Open Access Article
Open Access ArticleUse of deep eutectic solvents in environmentally-friendly dye-sensitized solar cells and their physicochemical properties: a brief review
Khatereh A. Pishro
 and
Mario Henrique Gonzalez
and
Mario Henrique Gonzalez
 *
*
São Paulo State University (UNESP), Institute of Biosciences, Humanities and Exact Sciences (IBILCE), National Institute for Alternative Technologies of Detection, Toxicological Evaluation and Removal of Micropollutants and Radioactives (INCT-DATREM), São José do Rio Preto, SP 15054-000, Brazil. E-mail: mario.gonzalez@unesp.br; Fax: +55 17 32212512; Tel: +55 17 32212512
First published on 2nd May 2024
Abstract
A novel way to mitigate the greenhouse effect is to use dye-sensitized solar cells (DSSCs) to convert carbon dioxide from the air into useful products, such as hydrocarbons, which can also store energy from the sun, a plentiful, clean, and safe resource. The conversion of CO2 can help reduce the impacts of greenhouse gas emissions that contribute to global warming. However, there is a major obstacle in using DSSCs, since many solar devices operate with organic electrolytes, producing pollutants including toxic substances. Therefore, a key research area is to find new eco-friendly electrolytes that can effectively dissolve carbon dioxide. One option is to use deep eutectic solvents (DESs), which are potential substitutes for ionic liquids (ILs) and have similar advantages, such as being customizable, economical, and environmentally friendly. DESs are composed of low-cost materials and have very low toxicity and high biodegradability, making them suitable for use as electrolytes in DSSCs, within the framework of green chemistry. The purpose of this brief review is to explore the existing knowledge about how CO2 dissolves in DESs and how these solvents can be used as electrolytes in solar devices, especially in DSSCs. The physical and chemical properties of the DESs are described, and areas are suggested where further research should be focused.
 Khatereh A. Pishro |
 Mario Henrique Gonzalez |
1. Introduction
One of the most important challenges in recent years concerns the design of alternative renewable energy technologies that can replace fossil fuels and reduce their environmental damage.1,2 According to the Paris Agreement, urgent actions are needed to lower the emissions of CO2 into the atmosphere, as they cause serious effects in the environment.3 One promising solution is to use solar cells to convert CO2 into valuable products such as hydrocarbons, which can also store solar energy, a plentiful, clean, and safe resource.4 The conversion of CO2 can help reduce greenhouse gas levels, while at the same time absorbing sunlight, contributing to mitigating global warming.5Artificial photosynthetic systems are devices that use sunlight as an energy source and water as an electron source to convert CO2 into useful chemicals (fuels or carbohydrates). This can be achieved by combining a photovoltaic (PV) cell that generates electrons and holes with an electrochemical cell (EC) that oxidizes water at the anode and reduces CO2 at the cathode.6–8 An electrolyte is a vital component in these devices, as it enables charge transport between the electrodes. Most of the electrolytes used are non-aqueous, including polar organic solvents (acetonitrile, valeronitrile, and others) and green solvents (ILs and DESs).9,10 Ionic liquids (ILs) and deep eutectic solvents (DESs) have been proposed for various industrial processes and applications, such as chemical synthesis,11 absorption,12 biomass pretreatment,13 extraction,14 electrolytes for electrochemistry (fuel cells and lithium batteries),15 and dye-sensitized solar cells (DSSCs).16–18 Scheme 1 provides an overview of DESs applications, based on their hydrophilic and hydrophobic characteristics.19
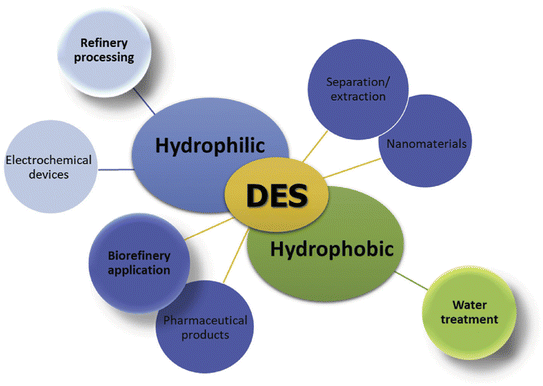 | ||
| Scheme 1 DES applications according to their individual hydrophilic and hydrophobic characteristics.19 | ||
Among the various technologies for converting solar energy, DSSCs offer the greatest potential for improving their properties, in terms of both environmental sustainability and energy conversion performance. DSSCs are a type of thin-film solar cell that has been widely studied for more than two decades, due to their simple preparation methodology, low toxicity, and low cost of preparation, and ease of production.20–24
DSSCs are based on a photoactive anode with an n-type semiconductor layer (usually TiO2) sensitized by an organometallic dye, a counter electrode, and a liquid electrolyte that completes the circuit and regenerates the dye. Liquid electrolytes25 have achieved a record efficiency of 14.7% and liquid DSSCs are low-cost solar cells with high power conversion efficiencies (PCEs). However, acetonitrile-based electrolytes and some other organic components used for DSSCs are harmful, while their volatility limits their performance and stability. To overcome this problem, the use of DESs has been suggested as an attractive way to improve liquid electrolytes, with the advantages of low toxicity, biodegradability, high thermal stability, non-flammability, no vapor pressure, and low production costs.18,26–28
The primary objective of this review is to examine a range of deep eutectic solvents (DESs) for their potential as innovative electrolytes in solar cells,29 focusing on the synthesis and properties of DESs, with particular attention given to their physicochemical attributes that facilitate the conversion of CO2 into value-added chemicals. The mechanism of these solar cells emulates the natural photosynthesis process and is termed “artificial photosynthesis”.30,31 The review also outlines prospective avenues for future research within this area.
2. Deep eutectic solvents (DESs)
2.1. DESs as green solvents
Green and sustainable solvents have influenced the recent development of the chemical industry. Volatile organic compounds (VOCs) are still widely used as solvents in industries, even though they are often toxic and highly flammable.32,33 Ionic liquids (ILs) are considered green solvents, due to their very low vapor pressure and high thermal stability, making them suitable for many processes. However, ILs have some drawbacks, such as high viscosity, high cost, and difficult synthesis.34 Deep eutectic solvents (DESs)35,36 are new types of green solvents, usually composed of Lewis or Brønsted acids and bases, with a strong hydrogen bond network, where the interactions act to reduce the lattice energy. A simple preparation pathway for DESs using HBD and HBA is shown in Scheme 2.37 A DES is a binary or ternary mixture of safe and cheap components that include at least one hydrogen bond donor (HBD) and one hydrogen bond acceptor (HBA), mixed in a specific molar ratio to form a eutectic mixture with a melting point much lower than that of any of the individual components.38 The term “deep” should only be used for those mixtures with a eutectic point temperature much lower than that of an ideal liquid mixture, which explains the decreased melting point, compared to those of the individual components.39,40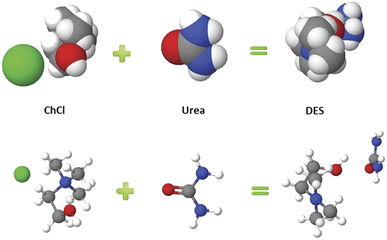 | ||
| Scheme 2 A DES preparation pathway composed of HBD and HBA, with strong hydrogen bonds to reduce the lattice energy.37 | ||
Binary DESs are mixtures of one kind of HBA and one kind of HBD, while ternary DESs are mixtures of three components, which have also been reported in the literature.41 Some HBDs and HBAs used for the preparation of DESs introduced in this review are shown in Scheme 3.
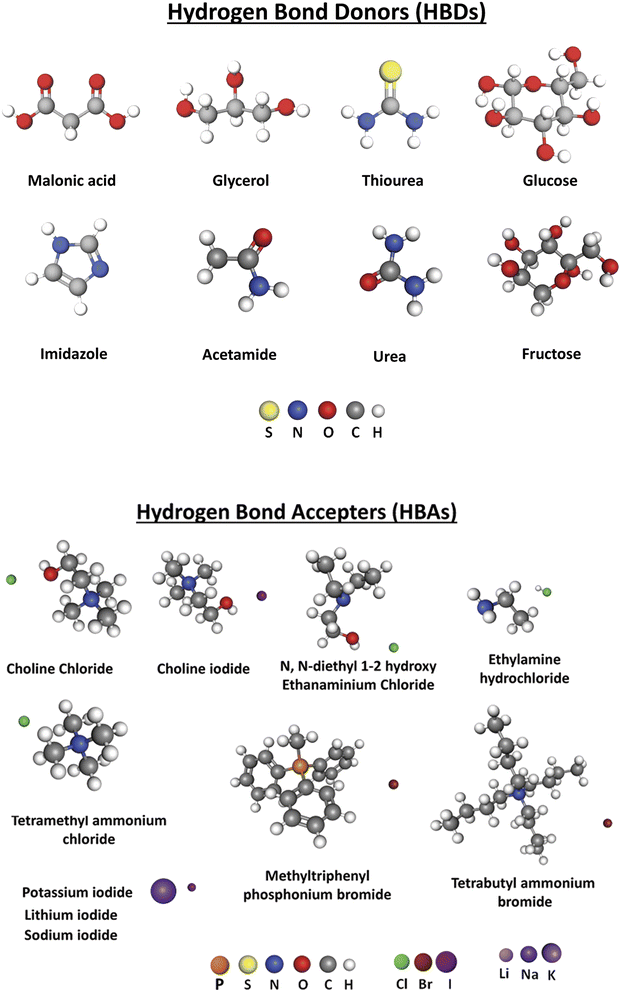 | ||
| Scheme 3 Some hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs) used in preparation of the DESs described in this review. | ||
The electrolytes commonly used in DSSCs are non-aqueous polar solvents containing organic compounds (such as acetonitrile). However, these solvents are volatile and toxic, which poses environmental and safety risks. Therefore, there is a need to develop new “green solvents” that have similar physicochemical properties, but are eco-friendly and more sustainable. Among the candidates for green solvents are DESs, which are HBA and HBD combinations that form eutectic mixtures with low melting points. The physicochemical properties of DESs depend on the type and ratio of HBA and HBD, resulting in different interactions (such as hydrogen bonding, π–π bonding, or anion exchange).51 These interactions affect the density, viscosity, melting/freezing point, and conductivity of DESs, which are important factors influencing their performance as electrolytes in DSSCs. In addition, other properties such as thermal stability, toxicity, and biodegradability affect the application potential of DESs.
The various uses of DESs in scientific and industrial fields are determined by their performance, which is directly influenced by their physicochemical properties. The relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents are depicted in Scheme 4, where temperature and moisture expand the liquid range of DESs.42
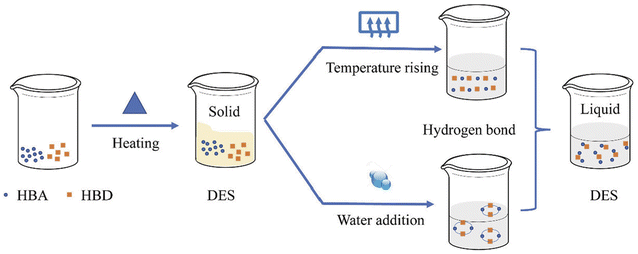 | ||
| Scheme 4 The relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents.42 | ||
In the following subsections, the basic physicochemical properties of DESs for use as effective electrolytes in DSSCs will be discussed and compared.
2.2. Properties and characteristics of DESs
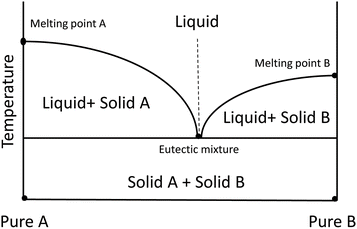 | ||
| Scheme 5 Eutectic point in a two-component (HBA and HBD) phase diagram.39 | ||
The DESs melting point influences the performance and stability of solar cells, as it affects the operating temperature range and the thermal degradation of the material.49 The melting point of the DESs, as well as the polarity, viscosity, and density of the solvent, all have an impact on the solubility of CO2 in DESs. The CO2 solubility in a DES is inversely proportional to the melting point of the DESs. To obtain the correct melting point and solubility of CO2 in DESs for solar cell applications, it is important to select a suitable mix and proportion of HBD and HBA. The DESs melting point also affects the physical and chemical properties of the perovskite materials used in common types of solar cells. Perovskite materials have a crystal structure analogous to that of the mineral perovskite, composed of calcium, titanium, and oxygen (CaTiO3). Increase of the heat treatment temperature can cause the absorption edge to present a red shift, the band gap to decrease, and the defect density of the perovskite material to increase. These changes can reduce the efficiency and stability of the solar cell. However, an appropriate heat treatment time and temperature can also passivate the defects and improve the solar cell performance.49 It is important to adjust the melting point and the heat treatment conditions of the DESs to optimize the properties of the perovskite material for solar cell applications.
The viscosity of DESs depends on the type and ratio of the HBD and HBA components, as well as the interactions between them. The stronger the interactions, such as hydrogen bonds or van der Waals forces, the higher the viscosity of the DESs. The viscosity of DESs affects their performance and applications in various fields, such as solar cells,50 electrochemistry,51 catalysis,52 and biotechnology.53 For example, the viscosity of DESs affects the solubility of CO2 in them, which is an important factor for the conversion of solar energy and CO2 to useful products such as hydrocarbons. The solubility of CO2 in DESs decreases as the viscosity increases. Therefore, selecting the best mix and proportion of the HBD and HBA parts is important for achieving suitable CO2 solubility and viscosity in DESs for solar cell uses.
The temperature and the ionic species in the DESs electrolyte mixtures affect their viscosity, which is an indicator of the fluidity of the electrolyte. Heydari Dokohani et al.54 used a DES based on choline chloride and ethylene glycol (ChCl–EG), adding different iodide salts to obtain electrolytes for dye-sensitized solar cells (DSSCs). Measurements were made of the viscosity of these electrolytes with added KI, 1-ethyl-3-methylimidazolium (EmimI), or KI–EmimI, at 293–365 K, which showed that the viscosity decreased at higher temperatures, improving DSSC performance under solar irradiation. In addition, the viscosity varied with the composition of the iodide salt, where the electrolytes with smaller cations had higher viscosity. The molecular weight of the ionic species also influenced the viscosity of the electrolytes. The simulated viscosity of the KI-containing electrolyte was 27.71 mPa s, which was close to the experimental value of 26.76 mPa s at 298 K. It was also found that the KI used in DESs preparation enhanced the DSSC performance, but also increased the viscosity of the solution.
The density of a DES determines its solubility properties, which are crucial for its use as a solvent for various organic reactions. Higher density results in higher solubility of polar and ionic compounds, because the molecules can form stronger interactions with the solute. However, density is not the only factor that affects the solubilization capacity of a DES. The solvation behavior is also influenced by the polarity, hydrogen bonding, molecular size, and shape of the components, as well as the nature of the solute. For instance, in organic oxidation reactions, DESs can break down both lipophilic and hydrophilic organic species, making the oxidation of organic compounds by hydrogen peroxide more effective.58
DESs have been used as electrolytes for DSSCs, a type of low-cost and environmentally friendly photovoltaic device that uses a dye to absorb light and generate electricity. The density of a DES is one of the physical properties that affect its performance in this application. Different studies have investigated the effect of density on the performance of DSSCs with DESs-based electrolytes. For example, one study used a DES composed of choline chloride and urea (Reline)59 as an electrolyte for carbon-based electrochemical capacitors. The study found that the specific capacitance increased from 100 to 157 F g−1 at a 1 A g−1 current load, by adding 1 wt% water to the DESs system, due to the decrease in viscosity and the increase in ionic conductivity.59
Another study used two DESs composed of choline chloride and ethylene glycol or urea as electrolytes for DSSCs. The devise using choline chloride and ethylene glycol showed an increase in the short circuit current (Isc) in opposite, the devices using choline chloride and urea electrolyte exhibited improved open circuit voltage values (Voc).,60 due to the different molecular structures and the ways that the DESs parts interacted with the dye and the redox mediator.
Various studies have explored how conductivity affects solar cells with electrolytes based on DESs. For instance, one study used a choline chloride and ethylene glycol DES as an electrolyte for DSSCs and found that the DSSCs with the DESs electrolyte exhibited higher power conversion efficiency, compared to DSSCs with a traditional liquid electrolyte, because the DES had higher conductivity and a lower recombination rate.62 Investigation was made of perovskite solar cells (PSCs), a new and efficient type of photovoltaic device that uses a perovskite material to absorb light and transport charge, using a DES composed of choline chloride and urea as the electrolyte. It was found that PSCs with the DESs electrolyte had better open-circuit voltage and fill factor, compared to PSCs with a usual solid-state electrolyte, because the DES provided higher conductivity and a smoother interface.62
The conductivity of DESs is affected by temperature, the alkyl chain length of the cation, the HBD to HBA molar ratio, and viscosity. For instance, adding a 33% mole fraction of choline chloride to a DES based on choline chloride and glycerol increased its conductivity from 1.047 to more than 1 mS cm−1,63 with the DESs conductivity being slightly affected by the cation alkyl chain length. In addition, the conductivity of DESs increases with temperature, because the hydrogen bond network breaks and the ionic mobility increases.64
Viscosity affects the conductivity of DESs, with higher viscosity leading to lower conductivity, and for some electrochemistry applications, the conductivity of DESs should not be too low (lower than 1 mS cm−1 at ambient temperature).65 Hence, DESs are a good choice for electrochemical applications, including as electrolytes for solar cells, because they have low viscosity and high conductivity.63,66
The acidity of the DESs can affect the redox potential of the mediator by changing the equilibrium between I− and I3−. Higher acidity, with lower pH, favors the formation of I3− and increases the redox potential.59 Another way that acidity affects the DES electrolyte is by influencing the stability and corrosion of the electrode materials and the dye sensitizer. The electrode materials are usually titanium dioxide (TiO2) for the photoanode and platinum (Pt) for the counter electrode. The dye sensitizer is usually a ruthenium complex that absorbs light and injects electrons into the TiO2. The acidity of the DESs can affect the stability and corrosion of these materials by changing the chemical reactions that occur on their surfaces.68 At higher acidity, there is a higher concentration of protons (H+) available to react with the electrode materials and the dye sensitizer, leading to degradation or dissolution. For example, higher acidity can cause the oxidation of Pt to PtO or PtO2, which reduces the catalytic activity of the counter electrode.69. Higher acidity can also break down the ruthenium dye, which makes the photoanode less effective in absorbing light and injecting electrons.69 Furthermore, higher acidity can cause hydrolysis of the ruthenium dye, which reduces light absorption and the electron injection efficiency of the photoanode.70 Therefore, the acidity of the DES electrolyte is an important factor affecting the performance and durability of the solar cell. The optimal acidity of the DES electrolyte depends on the type and composition of the DES, the type and concentration of the mediator, and the type and structure of the electrode materials and the dye sensitizer. The acidity of the DES electrolyte can be adjusted by changing the ratio of the hydrogen bond donor (HBD) and the hydrogen bond acceptor (HBA) in the DES, or by adding water or other additives to the DES.71,72
3. Preparation of DESs
3.1. Selection of HBDs and HBAs
The main methods described in the literature for synthesis of DESs are based on stirring and heating, freeze drying, evaporation, and microwave-assisted synthesis.79 The most popular method involves mixing and heating two or three components, until a homogeneous liquid is formed80 Among the different components used in the mixture, attention is increasingly being focused on those suitable for the synthesis of hydrophobic DESs that are stable in the aquatic environment.43,46 The DES type Cat+X− + zRZ, such as ChCl+urea, is especially popular due to its hydrophobicity and stability in aqueous mixtures.43 The ultrasound-assisted and microwave-assisted DESs synthesis techniques are faster and more efficient than the use of stirring and heating at 50 °C. However, the stirring and heating synthesis method has the advantages of simplicity and the ability to produce larger volumes of solvents.81 The formation of a eutectic solvent occurs due to hydrogen bonding of the precursors, together with intermolecular interactions involving van der Waals and electrostatic forces between HBDs and HBAs. The NMR and FTIR spectra of the synthesized DESs show bands typical of the functional groups of the precursors that were used.The selection of HBDs and HBAs is based on the desired properties of the DESs, including aspects such as solubility, conductivity, viscosity, surface tension, volatility, toxicity, and biodegradability. Certain HBDs and HBAs are mixed together in a fixed molar ratio to make Natural Deep Eutectic Solvents (NADES), which are a type of eutectic mixture. These solvents are considered “natural” because their constituents are naturally found in plants. The main components of NADES include sugars, organic acids, bases, and amino acids.79,86 The introduction of ultrasound and microwave-assisted synthesis methods81 resulted in a faster and more efficient process, while the stirring and heating methods enabled the production of larger volumes of solvents. Infrared spectroscopy (ATR-FTIR) and thermogravimetric analysis (TGA) were used to characterize the prepared NADES and identify intermolecular interactions.81 Hydrogen bonds were observed between the precursor molecules, regardless of the synthesis method, and they revealed interactions between the precursor components and decomposition temperatures exceeding 150 °C.81
The Scheme 6 demonstrates that various synthesis methods can yield NADES with consistent properties, despite differences in efficiency and equipment requirements. Researchers can choose the most suitable method based on their specific needs and constraints, and all synthesis methods yield NADES with similar physicochemical characteristics.81
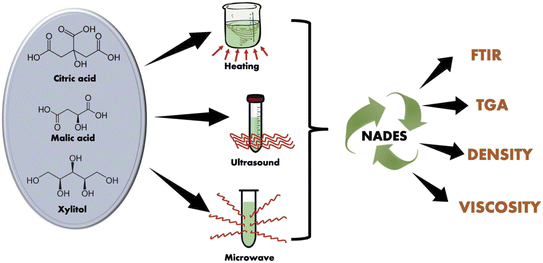 | ||
| Scheme 6 The synthesis of NADES using different methods.81 | ||
Examples of HBAs are choline chloride, ethylamine hydrochloride, tetramethylammonium chloride, tetrabutyammounium chloride, N,N-diethyl 1-2 hydroxy ethanamidium chloride, and methyltriphenylphosphonium bromide, while examples of HBDs are malonic acid, citric acid, acetic acid, urea, thiourea, glycerol, ethylene glycol, acetamide, fructose, glucose, benzamide and imidazole. Some of the HBDs and HBAs structures presented in this review are depicted in Scheme 2. The choice of the HBDs and the HBAs also depends on the application of the DESs, for example, as catalysts, solvents, electrolytes, or extraction agents,82–84 in addition to the nature of the analyte to be evaluated or determined.
3.2. Biodegradability and low toxicity of DESs
DESs are a class of alternative electrolytes that have low toxicity and high biodegradability, which makes them suitable for use as electrolytes in solar cells. DESs are formed by mixing a hydrogen bond donor and acceptor, usually derived from biological sources such as carboxylic acids, alcohols, amines, amides, and ammonium salts. The toxicological properties of DESs depend on the components used, so the choice of benign materials can produce solvents with low toxicity. In addition, microorganisms easily biodegrade DESs, reducing the environmental impact of the waste generated. DESs can improve the performance and durability of solar cells, as they offer a stable electrochemical window, good solubility of CO2, low surface tension, and high conductivity.59,85As petroleum resources are dwindling worldwide, future work should focus on exploring the potential of using DESs as new “green” media for solar devices, fully replacing the hazardous and toxic VOCs that are still widely used. As discussed, many components of DESs are from natural sources,86,87 leading to the introduction of natural deep eutectic solvents (NADES) that present extremely low toxicity and high biodegradability.
Entropy plays a crucial role in NADES formation; when components are mixed in the right proportion, their molecular interactions lead to a decrease in entropy, resulting in an eutectic point. Heating is often employed during NADES preparation; it aids in dissolving the components and promotes molecular interactions and eutectic formation. As Scheme 7 demonstrates, when entropy rises, the strength of intermolecular bonding decreases, which subsequently impacts the relevant physical parameters.86
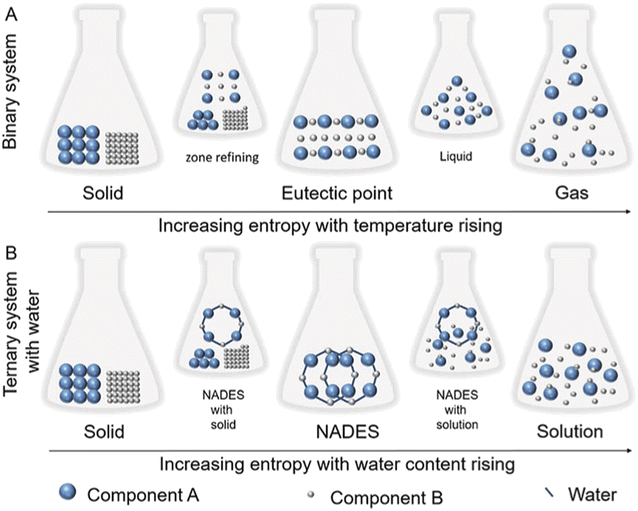 | ||
| Scheme 7 Kinetic energy models for a binary NADES ((A) temperature administration and (B) water content administration).86 | ||
3.3. Adding water to DESs
The addition of water to DESs used as electrolytes in solar cells can affect their conductivity and stability. Water can increase ion mobility and enhance the conductivity of DES electrolytes, resulting in improved performance of the solar cell.59 The substance Reline, composed of choline chloride (HBA) and urea (HBD), serves as one example. Electrochemical capacitors can use Reline as a green electrolyte to store electrical energy. Adding 1 wt% water to Reline can increase the specific capacitance from 100 to 157 F g−1 at a 1 A g−1 current load. This means that more charge can be stored per unit mass. The addition of water can strengthen the hydrogen bonds between the NH and OH groups of the HBD molecules, which can enhance the charge transfer process. Another example of a DES is the mixture of choline chloride and lactic acid, which can also be used as an electrolyte for electrochemical devices. Adding water to this DES does not affect the interactions between the HBA and HBD molecules, but instead forms solvated clusters of the DES. This may affect the diffusion and mobility of the ions in the electrolyte, as well as the stability of the electrode materials.88 However, excessive amounts of water can also cause phase separation and decrease the stability of the DES electrolyte, leading to reduced efficiency of the solar cell. Therefore, the effect of adding water to DESs used as solar cell electrolytes will depend on the type and composition of the DES, as well as the amount of water added. Water can modify the structure and dynamics of the DES molecules, which in turn can influence the performance and efficiency of the solar cell.3.4. Solubility of CO2 in DESs
The solubility of CO2 in DESs is an important factor for using them as electrolytes in solar cells, as it affects the conductivity, stability, and performance of the devices. CO2 is a greenhouse gas that solar cells can capture and utilize to produce electricity and reduce emissions. However, the solubility of CO2 in DESs depends on several factors, such as the type and composition of the DES, the temperature and pressure of the system, and the presence of water or other additives.The PC-SAFT equation of state has been used to estimate the solubility of CO2 in 109 different DESs, covering a wide range of temperatures and pressures.89 It was found that the PC-SAFT model could provide accurate and reliable predictions of CO2 solubility in DESs. It was also possible to propose generalized correlations to predict model parameters for new and upcoming DESs.
Amine-based DESs are a type of green and sustainable solvents that can be used as electrolytes for solar cells. Amine-based DESs consist of a hydrogen bond acceptor (HBA) that is typically an amine, together with a hydrogen bond donor (HBD) that is usually an organic acid or a salt. They have a low melting point, high solubility, and high conductivity, which are desirable properties for solar cell electrolytes. They can also capture and utilize CO2, a greenhouse gas, to produce electricity and reduce emissions.
An amine-based DES used as a DSSC electrolyte was able to achieve a power conversion efficiency of 6.8%, comparable to that of a conventional ionic liquid electrolyte, with the additional advantages of high electrochemical stability and low viscosity, which are advantageous for DSSCs performance.59 A mixture of choline chloride and urea is another example of an amine-based DES that can also serve as an electrolyte for electrochemical capacitors (ECs), which are devices that store electrical energy by accumulating charges on the electrodes. In another study, DESs60 increased the specific capacitance and the voltage window of the ECs, enabling them to store more energy per unit mass and operate at higher voltages. It was also shown that adding a small amount of water to the DES could further enhance the charge transfer process and the cycle stability of the ECs.
Water is an eco-friendly and renewable solvent, but many reactions are slow in water. CO2 has low solubility in water (0.033 M) under ambient conditions,90 which limits the efficiency of its adsorption and conversion in solar cells. A common technique for CO2 capture is using amine solvents, which have high selectivity and capacity, but also have drawbacks such as high corrosiveness91 and high energy consumption,92 which hinders their use in solar cells.93,94
DESs have attracted attention in both experimental and theoretical studies, due to their ability to efficiently capture CO2. In particular, a combination of ethanolamine hydrochloride (HBA) and tetraethylenepentamine (HBD) in a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, referred to as EAHC-TEPA, showed remarkable CO2 solubility, among several DESs investigated.95 Haider and Kumar96 developed amine-based DESs with bulky structures that exhibited superior CO2 uptake, while maintaining minimal CH4 absorption, demonstrating a pronounced preference for CO2.
9, referred to as EAHC-TEPA, showed remarkable CO2 solubility, among several DESs investigated.95 Haider and Kumar96 developed amine-based DESs with bulky structures that exhibited superior CO2 uptake, while maintaining minimal CH4 absorption, demonstrating a pronounced preference for CO2.
Studies have explored the integration of CO2 sequestration and transformation using amine-based solvents that can absorb CO2 and serve as electrolytes for electrochemical conversion. Pérez-Gallent et al.97 found that using both chemical and physical absorption solvents in combination led to high faradaic efficiencies (F.E.) of up to 50%, together with a 30% carbon conversion rate. An aqueous amine-based DES, [monoethanolamine hydrochloride][methyldiethanolamine] ([MEAHCl][MDEA]), achieved a high F.E. of 71% for production of CO at a potential of −1.1 V vs. RHE at an Ag electrode, which was 33% higher than for [MEAHCl][MEA]. Elsewhere, an innovative DES composed of choline chloride and ethylene glycol was utilized as an electrolyte in place of dimethyl formamide (DMF), enhancing the electrochemical CO2 reduction efficiency, due to its favorable CO2 solubility.98 In a similar vein, [MEAHCl][MDEA], as a novel aqueous amine-based deep eutectic solvent, was formulated as an electrolyte for the reduction of CO2 to CO, showing a high faradaic efficiency of 71% for CO production at −1.1 V vs. RHE at an Ag electrode, exceeding the performance of [MEAHCl][MEA] by 33%.99
Building on these studies, other work developed a DES by combining choline chloride and ethylene glycol, for use as an electrolyte.100 This new formulation replaced dimethylformamide (DMF) and contributed to advancements in DSSCs technology, showing improved efficiency in the electrochemical reduction of CO2, attributed to enhanced CO2 solubility. Notably, the use of an Au electrode resulted in CO as the primary product, achieving a faradaic efficiency of 81.8%. Despite these findings, amine-based solvents remain the preferred choice for designing DESs with high CO2 solubility.
In addition, imidazole and its derivatives have long been recognized as potent components in ionic liquids (ILs) and DESs for enhancing CO2 solubility.101,102 In a study examining the effects of modifying the imidazole substituent groups, considering the compounds 2-methylimidazole (2-MeIM), 2-ethyl-4-methylimidazole (2-Et-4-MeIM), and 1,2,4,5-tetramethylimidazole (1,2,4,5-MeIM), the addition of 2 M 1-propyl-3-methylimidazolium iodide ([PMIM]I) improved the capacity of the DESs to capture CO2.103 Boualavong and Gorski104 employed a chemical reaction equilibrium model to explore how variations in the substituent groups of imidazole affected CO2 capture efficiency and energy requirements. The authors proposed a method to reduce the energy consumption of electrochemical capture technologies by doubling the number of CO2 molecules captured per electron transferred.
