Open Access Article
Open Access ArticleCarbon nanomaterials as carriers for the anti-cancer drug doxorubicin: a review on theoretical and experimental studies
K.
Gayathri
 ab and
R.
Vidya
ab and
R.
Vidya
 *ab
*ab
aCentre for Materials Informatics(C-mAIn), Sir. C.V. Raman Science Block, Anna University, Sardar Patel Road, Guindy, Chennai 600 025, India. E-mail: vidyar@annauniv.edu; vidyapl.ravi@gmail.com
bDepartment of Physics, Anna University, Sardar Patel Road, Guindy, Chennai 600 025, India
First published on 26th April 2024
Abstract
The incidence of cancer is increasing worldwide in a life-threatening manner. In such a scenario, the development of anti-cancer drugs with minimal side effects and effective drug delivery systems is of paramount importance. Doxorubicin (DOX) is one of the powerful anti-cancer drugs from the chemical family anthracycline, which is used to treat a wide variety of cancers, including breast, prostate, ovarian, and hematological malignancies. However, DOX has been associated with many side effects, including lethal cardiotoxicity, baldness, gastrointestinal disturbances and cognitive function impairment. Even though DOX is administered in liposomal formulations to reduce its toxicity and enhance its therapeutic profile, the liposomal formulations themselves have certain therapeutic profile limitations such as “palmar-plantar erythrodysesthesia (PPE)”, which shows severe swelling and redness in the skin, thus restricting the dosage and reducing patient compliance. In contemporary chemotherapy research, there is a great interest in the utilization of nanomaterials for precise and targeted drug delivery applications, especially using carbon-based nanomaterials. This review provides a comprehensive overview of both experimental and theoretical scientific works, exploring diverse forms of carbon-based materials such as graphene, graphene oxide, and carbon nanotubes that function as carriers for DOX. In addition, the review consolidates information on the fate of the carriers after the delivery of the payload at the site of action through different imaging techniques and the various pathways through which the body eliminates these nanomaterials. In conclusion, the review presents a detailed overview of the toxicities associated with these carriers within the human body, contributing to the development of enhanced drug delivery systems.
Introduction
In recent years, anti-cancer drugs have been among the top-selling drugs in the market, which indicates the severity of cancer on a global scale. Oncology drugs always have an increasing trends in drug approval.1 Among the 37 novel drugs that were approved by the Food and Drug Administration (FDA) in 2022, 10 are oncology drugs, accounting for 27% of the overall approval. Cancer is a group of more than hundreds of diseases in multi-cellular organisms and is characterized by the abnormal growth of cells due to the alteration or mutations in gene sequences leading to the dysregulation of cell death and proliferation, which ends up in the formation of a group of cells that can invade, metastasize distant organs and cause significant morbidity in the host. The metastatic potential, invasiveness, and morphology of the different cancers are also different, which makes the prognosis of the disease harder.2 The incidence of cancer cases around the world is increasing rapidly, especially in India. It is also predicted to increase from 1.46 million in 2022 to 1.57 million in 2025. Among the different types of cancers, breast, cervical, and ovarian cancers are the top three cancers with the highest incidence in females, whereas lung, mouth, and prostate cancers are the ones with the highest incidence in males. Mouth, tongue, and lung cancers are usually related to tobacco usage. Overall cancer statistics around the world suggest that the detection of carcinogens, diagnosis of the disease in earlier stages, and development of appropriate treatment modalities are essential to fight against this deadly disease.3 Researchers around the world are focusing on finding new therapeutics and ways to deliver them to the diseased regions in the body and developing effective drug delivery systems. Doxorubicin (DOX), with the brand name adriamycin, is an effective anti-neoplastic drug administered for a variety of cancers. Many different nanomaterials have been proposed as carriers for DOX in the past few decades. Here, we have given a consolidated report of theoretical and experimental scientific works on carbon-based drug delivery systems for DOX in cancer therapy (Fig. 1).Story of doxorubicin from soil bacteria to therapeutics and survival in medical history
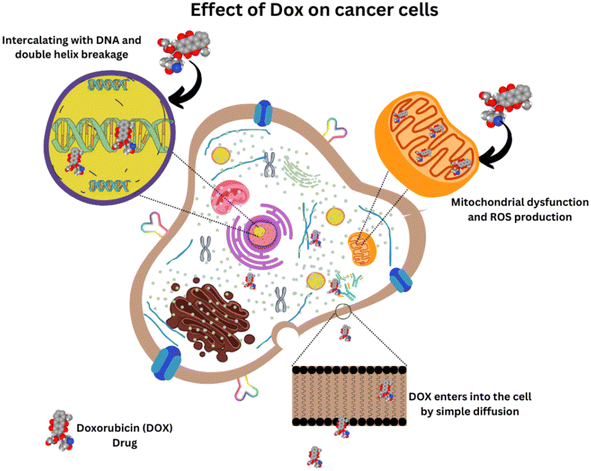
DOX is one of the interesting and effective anthracycline drugs, which is administered for different types of cancer, including breast, ovarian, cervical, bladder, and also for haematological malignancies via an intravenous route. DOX was extracted from Streptomyces peucetius var. caesius in 1970 and appeared as a red-colored product. Even though the entire mechanism of action of the drug is under debate, it has been found to kill cancer cells by intercalating with DNA and inducing an apoptosis mechanism in cancer tissues.4–6 A molecular dynamics study by Esra Şahin Akdenizet et al., in which the interaction of DOX with purine bases was analysed using Density Functional Theory (DFT) and Molecular Dynamics (MD) simulations in an aqueous phase, showed that the complexation energy of DOX with purine bases have negative values, indicating that the complex formation is spontaneous, and stable interactions are present in the system, which justifies the proposed mechanism of DOX toxicity.7 Unfortunately, its clinical uses are restricted due to its terrible side effects like acute, lethal, age and dose-dependent cardiotoxicity with 50% mortality rate, neurological disturbances by affecting the cognitive function of the brain and causing toxicities in the kidney, liver, etc., and also drug resistance like most of the chemotherapeutic drugs. However, the co-administration of other drugs helped to reduce its drug toxicity.8Many of the drugs, including DOX, fail to penetrate inside the tumor cells and reach only the periphery of the cancer islets. The cancer tissues obtained from incision biopsy proved to have heterogeneous drug distribution revealed by autofluorescence imaging techniques and the drug rarely eliminates all the cancer cells in the patients diagnosed with locally advanced breast cancer.9 Drug resistance is one of the major problems encountered by many drugs in the body, which is mostly due to the drug efflux proteins expressed in the membrane of the cell. The interaction study of DOX with a model cell membrane in the presence and absence of highly ordered cholesterol regions revealed that the drug resistance mechanism against DOX is due to its affinity towards the highly ordered cholesterol-rich environment where the efflux protein is present.10
An effective drug delivery system that can localize itself in the tumor region with minimized side effects has been the focus of scientists around the world. In particular, nanomaterials can effectively accumulate in the tumor microenvironment by utilizing the Enhanced Permeation and Retention (EPR) effect due to the leaky vasculature near the tumor and the insufficient lymphatic drainage in the tumor.11 Further, this review explores the possible ways of delivering this effective drug to the tumor region by utilizing the amazing properties of nanomaterials, especially multi-functional carbon nanomaterials.
General approaches for efficient drug delivery
Before starting with the carbon-based drug delivery systems, we recollect the other formulations in DOX drug delivery. To achieve the controlled and sustained drug release, which increases the therapeutic window of the drug candidate, different strategies have been followed. PEGylation is one such strategy to enhance the efficacy of the drug. PEG (Poly Ethylene Glycol) is a hydrophilic polymer that has been widely used as a conjugating ligand in many drug delivery systems for prolonged systemic circulation of the drug, reduced uptake by Reticuloendothelial system (RES) and reduced protein immunogenicity.12 Generally, DOX is available in three different formulations known as (i) PEGylated liposomal DOX(PLD), (ii) non-pegylated liposomal DOX(NPLD) and (iii) non-liposomal DOX formulations. PEGylated liposomal DOX is known as Doxil/Caelyx. In this formulation, DOX is encapsulated in liposomes and coated further with PEG chains, keeping the drug encapsulated until it reaches the target site and proved to have prolonged circulation and decreased uptake by RES and was approved by FDA. However, this formulation has “palmar-plantar erythrodysesthesia (PPE)” or “foot and hand syndrome”, whose severity increases with dosage. One of the types of NPLD, known as Myocet, has a specific composition of liposomes and a unique manufacturing process without any PEG coating. This offers the benefits of Doxil without major side effects and possesses a better pharmacological profile than Doxil and conventional DOX. Nowadays, Myocet is used for metastatic breast cancer. The performance of NPLD was better than that of the free drug and had reduced cardiotoxicity. But, equal doses of Myocet and another anthracycline drug, Epirubicin, showed no difference in cardiotoxicity. Non-liposomal DOX formulation has a large volume of distribution (Vd), which leads to the accumulation of a significant amount of drug in normal tissues, resulting in side effects.13,14 In another formulation, DOX conjugated to PEG via cleavable disulfide linkages, which self-assembled in micelles, are reported to have good drug loading efficiency, better tumor inhibition in MCF-7 cell lines, and controlled release profiles.15Gold nanoparticles are also often used in drug delivery systems. Hanane Moustaoui et al. experimentally synthesized stable DOX-Au nanoparticles coated with biocompatible PEG polymers and further linked with polygonal antibodies, which target human pancreatic cancer cells and showed drug release in acidic environments.16
Nanomaterials in drug delivery
Generally, all drugs suffer from a lot of issues from the time of administration to the time of reaching their site of action, including the difficulties in crossing biological barriers like plasma membrane, nuclear membrane, development of Multi-Drug Resistance (MDR) condition where the tumor becomes irresponsive to drugs (not only for structurally similar drugs but also for a wide class of drugs), clearance by the liver and kidney via the Reticulo-Endothelial System (RES).17 Since most anticancer drugs, including DOX, target the genetic material to get the fullest of its therapeutic potential, enhanced circulation, strategies to multidrug resistance mechanism in the tumor region, and increased half-lives are necessary. PEGylation is an important strategy to enhance the circulation period and reduce the clearance of the drug from the body.18 Multi-functional nanomaterial-based delivery of drugs can achieve prolonged circulation, specificity towards the target, and reduced off-target effects. They can also induce an immune response in cancer cells after accumulating in them. Usually, an average measurement scheme known as the hydrodynamic radius is used to denote the size of macromolecules like proteins when they drift through fluids.19 When the hydrodynamic radius is increased approximately to 10 nm by grafting the drug carrier conjugates with biocompatible polymers like PEG and chitosan, the above-mentioned issues can be reduced to a certain extent. These colloidal objects can be easily accumulated in the tumor environment through their leaky vasculature, as described in the EPR effect.11,20,21Importantly, the attenuation of the resistance of cancer cells towards drugs can be achieved by combining a wide variety of functionalized nanomaterials with conventional drugs. Multifunctional nanomaterials have the potential to overcome drug resistance using increased half-lives in the body and delivering multiple drugs (as the resistance to drugs is the result of simultaneous activation of various pathways in cancer cells) at the tumor site and blocking various pathways involved in the MDR mechanism.22,23 The MDR mechanism is reported to be the result of the following events in tumor cells, as stated here: reduced drug uptake, increased efflux, activation of DNA repair mechanism, inherent detoxification mechanism of the body, inactivation of apoptotic pathways, and most importantly, the activation of anti-apoptotic defence mechanism. The nanomaterial-assisted delivery of drugs by means of encapsulation, attachment, and conjugation of therapeutic small molecules can pave the way to overcome MDR in tumors and enhance the efficacy of the treatment. Receptor-mediated endocytosis and attenuation of MDR by nanomaterials conjugated with ligands such as RGD (arginyl-glycyl-aspartic acid) peptide, Epidermal Growth Factor Receptor (EGFR), biotin, folate, and transferrin have been reported in the literature.24 The stem-cell-like nature of cancer cells plays a crucial role in MDR. A very recent study by Jinyuan Liu et al. has demonstrated a novel strategy to attenuate the stemness of the cancer cell, thereby reducing its drug resistance. They have prepared MoS2 nanosheets coated with protein A and Bovine Serum Albumin (BSA) and further coated with CD44 antibodies to target the CD44 receptor (biomarker for cancer stem cells and a highly expressed transmembrane protein in various cancers) on cancer stem cells. The nanosheets were incubated with a triple-negative breast cancer cell line (MDA-MB-231), followed by the laser treatment to attenuate the stemness by utilizing the photothermal conversion efficiency of MoS2 nanosheets. They found that this laser treatment has reduced stemness, invasive potential, and drug resistance. In order to understand the sensitivity and response of DOX, an analysis was carried out before and after the laser treatment. It was shown that the response of cancer cells to DOX has been increased, thereby enabling better clinical outcome.25 Graphene oxide (GO) and its derivatives functionalized with various biocompatible polymers like PEG and Dextran are shown to have increased cellular uptake, exhibiting potent cytotoxic effects and overcoming drug resistance by activating various apoptotic pathways.26
Combinatorial approaches
Combinatorial approaches have been followed to increase the effectiveness of the drug and reduce the side effects as well. Generally, there are a few drugs, such as Vincristine, Probucol, and Dexrazoxane, which are co-administered with DOX to reduce its side effects.8Especially, Dexrazoxane has been widely used against DOX-induced cardiotoxicity and tissue necrosis caused by accidental extravasation of DOX in patients. However, repeated administration in paediatric patients is reported to end up in second primary malignancies in rare cases.27 By combining prodrug strategy and combinatorial therapy, Yumin Zhang et al. have prepared PEG-conjugated DOX particles and encapsulated curcumin inside the core. The internalization and cellular uptake of nanoparticles (NPs) in HepG2 cell lines were confirmed by fluorescence imaging and flow cytometry. The cytotoxicity studies showed that the anti-tumour activity of PEG–DOX–Cur is more than that of free DOX, and free curcumin with enhanced tumour penetration is achieved using this complex, and the drug release is found to be pH dependent.28
Very recently, Ling Zhang et al. have done a theoretical study based on the Density Functional Tight Binding method (DFTB) to understand the loading and release of DOX with camptothecin (CPT), which is another powerful drug mainly used for colorectal cancer and studied the effect of chirality, diameter and molecular species on SWCNTs. The Gibbs free energy of solvation calculations showed that the CNT is more soluble after the drugs are adsorbed, implying that more specific drug delivery systems can be developed by this method.29 Computational simulations can play a major role in developing combinatorial drug delivery systems by offering the possibility of a wide range of combinations of drugs and carriers before starting an experiment.
Carbon-based materials for drug delivery
Many different nanoparticles have been proposed as drug delivery carriers in the last two decades for various life-threatening diseases, including cancer, Alzheimer's and rheumatoid disease, and even for gene delivery and anti-body delivery in some cases.30 These particles include liposomes, self-assembled micelles,31 protein–drug conjugates,32 dendrimers33 which are highly dense branched niosomes, polymeric nanoparticles, biodegradable polymers,34 hybrid nanoparticles and inorganic nanoparticles targeted via various routes including oral, intravenous, intramuscular, transdermal, pulmonary, nasal, ocular and vaginal routes depending upon the pharmacokinetics of the particles and disease state of the patient.34 Beyond these particles, magnetic nanoparticles,35 metal–organic frameworks,36 cell-based carriers that have excellent biocompatibility, low immunogenicity, and longer circulation periods17 are also available.Carbon is one of the most interesting elements in the periodic table, and it can exist in a wide variety of substances. Carbon, along with hydrogen, oxygen, nitrogen, and some other elements, forms most of the important biomolecules in our body, including, the genetic material and amino acids making up the proteins that are essential for the functioning of the cell.37 Carbon can form a wide variety of covalently bonded substances, including carbon nanotube (CNT), graphene family of materials and many organic molecules that are used as therapeutics. They possess excellent optical, mechanical, electrical properties, high aspect ratio, and multiple sites for drug loading.21,38
Among the different nanomaterials proposed as drug delivery carriers for cancer drugs, carbon-based materials like graphene, graphene oxide (GO), and fullerenes that are functionalized with various biocompatible polymers and biocompatible targeting moieties39 possess improved biocompatibility, dispersibility in an aqueous biological medium, reduced immunogenicity from the body and high loading capacity of various drugs.40 The carbon-based materials have also been proposed for imaging purposes.41–45
Theoretical studies on the drug carrier interactions
A plethora of computational approaches have been used in the property prediction of materials using different levels of theory, from molecular dynamics simulations to the accurate first principle density functional calculations. These techniques have their own restrictions in terms of accuracy and computational time. Interestingly, there always exists a trade-off between the computational cost and the accuracy; thus, different levels of theory employed in different methods can give different levels of accuracy. However, computational techniques have played a major role in predicting the properties of materials in the last few decades and provided theoretical insights for experimental observations. Computational techniques like DFT and MD simulations46,47 are able to give important predictions in the field of drug discovery as well.48 Here, the computational simulations to understand the interactions of DOX with different moieties are presented.Achieving a sustained drug release profile and increased circulation period is one of the major concerns in drug delivery systems. Computational investigations on the physiochemical properties of doxorubicin dialdehyde conjugated with starch nanoparticles revealed that this complex can increase the anti-cancer activity and water solubility of doxorubicin with sustained release of the drug.49 Bio conjugation is often used to deliver these drugs effectively. S. M. Hassini et al. have investigated the physiochemical properties of doxorubicin and daunorubicin conjugated with biocompatible polymer PEG and folate nanoparticles and found that these complexes have increased hydrophilicity than the free drug.50
Theoretical studies on the interaction of carbon nanomaterials (CNMs) with DOX
DOX with graphene and graphene oxide
Graphene and its derivatives are widely studied as the carrier for various anti-cancer drugs, which are two-dimensional systems with one atom thickness51 having more reaction sites for drug loading and surface modifications, yielding desired properties. GO has different functional groups like carboxyl, epoxide and hydroxyl groups that tend to have colloidal stability, unlike pristine graphene, which is highly hydrophobic and agglomerate in hydrophilic solvents.52 The preliminary DFT and Ab initio molecular dynamics studies on the interaction of pristine graphene and DOX have revealed that the DOX molecule is adsorbed on the graphene surface with a binding energy of 0.5 eV and the complex showed temperature-dependent release of the drug from the carrier.53Molecular dynamics studies to understand the interaction between the pristine and functionalized graphene sheets with the drug in varying oxygen densities and pH conditions showed that the drug diffusion coefficient increases with decreasing pH (the tumour region is usually acidic). This study revealed that functionalized graphene can be used to design potential drug delivery systems.54
M. M. Mirhosseini et al. have studied the interaction of DOX with pristine and functionalized forms of graphene with four different functional groups like hydroxyl (–OH), carboxyl (–COOH), methyl (–CH3) and amine (–NH2) groups and also studied the effect of porosity in the drug loading capacity of the carrier. The carboxyl functionalized system showed intense interaction with the drug, and they have also concluded that the solubility and binding energy are temperature dependent and the importance of functionalization is due to the fact that pristine graphene may form a chemical bond which could possibly deactivate or change the properties of the drug.55 Different chitosan functionalized nanocarriers have been proven to be pH-responsive and have high drug-loading capacities. J. W. Shen et al. have investigated the drug loading and release behaviour of graphene non-covalently functionalized with a biocompatible polymer, chitosan, using molecular dynamics simulations and found that drug adsorption and release profile of the drug is dependent on the various protonation states of amine groups found in chitosan binding on the graphene surface. This pH responsive drug loading and release profile can lead to novel graphene-chitosan based drug delivery systems.56
Even though the influence of different functional groups on the binding energy of DOX and GO has been investigated in previous works,48 the affinity of DOX towards pristine graphene and GO with oxygen-containing functional groups (hydroxyl and epoxide) is investigated using DFT and MD simulations.
Graphene displayed more binding affinity for DOX since it contains more reactive sites. Among the functionalized systems, GO functionalized only with the hydroxyl group exhibits more binding affinity for the drug, followed by GO with hydroxyl and epoxy and GO with epoxy alone (Fig. 2). The results suggest that increased oxygen density on the surface affects the binding affinity of the drug, and optimization of functional groups renders controllability over drug loading, which can lead to a better drug delivery system.57
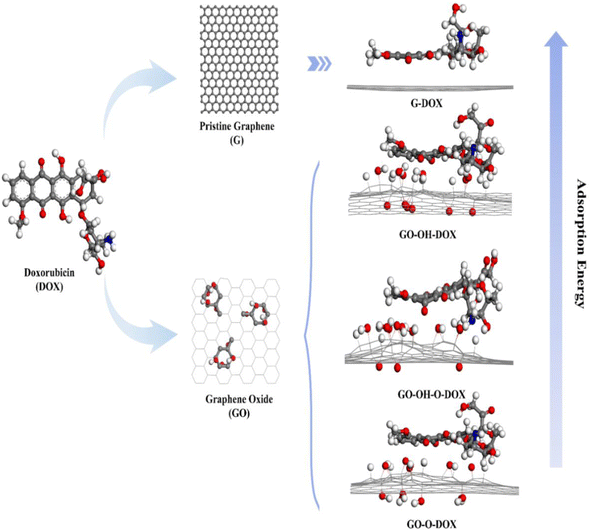 | ||
| Fig. 2 A schematic representation of the binding affinity of DOX with graphene and its derivatives. “Reprinted with permission from J. Song et al., “Controllability of graphene oxide doxorubicin loading capacity based on density functional theory”, Nanomaterials, vol. 12, no. 3, pp. 1–10, 2022, doi: https://doi.org/10.3390/nano12030479. Copyright license link: https://creativecommons.org/licenses/by/4.0/”. | ||
The poly-dispersity index is an important parameter in determining the properties of polymers. Even though many studies are available on graphene and GO conjugated with PEG polymers, Mina Mahdavi et al. have investigated the loading of DOX on GO surface conjugated with PEG chains (PEGGO) of different lengths and their effect on the adsorption pattern of the drug on the surface of the carrier (Fig. 3). During the adsorption of the drug on GO surface, uniform distribution on the surface and minimal self-aggregation of the drug is observed. In DOX/sh-PEGGO(sh-short) system, due to the reduced surface area, most of the drug molecules tend to move to the corner of the sheet and interact with carboxylic functional groups and an increase in self-aggregation is found and only a few drug molecules interact with PEG chains. But, when the length of the PEG chains is increased, more DOX molecules tend to interact with PEG chains. Here, due to the reduced carrier surface, the long polymer chains are capable of trapping drug molecules in them. These different distributions may lead to the development of an efficient drug delivery system using PEG conjugation of varying chain lengths. These simulations have been carried out in an aqueous environment, with a pH level of 7.4 at 310 K, to mimic the human body conditions.58
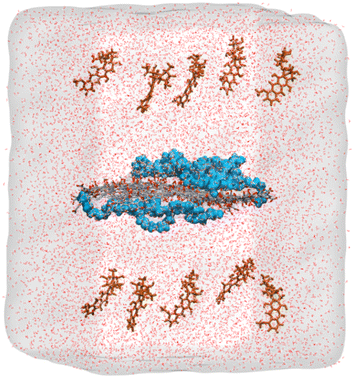 | ||
| Fig. 3 Initial configuration of the molecules inside the simulation box containing water solvated GO embedded with short PEG chains and drug molecules. DOX molecules on both sides of the carrier, which is placed in the middle of the box and PEG chains on both sides of the GO sheet are represented as brown and blue colors, respectively. Oxygen and carbon atoms in GO are represented in red and grey, respectively. “Reprinted with permission from M. Mahdavi, A. Fattahi, E. Tajkhorshid, and S. Nouranian, “Molecular insights into the loading and dynamics of doxorubicin on PEGylated graphene oxide nanocarriers”, ACS Appl. Bio Mater., vol. 3, no. 3, pp. 1354–1363, 2020, doi: https://doi.org/10.1021/acsabm.9b00956. Copyright-2023-American Chemical Society”. | ||
DOX delivery with carbon nanotube
Even though the flat and one-atom-thick layered materials like graphene and GO have the advantages of a high surface-to-volume ratio and more sites for functionalization over the CNTs, the CNTs have a needle-like structure that enhances their permeability in the cell membrane, which is not constrained by the type of functional groups on CNT and type of cells being targeted.38,59Tahereh Arabian et al. have investigated the adsorption mechanism of DOX on CNT functionalized with folic acid, which is an important targeting moiety and tryptophan, using MD simulations at two different pH conditions representing the normal and tumour environment. From the Solvent Accessible Surface Area (SASA) and solvation free energy calculations, it was found that the surface functionalization increases the hydrogen bond interaction of the carrier with water molecules, which gives better solubility in the aqueous phase, and the adsorption energy calculations showed that the drug molecules are adsorbed on the surface of the functionalized CNT(f-CNT). The system is proved to have pH-responsive drug release behaviour.60
S. Karimzadeh et al. have investigated the ability of pristine, carboxyl and hydroxyl functionalized carbon nanotubes to carry DOX molecules in aqueous and gaseous environments using the DFT calculations. The most stable configurations of free drugs and complexes of drugs formed with pristine and functionalized CNTs are optimized. The structural analysis of the complex suggests that CNT can act as a carrier and protect the drug from degradation. The negative binding energies of the complexes showed that the complex formation is an exothermic reaction in both phases. The Molecular Electrostatic Potential (MEP) surface provides a visualization of the charge states of the complexes. This work also concludes that the drug adsorption process is more favorable in functionalized carriers than in the pristine form.61
Jianghao Zhao et al. have studied the effect of the curvature of CNTs, surface modification with different functional groups and doping with boron and nitrogen atoms on drug adsorption properties of CNTs by having benzene as a model drug since it possesses the main chemical fragment of DOX using quantum chemical calculations employing Dispersion corrected Density Functional Theory (D-DFT) and PBE functional proposed by Perdew, Burke and Ernzerhof to accurately describe the molecular interactions. They reported that the interactions between the systems are stronger in functionalized carriers than in the pristine form and even stronger in doped systems. Adsorption energy calculations showed that doping leads to stable drug carrier interactions due to the introduction of new energy levels in the system. These findings may lead to the development of better CNT-based drug delivery systems for anthracycline. However, the simulation, including the entire fragment of the drug, will yield more accurate results compared to the single fragment.62
In most DFT and MD simulations, only a limited number of drug molecules on the surface are simulated to understand their adsorption behavior. There is a need to understand the adsorption behavior of molecules when the number of molecules in the simulation system increases.
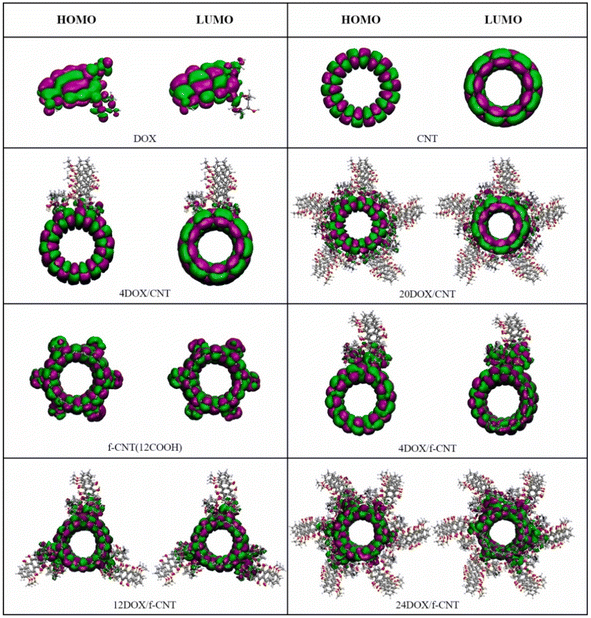
Cuihong Wang et al. have investigated the adsorption behavior by varying the number of drug molecules with a higher limit of 20 DOX molecules on pristine CNT and 24 DOX molecules on carboxyl functionalized CNT (f-CNT) using Density Functional Tight Binding (DFTB) method. The interaction energies showed that the adsorption of DOX on f-CNT is more favorable than that on CNT, and the f-CNT and DOX complex is more stable when the number of DOX molecules is increased with increasing hydrogen bonds (Fig. 3). The results support the high loading capacity of f-CNTs. Further MD simulations of these complexes confirm the stability at physiological conditions. Thus, this work sheds light on the interaction mechanism of the increased number of drug molecules on carriers to understand the practical condition of the problem (Fig. 4).63
Majid Pakdel et al. have studied the adsorption and encapsulation of DOX in Single-Walled CNTs (SWCNTs) of different diameters. They simulated the permeability of the loaded carrier through the POPC(1-palmitoyl-2-oleoylphosphatidylcholine) lipid membrane using the GROMACS5.1.2 MD Simulation package. The authors also studied the effect of nicotine molecules on the penetration of the loaded carrier through the membrane. They have concluded that DOX encapsulation efficiency increases with the increase in the diameter of SWCNT. The adsorption on the outside and inside of the SWCNT is mainly due to the π–π stacking interactions, which is mainly because of the presence of aromatic rings in the drug. The investigation of the effect of nicotine concentration outside the membrane on the permeability of the loaded carrier showed that the presence of nicotine decreases the membrane permeability of the drug. The results suggest that Nicotine intake by any means can drastically decrease the efficacy of cancer treatment.64
Azadeh Kordzadeh et al. studied the drug loading and release of DOX in carboxyl (COO) and folic acid (FA) functionalized SWCNTs using MD simulations. The simulations were carried out in GROMACS 5.1.4 MD Simulation package with GROMOS54A7 force field and further modelled the interaction of CNT–COO–FA with model cell membrane designed with dipalmitoylphosphatidylcholine (DPPC) lipid bilayer embedded with folate receptor to mimic the real cancer cell membrane where folate receptors are overexpressed. From the MD simulations, the Lennard-Jones electrostatic interaction potential between the drug molecules and CNT–COO–FA was found to be greater than CNT–COO, suggesting that adding a targeting agent can improve the loading efficiency too. The drug release behaviour has also been studied, and the results of the simulations showed a higher density of drug release from the CNT–COO–FA carrier than the other one, which is simulated in acidic pH. These results can lead to the development of more specific drug delivery systems.65
Reza Maleki et al. investigated the loading and release of carboxyl functionalized SWCNT and multi-walled CNT (MWCNT) and compared their drug delivery performance. The simulations were done in GROMACS 5.1 with the OPLS-AA force field. They found that drug loading can be achieved in neutral pH, where electrostatic interactions play a major role. Since the drug–carrier complex has more hydrogen bonds, the drug loading is efficient and the drug release is pH dependent. The total interaction energies showed that MWCNT can be a more efficient adsorbent for DOX than the SWCNT and is also able to achieve sustained release due to its strong adsorbing properties in acidic pH found in cancer tissues and the carboxyl functionalization increased the adsorbing properties of the carrier and their solubility in the bloodstream.66
Experimental studies on the drug carrier interactions
DOX delivery with graphene oxide
Liming Zhang et al. synthesized nanoscale graphene oxide (NGO) and functionalized it with sulfonic acid groups to render stability in physiological pH and covalent conjugation of folic acid via covalent reactions. The formation of this molecular complex is confirmed with FT-IR and UV-vis spectra and two anti-cancer drugs DOX and CPT were loaded on the FA-NGO complex. The toxicity of FA-NGO complex was tested and no toxic effects were found in the MCF-7 breast cancer cell lines. The anti-cancer activity of FA-NGO particles loaded with DOX and CPT, which were incubated in MCF-7 breast cancer cell lines at the concentration of approximately 20 ng mL−1, showed obvious cytotoxicity, whereas the FA-NGO/DOX and FA-NGO/CPT showed no obvious toxicity at the concentrations 2000 ng mL−1 and 2–20 ng mL−1, respectively. Here, the co-loading of drugs and folic acid conjugation to the carrier allowed the co-loaded drugs to show more toxicity towards the cancer cell lines than their individual performance.67Zonghua Wanga et al. prepared the GO nanoparticles decorated with Fe3O4 nanoparticles and conjugated them with folic acid via biocompatible polymer chitosan (CHI) and loaded DOX particles as a triple functionalized system enabling active targeting via folic acid and passive targeting via Fe3O4 particles that enable efficient targeting of folic acid receptors overexpressed in the brain, kidney, lung, and breast cancer cells. Further, they investigated the loading efficiency and drug release behaviour in different physiological pH. The loading of DOX on Fe3O4/GO–CHI–FA is 0.98 mg mg−1 at pH = 7.4, which is higher than the Fe3O4/GO, which is (0.86 mg mg−1). The loading capacity of Fe3O4/GO–CHI–FA (0.74 mg mg−1) also decreased in the acidic pH. The release behaviour also showed strong pH dependence and controlled release, which is favourable for in vivo systems.68
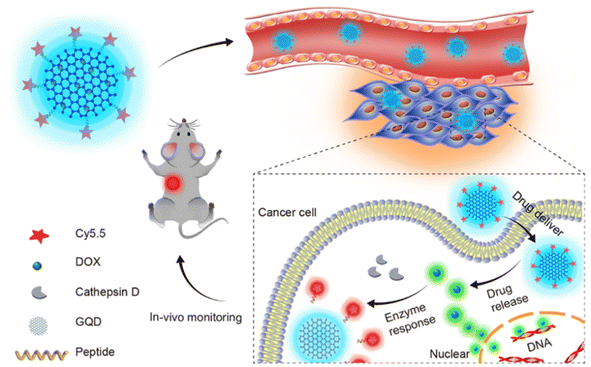
Graphene quantum dots loaded with DOX and linked with Cy5.5 dye through cathepsin D responsive peptide(Fig. 5) have been used as a theranostic agent in treating cancer in mice model by utilizing the EPR effect and this has been proven as a probe for monitoring the drug release and response in vivo conditions.69
Xubo Zhao et al. successfully synthesized GO nanoparticles loaded with DOX coated with biocompatible polymer CS-DMMA (chitosan/dimethyl maleic anhydride modified chitosan), which was synthesized via self-assembly with charge-reversal property. This aids the system with both prolonged circulation and increased cellular uptake rate, as well as a pH responsive nature for releasing the drug inside the cells. The biocompatibility of GON/CS/CS-DMMA had been verified with WST-1 assay in HepG2 cells, yielding 96% cell viability during different testing concentrations, and the cellular uptake of the drug has been confirmed with confocal laser scanning microscopy, and the toxicity of the DOX loaded system had also been observed in cells after 24 hours of incubation at pH 6.5. In this process, good drug encapsulation efficiency and drug loading capacity have been achieved (Fig. 6).70
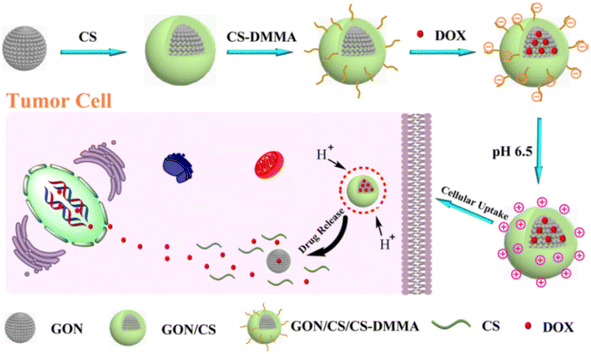 | ||
| Fig. 6 Schematic illustration of the fabrication and drug release of the GON/CS/CS-DMMA by pH trigger. Reprinted with permission from “X. Zhao et al., “Design and development of graphene oxide nanoparticle/chitosan hybrids showing pH-sensitive surface charge-reversible ability for efficient intracellular doxorubicin delivery,” ACS Appl. Mater. Interfaces, vol. 10, no. 7, pp. 6608–6617, 2018, doi: https://doi.org/10.1021/acsami.7b16910. Copyright-2023-American Chemical Society. | ||
Nanocomposites have recently been explored as drug delivery carriers. Mohamed. L. Salim et al. have prepared superparamagnetic graphene oxide hybrid nanocomposites (GO/Fe3O4) as drug delivery carriers and investigated the behaviour of DOX passively loaded on GO/Fe3O4 hybrid and actively loaded on the same composite with folic acid conjugated with GO and the loading of the drug was checked with UV-vis spectrum. The loading efficiency of active and passively loaded complexes was 94% and 92–94%, respectively. The composites were characterized by Transmission Electron Microscopy (TEM), Fourier Transform Infrared Spectroscopy (FT-IR) and thermogravimetric analysis (TGA) analysis. Anti-tumour and cardiotoxic effects were studied with EAC breast cancer cell lines, and the complexes are shown to have high loading capacities. In vivo studies using EAC cell lines showed that actively loaded conjugates have higher therapeutic effects than the free drug and passively loaded conjugates. Since DOX is known for its cardiotoxicity, both complexes induced significant cardiotoxicity, as revealed by the cardiotoxicity evaluation. Due to the fact that GO has excellent photothermal conversion properties, it was tested for hyperthermia applications and both conjugates showed anti-tumour effects on cell lines. However, the active complex with IR exposure is found to exhibit significantly lesser toxicity. The study concludes that GO/Fe3O4/FA/DOX + IR can lead to a system giving anti-tumour effect with less toxicity.71
S. M. Mousavi et al. have synthesized GO and biocompatible copolymer N-phthaloylchitosan-graft-poly(methylmethacrylate-block-poly (ethyleneglycol) methacrylate-random-dimethylaminoethyl methacrylate) represented as CS-P(MMAb-(PEGMA-ran-DMAEMA)) through RAFT (Reversible Addition Fragmentation chain Transfer) polymerization. GO was functionalized with this copolymer using covalent interaction. Finally, the anti-cancer drug was loaded on this complex using the membrane dialysis method. FT-IR spectra have been obtained to identify the chemical components of the drug–carrier complex and Field Emission Scanning Electron Microscopy (FE-SEM) analysis showed the structural morphology of the complex and the average size was determined to be 110 nm, which can effectively use the EPR effect followed by the investigation of optical properties using UV-vis spectroscopy. The particle size and zeta potential were analysed using Dynamic Light Scattering (DLS). The drug encapsulation efficiency of the nanocomposite was calculated to be 81% and the drug release mechanism is controlled by pH levels at physiological temperatures.72
To preserve the properties of the drug, they are loaded on the carrier through non-covalent interactions. In the current work, DOX is covalently linked with the carboxylic groups of functionalized graphene. The Carbon Nuclear Magnetic Resonance (C-NMR) spectra identify the functional groups present in the GO-DOX complex, which is further confirmed using IR spectra, and the surface chemistry is provided by the XPS spectra. Further characterization techniques like X-ray diffraction (XRD), Raman spectroscopy, elemental analysis and UV-vis spectrum confirm the formation of the GO-DOX complex and high-resolution transmission electron microscopy (HRTEM) is used to see the morphology of the particles. Hemo-compatibility analysis confirmed that the complex has dose-dependent hemolysis properties and produces no significant effects up to 200 μM. Cytotoxicity analysis showed that GO-DOX showed toxicity in various cell lines, including the lung carcinoma cell line, ovarian cancer cell line, liver adenocarcinoma cell lines and a few other cancer cell lines. The complex showed reduced toxicity in healthy non-cancer cell line HEK293. The interaction with HSA (Human Serum Albumin) confirms its binding site and DNA interaction showed its genotoxicity, which confirmed its reinforced mechanism of action in literature.73
DOX delivery with carbon nanotubes
In most cases, the drugs are attached to the carrier via non-covalent interactions to preserve their properties. Dorota Matyszewska et al. have synthesized and characterized SWCNTs and conjugated them with DOX via the hydrazone bond, which is easily cleavable in acidic pH where it has to be released. The formation of the hydrazone bond is confirmed by FT-IR, Raman and TGA analyses. To understand the interactions of SWCNT-DOX with biological systems, the Langmuir technique was used. Here, 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanol (DPPTE) is a type of synthetic lipid or phospholipid used as a model membrane and it was found that the model layers changed their properties under the influence of nanostructures. The strong influence of CNT and DOX conjugate over the model membrane suggests the strong possibility for the system to cross the membrane for effective drug delivery.74Shuoye Yang et al. have investigated the drug loading, release and cytotoxicity of SWCNT functionalized with PEG and PEI (Polyethyleneimine) and compared the efficiency of the three different complexes like COOH functionalized, PEG functionalized and PEG–PEI functionalized SWCNTs, which is shortened by acidification using different acids to decrease the size and increase the dispersibility of the complex in aqueous environment. These conjugates loaded with DOX were tested in human breast cancer cell lines MCF-7 and the cellular internalization was investigated via fluorescence imaging and flow cytometry, which revealed that the CNT–PEG–PEI/DOX showed enhanced intracellular penetration and more anti-tumour activity than the other complexes and showed enhanced drug release in the acidic pH.75
DOX delivery with multi-walled carbon nanotube
Farahani et al. prepared the MWCNTs–PEG complex, loaded DOX and optimized adsorption with different parameters like pH, contact time, adsorbent dose, and initial concentration. The MWCNT–PEG complex was characterized by FT-IR, which confirmed the ester linkage between MWCNT and PEG. SEM and Raman analyses confirmed the conjugation of PEG chains on the tubes. TGA analysis was also carried out to characterize and confirm the degree of functionalization by the PEG chains. Finally, DOX was loaded on the prepared conjugates. DOX adsorption was studied using the Analysis of Variation (ANOVA) technique. The results have concluded that the adsorption of DOX on MWCNT–PEG conjugation is spontaneous and the release is pH dependent, thereby confirming that the drug would be released in an acidic tumour environment.59In most nano drug delivery systems, PEG conjugation is used for a wide variety of applications, rendering solubility, reduced toxicity and controlled drug release. Here, Chanchal Kiran Thakur et al. have presented a simple and cost-effective method for functionalizing MWCNTs with carbohydrate ligands such as galactose (GA), mannose (MA) and lactose (LA) using lysine as a chemical linker and loading DOX. Carbohydrate ligands are hydrophilic, biocompatible, biodegradable, and nontoxic in nature. These complexes are characterized by FT-IR, NMR, Raman spectroscopy and XRD analysis. After DOX was loaded on these complexes, the particle sizes of DOX-loaded complexes were measured. DOX loading efficiency of carboxylated MWCNTs was 90.83 ± 0.21%. Interestingly, the DOX loading efficiencies of lysine-modified LyMWCNTs and ligand-modified CNTs like GAMWCNTs, MAMWCNTs and LAMWCNTs are 93.30 ± 0.22%, 96.78 ± 0.017%, 97.29 ± 0.06%, respectively, which showed the enhancement of drug loading efficiency due to the ligands conjugation. An in vitro drug release study indicates that drug release is more favourable in acidic pH, like all previously reported works. In vitro cytotoxicity studies on human breast cancer cell lines MDA-MB-231 and MCF-7, showed that MWCNT conjugated with ligands are not toxic to the cell lines with a 90% viability rate, whereas the DOX-loaded complexes showed enhanced cytotoxicity and anti-cancer efficacy. Fluorescence microscopy was used to investigate the cellular uptake of NPs. The study concludes that DOX loaded on carbohydrate ligand attached MWCNTs could actively target breast cancer cells since the ligands can effectively target lectin receptors overexpressed in breast cancer cells, thus leading to a more possibility of developing a better drug delivery system for breast cancer.76
Pravin S. Uttekar et al. presented a simple method to prepare folic acid and ethylene diamine (EDA) conjugated MWCNTs, which are treated with blended acid. This is done to introduce a carboxyl group on the tube surface loaded with DOX via π–π stacking interaction targeted against folate receptor overexpressed breast cancer cell lines MCF-7. The FT-IR spectra proved the functionalization of MWCNTs and UV-vis spectroscopy confirmed the successful loading of DOX in nano conjugate and further confirmed by NMR spectra of the drug and nano conjugate complex. Surface morphology was studied using Scanning Electron Microscope (SEM) images. The elemental concentrations are investigated using Energy Dispersive X-ray spectroscopy. Further, XRD was used to analyse the structural features of the conjugates. The in vitro anticancer activity of the prepared nano conjugate was found to increase, and the carrier may enter into the cell by means of folate-mediated endocytosis.77
Dendrimer reinforced CNMs in DOX delivery
A lot of strategies are proposed to increase the loading efficiency and targeting capability of nanocarriers. Dendrimers are one such carrier as they have hyper-branched structures that render the ability to trap different kinds of molecules, including poorly soluble drugs for drug delivery.27 Kyriaki-Marina Lyra et al. have prepared acid-treated MWCNTs conjugated with guanidinylated derivatives of hyperbranched polyethyleneimine with two different molecular weights 5000 Da (GPEI5K) and 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da (GPEI25K) on the side walls. The formation of conjugates was confirmed and characterized by spectroscopic techniques like FT-IR, NMR, Raman, X-ray Photoelectron Spectroscopy (XPS) etc. The DOX loading on GPEI5K-oxCNTs and GPEI25K-oxCNTs were 44.5% and 51.7%, respectively. The corresponding efficiencies were 78.7% and 99.5% with the initial DOX concentration of 2 mg mL−1 and the DOX-loaded systems exhibited pH-triggered release. However, the release rate was faster in the GPEI25K-oxCNTs system loaded with DOX. The cytotoxic activity of the free drug and the other two complexes have been studied in human cancer cell lines PC3 and DU145 and non-cancerous cell lines HEK293. The free drug has more cytotoxicity in healthy cells and among the cancerous cell lines, DU145 was found to be more sensitive to free DOX when compared to PC3. During the administration of the oxCNTs@GPEI5K-DOX system, greater cytotoxicity is observed in DU145, whereas PC3 was found to be more resistant to the complex and exhibits cytotoxicity only at higher concentrations. But interestingly, the HEK293 cell line exhibited greater viability or almost no cytotoxic effect for this complex, indicating that the target specificity has been improved by this complex. Moreover, the oxCNTs@GPEI25K-DOX showed higher cytotoxicity in all the cell lines, especially in DU145 and HEK293. The cellular uptake and internalization were investigated via confocal microscopy, revealing that oxCNTs@GPEI25K-DOX internalizes more than oxCNTs@GPEI5K-DOX due to its higher polymeric contents, but it lacks the specificity towards the cancerous cell lines. Overall, the work concludes that the oxCNTs@GPEI5K-DOX system possesses unique selectivity toward the cell lines and sustained release profile than the other system, thus offering a possibility for becoming a novel delivery system for DOX and this strategy can be utilized for other potent anti-cancer drugs too.78
000 Da (GPEI25K) on the side walls. The formation of conjugates was confirmed and characterized by spectroscopic techniques like FT-IR, NMR, Raman, X-ray Photoelectron Spectroscopy (XPS) etc. The DOX loading on GPEI5K-oxCNTs and GPEI25K-oxCNTs were 44.5% and 51.7%, respectively. The corresponding efficiencies were 78.7% and 99.5% with the initial DOX concentration of 2 mg mL−1 and the DOX-loaded systems exhibited pH-triggered release. However, the release rate was faster in the GPEI25K-oxCNTs system loaded with DOX. The cytotoxic activity of the free drug and the other two complexes have been studied in human cancer cell lines PC3 and DU145 and non-cancerous cell lines HEK293. The free drug has more cytotoxicity in healthy cells and among the cancerous cell lines, DU145 was found to be more sensitive to free DOX when compared to PC3. During the administration of the oxCNTs@GPEI5K-DOX system, greater cytotoxicity is observed in DU145, whereas PC3 was found to be more resistant to the complex and exhibits cytotoxicity only at higher concentrations. But interestingly, the HEK293 cell line exhibited greater viability or almost no cytotoxic effect for this complex, indicating that the target specificity has been improved by this complex. Moreover, the oxCNTs@GPEI25K-DOX showed higher cytotoxicity in all the cell lines, especially in DU145 and HEK293. The cellular uptake and internalization were investigated via confocal microscopy, revealing that oxCNTs@GPEI25K-DOX internalizes more than oxCNTs@GPEI5K-DOX due to its higher polymeric contents, but it lacks the specificity towards the cancerous cell lines. Overall, the work concludes that the oxCNTs@GPEI5K-DOX system possesses unique selectivity toward the cell lines and sustained release profile than the other system, thus offering a possibility for becoming a novel delivery system for DOX and this strategy can be utilized for other potent anti-cancer drugs too.78
Increasing the targeting nature of the drug is a major criteria in designing an efficient drug delivery system. Yan Yan et al. have synthesized acid-treated and carboxyl functionalized MWCNTs conjugated with polyethyleneimine (PEI) consisting of rich amine groups and hyperbranched structure, which will enhance the modifiability and stability of the complex. Subsequently, the COOH–PEG–FA complex is formed and is conjugated with MWCNT–PEI and finally, MWCNT–PEI–PEG–FA is prepared and labelled with fluorescein isothiocyanate (FI). Finally, terminal amines and unreacted products were removed from the FA-targeted multifunctional MWCNTs. The prepared complex is characterized by TEM and TGA analysis and UV-vis spectroscopy techniques. The origin of different absorbance peaks after each reaction in forming the nano complex in the UV-vis spectrum and changes over the surface of the nano complex (NC) after each step in zeta potential analysis confirmed the successful synthesis of the desired complex.79
Doxorubicin hydrochloride is loaded on the multifunctional carrier in an aqueous solution of pH 9.5 and the DOX loading is confirmed by UV-vis spectroscopy and the unbound DOX molecules were removed by centrifugation. The release kinetics have been studied in two different pH values (7.4 and 5). They found that the system undergoes a pH-responsive drug release profile, and it is notable that 58.3% of DOX is released in acidic pH, whereas only 10.1% is released in normal physiological pH (7.4) for the first 10 h, showing a prolonged release in a normal environment and rapid release in the tumor environment showing reduced toxicity in healthy cells. DOX loading efficiency and loading percentages are calculated to be 70.4% and 26.0% and they possessed colloidal stability in water, PBS and cell culture medium. Here, to understand the cancer cell inhibition, HeLa cells were cultivated in the laboratory and injected into nude mice. The IC50 of free DOX and DOX conjugated with the NC is 3.45 and 3.53 mg L−1, which shows that the conjugation does not affect the efficacy of the drug. FA-targeted NCs showed 90% viability in HeLa cells, proving that the NC is non-toxic to HeLa cells.
The quantification of cellular uptake of DOX conjugated with NCs showed that the uptake is mainly via the folic acid receptor-mediated pathway, and it is also confirmed by confocal microscopy, which showed strong signals of accumulation of NCs with DOX in the cytoplasm. In vivo studies showed that tumor growth is suppressed in the mice treated with NC loaded with DOX than the free DOX treated mice. Here the prolonged circulation by PEGylation and targeted delivery via FA conjugation is achieved. The weight measurements of saline-treated, free DOX treated, and DOX/NC treated mice showed that the side effects were reduced due to this formulation. Histological examination of tissues showed that the DOX-NCs possessed higher apoptotic cells (73.3%) than the free DOX, MWCNT treated, and untreated tissues. Bio-distribution studies showed that no organ toxicity was found in the treated mice. This versatile nano platform has a greater possibility of being converted into a powerful DOX delivery system.79
Experimental analysis combined with theoretical calculations
Comparing the theoretical simulations with the experimentally obtained results is always advantageous for better understanding the mechanism involved in the drug adsorption and delivery process. The simultaneous theoretical simulations can provide explanations for experimental observations. Here, some of the interesting literature in which theoretical and experimental approaches are combined are presented: Hakim Vovusha et.al. has done a combined study to understand the binding characteristics of DOX with graphene and GO. The structures for simulations were designed based on the structural data available from the experiments. The presence of different functional groups like carboxyl, carbonyl, epoxide and hydroxyl was confirmed with XPS, and the size was validated to be in the range of hundred nanometers using SEM analysis and the loading of DOX on the GO surface was confirmed with fluorescence spectroscopy. Simulations have been carried out using the Vienna-Ab initio Simulation Package (VASP) with Perdew–Burke and Ernzerhof (PBE) functional with structural vacancies introduced and substituted with functional groups to saturate the dangling bonds. Two different orientations of the drug with GO surface were taken for the optimization procedure and the one with minimal energy, having the ring structures facing the GO, was used for further analysis. The calculations suggest that DOX binds more favorably to graphene than GO due to the fact that DOX interacts strongly with sp2 electron clouds than the sp3 electron clouds present in functionalized graphene and the binding was confirmed using fluorescence spectroscopy. Despite the strong affinity of the drug towards the carrier, GO is chosen for further applications due to its biocompatibility and dispersibility in biological systems after functionalization.80Somayeh Sohrabi et al. have done combined theoretical and experimental studies to investigate the properties of PEG–PLGA–riboflavin conjugated DOX loaded CNT and CNT doped with bromine, nitrogen and phosphorous nanoparticles using DFT and MD simulations. Here, PLGA is chosen for its amphiphilic nature to encapsulate hydrophobic DOX, PEG for enhanced circulation and kinetic stability, and riboflavin for its targeting capacity. DFT and MD Simulations have been done using the Gaussian 09-B3LYP function and GROMACS 2019.5 (OPLS-AA force field), respectively. The simulation results suggest the formation of micelles of these complexes, which is suitable for the delivery and SASA of doped systems, remain more stable than the other systems and provide more surface for drug loading.81
Magnetic nanoparticles are of great interest as drug delivery carriers because of their controllability in tumor accumulation and drug release profiles using external magnetic fields. Sohelia Javadian et al. have synthesized a non-toxic magnetite (Fe3O4) nanoparticle functionalized with biocompatible PEG polymers to improve the hydrophilicity and solubility in an aqueous environment and further decorated with graphene quantum dots to enhance the loading capacity and introduce optical properties to the nanocomposite and finally DOX was loaded in the carrier. Characterization techniques like FT-IR, XRD, and TEM are used to confirm the chemical composition, crystalline nature, and morphology of the prepared nanocomposites. Other techniques like UV-vis spectroscopy, vibrating sample magnetometer (VSM) analysis, dynamic light scattering analysis, and TGA analysis were used to investigate other physiochemical properties. Drug release profiles in both acidic (pH = 5.5) and physiological pH = 7.4 conditions were studied and the composite was shown to have a controlled release rate. The cytocompatibility of the nanocomposite was checked via MTT assay in MCF-7 human breast cancer cell lines, which showed negligible toxicity for all the tested concentrations. The cytotoxicity of the nanocomposite loaded with the drug was also confirmed. Further DFT simulation confirmed the experimental observations. Molecular Electrostatic Potential (MEP) maps and HOMO–LUMO energy calculation throws more light on the energetics of the system. This study can lead to the development of novel magnetic drug delivery systems with controlled release profiles and biocompatibility in biological systems.82
Comparison of different CNMs in drug delivery
From the reported literature in the past decades, it is clear that different forms of carbon nanomaterials show interestingly different properties in terms of drug loading and releasing efficiency and toxicological profiles.A. M. Sawy et al.83 have investigated the five different carbon nanomaterials (CNMs), namely SWCNTs and MWCNTs functionalized with oxygen-containing functional groups, GO, and Graphene Quantum Dots (GQD1 & GQD2) synthesized via different methods (modified Hummer's method and Chemical Vapor Deposition-CVD). Surface and morphological analysis of CNMs revealed that the hydrodynamic size distributions of GQDs1, GQDs2, GO, SWCNTs, MWCNTs are 30.14 ± 3.7 nm, 52.7 ± 4.3 nm, 91.99 ± 5.4 nm, 125.5 ± 1.33 nm and 175.6 ± 3.2 nm, respectively. DOX is loaded on these carriers and the loading was confirmed using the UV-vis spectra and photoluminescence spectra (PL). Loading and release profiles were studied in physiological pH = 7.4 and endosomal pH = 5 (tumor cell). The GO showed the highest loading capacity of 2.85 mg mg−1 and MWCNTs showed the lowest loading capacity of 0.94 mg mg−1. Other carriers such as GQD1, SWCNTs, GQD2 have loading capacities of 1.66, 1.6, and 1.14 mg mg−1, respectively.
