 Open Access Article
Open Access ArticleSafe nanomaterials: from their use, application, and disposal to regulations
Jorge Antonio
Chávez-Hernández
a,
Aída Jimena
Velarde-Salcedo
a,
Gabriela
Navarro-Tovar
ab and
Carmen
Gonzalez
*a
aFacultad de Ciencias Quimicas, Universidad Autonoma de San Luis Potosi, Manuel Nava 6, Zona Universitaria, 78210, San Luis Potosí SLP, Mexico. E-mail: gonzalez.castillocarmen@uaslp.mx; Tel: +5211-52-444-8262300, ext. 6459
bConsejo Nacional de Humanidades, Ciencias y Tecnologias, Insurgentes Sur 1582, Credito Constructor, Benito Juarez, 03940, Mexico City, Mexico
First published on 15th January 2024
Abstract
Nanomaterials are structures with a wide range of applications in the medical, pharmaceutical, food, textile, and electronic industries, reaching more customers worldwide. As a relatively new technological field, the information about the associated risk of nanomaterials in environmental and human health must be addressed and consolidated to develop accurate legislations, frameworks, and guidelines to standardise their use in any field. This review aims to display and context the global applications of nanomaterials, their final disposal, as well as the perspective of the current efforts formulated by various countries (including Mexico and Latin American countries), international official departments and organisations directed to implement regulations on nanomaterials, nanotechnology, and nanoscience matters. In addition, the compiled information includes the tools, initiatives, and strategies to develop regulatory frameworks, such as life cycle assessments, risk assessments, technical tools, and biological models to evaluate their effects on living organisms. Finally, the authors point out the importance of implementing global regulations to promote nanotechnological research according to a precautionary principle focused on an environmental and health protection approach to ensure the use and application of nanotechnologies safely, and responsibly.
Introduction
Today, nanotechnology leads to numerous nano-based products available in the market, breakthroughs around technology, and synthesis methods. Thus, nanotechnology brings to the market high-tech characterisation equipment, and it has accelerated growth in various areas of science and industry.Historically, the visualisation of nanotechnology can be tracked to 1959, when physicist Richard Feynman proposed his famous lecture: “There's plenty of room at the bottom”,1 where he first stated direct manipulations of atoms to synthesise materials for the first time. Since then, fundamental advances in the development of nanotechnology have taken place; for example, the molecular beam epitaxy growth technique by Günther in 1968.2 Furthermore, Norio Taniguchi and Kim Erick Drexler in 1974 and 1986, respectively, used the term «nanotechnology» to describe materials at a nanoscale and their applications.3 Then, the first NMs were obtained, when a patented process for the synthesising fullerenes was granted to Robert Curl, Richard Smalley, and Harold Kroto in 1978,4 and Sumio Lijima made a breakthrough in describing the procedure to obtain carbon nanotubes (CNTs) in 1991.3 Later, Xu et al. (2004) discovered carbon dots with a size below 10 nm when they were developing an electrophoretic method for single-walled CNTs5 suitable for applications such as bioimaging, drug delivery, and catalysis due to their enhanced optical and electronic properties and low toxicity.6
The knowledge of the matter manipulation in a nanoscale, confers a great impact with economic relevance in different areas of industry and research. For the last 20 years, nanotechnology stands out with the registration of products and manufactured patents related to nanomaterials (NMs): cosmetics, toothpaste, filtration systems, photovoltaic cells, electronic devices, medical products, among others.7 In 2020, this impact reached a vaccine for the SARS CoV-2 virus, where two large pharmaceutical companies, Pfizer and Moderna, developed a vaccine using nanoliposomes (lipidic nanostructures) as a vehicle for the mRNA sequence needed for the antigenic spike protein of this virus,8 and in 2023, Alexey Ekimov, Moungi Bawendi, and Louis Eugene Brus won The Nobel Prize in Chemistry for obtaining and studying quantum dots of different elements for current electronical and optical applications9 (Fig. 1).
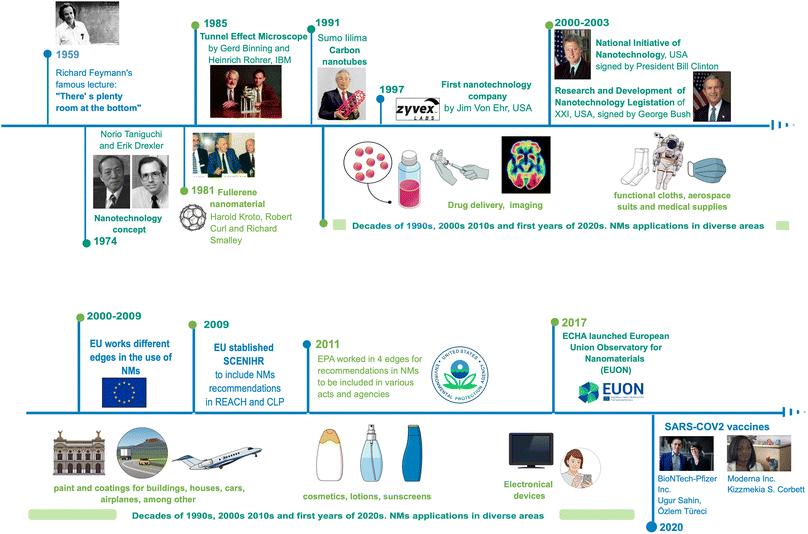 | ||
| Fig. 1 The history and development of Nanotechnology (1959–2020). Original creation (using Mind the Graph program) based on information obtained from ref. 1–9, 106, 109, 133–137, 142–144. | ||
According to the National Science Foundation workshop report “Nanotechnology Research Directions”, nanotechnology is defined as «the application of scientific knowledge to manipulate and control matter predominantly in the nanoscale to use size- and structure-dependent properties and phenomena distinct from those associated with individual atoms or molecules, or extrapolation from larger sizes of the same material».10 From this, crucial concepts emerge such as nanomaterial (NM), which is «a material with any external dimension in the nanoscale or having an internal structure or surface structure in the nanoscale»,11 and nanoparticles (NPs) defined as «nano-objects with all external dimensions in the nanoscale where the lengths of the longest and the shortest axes of the nano-object do not differ significantly».12 However, other definitions are suggested. The European Commission (EC) has worked on definitions, compiling information, and proposing regulations for about two decades and defines NM as «a natural, incidental or manufactured material consisting of solid particles that are present, either on their own or as identifiable constituent particles in aggregates or agglomerates, and where 50% or more of these particles in the number-based size distribution fulfil at least one of the following conditions: (a) one or more external dimensions of the particle are in the size range 1 nm to 100 nm; (b) the particle has an elongated shape, such as a rod, fibre or tube, where two external dimensions are smaller than 1 nm and the other dimension is larger than 100 nm; (c) the particle has a plate-like shape, where one external dimension is smaller than 1 nm and the other dimensions are larger than 100 nm».13
If the NM is synthesised, the process can be performed by “top down” or “bottom up” techniques. In the first group, a raw material in a micro or macro scale is breaking down to the nanoscale, while “bottom up” techniques refer to assembling atoms and molecules into nanoscale arrangements.14 Those synthesis are selected according with the chemical nature of the NM. In this respect, NMs are classified in (1) carbon-based NMs (e.g. graphene and carbon nanotubes); (2) metallic-based NMs (e.g. gold and silver NPs; (3) quantum dots (e.g. Cerium quantum dots); (4) dendrimers, which are polymeric branched nanostructures; and (5) composites, for a combination of inorganic and organic structures.15
The development of new NM-derived products and applications are associated with increasing numbers of requested and approved patents. In 1991, 224 patents were registered worldwide while in 2008, the number of patents increased to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 776.16 The data obtained from the United States Patent and Trademark Office (USPTO) until 2023 ranks the United States as the leading country in nanotechnology patenting, followed by South Korea, China, Japan, and Taiwan. Moreover, according to the European Patent Office (EPO), the United States is also the leading country in patenting, followed by Germany, France, Japan, and South Korea. The most recent update of nanotechnology Japan patenting dates to March 2023 and it shows that the United States still leads in nanotechnology patenting (Fig. 2A). Currently, the Statnano platform17 compiles information converging from nanotechnology, biotechnology, informatics, and cognitive science technologies. This platform states that 3910 companies in 68 countries offer 11
776.16 The data obtained from the United States Patent and Trademark Office (USPTO) until 2023 ranks the United States as the leading country in nanotechnology patenting, followed by South Korea, China, Japan, and Taiwan. Moreover, according to the European Patent Office (EPO), the United States is also the leading country in patenting, followed by Germany, France, Japan, and South Korea. The most recent update of nanotechnology Japan patenting dates to March 2023 and it shows that the United States still leads in nanotechnology patenting (Fig. 2A). Currently, the Statnano platform17 compiles information converging from nanotechnology, biotechnology, informatics, and cognitive science technologies. This platform states that 3910 companies in 68 countries offer 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 171 products using nanotechnology products and developments (Fig. 2B). Companies such as Intel Corporation have registered 189 products using nanotechnology, and Texas Instruments Inc. has registered 60 products that employ nanotechnologies.18
171 products using nanotechnology products and developments (Fig. 2B). Companies such as Intel Corporation have registered 189 products using nanotechnology, and Texas Instruments Inc. has registered 60 products that employ nanotechnologies.18
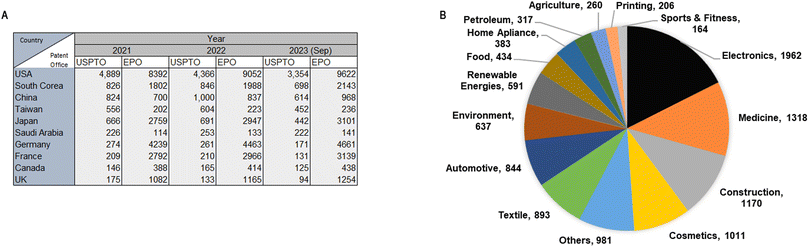 | ||
| Fig. 2 (A) Top ten patenting countries (2019–2023) according with the United States Patent and Trademark Office and the European Patent Office; (B) nanotechnology products classified (2019–2023) according with industrial areas.18 Creation adapted and based on information obtained from http://www.statnano.com/ (accessed on December 14th, 2023).18 | ||
On the other hand, funding for nanotechnology research increased from 432 million dollars to 4100 million dollars between 1997 and 2005.16 NM-related products have attracted more investments, and between 2007 and 2011, only the European Union reported 970 million dollars as an investment in NMs research. That approximation is about a quarter of a trillion dollars worldwide, and it is expected to exceed 125 billion dollars by 2024.19 The Statnano database has registered 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 040
040![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 research papers about NMs, 229
200 research papers about NMs, 229![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 774 patents and 4326 products.18 The United States alone had registered 2300 products containing NMs during the first decade of the 21st century.20 The Woodrow Wilson International Centre for Scholars, a non-partisan organisation forum, conducted independent research, and registered 54 nanoproducts in 2005; by 2009 this number increased to 1015 products.21 By 2020, the Woodrow Wilson Database reported 1833 nanotechnological products worldwide.22 Nanowerk Nanomaterial Database Inventory, the global leading portal database of nanosciences and nanotechnology products, news, links with nanotechnologies industries/laboratories, industrial reports, magazines, and journals, registered an increase in the number of manufactured nanoproducts from 1917 in 2008 up to 2238 products in 2009, and a total of 3600 products in 2018.2,21
774 patents and 4326 products.18 The United States alone had registered 2300 products containing NMs during the first decade of the 21st century.20 The Woodrow Wilson International Centre for Scholars, a non-partisan organisation forum, conducted independent research, and registered 54 nanoproducts in 2005; by 2009 this number increased to 1015 products.21 By 2020, the Woodrow Wilson Database reported 1833 nanotechnological products worldwide.22 Nanowerk Nanomaterial Database Inventory, the global leading portal database of nanosciences and nanotechnology products, news, links with nanotechnologies industries/laboratories, industrial reports, magazines, and journals, registered an increase in the number of manufactured nanoproducts from 1917 in 2008 up to 2238 products in 2009, and a total of 3600 products in 2018.2,21
On the other hand, the technology consultancy Future Markets Inc. projected in 2012 a total worldwide production of 250![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 300
000 to 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 metric tons of NPs obtained by chemical synthesis. Meanwhile, the EC reported in the same year 1
000 metric tons of NPs obtained by chemical synthesis. Meanwhile, the EC reported in the same year 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 tons worldwide;23 among them, the most produced NMs (2010–2012) were NPs of titanium dioxide (TiO2), silicon dioxide (SiO2), aluminium oxides (AlxOx), zinc oxide (ZnO), iron oxides (FexOy), cerium oxides (CexOy), silver (AgNPs), and CNTs.20
000 tons worldwide;23 among them, the most produced NMs (2010–2012) were NPs of titanium dioxide (TiO2), silicon dioxide (SiO2), aluminium oxides (AlxOx), zinc oxide (ZnO), iron oxides (FexOy), cerium oxides (CexOy), silver (AgNPs), and CNTs.20
Nowadays, nanotechnology has a significant participation in global markets; the sustained increase and past and future growth clearly show its importance. For example, the valued of nanotechnology was up to 2 trillion USD in 2015 (ref. 24); meanwhile, other projections assessed 2020 a value in the global economy of up to 3 trillion USD.25 Inkwood Research projected that the NM market would reach an approximate value of up to 13 billion USD, and Mordor Intelligence forecasted a value in markets of NMs up to 11.3 billion USD in 2020.2 This economic data was before 2020; therefore, the current monetary yields could be far more today, and the impact of the SARS-CoV2 global pandemic must be considered.
By the immediate antecedents related to the accelerated increase in products containing NMs, this review explores and make an analysis of the current applications and uses of a series of products based on NMs. At the same time, the authors contextualise aspects related to the disposal, regulations, legislations, and worldwide advances of these materials in the nano scale within the last years. Some publications have recognised the importance of the regulation of NMs in fields such as cosmetics or nanomedicine26–28 or address them in a broader approach,29,30 analysing aspects such as funding, patenting, economic participation of NMs in global markets, but also the impact that confer the release of NMs in the environment and health risks. In addition, this review emphasises the current situation in which countries from different continents face the situation of NMs regulation, including countries that have designed initiatives with different degrees to contribute to the good use of nanotechnologies, which gives a broader view to the reader of the importance of developing regulations on NMs. It is also stablished the importance of the development of these regulations and how they should be strongly related with the Sustainable Development Goals (SDG).
Global applications of NMs
The United Nations (UN) has developed actions to contribute to NMs-related environmental safety. In 2015, all the countries that belong to the UN approved the 17 Sustainable Development Goals (SDG), part of the Sustainable Development Agenda 2030 that included objectives related to human health and environmental safety.31 To the date, uses and application with NMs are diverse as Fig. 1 represents. Those applications may cover relevant targets in the SDG. For instance, objective 3 good health and well being, considers reducing by one third premature mortality from non-communicable diseases through prevention and treatment and promote mental health and well-being. NMs are being applied for the improvement of diagnosis, drug delivery and vaccines (communicable and non-communicable diseases). NMs are also considered for the removal and degradation of water pollutants to enhance water quality, which is part of the targets in objective 6 clean water and sanitation. Interestingly, a recent use of NMs is as nano pesticides to replace hazardous chemical currently applied in crops, and this is also a target in the objective 6 for reducing water contamination. Moreover, the use and applications of NMs in solar cells and other devices for renewal energies may reduce the costs of those systems, as considered in objective 7 affordable and clean energy. On the other hand, the commercial use of NMs for painting and coating impact in the development of quality, reliable, sustainable, and resilient infrastructure as set in objective 9 industry innovation and infrastructure.Regarding objective 3 good health and well being, most of the NMs applications are lipid-based NMs. Previously, it has been mentioned the BioNTech-Pfizer and Moderna SARS-CoV2 vaccines were a breakthrough in 2020 when the COVID-19 pandemic stroked. Those vaccines contain a nucleoside-modified mRNA encoding for SARS-CoV-2 spike protein encapsulated in liposomes assembly with polymer-phospholipids, allowing the mRNA protection and enhancing the immune response.8 However, this vaccine has not been the first biomedical NMs applications. In 1995, the FDA approved the use of Doxil®, a liposomal doxorubicin drug for Kaposi's sarcoma, ovarian cancer, and myeloid melanoma. Later, in 1997, AmBisome®, a liposomal amphotericin B against opportunistic fungal pathogens, was released to the market, reducing the toxicity of the drug in patients.32 That was the starting point for several liposomal formulations for drug delivery.33 Moreover, in 2013, MagForce Nanotechnologies AG started the commercialisation of NanoTherm® AS1 (12 nm Fe2O3 with a lipidic shell), for thermal therapy in patients with brain tumours. The Fe2O3 NPs are activated by a magnetic field that changes its polarity 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times per second and release heat into the tumour (intracranial administration of NanoTherm® is required).34,35
000 times per second and release heat into the tumour (intracranial administration of NanoTherm® is required).34,35
Another application of NMs is in the food industry. ZnONPs, SiO2 NPs, and TiO2 NPs are used in the food industry as thickeners, dyes, clarifying agents, and dust fluidity controllers. In contrast, AgNPs and ZnONPs enhance the shelf time of several products due to their bacteriostatic properties; FeONPs are used to fortify foods.36,37 Organic polymeric NPs such as pectin have also been used to elaborate films in food packages containing aqueous and alcoholic food extracts, and their synthesis is carried out from polymers obtained from natural sources and biodegradable synthetic polymers such as starch, pectin wheat proteins, zein, sunflower seeds proteins, among others.38 Polymeric NPs have been employed in the nanoencapsulation of functional foods (nutraceuticals) to protect them from the gastrointestinal tract,39 or as drug delivery vehicles to produce nanoemulsions of omega-3 fatty acids,40,41 and oil-in-water nanoemulsions (such as triglycerides with either vitamin D, astaxanthin, or β-carotene).42–44
Relevant mention is the use of NMs in cosmetics since the definition of cosmetics is typically wide in many countries. Cosmetics are interpreted as beauty products such as lipsticks and eyeliners, but according to The European Union Cosmetics Directive (EUCD) a cosmetic is “any substance or preparation intended to be placed in contact with various external parts of the human body (epidermis, hair, nails, lips, teeth…changing their appearance and/or correcting body odours and/or protecting them in good condition”.45 Thus, since cosmetics are not aimed at first to be drug formulations, less legislations applied in their production and commercialisation; hence, more NMs can be found in cosmetic formulations, including TiO2 as sunscreen (e.g. Phytorx UV defense46), nano-hydroxyapatite for dental desensitiser and polish remineralisation of teeth (e.g. Kinder Karex47), AuNPs as antimicrobial activity in facemask (e.g. gold radiance peel off mask48). However, the long-term use of NMs in cosmetics has not been studied and toxic effects could be found in evaluations. Furthermore, the released of NMs in the environment can alter the trophic chain.
