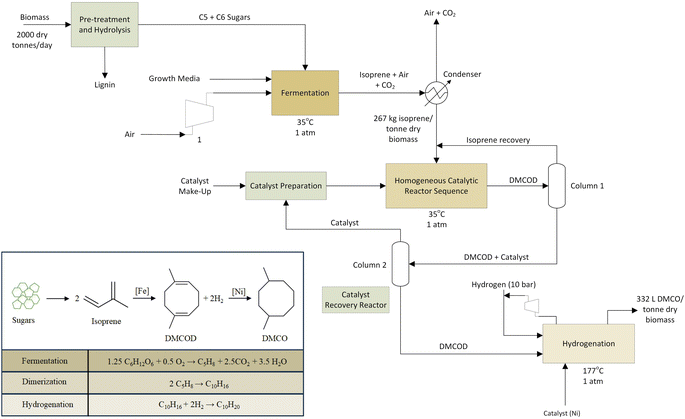
The fermenter, operating under aerobic conditions, was modelled using UniSim chemical process simulation.63 Dextrose, a native UniSim component, that has been used to model 6 carbon sugars was used as a feed along with air to supply oxygen. The feed temperature was considered at room temperature of 20 °C to match the average yearly temperature in Iowa, US. The direct fermentation (DF) using genetically engineered E. coli converts sugar to isoprene using parameter conditions outlined in the dual MEP/MVA pathway54 according to stoichiometry (Fig. 3).
The fermentation process was modeled based on pilot-scale experiments operating on a 100 L reactor at Technology Holding LLC laboratories to determine the initial operating parameters and ensure that isoprene production is maintained in an environment to maximize its yield. Isoprene yields used in the process simulation and LCI are taken from experiments on the 100 L reactor and scaled-up through process simulation. The energy requirements for fermentation are dominated by the heat required to raise the feed temperature (20 °C) to the reactor temperature (35 °C). The fermenter reactor temperature was set at 35 °C as isoprene changes to the vapor phase at 34.1 °C.65 Using data from fermentation experiments, the heat duty required for this step is calculated using optimized process simulation in the UniSim design suite.63 Direct CO2 emissions from the fermentation step are calculated using the net stoichiometric fermentation reaction (Fig. 3). Total GHG emissions of bio-conversion are related to biogenic carbon uptake by the feedstock along with boiler lignin combustion and fuel combustion, and are balanced by the CO2 uptake during plant growth and are assumed to be netted out to zero. After exiting the fermenter, isoprene is recovered via a condenser and flash separator. The condenser modeling allows for heat duty calculations; however, in the refinery alternative equipment such as a gas absorption unit may be used to separate CO2 and vapor impurities from the isoprene gas.
Inlet feed stream temperatures and reactor temperature were set at 20 °C and 35 °C to concur with previous studies.35,54 Isoprene has a boiling point of 34.1 °C and the reaction proceeds to allow dilute isoprene in the tops product. The rate of production of isoprene is considered as 1 kg of isoprene in a 1000 L reactor formed in 1 hour according to a titer value of 60 g L−1 broth.55 A feed of 3.54 kg input of sugars is required to produce 1 kg of isoprene. The fermenter product stream consists of 26.7% isoprene by mass and the recovery of isoprene is modelled in UniSim with a condenser unit process. A small distillation duty is incorporated from the downstream cooling of DMCO. The UniSim model provided mass and energy balances required for the LCI model.
2.3 Isoprene upgrading to DMCO
In the dimerization step, isoprene is pre-heated to enter the homogeneous catalytic reactor sequence in vapor form at 35 °C corresponding to the reactor temperature. A 2% isoprene recovery recycle is assumed and an optimal 97% conversion to DMCOD.51 An iron-based catalyst is used to facilitate the dimerization step at a 0.025 mol% loading.51 The dimerization step is modeled in a conversion reactor in UniSim and is considered to behave as a continuous stirred tank reactor.The DMCOD product stream is then fed to the hydrogenator with 2 mol of hydrogen for every mol of DMCOD using a nickel catalyst at 1% w/w loading. Atmospheric hydrogen is compressed to 10 bar and fed to the hydrogenator. The hydrogenation step takes place at 177 °C and the exit gas stream of DMCO is cooled for storage by process cooling water. An 80% heat recovery from the cooling of DMCO for pre-heating the inlet streams of the hydrogenator is applied. The majority of the hydrogen is reclaimed and returned to the hydrogenation unit. Section 2.4 will discuss sensitivity for the catalytic upgrading steps.
It is assumed that all utility steps are supplied by combustion of the lignin by-product in the pre-treatment step and a surplus of electricity supply to the grid has been calculated, which is assumed to offset emissions using system expansion credits. The effect of catalysts on the biofuel's life-cycle GHG emissions hinges on their production emissions and consumption rate. Some catalysts, despite a high production GHG footprint, can have minimal impact if they last long and have low consumption rates, especially in a farm-to-wheels context. RANEY® nickel catalysts used in this process are considered to have an influence on the life-cycle GHG emissions of biofuels and therefore sensitivity for catalyst loading in the overall lifecycle is included.66 Based on Dros et al.(2015)67 the following best, worst and base case sensitivities were considered for the catalyst recovery loading:
(1) Best case: low catalyst loss 0.1 g per kg DMCO.
(2) Worst case: high catalyst loss 5 g per kg DMCO.
(3) Base case: 1 g per kg DMCO.