Theoretical first principle calculations to understand the adsorption mechanism between GQDs and GO have been performed with the GGA (Perdew–Burke–Ernzerhof)-exchange-correlation functional. The adsorption energy for the DOX@GQDs system is 1.75 eV. Two different GOs, one decorated with epoxy groups (GO1) and the other with hydroxyl groups (GO2). Both structures, in one case, have single layers and in another case, they have two layers like sandwich structures that give four systems. The binding energies of single-layered systems GO1 and GO2 are 1.66 eV and 2.07 eV, respectively. The binding energies of sandwiched GO1 and GO2 are 3.72 eV and 3.09 eV, respectively. These theoretical simulations also confirmed the experimentally proved loading capacity of GO was higher than that of the other CNMs. The authors have further investigated the toxicity profile of CNMs before and after loading of DOX in MCF-7 cell lines at different concentrations. No obvious toxic effects were observed up to the dose of 200 μg mL−1 and only GQDs2 showed a slightly high percentage (40%) of cell death, which may be due to the preparation impurities. Significantly, after the loading of DOX on CNMs, a low percentage of cellular viability was observed and the half–maximal inhibitory concentration of DOX@GQDs1, DOX@GQDs2, DOX@SWCNTs, DOX@GO, DOX@MWCNTs, and free DOX was reported to be 2.85, 3.9, 5.06, 5.7, 8.15, and 8.14 μg mL−1 after the incubation time of 24 hours. Biocompatibility was also checked with human normal, human lung fibroblast cell lines (WI-38) with MTT assay. Cellular uptake of different NMs was confirmed with confocal laser microscopy and imaging using MCF-7 cell lines. Structural changes, necrotic and apoptotic cell death in the cell lines have been investigated through TEM analysis. Animal experiments and histological assessments of the kidney and liver showed no toxic effects of GQDs and confirmed biosafety levels. These results gave a new understanding towards the various functional aspects of CNMs.83
In another study, Rosa Garriga et al. analyzed the performance of different types of carbon nanomaterials, including Carbon Nano-Horns (CNH-100 nm sized dahlia flower-like assemblies), carbon nanotubes (CNT-up to 1 μm in length and 10 nm in diameter), graphene oxide (GO-both GO and rGO have flakes up to 1 μm in length with high exfoliation degree, i.e., very few layers), reduced graphene oxide (rGO), Carbon Nano Platelets (CNP-they have less exfoliation degree than the previous two, i.e. more aggregated form) and Nano Diamonds (ND-5 nm minimum diameter). Among all the structures, CNP has the highest surface area of 701.8 m2 g−1. These carriers are loaded with two anticancer drugs, doxorubicin and CPT, and their potential was tested against two cancerous cell lines, namely human colorectal adenocarcinoma cells (Caco-2) and human breast adenocarcinoma cells (MCF-7). Pluronic F127 was used to prepare a dispersed solution of these nanomaterials. To assess the potential toxicity of these carbon materials, a cell viability assay was performed in both cell lines with all the above-mentioned six nanomaterials and administered in two different doses, 0.6 μg mL−1 and 3 μg mL−1 and cell viability was measured after 24 hours and 72 hours. The nano diamonds produced the least toxicity, while the CNP produced more toxicity. The cell viability of the materials followed the given pattern of CNP < CNH < RGO < CNT < GO < ND. From the cell viability assay, it was found that the colorectal cancer cell lines were more vulnerable to the toxicity of these nanomaterials. When the same assay was done for the healthy human dermal fibroblast cells, the healthy cells also experienced a similar toxic effect as that experienced by MCF-7 cell lines and lesser than that of Caco-2 cells.
This result suggests the potential toxic effects of these nanomaterials on healthy tissues, which also end up in undesirable biological effects in the human body similar to the chemotherapeutic drugs. So, great concern has to be given to the chemical modification of the carbon nanomaterials to reduce their side effects before using them as a carrier for the future clinical translation of this interesting class of materials. Since the production of Reactive Oxygen Species (ROS) and oxidative stress-induced damages are the commonly accepted mechanism of toxicity of carbon nanomaterials, here, the authors analyzed the ROS production of cell lines and the Caco-2 was observed to undergo the production of more ROS by CNP compared to that of MCF-7 after 24 hours of incubation. Cell death assay by flow cytometry analysis showed that the CNH produced more late apoptosis/necrosis for a dosage of 3 μg mL−1 after the incubation of 72 hours. For the drug carrier complex preparation, only four of the above-tested nanomaterials ND, GO, CNT and rGO were chosen for the testing since the other two carriers showed higher toxic effects. The concentrations of these materials were fixed to be 0.6 μg mL−1 as used in the assays to make sure that the difference in cellular viability may be attributed to the drug and the drug concentrations were fixed to be 0.2 μg mL−1, 0.8 μg mL−1. From the assays, it was found that both the CPT and DOX showed more cytotoxic effects than the inherent toxic effects of the carrier in both cell lines. Compared to the DOX, CPT is found to be more cytotoxic in both cell lines. The drug-loaded carriers also showed better cytotoxicity against cancer cell lines than the free drug. These findings threw more insights into the delivery of drugs, potential toxic effects of carriers, improved performance of the drugs by loading them on carriers and show the path for the future development of effective drug delivery systems.84
Combinatorial approaches for DOX delivery
Since drug resistance is often experienced by many chemotherapeutic drugs, combined therapy is adapted to overcome this issue, which can increase the synergic effects of the drugs. Abutaleb Alinejad et al. have investigated the nature of the interactions between folic acid-conjugated graphene co-loaded with two anti-cancer drugs, DOX and CPT, using DFT and MD simulations. The results confirmed that the formation of the FA–G/DOX/CPT molecular complex is a spontaneous process. The drugs interacted and formed the complex via π–π stacking interactions with the carrier surface. The conjugation of folic acid further enhanced the stability of complex formation and rendered the system with better targeting ability. From the simulation results, it is found that the adsorption of DOX on the carrier is preferable to that of CPT and the presence of folic acid stabilizes the drug–carrier complex. Importantly, no changes in the chemical structures of drugs were observed, which indicates the preservation of the activity of the drug.85H. Hashemzadeh et al. have investigated the co-loading, diffusion and release properties of DOX and paclitaxel (PTX), another powerful chemotherapy drug, in the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 on the surface of pristine graphene and graphene oxide surface functionalized with oxygen-containing functional groups such as hydroxyl and epoxy functional groups through MD simulations. The free energy calculations of the systems suggested that the binding energies of DOX and PTX were greater in graphene than in the GO system, which is due to the increased oxygen densities found on the surface of GO. It is also observed that there is no competition between the drugs during the process of loading in both the carriers and hydrogen bond interactions also play a major role in the drug binding mechanism. From the investigation of interactions of the Drug Delivery System (DDS) with the cell membrane, it is found that graphene-based DDS cannot diffuse into the membrane spontaneously, but the GO-based DDS can easily penetrate the membrane for intracellular delivery.86
1 on the surface of pristine graphene and graphene oxide surface functionalized with oxygen-containing functional groups such as hydroxyl and epoxy functional groups through MD simulations. The free energy calculations of the systems suggested that the binding energies of DOX and PTX were greater in graphene than in the GO system, which is due to the increased oxygen densities found on the surface of GO. It is also observed that there is no competition between the drugs during the process of loading in both the carriers and hydrogen bond interactions also play a major role in the drug binding mechanism. From the investigation of interactions of the Drug Delivery System (DDS) with the cell membrane, it is found that graphene-based DDS cannot diffuse into the membrane spontaneously, but the GO-based DDS can easily penetrate the membrane for intracellular delivery.86
Azadeh Khoshoei et al. have investigated the co-loading and pH sensitive release of DOX and PTX on functionalized SWCNT and graphene grafted with trimethyl chitosan (TMC) using classical MD simulations. The idea behind this simulation is that the carboxylic group can introduce strong electrostatic interactions between the drug and carrier, while the hydrophilicity and solubility of the carbon-based materials can be increased, and controlled release in acidic pH can also be achieved by attaching TMC. The results of simulations suggest that the electrostatic energies are favorable such that DOX can be loaded efficiently on both SWCNT and graphene and released successfully in acidic pH. Adding TMC provides more hydrogen bond interactions between the drug and carrier and also aids in releasing the drug in acidic pH.87
Drug loading, release and dual responsive behaviour of C60 functionalized(f-C60) with carboxyl groups and conjugated with biocompatible copolymer dimethyl acrylamide-trimethyl chitosan (DMAA-TMC) loaded with two anti-cancer drugs DOX and PTX have been explored by the authors using DFT and MD simulations. The simulations were performed in acidic and neutral environments. The adsorption energies showed that the drug–carrier complex is favourable for adsorption, and the release of DOX and PTX was found to be pH dependent. The negative electrostatic interaction in neutral conditions confirms the stability of the complex and the electrostatic interaction energy becomes zero in acidic pH, denoting the release of the drug in the cancerous tissues. The combination therapy using f-C60 can be more effective than the free drugs in the body.88
Peiwei Gong et al. have successfully synthesized fluorinated graphene oxide (FGO) conjugated with targeting moiety, folic acid linked with biodegradable polymer PEG, and loaded two drugs DOX and CPT and confirmed the complex formation with FT-IR spectral analysis and further validated with XPS measurements. Structural analysis using XRD measurements confirmed the successful chemical modification process. The prepared FGO is found to have excellent photothermal properties, which can be used to increase the toxicity of the complex towards the cancer cells. In addition, it possesses very good photoluminescence properties that can be utilized to monitor the drug delivery system inside the body. Flow cytometry measurement confirmed the uptake of DOX by cells, and drug release inside the cells is further validated with laser scanning confocal microscopy. Biocompatibility of FGO–PEG–FA was confirmed with normal cells and HeLa cells, showing negligible cytotoxic effects with good cell viability. In addition to these, DFT calculations depicting various interactions in the systems have been visualized. This work opens up various possibilities and effective methodologies for the co-administration of two drugs that reduce drug resistance and improve efficacy in treating the disease.89
An experimental work by Shahram Astani et al. on the co-administration of two powerful anti-cancer drugs, DOX and Cisplatin (CIS), by using a reduced graphene oxide carrier presents a promising pH-responsive DDS. Here, poly-2-hydroxyethyl methacrylate (PHEMA) polymer, which is both biocompatible and hemo-compatible, is attached with rGO through the Atom Transfer Radical Polymerization (ATRP) method. Then, the hydroxyl groups in PHEMA are converted to succinyl oxy groups by polyesterification with succinic anhydride to produce poly-2-succinyloxyethylmethacrylate (PSEMA), followed by the adsorption of iron oxide nanoparticles on the surface. Then, DOX and CIS are adsorbed on the surface through various interactions. The prepared nanocomposite was characterized by various techniques like FT-IR and FESEM to understand its chemical structure and morphology. The thermal behaviour of the composite was examined by TGA experiments. The intracellular uptake of Rhodamine-b labelled nanocomposite on MCF-7 breast cancer cell lines was observed with fluorescence microscopy. The nanocomposite without the drugs in varying concentrations incubated with cells has no significant cytotoxicity in the cell lines, showing their biocompatibility. However, the dual drug loaded on the nanocomposite showed higher cell death compared to that of free drugs, which showed the effectiveness of combination chemotherapy. So, combination therapy results in high efficiency and reduction of dosage of drugs, which in turn can effectively decrease the potential side effects of the administration of free drugs.90
Toxicology of the carbon-based nanomaterials
The toxicity of graphene-based materials is reported to be dependent on various factors like size, shape, functional groups present on the surface, chemical composition, oxidation states, route and dose of administration, and synthesis methods. The cytotoxic effects and immune response of a wide range of graphene materials like graphene nanoribbons, GO nanoplatelets, GO nano onions are found to be dose, shape, size, and functional group dependent in various human and animal cell lines. The primary mechanism of GO-induced toxicity is proposed to be via the production of ROS-induced cell death due to the agglomeration of GO composite in a particular biological environment. Regardless of its chemical composition, the sharp edges of the graphene sheet itself induce destabilization of the lipid membrane and cause physical damage to the organelle in a cell.91As mentioned earlier, the toxicology of carbon-based nanomaterials is dependent on various factors and different for various cell lines. Especially when it comes to carbon nanotubes, both single and multi-walled carbon nanotubes are dependent on length, diameter, rigidity, and surface modifications and the immune response for the CNTs is also reported to be desirable when they are modified efficiently. These aspects should be taken care of while designing the drug delivery vectors.92,93
The toxicity and biological distribution profile of SWCNTs and MWCNTs are also dependent on the structure, length of the tube, agglomeration state, purity, and chemical functionalization. The toxicity data obtained is also inconsistent. This may be due to the experimental conditions under which the results are obtained, the specific cell line in which the biological response against the substance is analyzed, dosages, incubation time, etc.94
Kai-Ping Wen et al. synthesized GO nanoparticles functionalized with poly sodium 4-styrenesulfonate (PSS) with a lateral size of 500 nm and thickness of about 2 nm characterized by AFM imaging and studied their biodistribution profile and investigated its toxicity over a period of six months. Three different dosages (4 mg kg−1, 6 mg kg−1, 16 mg kg−1) were administered to three groups of mice and the fourth group was a control group administered with PBS (phosphate-buffered saline). The biochemical analysis and histological examinations showed that the nanoparticles accumulated mainly in the lung, liver, bladder and spleen (Fig. 7). The high-dose injected mice showed acute inflammations and all other groups showed chronic inflammation. Considering these results, one may understand that nanoparticles with hundreds of lateral dimensions in nm could possibly cause severe damage to the organs. So, the size distribution of prepared nanoparticles plays a major role in their systemic toxicity and excretion mechanism, which should be considered during the design of drug delivery systems.95
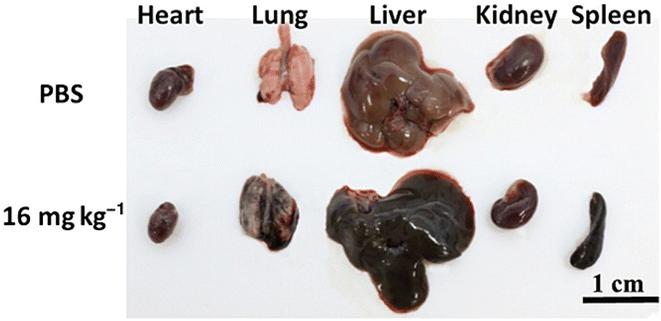 | ||
| Fig. 7 Images of major organs taken at day 14 from the control mouse (top row) and the mouse injected with 16 mg kg−1 nano-graphene oxide-poly sodium 4-styrenesulfonate (NGO-PSS). Reprinted with permission from “K. P. Wen, Y. C. Chen, C. H. Chuang, H. Y. Chang, C. Y. Lee, and N. H. Tai, “Accumulation and toxicity of intravenously-injected functionalized graphene oxide in mice”, J. Appl. Toxicol., vol. 35, no. 10, pp. 1211–1218, 2015, doi: https://doi.org/10.1002/jat.3187. License number: 5622900437481. | ||
Wei Chen et al. have studied the renal clearance mechanism and dose-dependent injury caused by GO in various renal compartments. They have prepared two different-sized GO (small-GO-70 nm, large-GO-311 nm) PEGylated and labelled with radio and fluorescent tags with similar surface chemistry and radio labeling efficiency. The s-GO and l-GO were given via intravenous injection with the dosage of 1 mg kg−1, 5 mg kg−1, and 15 mg kg−1. Using various imaging techniques, they have demonstrated that the s-GO could be excreted via glomerular filtration and tubular secretion, while the l-GO is excreted via tubular secretion. The renal clearance of l-GO is much faster than that of s-GO. The interesting fact is revealed by histopathological analysis which showed that no changes were observed in renal tubules for the first two doses, while the third dose induced injuries, including necrosis of renal tubular epithelial cells, cast formation, and dilatation for both different-sized GO and also causes structural changes in the Glomerular Filtration Barrier (GFB), tubular injury, renal vasodilation and necrosis in renal compartments. The biomarker analysis also supports the observed impairments. The study suggests that the high doses and high lateral dimensions will play a major role in its biological interactions and should be taken care of while designing graphene-based drug delivery systems.96
The fate of the carrier after the delivery
The journey of the drug delivery system from the point of administration via an appropriate route to reaching the site of action is very complex and not yet fully understood. In order to understand it, different imaging and tracing techniques are necessary.97 It is very important to understand the fate of the nanocarrier inside the body after delivering the payload to the intended site of action and the toxic effects they introduce in various organs of the body, which determine the treating potential and ability of the suitable candidates for clinical translation.98 Different imaging techniques, ranging from macroscopic to subcellular levels, are available to track the foreign object inside the human body. These imaging techniques range from Computed Tomography (CT), Magnetic Resonance Imaging (MRI), Magnetic Particle Imaging (MPI), ultrasound imaging, Positron Emission Tomography (PET), Single Photon Emission Computed Tomography (SPECT), Optical Coherence Tomography (OCT) and Multispectral Optoacoustic Tomography (MSOT) and there are other excellent imaging techniques like confocal microscopy. Especially for tracking carbon-based materials like GO and rGO transmission electron microscopy (TEM), Raman Spectroscopy and labelling them with different isotopes are possible.30,99 To learn about the broad spectrum of imaging techniques, the readers are suggested to refer to an excellent review on imaging techniques for nanomaterials by Ran Li et al. and Zhuang Liu et al.100,101The kidney plays a major role in the excretion of water and metabolically active substances from the body. The main excretion pathway of many nanomaterials, including GO materials and metabolic products of drugs, is renal clearance.96 The in vivo circulation period mainly affects the therapeutic efficacy of the cargo. The barriers that are encountered by the drug injected in the blood are the Mononuclear Phagocytic System (MPS), which engulfs the foreign entities, physiological hemodynamics, extravasation at the specific site, crossing the cell lipid membrane and endosomal compartmentalization, which could potentially degrade and inactivate the drug. Generally, the physiochemical properties of the nanoparticles, such as size, shape, surface chemistry, elasticity and porosity, play a major role in the life of nanomaterials inside the body.102
Shanshan Liang et al.99 have investigated the in vivo fate of GO modified with polyvinylpyrrolidone (PVP) and labelled with rare earth elements lanthanum (La) and cerium (Ce) and the prepared nanoparticles (NPs) were injected intravenously to sample mice and the tissues of various organs including the brain, heart, liver, kidney, spleen, stomach, small intestine and testis were collected. To understand the excretion of particles, stool and urine samples were also collected. The distribution of La/Ce–GO–PVP in organs was imaged with Laser Ablation Inductively Coupled Plasma Mass Spectrometry. The pharmacokinetics profiles showed that the NPs were rapidly accumulated into tissues and organs from the blood and slowly cleared from the organs. Biodistribution analysis showed that NPs accumulate higher in MPS-rich organs like the liver, spleen, lungs, and kidney with a slow clearance rate. Further TEM analysis also showed that the GO sheets rolled as fibrous nanoparticles and deposited in the alveolar septa and cytosol of alveolar macrophages and also accumulated in the liver and spleen with reconfigured shapes. However, no serious pathological effects were observed in the organs where NPs accumulated.
TEM images of the kidney showed that the NPs formed a cluster of particles with a width of 20–401 nm and length of 100–200 nm deposited on the glomerular capsule. The deposition of GO in the glomerulus and renal tube suggests that they can be excreted through urine. The concentration of La and Ce was measured in fecal and urine samples, which were respectively 7–10 ng g−1 and 31–36 ng g−1 after 12 hours of post-i.v injection, suggesting that the urinary excretion is more predominant than the fecal excretion. This has further been confirmed with TEM analysis of urine in which the edge deformed thin nanosheets were detected and the Energy Dispersive X-ray spectroscopy (EDX) confirmed the elemental composition. This study leads to an overall behavior and excretion of NPs in animals and gives an understanding that the deforming property of GO enables it to cross different biological barriers to enter different organs.99
The tissue distribution and accumulation of radio labelled (In) and DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) functionalized GO have been prepared and characterized via different characterization techniques and administered via intravenous route and the pharmacokinetics were studied. The study revealed the extensive urinary excretion of intact GO sheets followed by rapid clearance from the blood and accumulation of particles in the liver and mainly in the spleen.103
Dhifaf A. Jasim synthesized graphene sheets functionalized with a PEGylated DOTA (1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid) in three different sizes, large (1–35 μm), small GO (30 nm to 1.9 μm) and ultra-small (10–550 nm), and investigated the role of lateral dimensions of graphene sheets in their excretion and found that graphene sheets with larger lateral dimensions have slower excretion rates than the other two sheets. The excretion of the graphene sheets was confirmed in the pooled fecal and urine samples and a large amount of ultra-small particles were found than in the other two structures, which implied the significance of the size of the particle in the excretion mechanism. The thickness of the same material is also found to have a role in excretion where thinner material is able to be excreted rapidly via the urinary pathway with lesser in vivo accumulation, whereas thicker materials are found to accumulate in the liver and spleen and slow excretion via renal clearance increasing the possibility of toxicity in the kidney.104
The peroxidase-mediated biodegradation of CNTs is also reported in the literature through test tube experiments, as well as in vitro and in vivo degradation pathways of functionalized CNTs. The pristine CNTs were not reported to be degraded by this peroxidase-mediated biodegradation since the tube orients itself long away from the active site of the enzyme, whereas the functionalized SWCNTs are orienting themselves properly with the active site residue and undergo degradation. The above findings are helpful in concluding that the functionalization of nanomaterials is not only necessary to enhance their characteristics but also to mediate its biodegradation process, which is essential for its elimination after the delivery of the payload at the site of action.105
Other excretion pathways
Most of the nanoparticles are reported to have urinary excretion from the animal model studies. Usually, some other pathways have also been reported in the literature, including the hepatobiliary system (HBS)-feces pathway, which is quite slower than renal excretion and reported for larger particles. Baoquan Zhao et al. used zebra fish and mice with ligated Common Bile Duct (CBD) and administered activated carbon nanoparticles (ACNP) with diameters of 30, 60, 100 and 200 nm via intravenous injection and observed the new excretion pathway through the intestine without involving the hepatobiliary pathway with the ligated bile duct. They have explored a novel excretion pathway called the Intestinal Goblet Cells Secretion Pathway (IGCSP) in which ACNP is excreted along with the mucin (suspension of complex glycoproteins that helps in the elimination of toxins and other food waste from the intestine106) secreted by the intestinal Goblet cells. So, in addition to the renal and HBS pathway of excretion, IGCSP also gives the possibility of the excretion of nanomaterials by which the nanomaterials can circulate longer inside the body due to the slow excretion rate.107Zhuang Liu et al. have studied the long-term fate of SWCNTs functionalized with PEG chains of various types using Raman spectroscopy, which directly confirms the presence of SWCNTs in organs, blood samples and fecal samples. Here, SWCNTs functionalized with two different linear PEG chains and one branched PEG chain having size distributions of SWNT-l-2kPEG-104 ± 49 nm, SWNT-l-5kPEG-101 ± 51 nm, SWNT-br-7kPEG-95 ± 46 nm and given to the mice through intravenous route in the dose level of 200 μL of saline solution containing 0.1 mg mL−1 nanotube concentration. The blood circulation span of SWNT-l-2kPEG is approximately 1.2 h, whereas the increase of PEG chain length from 2 kDa to 5 kDa gives the circulation span of approximately 5 h. However, a further increase in the linear PEG chain length had a minor effect, whereas the branched PEG chains of 7 kDa had a circulation of approximately 15 h. These results lead to a conclusion that PEG chains, importantly branched PEG structures, give rise to longer circulation time and biological inertness, which helps to escape from RES in the body. Further, Raman spectroscopic analysis of the sacrificed mice organ tissues, it is found that the NPs are dominant in the liver and spleen and the 5 & 7 kDa PEG-coated tubes were less than the 2 kDa PEG-coated tube, denoting the fact that the higher degree of PEGylation affords lower RES uptake, which is an essential aspect of NPs in drug delivery applications. In the case of other organs, no obvious signals were detected suggesting the uptake is below the level of 1–2% injected drug per gram of the tissues. Another dose of 0.5 mg mL−1 also showed the same pattern of organ uptake, and additionally, NPs were also detected in the bones, kidney, intestine, stomach and lungs of mice. The results suggest the urinary and biliary excretion of NPs. The tissue analysis of organs also showed that the 5 and 7 kDa NPs were cleared faster than the 2 kDa NPs, ensuring favorable behavior in the body. Raman spectroscopic analysis of urine and fecal samples also confirmed the excretion pathway having appreciable signals from fecal samples and very low signals in urine samples, revealing that the excretion happens mainly via the biliary pathway. Necropsy, histology and blood chemistry analysis after three months post-injection showed no obvious toxicity in the organs. This work reveals the importance of PEGylation in designing biocompatible carriers and the biliary pathway as the major excretion pathway of SWNTs.101
Summary
• This review analyses the recent trends in doxorubicin delivery and mainly emphasizes the importance of simulation techniques, which can speed up the selection of a combination of compounds for targeted delivery of DOX, followed by clinical trials and regulatory approval process.• Currently, three kinds of DOX formulations are administered to the patients.
• Conventional free drug form has many side effects, including congestive heart failure and other related side effects in the heart, cognitive function deficiency, liver and kidney injury.
• Dox extravasation during administration also causes local tissue necrosis.
• PEGylated liposomal form of the drug known as Doxil/Caelyx is administered specifically for the treatment of AIDS-related Kaposi's sarcoma, metastatic breast cancer, advanced ovarian cancer and a few other cases. Despite its advantages like increased efficacy and sustained release, its use is restricted by the “palmar-plantar erythrodysesthesia(PPE)”.
• It is noteworthy that NPLD, known as Myocet, is free from PPE and gives the benefits of PEGylated Dox without many side effects.
• In addition to these formulations, a few drugs are co-administered with DOX to reduce the various side effects. These drugs are Vincristine, Probucol, and Dexrazoxane. However, the adverse reactions are not reduced to a significant extent. The researchers have been persistently examining new materials for precise DOX delivery, aiming to unlock its full therapeutic potential while minimizing the associated side effects.
• In the past decades, nanoparticles like micelles, protein–drug conjugates and dendrimers have been proposed as carriers for chemotherapy drugs. Among all these carriers, carbon-based nanomaterials like graphene and GO are being extensively researched.
• Both theoretical modelling of materials to understand the drug–carrier complex interactions and suitability of the materials and experimental studies have been done simultaneously.
Theoretical (DFT & MD) and experimental studies on the interaction of DOX on the surface of graphene and its derivatives
• The adsorption of the drug on the carrier surface is confirmed and is found to have a temperature-dependent release from the carrier behavior.• The functional groups present on the carrier surface have an influence over the drug carrier interactions. In particular, oxygen density on the carrier surface is an important factor. These findings should be taken care of while tailoring the carrier for the drug in further research.
• Chitosan-functionalized graphene showed pH-dependent release of the drug molecules, which can aid the drug delivery by having pH as a controlling parameter.
• The drug delivery and adsorption mechanism is also found to have a relation with the length of the PEG chains linked with the carrier. From these simulations, it is clear that the chemical composition of the carrier plays a major role in drug adsorption and release behaviour.
• The targeting efficiency of the folic acid conjugation has also been confirmed by various cancerous cell line studies, including MCF-7, HepG2, EAC and healthy cell lines.
• GO functionalized with magnetic nanoparticles was also found to have better targeting ability and lesser cardiotoxicity with IR exposure.
Theoretical DFT and MD simulations on the interaction of DOX on the surface of CNT and its derivatives
• In all the reviewed scientific works, the adsorption of the drug on the CNT surface is confirmed and found to be more favorable.• CNTs functionalized with folic acid and tryptophan have a pH-dependent release, showing that adding the targeting ligands not only targets the tumour but also stabilizes the system with good physiochemical properties.
• A DFT study with a simulation involving a large number of drug molecules confirms that the additional drug molecules stabilize the system and suggests the CNTs as a carrier with high loading capacity.
• When comparing the carboxyl-functionalized SWCNT and MWCNT, the second one possesses superior properties in terms of adsorption and pH-dependent release.
• Another interesting MD simulation on SWCNTs penetrating the model lipid membrane in the presence of nicotine molecules highlights that nicotine intake decreases the efficacy of the treatment.
• CNTs functionalized with PEG chains, carbohydrate ligands, and folic acid are found to have better anti-tumor effects in cell line studies.
Toxicity and fate of the carrier after the delivery of the payload
• The toxicity profile of carbon-based nanomaterials is dependent on various factors like size, surface chemistry, and dosage. However, they mainly accumulate in the major organs like the heart, lungs, liver, kidney, and spleen and produce acute and chronic inflammations.• They cause modifications in the organs during their excretion.
• These particles have different excretion pathways, including peroxidase-mediated, HBS, and IGCSP pathways, and functionalization is found to have an important role in their in vivo degradation.
• Most of the research works in which the toxicity profile is analysed come under the range of 70–700 nm, which exhibited noticeable toxic effects. In general, when the nanomaterial has its dimension in several hundreds of nm, it shows inflammations in the major organs.
• However, the toxicity can be reduced by tailoring the size and optimizing the functional groups present on the surface.
In conclusion, this review provides an exhaustive description of DOX administration, functionalization strategies, characterization techniques involved in the process, and throws light on the fate of the carrier and the toxicological aspects to be considered while designing drug delivery systems.
Conclusion and future directions
Cancer has become one of the major health threats for humankind, with increasing new incidences and mortality rates in the current scenario. Despite the development of therapeutic modalities for early detection and treatment options, we witness poor prognosis and recurrence in patients. Chemotherapy is one of the most powerful treatment options for cancer. However, its applications in cancer therapy are restricted due to the lack of specificity of the drugs towards the cancer cells, which results in severe side effects in healthy cells. Therefore, targeted delivery of therapeutics in tumors is of great clinical importance in the current context of cancer. Nanomaterials could serve as a carrier for these drugs to selectively deliver them in the tumor region. This targeted delivery of drugs using nanomaterials not only reduces the side effects of the drugs but also can improve the circulation half-life in blood, improve the stability of the drug, and ability to deliver multiple drugs, thus enabling combination therapy, and most importantly, can tackle multi-drug resistance of the cancer cells by simultaneously switching off the involved pathways in the cell by delivering multiple drugs.A wide variety of nanomaterials for the delivery of chemotherapeutic drugs are being researched extensively and few liposomal formulations have been approved for several cancers. Among them, carbon-based nanomaterials possess excellent physiochemical properties. Carbon nanomaterials like CNTs, graphene, and its derivatives are promising nanomaterials for drug delivery applications. Even though these materials are reported to have toxicity in biological systems, the scientific findings are often incongruous and sometimes vary with their size. This leads to the conclusion that their properties can be tailored using their size and degree of functionalization with biodegradable moieties to improve their biocompatibility. A systemic approach for the optimization of structural features of these nanomaterials with desired biocompatibility is the most needed aspect in using these materials for biological applications.
In most scientific literature, cell line studies to evaluate the cytotoxic effects of the prepared drug–carrier complex have been investigated only with malignant cell samples. In real conditions, after their administration in patients, the drug carrier complex tends to interact with different cell types and may produce different effects. However, to understand the potential side effects that may arise and to fully understand their character, the prepared materials have to be tested on healthy cell lines as well.
Computer simulations based on DFT and molecular dynamics approaches can speed up the optimization of properties of these materials and reduce the research and development cost of cancer research. A simultaneous experimental approach to synthesize the predicted materials and the consequent characterization of properties can lead to the development of better drug delivery systems. With all the suggested ways, these carbon-based nanomaterials could be utilized as a carrier for many chemotherapeutic drugs in the future.
Conflicts of interest
The authors declare no potential conflicts of interest.Acknowledgements
The authors would like to thank the financial support provided by the Department of Science and Technology—Science and Engineering Research Board, India, through the project “Engineering Two-dimensional Nanostructure for Drug–carrier Applications”. Project Number: CRG/2020/006330.References
- A. Mullard, 2022 FDA approvals, Nat. Rev. Drug Discovery, 2023, 22(2), 83–88, DOI:10.1038/d41573-023-00001-3.
- R. W. Ruddon, Cancer Biology, Oxford University Press, 4th edn, 2007 Search PubMed.
- A. Aggarwal, et al., Clinical & immunological erythematosus patients characteristics in systemic lupus Maryam, J. Dent. Educ., 2012, 76(11), 1532–1539, DOI:10.4103/ijmr.IJMR.
- C. Carvalho, et al., Doxorubicin: The Good, the Bad and the Ugly Effect, Curr. Med. Chem., 2009, 16(25), 3267–3285, DOI:10.2174/092986709788803312.
- C. F. Thorn, C. Oshiro, S. Marsh, T. Hernandez-Boussard, H. McLeod, T. E. Klein and R. B. Altman, et al., Doxorubicin pathways: pharmacodynamics and adverse effects, Pharmacogenet. Genomics, 2011, 27(2), 440–446, DOI:10.1097/FPC.0b013e32833ffb56.
- O. Tacar, P. Sriamornsak and C. R. Dass, Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems, J. Pharm. Pharmacol., 2013, 65(2), 157–170, DOI:10.1111/j.2042-7158.2012.01567.x.
- E. Şahin Akdeniz and C. Selçuki, Investigation of interactions of doxorubicin with purine nucleobases by molecular modeling, J. Mol. Model., 2022, 28, 69, DOI:10.1007/s00894-022-05031-z.
- K. Chatterjee, J. Zhang, N. Honbo and J. S. Karliner, Doxorubicin cardiomyopathy, Cardiology, 2010, 115(2), 155–162, DOI:10.1159/000265166.
- J. Lankelma, et al., Doxorubicin gradients in human breast cancer, Clin. Cancer Res., 1999, 5(7), 1703–1707 CAS.
- A. C. Alves, et al., Influence of doxorubicin on model cell membrane properties: Insights from in vitro and in silico studies, Sci. Rep., 2017, 7(1), 1–11, DOI:10.1038/s41598-017-06445-z.
- Y. Matsumura and H. Maeda, A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs, Cancer Res., 1986, 46(8), 6387–6392 CAS.
- A. Kolate, D. Baradia, S. Patil, I. Vhora, G. Kore and A. Misra, PEG – A versatile conjugating ligand for drugs and drug delivery systems, J. Controlled Release, 2014, 192, 67–81, DOI:10.1016/j.jconrel.2014.06.046.
- S. Rivankar, An overview of doxorubicin formulations in cancer therapy, J. Cancer Res. Ther., 2014, 10(4), 853–858, DOI:10.4103/0973-1482.139267.
- Y. Barenholz, Doxil – The first FDA-approved nano-drug: Lessons learned, J. Controlled Release, 2012, 160(2), 117–134, DOI:10.1016/j.jconrel.2012.03.020.
- D. Wang, X. Zhang and B. Xu, PEGylated Doxorubicin Prodrug-Forming Reduction-Sensitive Micelles With High Drug Loading and Improved Anticancer Therapy, Front. Bioeng. Biotechnol., 2021, 9, 1–9, DOI:10.3389/fbioe.2021.781982.
- H. Moustaoui, et al., Tunable Design of Gold(III)-Doxorubicin Complex-PEGylated Nanocarrier. the Golden Doxorubicin for Oncological Applications, ACS Appl. Mater. Interfaces, 2016, 8(31), 19946–19957, DOI:10.1021/acsami.6b07250.
- L. Yang, Y. Yang, Y. Chen, Y. Xu and J. Peng, Cell-based drug delivery systems and their in vivo fate, Adv. Drug Delivery Rev., 2022, 187, 114394, DOI:10.1016/j.addr.2022.114394.
- Z. Jing, Q. Du, X. Zhang and Y. Zhang, Nanomedicines and nanomaterials for cancer therapy: Progress, challenge and perspectives, Chem. Eng. J., 2022, 446(P3), 137147, DOI:10.1016/j.cej.2022.137147.
- S. F. Sun, Physical Chemistry of Macromolecules: Basic Principles and Issues, 2nd edn, 2004 Search PubMed.
- J. A. Hubbell and A. Chilkoti, Nanomaterials for Drug Delivery, Science, 2012, 337, 6092, DOI:10.1126/science.1219657.
- M. Kakran and L. Li, Carbon nanomaterials for drug delivery, Key Eng. Mater., 2012, 508, 76–80, DOI:10.4028/www.scientific.net/KEM.508.76.
- Z. Su, et al., Novel nanomedicines to overcome cancer multidrug resistance, Drug Resistance Updates, 2021, 58, 100777, DOI:10.1016/j.drup.2021.100777.
- L. Cao, Y. Zhu, W. Wang, G. Wang, S. Zhang and H. Cheng, Emerging Nano-Based Strategies Against Drug Resistance in Tumor Chemotherapy, Front. Bioeng. Biotechnol., 2021, 9, 1–17, DOI:10.3389/fbioe.2021.798882.
- Z. Gao, L. Zhang and Y. Sun, Nanotechnology applied to overcome tumor drug resistance, J. Controlled Release, 2012, 162(1), 45–55, DOI:10.1016/j.jconrel.2012.05.051.
- J. Liu, S. Smith and C. Wang, Photothermal Attenuation of Cancer Cell Stemness, Chemoresistance, and Migration Using CD44-Targeted MoS2 Nanosheets, Nano Lett., 2023, 23(5), 1989–1999, DOI:10.1021/acs.nanolett.3c00089.
- M. Ashrafizadeh, et al., Doxorubicin-loaded graphene oxide nanocomposites in cancer medicine: stimuli-responsive carriers, co-delivery and suppressing resistance, Expert Opin. Drug Delivery, 2022, 19(4), 355–382, DOI:10.1080/17425247.2022.2041598.
- S. W. Langer, Dexrazoxane for the treatment of chemotherapy-related side effects, Cancer Manage. Res., 2014, 6, 357–363, DOI:10.2147/CMAR.S47238.
- Y. Zhang, et al., Co-delivery of doxorubicin and curcumin by pH-sensitive prodrug nanoparticle for combination therapy of cancer, Sci. Rep., 2016, 6, 1–12, DOI:10.1038/srep21225.
- L. Zhang, C. Wang, Y. Jiang, S. Zhang, D. Ye and L. Liu, Drug delivery mechanism of doxorubicin and camptothecin on single-walled carbon nanotubes by DFT study, Appl. Surf. Sci., 2023, 614(2022), 156242, DOI:10.1016/j.apsusc.2022.156242.
- P. M. Perrigue, R. A. Murray, A. Mielcarek, A. Henschke and S. E. Moya, Degradation of drug delivery nanocarriers and payload release: A review of physical methods for tracing nanocarrier biological fate, Pharmaceutics, 2021, 13(6), 770, DOI:10.3390/pharmaceutics13060770.
- M. Ghezzi, S. Pescina, C. Padula, P. Santi, E. Del Favero, L. Cantù and S. Nicoli, Polymeric Micelles in Drug Delivery: An Insight of the Techniques for Their Characterization and Assessment in Biorelevant Conditions, J. Controlled Release, 2021, 332(10), 312–336, DOI:10.1016/j.jconrel.2021.02.031.
- E. Miele, G. P. Spinelli, E. Miele, F. Tomao and S. Tomao, Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer, Int. J. Nanomed., 2009, 4(1), 99–105, DOI:10.2147/ijn.s3061.
- L. P. Mendes, J. Pan and V. P. Torchilin, Dendrimers as nanocarriers for nucleic acid and drug delivery in cancer therapy, Molecules, 2017, 22(9), 1–21, DOI:10.3390/molecules22091401.
- K. Dhaliwal, Biodegradable Polymers and their Role in Drug Delivery Systems, Biomed. J. Sci. Tech. Res., 2018, 11(1), 8315–8320, DOI:10.26717/bjstr.2018.11.002056.
- S. C. McBain, H. H. P. Yiu and J. Dobson, Magnetic nanoparticles for gene and drug delivery, Int. J. Nanomed., 2008, 3(2), 169–180, DOI:10.2147/ijn.s1608.
- Y. He, et al., Pulmonary Targeting Crosslinked Cyclodextrin Metal–Organic Frameworks for Lung Cancer Therapy, Adv. Funct. Mater., 2020, 31(3), 2004550, DOI:10.1002/adfm.202004550.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Molecular Biology of the cell, Garland Science, New York, 2008 Search PubMed.
- B. S. Wong, et al., Carbon nanotubes for delivery of small molecule drugs, Adv. Drug Delivery Rev., 2013, 65(15), 1964–2015, DOI:10.1016/j.addr.2013.08.005.
- Z. Guo, et al., Surface Functionalization of Graphene-Based Materials: Biological Behavior, Toxicology, and Safe-By-Design Aspects, Adv. Biol., 2021, 5(9), 2100637, DOI:10.1002/adbi.202100637.
- S. Goenka, V. Sant and S. Sant, Graphene-based nanomaterials for drug delivery and tissue engineering, J. Controlled Release, 2014, 173(1), 75–88, DOI:10.1016/j.jconrel.2013.10.017.
- Z. Liu, S. Tabakman, K. Welsher and H. Dai, Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery, Nano Res., 2009, 2(2), 85–120, DOI:10.1007/s12274-009-9009-8.
- W. F. Lai and W. T. Wong, Use of graphene-based materials as carriers of bioactive agents, Asian J. Pharm. Sci., 2021, 16(5), 577–588, DOI:10.1016/j.ajps.2020.11.004.
- D. J. Lim, M. Sim, L. Oh, K. Lim and H. Park, Carbon-based drug delivery carriers for cancer therapy, Arch. Pharmacal Res., 2014, 37(1), 43–52, DOI:10.1007/s12272-013-0277-1.
- M. Orecchioni, R. Cabizza, A. Bianco and L. G. Delogu, Graphene as cancer theranostic tool: Progress and future challenges, Theranostics, 2015, 5(7), 710–723, DOI:10.7150/thno.11387.
- K. Santhosh Kumar, M. D. Modak and P. Paik, Graphene Oxide for Biomedical Applications, J. Nanomed. Res., 2017, 5(6), 00136, DOI:10.15406/jnmr.2017.05.00136.
- R. Shaheena, Role of DFT in Drug Design: A Mini Review, Drug Des.: Open Access, 2022, 11(4), 1–4, DOI:10.35248/2169-0138.
- H. Tandon, T. Chakraborty and V. Suhag, A Brief Review on Importance of DFT In Drug Design, Res. Med. Eng. Sci., 2019, 7(4), 791–795, DOI:10.31031/RMES.2019.07.00068.
- V. T. Sabe, et al., Current trends in computer aided drug design and a highlight of drugs discovered via computational techniques: A review, Eur. J. Med. Chem., 2021, 224, 113705, DOI:10.1016/j.ejmech.2021.113705.
- S. M. Hassani and S. Bagheri, Computational procedure for determining physicochemical properties of doxorubicin-PLGA nanoparticles and daunorubicin-PLGA nanoparticles, Int. J. PharmTech Res., 2012, 4(3), 1242–1246 CAS.
- S. M. Hassani and S. Bagheri, Theoretical Study on Physicochemical and Geometrical Properties of Doxorubicin-PEG-FOL Nanoparticles and Daunorubicin-PEG-FOL Nanoparticles, Adv. Biores., 2012, 3, 45–48 CAS.
- K. S. Novoselov, Electric Field Effect in Atomically Thin Carbon Films, Science, 2004, 306(5696), 666–669, DOI:10.1126/science.1102896.
- S. Park, et al., Graphene Oxide in a Wide organic solvent, Nano Lett., 2009, 9, 1593–1597 CrossRef CAS PubMed.
- M. Z. Tonel, M. O. Martins, I. Zanella, R. B. Pontes and S. B. Fagan, A first-principles study of the interaction of doxorubicin with graphene, Comput. Theor. Chem., 2017, 1115, 270–275, DOI:10.1016/j.comptc.2017.07.004.
- M. Mahdavi, F. Rahmani and S. Nouranian, Molecular simulation of pH-dependent diffusion, loading, and release of doxorubicin in graphene and graphene oxide drug delivery systems, J. Mater. Chem. B, 2016, 4(46), 7441–7451, 10.1039/C6TB00746E.
- M. M. Mirhosseini, M. Rahmati, S. S. Zargarian and R. Khordad, Molecular dynamics simulation of functionalized graphene surface for high efficient loading of doxorubicin, J. Mol. Struct., 2017, 1141, 441–450, DOI:10.1016/j.molstruc.2017.04.007.
- J. W. Shen, et al., Molecular dynamics study on the adsorption and release of doxorubicin by chitosan-decorated graphene, Carbohydr. Polym., 2020, 248, 1–7, DOI:10.1016/j.carbpol.2020.116809.
- J. Song, et al., Controllability of Graphene Oxide Doxorubicin Loading Capacity Based on Density Functional Theory, Nanomaterials, 2022, 12(3), 1–10, DOI:10.3390/nano12030479.
- M. Mahdavi, A. Fattahi, E. Tajkhorshid and S. Nouranian, Molecular Insights into the Loading and Dynamics of Doxorubicin on PEGylated Graphene Oxide Nanocarriers, ACS Appl. Bio Mater., 2020, 3(3), 1354–1363, DOI:10.1021/acsabm.9b00956.
- B. V. Farahani, G. R. Behbahani and N. Javadi, Functionalized multi walled carbon nanotubes as a carrier for doxorubicin: Drug adsorption study and statistical optimization of drug loading by factorial design methodology, J. Braz. Chem. Soc., 2016, 27(4), 694–705, DOI:10.5935/0103-5053.20150318.
- T. Arabian, S. Amjad-Iranagh and R. Halladj, Molecular dynamics simulation study of doxorubicin adsorption on functionalized carbon nanotubes with folic acid and tryptophan, Sci. Rep., 2021, 11(1), 1–11, DOI:10.1038/s41598-021-03619-8.
- S. Karimzadeh, B. Safaei and T. C. Jen, Theorical investigation of adsorption mechanism of doxorubicin anticancer drug on the pristine and functionalized single-walled carbon nanotube surface as a drug delivery vehicle: A DFT study, J. Mol. Liq., 2021, 322, 114890, DOI:10.1016/j.molliq.2020.114890.
- J. Zhao, et al., Molecular insight into the enhancement of benzene–carbon nanotube interactions by surface modification for drug delivery systems (DDS), Appl. Surf. Sci., 2017, 416, 757–765, DOI:10.1016/j.apsusc.2017.04.186.
- C. Wang, et al., A DFT Study on the High-density Assembly of Doxorubicin Drug Delivery by Single-walled Carbon Nanotubes, Phys. E, 2021, 134, 114892 CrossRef CAS.
- M. Pakdel, H. Raissi and M. Shahabi, Predicting doxorubicin drug delivery by single-walled carbon nanotube through cell membrane in the absence and presence of nicotine molecules: a molecular dynamics simulation study, J. Biomol. Struct. Dyn., 2020, 38(5), 1488–1498, DOI:10.1080/07391102.2019.1611474.
- A. Kordzadeh, M. Zarif and S. Amjad-Iranagh, Molecular dynamics insight of interaction between the functionalized-carbon nanotube and cancerous cell membrane in doxorubicin delivery, Comput. Methods Programs Biomed., 2023, 230, 107332, DOI:10.1016/j.cmpb.2022.107332.
- R. Maleki, H. H. Afrouzi, M. Hosseini, D. Toghraie, A. Piranfar and S. Rostami, pH-sensitive loading/releasing of doxorubicin using single-walled carbon nanotube and multi-walled carbon nanotube: A molecular dynamics study, Comput. Methods Programs Biomed., 2020, 186, 105210, DOI:10.1016/j.cmpb.2019.105210.
- L. Zhang, J. Xia, Q. Zhao, L. Liu and Z. Zhang, Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs, Small, 2010, 6(4), 537–544, DOI:10.1002/smll.200901680.
- Z. Wang, et al., Fabrication and characterization of a triple functionalization of graphene oxide with Fe3O4, folic acid and doxorubicin as dual-targeted drug nanocarrier, Colloids Surf., B, 2013, 106, 60–65, DOI:10.1016/j.colsurfb.2013.01.032.
- H. Ding, et al., Beyond a Carrier: Graphene Quantum Dots as a Probe for Programmatically Monitoring Anti-Cancer Drug Delivery, Release, and Response, ACS Appl. Mater. Interfaces, 2017, 9(33), 27396–27401, DOI:10.1021/acsami.7b08824.
- X. Zhao, et al., Design and Development of Graphene Oxide Nanoparticle/Chitosan Hybrids Showing pH-Sensitive Surface Charge-Reversible Ability for Efficient Intracellular Doxorubicin Delivery, ACS Appl. Mater. Interfaces, 2018, 10(7), 6608–6617, DOI:10.1021/acsami.7b16910.
- M. L. Salem, A. Gemeay, S. Gomaa, M. A. Aldubayan and L. Assy, Superparamagnetic graphene oxide/magnetite nanocomposite delivery system for doxorubicin-induced distinguished tumor cell cycle arrest and apoptosis, J. Nanopart. Res., 2020, 22, 219, DOI:10.1007/s11051-020-04932-5.
- S. M. Mousavi, M. Babazadeh and M. Nemati, et al., Doxorubicin-loaded biodegradable chitosan–graphene nanosheets for drug delivery applications, Polym. Bull., 2022, 79, 6565–6580, DOI:10.1007/s00289-021-03783-x.
- A. O. E. Abdelhalim, et al., Graphene oxide conjugated with doxorubicin: Synthesis, bioactivity, and biosafety, J. Mol. Liq., 2022, 359, 119156, DOI:10.1016/j.molliq.2022.119156.
- D. Matyszewska, E. Napora, K. Żelechowska, J. F. Biernat and R. Bilewicz, Synthesis, characterization, and interactions of single-walled carbon nanotubes modified with doxorubicin with Langmuir–Blodgett biomimetic membranes, J. Nanopart. Res., 2018, 20(5) DOI:10.1007/s11051-018-4239-x.
- S. Yang, et al., PEG/PEI-functionalized single-walled carbon nanotubes as delivery carriers for doxorubicin: synthesis, characterization, and in vitro evaluation, Beilstein J. Nanotechnol., 2020, 11, 1728–1741, DOI:10.3762/BJNANO.11.155.
- C. K. Thakur, R. Neupane, C. Karthikeyan, C. R. Ashby Jr, R. J. Babu, S. H. S. Boddu, A. K. Tiwari and N. S. H. N. Moorthy, et al., Lysinated Multiwalled Carbon Nanotubes with Carbohydrate Ligands as an Effective Nanocarrier for Targeted Doxorubicin Delivery to Breast Cancer Cells, Molecules, 2022, 27(21), 7461, DOI:10.3390/molecules27217461.
- P. S. Uttekar, S. H. Lakade, V. K. Beldar and M. T. Harde, Facile synthesis of multi-walled carbon nanotube via folic acid grafted nanoparticle for precise delivery of doxorubicin, IET Nanobiotechnol., 2019, 13(7), 688–696, DOI:10.1049/iet-nbt.2018.5421.
- K. M. Lyra, et al., Multi-walled carbon nanotubes decorated with guanidinylated dendritic molecular transporters: An efficient platform for the selective anticancer activity of doxorubicin, Pharmaceutics, 2021, 13(6), 858, DOI:10.3390/pharmaceutics13060858.
- Y. Yan, et al., Stacking of doxorubicin on folic acid-targeted multiwalled carbon nanotubes for in vivo chemotherapy of tumors, Drug Delivery, 2018, 25(1), 1607–1616, DOI:10.1080/10717544.2018.1501120.
- H. Vovusha, et al., Binding Characteristics of Anticancer Drug Doxorubicin with Two-Dimensional Graphene and Graphene Oxide: Insights from Density Functional Theory Calculations and Fluorescence Spectroscopy, J. Phys. Chem. C, 2018, 122(36), 21031–21038, DOI:10.1021/acs.jpcc.8b04496.
- S. Sohrabi, M. Khedri, R. Maleki and M. Keshavarz Moraveji, Molecular engineering of the last-generation CNTs in smart cancer therapy by grafting PEG-PLGA-riboflavin, RSC Adv., 2020, 10(67), 40637–40648, 10.1039/d0ra07500k.
- S. Javadian, K. Najafi, S. M. Sadrpoor, F. Ektefa, N. Dalir and M. Nikkhah, Graphene quantum dots based magnetic nanoparticles as a promising delivery system for controlled doxorubicin release, J. Mol. Liq., 2021, 331, 115746, DOI:10.1016/j.molliq.2021.115746.
- A. M. Sawy, et al., Insights of doxorubicin loaded graphene quantum dots: Synthesis, DFT drug interactions, and cytotoxicity, Mater. Sci. Eng., C, 2021, 122, 111921, DOI:10.1016/j.msec.2021.111921.
- R. Garriga, et al., Toxicity of carbon nanomaterials and their potential application as drug delivery systems: In vitro studies in caco-2 and mcf-7 cell lines, Nanomaterials, 2020, 10(8), 1–21, DOI:10.3390/nano10081617.
- A. Alinejad, H. Raissi and H. Hashemzadeh, Understanding co-loading of doxorubicin and camptothecin on graphene and folic acid-conjugated graphene for targeting drug delivery: classical MD simulation and DFT calculation, J. Biomol. Struct. Dyn., 2020, 38(9), 2737–2745, DOI:10.1080/07391102.2019.1645044.
- H. Hashemzadeh and H. Raissi, Understanding loading, diffusion and releasing of Doxorubicin and Paclitaxel dual delivery in graphene and graphene oxide carriers as highly efficient drug delivery systems, Appl. Surf. Sci., 2020, 500, 144220, DOI:10.1016/j.apsusc.2019.144220.
- A. Khoshoei, E. Ghasemy, F. Poustchi, M. A. Shahbazi and R. Maleki, Engineering the pH-Sensitivity of the Graphene and Carbon Nanotube Based Nanomedicines in Smart Cancer Therapy by Grafting Trimetyl Chitosan, Pharm. Res., 2020, 37(8), 1–13, DOI:10.1007/s11095-020-02881-1.
- E. Alimohammadi, R. Maleki, H. Akbarialiabad and M. Dahri, Novel pH-responsive nanohybrid for simultaneous delivery of doxorubicin and paclitaxel: an in-silico insight, BMC Chem., 2021, 15(1), 1–11, DOI:10.1186/s13065-021-00735-4.
- P. Gong, et al., Multifunctional fluorescent PEGylated fluorinated graphene for targeted drug delivery: An experiment and DFT study, Dyes Pigm., 2019, 162, 573–582, DOI:10.1016/j.dyepig.2018.10.031.
- S. Astani, R. Salehi, B. Massoumi and A. Massoudi, Co-delivery of cisplatin and doxorubicin by carboxylic acid functionalized poly (hydroxyethyl methacrylate)/reduced graphene nanocomposite for combination chemotherapy of breast cancer cells, J. Biomater. Sci., Polym. Ed., 2021, 32(5), 657–677, DOI:10.1080/09205063.2020.1855393.
- G. Lalwani, M. D'Agati, A. M. Khan and B. Sitharaman, Toxicology of graphene-based nanomaterials, Adv. Drug Delivery Rev., 2016, 105, 109–144, DOI:10.1016/j.addr.2016.04.028.
- S. Toyokuni, Genotoxicity and carcinogenicity risk of carbon nanotubes, Adv. Drug Delivery Rev., 2013, 65(15), 2098–2110, DOI:10.1016/j.addr.2013.05.011.
- H. Dumortier, When carbon nanotubes encounter the immune system: Desirable and undesirable effects, Adv. Drug Delivery Rev., 2013, 65(15), 2120–2126, DOI:10.1016/j.addr.2013.09.005.
- L. Meng, X. Zhang, Q. Lu, Z. Fei and P. J. Dyson, Single walled carbon nanotubes as drug delivery vehicles: Targeting doxorubicin to tumors, Biomaterials, 2012, 33(6), 1689–1698, DOI:10.1016/j.biomaterials.2011.11.004.
- K. P. Wen, Y. C. Chen, C. H. Chuang, H. Y. Chang, C. Y. Lee and N. H. Tai, Accumulation and toxicity of intravenously-injected functionalized graphene oxide in mice, J. Appl. Toxicol., 2015, 35(10), 1211–1218, DOI:10.1002/jat.3187.
- W. Chen, et al., Renal clearance of graphene oxide: glomerular filtration or tubular secretion and selective kidney injury association with its lateral dimension, J. Nanobiotechnol., 2023, 21(1), 1–18, DOI:10.1186/s12951-023-01781-x.
- W. Wu and T. Li, Unraveling the in vivo fate and cellular pharmacokinetics of drug nanocarriers, Adv. Drug Delivery Rev., 2019, 143, 1–2, DOI:10.1016/j.addr.2019.08.003.
- W. Wu and T. Li, Deepening the understanding of the in vivo and cellular fate of nanocarriers, Adv. Drug Delivery Rev., 2022, 189, 114529, DOI:10.1016/j.addr.2022.114529.
- S. Liang, et al., In vivo pharmacokinetics, transfer and clearance study of graphene oxide by La/Ce dual elemental labelling method, NanoImpact, 2020, 17, 100213, DOI:10.1016/j.impact.2020.100213.
- R. Li, T. S. C. Ng, M. A. Garlin, R. Weissleder and M. A. Miller, Understanding the In Vivo Fate of Advanced Materials by Imaging, Adv. Funct. Mater., 2020, 30(37), 1910369, DOI:10.1002/adfm.201910369.
- Z. Liu, C. Davis, W. Cai, L. He, X. Chen and H. Dai, Circulation and long-term fate of functionalized, biocompatible single-walled carbon nanotubes in mice probed by Raman spectroscopy, Proc. Natl. Acad. Sci. U. S. A., 2008, 105(5), 1410–1415, DOI:10.1073/pnas.0707654105.
- Z. Zhao, A. Ukidve, V. Krishnan and S. Mitragotri, Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers, Adv. Drug Delivery Rev., 2019, 143, 3–21, DOI:10.1016/j.addr.2019.01.002.
- D. A. Jasim, C. Ménard-Moyon, D. Bégin, A. Bianco and K. Kostarelos, Tissue distribution and urinary excretion of intravenously administered chemically functionalized graphene oxide sheets, Chem. Sci., 2015, 6(7), 3952–3964, 10.1039/c5sc00114e.
- D. A. Jasim, et al., Thickness of functionalized graphene oxide sheets plays critical role in tissue accumulation and urinary excretion: A pilot PET/CT study, Appl. Mater. Today, 2016, 4, 24–30, DOI:10.1016/j.apmt.2016.04.003.
- G. P. Kotchey, Y. Zhao, V. E. Kagan and A. Star, Peroxidase-mediated biodegradation of carbon nanotubes in vitro and in vivo, Adv. Drug Delivery Rev., 2013, 65(15), 1921–1932, DOI:10.1016/j.addr.2013.07.007.
- N. E. Helwig, S. Hong, and E. T. Hsiao-wecksler, Gray's Anatomy: the Anatomical Basis of Clinical Practice, Elsevier Publications, 41st edn, 2016 Search PubMed.
- B. Zhao, et al., Secretion of intestinal goblet cells: A novel excretion pathway of nanoparticles, Nanomed. Nanotechnol. Biol. Med., 2014, 10(4), 839–849, DOI:10.1016/j.nano.2013.10.004.
| This journal is © The Royal Society of Chemistry 2024 |