Dual-ion conductors: from liquid to solid
Tao
Yu
ab,
Wenjie
Ning
ab,
Haoyu
Li
ab,
Shaohua
Guo
 *ab and
Haoshen
Zhou
*ab and
Haoshen
Zhou
 *a
*a
aCollege of Engineering and Applied Sciences, Jiangsu Key Laboratory of Artificial Functional Materials, National Laboratory of Solid State Microstructures, Collaborative Innovation Center of Advanced Microstructures, Frontiers Science Center for Critical Earth Material Cycling, Nanjing University, Nanjing, 210023, China. E-mail: shguo@nju.edu.cn; hszhou@nju.edu.cn
bLab of Power and Energy Storage Batteries, Shenzhen Research Institute of Nanjing University, Shenzhen, 518057, P. R. China
First published on 5th March 2024
Abstract
The traditional working principle within lithium-ion batteries relies on Li+ shuttling between the cathode and anode, namely the rocking-chair mechanism. A single working ion constrains the possibilities for battery design and the selection of electrode materials, while realizing multiple working ions offers the potential to break through the fundamental principles of traditional battery construction. Accordingly, it is necessary to develop dual-ion conductors to enable the migration of multiple working ions. This focus article starts by introducing traditional dual-ion batteries based on liquid electrolytes and their pros and cons. Then, solidifying liquid dual-ion conductors is expected to overcome these drawbacks, so the development of solid dual-ion conductors is discussed in detail. Specifically, basic design principles of solid dual-ion conductors are briefly proposed, including constructing continuous ion transport channels and choosing appropriately sized ion carriers. The potential applications of solid dual-ion conductors are also summarized, such as stabilizing the electrode/electrolyte interface and activating additional redox couples. The goal of this article is to inspire researchers in the development of dual-ion conductors and to contribute to the advancement of all-solid-state batteries.
Introduction
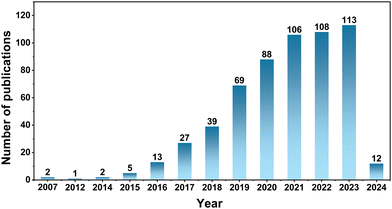
Due to the widespread use of portable devices, the application of secondary batteries has also become increasingly common.1,2 At present, single charge carrier-based batteries, especially Li+-based, are the most widely used and studied systems, while relying on single ion carrier provides stringent requirements for the selection of both cathode and anode materials. To increase the flexibility of battery design, researchers have developed a series of dual-ion batteries based on two carriers, where the two carriers will participate in electrochemical reactions of the cathode and anode materials.3–5 The dual-ion strategy could expand the range of electrode materials, introduce new electrochemical mechanisms and take advantage of different carriers. As a key part in the secondary batteries with various working principles, the electrolyte determines the successful operation of the electrodes and the realization of promising performance. Thus, the crucial prerequisite for constructing dual-ion batteries is designing suitable dual-ion conductors which can transport two types of charge carriers.Dual-ion batteries based on liquid electrolytes have been made practical through introducing the corresponding soluble salts, such as Li+–PF6−, K+–PF6− and Li+–Cu2+ into the dual-ion batteries.4,6,7 However, the anions and cations in liquid electrolytes can move freely, which will affect the compatibility between the electrolyte and the electrode.8 Solid electrolytes, with their unique ion transfer mechanism, can restrict the area of ion transport, thereby avoiding ion shuttle effects and consequently reducing interface side reactions.9Fig. 1 exhibits the timeline of the development of dual-ion batteries. In 2007, West et al. discovered that fluoride-anion receptor complexes can reversibly intercalate into graphite layers, laying the groundwork for the development of liquid dual-ion conductors.10 Subsequently, researchers have gradually initiated studies on dual-ion batteries. Recent statistics on the number of related papers are shown in Fig. 2, indicating the strong interest of researchers in dual-ion batteries.
 | ||
| Fig. 1 The timeline of the development of dual-ion batteries.3–5,7,10–25 | ||
In our recent work, we constructed a Li+/Cu+ dual-ion conductor based on solid electrolytes for the first time.26 The universal anion framework of the dual-ion conductor enables the rapid transport of Li+ and Cu+, which is the critical foundation for a dual-ion conductor. Thanks to the ion-hopping transport mechanism of solid electrolytes,9 dual-ion conductors can restrict the range of ion transport, and thus dual-ion conductors can prevent the ion crossover between the cathode and anode materials.
In this focus article, we primarily outlined the development of dual-ion conductors and briefly analyzed the advantages and disadvantages of liquid and solid dual-ion conductors. Additionally, we analyzed the design principles of solid dual-ion conductors, emphasizing the importance of matching ion radius with ion transport channels. The potential applications of solid dual-ion conductors are also discussed, which can improve the ion transport at the interface between electrolytes and electrodes and activate the extra redox reaction of active materials.
Batteries based on a liquid dual-ion conductor
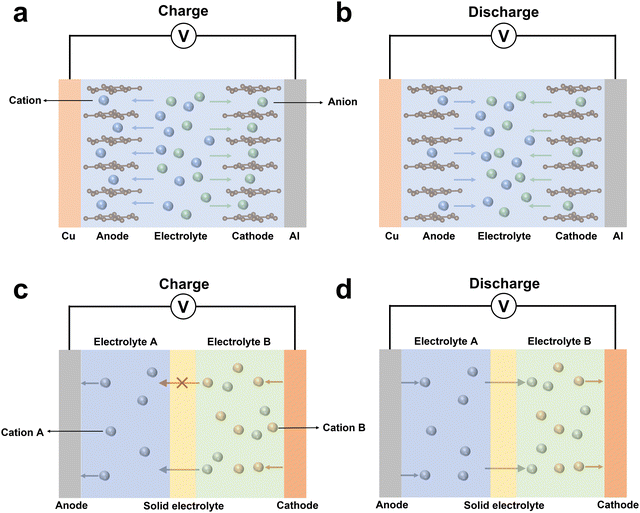
Over the past decade, various dual-ion batteries have been developed using liquid electrolytes, which can be divided into two types based on the working ions.7,27,28 The working ions for the first type of dual-ion batteries consist of anions and cations, while the working ions consist of two types of cations for the other dual-ion batteries.For the first type of dual-ion batteries based on anions and cations, the fundamental working principle is shown in Fig. 3a and b.29 During the charging process, the anions and cations in the electrolyte are embedded into the cathode and anode, respectively. And during discharge, the embedded anions and cations can be released from the cathode and anode. The anode materials are analogous to the ones in conventional “rocking-chair” batteries, while the cathode materials are required to enable the insertion of anions. This type of battery is advantageous due to its high discharge voltage and low cost, as it does not require expensive transition metals. However, it also has unavoidable shortcomings, including the large amount of electrolyte needed and poor cycling stability.
For the second type of dual-ion battery, the working ions consist of two cations (Fig. 3c and d), in which one is usually an alkali metal ion, while the other is a transition metal ion such as Cu2+.8 These batteries typically require using composite electrolytes to block direct contact between the transition metal ions and anode materials, in order to prevent serious side reactions between the two.7,8 Only alkali metal ions are allowed to pass through the solid electrolyte layer in the composite electrolytes. This type of battery can release a significantly large specific capacity, as demonstrated by previous research papers. Wang et al. achieved the Cu2+/Cu redox reaction with a specific capacity over 800 mA h g−1.8 However, its composite structure limits its practical application.
The design criteria for solid dual-ion conductors
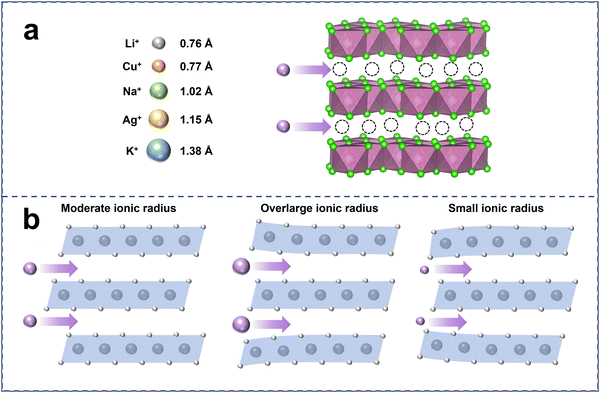
Solidifying liquid dual-ion conductors can localize certain ions in specific regions, thereby reducing unwanted interfacial side reactions between the ions and the electrodes.30 However, building solid dual-ion conductors is far more challenging than building liquid dual-ion conductors. Due to the poor selectivity of polymer solid-state electrolytes for ion transport, they cannot completely hinder the interface side reactions between ions and the electrode. The ion transport mechanism in inorganic solid-state electrolytes leads to their excellent selectivity for ion transport,31 making them well-suited for use as dual-ion conductors to construct batteries and completely suppress side reactions between ions and electrodes.The ion transport in inorganic solid electrolytes requires continuous ion transport channels with appropriately sized charge carriers (Fig. 4a). Currently, most ion conductors are based on monovalent cations because higher valence cations experience excessive Coulomb interaction, making their transmission difficult. The five common cations suitable for ion transport are: Li+ (0.76 Å), Cu+ (0.77 Å), Na+ (1.02 Å), Ag+ (1.15 Å), and K+ (1.38 Å).32,33 The dual-ion conductor requires a shared anion framework for the simultaneous transport of two different cations. Therefore, the ion radius of these two cations should not differ significantly, as otherwise the anion framework of the electrolyte will be unable to accommodate both cations simultaneously.
Fig. 4b illustrates our understanding of the relationship between carrier ion radius and electrolyte framework. Suitable ion radius is essential for fast ion migration without affecting the electrolyte framework structure. Cations with overlarge ion radius can not only cause lattice distortion in electrolytes, but may also block the continuous ion transport channels, preventing the conduction of carrier ions with appropriate radius. A small ionic radius is also undesirable because it may not provide enough support for the lattice, leading to lattice collapse and subsequently damaging the ion transport channels. In our previous work, we successfully constructed a dual-ion conductor based on Li+ and Cu+, leveraging the close proximity of their ionic radii as a fundamental factor.26 Additionally, the electronegativity of the elements may be a crucial factor affecting ion transport. Cations not only experience the influence of coulombic forces within the electrolyte framework, but also undergo bonding interactions between ions. The difference in electronegativity between the cations and anions affects the strength of the ionic bonds between them, and thus a smaller electronegativity difference between them may result in lower ion migration barriers.
The potential applications for a solid dual-ion conductor
Solid dual-ion conductors have the capability to simultaneously transport multiple ions, making them more functional than traditional single-ion solid electrolytes. In this chapter, we will explore several potential applications of solid dual-ion conductors based on our understanding.Firstly, solid dual-ion conductors have the potential to improve the anode/electrolyte interface (Fig. 5a). These dual-ion conductors are capable of transporting monovalent transition metals, such as Cu+ and Ag+, which can chemically react with the anode materials to form an alloy modification layer. The alloy-modified layer has a higher ion surface migration rate, which can inhibit the growth of lithium dendrites.34 Additionally, it effectively reduces the reactivity of the lithium metal anode, thereby suppressing the interfacial side reactions between the lithium metal and the electrolyte.
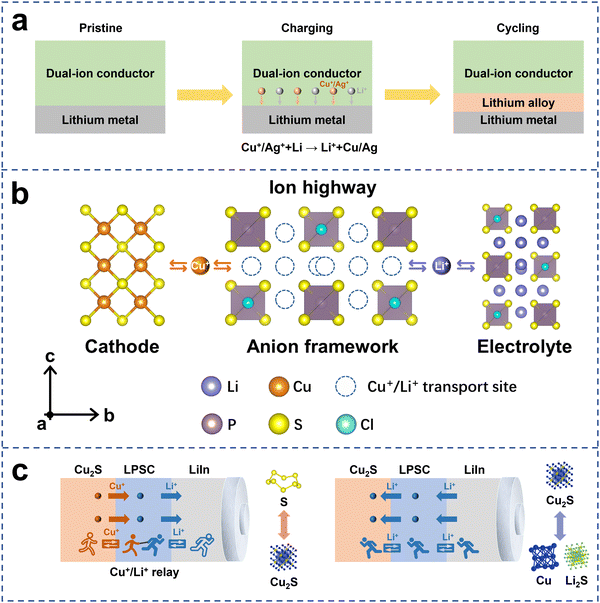 | ||
| Fig. 5 The potential applications for a solid dual-ion conductor. (a) Solid dual-ion conductors can improve the anode/electrolyte interface by constructing an in situ alloy modification layer. (b) Solid dual-ion conductors can improve ion transport at the cathode/electrolyte interface by constructing an ion highway that connects the active materials and the electrolytes. (c) Solid dual-ion conductors can activate additional redox reactions in some active materials through the migration of transition metal ions. Reproduced with permission.26 Copyright 2023, AAAS. | ||
Secondly, dual-ion conductors can also enhance the interfacial compatibility between the cathode active materials and electrolytes, improving ion transport at the interface and thereby significantly enhancing the kinetic performance of all-solid-state batteries (ASSBs).26 In our previous work, we constructed a dual-ion conductor to create an ion highway connecting the active material Cu2S with the electrolyte (Fig. 5b), increasing the number of mobile charge carriers and significantly enhancing the kinetic performance of all-solid-state batteries.
Thirdly, dual-ion conductors can activate additional redox reactions in some cathode active materials by facilitating the migration of transition metal ions, thereby significantly increasing the discharge specific capacity and energy density of all-solid-state batteries.26 In traditional single-ion electrolytes, the Cu2S cathode can only achieve the redox reaction of Cu+/Cu through the transport of lithium ions, resulting in limited discharge capacity. However, we successfully activated the valence change of S in the Cu2S cathode using a dual-ion conductor (Fig. 5c), thereby enabling a four-electron reaction in the Cu2S cathode and achieving an actual discharge capacity of over 600 mA h g−1.
Fig. 6 shows the electrochemical performance of ASSBs based on a Li+/Cu+ dual-ion conductor. First, the charge and discharge curve of the first three cycles (Fig. 6a) shows the excellent reversibility of the battery system and ultrahigh specific capacity (∼600 mA h g−1), in which there are two voltage platforms with similar specific capacity. Besides, due to the improved cathode/electrolyte interface via a dual-ion conductor, the ASSB displays a magnificent rate performance (Fig. 6b), in which the ASSB can offer a decent capacity (231.6 mA h g−1) under a superhigh current density (20 mA cm−2). The excellent kinetic performance of the ASSB endows the battery system with a low overpotential (Fig. 6c), which has obvious advantages compared with other multi-electron systems. Moreover, the cycling stability of this battery system is conspicuous (Fig. 6d), which can maintain 93.2% capacity after 1500 cycles.
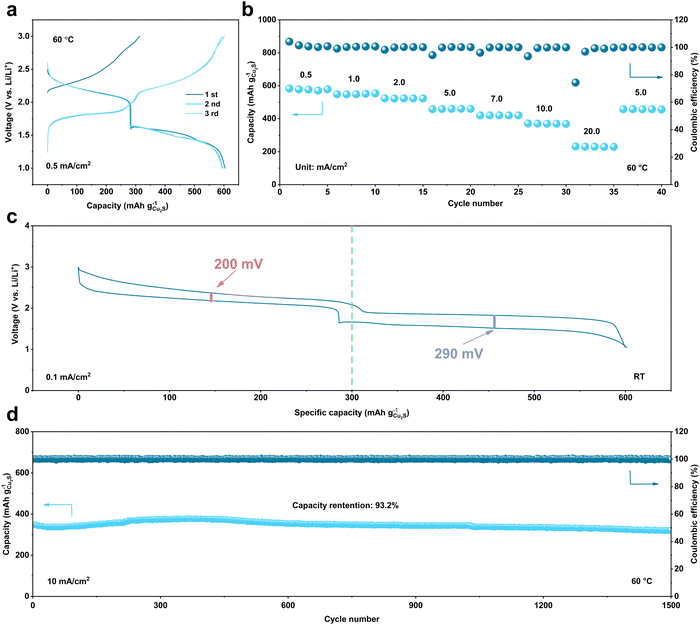 | ||
| Fig. 6 Electrochemical performance of the ASSBs based on a Li+/Cu+ dual-ion conductor. (a) The voltage curves of the first three cycles of LiIn‖Cu2S ASSBs under 0.5 mA cm−2 at 60 °C. (b) The rate performance of LiIn‖Cu2S ASSBs at 60 °C. (c) The overpotential curve of LiIn‖Cu2S ASSBs under 0.1 mA cm−2 at room temperature (RT). (d) The cycling performance of LiIn‖Cu2S ASSBs under 10 mA cm−2 at 60 °C. Reproduced with permission.26 Copyright 2023, AAAS. | ||
Conclusions and outlook
In this focus article, we mainly introduce the functional design of dual-ion electrolytes. We first discuss the development history of liquid dual-ion electrolytes from two categories based on their working ions, and then analyze the advantages and disadvantages of the corresponding batteries. Solid-state transformation of liquid batteries is a key method to enhance battery safety and energy density,35–38 but developing solid dual-ion conductors is much more difficult than liquid dual-ion conductors. Thus, based on our recent work, we discussed the design principle and potential application of solid-state dual-ion conductors.The construction of solid dual-ion conductors requires continuous ion transport channels and two types of charge carriers with suitable ion radii, which makes developing solid dual-ion conductors quite challenging.
In addition, the multifunctionality of solid dual-ion conductors offers numerous potential applications. For one thing, they are capable of in situ construction of alloy-modified layers, thus improving the anode/electrolyte interface. Solid dual-ion conductors can enhance ion transport at the cathode/electrolyte interface, thereby improving the kinetic performance of all-solid-state batteries. For another, they have the potential to activate additional redox couples in active materials by enabling the migration of transition metal ions, facilitating redox reactions that were previously inaccessible. This capability significantly increases the discharge specific capacity and energy density of all-solid-state batteries.
However, significant challenges must be overcome to achieve industrialization of such batteries. The primary requirements for industrialization include reducing the content of inactive electrochemical materials like electrolytes, and increasing the mass loading of the cathode layer. In this scenario, the electrolyte layer may not completely block the shuttling of Cu+ to the anode side, leading to irreversible side reactions with anode materials. Developing a single-ion conductor that exclusively transports lithium ions without transferring cuprous ions could be crucial in overcoming this challenge.
In summary, the research on solid dual-ion conductors is still in an early stage, and with continued efforts in this field, we will gain a deeper understanding of solid dual-ion conductors. We believe that the development of solid dual-ion conductors will provide new impetus for the advancement of all-solid-state batteries.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Key R&D Program of China (no. 2021YFB3800300), the National Natural Science Foundation of China (no. 22075132 and 22209069), the Natural Science Foundation of Jiangsu Province, China (no. BK20211556, BK20220783, and BK20230789), Jiangsu Province Carbon Peak and Neutrality Innovation Program (Industry tackling on prospect and key technology, no. BE2022002-2), the Shenzhen Science and Technology Innovation Committee (no. RCYX20200714114524165 and JCYJ20210324123002008), and Guangdong Basic and Applied Basic Research Foundation (2023A1515011437, 2022A1515110736 and 2022A1515010026). We also thank Fundamental Research Funds from the Central Universities and Frontiers Science Center for Critical Earth Material Cycling Fund. The authors are grateful to the High Performance Computing Center (HPCC) of Nanjing University for doing the numerical calculations in this paper on its blade cluster system.References
- M. Armand and J. M. Tarascon, Building better batteries, Nature, 2008, 451, 652–657 CrossRef CAS PubMed.
- J. M. Tarascon and M. Armand, Issues and challenges facing rechargeable lithium batteries, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- X. Zhang, Y. Tang, F. Zhang and C. S. Lee, A novel aluminum-graphite dual-ion battery, Adv. Energy Mater., 2016, 6, 1502588 CrossRef.
- B. Ji, F. Zhang, X. Song and Y. Tang, A novel potassium-ion-based dual-ion battery, Adv. Mater., 2017, 29, 1700519 CrossRef PubMed.
- M. Wang, C. Jiang, S. Zhang, X. Song, Y. Tang and H.-M. Cheng, Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage, Nat. Chem., 2018, 10, 667–672 CrossRef CAS PubMed.
- X. Tong, F. Zhang, B. Ji, M. Sheng and Y. Tang, Carbon-coated porous aluminum foil anode for high-rate, long-term cycling stability, and high energy density dual-ion batteries, Adv. Mater., 2016, 28, 9979–9985 CrossRef CAS PubMed.
- H. Wang, C. Wang, M. Zheng, J. Liang, M. Yang, X. Feng, X. Ren, D. Y. W. Yu, Y. Li and X. Sun, A shuttle-free solid-state Cu−Li battery based on a sandwich-structured electrolyte, Angew. Chem., Int. Ed., 2023, 62, e202214117 CrossRef CAS PubMed.
- Y. Wang and H. Zhou, A new type rechargeable lithium battery based on a Cu-cathode, Electrochem. Commun., 2009, 11, 1834–1837 CrossRef CAS.
- X. He, Y. Zhu and Y. Mo, Origin of fast ion diffusion in super-ionic conductors, Nat. Commun., 2017, 8, 15893 CrossRef CAS PubMed.
- W. C. West, J. F. Whitacre, N. Leifer, S. Greenbaum, M. Smart, R. Bugga, M. Blanco and S. R. Narayanan, Reversible intercalation of fluoride-anion receptor complexes in graphite, J. Electrochem. Soc., 2007, 154, A929 CrossRef CAS.
- T. Placke, O. Fromm, S. F. Lux, P. Bieker, S. Rothermel, H.-W. Meyer, S. Passerini and M. Winter, Reversible intercalation of bis(trifluoromethanesulfonyl)imide anions from an ionic liquid electrolyte into graphite for high performance dual-ion cells, J. Electrochem. Soc., 2012, 159, A1755–A1765 CrossRef CAS.
- M. L. Aubrey and J. R. Long, A dual-ion battery cathode via oxidative insertion of anions in a metal–organic framework, J. Am. Chem. Soc., 2015, 137, 13594–13602 CrossRef CAS PubMed.
- J. Gao, S. Tian, L. Qi and H. Wang, Intercalation manners of perchlorate anion into graphite electrode from organic solutions, Electrochim. Acta, 2015, 176, 22–27 CrossRef CAS.
- É. Deunf, P. Jiménez, D. Guyomard, F. Dolhem and P. Poizot, A dual-ion battery using diamino-rubicene as anion-inserting positive electrode material, Electrochem. Commun., 2016, 72, 64–68 CrossRef.
- X. Shi, W. Zhang, J. Wang, W. Zheng, K. Huang, H. Zhang, S. Feng and H. Chen, (EMIm)+(PF6)− ionic liquid unlocksoptimum energy/power density for architecture of nanocarbon-based dual-ion battery, Adv. Energy Mater., 2016, 6, 1601378 CrossRef.
- M. Sheng, F. Zhang, B. Ji, X. Tong and Y. Tang, A novel tin-graphite dual-ion battery based on sodium-ion electrolyte with high energy density, Adv. Energy Mater., 2017, 7, 1601963 CrossRef.
- S. Dong, Z. Li, I. A. Rodríguez-Pérez, H. Jiang, J. Lu, X. Zhang and X. Ji, A novel coronene//Na2Ti3O7 dual-ion battery, Nano Energy, 2017, 40, 233–239 CrossRef CAS.
- G. Chen, F. Zhang, Z. Zhou, J. Li and Y. Tang, A flexible dual-ion battery based on PVDF-HFP-modified gel polymer electrolyte with excellent cycling performance and superior rate capability, Adv. Energy Mater., 2018, 8, 1801219 CrossRef.
- X. Wu, Y. Xu, C. Zhang, D. P. Leonard, A. Markir, J. Lu and X. Ji, Reverse dual-ion battery via a ZnCl2 water-in-salt electrolyte, J. Am. Chem. Soc., 2019, 141, 6338–6344 CrossRef CAS PubMed.
- A. Yu, Q. Pan, M. Zhang, D. Xie and Y. Tang, Fast rate and long life potassium-ion based dual-ion battery through 3D porous organic negative electrode, Adv. Funct. Mater., 2020, 30, 2001440 CrossRef CAS.
- Z. Li, X. Li and W. Zhang, A high-performance graphite-graphite dual ion battery based on AlCl3/NaCl molten salts, J. Power Sources, 2020, 475, 228628 CrossRef CAS.
- S. Wang, Z. Yuan, X. Zhang, S. Bi, Z. Zhou, J. Tian, Q. Zhang and Z. Niu, Non-metal ion co-insertion chemistry in aqueous Zn/MnO2 batteries, Angew. Chem., Int. Ed., 2021, 60, 7056–7060 CrossRef CAS PubMed.
- Z. Huang, Y. Hou, T. Wang, Y. Zhao, G. Liang, X. Li, Y. Guo, Q. Yang, Z. Chen, Q. Li, L. Ma, J. Fan and C. Zhi, Manipulating anion intercalation enables a high-voltage aqueous dual ion battery, Nat. Commun., 2021, 12, 3106 CrossRef CAS PubMed.
- J. Li, C. Han, X. Ou and Y. Tang, Concentrated electrolyte for high-performance Ca-ion battery based on organic anode and graphite Cathode, Angew. Chem., Int. Ed., 2022, 61, e202116668 CrossRef CAS PubMed.
- G. Wang, M. Zhu, G. Chen, Z. Qu, B. Kohn, U. Scheler, X. Chu, Y. Fu, O. G. Schmidt and X. Feng, An anode-free Zn–graphite battery, Adv. Mater., 2022, 34, 2201957 CrossRef CAS PubMed.
- T. Yu, H. Li, Y. Liu, J. Li, J. Tian, Z. Liu, Y. Rao, S. Guo and H. Zhou, A prototype of dual-ion conductor for all-solid-state lithium batteries, Sci. Adv., 2023, 9, eadj8171 CrossRef CAS PubMed.
- D. Zhang, K. Yan, F. Wu and C. Zhang, A high power density dual-electrolyte lithium-silver battery with Celgard® 2325 separator, Electrochim. Acta, 2014, 116, 429–433 CrossRef CAS.
- M. Wang and Y. Tang, A review on the features and progress of dual-ion batteries, Adv. Energy Mater., 2018, 8, 1703320 CrossRef.
- T. Placke, A. Heckmann, R. Schmuch, P. Meister, K. Beltrop and M. Winter, Perspective on performance, cost, and technical challenges for practical dual-ion batteries, Joule, 2018, 2, 2528–2550 CrossRef CAS.
- Z. Cheng, H. Pan, F. Li, C. Duan, H. Liu, H. Zhong, C. Sheng, G. Hou, P. He and H. Zhou, Achieving long cycle life for all-solid-state rechargeable Li-I2 battery by a confined dissolution strategy, Nat. Commun., 2022, 13, 125 CrossRef CAS PubMed.
- Y. Wang, W. D. Richards, S. P. Ong, L. J. Miara, J. C. Kim, Y. Mo and G. Ceder, Design principles for solid-state lithium superionic conductors, Nat. Mater., 2015, 14, 1026–1031 CrossRef CAS PubMed.
- X. Li, Y. Xu, C. Zhao, D. Wu, L. Wang, M. Zheng, X. Han, S. Zhang, J. Yue, B. Xiao, W. Xiao, L. Wang, T. Mei, M. Gu, J. Liang and X. Sun, The universal super cation-conductivity in multiple-cation mixed chloride solid-state electrolytes, Angew. Chem., Int. Ed., 2023, 62, e202306433 CrossRef CAS PubMed.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32, 751–767 CrossRef.
- J. Wan, Y.-X. Song, W.-P. Chen, H.-J. Guo, Y. Shi, Y.-J. Guo, J.-L. Shi, Y.-G. Guo, F.-F. Jia, F.-Y. Wang, R. Wen and L.-J. Wan, Micromechanism in all-solid-state alloy-metal batteries: regulating homogeneous lithium precipitation and flexible solid electrolyte interphase evolution, J. Am. Chem. Soc., 2020, 143, 839–848 CrossRef PubMed.
- H. Pan, M. Zhang, Z. Cheng, H. Jiang, J. Yang, P. Wang, P. He and H. Zhou, Carbon-free and binder-free Li–Al alloy anode enabling an all-solid-state Li–S battery with high energy and stability, Sci. Adv., 2022, 8, eabn4372 CrossRef CAS PubMed.
- T. Yu, B. Ke, H. Li, S. Guo and H. Zhou, Recent advances in sulfide electrolytes toward high specific energy solid-state lithium batteries, Mater. Chem. Front., 2021, 5, 4892–4911 RSC.
- W. G. Z. Jürgen Janek, Challenges in speeding up solid-state battery development, Nat. Energy, 2023, 8, 230–240 CrossRef.
- Y. Liu, T. Yu, S. Guo and H. Zhou, Designing high-performance sulfide-based all-solid-state lithium batteries: from laboratory to practical application, Acta Phys. - Chim. Sin., 2023, 39, 2301027 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |