Open Access Article
Open Access ArticleRobust myco-composites: a biocomposite platform for versatile hybrid-living materials†
Sabrina C.
Shen‡
ab,
Nicolas A.
Lee‡
ac,
William J.
Lockett
ade,
Aliai D.
Acuil
af,
Hannah B.
Gazdus
cg,
Branden N.
Spitzer
ab and
Markus J.
Buehler
 *afgh
*afgh
aLaboratory for Atomistic and Molecular Mechanics (LAMM), Massachusetts Institute of Technology, 77 Massachusetts Ave. 1-165, Cambridge, MA 02139, USA. E-mail: mbuehler@MIT.EDU
bDepartment of Materials Science and Engineering, Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA 02139, USA
cSchool of Architecture and Planning, Media Lab, Massachusetts Institute of Technology, 75 Amherst Street, Cambridge, MA 02139, USA
dMIT Center for Art, Science & Technology (CAST), Massachusetts Institute of Technology, 77 Massachusetts Ave. 10-183, Cambridge, MA 02139, USA
eDepartment of Media, Culture, and Communication, New York University, 239 Greene Street, New York, NY 10003, USA
fDepartment of Civil and Environmental Engineering, Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA 02139, USA
gDepartment of Mechanical Engineering, Massachusetts Institute of Technology, 77 Massachusetts Ave., Cambridge, MA 02139, USA
hCenter for Computational Science and Engineering, Schwarzman College of Computing, 77 Massachusetts Ave., Cambridge, MA 02139, USA
First published on 17th January 2024
Abstract
Fungal mycelium, a living network of filamentous threads, thrives on lignocellulosic waste and exhibits rapid growth, hydrophobicity, and intrinsic regeneration, offering a potential means to create next-generation sustainable and functional composites. However, existing hybrid-living mycelium composites (myco-composites) are tremendously constrained by conventional mold-based manufacturing processes, which are only compatible with simple geometries and coarse biomass substrates that enable gas exchange. Here we introduce a class of structural myco-composites manufactured with a novel platform that harnesses high-resolution biocomposite additive manufacturing and robust mycelium colonization with indirect inoculation. We leverage principles of hierarchical composite design and selective nutritional provision to create a robust myco-composite that is scalable, tunable, and compatible with complex geometries. To illustrate the versatility of this platform, we characterize the impact of mycelium colonization on mechanical and surface properties of the composite. We found that our method yields the strongest mycelium composite reported to date with a modulus of 160 MPa and tensile strength of 0.72 MPa, which represents over a 15-fold improvement over typical mycelium composites, and further demonstrate unique applications with fabrication of foldable bio-welded containers and flexible mycelium textiles. This study bridges the gap between biocomposite and hybrid-living materials research, opening the door to advanced structural mycelium applications and demonstrating a novel platform for development of diverse hybrid-living materials.
New conceptsThis study advances the field of biocomposite design for additive manufacturing and engineered living materials, yielding both the strongest mycelium composite to date and a novel fabrication method for hybrid-living materials. Together, these greatly enhance the versatility of mycelium-based composites, enabling them to engage a space previously occupied only by conventional or synthetic structural materials while maintaining beneficial attributes of mycelium such as extreme hydrophobicity and self-healing capabilities. We developed a unique composite material that is not only compatible with high-resolution 3D-printing, overcoming constraints imposed by conventional mold-based manufacturing methods, but also biocompatible with mycelium, composed entirely of organic waste residues, and possessing mechanical properties suitable for structural applications. Through systematic analysis, we have achieved a foundational understanding of the biocomposite design process and important considerations for extending this platform to other classes of engineered hybrid-living materials. Here, the versatility of the method is demonstrated through the creation of self-sealing mycelium containers and flexible textile-like 3D prints, highlighting the unique properties of mycelium materials that can be leveraged for a variety of applications. There is a tremendous design space constituted by biocomposite composition, organism or species selection, and processing and growth parameters, forming the potential to further tune or create optimized hybrid-living composites for new and various applications. Our findings present the start of this new class of engineered living materials and open up abounding possibilities for the creation of similar materials that are not only sustainable, but can provide unique functionalities to solve societal issues at scale. |
1. Introduction
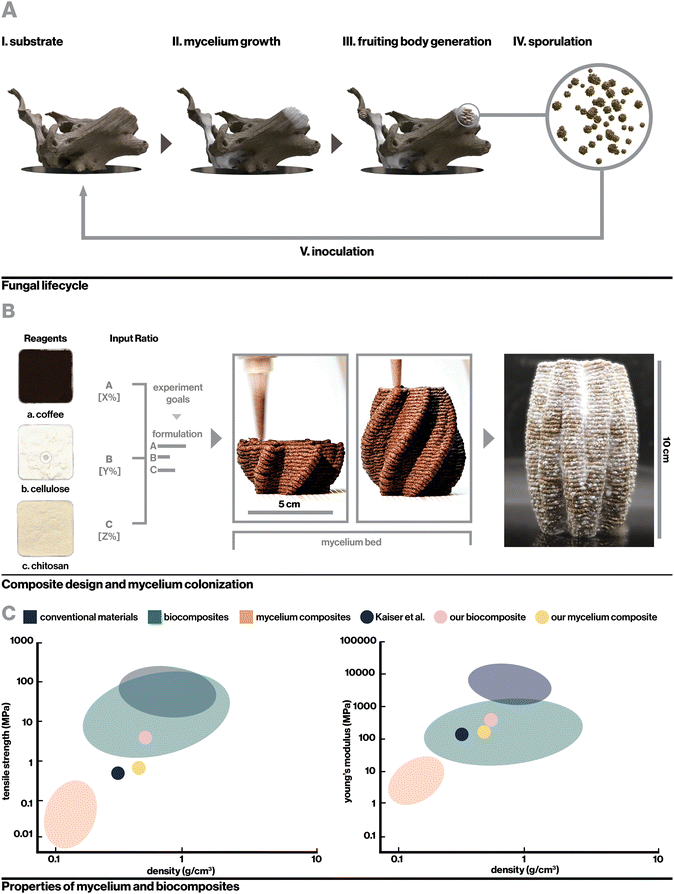
Living organisms have universally evolved the ability to transform abundant energy and materials from their environments into functional materials with extraordinary properties.1–4 Recently, strategies to harness the abilities of various non-human organisms in materials engineering including the creation of living biosensors, engineered drug-releasing organisms, and living functional materials have received increased attention as a potential means of creating more sustainable materials with enhanced functionalities.5 One such strategy is the creation of engineered hybrid-living materials, composed of live organisms grown on biotic or synthetic scaffolds, which enables engineers to harness material deposition and regenerative abilities of organisms while tuning factors such as form and substrate. This has led to innovative technologies including self-healing bacterial concrete,6 bacterial cellulose composites,7 and mycelium composites with proposed applications ranging from packaging to medical devices and construction materials.8,9Mycelium, the filamentous root structure of fungi, has particularly been investigated for its rapid growth rate and ability to upcycle cheap lignocellulosic waste (Fig. 1A). Mycelial hyphae quickly grow through loose substrates such as mulch or sawdust, binding them into solid, lightweight foams with potential as low-cost alternatives to synthetic packaging foams and insulators.10–14 For example, with a modulus of 1.14 MPa, a hemp-based mycelium foam produced by Ecovative Design LLC has comparable mechanical properties to expanded polystyrene foam (EPF) and has been suggested as a more sustainable alternative.15–17 As a broader platform for sustainable materials, however, the scope of mycelium applications has been limited. This is due in large part to biological, engineering, and design constraints associated with growing mycelium in a molded formwork, which most reported manufacturing methods require.18–20 Compressing a loose substrate into a fixed formwork inherently leads to a gradient in temperature, humidity, and oxygenation based on the relative distance between any point in the substrate and the edge of the mold, which can restrict hyphal proliferation into the interior of larger forms. Furthermore, molds need to be able to be opened and closed in a manner that allows for the clean removal of molded substrate, which precludes the creation of complex geometric features including enclosed voids and fine details. Finally, while additive manufacturing systems that implement mechanical or pneumatic extrusion to deposit layers of mycelium substrate have been proposed, they generally suffer from limitations in scale and application due to the low stiffness of extruded material21 or low resolution of printing resulting in the ability to only create crude geometries.22–24