Future research should address the existing gaps in assessment of the efficiency and energy requirements for solar cells incorporating imidazole (IM) or its derivatives such as 2-MeIM, 2-Et-4-MeIM, and 1,2,4,5-MeIM into the formulated DESs composed of hydrogen bond donors and acceptors. Table 1 shows some of the reported DESs prepared using different HBDs and HBAs, together with their solubilities at various temperatures and pressures.
| DES components and absorption conditions | ||||||
|---|---|---|---|---|---|---|
| ILs/HBA | HBD | Molar ratio | Solubility (mol mol−1) | T (K) | P (bar) | Reference |
| ChCl | Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
0.201 | 313.15 | 118.4 | 105 |
| ChCl | Ethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.0347 | 298 | 10 | 106 |
| ChCl | EG | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.5396l | 303.15 | 58.6 | 107 |
| ChCl | Urea | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.6 | 303.15 | 60 | 108 |
| ChCl | TEG | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.0667 | 293 | 5 | 109 |
| ChCl | Lactic acid | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.0248 | 348 | 19.27 | 110 |
| ChCl | Glycerol + DBN | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.111 | Ambient | Ambient | 111 |
| ChCl | Urea + water | 50% | 0.111 | 313 | 7.8 | 112 |
| TBAB | Ethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.0407 | 298 | 10 | 106 |
| MTPPBr | Ethanolamine | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 6 |
0.0518 | 298 | 10 | 106 |
| BMIMCl | MEA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0.214 | Ambient | Ambient | 102 |
| MEA·Cl | EDA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.2698 | Ambient | Ambient | 113 |
| TBAB | MDEA | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0.29 | 303.15 | 10 | 114 |
| [DETA][IM] | EG | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.2235 | 298.15 | 1.0 | 115 |
| [bmim][tf2N] | DBU | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.0 | 298 | 1.0 | 116 |
| ChCl | 1,4-Butanediol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.0164 | 298 | 5.14 | 117 |
| ChCl | 2,3-Butanediol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
0.0188 | 298 | 5.08 | 117 |
| ChCl | Glycerol | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.8589 | 303 | 58 | 118 |
| TEG | DBU | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
1.04 | 298 | 1.0 | 119 |
As shown in Table 1, the solubility of CO2 in DESs is influenced by both temperature and pressure. Generally, the solubility of a gas such as CO2 decreases with increase in temperature and decrease in pressure.120 This is because higher temperatures provide more kinetic energy to the gas molecules, making them more likely to escape from the solvent. DESs, which are a type of ionic liquid, have been found to have good solubility for gases, including CO2, which can be advantageous for applications such as in solar cells. The solubility is typically expressed as the mass of the gas per unit mass of solvent, at a given temperature and partial pressure of the gas.121 For solar cells, specifically DSSCs, DESs can be used as electrolytes. Researchers have developed choline chloride-based DESs as effective electrolytes for these types of solar cells. They offer benefits such as easy preparation, low cost, biodegradability, and non-toxicity, which are important for large-scale production and commercialization.60
The use of DESs as electrolytes in solar cells can potentially improve the open-circuit voltage and short-circuit current, depending on their molecular structures. This makes them an interesting area of research for enhancing the efficiency and eco-friendliness of solar cell technologies.60 In particular, the CO2 solubility in DESs can have significant implications for their use as green and sustainable electrolytes in solar cells. Amine-based DESs are promising candidates for solar cell electrolytes, offering benefits such as low cost, high efficiency, high stability, and environmental friendliness. However, understanding the underlying mechanisms and optimizing the properties and performance of these DESs requires further experimental and theoretical studies.
4. DESs as electrolytes in dye-sensitized solar cells (DSSCs)
4.1. Preparation of DSSCs and their advantages
DSSCs are devices that convert light into electrical energy by receiving photons from sunlight which excite the electrons of the dye molecule, and are then injected into the TiO2 layer.A DSSC (as shown in Scheme 8) classically consists of the following components:78 (1) a photo-sensitized anode, usually made of a semiconductor material such as titanium dioxide (TiO2), which has dye molecules on top of it that absorb sunlight and generate electrons; (2) an electrolyte that may be liquid or solid, usually possessing an iodide (I−) and tri-iodide (I3−) couple that assists in moving electrons from the dye to the cathode; (3) a counter electrode, typically composed of platinum or other conductive material, which collects electrons and returns them to the electrolyte.
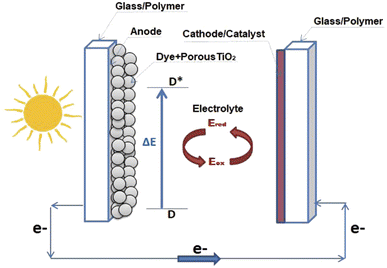 | ||
| Scheme 8 Schematic illustration of the structure of a dye-sensitized solar cell device.78 | ||
The iodide and triiodide ions constitute the redox couple commonly used in DSSC electrolytes, playing a crucial role in the operation of these solar cells by facilitating the transport of charge and contributing to the overall efficiency of the device. The iodide ion (I−) acts as a charge carrier, while the triiodide ion (I3−) helps the oxidized dye molecules to regenerate, completing the electrical circuit of the cell.122 This redox couple is widely adopted due to its favorable recombination kinetics and ability to improve the performance of the solar cell.
The operation of a DSSC involves the dye molecules absorbing photons from sunlight and becoming excited, with subsequent injection of electrons into the conduction band of the semiconductor anode. From there, the electrons flow through an external circuit to do work (such as powering a device), before returning to the counter electrode. The redox mediator in the electrolyte then completes the circuit by regenerating the dye molecules.
The fabrication of DSSCs is mostly performed according to a well-established protocol,123 to prevent metal contamination. The glass or Teflon containers are firstly cleaned with ethanol (EtOH) and a 10% hydrochloric acid (HCl) solution. Throughout the fabrication process, only plastic tools (such as spatulas and tweezers) are utilized. Fluorine-doped tin oxide (FTO) glass substrates are cleaned by immersion for 15 min in an ultrasonic bath with detergent, thorough rinsing with deionized water and EtOH, and exposure to a UV-O3 for 18 min. The UV-O3 method, which combines ultraviolet (UV) light and ozone (O3) for surface cleaning and treatment, is particularly effective for removing organic contaminants from surfaces, without damaging the sample. After cleaning, the substrates are soaked for 30 min in a 40 mM TiCl4 solution, at 70 °C, followed by rinsing. A transparent layer of TiO2 (determined by measurement of an area of 0.20 cm2) is applied onto the substrate using a screen-printing technique, with a 20 nm particle size paste. After applying the TiO2 layer, the material is submitted a series of drying and heat treatments at progressively higher temperatures. After sintering, the layer is treated again with TiCl4 and heated to 500 °C for 30 min. For the preparation of counter electrodes, a 1 mm hole is drilled into an FTO plate, followed by application of the same cleaning process used for the FTO glass substrates. A 10 μL H2PtCl6 solution in EtOH is deposited onto the plate, and then heat-treated at 500 °C for 30 min. At the time a hot-melt ionomer resin spacer is inserted between the dye-coated TiO2 electrode and the counter electrode. Subsequent, the electrolyte solution is introduced into the cell through the drilled hole, using a vacuum backfilling method, and the hole is sealed. Light absorption is enhanced by attaching a reflective foil to the rear side of the counter electrode, directing any unabsorbed light back towards the photoanode.
A survey of commonly used photocatalyst substances124,125 revealed that they fall into three main categories: semiconductor materials, graphene-based nanocatalysts, and organometallic complexes. The semiconductor group includes a diverse range of inorganic binary compounds, including (but not limited to) TiO2, ZnO, CdS, and SiC. The performance of these semiconductor photocatalysts depends on a few critical factors: (1) the band gap width of the semiconductor, which determines the photon absorption spectrum for generation of electron–hole pairs; (2) efficient separation and transport of the electron–hole pairs to the surface of the material; and (3) the number and accessibility of active sites that are available for the photocatalytic reactions to occur.
DSSCs offer several advantages that make them an attractive option for solar energy conversion, including: (1) low manufacturing cost, since DSSCs can be produced at a lower cost than traditional silicon-based solar cells;123 (2) absorption of fluorescent light, in addition to sunlight, which is beneficial for indoor applications;123 (3) flexibility in the design and application of the solar cells, as a result of the thin-film nature of DSSCs;126 (4) good performance under conditions of low light, making them useful in less sunny climates;127 (5) use of eco-friendly materials in the construction of DSSCs, contributing to a greener production process;128 (6) versatility for use in a wide range of environments and ability to be integrated into various solar products.128
The unique properties of DSSCs make them an innovative solution for sustainable and adaptable energy generation. They are highly suitable for powering portable devices and are ideal for low-density power needs where conventional solar panels might be limited in terms of efficiency or cost-effectiveness. Standard roll-printing methods can facilitate the production of DSSCs, enhancing their appeal by features such as partial flexibility, translucency, and the use of economical materials.
Nonetheless, DSSCs still have some drawbacks, such as the requirement for expensive components including platinum and ruthenium, while the liquid electrolyte can cause problems that make them less durable and unable to function in all weather conditions. Despite these hurdles, DSSCs stand out as a viable and cost-effective alternative to traditional silicon-based photovoltaic cells, particularly for applications that demand flexibility and affordability.
4.2. Application of DSSCs in CO2 conversion
DSSCs have recognized potential in various applications, including the conversion of CO2 to obtain useful products.129 The literature lacks specific details on the application of DSSCs in CO2 conversion, but nonetheless highlights the versatility and benefits of DSSCs for use in energy harvesting and low-carbon technologies. DSSCs can be integrated into systems that aim to reduce CO2 levels by converting the gas into valuable chemicals or fuels by photocatalytic processes. The ability of DSSCs to effectively harness solar energy makes them suitable for powering reactions that can transform CO2 into compounds such as formic acid, methanol, or other carbon-based products. This process not only helps in mitigating CO2 emissions, but also provides a sustainable pathway for chemical synthesis.This process essentially mimics photosynthesis,78 using solar energy to convert CO2 into organic compounds. The efficiency and selectivity of the CO2 conversion depend on various factors, including the type of dye used, the semiconductor material, and the nature of the redox couple. Research is ongoing to optimize these aspects and improve the overall efficiency of the process. DSSCs offer a promising route for sustainable energy production and CO2 utilization, contributing to the reduction of greenhouse gases and the generation of renewable fuels.
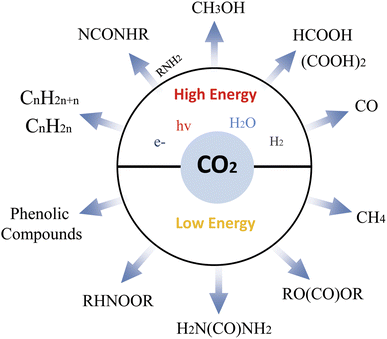
Improving the capability of solar cells to convert solar energy and CO2 into high-value products such as HCOOH, CO, CH2O, CH3OH, and CH4 is of paramount importance.130 The family of carboxylic acids is varied, including compounds such as formic acid, acetic acid, benzoic acid, acetylenic acid, amino acids, and lactic acid, which can be produced by different photocatalytic reaction pathways. Scheme 9 shows different chemical produces from CO2
Any chemical transformation of CO2 requires energy, but there are distinct processes with varying energy requirements. Low-energy processes incorporate the entire CO2 molecule into an organic or inorganic substrate. For instance, consider the series: CO2 → HCOOH (formic acid) → CO → CH2O (formaldehyde) → CH3OH (methanol) → CH4 (methane). These reactions primarily yield products used in the chemical industry. In high-energy processes, the carbon atom's oxidation state is reduced from +4 in CO2 to −4 in methane (CH4). These processes have the potential to produce fuel.130 If we focus solely on chemical synthesis, we'll recycle limited amounts of CO2. However, if we prioritize fuel we could potentially recycle larger volumes of CO2. The ongoing processes developments relevant to the chemical industry, and reactions pertinent to the energy sector. Ultimately, finding sustainable energy solutions will be a key to unlocking the full potential of CO2 conversion.130
However, the large-scale synthesis of carboxylic acids faces challenges, especially when it involves the conversion of lignocellulosic biomass, which requires hydrolysis and electrophilic attack by metal–organic reagents. These processes often have drawbacks such as high energy consumption, low efficiency, and poor product selectivity.134,135 To address these issues, the adoption of green and efficient methods is crucial for the production of diverse carboxylic acids by CO2 reduction. Carboxylic acids are attractive products, with high commercial value and advantages including low toxicity, high energy density, and greater energy value per kilowatt-hour, compared to other products. These acids are utilized in a wide range of industries, from rubber production to the manufacture of flavorings, cosmetics, pharmaceuticals, and pesticides.
4.3. Mechanism of CO2 conversion in DSSCs
In DSSCs, the conversion of CO2 is facilitated by a photoelectrochemical process, with the DSSC acting as a catalyst for the transformation of CO2 into useful substances. Sunlight is captured by the dye sensitizer, which then energizes the electrons of the dye. These energized electrons are propelled into the conduction band of the semiconductor (typically TiO2), which constitutes the anode. The electrons then traverse the external circuit and reach the cathode, where they participate in reduction reactions. At this stage, the electrons convert CO2 into a variety of carbon-based compounds, such as hydrocarbons or alcohols. Concurrently, the dye molecules, now oxidized, are regenerated by acquiring electrons from the redox couple of the electrolyte solution. Electrons flowing from the cathode replenish the redox couple, closing the electrical loop.123Enhancing the efficiency of solar cells is crucial for optimizing the conversion of solar energy and CO2 into valuable products such as HCOOH, CO, CH2O, CH3OH, and CH4.130 Among these products, carboxylic acids, especially formic acid (HCOOH),133 are of particular interest, due to their commercial significance and attractive characteristics including low toxicity, high energy density, and greater energy value per kWh, compared to other products. The versatility of formic acid enables its use in many industrial applications, including those mentioned above.
Carboxylic acids encompass a diverse array of compounds, including formic acid, acetic acid, benzoic acid, acetylenic acid, amino acid, and lactic acid. In photocatalytic processes, these acids can be synthesized according to various pathways (see Scheme 10). However, the industrial production of carboxylic acids has been hindered by the fact that methods involving the breaking down of lignocellulosic biomass by hydrolysis and electrophilic attack with metal–organic reagents have high energy costs, low efficiency, and poor product selectivity.131,132 To address these issues, there is an urgent need for sustainable and effective techniques to generate a range of carboxylic acids by CO2 reduction. Honda et al. accomplished the photocatalytic reduction of CO2 to hydrocarbons in 1970.134 Subsequent research has continued to advance the field of photocatalytic CO2 reduction.135–137
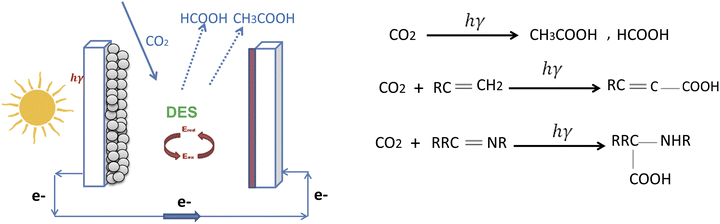 | ||
| Scheme 10 Different carboxylic acid reaction paths in solar cells.130 | ||
Green methods for producing carboxylic acids offer several significant benefits: (1) they are environmentally sustainable, since these methods typically avoid the use of heavy metals and strong acids, reducing the environmental impact; (2) green techniques often require less energy, contributing to lower production costs and a smaller carbon footprint; (3) cost-effectiveness is improved by the absence of byproducts and the ability to recover and recycle solvents; (4) harmful emissions are reduced in processes that do not generate nitrogen oxides (NOx) or other greenhouse gases that have climate implications; (5) the use of renewable feedstocks contributes to the long-term sustainable production of carboxylic acids. These advantages align with the global shift towards more sustainable and eco-friendly industrial processes.138
The process of converting CO2 into carboxylic acids by photocatalysis, shown in Scheme 10, begins when the catalysts, typically semiconductor materials, are subjected to light irradiation. This exposure causes electrons within the semiconductor to become excited, leaving behind positively charged vacancies known as holes. These photogenerated electron–hole pairs are crucial to the process, as they are separated and directed towards the active sites of the catalyst.
