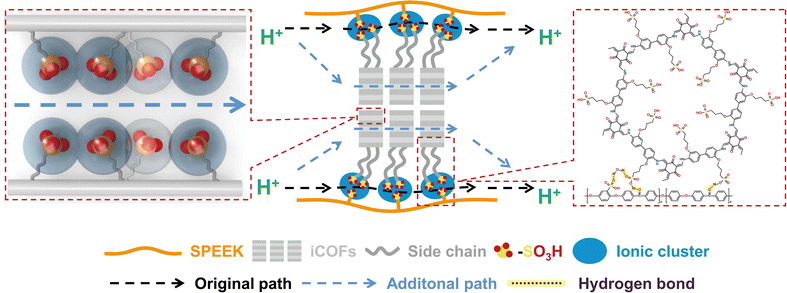
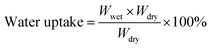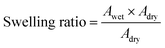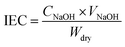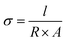Covalent organic frameworks with flexible side chains in hybrid PEMs enable highly efficient proton conductivity†
Ziwen
Liu‡
ab,
Xiao
Pang‡
ab,
Benbing
Shi
ab,
Na
Xing
ab,
Yawei
Liu
 c,
Bohui
Lyu
ab,
Leilang
Zhang
ab,
Yan
Kong
ab,
Sijia
Wang
ab,
Zhong
Gao
ab,
Rou
Xue
ab,
Tianyu
Jing
ab,
Changkun
Liu
ab,
Qinhuidan
Bai
ab,
Hong
Wu
c,
Bohui
Lyu
ab,
Leilang
Zhang
ab,
Yan
Kong
ab,
Sijia
Wang
ab,
Zhong
Gao
ab,
Rou
Xue
ab,
Tianyu
Jing
ab,
Changkun
Liu
ab,
Qinhuidan
Bai
ab,
Hong
Wu
 *ab and
Zhongyi
Jiang
*ab and
Zhongyi
Jiang
 *abd
*abd
aKey Laboratory for Green Chemical Technology of Ministry of Education, School of Chemical Engineering and Technology, Tianjin University, Tianjin 300072, China. E-mail: wuhong@tju.edu.cn; zhyjiang@tju.edu.cn
bHaihe Lab Sustainable Chem Transformations, Tianjin 300192, P. R. China
cBeijing Key Laboratory of Ionic Liquids Clean Process, CAS Key Laboratory of Green Process and Engineering, Institute of Process Engineering, Chinese Academy of Sciences, Beijing, 100190, China
dJoint School of National University of Singapore and Tianjin University, International Campus of Tianjin University, Binhai New City, Fuzhou, 350207, China
First published on 26th October 2023
Abstract
Electrochemical hydrogen compression (EHC) is an emerging energy conversion technology. Proton exchange membranes (PEMs) with high proton conductivity and high mechanical strength are highly required to meet the practical requirements of EHC. Herein, ionic covalent organic frameworks (iCOFs) with tunable side chains were synthesized and introduced into the sulfonated poly (ether ether ketone) (SPEEK) matrix to fabricate hybrid PEMs. In our membranes, the rigid iCOFs afford ordered proton conduction channels, whereas the flexible side chains on iCOFs afford abundant proton conduction sites, adaptive hydrogen bonding networks, and high local density short hydrogen bonds for highly efficient proton transport. Moreover, the hydrogen bond interactions between the side chains on iCOFs and the SPEEK matrix enhance the mechanical stability of membranes. As a result, the hybrid PEM acquires an enhanced proton conductivity of 540.4 mS cm−1 (80 °C, 100%RH), a high mechanical strength of 120.41 MPa, and a superior performance (2.3 MPa at 30 °C, 100%RH) in EHC applications.
New conceptsHybrid PEMs have been developed to overcome the trade-off between the proton conductivity and mechanical stability of PEMs. Filler design is the core for hybrid PEMs. In this work, we designed a kind of rigid-flexible filler, i.e., ionic covalent organic frameworks (iCOFs) with rigid frameworks and tunable flexible side chains, and then introduced into the SPEEK matrix to fabricate hybrid PEMs. In our membrane, the rigid iCOFs afforded ordered proton-conducting highways and intrinsic chemical and mechanical stabilities, whereas the flexible side chains afforded abundant conduction sites for highly efficient proton conductivity. Through molecular simulation calculations, it is found that the moderate-length side chains endow the hydrogen bond network with lower proton transfer resistance. Moreover, the hydrogen bond interactions between the iCOF filler and the polymer matrix elevate the mechanical stability of hybrid PEMs. Through the rational synergy of rigidity and flexibility, the hybrid membrane exhibits a proton conductivity of 540.4 mS cm−1 at 80 °C 100% RH (the highest value of hybrid PEMs ever reported) and a high mechanical strength of 120.41 MPa simultaneously. This work may offer some inspirations for the filler design of hybrid membranes. |
Introduction
EHC, an emerging energy conversion technology, has attracted a growing amount of interest for its low noise, low energy consumption, and rich application scenario.1–3 PEMs, the key components of EHC, must satisfy a number of criteria to meet the practical application requirements, including high proton conductivity, high mechanical strength, and low H2 permeability4,5 to elevate the power output and ensure long-term stability. Nevertheless, because of the random distribution and electrostatic repulsion of proton-conducting groups, polymeric proton conductors often suffer from the pronounced trade-off6–8 between proton conductivity and mechanical properties. The hybrid PEMs comprising of the polymeric matrix and fillers have been recognized as an effective solution.9,10In recent years, designing fillers with abundant and ordered proton conduction groups, inherent thermal and chemical stabilities, and good interfacial compatibility with the polymer matrix has become an active pursuit. For instance, rigid fillers are represented by inorganic materials such as CNTs,11 GO,12 and MOFs,13 in which their rigid structures provide ordered channels for proton conduction and inherent good thermal, chemical, and mechanical stabilities. Alternatively, flexible fillers comprised of organic polymers, such as PES,14 PVA,15 and PAA,16 in which their flexible main chains and/or side chain segments endow the polymer matrix with abundant transport groups as well as good interfacial compatibility.
The emergent crystalline microporous materials bring about wonderful opportunities to explore disruptive fillers. Ionic covalent organic frameworks (iCOFs)17–20 featuring robust skeletons, abundant and uniformly distributed functional groups, and superior thermal and mechanical stability are considered as ideal candidates for fabricating next-generation fillers. In particular, the diversity and designability of ligands and linkage bonds offer unlimited possibilities for the preparation of fillers with moderate rigidity and flexibility.
Herein, rigid-flexible iCOFs with tunable side chain lengths (TpBd-Cx-SO3H) were designed, synthesized, and introduced into the SPEEK matrix to prepare hybrid PEMs. Specifically, in our hybrid PEMs, the rigid iCOFs afford ordered proton conduction channels, while the flexible side chains on iCOFs afford abundant –SO3H groups, adaptive hydrogen bonding networks, and high local density short hydrogen bonds for highly efficient proton conductivity. Additionally, the flexible side chains on iCOFs enhance the hydrogen bond interactions between fillers and the polymer matrix. Through the synergistic effect of rigidity and flexibility, the resulting SPEEK/TpBd-C3-SO3H hybrid membrane exhibits a proton conductivity of up to 540.4 mS cm−1 at 80 °C 100% RH and a high mechanical strength of 120.41 MPa simultaneously. Consequently, the membrane exhibits superior performance (2.3 MPa at 30 °C, 100%RH) in EHC application.
Results and discussion
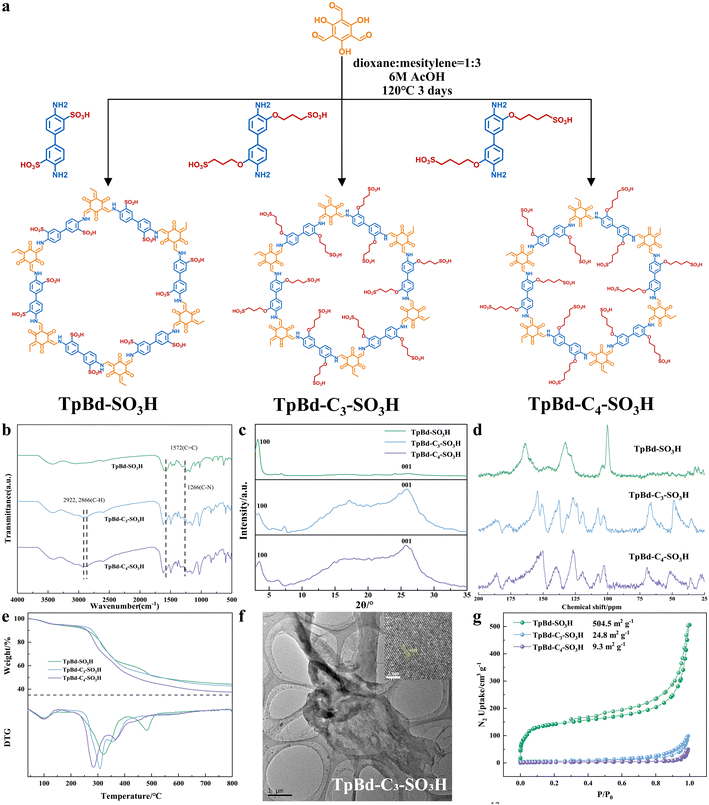
The TpBd-Cx-SO3H iCOFs (Fig. 1a) were synthesized in a typical solvothermal method. Amine monomers with different side chain lengths and –SO3H groups were synthesized based on the ring-opening reaction of sultones (Fig. S1 and S2, ESI†), as verified by 1H-NMR (Fig. S3, ESI†). The digital photos of iCOFs are shown in Fig. S4 (ESI†). The chemical structure of iCOFs was characterized by FTIR (Fig. S6, ESI† and Fig. 1b) and solid-state 13C-NMR (Fig. S7, ESI† and Fig. 1d) spectra. The appearance of the characteristic peak of C–H (2922, 2866 cm−1)21 compared to Bd-SO3H belonged to side chains of Bd-C3-SO3H and Bd-C4-SO3H. The disappearance of the characteristic peaks of N–H (3425 cm−1) for the amine monomer, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (2896 cm−1) and C
O (2896 cm−1) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1639 cm−1) for the aldehyde monomer Tp, and the appearance of C
O (1639 cm−1) for the aldehyde monomer Tp, and the appearance of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C (1572 cm−1) and C–N (1266 cm−1) for iCOFs proved the completion of the Schiff base polycondensation of iCOFs. In addition, the characteristic O
C (1572 cm−1) and C–N (1266 cm−1) for iCOFs proved the completion of the Schiff base polycondensation of iCOFs. In addition, the characteristic O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1076, 1027 cm−1) peak of –SO3H was also observed.22 Taking the 13C-NMR spectra of TpBd-C3-SO3H (Fig. S7b, ESI†) as an example, the appearance of characteristic peaks at ∼ 186.9 ppm (C
O (1076, 1027 cm−1) peak of –SO3H was also observed.22 Taking the 13C-NMR spectra of TpBd-C3-SO3H (Fig. S7b, ESI†) as an example, the appearance of characteristic peaks at ∼ 186.9 ppm (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), ∼126.6 ppm (C
O), ∼126.6 ppm (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), and ∼175.8 ppm (C–N) indicated the occurrence of the enol–keto intercalation reaction and the presence of a keto structure.23
C), and ∼175.8 ppm (C–N) indicated the occurrence of the enol–keto intercalation reaction and the presence of a keto structure.23
Scanning electron microscopy (SEM) images (Fig. S8, ESI†) revealed that all three iCOFs presented a layer-like morphology of about 2–3 μm.24 The crystalline structures of the iCOFs were characterized by powder X-ray diffraction (PXRD) and transmission electron microscopy (TEM). The main peaks (Fig. 1c) of the three iCOFs at 3.28°, 3.34°, and 3.5° corresponded to the 100 planes, which indicated the high crystallinity of iCOFs and were highly consistent with the simulated reversed AA stacking PXRD patterns (Fig. S9 and Table S1, ESI†). Other peaks at 26.08°, 25.86°, and 25.74° corresponded to the 001 planes, respectively. Adding flexible side chains to the rigid iCOF backbone increased the area of the amorphous region. In the TEM image (Fig. 1f), the nanosheets showed a transparent sheet structure with a size of about 2–3 μm. Furthermore, in the high-resolution transmission electron microscope (HR-TEM), the nanosheets possessed obvious lattice stripes (Fig. 1f) assigned to the 001 planes, and the lattice stripes were in a parallel lattice shape with a lattice stripe spacing of about 0.34 nm. The high crystallinity could be further confirmed by selected area electron diffraction (SAED) (Fig. S10, ESI†).
The thermal stability of iCOFs was characterized by thermogravimetric analysis (TGA). There were three main stages (Fig. 1e) of weight loss. Take TpBd-SO3H as an instance, the first stage before 150 °C was caused by the bound and free water reduction. The second stage, from 270 °C to 450 °C, was explained by the degradation of sulfonic acid groups. The third stage above 800 °C was attributed to the decomposition of the organic backbone of iCOFs. The initial thermal decomposition temperatures did not decrease (270 °C, 275 °C, and 255 °C), which could ensure structural stability.
The pore properties of iCOFs were tested by nitrogen adsorption measurement at 77 K. All iCOFs showed rapid N2 uptake at low pressure and exhibited reversible type-IV adsorption isotherms, indicating their mesoporous structure. The surface areas (Fig. 1g) of three iCOFs were calculated using the Brunauer–Emmett–Teller (BET) model to be 504.5, 24.8, and 9.3 m2 g−1, respectively, and the incorporation of the side chains occupied some pore space and restricted the entrance of the probe molecules into the free pore space. The pore size distributions (PSDs) of the three iCOFs were obtained based on the nonlocal density functional theory (NLDFT), with pore sizes of 2.14 nm, 2.19 nm, and 2.19 nm, respectively (Fig. S11, ESI†), which were consistent with their theoretical pore sizes (calculated from 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = λ, about 2.6 nm).
θ = λ, about 2.6 nm).
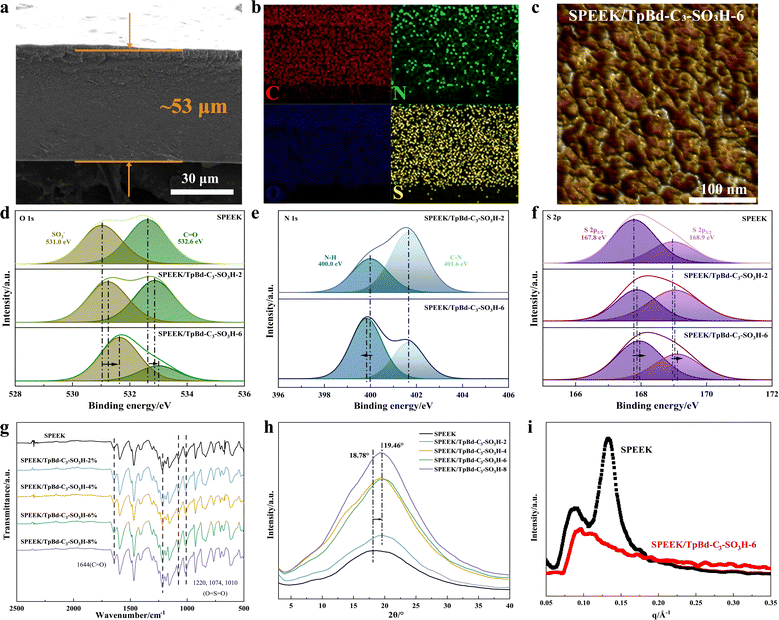
With the addition of the iCOF filler, the color of hybrid PEMs (Fig. S12, ESI†) deepened and became dark red. The cross-section (Fig. 2a) of membranes was compact without defects by SEM. Energy dispersive spectroscopy (EDS) mapping of the SPEEK/TpBd-C3-SO3H-6 membrane revealed the homogeneous distribution (Fig. 2b) of C, N, O, and S elements in the membrane. The presence of nitrogen indicated the homogeneous dispersion of iCOFs in the membrane due to the good interfacial compatibility of iCOFs and SPEEK. The hydrophilic/hydrophobic morphology of SPEEK/TpBd-C3-SO3H-6 was characterized by the atomic force microscopy (AFM) phase image (Fig. 2c and Fig. S13, ESI†). The phase image exhibited a clear hydrophilic/hydrophobic phase,25 with dark and bright regions corresponding to the flexible structure of the hydrophilic ion clusters containing the –SO3H and the rigid structure of the hydrophobic backbones, respectively. After the addition of iCOF, the size of the hydrophilic ion clusters became smaller and the distribution became more uniform, which contributed to the construction of continuous and fast proton conduction channels.
The chemical structures of hybrid PEMs were explored by FTIR spectra. The FTIR spectra of all membranes (Fig. 2g) did not differ greatly with the increase of iCOF filler content. O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S
S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1220, 1074, and 1010 cm−1) of the sulfonic acid group, and C
O (1220, 1074, and 1010 cm−1) of the sulfonic acid group, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (1644 cm−1) pertained to SPEEK. The characteristic peaks (Fig. S14d, ESI†) of C–H (2922 and 2851 cm−1) proved the addition of iCOFs into the hybrid PEMs. The blue shift (1647.8 cm−1 to 1642.1 cm−1) of the C
O (1644 cm−1) pertained to SPEEK. The characteristic peaks (Fig. S14d, ESI†) of C–H (2922 and 2851 cm−1) proved the addition of iCOFs into the hybrid PEMs. The blue shift (1647.8 cm−1 to 1642.1 cm−1) of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O characteristic peak (Fig. S14b, ESI†) was due to the involvement of C
O characteristic peak (Fig. S14b, ESI†) was due to the involvement of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the formation of hydrogen bonds, resulting in the averaging of the electron cloud density.
O in the formation of hydrogen bonds, resulting in the averaging of the electron cloud density.
The hydrogen bond interactions between SPEEK and iCOFs were further verified by XPS spectra (Fig. 2d–f). The O 1s XPS spectra of SPEEK showed two peaks at 531.0 eV and 532.6 eV, corresponding to SO3− and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, respectively. The binding energies of these two peaks increased to 531.6 eV and 533.0 eV as the content of iCOFs increased, respectively. The higher binding energies were attributed to the decrease in the electron density around the O atom due to the formation of hydrogen bonds26 between the O and H atoms in the hybrid PEMs. Meanwhile, the N 1s XPS spectra of SPEEK/TpBd-C3-SO3H-2 showed the presence of both N–H and C–N peaks at 400.0 eV and 401.6 eV, respectively. Compared to SPEEK/TpBd-C3-SO3H-2, the binding energy of the N–H peak of SPEEK/TpBd-C3-SO3H-6 was reduced to 399.8 eV, indicating that that the N–H group in iCOFs accepted electrons from SPEEK to form hydrogen bonds. The S 2p XPS spectrum of SPEEK could be deconvoluted into two peaks, s2p3/2 and s2p1/2, which possessed 167.8 eV and 168.9 eV, respectively. The corresponding peaks of SPEEK/TpBd-C3-SO3H-6 rose to 168.0 eV and 169.1 eV, further demonstrating the existence of hydrogen bond interactions. The nanophase separation structure size of the membranes was probed by XRD (the backbones formed hydrophobic domains) and SAXS (the –SO3H groups aggregated into ionic clusters). A very broad crystalline peak27 was typically observed in the XRD patterns (Fig. 2h) of SPEEK at around 2θ = 19°. The intensity of this peak and crystalline regions in the hybrid PEMs increased compared to SPEEK, contributing to maintaining structural stability. In addition, the peak position of the hybrid membrane shifted from 18.78° to 19.46°, indicating dilation in the structure, which might increase its water retention and hence proton conductivity. For the SAXS of SPEEK, two peaks28 (Fig. 2i) appeared; the ionomer peak at q = 0.089 Å−1 corresponded to the size of the hydrophilic ionic clusters formed by –SO3H surrounded by H2O, and the SO3 peak at q = 0.134 Å−1 reflected the average separation distance between –SO3H on the polymer backbone, with a sharp peak indicating a very uniform distribution of –SO3H. With the addition of iCOFs, the ionomer peak shifted from 0.089 Å−1 to 0.097 Å−1, and the disappearance of the SO3 peak might be due to the formation of more ionic clusters. The Bragg spacing, calculated from d = 2π/q for the average size of hydrophilic ionic clusters, decreased from 7.06 nm to 6.48 nm, indicating the smaller size and spacing of the ionic cluster. The smaller ion cluster size enhanced the phase separation behavior between the hydrophilic sulfonated groups and the hydrophobic polymer backbone, promoting the interconnection between ion clusters and facilitating fast proton conduction. Smaller ionic clusters could also inhibit membrane swelling by reducing water uptake.
O groups, respectively. The binding energies of these two peaks increased to 531.6 eV and 533.0 eV as the content of iCOFs increased, respectively. The higher binding energies were attributed to the decrease in the electron density around the O atom due to the formation of hydrogen bonds26 between the O and H atoms in the hybrid PEMs. Meanwhile, the N 1s XPS spectra of SPEEK/TpBd-C3-SO3H-2 showed the presence of both N–H and C–N peaks at 400.0 eV and 401.6 eV, respectively. Compared to SPEEK/TpBd-C3-SO3H-2, the binding energy of the N–H peak of SPEEK/TpBd-C3-SO3H-6 was reduced to 399.8 eV, indicating that that the N–H group in iCOFs accepted electrons from SPEEK to form hydrogen bonds. The S 2p XPS spectrum of SPEEK could be deconvoluted into two peaks, s2p3/2 and s2p1/2, which possessed 167.8 eV and 168.9 eV, respectively. The corresponding peaks of SPEEK/TpBd-C3-SO3H-6 rose to 168.0 eV and 169.1 eV, further demonstrating the existence of hydrogen bond interactions. The nanophase separation structure size of the membranes was probed by XRD (the backbones formed hydrophobic domains) and SAXS (the –SO3H groups aggregated into ionic clusters). A very broad crystalline peak27 was typically observed in the XRD patterns (Fig. 2h) of SPEEK at around 2θ = 19°. The intensity of this peak and crystalline regions in the hybrid PEMs increased compared to SPEEK, contributing to maintaining structural stability. In addition, the peak position of the hybrid membrane shifted from 18.78° to 19.46°, indicating dilation in the structure, which might increase its water retention and hence proton conductivity. For the SAXS of SPEEK, two peaks28 (Fig. 2i) appeared; the ionomer peak at q = 0.089 Å−1 corresponded to the size of the hydrophilic ionic clusters formed by –SO3H surrounded by H2O, and the SO3 peak at q = 0.134 Å−1 reflected the average separation distance between –SO3H on the polymer backbone, with a sharp peak indicating a very uniform distribution of –SO3H. With the addition of iCOFs, the ionomer peak shifted from 0.089 Å−1 to 0.097 Å−1, and the disappearance of the SO3 peak might be due to the formation of more ionic clusters. The Bragg spacing, calculated from d = 2π/q for the average size of hydrophilic ionic clusters, decreased from 7.06 nm to 6.48 nm, indicating the smaller size and spacing of the ionic cluster. The smaller ion cluster size enhanced the phase separation behavior between the hydrophilic sulfonated groups and the hydrophobic polymer backbone, promoting the interconnection between ion clusters and facilitating fast proton conduction. Smaller ionic clusters could also inhibit membrane swelling by reducing water uptake.
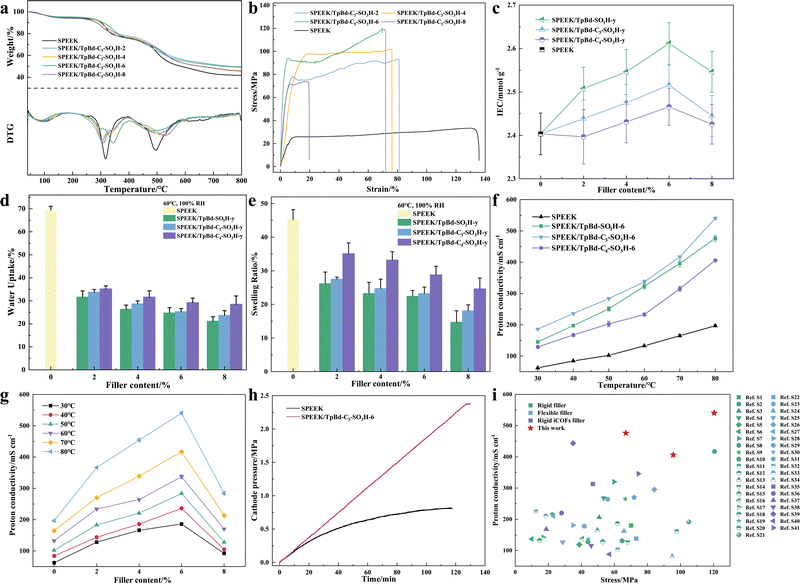
High thermal stability is important for the service lifespan of hybrid PEMs. All hybrid PEMs exhibited three typical weight loss stages (Fig. 3a and Fig. S15, ESI†). The initial weight loss stage was below 150 °C and corresponded to the evaporation of free and bound water. Subsequent pyrolysis of the –SO3H led to a weight loss of the membrane at 280 °C. The weight loss above 440 °C could be attributed to the degradation of the SPEEK matrix. The residual weight of the hybrid PEMs rose after the corresponding stages, possibly due to the incomplete decomposition of iCOFs.
The stress–strain curves of the SPEEK/TpBd-C3-SO3H-y hybrid PEMs are shown in Fig. 3b and Fig. S16 (ESI†). The mechanical strength was enhanced compared to SPEEK, and SPEEK/TpBd-C3-SO3H-6 possessed the highest mechanical strength, 120.41 MPa, an increase of 261.27% compared to 33.33 MPa for SPEEK. Hybrid PEMs have high mechanical strength for the following reasons: Firstly, iCOFs had high mechanical strength due to their rigid frameworks; secondly iCOFs showed abundant interactions with SPEEK, which limit the movement of the chain segments of SPEEK; thirdly, the flexible side chains on the rigid skeleton of iCOFs fortified the hydrogen bond interactions with SPEEK, further enhancing the mechanical strength. However, the longer side chains resulted in a smaller increase in the mechanical strength (95.64 MPa for SPEEK/TpBd-C4-SO3H-6) on the contrary, probably due to the weakened steric hindrance effect, which had a limiting impact on the migration of SPEEK polymer chains. In addition, the mechanical strength of the membranes showed a trend of increasing and then decreasing with the increase of iCOF filler content, which might be due to the slight aggregation of fillers, reducing the interactions between iCOFs and SPEEK. Moreover, the elongation at break decreased, indicating that the membranes strongly resisted deformation (Table S2 and Fig. S17, ESI†). At the same time, flexible side chains enhanced the processability of the membranes.
The dimensional stability of the membranes was characterized by testing the water uptake (WU) and swelling ratio (SR) of the membranes at 30 °C and 60 °C at saturated humidity (Fig. 3d, e and Fig. S19, ESI†). The WU and SR decreased overall with increasing iCOF content due to the smaller size of the hydrophilic ion clusters of hybrid PEMs and the rich hydrogen bond interactions between iCOFs and SPEEK. Compared with pristine SPEEK, SR decreased from 45.2% to 14.7%, 18.1%, and 24.6% when the filler content reached 8 wt%, with a decrease of 67.4%, 59.9%, and 45.5%, respectively, and the dimensional stability of the membrane was greatly improved (Table S3, ESI†). In addition, WU could reach about 30% accordingly, and the good water retention performance made the membrane possess more water, which was conducive to efficient proton conduction. The water contact angles (Fig. S18, ESI†) of the membranes were measured, and the hydrophilicity of the membranes increased with the introduction of both TpBd-SO3H and TpBd-C3-SO3H compared to SPEEK and decreased with the introduction of TpBd-C4-SO3H, probably because the longer side chains interfered with the formation of hydrophilic ion clusters in the membranes. The high mechanical strength and dimensional stability made the membranes maintain stable structural dimensions when applied in EHCs.
Fig. 3f and g show the proton conductivity of the membranes at different temperatures under 100% RH. All the hybrid PEMs exhibited higher proton conductivity (Fig. S20 and S23, ESI†) compared to the pristine SPEEK membrane. Among them, SPEEK/TpBd-C3-SO3H-6 reached the highest proton conductivity of 540.4 mS cm−1 at 80 °C, which was 2.75 times higher than that of the pristine SPEEK (196.6 mS cm−1). With the addition of the iCOF filler, the activation energy Ea (Table S5, ESI†) decreased from 0.21 eV to 0.18 eV, specifically, as explained by the following aspects. Firstly, abundant –SO3H groups loaded on side chains were introduced into the membrane, and the IEC increased from 2.40 to the highest 2.61 (Fig. 3c, Table S4, ESI†), which shortened the distance between the adjacent proton hopping sites and facilitated the formation of short hydrogen bonds. Secondly, ordered proton transport channels were constructed in the frameworks of iCOFs, in which protons could migrate by the Grotthuss mechanism29 (Ea < 0.4 eV) which uniformed the distribution of –SO3H groups and promoted the continuity of hydrogen bond networks. Thirdly, the high hydronium density (TpBd-C3-SO3H) and the flexibility of the side chains allowed hydrogen bond reorganization (Fig. 5e), and the hydrogen bonding networks were more adaptive to achieve rapid proton transport within the iCOF channel. In addition, the –SO3H on the flexible side chains were more inclined to arrange into clusters (Fig. 4), optimizing the phase separation structure of hydrophilic ion clusters from hydrophobic polymer segments through hydrogen bond interactions with SPEEK, further facilitating proton conduction. The longer side chains did not further improve the proton conductivity, probably due to the high proton dissociation energy30 and the spatial hindrance effect that prevented the formation of the nanophase separation structure. In sum, the proton conductivity and mechanical stability are much higher than those reported in the literature (Fig. 3i and Fig. S21, ESI†).
Furthermore, molecular dynamics (MD) simulation was carried out to elucidate the hydrogen bonding networks in the iCOF channel. The distribution of water molecules in the iCOF channel (Fig. S24, ESI†) indicated that the flexibility of the side chains made –SO3H more inclined to cluster. The clustering of –SO3H reduced the number of water molecules in the iCOF channel, which decreased the number of hydrogen bonds (Fig. S25, ESI†), increasing the resistance to proton transfer in-plane. Then, the intensity of hydrogen bonds was simulated, and the results of the O–O distance distribution for hydrogen bonds (Fig. 5a–c) showed that hydrogen bonds were mainly divided into two types: typical hydrogen bonds (O–O distance >2.55 Å) and short hydrogen bonds (O–O distance <2.55 Å). With the increase of side chain length, the proportion of short hydrogen bonds within the iCOF channel increased. The local density of hydrogen bonds between water molecules in the radial direction (Fig. 5d–f) was analyzed, and the density of short hydrogen bonds (green lines) within the clusters increased, while the density of typical hydrogen bonds possessed no significant changes. The strength of hydrogen bonds was affected by the –SO3H group distance. Due to the formation of clusters, the spacing of –SO3H groups decreased further and the short hydrogen bonds were stronger. Short hydrogen bonds allowed protons to be more favorably shared between donor and acceptor,31,32 which led to a lower resistance (Fig. S22, ESI†) to proton transfer through-plane. Therefore, the synergy of interconnected hydrogen bonding networks and high local density short hydrogen bonds afforded SPEEK/TpBd-C3-SO3H the highest proton conductivity.
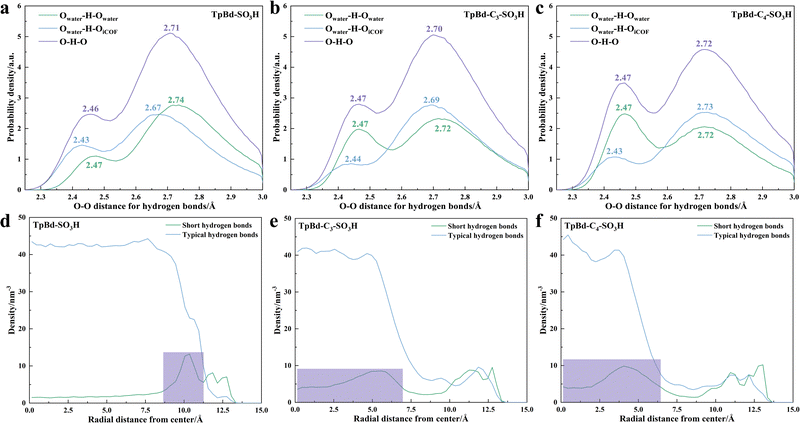 | ||
| Fig. 5 (a)–(c) O–O distance in water and wet TpBd-Cx-SO3H from MD simulations; (d)–(f) local density of hydrogen bonds between water molecules in the radial direction. | ||
The EHC performance of hybrid PEMs was tested under constant current at 30 °C, 100% RH. As shown in Fig. 3h, the cathode-side outlet pressure of SPEEK/TpBd-C3-SO3H-6 was 2.35 MPa, which was 193.8% higher than that of SPEEK of 0.80 MPa, attributed to the enhanced proton conductivity and strong mechanical stability of hybrid PEMs. The high proton conductivity promotes the fast conduction of hydrated protons from the anode side to the cathode side. Strong mechanical stability ensures high-pressure operation as well as resistance to swelling deformation. Moreover, the single fuel cell performance (Fig. S26, ESI†) of hybrid PEMs was also tested at 25 °C, 100% RH. The single fuel cell with SPEEK/TpBd-C3-SO3H-6 exhibited higher open voltage (0.996 V) and power density (93.63 mW cm−2) than that with pristine SPEEK (0.942 V, 35.73 mW cm−2), benefiting from higher proton conductivity of SPEEK/TpBd-C3-SO3H-6. By further optimizing the preparation process of the MEA, the hybrid PEM is expected to be practically applied in EHC.
Conclusions
In summary, we explore rigid-flexible iCOF fillers for solving the trade-off between proton conductivity and mechanical stability in PEMs. In our hybrid PEMs, the rigid frameworks of iCOFs provide ordered proton transport channels, while the flexible side chains of iCOFs aggregate abundant –SO3H groups into ion clusters, thus facilitating the formation of adaptive hydrogen bonding networks and high local density short hydrogen bonds. Additionally, the flexible side chains of iCOFs enhance the hydrogen bond interactions between fillers and the matrix, so as to enhance the mechanical strength of the membranes. Through the optimal interplay of the rigid backbone and flexible side chains of iCOFs, our membrane exhibits 540.4 mS cm−1 at 80 °C 100% RH and a high mechanical strength of 120.41 MPa simultaneously. The EHC cathode-side outlet pressure of SPEEK/TpBd-C3-SO3H-6 is triple that of the pristine membrane. Our work may inspire the rational design of fillers for hybrid PEMs through the synergy of rigidity and flexibility.Experimental section
Materials
Polyether-ether-ketone (PEEK) was purchased from Nanjing Yuanbang Engineering Plastics Co., Ltd. 3,3′′-Disulfonic acid (Bd-SO3H) was obtained from Henan Alpha Technology Co. Ltd. 3,3′′-Dihydroxybenzidine was purchased from Energy-Chemical Co., Ltd. 1,3-Propane sulfonide and 1,4-butane sulfonide were obtained from Tianjin Sciens Biochemical Technology Co., Ltd. 1,3,5-Trihydroxy homobenzaldehyde (Tp) was purchased from Jilin Chinese Academy of Sciences-Yanshen Technology Co., Ltd (Jilin, China). Glacial acetic acid, methanol, mesitylene, N,N-dimethylformamide (DMF), and 1,4-dioxane were purchased from Aladdin Reagents. All the reagents were used as obtained without further purification. Deionized water (18.2 MΩ) was used throughout the experiment.Synthesis of TpBd-Cx-SO3H iCOF powders
In a typical solvothermal method, a Pyrex tube was loaded with 0.6 mmol (Bd-SO3H: 206.62 mg, Bd-C3-SO3H: 276.06 mg, Bd-C4-SO3H: 292.878 mg) of ionic amine monomers, 0.4 mmol of aldehydes (Tp: 84 mg), 0.8 mL of 6M aqueous acetic acid and a solvent mixture of mesitylene (4.5 mL) and dioxane (1.5 mL). After ultrasonication for 15 min, the tube was flash-frozen in a liquid N2 bath, degassed by three freeze–pump thaw cycles, and then sealed off for high-temperature treatment (120 °C, 96 h). A crude product TpBd-Cx-SO3H (x = 0, 3, 4) was collected and washed with DMF/water/methanol to be vacuum-dried at 80 °C for 48 h.Preparation of membranes
SPEEK (DS = 84%, Fig. S5, ESI†) was synthesized according to the literature reported.33 DMF (15mL) and TpBd-Cx-SO3H iCOFs (300 mg) prepared by the above method were mixed and added into the ball mill and milled for 12 h to make homogenous (20 mg mL−1). A determined amount of SPEEK was pre-dissolved in DMF. Mixed the two solutions according to the mass proportion of TpBd-Cx-SO3H: SPEEK = 0.02, 0.04, 0.06, and 0.08. The hybrid PEMs were fabricated using the flow-casting method, kept under 60 °C for 12 h, and subsequently heated to 80 °C for 12 h. The resulting membranes were named as SPEEK/TpBd-Cx-SO3H-y (x = 0, 3, 4; y = 2, 4, 6, 8). The membrane thicknesses were within the 80–90 μm range.Characterization studies
Nuclear magnetic resonance spectroscopy (NMR, VARIAN INOVA 500 MHZ) was performed to characterize the chemical structure of SPEEK and iCOF powders. X-ray photoelectron spectroscopy (XPS, ESCALAB-250Xi) was performed to measure valence states of elements of hybrid PEMs. Atomic Force Microscopy (AFM, Dimension FastScanTM) was used to characterize the phase separation of hybrid PEMs through phase images. Chemical structures of iCOF powders and hybrid PEMs were measured by Fourier transform infrared (FTIR, BRUKER Vertex70) spectroscopy. Thermal gravimetric (TG, NETZSCHTG 209 F3) analysis was utilized to measure the thermal stability of iCOF powders and hybrid PEMs at a heating rate of 10 °C min−1 (N2, 40–800 °C). The iCOF powder morphologies and hybrid PEM cross-section morphologies were detected by scanning electron microscopy (SEM, Nano SEM 430, 15 KV). The element distribution in hybrid PEMs was observed by energy dispersive system (EDS) mapping. Transmission electron microscopy (TEM) images of iCOF powders were revealed on a JEM-2100F TEM. The amorphous properties of the dry membranes were measured by X-ray diffraction (XRD, Cu target, λ = 0.15418 nm, 5–40°, 8° min−1), and the ionic clusters of the wet membranes were tested through small-angle X-ray scattering (SAXS, λ = 0.15418 nm, 0.5–5°, 0.5° min−1).Water uptake and swelling ratio
All membrane weights (Wdry, g) and surface area (Adry, cm2) were recorded after drying in a vacuum at 100 °C. Then the membranes were placed in a thermostat under 100% relative humidity (RH) at different temperatures for 24 h to absorb water until balanced. The weight (Wwet, g) and area (Awet, cm2) of the wet membranes were weighed after removing water from the surface of the samples. The water uptake and swelling ratio were calculated by following formulas, respectively.IEC and proton conductivity
The IEC values (mmol g−1) of hybrid PEMs were measured by the acid–base titration method. Firstly, the samples were dried in a vacuum and recorded weight (Wdry, g). Then, the dry samples were steeped in NaCl solution (2.0 M) and stirred for 48 h until Na+ completely replaced H+. The solution was titrated with NaOH (0.01 M) standard solution indicated by phenolphthalein. The calculation formula of IEC is as follows:where CNaOH (mol L−1) and VNaOH (L) are the molar concentration and the consumed volume of the NaOH solution.
The proton conductivity of hybrid PEMs under different temperatures was measured by two-electrode AC impedance. Hybrid PEMs were soaked in deionized water for 24 h to remove excess acid before performing proton conductivity measurements. PGSTAT20 (IVIUM, The Netherlands) was used to test the impedance value of the sample. The disturbance voltage is 15 mV, and the frequency range is 1 × 106–1 Hz. Then, the membrane sample (about 5 mm × 10 mm) was placed between the electrodes of platinum sheets in a constant temperature and humidity chamber. The AC impedance spectrum was measured when the temperature and humidity reached stability. The proton conductivity of the membrane was calculated by the formula given below.
Mechanical properties
Electronic universal testing equipment was operated to test the mechanical strength of dry membranes using Yangzhou Zhongke WDW-0. Before the test, all as-prepared membranes were dried to constant weight, and then each membrane was cut into a sample strip with a size of 1.5 mm × 30 mm and measured the thickness. The samples were mechanically stretched at a rate of 2 mm min−1 to characterize their mechanical properties.Electrochemical hydrogen compression performance
The Pt/C catalyst (40 wt% Pt), deionized water, Nafion solution and isopropyl alcohol, were mixed in proportion. The catalyst ink was obtained by ultrasonication for 1 h, and then was uniformly painted on a certain area of hybrid PEMs to make Pt loading density of 0.5 mg cm−2. The front and back sides of the membrane sample were painted with catalyst to prepare membrane-electrode assembly (MEA). The anode side of the single cell was supplied with 1 L min−1 H2, while the cathode side of the single cell was connected with a pressure sensor and a storage tank. The hydrogen compression performance was tested at a constant current (0.5 A).Author contributions
Ziwen Liu and Xiao Pang contributed equally to this work. Zhongyi Jiang, Ziwen Liu, Xiao Pang, Hong Wu: supervision, conceptualization, methodology, and writing. Yawei Liu, Bohui Lyu: molecular simulation. Benbing Shi, Na Xing: investigation, data curation, methodology, and writing. Leilang Zhang, Yan Kong, Sijia Wang, Zhong Gao: validation. Rou Xue, Tianyu Jing, Changkun Liu, Qinhuidan Bai: investigation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge financial support from the National Natural Science Foundation of China (U20B2024, 22378300, and 21838008).References
- K. Jiao, J. Xuan, Q. Du, Z. Bao, B. Xie, B. Wang, Y. Zhao, L. Fan, H. Wang, Z. Hou, S. Huo, N. P. Brandon, Y. Yin and M. D. Guiver, Nature, 2021, 595, 361–369 CrossRef CAS PubMed.
- M. Wang, J. H. Park, S. Kabir, K. C. Neyerlin, N. N. Kariuki, H. Lv, V. R. Stamenkovic, D. J. Myers, M. Ulsh and S. A. Mauger, ACS Appl. Energy Mater., 2019, 2, 6417–6427 CrossRef.
- J. Zou, N. Han, J. Yan, Q. Feng, Y. Wang, Z. Zhao, J. Fan, L. Zeng, H. Li and H. Wang, Electrochem. Energy Rev., 2020, 3, 690–729 CrossRef CAS.
- B. Zhu, Y. Sui, P. Wei, J. Wen, H. Cao, C. Cong, X. Meng and Q. Zhou, J. Membr. Sci., 2021, 628, 119214 CrossRef CAS.
- S. J. Hong, S. J. Yoon, T. H. Kim, J. Y. Lee, S. G. Oh, Y. T. Hong, S. So and D. M. Yu, Langmuir, 2021, 37, 3694–3701 CrossRef CAS.
- B. Shi, X. Pang, H. Wu, J. Shen, J. Guan, X. Wang, C. Fan, L. Cao, T. Zhu, Z. Yin, Y. Kong, Y. Liu, S. Wang and Z. Jiang, J. Mater. Chem. A, 2022, 10, 16995–17000 RSC.
- S. Wang, T. Zhu, B. Shi, C. Fan, Y. Liu, Z. Yin, Z. Gao, Z. Zhang, H. Wu and Z. Jiang, J. Membr. Sci., 2023, 666, 121147 CrossRef CAS.
- F. Sun, L.-L. Qin, J. Zhou, Y.-K. Wang, J.-Q. Rong, Y.-J. Chen, S. Ayaz, Y. U. Hai-Yin and L. Liu, J. Membr. Sci., 2020, 611, 118381 CrossRef CAS.
- J. Wang, Y. Dai, S. Xu, H. Jiang and R. He, J. Power Sources, 2021, 506, 230217 CrossRef CAS.
- F. Chen, W. Dong, F. Lin, W. Ren and X. Ma, Compos. Commun., 2021, 24, 100676 CrossRef.
- F. Zhong, Z. Zeng, Y. Liu, R. Hou, X. Nie, Y. Jia, J. Xi, H. Liu, W. Niu and F. Zhang, Polymer, 2022, 249, 124839 CrossRef CAS.
- Q. Peng, Y. Li, M. Qiu, B. Shi, X. He, C. Fan, X. Mao, H. Wu and Z. Jiang, Ind. Eng. Chem. Res., 2021, 60, 4460–4470 CrossRef CAS.
- H. Sun, B. Tang and P. Wu, ACS Appl. Mater. Interfaces, 2017, 9, 35075–35085 CrossRef CAS.
- Y. Yagizatli, A. Sahin and I. Ar, Int. J. Hydrogen Energy, 2022, 47, 40445–40461 CrossRef CAS.
- S. Feng, J. Liu, B. Gao, L. Bo and L. Cao, J. Environ. Chem. Eng., 2022, 10, 107025 CrossRef.
- C. Meemuk and S. Chirachanchai, Int. J. Hydrogen Energy, 2018, 43, 6701–6710 CrossRef CAS.
- Z. Yin, H. Geng, P. Yang, B. Shi, C. Fan, Q. Peng, H. Wu and Z. Jiang, Int. J. Hydrogen Energy, 2021, 46, 26550–26559 CrossRef CAS.
- X. Sun, J.-H. Song, H.-Q. Ren, X.-Y. Liu, X.-W. Qu, Y. Feng, Z.-Q. Jiang and H.-L. Ding, Electrochim. Acta, 2020, 331, 135235 CrossRef CAS.
- J. Yao, G. Xu, Z. Zhao, J. Guo, S. Li, W. Cai and S. Zhang, Int. J. Hydrogen Energy, 2019, 44, 24985–24996 CrossRef CAS.
- X. Meng, Y. Lv, L. Ding, L. Peng, Q. Peng, C. Cong, H. Ye and Q. Zhou, Nanomaterials, 2022, 12, 3518 CrossRef CAS PubMed.
- M. Saminathan, S. Kanagarajan, R. Chandrasekaran, A. Sivasubramaniyan, R. Raja and P. Alagusundaram, J. Chin. Chem. Soc., 2019, 67, 1100–1112 CrossRef.
- H. Dong, Z. Zhu, K. Li, Q. Li, W. Ji, B. He, J. Li and X. Ma, J. Membr. Sci., 2021, 635, 119440 CrossRef CAS.
- S. Kandambeth, A. Mallick, B. Lukose, M. V. Mane, T. Heine and R. Banerjee, J. Am. Chem. Soc., 2012, 134, 19524–19527 CrossRef CAS PubMed.
- Y. Guo, X. Zou, W. Li, Y. Hu, Z. Jin, Z. Sun, S. Gong, S. Guo and F. Yan, J. Mater. Chem. A, 2022, 10, 6499–6507 RSC.
- L. Sun, J. Guo, J. Zhou, Q. Xu, D. Chu and R. Chen, J. Power Sources, 2012, 202, 70–77 CrossRef CAS.
- P. Li, N. Zhang, X. Li and S. Tang, Green Energy Environ., 2023, 8, 915–926 CrossRef CAS.
- B. Liu, B. Hu, J. Du, D. Cheng, H. Y. Zang, X. Ge, H. Tan, Y. Wang, X. Duan, Z. Jin, W. Zhang, Y. Li and Z. Su, Angew. Chem., Int. Ed., 2021, 60, 6076–6085 CrossRef CAS PubMed.
- H. Mendil-Jakani, I. Zamanillo Lopez, P. M. Legrand, V. H. Mareau and L. Gonon, Phys. Chem. Chem. Phys., 2014, 16, 11228–11235 RSC.
- Y. Yin, Z. Li, X. Yang, L. Cao, C. Wang, B. Zhang, H. Wu and Z. Jiang, J. Power Sources, 2016, 332, 265–273 CrossRef CAS.
- B. Shi, X. Pang, B. Lyu, H. Wu, J. Shen, J. Guan, X. Wang, C. Fan, L. Cao, T. Zhu, Y. Kong, Y. Liu and Z. Jiang, Adv. Mater., 2023, 35, e2211004 CrossRef PubMed.
- S. Zhou and L. Wang, Chem. Sci., 2019, 10, 7734–7745 RSC.
- B. Shi, X. Pang, S. Li, H. Wu, J. Shen, X. Wang, C. Fan, L. Cao, T. Zhu, M. Qiu, Z. Yin, Y. Kong, Y. Liu, M. Zhang, Y. Liu, F. Pan and Z. Jiang, Nat. Commun., 2022, 13, 6666 CrossRef CAS PubMed.
- L. Xin, D. Zhang, K. Qu, Y. Lu, Y. Wang, K. Huang, Z. Wang, W. Jin and Z. Xu, Adv. Funct. Mater., 2021, 31, 2104629 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01604h |
| ‡ These authors are co-first authors in this work. |
| This journal is © The Royal Society of Chemistry 2024 |