Recently, the use of nanotechnology in water treatment systems (objective 6 clean water and sanitation) has been explored to improve the quality of treated water, using metallic and oxide metallic NPs due to their catalytic and/or antimicrobial activity and non-metallic NMs such as CNTs due to properties, such as high absorption surface area, layered structure and hollowness.49 In this sense, CNTs employed for wastewater treatments have demonstrated to remove heavy metals (lead or cadmium)50 and decreased microbial pollution.21 Fe2O3 NPs in batch systems have removed heavy metals such as arsenic (As III), copper (Cu II), aluminium (Al III), cadmium (Cd II), cobalt (Cd II) and nickel (Ni II).51 On the other hand, fullerenes (C60) have showed antimicrobial activity in a Bacillus subtilis demonstrating that fullerenes could be effective antimicrobial agents.52
Nano pesticides also represent an emerging research area in agriculture and environmental protection science and technology. They are chemicals used to control pests, diseases, or harmful microorganisms in agriculture and crop protection that can offer substantial benefits, such as increased effectiveness in controlling pests and diseases, reducing the use of conventional chemicals, controlled release of active ingredients, and minimizing environmental impact. There are two types of nano pesticides, the first one elaborated with Ag, Ti, and Cu as active ingredients, and the second one elaborated based on nanocarriers like nano-polymer and nano-clay to house as the active ingredients. These products act through various mechanisms, such as the controlled release of active ingredients, the inhibition of specific biological processes in pests or microorganisms, or through the modification of the surfaces properties to prevent the adhesion of microorganisms.53,54
Moreover, NMs can contribute to objective 7 affordable and clean energy by reducing environmental pollution, as they have improved the usage of renewable energy in solar cells, hydrogen production, batteries, and supercapacitors.55 Potential candidates are semiconductor materials such as silicon or gallium arsenide, which at the nanoscale present a decreased electronic and thermal conductivity and can be used in solar cells with high photoelectric conversion efficiency by catalysing the decomposition of inorganic and organic substances by solar energy.56 On the other hand, graphite and graphite-containing NMs has also been incorporated to solar panels due to their high charge and discharge rates.57 Due to the reduced scale, and therefore a faster ion and electron transport, NMs can improve the power of rechargeable batteries. Graphene and metallic-based nanocomposites have been proposed as an alternative to lithium in rechargeable batteries;58 these materials have also been used for the fabrication of electrodes in supercapacitors.59 The increased active surface area of NPs has also been exploited in catalysis and energy-conversion systems.60
The industry of paintings and coatings have applied NMs in recent years, looking for the improvement of various properties such as anticorrosion, durability, biodegradability, antimicrobial properties,61 and waterproofing in textiles62 addressing the objective 9 industry innovation and infrastructure. NMs have been used for coatings on sealed porous surfaces with a thinner layer, and various companies have applied those and other properties to commercial products. The company Niasol has products for car, furniture, and shoe protection, repelling dirt, and water from surfaces.63 Aculon sells about 100 hydrophobic nanocoating products with over 35 patents, finding applications in medical devices and electronics.64 Nanocar, which additional to the nanocoatings for car protection, it produces antimicrobial sprays for surfaces.65 Importantly, none of the recent nanotechnological companies selling paintings and coatings disclosure the use NMs in their formulations, they only state the functionality.
In contrast, NMs in commercial products and their potential applications in several fields highlights their relevance in human history, as the SDG in the Agenda 2030 stablishes. However, due to the NMs small size is crucial to consider their implications in human health and environment, faced critical challenges including the safety and toxicity of NPs, risk assessment for human health and the environment, stability and controlled release of active ingredients, regulatory concerns, in addition to the public agreement, topics that will be discuss in the following sections.
Where NMs are going after their shelf-life?
Nanotechnology-derived products are imminently incorporated in products and services under a global market that increases daily.Due to their extended production and distribution, NMs may be released to air, soil, and water. Rains can also deposit NMs to water bodies and soils, and changes in temperature, pH or either interaction with other NMs can form aggregates or ionised NMs with the subsequent uptake by different animal and vegetable species through the trophic chain (Fig. 3).
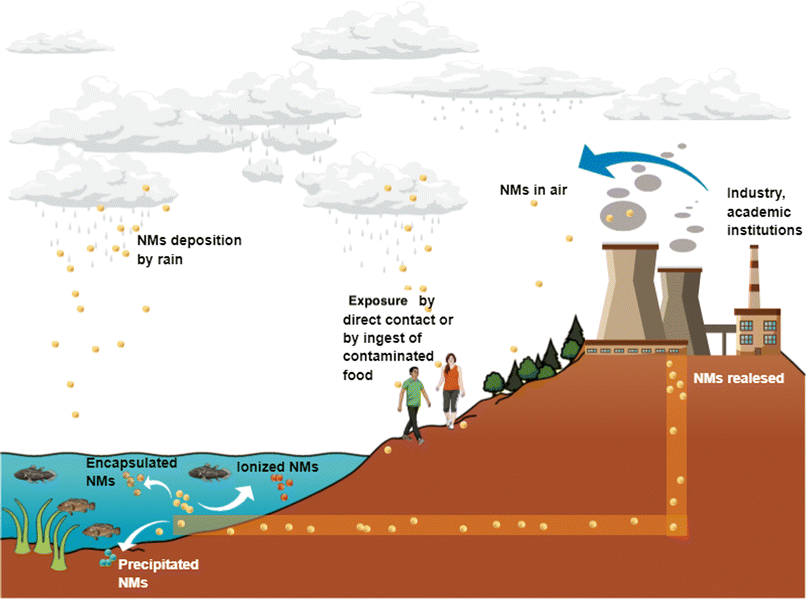 | ||
| Fig. 3 Nanoparticles released in water bodies can be taken up by aquatic fauna and enter the human body by trophic transfer. Air pollution by nanoparticles is also a concern due to nanoparticles could be deposited in water bodies through rainfall. Original creation (using Mind the Graph program) based on information obtained from ref. 23, 66–74. | ||
Thus, contact with NMs by air, water, soil, or food may activate different cellular pathways in living organisms, including humans, and this topic is worthy of being studied in detail.66 Even with the benefits that NMs provide due to their novel properties, the other side of the coin is how the waste is treated for disposal to avoid environmental pollution and diseases. Particular attention is required for products containing NMs, in which ions or individual NPs can be released from the whole matrix or from some matrix fragments. For instance, painting and coating containing NPs and applied in outdoor buildings and monuments for stone's consolidation can be altered when exposing to different weather conditions.67 Zuin et al. (2014) and Zhang et al. (2017) tested nano-based paints containing TiO2 and Ag NPs according to Standard ISO 2812-2:2007 for paints and varnishes finding the release of NPs as free forms and ionic forms.68 Other evidence of NPs and ion release is reported by Pantano et al. (2018), who analysed wood preservatives containing CuO NPs by European Standard EN84:1997, which specifies a method for the leaching of compounds (durability in rainwater).69
Hence, NMs are now grouped in a new pollutant classification known as «emerging pollutants», which can be defined as «those compounds from different origins and chemical nature whose presence in the environment is not considered significant in terms of distribution or concentration»,70 and their «concentration ranges are parts per million (ppm) to parts per trillion (ppt)»71 Some reports alert that the concentrations of several NMs such as TiO2 NPs, AgNPs, CNTs, and fullerenes in freshwater and wastewater treatment plants and effluents have reached worrying levels ranging from 0.0005–0.0008 μg L−1 (or ppb) of CNTs, 0.03–0.08, 0.04–0.34 μg L−1, both ranges valued for AgNPs, 0.0005–19 μg L−1 for fullerenes and 0.7–16 μg L−1 for TiO2 NPs.72
Furthermore, other concepts around this topic emerge that are also relevant and have their implications, such as «nanopollutants» for referring to «those NMs that are directly released in the environment», or «nanowaste», which englobes «those nanomaterials that were collected as waste coming from production activities, disposal due to end of its useful life of solid waste containing nanomaterials».23 Nanopollutants suspended in the air, for example, composed mainly of heavy metals and produced by industrial facilities, waste incinerating plants, and fossil fuel burning, can reach different environments by spreading over long distances to enter terrestrial and aquatic ecosystems. Nanopollutants have also been spread indirectly by the surface runoff from soil to waterbodies with their subsequent use of biosolids on the ground. It has been reported that NPs can permeate soil when fertilisers or plant protection products are applied and into aquatic environments through effluent discharge from wastewater plants.73 Thus, using NMs faces complex challenges before they become safe to use and prevent contributing to the problem of pollution.
Using NMs in water treatment systems is a helpful tool that increases water quality. Nevertheless, the regulatory gaps and the subsequent lack of acceptable concentration limits for NMs cannot guarantee their biosafety, including when they are released to water bodies. AgNPs could lead to possible toxic effects starting with bioaccumulation, causing cytotoxicity, tissue damage, oxidative stress, and genotoxicity in aquatic species.74 Carbon-based NPs could be a safer alternative to metallic NPs for wastewater treatment, with less harmful effects on humans and the environment; however, side effects must be further studied in the short, medium, and long term.
Since NMs are tiny and have unique properties, the same elimination approach used for other conventional materials cannot simply be applied. Then some general considerations and strategies being studied are listed: (1) recycling and reuse: depending on the type of NM and its application, it may be possible to recover and recycle them for reuse in other processes or products. This requires proper management and effective separation of NMs during the recycling process;75,76 (2) waste treatment: Sometimes, NMs can end in solid or liquid waste. For appropriate direction, it is essential to consider waste treatment methods that are safe and effective in eliminating NMs without causing negative impacts on the environment or human health;76,77 (3) research on degradability: the degradability of NMs is being investigated to determine whether they can naturally decompose into more uncomplicated and less harmful components over time.78 These approaches will help us understand NMs persistence and long-term effects on the environment and contribute to consolidate regulations and guidelines or elaborate new ones that include specific protocols and procedures directed to eliminate the NMs, reducing the damage to the environment and health.
International regulatory framework for NMs
If the incorporation of NMs to the environment and biological systems is possible, due to the accelerated increase of nanotechnology, it is necessary an adequate regulatory framework and adapting regulations that consider the particularities that emerge in the production of NMs and the industries in which they are applied.Accordingly, in 2017, the World Health Organisation (WHO) published the “WHO guidelines on protecting workers from potential risks of manufactured nanomaterials” aimed to establish guidelines for policymakers in the occupational field,79 and to address goals such as good health and well-being, decent work and economic growth, responsible consumption and production, life below water and life on land. Nowadays, the SDGs are a major axis in the development of new policies, such as environmental legislations (including NMs), and are being approached in universities and educational centres. Importantly, regulations regarding the production, use, application, and disposal of NMs impact on objective 12 responsible consumption and production of SDG, since it targets to promote public procurement practices that are sustainable, ensure that people have the relevant information and awareness for sustainable development and lifestyles in harmony with nature.
Institutions such as the United Nations for Training and Research (UNITAR) provide solutions through developing human resources with knowledge and skills in environmental matters. On the other hand, the United Nations Environmental Program (UNEP) is the foremost environmental authority in the world that promotes, overlooks, and qualifies nations for the sustainable development of the countries. To date, UNEP offers more than 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 documents related to environmental issues, including nanopollutants, in various areas, such as biosafety, energy, technology, water, etc.,80,81 (Fig. 4). So far, UNITAR's achievements can be quantified as follows: since 2010, UNITAR has organised 7172 courses and learning events and reached 780
000 documents related to environmental issues, including nanopollutants, in various areas, such as biosafety, energy, technology, water, etc.,80,81 (Fig. 4). So far, UNITAR's achievements can be quantified as follows: since 2010, UNITAR has organised 7172 courses and learning events and reached 780![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 534 beneficiaries.81
534 beneficiaries.81
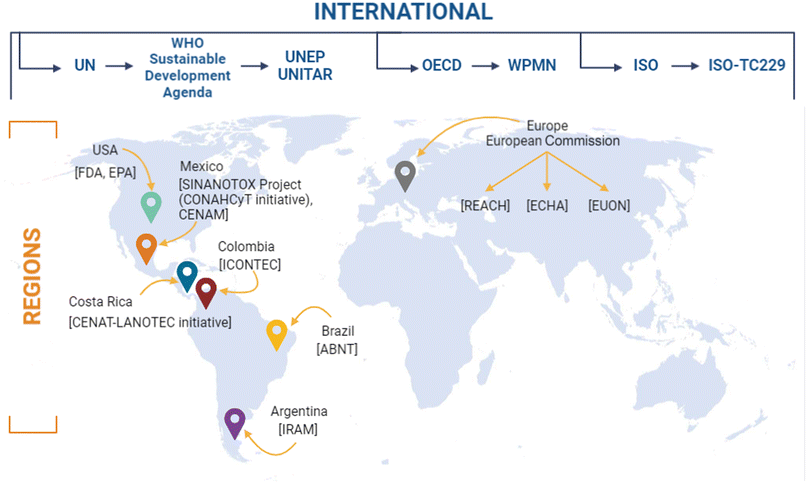 | ||
| Fig. 4 National and international authorities, agencies and initiatives developing frameworks, legislations, and guidelines for nanomaterials. UN, United Nations; WHO, World Health Organisation; UNEP, United Nations Environment Program; UNITAR, United Nations Institute for Training and Research; OECD, Organisation for Economic Cooperation and Development; WPMN, Working Party on Manufactured Nanomaterials; ISO, International Organisation for Standardisation; ISO-TC229, International Organisation for Standardisation Technical Committee 229; USA, United States of America; FDA, Food and Drug Administration; EPA, Environmental Protection Agency; SINANOTOX, National Nanotoxicological Evaluation System Initiative; CONAHCyT, National Council of Humanities, Sciences and Technologies; CENAM, National Centre of Metrology; CENAT, National Centre of High Technology; LANOTEC, National Laboratory of Nanotechnology; ICONTEC, Colombian Institute of Technical Norms; ABNT, Brazilian Association of Technical Norms; IRAM, Argentinian Institute of Normalisation and Certification; REACH, Registration, Evaluation, Authorisation, and Restriction Chemicals; ECHA, European Chemical Agency; EUON, European Union Observatory for Nanomaterials. Original creation (using Excel and Biorender programs) based on information obtained from ref. 12, 31, 79–81, 94, 98, 115, 124–137, 143, 144,159, 180, 189, 202–204, 269. | ||
In 2004, the international organisation erosion technology and concentration group pointed out the non-legal development of nanotechnology due to the considerable number of manufactured products.82 In this respect, the reports regarding the impact of NMs on health and the environment have increased at least during the last 20 years,83–92 showing the harmful effects in organs of different species (rats, fish, humans) due to the exposure and intake of NMs that could lead to a physiological misfunction and a lowered life quality. Consequently, today, the issue of regulation on emerging NMs through socio-economical politics is an aspect that has been evolving, perfecting, and advancing gradually during the last few years. However, many actions must be taken to reach the legal order and global harmonisation.
The momentum of nanotechnology development, establishment, and consolidation paths varies and differs between different countries. In some cases, there are no points of comparison regarding the aspects to be regulated or the standard type. The United States and Europe are the ones who have leadership in the establishment of both mandatory and soft regulations with specific considerations, where government agencies, public and private organisations have joined through initiatives, proposals, and generation of documents that contribute to the proper governance of nanotechnologies that include the regulation of NMs and even more, to achieve harmonisation at a global level, with the expectative to reach a delicate balance between the flexibility and control in the use and application of the regulatory measured to be beneficial in the aspects in where the social sectors are moving, such as the economic level in matters of exportation and importation products made with a nanotechnology base, but at the same time protect and safeguard the living beings and the environment.
Most of the topics discussed in turn to future regulations include: the common language related to the nomenclature, terminology, and classification, the standardised forms in its measurement, characterisation, traceability, aging of the NM, types, and levels of toxicological evaluation in the short, medium, and long term, the forms, and protocols for the disposition of them, as well as their impact on health and the environment. In addition to the products and processes generated based on NMs, their possible and potential combinations, risks, labelling for consumer awareness, work exposure, and the openness to dialogue on social and ethical aspects, several controversial points are being addressed at the national and international levels, which have promoted and motivated various sectors to propose and generate documents that lead their regulation.
To date, several countries have generated regulations on nanotechnology. The USA and Europe are actively working in the elaboration of different documents through their corresponding instruments. Considering that there are two forms of regulation: (a) mandatory and (b) voluntary or soft, the second one is mainly used while the obligatory frameworks develop,93–96 applying them as an essential reference in emerging technologies, such as nanotechnologies, where more information is necessary in specific areas that permit conduce to the generation of mandatory frameworks.94 Regulation can also be classified as horizontal and vertical. The horizontal one is defined as that regulation which affects two or more sectors, in contrast to the vertical regulation (or specific), in where dispositions affect a particular sector.95
USA and Europe have tried to include and cover several aspects of the NMs and their derivatives by applying horizontal regulation (or primary) forms. Even though the horizontal mandatory regulations cover the NMs, in this concern, it is essential to mention that the USA and Europe have different points of view on generating specific regulations for NMs. For instance, in Europe, the creation of a “NanoAct” could be elaborated from the previous instruments and regulations or the proposal of a particular and detailed regulatory framework named REACT NOW, whose acronym is Registration, Evaluation, Authorisation, Categorisation, and Tools to Evaluate Nanomaterials-Opportunities and Weaknesses.94,97,98
On the other hand, the USA regulation in the matter of NMs is conducted to ensure that products are safe for use and consumption, it focuses on occupational safety and health, establishing regulations to protect workers who handle NMs in work environments, and monitoring their exposure to the environment.94,99,100 Related to the voluntary or soft regulations of NMs, they are more flexible, and autoregulatory, represent an alternative to the laws and traditional regulatory politics, are complementary at the normative, and in several times and cases they become mandatory.94,95,101,102
Due to the controversy that NMs and their derivatives display in terms of commercialisation and the impact on health and the environment, soft regulation represents an essential and viable alternative for the industries to display to the consumer the degree of compromise and responsibility in the use of products based on NMs and prevented for new regulations.103 In this concern, there are four types of soft regulation by the nanotechnologies,93–95,104 which are described briefly:
(1) Registers; (2) labelled; (3) codes of conduct; (4) risk management systems; (5) guidelines; (6) technical standards.
Registers
As Saldívar Tanaka L., 2020 describes, the registers are systems that collect helpful information for public authorities to act more appropriately in risk management associated with protecting workers, consumers, and the environment. The information compiled in these systems is related to the potential hazard and possible exposure to humans and the environment.95 The United Kingdom, through the Department for Environment, Food and Rural Affairs (DEFRA), implemented this voluntary register to understand the properties of the NMs contained in products of importation.105 Through the EPA, the USA implemented the Nanoscale Materials Stewardship Program (NMSP), which was designed for those companies to voluntarily report information on the NMs related to the development, manufacturing, transportation, and risk management practices.106 In contrast, other European countries, such as France, Belgium, Norway, Denmark, and Sweden, have established obligatory registers for the NMs that cover aspects of the collection of information related to the production, distribution, and importation of the nanoscale substances (France, through the French Agency for Food, Environmental and Occupational Health & Safety-ANSES);107 or the registration of products based on NM in the Swedish Chemical Agency for Chemicals-KEMI implemented by Sweden. KEMI is a central supervisory authority of the Ministry of the Environment. It is responsible for “a non-toxic environment”108 to generate databases such as Nano DataBase through Danish Enviroment Pretction Agency (DEPA) or the RiskCat as a tool for understanding the risk of products based on NMs and the correspondent risk management (created by Denmark).Labelled
Product labelling is an essential tool to provide complete and accurate information to consumers related to ensuring safety and health, compliance with regulations, and enabling informed decision-making. In the case of nanotechnology-based products, several countries have implemented regulations; some examples of these countries include essential aspects: (1) European Union (EU): The EU has established labelling requirements for NM products. Manufacturers must indicate on labelling whether a product contains NMs and provide additional information on their use. The voluntary tool is the EU Ecolabel for detergents and cleaning products.109 However, in Europe, there are also mandatory mechanisms for this purpose, such as regulations No. 1223/2009![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 110 by cosmetics to ensure the safety of this kind of products and protect consumers' health. It establishes specific requirements in terms of allowed ingredients, labelling, product notification, safety testing, and market oversight No. 1169/2011,111 which contains essential considerations related to food information; the primary purpose is protect the interests of consumers by providing them with clear and complete information about the food they consume. This allows them to make informed decisions, especially regarding food safety, allergies, and nutritional information; (2) Australia: the Therapeutic Goods Regulatory Authority of Australia (TGA) has established guidelines for labelling medicines containing NMs;112 (3) Canada: Health Canada is the agency responsible for health that has developed guidelines for labelling consumer products based on NMs. These guidelines guide how manufacturers can label and communicate the presence of NMs in their products;113 (4) South Korea: The Korean Ministry of Food and Product Safety (MFDS) has established regulations for labelling products containing NMs. These regulations require manufacturers to indicate the presence of NMs in products;114 (5) Mexico: It applies the Mexican technical standard non-mandatory NMX-R13830-SCFI-2014, which is equal to the ISO/TS1383 guide for voluntary labelling of consumer products containing nanoobjects.115
110 by cosmetics to ensure the safety of this kind of products and protect consumers' health. It establishes specific requirements in terms of allowed ingredients, labelling, product notification, safety testing, and market oversight No. 1169/2011,111 which contains essential considerations related to food information; the primary purpose is protect the interests of consumers by providing them with clear and complete information about the food they consume. This allows them to make informed decisions, especially regarding food safety, allergies, and nutritional information; (2) Australia: the Therapeutic Goods Regulatory Authority of Australia (TGA) has established guidelines for labelling medicines containing NMs;112 (3) Canada: Health Canada is the agency responsible for health that has developed guidelines for labelling consumer products based on NMs. These guidelines guide how manufacturers can label and communicate the presence of NMs in their products;113 (4) South Korea: The Korean Ministry of Food and Product Safety (MFDS) has established regulations for labelling products containing NMs. These regulations require manufacturers to indicate the presence of NMs in products;114 (5) Mexico: It applies the Mexican technical standard non-mandatory NMX-R13830-SCFI-2014, which is equal to the ISO/TS1383 guide for voluntary labelling of consumer products containing nanoobjects.115
Even with these labelling initiatives, several concerns arise, such as industrial secrecy, which is another controversial point116 and a limitation for the total reliability and transparency to the user.
Codes of conduct
The codes of conduct in NM regulation are sets of principles and guidelines related to ethical and practical standards for their responsible use and management. They have been developed by different organisations, such as government agencies, academic institutions, and industry associations, to point out the ethics, safety, responsibility, and sustainability related to the use and development of NMs and their derivatives, covering essential topics associated with:10,12,23,24,93,95,117(1) Occupational safety and health, related to the safety in workplaces.
(2) The manufacturing practices of NMs, which ensure that safety processes protecting the workers, environment, and consumers, are minimised.
(3) Promoting adequate transparency and communication about the presence of NMs in products allows consumers and end users to make informed decisions.
(4) The identification of possible risks of NMs, like potential hazards and implementation of mitigation measures.
(5) The impulse at the responsible and open research in the field of nanotechnologies.
It is important to note that codes of conduct regulating NMs are generally voluntary and do not have mandatory legal force. However, organisations in the different sectors must implement and follow these codes to manifest their commitment, responsability and safe practices.
Risk management systems
They are processes constituted to identify, evaluate, and control the possible risks associated with these technologies and apply them for the use and application of nanotechnologies and NMS.These systematic processes are designed to help identify, manage, and reduce the potential risks to the health and environment to ensure that NMs are safe, during their life cycle, in addition to the responsible use during and after their development, through the appropriate continuous monitoring and, if it is the case, by taking precautionary measures, through the precautionary principle, based on taking preventive measures to protect human health and the environment, even in the absence of complete scientific certainty, to avoid possible risks and promote safe and sustainable use of NMs.
This involves identifying and evaluating potential risks, implementing appropriate control measures, and using continuous monitoring to ensure that established security standards are met. These systems are important because NMs and nanotechnologies are constantly growing in various fields, such as medicine, electronics, and energy, among others.
Some initiatives that have proposed and developed risk management systems on this topic we can mention: nano risk framework;118 Certifiable Nanospecific Risk Management and Monitoring System (CENARIOS);119 precautionary matrix for synthetic nanomaterials.10,95,120
Guidelines
They are documents that provide recommendations about the use of NMs in a responsible and safe way in the different sectors and applications. These documents are elaborated and developed by different instances like government agencies, experts in the development of NMs and nanotechnologies, and international organisations.Examples of guidelines are:
Foresight guidelines for responsible nanotechnology development, elaborated by the Foresight Institute;121 guidance on the risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain revised by the European Food Safety Authority (EFSA);95,122 guidance on the safety assessment on nanomaterials in cosmetics with the Scientific Committee on Consumer Safety (SCCS) and published by the European Commission;123 guidelines of the testing programme of WPMN124 by the OECD. In addition, a compilation of “the current status of projects (aimed) to develop or adapt OECD Test Guideline (TGs) and Guidance Documents (GDs) for nanomaterials”, has elaborated in collaboration to the Federal Institute for Occupational Safety and Health (BAuA), Germany, The National Institute for Public Health, and the Environment (RIVM), Netherlands and Organisation for Economic Co-operation and Development (OECD). In a status report in July 2022, the authors included a list of OECD documents completed and OECD projects under development applicable for nanomaterials, including since documents related to the physicochemical properties of the NMs to toxicity tests, for instance some listed guidelines included in the development or revisions of oecd Test Guideline (TG) and Guidance Documents (GD) applicable for nanomaterials, 2022,125 which literally mentions:
“Related to the physicochemical properties of the NMs (OECD documents completed):
(a) Test guideline No. 124 on determination of the volume specific surface area of manufactured nanomaterials.
(b) Test guideline No. 125 on particle size and size distribution of manufactured nanomaterials.
(c) Test guideline No. 318: dispersion stability in simulated environmental media.
(d) Guidance document 318 for testing of dissolution and dispersion stability of nanomaterials and the use of the data for further environmental testing and assessment strategies.
Related to the toxicity of the NMs (OECD documents completed):
(a) Test guideline No. 412: subacute inhalation toxicity: 28-day study.
(b) Test guideline No. 413: subchronic inhalation toxicity: 90 days study.
(c) Guidance document No. 39 on inhalation toxicity studies
(d) Study report and preliminary guidance on the adaptation of in vitro mammalian cell based genotoxicity TGs for testing of NMs.”
This document is very important compilations that also summarise those documents that are in development, as well as other activities related to the evaluation of the NMs. Then this list of guidelines and guidance's can be updated constantly or adapted since the use and application of the NMs in different sectors is increasing every day, which importance may help in minimise the possible risks associated with them in aspects related to the risk assessment, safe selection, and handling of nanomaterials, safe work practices, health, and environmental protection, labelling and risk communication.
In this context, is important to mention that the first initiative was the “special session of the potential implications of manufactured nanomaterials for human health and environmental safety” held by OECD's Chemicals Committee in 2005![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 126 which intended to identify human health and environment safety issues associated with manufactured NMs. Its significant achievement was the establishment of the WPMN in 2006, which is responsible for coordinating methods and developments that allow the elaboration of the appropriate NMs regulatory frameworks or the applicability of test guidelines. The efforts of countries and international and non-governmental organisations are conducted by the Steering Groups of Priority Area 9, which belongs to a nine-priority strategic area plan for regulatory science, establishing the test guidelines that involve the participation of the OECD.127,128 Also, OECD-WPMN has developed the “OECD Series on the safety of manufactured nanomaterials” in its open access series ENV/JM/MONO (Environmental Directorate. Joint Meeting of The Chemicals Committee and The Working Party on Chemicals, Pesticides and Biotechnology), a valuable tool for the development of standards, including ISO.129 These series consist of numerous reports about NMs guidance documents, health and environmental reports, risk and life cycle analysis, chemical, and physical properties, etc., aiming to develop future NMs regulations and legislations.
126 which intended to identify human health and environment safety issues associated with manufactured NMs. Its significant achievement was the establishment of the WPMN in 2006, which is responsible for coordinating methods and developments that allow the elaboration of the appropriate NMs regulatory frameworks or the applicability of test guidelines. The efforts of countries and international and non-governmental organisations are conducted by the Steering Groups of Priority Area 9, which belongs to a nine-priority strategic area plan for regulatory science, establishing the test guidelines that involve the participation of the OECD.127,128 Also, OECD-WPMN has developed the “OECD Series on the safety of manufactured nanomaterials” in its open access series ENV/JM/MONO (Environmental Directorate. Joint Meeting of The Chemicals Committee and The Working Party on Chemicals, Pesticides and Biotechnology), a valuable tool for the development of standards, including ISO.129 These series consist of numerous reports about NMs guidance documents, health and environmental reports, risk and life cycle analysis, chemical, and physical properties, etc., aiming to develop future NMs regulations and legislations.
Standards
The OECD defines standard as legal instruments and other sets of policy principles that are committed to stablish the fundamentals of how certain processes must be done;130 ISO also defines standard, describing it as “a formula that describes the best way of doing something”.131 Both organisations agreed that these standards are elaborated in accordance with the opinion and consultancy of group of experts in the topics of different areas. In Latin America, standards on NMs are elaborated in accordance with the ISO standards, such as the NMX in Mexico, that are the first approach of regulating NMs based on an international document emitted by a regulatory authority such as ISO.94In 2005, ISO established the Technical Committee 229 (ISO/TC 229) of nanotechnologies composed of 33 permanent countries and 16 observing countries. This committee has established four working groups headed by the United States, Canada, Japan, and China. Furthermore, ISO's primary goal is to contribute to the fundaments for the management, RA, and standardisation of nanotechnologies worldwide. Nowadays, ISO/TC 229 is composed of 39 permanent members, including all the countries with the most remarkable advances in nanotechnology and nanoscience's and 17 observing countries.132 The standards developed by ISO/TC 229 are focused on issues such as vocabulary, characterisation, specifications, and methodology guidance of NMs. Nowadays, to reach a more accurate regulation on NMs, ISO/TC 229 is developing a new ISO framework, “ISO/WD TS 12901-2: occupational risk management applied to engineered nanomaterials-part 2: use of the control banding approach” that is going to replace the previous framework “ISO/TS 12901-2:2014, nanotechnologies-occupational risk management applied to engineered nanomaterials-part 2:Use of the control banding approach”, for controlling the risks associated with occupational exposures to nano-objects, and their aggregates and agglomerates greater than 100 nm (NOAA)”,132 which means that previous solutions developed for control chemical occupational exposures can be suggested to be applied in other cases with similar exposure situations.
Other authors have suggested a nanotechnology regulation with at least two approaches: (1) modifying the current regulations and (2) considering NMs as a separate category of potential pollutants.133 Both proposals are closely related to the points made by Handy & Shaw (2007) about the assignment of unique numbers for existing chemicals such as CAS numbers (Chemicals Abstracts Service).134 For new materials without CAS numbers, it will be required to carry out tests considering that some NMs have the same chemical formula as the other bulk materials; thus, NMs must be considered “new substances.” In this situation, the Royal Society and The Royal Academy of Engineering in the United Kingdom favoured treating chemicals in nanoforms as new substances under the Registration, Evaluation, Authorisation, and Restriction Chemicals (REACH) criteria. Similarly, The Danish Board of Technology (2006) proposed classifying NMs separately from existing chemicals.135
The United States, Australia, and the United Kingdom have been at the forefront of generating information, leading to the development of regulatory frameworks for nanotechnology. Additionally, some other countries have made valuable contributions to this matter. According to the Statnano database, the top ten countries with nanotechnology standards are China, the United Kingdom, Iran, the Netherlands, the United States, Denmark, Sweden, Taiwan, Bulgaria, and France18 (Fig. 5).
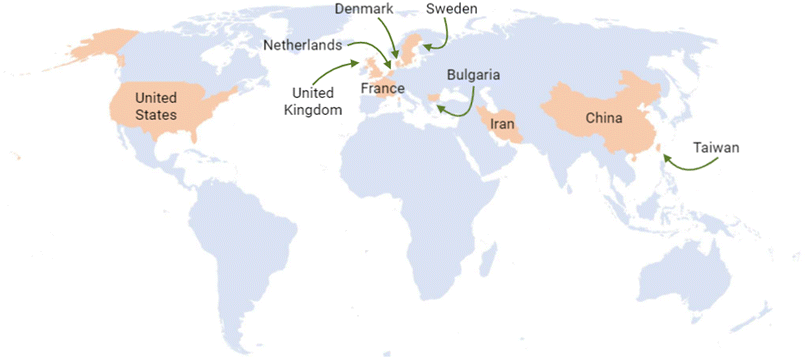 | ||
| Fig. 5 Top ten countries with nanotechnology standards to date.17,18 Original creation (using Excel and Biorender programs) based on information obtained from http://www.statnano.com/ (accessed on December 6th and 14th, 2023).17,18 | ||
For the above, the development of international regulations around NMs would provide the tools for controlling environmental spoilage, keeping the integrity of terrestrial and aquatic ecosystems, and preventing or reducing their effects on animal, plant, and human health. Future regulations must focus on a progressive decrease in NM waste and the rational use of resources in the synthesis without affecting technological progress and improving life quality (Fig. 3).
Regulatory frameworks by regions
Europe
The European Chemical Agency (ECHA) is the EC regulation committee for the registration of manufactured or imported substances in ranges above one ton per year that includes carcinogenic, mutagenic, reproductive, persistent, bioaccumulative, and toxic NMs136,137 which aligns previous European Union legislation with the Globally Harmonised System (GHS).137 Other databases and systems for reporting and tracking NMs in Europe include the European Association for Coordination of Consumer Representation in Standardisation, the Bureau Europeén des Unions de Consommateurs, the German Environmental NGO Bund für Umwelt und Naturschutz Deutschland, the Grenelle II Act (by France), and the Nanotechnology Action Plan for the Safety of NMs (by Austria).136,138The EC has published some guidelines and approaches to arrange scientific committees to address the regulatory problem of NMs. For example, in 2005, the EC published recommendations in the document “action plan for Europe 2005–2009” that aimed to reinforce the leading position of Europe in nanoscience's and nanotechnology but also highlighted the importance of environmental, health, social, and safety issues139,140 and a review of the European legislation recapping the risks involved with the production and use of NMs including aspects such as health, safety, and environment of any product containing NMs.138 By 2007, the EC included an approach that took advantage of existing laws and legislative structures to carry out a series of actions, including studies, recommendations, commissions, trials, and interventions to lead a regulatory framework.135 These actions preceded the creation of organisations such as ECHA.
In 2009, the EC recommended all users related to nanotechnological research, including funding organisations, industrial, academic, and research groups. Those recommendations emphasise the limitations and suggest solutions for better study and work-related practices following the precautionary principle, avoiding contact, and including NMs if safety risk information is unavailable. The recommendations include implementing safety, environmental, and hygiene measures for students, researchers, and workers.7
Also, the EC created the Scientific Committee on Emerging and Newly Identified Health Risk (SCENIHR) to assess the associated risk of working, using, or conducting research with NMs. In 2008, The SCENIHR set up an advisor structure of Scientific Committees and experts in different areas advising consumer safety, public health, environmental risk, and emerging and newly identified health risk based on risk assessments according to their area of experience.138 The SCENIHR considers that using NMs in medical devices is an area of potential risk, especially when those materials will be incorporated into cell therapy, tissue engineering, or blood products; adverse effects may include fertility reduction, and cancer, among others.134 In 2014, the SCENIHR released an expert public opinion on using AgNPs regarding safety, health, and environmental effects and their role in antimicrobial resistance.141 This document suggests scientific evidence cannot elucidate the mechanisms of action regarding microorganisms' AgNPs resistance,142 therefore, using AgNPs in several products should be further evaluated regarding human and environmental health towards broader regulations.
Later in 2011, the EC recommended that NMs be considered in the European regulations, including REACH and the European Classification, Labelling, and Packaging of Substances and Mixtures (CLP), which have been in force since 2009. CLP regulation aligns the previous European Union legislation with GHS and relates to the REACH legislation.141 Meanwhile, the ECHA and the European Union permanent members made public some documents that could ease the registration of NMs in REACH.137
In 2017, the EC, through ECHA, launched the European Union Observatory for Nanomaterials (EUON), providing information about NMs used in the European Union markets. This initiative also conducts research and innovation on NMs, includes information for new regulation, and serves as an ECHA collaborative tool jointly with REACH and the Organisation for Economic Cooperation and Development (OECD) test guidelines and alternative methods. Besides, EUON's platform describes the importance of learning the risks of NMs to human health and their potential environmental damage. It also considers the risk of exposure in working places and for workers as a primary subject.143,144 To provide valuable and complete information about NMs, EUON looks beyond the recommendations emitted in 2009 by the EC and proposes actions that are addressed to the public, students, researchers, workers, and industry, emphasising the importance of issues such as visual content targeted at the public, promote communication networks and the social, environmental, and economic implications of NMs.145
Some existing regulatory frameworks and legislation that include NMs can be applied to regulate their use, such as the guidelines of the Health & Safety Executive, e.g., (1) the Approved Code of Practice (ACOP) under the Control of Substances Hazardous to Health Regulations (COSHH), 2) the risk assessment guideline of the COSHH, and 3) the ACOP of the dangerous substances and explosive atmosphere regulations.146 In addition, other European agencies are responsible for the regulation of cosmetic products, such as the European Union Cosmetic Products Notification Portal and the REACH Scientific Committee on Consumers Safety, which has emitted opinions regarding the use of silica, hydrated silica, and silica surface modified with alkyl silicates (nanoform) in cosmetic products, commenting that evidence by far point out there is still not sufficient evidence in the safe use of those materials.147
Other approaches for developing regulatory frameworks include risk and NMs exposure assessments. In this respect, the REACH system aims to ensure that “new” chemical substances will not be marketed without convincing safety information under the premise «no information, no market».146 However, it is essential to address that due to the lack of a mandatory regulation on NMs, the information about the risk and exposure of NMs cannot be assured. On the other hand, the European Food Safety Authority stated the possibility of applying food regulations to use NMs and nanotechnologies (including guidelines for food products with NMs).138 In 2013, the group assessing already registered nanomaterials, -belonging to ECHA-focused on studying the exposure of NMs and their risks, recognised the usefulness of the manuals of the International Uniform Chemical Information Database for evaluating NMs.127
Until 2007, it was considered impractical to regulate nanotechnology in a European context due to the challenge of legislation for clear and specific regulatory processes.135 One of the clearest examples can be found in the pharmaceutical industry, where current legislation is insufficient for nano pharmaceuticals due to the particularity of their properties (morphology, composition, and surface properties), production methods, quality measurement, and good manufacturing practices (including quality control).148 Another industry in the European context that has also been subject to regulations is the cosmetic industry. In 2013, the European Union cosmetic regulation 1223/2001 was replaced by directive 76/768/EEC,136 generating a renewed concern about how NMs used in cosmetic products could impact human and environmental health, unlike directive 76/768/EEC, which only regulated cosmetics in a human health approach based on composition, labelling, packaging, and economic and technological requirements but not attending environmental issues.149
Other institutions that have published guidelines for the safe management of NMs include the Health & Safety Executive in the United Kingdom, which published an approach for the management of CNTs used in electronic devices, sensors, batteries, medical applications, etc.,150 since exposure levels to multi-walled CNTs were considered a serious concern due to their effects in lungs such as injuries, epithelioid granulomas, mechanical blockage of upper airways, inflammation, and fibrosis.134 On the other hand, the British Standard Institute published in 2009 a guideline for the safe management of NMs in terms of risk management, information needs, hazard assessments, exposure measurement, disposal, and control methods.7 Furthermore, the European Centre for Ecotoxicology of Chemical Task Force on Nanomaterials made significant progress on functional conceptualisation by grouping NMs according to their environmental prevalence. This is a decision-making framework for NMs classification and testing (DF4 nano grouping), which is a risk assessment (RA) tool consisting of three levels to assign four significant groups abbreviated as MGs (main groups), including soluble NMs (MG1's), persistent high aspect ratio NMs (MG2's), passive NMs (MG3's) and active NMs (MG4's).73
All these efforts and actions aimed at better and most effective management of NMs have impacted how NMs are used and treated. The frameworks, guidelines, committees, and organisations created over the last 20 years have led to the sustainable and responsible management of NMs; nevertheless, it is essential to establish mandatory enforcement of the proposed actions and plans. The regulatory gaps and the lack of information about the risks of NMs allow environmental and health issues to persist. Nonetheless, it is still necessary to compile all the information to generate global and unified criteria for regulations on NMs, which all industries or research groups related could use as a guideline for a responsible and sustainable standard. Thus, the European Union has come with strong efforts to face the challenges in the production, use, and disposal of NMs; however, the regulations have yet to reach a level of law. This may be related to regulatory gaps, in other words, the heterogeneous definition of NMs, and to the authors' perspective, there are still isolated efforts and committees of different organisations and governments that need to agree and join the information.
In the United Kingdom (UK) there are some regulatory authorities that participates in the nanotechnology regulation in different areas such as cosmetic, food, agroindustry's, etc. The UK Office for Product Safety and Standards (OPSS) regulates consumer products except food, medicine, and vehicles, participating in regulation of nanomaterials applied on cosmetic products.151 The Department of Environment, Food & Rural Affairs (DEFRA) is the governmental organisation in charge of the improvement and environmental conservation that has emitted documents about the labelling of food containing nanomaterials and the potential risk of NMs in the environment.105 The Health and Safety Executive (HSE) is the British national regulator for laboral safety in workplaces that in 2013 emitted a guidance document about the control of exposure to manufactured nanomaterials and has published other documents. Other organisations in UK responsible for NMs regulations are the Health Security Agency (HSA) and the Food Standard Agency (FSA).152,153
In Germany, organism such as the German Federal Institute for Risk Assessment, the Federal Ministry for Food and Agriculture, The German Chemical Industry Association and The German Environment Agency have developed recommendations and legislations about NMs used in food, cosmetics, etc.,154–157 while in Spain, the only known regulatory authority on NM Superiore di Sanità is the institution that has published research and document about risk assessments, characterisation and toxicological assays of NMs.158
The United States of America
As mentioned previously, the United States is the leading country in nanotechnology research and one of the countries where a wide variety of consumer products comprise many NMs and NPs. Through national institutions and initiatives, the United States authorities have been responsible for developing regulatory frameworks and legislations that address the use and necessary restrictions of NMs and nanotechnology in different industries. In this respect, the most critical regulatory authorities are the Food & Drug Administration (FDA), the Environmental Protection Agency (EPA), the National Institute of Occupational and Safety Health (NIOSH), and the Institute for Food and Agricultural Standards (IFAS). These organisations have been responsible for developing protocols in the United States about the risks of NMs and nanoproducts.136,159The EPA classifies NMs according to their chemical properties under the Toxic Substances Control Act (TSCA) jurisdiction that prompts them to collect, publish, and review the tests made on chemical substances. In 2011, EPA worked in a 4-edge regulatory approach that included: (1) pre-manufacturing notifications, (2) a significant new use rule, (3) an information collection rule, and (4) a test rule.133 Also, manufacturers must provide detailed information about NMs, a provision previously voluntary through the Nanoscale Materials Stewardship Program.137 Under this approach, EPA gives a 90 days deadline for manufacturers to submit information about their NMs.127 By 2015, EPA had revised more than 160 new “nanoscale” chemical substances issuing actions aimed at the use and limitations of NMs in terms of limiting emissions to the environment and, with it, the use of protective equipment and the test applications to generate information about environmental and health effects.137
The FDA regulatory approaches, however, are restricted and only address terminology or classification issues around handling nanoproducts but not nanotechnology, which means that the processes to produce NMs are not included in the FDA regulatory initiatives. Furthermore, regulations emitted by this governmental agency only reach a limited class of products, regulating only those the sponsor requests.146,160 In June 2001, the FDA published the guidelines for NMs identification that are valid in all industries that use NMs in food, medical devices, drugs, cosmetics, and other products entitled “draft guidance for industry considering whether an FDA-regulated products involves the application of nanotechnology”.133 Among the actions undertaken by the FDA include the creation in 2006 of the FDA Nanotechnology Task Force to determine the regulatory approaches for the development of new products that incorporate NPs, as well as cooperation programs with the National Institute of Health/National Institute of Environmental Health Sciences.133,146 In addition, by 2013, the FDA launched the “nanotechnology regulatory science research plan”, which addresses the execution and management of various NPs and nanoproducts.148 With this plan, the FDA increases collaboration with external and international stakeholders to contribute to the solution of emerging challenges, regulatory science knowledge, facilitate innovation and coordination of policy to fulfil FDA's mission.161
Other regulatory organisations that endeavour to develop frameworks, regulations, and standardisations on NMs used in different areas aiming for their safe and responsible use are the IFAS,71 the National Cancer Institute through the Nanotechnology Characterisation Laboratory,73 the American Society for Testing and Materials, the Institute of Electrical and Electronics Engineers,133 and the NIOSH.159 In addition, in 2000, during Bill Clinton's administration, the National Nanotechnology Initiative (NNI) was created to span around 20 agencies and departments (including EPA) to work on the research and development (R&D) of NMs, and their impact on the environment, health, and safety;127,146,161–165 and in 2003, President George W. Bush signed the Nanotechnology Research and Development Act.6 Since its creation, NNI has strengthened nanotechnology development, increasing research capacity by supporting postdoctoral researchers, graduate students from a broad range of scientific areas, imparting courses, and investing in the technician training,166 boosting the development of high training human resources.
Agencies in charge of NM regulatory framework development are ruled under federal laws. As previously mentioned, EPA is governed under TSCA, and the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA).137 Other federal laws that can be useful for regulatory framework development include the Clean Air Act, the Comprehensive Environmental Recovery Compensation and Liability Act, the Resource Conservation and Recovery Act,133 the Federal Food, Drug, and Cosmetic Act, the Personal Care Products Council, and the Voluntary Cosmetic Registration Program.136 Although these regulatory agencies can settle a starting point for broader regulations, some of them, such as FIFRA have limitations on NMs, therefore, research about NM effects is needed for developing regulatory frameworks that can be applied according to the needs of the products using NMs.
It can be pointed out some parallelism between the United States and the European Union efforts to regulate NMs; both have legislation for the registration and management of chemical substances, including NMs, REACH in the case of the European Union and TSCA in the United States, besides, in both cases, frameworks and guidelines have been developed, not only for NMs but for products containing NMs such as food, pharmaceutics, and cosmetics that are consumed by humans and could be dangerous for human health and environmentally risky. Despite this, definitions of NMs are still different, so a homologated purpose and legislation are still off the table.
Nevertheless, with the many initiatives and agencies in charge of developing frameworks and guidelines for the safe management and use of NMs, the non-mandatory application is still an issue that needs to be solved due to the vast fields of applications of NMs. Therefore, governmental, and non-governmental agencies should coordinate among themselves to develop mandatory frameworks, considering the urgency of the significant number of products using NMs and the waste generated that could lead NMs to natural environments.
Other countries in the world
Other countries with outstanding R&D of nanotechnologies and NMs have developed or started developing projects, guidelines, RA, and/or regulatory frameworks that allow the responsible and controlled application of NMs, such as the Asian countries. Japan, in the last 20 years has focused its efforts on creating research platforms, testing methods of standardisation, data collection, and RA of NMs supported by governmental agencies such as the Ministry of Education, Culture, Sports, Science and Technology, the Ministry of Economy, Trade, and Industry, the Ministry of Health, Labour and Welfare, the Ministry of Environment, the National Institute of Material Science and the National Institute of Advanced Industrial Science and Technology Council for Science, Technology, and Innovation.26,127,137,146 China and South Korea have also worked in the development of regulatory policies for NMs using regulatory frameworks such as the Act of Registration and Evaluation of Chemicals or the Development Promotion Act, the involvement of some government departments as well as cooperation with international organisations (in the case of Korea)127 or the creation of agencies, such as the Chinese Academy of Sciences, or the National Centre for Nanoscience and Technology in China.146 These organisations/initiatives address guidance and regulatory issues such as terminology, safety requirements, and trade regulations useful for nanotechnology development and boosting China and Korea as technological leaders in this field.In Canada, regulations are led by the Canadian Environmental Protection Act, the Pest Control Product Act, and the Fertiliser, Feeds, Food, and Drug Act;137 however, these legislations are not specific to NMs. NMs are regulated by different government organisations and frameworks; for cosmetics, the Cosmetic Regulation Act (1977) and Food and Drug Act (1985) regulates nanomaterials in cosmetic products;167 NMs in food products are regulated by the Canadian Food Inspection Agency (CFIA) and the Public Health Agency of Canada (PHAC)168 and the nanotechnology-based agri-products are regulated by the Canada Environmental Protection Act 1999 (CEPA), the Food and Drug Act, The Pest Control Product Act (PCPA)and the Canada Agricultural Products Act (CAPA).169 In Australia, there are at least four national chemical schemes that are relevant for NM regulation: Food Standards Australia-New Zealand, the National Industrial Chemicals Assessment Scheme, Therapeutic Good Administration, and the Australian Pesticides and Veterinarian Medicines Authority,159 all these legislations are applicable for food products containing NMs, particularly nanosilver. It is worth mentioning that Canadian and Australian regulatory initiatives are non-specific for NMs and were designed to regulate a wide range of products, which consider NMs or products containing NMs, so the importance of designing and developing specific regulations for NMs cannot be ignored.
Those regulatory initiatives and policies are based on other international or foreign frameworks; their criteria or at least some regulations have coincident points that encompass each country's needs and environmental or health goals. Therefore, the cooperation between countries, governmental institutions, private companies, and non-governmental plays a key role due to the synergic that can be accomplished (Table 1).
| Country | Framework |
|---|---|
| United States | Toxic substances control act |
| Draft guidance for industry considering whether an FDA-regulated products involves the applications of nanotechnology | |
| Nanotechnology regulatory science research plan | |
| National nanotechnology initiative | |
| Nanotechnology research and development act | |
| Federal insecticide, fungicide and rodenticide act | |
| Clean air act | |
| Comprehensive environmental recovery, compensation and liability act | |
| Resource conservation and recovery act | |
| Federal food, drug, and cosmetic act | |
| Voluntary cosmetic registration program | |
| European countries | Globally harmonised system |
| Action plan for Europe 2005–2009 | |
| Scientific committee on emerging and newly identified risk | |
| European classification, labelling and packaging of substances and mixtures | |
| Approved code of practice | |
| Risk assessment guideline of the control of substances, hazardous to health regulations | |
| ACOP of the dangerous, substances and explosive atmosphere regulation | |
| EU cosmetic regulation 1223/2001 | |
| Directive 76/768/EEC | |
| China | Act of registration and evaluation of chemicals |
| Development promotion act | |
| Canada | Canadian environmental protection act |
| Pest control product act | |
| Fertiliser, feeds, food and drug act | |
| Australia | Food standards Australia-New Zealand |
| National industrial chemical assessment scheme | |
| Therapeutic good administration | |
| Australian pesticides and veterinarian medicines authority | |
| Korea | Act of registration and evaluation of chemicals |
| Development promotion act |
In the race for nanotechnology development leadership, Mexico and other Latin American countries recently started to develop this area through the newest economic and technological tendencies that would allow them to create their technologies. Unlike the leading countries in nanotechnology, the development of NMs in Mexico is not considered an emerging area. In terms of funding, the public amounts invested in nanotechnology research in Mexico are minimal compared to the investment in countries such as the United States, China, and Germany. In perspective, Camarillo et al. (2019) estimated that nanotechnology investment in Mexico was only US60 million in the period 2005–2010,170 while in the United States, only in 2005, the funding from the NNI was USD 982 million.171 This valuation contrasts with Anzaldo et al. (2014), who pointed out that public investment in México until 2009 amounted to USD 128 million.172 Nevertheless, these are not isolated facts since the estimate for 2011 in public investment in Mexico for science and technology was equivalent to 0.43% Gross Domestic Product (GDP). Meanwhile, in many OECD country members, the investment amount is around 2% GDP.173 Furthermore, regarding public funding distribution to nanotechnologies, 37% was captured by private companies, followed by the National Council of Humanities Sciences and Technologies (CONAHCyT) research centres (17%) and public universities (16%).172
The balance recorded from 1994 to 2020 by the country is that 559 Mexican nanotechnological patents were registered in the World Intellectual Property Organisation (WIPO) Pantescope.174 By 2012, 86 companies used nanotechnology in their products175 and by 2014 the number of companies went up to 188,170 including Investigación y Desarrollo de Nanomaterials SA de CV, Nanomaterial SA de CV and Nanobio, Tronics SA de CV, Nano AZT, Nanomat S.A de CV19 and Nanoquem176 in Apodaca, Nuevo León as examples.
Since the early 21st century, Mexico has considered nanotechnology a strategic area in the “National Development Plan 2001–2006”.177 Thus, the nanotechnology area should be developed, boosted, and managed mainly by decentralised public organisms for being considered as a matter of “national security interest” or a social benefit. The first nanotechnological initiative was the National Technical Committee for Standardisation of Nanotechnologies which is jointly coordinated by the Ministry of Economy and the National Centre of Metrology, in charge of issuing Mexican non-mandatory regulations in nanotechnology and validating existing norms that have their equivalents in ISO standards;170,177–179 however, this committee does not have official attributions for the development of mandatory guidelines or regulations.
In 2012, the “High-level Council for Regulatory Cooperation,” a binational Mexico-United States initiative (2012), was created and presented the document “Working Group on Regulations for Nanotechnology”177 that established the guidelines for developing future regulations on nanotechnology or products containing NMs. Similarly, in 2014, the valid declaration of some Mexican standards related to nanotechnology was issued, and later (2017–2019), another three standards were published. In recent years, at least three Mexican non-mandatory standards (2019–2020) and standard projects approved as listed in Table 2.
| Mexican standards with declaration of validity and projects (2014–2020) | Description | Year of issuance of declaration of validity | Reference |
|---|---|---|---|
| NMX-R-10867-SCFI-2014 | For single-layer carbon nanotubes characterisation by near-infrared photoluminescence spectroscopy | 2014 | 267 |
| NMX-R-10929-SCFI-2014 | Multi-layer carbon nanotubes samples characterisation | 2014 | 267 |
| NMX-R-27687-SCFI-2014 | Terminology and definitions for nano-objects-particles, nanofiber, and nano silver | 2014 | 267 |
| NMX-R-80004-1-SCFI-2020 | Nanotechnologies vocabulary-part 1: core concepts | 2022 | 267 |
| NMX-R-80004-3-SCFI-2020 | Nanotechnologies vocabulary-part 3: carbon nano-objects | 2022 | 267 |
| PROY-NMX-R-12901-1-SCFI-2020 | Nanotechnologies-occupational risk management applied to manufactured nanomaterials. Part 1: principles and approaches | 2020 (project) | 268 |
| NMX-R-80004-5-SCFI-2015 | Nanotechnologies vocabulary-part 5: nano/bio interface | 2017 | 268 |
| NMX-R-12901-2-SCFI-2016 | Nanotechnologies-occupational risk management applied to manufactured nanomaterials. Part 2: using the band control approach | 2019 | 268 |
| NMX-R-80004-4-SCFI-2019 | Nanotechnologies-vocabulary-part 4: nanostructured materials | 2020 | 268 |
| NMX-R-80004-6-SCFI-2015 | Nanotechnologies-vocabulary-part 6: nano-object characterisation | 2019 | 268 |
| NMX-R-13121-SCFI-2019 | Nanotechnologies – risk assessment on nanomaterials | 2020 | 268 |
| PROY-NMX-R-21363-SCFI-2019 | Nanotechnologies-measuring particle size and shape distributions using transmission electron microscopy | 2020 (project) | 268 |
| PROY-NMX-R-80004-1-SCFI-2019 | Nanotechnologies-vocabulary-part 1: basic concepts (will cancel the NMX-R-80004-1-SCFI-2014) | 2020 (project) | 268 |
In 2015 the National Nanotoxicological Evaluation System initiative (SINANOTOX) was created, belonging to the Thematic Network of Nanoscience's and Nanotechnology of the CONAHCyT. This initiative is a shared effort of several research groups in Mexican Universities and research centres that aims to create a platform and protocols to evaluate and analyse the impact of NMs for multiple purposes in the Mexican territory. SINANOTOX offers a system of evaluation in various biological models, such as rodents, zebrafish, Caenorhabditis elegans, cell cultures, and isolated tissue systems, among others, offering valid information regarding the health and environmental safety of NMs produced by public and private sectors.180 In Mexico, nanotechnology is developing in a lax legal regulatory scheme if we consider that existing approved standards and their validity provide security for producers and consumers. Nevertheless, the application and compliance are not mandatory. Mexico's situation regarding the regulation of NMs is still developing and requires more support for its optimal development. Therefore, SINANOTOX expects to settle the bases for the future development of regulatory frames to collaborate to the safe obtention, use, and disposal of products derived from nanotechnology in the following years.
Besides Mexico, only Brazil participates as a permanent member of ISO/TC 229, an organisation in charge of worldwide standardisation, management, and risk assessment of nanotechnologies. Argentina and Jamaica were the other two Latin American countries participating in the same group as observer countries in 2005.127,181 In 2021, Mexico, Brazil, and Colombia participated as permanent members, while Argentina, Peru, and Costa Rica participated as observing members.132 Standardisation agencies from Latin America also participate in this committee, as shown in Table 3. Their presence in this committee has allowed these countries to develop national frameworks and legislative initiatives that serve as starting points for broader regulations on NMs.
| Country | Standardisation agency |
|---|---|
| Brazil | Brazilian Association of Technical Standards (ABNT) |
| Colombia | Colombian Institute of Technical Standards and Certification (ICONTEC) |
| Mexico | General Directorate of Standards (DGN) of the Ministry of Economy through National Centre of Metrology (CENAM) |
| Argentina | Argentine Normalisation and Certification Institute (IRAM) |
| Peru | National Institute of Quality (INACAL) |
Brazil has a remarkable share in the nanotechnology market and has also worked in a regulatory system of NMs for some years, creating government initiatives dating from 2004 to establish regulatory frameworks that allow the responsible use of NMs, such as the Brazilian Nanotechnology Initiative. However, there are some regulatory policies in Brazil that are more related with an industrial approach taking aside the environmental issues.182–185
Argentina has carried out actions to standardise the use of NMs, taking into consideration, as in the case of Brazil and many other countries, the guidelines established by regulatory authorities and some other regulatory guidelines, such as those shown by Mercosur, a trademark initiative of South America has led to the publication of 1417 articles related with food additives by the Argentinean Food Code, including NMs.186–188 To reach a broader legislation that ensures environmental and health safety, responsible use, and life quality improvement, investment in research is a cornerstone to achieving broad regulations in Latin America.
Other countries in Latin America that have started to develop the nanotechnology area are Costa Rica, Uruguay, and Venezuela. In 1999, Costa Rica created the National Centre of High Technology (CENAT), and by 2004 the National Laboratory of Nanotechnology was created (LANOTEC),189 which is also attached to CENAT, and among its activities included: R&D, innovation and entrepreneurship, normalisation, teaching, and divulgation. By 2020, LANOTEC had published 26 articles, 13 projects, and 81 technology transfers.190 Uruguay established the nanotechnology laboratory “NanoMat” from Technological Polo Institute of Pando aiming for R&D of materials with industrial applications; Venezuela, in 2005 established the “National Plan of Science, Technology, and Innovation 2005–2030” recognising the importance of nanotechnology although is not considered as a priority, and to date, four institutions communicate nearly 80% of nanotechnology-related publications: the Venezuelan Institute of Scientific Research, the Central University of Venezuela, Simón Bolívar University and Los Andes University.189
These series consist of numerous reports about NMs guidance documents, health and environmental reports, risk and life cycle analysis, chemical, and physical properties, etc., aiming to develop future NMs regulations and legislations.
Challenges and advances towards a standardised regulatory process
Beyond the efforts of countries to generate regulatory frameworks and legislations for NMs and nanotechnologies, some challenges can emerge, limiting adequate regulations. Conceptualisation, characterisation techniques, toxicity/ecotoxicity tests, and even synthesis methods are still not homologated among countries.191Concerning the NM and NP concept, while it is true there is an agreement with the size ranges from 1 to 100 nm, there may be some variations such as the number of dimensions less than 100 nm, accumulation, and if they are biologically active or not, their solubility and even their biopersistence. Thus, Table 4 compiles NM definitions according to four organisms, where slight variations are translated in the first challenge for applying regulations to nanotechnological products.
| FDA | Material that has at least one dimension in the range of approximately 1 to 100 nm and exhibit dimension-dependent phenomena |
| OECD | Manufactured nanoparticle refers to particles that have a physical size of less than 100 nm in at least two dimensions that are deliberately produced rather than merely emerging as a by-product in activities not targeted to produce these particles |
| EC | A natural, incidental, or manufactured material containing particles, in an unbound state or as an aggregate or as an agglomerate and where, for 50% or more if the particles in the number size distribution, one or more external dimensions is the size range 1 to 100 nm |
| ISO | A material with any external dimension in the nanoscale or having internal structure or surface structure in the nanoscale |
| Health Canada | Any manufactured product, material, substance, ingredient, device, system, or structure if: (1) it is at or within, the nanoscale in at least one spatial dimension, or (2) it is smaller or larger than the nanoscale in all spatial dimensions and exhibits one or more nanoscale phenomena |
Other conceptual variations persist in the NM classifications; today, it is known that the most accepted category is the one proposed by the EPA that groups them into four main types: carbon-based, metal-based NPs, dendrimers, and composites. However, there are other classifications proposed by ISO or the research group of Bystrzejewska, based on aspects such as generations sources, properties, dimensioning, uniformity, morphology, and aggregation.2,23,25,127,138,160,192–201
Importantly none of these definitions are legal. Thus, although the particle size is relevant for nanotechnological products, it is insufficient for mandatory regulations.
Another challenge is that the characteristics of NMs could vary during different stages in their life cycle, ranging from material acquisition, purification, manufacture, commercial use, and final disposal. Thus, regulations must follow every step, and life cycle assessment (LCA) constitutes an important tool.
LCA is a standardised method that assesses the possible impacts that various products, services, and processes could exert on the environment. LCA consists of four phases according to provisions of ISO 14040: (a) definitions of goals and scopes; (b) inventory analysis; (sc) impact assessments, and (sd) interpretation.202–204 These assessments reinforce the protocol arsenal for issuing regulations and legislation for responsible control, production, and consumption of NMs and nanotechnologies. However, these assessments may still have some limitations; one includes conceptualisation since depending on the interpretation of the term “life cycle” or the scientific area where it is being used, its application may vary.205 Salieri et al. (2017) compiled three aspects to consider about the use of LCAs: (1) the lack of inventory information on average life cycle, (2) characterisation factors, and (3) the need for an adequate functional unit for NMs.203 We can point to the work by Khanna et al. (2008) as an example of how a LCA is designed: they designed an LCA for carbon nanofibers which due to the lack of information about requirements, emissions, and outputs from the carbon nanofibers synthesis processes they were only able to assume its boundaries pointing that this could be an error and uncertainty factor in the LCA.206
LCAs have gained greater relevance at a practical level. Some reports carry out a partial LCA (cradle to gate), which generates information related to the possible environmental impact of NMs but only during their early stages (NMs acquisition to their synthesis).207–216 Another type of LCA is the “cradle to grave”, which can be considered a broader analysis since it spans from the material acquisition to their final disposal, including reuse and recycling processes.204 Temizel-Sekeryan & Hicks (2021) performed an LCA on AgNPs embedded in textile polyester and found that the released silver impact on human and environmental health is not significant.217
Together with LCAs, another helpful tool is the RA which can be defined as «a quantitative and qualitative assessment of the risk involved to health and/or environment due to the presence of a particular pollutant or a mixture of pollutants».218 Based on the National Academy of Sciences of the United States, for chemical substances, RA is performed in four steps, including (1) hazard identification; (2) hazard characterisation; (3) exposure assessment, and (4) risk characterisation;219 but since NMs have different properties due to their nanoscale, RA for these substances must be modified considering their particularities. The earliest background of RA is the regulatory framework of the International Risk Governance Council in 2006, which is defined as «a regulatory framework for analysis and management related to NMs» that proposes a risk management and RA multi-criteria decision analysis-based framework as a strategy for NM regulation.220
On the other hand, the EC bases the performance of NMs RA in the food industry and agriculture on the European Food Safety Authority documents221 or in the procedures established by REACH.7 Some of the most recent research papers consulted oriented to RA have different approaches ranging from graphic and probabilistic methods,222–224 environmental studies using model organisms such as Daphnia Magna, Vibrio fischeri, Chlorella sp. or Drosophila melanogaster,225,226 occupational risk-oriented studies222,227 or the everyday use of RA and LCA.218,228 These approaches have proven useful in predicting certain properties and possible effects of NMs in the environment and living organisms when a RA is developed. It is important to address that despite being a valuable tool for RA, these approaches have limitations, thus, a joint application of these strategies is advisable, through a global consensus.
Current regulatory frameworks and legislations have increasingly used both LCAs and RA due to the information they provide about toxic effects on health, the environment, and processes or stages that NMs go through before their final disposal. However, it is necessary to mention that the LCAs and RA must be performed on NMs and not in manufactured products since the NMs life cycles may differ. This could have other implications at a regulatory level since the LCA and RA approaches must be well established, and ambiguity must be avoided when proposing and designing regulatory frameworks and legislations, both national and international. The protocolary establishment of LCAs and RA in developing the regulatory framework worldwide provides an essential tool that opens the way to more precise and assertive legislation. Their usefulness can be synergistic if applied jointly.
Technological progress in the last 70 years has contributed to expanding researchers' technical capacity, allowing the generation of relevant information in all areas. Without a doubt, nanotechnology is one of them. In this sense, regulations in nanotechnology aim to asset sophisticated devices and equipment that provide morphological, chemical, and physical data of NMs, as well as regulations in the studies focused on toxicological and/or eco-toxicological analysis.
The set of tools used for NMs characterisation has been well documented, especially in research papers that have yielded a large amount of information about the effect of NMs on living organisms or the environment.84,88,229–236
Electron microscopy in its different variants (such as transmission electron microscopy, scanning electron microscopy, scanning transmission electron microscopy, and high-resolution transmission microscopy) is the primary tool for knowing the morphology of NMs. This equipment and techniques may include accessory features that enable information generation, such as the selected area diffraction pattern, or other coupled technologies, such as X-ray diffraction. Besides the morphological characteristic, chemical element analysis provides relevant data in the study of NMs. This information is provided by chromatography liquid, gas, high-performance chromatography, coupled to mass spectrometry, ultraviolet-visible spectroscopy, dynamic light scattering equipment, Fourier transform infrared spectroscopy, and X-ray diffraction.237–241
These and other techniques have already been compiled in terms of regulation, as in the review of Karamanidou et al. (2021), which refers to the different approaches, methodologies, and regulatory frameworks used in the cosmetics industry as well as the information the sector provides about the used NMs.201 Paterson et al. (2011) also addressed the technical aspects of monitoring anthropogenic NPs and included the analysis techniques for their characterisation;242 Linsinger et al. (2012) pointed out the importance of validation methods for NPs quantification.198 Jointly, the methodologies, analysis techniques, and validation methods provide an important source of information that could help to appraise the impact and possible effects of NMs on human and environmental health.
The research on nanotoxicology matters includes a wide range of NMs and NPs. It cooperatively addresses the use of high-tech equipment and their evaluation through biological models that include cell cultures (Caco-2 colon cancer cell lines, epithelial cell lines, and fibroblasts),66,83,231,243–245 murine models (like rats or mice),88,89,245–252 aquatic models (Danio rerio, Daphnia magna, Oncorhynchus mykiss, Xenopus laevis, Tisbebattagliai, Ceramium tenuicorne),84,229,253–257 insects (Drosophila melanogaster),225 plants (Medicago sativa, Triticum aestivum)236,258 and microbiological models (Chlorella Vulgaris, Vibrio fischeri).226,259 These models for toxicological and eco-toxicological studies of NMs have specific characteristics such as specimen availability due to the relatively short gestation periods, genetic homology with humans, broad or complete genomic studies, and similarity in their biological processes with humans or other relevant species.
It is known that murine models are used in nanotoxicity studies due to their capability to replicate physiological effects in humans, allowing a better comprehension of health and disease processes. This model is used in many experimental approaches, such as cytotoxicity, hepatotoxicity, nephropathy, dermal toxicity, immunotoxicity, and cardiovascular toxicity. The effects that NMs produce in the murine models can be easily monitored so that researchers can have a better perspective of the effects produced due to NMs exposure.260 More recently, aquatic models have gained great relevance in eco-toxicology, particularly zebrafish, since it is easy to handle due to their small size, the transparency of their embryos, and external fertilisation and development. Also, other aquatic organisms are used (algae, small crustaceans, bacteria) in eco-toxicological studies due to their capability to react to environmental changes in which they live, changes caused by pollutants of different kinds, NMs among them.192,261–264
For years, OECD has included some model organisms, including Daphnia sp. Chlorella sp., algae, fish, rats, and in vitro methods for toxicity and ecotoxicity studies of NMs, which also have been applied in research papers and collected in review articles,128,227,246,265,266 which is of great relevance since, unlike 20 years ago, nowadays there are protocols to study the effects of NMs on health and environment. Some examples of experimental approaches are shown in Table 5.
| Approach | Biologic model | Nanoparticles | Findings | Author |
|---|---|---|---|---|
| In vitro | Caco-2 cells | AgNPs | Caco-2 and SW480 cell lines exposed to NPs produce cell stress and cell death | 243 |
| SW480 cells | ZnONPs | |||
| TiO2 NPs | ||||
| Buccal epithelial cells (TR146) | AgNPs | NPs (100 mg L−1) concentration in potable water produced cytotoxicity in oral and gastrointestinal systems, decreasing from ZnONPs to AgNPs to TiO2 NPs | 83 | |
| Human colon mucosal epithelial cells (NCM460) | ZnONPs | |||
| TiO2 NPs | ||||
| Caco-2 | AgNPs | Cell viability decreased after 24 hours exposure to concentrations of ZnONPs (3, 6, and 12 mM) and AgNPs (0.5, 1.5, and 3 mM) producing cell shape alterations, abnormal nuclear structure, membrane blebbing, and cytoplasmic deterioration | 66 | |
| ZnONPs | ||||
| In vivo | Wistar rats (male/pregnant female) | AgNPs | Descendants of pregnant rats exposed to AgNPs showed an increase of glutathione peroxidase, malondialdehyde and caspase 9, on the other hand levels of glutathione decreased. Oxidative stress and apoptosis in the offspring were observed | 89 |
| Wistar rats | AgNPs | After oral administration for 55 days, silver was found in small intestine, kidney, liver, and brain. It was also determined that AgNPs exposure changed urea nitrogen, total proteins and mean corpuscular haemoglobin in blood | 248 | |
| Wistar rats | VO2 NPs | Exposure to VO2 NPs and VO5 NPs by inhalation showed that VO2 NPs were more toxic than VO5 NPs. VO2 NPs caused an increment in the levels of oxidative stress markers such as malondialdehyde and reduced glutathione | 272 | |
| VO5 NPs | ||||
| Ex vivo | Zebrafish (Danio rerio) | AgNPs | After AgNPs exposure (20 and 110 nm), high silver concentrations of 20 nm AgNPs were found in gills and intestine deposited in the basolateral membranes, disrupting Na+/K+ ionic channels | 91 |
| Wistar rat | AgNPs | AgNPs administration in trachea isolated rings AgNPs produced a contractile effect when is administrated simultaneously with acetylcholine increasing smooth muscle contractility. Nitric oxide production due to AgNPs administration was associated with a change in the muscle tone | 273 | |
| Wistar rat | AgNPs | Perfused isolated hearts from spontaneously hypertensive rats exposed to AgNPs at cumulative and individual administrations (10 and 100 μg mL−1) produced persistent vasoconstriction due to inducible nitric oxide synthase and endothelial nitric oxide synthase reduced production | 274 |
Conclusions
The regulatory area around NMs has had significant advances worldwide and goes hand in hand with the development of nanotechnology and nanoscience. In the last 20 years, the interest in NMs effects can be noticed, and this topic concerns not only the scientific community but also the public demanding safer products.The progress achieved so far results from the joint efforts of governments, international organisations, non-governmental organisations, research, and academic groups genuinely concerned about the possible harmful implications of these products. However, it is not surprising that due to the wide variety of NPs, NMs, and nanotechnologies, the development of the regulatory framework is a dynamic and extensive process, as is the nanotechnological development, and even with the current achievements, a long way is ongoing.
An ethical approach is needed to develop safe and accurate legislation for NMs. Besides the theoretical knowledge, frameworks, and legislation, consumers must be aware of the benefits and risks of using NMs. Therefore, it is necessary a clear communication between scientific-academic institutions, governments, and society. The use of NMs is exponential, and in 2020 this was evident with the emergency use and later commercialisation of vaccines against SARS-CoV-2 Pfizer and Moderna, both vaccines using nanoliposomes as carriers. Thus, the public debate and the information flow about nanotechnology benefits, risks, and profits would be helpful tools for people to become aware of these matters. However, not only R&D of NMs and nanotechnology issues should be regulated ethically but also patenting; the need for proper regulation about patenting must include the same aspects, including the application of methodologies and techniques with high-quality standards, RA and communication, risk/profit evaluation, and human health and environmental risk. The purpose of the NM regulations, beyond established methodologies and techniques, must aim to guarantee the safety and usefulness of nanotechnological products to customers to support the public opinion and keep improving scientific development, people's life, and environmental quality.
The actions undertaken by organisations such as the OECD and ISO, and the mutually efforts of the United States institutions such as FDA/EPA and the European Union are steps towards the unifying regulatory system as a basis for the regulatory system.
It is necessary to mention that all these regulatory matters can be embedded or be related to some of the SDG: (a) good health and well-being (3); (b) clean water and sanitation (6); (c) affordable and clean energy (7); (d) climate action (13); (e) life bellow water (14) and (f) life on land (15). Therefore, the development of regulatory frameworks, legislations, and guidelines can be considered as actions and strategies towards achieving the SDG in all countries since they are considered a “call for action by all countries – poor, rich and middle-income – to promote prosperity while protecting the planet”.31
Nowadays, most of the legislation around chemicals in the most industrialised countries is based on the OECD program's results.143 Still, in the authors point of view, it is necessary to link the regulatory frameworks and initiatives from countries and organisations around the world. As it was mentioned before, CLP serves as a complementary tool for REACH and OECD. Still, also, OECD and UNITAR in 2006 launched Awareness-Raising Workshops on Nanotechnology/Manufactured Nanomaterials for Developing and Transition Countries in a joint effort.201 We can settle parallelism between the different international organisations developing and promoting actions for NMs regulations, frameworks, and legislations that are all useful tools for sustainable development that guarantee improvement in the life quality of all human beings.
Mexico already has some standards that can be the starting point for broader regulation, but these regulatory frameworks are more conceptual guidelines and are still not mandatory. In addition, its participation in international groups such as ISO/TC 229, will make it possible for regulatory frameworks emanating from these groups, could serve for elaboration, improvement, and expansion of regulatory frameworks and legislations in this country and complement other existing standards. In Latin America, we must also observe the achievements and advances in other countries due to commercial exchange in this region based on boosting R&D of NMs, nanotechnology, and products containing NMs that are the newest economic, industrial, and trade tendencies.
Monitoring the development of nanotechnology and nanoscience is a priority, looking forward and having as a reference the increasingly hostile environmental conditions largely to man's action. The regulatory frameworks that deal with NMs will be a helpful tool that to reduce and, if necessary, amend the damage generated by anthropogenic waste released into the environment. In the same way, although continuous exposure to NMs, directly or indirectly, can have consequences that alter human beings' optimal health, regulatory frameworks can be handy in reducing and/or preventing these harmful effects.
In all its branches, advances in science and technology have proven helpful for human progress. Without a doubt, nanoscience is not an exception. Therefore, it can be expected that the trend to incorporate NMs will continue its growing inertia maintained over the last 20 years. The need to develop legislation and regulatory frameworks that guarantee consumers' and workers' environmental safety, and health will continue to become evident. Likewise, it is essential to permanently incorporate informative and educational programs to communicate aspects related to the science of NMs and the uses and applications of nanotechnology in all sectors of society, and thereby raising awareness about its use, management, and disposal.
In summary, in the globalised world, the position regarding NMs regulations may vary between countries and regions. However, there is a growing consensus on the need to establish adequate regulations to ensure the protection and safety of health and the environment impact of NMs. Many countries and international organisations identify that NMs have unique characteristics and properties that can have both positive and potentially negative impacts. It is, therefore, crucial to have effective regulations that address the risks associated with using and exposing NMS while promoting innovation and responsible development of this technology.
The position of the global world regarding regulations in this matter tends to favour adopting measures to guarantee this technology's safety and sustainability while promoting innovation and responsible development and achieve a harmonised system in the near future.
Abbreviations
| ACOP | Approved code of practice |
| AgNPs | Silver nanoparticles |
| AuNPs | Gold nanoparticles |
| CAA | Clean Air Act |
| CAS | Chemical Abstracts Service |
| CENARIOS | Certifiable Nanospecific Risk Management and Monitoring System |
| CENAT | National Centre of High Technology (Centro Nutrición Alta Tecnología) |
| CLP | European classification, labelling and packaging of substances and mixtures |
| CNTs | Carbon nanotubes |
| CONAHCyT | National Council of Humanities, Sciences and Technologies (Consejo Nacional de Humanidades, Ciencias y Tecnologías) |
| COSHH | Control of Substances Hazardous to Health Regulations |
| DEFRA | Department of Environment, Food & Rural Affairs |
| EC | European Commission |
| ECHA | European Chemical Agency |
| EPA | United States Environmental Protection Agency |
| EPO | European Patent Office |
| EUON | European Union Observatory for Nanomaterials |
| FDA | United States Food and Drug Administration |
| FIFRA | Federal Insecticide, Fungicide, and Rodenticide Act |
| GDP | Gross Domestic Product |
| GHS | Globally Harmonised System |
| IFAS | Institute for Food and Agricultural Standards |
| ISO | International Organisation for Standardisation |
| ISO/TC 229 | International Organisation for Standardisation Technical Committee 229 |
| KEMI | Swedish Chemical Agency for Chemicals (Kemikalieninspektionen) |
| LANOTEC | National Laboratory of Nanotechnology (Laboratorio Nacional de Nanotecnología) |
| LCA | Life cycle assessment |
| MGs | Main groups |
| NIOSH | National Institute of Occupational and Safety Health |
| NM | Nanomaterial |
| NMX | Mexican Standard |
| NNI | National Nanotechnology Initiative |
| NPs | Nanoparticles |
| OECD | Organisation for Economic Cooperation and Development |
| R&D | Research and development |
| RA | Risk assessment |
| REACH | Registration, Evaluation, Authorisation, and Restriction Chemicals |
| SCENIHR | Scientific Committee on Emerging and Newly Identified Health Risk |
| SDG | Sustainable Development Goals |
| SINANOTOX | National Nanotoxicological Evaluation System Initiative (Sistema Nacional de Evaluación Nanotoxicológica) |
| TC | Technical Committee |
| TGA | Therapeutic Goods Regulatory Authority of Australia |
| TSCA | Toxic Substances Control Act |
| UK | United Kingdom |
| UN | United Nations |
| UNEP | United Nations Environmental Program |
| UNITAR | United Nations for Training and Research |
| USA | United States of America |
| USPTO | United States Patent and Trademark Office |
| WPMN | Working Party on Manufactured Nanomaterials |
| ZnONPs | Zinc oxide nanoparticles |
Author contributions
The authors of this article made the following contributions: conceptualisation (ideas and aims) C. G.; investigation: J. A. C. H, A. J. V. S., G. N. T., C. G.; project administration (coordination responsibility and planning): C. G.; validation (data verification): A. J. V. S., G. N. T., C. G.; writing (draft, review, and editing): J. A. C. H., A. J. V. S., G. N. T., C. G. All authors approve the final manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the CONAHCyT with the grant Problemas Nacionales CONAHCyT PN-2017-01-4710. The authors appreciate the involvement of the scholarship recipient Osvaldo Hernández in the preparation of some images, who received a financial scholarship from the Autonomous University of San Luis Potosi.Notes and references
- R. Feynman, Eng. Sci., 1960, 22–36 Search PubMed.
- F. Matteucci, R. Giannantonio, F. Calabi, A. Agostiano, G. Gigli and M. Rossi, AIP Conf. Proc., 1990, 1, 020001 Search PubMed.
- J. C. García-Gallegos, in Tendencias de innovación en la ingeniería de alimentos, ed. M. E. Ramirez-Ortiz, OmniaScience, Barcelona, España, 2015, pp. 255–287 Search PubMed.
- H. W. Kroto, J. R. Heath, S. C. ÓBrien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 163 CrossRef.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- S. Bayda, M. Adeel, T. Tuccinardi, M. Cordani and F. Rizzolio, Molecules, 2020, 25, 1–15 Search PubMed.
- A. Seaton, L. Tran, R. Aitken and K. Donaldson, J. R. Soc., Interface, 2010, 6(7), S119–S129 Search PubMed.
- R. Patel, M. Kaki, V. S. Potluri, P. Kahar and D. Khanna, Hum. Vaccines Immunother., 2022, 18, 2002083 CrossRef PubMed.
- The Nobel Prize, Press release, The Nobel Prize in Chemistry, 2023, October 4th, https://www.nobelprize.org/prizes/chemistry/2023/press-release/(accessed Nov 20th, 2023) Search PubMed.
- M. C. Roco, J. Nanopart. Res., 2011, 13, 427–445 CrossRef.
- International Organisation for Standardisation, ISO/TS 80004-1:2015, Nanotechnologies —Vocabulary— Part 1: Core terms, Geneva, Switzerland, 2015, https://www.iso.org/standard/68058.html, (accessed Jul 19th, 2023) Search PubMed.
- International Organisation for Standardisation, ISO/TS 80004-2:2015. Nanotechnologies - Vocabulary - Part 2: Nano-objects (ISO/TS 80004-2:2015), Geneva, Switzerland, 2015, https://www.iso.org/standard/54440.html (accessed 19 Jul 2023) Search PubMed.
- European Commission, Commission Recommendation of 10 June 2022 on the definition of nanomaterial (Text with EEA relevance) 2022/C 229/01, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32022H0614(01) (accessed Dec 8th, 2023) Search PubMed.
- N. Abid, A. M. Khan, S. Shujait, K. Chaudhary, M. Ikram, M. Imran, J. Haider, M. Khan, Q. Khan and M. Maqbool, Adv. Colloid Interface Sci., 2022, 300, 102597 CrossRef CAS PubMed.
- Environmental Protection Agency, Technical Fact Sheet – Nanomaterials, January 2014, https://19january2017snapshot.epa.gov/sites/production/files/2014-03/documents/ffrrofactsheet_emergingcontaminant_nanomaterials_jan2014_final.pdf (accessed Nov 15th, 2023) Search PubMed.
- S. S. Patil, U. U. Shedbalkar, A. Truskewycz, B. A. Chopade and A. S. Ball, Environ. Technol. Innovation, 2016, 5, 10–21 CrossRef.
- StatNano, (http://www.statnano.com) (accessed Dec 6th, 2023).
- Nanotechnology Products Database, Statnano, https://product.statnano.com/(accessed Jul 19th, 2023) Search PubMed.
- A. Nanda, S. Nanda, T. A. Nguyen, S. Rajendran, and Y. Slimani, Nanocosmetics: Fundamentals, Applications and Toxicity, Elsevier, 2020 Search PubMed.
- L. M. Skjolding, S. N. Sørensen, N. B. Hartmann, R. Hjorth, S. F. Hansen and A. Baun, Angew. Chem., Int. Ed., 2016, 55, 15224–15239 CrossRef CAS PubMed.
- N. Musee, M. Thwala and N. Nota, J. Environ. Monit., 2011, 13, 1164–1183 RSC.
- The Project on Emerging Nanotechnologies, a project of the Woodrow Wilson International Centre for Scholars, https://www.nanotechproject.tech/inventories/map/data/states/dc/3934/(accessed Jul 10th, 2023) Search PubMed.
- P. Cervantes-Avilés, E. Souza-Brito, A. Bernal-Martínez, J. Antonio Reyes-Aguilera, G. de la Rosa and G. Cuevas-Rodríguez, Rev. Mex. Ing. Quim., 2017, 16, 247–260 Search PubMed.
- G. C. Delgado, Technol. Soc., 2010, 32, 137–144 CrossRef.
- M. I. El Sabry, K. W. McMillin and C. M. Sabliov, Ann. Anim. Sci., 2018, 18, 319–334 CrossRef.
- R. Foulkes, E. Man, J. Thind, S. Yeung, A. Joy and C. Hoskins, Biomater. Sci., 2020, 8, 4653–4664 RSC.
- G. Fytianos, A. Rahdar and G. Z. Kyzas, Nanomaterials, 2020, 10, 1–16 CrossRef PubMed.
- C. Ferraris, C. Rimicci, S. Garelli, E. Ugazio and L. Battaglia, Pharmaceutics, 2021, 13, 1408 CrossRef CAS PubMed.
- P. Isigonis, A. Afantitis, D. Antunes, A. Bartonova, A. Beitollahi, N. Bohmer, E. Bouman, Q. Chaudhry, M. R. Cimpan, E. Cimpan, S. Doak, D. Dupin, D. Fedrigo, V. Fessard, M. Gromelski, A. C. Gutleb, S. Halappanavar, P. Hoet, N. Jeliazkova, S. Jomini, S. Lindner, I. Linkov, E. M. Longhin, I. Lynch, I. Malsch, A. Marcomini, E. Mariussen, J. M. Fuente, G. Melagraki, F. Murphy, M. Neaves, R. Packroff, S. Pfuhler, T. Puzyn, Q. Rahman, E. R. Pran, E. Semenzin, T. Serchi, C. Steinbach, B. Trump, I. V. Vrček, D. Warheit, M. R. Wiesner, E. Willighagen and M. Dusinska, Small, 2020, 16, 2003303 CrossRef CAS PubMed.
- J. Allan, S. Belz, A. Hoeveler, M. Hugas, H. Okuda, A. Patri, H. Rauscher, P. Silva, W. Slikker, B. Sokull-Kluettgen, W. Tong and E. Anklam, Regul. Toxicol. Pharmacol., 2021, 122, 104885 CrossRef CAS PubMed.
- World Health Organisation, Sustainable Development Goals, https://www.who.int/europe/about-us/our-work/sustainable-development-goals (accessed Jul 19th, 2023) Search PubMed.
- N. R. H. Stone, T. Bicanic, R. Salim and W. Hope, Drugs, 2016, 76, 485–500 CrossRef CAS PubMed.
- P. Liu, G. Chen and J. Zhang, Molecules, 2022, 27, 1372 CrossRef CAS PubMed.
- K. Maier-Hauff, F. Ulrich, D. Nestler, H. Niehoff, P. Wust, B. Thiesen, H. Orawa, V. Budach and A. Jordan, J. Neuro-Oncol., 2011, 103, 317–324 CrossRef PubMed.
- Z. W. Tay, P. Chandrasekharan, B. D. Fellows, I. R. Arrizabalaga, E. Yu, M. Olivo and S. M. Conolly, Cancers, 2021, 13, 5285 CrossRef CAS PubMed.
- D. J. McClements and H. Xiao, npj Sci. Food, 2017, 1, 1–13 CrossRef PubMed.
- P. Chaudhary, F. Fatima and A. Kumar, J. Inorg. Organomet. Polym. Mater., 2020, 30, 5180–5192 CrossRef CAS PubMed.
- F. V. Leimann, O. H. Goncalves, L. S. Sakanaka, S. B. Azevedo, M. V Lima, F. Barreiro, and M. Shirai, in Food Packaging and Preservation, Elsevier Inc., 2018, pp. 87–135 Search PubMed.
- J. Jampilek, J. Kos and K. Kralova, Nanomaterials, 2019, 9(2), 296 CrossRef CAS PubMed.
- T. K. Dey, P. Banerjee, R. Chatterjee and P. Dhar, Colloids Surf., A, 2018, 538, 36–44 CrossRef CAS.
- V. Gökmen, B. A. Mogol, R. B. Lumaga, V. Fogliano, Z. Kaplun and E. Shimoni, J. Food Eng., 2011, 105, 585–591 CrossRef.
- M. Guttoff, A. H. Saberi and D. J. Mcclements, Food Chem., 2015, 171, 117–122 CrossRef CAS PubMed.
- X. Liu, R. Zhang, D. J. McClements, F. Li, H. Liu, Y. Cao and H. Xiao, Food Biophys, 2018, 13, 412–421 CrossRef.
- L. Salvia-Trujillo and D. J. McClements, J. Agric. Food Chem., 2016, 64, 4639–4647 CrossRef CAS PubMed.
- V. Gupta, S. Mohapatra, H. Mishra, U. Farooq, K. Kumar, M. J. Ansari, M. F. Aldawsari, A. S. Alalaiwe, M. A. Mirza and Z. Iqbal, Gels, 2022, 8, 173 CrossRef CAS PubMed.
- Lotus Professional®, https://lotus-professional.com/products/lotus-phytorx-silk-matte-uv-defence-sunblock-spf-50 (accessed Nov 4th, 2023) Search PubMed.
- Karex, Dr Wolff USA Distribution Inc., https://buy.karex.com, (accessed Dec 6th, 2023).
- SeneGence®, https://web.senegence.com/en_us/3369a-golden-radiance-peeloff-mask.html?distributor_id=204049 (accessed Nov 4th, 2023) Search PubMed.
- S. Singh, V. Kumar, R. Romero, K. Sharma and J. Singh, Nanotechnol. Life Sci., 2019, 1, 395–418 Search PubMed.
- T. Esakkimuthu, D. Sivakumar and S. Akila, Pollut. Res., 2014, 33, 567–571 CAS.
- K. A. Al-Saad, M. A. Amr, D. T. Hadi, R. S. Arar, M. M. Al-Sulaiti, T. A. Abdulmalik, N. M. Alsahamary and J. C. Kwak, Arab J. Nucl. Sci. Appl., 2012, 45, 335–346 Search PubMed.
- D. Y. Lyon, L. K. Adams, J. C. Falkner and P. J. J. Alvarez, Environ. Sci. Technol., 2006, 40, 4360–4366 CrossRef CAS PubMed.
- M. C. Camara, E. V. Ramos Campos, R. A. Monteiro, A. E. Santo Pereira, P. L. de Freitas Proenca and L. Fernandes Fraceto, J. Nanobiotechnol., 2019, 17, 100 CrossRef PubMed.
- Q. H. Wang, K. Kalantar-Zedeh, A. Kis, J. N. Coleman and M. S. Strano, Nat. Nanotechnol., 2012, 7, 699–712 CrossRef CAS PubMed.
- G. Q. Lu, Prog. Nat. Sci.: Mater. Int., 2018, 28, 97–98 CrossRef CAS.
- C. Song, C. Yin and H. Qu, Microelectron. J., 2021, 108, 104988 CrossRef CAS.
- A. A. Inada, S. Arman and B. Safaei, J. Energy Storage, 2022, 55, 105661 CrossRef.
- M. Cheng, L. Chen and J. Guo, Energy Rep., 2023, 9, 1696–1704 CrossRef.
- A. Nayak, B. Bhushan, S. Kotnala, N. Kukretee, P. Chaudhary, A. R. Triphaty, K. Ghai and S. L. Mudliar, Mater. Today: Proc., 2023, 73, 227–232 CAS.
- M. T. McDowell, H. Xiong, M. Nazemi, J. Peng, J. L. Lutkenhaus, R. Wang, A. Dijire, A. Sankaran, J. Leem and Y. Gogotsi, Cell Rep., 2023, 4, 101605 Search PubMed.
- K. R. Sharma, in Anti-Abrasive Nanocoating, ed. M. Aliofkhazraei, Woodhead Publishing, 1st Edition, 2015, ch. 6, pp. 137–153 Search PubMed.
- M. Joshi and B. Adak, in Comprehensive Nanoscience and Nanotechnology, Elsevier, 2nd Edition, 2019, ch. 5.10, pp. 253–290 Search PubMed.
- Nasiol® Artekya Ldt.Co., https://www.nasiol.com/pershoes-waterproof-spray/, (accessed Nov 4th, 2023).
- Aculon®, https://www.aculon.com (accessed Nov 4th, 2023).
- NanoCare® Deutschland AG, https://nano-care.com/antimicrobial-coating/, (accessed Nov 4th, 2023).
- X. Mao, T. H. D. Nguyen, M. Lin and A. Mustapha, J. Food Sci., 2016, 81, T2107–T2113 CrossRef CAS PubMed.
- A. Brunelli, L. Calgaro, E. Semenzin, V. Cazzagon, E. Giubilato, A. Marcomini and E. Badetti, Environ. Sci. Eur., 2021, 33, 48 CrossRef.
- X. Zhang, M. Wang, S. Guo, Z. Zhang and H. Li, J. Nanopart. Res., 2017, 338, 338 CrossRef.
- D. Pantano, N. Neubauer, J. Navratilova, L. Scifo, C. Civadi, V. Stone, F. von der Kammer, P. Müller, M. S. Sobrido, B. Angeletti, J. Rose and W. Wohlleben, Environ. Sci. Technol., 2018, 52, 1128–1138 CrossRef CAS PubMed.
- M. Gil Garzón, A. Soto, J. Usma Gutierrez and O. Gutiérrez Florez, Prod. más Limpia, 2013, 7, 52–73 Search PubMed.
- C. García Gómez, P. Gortáres Moroyoqui and P. Drogui, Rev. Química Viva, 2011, 1, 96–105 Search PubMed.
- G. E. Batley, J. K. Kirby and M. J. McLaughlin, Acc. Chem. Res., 2013, 46, 854–862 CrossRef CAS PubMed.
- R. Gupta and H. Xie, J. Environ. Pathol., Toxicol. Oncol., 2018, 37, 209–230 CrossRef PubMed.
- A. Bruneau, P. Turcotte, M. Pilote, F. Gagné and C. Gagnon, Aquat. Toxicol., 2016, 174, 70–81 CrossRef CAS PubMed.
- M. A. Elkodous, H. M. El-Husseiny, G. S. El-Sayyad, A. H. Hashem, A. S. Doghish, D. Elfadil, Y. Radwan, H. M. El-Zeiny, H. Bedair, O. A. Ikhdair, H. Hashim, A. M. Salama, H. Alshater, A. A. Ahmed, M. G. Elsayed, M. Nagy, N. Y. Ali, M. Eladahmady, A. M. Kamel, M. A. Elkodous, I. Maallem, M. B. Sh. Kaml, N. Nasser, A. A. Nouh, F. M. Safwat, M. M. Alshal, S. K. Ahmed, T. Nagib, F. M. El-sayed, M. Almahdi, Y. Adla, N. T. ElNashar, A. M. Hussien, A. S. Salih, S. A. Mahmoud, S. Magdy, D. I. Ahmed, F. M. S. Hassan, N. A. Edward, K. S. Milad, S. R. Hasala, M. M. Arafa, A. Hegazy, G. Kawamura, W. K. Tan and A. Matsuda, Nanotechnol. Rev., 2021, 10, 1662–1739 CrossRef CAS.
- K. Dericiler, I. Berktas, S. Dogan, Y. Z. Menceloglu and B. S. Okan, in Micro and Nano Technologies, ed. M. Rai and T. A. Nguyen, Elsevier, 1st Edition, 2022, ch. 8, pp. 147–174 Search PubMed.
- C. Yu, S. Kim, M. Jang, C. M. Park and Y. Yoon, Environ. Eng. Res., 2022, 27, 210339 CrossRef.
- EUON European Union Observatory for Nanomaterials, Nanomaterials in Environment, https://euon.echa.europa.eu/es/nanomaterials-in-the-environment (accessed on Dec 5th, 2023) Search PubMed.
- WHO guidelines on protecting workers from potential risks of manufactured nanomaterials, 2017, https://www.who.int/publications/i/item/9789241550048 (accessed Jul 19th, 2023) Search PubMed.
- United Nations Environment Programme, https://www.unep.org/, (accessed Jul 19th, 2023) Search PubMed.
- United Nations Institute for Training and Research, https://unitar.org/about/unitar/institute (accessed Jul 19th, 2023) Search PubMed.
- G. C. Delgado-Ramos, Rev. Tecnol. e Soc., 2007, 3(4), 77–101 Search PubMed.
- M. Giovanni, C. Y. Tay, M. I. Setyawati, J. Xie, C. N. Ong, R. Fan, J. Yue, Z. Lifeng and D. T. Leong, Environ. Toxicol., 2015, 30, 1459–1469 CrossRef CAS PubMed.
- T. Tang, Z. Zhang and X. Zhu, Int. J. Environ. Res. Public Health, 2019, 16(4), 523 CrossRef CAS PubMed.
- T. H. Cheng, F. C. Ko, J. L. Chang and K. A. Wu, Ann. Thorac. Surg., 2012, 93, 666–669 CrossRef PubMed.
- A. Shvedova, V. Castranova, E. Kisin, A. Murray, V. Gandelsman and P. Baron, J. Toxicol. Environ. Health, Part A, 2011, 66, 1909–1926 CrossRef PubMed.
- L. Ma-Hock, S. Treumann, V. Strauss, S. Brill, F. Luizi, M. Mertler, K. Wiench, A. O. Gamer, B. van Ravenzwaay and R. Landsiedel, Toxicol. Sci., 2009, 112, 468–481 CrossRef CAS PubMed.
- B. Shahare, M. Yashpal and G. Singh, Toxicol. Mech. Methods, 2013, 23, 161–167 CrossRef CAS PubMed.
- M. Fatemi, J. Moshtaghian, K. Ghaedi and N. J. Dinani, Iran. J. Pharm. Res., 2017, 16, 685–693 CAS.
- S. Abarghoei, A. Hedayati, R. Ghorbani, H. K. Miandareh and T. Bagheri, Int. J. Environ. Sci. Technol., 2016, 13, 1753–1760 CrossRef CAS.
- O. J. Osborne, S. Lin, C. H. Chang, Z. Ji, X. Yu, X. Wang, S. Lin, T. Xia and A. E. Nel, ACS Nano, 2015, 9, 9573–9584 CrossRef CAS PubMed.
- T. Ostaszewska, M. Chojnacki, M. Kamaszewski and E. Sawosz-Chwalibóg, Environ. Sci. Pollut. Res., 2016, 23, 1621–1633 CrossRef CAS PubMed.
- T. Fleischer, J. B. Jahnel and S. B. Seitz, NanoSafety - Risk Governance of Manufactured Nanoparticles, Final Re- Port, Science and Technology Options Assessment (STOA), 2012 Search PubMed.
- L. Saldivar Tanaka, Mundo Nano, 2019, 12, 1–21 Search PubMed.
- L. Saldivar Tanaka, Mundo Nano, 2020, 13, 1–27 Search PubMed.
- Ni. Bryndum, Al. Lang, C. Mandl, M. V. Nielsen and B. Bedsted, The Res-AGorA Co-construction Method, 2016 Search PubMed.
- S. F. Hansen, Nat. Publ. Gr., 2017, 12, 714–716 CAS.
- EU, Regulation for Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), https://osha.europa.eu/en/themes/dangerous-substances/reach (accessed Nov 15th, 2023) Search PubMed.
- The Nanotechnology Challenge: Creating Legal Institutions for Uncertain Risks, ed. D. Dana, Cambridge University Press, 2012 Search PubMed.
- R. Clift, in Nanotechnology: Risk, Ethics and Law, ed. G. Hunt and M. Mehta, Routledge, London, 1st edn, 2006, pp. 140–153 Search PubMed.
- M. Fredriksson, P. Blomqvist and U. Winblad, Scan. Polit. Stud., 2012, 35, 48–70 CrossRef.
- S. Arnaldi, Mundo Nano, 2014, 7, 6–27 Search PubMed.
- C. Meili and M. Widmer, in International Handbook on Regulating Nanotechnologies, ed. G. A. Hodge, D. M. Bowman and A. D. Maynard, 1st edn, 2010, pp. 446–461 Search PubMed.
- E. Kica and D. M. Bowman, Jurimetrics, 2012, 53, 11–56 Search PubMed.
- Department of Environment, Food and Rural Affairs (DEFRA), https://www.gov.uk/government/organisations/department-for-environment-food-rural-affairs (accessed 1 Nov 1st, 2023).
- Environmental Protection Agency, Nanoscale Materials Stewardship Program, Federal Register (Federal Communications Commissions), US 2008, 73, pp. 4861–4866 Search PubMed.
- French Agency for Food, Environmental and Occupational Health & Safety (ANSES), https://www.anses.fr/en/content/nanomaterials (accessed Nov 15th, 2023) Search PubMed.
- Swedish Chemistry Agency (KEMI), https://www.kemi.se/en/rules-and-regulations/agency-regulations-kifs, (accessed Nov 15th, 2023) Search PubMed.
- A. Boyano, R. Kaps, G. Medyna and R. Villalba, User Manual: EU Ecolabel for Detergents and Cleaning Products, Publications Office of the European Union, Luxembourg, 2018 Search PubMed.
- The European Parliament and the Council of the European Union, Regulation (EC) No. 1223/2009, 2009, L 342/59-L 342/209 Search PubMed.
- The European Parliament and the Council of the European Union, Regulation (EC) No. 1169/2011, 2011, L304/18- L 304/62 Search PubMed.
- Department of Health and Aged Care, Therapeutic Goods Administration, Australian Government (https://www.tga.gov.au/(accessed Dec 5th, 2023) Search PubMed.
- Health Canada, Government of Canada(https://www.canada.ca/en/health-canada.html (accessed Dec 5th, 2023) Search PubMed.
- Ministry of Food and Drug Safety, Republic of Korea, (https://www.mfds.go.kr/eng/index.do, (accessed Dec 5th, 2023) Search PubMed.
- Norma NMX-R-13830-SCFI-2014, https://www.sinec.gob.mx/SINEC/Vista/Normalizacion/DetalleNMX.xhtml?pidn=WXNuZU04czBZcVg2dWo0MFczTndSdz09 (accessed Dec 5th, 2023) Search PubMed.
- German Advisory Council on the Enviromental, SRU, Precautionary Strategies for Managing Nanomaterials. Chapter 7: Conclusions and Recommendations, 2011, pp. 1–38 Search PubMed.
- Recomendación de la Comisión de 7 de febrero de 2008 sobre un código de conducta para una investigación responsable en el campo de las nanociencias y las nanotecnologías, Agencia Estatal Boletion Oficial del Estado, 2008, DOUE-L-2008-80735 https://www.boe.es/buscar/doc.php?lang=en%26id=DOUE-L-2008-80735 (accessed Nov 19th, 2023) Search PubMed.
- M. Janin and M. Reardon, Enviromental Defense Fund, Media Advisory, Jun 15, 2007, https://www.edf.org/media/environmental-defense-and-dupont-jointly-launch-nano-risk-framework-evaluate-and-address) (accessed Nov 4th, 2023) Search PubMed.
- The Innovation Society, Cenarios ®(http://innovationsgesellschaft.ch/en/competences/risk-management/cenarios/(accessed Dec 6th, 2023) Search PubMed.
- Schweizerisch Eidgenossenschft, Federal Office of Public Health, Division of Chemical Products, (https://www.bag.admin.ch/bag/en/home/gesund-leben/umwelt-und-gesundheit/chemikalien/nanotechnologie/sicherer-umgang-mit-nanomaterialien/vorsorgeraster-nanomaterialien-webanwendung.html#:%7E:text=The-precautionary-matrix-enables-the,precautions%E2%80%9D-when-.) (accessed Dec 6th, 2023) Search PubMed.
- N. Jacobstein, Foresight Institute and IMM, Foresight Guidelines for Responsible Nanotechnology Development, 2006, http://www.imm.org/policy/guidelines/, (accessed Dec 6th, 2023) Search PubMed.
- European Food Safety Authority (EFSA), https://www.efsa.europa.eu/en (accessed Nov 15th, 2023) Search PubMed.
- European Commission, Scientific Committee on Customer Safety (SCCS), Guidance on the Safety Assessment of Nanomaterials in Cosmetics (2nd revision)https://health.ec.europa.eu/publications/sccs-guidance-safety-assessment-nanomaterials-cosmetics-2nd-revision_en, 2023, (accessed Nov 15th, 2023) Search PubMed.
- Organisation for Economic Co-operation and Development, Safety of manufactured nanomaterials, https://www.oecd.org/science/nanosafety/(accessed 01 Nov 2023) Search PubMed.
- E. Heunisch, F. Cassee, E. Bleeker, T. Kuhlbusch and M. Gonzalez, Development or Revisions of Oecd Test Guideline (Tg) and Guidance Documents (Gd) Applicable for Nanomaterials, 2022 Search PubMed.
- Organisation for Economic Co-operation and Development, Nanosafety at the OECD: The First Five Years 2006-2010, 2011, https://www.oecd.org/science/nanosafety/47104296.pdf (accessed 19 Jul 2023) Search PubMed.
- H. G. Park and M. K. Yeo, Mol. Cell. Toxicol., 2016, 12, 223–236 CrossRef CAS.
- K. Rasmussen, H. Rauscher, P. Kearns, M. González and J. Riego, Sintes, Regul. Toxicol. Pharmacol., 2019, 104, 74–83 CrossRef PubMed.
- Organisation for Economic Co-operation and Development, Publications in the Series on the Safety of Manufactured Nanomaterials, https://www.oecd.org/science/nanosafety/publications-series-safety-manufactured-nanomaterials.htm (accessed 19 Jul 2023) Search PubMed.
- Organisation for Economic Co-operation and Development, OECD legal intstruments, https://legalinstruments.oecd.org/en/about#:%7E:text=An/OECD/standard/sets/out,instruments, (accessed Nov 16th, 2023) Search PubMed.
- International Organisation for Standardisation, Standards, https://www.iso.org/standards.html#:%7E:text=ISO-standards-are-internationally-agreed,a-huge-range-of-activities (accessed Nov 19th, 2023) Search PubMed.
- International Organisation for Standardisation, ISO/TC229, https://www.iso.org/(accessed Jul 19th, 2023) Search PubMed.
- J. Wang, J. D. Gerlach, N. Savage and G. P. Cobb, Sci. Total Environ., 2013, 442, 56–62 CrossRef CAS PubMed.
- R. D. Handy and B. J. Shaw, Health Risk Soc., 2007, 9, 125–144 CrossRef.
- A. Franco, S. F. Hansen, S. I. Olsen and L. Butti, Regul. Toxicol. Pharmacol., 2007, 48, 171–183 CrossRef CAS PubMed.
- J. Jeevanandam, A. Barhoum, Y. S. Chan, A. Dufresne and M. K. Danquah, Beilstein J. Nanotechnol., 2018, 9, 1050–1074 CrossRef CAS PubMed.
- K. Hegde, S. Kaur Brar, V. Mausam and R. Y. Surampalli, Nanotechnol. Environ. Eng., 2016, 1, 1–12 CrossRef.
- H. Stamm, N. Gibson and E. Anklam, Food Addit. Contam.: Part A, 2012, 29, 1175–1182 CrossRef CAS PubMed.
- European Parliament and Commission of the European Communities, Procedure Nanosciences and nanotechnologies: An action plan for Europe 2005-2009, 2009, https://www.europarl.europa.eu/doceo/document/A-6-2006-<?pdb_no 0216?>0216<?pdb<?db_id PDB?> END?>_EN.html (accessed 19 Jul 2023).
- European Union, Commission Decision of 5 August 2008 setting up an advisory structure of Scientific Committees and experts in the field of consumer safety, public health, and the environment and repealing Decision 2004/210/EC, 2008. https://eur-lex.europa.eu/eli/dec/2008/721/oj (accessed 19 Jul 2023) Search PubMed.
- European Union, European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending, Regulation (EC) No 1272/2008, 2008, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32008R1272 (accessed 19 Jul 2023) Search PubMed.
- SCENIHR, SCENIHR & European Commission, Opinion on: ‘Nanosilver: safety, health and environmental effects and role in antimicrobial resistance’, 2014, https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_039.pdf (accessed Jul 19th, 2023).
- E. T. Ballester, J. Holmqvist, A. Elwan, A. Sumrein and T. Multasuo, INTERIM: Ex-post evaluation of the European Union Observatory for Nanomaterials (EUON), T. Blagoeva and J. Sheridan, European Chemicals Agency, 2017, https://euon.echa.europa.eu/documents/2435000/3268573/050719_evaluation_nano_observatory_en.pdf/04466705-48a8-b759-b719-5a503b180034?t=1570640129520 (accessed Jul 19th, 2023) Search PubMed.
- EUON, European Union Observatory for Nanomaterials, https://euon.echa.europa.eu/, (accessed Jul 19th, 2023) Search PubMed.
- EUON, Workshop Report: Stakeholder Dialogue meeting European Union Observatory for Nanomaterials, Helsinki, 2017https://euon.echa.europa.eu/documents/2435000/2604302/workshop_report_en.pdf/4fddbb2d-bc12-244d-d997-29d855c2cadd?t=1505819178682 Search PubMed.
- C. F. Chau, S. H. Wu and G. C. Yen, Trends Food Sci. Technol., 2007, 18, 269–280 CrossRef CAS.
- P. H. Hoet, Regul. Toxicol. Pharmacol., 2016, 74, 79–80 CrossRef PubMed.
- E. B. Souto, G. F. Silva, J. Dias-ferreira, A. Zielinska, F. Ventura, A. Durazzo, M. Lucarini, E. Novellino and A. Santini, Pharmaceutics, 2020, 12, 1–14 Search PubMed.
- European Union, European Parliament and of the Council of 30 November 2009 on cosmetic products (recast)https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02009R1223-20221217#tocId26 (accessed 19 Jul 2023) Search PubMed.
- K. S. Ibrahim, Carbon Lett., 2013, 14, 131–144 CrossRef.
- Office for Product Safety and Standards (OPSS), https://www.gov.uk/government/organisations/office-for-product-safety-and-standards (accessed 1 November 2023) Search PubMed.
- UK Health Security Agency (UKHSA), https://www.gov.uk/government/organisations/uk-health-security-agency (accessed 1 November 2023) Search PubMed.
- Food Standard Agency (FSA), https://www.food.gov.uk/(accessed 1 November 2023) Search PubMed.
- German Environment Agency (UBA), https://www.umweltbundesamt.de/en (accessed 1 November 2023) Search PubMed.
- Federal Ministry of Food and Agriculture (BMEL), https://www.bmel.de/EN/Home/home_node.html (accessed 1 November 2023) Search PubMed.
- German Chemical Industry Association (VCI), https://www.vci.de/startseite.jsp (accessed 1 November 2023).
- German Federal Institute for Risk Assessment (BfR), https://www.bfr.bund.de/en/home.html (accessed Nov 1st, 2023).
- Instituto Superiore di Sanità, https://www.iss.it/en/home (accessed Nov 1st, 2023).
- R. Sood and D. S. Chopra, Appl. Clin. Res. Clin. Trials Regul. Aff., 2018, 5, 74–79 Search PubMed.
- C. Gourish, S. Nimrata and S. Vishal, J. Dispersion Sci. Technol., 2013, 3, 138–141 Search PubMed.
- Food and Drug Administration Nanotechnology: Over a Decade of Progress and Innovation, FDA, 2020. https://www.fda.gov/media/140395/download (accessed Jul 19th, 2023) Search PubMed.
- Environmental Protection Agency, EPA Needs to Manage Nanomaterial Risks More Effectively, EPA, 2011. https://www.epa.gov/sites/default/files/2015-09/documents/20121229-12-p-0162.pdf (accessed Jul 19th, 2023) Search PubMed.
- J. E. M. Allan, Global Summit on Regulatory Science 2019 Nanotechnology and Nanoplastics, ed. B. Sokull Kluettgen and A. K. Patri, EUR 30195 EN, Publications Office of the European Union, Luxembourg, 2020 Search PubMed.
- C. W. Lam, J. T. James, R. McCluskey, S. Arepalli and R. L. Hunter, Crit. Rev. Toxicol., 2006, 36, 189–217 CrossRef CAS PubMed.
- J. F. Sargent, Adv. Nanotechnol., 2012, 9, 123–136 Search PubMed.
- L. E. Friedersdorf, IEEE Nanotechnol. Mag., 2020, 14, 13–20 Search PubMed.
- M. Ferreira, A. Matos, A. Couras, J. Marto and H. Ribeiro, Cosmetics, 2022, 9, 72 CrossRef.
- N. Basavegowda, T. K. Mandal and K. H. Baek, Food Bioprocess Technol., 2020, 13, 30–44 CrossRef CAS.
- R. Kumari, K. Suman and S. Karmakar, Front. Genome Ed, 2023, 5, 1200987 CrossRef PubMed.
- E. Camarillo Abad, R. Blome Fernández, P. Castellanos Andrade and J. Campos Delgado, Mundo Nano, 2019, 12, 73–88 Search PubMed.
- D. Maclurcan, J. Nanotechnol., 2005, 1, 1–19 Search PubMed.
- M. Anzaldo, M. Chauvet and L. A. Maldonado, Interciencia, 2014, 39, 8–15 Search PubMed.
- G. Foladori, E. A. Figueroa, E. Z. Lau, R. Appelbaum, E. Robles-Belmont, L. L. Villa Vázquez, R. Parker and V. Leos, Revista Iberoamericana de Ciencia, Tecnología y Sociedad, 2017, 12, 51–64 Search PubMed.
- World Intellectual Property Organisation, Patentscope, https://patentscope.wipo.int/, (accessed Jul 7th, 2022) Search PubMed.
- D. A. López de la Mora, Rev. Med., 2019, 10, 221–228 Search PubMed.
- Xignux, Nanoquem, https://nanoqem.com/, (accessed Jul 11th, 2023) Search PubMed.
- G. Foladori and E. Zayayo, in Nanomedicina: Aspectos Regulatorios Y Socioeconómicos, ed. L. A. Vidal Correa, UNAM, 2014, vol. 7, pp. 123–146 Search PubMed.
- M. Anzaldo Montoya and M. Chauvet, J. Responsible Innov., 2016, 3, 135–153 CrossRef.
- V. J. Lizardi-Nieto and N. Gonzalez-Rojano, Mundo Nano, 2016, 9(16), 168–178 Search PubMed.
- Sistema Nacional de Evaluación Nanotoxicológica “SINANOTOX”. Red Temática de Nanociencias y Nanotecnología CONAHCyT, 2018, https://sinanotox.com (accessed Jul 15th, 2023).
- A. Kübra Demirbaş and S. Çevik, Open J. Nano, 2020, 5, 1–16 Search PubMed.
- L. da S. Sant'Anna, M. S. M. de Alencar and A. P. Ferreira, Scientometrics, 2014, 100, 675–686 CrossRef.
- A. R. G. Ledesma and M. F. L. de Almeida, J. Technol. Manag. Innov., 2013, 8, 39–52 CrossRef.
- M. Rai and J. K. Biswas, Nanomaterials: Ecotoxicity, Safety, and Public Perception, Springer, Switzerland, 2018 Search PubMed.
- W. Engelmann and R. von Hohendorff, J. Hazardous, Toxic, Radioact. Waste, 2016, 20, 1–5 Search PubMed.
- B. Magnuson, I. Munro, P. Abbot, N. Baldwin, R. Lopez-Garcia, K. Ly, L. McGirr, A. Roberts and S. Socolovsky, Food Addit. Contam.: Part A, 2013, 30, 1147–1220 CrossRef CAS PubMed.
- G. Foladori, F. Bejarano and N. Invernizzi, Trab. Educ. Saúde, 2013, 11, 145–167 CrossRef.
- ANMAT, Código Alimentario Argentino, https://www.argentina.gob.ar/anmat/codigoalimentario (accessed Jul 11th, 2023) Search PubMed.
- A. Chiancone, M. S. López and J. R. Vega Baudrit, Mundo Nano, 2021, 15, 1e–25e Search PubMed.
- Laboratorio Nacional de Nanotecnología in Centro Nacional de Alta Tecnología, https://lanotec.cenat.ac.cr/es (accessed Jul 19th, 2023) Search PubMed.
- A. Pavlicek, F. Part, G. Rose, A. Praetorius, M. Miernicki, A. Gazsó and M. Huber-Humer, NanoImpact, 2021, 21, 100276 CrossRef CAS PubMed.
- M. A. Saifi, W. Khan and C. Godugu, Pharm. Nanotechnol., 2018, 6, 3–16 CrossRef CAS PubMed.
- S. P. Forster, S. Olveira and S. Seeger, Int. J. Nanotechnol., 2011, 8, 592–613 CrossRef.
- D. M. Hariyadi, U. Athiyah and Y. V. Pathak, in Nanomedicine and Nanosafety: Recent Trends and Clinical Evidences, Springer, 2020, pp. 561–578 Search PubMed.
- A. C. Santos, F. Morais, A. Simões, I. Pereira, J. A. D. Sequeira, M. Pereira-Silva, F. Veiga and A. Ribeiro, Expert Opin. Drug Delivery, 2019, 16, 313–330 CrossRef CAS PubMed.
- F. P. de Albuquerque, A. C. Preisler, L. F. Fraceto, H. C. Oliveira and V. L. S. S. de Castro, in Nanopesticides, Elsevier, 2020, pp. 281–298 Search PubMed.
- C. Giannakou, M. V. D. Z. Park, I. E. M. Bosselaers, W. H. de Jong, J. W. van der Laan, H. van Loveren, R. J. Vandebriel and R. E. Geertsma, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2020, 12, 1–16 Search PubMed.
- T. P. J. Linsinger, Q. Chaudhry, V. Dehalu, P. Delahaut, A. Dudkiewicz, R. Grombe, F. Von Der Kammer, E. H. Larsen, S. Legros, K. Loeschner, R. Peters, R. Ramsch, G. Roebben, K. Tiede and S. Weigel, Food Chem., 2013, 138, 1959–1966 CrossRef CAS PubMed.
- F. Poças and R. Franz, in Nanomaterials for Food Packaging: Materials, Processing Technologies, and Safety Issues, 2018, pp. 277–300 Search PubMed.
- A. D. Romig, A. B. Baker, J. Johannes, T. Zipperian, K. Eijkel, B. Kirchhoff, H. S. Mani, C. N. R. Rao and S. Walsh, Technol. Forecast. Soc. Change, 2007, 74, 1634–1642 CrossRef.
- T. Karamanidou, V. Bourganis, G. Gatzogianni and A. Tsouknidas, Metals (Basel), 2021, 11, 1–15 Search PubMed.
- Y. Pu, F. Tang, P. M. Adam, B. Laratte and R. E. Ionescu, Environ. Sci. Technol., 2016, 50, 9370–9379 CrossRef CAS PubMed.
- B. Salieri, D. A. Turner, B. Nowack and R. Hischier, NanoImpact, 2018, 10, 108–120 CrossRef.
- D. E. Meyer, M. A. Curran and M. A. Gonzalez, J. Nanopart. Res., 2011, 13, 147–156 CrossRef CAS.
- C. Som, M. Berges, Q. Chaudhry, M. Dusinska, T. F. Fernandes, S. I. Olsen and B. Nowack, Toxicology, 2010, 269, 160–169 CrossRef CAS PubMed.
- V. Khanna, B. R. Bakshi and L. J. Lee, J. Ind. Ecol., 2008, 12, 394–410 CrossRef CAS.
- F. Afkhami, S. J. Pourhashemi, M. Sadegh, Y. Salehi and M. J. K. Fard, J. Dent., 2015, 43, 1573–1579 CrossRef CAS PubMed.
- A. Bafana, S. V. Kumar, S. Temizel-Sekeryan, S. A. Dahoumane, L. Haselbach and C. S. Jeffryes, Sci. Total Environ., 2018, 636, 936–943 CrossRef CAS PubMed.
- D. M. A. Crista, J. C. G. E. da Silva and L. P. da Silva, Nanomaterials, 2020, 10, 1–15 Search PubMed.
- Y. Deng, L. Ma, T. Li, J. Li and C. Yuan, ACS Sustain. Chem. Eng., 2019, 7, 599–610 CrossRef CAS.
- S. Feijoo, S. González-García, Y. Moldes-Diz, C. Vazquez-Vazquez, G. Feijoo and M. T. Moreira, J. Cleaner Prod., 2017, 143, 528–538 CrossRef CAS.
- D. Koch, M. Paul, S. Beisl, A. Friedl, B. Mihalyi and E. Stage, J. Cleaner Prod., 2020, 245, 118760 CrossRef CAS.
- W. Leng, P. Pati and P. J. Vikesland, Environ. Sci. Nano, 2015, 2, 440–453 RSC.
- S. Middlemas, Z. Z. Fang and P. Fan, J. Cleaner Prod., 2015, 89, 137–147 CrossRef CAS.
- P. Pati, S. McGinnis and P. J. Vikesland, Environ. Eng. Sci., 2014, 31, 410–420 CrossRef CAS.
- M. Tsang, G. Philippot, C. Aymonier and G. Sonnemann, Green Chem., 2016, 18, 4924–4933 RSC.
- S. Temizel-Sekeryan and A. L. Hicks, NanoImpact, 2021, 22, 100319 CrossRef CAS PubMed.
- J. B. Guineé, R. Heijungs, M. G. Vijver and W. J. G. M. Peijnenburg, Nat. Nanotechnol., 2017, 12, 727–733 CrossRef PubMed.
- K. G. Steinhäuser, P. G. Sayre and B. Nowack, NanoImpact, 2018, 10, 68–69 CrossRef.
- I. Linkov, F. K. Satterstrom, J. Steevens, E. Ferguson and R. C. Pleus, J. Nanopart. Res., 2007, 9, 543–554 CrossRef.
- P. Laux, J. Tentschert, C. Riebeling, A. Braeuning, O. Creutzenberg, A. Epp, V. Fessard, K. H. Haas, A. Haase, K. Hund-Rinke, N. Jakubowski, P. Kearns, A. Lampen, H. Rauscher, R. Schoonjans, A. Störmer, A. Thielmann, U. Mühle and A. Luch, Arch. Toxicol., 2018, 92, 121–141 CrossRef CAS PubMed.
- J. R. A. Schmidt, D. J. Nogueira, S. M. Nassar, V. P. Vaz, M. L. N. da Silva, D. S. Vicentini and W. G. Matias, Nanotoxicology, 2020, 14, 1258–1270 CrossRef CAS PubMed.
- A. Spinazzè, C. Zellino, F. Borghi, D. Campagnolo, S. Rovelli, M. Keller, G. Fanti, A. Cattaneo and D. M. Cavallo, Nanomaterials, 2021, 11, 1–11 CrossRef PubMed.
- H. J. P. Marvin, Y. Bouzembrak, E. M. Janssen, M. van der Zande, F. Murphy, B. Sheehan, M. Mullins and H. Bouwmeester, Nanotoxicology, 2017, 11, 123–133 CrossRef CAS PubMed.
- E. Demir, Nanotoxicology, 2020, 14, 1271–1279 CrossRef CAS PubMed.
- N. Erdinçmer and D. T. Sponza, Asian Basic Appl. Res. J., 2020, 2, 37–42 CrossRef.
- M. Pelin, S. Sosa, M. Prato and A. Tubaro, Nanoscale, 2018, 10, 15894–15903 RSC.
- J. A. Shatkin and B. Kim, Environ. Sci. Nano, 2015, 2, 477–499 RSC.
- R. Pecoraro, F. Marino, A. Salvaggio, F. Capparucci, G. Di Caro, C. Iaria, A. Salvo, A. Rotondo, D. Tibullo, G. Guerriero, E. M. Scalisi, M. Zimbone, G. Impellizzeri and M. V. Brundo, Front. Physiol., 2017, 8, 1011 CrossRef PubMed.
- T. Garcia, D. Lafuente, J. Blanco, D. J. Sánchez, J. J. Sirvent, J. L. Domingo and M. Gómez, Food Chem. Toxicol., 2016, 92, 177–187 CrossRef CAS PubMed.
- L. Vila, A. García-Rodríguez, C. Cortés, R. Marcos and A. Hernández, Food Chem. Toxicol., 2018, 116, 1–10 CrossRef CAS PubMed.
- S. Bao, W. Tang and T. Fang, Chemosphere, 2020, 249, 126172 CrossRef CAS PubMed.
- X. Luo, S. Xu, Y. Yang, Y. Zhang, S. Wang, S. Chen, A. Xu and L. Wu, Chemosphere, 2017, 168, 648–657 CrossRef CAS PubMed.
- L. Pinďáková, V. Kašpárková, K. Kejlová, M. Dvořáková, D. Krsek, D. Jírová and L. Kašparová, Int. J. Pharm., 2017, 527, 12–20 CrossRef PubMed.
- O. D. Hendrickson, S. G. Klochkov, O. V. Novikova, I. M. Bravova, E. F. Shevtsova, I. V. Safenkova, A. V. Zherdev, S. O. Bachurin and B. B. Dzantiev, Toxicol. Lett., 2016, 241, 184–192 CrossRef CAS PubMed.
- C. Larue, H. Khodja, N. Herlin-Boime, F. Brisset, A. M. Flank, B. Fayard, S. Chaillou and M. Carrière, J. Phys.: Conf. Ser., 2011, 304, 1–7 CrossRef.
- Z. Guerra Que, H. Perez Vidal, M. A. Lunagómez Rocha, J. C. Arévalo Pérez, I. Cuahutémoc López, D. De la Cruz Romero, A. E. Espinosa De los Monteros Reyna, J. G. Pacheco Sosa, A. A. Silahua Pavón and J. S. Ferráez Hernández, in Silver Nanoparticles-Fabrication, Characterisation and Applications, InTech, 2018 Search PubMed.
- G. Singh, C. Stephan, P. Westerhoff, D. Carlander and T. V. Duncan, Compr. Rev. Food Sci. Food Saf., 2014, 13, 693–704 CrossRef CAS PubMed.
- E. Vamanu, M. Ene, B. Biță, C. Ionescu, L. Crăciun and I. Sârbu, Nutrients, 2018, 10(5), 1–12 CrossRef PubMed.
- K. Saravanakumar, R. Chelliah, S. Shanmugam, N. B. Varukattu, D. H. Oh, K. Kathiresan and M. H. Wang, J. Photochem. Photobiol., B, 2018, 185, 126–135 CrossRef CAS PubMed.
- J. L. Wang, E. Alasonati, M. Tharaud, A. Gelabert, P. Fisicaro and M. F. Benedetti, Water Res., 2020, 176, 115722 CrossRef CAS PubMed.
- G. Paterson, A. MacKenac and K. V. Thomasa, Anal. Methods, 2011, 3, 1461–1467 RSC.
- T. E. Abbott Chalew and K. J. Schwab, Cell Biol. Toxicol., 2013, 29, 101–116 CrossRef CAS PubMed.
- J. Pelka, H. Gehrke, M. Esselen, M. Türk, M. Crone, S. Bräse, T. Muller, H. Blank, W. Send, V. Zibat, P. Brenner, R. Schneider, D. Gerthsen and D. Marko, Chem. Res. Toxicol., 2009, 22, 649–659 Search PubMed.
- L. Wang, D. K. Nagesha, S. Selvarasah, M. R. Dokmeci and R. L. Carrier, J. Nanobiotechnol., 2008, 6, 1–15 CrossRef PubMed.
- A. Kulkarni, G. S. Kumar, J. Kaur and K. Tikoo, Inhalation Toxicol., 2014, 26, 772–788 CrossRef CAS PubMed.
- T. Liu, L. Li, C. Fu, H. Liu, D. Chen and F. Tang, Biomaterials, 2012, 33, 2399–2407 CrossRef CAS PubMed.
- L. F. Espinosa Cristobal, G. A. Martinez Castañon, J. P. Loyola Rodriguez, N. Patiño Marin, J. F. Reyes Macías, J. M. Vargas Morales and F. Ruiz, J. Nanopart. Res., 2013, 15(1702), 1–12 Search PubMed.
- H. Rosas-Hernández, S. Jiménez-Badillo, P. P. Martínez-Cuevas, E. Gracia-Espino, H. Terrones, M. Terrones, S. M. Hussain, S. F. Ali and C. González, Toxicol. Lett., 2009, 191, 305–313 CrossRef PubMed.
- K. Williams, J. Milner, M. D. Boudreau, K. Gokulan, C. E. Cerniglia and S. Khare, Nanotoxicology, 2015, 9, 279–289 CrossRef CAS PubMed.
- H. Guo, J. Zhang, M. Boudreau, J. Meng, J. jie Yin, J. Liu and H. Xu, Part. Fibre Toxicol., 2015, 13, 1–13 CrossRef PubMed.
- M. A. Ramírez-Lee, H. Rosas-Hernández, S. Salazar-García, J. M. Gutiérrez-Hernández, R. Espinosa-Tanguma, F. J. González, S. F. Ali and C. González, Toxicol. Lett., 2013, 1, 1–11 Search PubMed.
- A. Macken, H. J. Byrne and K. V. Thomas, Ecotoxicol. Environ. Saf., 2012, 86, 101–110 CrossRef CAS PubMed.
- A. C. Carew, M. E. Hoque, C. D. Metcalfe, C. Peyrot, K. J. Wilkinson and C. C. Helbing, Aquat. Toxicol., 2015, 159, 99–108 CrossRef CAS PubMed.
- S. A. Johari, M. R. Kalbassi, I. J. Yu and J. H. Lee, Comp. Clin. Pathol., 2015, 24, 995–1007 CrossRef CAS.
- Y. Wu and Q. Zhou, Environ. Toxicol. Chem., 2013, 32, 165–173 CrossRef CAS PubMed.
- H. J. Yen, J. L. Horng, C. H. Yu, C. Y. Fang, Y. H. Yeh and L. Y. Lin, Aquat. Toxicol., 2019, 215, 105273 CrossRef CAS PubMed.
- A. T. Harris and R. Bali, J. Nanopart. Res., 2008, 10, 691–695 CrossRef CAS.
- X. Hu, S. Ouyang, L. Mu, J. An and Q. Zhou, Environ. Sci. Technol., 2015, 49, 10825–10833 CrossRef CAS PubMed.
- B. Siddhardha, M. Dyavaiah and K. Kasinathan, Model Organisms to Study Biological Activities and Toxicity of Nanoparticles, Springer, 2020 Search PubMed.
- D. A. Mosselhy, W. He, D. Li, Y. Meng and Q. Feng, J. Nanopart. Res., 2016, 18, 222 CrossRef.
- C. Chakraborty, A. R. Sharma, G. Sharma and S. S. Lee, J. Nanobiotechnol., 2016, 14, 1–13 CrossRef PubMed.
- R. de Lima, A. B. Seabra and N. Durán, J. Appl. Toxicol., 2012, 32, 867–879 CrossRef PubMed.
- F. V. Leimann, O. H. Goncalvez, L. Sakanaka, A. S. B. Azevedo, M. V. Lima, F. Barreiro, and M. Shirai, in Food Packaging and Preservation, Academic Press, 2018, pp. 87–135 Search PubMed.
- S. Park, J. Woodhall, G. Ma, J. G. C. Veinot, M. S. Cresser and A. B. A. Boxall, Nanotoxicology, 2014, 8, 583–592 CrossRef CAS PubMed.
- B. Ertit Taştan, İ. Kars Durukan and M. Ateş, J. Polytech., 2019, 0900, 1073–1079 Search PubMed.
- SINEC, Catálogo de Normas, https://www.sinec.gob.mx/SINEC/Vista/Normalizacion/BusquedaNormas.xhtml, (accessed 6 July 2023) Search PubMed.
- SEGOB, Diario Oficial de la Federacion (DOF), https://www.dof.gob.mx/index.php, (accessed October 25th, 2022) Search PubMed.
- G. Foladori, Econ. Labour Relations Rev., 2017, 28, 538–554 CrossRef.
- C. Lauterwasser, Organisation for Economic Co-operation and Development, International Futures Programme, Small sizes that matter: Opportunities and risks of Nanotechnologies, Allianz, 2007. https://www.oecd.org/science/nanosafety/44108334.pdf (accessed Jul 19th, 2023) Search PubMed.
- Z. Ferdous and A. Nemmar, Int. J. Mol. Sci., 2020, 21(7), 2375 CrossRef CAS PubMed.
- A. Kulkarni, G. S. Kumar, J. Kaur and K. Tikoo, Inhalation Toxicol., 2014, 26, 772–788 CrossRef CAS PubMed.
- C. González, S. Salazar-García, G. Palestino, P. P. Martínez-Cuevas, M. A. Ramírez-Lee, B. B. Jurado-Manzano, H. Rosas-Hernández, N. Gaytán-Pacheco, G. Martel, R. Espinosa-Tanguma, A. S. Biris and S. F. Ali, Toxicol. Lett., 2011, 207, 306–313 CrossRef PubMed.
- M. A. Ramírez Lee, P. Aguirre Bañuelos, P. P. Martínez Cuevas, R. Espinosa Tanguma, E. Chi Ahumada, G. A. Martínez Castañón and C. González, Nanomedicine Nanotechnology, Biol. Med., 2018, 14, 385–395 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
