Open Access Article
Open Access ArticleRecent advances of nanoparticles on bone tissue engineering and bone cells
Gejing
Zhang
abc,
Chenxiao
Zhen
abc,
Jiancheng
Yang
d,
Jianping
Wang
abc,
Shenghang
Wang
ae,
Yanwen
Fang
f and
Peng
Shang
 *bc
*bc
aSchool of Life Sciences, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, China
bResearch & Development Institute of Northwestern Polytechnical University, Shenzhen, 518057, China. E-mail: shangpeng@nwpu.edu.cn
cKey Laboratory for Space Bioscience and Biotechnology, Institute of Special Environment Biophysics, Northwestern Polytechnical University, Xi'an, Shaanxi 710072, China
dDepartment of Osteoporosis, Honghui Hospital, Xi'an Jiaotong University, Xi'an 710054, China
eDepartment of Spine Surgery, Affiliated Longhua People's Hospital, Southern Medical University (Longhua People's Hospital), Shenzhen, 518109, China
fHeye Health Technology Co., Ltd, Huzhou 313300, China
First published on 12th February 2024
Abstract
With the development of biotechnology, biomaterials have been rapidly developed and shown great potential in bone regeneration therapy and bone tissue engineering. Nanoparticles have attracted the attention of researches and have applied in various fields especially in the biomedical field as the special physicochemical properties. Nanoparticles were found to regulate bone remodeling depending on their size, shape, composition, and charge. Therefore, in-depth research was necessary to provide the basic support to select the most suitable nanoparticles for bone relate diseases treatment. This article reviews the current development of nanoparticles in bone tissue engineering, focusing on drug delivery, gene delivery, and cell labeling. In addition, the research progress on the interaction of nanoparticles with bone cells, focusing on osteoblasts, osteoclasts, and bone marrow mesenchymal stem cells, and the underlying mechanism were also reviewed. Finally, the current challenges and future research directions are discussed. Thus, detailed study of nanoparticles may reveal new therapeutic strategies to improve the effectiveness of bone regeneration therapy or other bone diseases.
Introduction
Medicine is defined as the applied science of detecting and diagnosing, treating, and preventing diseases. Nanomedicine is different from other types of medicine in that it refers to the development and application of nanoscale materials and technologies, and is an interdisciplinary field involving the interaction of nanoscience, nanoengineering, nanotechnology and life sciences.1 Nanoparticles (NPs) are main components of nanomedicine. Currently, nanoparticles can be divided into organic nanoparticles and inorganic nanoparticles based on their composition. Such as organic nanoparticles include lipid-based nanoparticles, polymeric-based nanoparticles, dendrimers, chitosan, and inorganic nanoparticles include metal nanoparticles carbon-based nanoparticles, magnetic-based nanoparticles, silica-based nanoparticles, calcium phosphate-based nanoparticles, quantum dot etc.2,3 With the development of biotechnology, the properties of nanoparticles have been greatly improved and have been used in several fields. However, the properties of nanoparticles depend mainly on the methods of synthesis, purification, and characterization.4,5In recent years, some functional bio-nanomaterial molecules have been used in bioengineering and tissue engineering.6 The research of nanoparticles is mainly focus on the field of bone tissue engineering, as the drug delivery, gene delivery, cell labeling, and especially in some experimental studies related to bone regeneration methods. Angiogenesis and osteogenesis are critical stages of bone regeneration, both of which require the regulation of multiple growth factors. The mechanical properties, biocompatibility, and bone integration properties of biomaterials are the priority factors for bone tissue regeneration engineering. To better mimic the nanostructures in the natural bone extracellular matrix (ECM), nanofibers, nanotubes, nanoparticles, and hydrogels have emerged as effective candidates to produce resemble the ECM and tissue scaffolds.7,8 For example, carbon nanotubes of tubular nanomaterials accelerate tissue healing and bone regeneration through orchestrated cell and tissue-regulatory responses.9 And nanoparticles as a carrier material for bone implants improved the osseointegration of the implants and reduced the risk of infection.10 Nanoparticles were found to regulate bone remodeling depending on their size, shape, composition, and charge in vitro. In the meantime, the biocompatibility, low toxicity, biodegradability, and precise targeting of nanoparticles are the key factors to evaluate safety in vivo.6,11 In addition, nanoparticles have made breakthroughs in cancer diagnosis and treatment, and it have developed targeted cell markers for nanoparticles used in the treatment of cancer.12 Therefore, in-depth research was necessary to provide the basic support to select the most suitable nanoparticles for bone relate diseases treatment.
This article reviews the current development of nanoparticles in bone tissue engineering, and the research progress on the interaction of nanoparticles with bone cells, focusing on osteoblasts, osteoclasts, and bone marrow mesenchymal stem cells (BMSCs), and the underlying mechanism were also reviewed. The physicochemical properties of nanoparticles change depending on their size, dimensions, and surface markers, which affects their function. Therefore, to enhance the role of nanoparticles in bone-related diseases, further studies on the composition of nanoparticles are needed to reveal new therapeutic strategies to improve the effectiveness of bone regeneration therapy or other bone diseases.
Different types of nanoparticles
At present, various nanoparticles have been used in bone tissue engineering related experimental studies. The function of nanoparticles can be enhanced by continuously improving the bioavailability of nanoparticles. Currently commonly used nanoparticles and their advantages and disadvantages are shown in Table 1.| Type of nanoparticles | Advantages | Disadvantages | Applications | Ref. |
|---|---|---|---|---|
| Liposomes | Biocompatible and biodegradable | Rapid absorbed and removing from the bloodstream | Bone regeneration | 13–15 |
| Osteoporosis | ||||
| Reducing drug toxicity | Drug/gene delivery | |||
| Cell labeling | ||||
| Polymeric NPs | Biocompatible and biodegradable | Scale-up issues | Bone regeneration | 21 |
| Easy to synthesize and functionalize | Osteoporosis | |||
| Synthesis flexibility | Drug/gene delivery | |||
| Cell labeling | ||||
| Dendrimers | Good biocompatible | Low drug retention | Bone regeneration | 26–28 |
| Large number of surface functional | Size-dependent toxicity | Drug/gene delivery | ||
| Monodispersity | Cell labeling | |||
| Gold NPs | Good biocompatible | Biosafety need to improving by long-term cytotoxicity | Bone regeneration | 30 |
| Easy functionalization | Osteoporosis | |||
| Lower cytotoxicity | Drug/gene delivery | |||
| Unique optical property | Cell labeling | |||
| Magnetic NPs | Good biocompatible | Potential toxic | Bone regeneration | 37 and 38 |
| Easy functionalization | Osteoporosis | |||
| Stability and monodispersity | Drug/gene delivery | |||
| Cell labeling | ||||
| Bioactive glasses NPs | Good biocompatibility | Complex synthesis process | Bone regeneration | 14 |
| Bioactive | Wound healing | |||
| Biostability | Bone grafting | |||
| Osteoconductivity | ||||
| Silica NPs | Biocompatible and biodegradable | Toxicity | Bone regeneration | 46–48 |
| Chemical stability | Osteoporosis | |||
| Uniform morphology | Drug/gene delivery | |||
| Cell labeling | ||||
| Hydroxyapatite NPs | Good biodegradability | Not easy to process | Bone regeneration | 52 and 53 |
| Osteoporosis | ||||
| Biocompatibility and osteoconductive capabilities | Potential toxic | Drug/gene delivery | ||
| Cell labeling | ||||
| Quantum dots | Wide absorption spectrum and narrow emission spectrum | Toxicity | Bone regeneration | 63–65 |
| Good photostability | Drug/gene delivery | |||
| Multi-color imaging | Cell labeling |
Organic NPs
In general, liposomes are used as carriers for delivery systems. For example several liposomal formulations 3β-(N-[N′,N′-dimethyl aminoethane]-carbamoyl) cholesterol (DCChol) and dimethyl diocta decylammonium (DDA) have been used for delivery of antibodies in cancer immunotherapy.18 Therefore, it is necessary to integrate the desired molecules into the liposome. Considering the properties of the loaded substance, hydrophilic molecules can be incorporated and retained within the liposome via an aqueous solution, and hydrophobic molecules must be mixed with an organic solvent and bound to the hydrophobic sits.14 The size of liposomes directly impacts the circulation half-life, and a disadvantage of the liposomes is that they are rapidly absorbed by the reticuloendothelial system (RES), and removes them from the bloodstream. To solve this problem, the researcher found that it can be combined with the hydrophilic polyethylene glycol (PEG) lipid to reduce the absorption by RES and increase the time in the bloodstream.3,13 Most bone-targeting liposomes are conducted based on the binding interactions between the cationic and negatively charged phosphates in bone tissue. Such as, bone-targeting liposomes with targeted fragments of phosphorylated cholesterol are being developed to accelerate fracture healing. In addition, specific ligands on the liposomes enabled them to locate target site and promote osteogenic differentiation.19 In short, it is need to find further strategies to overcome the shortcomings and give full play to its advantages in the field of bone tissue engineering in the future.
Polymeric NPs can bind different types of molecules and have high drug-carrying capacity. To data, these vectors have been used for molecular transport and delivery in areas such as inflammation, cancer, and tissue regeneration.20,22,23 Furthermore, the method of synthesizing polymeric NPs depends on the types of molecules loaded. In the case of small molecule ligands, it can be attached prior to self-assembly, and if the macromolecule ligands, it is usually linked to the surface of assembled nanoparticles.14 At present, chitosan is one of the most common used polymers in drug delivery filed as the good biocompatibility, biodegradability, non-toxicity and safety.3 Moreover, PLGA nanoparticles is one of the most successful polymers due to its good biodegradability and biocompatibility, sustained release, and other advantages.24 Since different polymeric NPs are produced depending on the type of drug-loaded. Currently, drug-loaded polymeric NPs delivery systems have rarely been studied in clinical trials. Therefore, it is necessary to further study about the toxicity and drug-loading mechanism of polymeric NPs are needed in vivo, which provide a valuable basis for clinical application.
Dendrimers are a new type of polymeric NPs with good biocompatibility, monodispersity, and multiple surfaces functional groups. However, they suffer from size-dependent toxicity (cationic dendritic macromolecules) and poor drug retention. Typically, dendrimers are delivered to target sites for drug targeting by binding to peripheral moieties to enhance drug solubility.25,26 Compared with traditional polymeric NPs, dendrimers have distinct advantages in drug delivery system. For example, high-efficiency drug loading capacity, precise peripheral size control, good targeting and multivalency of bind drugs. Therefore, dendrimers have become ideal carriers for studying the influence of polymer size and charge on biological effects such as cytotoxicity, biological distribution and retention time.28
Inorganic NPs
Currently, gold NPs are the most widely used inorganic nanoparticles due to their unique physicochemical properties. For example, the surface plasmon resonance (SPR) effect, which is an optical phenomenon caused by the interaction of electromagnetic waves with electrons in metals. The shape, size, charge, ligand, and surface temperature of nanoparticles will affect SPR effect, and this unique property makes them valuable in biomedical therapeutics and bio-diagnostic tools.14,31 Moreover, it also has significant advantages in bioimaging, which can be absorbed in the near-infrared range and improve the visualization of deep tissues through imaging techniques.32,33 Although gold nanoparticles have low toxicity and safety compared with other metal nanoparticles, long-term cytotoxicity, biocompatibility and biodistribution tests are still needed before application in vivo to improve the efficiency.
MNPs have good biocompatibility, low cost, stability and monodispersity. Moreover, it also has good targeting properties, which can be precisely targeted to the target location under the external magnetic field. Therefore, they can be used as good MRI contrast agents and an effective carrier for tumor drug delivery.37,38 For example, a study has shown that dimercaptosuccinic acid (DMSA) coated (SPIONs) effectively delivered IFN-γ (an anti-tumorigenic cytokine) at the tumor site under the external magnetic field to inhibit tumor growth.39 In recent years, cell-free therapies have received more attention, but the high heterogeneity of cell-free therapeutic-based EVs has limited their current clinical translation. Magnetic nanomaterials also play an important role in facilitating the separation, delivery, monitoring, and imaging of EVs for biomedical application. For example, MNPs can increase MRI in vivo for the tracking of EVs, combined with magnetic hyperthermia to control the spatiotemporal release of biomolecules, and thus precisely deliver EVs to realize the therapeutic potential of drugs.40 Although MNPs have been applied in various research fields, it still faces great challenges in practical applications. MNPs have showed a potential toxicity, so surface-modified coatings (such as nickel ferrite) are necessary to ensure safety and efficacy in clinical applications.
The new generation of mesoporous bioactive glass (MBG) has a higher specific surface area and allows biomolecular adsorption, which provides a new material for bone regeneration.42 Patel K. D. et al.43 found that the combinatory cues provided by nanotopology (25 nm roughness) and ions released from of MBG nanoparticles could effectively stimulate osteoblast differentiation and enhance the expression of bone-associated genes (ALP, OPN, and OCN). In addition, boron is a necessary trace element that plays an important role in the human body. Borate bioactive glasses (BBGs) are produced by replacing silica ions with boron ions in the glass networks, and is mainly focused on the bone regeneration and wound healing applications, which an effective biological material.44
Silica NPs can be divided into mesoporous silica NPs (MSNPs) and core/shell silica particles (C/S NPs) based on their applications. For example, C/S NPs are mainly used for molecular imaging because their unique shell structure can defend the imaging agent inside the nanoparticles, which enables the nanoparticles to precisely target to the sites.49 Compared to other silica NPs, MSNPs have good drug delivery and release and biomedical applications. MSNPs with large surface area and pores are conductive to drug adsorption and loading, adjustable-sized pores control drug release, and easily functionalized surfaces contribute to drug targeting control, improving drug efficacy, and reducing toxicity.50 In addition, studies have shown that dietary silica intake are positively correlated with human bone mineral density (BMD), and MSNPs can regulate the process of bone remodeling, which have a certain impact on the development of bone.3 Singh R. K. et al. developed novel nanofibrous hybrid scaffolds of polycaprolactone shelled with mesoporous silica (PCL@MS). The results have shown that growth, proliferation, and the osteogenic differentiation of rat mesenchymal stem cells were significantly improved on the scaffolds, and the hybrid scaffolds was a novel nanobiomatrix platform for bone regeneration.51
Although pure hydroxyapatite has excellent biocompatibility and bioactivity, it has poor mechanical properties and cannot be used as load-bearing implant materials, which is related to the physicochemical properties. However, hydroxyapatite nanoparticles overcame the traditional hydroxyapatite difficult plasticity, brittleness, slow degradation, with high chemical activity, which is conducive to cell attachment and growth, enabling bone cells to secrete varieties of osteogenic differentiation factors, and they also provide crystal nucleus for bone cell calcification and plays the role of osteoconductivity. In addition, HA NPs could improve the performance of scaffolds and increase bone mineral deposition in bone tissue engineering. When calcium and phosphorus are implanted in the body, they will be released from the surface of the material and absorbed by tissues.56 In addition, HA NPs have also been applied in cancer therapy to inhibit tumor growth and metastasis through the release of loaded drugs.57,58
Applications of nanoparticles in bone regeneration
In recent years, the discovery of novel biologically active compounds that could be used to treat diseases has degraded, with fewer new drugs entering the market every year. At present, NPs have become a focus of interest as a versatile and multifaceted drug delivery vehicle. NPs have good pharmacokinetic properties, sustained release, and target specific cells or tissues to enhance the efficacy of existing drugs through aggressive targeting and enhanced permeability and retention effect. In recent years, nanoparticles have been found to be effective drug carriers for the treatment of skeletal-related diseases (osteoporosis, osteoarthritis, osteosarcoma, and bone defect/repair) and have been applied in bone tissue engineering (drug/gene delivery and cell labeling/MRI) (Fig. 1). As the drug and gene delivery system as a carrier can be more accurately targeted to specific tissues, thereby improving the efficiency of treatment. Cell labeling can more accurately and permanently perform in vivo cell tracking and monitoring, and the application of diagnostic techniques can improve disease prevention functions. As shown in Table 2, the experimental study of nanoparticles in the field of bone tissue engineering.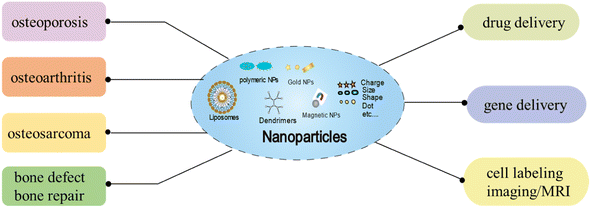 | ||
| Fig. 1 Schematic illustration of the effects of NPs on different type of bone diseases, and the applications of NPs in bone regeneration as drug/gene delivery, and cell labeling/imaging (MRI). | ||
| Application | Type of NP | Outcome | Ref. |
|---|---|---|---|
| Drug delivery | CS-PEI NPs | CS-PEI loaded with hBMP-2 promoted the proliferation and differentiation of MC3T3-E1 cells in vitro and accelerated new bone formation in vivo | 71 |
| Mesoporous silica NPs | Mesoporous silica NPs loaded with BMP-2 promoted the repair of bone defects and open fractures, and promoted the osteogenic differentiation of BMSCs | 72 and 73 | |
| Nanoparticle-embedded electrospun nanofiber scaffold | The scaffold encapsulated with BMP-2 and DXMS promote the repair of critical-sized rat calvarial defect | 74, 75 | |
| Biopolymer NPs | PLA and PHA loaded with BMP7 enhanced osteogenic differentiation of ADSCs | 76 and 77 | |
| Liposomes | Liposomes loaded with DXMS induced osteogenic differentiation of hBMSCs | 78 and 79 | |
| IONPs | IONPs loaded with alendronate inhibited osteoclastogenesis and alleviated OVX-induced mice osteoporosis | 80 | |
| HA NPs | HA NPs loaded with BMP-2 heightened osteogenic differentiation of BMSCs in vitro bone formation of rat calvaria defect in vivo; HA NPs loaded with risedronate inhibited OVX-induced reduction of bone density and mechanical properties in mice | 74 and 81 | |
| Gene delivery | Mesoporous silica NPs | Mesoporous silica nanoparticles with BMP2 plasmid DNA (pDNA) increased transfection efficiency and osteogenic differentiation of MSCs | 83 |
| Bioactive glass | BGN loaded with BMP2 plasmid DNA increased osteogenic differentiation of MSCs and promoted bone regeneration at the rat calvaria defect model | 84 | |
| Ionizable lipid nucleic acid NPs | Ionizable lipid nucleic acid NPs loaded BMP-9 gene delivery to BMSCs to promote osteogenic differentiation and increase bone density in OVX mice | 85 | |
| Lipopolysaccharide amine nanovesicles and nanopolymersomes | Lipopolysaccharide amine nanovesicles and nanopolymersomes, loaded gene pBMP-2 could induce osteogenic differentiation of BMSCs and MC3T3-E1 cells in vitro | 86 and 87 | |
| Gold NPs | Gold NPs mediated c-myb gene delivery to promote osteogenic differentiation of MC3T3-E1 cells and inhibite osteoclast differentiation of BMMs, and facilitate osseointegration of dental implants in OVX rat; gold NPs mediated PPARγ gene on implants improves osseointegration in diabetes mellitus rat model | 88 and 89 | |
| Gold nanorods | Gold nanorods mediated BMP-2 peptide delivery enhanced chondrogenesis | 90 | |
| IONPs | IONPs enhanced transfection efficiency of miR-21 into BMSCs and HUVECs, promoted osteogenic differentiation of MSCs and angiogenesis of HUVECs | 91 | |
| Cell labeling | fNPs | fNPs labeled MSCs on the periosteal side of tibial defects promoted tibial defect repair and increased vascular maturity in mice by NIR-II live imaging | 94 |
| SPIO@SiO2–NH2 | The proliferation, migration, and differentiation potentials of BMSCs could be tracked by SPIO@SiO2–NH2 via MRI imaging | 95 | |
| SPIONs | SPIONs serve as good MRI contrast agents to track MSCs biodistribution in the whole body | 93, 96 and 97 | |
| UCNPs | UCNPs could be labeled and tracked the osteogenic differentiation and chondrogenic differentiation of BMSCs in vitro | 99 | |
| Magnetic NPs | The migratory activity of hBMSCs labelled with 1.0 μg μL−1 silica-coated magnetic NPs incorporating rhodamine B isothiocyanate was reduced | 100 | |
| Gold NPs | Gold NPs-labeled stem cells could be monitored and tracked therapeutic processes in vivo by ultrasound-guided photoacoustic imaging | 101 | |
| Au NPs | PDL-FITC AuNPs could identify M1 macrophages in different cell populations by labeling RAW 264.7 cells and BMDMs | 102 | |
| QDs | QDs could label hASCs and tracked osteogenic differentiation of labeled stem cells | 103 and 104 |
Drug delivery
With the development of bone biology research, several different drugs are currently available for therapeutic intervention. However, some drugs are blocked in delivery by gastrointestinal (oral) related enzymes and may be cleared from the body, making it difficult to reach specific tissue and diminishing the effects of the drug. Higher or more frequent drug doses to ensure the effectiveness of the treatment. However, higher drug concentrations may also have toxic effects on other organs and cause a series of adverse reactions. Research have shown that people develop new drug vectors to treat complex diseases by improving technology. Nanomaterials have unique structure, which can improve cell uptake and blood circulation, enable continuous controlled release of drugs, prolong the retention time of drugs in the body, and reduce the toxicity of drugs. In general, loading specific targeting ligands on the surface of nanoparticles is the most common form, and these targeting ligands can be in the form of small molecules, antibodies, peptides.67,68 Typically, the drug is dissolved, captured, adsorbed, or covalently attached to the surface of nanocarrier, and it also can be encapsulated into it. The nanomedicine delivery system will be implemented by using active or passive targeting mechanisms. Once, the drug is released from the nanocarriers after they reach the target location by identifying specific ligands. In general, the rate of drug release is related to the physiological environment (such as temperature, pH, osmolality, and enzymatic activity), the solubility of the drug, and the degree of drug diffusion through the nanoparticle matrix.69,70Usually, nanoparticles were combined with scaffolds such as protein hydrogels or biodegradable polymer matrices to promote application in bone tissue. On the one hand, growth factors could be delivered by nanoparticles to promote the osteogenic process. On the other hand, specific inhibitors could be released locally to regulate the function of osteoclasts, and the balance of bone remodeling was regulated.6,14 Studies have shown that bone morphogenetic proteins (BMPs), members of the transforming growth factor (TGF)-β superfamily, were suitable for promoting osteogenic differentiation. Among the many BMPs, BMP-2 and BMP-7 are the most used and have been approved by FDA for clinical applications. Zhao et al.71 found that chitosan-polyethyleneimine (CS-PEI) nanoparticle loaded with hBMP-2 could effectively promote the proliferation and differentiation of MC3T3-E1 cells in vitro without cytotoxicity. In addition, the CS-PEI/hBMP-2 nanoparticle significantly accelerated new bone formation at the bone defect area 12 weeks after implantation in vivo. Yi et al.72 found that mesoporous silica nanoparticles-chitosan-loaded BMP-2 could effectively promote the repair of bone defects in chronic osteomyelitis and promote the osteogenic differentiation of BMSCs. Shen et al.73 found that Cefazolin/BMP-2 loaded mesoporous silica nanoparticles significantly promoted the repair of open fractures and reduced inflammation with bone defects in mice, and increased the osteogenic differentiation ability of BMSCs in vitro. Qiu et al.74 prepared silk fibroin/chitosan scaffolds containing mesoporous hydroxyapatite nanoparticles (mHANPs) of BMP-2 (SCH-L). The results showed the interaction of BMP-2/m HANPs heightened the binding ability of BMP-2 to cellular receptors, and the osteogenic differentiation of BMSCs in vitro and bone formation of rat calvaria defect in vivo were significantly promoted with the SCH-L scaffold. It has been shown that nanoparticle-embedded electrospun nanofiber scaffold encapsulated with BMP-2 and dexamethasone (DXMS) promote the repair of critical-sized rat calvarial defect.75 In contrast to the BMP2, BMP7 plays an important role in the late stages of bone formation. It has been shown that biopolymer nanoparticle loaded with BMP7 could release BMP7 with long-acting and promoted osteogenic differentiation of adipose mesenchymal stem cells (ADSCs). Polylactic acid (PLA) and polyhydroxyalkanoate (PHA) nanoparticles loaded with BMP2 and BMP7, respectively, and modified with soybean lecithin (SL) as biosurfactants, enhanced osteogenic differentiation process of ADSCs in simulated microgravity.76,77 In addition, a microporous silica nanoparticle for loading DXMS and ECM-derived peptides-poly(N-isopropylacrylamide-b-(2-(dimethylamino)ethyl methacrylate) promoted osteoblast mineralization and ectopic bone formation. DXMS-loaded liposomes induced osteogenic differentiation of hBMSCs.78,79
In addition to affecting osteoblasts, nanoparticles loaded with drugs could also manipulate of osteoclasts. Currently, bisphosphonates, a clinical anti-osteoporosis drug, reduce the risk of osteoporosis by inhibiting osteoclast activity. However, the bioavailability of oral bisphosphonates is poor. Therefore, bisphosphonate-loaded nanoparticles are feasible for local bone regeneration. It has been shown that IONPs loaded with alendronate could inhibit osteoclastogenesis and alleviated OVX-induced mice osteoporosis.80 HA NPs loaded with risedronate could be effectively used for bone-targeted drug delivery and inhibited OVX-induced reduction of bone density and mechanical properties in mice.81 In conclusion, nanoparticle bone-targeted drug delivery systems have good prospects for application.
Gene delivery
Gene delivery is a promising application of nanoparticles due to the long-term expression and longer therapeutic effect. It can use the viral or the plasmid vehicles for the delivery of genetic material, and so that it does not degrade once internalized by cells. Nanoparticles emerged as a strategic tool for gene delivery, mainly due to their size and simple functionalization. Such as the application of liposomes, gold nanoparticles and silica nanoparticles, and so on. Nanoparticles are used as gene delivery carriers to absorb DNA, RNA, dsRNA(double-stranded), oligonucleotides, and other bioactive molecules on the surface of nanoparticles or wrapped inside by electrostatic action. The specific ligands modified on the surface of nanoparticles interact with the receptors on the cell surface targeting specific tissue cells. When the nanoparticles absorbed by the cells through endocytosis, these active molecules were released through a series of complex processes according to the changes in the microenvironment of the organism, thus playing the role of gene delivery and increasing the expression of genes at the target location.82Kim et al.83 prepared a complex of mesoporous silica nanoparticles with BMP2 plasmid DNA (pDNA) to tested its transfection efficiency in MSCs. The results showed significant intracellular uptake of the complex BMP2 pDNA/MSN-NH2 and increased transfection efficiency, and the osteogenic differentiation of the MSCs was promoted. Bioactive glass nanoparticles (BGN) surface aminated by adding 15% calcium silica could be loaded with BMP2 plasmid DNA and internalized into MSCs to increased osteogenic differentiation, and bone regeneration at the rat calvarium critical-sized defect model was promoted.84 Novel ionizable lipid nucleic acid nanoparticles for systemic BMP-9 gene delivery to BMSCs to promote osteogenic differentiation of BMSCs and increase bone density in OVX mice.85 Lipopolysaccharide amine nanovesicles loaded with the gene pBMP-2-green fluorescent protein complex could significantly induce osteogenic differentiation of BMSCs.86 Lipopolysaccharide-amine nanopolymersomes mediated Noggin small interfering (si)RNA (siNoggin) and pBMP-2 to transfect MC3T3-E1 cells, respectively. The results showed that osteoblast differentiation was promoted in vitro.87 Chitosan gold nanoparticles mediated gene delivery of c-myb promote osteogenic differentiation of MC3T3-E1 cells, inhibit osteoclast differentiation of bone marrow macrophages (BMMs), and facilitate implant osseointegration of dental implants in ovariectomized rat.88 Gold nanoparticle-mediated PPARγ gene on implants improves osseointegration in diabetes mellitus rat model.89 Hyaluronic acid-encapsulated gold nanorods mediated BMP-2 peptide delivery could enhance chondrogenesis.90 In addition, the gene delivery mediated by IONPs can achieve better tissues targeting and reduce free diffusion of particles under the external magnetic field. Electromagnetic field and IONPs enhanced transfection efficiency of miR-21 into BMSCs and human umbilical endothelial cells (HUVECs), and osteogenic differentiation of MSCs and angiogenesis of HUVECs were promote.91 In conclusion, nanoparticles can minimize toxicity and improve in vivo stability due to their good biosafety, surface modifiability and degradability. Nanoparticle-based gene delivery can effectively deliver target genes into the specific cells and affect cell proliferation and differentiation, thus promoting bone regeneration. Currently, it has good prospects for application in the field of bone tissue engineering.
Cell labeling
Due to their regenerative potential, stem cells are used in the field of bone tissue engineering or regenerative therapies. Nanoparticles provide visualization and tracking opportunities for stem cell labeling and imaging, and guide stem cells to different target locations, thus assessing the fate and involvement of the transplanted cells in tissue regeneration. Fluorescent nanoparticles are organic fluorescent dyes (including fluorescein and rhodamine dyes) adsorbed on the surface of nanoparticles or wrapped inside by chemical or physical methods, which improves the stability of the dye molecules in the biological environment and prevents the diffusion of organic dye molecules in biological tissues. The connectivity proteins or biomolecules modified on the surface of nanoparticles bind to the specific receptors on the cell surface to enter the cell, which realizes the specific biomarkers and fluorescence imaging diagnosis of cells and living tissues, thus enabling dynamic tracking of the cell status.92 For example, nanoparticles for labeling MSCs have SPIONs, fluorescently labeled mesoporous silica nanoparticles, gold nanoparticles, or quantum dots et al.6,14,93It has been shown that local implantation of fluorescent nanoparticles (fNPs)-labeled MSCs on the periosteal side of tibial defects could promote tibial defect repair and increase the number of stem cell and vascular maturity in mice by NIR-II live imaging94 (Fig. 2). Silica-coating and amine-modified SPIONs (SPIO@S–N) increased migration capacity while retained proliferation and differentiation potential of BMSCs. As an ideal tracking marker, the P/T scaffold facilitated homing of MSCs in rabbit bone defect model, and this process could be traced by SPIO@SiO2–NH2via MRI imaging.95 In addition, MSCs-labeled with SPIONs serve as good MRI contrast agents to track their biodistribution in the whole body.93,96,97 Dextran-coated doped with Yb3+/Ho3+ fluorapatite crystals for labeling and tracking chondrogenic differentiation of BMSCs process in vitro and in vivo.98 Polyacrylic acid (PAA) and polyallylamine hydrochloride (PAH) modified upconverted fluorescent nanoparticles (UCNPs) could be well labeled and tracked the osteogenic differentiation of rabbit BMSCs in vitro.99 However, the migratory activity of hBMSCs labelled with 1.0 μg μL−1 silica-coated magnetic nanoparticles incorporating rhodamine B isothiocyanate was reduced by reducing membrane fluidity and altering the cytoskeleton. This study suggested that optimal nanoparticle concentrations are critical for stem cell labeling and migration.100
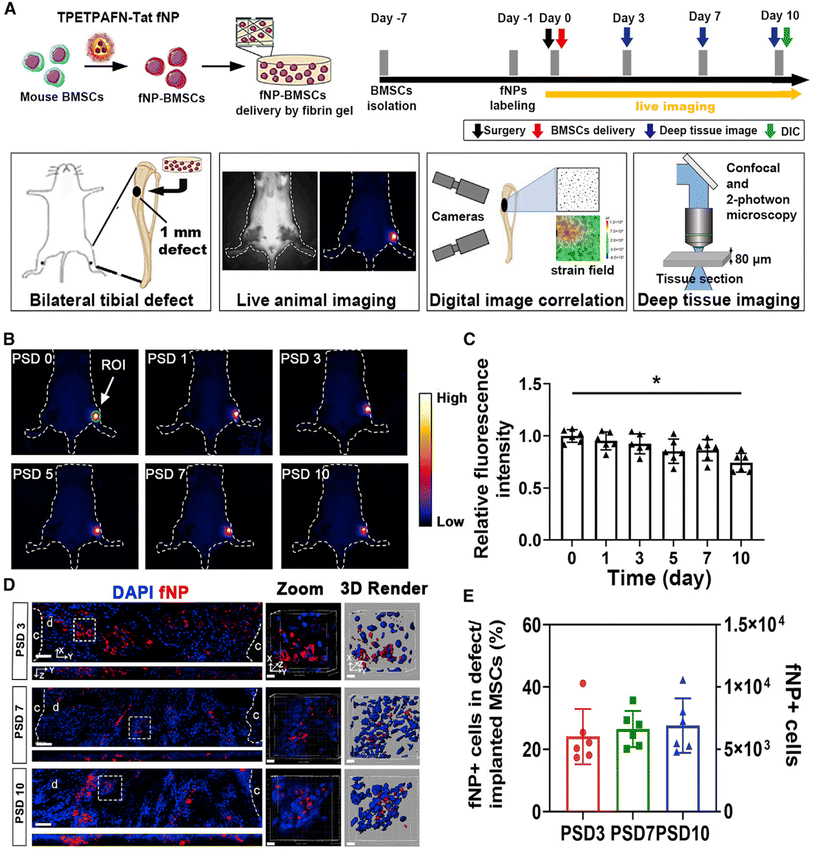 | ||
| Fig. 2 Long-term tracking of implanted MSCs labeled by NPs in tibial bone defect during bone repair. (A) Schematic of NPs labeling and MSC transplantation, (B) quantitative analysis of areas of interest, (C) fluorescence intensity analysis of defect area, (D) implanted cells (fNP in the defect) on PSD 3, 7, and 10 were observed by confocal microscopy, and (E) the number of MSCs from (C) at each time point.94 Reprinted with permission from ref. 94. Copyright 2022, Stem Cell Reports. | ||
In addition, ultrasound-guided photoacoustic imaging of gold nanoparticle-labeled stem cells could monitor and track therapeutic processes in vivo. It was shown that function and imaging properties of AuNP-labeled MSCs were retained after freezing and storage.101 Hernandez et al.102 prepared a fluorescein isothiocyanate-conjugated poly D-lysine (PDL-FITC)-modified reactive oxygen species (ROS)-sensitive AuNP. PDL-FITC AuNPs were loaded into RAW264.7 macrophages and primary BMDMs for labeling helped to identify M1 macrophages in different cell populations. Histidine conjugated β-cyclodextrin loaded with Dex attached to QDs nanoparticle could effectively label human adipose stem cells (hASCs). And osteogenic differentiation of labeled stem cells was promoted by monitoring in temperature-sensitive chitosan hydrogel scaffolds.103,104 In conclusion, the application of nanoparticles in stem cell labeling and tracking supports the prognostic monitoring and tracking of stem cell therapies in clinical. In the field of bone tissue engineering, this technology has great potential.
Research progress on the interaction of nanoparticles with bone cells
During bone regeneration, normal bone remodeling is maintained through the coupling of bone formation by osteoblasts and bone resorption by osteoclasts. With the development of biotechnology, the application of nanoparticles in bone regeneration is becoming more and more widespread. However, the possible particle uptake and potential effects of nanoparticles on bone cells activity and functions, such as differentiation potential of MSC, mineralization by osteoblasts or regulation of resorptive activity by osteoclasts, are required to investigate before any nanoparticles applied in the field of bone research. Therefore, this section reviews the experimental study on the interaction of nanoparticles with bone cells.Bone marrow mesenchymal stem cells
BMSCs are multifunctional differentiation cells derived from bone marrow, which can be differentiated into osteoblasts, adipocytes, and chondrocytes during the special environment of bone regeneration, and are widely used in tissue engineering and biomedical fields.105Studies have shown the absorption behavior of BMSCs for nanoparticles primarily depended on the shape of the nanoparticles, charge, cell type, microenvironment, as well as the chemical properties.106 Thereby, it is generally difficult to predict exactly the uptake rules of nanoparticles. In the aspect of shape and size, Li et al.107 prepared bovine serum albumin (BSA)-coated Au nanospheres, Au nanostars and Au nanorods with diameters of 40, 70 and 110 nm. The results found that sphere-40, sphere-70, and rod-70 significantly promoted osteogenic differentiation ALP activity and calcium deposition of hBMSC, while rod-40 reduced osteogenic differentiation, which may be related to the activation of Yes-associated protein (YAP). With regard to charge, mesoporous silica microspheres (MSNs) uptake by hMSCs could be modulated by positive surface charge.108 Positively charged polymers promote internalization of genetic material with high transfection efficiency, suggesting that positive charged particles polymer interact with negative charge of BMSCs membranes by bind to each other, and promoting uptake of nanoparticle.109 Positively charged AuNPs promoted higher uptake by hMSCs.110 However, there are also negatively charged polymeric nanoparticles, such as carboxyl-or phosphate-functionalized particles were also susceptible internalized by MSC.111 Moreover, Yan et al. found that positively charged CQDs were more cytotoxic and lower photoluminescence (PL) but they have higher uptake and labeling efficiency compared to negative CQDs. The relatively weak positive surface charge gives CQDs good biocompatibility and labeling efficiency in hUCMSCs.112
In regards to the uptake mechanisms of nanoparticles, they can enter cells rely on diverse endocytosis pathways. Such as pinocytosis, micropinocytosis, receptor-mediated endocytosis and clathrin. PLGA-PEI PCS NPs was transported to the lysosomes of MSCs through clathrin-mediated endocytosis.113 Ag-NP particles were internalized to hMSC in a concentration-dependent manner with clathrin-dependent endocytosis and macropinocytosis.114 Hydroxyapatite nanoparticles of different sizes could be uptake by hWJ-MSCs through clathrin and caveolin-mediated endocytosis and macropinocytosis.115
After illuminate the mechanism of nanoparticles into cells, further research for the BMSCs differentiation potential is crucial. The process of osteogenic differentiation of BMSCs is a complex and involves the activation of several signaling pathways as the BMP/Smad, PI3K/Akt/mTOR, MAPK, Wnt/β-catenin.116 Exosomal miR-1260a and miR-21-5p derived from BMSCs preconditioned with Fe3O4 nanoparticles and SMF could improve osteogenic differentiation of BMSCs and enhance wound healing.117,118 Exosomes derived from BMSCs inhibited mitochondrial dysfunction-induced apoptosis of chondrocytes through p38, ERK, and Akt pathways.119In vitro research shows that IOPNs promoted osteogenic differentiation of BMSCs by activating MAPK pathway, increased the expression of ALP, BMP2 and Runx2.120 Electromagnetic feld (EMF) and IONPs enhanced magnetofection efficiency of miR-21 into BMSCs and HUVECs, which improved the osteogenesis and angiogenesis and contributes to the intervertebral fusion.91 The osteogenic differentiation of BMSCs was facilitated by HA NPs and wedelolactone with increased formation of ALP and mineralization and upregulation of osteogenic related genes.121 Tantalum NPs could promote osteogenic differentiation of BMSCs and induce bone regeneration by activating the BMP2/Smad4/Runx2 signaling pathway.122 In addition, BGN inhibited osteoclast differentiation and osteoporotic bone loss by activating lncRNA NRON expression derived from BMSCs.123 And it has been proved zinc silicate/nano-hydroxyapatite/collagen scaffolds could promote angiogenesis of aortic endothelial cells and bone regeneration of BMSCs via the p38 MAPK pathway in activated monocytes.124 Au NPs promoted osteogenic differentiation of BMSCs through activation p38 MAPK pathway, and increased the expression of Runx2, ALP and OCN.125 In addition, a polydopamine-mediated graphene oxide (PGO) and hydroxyapatite nanoparticle (PHA)-incorporated conductive alginate/gelatin (AG) scaffold increased the cell adhesion via RhoA/ROCK signaling pathways and improved osteogenic differentiation of BMSCs.126 Mesoporous silica nanoparticle (MSN)-incorporated PDLLA (poly (DL-lactide))-PEG-PDLLA (PPP) thermosensitive hydrogel markedly enhanced the migration and osteogenic capacities of rBMSCs under high glucose conditions in vitro and significantly promote periodontal bone regeneration under type 2 DM in vivo.127 3D-printed bio-scaffolds composed of Sr-containing mesoporous bioactive glass nanoparticles (Sr-MBGNs) and gelatin methacrylate (GelMA) promoted the osteoblast differentiation of BMSCs harvested from type II diabetic rats via the Kindlin-2/PTH1R/OCN axis.128 Chen et al.129 constructed a nano platform by modifying BMSCs-derived EXOs using the bone-targeting peptide SDSSD and encapsulated capreomycin (CAP) within a shell. And the results showed the constructed NPs induced ferroptosis in osteosarcoma cells by activate Keap1/Nrf2/GPX4 signaling pathway.
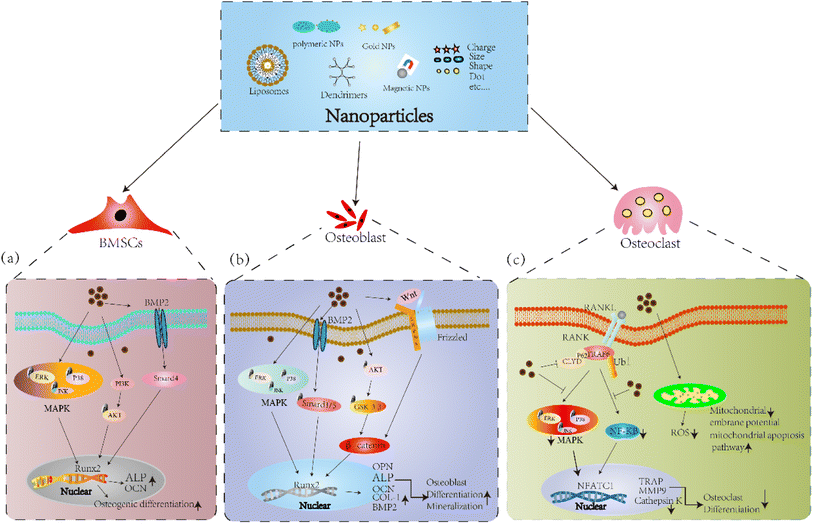
In general, different shapes, sizes and charges affect the absorption mechanism of BMSCs, meanwhile greatly affect the differentiation potential of BMSCs. These in vitro studies indicate NPs, NPs-loaded scaffolds accelerate osteogenic differentiation of BMSCs through the BMP-2/Smad, PI3K-Akt, and MAPK signaling pathways (Fig. 3a). Therefore, it is necessary to continue deeply research nanoparticles to ensure safer and more effectively targeting to objective sites without affecting the differentiation potential of BMSCs.
Osteoblast
Osteoblasts are derived from marrow mesenchymal stem cells and are primarily responsible for bone formation. Osteoblast play a key role in the reconstruction and maintenance of bones.130 The effect of nanoparticles on osteoblasts is like BMSCs, such as hydroxyapatite, polymers, and calcium phosphate particles. Shape, size and charge will affect the uptake mechanism and potential function of osteoblasts.6With respect to the shape and size, Steckiewicz et al.131 examined the cytotoxicity of AuNPs stars (≈215 nm), AuNPs rods (≈39 nm length, 18 nm width) and AuNPs spheres (≈6.3 nm) on human osteoblast(hFOB1.19) and osteosarcoma cells (143B, MG63). The results have proven that AuNPs stars were the most cytotoxic against osteosarcoma cells and had a good anti-cancer potential. AuNPs spheres were the least toxic and safest. Previous studies have proved that 20 nm HANPs have a good effects on promotion of cell growth and inhibition of cell apoptosis of human osteoblast-like MG-63 cells.132 Juhl et al.133 found that compared with 200 nm and 900 nm carbonated hydroxyapatite (CHA), 500 nm CHA were more conducive to inducing the differentiation of human osteoblasts hFOB 1.19 and did not affect cell viability. About the particle charge, HANPs with positive charge were more easily internalized and promoted cell proliferation activity of MC3T3-E1 cells compared to negative charge. The underlying mechanism may be attributed to the interaction of positively charged nanoparticles with negatively charged cell membranes.134
Apart from particle uptake and potential effects on proliferation activity, different nanoparticles simultaneously affect the mineralization and differentiation of osteoblast cells. The expression of ALP and deposition of calcium salts were increased, and the expression of osteoblast marker BMP-2, OCN, Col-1 and Runx-2 were upregulated by AuNPs through ERK/MAPK signaling pathway.135 HANPs facilitated the expression of osteoblast related genes and proteins, and the BV/TV, BMD were improved in a zebrafish and within sagittal suture during expansion in rats.136,137 In addition, HANPs modulated osteoblast cell line MC3T3-E1 differentiation through autophagy induction via mTOR signaling pathway.138 Moreover, bioactive silica nanoparticles promoted osteoblast differentiation and mineralization through stimulation of autophagy and direct association with LC3 and p62, and enhanced BMD of young rats.139 Sun et al.140 designed ROS scavenging and responsive prolonged oxygen-generating hydrogels (CPPL/GelMA, an antioxidant enzyme catalase (CAT) and ROS-responsive oxygen-releasing nanoparticles (PFC@PLGA/PPS) co-loaded liposome (CCP-L) and GelMA hydrogels), which founded the osteogenic differentiation of MC3T3-E1 cell was promoted and showed excellent bone regeneration effect in a mice skull defect model via the Nrf2-BMAL1-autophagy pathway. Novel PEEK scaffolds modified with molybdenum disulfide (MoS2) nanosheets and hydroxyapatite (HA) nanoparticles significantly reduced the viability of MG63 osteosarcoma cells and increased the mineralization of MC3T3-E1 cells, and promoted the osteogenesis capacity in bone defect repair.141 At present, the application of magnetic nanoparticles in bone remodeling has received more attention due to good biosafety. Research have shown the MNPs coated with citric acid (MG@CA) have a good biocompatibility for ECs and MC3T3-E1 cells.142 Tran et al.143,144 Showed HA-coated Fe3O4 magnetic nanoparticles enhanced ALP activity, collagen synthesis and calcium deposition of osteoblast cells through increased amounts of fibronectin, a protein known to increase osteoblast functions. In addition, IONPs calcium phosphate improved osteogenic behavior of hDPSCs by activating the WNT/β-catenin signaling.145 Yu et al.146 developed a novel polysaccharide-based iron oxide nanoparticle (Fe2O3@PSC), which showed the ability to scavenge ROS and promote osteogenic differentiation of MC3T3-E1 cells through activating Akt-GSK-3β-β-catenin signaling. Study have shown that IONPs could be rapidly magnetized under exposed to an SMF, and the combination of IOPNs and SMF have a synergistically enhance or inhibit effect on the differentiation of osteoblasts and osteoclasts.147 Marycz et al.148 showed α-Fe2O3/γ-Fe2O3 nanocomposite (IOs) combined with 0.2 T SMF enhance the expression of osteogenic marker OPN, OCN, and Coll-1 in MC3T3 osteoblasts by activating integrin alpha-3 (INTa-3). IONPs-loaded bovine serum albumin (Fe3O4/BSA) particles exposed to 1 T SMF enhanced ALP activity and the expressions of COL-1 and OCN, and increased the osteogenic differentiation of MSCs.149 However, 50 nm silver NPs exhibited strong cytotoxic effects on osteoblasts, but weak cytotoxic effects were observed for silver microparticles (3 μm). Such adverse effects may have deleterious effects on the biocompatibility of orthopedic implants and requires detailed evaluation prior to clinical use of orthopedic implants with silver nanoparticle coatings.150 In summary, these results in vitro and in vivo suggest that NPs and NPs-loaded scaffolds promote osteogenic differentiation via the BMP-2/Smad, MAPK, Akt-GSK-3β-β-catenin, and Wnt/β-catenin signaling pathways (Fig. 3b).
To date, studies on the interaction between nanoparticles and osteoblasts are limited. Therefore, it is necessary to further evaluate the properties of nanoparticles to find more ways for bone-related diseases to achieve a positive balance in the process of bone remodeling.
Osteoclast
Osteoclasts are differentiated from mononuclear macrophages under the induction of macrophage colony-stimulating factor(M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL), and are the component of bone tissue and mainly perform bone resorption.151 Studies have shown that high expression of osteoclasts has a negative impact on bone tissue. Compared with osteoblasts and BMSCs, there are fewer research on the effects of nanoparticles on osteoclasts, and the underlying mechanism needs to be further clarified.Studies have shown that AuNPs decreased the expression of osteoclast differentiation marker NFATC1, c-Fos and TRAP, inhibited osteoclast formation by suppression RANKL-induced signaling pathway, and prevented OVX-induced bone loss. Moreover, bisphosphonate-conjugated AuNPs showed more significant inhibition.152,153 Silica nanoparticles restrained bone resorption through inhibiting NF-κB signaling pathway and phosphorylation of MAPK signaling pathway, osteoblasts activity and bone mineral density (BMD) were enhanced in vivo and prevented osteoporosis and fracture.154–156 Yang et al.123 shown BGN induced the expression of extracellular vesicles secreted by BMSCs, which could suppress osteoclast differentiation in vitro and alleviated osteoporotic bone loss in vivo. Moreover, studies have shown that Ferucarbotran and Feraheme inhibited the differentiation of osteoclast and OVX induced bone loss by regulating TRAF6-p62-CYLD signaling complex. Then, they showed hydroxyapatite coated SPIO (SPIO@HA) significantly prevented the bone loss of OVX mice and increased BMD though activating MSC osteogenic differentiation via TGF-β, PI3K-AKT and calcium signaling pathway regulation.157,158 Similarly, Fe2O3@PSC resisted osteoclast differentiation of Raw264.7 cells by scavenging ROS and blocking the MAPK and NF-κB pathways in vitro and prevent iron accumulation (IA)-related osteoporosis in vivo.146 Zheng et al.80 prepared Fe2O3@PSC loaded with alendronate, a new bone targeting IONP(BTNPs), which verified BTNPs revised bone loss caused by OVX, and the effects of BTNPs were more pronounced than alendronate alone. Marycz et al.148 found α-Fe2O3/γ-Fe2O3 combined with SMF inhibited osteoclasts activity, and diminished the mRNA expression levels of MMP9. Moreover, α-Fe2O3/γ-Fe2O3 combined with SMF increased the expression of BAX, p21, Casp-3 in osteoclasts and decreased mitochondrial membrane potential, which revealed mitochondrial dysfunction was associated with osteoclast apoptosis. In addition, our previous studies showed that 1–2 T SMF and Ferumoxytol prevented the damage to bone microstructure in HLU mice. And the osteoclast differentiation was suppressed by decreasing the levels of ROS and blocking NF-κB and MAPK signaling pathways.159 Chen et al.160 designed a novel nano-fluorescent carbon quantum dots (N-CDs), the results showed the osteoclastogenesis and bone resorption was attenuated via downregulating ROS level by impaired the activation of NF-κB and MAPK pathways. Therefore, NPs can inhibit osteoclast differentiation via the inhibition of MAPK and NF-κB signaling pathways, and decrease the levels of ROS (Fig. 3c).
In short, the differentiation process and potential mechanism of osteoclasts induced by different nanoparticles need to further study, so that to choose suitable and effective nanoparticles to provide a theoretical basis for the treatment of osteoporosis and other bone-related diseases.
Conclusion and future clinical prospects of NPs
In summary, this review discusses the different types of nanoparticles and application in the bone tissue engineering and the potential effects bone cells applications (Fig. 3). Currently, nanoparticles are at the forefront of nanotechnology. Some studies have shown that nanoparticles affected the activity of bone tissue-related cells such as BMSCs, osteoblasts and osteoclasts, and it will affect bone growth, resorption, and repair. However, the potential effects of nanoparticles on cells are different, depending on the different materials and properties. For example, magnetic nanoparticles can be targeted under an external magnetic field, reducing damage to normal tissue, and improving the precise delivery and treatment of drugs. In addition, SMF as a non-invasive physical therapy, some medical devices based on SMF have been used in the treatment of orthopedic related diseases, such as osteoporosis, fracture and et al. The combination of magnetic nanoparticles and SMF is a non-invasive, convenient, and inexpensive form of therapy for preventing osteoporosis and enhancing bone regeneration, which has the potential value for clinical application in the future.Overall, nanoparticles have shown great potential as enhanced bone regeneration and tissue engineering. However, previous researches have mainly focused on animal and cell experiments, and few clinical studies have been conducted. The toxicity detection and safety evaluation are the primary evaluation criteria in clinical therapy. At present, most of the applications of nanoparticles in bone tissue have mainly focused on the study of biological effect, and the toxic dose in vivo have not been studied in more detail. Therefore, further research on the absorption, distribution, and metabolic pathways of nanoparticles are needed to understand their optimal use. Further explore the potential risks of nanoparticles to bone-associated cells to assess the impact of these risks on bone health and discover the underlying mechanisms, thereby providing a better theoretical basis for clinical translation application.
Author contributions
PS and GZ defined the focus of the review. GZ and CZ summarized studies. GZ drafted the manuscript. JY and JW participated in some parts of the final manuscript. GZ, SW, and PS revised the manuscript. All authors reviewed the final version of the manuscript. All authors read and approved the final manuscript.Conflicts of interest
The authors declare no conflicts of interest for this work.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52037007), and the fellowship of China Postdoctoral Science Foundation (2022M712599), and Heye Health Technology Chongming Project HYCMP-2024004.References
- B. Pelaz, C. Alexiou, R. A. Alvarez-Puebla, F. Alves, A. M. Andrews, S. Ashraf, L. P. Balogh, L. Ballerini, A. Bestetti and C. Brendel, et al. , ACS Nano, 2017, 11, 2313–2381 CrossRef CAS PubMed.
- M. Goldberg, R. Langer and X. Q. Jia, J. Biomater. Sci., Polym. Ed., 2007, 18, 241–268 CrossRef CAS PubMed.
- M. Vallet-Regí, P. Mora-Raimundo and M. Manzano, AIMS Bioeng., 2017, 4, 259–274 Search PubMed.
- O. Salata, J. Nanobiotechnol., 2004, 2, 3 CrossRef PubMed.
- A. Hasan, M. Morshed, A. Memic, S. Hassan, T. J. Webster and H. E. Marei, Int. J. Nanomed., 2018, 13, 5637–5655 CrossRef CAS PubMed.
- A. Tautzenberger, A. Kovtun and A. Ignatius, Int. J. Nanomed., 2012, 7, 4545–4557 CrossRef CAS PubMed.
- N. Akiyama, K. D. Patel, E. J. Jang, M. R. Shannon, R. Patel, M. Patel and A. W. Perriman, J. Mater. Chem. B, 2023, 11, 6225–6248 RSC.
- T. Gong, J. Xie, J. Liao, T. Zhang, S. Lin and Y. Lin, Bone Res., 2015, 3, 15029 CrossRef CAS PubMed.
- K. D. Patel, T. H. Kim, N. Mandakhbayar, R. K. Singh, J. H. Jang, J. H. Lee and H. W. Kim, Acta Biomater., 2020, 108, 97–110 CrossRef CAS PubMed.
- G. G. Walmsley, A. McArdle, R. Tevlin, A. Momeni, D. Atashroo, M. S. Hu, A. H. Feroze, V. W. Wong, P. H. Lorenz, M. T. Longaker and D. C. Wan, Nanomedicine, 2015, 11, 1253–1263 CrossRef CAS PubMed.
- S. Iman Roohani-Esfahani and H. Zreiqat, Nanomedicine, 2017, 12, 419–422 CrossRef PubMed.
- S. Fathi Karkan, M. Mohammadhosseini, Y. Panahi, M. Milani, N. Zarghami, A. Akbarzadeh, E. Abasi, A. Hosseini and S. Davaran, Artif. Cells, Nanomed., Biotechnol., 2016, 45, 1–5 Search PubMed.
- G. Bozzuto and A. Molinari, Int. J. Nanomed., 2015, 10, 975–999 CrossRef CAS PubMed.
- S. Vieira, S. Vial, R. L. Reis and J. M. Oliveira, Biotechnol. Prog., 2017, 33, 590–611 CrossRef CAS PubMed.
- A. Madni, M. Sarfrza, M. Rehman, M. Ahmad, N. Akhtar, S. Ahmad, N. Tahir, S. Liaz, R. AI-Kassas and R. Löbenberg, J. Pharm. Pharm. Sci., 2014, 17, 401–426 Search PubMed.
- M. L. Immordino, F. Dosio and L. Cattel, Int. J. Nanomed., 2006, 1, 297–315 CrossRef CAS PubMed.
- E. Yuba, N. Tajima, Y. Yoshizaki, A. Harada, H. Hayashi and K. Kono, Biomaterials, 2014, 35, 3091–3101 CrossRef CAS PubMed.
- N. Thakur, S. Thakur, S. Chatterjee, J. Das and P. C. Sil, Front. Chem., 2020, 8, 597806 CrossRef CAS PubMed.
- M. Kang, C. S. Lee and M. Lee, Bioengineering, 2021, 8, 137 CrossRef CAS PubMed.
- A. Zielinska, F. Carreiro, A. M. Oliveira, A. Neves, B. Pires, D. N. Venkatesh, A. Durazzo, M. Lucarini, P. Eder, A. M. Silva, A. Santini and E. B. Souto, Molecules, 2020, 25, 3731 CrossRef CAS PubMed.
- M. Elsabahy and K. Wooley, Chem. Soc. Rev., 2012, 41, 2546–2561 RSC.
- X. Xiao, F. Teng, C. Shi, J. Chen, S. Wu, B. Wang, X. Meng, A. Essiet Imeh and W. Li, Front. Bioeng. Biotechnol., 2022, 10, 1024143 CrossRef PubMed.
- Y. Zhang, J. Chen, L. Shi and F. Ma, Mater. Horiz., 2023, 10, 361–392 RSC.
- F. Danhier, E. Ansorena, J. M. Silva, R. Coco, A. Le Breton and V. Preat, J. Controlled Release, 2012, 161, 505–522 CrossRef CAS PubMed.
- E. Abbasi, S. F. Aval, A. Akbarzadeh, M. Milani, H. T. Nasrabadi, S. W. Joo, Y. Hanifehpour, K. Nejati-Koshki and R. Pashaei-Asl, Nanoscale Res. Lett., 2014, 9, 247 CrossRef PubMed.
- Y. Kim, E. J. Park and D. H. Na, Arch. Pharm. Res., 2018, 41, 571–582 CrossRef CAS PubMed.
- A. S. Chauhan, Molecules, 2018, 23, 938 CrossRef PubMed.
- B. Klajnert and M. Bryszewska, Acta Biochim. Pol., 2001, 48, 199–208 CrossRef CAS PubMed.
- R. Herizchi, E. Abbasi, M. Milani and A. Akbarzadeh, Artif. Cells, Nanomed., Biotechnol., 2016, 44, 596–602 CrossRef CAS PubMed.
- W. Wang, J. Wang and Y. Ding, J. Mater. Chem. B, 2020, 8, 4813–4830 RSC.
- J. H. Lee and J. W. Choi, Curr. Drug Targets, 2018, 19, 271–278 CAS.
- A. Astolfo, E. Schultke, R. H. Menk, R. D. Kirch, B. H. Juurlink, C. Hall, L. A. Harsan, M. Stebel, D. Barbetta, G. Tromba and F. Arfelli, Nanomedicine, 2013, 9, 284–292 CrossRef CAS PubMed.
- E. H. Jeong, G. Jung, C. A. Hong and H. Lee, Arch. Pharm. Res., 2014, 37, 53–59 CrossRef CAS PubMed.
- A. Farzin, S. A. Etesami, J. Quint, A. Memic and A. Tamayol, Adv. Healthcare Mater., 2020, 9, e1901058 CrossRef PubMed.
- M. Duan, J. G. Shapter, W. Qi, S. Yang and G. Gao, Nanotechnology, 2018, 29, 452001 CrossRef PubMed.
- M. Colombo, S. Carregal-Romero, M. F. Casula, L. Gutierrez, M. P. Morales, I. B. Bohm, J. T. Heverhagen, D. Prosperi and W. J. Parak, Chem. Soc. Rev., 2012, 41, 4306–4334 RSC.
- D. K. Kim, Y. Zhang, W. Voit, K. V. Rao, J. Kehr, B. Bjelke and M. Muhammed, Scr. Mater., 2001, 44, 1713–1717 CrossRef CAS.
- R. Tietze, J. Zaloga, H. Unterweger, S. Lyer, R. P. Friedrich, C. Janko, M. Pottler, S. Durr and C. Alexiou, Biochem. Biophys. Res. Commun., 2015, 468, 463–470 CrossRef CAS PubMed.
- R. Mejias, S. Perez-Yague, L. Gutierrez, L. I. Cabrera, R. Spada, P. Acedo, C. J. Serna, F. J. Lazaro, A. Villanueva, P. Morales Mdel and D. F. Barber, Biomaterials, 2011, 32, 2938–2952 CrossRef CAS PubMed.
- L. Yang, K. D. Patel, C. Rathnam, R. Thangam, Y. Hou, H. Kang and K. B. Lee, Small, 2022, 18, e2104783 CrossRef PubMed.
- D. S. Brauer, Angew Chem. Int. Ed. Engl., 2015, 54, 4160–4181 CrossRef CAS PubMed.
- V. Lalzawmliana, A. Anand, M. Roy, B. Kundu and S. K. Nandi, Mater. Sci. Eng., C, 2020, 106, 110180 CrossRef CAS PubMed.
- K. D. Patel, J. O. Buitrago, S. P. Parthiban, J. H. Lee, R. K. Singh, J. C. Knowles and H. W. Kim, ACS Appl. Bio Mater., 2019, 2, 5190–5203 CrossRef CAS PubMed.
- D. Ege, K. Zheng and A. R. Boccaccini, ACS Appl. Bio Mater., 2022, 5, 3608–3622 CrossRef CAS PubMed.
- A. M. Mebert, C. J. Baglole, M. F. Desimone and D. Maysinger, Food Chem. Toxicol., 2017, 109, 753–770 CrossRef CAS PubMed.
- Y. Wang, Q. Zhao, N. Han, L. Bai, J. Li, J. Liu, E. Che, L. Hu, Q. Zhang, T. Jiang and S. Wang, Nanomedicine, 2015, 11, 313–327 CrossRef CAS PubMed.
- Y. Huang, P. Li, R. Zhao, L. Zhao, J. Liu, S. Peng, X. Fu, X. Wang, R. Luo, R. Wang and Z. Zhang, Biomed. Pharmacother., 2022, 151, 113053 CrossRef CAS PubMed.
- S. W. Ha, M. Viggeswarapu, M. M. Habib and G. R. Beck Jr, Acta Biomater., 2018, 82, 184–196 CrossRef CAS PubMed.
- X. Wu, M. Wu and J. X. Zhao, Nanomedicine, 2014, 10, 297–312 CrossRef CAS PubMed.
- S. H. Wu, C. Y. Mou and H. P. Lin, Chem. Soc. Rev., 2013, 42, 3862–3875 RSC.
- R. K. Singh, G. Z. Jin, C. Mahapatra, K. D. Patel, W. Chrzanowski and H. W. Kim, ACS Appl. Mater. Interfaces, 2015, 7, 8088–8098 CrossRef CAS PubMed.
- M. U. Munir, S. Salman, A. Ihsan and T. Elsaman, Int. J. Nanomed., 2022, 17, 1903–1925 CrossRef PubMed.
- A. Szczes, L. Holysz and E. Chibowski, Adv. Colloid Interface Sci., 2017, 249, 321–330 CrossRef CAS PubMed.
- F. Vazquez-Hernandez, S. Mendoza-Acevedo, C. O. Mendoza-Barrera, J. Mendoza-Alvarez and J. P. Luna-Arias, Mater. Sci. Eng., C, 2017, 71, 909–918 CrossRef CAS PubMed.
- S. Lara-Ochoa, W. Ortega-Lara and C. E. Guerrero-Beltran, Pharmaceutics, 2021, 13, 1642 CrossRef CAS PubMed.
- Y. Cai, Y. Liu, W. Yan, Q. Hu, J. Tao, M. Zhang, Z. Shi and R. Tang, J. Mater. Chem., 2007, 17, 3780–3787 RSC.
- S. Kargozar, S. Mollazadeh, F. Kermani, T. J. Webster, S. Nazarnezhad, S. Hamzehlou and F. Baino, J. Funct. Biomater., 2022, 13, 100 CrossRef CAS PubMed.
- L. Zhao, W. Zhao, Y. Liu, X. Chen and Y. Wang, Med. Sci. Monit., 2017, 23, 4723–4732 CrossRef PubMed.
- J. C. Bonilla, F. Bozkurt, S. Ansari, N. Sozer and J. L. Kokini, Trends Food Sci. Technol., 2016, 53, 75–89 CrossRef CAS.
- C. T. Matea, T. Mocan, F. Tabaran, T. Pop, O. Mosteanu, C. Puia, C. Iancu and L. Mocan, Int. J. Nanomed., 2017, 12, 5421–5431 CrossRef CAS PubMed.
- S. Pleskova, E. Mikheeva and E. Gornostaeva, Adv. Exp. Med. Biol., 2018, 1048, 323–334 CrossRef CAS PubMed.
- V. G. Reshma and P. V. Mohanan, J. Lumin., 2019, 205, 287–298 CrossRef CAS.
- N. Le, M. Zhang and K. Kim, Int. J. Mol. Sci., 2022, 23, 10763 CrossRef CAS PubMed.
- D. Bera, L. Qian, T.-K. Tseng and P. H. Holloway, Materials, 2010, 3, 2260–2345 CrossRef CAS.
- Q. Xu, J. Gao, S. Wang, Y. Wang, D. Liu and J. Wang, J. Mater. Chem. B, 2021, 9, 5765–5779 RSC.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. Gambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS PubMed.
- S. A. A. Rizvi and A. M. Saleh, Saudi Pharm. J., 2018, 26, 64–70 CrossRef PubMed.
- Y. Chen, X. Wu, J. Li, Y. Jiang, K. Xu and J. Su, Front. Pharmacol, 2022, 13, 909408 CrossRef CAS PubMed.
- A. Z. Wilczewska, K. Niemirowicz, K. H. Markiewicz and H. Car, Pharmacol. Rep., 2012, 64, 1020–1037 CrossRef CAS PubMed.
- R. Singh and J. W. Lillard Jr, Exp. Mol. Pathol., 2009, 86, 215–223 CrossRef CAS PubMed.
- L. Zhao, K. Zhang, W. Bu, X. Xu, H. Jin, B. Chang, B. Wang, Y. Sun, B. Yang, C. Zheng and H. Sun, RSC Adv., 2016, 6, 34081–34089 RSC.
- M. Yi, Y. Nie, C. Zhang and B. Shen, J. Immunol. Res., 2022, 2022, 4450196 Search PubMed.
- M. Shen, L. Wang, L. Feng, C. Xu, Y. Gao, S. Li, Y. Wu and G. Pei, Oxid. Med. Cell. Longevity, 2022, 2022, 8385456 Search PubMed.
- Y. Qiu, X. Xu, W. Guo, Y. Zhao, J. Su and J. Chen, ACS Biomater. Sci. Eng., 2020, 6, 2323–2335 CrossRef CAS PubMed.
- L. Li, G. Zhou, Y. Wang, G. Yang, S. Ding and S. Zhou, Biomaterials, 2015, 37, 218–229 CrossRef CAS PubMed.
- R. Chen, J. Yu, H. L. Gong, Y. Jiang, M. Xue, N. Xu, D. X. Wei and C. Shi, J. Tissue Eng. Regener. Med., 2020, 14, 964–972 CrossRef CAS PubMed.
- X. H. Zhao, X. L. Peng, H. L. Gong and D. X. Wei, Biomed. Mater., 2021, 16, 044102 CrossRef CAS PubMed.
- N. Shao, Y. Guan, S. Liu, X. Li, D. Zhou and Y. Huang, Macromol. Biosci., 2019, 19, e1900255 CrossRef PubMed.
- N. Monteiro, A. Martins, D. Ribeiro, S. Faria, N. A. Fonseca, J. N. Moreira, R. L. Reis and N. M. Neves, J. Tissue Eng. Regener. Med., 2015, 9, 1056–1066 CrossRef CAS PubMed.
- L. Zheng, Z. Zhuang, Y. Li, T. Shi, K. Fu, W. Yan, L. Zhang, P. Wang, L. Li and Q. Jiang, Bioact. Mater., 2022, 14, 250–261 CAS.
- H. Sahana, D. K. Khajuria, R. Razdan, D. R. Mahapatra, M. R. Bhat, S. Suresh, R. R. Rao and L. Mariappan, J. Biomed. Nanotechnol., 2013, 9, 193–201 CrossRef CAS PubMed.
- H. Chen, Z. Li, X. Li, J. Lu, B. Chen, Q. Wang and G. Wu, Drug Des., Dev. Ther., 2023, 17, 3605–3624 CrossRef CAS PubMed.
- T. H. Kim, M. Kim, M. Eltohamy, Y. R. Yun, J. H. Jang and H. W. Kim, J. Biomed. Mater. Res., Part A, 2013, 101, 1651–1660 CrossRef PubMed.
- T. H. Kim, R. K. Singh, M. S. Kang, J. H. Kim and H. W. Kim, Nanoscale, 2016, 8, 8300–8311 RSC.
- I. Vhora, R. Lalani, P. Bhatt, S. Patil and A. Misra, Int. J. Pharm., 2019, 563, 324–336 CrossRef CAS PubMed.
- J. Li, Y. Chen, W. Teng and Q. Wang, Zhongguo Xiufu Chongjian Waike Zazhi, 2018, 32, 1469–1476 Search PubMed.
- M. Huang, X. Zhang, J. Li, Y. Li, Q. Wang and W. Teng, Int. J. Nanomed., 2019, 14, 4229–4245 CrossRef CAS PubMed.
- J. S. Takanche, J. E. Kim, J. S. Kim, M. H. Lee, J. G. Jeon, I. S. Park and H. K. Yi, Artif. Cells, Nanomed., Biotechnol., 2018, 46, S807–S817 CrossRef CAS PubMed.
- Y. H. Lee, J. S. Kim, J. E. Kim, M. H. Lee, J. G. Jeon, I. S. Park and H. K. Yi, Nanomedicine, 2017, 13, 1821–1832 CrossRef CAS PubMed.
- K. Sansanaphongpricha, P. Sonthithai, P. Kaewkong, B. Thavornyutikarn, S. Bamrungsap, W. Kosorn, T. Thinbanmai and N. Saengkrit, Nanotechnology, 2020, 31, 435101 CrossRef CAS PubMed.
- T. Wang, H. Zhao, S. Jing, Y. Fan, G. Sheng, Q. Ding, C. Liu, H. Wu and Y. Liu, J. Nanobiotechnol., 2023, 21, 27 CrossRef CAS PubMed.
- A. Peserico, C. Di Berardino, V. Russo, G. Capacchietti, O. Di Giacinto, A. Canciello, C. Camerano Spelta Rapini and B. Barboni, Nanomaterials, 2022, 12, 1414 CrossRef CAS PubMed.
- M. Ma, Y. Shu, Y. Tang and H. Chen, Nano Today, 2020, 34 CrossRef.
- C. Yang, Z. Li, Y. Liu, R. Hou, M. Lin, L. Fu, D. Wu, Q. Liu, K. Li and C. Liu, J. Biomed. Mater. Res., Part A, 2022, 17, 2318–2333 CAS.
- D. Yao, N. N. Liu and B. W. Mo, Cytotechnology, 2020, 72, 513–525 CrossRef CAS PubMed.
- K. J. Mehta, Stem Cell Rev. Rep., 2022, 18, 2234–2261 CrossRef CAS PubMed.
- A. M. Demin, A. V. Mekhaev, O. F. Kandarakov, V. I. Popenko, O. G. Leonova, A. M. Murzakaev, D. K. Kuznetsov, M. A. Uimin, A. S. Minin, V. Y. Shur, A. V. Belyavsky and V. P. Krasnov, Colloids Surf., B, 2020, 190, 110879 CrossRef CAS PubMed.
- X. Hu, J. Zhu, X. Li, X. Zhang, Q. Meng, L. Yuan, J. Zhang, X. Fu, X. Duan, H. Chen and Y. Ao, Biomaterials, 2015, 52, 441–451 CrossRef CAS PubMed.
- Y. Ma, Y. Ji, M. You, S. Wang, Y. Dong, G. Jin, M. Lin, Q. Wang, A. Li, X. Zhang and F. Xu, Acta Biomater., 2016, 42, 199–208 CrossRef CAS PubMed.
- T. H. Shin, D. Y. Lee, A. A. Ketebo, S. Lee, B. Manavalan, S. Basith, C. Ahn, S. H. Kang, S. Park and G. Lee, Nanomaterials, 2019, 9 CAS.
- M. K. Laffey, K. P. Kubelick, E. M. Donnelly and S. Y. Emelianov, Tissue Eng., Part C, 2020, 26, 1–10 CrossRef CAS PubMed.
- D. S. Hernandez, H. C. Schunk, K. M. Shankar, A. M. Rosales and L. J. Suggs, Nanoscale Adv., 2020, 2, 3849–3857 RSC.
- G. Kundrotas, V. Karabanovas, M. Pleckaitis, M. Juraleviciute, S. Steponkiene, Z. Gudleviciene and R. Rotomskis, J. Nanobiotechnol., 2019, 17, 39 CrossRef PubMed.
- V. Jahed, E. Vasheghani-Farahani, F. Bagheri, A. Zarrabi, H. H. Jensen and K. L. Larsen, Nanomedicine, 2020, 27, 102217 CrossRef CAS PubMed.
- J. Liu, J. Gao, Z. Liang, C. Gao, Q. Niu, F. Wu and L. Zhang, Stem Cell Res. Ther., 2022, 13, 429 CrossRef CAS PubMed.
- X. Yang, Y. Y. Li, X. J. Liu, W. He, Q. L. Huang and Q. L. Feng, Biomater. Transl., 2020, 1, 58–68 Search PubMed.
- J. Li, J. J. Li, J. Zhang, X. Wang, N. Kawazoe and G. Chen, Nanoscale, 2016, 8, 7992–8007 RSC.
- T.-H. Chung, S.-H. Wu, M. Yao, C.-W. Lu, Y.-S. Lin, Y. Hung, C.-Y. Mou, Y.-C. Chen and D.-M. Huang, Biomaterials, 2007, 28, 2959–2966 CrossRef CAS PubMed.
- D. Y. Kim, J. S. Kwon, J. H. Lee, L. M. Jin, J. H. Kim and M. S. Kim, J. Biomed. Nanotechnol., 2015, 11, 522–530 CrossRef CAS PubMed.
- J. E. J. Li, N. Kawazoe and G. Chen, Biomaterials, 2015, 54, 226–236 CrossRef CAS PubMed.
- X. Jiang, A. Musyanovych, C. Röcker, K. Landfester, V. Mailänder and G. U. Nienhaus, Nanoscale, 2011, 3, 2028–2035 RSC.
- J. Yan, S. Hou, Y. Yu, Y. Qiao, T. Xiao, Y. Mei, Z. Zhang, B. Wang, C.-C. Huang, C.-H. Lin and G. Suo, Colloids Surf., B, 2018, 171, 241–249 CrossRef CAS PubMed.
- D. J. Park, W. S. Yun, W. C. Kim, J. E. Park, S. H. Lee, S. Ha, J. S. Choi, J. Key and Y. J. Seo, J. Nanobiotechnol., 2020, 18, 178 CrossRef CAS PubMed.
- C. Greulich, J. Diendorf, T. Simon, G. Eggeler, M. Epple and M. Köller, Acta Biomater., 2011, 7, 347–354 CrossRef CAS PubMed.
- X. Shi, K. Zhou, F. Huang, J. Zhang and C. Wang, Int. J. Nanomed., 2018, 13, 1457–1470 CrossRef CAS PubMed.
- H. Sadeghzadeh, H. Dianat-Moghadam, A. R. Del Bakhshayesh, D. Mohammadnejad and A. Mehdipour, Stem Cell Res. Ther., 2023, 14, 194 CrossRef PubMed.
- D. Wu, X. Chang, J. Tian, L. Kang, Y. Wu, J. Liu, X. Wu, Y. Huang, B. Gao, H. Wang, G. Qiu and Z. Wu, J. Nanobiotechnol., 2021, 19, 209 CrossRef CAS PubMed.
- D. Wu, L. Kang, J. Tian, Y. Wu, J. Liu, Z. Li, X. Wu, Y. Huang, B. Gao, H. Wang, Z. Wu and G. Qiu, Int. J. Nanomed., 2020, 15, 7979–7993 CrossRef CAS PubMed.
- H. Qi, D. P. Liu, D. W. Xiao, D. C. Tian, Y. W. Su and S. F. Jin, Vitro Cell. Dev. Biol.: Anim., 2019, 55, 203–210 CrossRef PubMed.
- Q. Wang, B. Chen, M. Cao, J. Sun, H. Wu, P. Zhao, J. Xing, Y. Yang, X. Zhang, M. Ji and N. Gu, Biomaterials, 2016, 86, 11–20 CrossRef CAS PubMed.
- P. Dong, D. Zhu, X. Deng, Y. Zhang, J. Ma, X. Sun and Y. Liu, J. Biomed. Mater. Res., Part A, 2019, 107, 145–153 CrossRef CAS PubMed.
- G. Zhang, W. Liu, R. Wang, Y. Zhang, L. Chen, A. Chen, H. Luo, H. Zhong and L. Shao, Int. J. Nanomed., 2020, 15, 2419–2435 CrossRef PubMed.
- Z. Yang, X. Liu, F. Zhao, M. Yao, Z. Lin, Z. Yang, C. Liu, Y. Liu, X. Chen and C. Du, Biomaterials, 2022, 283, 121438 CrossRef CAS PubMed.
- Y. Song, H. Wu, Y. Gao, J. Li, K. Lin, B. Liu, X. Lei, P. Cheng, S. Zhang, Y. Wang, J. Sun, L. Bi and G. Pei, ACS Appl. Mater. Interfaces, 2020, 12, 16058–16075 CrossRef CAS PubMed.
- C. Q. Yi, D. D. Liu, C. C. Fong, J. C. Zhang and M. S. Yang, ACS Nano, 2010, 4, 6439–6448 CrossRef CAS PubMed.
- Y. Li, L. Yang, Y. Hou, Z. Zhang, M. Chen, M. Wang, J. Liu, J. Wang, Z. Zhao, C. Xie and X. Lu, Bioact. Mater., 2022, 18, 213–227 CAS.
- H. Wang, X. Chang, Q. Ma, B. Sun, H. Li, J. Zhou, Y. Hu, X. Yang, J. Li, X. Chen and J. Song, Bioact. Mater., 2023, 21, 324–339 CAS.
- Z. Xu, X. Qi, M. Bao, T. Zhou, J. Shi, Z. Xu, M. Zhou, A. R. Boccaccini, K. Zheng and X. Jiang, Bioact. Mater., 2023, 25, 239–255 CAS.
- W. Chen, Z. Li, N. Yu, L. Zhang, H. Li, Y. Chen, F. Gong, W. Lin, X. He, S. Wang, Y. Wu and G. Ji, J. Nanobiotechnol., 2023, 21, 355 CrossRef CAS PubMed.
- D. B. BuRRa and M. ALLEN, Science & Technology Books, 2014 Search PubMed.
- K. P. Steckiewicz, E. Barcinska, A. Malankowska, A. Zauszkiewicz-Pawlak, G. Nowaczyk, A. Zaleska-Medynska and I. Inkielewicz-Stepniak, J. Mater. Sci.: Mater. Med., 2019, 30, 22 CrossRef PubMed.
- Z. Shi, X. Huang, Y. Cai, R. Tang and D. Yang, Acta Biomater., 2009, 5, 338–345 CrossRef CAS PubMed.
- O. J. t. Juhl, S. M. Latifi and H. J. Donahue, J. Biomed. Mater. Res., Part B, 2021, 109, 1369–1379 CrossRef CAS PubMed.
- L. Chen, J. M. McCrate, J. C. Lee and H. Li, Nanotechnology, 2011, 22, 105708 CrossRef PubMed.
- D. Zhang, D. Liu, J. Zhang, C. Fong and M. Yang, Mater. Sci. Eng., C, 2014, 42, 70–77 CrossRef CAS PubMed.
- D. K. Khajuria, V. B. Kumar, D. Gigi, A. Gedanken and D. Karasik, ACS Appl. Mater. Interfaces, 2018, 10, 19373–19385 CrossRef CAS PubMed.
- W. Liang, P. Ding, G. Li, E. Lu and Z. Zhao, Drug Des., Dev. Ther., 2021, 15, 905–917 CrossRef PubMed.
- R. Wang, H. Hu, J. Guo, Q. Wang, J. Cao, H. Wang, G. Li, J. Mao, X. Zou, D. Chen and W. Tian, J. Biomed. Nanotechnol., 2019, 15, 405–415 CrossRef CAS PubMed.
- S. W. Ha, M. N. Weitzmanna and G. R. Beck-Jr, ACS Nano, 2014, 8, 5898–5910 CrossRef CAS PubMed.
- H. Sun, J. Xu, Y. Wang, S. Shen, X. Xu, L. Zhang and Q. Jiang, Bioact. Mater., 2023, 24, 477–496 CAS.
- W. Dai, Y. Zheng, B. Li, F. Yang, W. Chen, Y. Li, Y. Deng, D. Bai and R. Shu, Colloids Surf., B, 2023, 228, 113384 CrossRef CAS PubMed.
- M. G. Montiel Schneider, P. Azcona, A. Campelo, V. Massheimer, M. Agotegaray and V. Lassalle, IEEE Trans. NanoBiosci., 2023, 22, 11–18 Search PubMed.
- N. Tran and T. J. Webster, Acta Biomater., 2011, 7, 1298–1306 CrossRef CAS PubMed.
- N. Tran, D. Hall and T. J. Webster, Nanotechnology, 2012, 23, 455104 CrossRef PubMed.
- Y. Xia, Y. Guo, Z. Yang, H. Chen, K. Ren, M. D. Weir, L. C. Chow, M. A. Reynolds, F. Zhang, N. Gu and H. H. K. Xu, Mater. Sci. Eng., C, 2019, 104, 109955 CrossRef CAS PubMed.
- P. Yu, L. Zheng, P. Wang, S. Chai, Y. Zhang, T. Shi, L. Zhang, R. Peng, C. Huang, B. Guo and Q. Jiang, Int. J. Biol. Macromol., 2020, 165, 1634–1645 CrossRef CAS PubMed.
- J. Yang, J. Wu, Z. Guo, G. Zhang and H. Zhang, Cells, 2022, 11 Search PubMed.
- K. Marycz, P. Sobierajska, M. Roecken, K. Kornicka-Garbowska, M. Kepska, R. Idczak, J. M. Nedelec and R. J. Wiglusz, J. Nanobiotechnol., 2020, 18, 33 CrossRef CAS PubMed.
- P. Jiang, Y. Zhang, C. Zhu, W. Zhang, Z. Mao and C. Gao, Acta Biomater., 2016, 46, 141–150 CrossRef CAS PubMed.
- C. E. Albers, W. Hofstetter, K. A. Siebenrock, R. Landmann and F. M. Klenke, Nanotoxicology, 2013, 7, 30–36 CrossRef CAS PubMed.
- W. J. Boyle, W. S. Simonet and D. L. Lacey, Nature, 2003, 423, 337–342 CrossRef CAS PubMed.
- D. N. Heo, W. K. Ko, H. J. Moon, H. J. Kim, S. J. Lee, J. B. Lee, M. S. Bae, J. K. Yi, Y. S. Hwang, J. B. Bang, E. C. Kim, S. H. Do and Il. K. Kwon, ACS Nano, 2014, 8, 12049–12062 CrossRef CAS PubMed.
- D. Lee, D. N. Heo, H. J. Kim, W. K. Ko, S. J. Lee, M. Heo, J. B. Bang, J. B. Lee, D. S. Hwang, S. H. Do and I. K. Kwon, Sci. Rep., 2016, 6, 27336 CrossRef CAS PubMed.
- G. R. Beck Jr, S. W. Ha, C. E. Camalier, M. Yamaguchi, Y. Li, J. K. Lee and M. N. Weitzmann, Nanomedicine, 2012, 8, 793–803 CrossRef PubMed.
- M. N. Weitzmann, S. W. Ha, T. Vikulina, S. Roser-Page, J. K. Lee and G. R. Beck Jr, Nanomedicine, 2015, 11, 959–967 CrossRef CAS PubMed.
- X. Sun, J. Zhang, Z. Wang, B. Liu, S. Zhu, L. Zhu and B. Peng, Theranostics, 2019, 9, 5183–5199 CrossRef CAS PubMed.
- L. Liu, R. Jin, J. Duan, L. Yang, Z. Cai, W. Zhu, Y. Nie, J. He, C. Xia, Q. Gong, B. Song, J. M. Anderson and H. Ai, Acta Biomater., 2020, 103, 281–292 CrossRef CAS PubMed.
- M. Li, S. Fu, Z. Cai, D. Li, L. Liu, D. Deng, R. Jin and H. Ai, Regener. Biomater., 2021, 8, rbab027 CrossRef CAS PubMed.
- G. Zhang, C. Zhen, J. Yang, Z. Zhang, Y. Wu, J. Che and P. Shang, J. Orthop. Translat., 2023, 38, 126–140 CrossRef PubMed.
- R. Chen, G. Liu, X. Sun, X. Cao, W. He, X. Lin, Q. Liu, J. Zhao, Y. Pang, B. Li and A. Qin, Nanoscale, 2020, 12, 16229–16244 RSC.
| This journal is © The Royal Society of Chemistry 2024 |