Once at the surface, the photogenerated electrons assist in the reduction of CO2, transforming it into various useful fuels (CO, CH4, CH3OH, and others). The presence of water (H2O) can influence the reaction, but is not always necessary. The number of electrons participating in the chemical reaction determines the specific products formed during this process. For instance, the formation of CO or formic acid (HCOOH) requires two electrons (2e−), while the production of methanol (CH3OH) necessitates six electrons (6e−), and methane (CH4) production requires eight electrons (8e−).139,140
This photo-electrocatalytic process is a promising green technology for CO2 reduction, as it utilizes sunlight, a renewable energy source, and can potentially lead to the creation of valuable chemicals and fuels. The efficiency and selectivity of the process can be influenced by the choice of semiconductor material, the design of the catalyst, and the reaction conditions. Studies continue to explore and optimize these factors to enhance the conversion rates and the yields of desired products.141
4.4. DESs as electrolytes in DSSCs
Deep eutectic solvents (DESs) are used as electrolytes in solar cells due to several compelling benefits. DESs are biodegradable and nontoxic, making them environmentally friendly, as well as cost-effective and able to enhance certain performance metrics of solar cells, such as the open-circuit voltage (Voc) and the short-circuit current (Isc), depending on their molecular structure. They are inexpensive to produce and can be prepared on a large scale, which is advantageous for commercialization. Furthermore, they are stable and can contribute to the long-term stability of solar cells, which is crucial for practical applications. In addition, DESs can dissolve a wide range of substances and their physicochemical properties can be tuned by including different hydrophilic or hydrophobic components. These properties make DESs suitable for use in DSSCs and other photovoltaic devices, aligning with the goals of producing low-cost and eco-friendly materials for large-scale production.60,141As discussed above, DSSCs consist of an anode made of a thin titanium dioxide (TiO2) layer that is sensitized by a suitable dye.142,143 A cathode containing Pt nanoparticles and a liquid electrolyte solution with a redox couple facilitate dye regeneration and complete the electrical circuit. The affinity of the dye for water (hydrophilicity) or its repulsion of water (hydrophobicity), as well as the choice of metal for dye optimization, are critical factors in enhancing the efficiency of photochemical cells and have been the subject of extensive research.25,144,145 Volatile organic compounds (VOCs), such as acetonitrile or nitriles, are commonly used as solvents in electrolyte solutions for DSSCs. However, drawbacks of VOCs include flammability, volatility, and possible toxicity. This can hinder the wider adoption of DSSCs as environmentally sustainable sources of renewable energy.
In the DSSC, the dye molecule becomes excited when exposed to light, transferring an electron to the TiO2 and a hole to the redox couple, for the process to work. This process resets the dye to its original state, allowing the device to continue functioning and prepare for the next cycle. The I−/I3− redox couple is the one most used in DSSCs, although other redox couples such as CO2+/CO3+ or Cu+/Cu2+ have also been explored for their potential benefits.146,147
4.5. Efficiency of DSSCs (J/V curve)
The efficiency of DSSCs is often represented by the J/V curve, which is a plot of the current density (J) vs. the voltage (V), under illumination. The J/V curve measurement is a crucial technique for characterizing solar cells, involving determination of the current density (J) generated by the cell as the applied voltage (V) is varied, under a solar light source. This curve, obtained under illumination, enables determination of all the key parameters of a photovoltaic (PV) device, such as the power conversion efficiency (PCE) as a very important factor, the short-circuit current density (Jsc), the open-circuit voltage (Voc), and the fill factor (FF). The Jsc value represents the peak current output of the cell, observed at zero voltage, while the Voc represents the highest voltage achievable, measured when the current output is zero. FF is a measure of the power output efficiency of the cell, calculated as the ratio of the actual power output (Pout) to the theoretical maximum power (Jsc × Voc). PCE is defined as the ratio of the power output (Pout) to the power input (Pin), with the output power density being the product of Jsc, Voc, and FF. Therefore, PCE can be expressed by the following equation:
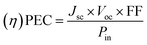 | (1) |
Several strategies can be used to improve the efficiency of DSSCs, such as optimizing the dye molecules, using dyes with broader light absorption spectra (including the visible and near-infrared ranges) to capture more sunlight, improving the redox mediator, or using solid-state hole transport materials (HTMs) for better charge transport and reduced recombination. Improvement of photoanodes can be achieved using nanostructured materials (such as TiO2) to increase the surface area available for dye adsorption and light harvesting. Counter electrodes composed of alternative carbon-based or platinum materials can present enhanced catalytic activity and stability. By focusing on these areas, studies aim to extend the limits of DSSC performance, making them more competitive with traditional silicon-based solar cells.78,148,149
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels of the sensitizer play a pivotal role in DSSCs, and understanding the interplay between HOMO/LUMO levels, charge distribution, and the sensitizer's mechanism is essential for designing efficient DSSCs to offer promise for clean, renewable energy production and contribute to sustainable development. The HOMO represents the energy level of the highest electron that can be excited, while the LUMO represents the lowest energy level of an unoccupied electron. The sensitizer enables electron injection into the semiconductor. Efficient sensitizers have a small energy gap between their HOMO and LUMO levels. The small distance between the two entities results in enhanced charge separation during the process of photo excitation.150 Sensitizers are crucial because they absorb sunlight and generate excited electrons. They must have appropriate HOMO and LUMO levels to match the redox potentials of the semiconductor and the electrolyte. Sensitizers can be organic (metal-free) or inorganic (such as ruthenium-based dyes). Organic sensitizers are chosen because they are not toxic, readily available, and cost-effective.151
Theoretical studies, especially using Density Function Theory (DFT), provide valuable insights for advancing solar cell technology by considering HOMO/LUMO levels, charge distribution, and the sensitizer's mechanisms. In the charge distribution mechanism, when sunlight hits the DSSC, the sensitizer (usually an organic dye) absorbs photons and gets excited. The excited sensitizer transfers an electron from its HOMO to its LUMO level. This electron then moves to the semiconductor (often titanium dioxide, TiO2) through the electrolyte. The difference in chemical potential between the electrolyte and the semiconductor drives this charge transfer.
Researchers employ DFT to investigate the electronic structure and properties of sensitizers. DFT calculations show that HOMO and LUMO are spread out in space at the interface. This helps excitation break apart and lines up the energy levels in a way that is good for charge transport. An investigation was conducted on graphene-based sensitizers using Density Functional Theory (DFT) and Time-Dependent Density Functional Theory (TD-DFT) in order to comprehend their influence on photophysical properties.150 Additional research investigates potential sensitizers for DSSCs, such as nanocomposites of graphene quantum dot hybrids and their Cu-metallated macrocycles.151 Besides, other study examined the structural and electrical characteristics, as well as the absorption spectra, of supramolecular triads consisting of fullerene and porphyrine in DSSCs.152 The HOMO and LUMO states of the C60-P-Mp triad are primarily located on the Mp unit, with the C60 cage unit acting as the donor and the C60 fullerene unit acting as the acceptor. This pattern is observed in the C60–P–ZnP double complex, C60-P-FeP, and C60–P–ZnP complexes. When the position of porphyrine and ZnP changes, the HOMO density is mostly on the C60 fullerene and linkage groups. On the other hand, the LUMO density is mostly in the porphyrine moiety.152
Optimizing the band gap, maximizing Jsc, and achieving an appropriate Voc are essential for enhancing the overall performance of DSSCs. Researchers continually explore new materials and sensitizers to improve these parameters and make solar energy conversion more efficient.152,153 There is a difference in energy between the molecular orbitals that are most full (HOMO) and least full (LUMO) in the semiconductor material, which is usually titanium dioxide (TiO2). This is called the band gap (Eg). A smaller band gap allows the semiconductor to absorb a broader range of photons from sunlight.
As explained, when a photon with energy greater than the band gap is absorbed, an electron is excited from the valence band (HOMO) to the conduction band (LUMO). The band gap influences the efficiency of charge separation and electron injection into the semiconductor. Short-circuit current density (Jsc) represents the maximum current density generated by the DSSC under short-circuit conditions (i.e., when no external load is connected), and it depends on the following factors: (a) photon absorption: a larger band gap results in a lower JSC because fewer photons are absorbed. (b) Sensitizer efficiency: optimizing DSSC performance, ensuring effective light absorption, and enhancing the overall efficiency of these solar cells. (c) Efficient sensitizers absorb more photons, generate more excited electrons, and (f) charge transport: more electrons moving from the sensitizer to the semiconductor is what causes higher JSC. This is directly related to the amount of photons absorbed and how well charges are transferred. Furthermore, open-circuit voltage (Voc) is the voltage across the DSSC terminals when no current flows (i.e., under open-circuit conditions), and it is dependent upon the following factors: (a) recombination losses: (b) reducing charge recombination improves Voc, which is directly related to the energy alignment between the sensitizer and the redox couple; (c) energy levels: (d) the difference between the sensitizer's HOMO/LUMO levels and the electrolyte's redox potential; and (e) electron injection efficiency: higher Voc is the result of efficient electron injection.152
The Light Harvesting Efficiency (LHE) of DSSCs is a key factor in their performance. LHE measures the efficiency of a DSSC in capturing and converting incoming solar energy into useful electrical energy. It denotes the proportion of photons that are absorbed and contribute to the production of photocurrent.153 A higher LHE results in improved utilization of sunshine, which in turn leads to an improvement in power conversion efficiency (PCE). Effective light harvesting maximizes the utilization of the sensitizer's absorption spectrum. The sensitizer of choice determines the absorption spectrum. Sensitizers with a wide range of absorption spectra improve LHE. It is very important that electrons move efficiently from the excited sensitizer to the semiconductor material, like TiO2. Maximizing LHE involves minimizing charge recombination and enhancing light trapping within the DSSCs.152 In order to enhance LHE, it is necessary to fine-tune the sensitizing properties, developing sensitizers with ideal HOMO–LUMO energy levels and absorption characteristics.153 In addition, the integration of nanostructures such as nanowires and nanoparticles can be employed to improve the capture and absorption of light.154 Light harvesting can be made even more effective by using different sensitizers that have absorption spectra that work well with each other and by making the path length of photons longer inside the cell.155
The calculated LHE for triad complexes using ZINDO/S method reported,152 the highest value of oscillator strength (13.8) is related to the C60–P–NiP in 545.1 nm wavelengths while lowest value (0.46) belongs to the C60–P–TiP in 478.5 nm.
The study on the same evaluation for Zn complexes152 revealed that the oscillator strength value for C60–P–ZnP (5.20) is higher than C70–P–ZnP (3.95), when the position of porphyrine and ZnP changed in C60–P–ZnP complex and the LHE and oscillator strength was enhanced. The same trend is observed in doubly C60–P–ZnP complex. In this case the oscillator strength value is 8.20, and consequently the LHE increase sharply. In conclusion, the LHE was improved by increasing the conjugation, and it is exhibited that doubly complex with longer π conjugations showed oscillator strength and light harvesting efficiency, thus leading to higher triad efficiency, and stability. Similar estimation has also been carried out for the same triad and reported.156
ILs/DESs have been used as electrolytes in DSSCs since 2009,17 including a quaternary ammonium salt-derivative ionic liquid called G.CI, which is a eutectic mixture of glycerol (GLY) and choline iodide (CI), in a specific ratio, with 0.5 M of a mixture of N-methylbenzimidazole and 1-propyl-3-methylimidazolium iodide ([PMIM]I). The high cell performance made the G.CI DES a strong candidate for use in the future development of DSSC electrolytes.
Other studies157,158 have investigated the development of novel eco-friendly DESs for use as electrolytes in DSSCs, such as DESs formulated from chlorine chloride (ChCl) and ethylamine hydrochloride (MEA·Cl) as hydrogen bond acceptors (HBAs), combined with hydrogen bond donors (HBDs) such as glycerol (Gly), ethylene glycol (EG), and imidazole (IM), in varying HBD to HBA molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, and 1
3, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6).
6).
The findings of the present review, considering the existing literature, indicate the need for further research to fully understand the processes involved in the use of these DESs, with a focus on the HBDs and HBAs illustrated in Table 2 summarizes the DESs used as electrolytes in DSSCs, reported in the literature.
| DESs | Iodide source | Dye/other adsorbent | TiO2 thickness | Dilution | Remark | Advantages | Ref. |
|---|---|---|---|---|---|---|---|
| Choline chloride + glycerol | PMII and G.CI 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v 7 v/v |
D149 | 6 μm T + 5 μm S | 15% w/w water | Pioneering work that opened new possibilities for ionic liquid-based DESs in DSSCs | Introduced a new type of DES for DSSCs, potentially enhancing electrolyte performance | 17 |
| Aqueous choline chloride-based DESs | KI 2 M | PTZ-TEG/GlcA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
5 μm T | 40% w/w water | Showcased the practicality of aqueous DES in real-world applications | Demonstrated effective use of aqueous DES as an electrolyte solution in DSSCs | 16 |
| PMII 2 M | 2.5 μm T | ||||||
| Menthol-based hydrophobic eutectic solvent, DL-menthol/AcOH | DMII 1.0 M | PTZ-Glu/GlcA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
2.5 μm T | 10% v/v EtOH | Focused on sustainability and environmental friendliness | Designed eco-sustainable DSSCs with improved electrolyte medium | 47 |
| Sugar-based natural DESs; choline chloride-Gly, -Glu, -Sorb, -Fru, -Man | PMII 2 M | PTZ-Glu/GlcA 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
2.5 μm T | 20% w/w water | Highlighted the performance benefits of using natural components | Used eco-friendly DES for better electrolyte solutions in DSSCs, showing active role in performance increase | 159 |
| 30% w/w water | |||||||
| 40% w/w water | |||||||
| Choline chloride + ethylene glycol (EG) | KI 0.6 M | N719 | T | 5% v/v water | Offered a comprehensive understanding of DES behavior in DSSCs | Provided insights from both experiment and simulation on DES in DSSCs | 54 |
| KI 0.3 M | |||||||
| EmimI 0.3 M | |||||||
| EmimI 0.6 M | |||||||
| Canonical choline chloride-based DESs | Coupled redox mediator (e.g., I−/I3−) | N719 | T | Up to 40% w/w water | Assessed the benchmark DES, setting a standard for comparison | Evaluated the performance of canonical DES as DSSC electrolytes | 50 |
| Alkali iodide DES; LiI/EG | Used in prepared DESs | N719 | 10 μm T + 5 μm S | No diluting agent | Investigated new DES formulations for potential improvements | Explored alternative DES electrolytes for DSSCs | 160 |
| NaI/EG | |||||||
| KI/EG | |||||||
| Choline chloride + urea | Coupled redox mediator (e.g., I−/I3−) | N719 | T | No diluting agent | Improved open circuit voltage (Voc) | Simple preparation, biodegradable, low-cost | 60 |
| Choline chloride + ethylene glycol | Coupled redox mediator (e.g., I−/I3−) | N719 | T | No diluting agent | Increase in short circuit current (Isc) | Nontoxic, available materials, comparative conversion efficiency | 60 |
| Betaine + glycerol | Coupled redox mediator (e.g., I−/I3−) | N719 | T | No diluting agent | Comparable efficiency to conventional electrolytes | Renewable, biodegradable, non-corrosive | 60 |
| Choline chloride + phenol | Redox mediator (e.g., I−/I3−) | N719 | T | No diluting agent | Improved Voc values by 10–40 mV, higher short circuit current (Jsc) not observed with 4-TBP | Eco-friendly, low cost, simple synthesis, used new efficient and eco-friendly additives | 77 |
The key parameters to assess in DSSC applications include the open-circuit voltage (Voc), the short-circuit current density (Jsc), the fill factor (FF), and the overall power conversion efficiency (η). Certain DESs are emerging as promising electrolytes for DSSC applications, due to their ability to achieve higher Voc values (up to 140 mV), maintain comparable FF, but exhibit lower Jsc, when compared to a standard electrolyte, under identical experimental conditions. The use of these DESs in DSSCs requires determination of their total energy efficiency and viability as sustainable electrolytes.
Another crucial metric is the external quantum efficiency of a DSSC, which quantifies the efficiency of conversion from incident photons to the current generated. This efficiency is known as the incident photon-to-current conversion efficiency (IPCE) and varies with the wavelength (λ). For this assessment, the photocurrent is measured under open-circuit conditions, during exposure of the cell to monochromatic light. The IPCE is calculated for each point by determining the ratio of the number of electrons harvested to the number of incident photons, at a specific wavelength (λ).17,161
In the context of DSSCs, it is essential to consider the following aspects: robustness of the absorption coefficients and the capacity of the designed DESs to dissolve CO2; significant molar extinction coefficients; and toxicity and ecological consequences. Furthermore, it is essential to undertake a thorough analysis of the physicochemical characteristics of DESs, the underlying absorption mechanisms, and the influence of the molar quantities of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs) utilized in the formulation of DESs.
In a study by Nguyen et al.,18 a novel electrolyte for DSSCs was formulated by blending a choline chloride-phenol DES with acetonitrile (ACN), in varying proportions. The most favorable outcomes were obtained using an electrolyte composed of 20% DES in ACN, together with 0.03 M iodine, 0.6 M tetrabutylammonium iodide, 0.1 M guanidinium thiocyanate, and 0.5 M 4-tert-butylpyridine. This specific composition was instrumental in enhancing the stability and photovoltaic efficiency of the DSSCs. The improvement was attributed to the interaction of the choline and phenol components of the DES with the TiO2 surface, which was more effective than in cells without DES.
One of the pioneering studies to demonstrate the potential of DESs as solvents in DSSCs was the work of Jhong et al.,17 who employed an electrolyte solution consisting of 0.2 M iodine and 0.5 M N-methylbenzimidazole, mixed with 1-propyl-3-methylimidazolium iodide and a choline iodide/glycerol DES. This solution was used in conjunction with the organic sensitizer D149. Building on this research, Boldrini et al.16 introduced an aqueous electrolyte based on a choline chloride-glycerol DES, which included iodine, potassium iodide, or PMII, and additional compounds such as guanidinium thiocyanate, 4-picoline, or pyridine, paired with a phenothiazine-based organic dye as the photosensitizer.
Many investigations have aimed at the development of highly efficient DSSCs. A binary solution of a deep eutectic solvent (DES) with acetonitrile (ACN) showed the most promising results, among these studies.25,162,163 It was found that this mixture greatly improved the diffusion of the redox couple (I−/I3−) and the electrochemical properties of the electrolyte solutions, resulting in satisfactory functioning of the device. Studies have also tested the use of additives including PMII, GuSCN, pyridine, 4-tBP, and ACN to further enhance the performance of DSSCs.16,18 However, despite the promising results, potential toxicological issues and high volatility may limit the practical applications of these additives. Therefore, there is a need for safer and more efficient alternatives for large-scale practical applications. Continued research is necessary to identify and develop new additives that can improve the performance of DSSCs, without compromising safety or practicality. With further advancements in this field, DSSCs have the potential to become a highly efficient and sustainable source of renewable energy.
To better understand the movement of electron carriers in electrolyte solutions and their interactions with solvents and surfaces, molecular dynamics (MD) simulations have been utilized in conjunction with experimental methods to investigate the effects of electrolytes on DSSC efficiency.164–166
One area of focus was the behavior of a DES composed of choline chloride and ethylene glycol (ChCl–EG), at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, as solvent in DSSC electrolytes. The aim was to examine the photovoltaic properties of a DES-based electrolyte for DSSCs. The ChCl–EG DES was chosen due to its relatively high ionic conductivity of 7.61 mS cm−1 at 298 K, attributed to its lower viscosity than other DESs that have been studied. Elsewhere, a 40% water content of a DES consisting of choline chloride and glycerol (ChCl/Gly, 1
2 molar ratio, as solvent in DSSC electrolytes. The aim was to examine the photovoltaic properties of a DES-based electrolyte for DSSCs. The ChCl–EG DES was chosen due to its relatively high ionic conductivity of 7.61 mS cm−1 at 298 K, attributed to its lower viscosity than other DESs that have been studied. Elsewhere, a 40% water content of a DES consisting of choline chloride and glycerol (ChCl/Gly, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mol mol−1) was reported to provide an effective electrolyte solvent for DSSCs.16 This study found that the best DSSC performance was obtained using a 2 M concentration of 1-propyl-3-methylimidazolium iodide ([PMIM]I) and 0.1 M of guanidinium thiocyanate (GuSCN) in a 40% aqueous solution of ChCl:Gly, resulting in a power conversion efficiency (PCE) of 1.7%. Further research is necessary to explore the potential of DESs with other additives for improving DSSC performance in a safe and practical manner.
2 mol mol−1) was reported to provide an effective electrolyte solvent for DSSCs.16 This study found that the best DSSC performance was obtained using a 2 M concentration of 1-propyl-3-methylimidazolium iodide ([PMIM]I) and 0.1 M of guanidinium thiocyanate (GuSCN) in a 40% aqueous solution of ChCl:Gly, resulting in a power conversion efficiency (PCE) of 1.7%. Further research is necessary to explore the potential of DESs with other additives for improving DSSC performance in a safe and practical manner.
A recent study161 investigated the use of different alkali metal iodide-based deep eutectic solvents (DESs) as electrolytes for DSSCs. These DESs included LiI:nEG, NaI:nEG, and KI:nEG, with varying amounts of iodine added to improve their performance. The results showed that the open-circuit voltage (Voc) under illumination increased with increasing cation radius from Li+ to K+. When compared to a reference electrolyte in the same experiment, the DESs had higher Voc (up to 140 mV), similar fill factor values, but lower current density values. After optimization, the DES electrolytes could be used in DSSCs as a promising alternative to electrolytes based on volatile organic compounds. A mixture of choline chloride (ChCl, 1 mol) and ethylene glycol (EG, 2 mol) in combination with lithium iodide (LiI), 1-ethyl-3-methylimidazolium iodide (EMIM), MeCN, and iodine was used to develop a cost-effective and sustainable DES-based electrolyte solution for DSSCs with TiO2 and Pt electrodes. Different iodine amounts were added to the original DES electrolyte to improve its electrolytic performance. On illumination, Voc increased with increase of the cation radius from Li+ to K+. When compared to a reference electrolyte, under the same experimental conditions, these DESs presented higher Voc values (up to 140 mV) and similar FF values. However, they had lower current density values. Improvement of these DES electrolytes could enable their use in DSSCs as a viable alternative to VOC-based electrolytes. Subsequently, a cost-effective and sustainable DES electrolyte was successfully developed, consisting of a mixture of choline chloride (ChCl, 1 mol) and ethylene glycol (EG, 2 mol) in combination with lithium iodide, 1-ethyl-3-methylimidazolium iodide (EMIM), MeCN, and iodine, for use in a DSSC with TiO2 and Pt electrodes.161
4.6. Challenges, opportunities and future directions
The utilization of DESs in environmentally-friendly DSSCs presents both opportunities and challenges, such as: (1) traditional liquid electrolytes have drawbacks such as volatility and environmental hazards; on the other hand, DESs electrolytes face viscosity issues, and high viscosity in DES can hinder ionic diffusion of redox mediators, affecting conversion efficiency. (2) Finding suitable DES-based electrolytes remains a challenge for large-scale DSC production. (3) DES stability at elevated temperatures is crucial to study to prevent thermal degradation of components. (4) Knowing when DES changes phases (solid, liquid, or gel) is important for getting the most out of their use in DSSCs. The density and viscosity of DES affect how they flow inside the solar cell. High ionic conductivity is desirable for efficient charge transfer. (5) The properties of surface tension and polarity influence DES wetting behavior on the TiO2-loaded dye-sensitized photoanode. The advantages included: (1) they can be synthesized from inexpensive, nontoxic starting materials, making them eco-friendly and offering a low-cost alternative to conventional ionic liquids. (2) DES-based solar cell devices exhibit competitive conversion efficiency compared to popular ionic liquids, which increase the efficiency of the cells. Because of the advantages of utilizing deep eutectic solvents (DESs) in environmentally friendly DSSCs, researchers continue to explore DES properties and address challenges to enhance their performance.167,168Addressing the mentioned challenges requires a combination of experimental investigations, computational modeling, and innovative thinking. Possible strategies that could be used to address these challenges are as follows: (1) mixing DESs with ionic liquids with low viscosity or adding items (like salts or co-solvents) to make them less viscous or less conductive and more stable at high temperatures. (2) Selecting suitable HBDs and HBAs (e.g., choline chloride, urea) to achieve optimal ionic conductivity and viscosity and improved thermal stability by altering hydrogen bond interactions or introducing functional groups. (3) Using nanostructured materials (e.g., mesoporous TiO2) to accommodate DESs phase changes. (4) Applying thin coatings (e.g., graphene, polymers) to improve DESs adhesion and stability. (5) Modifying TiO2 electrodes with hydrophilic or DES-compatible functional groups to enhance wetting behavior. (5) Optimizing ion migration pathways within the cell to minimize DESs viscosity effects. (6) Investigating DESs recyclability and exploring methods for reusing or regenerating DESs. (7) Engaging industry partners to bridge the gap between fundamental research and practical applications of DESs.
The perspectives related to the use of DESs in DSSCs can be described as follows: (1) combining DESs with polymers to create hybrid materials. These blends could offer improved mechanical strength, thermal stability, and ion transport properties, or combine graphene into DESs to enhance electrical conductivity and provide a conductive network within the electrolyte. (2) Developing DESs that responds to environmental cues (e.g., temperature, light) by altering its properties. This adaptability could enhance DSSCs performance under varying conditions. (3) Investigating DESs for super-capacitor applications. Their unique properties may lead to high-capacity, environmentally friendly energy storage devices. (4) Utilizing DESs as thermoelectric materials for converting sun heat and CO2 into added-value products. (5) Developing efficient methods to recycle DESs after their useful life in DSSCs and promoting DES-based technologies beyond solar cells, such as in sustainable chemical processes or green manufacturing.
The present review summarizes the use of various eutectic mixtures as strong electrolytes in DSSCs, considering the different factors affecting the performance of these solar cells. Deep eutectic solvents (DESs) are more viscous than volatile organic compound (VOC) electrolytes, but they have been shown to greatly improve cell efficiency in thin-film DES-DSSCs by raising the photovoltage. DESs could make a major contribution to the development of long-lasting and environmentally-friendly liquid DSSCs, due to their advantages in terms of performance metrics, providing higher voltage and lower recombination resistance. This would support a shift towards a more environmentally aware approach to industrial progress.
5. Conclusions
Solar devices based on deep eutectic solvents (DESs) are at the forefront of sustainable and environmentally friendly green technologies. These innovative systems have the potential to significantly enhance the availability of clean and renewable energy sources within both the scientific realm and the broader societal context over the coming years. However, this potential can only be achieved by a dedicated investment in fundamental research. A comprehensive understanding of the intricate role played by DESs is crucial, especially concerning their intermolecular interactions involving the diverse components of solar cell structures. Such knowledge is essential for harnessing their positive impact on charge transfer processes at the molecular level. The application of DESs across diverse scientific disciplines is an emerging and exciting area of technology, with great potential for large-scale industrial application. Contrary to initial assumptions, on an industrial level, the transformation of two solid substances into a liquid form presents certain complexities. DESs are highly adaptable mixtures, comprising a variety of hydrogen bond acceptors (HBAs) and hydrogen bond donors (HBDs). Consequently, for the transition of DESs into a tangible industrial entity, researchers have been concentrating on developing a variety of scalable preparation techniques.As discussed in this review, the physicochemical properties of DESs have a major impact on their performance as electrolytes in dye-sensitized solar cells (DSSCs). DESs are known for their low volatility, strong solubilization capacity, and excellent ionic conductivity, which are crucial for the efficient operation of DSSCs. Their tunable nature allows the optimization of these properties to enhance the charge transport and stability of solar cells. In addition, the ability to tailor DESs by adjusting the HBA/HBD ratio provides a pathway for fine-tuning the polarity and viscosity of the solvent, further improving DSSC performance. In brief, the unique physicochemical properties of DESs offer a promising avenue for the development of high-efficiency and stable DSSCs, making them a valuable component in the advancement of renewable energy technologies.
This review highlights the increasing allure of DESs as key elements in DSSCs. Recently, researchers have evaluated numerous DESs as electrolyte solvents for DSSCs, alongside other high-voltage open-circuit electrolyte components. DESs have been used as solvents with or without added water, as well as in conjunction with ionic liquids (ILs) or VOCs. A notable feature that can be seen from the existing studies is the overall enhancement in the long-term stability of solar devices, even when DESs constitute a small fraction of the electrolyte solution. Although the efficiencies reported for DES-only DSSCs have not been as high as expected, the burgeoning interest and continuous performance advancements suggest that superior efficiencies may soon be achievable. However, using DESs in mixtures with conventional solvents to achieve higher efficiencies introduces additional VOC-related concerns, including flammability, toxicity, and volatility.
A thorough review of the literature showed that the ability of CO2 to dissolve in DESs can greatly improve the capacity of solar cells to convert CO2 into useful products. In the synthesis of DESs, amine-based DESs have emerged as an excellent option for selecting hydrogen bond donors (HBDs), with their corresponding salts as hydrogen bond acceptors (HBAs). In addition, researchers have recognized ethylene glycol as an effective HBD that increases the viscosity of DESs and enhances CO2 solubility. The addition of potassium iodide (KI) to DESs formulations can further improve the performance of DSSCs.
This review highlights that the addition of water can alter the molecular structure and dynamics of DESs, consequently affecting solar cell performance and efficiency. Also uncovered is the significance of CO2 solubility in DESs for the capture and utilization of CO2 by solar cells. Certain DESs demonstrate a high capacity and selectivity for CO2 absorption, while the presence of water can further enhance the absorption kinetics and capacity. Amine-based DESs are shown to be eco-friendly solvents suitable for use as electrolytes in various solar cell applications. These DESs have the potential to reach or exceed the power conversion efficiency, specific capacitance, and voltage of traditional solvents. However, it is important to acknowledge the drawbacks of DESs, such as their potential corrosiveness and the energy required for their production and use. These factors must be considered when evaluating the overall sustainability of DES-based technologies.
To conclude, the design of a specific eutectic mixture for a particular application requires a more comprehensive understanding than has so far been achieved. At the fundamental level, it is important to elucidate the structure and dynamics of DESs, considering the intermolecular interactions, the networks of bonds within HBDs and HBAs, and the roles that they play. In this way, it will soon be possible to design DESs with optimal chemical and physical characteristics for maximizing the performance of fully eco-friendly and sustainable solar devices.