Table S1 (ESI†) includes a comprehensive list of existing mycelium composites including substrate composition, fungal species, and the reported mechanical properties. The highest reported moduli (153 MPa and 9.67 MPa) were formed using Ganoderma lucidum to bind “mycocrete” and beech sawdust respectively in composites that were packed into molded formworks during incubation. The same composites also reported superior ultimate tensile strength (0.52 MPa and 0.17 MPa), while similar composites formed by Ecovative fungi grown on cotton fiber substrate also reported relatively strong strength under tension with an ultimate tensile strength of 0.2 MPa. The mechanical properties of these fall within the range of lightweight foams such as EPF,19 but are far weaker than conventional structural materials such as timber or particleboard.25 Notably, some mycelium-based materials that require extensive post-processing such as heat pressing achieve good mechanical properties, but are outside of the scope of this work due to the required post-processing, which typically disables the living mycelium and limits fabrication to sheets and bricks.26,27
In this work, we propose an approach to overcome the shortcomings associated with the manufacturing process and mechanical properties of conventional mycelium composites by utilizing a novel indirect inoculation method to induce fungal growth within mechanically robust 3D-printed biocomposites rather than bulky biomass residues (Fig. 1B). Importantly, this method allows for the realization of more complex geometries than mold-dependent methods and achieves great mechanical properties without requiring heat-pressing or extensive post processing. We use rational design and evaluation of key parameters in biocomposite formulation including component selection, hierarchically-induced anisotropy resulting from the additive manufacturing process,28 and selective nutritional density to realize a rigid, printable, and biocompatible printing material, then characterize the surface and bulk impacts of mycelium colonization. The resulting mycelium composite achieves Young's modulus up to 160 MPa, over a 15-fold improvement compared to typical mycelium composites, to demonstrate the first 3D-printable mycelium composite with mechanical properties suitable for structural applications (Fig. 1C and Table S1, ESI†). The platform described here elegantly leverages the strengths of biocomposite 3D printing technologies with high resolution, mechanical properties comparable to engineering materials, and capacity for creating complex geometries, while harnessing advantages of mycelium to achieve lower density, improved hydrophobicity, and generative capabilities. We further demonstrate the versatility of this method and the unique properties of mycelium materials that can be leveraged with the creation of self-sealing mycelium containers and flexible textile-like 3D prints and consider the broad extensibility of this platform to other applications and classes of engineered hybrid-living materials.
2. Results
2.1. Biocomposite design
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 chitosan to cellulose ratio yields pliable material,29 we found that a higher 1
8 chitosan to cellulose ratio yields pliable material,29 we found that a higher 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 ratio with lower hydration better maintained smooth printing and vertical layer integrity in high-resolution 3D-printing. This may be attributable to strong chitosan intermolecular forces,33 including hydrogen bonding and electrostatic interactions, which can thicken the gel and enhance adhesion between printed layers. Decreasing water content minimized deformation and time spent in drying stages to a limit, where mixtures were too stiff to print.
2.5 ratio with lower hydration better maintained smooth printing and vertical layer integrity in high-resolution 3D-printing. This may be attributable to strong chitosan intermolecular forces,33 including hydrogen bonding and electrostatic interactions, which can thicken the gel and enhance adhesion between printed layers. Decreasing water content minimized deformation and time spent in drying stages to a limit, where mixtures were too stiff to print.
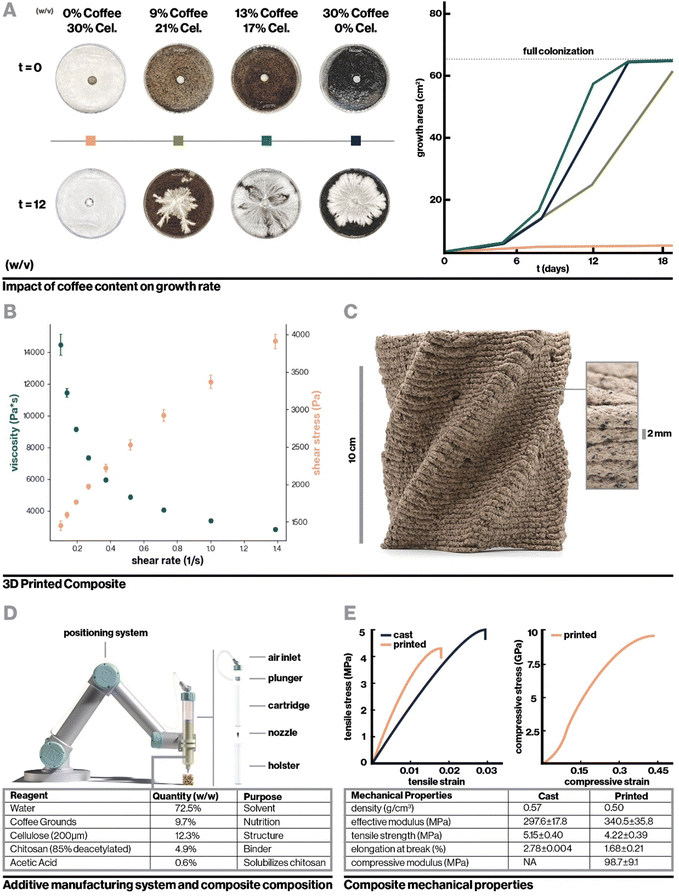
To incentivize fungal growth on the composite, locally-sourced spent coffee grounds were incorporated to serve as a low-cost and sustainable source of nutrition.34 As shown in Fig. 2A, with hydration level held constant, the addition of coffee increased biocompatibility with fungal mycelium as measured by hyphal growth rate. However, very high coffee concentrations resulted in slowed mycelium colonization by incentivizing dense, localized growth, likely the result of mycelium finding adequate nutrition in a smaller area.21 Furthermore, to retain a high enough proportion of chitosan binder for printability, composites containing high concentrations of coffee required a reduction in cellulose content, which negatively impacted vertical layer integrity during 3D-printing. This can be seen in Videos S1–S3 and Fig. S1 (ESI†), which display the 3D-printing of chitosan-bound composites with only coffee, coffee and cellulose, and only cellulose respectively. Approximately 13% (w/v) coffee grounds was found to optimally balance robust mycelium growth and structural integrity during 3D printing. The optimized biocomposite was found to be compatible with several fungal species including the gourmet mushrooms Turkey Tail (Trametes versicolor) and Oyster (Pleurotus ostreatus),35 and a strain engineered by Ecovative, a mycelium materials company36 (Fig. S2b, ESI†).
Pectin, a common fruit-derived carbohydrate that readily forms a weak hydrocolloidal gel in aqueous solution with hydrogen bonding and hydrophilic interactions,37–39 was also considered as a binder and was hypothesized to enhance mycelial growth by providing nutrition without the negative structural effects of coffee grounds. While pectin-based composites showed robust initial mycelium growth, growth slowed dramatically within days and contamination by other species was frequently observed afterwards, indicating that competition with contaminant species for the highly accessible nutrition in pectin stalled fungal growth (Fig. S2a, ESI†).
For the optimized biocomposite containing chitosan, cellulose, and coffee grounds, flow properties were characterized with rotational rheometry. The biocomposite was found to be shear thinning, which is advantageous for extrusion-based additive manufacturing, as demonstrated by increasing shear stress and decreasing viscosity with increasing shear rate (Fig. 2B). Mixtures of chitosan alone and chitosan blended with cellulose were similarly found to be shear thinning (Fig. S3a and b, ESI†). Each addition of a solid filler, first cellulose then cellulose and coffee, dramatically increased the viscosity of the mixture. By extrapolating the measured shear rate and shear stress to where shear rate is zero (Fig. S3c, ESI†),40 yield stresses were roughly approximated to be 5 Pa, 6825 Pa, and 1442 Pa for chitosan, chitosan with cellulose, and the final biocomposite, respectively. Interestingly, the yield stress for chitosan with cellulose was higher than the biocomposite despite having lower viscosity, which may be attributable to having only fine fiber fillers that can pack densely to resist deformation. The viscosity measurements and yield stress for the biocomposite are notably higher than those of materials previously used in biocomposite additive manufacturing.41 These properties were sufficient to enable extrusion-based printing with pressure of 500 kPa while resisting gravity-driven shape distortion in complex geometries.
Fig. 2C shows the printed biocomposite along with the final composition that achieved excellent printing stability and robust mycelial growth, with the ability to accommodate overhanging geometric features, consistent vertical layering, and full colonization of 10 cm tall structures within one to two weeks. As a whole, key parameters for biocomposite design were found to be multiscale structural reinforcement, in this case involving intermolecular chitosan interactions and microscale cellulose fiber reinforcement, and appropriate nutritional density accessible only to the target organism. For instance, the relatively low nutrient accessibility in spent coffee grounds allows for the growth of fungal mycelium, but reduces risk of contamination from other fungal and bacterial species that can quickly proliferate in more nutrient-dense environments. An additional interesting consideration is that while coffee grounds negatively impacted structural stability, their porous nature may enhance oxygen diffusion, which is critical for mycelial proliferation.42,43 Similar design principles can be applied to biocomposite substrates for other classes of engineered hybrid-living materials, such as with cellulose or mineral-depositing bacteria.44
For mechanical testing, the resultant properties of both printed and cast composites colonized and uncolonized by mycelium were compared. The bulk mechanical properties of the composite varied slightly as compared to 3D-printed material, as summarized in Fig. 2D. Interestingly, in tension, the fully-infilled printed composite demonstrated an increased Young's modulus compared to cast samples, even with slightly lower density. This is likely attributable to the alignment of cellulose fibers, explored in detail below. Meanwhile, the modulus measured for printed material in compression was significantly reduced as compared to the bulk tensile modulus. Comparison to bulk compressive modulus is more ideal, however cast compression samples were prohibitively difficult to fabricate due to inconsistent drying rates in samples with large interior solid spaces. Nevertheless, this decrease in modulus was expected as a consequence of the 3D printing toolpath since tensile samples were infilled at a 45° angle to the test vector, while compression samples were crushed perpendicularly to their vertical layers. This means that effects of mechanical defects such as imperfect infill and layer adhesion were much more pronounced in compression tests. Similar mechanical defects provide rationale for the printed composite's decreased tensile strength and elongation at break compared to bulk material in tension. Nevertheless, both the printed and cast composites achieve mechanical properties comparable to or exceeding current state-of-the-art biocomposites despite being optimized for biocompatibility and printability rather than solely mechanical properties. At approximate stiffness of 340 MPa and tensile strength of 4.2 MPa for the printed composite, this material exceeds the mechanical properties of rigid polymer foams such as EPF which has modulus ranging from 6.5–265 MPa,17 and approaches the mechanical properties of conventional structural materials. For example, in the transverse direction, wood sections have modulus beginning at 500 MPa.45 In applications where objectives other than biocompatibility or printability are paramount, similar biocomposites can be preferentially tuned such as by increasing the relative proportion of cellulose to enhance mechanical strength.29
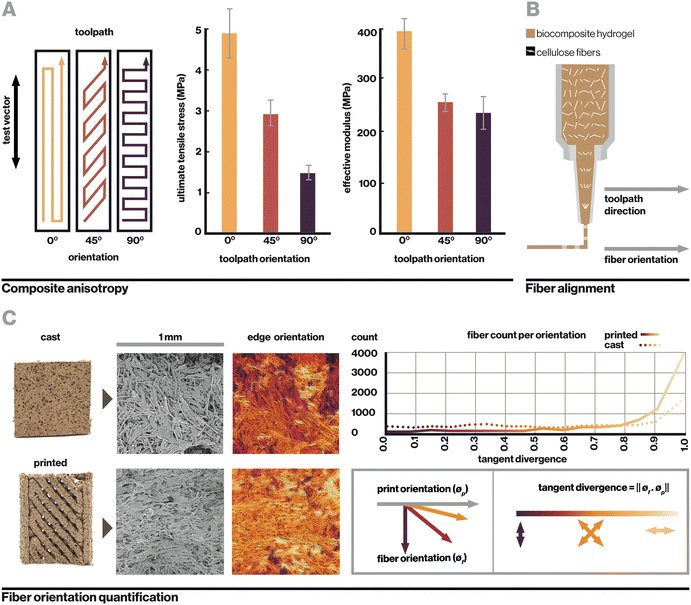
Evidently, sample geometry and print toolpath play a significant role in mechanical performance. This is analogous to hierarchical materials found in nature such as hardwood grain or complex fibers in spider silk, where multiple levels of organization create extraordinary mechanical, optical, and other material properties.1–3 Within this manufacturing system, the biocomposite was found to contain two notable levels of hierarchy, toolpath orientation and cellulose fiber alignment, which yield anisotropic mechanical properties based on toolpath orientation relative to the direction of applied strain (Fig. 3A). Toolpath orientation refers to the direction of the nozzle's movement relative to the vector of testing. Here, even in fully-infilled 3D prints, we observe that a 0° (parallel) toolpath orientation yields the highest observed tensile strength of 4.88 MPa, while a 90° (perpendicular) orientation yields the lowest at 1.45 MPa.
In uncolonized composites, failure under tension generally occurred along the lines between toolpaths or at edges where toolpaths were joined. At the macroscale, toolpath orientation creates anisotropy from relatively weak adhesion between parallel paths, which is a common phenomenon with extrusion-based 3D printing.28,46 At the microscale, another contributor is the alignment of cellulose fibers, which straighten from shear stress as they are forced through a small extrusion nozzle, resulting in anisotropy within a single printed filament (Fig. 3B). Fig. 3C shows visible alignment of cellulose fibers in an SEM image of printed material, which can be quantified by calculating tangent divergence of fiber edges using eqn (1) where ϕf is the 2D vector tangent measured on a fiber's edge and ϕp is the vector representing the print direction. This provides a metric of how aligned each fiber is with the print orientation, with a value of 1 or 0 indicating a parallel or perpendicular fiber respectively. Printed samples demonstrate a higher number of fibers aligned with the print direction. Tangent divergence was more evenly spread from 0–1 in the cast material, indicating a higher level of isotropy, however the small peak near 1 may have resulted from the scraping motion used to level cast material that can cause some surface alignment.
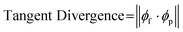 | (1) |
In this system, anisotropic effects from toolpath orientation and cellulose fiber alignment are coupled as a result of the extrusion method; that is, they are both aligned with the printing vector. Their joint effect results in printed composites that are strongest when strained along the axis of toolpath orientation and fiber alignment, weakest when strained in the perpendicular direction. These are important considerations in the design of printed objects and fabrication processes.
2.2. Indirect inoculation
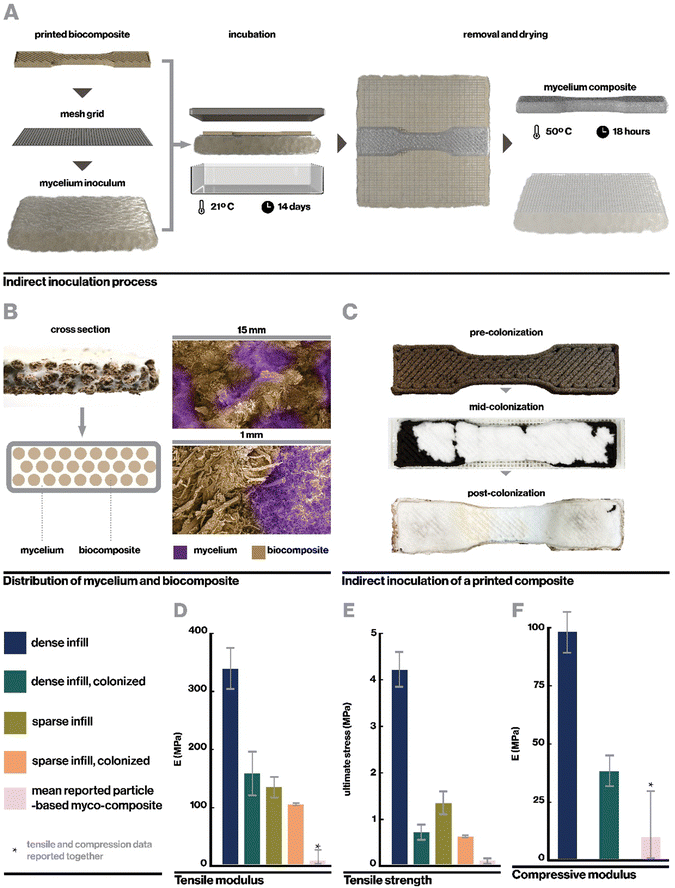
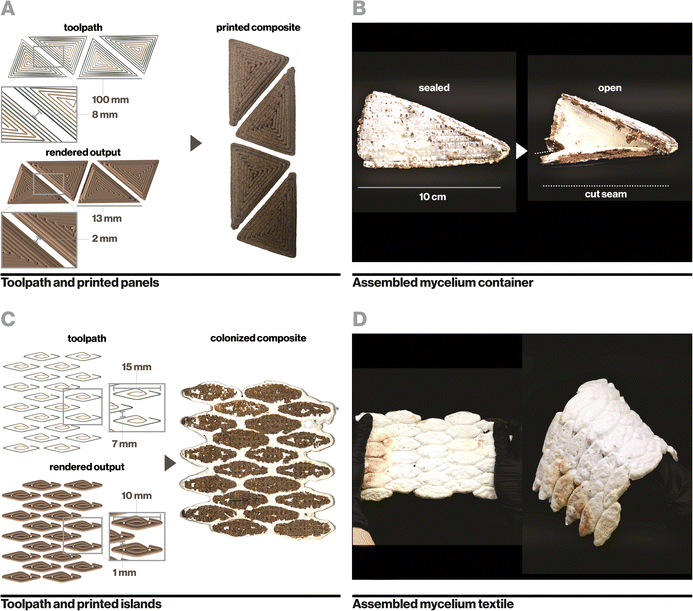
Ecovative mycelium demonstrated faster growth and better contamination resistance than other species and so was used in subsequent experiments. Biocomposite samples were colonized with mycelium using an indirect inoculation method where printed samples were incubated atop a bed of living mycelium until fully colonized as indicated by development of a full mycelium skin around the sample, typically a period of 14 days (Fig. 4A). The removed samples could then be dried in order to stall fungal growth or kept hydrated in order to retain the behaviors of active mycelium.Indirect inoculation provides a means of achieving rapid and robust mycelium colonization in high-resolution 3D printed forms. In sparsely infilled samples, void spaces were completely filled with mycelium hyphae (Fig. 4B), but even at fully dense infills (Fig. 4C), mycelium grew around and directly through the biocomposite to colonize it completely. It was empirically observed that mycelium grew readily along toolpaths, likely due to the ease of growing along a continuous filament with consistent nutrition, however the mycelium also spread between adjacent toolpaths quickly due to its ability to bridge small gaps of approximately 1 mm in our sample geometries.21 The impact of toolpath geometry and complex forms on mycelium growth behavior, especially with large gaps, may affect the resulting properties of a material and therefore has implications for toolpath design and warrants an independent future study.21
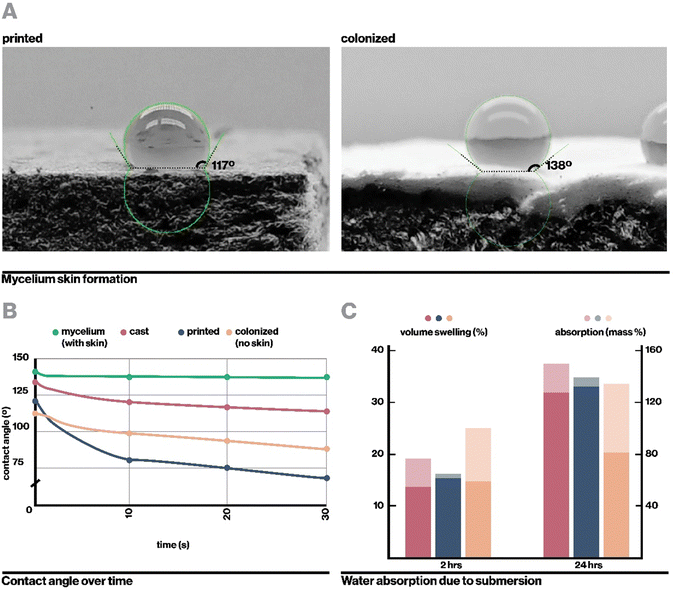
Upon full colonization, a dense mycelium skin formed around each sample. This mycelium skin appeared denser than mycelium grown in interstitial spaces, and was thick enough that it could be carefully peeled away from the rest of the sample. Growth of mycelium skin at the interface of a substrate and hair has been commonly observed in literature, and is proposed to function to manage moisture levels within the core of mycelium composites.21,47
Nevertheless, indirect inoculation has some inherent limitations. Because colonization occurs from a fixed plane on only one side of the biocomposite print, despite rapid growth, full colonization theoretically takes longer than direct inoculation methods where inoculum is mixed into composite material before printing, allowing for growth from every point within the substrate simultaneously. However, in our preliminary experiments, direct inoculation resulted in slow or stalled growth, likely due to the high shear stress experienced by mycelium when extruded through a thin nozzle, which is known to weaken living components.48 Other studies have demonstrated successful mycelium colonization after printing with direct inoculation, however these generally use low resolutions and less rigid printing substrates21,49,50 with poor mechanical properties,21 such as with agar alone or agar with coffee grounds.21,50 With yield stress of 1442 Pa, our biocomposite necessarily experiences higher shear stresses during extrusion than agar-based materials, which have yield stress around 400 Pa.21
It is important to note that in the theoretical case of fabricating very tall objects, indirect inoculation may yield composites where mycelium has more time to feed on regions closer to the inoculum than those it reaches later in the incubation process. This could inherently lead to the creation of inhomogeneous composites. In such a scenario, direct inoculation may yield composites with a greater degree of homogeneity due to colonization proceeding from multiple points in the printed composite rather than only from the inoculum bed. When fabricating large-scale objects that do not require such high-resolution, this challenge can potentially be overcome by using direct inoculation methods with a larger nozzle size that reduces shear stress on the living mycelium.51,52
2.3. Impacts of mycelium colonization
Here, samples were dried at 50 °C for 18 hours, which enabled full dehydration with minimal warping. Drying additionally served a second purpose to neutralize the mycelium in colonized materials, preventing further growth such that the properties of the composite should no longer change significantly over time. The resulting mycelium composite was substantially denser than previously reported mycelium composites as reported in Table S1 (ESI†), likely due to the use of a rigid 3D-printed substrate rather than loose biomass (Fig. 1C and D). At 160 MPa for the fully infilled material, our mycelium composites were also stronger than any other mycelium composite reported in literature, with over a fifteen-fold increase in modulus compared to the average of reported materials (Fig. 4D) excepting one by Kaiser et al., who similarly used a strong biocomposite substrate rather than loose particle biomass.53 However, Kaiser et al. developed a thick and coarse “mycocrete” paste that was packed into molds rather than extrudable in high-resolution printing. In this work, tensile strength was measured at 0.72 MPa (Fig. 4E), similarly exceeding the highest performing mycelium composite in literature.
The mechanical properties of the mycelium-colonized composite are also shown in Fig. 4D–F. In comparison to the uncolonized biocomposite, growth of mycelium actually weakens the material. This is contrary to what is typically observed in particulate-based mycelium composites, where mycelium serves as a binder and enhances mechanical properties.54–56 However, it makes sense in the context of a biocomposite substrate, where mycelium lends new properties but is actively digesting the organic material that makes up the pre-formed and already rigid material. This indicates a tradeoff between mycelium growth and its associated functions and mechanical properties in this class of materials, which presents an important consideration when designing materials for specific applications. For instance, our uncolonized composite has a young's modulus similar to that of low-density polyethylene (LDPE)57 while maintaining lower density, and may have potential applications as a rigid core material. The colonized material, by contrast, has density and modulus more similar to EPF foam58 and may find application as a sustainable alternative to styrofoams.
Kaiser et al. reported a tensile modulus of 153 MPa but did not report a tensile modulus of their base composite formulation due to slippage at clamps. They did report a base compressive modulus of 8.26 MPa, which was increased to 10.01 MPa after inoculation with mycelium. While this is notably weaker than our measured compressive modulus at 38.3 MPa, future work can investigate relationships between particulate-based and biocomposite-based substrates, and impacts of mycelium growth, to optimize for enhanced mechanical properties.
Interestingly, colonized samples of dense and sparse prints show relatively similar mechanical properties, especially compared to the large contrast in mechanical properties between uncolonized dense and sparse samples. A potential explanation for this is that in colonized materials, mycelium serves to homogenize the materials slightly and dominates the mechanical response. Whereas in uninoculated composites mechanical properties are entirely dependent on chemical or physical bonds within the composite itself, in colonized composites, mycelium may have broken some of these bonds with its enzymatically-driven growth and formed its own bridges and bonds with and between the substrate material.59 Similarly, by filling interstitial spaces between filaments of biocomposite, the mycelium may also reduce the effects of filament sparsity. Additional detail regarding the mechanical behavior of the colonized myco-composite can be found in Fig. S4 (ESI†).
In a similar vein, the fully colonized composite exhibited lower water absorption and volume swelling than non-colonized samples after being submerged for 24 hours despite initially swelling relatively rapidly (Fig. 5C), likely due to the presence of hydrophobic mycelial hyphae. The initial water absorption and swelling may be attributable to higher porosity. As indirect inoculation proceeds from an inoculation surface from which the composite must be detached, we could not fabricate samples that are fully covered by the mycelium skin on all sides. Indeed, even direct inoculation methods require a surface for the sample to rest on, which will not form a mycelium skin. However, we anticipate from the existing data that full coverage with mycelium skin further reduce rates of water absorption and volume swelling.56
Similarly, small islands of biocomposite were printed with precise spacing (Fig. 6C) and colonized to yield a flexible textile-like material that could bend and stretch in multiple directions (Fig. 6D). During flexing and stretching, biocomposite regions remained rigid while mycelium flexed. This process yields a simplified manner of constructing textile-like mycelium materials compared to existing mycelium-based leather alternatives that generally require intensive processes of cutting and laminating thin-sheets of pure mycelium.65 Leveraging additive manufacturing and indirect inoculation further enables precise engineering of rigid and flexible regions in a textile, which determines how it moves, folds, and drapes.66,67 Video documentation of sealed mycelium containers (Video S4, ESI†) and flexible mycelium textiles (Video S5) can be found in the ESI.†
3. Conclusion
We have described a rapid and reliable means to create robust myco-composites that require no external mold, no heat-pressing or extensive post processing, and achieve good hydrophobicity and mechanical properties superior to existing myco-composites. This platform leverages a biocomposite derived entirely from existing organic waste streams and designed for biocompatibility with fungal indirect inoculation through selective nutritional provision, as well as for excellent printability, which can enhance mechanical properties through cellulose fiber and toolpath alignment. Compatibility with additive manufacturing further enables broad versatility in applications, such as the creation of complex geometries, including gap-bridging geometries that leverage both the stiffness of the colonized myco-composite and the extended generative capabilities of mycelium alone. This provides a means to lend the strengths of biocomposites and mycelium materials to one another while countervailing their weaknesses, bridging the gap between the two research fields.Mechanically strong myco-composites with water resistant exteriors could hold promise as advanced structural materials, while lightweight forms with 3D-printed sparse infills can leverage the insulative properties of mycelium as potential packaging systems,68 and flexible textile-like behaviors can be achieved by harnessing the generative behaviors of mycelium. In this work, we used Ecovative mycelium and achieved greater mechanical properties than any reported mycelium composites in literature, including other biocomposite-based materials53 and Ecovative-based materials.69,70 However, mycelium growth and characteristics are highly dependent on the species and strain as well as on environment. We present a successful platform for creating robust myco-composites, and future work with this platform utilizing other species of fungi, especially more widely available or “wild-type” species, would be beneficial. Some interesting species include Trametes versicolor and Pleurotus ostreatuss, which were included in preliminary experimentation for this study, and Ganoderma lucidum, which has been successfully used in other applications of mycelium materials.71
Following the tremendous design space constituted by composite formulation, organism and species selection, and processing parameters, there is high potential for continued exploration of novel hybrid-living materials for further enhanced mechanical or other objective properties. With sufficient data, a broad range of previously unexplored applications may become feasible. Such research efforts typically require substantial wrought experimentation, however computation-assisted approaches to biomateriomics may accelerate the rate of material discovery or provide an avenue for more rapid optimization of specific properties.72,73 This lays the foundation for future advances that could enable scalable, sustainable manufacturing with hybrid-living biocomposites.
4. Experimental section/methods
4.1. Mycelium cultivation
Malt extract agar plates were prepared for mycelium cultivation. 2 wt% malt extract (Briess Malt & Ingredients Co., WI) and 2 wt% agar (Spectrum Chemical, NJ) were dissolved in boiling filtered water, then sterilized in a 6-quart pressure cooker at 15 psi for 45 minutes. While warm, 20 mL each of agar solution was pipetted into 45 mm diameter Petri dishes and allowed to cool completely before sealing with parafilm and refrigerated until use.P. ostreatus (oyster mushroom) and T. versicolor (turkey tail) were purchased as liquid mycelium cultures from liquidfungi.com (Holiday, FL) and shipped to Cambridge, MA in insulated packaging. Ecovative culture was purchased as bulk solid inoculated hemp substrate (Grow-It-Yourself material) from Ecovative Design (Green Island, NY). 0.2 mL of liquid cultures or small pieces of solid inoculated material were used to propagate each species onto prepared agar plates. Petri dishes were fully colonized in approximately 1 week, after which they were refrigerated at 3 °C to preserve the living mycelium. Subsequent propagations of the mycelium were inoculated with prior-generation colonized agar dishes.
4.2. Composite design
To explore principles of composite design for printability and biocompatibility with mycelial growth, parameters including coffee content, hydration level, and acidity were varied individually. To assess biocompatibility, for each material of interest, 25 g of composite was pressed into a 45 mm diameter Petri dish. A 1 cm diameter cylindrical core of material was removed from the center and replaced with a mycelium-colonized agar plug of the same size, from which mycelium generally grew radially outward. Growth rate was then evaluated over time as measured by the surface area of each Petri dish covered by mycelium. These experiments were performed with replicates of 3 to assess variability.To assess printability, each composite of interest was printed in the same geometry where qualitative features such as vertical layer integrity, overhang stability, and print consistency could be observed. These included graded overhangs up to 1 cm and total print height up to 10 cm.
4.3. Rheology
Rheological analysis of the biocomposite was performed with a Discovery RH20 machine. To interrogate the effects of each constituent, flow behavior was determined for the 8% chitosan solution, for the 8% chitosan solution with 20 wt% cellulose, and finally for the final biocomposite with chitosan, cellulose, and coffee grounds. Dynamic shear viscosity was measured over different shear rates (0.1–5 s−1) with a 25.0 mm parallel plate and a 2 mm gap at ambient temperature and humidity. These test parameters were held constant across all samples, and were selected to accommodate for the thick nature of the biocomposite. With smaller gaps or shear viscosities above approximately 2 s−1, the biocomposite could not deform at a fast enough rate and instead tore and peeled away from the testing plate. Rheological tests were performed in triplicate for each material.4.4. Composite mixing & printing
Used coffee grounds were obtained in bulk from local waste streams (Dunkin’, Boston, MA) and dried under forced air for at least 72 hours prior to being powderized in a bladed grinder until particles were approximately 150 μm in diameter. Prior to composite mixing, coffee grounds were hydrated to 82% w/v (450 g coffee:550 g water) with filtered water based on accepted practices for mycelium growth56 and steam sterilized at 15 psi for 120 minutes.Hydrated biocomposite pastes were mixed at 21 °C in a sterile laboratory setting. For each batch of composite, 80 g of 85% deacetylated chitosan from Bulk Supplements (Henderson, NV) was combined with 990 mL of 21 °C filtered water in a mechanical blender (Blendtec, Orem, UT) and pulsed once to homogenize the mixture. 10 ml of pure glacial acetic acid (VWR, Radnor, PA) was added to the mixture in order to form a gel. The mixture was then pulsed for 15 seconds. The resulting chitosan gel was transferred to an industrial mixer (Atosa, Westboro, MA) and combined with 200 g of 200 μm de-lignated cellulose fibers (Creafil, Chestertown, MD) on a medium speed for 2 minutes. The mixture was then scraped from the sides of the mixing bowl and mixed again on a high speed for 3 minutes to form a smooth and homogeneous paste. 350 g of hydrated coffee grounds was then combined into the cellulose–chitosan mixture and mixed on low for 30 seconds. The bowl was scraped down and then mixed again for 30 seconds on a medium setting to form a homogenous paste.
Preliminary experiments with pectin binders followed a similar protocol but combined 200 g (20% w/v) pectin (VWR, Radnor, PA) instead of chitosan with water before adding acetic acid. Experiments with both pectin and chitosan stacked the binders, combining both 20% w/v pectin and 8% w/v chitosan. Due to the nonlinear behavior of combinations of pectin and chitosan,74,75 these chitosan, pectin, and chitosan and pectin blended solutions empirically yielded similar viscosity solutions.
For additive manufacturing, the hydrated biocomposite paste was loaded into 300 mL cartridges and sealed with a polyethylene piston and 1 mm nozzle. Pastes were printed via pneumatic extrusion at ambient conditions, approximately 21 °C and 30% humidity. Dogbone geometries were printed with 45° diagonal crosshatch infills. Extended dehydration processes were implemented to counteract reported issues of deformation during the drying process. Un-inoculated composites were printed onto an acrylic grid and placed on a wire rack covered with a tarp to ensure even dehydration across the print. After 24 hours, prints were transferred to a dehydrator and dried at 40 °C for 18 hours. Dried prints were rested at ambient conditions for at least 24 hours prior to mechanical testing in order to allow for equilibration with ambient humidity.
Cast composites were loaded into silicone molds and allowed to rest on the tarp covered wire rack for 24 hours of slow drying, then dehydrated at 40 °C for 18 hours. Samples were removed from their silicone molds approximately 2 hours into the dehydration process. Dried samples were rested at 21 °C and 30% humidity for at least 24 hours prior to mechanical testing.
Mechanical tests were performed in replicates of 5. Data from samples with poor test quality were excluded, such as samples that broke within the grips rather than in the gauges, resulting in data collected at least in triplicate for each condition.
4.5. Additive manufacturing system
The digital fabrication system consisted of a 3-axis gantry positioning system with a print area of 300 cm × 150 cm × 10 cm fitted with a pneumatic dispensing system (Nordson Medical,76 Salem, NH). The composite was typically extruded at 500 kPa with feed rate of 1250 mm min−1. Extrusion pressure and feed rate were manually adjusted during printing to achieve consistent fabrication with 2.5 mm extrusion width, 2 mm layer height, and 0.5 mm system resolution.4.6. Indirect inoculation
Purchased Ecovative substrate (inoculated hemp) was pressed flat into containers and incubated at room temperature for a period of five days prior to the insertion of 3D printed media. The remainder of the substrate was refrigerated at 3 °C until use.Prints for indirect inoculation were extruded onto photopolymer 3D printed (Stratasys Inc, Revohot, Israel) grids. Printing onto an inert grid enabled easy transfer of printed samples while allowing mycelium through the grid and into samples. Furthermore, leaving the grid between the samples and the mycelium inoculum enabled clean removal of the samples after full colonization. Printed samples were immediately transferred into airtight containers filled with Ecovative substrate and left to be colonized for 14 days prior to removal and dehydration at 40 °C for 24 hours. Before mechanical testing, dried prints were allowed to rest for at least 24 hours at 21 °C and 30% humidity.
4.7. Mechanical testing
Tensile testing was performed at ambient conditions using an Instron Universal Testing Machine (Instron, Norwood, MA) with a 5 kN load cell. The ASTM d1037 dimensions for tensile testing were modified to use a gauge width of 24 mm in order to ensure more consistent breakage within the gauge. Test samples were strained at a rate of 1 mm min−1 until failure. Tests that exhibited breakage in the grips were discarded from statistical analysis.Compression testing was performed using an Instron Universal Testing Machine (Instron, Norwood, MA) with a 100 kN load cell. The test samples were walled cylinders with 5 cm diameter and 1 cm wall thickness. Concentric circles were printed in order to achieve the 1 cm thickness. Tests were compressed at a rate of 2 mm min−1.
For each parameter evaluated, 3–5 samples were included in final analysis.
4.8. Hydrophobicity, wettability, and absorbance
Hydrophobicity was measured through static advancing contact angle measurements, adapted from ASTM D7334. For each of cast, printed, colonized, and colonized without mycelium skin specimens, three 5 μL droplets of distilled water, dyed red with food coloring for visibility, were manually deposited on the surface with a micropipette under video recording. For each droplet, contact angle was measured at timesteps 0, 1, 10, and 30 seconds after deposition. Contact angle was measured with the Drop Shape Analysis plugin on ImageJ77 (Bethesda, Maryland) 3 times each for each droplet and averaged.Water absorbance and swelling experiments were adapted from ASTM D1037. At least 3 test specimens for each cast, printed, and printed and inoculated settings were cut into approximately 5 cm by 5 cm by 0.5 cm squares, then sanded smooth with 60 grit sandpaper. They were dehydrated overnight at 40 °C, then submerged under 1 inch of room temperature water. Volume and mass were measured for each sample prior to submersion, and after blotting at 2 hours and 24 hours of submersion.
Author contributions
Authors S. S. and N. L. contributed equally to this work. Conceptualization: S. S., N. L., W. L., M. B. Data collection: S. S., N. L., A. A., H. G., B. S. Data curation and visualization: S. S., N. L., B. S. Writing – original draft: S. S., N. L., W. L., A. A., B. S. Writing – review & editing: S. S., N. L., M. B.Conflicts of interest
The authors declare no competing interests.Acknowledgements
We extend our thanks to Dr James C. Weaver for his assistance with SEM imaging. We also extend our appreciation to the Dunkin’ employees at the Stratton Student Center location (84 Massachusetts Ave) for their efforts in collecting and donating spent coffee grounds. We acknowledge the National Science Foundation Graduate Research Fellowship Program (1745302) and the MathWorks Engineering Fellowship for support of this work. We further acknowledge support from the MIT Reed Fund and Google.References
- P. Fratzl and R. Weinkamer, Prog. Mater. Sci., 2007, 52, 1263–1334 CrossRef CAS.
- K. Guo and M. J. Buehler, Matter, 2019, 1, 302–303 CrossRef.
- D. J. Buss, R. Kröger, M. D. McKee and N. Reznikov, J. Struct. Biol. X, 2022, 6, 100057 CAS.
- M. M. Ito, A. H. Gibbons, D. Qin, D. Yamamoto, H. Jiang, D. Yamaguchi, K. Tanaka and E. Sivaniah, Nature, 2019, 570, 363–367 CrossRef CAS PubMed.
- A. Rodrigo-Navarro, S. Sankaran, M. J. Dalby, A. del Campo and M. Salmeron-Sanchez, Nat. Rev. Mater., 2021, 6, 1175–1190 CrossRef.
- M. R. Binelli, P. A. Rühs, G. Pisaturo, S. Leu, E. Trachsel and A. R. Studart, Biomater. Adv., 2022, 141, 213095 CrossRef CAS PubMed.
- M. Nodehi, T. Ozbakkaloglu and A. Gholampour, J. Build. Eng., 2022, 49, 104038 CrossRef.
- M. Jones, A. Mautner, S. Luenco, A. Bismarck and S. John, Mater. Des., 2020, 187, 108397 CrossRef CAS.
- R. Abhijith, A. Ashok and C. R. Rejeesh, Mater. Today Proc., 2018, 5, 2139–2145 CrossRef CAS.
- M. R. Islam, G. Tudryn, R. Bucinell, L. Schadler and R. C. Picu, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- A. Livne, H. A. B. Wösten, D. Pearlmutter and E. Gal, ACS Sustainable Chem. Eng., 2022, 10, 12099–12106 CrossRef CAS.
- Z. Zimele, I. Irbe, J. Grinins, O. Bikovens, A. Verovkins and D. Bajare, J. Renewable Mater., 2020, 8(9), 1067–1076 CAS.
- J. A. López Nava, J. Méndez González, X. Ruelas Chacón and J. A. Nájera Luna, Mater. Manuf. Processes, 2016, 31(8), 1085–1090 CrossRef.
- Z. Yang, F. Zhang, B. Still, M. White and P. Amstislavski, J. Mater. Civ. Eng., 2017, 29(7) DOI:10.1061/(ASCE)MT.1943-5533.0001866.
- R. Abhijith, A. Ashok and C. R. Rejeesh, Mater. Today Proc., 2018, 5, 2139–2145 CrossRef CAS.
- technical-data – Mushroom Packaging, https://mushroompackaging.com/pages/technical-data, (accessed 10 December 2023).
- Overview of materials for Expanded Polystyrene (EPS), https://www.matweb.com/search/datasheet.aspx?matguid=5f099f2b5eeb41cba804ca0bc64fa62f, (accessed 10 December 2023).
- E. Elsacker, A. Søndergaard, A. Van Wylick, E. Peeters and L. De Laet, Constr. Build. Mater., 2021, 283, 122732 CrossRef.
- E. Elsacker, S. Vandelook, A. Van Wylick, J. Ruytinx, L. De Laet and E. Peeters, Sci. Total Environ., 2020, 725, 138431 CrossRef CAS PubMed.
- G. Simondon and T. Adkins, Individuation in light of notions of form and information, 2020 Search PubMed.
- S. Gantenbein, E. Colucci, J. Käch, E. Trachsel, F. B. Coulter, P. A. Rühs, K. Masania and A. R. Studart, Nat. Mater., 2022, 22, 128–134 CrossRef PubMed.
- Tree Column | Blast Studio, https://www.blast-studio.com/tree-column, (accessed 9 January 2023).
- A. Bhardwaj, A. M. Rahman, X. Wei, Z. Pei, D. Truong, M. Lucht and N. Zou, J. Manuf. Mater. Process., 2021, 5, 112 CAS.
- E. Soh, Z. Y. Chew, N. Saeidi, A. Javadian, D. Hebel and H. Le Ferrand, Mater. Des., 2020, 195, 109058 CrossRef CAS.
- Particleboard | Composite Panels | Timber Products, https://timberproducts.com/composite-panels-particleboard/, (accessed 30 January 2023).
- R. Liu, L. Long, Y. Sheng, J. Xu, H. Qiu, X. Li, Y. Wang and H. Wu, Ind. Crops Prod., 2019, 141, 111732 CrossRef CAS.
- M. Nussbaumer, D. Van Opdenbosch, M. Engelhardt, H. Briesen, J. P. Benz and T. Karl, Environ. Technol. Innov., 2023, 30, 103063 CrossRef CAS.
- G. Ma, Z. Li, L. Wang, F. Wang and J. Sanjayan, Constr. Build. Mater., 2019, 202, 770–783 CrossRef.
- N. D. Sanandiya, Y. Vijay, M. Dimopoulou, S. Dritsas and J. G. Fernandez, Sci. Rep., 2018, 8, 1–8 CAS.
- J. P. Martínez, M. P. Falomir and D. Gozalbo, eLS, 2014 DOI:10.1002/9780470015902.A0000694.PUB3.
- A. El Hadrami, L. R. Adam, I. El Hadrami and F. Daayf, Mar. Drugs, 2010, 8, 968 CrossRef CAS PubMed.
- L. H. Carvalho, E. L. Canedo, S. R. Farias Neto, A. G. B. de Lima and C. J. Silva, Adv. Struct. Mater., 2013, 36, 37–62 Search PubMed.
- C. Lim, D. S. Hwang and D. W. Lee, Carbohydr. Polym., 2021, 259, 117782 CrossRef CAS PubMed.
- R. M. Escaleira, M. J. Campos and M. L. Alves, Adv. Sci., Technol. Innov., 2021, 261–266 Search PubMed.
- N. M. Majib, S. T. Sam, N. D. Yaacob, N. M. Rohaizad and W. K. Tan, Polymers, 2023, 15, 873 CrossRef CAS PubMed.
- P. Q. Nguyen, N. M. D. Courchesne, A. Duraj-Thatte, P. Praveschotinunt and N. S. Joshi, Adv. Mater., 2018, 30, 1704847 CrossRef PubMed.
- N. A. Lee, R. E. Weber, J. H. Kennedy, J. J. Van Zak, M. Smith, J. Duro-Royo and N. Oxman, 3D Print Addit. Manuf., 2020, 7, 205–215 CrossRef PubMed.
- U. Einhorn-Stoll, Food Hydrocoll., 2018, 78, 109–119 CrossRef CAS.
- D. Gawkowska, J. Cybulska and A. Zdunek, Polymers, 2018, 10(7), 762 CrossRef PubMed.
- Malvern Instruments Worldwide, A Basic Introduction to Rheology, 2016, https://cdn.technologynetworks.com/TN/Resources/PDF/WP160620BasicIntroRheology.pdf Search PubMed.
- N. D. Sanandiya, Y. Vijay, M. Dimopoulou, S. Dritsas and J. G. Fernandez, Sci. Rep., 2018, 8, 8642 CrossRef PubMed.
- V. Bejenari, A. Marcu, A. M. Ipate, D. Rusu, N. Tudorachi, I. Anghel, I. E. Şofran and G. Lisa, J. Mater. Res. Technol., 2021, 15, 4437–4451 CrossRef CAS.
- S. H. Zein, B. A. Gyamera and V. K. Skoulou, Mater. Lett., 2017, 193, 46–49 CrossRef CAS.
- M. Pommet, J. Juntaro, J. Y. Y. Heng, A. Mantalaris, A. F. Lee, K. Wilson, G. Kalinka, M. S. P. Shaffer and A. Bismarck, Biomacromolecules, 2008, 9, 1643–1651 CrossRef CAS PubMed.
- Materials Data Book, Cambridge University Engineering Department, UK, 2011 Search PubMed.
- M. Somireddy and A. Czekanski, Mater. Des., 2020, 195, 108953 CrossRef CAS.
- E. Soh and H. Le Ferrand, Mater. Des., 2023, 225, 111530 CrossRef CAS.
- H. Q. Xu, J. C. Liu, Z. Y. Zhang and C. X. Xu, Mil. Med. Res., 2022, 9, 1–15 Search PubMed.
- A. Bhardwaj, J. Vasselli, M. Lucht, Z. Pei, B. Shaw, Z. Grasley, X. Wei and N. Zou, Manuf. Lett., 2020, 24, 96–99 CrossRef.
- E. Soh, J. H. Teoh, B. Leong, T. Xing and H. Le Ferrand, Mater. Des., 2023, 236, 112481 CrossRef.
- Z. Wang, J. Xue, H. Sun, M. Zhao, Y. Wang, J. Chu and Y. Zhuang, Process Biochem., 2020, 92, 120–129 CrossRef CAS.
- A. Blaeser, D. F. Duarte Campos, U. Puster, W. Richtering, M. M. Stevens and H. Fischer, Adv. Healthcare Mater., 2016, 5, 326–333 CrossRef CAS PubMed.
- R. Kaiser, B. Bridgens, E. Elsacker and J. Scott, Front. Bioeng. Biotechnol., 2023, 11, 1229693 CrossRef PubMed.
- M. R. Islam, G. Tudryn, R. Bucinell, L. Schadler and R. C. Picu, J. Mater. Sci., 2018, 53, 16371–16382 CrossRef CAS.
- J. M. C. Ongpeng, E. Inciong, V. Sendo, C. Soliman and A. Siggaoat, Appl. Sci., 2020, 10, 5303 CrossRef CAS.
- E. Elsacker, S. Vandelook, J. Brancart, E. Peeters and L. De Laet, PloS One, 2019, 14, e0213954 CrossRef CAS PubMed.
- Overview of materials for Low Density Polyethylene (LDPE), Blow Molding Grade, https://www.matweb.com/search/datasheet_print.aspx?matguid=b34a78d271064c4f85f28a9ffaf94045, (accessed 19 December 2023).
- Physical and mechanical property of EPS, https://www.intcorecycling.com/Physical-and-mechanical-property-of-EPS.html, (accessed 12 January 2023).
- G. G. de Lima, Z. C. P. Schoenherr, W. L. E. Magalhães, L. B. B. Tavares and C. V. Helm, Bioresour. Bioprocess., 2020, 7, 1–17 CrossRef.
- M. B. Linder, G. R. Szilvay, T. Nakari-Setälä and M. E. Penttilä, FEMS Microbiol. Rev., 2005, 29, 877–896 CrossRef CAS PubMed.
- J. Zhao, Encyclopedia Tribol., 2013, 295–298 Search PubMed.
- E. Elsacker, M. Zhang, M. Dade-Robertson, E. Elsacker, M. Dade-Robertson and M. Zhang, Adv. Funct. Mater., 2023, 33, 2301875 CrossRef CAS.
- M. Jones, T. Bhat, E. Kandare, A. Thomas, P. Joseph, C. Dekiwadia, R. Yuen, S. John, J. Ma and C.-H. Wang, Sci. Rep., 2018, 8, 17583 CrossRef PubMed.
- H. Schritt, S. Vidi and D. Pleissner, J. Clean. Prod., 2021, 313, 127910 CrossRef.
- S. Vandelook, E. Elsacker, A. Van Wylick, L. De Laet and E. Peeters, Fungal Biol. Biotechnol., 2021, 8, 1–10 CrossRef PubMed.
- Colored Wooden Rugs by Elisa Strozyk, https://design-milk.com/colored-wooden-rugs-by-elisa-strozyk/?media_id=158111, (accessed 30 April 2023).
- Flexible Yet Rigid: A 3D- Printed Textile—Digital Craft Lab @ CCA, https://digitalcraft.cca.edu/research/2021/8/17/flexible-yet-rigid-a-3d-printed-textile, (accessed 30 April 2023).
- Y. Hu, K. Xiao, L. Yan, I. F. Ridzqo, D. Susanto, T. H. Panjaitan, Y. Xing, M. Brewer, H. El-Gharabawy, G. Griffith and P. Jones, IOP Conf. Ser. Earth Environ. Sci., 2018, 121, 022032 CrossRef.
- A. R. Ziegler, S. G. Bajwa, G. A. Holt, G. McIntyre and D. S. Bajwa, Appl. Eng. Agric., 2016, 32, 931–938 Search PubMed.
- G. A. Holt, G. McIntyre, D. Flagg, E. Bayer, J. D. Wanjura and M. G. Pelletier, J. Biobased Mater. Bioenergy, 2012, 6, 431–439 CrossRef CAS.
- M. Haneef, L. Ceseracciu, C. Canale, I. S. Bayer, J. A. Heredia-Guerrero and A. Athanassiou, Sci. Rep., 2017, 7, 1–11 CrossRef PubMed.
- N. A. Lee, S. C. Shen and M. J. Buehler, Matter, 2022, 5, 3597–3613 CrossRef CAS PubMed.
- S. C. Shen, E. Khare, N. A. Lee, M. K. Saad, D. L. Kaplan and M. J. Buehler, Chem. Rev., 2023, 123(5), 2242–2275 CrossRef CAS PubMed.
- R. D. Baron, L. L. Pérez, J. M. Salcedo, L. P. Córdoba and P. J. D. A. Sobral, Int. J. Biol. Macromol., 2017, 98, 676–683 CrossRef CAS PubMed.
- N. A. Lee, R. E. Weber, J. H. Kennedy, J. J. Van Zak, M. Smith, J. Duro-Royo and N. Oxman, 3D Print Addit. Manuf., 2020, 7, 205–215 CrossRef PubMed.
- Home | Nordson MEDICAL, https://www.nordsonmedical.com/, (accessed 12 January 2023).
- ImageJ, https://imagej.net/ij/, (accessed 9 January 2023).
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh01277h |
| ‡ Equal contribution. |
| This journal is © The Royal Society of Chemistry 2024 |