Previous findings have shown that transporting sugar with a conventional diesel-powered truck increases its GHG footprint, contributing to a 4.4 gCO2e MJ−1 per 100 km increase in the current technological state and a 1.7 gCO2e MJ−1 per 100 km increase in the optimal future case.36 We use a conservative value of 2 gCO2e MJ−1 to cover the return journeys of GHG emission feedstock transportation and an additional 5 gCO2e MJ−1 for the worst case catalyst make-up scenario.35,67
2.4 Economic assumptions
A process model for the production of DMCO was developed and used as the basis for the LCA and TEA. Mass and energy balance outputs from the UniSim Process Simulation models combined with NREL's Technical report were used to evaluate all capital and operating costs to establish an overall cost of production value.63,68 The feed-rate of lignocellulose has been chosen based on previous literature and allows for a straightforward benchmark against other biomass TEA models.35,62 All capital costs for DMCO fuel production were estimated based on prior literature studies,36,62 NREL's Excel costing spreadsheet68 (Table S4†) or standard engineering estimates utilizing product and process design principles.69 Likewise, all operating costs were calculated by leveraging the framework of NREL's Microsoft Excel economic spreadsheet for the corn stover to hydrocarbon fuel pathway with changes outlined in the ESI, Tables S5 and S6.†62 Key assumptions used in the TEA are outlined in Table 1 and all monetary values are given for the year 2018 in USD $.| Item | Scale |
|---|---|
| Plant life | 30 years |
| Internal rate of return | 5% |
| Plant depreciation | 200% declining balance (DDB) |
| Plant recovery period | 7 years |
| Vapor plant depreciation | 150% DB |
| Vapor plant recovery period | 20 years |
| Taxes | 21% |
| Financing | 40% |
| Loan terms | 10 years, 8% interest |
| Construction time | 3 years |
| First year expenditure | 8% |
| Second year expenditure | 60% |
| Third year expenditure | 32% |
| Working capital | 5% of fixed capital investment |
| Start-up time | 3 months |
| Revenues during start-up | 50% |
| Variable costs during start-up | 75% |
| Fixed costs during start-up | 100% |
The UniSim process simulation results were used to establish variable operating requirements associated with raw materials, waste management, electricity requirement, and process byproducts. While the economic analysis maintains a majority of cost assumptions used by Humbird et al. (2011)62 several changes were made to the model to account for the downstream catalytic upgrading to DMCO. We assume a conservative value for hydrogen supply of $1.61 per kg.68 These changes are summarized in Table 2.
2.5 Sensitivity analysis
Performing a sensitivity analysis is critical for assessing the extent to which key uncertain parameters impact the project's economic feasibility and environmental impact.70 Certain parameters were chosen that were likely to have a significant impact. Based on previous research,16,21,35 data were gathered from several sources, including experimental findings, existing literature, and model simulations, to delineate the upper and lower limits of these parameters (Tables S1 and S2†). Three scenarios were examined related to the life cycle GHG emissions, including the best case, single-point sensitivity analysis of hydrogen supply and varied catalyst loading based on GREET's 2022 model.71 Hydrogen supply for the hydrogenation step is produced from steam methane reforming with a GHG intensity that ranges from 8.2 to 11 g CO2e kg−1 H2 evaluated from the GaBi Professional database.72,733 Results and discussion
3.1 LCA results
The three scenarios for life cycle GHG emissions of corn stover to DMCO jet fuel are presented in Fig. 4 and Table 3 using the direct fermentation step of lignocellulosic sugars to isoprene. The best case scenario represents total life cycle emissions of 7.2 gCO2e MJ−1 DMCO fuel. Two additional scenarios were assessed to allow for sensitivity of H2 supplied for the hydrogenation reactor and the catalyst loading within the system with GHG emissions of 15.6 and 16.3, respectively. The hydrogen sensitivity has a ±1.4 gCO2e MJ−1 DMCO effect on the GHG emissions. When considering a high load of catalyst make-up, an additional 2 gCO2e MJ−1 DMCO has been calculated as compared to 0.4 and 0.04 for base and low catalyst make-up, respectively. The effect of both hydrogen sensitivity and catalyst make-up has a low impact on the GHG emission life cycle and substantiates Benavides et al.'s66 findings for possible catalyst life-cycle GHG emissions.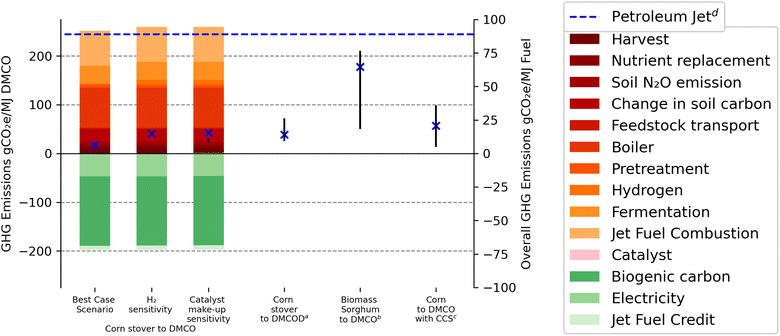 | ||
| Fig. 4 GHG emissions of corn stover to DMCO expressed in gCO2 MJ−1 DMCO, including best case, H2 supply and catalyst loading sensitivity. a Riazi et al. (2018).35b Baral et al. (2021).36c Batten et al. (2023).56d Prussi et al. (2021).10 89 CO2e MJ−1 petroleum jet. | ||
| Life cycle component | Baseline | H2 supply | Catalyst make-up |
|---|---|---|---|
| Harvest | 12.9 | 12.9 | 12.9 |
| Nutrient replacement | 5.1 | 5.1 | 5.1 |
| Soil N2O emission | 7.0 | 7.0 | 7.0 |
| Change in soil carbon | 25.5 | 25.5 | 25.5 |
| Biogenic carbon | −189.6 | −189.6 | −189.6 |
| Feedstock transport | 2.0 | 2.0 | 7.0 |
| Pretreatment | 6.0 | 6.0 | 6.0 |
| Chemicals | 2.0 | 12.1 | 8.9 |
| Fermentation | 36.9 | 36.9 | 36.9 |
| Boiler | 82.9 | 82.9 | 82.9 |
| Electricity | −47.1 | −47.1 | −47.1 |
| Jet fuel combustion | 71.6 | 71.6 | 71.6 |
| Jet fuel credit | −7.9 | −7.9 | −7.9 |
| Total | 7.2 | 15.6 | 16.3 |
Combustion of the jet fuel has been included to represent the well-to-wake model and allow for comparison to previous pathways for jet fuel production from biomass sources. Pathways similar to this study that involved production of renewable jet fuel with catalytic upgrading (DMCOD and DMCO) are included in Fig. 4 from Riazi et al.35 of 14.9 gCO2e MJ−1 DMCO and Baral et al.'s36 range of 18.3–61 gCO2e MJ−1 DMCO. Major differences in life cycle emissions are attributed to the feedstock source. For example, Baral et al.36 examined the use of biomass sorghum, which is a dedicated annual crop that requires a large annual input of nutrients to attain high annual yield, whereas agricultural residues assumed here and in the work by Riazi et al.35 would require significantly lower nutrient replacement quantities, but would also involve SOC loss, which raises GHG emissions. The work by Riazi et al.19 only modeled indirect fermentation to DMCOD, the pre-hydrogenation jet fuel blendstock. However, the value of 14.9 gCO2e incorporates the hydrogenation step modeled in the current work and the sensitivity includes upper and lower bounds (10.1 to 26.2 gCO2e) based on parameters that could affect life cycle GHG emission results. The CORSIA baseline for petroleum jet GHG emissions (89 gCO2e) includes crude oil recovery, transportation and refining, jet fuel transportation, and jet fuel combustion.2 Prior literature37 reported a benchmark of petroleum jet of 85 gCO2e MJ−1 petroleum jet fuel that included 12.4 gCO2e and 72.9 gCO2e for well-to-pump and pump-to-wake, respectively.
The GHG emissions have greater savings than previous studies involving the indirect fermentation to 1-methyl-3-butenol35,36 and confirm the engineering logic that by removing process units through process intensification, the environmental impact, in this case GHG emissions, would decrease overall. Table 3 outlines the results shown in Fig. 4.
In the corn stover-to-DMCO pathway, DMCO achieves GHG emissions savings relative to current petroleum jet. The corn stover feedstock is used to make DMCO jet fuel that is converted into fuel with a small penalty (25.5 gCO2e MJ−1) related to SOC loss (Table 3). This results in a relatively low GHG footprint per unit of lignocellulosic sugar and consequently a modest GHG benefit from increased sugar-to-fuel yields. Biogenic CO2 is defined as CO2 emissions directly resulting from combustion, decomposition, or processing of biologically based materials other than fossil fuels, peat, and mineral sources of carbon through combustion, digestion, fermentation, or decomposition processes. Here it is calculated according to the carbon in the feedstock that has been removed through harvesting the corn stover.
To further reduce net GHG contributions, the 60% yield of feedstock to sugar must be improved as this would increase yields in the final fuel product. Improved yields arising from dilute acid pre-treatment have been shown to reach as high as 66.8%.74 However, the 40% of biomass that is removed during pre-fermentation processes (pre-treatment and enzymatic hydrolysis) is mostly used in the production of steam and electricity in the lignin boiler. This use of renewable biomass originating in the feedstock translates into a GHG emissions credit from displaced electricity from the regional electricity grid. This electricity credit is calculated from the surplus electricity sold back to the grid. Yet, this credit is uncertain; in particular, as regional electricity grids decarbonize, the credit will also decline and in the worst case, it could displace current marginal sources of electricity supplied from other renewable sources. Thus, in the long term, such a pronounced electricity credit (−47 gCO2e MJ−1) will reach a lower limit that would raise the net AFCI of a fuel like DMCO. The jet-fuel credit calculation of −7.9 gCO2e MJ−1 DMCO is based on the improved net heat of combustion as compared to the minimum requirements for jet-A fuel according to ATSM standards. A sample calculation is given in the ESI.†
Table S7† gives a comparative analysis of various biofuel pathways including alcohol-to-jet (ATJ), HEFA fuels, FT synthesis, STJ catalytic upgrading, STJ direct sugar biological to hydrocarbons, and fast pyrolysis. DMCO, produced from an STJ catalytic upgrading pathway, emerges as a highly promising jet fuel. The environmental performance underscores the potential of DMCO as a sustainable alternative for current aviation fuels, demonstrating an opportunity to reduce carbon emissions in the aviation sector.
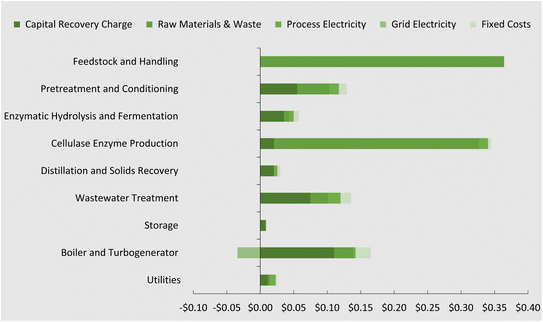
3.2 Economic analysis
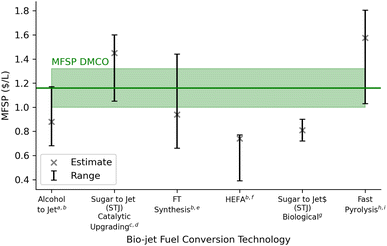
Fig. 5 presents the contribution to the overall cost (minimum selling price) by process area according to capital, operational, and fixed costs. The tabulated results can be found in Table S3 of the ESI.† Compared with previous results obtained by Humbird et al.62 for ethanol, this process showed an improvement on the boiler and turbogenerator contribution, due largely to the increased sale price of electricity to the grid ($0.069 kWh−1). All other process areas have increased in cost and this can be attributed to yearly price increases that were considered.The minimum fuel selling price (MFSP) of DMCO jet-fuel computed from the TEA is $1.16 per litre ±0.16. This is representative according to a breakeven net present value of 0 to the biorefinery capital and operational costs over a 30 year lifetime. Generally, this value is in the range for novel technology and is not far from NREL's reporting values for ethanol production. Jet-fuel prices today are on the rise in the post-COVID-19 era and the price has increased to $0.87 per L ($3.28 per gallon) from $0.51 per L ($1.91 per gallon) since January 2019 (Fig. S1†).75 If these rising trends continue, biomass-to-DMCO through the direct fermentation pathway of isoprene may become competitive with petroleum jet-fuel. In addition to this, the MFSP is mainly affected by the biorefinery yield and cost of feedstock. Sorunmu et al.30 examined a range of bio-jet fuels that could be competitive with a social cost of carbon as high as 200 USD per ton CO2; this would further render the DMCO route competitive.
The most recent TEA reporting related to this process was done by Baral et al. (2021)36 who looked at a similar pathway for DMCO production from sorghum biomass. It was reported at the current state of technology of $9 per L DMCO jet fuel and best-case scenario of $1.5 per L DMCO jet fuel when considering optimal conditions and using a similar Ni catalyst to that employed in this research. This reduction in cost can be attributed to the indirect fermentation pathway that they considered from Riazi et al.35 of the intermediate compound 1-methyl-3-butanol. This indirect fermentation pathway requires two additional steps post-fermentation already mentioned in the life cycle assessment of separation and dehydrogenation before isoprene can be dimerized to DMCOD and subsequently hydrogenated to DMCO. Additionally, annual crops such as biomass sorghum can raise the feedstock supply cost by up to $115 per tonne23 as can alternative pre-treatments (e.g. integrated high-gravity ionic liquid) that produce higher sugar yields but at a higher cost compared to dilute-acid pretreatment for corn stover.
For a closer comparison, NREL's 2016 technical report estimated a sugar-to-hydrocarbon fuel (jet fuel precursor) MFSP in the range of $1 to 2.4 per L Jet Aeq with an average value of $1.5 per L Jet Aeq.76 The actual cost for jet fuel would be higher given that not all hydrocarbons have the desired chain length to meet jet fuel standards. For example, Jet A-1 typically has hydrocarbons with carbon chain lengths in the C9 to C16 range. Hydrocarbons that are too short (e.g., methane or ethane) or too long (e.g., heavy waxes) are not suitable for jet fuel. When producing jet fuel from feedstocks like biomass or waste oils, not all of the hydrocarbons present may meet these stringent requirements. Therefore, additional refining, upgrading, or treatment processes are often needed to ensure that the final product meets aviation fuel standards. These processes can add complexity and cost to the production of jet fuel, making it more expensive than simply converting all hydrocarbons in a feedstock into jet fuel. Lundberg et al.77 conducted an economic analysis on the minimum selling price (MSP) of isoprene, the intermediate compound for producing DMCO. The significant cost associated with mesaconic acid in Lundberg's system played a pivotal role in the high price of isoprene production. Using this different bio-sourced pathway to produce isoprene, the MSP of isoprene was evaluated at $4.07 per kg ($2.89 per L). This research pathway reduces large costs in purchasing the raw material in the indirect fermentation pathway to isoprene of mesaconic acid, and thus can result in a better economic outcome for DMCO production.
The enzyme cost contribution modeled here is lower than expected for an enzyme preparation purchased from a separate, non-adjacent production facility. Transportation of the enzyme to the biorefinery facility adds to enzyme costs, even if production costs could be reduced. Here, by adding the enzyme production facility to the biorefinery, certain infrastructure can be shared (e.g. utilities and buildings) that can further reduce overall cost.68
3.3 MFSP sensitivity analysis
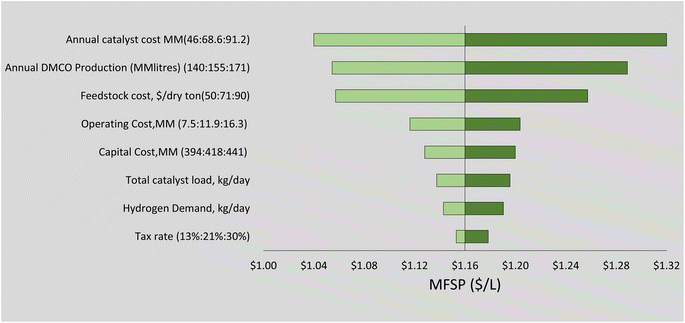
Sensitivity analysis was carried out to assess the effect of some process parameters previously identified as contributing economic hotspots that affect DMCO fuel MFSP. These factors include feedstock price and handling, dimerization catalyst and hydrogen production for supply in the hydrogenation step.Feedstock cost can have a large effect on the total production costs and can increase or decrease over time depending on technological developments and market fluctuations.78,79 Our results show it is by far the single most influential parameter in determining the minimum selling price of DMCO. A single variable sensitivity analysis was carried out increasing the cost of feedstock and handling at $5 increments shown in Fig. 7. For every $5 increment added to the feedstock price, an addition of 9 cents to the MFSP can be seen. The feedstock could be sourced from commercial sugars; however, additional investigation may be warranted in the near future to estimate economic feasibility.
The dimerization step involves a catalyst that has an optimal case cost of $10.26 per kg (13% wt).36 Both the dimerization and hydrogenation steps require more in-depth research to ensure estimated high yields, and catalyst recovery and selectivity can reduce the stage costs. A heterogeneous catalyst with a long lifetime could reduce costs and a single variable sensitivity analysis was carried out to examine the effect of underestimated catalyst cost on MFSP (Fig. 6).
The sensitivity of the nine model parameters tested on MFSP is considered in the TEA and is illustrated in Fig. 6 and compared with literature estimates of bio-jet fuel from biochemical and thermochemical technological platforms (Fig. 6). The upper and lower bounds in the sensitivity analysis were chosen using prior literature68,71 and engineering logic. Fig. 6 shows that the annual catalyst cost has the greatest effect on the MFSP. The sensitivity of hydrogen and catalyst supply on life cycle GHG impact (Fig. 4) showed little variance; however, these parameters strongly affect process economics (Fig. 6). The hydrogen production supply has been shown in terms of the minimum required to begin the hydrogenation reaction and make-up hydrogen if needed in the recycle stream. The sensitivity analysis showed that an additional 5% of hydrogen supplied in the hydrogenation stage affects the MFSP with an increase of 2 cents per L. A transition to green hydrogen supply by 2050 could reduce these costs further with current projected optimistic and pessimistic values of $0.65–$1.25 USD per kg hydrogen, respectively.80
The main contributors to variable costs were feedstock ($0.36 per litre), catalytic upgrading ($0.22 per litre) and enzyme hydrolysis with fermentation ($0.16 per litre). It is possible in the future that the cost of feedstock supply to biorefineries would decrease as it is the single most influential parameter in determining the MSP of DMCO. However, it is more feasible to consider optimizing the process areas of the biorefinery to reduce operating costs and MFSP. The catalytic upgrading and hydrogenation area in the biorefinery are a challenge as a result of high catalyst costs. These steps require additional research to ensure high yield and selectivity to lower the loading requirements of the metal catalyst. This will reduce the upfront catalyst and catalytic upgrading costs, in this case the iron and RANEY® Ni catalysts where prices were $10.26 per kg and $14.5 per kg, respectively. These catalysts were assumed to give isoprene-to-DMCOD and DMCOD-to-DMCO conversion yields of 98%. A reduction in the yields would provide sensitivity in the real viability of the process. For improvement in the fermentation step, optimizing the titer rate and yield for direct fermentation to isoprene could yield better results.55 These coincide with many TEA fuel analyses derived from biomass (Fig. 7).
Fig. 7 shows the MFSP contrast of DMCO production to other jet fuel pathways derived from biomass feedstock and demonstrates similar economic viability in terms of price. While the costs of producing fuels from biomass feedstock can be influenced by factors such as feedstock availability, processing technologies, and market demand, DMCO stands out as a promising alternative due to its specific suitability for the aviation industry. DMCO's cyclic structure and molecular branching give it appropriate fuel properties, with a density of 0.827 kg L−1 (6.7% higher than Jet A) and a gravimetric net heat of combustion at 43.82 MJ kg−1 (2.4% higher than Jet A). Its volumetric net heat of combustion is 36.22 MJ L−1 (9.2% greater than Jet A).51 With additional refining processes and strict adherence to aviation specifications, DMCO could ensure similar performance and safety in aircraft engines. These refined qualities, combined with efficient production and distribution systems, contribute to DMCO's competitiveness in terms of its minimum selling price when compared to other fuels derived from biomass feedstock. DMCO can potentially and cost-effectively meet the need for high performance and environmentally sustainable alternative jet fuel.
Finally, within the SAF framework, tax rate incentives can be used as a financial tool for short and medium-term policy measures to incentivize the aviation market.81 In the context of environmental policies, including those aimed at reducing carbon emissions, implementing tax schemes can be an effective strategy to drive immediate market responses.82 Imposing higher taxes on the aviation industry can encourage airlines to adopt cleaner fuels. However, it is essential to recognize the limitations of tax rates as a long-term solution for considered sustainable fuels. While they can drive short-term changes, relying solely on a tax incentive may not ensure the sustained viability of the introduction of SAFs. Long-term success often requires a more comprehensive approach, involving regulatory frameworks, technological innovation, and industry collaboration.83 In a decarbonized aviation world, reduced tax rates may have limited relevance. Today they can help spur a transition towards cleaner energy sources, like adopting low GHG impact fuels like DMCO, to meet goals for greener aviation. Dependence on tax incentives alone may not provide the necessary foundation for the sustained growth and viability of decarbonization efforts.
3.4 Life cycle impacts of DMCO production
While the focus of this study is the techno-economic and life cycle GHG emission impact of DMCO jet fuel, multiple other life cycle impact assessment (LCIA) metrics that affect human health and the environment are critical for investment decisions. Our previous research84 evaluated nine midpoint LCIA metrics using ReCiPe 2016 (ref. 85) for a biorefinery producing isoprene, corresponding with all cradle-to-isoprene process steps in the DMCO life cycle, including the lignin-fed boiler, which was the source of elevated air particulate matter (PM2.5), an indicator of air quality, and slightly high terrestrial acidification, which is also attributed to the lignin boiler. Batten et al.84 used the biorefinery mass balance audit from an Aspen Plus simulation by Humbird et al.62 to predict air pollutant emissions for a corn stover-to-ethanol biorefinery that was not equipped with air pollution controls. However, as noted in an earlier analysis by Spatari et al.,86 a biorefinery would not be permitted to emit uncontrolled VOC, CO or NOx emissions and would be required to invest in air pollutant controls, which would raise capital costs, and possible air quality impacts could be mitigated. Findings by Batten et al.84 agree with the biofuel LCA review by Jeswani et al.,11 which concluded that the use of agricultural residue feedstocks may increase eutrophication relative to petroleum-based fuels; however, this can be mitigated through implementing nutrient best-management practices, which would also help to reduce impacts related N2O emissions, the dominant greenhouse gas from agricultural feedstocks.874 Conclusion
We applied process modeling and simulation to estimate the cost and life cycle GHG emissions for converting corn stover to DMCO jet-fuel, an early-stage jet fuel blendstock. Although infrastructure compatible fuels like DMCO are foreseen to match the expanding liquid fuel markets in aviation, the performance of the processes evaluated herein to produce DMCO was slightly more costly compared to ATJ and HEFA jet fuels that are commercial today and can compete with conventional jet fuels produced from petroleum. However, the stronger environmental performance compared to other alternatives made from biomass yields a promising direction for biomass-derived aviation fuel. The higher process yield shown from the combined pathway of direct fermentation of sugars to isoprene improves the isoprene-to-DMCO environmental performance while reducing costs. In addition, improving process yields would further improve the MFSP of DMCO and in parallel reduce GHG emissions. However, this may not be sufficient to achieve a cost-competitive fuel compared to petroleum-derived jet fuel. Reductions in biorefinery capital cost and scale-up capability should be further studied to improve the economic viability of DMCO.Author contributions
Rahamim Batten: conceptualization, methodology, investigation, formal analysis, data curation, writing – original draft, writing – review and editing. Mukund Karanjikar: conceptualization and investigation. Sabrina Spatari: conceptualization, methodology, investigation, resources, supervision, project administration, funding acquisition, writing – review and editing, visualization.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was supported by the Israel Science Foundation (ISF 1484/20) and the U.S. Department of Energy Co-operative Agreement DE-EE00008504. Rahamim Batten acknowledges support from the Israeli Smart Transportation Research Center (ISTRC). Sabrina Spatari acknowledges support from the Grand Technion Energy Program (GTEP) and the Technion Women's Advancement Chair.Notes and references
- U.S. Energy Information Administration, EIA – Independent Statistics and Analysis, https://www.eia.gov/opendata/, accessed Apr 26, 2023 Search PubMed.
- U.S. Energy Information Administration, International Energy Outlook, 2019 Search PubMed.
- F. Shen, X. Xiong, J. Fu, J. Yang, M. Qiu, X. Qi and D. C. W. Tsang, Renewable Sustainable Energy Rev., 2020, 130, 109944 CrossRef CAS.
- J. Baeyens, Q. Kang, L. Appels, R. Dewil, Y. Lv and T. Tan, Prog. Energy Combust. Sci., 2015, 47, 60–88 CrossRef.
- A. Limayem and S. C. Ricke, Prog. Energy Combust. Sci., 2012, 38, 449–467 CrossRef CAS.
- S. I. Mussatto, G. Dragone, P. M. R. Guimarães, J. P. A. Silva, L. M. Carneiro, I. C. Roberto, A. Vicente, L. Domingues and J. A. Teixeira, Biotechnol. Adv., 2010, 28, 817–830 CrossRef CAS PubMed.
- S. Yeh, J. Witcover, G. E. Lade and D. Sperling, Energy Policy, 2016, 97, 220–234 CrossRef.
- Overview for Renewable Fuel Standard — US EPA, https://www.epa.gov/renewable-fuel-standard-program/overview-renewable-fuel-standard, accessed Aug 29, 2023 Search PubMed.
- International Civil Aviation Organization, International Energy Outlook 2019, 2022, accessed: 05 February 2024 Search PubMed.
- M. Prussi, U. Lee, M. Wang, R. Malina, H. Valin, F. Taheripour, C. Velarde, M. D. Staples, L. Lonza and J. I. Hileman, Renewable Sustainable Energy Rev., 2021, 150, 111398 CrossRef CAS.
- H. K. Jeswani, A. Chilvers and A. Azapagic, Proc. R. Soc. A, 2020, 476, 20200351 CrossRef PubMed.
- N. R. Baral, S. K. Mishra, A. George, S. Gautam, U. Mishra and C. D. Scown, Renewable Sustainable Energy Rev., 2022, 169, 112857 CrossRef CAS.
- J. Sheehan, A. Aden, K. Paustian, K. Killian, J. Brenner, M. Walsh and R. Nelson, J. Ind. Ecol., 2003, 7, 117–146 CrossRef CAS.
- S. Spatari, Y. Zhang and H. L. MacLean, Environ. Sci. Technol., 2005, 39, 9750–9758 CrossRef CAS PubMed.
- R. E. Bailis and J. E. Baka, Environ. Sci. Technol., 2010, 44, 8684–8691 CrossRef CAS PubMed.
- G. Pourhashem, P. R. Adler, A. J. McAloon and S. Spatari, Environ. Res. Lett., 2013, 8, 025021 CrossRef CAS.
- C. W. Murphy and A. Kendall, GCB Bioenergy, 2015, 7, 1019–1033 CrossRef CAS.
- M. Zhao, D. Shi, X. Lu, H. Zong, B. Zhuge and H. Ji, Bioresour. Technol., 2019, 273, 634–640 CrossRef CAS PubMed.
- V. Larnaudie, M. D. Ferrari and C. Lareo, Renewable Energy, 2021, 176, 606–616 CrossRef CAS.
- B. Neupane, N. V. S. N. M. Konda, S. Singh, B. A. Simmons and C. D. Scown, ACS Sustain. Chem. Eng., 2017, 5, 10176–10185 CrossRef CAS.
- S. Spatari, D. M. Bagley and H. L. MacLean, Bioresour. Technol., 2010, 101, 654–667 CrossRef CAS PubMed.
- J. B. Dunn, S. Mueller, H. Kwon and M. Wang, Biotechnology and Biofuels, 2013, 6, 51 CrossRef CAS PubMed.
- M. Yang, N. Baral, A. Anastasopoulou, H. Breunig and C. Scown, Environ. Sci. Technol., 2020, 54, 12810–12819 CrossRef CAS PubMed.
- C. Fertitta-Roberts, S. Spatari, D. A. Grantz and G. D. Jenerette, GCB Bioenergy, 2017, 9, 1764–1779 CrossRef CAS.
- I. Gelfand, S. K. Hamilton, A. N. Kravchenko, R. D. Jackson, K. D. Thelen and G. P. Robertson, Environ. Sci. Technol., 2020, 54, 2961–2974 CrossRef CAS PubMed.
- M. R. Kubis and L. R. Lynd, Sustainable Energy Fuels, 2023, 7, 3842–3852 RSC.
- L. R. Lynd, G. T. Beckham, A. M. Guss, L. N. Jayakody, E. M. Karp, C. Maranas, R. L. McCormick, D. Amador-Noguez, Y. J. Bomble, B. H. Davison, C. Foster, M. E. Himmel, E. K. Holwerda, M. S. Laser, C. Y. Ng, D. G. Olson, Y. Román-Leshkov, C. T. Trinh, G. A. Tuskan, V. Upadhayay, D. R. Vardon, L. Wang and C. E. Wyman, Energy Environ. Sci., 2022, 15, 938–990 RSC.
- S. De Jong, et al. , Biotechnol. Biofuels, 2017, 10, 64 CrossRef PubMed.
- P. Suresh, et al. , Environ. Sci. Technol., 2018, 52, 12055–12065 CrossRef CAS PubMed.
- Y. E. Sorunmu, P. Billen, Y. Elkasabi, C. A. Mullen, N. A. Macken, A. A. Boateng and S. Spatari, ACS Sustain. Chem. Eng., 2017, 5, 8804–8814 CrossRef CAS.
- E. I. Fitriasari, W. Won and J. J. Liu, Sustainable Energy Fuels, 2023, 3625–3636 RSC.
- F. Habermeyer, V. Papantoni, U. Brand-Daniels and R. U. Dietrich, Sustainable Energy Fuels, 2023, 4229–4246 RSC.
- M. D. Staples, et al. , Energy Environ. Sci., 2014, 7, 1545–1554 RSC.
- E. Budsberg, J. T. Crawford, H. Morgan, W. S. Chin, R. Bura and R. Gustafson, Biotechnol. Biofuels, 2016, 9, 1–13 Search PubMed.
- B. Riazi, M. Karanjikar and S. Spatari, ACS Sustain. Chem. Eng., 2018, 6, 14414–14422 CrossRef CAS.
- N. R. Baral, M. Yang, B. G. Harvey, B. A. Simmons, A. Mukhopadhyay, T. S. Lee and C. D. Scown, ACS Sustain. Chem. Eng., 2021, 9, 11872–11882 CrossRef CAS.
- J. Han, L. Tao and M. Wang, Biotechnol. Biofuels, 2017, 10, 201 RSC.
- K. Cox, M. Renouf, A. Dargan, C. Turner and D. Klein-Marcuschamer, Biofuels, Bioprod. Biorefin., 2014, 8, 579–593 CrossRef CAS.
- A. Gollakota, A. Thandlam and C. Shu, Liq. Biofuels, 2021, 183–213 CAS.
- A. H. Tanzil, K. Brandt, X. Zhang, M. Wolcott, E. Silva Lora, C. Stockle and M. Garcia-Perezand, Fuel, 2022, 321, 123992 CrossRef CAS.
- R. W. Stratton, H. M. Wong and J. I. Hileman, Environ. Sci. Technol., 2011, 45, 4637–4644 CrossRef CAS PubMed.
- S. De Jong, K. Antonissen, R. Hoefnagels, L. Lonza, M. Wang, A. Faaij and M. Junginger, Biotechnol. Biofuels, 2017, 10, 64 CrossRef PubMed.
- G. Seber, R. Malina, M. N. Pearlson, H. Olcay, J. I. Hileman and S. R. H. Barrett, Biomass Bioenergy, 2014, 67, 108–118 CrossRef CAS.
- D. R. Shonnard, L. Williams and T. N. Kalnes, Environ. Prog. Sustainable Energy, 2010, 29, 382–392 CrossRef CAS.
- Y. Elkasabi, V. Wyatt, K. Jones, G. D. Strahan, C. A. Mullen and A. A. Boateng, Energy Fuels, 2020, 34, 483–490 CrossRef CAS.
- M. Laser, H. Jin, K. Jayawardhana and L. R. Lynd, Biofuels, Bioprod. Biorefin., 2009, 3, 195–218 CrossRef CAS.
- T. R. Brown, Bioresour. Technol., 2015, 178, 166–176 CrossRef CAS PubMed.
- N. R. Baral, O. Kavvada, D. Mendez-Perez, A. Mukhopadhyay, T. S. Lee, B. A. Simmons and C. D. Scown, Energy Environ. Sci., 2019, 12, 807 RSC.
- S. Geleynse, K. Brandt, M. Garcia-Perez, M. Wolcott and X. Zhang, ChemSusChem, 2018, 11, 3728–3741 CrossRef CAS PubMed.
- I. Ganguly, F. Pierobon, T. C. Bowers, M. Huisenga, G. Johnston and I. L. Eastin, Biomass Bioenergy, 2018, 108, 207–216 CrossRef CAS.
- K. E. Rosenkoetter, C. R. Kennedy, P. J. Chirik and B. G. Harvey, Green Chem., 2019, 21, 5616–5623 RSC.
- J.-D. Woodroffe and B. G. Harvey, Energy Fuels, 2022, 36, 2630–2638 CrossRef CAS.
- C. J. Walkling, D. D. Zhang and B. G. Harvey, Fuel, 2024, 356, 129554 CrossRef CAS.
- C. Yang, X. Gao, Y. Jiang, B. Sun, F. Gao and S. Yang, Metab. Eng., 2016, 37, 79–91 CrossRef CAS PubMed.
- G. M. Whited, F. J. Feher, D. A. Benko, M. A. Cervin, G. K. Chotani, J. C. McAuliffe, R. J. LaDuca, E. A. Ben-Shoshan and K. J. Sanford, Ind. Biotechnol., 2010, 152–163 CrossRef CAS.
- R. Batten, O. Galant, M. Karanjikar and S. Spatari, Fuel, 2023, 333, 126294 CrossRef CAS.
- ISO – ISO 14040:2006 – Environmental management — Life cycle assessment — Principles and framework, https://www.iso.org/standard/37456.html, accessed Jul 9, 2023 Search PubMed.
- ISO 14040: Environmental Management — Life Cycle Assessment — Principles and Framework, https://www.iso.org/standard/37456.html, 2006, report no. ISO 14040:2006(E) Search PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- SimaPro 8.4, Pre Consultants, T. N., 2018 Search PubMed.
- P. R. Adler, J. G. Mitchell, G. Pourhashem, S. Spatari, S. J. Del Grosso and W. J. Parton, Ecol. Appl., 2015, 25, 1142–1156 CrossRef PubMed.
- D. Humbird, R. Davis, L. Tao, C. Kinchin, D. Hsu, A. Aden, P. Schoen, J. Lukas, B. Olthof, M. Worley, D. Sexton and D. Dudgeon, Process Design and Economics for Conversion of Lignocellulosic Biomass to Ethanol, NREL Tech. Rep., NREL/TP-5100-51400 Technical Report, May 2011 Search PubMed.
- UniSim Process Design Suite, Honeywell, 2021 Search PubMed.
- H. L. MacLean and S. Spatari, Environ. Res. Lett., 2009, 4, 014001 CrossRef.
- Kirk-Othmer Encyclopedia of Chemical Technology, Wiley, 2000 Search PubMed.
- P. T. Benavides, D. C. Cronauer, F. Adom, Z. Wang and J. B. Dunn, Sustain. Mater. Technol., 2017, 11, 53–59 CAS.
- A. B. Dros, O. Larue, A. Reimond, F. De Campo and M. Pera-Titus, Green Chem., 2015, 17, 4760–4772 RSC.
- R. E. Davis, N. J. Grundl, L. Tao, M. J. Biddy, E. C. Tan, G. T. Beckham, D. Humbird, D. N. Thompson and M. S. Roni, Process Design and Economics for the Conversion of Lignocellulosic Biomass to Hydrocarbon Fuels and Coproducts, NREL Tech. Rep., NREL/TP-5100-71949 Technical Report November, 2018 Search PubMed.
- W. Seider, D. Lewin, J. Seader, S. Widagdo, R. Gani and K. Ng, Product and Process Design Principles: Synthesis, Analysis, and Evaluation, 2017 Search PubMed.
- A. Saltelli, M. Ratto, S. Tarantola and F. Campolongo, Chem. Rev., 2005, 105, 2811–2828 CrossRef CAS PubMed.
- R. K. Iyer and J. C. Kelly, Life-Cycle Inventory of Critical Materials: Nickel, Copper, Titanium, and Rare-Earth Elements, Argonne National Laboratory (ANL), ANL/ESIA-22/8178670 Technical Report December, 2022 Search PubMed.
- S. Spatari, M. Betz, H. Florin, M. Baitz and M. Faltenbacher, Int. J. Life Cycle Assess., 2001, 6(2), 81–84 CrossRef.
- Sphera, Gabi 9, Life Cycle Assessment LCA Software, 2020, http://www.gabi-software.com/international/index/, accessed on July 9, 2023 Search PubMed.
- D. Aboagye, N. Banadda, R. Kambugu, J. Seay, N. Kiggundu, A. Zziwa and I. Kabenge, J. Ecol. Environ., 2017, 41, 1–11 CrossRef.
- B. of Transportation Statistics, U.S. Airlines' January 2023 Fuel Cost per Gallon up 4.3% from December 2022; Aviation Fuel Consumption Down 0.7% from Pre-pandemic January 2019, 2023, https://www.bts.gov/newsroom/us-airlines-january-2023-fuel-cost-gallon-43-december-2022-aviation-fuel-consumption-down, accessed: 06 September 2023 Search PubMed.
- W.-C. Wang, L. Tao, J. Markham, Y. Zhang, E. Tan, L. Batan, E. Warner and M. Biddy, Review of Biojet Fuel Conversion Technologies, U.S. Department of Energy Technical Report, July 2016 Search PubMed.
- D. J. Lundberg, K. Zhang and P. J. Dauenhauer, ACS Sustain. Chem. Eng., 2019, 7, 5442–5452 Search PubMed.
- S. Kim and B. E. Dale, Biofuels, Bioprod. Biorefin., 2016, 10, 819–832 CrossRef CAS.
- R. Rajendran and G. S. Murthy, Biotechnol. Biofuels, 2017, 10, 268 CrossRef PubMed.
- IRENA, Global Hydrogen Trade to Meet the 1.5 °C Climate Goal: Part III – Green Hydrogen Cost and Potential, International Renewable Energy Agency, Abu Dhabi, 2022 Search PubMed.
- K. Santos and L. Delina, Energy Research & Social Science, 2021, 77, 102074 Search PubMed.
- A. H. Bhatt, Y. M. Zhang and A. Milbrandt, et al. , Energy Convers. Manage., 2023, 275, 116441 CrossRef CAS.
- R. Stavins, Environmental and Energy Policy and Economics, 2020, 1, pp. 8–64 Search PubMed.
- R. Batten, M. Karanjikar and S. Spatari, Fermentation, 2021, 7, 204 CrossRef CAS.
- M. A. Huijbregts, Z. J. Steinmann, P. M. Elshout, G. Stam, F. Verones, M. Vieira, M. Zijp, A. Hollander and R. Van Zelm, Int. J. Life Cycle Assess., 2017, 22, 138–147 CrossRef.
- S. Spatari and H. L. MacLean, Environ. Sci. Technol., 2010, 44(22), 8773–8780 CrossRef CAS PubMed.
- P. R. Adler, S. J. Del Grosso, D. Inman, R. E. Jenkins, S. Spatari and Y. M. Zhang, Mitigation Opportunities for Life-Cycle Greenhouse Gas Emissions during Feedstock Production across Heterogeneous Landscapes, Managing Agricultural Greenhouse Gases: Coordinated Agricultural Research through Gracenet to Address Our Changing Climate, ed. M. Liebig, A. J. Franzluebbers and R. F. Follet, Elsevier, 2012, pp. 203–219 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se01470c |
| This journal is © The Royal Society of Chemistry 2024 |