List of abbreviations
| ILs | Ionic liquids |
| DES | Deep Eutectic Solvents |
| HBD | Hydrogen Bond Donor |
| HBA | Hydrogen Bond Acceptor |
| DSSCs | Dye-sensitized solar cells |
| EC | Electrochemical Cell |
| PCEs | Power Conversion Efficiencies |
| VOCs | Volatile Organic Compounds |
| ChCl | Choline Chloride |
| EG | Ethylene Glycol |
| EmimI | 1-Ethyl-3-methylimidazolium |
| Reline | Choline chloride and urea |
| Isc | Short circuit current |
| Voc | Open circuit voltage values |
| PSCs | Perovskite Solar Cells |
| I−/I3− | Iodide/triiodide |
| TiO2 | Titanium dioxide |
| NADES | Natural Deep Eutectic Solvents |
| ECs | Electrochemical Capacitors |
| F.E. | Faradaic Efficiencies |
| MEAHCl | Monoethanolamine hydrochloride |
| MDEA | Methyldiethanolamine |
| DMF | Dimethyl formamide |
| IM | Incorporating imidazole |
| FTO | Fluorine-doped tin oxide |
| FF | Fill Factor |
| LUMO | Lowest unoccupied molecular orbital |
| HOMO | Highest occupied molecular orbital |
| Eg | band gap |
| [PMIM]I | 1-Propyl-3-methylimidazolium iodide |
| IPCE | Incident Photon-to-Current Conversion Efficiency |
| ACN | Acetonitrile |
| EMIM | 1-Ethyl-3-methylimidazolium iodide |
| MEA·Cl | Ethylamine hydrochloride |
| DBN | 1,5-Diazabicyclo[4.3.0]-non-5-ene |
| DBU | 1,8-Diazabicyclo-[5.4.0]undec-7-ene |
| TBAB | Tetrabutylammonium bromide |
| MTPPBr | Methyltriphenylphosphonium bromide |
| BMIMCl | 1-Butyl-3-methylimidazolium chloride |
| MEA | Monoethanolamine |
| MDEA | Methyldiethanolamine |
| [bmim][tf2N] | 1-Butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| KI | Potassium iodide |
| LiI | Lithium iodide |
| NaI | Sodium iodide |
| Fr | Fructose |
| Glu | Glucose |
| MA | Malonic acid |
| Gly | Glycerol |
| VOC | Volatile Organic Compound |
Author contributions
Khatereh A. Pishro: formal analysis, investigation, writing – review & editing, original draft. Mario Henrique Gonzalez: supervision, conceptualization, project administration, resources, writing – review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful for the financial support provided by the São Paulo Research Foundation (FAPESP, grant numbers #2015/08893-4, #2017/18531-3, #2019/22113-8, and #2021/14759-5) and the National Institute for Alternative Technologies of Detection, Toxicological Evaluation and Removal of Micropollutants and Radioactives (INCT-DATREM) (FAPESP, grant number #2014/50945-4; CNPq, grant number #465571/2014-0).References
- N. Armaroli and V. Balzani, Solar electricity and solar fuels: status and perspectives in the context of the energy transition, Chem.–Eur. J., 2016, 22(1), 32–57 CrossRef CAS PubMed.
- M. S. Su’ait, M. Y. A. Rahman and A. Ahmad, Review on polymer electrolyte in dye-sensitized solar cells (DSSCs), Sol. Energy, 2015, 115, 452–470 CrossRef.
- A. Yamasaki, An overview of CO2 mitigation options for global warming—emphasizing CO2 sequestration options, J. Chem. Eng. Jpn., 2003, 36(4), 361–375 CrossRef CAS.
- S. C. Roy, O. K. Varghese, M. Paulose and C. A. Grimes, Toward Solar Fuels: Photocatalytic Conversion of Carbon Dioxide to Hydrocarbons, ACS Nano, 2010, 4(3), 1259–1278, DOI:10.1021/nn9015423.
- S. Pye, F. G. N. Li, J. Price and B. Fais, Achieving net-zero emissions through the reframing of UK national targets in the post-Paris Agreement era, Nat. Energy, 2017, 2(3), 17024, DOI:10.1038/nenergy.2017.24.
- M. Schreier, F. Héroguel, L. Steier, S. Ahmad, J. S. Luterbacher, M. T. Mayer, J. Luo and M. Grätzel, Solar conversion of CO2 to CO using Earth-abundant electrocatalysts prepared by atomic layer modification of CuO, Nat. Energy, 2017, 2(7), 1–9 Search PubMed.
- M. Schreier, L. Curvat, F. Giordano, L. Steier, A. Abate, S. M. Zakeeruddin, J. Luo, M. T. Mayer and M. Grätzel, Efficient photosynthesis of carbon monoxide from CO2 using perovskite photovoltaics, Nat. Commun., 2015, 6(1), 7326 CrossRef CAS PubMed.
- J. L. White, J. T. Herb, J. J. Kaczur, P. W. Majsztrik and A. B. Bocarsly, Photons to formate: Efficient electrochemical solar energy conversion via reduction of carbon dioxide, J. CO2 Util., 2014, 7, 1–5 CrossRef CAS.
- J.-P. Correa-Baena, A. Abate, M. Saliba, W. Tress, T. J. Jacobsson, M. Grätzel and A. Hagfeldt, The rapid evolution of highly efficient perovskite solar cells, Energy Environ. Sci., 2017, 10(3), 710–727 RSC.
- C. M. Gabardo, A. Seifitokaldani, J. P. Edwards, C.-T. Dinh, T. Burdyny, M. G. Kibria, C. P. O'Brien, E. H. Sargent and D. Sinton, Combined high alkalinity and pressurization enable efficient CO 2 electroreduction to CO, Energy Environ. Sci., 2018, 11(9), 2531–2539 RSC.
- W. Miao and T. H. Chan, Ionic-liquid-supported synthesis: A novel liquid-phase strategy for organic synthesis, Acc. Chem. Res., 2006, 39(12), 897–908 CrossRef CAS PubMed.
- W. M. Budzianowski, Energy Efficient Solvents for CO2 Capture by Gas-Liquid Absorption: Compounds, Blends and Advanced Solvent Systems, Springer, 2016 Search PubMed.
- Y.-L. Loow, T. Y. Wu, G. H. Yang, L. Y. Ang, E. K. New, L. F. Siow, J. M. Jahim, A. W. Mohammad and W. H. Teoh, Deep eutectic solvent and inorganic salt pretreatment of lignocellulosic biomass for improving xylose recovery, Bioresour. Technol., 2018, 249, 818–825 CrossRef CAS PubMed.
- S. P. Ventura, F. A. e Silva, M. V. Quental, D. Mondal, M. G. Freire and J. A. Coutinho, Ionic-liquid-mediated extraction and separation processes for bioactive compounds: past, present, and future trends, Chem. Rev., 2017, 117(10), 6984–7052 CrossRef CAS PubMed.
- A. Sharma, R. Sharma, R. C. Thakur and L. Singh, An overview of deep eutectic solvents: Alternative for organic electrolytes, aqueous systems & ionic liquids for electrochemical energy storage, J. Energy Chem., 2023, 82, 592–626, DOI:10.1016/j.jechem.2023.03.039.
- C. L. Boldrini, N. Manfredi, F. M. Perna, V. Trifiletti, V. Capriati and A. Abbotto, Dye-Sensitized Solar Cells that use an Aqueous Choline Chloride-Based Deep Eutectic Solvent as Effective Electrolyte Solution, Energy Technol., 2017, 5(2), 345–353, DOI:10.1002/ente.201600420.
- H.-R. Jhong, D. S.-H. Wong, C.-C. Wan, Y.-Y. Wang and T.-C. Wei, A novel deep eutectic solvent-based ionic liquid used as electrolyte for dye-sensitized solar cells, Electrochem. Commun., 2009, 11(1), 209–211, DOI:10.1016/j.elecom.2008.11.001.
- P. T. Nguyen, T. D. T. Nguyen, V. S. Nguyen, D. T. X. Dang, H. M. Le, T. C. Wei and P. H. Tran, Application of deep eutectic solvent from phenol and choline chloride in electrolyte to improve stability performance in dye-sensitized solar cells, J. Mol. Liq., 2019, 277, 157–162 CrossRef CAS.
- C. Florindo, F. Lima, B. D. Ribeiro and I. M. Marrucho, Deep eutectic solvents: overcoming 21st century challenges, Curr. Opin. Green Sustainable Chem., 2019, 18, 31–36 CrossRef.
- J. Y. Kim, J.-W. Lee, H. S. Jung, H. Shin and N.-G. Park, High-efficiency perovskite solar cells, Chem. Rev., 2020, 120(15), 7867–7918 CrossRef CAS PubMed.
- H. Li and W. Zhang, Perovskite tandem solar cells: from fundamentals to commercial deployment, Chem. Rev., 2020, 120(18), 9835–9950 CrossRef CAS PubMed.
- R. Li, C. Li, M. Liu, P. Vivo, M. Zheng, Z. Dai, J. Zhan, B. He, H. Li and W. Yang, Hydrogen-bonded dopant-free hole transport material enables efficient and stable inverted perovskite solar cells, CCS Chem., 2022, 4(9), 3084–3094 CrossRef CAS.
- R. Li, M. Liu, S. K. Matta, A. Hiltunen, Z. Deng, C. Wang, Z. Dai, S. P. Russo, P. Vivo and H. Zhang, Sulfonated Dopant-Free Hole-Transport Material Promotes Interfacial Charge Transfer Dynamics for Highly Stable Perovskite Solar Cells, Adv. Sustainable Syst., 2021, 5(12), 2100244 CrossRef CAS.
- A. W. Ho-Baillie, J. Zheng, M. A. Mahmud, F.-J. Ma, D. R. McKenzie and M. A. Green, Recent progress and future prospects of perovskite tandem solar cells, Appl. Phys. Rev., 2021, 8(4), 041307 CAS.
- K. Kakiage, Y. Aoyama, T. Yano, K. Oya, J.-i. Fujisawa and M. Hanaya, Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes, Chem. Commun., 2015, 51(88), 15894–15897 RSC.
- G. P. Salvador, D. Pugliese, F. Bella, A. Chiappone, A. Sacco, S. Bianco and M. Quaglio, New insights in long-term photovoltaic performance characterization of cellulose-based gel electrolytes for stable dye-sensitized solar cells, Electrochim. Acta, 2014, 146, 44–51 CrossRef CAS.
- C. L. Boldrini, N. Manfredi, F. M. Perna, V. Trifiletti, V. Capriati and A. Abbotto, Dye-sensitized solar cells that use an aqueous choline chloride-based deep eutectic solvent as effective electrolyte solution, Energy Technol., 2017, 5(2), 345–353 CrossRef CAS.
- D. V. Wagle, H. Zhao and G. A. Baker, Deep eutectic solvents: sustainable media for nanoscale and functional materials, Acc. Chem. Res., 2014, 47(8), 2299–2308 CrossRef CAS PubMed.
- S. Choudhury, U. Mahanta, R. Prasanna Venkatesh and T. Banerjee, Ionic liquid derived novel deep eutectic solvents as low viscous electrolytes for energy storage, J. Mol. Liq., 2022, 366, 120245, DOI:10.1016/j.molliq.2022.120245.
- J. Kargul and M. Kiliszek, Artificial Photosynthesis, in Bioelectrochemical Interface Engineering, 2019, pp. 271–309 Search PubMed.
- A. Prajapati and M. R. Singh, Assessment of Artificial Photosynthetic Systems for Integrated Carbon Capture and Conversion, ACS Sustain. Chem. Eng., 2019, 7(6), 5993–6003, DOI:10.1021/acssuschemeng.8b04969.
- C. J. Clarke, W.-C. Tu, O. Levers, A. Bröhl and J. P. Hallett, Green and Sustainable Solvents in Chemical Processes, Chem. Rev., 2018, 118(2), 747–800, DOI:10.1021/acs.chemrev.7b00571.
- B. H. Lipshutz, F. Gallou and S. Handa, Evolution of Solvents in Organic Chemistry, ACS Sustain. Chem. Eng., 2016, 4(11), 5838–5849, DOI:10.1021/acssuschemeng.6b01810.
- S. Mallakpour and M. Dinari, Ionic Liquids as Green Solvents: Progress and Prospects, in Green Solvents II: Properties and Applications of Ionic Liquids, ed. Mohammad, A. and Inamuddin, D., Springer, Netherlands, 2012, pp. 1–32 Search PubMed.
- D. A. Alonso, A. Baeza, R. Chinchilla, G. Guillena, I. M. Pastor and D. J. Ramón, Deep eutectic solvents: the organic reaction medium of the century, Eur. J. Org Chem., 2016, 2016(4), 612–632 CrossRef CAS.
- F. M. Perna, P. Vitale and V. Capriati, Deep eutectic solvents and their applications as green solvents, Curr. Opin. Green Sustainable Chem., 2020, 21, 27–33, DOI:10.1016/j.cogsc.2019.09.004.
- T. Aissaoui, I. M. AlNashef, U. A. Qureshi and Y. Benguerba, Potential applications of deep eutectic solvents in natural gas sweetening for CO2 capture, Rev. Chem. Eng., 2017, 33(6), 523–550 CAS.
- E. L. Smith, A. P. Abbott and K. S. Ryder, Deep Eutectic Solvents (DESs) and Their Applications, Chem. Rev., 2014, 114(21), 11060–11082, DOI:10.1021/cr300162p.
- B. B. Hansen, S. Spittle, B. Chen, D. Poe, Y. Zhang, J. M. Klein, A. Horton, L. Adhikari, T. Zelovich and B. W. Doherty, Deep eutectic solvents: A review of fundamentals and applications, Chem. Rev., 2020, 121(3), 1232–1285 CrossRef PubMed.
- M. A. Martins, S. P. Pinho and J. A. Coutinho, Insights into the nature of eutectic and deep eutectic mixtures, J. Solution Chem., 2019, 48, 962–982 CrossRef CAS.
- Y.-T. Liu, Y.-A. Chen and Y.-J. Xing, Synthesis and characterization of novel ternary deep eutectic solvents, Chin. Chem. Lett., 2014, 25(1), 104–106 CrossRef CAS.
- M. Zhang, et al., Insights into the relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents, Environ. Sci. Pollut. Res., 2021, 28(27), 35537–35563 CrossRef CAS PubMed.
- C. Florindo, F. S. Oliveira, L. P. N. Rebelo, A. M. Fernandes and I. M. Marrucho, Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids, ACS Sustain. Chem. Eng., 2014, 2(10), 2416–2425 CrossRef CAS.
- D. J. van Osch, L. F. Zubeir, A. van den Bruinhorst, M. A. Rocha and M. C. Kroon, Hydrophobic deep eutectic solvents as water-immiscible extractants, Green Chem., 2015, 17(9), 4518–4521 RSC.
- M. Chen, A. G. Edlow, T. Lin, N. A. Smith, T. F. McElrath and C. Lu, Determination of bisphenol-A levels in human amniotic fluid samples by liquid chromatography coupled with mass spectrometry, J. Sep. Sci., 2011, 34(14), 1648–1655 CrossRef CAS PubMed.
- O. S. Hammond, D. T. Bowron and K. J. Edler, The effect of water upon deep eutectic solvent nanostructure: an unusual transition from ionic mixture to aqueous solution, Angew. Chem., 2017, 129(33), 9914–9917 CrossRef.
- C. L. Boldrini, N. Manfredi, F. M. Perna, V. Capriati and A. Abbotto, Designing eco-sustainable dye-sensitized solar cells by the use of a menthol-based hydrophobic eutectic solvent as an effective electrolyte medium, Chem.–Eur. J., 2018, 24(67), 17656–17659 CrossRef CAS PubMed.
- M. Zhang, X. Zhang, Y. Liu, K. Wu, Y. Zhu, H. Lu and B. Liang, Insights into the relationships between physicochemical properties, solvent performance, and applications of deep eutectic solvents, Environ. Sci. Pollut. Res., 2021, 28(27), 35537–35563, DOI:10.1007/s11356-021-14485-2.
- Q. Meng, Y. Chen, Y. Y. Xiao, J. Sun, X. Zhang, C. B. Han, H. Gao, Y. Zhang and H. Yan, Effect of temperature on the performance of perovskite solar cells, J. Mater. Sci.: Mater. Electron., 2021, 32(10), 12784–12792, DOI:10.1007/s10854-020-03029-y.
- D. J. Boogaart, J. B. Essner and G. A. Baker, Evaluation of canonical choline chloride based deep eutectic solvents as dye-sensitized solar cell electrolytes, J. Chem. Phys., 2021, 155(6), 061102 CrossRef CAS PubMed.
- N. Chaabene, K. Ngo, M. Turmine and V. Vivier, New hydrophobic deep eutectic solvent for electrochemical applications, J. Mol. Liq., 2020, 319, 114198 CrossRef CAS.
- S. Khandelwal, Y. K. Tailor and M. Kumar, Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations, J. Mol. Liq., 2016, 215, 345–386 CrossRef CAS.
- Y. P. Mbous, M. Hayyan, A. Hayyan, W. F. Wong, M. A. Hashim and C. Y. Looi, Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges, Biotechnol. Adv., 2017, 35(2), 105–134 CrossRef CAS PubMed.
- M. Heydari Dokoohaki, F. Mohammadpour and A. R. Zolghadr, Dye-Sensitized Solar Cells Based on Deep Eutectic Solvent Electrolytes: Insights from Experiment and Simulation, J. Phys. Chem. C, 2021, 125(28), 15155–15165 CrossRef CAS.
- A. K. Halder, R. Haghbakhsh, I. V. Voroshylova, A. R. C. Duarte and M. N. D. S. Cordeiro, Density of Deep Eutectic Solvents: The Path Forward Cheminformatics-Driven Reliable Predictions for Mixtures, Molecules, 2021, 26(19), 5779 CrossRef CAS PubMed.
- V. Alizadeh, L. Esser and B. Kirchner, How is CO2 absorbed into a deep eutectic solvent?, J. Chem. Phys., 2021, 154(9), 094503 CrossRef CAS PubMed.
- T. El Achkar, H. Greige-Gerges and S. Fourmentin, Basics and properties of deep eutectic solvents: a review, Environ. Chem. Lett., 2021, 19(4), 3397–3408, DOI:10.1007/s10311-021-01225-8.
- G. Di Carmine, A. P. Abbott and C. D'agostino, Deep eutectic solvents: alternative reaction media for organic oxidation reactions, React. Chem. Eng., 2021, 6(4), 582–598 RSC.
- S. Azmi, M. F. Koudahi and E. Frackowiak, Reline deep eutectic solvent as a green electrolyte for electrochemical energy storage applications, Energy Environ. Sci., 2022, 15(3), 1156–1171 RSC.
- D. Nguyen, T. Van Huynh, V. S. Nguyen, P.-L. D. Cao, H. T. Nguyen, T.-C. Wei, P. H. Tran and P. T. Nguyen, Choline chloride-based deep eutectic solvents as effective electrolytes for dye-sensitized solar cells, RSC Adv., 2021, 11(35), 21560–21566 RSC.
- A. Onno, C. Chen, P. Koswatta, M. Boccard and Z. C. Holman, Passivation, conductivity, and selectivity in solar cell contacts: Concepts and simulations based on a unified partial-resistances framework, J. Appl. Phys., 2019, 126(18), 183103 CrossRef.
- L. M. Shaker, A. Alamiery, W. N. R. W. Isahak and W. K. Al-Azzawi, Corrosion in solar cells: challenges and solutions for enhanced performance and durability, J. Opt., 2023, 1–15 Search PubMed.
- A. P. Abbott, R. C. Harris and K. S. Ryder, Application of hole theory to define ionic liquids by their transport properties, J. Phys. Chem. B, 2007, 111(18), 4910–4913 CrossRef CAS PubMed.
- Y. Marcus, Properties of Deep Eutectic Solvents, in Deep Eutectic Solvents, ed. Marcus, Y., Springer International Publishing, 2019, pp. 45–110 Search PubMed.
- Q. Zhang, K. D. O. Vigier, S. Royer and F. Jérôme, Deep eutectic solvents: syntheses, properties and applications, Chem. Soc. Rev., 2012, 41(21), 7108–7146 RSC.
- Y. Hou, Y. Gu, S. Zhang, F. Yang, H. Ding and Y. Shan, Novel binary eutectic mixtures based on imidazole, J. Mol. Liq., 2008, 143(2–3), 154–159 CrossRef CAS.
- Y.-l. Yang and Y. Kou, Determination of the Lewis acidity of ionic liquids by means of an IR spectroscopic probe, Chem. Commun., 2004,(2), 226–227 RSC.
- W. N. Sanders and J. E. Berger, Measurement and significance of the Hammett acidity function in nonhydroxylic solvents, Anal. Chem., 1967, 39(12), 1473–1476 CrossRef CAS.
- H. Lin, F. Hao and J. Li, Electrolyte-dependent photovoltaic responses in dye-sensitized solar cells, Front. Optoelectron, 2011, 4, 45–52 CrossRef.
- J. Wu, Z. Lan, J. Lin, M. Huang, Y. Huang, L. Fan, G. Luo, Y. Lin, Y. Xie and Y. Wei, Counter electrodes in dye-sensitized solar cells, Chem. Soc. Rev., 2017, 46(19), 5975–6023 RSC.
- G. Boschloo, Improving the performance of dye-sensitized solar cells, Front. Chem., 2019, 7, 77 CrossRef CAS PubMed.
- H. S. Salehi, O. A. Moultos and T. J. Vlugt, Interfacial properties of hydrophobic deep eutectic solvents with water, J. Phys. Chem. B, 2021, 125(44), 12303–12314 CrossRef CAS PubMed.
- J. Xue, J. Wang, D. Feng, H. Huang and M. Wang, Processing of Functional Composite Resins Using Deep Eutectic Solvent, Crystals, 2020, 10(10), 864 CrossRef CAS.
- L. E. Crandon, K. M. Boenisch, B. J. Harper and S. L. Harper, Adaptive methodology to determine hydrophobicity of nanomaterials in situ, PLoS One, 2020, 15(6), e0233844 CrossRef CAS PubMed.
- H. Lin, F. Hao and J. Li, Electrolyte-dependent photovoltaic responses in dye-sensitized solar cells, Front. Optoelectron, 2011,(1), 45–52, DOI:10.1007/s12200-011-0208-z.
- A. Ebenezer Anitha and M. Dotter, A Review on Liquid Electrolyte Stability Issues for Commercialization of Dye-Sensitized Solar Cells (DSSC), Energies, 2023, 16(13), 5129 CrossRef CAS.
- D. Nguyen, M. T. Nguyen, T. T. D. Nguyen, V. T. Huynh, B. P. N. Nguyen and P. T. Nguyen, Deep eutectic solvent based on choline chloride and phenol as electrolyte additives in dye-sensitized solar cells: a comparison with 4-tert-butylpyridine, J. Aust. Ceram. Soc., 2022, 58(3), 913–921 CrossRef CAS.
- K. Sharma, V. Sharma and S. S. Sharma, Dye-Sensitized Solar Cells: Fundamentals and Current Status, Nanoscale Res. Lett., 2018, 13(1), 381, DOI:10.1186/s11671-018-2760-6.
- Y. Dai, J. van Spronsen, G.-J. Witkamp, R. Verpoorte and Y. H. Choi, Natural deep eutectic solvents as new potential media for green technology, Anal. Chim. Acta, 2013, 766, 61–68 CrossRef CAS PubMed.
- A. P. Abbott, D. Boothby, G. Capper, D. L. Davies and R. K. Rasheed, Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids, J. Am. Chem. Soc., 2004, 126(29), 9142–9147 CrossRef CAS PubMed.
- A. P. R. Santana, J. A. Mora-Vargas, T. G. S. Guimarães, C. D. B. Amaral, A. Oliveira and M. H. Gonzalez, Sustainable synthesis of natural deep eutectic solvents (NADES) by different methods, J. Mol. Liq., 2019, 293, 111452, DOI:10.1016/j.molliq.2019.111452.
- M. Shaibuna, A. Abbas, M. J. K. Kuniyil and K. Sreekumar, Sustainable synthesis of 1, 8-dioxooctahydroxanthenes in deep eutectic solvents (DESs), New J. Chem., 2021, 45(18), 8335–8344 RSC.
- Z. Wang, S. Li, T. Li, T. Hu and X. Ge, Deep eutectic solvents (DESs) for green recycling of wasted lithium-ion batteries (LIBs): progress on pushing the overall efficiency, Min. Metall. Explor., 2022, 39(5), 2149–2165 Search PubMed.
- C. Zhang, Y. Fu, W. Gao, T. Bai, T. Cao, J. Jin and B. Xin, Deep Eutectic Solvent-Mediated Electrocatalysts for Water Splitting, Molecules, 2022, 27(22), 8098 CrossRef CAS PubMed.
- D. Yu, Z. Xue and T. Mu, Deep eutectic solvents as a green toolbox for synthesis, Cell Rep. Phys. Sci., 2022, 3(4), 1–23 Search PubMed.
- Y. Liu, J. B. Friesen, J. B. McAlpine, D. C. Lankin, S.-N. Chen and G. F. Pauli, Natural deep eutectic solvents: properties, applications, and perspectives, J. Nat. Prod., 2018, 81(3), 679–690 CrossRef CAS PubMed.
- A. Paiva, R. Craveiro, I. Aroso, M. Martins, R. L. Reis and A. R. C. Duarte, Natural deep eutectic solvents–solvents for the 21st century, ACS Sustain. Chem. Eng., 2014, 2(5), 1063–1071 CrossRef CAS.
- M. M. Nolasco, S. N. Pedro, C. Vilela, P. D. Vaz, P. Ribeiro-Claro, S. Rudić, S. F. Parker, C. S. Freire, M. G. Freire and A. J. Silvestre, Water in deep eutectic solvents: new insights from inelastic neutron scattering spectroscopy, Front. Phys., 2022, 10, 834571 CrossRef.
- S. Sarmad, Y. Xie, J.-P. Mikkola and X. Ji, Screening of deep eutectic solvents (DESs) as green CO 2 sorbents: from solubility to viscosity, New J. Chem., 2017, 41(1), 290–301 RSC.
- J. Kumełan, A. l. Pérez-Salado Kamps, D. Tuma and G. Maurer, Solubility of carbon dioxide in liquid mixtures of water+[bmim][CH3SO4], J. Chem. Eng. Data, 2011, 56(12), 4505–4515 CrossRef.
- S. Zhang, M. Du, P. Shao, L. Wang, J. Ye, J. Chen and J. Chen, Carbonic Anhydrase Enzyme-MOFs Composite with a Superior Catalytic Performance to Promote CO2 Absorption into Tertiary Amine Solution, Environ. Sci. Technol., 2018, 52(21), 12708–12716, DOI:10.1021/acs.est.8b04671.
- W. Lu, Y. Zhang, J. Zhang and P. Xu, Reduction of Gas CO2 to CO with High Selectivity by Ag Nanocube-Based Membrane Cathodes in a Photoelectrochemical System, Ind. Eng. Chem. Res., 2020, 59(13), 5536–5545, DOI:10.1021/acs.iecr.9b06052.
- P. Luis, Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives, Desalination, 2016, 380, 93–99, DOI:10.1016/j.desal.2015.08.004.
- S. Zhang, Y. Shen, L. Wang, J. Chen and Y. Lu, Phase change solvents for post-combustion CO2 capture: Principle, advances, and challenges, Appl. Energy, 2019, 239, 876–897, DOI:10.1016/j.apenergy.2019.01.242.
- K. A. Pishro, G. Murshid, F. S. Mjalli and J. Naser, Carbon dioxide solubility in amine-based deep eutectic solvents: Experimental and theoretical investigation, J. Mol. Liq., 2021, 325, 115133, DOI:10.1016/j.molliq.2020.115133.
- M. B. Haider and R. Kumar, Solubility of CO2 and CH4 in sterically hindered amine-based deep eutectic solvents, Sep. Purif. Technol., 2020, 248, 117055, DOI:10.1016/j.seppur.2020.117055.
- E. Pérez-Gallent, C. Vankani, C. Sánchez-Martínez, A. Anastasopol and E. Goetheer, Integrating CO2 Capture with Electrochemical Conversion Using Amine-Based Capture Solvents as Electrolytes, Ind. Eng. Chem. Res., 2021, 60(11), 4269–4278, DOI:10.1021/acs.iecr.0c05848.
- N. Ahmad, X. Wang, P. Sun, Y. Chen, F. Rehman, J. Xu and X. Xu, Electrochemical CO2 reduction to CO facilitated by MDEA-based deep eutectic solvent in aqueous solution, Renew. Energy, 2021, 177, 23–33 CrossRef CAS.
- A. Abdelaaziz, S. M. Bouzzine and M. Hamidi, Computational investigation of dyes-sensitized solar cells containing various π-bridges achieving high photovoltaic performance with triazatruxene-based dyes, J. Photochem. Photobiol., A, 2024, 447, 115192 CrossRef CAS.
- S. Imteyaz, C. M. Suresh, T. Kausar and P. P. Ingole, Carbon dioxide capture and its electrochemical reduction study in deep eutectic solvent (DES) via experimental and molecular simulation approaches, J. CO2 Util., 2023, 68, 102349 CrossRef CAS.
- Q. T. Vu, H. Yamada and K. Yogo, Exploring the Role of Imidazoles in Amine-Impregnated Mesoporous Silica for CO2 Capture, Ind. Eng. Chem. Res., 2018, 57(7), 2638–2644, DOI:10.1021/acs.iecr.7b04722.
- L. Cao, J. Huang, X. Zhang, S. Zhang, J. Gao and S. Zeng, Imidazole tailored deep eutectic solvents for CO 2 capture enhanced by hydrogen bonds, Phys. Chem. Chem. Phys., 2015, 17(41), 27306–27316 RSC.
- S. Evjen, A. Fiksdahl and H. K. Knuutila, High-capacity amine-imidazole solvent blends for CO2 capture, Ind. Eng. Chem. Res., 2019, 58(24), 10533–10539 CrossRef CAS.
- J. Boualavong and C. A. Gorski, Electrochemically Mediated CO2 Capture Using Aqueous Cu(II)/Cu(I) Imidazole Complexes, ACS ES&T Eng., 2021, 1(7), 1084–1093, DOI:10.1021/acsestengg.1c00068.
- X. Li, M. Hou, B. Han, X. Wang and L. Zou, Solubility of CO2 in a choline chloride+ urea eutectic mixture, J. Chem. Eng. Data, 2008, 53(2), 548–550 CrossRef CAS.
- E. Ali, M. K. Hadj-Kali, S. Mulyono, I. Alnashef, A. Fakeeha, F. Mjalli and A. Hayyan, Solubility of CO2 in deep eutectic solvents: Experiments and modelling using the Peng–Robinson equation of state, Chem. Eng. Res. Des., 2014, 92(10), 1898–1906 CrossRef CAS.
- R. B. Leron and M.-H. Li, Solubility of carbon dioxide in a choline chloride–ethylene glycol based deep eutectic solvent, Thermochim. Acta, 2013, 551, 14–19 CrossRef CAS.
- R. B. Leron, A. Caparanga and M.-H. Li, Carbon dioxide solubility in a deep eutectic solvent based on choline chloride and urea at T= 303.15–343.15 K and moderate pressures, J. Taiwan Inst. Chem. Eng., 2013, 44(6), 879–885 CrossRef CAS.
- G. Li, D. Deng, Y. Chen, H. Shan and N. Ai, Solubilities and thermodynamic properties of CO2 in choline-chloride based deep eutectic solvents, J. Chem. Thermodyn., 2014, 75, 58–62 CrossRef CAS.
- M. Francisco, A. van den Bruinhorst, L. F. Zubeir, C. J. Peters and M. C. Kroon, A new low transition temperature mixture (LTTM) formed by choline chloride+ lactic acid: Characterization as solvent for CO2 capture, Fluid Phase Equilib., 2013, 340, 77–84 CrossRef CAS.
- L. L. Sze, S. Pandey, S. Ravula, S. Pandey, H. Zhao, G. A. Baker and S. N. Baker, Ternary deep eutectic solvents tasked for carbon dioxide capture, ACS Sustain. Chem. Eng., 2014, 2(9), 2117–2123 CrossRef CAS.
- Y.-H. Hsu, R. B. Leron and M.-H. Li, Solubility of carbon dioxide in aqueous mixtures of (reline+ monoethanolamine) at T=(313.2 to 353.2) K, J. Chem. Thermodyn., 2014, 72, 94–99 CrossRef CAS.
- T. J. Trivedi, J. H. Lee, H. J. Lee, Y. K. Jeong and J. W. Choi, Deep eutectic solvents as attractive media for CO 2 capture, Green Chem., 2016, 18(9), 2834–2842 RSC.
- M. B. Haider, D. Jha, B. Marriyappan Sivagnanam and R. Kumar, Thermodynamic and kinetic studies of CO2 capture by glycol and amine-based deep eutectic solvents, J. Chem. Eng. Data, 2018, 63(8), 2671–2680 CrossRef CAS.
- C. Mukesh, S. G. Khokarale, P. Virtanen and J.-P. Mikkola, Rapid desorption of CO 2 from deep eutectic solvents based on polyamines at lower temperatures: An alternative technology with industrial potential, Sustainable Energy Fuels, 2019, 3(8), 2125–2134 RSC.
- C. Wang, S. M. Mahurin, H. Luo, G. A. Baker, H. Li and S. Dai, Reversible and robust CO 2 capture by equimolar task-specific ionic liquid–superbase mixtures, Green Chem., 2010, 12(5), 870–874 RSC.
- Y. Chen, N. Ai, G. Li, H. Shan, Y. Cui and D. Deng, Solubilities of carbon dioxide in eutectic mixtures of choline chloride and dihydric alcohols, J. Chem. Eng. Data, 2014, 59(4), 1247–1253 CrossRef CAS.
- R. B. Leron and M.-H. Li, Solubility of carbon dioxide in a eutectic mixture of choline chloride and glycerol at moderate pressures, J. Chem. Thermodyn., 2013, 57, 131–136 CrossRef CAS.
- Z.-Z. Yang, L.-N. He, Y.-N. Zhao, B. Li and B. Yu, CO 2 capture and activation by superbase/polyethylene glycol and its subsequent conversion, Energy Environ. Sci., 2011, 4(10), 3971–3975 RSC.
- B. Averill, General Chemistry: Principles, Patterns, and Applications, 2011 Search PubMed.
- Y. Marcus, Gas solubilities in deep eutectic solvents, Monatsh. Chem., 2018, 149, 211–217 CrossRef CAS.
- E. Tanaka and N. Robertson, Polyiodide solid-state dye-sensitized solar cell produced from a standard liquid I−/I 3− electrolyte, J. Mater. Chem. A, 2020, 8(38), 19991–19999 RSC.
- S. Tontapha, P. Uppachai and V. Amornkitbamrung, Fabrication of functional materials for dye-sensitized solar cells, Front. Energy Res., 2021, 9, 641983 CrossRef.
- W.-J. Ong, L.-L. Tan, S.-P. Chai, S.-T. Yong and A. R. Mohamed, Self-assembly of nitrogen-doped TiO 2 with exposed {001} facets on a graphene scaffold as photo-active hybrid nanostructures for reduction of carbon dioxide to methane, Nano Res., 2014, 7, 1528–1547 CrossRef CAS.
- V. P. Indrakanti, J. D. Kubicki and H. H. Schobert, Photoinduced activation of CO 2 on Ti-based heterogeneous catalysts: Current state, chemical physics-based insights and outlook, Energy Environ. Sci., 2009, 2(7), 745–758 RSC.
- E. Muchuweni, B. S. Martincigh and V. O. Nyamori, Recent advances in graphene-based materials for dye-sensitized solar cell fabrication, RSC Adv., 2020, 10(72), 44453–44469 RSC.
- R. Baby, P. D. Nixon, N. M. Kumar, M. Subathra and N. Ananthi, A comprehensive review of dye-sensitized solar cell optimal fabrication conditions, natural dye selection, and application-based future perspectives, Environ. Sci. Pollut. Res., 2022, 1–34 Search PubMed.
- J. A. Castillo-Robles, E. Rocha-Rangel, J. A. Ramírez-de-León, F. C. Caballero-Rico and E. N. Armendáriz-Mireles, Advances on Dye-Sensitized Solar Cells (DSSCs) Nanostructures and Natural Colorants: A Review, J. Compos. Sci., 2021, 5(11), 288 CrossRef CAS.
- L. I. Tomé, V. Baião, W. da Silva and C. M. Brett, Deep eutectic solvents for the production and application of new materials, Appl. Mater. Today, 2018, 10, 30–50 CrossRef.
- M. Aresta, A. Dibenedetto and A. Angelini, The use of solar energy can enhance the conversion of carbon dioxide into energy-rich products: stepping towards artificial photosynthesis, Philos. Trans. R. Soc., A, 2013, 371(1996), 20120111 CrossRef PubMed.
- X.-F. Wu and F. Zheng, Synthesis of carboxylic acids and esters from CO 2, Chemical Transformations of Carbon Dioxide 2018, pp. 1–60 Search PubMed.
- J. Iglesias, I. Martínez-Salazar, P. Maireles-Torres, D. M. Alonso, R. Mariscal and M. L. Granados, Advances in catalytic routes for the production of carboxylic acids from biomass: a step forward for sustainable polymers, Chem. Soc. Rev., 2020, 49(16), 5704–5771 RSC.
- J. Li, C. Meng, J. Gu, H. Wang, R. Dai, H. Sha and H. Zhu, High faradic efficiency of CO2 conversion to formic acid catalyzed by Cu2O hollow-dices, Carbon Neutrality, 2022, 1(1), 36, DOI:10.1007/s43979-022-00037-1.
- T. Inoue, A. Fujishima, S. Konishi and K. Honda, Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders, Nature, 1979, 277(5698), 637–638 CrossRef CAS.
- W. Tu, Y. Zhou and Z. Zou, Photocatalytic conversion of CO2 into renewable hydrocarbon fuels: state-of-the-art accomplishment, challenges, and prospects, Adv. Mater., 2014, 26(27), 4607–4626 CrossRef CAS PubMed.
- E. Fujita, Photochemical carbon dioxide reduction with metal complexes, Coord. Chem. Rev., 1999, 185, 373–384 CrossRef.
- Y. Yu, Z. Zhang, X. Yin, A. Kvit, Q. Liao, Z. Kang, X. Yan, Y. Zhang and X. Wang, Enhanced photoelectrochemical efficiency and stability using a conformal TiO2 film on a black silicon photoanode, Nat. Energy, 2017, 2(6), 1–7 Search PubMed.
- L. Reyes, C. Nikitine, L. Vilcocq and P. Fongarland, Green is the new black–a review of technologies for carboxylic acid recovery from black liquor, Green Chem., 2020, 22(23), 8097–8115 RSC.
- S. Samanta and R. Srivastava, Catalytic conversion of CO 2 to chemicals and fuels: the collective thermocatalytic/photocatalytic/electrocatalytic approach with graphitic carbon nitride, Mater. Adv., 2020, 1(6), 1506–1545 RSC.
- Q. Zhang, C. Yang, A. Guan, M. Kan and G. Zheng, Photocatalytic CO2 conversion: from C1 products to multi-carbon oxygenates, Nanoscale, 2022, 14(29), 10268–10285 RSC.
- M. Aresta, A. Dibenedetto and A. Angelini, The changing paradigm in CO2 utilization, J. CO2 Util., 2013, 3–4, 65–73 CrossRef CAS.
- C. L. Boldrini, A. F. Quivelli, N. Manfredi, V. Capriati and A. Abbotto, Deep Eutectic Solvents in Solar Energy Technologies, Molecules, 2022, 27(3), 709 CrossRef CAS PubMed.
- B. O'regan and M. Grätzel, A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films, Nature, 1991, 353(6346), 737–740 CrossRef.
- C. T. Li, R. Y. Y. Lin and J. T. Lin, Sensitizers for Aqueous-Based Solar Cells, Chem.–Asian J., 2017, 12(5), 486–496 CrossRef CAS PubMed.
- X. Jiang, T. Marinado, E. Gabrielsson, D. P. Hagberg, L. Sun and A. Hagfeldt, Structural Modification of Organic Dyes for Efficient Coadsorbent-Free Dye-Sensitized Solar Cells, J. Phys. Chem. C, 2010, 114(6), 2799–2805, DOI:10.1021/jp908552t.
- S. M. Feldt, E. A. Gibson, E. Gabrielsson, L. Sun, G. Boschloo and A. Hagfeldt, Design of Organic Dyes and Cobalt Polypyridine Redox Mediators for High-Efficiency Dye-Sensitized Solar Cells, J. Am. Chem. Soc., 2010, 132(46), 16714–16724, DOI:10.1021/ja1088869.
- K. Kakiage, Y. Aoyama, T. Yano, T. Otsuka, T. Kyomen, M. Unno and M. Hanaya, An achievement of over 12 percent efficiency in an organic dye-sensitized solar cell, Chem. Commun., 2014, 50(48), 6379–6381 RSC.
- M. Kokkonen, P. Talebi, J. Zhou, S. Asgari, S. A. Soomro, F. Elsehrawy, J. Halme, S. Ahmad, A. Hagfeldt and S. G. Hashmi, Advanced research trends in dye-sensitized solar cells, J. Mater. Chem. A, 2021, 9(17), 10527–10545 RSC.
- S. Zhang, J. Jin, D. Li, Z. Fu, S. Gao, S. Cheng, X. Yu and Y. Xiong, Increased power conversion efficiency of dye-sensitized solar cells with counter electrodes based on carbon materials, RSC Adv., 2019, 9(38), 22092–22100 RSC.
- Y. Li, X. Li and Y. Xu, Theoretical insights into the effect of pristine, doped and hole graphene on the overall performance of dye-sensitized solar cells, Inorg. Chem. Front., 2020, 7(1), 157–168 RSC.
- F. Gao, et al., Theoretical studies on the possible sensitizers of DSSC: nanocomposites of graphene quantum dot hybrid phthalocyanine/tetrabenzoporphyrin/tetrabenzotriazaporphyrins/cis-tetrabenzodiazaporphyrins/tetrabenzomonoazaporphyrins and their Cu-metallated macrocycles, Spectrochim. Acta, Part A, 2018, 195, 176–183 CrossRef CAS PubMed.
- M. Rezvani, M. D. Ganji, S. Jameh-Bozorghi and A. Niazi, DFT/TD-semiempirical study on the structural and electronic properties and absorption spectra of supramolecular fullerene-porphyrine-metalloporphyrine triads based dye-sensitized solar cells, Spectrochim. Acta, Part A, 2018, 194, 57–66 CrossRef CAS PubMed.
- M. T. Moghim, S. Jamehbozorgi, M. Rezvani and M. Ramezani, Computational investigation on the geometry and electronic structures and absorption spectra of metal-porphyrin-oligo-phenyleneethynylenes-[60] fullerene triads, Spectrochim. Acta, Part A, 2022, 280, 121488 CrossRef PubMed.
- J. Fina, et al., Enhancing Light Harvesting in Dye-Sensitized Solar Cells through Mesoporous Silica Nanoparticle-Mediated Diffuse Scattering Back Reflectors, Electron. Mater., 2023, 4(3), 124–135 CrossRef.
- S. Tomasi, S. Baghbanzadeh, S. Rahimi-Keshari and I. Kassal, Coherent and controllable enhancement of light-harvesting efficiency, Phys. Rev. A, 2019, 100(4), 043411 CrossRef CAS.
- M. D. Ganji, M. Rezvani, and S. Tanreh, Characterization and theoretical modeling of solar cells, Fundamentals of Solar Cell Design, 2021, pp. 169–215 Search PubMed.
- J. P. Bittner, N. Zhang, L. Huang, P. D. de María, S. Jakobtorweihen and S. Kara, Impact of deep eutectic solvents (DESs) and individual DES components on alcohol dehydrogenase catalysis: connecting experimental data and molecular dynamics simulations, Green Chem., 2022, 24(3), 1120–1131 RSC.
- H. Iftikhar, G. G. Sonai, S. G. Hashmi, A. F. Nogueira and P. D. Lund, Progress on Electrolytes Development in Dye-Sensitized Solar Cells, Materials, 2019, 12(12), 1998 CrossRef CAS PubMed.
- C. L. Boldrini, N. Manfredi, F. M. Perna, V. Capriati and A. Abbotto, Eco-friendly sugar-based natural deep eutectic solvents as effective electrolyte solutions for dye-sensitized solar cells, ChemElectroChem, 2020, 7(7), 1707–1712 CrossRef CAS.
- H. Cruz, A. L. Pinto, N. Jordão, L. A. Neves and L. C. Branco, Alkali Iodide Deep Eutectic Solvents as Alternative Electrolytes for Dye Sensitized Solar Cells, Sustainable Chem., 2021, 2(2), 222–236 CrossRef CAS.
- A. S. Steparuk, R. A. Irgashev, E. F. Zhilina, V. V. Emets, V. A. Grinberg, E. V. Krivogina, E. V. Belova, P. I. Lazarenko, G. L. Rusinov and S. A. Kozyukhin, Performance evaluation of dye-sensitized solar cells (DSSCs) based on metal-free thieno[3,2-b]indole dyes, J. Mater. Sci.: Mater. Electron., 2022, 33(9), 6307–6317, DOI:10.1007/s10854-022-07805-w.
- A. Yella, H.-W. Lee, H. N. Tsao, C. Yi, A. K. Chandiran, M. K. Nazeeruddin, E. W.-G. Diau, C.-Y. Yeh, S. M. Zakeeruddin and M. Grätzel, Porphyrin-sensitized solar cells with cobalt (II/III)–based redox electrolyte exceed 12 percent efficiency, Science, 2011, 334(6056), 629–634 CrossRef CAS PubMed.
- A. Yella, R. Humphry-Baker, B. F. E. Curchod, N. Ashari Astani, J. Teuscher, L. E. Polander, S. Mathew, J.-E. Moser, I. Tavernelli and U. Rothlisberger, et al., Molecular Engineering of a Fluorene Donor for Dye-Sensitized Solar Cells, Chem. Mater., 2013, 25(13), 2733–2739, DOI:10.1021/cm401593b.
- G. Cassone, G. Calogero, J. Sponer and F. Saija, Mobilities of iodide anions in aqueous solutions for applications in natural dye-sensitized solar cells, Phys. Chem. Chem. Phys., 2018, 20(18), 13038–13046 RSC.
- G. Cassone, F. Creazzo, P. V. Giaquinta, F. Saija and A. M. Saitta, Ab initio molecular dynamics study of an aqueous NaCl solution under an electric field, Phys. Chem. Chem. Phys., 2016, 18(33), 23164–23173 RSC.
- G. Cassone, F. Creazzo, P. V. Giaquinta, J. Sponer and F. Saija, Ionic diffusion and proton transfer in aqueous solutions of alkali metal salts, Phys. Chem. Chem. Phys., 2017, 19(31), 20420–20429 RSC.
- D. Nguyen, et al., Choline chloride-based deep eutectic solvents as effective electrolytes for dye-sensitized solar cells, RSC Adv., 2021, 11(35), 21560–21566 RSC.
- Y. Nahar and S. C. Thickett, Greener, Faster, Stronger: The Benefits of Deep Eutectic Solvents in Polymer and Materials Science, Polymers, 2021, 13(3), 447 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |