Open Access Article
Open Access ArticleEnhancing NIR-to-visible upconversion in a rigidly coupled tetracene dimer: approaching statistical limits for triplet–triplet annihilation using intramolecular multiexciton states†
Alexander T.
Gilligan‡
a,
Raythe
Owens‡
a,
Ethan G.
Miller
a,
Nicholas F.
Pompetti
a and
Niels H.
Damrauer
 *ab
*ab
aDepartment of Chemistry, University of Colorado Boulder, Boulder, Colorado 80309, USA. E-mail: niels.damrauer@colorado.edu
bRenewable and Sustainable Energy Institute (RASEI), University of Colorado Boulder, Boulder, Colorado 80309, USA
First published on 12th December 2023
Abstract
Important applications of photon upconversion through triplet–triplet annihilation require conversion of near-IR photons to visible light. Generally, however, efficiencies in this spectral region lag behind bluer analogues. Herein we consider potential benefits from a conformationally well-defined covalent dimer annihilator TIPS-BTX in studies that systematically compare function to a related monomer model TIPS-tetracene (TIPS-Tc). TIPS-BTX exhibits weak electronic coupling between chromophores juxtaposed about a polycyclic bridge. We report an upconversion yield ϕUC for TIPS-BTX that is more than 20× larger than TIPS-Tc under comparable conditions (0.16%). While the dimer ϕUC is low compared to bluer champion systems, this yield is amongst the largest so-far reported for a tetracenic dimer system and is achieved under unoptimized conditions suggesting a significantly higher ceiling. Further investigation shows the ϕUC enhancement for the dimer is due exclusively to the TTA process with an effective yield more that 30× larger for TIPS-BTX compared to TIPS-Tc. The ϕTTA enhancement for TIPS-BTX relative to TIPS-Tc is indicative of participation by intramolecular multiexciton states with evidence presented in spin statistical arguments that the 5TT is involved in productive channels. For TIPS-BTX we report a spin-statistical factor f = 0.42 that matches or exceeds values found in champion annihilator systems such as DPA. At the same time, the poor relative efficiency of TIPS-Tc suggests involvement of non-productive bimolecular channels and excimeric states are suspected. Broadly these studies indicate that funneling of photogenerated electronic states into productive pathways, and avoiding parasitic ones, remains central to the development of champion upconversion systems.
Introduction
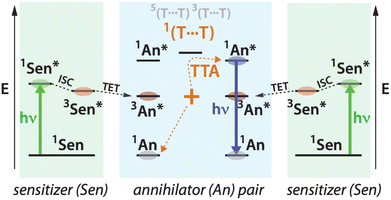
Generation of anti-stokes shifted electronic states relative to their excitation source and their application towards driving photophysical processes is an area of interest for both fundamental science and a blossoming scope of applications including solar energy conversion,1–7 photoredox catalysis,8–11 bioimaging and therapies,12–16 and 3D printing17,18 to name a few. One method of anti-stokes generation heralded for its promise in low excitation intensity scenarios is triplet–triplet annihilation upconversion (TTA-UC).2,19–24 TTA-UC generally comprises a system of molecules where one species with a low energy singlet (S1) state, referred to as the sensitizer (Sen), is first photoexcited and efficiently undergoes intersystem crossing into its triplet (T1) state. This is often driven by the heavy atom effect, for example in the palladium phthalocyanine species used here. Following collision in solution-phase diffusional systems that are common, the energy is transferred to the T1 of a harvesting molecule, referred to as the annihilator (An). Finally, collisions between two excited triplet annihilators (homofusion) or between a triplet sensitizer and a triplet annihilator (heterofusion) lead to the generation of an annihilator S1 that can fluoresce with photon energy greater than the excitation source (see Scheme 1). The conversion is mediated by an intermolecular multiexciton state and potentially other higher excited states within the collisional partners.While upconversion has been studied with many molecular partners, a prototypical champion system in solution to be emulated consists of the annihilator 9,10-diphenylanthracene (DPA) and a metal porphyrin species (Zn, Pd or Pt octaethylporphyrin for example). Indeed, a growing body of literature on DPA has reported upconversion quantum yields ϕUC of order ∼25%.25,26 However, usage of DPA restricts upconversion photoluminescence to the blue region of the visible spectrum and excitation must be in the green at ∼520 nm. For many applications these restrictions are prohibitive. For example, with photovoltaics upconversion would be valuable by way of harvesting sub-bandgap photons. The green visible spectrum is not useful for current solar-cell technologies of importance, ranging from organic to perovskite to crystalline silicon devices.4 For bioimaging and 3D printing, higher photon energy excitation sources run into issues of scattering and tissue penetration.27,28 It would therefore be beneficial if upconversion could move to redder emitting annihilators and excitation sources in so-called NIR-to-visible upconversion, a field that has recently been reviewed.23
A natural path forward for lowering S1 is to employ larger acenes such as substituted tetracenic species. This is approximately the limit in acene size before singlet fission – which is more exoergic in pentacenic materials29 – begins to significantly undermine TTA. Rubrene (5,6,11,12-tetraphenyltetracene) has been extensively explored due to its near-unity fluorescence quantum yield and shows a red-shifted peak emission compared to DPA, for example at ∼560 nm in room temperature toluene.30–33 Here we focus on solution phase upconversion systems rather than films that incorporate rubrene.34–41 An earlier (2010) exploration of TTA-UC for rubrene in solution analyzed statistical factors tied to the photophysical outcome of different spin channels in the triplet–triplet encounter complex (commonly referred to as f, vide infra).31 They reported favorable properties, exceeding ones seen for DPA, suggesting that a sizable amount of the spin-triplet encounter complex population is able to dissociate without individual triplet loss, in the same way as the spin-quintet encounter complexes. However, upconversion yields for rubrene in solution, even in optimized TTA-UC systems, are muted relative to DPA with values lower than a factor of five, often significantly so.23,30,32,42–46 More recent experiments on rubrene exploring the same spin statistics while using lower excitation peak intensities question the 2010 results and suggest they are skewed by measurement conditions.32 These authors see a statistical factor f at 15%32 that is a factor of four smaller than the earlier report (60%),31 and only modestly larger than expected if only the spin-singlet encounter complex channel is productive while the spin-triplet and spin-quintet encounter complexes decay fully to ground state (11.1%; i.e., 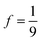 ). They suggest this lies at the heart of the less than ideal ϕUC properties for rubrene. It is noted that in terms of ϕUC, other tetracenic systems such as BPEN (5,12-bis(phenylethynyl)naphthacene),16 or TIPS-tetracene (vide infra and ref. 33, 47 and 48) do not fare better. Whether or not there is a common origin of poor performance in the statistical factor is to be determined, but it is clear that new strategies aimed to improve the upconversion limits of larger acenes would be valuable.
). They suggest this lies at the heart of the less than ideal ϕUC properties for rubrene. It is noted that in terms of ϕUC, other tetracenic systems such as BPEN (5,12-bis(phenylethynyl)naphthacene),16 or TIPS-tetracene (vide infra and ref. 33, 47 and 48) do not fare better. Whether or not there is a common origin of poor performance in the statistical factor is to be determined, but it is clear that new strategies aimed to improve the upconversion limits of larger acenes would be valuable.
We and others have considered potential benefits within dimers and oligomers comprising covalently fused annihilators.26,33,47,49–57 In principle, intramolecular multiexciton states in such systems could participate in excited state dynamics, perhaps in productive ways.33 From a technological perspective dimers and oligomers are also interesting in settings where one cannot rely on diffusion to bring pairs of triplet annihilator species together, for example at nanomaterial/molecular interfaces where the nanomaterial functions as a triplet sensitizer to the bound species. For dimers derived from anthracenic chromophores, studied in common diffusive solution phase experiments with a molecular sensitizer, there is not yet evidence for advantage derived from intramolecular multiexciton states.26,51 In these systems there is a strong energetic driving force for annihilation (ES1 < ET1 + ET1), and it appears that intermolecular encounter complexes provide sufficiently productive excited-state precursors. This does not mean that such systems will not be valuable in non-diffusive settings, only that they do not yet provide evidence for the relevance of intramolecular multiexciton states in mediating productive dynamics.
As a point of contrast, there is growing evidence that tetracenic dimers may behave differently, potentially offering opportunities to improve redder emitting platforms. There are now several reports showing benefits of covalently fused systems over monomer models, although these studies have not all specifically quantified TTA yields or use the same definitions for ϕUC, so direct comparisons are challenging. Wilson and coworkers in collaboration with members of our group considered a rigid norbornyl-bridged TIPS-tetracene dimer called TIPS-BT1′ compared to the monomer models TIPS-tetracene (TIPS-Tc) and rubrene and found upconversion brightness to be higher in the dimer at comparable annihilator concentrations.33 This work also found advantage for the dimer at comparable triplet flux, which is a surrogate for relative TTA yield. Congreve, Campos, and coworkers studied a series of non-rigid TIPS-tetracene dimers linked at chromophore end positions by varying p-phenylene spacers (n = 0, 1, 2, and 4).47 In two of their dimer systems (n = 1 and 2), they also found that upconversion brightness is larger than TIPS-Tc at comparable annihilator concentrations. At the highest concentration considered, the n = 1 and n = 2 dimers are brighter TTA-UC emitters than TIPS-Tc by a factor of approximately 4 and 2, respectively. Finally, Guldi, Tykwinski, and coworkers recently explored two non-rigid tetracene dimer types with either a meta-substituted diethynylphenylene bridge or a 1,3-diethynyladamantyl bridge relative to monomer models with bridge-specific substituents.56 They also found that the dimers are brighter TTA-UC emitters compared to the respective monomer models at comparable annihilator concentrations, with relative ratios of approximately 4 and 3 for the phenylene and adamantyl comparisons, respectively. They have also considered upconversion yields ϕUC, as we do here, and at the highest annihilator concentrations they consider, found ratios of 3.7 and 2.8 favoring the dimers for the phenylene and adamantyl comparisons, respectively. In the current work, we consider the behavior of another rigid dimer system, this time utilizing a larger fused polycyclic bridge comprising both saturated and unsaturated fragments constructed using Pd-catalyzed annulation chemistry.58,59 We measure upconversion yields and disentangle them into yields of contributing photophysical processes with an eye towards enabling direct comparisons between dimer versus monomer. The results suggest significant advantages for the dimer including a ∼20× enhancement of ϕUC and a ∼35× enhancement of ϕTTA relative to TIPS-Tc at comparable concentrations. These studies provide evidence that intramolecular multiexciton states play decisive roles controlling upconversion yield in these NIR-visible TTA-UC systems.
Results and discussion
Annihilator properties
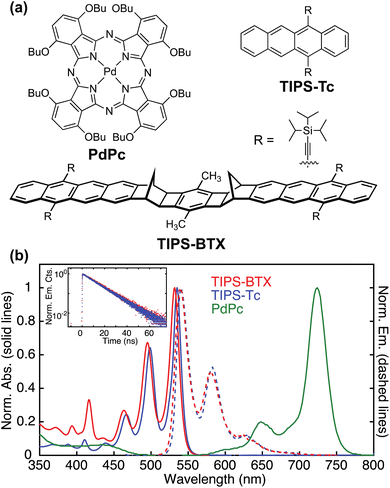
As described in the Introduction, this work seeks to compare TTA-UC properties of the monomer model TIPS-Tc relative to a new rigid dimer system TIPS-BTX that utilizes the same acene chromophore (see stick structures for both in Fig. 1). The synthesis of TIPS-BTX and the isolation of the syn-diastereomer were reported elsewhere,58,59 but briefly, it is prepared through a palladium-catalyzed arene-norbornene annulation reaction (CANAL) that generates the two cyclobutene rings flanking the central xylene unit. The lowest energy electronic absorption spectrum and its mirrored emission (short-axis transition dipole moments) are vibronically structured in the expected way for delocalized acene units with low excited state nuclear reorganization, with the 0–0 features determining the band maxima. For example, in Fig. 1(b) it is seen that the absorption and emission features closely match those of TIPS-Tc. The average of the 0–0 absorption and emission features indicate an S1 energy of 2.32 eV in TIPS-BTX60 and 2.30 eV for TIPS-Tc (Table 1). The similarity of absorption and emission features of TIPS-BTX relative to the monomer TIPS-Tc, particularly the lack of enhancement of the second vibronic component relative to the 0–0 band, is indicative of weak electronic coupling between the two chromophores of the dimer.61 In support of this we also observe an approximate doubling of the lowest energy transition in the dimer versus the monomer (Fig. S1†). An absorption spectrum for TIPS-BTX was also collected in room-temperature chloroform (Fig. S2†) which has a higher energy UV cutoff than toluene. These data show the intense UV transition (peaked at 296 nm) derived from the long-axis transition dipole moment of the chromophore units. No Davydov splitting is observed, as is expected from the long bridge including two norbornyl spacers as well as the near-collinear arrangement of the two chromophores of the dimer such that the higher energy transition of a split pair would be dark.| TIPS-Tc | TIPS-BTX | PdPc | |
|---|---|---|---|
| a Reported from a phosphorescence measurement of TIPS-Tc in a polystyrene thin film.78 b It is assumed that the dimer will have a T1 energy that is similar to TIPS-Tc (see (a)) given the nature of the chromophores. c Reported from a phosphorescence measurement of PdPc in toluene.33 d The energy of the intermolecular multiexciton state for the monomer is approximated to be twice the T1 energy. e The upper bound in this TT energy estimation relates to the T1 yield measurement made by TA (Fig. 2) See equilibrium analysis in ESI (section S6) for details. | |||
| S1 (eV) | 2.30 | 2.32 | 1.71 |
| T1 (eV) | 1.21a | ∼1.21b | 1.13c |
| TT (eV) | 2.42d | <2.41e | — |
| ϕ FL (%) | 74 | 72 ± 7 | — |
| τ 0;S (ns) | 12.4 | 12.7 ± 0.5 | — |
| τ 0;T (μs) | 290 | 410 | 3.42 |
Further photophysical characterization confirms similarities between the dimer and the monomer model. The quantum yield of fluorescence for TIPS-BTX was measured to be ϕFL = 72 ± 7%, comparable to that for TIPS-Tc at ϕFL = 74%. Regarding emissive singlet lifetimes, time-correlated single photon counting (TCSPC) measurements at 540 nm (coincident with the 0–0 transition) following excitation at 405 nm indicate mono-exponential decay for both molecules with lifetimes of τ0;S = 12.7 ± 0.5 ns and τ0;S = 12.4 ns for TIPS-BTX and TIPS-Tc, respectively (see inset in Fig. 1(b)). Results collected at 578 nm, corresponding to the 0–1 emission transition, were consistent.
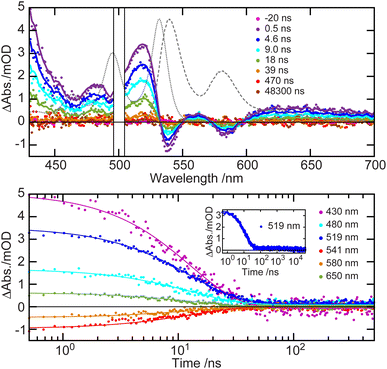
As photoluminescence measurements are insensitive to dark states that may be produced in the dynamical evolution away from the emissive state, a transient absorption (TA) experiment was performed over a time range spanning ∼500 ps to 100 μs. The TA spectra obtained for TIPS-BTX (Fig. 2) are comparable to those previously reported for TIPS-Tc,62 showing a strong ESA at ∼420 nm, negative features at ∼540 nm and ∼580 nm, and broad weak ESA ≥ 600 nm. Of the negative features, 580 nm corresponds to stimulated emission of the 0–1 band while 540 nm is a convolution of ground state bleach and stimulated emission of the 0–0 band. The ESA with peaks at 480 nm and 520 nm and valleys at 470 nm and 490 nm comes from contributions due to bleach of the vibronically structured ground-state absorption, a quality that is also observed in TIPS-Tc and other related dimers.62,63 These spectral features begin decaying on the order of nanoseconds with no other significant spectral evolution observed. Single wavelength kinetics traces extracted from the overall dataset exhibit decay to baseline at most wavelengths with a time scale of order 10 ns, consistent with the TCSPC measurements. However, there is minor ΔAbs persistence at long times at the 520 nm ESA maximum. The full wavelength/time dataset can be cleanly modeled using a biexponential decay function (Fig. 2), returning a major component with a lifetime of 12.9 ns (Fig. S4†) that matches well with the singlet lifetime observed via TCSPC (Fig. 1(b)). A long-lived component peaking at ∼520 nm is also observed, but it is significantly weaker (Fig. S4†). TIPS-Tc based dimers in singlet fission studies are known to have ESA intensity in this wavelength region heralding triplet character from either triplet multiexcitonic states (2S+1TT) or isolated triplets (T1).63–70 Given the long lifetime that approximately matches sensitization experiments (Fig. S6†) this weak feature observed in TIPS-BTX is best explained as arising due to formation of a small amount of isolated T1.
The observation of T1 population in such a dimer is an interesting one for fundamental reasons involving singlet fission, although we primarily address it here given its potential to complicate a comparative analysis (dimer versus monomer) of TTA-UC. First, we do not believe that T1 population arises due to intersystem crossing, on the basis that no T1 is observed in the monomer model TIPS-Tc.62 This then raises the likelihood of an origin in singlet fission, although there is underlying complexity. In related rigid tetracenic dimers such as TIPS-BT1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 and TIPS-BT1′,63 where the acene chromophores are separated by a single norbornyl bridge, we have observed formation of the multiexciton singlet 1TT in equilibrium with S1, but this equilibrated set of states decays to the ground state without observation of isolated T1. However, other non-rigid tetracenic dimer systems have shown long-lived T1 populations that may be presumed to originate from the multiexcitonic triplet (2S+1TT) manifold, likely following internal conversion via the 3TT,56,67,69 something that is more commonly seen in pentacene-based dimers (e.g. ref. 71–73). We suspect that TIPS-BTX is behaving in a similar way and that the difference in its photophysics relative to the other rigid dimers TIPS-BT1 and TIPS-BT1′ has origins in its smaller isotropic spin–spin exchange interaction J due to the significantly larger bridge. Detailed studies, including time resolved EPR measurements, will be needed to fully disentangle the dynamics. A cursory estimate of T1 yield is made for TIPS-BTX using information that includes the focal volume of the pump laser in the sample as well as a Δε measurement that was made for the T1 in the related rigid dimer system TIPS-BT1′ (see ESI for details†). We find an upper bound in T1 yield in TIPS-BTX of 6.5% and suggest that this yield is low enough to proceed with an analysis of TTA-UC that hinges upon comparisons between dimer and monomer.
62 and TIPS-BT1′,63 where the acene chromophores are separated by a single norbornyl bridge, we have observed formation of the multiexciton singlet 1TT in equilibrium with S1, but this equilibrated set of states decays to the ground state without observation of isolated T1. However, other non-rigid tetracenic dimer systems have shown long-lived T1 populations that may be presumed to originate from the multiexcitonic triplet (2S+1TT) manifold, likely following internal conversion via the 3TT,56,67,69 something that is more commonly seen in pentacene-based dimers (e.g. ref. 71–73). We suspect that TIPS-BTX is behaving in a similar way and that the difference in its photophysics relative to the other rigid dimers TIPS-BT1 and TIPS-BT1′ has origins in its smaller isotropic spin–spin exchange interaction J due to the significantly larger bridge. Detailed studies, including time resolved EPR measurements, will be needed to fully disentangle the dynamics. A cursory estimate of T1 yield is made for TIPS-BTX using information that includes the focal volume of the pump laser in the sample as well as a Δε measurement that was made for the T1 in the related rigid dimer system TIPS-BT1′ (see ESI for details†). We find an upper bound in T1 yield in TIPS-BTX of 6.5% and suggest that this yield is low enough to proceed with an analysis of TTA-UC that hinges upon comparisons between dimer and monomer.
As a final photophysical characterization of the annihilators, triplet lifetimes of TIPS-BTX and TIPS-Tc have been measured, as the rate constant for triplet decay is intimately tied to the probability/yield of TTA mediated by collisional interactions between excited state annihilator species. Using triplet sensitization experiments initiated by photoexcited anthracene (ϕISC = 0.71;74 see ESI for details†), modest differences are uncovered between dimer and the monomer model where TIPS-BTX exhibits a triplet lifetime of τ0;T = 410 μs (k0;T = 2.4 ×103 s−1) and where TIPS-Tc is slightly shorter at τ0;T = 290 μs (k0;T = 3.4 ×103 s−1)(Fig. S6†). We anticipate these lifetime differences are tied to the energy gap law where the cyclobutene/norbornyl bridge slightly raises the T1 energy in TIPS-BTX relative to TIPS-Tc as was seen for the S1 energies in Table 1. To put these triplet lifetime values in some context, they are long enough to expect opportunities for collision-mediated TTA, with some modest advantage expected for the dimer. However, both lifetimes remain more than an order of magnitude shorter than champion annihilator systems like DPA,75 and several times shorter than TIPS-anthracene.76 This general trend is understood as being due to the smaller amount of triplet energy stored in tetracenic versus anthracenic annihilator systems, with consequences in non-radiative excited state decay as predicted using the energy gap law. This highlights a need to identify strategies for improving TTA-UC in systems designed to process lower energy photons.
Sensitizer properties
Sensitization of TIPS-BTX and TIPS-Tc utilized the n-butoxide-substituted palladium phthalocyanine species shown in Fig. 1a abbreviated as PdPc, that was also previously used with another TIPS-tetracene derived rigid dimer system as mentioned in the Introduction.33 The intersystem crossing yield ϕISC has been reported to be 0.75 in room temperature deoxygenated benzene;77i.e. a solvent similar to the toluene used herein. Phosphorescence measurements33 indicate the triplet stores 1.13 eV and we measure a lifetime of 3.42 μs in room temperature deoxygenated toluene (Fig. S5†). Although a unity ϕISC is desirable, these sensitizer properties are adequate for exploration of dimer versus monomer trends. The electronic spectrum of PdPc is characterized by a strongly absorbing Q band observed from ∼600–750 nm (see Fig. 1(b)) with minimal absorption overlap with the S1 emission of the annihilators.Annihilator and sensitizer together in UC systems
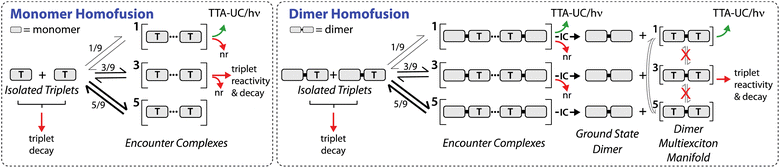
To explore TTA-UC property differences between dimer and monomer, a focus is placed on upconversion quantum yields (ϕUC). The expression shown in Eq. 1a is fundamentally bound at 1/2, because two absorption events are minimally required for the emission of one photon. This bounding occurs within ϕTTA (see Eq. S10†) a quantity that refers to annihilation exclusively through the singlet channel. The term f is a spin statistical factor used to account for different reactive pathways associated with singlet, triplet, and quintet character in encounter complexes between two triplets (S = 1 particles). It is simplified under conditions of strong exchange coupling between the two triplets such that overall spin in the encounter complex is a good quantum number (i.e., 2S+1(T⋯T)).79 The factor f is bound at 1, a rare situation where all spin channels are without loss, for example because both 5(T⋯T) and 3(T⋯T) are unreactive and simply re-dissociate to form isolated T1's, while the singlet encounter complex 1(T⋯T) engages in annihilation to S1 + S0.80 If, on the other hand, 5(T⋯T) and 3(T⋯T) are fully unproductive, leading to S0 for all species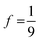 , reflecting only the statistical weight of the singlet channel. If the 5(T⋯T) is without loss but the 3(T⋯T) decays to a single T1 (for example due to annihilation to T2 + S0 followed by rapid internal conversion T2 → T1) then
, reflecting only the statistical weight of the singlet channel. If the 5(T⋯T) is without loss but the 3(T⋯T) decays to a single T1 (for example due to annihilation to T2 + S0 followed by rapid internal conversion T2 → T1) then 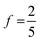 (i.e., 0.4) and ϕUC is bound at 20% (i.e.,
(i.e., 0.4) and ϕUC is bound at 20% (i.e., 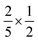 ). For now, we fold the f into
). For now, we fold the f into  and organize initial discussions around the simpler Eq. 1b.81 Further discussions about Eq. 1a will follow later. It is noted that when systems experience losses that are otherwise unaccounted for, the statistical factor f is the repository manifesting as a low value. This limitation of the framework is taken into account and guides the discussion later.82
and organize initial discussions around the simpler Eq. 1b.81 Further discussions about Eq. 1a will follow later. It is noted that when systems experience losses that are otherwise unaccounted for, the statistical factor f is the repository manifesting as a low value. This limitation of the framework is taken into account and guides the discussion later.82| ϕUC = fϕISCϕTETϕTTAϕFL | (Eq. 1a) |
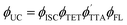 | (Eq. 1b) |
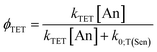 | (Eq. 1c) |
 | (Eq. 1d) |
The outer quantities of Eq. 1b are unimolecular and depend on the yield of ISC in the sensitizer and the yield of fluorescence in the annihilator (ϕISC and ϕFL, vide supra). The inner quantities on the other hand are bimolecular in nature (see typical UC schematic in Scheme 1). For the first, the yield of triplet–triplet energy transfer (ϕTET; Eq. 1c) involves photoexcited sensitizer and ground state annihilator, with dependence on the rate constant for TET (kTET), on the rate constant for triplet loss in the absence of interactions with annihilator (k0;T(Sen) = 1/τ0;T(Sen)), and on the ground state concentration of the annihilator ([An]). The second term derives from collisional interaction between a triplet excited state annihilator and another triplet. In principle this can occur either from the excited sensitizer (heterofusion) or from an excited-state annihilator species (homofusion). However, in the low relative sensitizer concentration regime that was investigated, homofusion is expected to be the dominant pathway. The simplest expression for ϕTTA under these conditions is Eq. 1d, which assumes the only loss pathways for 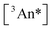 population is via unimolecular decay of the triplet (governed by the rate constant k0;T(An)) and bimolecular loss via annihilation of two
population is via unimolecular decay of the triplet (governed by the rate constant k0;T(An)) and bimolecular loss via annihilation of two 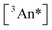 species producing one S1 and one S0, governed by the rate constant kTTA as well as
species producing one S1 and one S0, governed by the rate constant kTTA as well as 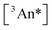 . As described more below, this expression can enable preliminary estimates of kTTA, but there are limits to its utility under conditions where other bimolecular channels contributing to triplet loss are active.
. As described more below, this expression can enable preliminary estimates of kTTA, but there are limits to its utility under conditions where other bimolecular channels contributing to triplet loss are active.
Determination of ϕTET
Stern–Volmer studies using nanosecond TA were performed to determine ϕTET between PdPc and each of the two annihilators. PdPc was photoexcited at 650 nm and monitored with a probe wavelength of 600 nm where the PdPc T1 exhibits a broad excited-state absorption. Unquenched T1 in PdPc decays to baseline monoexponentially with a fitted lifetime of τ0;T(Sen) = 3.42 μs (Fig. S5†), matching well with previous literature.33,77 On the other hand, samples prepared with either TIPS-BTX or TIPS-Tc show a reduction of the PdPc T1 lifetime that is proportional to annihilator concentration, i.e., a hallmark of dynamic quenching (Fig. 3 (top)). Fitting the plotted data to the classic Stern–Volmer equation (Eq. 2) allows for the determination of the constant KSV, and from this and the triplet lifetime of the sensitizer, the determination of a quenching rate constant kq.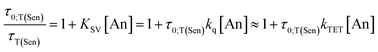 | (Eq. 2) |
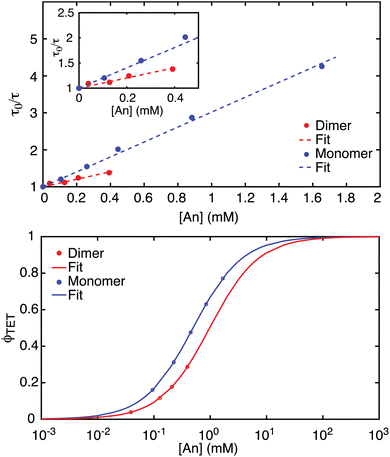 | ||
| Fig. 3 (Top) Stern–Volmer plot for TIPS-BTX and TIPS-Tc in room-temperature toluene. Slopes reflecting KSV along with sensitizer lifetime leads to values of kTET = 3.0 × 108 M−1 s−1 for TIPS-BTX and kTET = 5.9 × 108 M−1 s−1 for TIPS-Tc. The inset focuses on the lower annihilator concentration regime. (Bottom) Concentration dependent triplet energy transfer (TET) yields from Eq. 1c for TIPS-BTX and TIPS-Tc in room-temperature toluene. | ||
An assumption follows that Dexter energy transfer is the only operative pathway for quenching and kq is equated with kTET. From the data shown in Fig. 3 (top), a slope KSV of 1026 M−1 and 2019 M−1 are determined for TIPS-BTX and TIPS-Tc, respectively, demonstrating that the monomer engages in energy transfer with the sensitizer triplet excited state more readily than the dimer, consistent with its smaller size. These slopes along with τ0;T(Sen) = 3.42 μs indicate kTET = 3.0 × 108 M−1 s−1 for TIPS-BTX and kTET = 5.9 × 108 M−1 s−1 for TIPS-Tc.
With these kTET values in hand, the quantum yield ϕTET was calculated for all upconversion samples as [An] is varied for both monomer and dimer according to Eq. 1c. As expected from the quenching constants, TIPS-BTX at equimolar concentrations shows less efficient transfer of the triplet from the sensitizer to the annihilator as compared to TIPS-Tc (Fig. 3 (bottom)). This behavior has been seen in several previous dimeric systems26,33,47,51 and again is explained by the larger molecular volume of dimers slowing diffusion in the solvent medium.
Exploration of ϕUC and elucidation of  and kTTA
and kTTA
From the yield quantities discussed so far that depend in some way on annihilator properties (ϕFL and ϕTET), one can assume a modest advantage for the monomer TIPS-Tc over the dimer TIPS-BTX. From this perspective ϕUC is particularly interesting. Unlike for  , the quantification of ϕUC is experimentally straightforward using actinometry, and the value can then be used to determine
, the quantification of ϕUC is experimentally straightforward using actinometry, and the value can then be used to determine  viaEq. 1b (vide infra). As has been discussed elsewhere,21 there is complexity in reporting ϕUC because it is not simply dependent on the nature of the annihilator and sensitizer being used, but also on the experimental conditions of annihilator concentration [An] and photoexcitation fluence. The variable [An] influences ϕTET (Eq. 1c and Fig. 3 (bottom)) but it also impacts
viaEq. 1b (vide infra). As has been discussed elsewhere,21 there is complexity in reporting ϕUC because it is not simply dependent on the nature of the annihilator and sensitizer being used, but also on the experimental conditions of annihilator concentration [An] and photoexcitation fluence. The variable [An] influences ϕTET (Eq. 1c and Fig. 3 (bottom)) but it also impacts  by altering the concentration of triplets
by altering the concentration of triplets 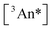 that are formed following collision with photoexcited sensitizer molecules. In a related way, photoexcitation fluence also controls
that are formed following collision with photoexcited sensitizer molecules. In a related way, photoexcitation fluence also controls 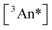 . In experiments described below, both variables are explored independently in dimer versus monomer comparisons.
. In experiments described below, both variables are explored independently in dimer versus monomer comparisons.
Upconversion samples of ∼1.3 μM PdPc sensitizer and annihilators of varying higher concentrations were prepared in deaerated toluene (Fig. S3†) and excited with a 730 nm diode laser with fluences ranging from ∼500–250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mW cm−2. For all dimer and monomer annihilator concentrations explored, we observe the expected crossover in the upconversion emission intensity from quadratic dependence on laser fluence to linear dependence (Fig. S7–S10†). At the highest concentrations explored for TIPS-Tc, this crossover occurs at 52 W cm−2 (see ESI, Fig. S8 and S9† for TIPS-Tc/TIPS-BTX fluence crossover points), comparable to the crossover observed by Pun et al. (44.5 W cm−2).47 The crossover for TIPS-BTX is observed to be 37 W cm−2 at the highest concentration investigated. While still lower than the crossover observed for TIPS-Tc, we expect this would be further reduced at higher annihilator concentration where we could expect increased triplet energy transfer efficiency.83 The crossover could be further reduced under optimal sample conditions, such as greater absorption at the excitation wavelength or when paired with an alternative sensitizer. We acknowledge that these crossing values are not insignificant, particularly when compared with those observed in anthracene system, nor expected solar fluxes. Still, this higher fluence region signals the strong annihilation regime where measurement of upconversion yield should be constant as laser fluence increases,84 thus allowing for independent interrogation of annihilator concentration effects (vide infra).
000 mW cm−2. For all dimer and monomer annihilator concentrations explored, we observe the expected crossover in the upconversion emission intensity from quadratic dependence on laser fluence to linear dependence (Fig. S7–S10†). At the highest concentrations explored for TIPS-Tc, this crossover occurs at 52 W cm−2 (see ESI, Fig. S8 and S9† for TIPS-Tc/TIPS-BTX fluence crossover points), comparable to the crossover observed by Pun et al. (44.5 W cm−2).47 The crossover for TIPS-BTX is observed to be 37 W cm−2 at the highest concentration investigated. While still lower than the crossover observed for TIPS-Tc, we expect this would be further reduced at higher annihilator concentration where we could expect increased triplet energy transfer efficiency.83 The crossover could be further reduced under optimal sample conditions, such as greater absorption at the excitation wavelength or when paired with an alternative sensitizer. We acknowledge that these crossing values are not insignificant, particularly when compared with those observed in anthracene system, nor expected solar fluxes. Still, this higher fluence region signals the strong annihilation regime where measurement of upconversion yield should be constant as laser fluence increases,84 thus allowing for independent interrogation of annihilator concentration effects (vide infra).
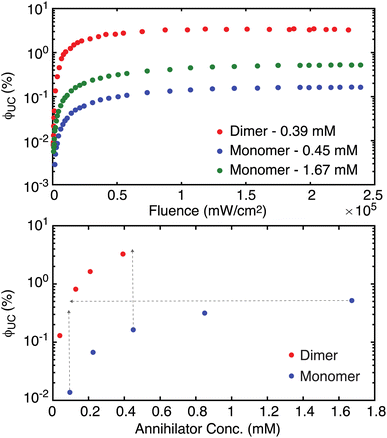
Fig. 4 (top) shows upconversion quantum yields measured versus laser fluence for TIPS-BTX at 0.39 mM and TIPS-Tc at 0.45 mM and at 1.67 mM. Upconversion emission spectra were corrected for self-absorption prior to integration and yields determined actinometrically against a directly excited TIPS-Tc reference in room temperature toluene (see ESI for details†). In the three cases, ϕUC saturates at higher fluences consistent with measurement in the strong annihilation regime. Critically, the dimer consistently outperforms the monomer. At comparable concentrations (0.39 mM for TIPS-BTX and 0.45 mM for TIPS-Tc) the dimer shows a maximum ϕUC (3.3%) that is more than 20 times that observed for the monomer (0.16%). But even when the monomer concentration is increased by more than a factor of three to 1.67 mM in order to affect an increase in 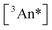 , the dimer outperforms the monomer (3.3% versus 0.52%; i.e., a factor of 6.3 improvement).
, the dimer outperforms the monomer (3.3% versus 0.52%; i.e., a factor of 6.3 improvement).
Having established boundaries for the strong annihilation regime, a concentration-dependent study of ϕUC for dimer versus monomer was also made using a fixed laser fluence well into this regime at 2.3 × 105 mW cm−2 for TIPS-BTX and at 2.4 × 105 mW cm−2 for TIPS-Tc. Neither sample reaches saturation of ϕUC as a function of annihilator concentration in the range employed here, but both appear on their way, particularly in the case of the monomer where we had access to larger amounts of compound during these studies (Fig. 4 (bottom)). As was seen for excitation fluence, this plot highlights significant advantages held by the dimer versus the monomer. For example, at common concentrations (see the vertical dashed arrows to guide the eye), the dimer outperforms the monomer by well over an order of magnitude, even though it is worse at TET (seen in Fig. 3 (bottom)). As another example, the same UC brightness measured as ϕUC for TIPS-Tc at 1.67 mM can be achieved for TIPS-BTX at approximately an order of magnitude lower concentration (see the horizontal dashed arrow to guide the eye). At the highest fluence and concentration investigated for the dimer, a steady-state triplet concentration is calculated to be 14 μM, a factor of 27× less than the ground state concentration. This argues strongly against advantage being gained by the dimer in this diffusional setting due to a double sensitization mechanism.26 While the maximum upconversion yield of 3.3% achieved at 0.39 mM TIPS-BTX is encouraging relative to monomer results, it does remain significantly below bluer upconverting champion systems like DPA that achieve yields ∼25%.25,26 But it is emphasized that our UC system as a whole is unoptimized, for example from the perspective of the sensitizer whose ϕISC is 75% and whose triplet lifetime limits ϕTET at 29% at the highest annihilator concentration that was used (Fig. 3 (bottom)).
As was suggested at the beginning of this section, the quantification of ϕUC as a function of annihilator concentration while in the strong annihilation regime enables determination of  using the other known quantities in Eq. 1b. These
using the other known quantities in Eq. 1b. These  are shown in Fig. 5 and like ϕUC highlight the advantage held by the dimer but now with considerations of poorer TET from the sensitizer due to the larger dimer size removed. These data also expose the overall quality of the dimers at negotiating productive TTA. As described earlier, the theoretical limit for
are shown in Fig. 5 and like ϕUC highlight the advantage held by the dimer but now with considerations of poorer TET from the sensitizer due to the larger dimer size removed. These data also expose the overall quality of the dimers at negotiating productive TTA. As described earlier, the theoretical limit for  is
is 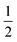 , corresponding to a case where the spin statistical factor f = 1. At the larger annihilator concentrations considered here, Fig. 5 indicates that TIPS-BTX is asymptotically approaching its maximal value of
, corresponding to a case where the spin statistical factor f = 1. At the larger annihilator concentrations considered here, Fig. 5 indicates that TIPS-BTX is asymptotically approaching its maximal value of  at the highest concentration considered (0.39 mM). The largest value that we measure is
at the highest concentration considered (0.39 mM). The largest value that we measure is  = 0.21, corresponding to f = 0.42 if annihilation from the singlet channel is unity. This value reflects 42% of the theoretical maximum of
= 0.21, corresponding to f = 0.42 if annihilation from the singlet channel is unity. This value reflects 42% of the theoretical maximum of 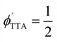 (a case that demands f = 1). While smaller than what is seen in perylene-based upconversion,80 this is on par with champion DPA systems.25,76,85 Juxtaposed relative to the monomer at a similar concentration of 0.45 mM where
(a case that demands f = 1). While smaller than what is seen in perylene-based upconversion,80 this is on par with champion DPA systems.25,76,85 Juxtaposed relative to the monomer at a similar concentration of 0.45 mM where  = 0.0062 (corresponding to 1.2% of the theoretical maximum), this is a compelling finding. Importantly, in the limit that the other efficiencies invoked by Eq. 1b are well characterized, any additional loss pathways that detract from productive TTA would be attributed to the spin statistical factor by this analysis,82 even when such parasitic pathways do not necessarily relate to spin statistics. In the case of TIPS-Tc presented here, we suspect a parasitic excimer state86 (discussed in more detail later) to form from the multiexciton collision complex, leading to the low observed yield.
= 0.0062 (corresponding to 1.2% of the theoretical maximum), this is a compelling finding. Importantly, in the limit that the other efficiencies invoked by Eq. 1b are well characterized, any additional loss pathways that detract from productive TTA would be attributed to the spin statistical factor by this analysis,82 even when such parasitic pathways do not necessarily relate to spin statistics. In the case of TIPS-Tc presented here, we suspect a parasitic excimer state86 (discussed in more detail later) to form from the multiexciton collision complex, leading to the low observed yield.
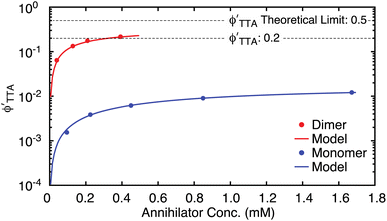 | ||
Fig. 5
 plotted against annihilator concentration for TIPS-Tc (blue) and TIPS-BTX (red). The results of a simplistic fitting model are included which assumes that annihilator triplet loss is only due to unimolecular decay and TTA via the singlet channel. See text and ESI† for discussion. plotted against annihilator concentration for TIPS-Tc (blue) and TIPS-BTX (red). The results of a simplistic fitting model are included which assumes that annihilator triplet loss is only due to unimolecular decay and TTA via the singlet channel. See text and ESI† for discussion. | ||
These  data can be subjected to a simple model, albeit with significant caveats alluded to below. The impetus for modeling flows from Eq. 1d which suggests that while annihilation yields are influenced by annihilator triplet lifetimes (where the dimer holds a modest advantage over the monomer (Table 1)), the rate constant kTTA might in principle serve as a fundamental measure of the probability of these photophysics for a given triplet concentration. If we make the likely too-simplistic (vide infra) assumptions that the dimer and monomer react similarly and that the only loss pathways for annihilator triplets is from unimolecular decay and TTA via the singlet channel, it is possible to obtain an expression for
data can be subjected to a simple model, albeit with significant caveats alluded to below. The impetus for modeling flows from Eq. 1d which suggests that while annihilation yields are influenced by annihilator triplet lifetimes (where the dimer holds a modest advantage over the monomer (Table 1)), the rate constant kTTA might in principle serve as a fundamental measure of the probability of these photophysics for a given triplet concentration. If we make the likely too-simplistic (vide infra) assumptions that the dimer and monomer react similarly and that the only loss pathways for annihilator triplets is from unimolecular decay and TTA via the singlet channel, it is possible to obtain an expression for 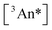 as a function of annihilator concentration, with kTTA as a single unknown (see Eq. S12†). This expression is then placed into Eq. 1d (or Eq. S10†), providing a fitting model for ϕTTAversus [An] that reveals kTTA. Fig. 5 shows this modeling with the result that kTTA = 6.5 × 107 M−1 s−1 for the dimer TIPS-BTX, nearly two orders of magnitude larger than kTTA = 6.7 × 105 M−1 s−1 obtained for the monomer TIPS-Tc. It is expected that kTTA is higher than stated here for the monomer, and instead TTA is in competition with parasitic second-order pathways. However, this is a behavior that is unaccounted for by a simple model, and further discussion will be focused on the apparent gains in efficiency made by the dimer TIPS-BTX.
as a function of annihilator concentration, with kTTA as a single unknown (see Eq. S12†). This expression is then placed into Eq. 1d (or Eq. S10†), providing a fitting model for ϕTTAversus [An] that reveals kTTA. Fig. 5 shows this modeling with the result that kTTA = 6.5 × 107 M−1 s−1 for the dimer TIPS-BTX, nearly two orders of magnitude larger than kTTA = 6.7 × 105 M−1 s−1 obtained for the monomer TIPS-Tc. It is expected that kTTA is higher than stated here for the monomer, and instead TTA is in competition with parasitic second-order pathways. However, this is a behavior that is unaccounted for by a simple model, and further discussion will be focused on the apparent gains in efficiency made by the dimer TIPS-BTX.
We propose a qualitative framework towards understanding the strong variation in  that favors the dimer TIPS-BTX over the monomer model TIPS-Tc. This is presented in Scheme 2 which aims to highlight differences in reactive pathways available to the two systems. Both monomer and dimer start from the perspective of a collisional encounter between two triplet (T1) excited state annihilator species (i.e., relevant for homofusion). These collisional encounters are ongoing in competition with nonradiative triplet decay to ground state. The schemes for both dimer and monomer also acknowledge the underlying spin statistics where, for example, there is a 5× higher probability that the encounter complex gains quintet character compared to that of a singlet.
that favors the dimer TIPS-BTX over the monomer model TIPS-Tc. This is presented in Scheme 2 which aims to highlight differences in reactive pathways available to the two systems. Both monomer and dimer start from the perspective of a collisional encounter between two triplet (T1) excited state annihilator species (i.e., relevant for homofusion). These collisional encounters are ongoing in competition with nonradiative triplet decay to ground state. The schemes for both dimer and monomer also acknowledge the underlying spin statistics where, for example, there is a 5× higher probability that the encounter complex gains quintet character compared to that of a singlet.
In the monomer system, the set of available pathways largely reflects expectations in place from studies of diffusional TTA-UC cases in the literature.84,87 For example, the route towards S1 emission (hν) is via TTA originating from a spin singlet encounter complex (summarized with the upward curved green arrow in Scheme 2 towards TTA-UC/hν). Encounter complexes with quintet character are not expected to confront loss pathways. Rather, the absence of an energetically accessible monomer-based quintet state means that re-dissociation is the most relevant outcome, forming isolated triplets that are again subjected to their own non-radiative losses.
The triplet encounter complex pathway could either dissociate in the same way just described for the quintet, or it could engage in its own reactivity such as annihilation to an energetically accessible triplet excited state followed by conversion to a single T1 plus heat. Although general, the diagram can address why monomers such as TIPS-Tc compete poorly relative to champion systems such as DPA. The pathway ‘triplet decay’ is important, with the Energy Gap Law driving shorter lifetimes for the lower energy T1 in TIPS-Tc compared to DPA. Also important and related are processes relevant to singlet fission (SF), the microscopic reverse of TTA (S1 → multiexciton singlet → T1 + T1). In TIPS-Tc the energy of two isolated triplets is expected to be comparable to the singlet multiexciton encounter complex or to a monomeric S1. In other words, while singlet fission is energetically relevant in TIPS-Tc, in DPA it is uphill. As SF transiently occurs in TIPS-Tc – in particular the final step where the multiexciton singlet encounter complex dissociates (the reverse equilibrium arrow in the singlet channel) – a pair of isolated T1 species are formed and again these are subject to their own nonradiative decay.
The reactive weight of triplet decay in this TIPS-Tc system compared to DPA means that one must work harder, in terms of excitation fluence, annihilator concentration, or both, to drive into the strong annihilation limit. However, once there, ϕTTA should not inherently be limited by smaller triplet lifetimes.83 This then suggests other effects driven by second order interactions between excited-state annihilators. These are symbolized by the downward curved red arrows in the Scheme 2 diagram signaling non-radiative decay from the singlet or triplet encounter complexes. Here we invoke observations made by Schmidt and coworkers studying excited-state dynamics of TIPS-Tc photoexcited in high-concentration solutions.86 These workers see evidence for singlet fission, but they also see evidence for a loss pathway via a spin-singlet excimer state.87 In the context of Scheme 2 we are implying that spin-singlet or spin-triplet encounter complexes could, for certain collision geometries, encounter non-radiative loss via excimer channels.
For the dimer system, the key distinction relative to the TIPS-Tc monomer lies in the fact that each spin-state encounter complex (1,3,5(T⋯T)) has an energetically accessible internal conversion (IC) pathway where energy reapportions, leading to formation of an intramolecular multiexciton state (1,3,5TT) along with one ground state dimer. In all cases this is competing against triplet dissociation. Thus, to the extent that the 1,3,5TT might then access productive outcomes, IC can provide a valuable alternative to annihilator triplet decay or non-radiative loss from encounter complexes. To this last point, while TIPS-BTX has additional steric bulk relative to the monomer TIPS-Tc, its two chromophores that are juxtaposed outward remain exposed to the environment and intermolecular excimeric geometries that would be loss-inducing should remain possible. Thus, the marked improvement in ϕTTA for TIPS-BTX relative to TIPS-Tc points to the relevance of intramolecular multiexciton states.
We reason that the singlet channel is likely to be productive although we have not observed the 1TT in TIPS-BTX directly (vide supra) and only know that the S1 radiates efficiently (ϕFL = 0.72) with observed decay in 12.7 ns when formed directly (Table 1). However, we can infer from a related norbornyl-bridged TIPS-pentacene dimer (TIPS-BP1′) where the 1TT (storing ∼1.7 eV) is formed quantitatively following visible photoexcitation. Its primary decay pathway involves non-radiative ground-state recovery in 102 ns in room temperature toluene.63 For a system like TIPS-BTX studied here that is similarly rigid compared to TIPS-BP1′ but that stores significantly more energy in its 1TT (∼2.41 eV; Table 1), our expectation is that direct 1TT → GS non-radiative decay will be slowed due to the energy gap law, thus affording ample time (>100 ns) for conversion to the S1 with access to its dominant (ϕFL = 0.72) radiative decay channel.
We also anticipate opportunities in the higher statistical weight quintet channel. Again, regarding the related TIPS-BP1′, we have recently shown using time-resolved EPR (trEPR) measurements that 5TT signatures are observable,88 a characteristic that has also been seen in other singlet fission systems including molecular dimers. This point is important because it means that the 1TT (formed first from the S1) is evolving in the multiexciton manifold to generate the 5TT. By converse, and therefore relevant to this current work, an intramolecular quintet (5TT) formed by IC in an upconversion system is coupled to the 1TT and through it to productive photoluminescence channels (see dotted equilibrium arrows in Scheme 2). Importantly and related, the trEPR studies of TIPS-BP1′ also reveal a valuable null result: they show no evidence for evolution of the 5TT into the triplet manifold, an observation that is highly unusual in the scope of SF systems that have been explored using trEPR (e.g. ref. 72, 73, 89 and 90). This avoidance is significant because the 3TT has access to spin-allowed loss pathways (3TT → T1 + heat). Thus, if avoidance of 5TT → 3TT on relevant time scales is a property built into these types of rigid dimer systems, of which TIPS-BTX is a member, it would afford an opportunity to harness the higher statistical weight quintet channel towards TTA while limiting exposure to non-radiative triplet biexciton loss followed by T1 decay. As noted in the introduction, non-rigid tetracenic dimers have been studied relative to TIPS-Tc with observation of an upconversion photoluminescence improvement of 6×47 to 10×56 at the highest annihilator concentrations used compared to the ∼20× increase seen here (Fig. 4). It is possible that rigid tetracenic dimers are able to exploit the 5TT in addition to the 1TT while non-rigid systems utilize only the latter.
The last point was about protecting the 5TT against loss pathways that would manifest through the triplet manifold. But this also implies that it is challenging to expect upconversion benefits in TIPS-BTX when triplet encounter complexes are initially formed, even if internal conversion to an intramolecular 3TT outcompetes re-dissociation of the 3(T⋯T) or direct non-radiative losses to T1via a triplet excimer state (vide supra). In TIPS-BTX, 3TT → T1 would release ∼1.2 eV of heat (Table 1). While this conversion is likely slowed by Marcus inverted-region reactivity owing to expected small reorganization energy in the process (of order 200 meV),63 it is unlikely slower than spin conversion to 5TT (where it might then access the singlet manifold), a process which is governed by minor terms in the already subtle spin Hamiltonian.88 Note that 1TT → S0 in TIPS-BP1′ is also spin allowed while releasing an even larger ∼1.7 eV of heat within the Marcus inverted region. Such decay takes ∼100 ns in room temperature toluene solutions.63
As noted earlier, Fig. 5 indicates that TIPS-BTX reaches a value of  that would correspond f = 0.42 (a lower limit achieved when ϕTTA is at its maximum of
that would correspond f = 0.42 (a lower limit achieved when ϕTTA is at its maximum of 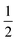 ), with a modest increase in f expected had higher TIPS-BTX concentrations been available. In other words, these data suggest that f is not bound at
), with a modest increase in f expected had higher TIPS-BTX concentrations been available. In other words, these data suggest that f is not bound at 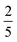 for this dimer, the scenario that would manifest if quintet encounter complexes simply dissociated while triplet encounter complexes each decayed to a single T1. This finding is consistent with participation by the intramolecular 5TT in a productive manner for upconversion as was suggested above in the context of Scheme 2. A statistical analysis that considers the singlet and quintet channels to be entirely productive while triplet encounter complexes decay to T1 would have f =
for this dimer, the scenario that would manifest if quintet encounter complexes simply dissociated while triplet encounter complexes each decayed to a single T1. This finding is consistent with participation by the intramolecular 5TT in a productive manner for upconversion as was suggested above in the context of Scheme 2. A statistical analysis that considers the singlet and quintet channels to be entirely productive while triplet encounter complexes decay to T1 would have f = 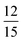 = 0.8 (see ESI for discussion†) and we anticipate this is the limit achievable by dimer systems.
= 0.8 (see ESI for discussion†) and we anticipate this is the limit achievable by dimer systems.
Conclusion
In this work we have compared the NIR-to-visible upconversion efficacy of a new rigid dimer system TIPS-BTX relative to the monomer model TIPS-Tc, seeking to quantify differences in terms of fundamental parameters underlying upconversion yields. A key observation is that the dimer consistently and markedly outperforms the monomer. For example, at comparable concentrations (0.39 mM TIPS-BTX versus 0.45 mM TIPS-Tc), the dimer reaches an upconversion yield that is more than 20× larger than the monomer. When we disentangle the contribution to the yield due specifically to TTA, the dimer is found to be more than 30× more effective. To understand this, we need to look from the perspective of both types of annihilators. On one hand the monomer TIPS-Tc is highly ineffective. But the similarity of its unimolecular photophysics and single-exciton state structure compared to the dimer TIPS-BTX suggests that TIPS-Tc triplets engage in deleterious second order processes, likely from both singlet and triplet collision-complex channels. We anticipate that these involve lower-energy excimer states that steal excited state population against TTA. In the nearly co-planar dimer TIPS-BTX, the two opposing chromophores remain exposed to the environment (they are not significantly sterically crowded by the bridge), and it is difficult to imagine that excimer channels would be so significantly undermined. From this lens, the marked improvement in for TIPS-BTX, to the point that it matches or exceeds what is seen in champion annihilator systems such as DPA, heralds internal conversion and subsequent participation by intramolecular multiexciton states in productive TTA. This is important, particularly under conditions where non-diffusive constructs are implemented, for example with upconversion dimers covalently decorating nanomaterial triplet sensitizers such as quantum dots. In this same context, we see the emergence of evidence that the quintet channel is productive in this rigid dimer class. First, the greater than 20× improvement in ϕUC compared to TIPS-Tc is more than 2× larger than the advantage reported for a non-rigid dimer compared to the same monomer.47,56 This could reflect a situation where dimer rigidity prevents intramolecular 5TT → 3TT → T1 loss channels while allowing for 5TT → 1TT → S1 productive channels. Second, we calculate a spin-statistical value of f = 0.42 for TIPS-BTX with evidence that f would increase in studies with higher concentrations of the dimer. A definitive demonstration of f > 0.4 (i.e.,
for TIPS-BTX, to the point that it matches or exceeds what is seen in champion annihilator systems such as DPA, heralds internal conversion and subsequent participation by intramolecular multiexciton states in productive TTA. This is important, particularly under conditions where non-diffusive constructs are implemented, for example with upconversion dimers covalently decorating nanomaterial triplet sensitizers such as quantum dots. In this same context, we see the emergence of evidence that the quintet channel is productive in this rigid dimer class. First, the greater than 20× improvement in ϕUC compared to TIPS-Tc is more than 2× larger than the advantage reported for a non-rigid dimer compared to the same monomer.47,56 This could reflect a situation where dimer rigidity prevents intramolecular 5TT → 3TT → T1 loss channels while allowing for 5TT → 1TT → S1 productive channels. Second, we calculate a spin-statistical value of f = 0.42 for TIPS-BTX with evidence that f would increase in studies with higher concentrations of the dimer. A definitive demonstration of f > 0.4 (i.e., 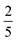 ) would be strong evidence for productive participation by 5TT and this issue should continue to be explored in rigid systems. Ultimately, we posit that rigid dimers could approach f =
) would be strong evidence for productive participation by 5TT and this issue should continue to be explored in rigid systems. Ultimately, we posit that rigid dimers could approach f = 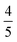 indicating productive use of both 1TT and the higher statistical weight 5TT.
indicating productive use of both 1TT and the higher statistical weight 5TT.
Data availability
Data are available upon reasonable request.Author contributions
NHD, ATG, and RO conceived of the studies and wrote the manuscript. ATG and RO made most of the measurements. NFP collected longer-time TA data for the dimer. EGM synthesized the dimer TIPS-BTX and oversaw analyses checking against sample degradation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Science Foundation (CHE-2102713). RO was supported by the NSF Graduate Research Fellowship Program (NSF-GRFP; DGE 2040434). Professor Tarek Sammakia was an essential collaborator in the synthesis of TIPS-BTX (ref. 58 and 59).Notes and references
- T. F. Schulze and T. W. Schmidt, Photochemical upconversion: present status and prospects for its application to solar energy conversion, Energy Environ. Sci., 2015, 8, 103–125 RSC.
- J. Zhou, Q. Liu, W. Feng, Y. Sun and F. Li, Upconversion Luminescent Materials: Advances and Applications, Chem. Rev., 2015, 115, 395–465 CrossRef CAS PubMed.
- Y. Zhou, C. Ruchlin, A. J. Robb and K. Hanson, Singlet Sensitization-Enhanced Upconversion Solar Cells via Self-Assembled Trilayers, ACS Energy Lett., 2019, 4, 1458–1463 CrossRef CAS.
- J. C. Goldschmidt and S. Fischer, Upconversion for Photovoltaics - a Review of Materials, Devices and Concepts for Performance Enhancement, Adv. Opt. Mater., 2015, 3, 510–535 CrossRef CAS.
- S. Fischer, A. Ivaturi, P. Jakob, K. W. Kramer, R. Martin-Rodriguez, A. Meijerink, B. Richards and J. C. Goldschmidt, Upconversion solar cell measurements under real sunlight, Opt. Mater., 2018, 84, 389–395 CrossRef CAS.
- T. Dilbeck and K. Hanson, Molecular Photon Upconversion Solar Cells Using Multilayer Assemblies: Progress and Prospects, J. Phys. Chem. Lett., 2018, 9, 5810–5821 CrossRef CAS PubMed.
- D. Beery, T. W. Schmidt and K. Hanson, Harnessing Sunlight via Molecular Photon Upconversion, ACS Appl. Mater. Interfaces, 2021, 13, 32601–32605 CrossRef CAS PubMed.
- M. Majek, U. Faltermeier, B. Dick, R. Perez-Ruiz and A. Jacobi von Wangelin, Application of Visible-to-UV Photon Upconversion to Photoredox Catalysis: The Activation of Aryl Bromides, Chem.–Euro. J., 2015, 21, 15496–15501 CrossRef CAS PubMed.
- B. D. Ravetz, A. B. Pun, E. M. Churchill, D. N. Congreve, T. Rovis and L. M. Campos, Photoredox catalysis using infrared light via triplet fusion upconversion, Nature, 2019, 565, 343–346 CrossRef CAS PubMed.
- F. Glaser, C. Kerzig and O. S. Wenger, Sensitization-initiated electron transfer via upconversion: mechanism and photocatalytic applications, Chem. Sci., 2021, 12, 9922–9933 RSC.
- J. C. Herrera-Luna, T. J. B. Zahringer, J. C. Herrera-Luna, M. C. Jimenez, C. Kerzig and R. Perez-Ruiz, A new green-to-blue upconversion system with efficient photoredox catalytic properties, Phys. Chem. Chem. Phys., 2023, 25, 12041–12049 RSC.
- Q. Liu, T. Yang, W. Feng and F. Li, Blue-Emissive Upconversion Nanoparticles for Low-Power-Excited Bioimaging in Vivo, J. Am. Chem. Soc., 2012, 134, 5390–5397 CrossRef CAS PubMed.
- X. J. Zhu, Q. Q. Su, W. Feng and F. Y. Li, Anti-Stokes shift luminescent materials for bio-applications, Chem. Soc. Rev., 2017, 46, 1025–1039 RSC.
- J. Park, M. Xu, F. Li and H.-C. Zhou, 3D Long-Range Triplet Migration in a Water-Stable Metal–Organic Framework for Upconversion-Based Ultralow-Power in Vivo Imaging, J. Am. Chem. Soc., 2018, 140, 5493–5499 CrossRef CAS PubMed.
- S. H. C. Askes and S. Bonnet, Solving the oxygen sensitivity of sensitized photon upconversion in life science applications, Nat. Rev. Chem, 2018, 2, 437–452 CrossRef.
- Q. Liu, M. Xu, T. Yang, B. Tian, X. Zhang and F. Li, Highly Photostable Near-IR-Excitation Upconversion Nanocapsules Based on Triplet–Triplet Annihilation for in Vivo Bioimaging Application, ACS Appl. Mater. Interfaces, 2018, 10, 9883–9888 CrossRef CAS PubMed.
- S. N. Sanders, T. H. Schloemer, M. K. Gangishetty, D. Anderson, M. Seitz, A. O. Gallegos, R. C. Stokes and D. N. Congreve, Triplet fusion upconversion nanocapsules for volumetric 3D printing, Nature, 2022, 604, 474 CrossRef CAS PubMed.
- J. T. Y. Wong, S. X. Wei, R. Meir, N. Sadaba, N. A. Ballinger, E. K. Harmon, X. Gao, G. Altin-Yavuzarslan, L. D. Pozzo, L. M. Campos and A. Nelson, Triplet Fusion Upconversion for Photocuring 3D-Printed Particle-Reinforced Composite Networks, Adv. Mater., 2023, 35, 2207673 CrossRef CAS PubMed.
- P. E. Keivanidis, S. Baluschev, T. Miteva, G. Nelles, U. Scherf, A. Yasuda and G. Wegner, Up-Conversion Photoluminescence in Polyfluorene Doped with Metal(II)–Octaethyl Porphyrins, Adv. Mater., 2003, 15, 2095–2098 CrossRef CAS.
- D. V. Kozlov and F. N. Castellano, Anti-Stokes delayed fluorescence from metal–organic bichromophores, Chem. Commun., 2004, 2860–2861 RSC.
- T. N. Singh-Rachford and F. N. Castellano, Photon upconversion based on sensitized triplet-triplet annihilation, Coord. Chem. Rev., 2010, 254, 2560–2573 CrossRef CAS.
- J. Zhao, S. Ji and H. Guo, Triplet–triplet annihilation based upconversion: from triplet sensitizers and triplet acceptors to upconversion quantum yields, R. Soc. Chem. Adv., 2011, 1, 937–950 CAS.
- P. Bharmoria, H. Bildirir and K. Moth-Poulsen, Triplet–triplet annihilation based near infrared to visible molecular photon upconversion, Chem. Soc. Rev., 2020, 49, 6529–6554 RSC.
- A. Ronchi and A. Monguzzi, Sensitized triplet–triplet annihilation based photon upconversion in full organic and hybrid multicomponent systems, Chem. Phys. Rev., 2022, 3, 041301 CrossRef CAS.
- Y. V. Aulin, M. van Sebille, M. Moes and F. C. Grozema, Photochemical upconversion in metal-based octaethyl porphyrin-diphenylanthracene systems, R. Soc. Chem. Adv., 2015, 5, 107896–107903 CAS.
- A. Olesund, V. Gray, J. Martensson and B. Albinsson, Diphenylanthracene Dimers for Triplet-Triplet Annihilation Photon Upconversion: Mechanistic Insights for Intramolecular Pathways and the Importance of Molecular Geometry, J. Am. Chem. Soc., 2021, 143, 5745–5754 CrossRef CAS PubMed.
- J. Zhou, Z. Liu and F. Y. Li, Upconversion nanophosphors for small-animal imaging, Chem. Soc. Rev., 2012, 41, 1323–1349 RSC.
- Q. Q. Dou, H. C. Guo and E. Y. Ye, Near-infrared upconversion nanoparticles for bio-applications, Mater. Sci. Eng. C, 2014, 45, 635–643 CrossRef CAS PubMed.
- M. B. Smith and J. Michl, Singlet Fission, Chem. Rev., 2010, 110, 6891–6936 CrossRef CAS PubMed.
- V. Yakutkin, S. Aleshchenkov, S. Chernov, T. Miteva, G. Nelles, A. Cheprakov and S. Baluschev, Towards the IR Limit of the Triplet-Triplet Annihilation-Supported Up-Conversion: Tetraanthraporphyrin, Chem.–Euro. J., 2008, 14, 9846–9850 CrossRef CAS PubMed.
- Y. Y. Cheng, T. Khoury, R. G. C. R. Clady, M. J. Y. Tayebjee, N. J. Ekins-Daukes, M. J. Crossley and T. W. Schmidt, On the efficiency limit of triplet-triplet annihilation for photochemical upconversion, Phys. Chem. Chem. Phys., 2010, 12, 66–71 RSC.
- E. Radiunas, S. Raisys, S. Jursenas, A. Jozeliunaite, T. Javorskis, U. Sinkeviciute, E. Orentas and K. Kazlauskas, Understanding the limitations of NIR-to-visible photon upconversion in phthalocyanine-sensitized rubrene systems, J. Mater. Chem. C, 2020, 8, 5525–5534 RSC.
- C. J. Imperiale, P. B. Green, E. G. Miller, N. H. Damrauer and M. W. B. Wilson, Triplet-Fusion Upconversion Using a Rigid Tetracene Homodimer, J. Phys. Chem. Lett., 2019, 10, 7463–7469 CrossRef CAS PubMed.
- M. F. Wu, D. N. Congreve, M. W. B. Wilson, J. Jean, N. Geva, M. Welborn, T. Van Voorhis, V. Bulovic, M. G. Bawendi and M. A. Baldo, Solid-State Infrared-to-Visible Upconversion Sensitized by Colloidal Nanocrystals, Nat. Photonics, 2016, 10, 31–34 CrossRef CAS.
- L. Nienhaus, M. Wu, N. Geva, J. J. Shepherd, M. W. B. Wilson, V. Bulović, T. V. Voorhis, M. A. Baldo and M. G. Bawendi, Speed Limit for Triplet-Exciton Transfer in Solid-State PbS Nanocrystal-Sensitized Photon Upconversion, ACS Nano, 2017, 11, 7848–7857 CrossRef CAS PubMed.
- S. Wieghold, A. S. Bieber, Z. A. VanOrman, L. Daley, M. Leger, J.-P. Correa-Baena and L. Nienhaus, Triplet Sensitization by Lead Halide Perovskite Thin Films for Efficient Solid-State Photon Upconversion at Subsolar Fluxes, Matter, 2019, 1, 705–719 CrossRef CAS.
- A. Abulikemu, Y. Sakagami, C. Heck, K. Kamada, H. Sotome, H. Miyasaka, D. Kuzuhara and H. Yamada, Solid-State, Near-Infrared to Visible Photon Upconversion via Triplet-Triplet Annihilation of a Binary System Fabricated by Solution Casting, ACS Appl. Mater. Interfaces, 2019, 11, 20812–20819 CrossRef CAS PubMed.
- Z. A. VanOrman and L. Nienhaus, Bulk Metal Halide Perovskites as Triplet Sensitizers: Taking Charge of Upconversion, ACS Energy Lett., 2021, 6, 3686–3694 CrossRef CAS.
- J. S. Lissau, M. Khelfallah and M. Madsen, Near-Infrared to Visible Photon Upconversion by Palladium(II) Octabutoxyphthalocyanine and Rubrene in the Solid State, J. Phys. Chem. C, 2021, 125, 25643–25650 CrossRef CAS.
- C. R. Conti, A. S. Bieber, Z. A. VanOrman, G. Moller, S. Wieghold, R. D. Schaller, G. F. Strouse and L. Nienhaus, Ultrafast Triplet Generation at the Lead Halide Perovskite/Rubrene Interface, ACS Energy Lett., 2022, 7, 617–623 CrossRef CAS.
- E. Radiunas, L. Naimovicius, S. Raisys, A. Jozeliunaite, E. Orentas and K. Kazlauskas, Efficient NIR-to-vis photon upconversion in binary rubrene films deposited by simplified thermal evaporation, J. Mater. Chem. C, 2022, 10, 6314–6322 RSC.
- F. Deng, J. R. Sommer, M. Myahkostupov, K. S. Schanze and F. N. Castellano, Near-IR phosphorescent metalloporphyrin as a photochemical upconversion sensitizer, Chem. Commun., 2013, 49, 7406–7408 RSC.
- F. Deng, W. F. Sun and F. N. Castellano, Texaphyrin sensitized near-IR-to-visible photon upconversion, Photochem. Photobiol. Sci., 2014, 13, 813–819 CrossRef CAS PubMed.
- Z. Huang, X. Li, M. Mahboub, K. M. Hanson, V. M. Nichols, H. Le, M. L. Tang and C. J. Bardeen, Hybrid Molecule–Nanocrystal Photon Upconversion Across the Visible and Near-Infrared, Nano Lett., 2015, 15, 5552–5557 CrossRef CAS PubMed.
- M. Mahboub, Z. Y. Huang and M. L. Tang, Efficient Infrared-to-Visible Upconversion with Subsolar Irradiance, Nano Lett., 2016, 16, 7169–7175 CrossRef CAS PubMed.
- Z. Huang, D. E. Simpson, M. Mahboub, X. Li and M. L. Tang, Ligand enhanced upconversion of near-infrared photons with nanocrystal light absorbers, Chem. Sci., 2016, 7, 4101–4104 RSC.
- A. B. Pun, S. N. Sanders, M. Y. Sfeir, L. M. Campos and D. N. Congreve, Annihilator dimers enhance triplet fusion upconversion, Chem. Sci., 2019, 10, 3969–3975 RSC.
- K. J. Fallon, E. M. Churchill, S. N. Sanders, J. Shee, J. L. Weber, R. Meir, S. Jockusch, D. R. Reichman, M. Y. Sfeir, D. N. Congreve and L. M. Campos, Molecular Engineering of Chromophores to Enable Triplet-Triplet Annihilation Upconversion, J. Am. Chem. Soc., 2020, 142, 19917–19925 CrossRef CAS PubMed.
- S. Baluschev, V. Yakutkin, T. Miteva, Y. Avlasevich, S. Chernov, S. Aleshchenkov, G. Nelles, A. Cheprakov, A. Yasuda, K. Müllen and G. Wegner, Blue-Green Up-Conversion: Noncoherent Excitation by NIR Light, Angew. Chem., Int. Ed., 2007, 46, 7693–7696 CrossRef CAS PubMed.
- D. Dzebo, K. Börjesson, V. Gray, K. Moth-Poulsen and B. Albinsson, Intramolecular Triplet–Triplet Annihilation Upconversion in 9,10-Diphenylanthracene Oligomers and Dendrimers, J. Phys. Chem. C, 2016, 120, 23397–23406 CrossRef CAS.
- C. Gao, S. K. K. Prasad, B. Zhang, M. Dvořák, M. J. Y. Tayebjee, D. R. McCamey, T. W. Schmidt, T. A. Smith and W. W. H. Wong, Intramolecular Versus Intermolecular Triplet Fusion in Multichromophoric Photochemical Upconversion, J. Phys. Chem. C, 2019, 123, 20181–20187 CrossRef CAS.
- H. Y. Liu, X. Y. Yan, L. Shen, Z. F. Tang, S. S. Liu and X. Y. Li, Color-tunable upconversion emission from a twisted intramolecular charge-transfer state of anthracene dimers via triplet-triplet annihilation, Mater. Horiz., 2019, 6, 990–995 RSC.
- M. Kanoh, Y. Matsui, K. Honda, Y. Kokita, T. Ogaki, E. Ohta and H. Ikeda, Elongation of Triplet Lifetime Caused by Intramolecular Energy Hopping in Diphenylanthracene Dyads Oriented to Undergo Efficient Triplet-Triplet Annihilation Upconversion, J. Phys. Chem. B, 2021, 125, 4831–4837 CrossRef CAS PubMed.
- W. J. Sun, A. Ronchi, T. H. Zhao, J. L. Han, A. Monguzzi and P. F. Duan, Highly efficient photon upconversion based on triplet-triplet annihilation from bichromophoric annihilators, J. Mater. Chem. C, 2021, 9, 14201–14208 RSC.
- F. Edhborg, H. Bildirir, P. Bharmoria, K. Moth-Poulsen and B. Albinsson, Intramolecular Triplet-Triplet Annihilation Photon Upconversion in Diffusionally Restricted Anthracene Polymer, J. Phys. Chem. B, 2021, 125, 6255–6263 CrossRef CAS PubMed.
- Y. Bo, Y. Hou, D. Thiel, R. Weiss, T. Clark, M. J. Ferguson, R. R. Tykwinski and D. M. Guldi, Tetracene Dimers: A Platform for Intramolecular Down- and Up-conversion, J. Am. Chem. Soc., 2023, 145, 18260–18275 CrossRef CAS PubMed.
- S. Mattiello, S. Mecca, A. Ronchi, A. Calascibetta, G. Mattioli, F. Pallini, F. Meinardi, L. Beverina and A. Monguzzi, Diffusion-Free Intramolecular Triplet-Triplet Annihilation in Engineered Conjugated Chromophores for Sensitized Photon Upconversion, ACS Energy Lett., 2022, 7, 2435–2442 CrossRef CAS.
- E. G. Miller, M. Singh, S. R. Parkin, T. Sammakia and N. H. Damrauer, Preparation of a Rigid and nearly Coplanar Bis-Tetracene Dimer through Application of the CANAL Reaction, ChemRxiv, 2021, DOI:10.26434/chemrxiv.14412200.v1.
- E. G. Miller, M. Singh, S. R. Parkin, T. Sammakia and N. H. Damrauer, Preparation of a Rigid and nearly Coplanar Bis-Tetracene Dimer through Application of the CANAL Reaction, J. Org. Chem., 2023, 88, 12251–12256 CrossRef CAS PubMed.
- We refer to the Frank-Condon allowed bright state as S1, while recognizing that a dark state (opposing short-axis transition dipole moments) would be lower in energy. However, Davydov splitting in this dimer is expected to be negligible given the chromophore separation. TD-DFT calculations support this, showing a < 2 meV separation between the lowest-energy dark transition to excited state 1 and the next transition that is strongly allowed to excited state 2 (see Table S4).
- F. C. Spano, The Spectral Signatures of Frenkel Polarons in H- and J-Aggregates, Acc. Chem. Res., 2010, 43, 429–439 CrossRef CAS PubMed.
- J. D. Cook, T. J. Carey, D. H. Arias, J. C. Johnson and N. H. Damrauer, Solvent-controlled branching of localized versus delocalized singlet exciton states and equilibration with charge transfer in a structurally well-defined tetracene dimer, J. Phys. Chem. A, 2017, 121, 9229–9242 CrossRef CAS PubMed.
- A. T. Gilligan, E. G. Miller, T. Sammakia and N. H. Damrauer, Using Structurally Well-Defined Norbornyl-Bridged Acene Dimers to Map a Mechanistic Landscape for Correlated Triplet Formation in Singlet Fission, J. Am. Chem. Soc., 2019, 141, 5961–5971 CrossRef CAS PubMed.
- J. Cook, T. J. Carey and N. H. Damrauer, Solution-Phase Singlet Fission in a Structurally Well-Defined Norbornyl-Bridged Tetracene Dimer, J. Phys. Chem. A, 2016, 120, 4473–4481 CrossRef CAS PubMed.
- T. Yamakado, S. Takahashi, K. Watanabe, Y. Matsumoto, A. Osuka and S. Saito, Conformational Planarization versus Singlet Fission: Distinct Excited-State Dynamics of Cyclooctatetraene-Fused Acene Dimers, Angew. Chem., Int. Ed., 2018, 57, 5438–5443 CrossRef CAS PubMed.
- S. Nakamura, H. Sakai, H. Nagashima, Y. Kobori, N. V. Tkachenko and T. Hasobe, Quantitative Sequential Photoenergy Conversion Process from Singlet Fission to Intermolecular Two-Electron Transfers Utilizing Tetracene Dimer, ACS Energy Lett., 2019, 4, 26–31 CrossRef CAS.
- Y. Matsui, S. Kawaoka, H. Nagashima, T. Nakagawa, N. Okamura, T. Ogaki, E. Ohta, S. Akimoto, A. Sato-Tomita, S. Yagi, Y. Kobori and H. Ikeda, Exergonic Intramolecular Singlet Fission of an Adamantane-Linked Tetracene Dyad via Twin Quintet Multiexcitons, J. Phys. Chem. C, 2019, 123, 18813–18823 CrossRef CAS.
- A. B. Pun, A. Asadpoordarvish, E. Kumarasamy, M. J. Y. Tayebjee, D. Niesner, D. R. McCamey, S. N. Sanders, L. M. Campos and M. Y. Sfeir, Ultra-fast intramolecular singlet fission to persistent multiexcitons by molecular design, Nat. Chem., 2019, 11, 821–828 CrossRef CAS PubMed.
- K. R. Parenti, G. Y. He, S. N. Sanders, A. B. Pun, E. Kumarasamy, M. Y. Sfeir and L. M. Campos, Bridge Resonance Effects in Singlet Fission, J. Phys. Chem. A, 2020, 124, 9392–9399 CrossRef CAS PubMed.
- S. Nakamura, H. Sakai, H. Nagashima, M. Fuki, K. Onishi, R. Khan, Y. Kobori, N. V. Tkachenko and T. Hasobe, Synergetic Role of Conformational Flexibility and Electronic Coupling for Quantitative Intramolecular Singlet Fission, J. Phys. Chem. C, 2021, 125, 18287–18296 CrossRef CAS.
- S. N. Sanders, E. Kumarasamy, A. B. Pun, M. T. Trinh, B. Choi, J. L. Xia, E. J. Taffet, J. Z. Low, J. R. Miller, X. Roy, X. Y. Zhu, M. L. Steigerwald, M. Y. Sfeir and L. M. Campos, Quantitative Intramolecular Singlet Fission in Bipentacenes, J. Am. Chem. Soc., 2015, 137, 8965–8972 CrossRef CAS PubMed.
- M. J. Y. Tayebjee, S. N. Sanders, E. Kumarasamy, L. M. Campos, M. Y. Sfeir and D. R. McCamey, Quintet Multiexciton Dynamics in Singlet Fission, Nat. Phys., 2017, 13, 182–188 Search PubMed.
- H. Sakai, R. Inaya, H. Nagashima, S. Nakamura, Y. Kobori, N. V. Tkachenko and T. Hasobe, Multiexciton Dynamics Depending on Intramolecular Orientations in Pentacene Dimers: Recombination and Dissociation of Correlated Triplet Pairs, J. Phys. Chem. Lett., 2018, 9, 3354–3360 Search PubMed.
- M. Montalti, A. Credi, L. Prodi and M. T. Gandolfi, Handbook of photochemistry, CRC Press, Boca Raton FL, 3rd edn, 2006 Search PubMed.
- F. Edhborg, A. Olesund and B. Albinsson, Best practice in determining key photophysical parameters in triplet-triplet annihilation photon upconversion, Photochem. Photobiol. Sci., 2022, 21, 1143–1158 Search PubMed.
- J. K. H. Pun, J. K. Gallaher, L. Frazer, S. K. K. Prasad, C. B. Dover, R. W. MacQueen and T. W. Schmidt, TIPS-anthracene: a singlet fission or triplet fusion material?, J. Photonics Energy, 2018, 8, 022006 Search PubMed.
- B. D. Rihter, M. E. Kenney, W. E. Ford and M. A. J. Rodgers, Synthesis and Photoproperties of Diamagnetic Octabutoxyphthalocyanines with Deep Red Optical Absorbency, J. Am. Chem. Soc., 1990, 112, 8064–8070 CrossRef CAS.
- H. L. Stern, A. J. Musser, S. Gelinas, P. Parkinson, L. M. Herz, M. J. Bruzek, J. Anthony, R. H. Friend and B. J. Walker, Identification of a Triplet Pair Intermediate in Singlet Exciton Fission in Solution, Proc. Natl. Acad. Sci. U.S.A., 2015, 112, 7656–7661 CrossRef CAS PubMed.
- D. G. Bossanyi, Y. Sasaki, S. Wang, D. Chekulaev, N. Kimizuka, N. Yanai and J. Clark, Spin Statistics for Triplet-Triplet Annihilation Upconversion: Exchange Coupling, Intermolecular Orientation, and Reverse Intersystem Crossing, JACS Au, 2021, 1, 2188–2201 CrossRef CAS PubMed.
- S. Hoseinkhani, R. Tubino, F. Meinardi and A. Monguzzi, Achieving the photon up-conversion thermodynamic yield upper limit by sensitized triplet-triplet annihilation, Phys. Chem. Chem. Phys., 2015, 17, 4020–4024 RSC.
- Y. Zhou, F. N. Castellano, T. W. Schmidt and K. Hanson, On the Quantum Yield of Photon Upconversion via Triplet-Triplet Annihilation, ACS Energy Lett., 2020, 5, 2322–2326 CrossRef CAS.
- V. Gray, A. Dreos, P. Erhart, B. Albinsson, K. Moth-Poulsen and M. Abrahamsson, Loss channels in triplet-triplet annihilation photon upconversion: importance of annihilator singlet and triplet surface shapes, Phys. Chem. Chem. Phys., 2017, 19, 10931–10939 RSC.
- Y. Murakami and K. Kamada, Kinetics of photon upconversion by triplet–triplet annihilation: a comprehensive tutorial, Phys. Chem. Chem. Phys., 2021, 23, 18268–18282 RSC.
- A. Haefele, J. Blumhoff, R. S. Khnayzer and F. N. Castellano, Getting to the (Square) Root of the Problem: How to Make Noncoherent Pumped Upconversion Linear, J. Phys. Chem. Lett., 2012, 3, 299–303 CrossRef CAS.
- T. W. Schmidt and F. N. Castellano, Photochemical Upconversion: The Primacy of Kinetics, J. Phys. Chem. Lett., 2014, 5, 4062–4072 CrossRef CAS PubMed.
- C. B. Dover, J. K. Gallaher, L. Frazer, P. C. Tapping, A. J. Petty, M. J. Crossley, J. E. Anthony, T. W. Kee and T. W. Schmidt, Endothermic singlet fission is hindered by excimer formation, Nat. Chem., 2018, 10, 305–310 CrossRef CAS PubMed.
- C. Ye, V. Gray, J. Martensson and K. Borjesson, Annihilation Versus Excimer Formation by the Triplet Pair in Triplet-Triplet Annihilation Photon Upconversion, J. Am. Chem. Soc., 2019, 141, 9578–9584 CrossRef CAS PubMed.
- R. D. Dill, K. E. Smyser, B. K. Rugg, N. H. Damrauer and J. D. Eaves, Entangled spin-polarized excitons from singlet fission in a rigid dimer, Nat. Commun., 2023, 14, 1180 CrossRef CAS PubMed.
- B. S. Basel, J. Zirzlmeier, C. Hetzer, S. R. Reddy, B. T. Phelan, M. D. Krzyaniak, M. K. Volland, P. B. Coto, R. M. Young, T. Clark, M. Thoss, R. R. Tykwinski, M. R. Wasielewski and D. M. Gulditla, Evidence for Charge-Transfer Mediation in the Primary Events of Singlet Fission in a Weakly Coupled Pentacene Dimer, Chem, 2018, 4, 1092–1111 CAS.
- Y. Kobori, M. Fuki, S. Nakamura and T. Hasobe, Geometries and Terahertz Motions Driving Quintet Multiexcitons and Ultimate Triplet–Triplet Dissociations via the Intramolecular Singlet Fissions, J. Phys. Chem. B, 2020, 124, 9411–9419 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sc04795d |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |