Smart stimuli-responsive strategies for titanium implant functionalization in bone regeneration and therapeutics
Jinkai
Zhang†
a,
Yu
Zhuang†
a,
Ruilong
Sheng†
 b,
Helena
Tomás
b,
João
Rodrigues
b,
Helena
Tomás
b,
João
Rodrigues
 *b,
Guangyin
Yuan
*c,
Xudong
Wang
*a and
Kaili
Lin
*b,
Guangyin
Yuan
*c,
Xudong
Wang
*a and
Kaili
Lin
 *a
*a
aDepartment of Oral & Cranio-Maxillofacial Surgery, Shanghai Ninth People's Hospital, College of Stomatology, Shanghai Jiao Tong University School of Medicine; National Clinical Research Center for Oral Diseases; Shanghai Key Laboratory of Stomatology & Shanghai Research Institute of Stomatology; Research Unit of Oral and Maxillofacial Regenerative Medicine, Chinese Academy of Medical Sciences, Shanghai 200011, China. E-mail: linkaili@sjtu.edu.cn; xudongwang70@hotmail.com
bCQM-Centro de Quimica da Madeira, Universidade da Madeira, Campus da Penteada, 9020-105, Funchal, Madeira, Portugal. E-mail: joaoc@staff.uma.pt
cSchool of Materials Science and Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: gyyuan@sjtu.edu.cn
First published on 4th October 2023
Abstract
With the increasing and aging of global population, there is a dramatic rise in the demand for implants or substitutes to rehabilitate bone-related disorders which can considerably decrease quality of life and even endanger lives. Though titanium and its alloys have been applied as the mainstream material to fabricate implants for load-bearing bone defect restoration or temporary internal fixation devices for bone fractures, it is far from rare to encounter failed cases in clinical practice, particularly with pathological factors involved. In recent years, smart stimuli-responsive (SSR) strategies have been conducted to functionalize titanium implants to improve bone regeneration in pathological conditions, such as bacterial infection, chronic inflammation, tumor and diabetes mellitus, etc. SSR implants can exert on-demand therapeutic and/or pro-regenerative effects in response to externally applied stimuli (such as photostimulation, magnetic field, electrical and ultrasound stimulation) or internal pathology-related microenvironment changes (such as decreased pH value, specific enzyme secreted by bacterial and excessive production of reactive oxygen species). This review summarizes recent progress on the material design and fabrication, responsive mechanisms, and in vitro and in vivo evaluations for versatile clinical applications of SSR titanium implants. In addition, currently existing limitations and challenges and further prospective directions of these strategies are also discussed.
Wider impactTitanium is the primary material to fabricate load-bearing implants or substitutes to rehabilitate bone-related disorders, which can considerably decrease quality of life and even endanger lives. However, it is far from rare to encounter failed cases in clinical practices, particularly with pathological factors involved. In recent years, smart stimuli-responsive (SSR) strategies have emerged to functionalize implants to accommodate the dynamic microenvironment of bone regeneration and remodeling. In this review, advances in SSR titanium implants that respond to external stimuli (such as light, magnetic field, electrical stimulation and ultrasound), or internal biochemical clues (such as pH value, specific enzyme and excessive production of reactive oxygen species), are summarized by category. The material design and fabrication, responsive mechanism, and biological evaluation of SSR titanium implants for versatile clinical applications are also discussed, which might arouse the broad interest of researchers from different fields, including material science and biotechnology, cranio-maxillofacial and orthopedic regenerative medicine. Finally, we discuss existing limitations and challenges, and propose potential prospective directions of these strategies. We believe this review article will provide a comprehensive insight into SSR titanium implants and would facilitate further advancements of this field. |
1. Introduction
Bone defects or fractures caused by various pathological processes (such as tumors, severe infection, inflammatory diseases, etc.) or traumatic events can dramatically decrease quality of life and even endanger lives. With the increasing and aging of the global population, there is a dramatic rise in the demand for bone implants or substitutes to rehabilitate bone-related disorders. It is expected that the number of total hip or knee replacements would rise to 572![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 3.48 million procedures in the United States by 2030, respectively.1 Moreover, the annual cost spent on treating defective healing of bone defects or fractures associated with trauma, tumors, and other pathologies in the United States, has boomed to 5 billion dollars.2 Though autologous grafts are considered the gold standard to restore bone defects, there are many inevitable disadvantages, including limited resources, additional injury, potential complications at donor sites, etc. Therefore, biomaterial-based bone regeneration has been revolving dramatically in the past several decades, which developed various material systems for different clinical scenarios.3–5
000 and 3.48 million procedures in the United States by 2030, respectively.1 Moreover, the annual cost spent on treating defective healing of bone defects or fractures associated with trauma, tumors, and other pathologies in the United States, has boomed to 5 billion dollars.2 Though autologous grafts are considered the gold standard to restore bone defects, there are many inevitable disadvantages, including limited resources, additional injury, potential complications at donor sites, etc. Therefore, biomaterial-based bone regeneration has been revolving dramatically in the past several decades, which developed various material systems for different clinical scenarios.3–5
For over half a century, titanium and its alloys have been used as the mainstream implant materials, attributed to its appropriate mechanical properties, high corrosion resistance, and favorable biocompatibility (Table 1). They can serve as prosthesis for total joint replacements, temporary internal fixation devices for bone fractures, or implants to restore segmental load-bearing bone defects and lost teeth.6 As the ability of implants to integrate with surrounding bone tissues (i.e., osseointegration) is crucial for the therapy, researchers have developed various strategies to improve the bioactivity of titanium surface through topographical, chemical, and/or biochemical modification.7 Our previous work employed multiple strategies, including elemental doping, topographical modification, surface coating, etc., to enhance the osseointegration of titanium implants in normal or pathological conditions.8–14 In particular, we conducted successive studies to evaluate and improve the osteogenic activity of 3D-printed Ti6Al4V surfaces via topographical modification.15,16 This series of studies verified that the hierarchical surface which was composed of inherently 3D-printed microscale structures, sub-micropits and nanotubes, could significantly enhance the osseointegration of implants.17 Though tremendous efforts have been made, it is far from rare to encounter failed titanium implantation in clinical practice due to insufficient osseointegration, which demands secondary surgical intervention, thus causing considerable pain and financial burdens to patients.18–20
| Tensile strength (MPa) | Yield strength (MPa) | Elastic modulus (GPa) | Young's modulus (GPa) | Wear resistance | Corrosion resistance | Biocompatibility | Bone-bonding ability | |
|---|---|---|---|---|---|---|---|---|
| Titanium and alloys | 690–110 | 585–1060 | 55–110 | 110 (Ti6Al4V) | Low | Higher than stainless steel and cobalt based alloys | Better than stainless steel and cobalt based alloys | Able to bond with bone without fibrous encapsulation |
In most cases, the failure could be ascribed to the incompatibility between implants and the specific microenvironment of bone defects, especially in pathological conditions, such as bacterial infection, chronic inflammation, tumor, diabetes mellitus, etc. Therefore, titanium implants with functionalized surfaces for repairing bone defects in pathological microenvironments are desiderated, which could, on the one hand, reverse the specific pathological microenvironment, and on the other hand, promote bone regeneration. One of the most frequently used strategies to functionalize titanium implants is loading bioactive molecules with therapeutic effects and/or pro-regenerative activity, including growth factors, pharmaceutical molecules (such as antibiotics to prevent infection and bisphosphonates for osteoporosis therapy), bioactive ions, etc. For instance, Aggarwal et al. reviewed researches on drug-loaded implants for eliminating implant-associated infection.21 As the study revealed, various drugs, including antibiotics (gentamicin, vancomycin, and tobramycin) and mental ions (Ag+, Cu2+, and Zn2+), have been used to inhibit the formation of bacterial colonies in situ by either impregnating themselves into the matrix substrate of an implant or externally forming an antibiotic layer of coating on implant surfaces. The release profiles, hemolytic behavior, antibacterial effects against different pathogens, and osteogenic activity of these versatile drugs were highlighted in this review. Although drug-loaded implants have been developed for a long time, many problems still need to be investigated and solved before their clinical applications. For example, the uncontrolled release of bioactive molecules could not maintain a long-term therapeutic effect, and more importantly, overdose accumulation of drugs will cause severe side effects on physiologically healthy cells and tissues.22
Bone regeneration and remodeling are long-term and dynamic processes that demand responsive biomaterials to spatially and temporally synchronize interactions between materials and surrounding tissues. In recent years, smart stimuli-responsive (SSR) biomaterials have received increasing attention from researchers in various fields.123–125 Other than traditional routines, stimuli-responsive biomaterials can detect externally applied stimuli or internal microenvironment changes and respond to them by changing the physical or chemical structures of materials, or catalyzing biochemical reactions, thus provoking biological effects on cells and tissues.126,127 Stimuli-triggered therapeutic effects of SSR materials could be activated externally by photostimulation (including visible light, ultraviolet (UV) light, and near-infrared (NIR) light),128,129 magnetic field (MF),130,131 electrical stimulation (ES),132,133 ultrasound (US),134,135 and so on. Otherwise, stimulation clues could exist inherently in the internal microenvironment. For examples, decreased pH values,136–138 excessive production of reactive oxygen species (ROS),28,139–141 and specific enzymes in infectious, inflammatory, tumorous, diabetic, and other pathological microenvironments,121,142 could be detected as stimulating factors to provoke transformation of SSR biomaterials. The on-demand provoking characteristic of SSR biomaterials could maximize the therapeutic efficiency and minimize side effects compared with traditional material designs.22,143
Progress on the design and construction of SSR biomaterials with both therapeutic and regenerative functions in recent years has been achieved rapidly and was reviewed in our previous work.144 Compared with polymers and other inorganic materials, the design and construction of non-degradable load-bearing SSR titanium implants are quite distinct. Herein, a specialized review on the progress of stimuli-responsive strategies conducted to functionalize titanium-based implants are presented (representative studies in recent years were displayed in Fig. 1.) This review summarizes the material design and fabrication, the responsive mechanisms of implants, in vitro and in vivo evaluations for versatile clinical applications (Table 2). In addition, currently existing limitations and challenges and further prospective directions of these strategies are also discussed.
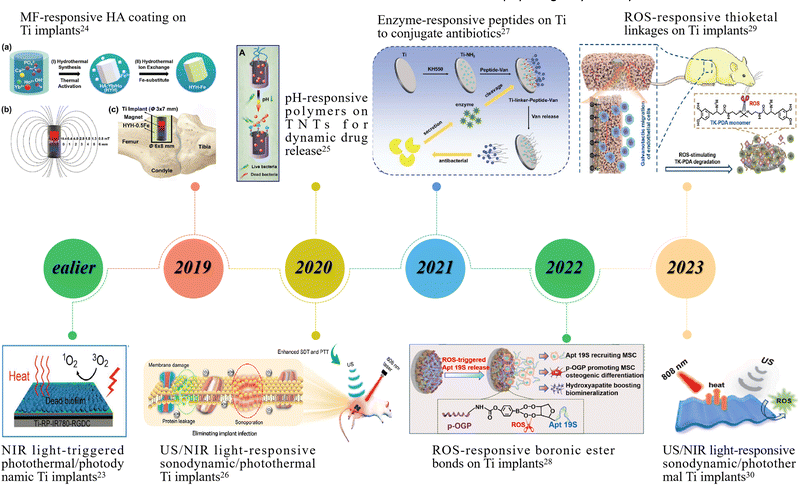 | ||
| Fig. 1 Representative studies on smart stimuli-responsive strategies applied for titanium implant functionalization in recent years. Image for ealier: reproduced with permission.23 Copyright 2018, Wiley-VCH; Image for 2019: reproduced with permission.24 Copyright 2019, Wiley-VCH; Image for 2020 (upper): reproduced with permission.25 Copyright 2020, Ivyspring INT; Image for 2020 (lower): reproduced with permission.26 Copyright 2020, American Chemical Society; Image for 2021: reproduced with permission.27 Copyright 2021, Wiley-VCH; Image for 2022: reproduced with permission.28 Copyright 2022, Elsevier; Image for 2023 (upper): reproduced with permission.29 Copyright 2023, American Chemical Society; Image for 2023 (lower): reproduced with permission.30 Copyright 2023, American Chemical Society. | ||
| Stimuli | Responsive materials or methods | Responsive effects | Biological applications |
|---|---|---|---|
| Photostimulation | (1) Chemical modification or elemental doping of nano-TiO226,32–44 | (1) Photocatalysis producing ROS (PDT) | (1) PDT and/or PTT for antibacterial or anti-tumor applications23,26,30,39–44,46–62,66,69 |
| (2) Incorporating photosensitive molecules (PDA, RP, IR780 and etc.)23,30,39,45–66 | (2) Photo-triggered hyperthermia (PTT) | (2) Photocatalysis-mediated controlled release36,37 | |
| (3) Fabricating heterojunctions67–69 | (3) Heterojunctions induced photoelectric effects | (3) Photoelectric and photothermal effects promoting osteogenesis57,67,68 | |
| Magnetic field (MF) | (1) Fe-doped HA coating or TiO224,70,71 | (1) in situ SMF | (1) SMF itself or SMF-induced mechanical stimulus promoting osteogenesis24,70,71,73–75 |
| (2) Fe3O4 NPs72–75 | (2) Mechanical stimulus generated by magnetic NPs | (2) Motions of magnetic NPs controlling drug release72 | |
| Electrical stimulation (ES) | (1) Chemical modification or elemental doping of TNTs76–79 | Direct effects of material-conducted external ES or material-generated internal ES | (1) ES-triggered drug release78,84,85 |
| (2) ES regulating cell functions and promoting osteogenesis76,77,80–83,86,87 | |||
| (2) Incorporating other electroactive or electroconductive materials (PPy, PTFE and BFO)80–87 | (3) ES-induced antibacterial effects79,86 | ||
| Ultrasound (US) | (1) Physical modification or elemental doping of TiO226,88,89 | (1) US-induced mechanical stimulus triggering piezoelectricity or surface hydrophilicity change | (1) US-induced ROS or hyperthermia preventing bacterial infection26,30,88,91,95,96 |
| (2) Incorporating other sonosensitizers (BaTiO3 and ICG)30,90–96 | (2) US-triggered ROS production (SDT) | (2) US-altered surface hydrophilicity controlling drug release89 | |
| (3) Fabricating heterojunctions (Ti–RP/BP)88 | (3) Heterojunctions induced hyperthermia | (3) US-triggered piezoelectricity promoting osteogenesis90,92–94 | |
| pH | Incorporating pH-sensitive materials: | (1) Collapsing or decomposition of coatings | (1) Triggered antibacterial or anti-tumor drug release25,97,98,100–103,107–113 |
| (1) Natural biomolecules (chitosan and silk fibroin)97–103 | (2) Conformation change of molecules | (2) Surface potential changes promoting cell adhesion and osteogenesis104–106 | |
| (2) Synthetic polymers (PMAA, DMMA and etc.)25,104,105 | (3) Cleavage of bonds/linkers | ||
| (3) Inorganic molecules (MOFs, ZnO and etc.)106–108 | |||
| (4) pH-sensitive bonds/linkers101,108–113 | |||
| ROS | Fabricating ROS-responsive bond-containing coatings: | Cleavage of chemical bonds | Triggered release of linked molecules to promote osteogenesis in osteoporotic microenvironment28,114,116 or orchestrate immunomodulation115 |
| (1) Boronic ester bonds28,114,115 | |||
| (2) 2,2′- [propane-2,2-diylbis (thio)] diacetic acid116 | |||
| Enzymes | Corresponding substrates of specific enzymes: | Enzyme-catalyzed decomposition of substrates | Enzyme-triggered release of linked molecules to prevent bacterial infection and/or promote osteogenesis27,66,117–122 |
| (1) Hyaluronic acid and its derivatives (hyaluronidase)66,117–120 | |||
| (2) MMP9-cleavable peptide (MMP-9)121 | |||
| (3) Poly-L-glutamic acid (Glutamyl endonuclease)122 | |||
| (4) A tailor-made peptide (S. aureus-specific SplB)27 |
2. External stimuli-responsive strategies
Light, magnetic field, electrical stimulation and ultrasound can be on-demand applied and controlled externally to deliver therapeutic and/or pro-regenerative signals to tissues and cells. Many studies construct SSR implants by additively fabricating a layer of responsive coating/film, or grafting responsive agents directly on implant surfaces, which is similar in nature to other SSR biomaterial systems. Particularly, the oxide layer formed on titanium surfaces in situ can be utilized and modified as the responsive center to construct external stimuli-responsive implants, which is an unique characteristic of titanium implants. In this section, we will introduce various external stimuli-responsive strategies for titanium implant functionalization.2.1. Photostimulation-responsive strategies
Photostimulation-responsive strategies are generally based on the photocatalytic activity and photothermal conversion property of photosensitive materials, which give birth to photodynamic therapy (PDT) and photothermal therapy (PTT), respectively. Nanostructured titanium dioxide (TiO2) is photosensitive, and its excellent photocatalytic activity has been widely used for air purification by degrading organic pollutants and sterilization.145 It is also reported that TiO2 and other titanium oxides possess photothermal conversion activity under illumination.146,147 However, the wide band gap of TiO2 restricts its excitation spectrum within UV range, which is harmful to healthy tissues, thus hindering its application for PDT and PTT in biomedical fields. To construct photostimulation-responsive titanium implants, considerable efforts have been made on chemical modification or elemental doping of TiO2, or alternatively, incorporating other photosensitive molecules via various fabricating methods.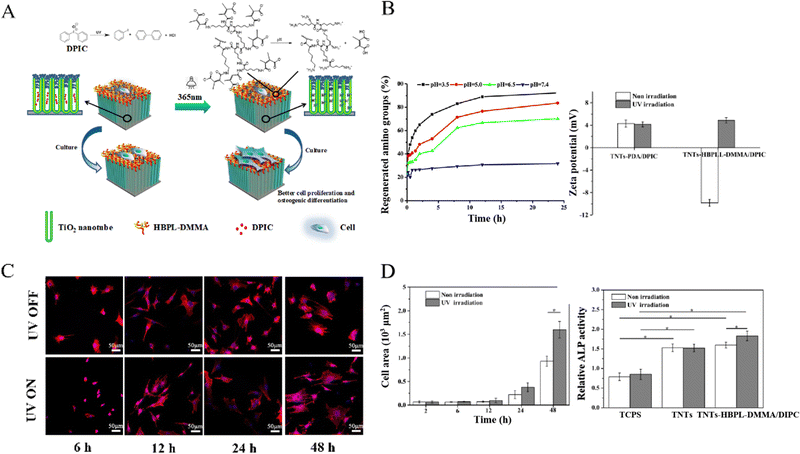 | ||
| Fig. 2 UV-triggered titanium surface charge reversal enhanced cell differentiation of BMSCs. (A) Schematic of the TNTs-HBPLL-DMMA/DPIC substrate showing cascade responses and mediation on cell adhesion and differentiation. (B) Variation of relative content of amino groups in different pH buffers (left) and surface zeta potential changes before and after UV irradiation (right). (C) Fluorescent images of cells on TNTs-HBPLL-DMMA/DPIC surfaces before and after UV irradiation for 30 s at different timepoints. (D) Cell spreading area and ALP activity of BMSCs before and after UV irradiation (*p ≤ 0.05). Reproduced with permission.104 Copyright 2019, American Chemical Society. | ||
Another visible light-responsive strategy of TiO2 is based on the photoelectric effect of heterojunctions formed at the interface of graphene and TiO2.67,68 For example, accumulated positive charges on the fabricated reduced graphene oxide (rGO)/TiO2 film under visible light irradiation, could induce a positive surface potential change, which promoted the osteogenic differentiation of BMSCs while maintaining good cell adhesion and proliferation.67 The pro-osteogenic effect of rGO/TiO2 film was presumed to mainly attributed to the activation of voltage-gated calcium channels and improved adsorption of osteogenic factors on implant surfaces. However, the tissue penetration depth of visible light is limited, which hinders the biomedical application of visible light-responsive strategies, especially for deep-sited tissue treatments.
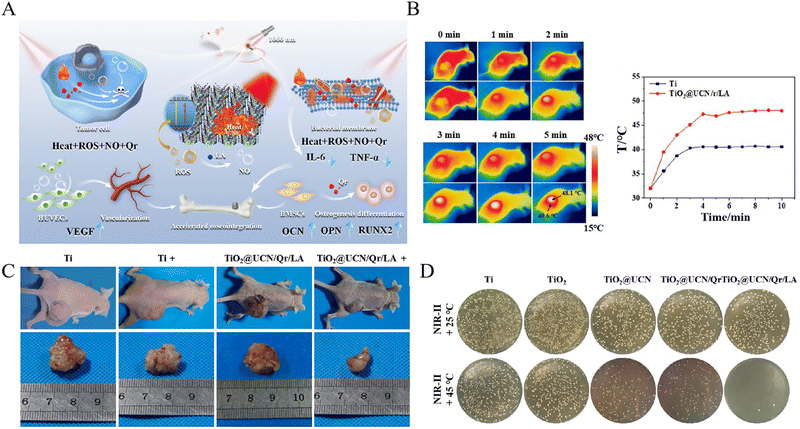 | ||
| Fig. 3 A NIR light-triggered multifunctional coating on titanium implants for repairing osteosarcoma-related bone defects. (A) Schematic of the multifunctional TiO2@UCN/Qr/LA coating to eliminate biofilms, inhibit bone tumor growth, and promote angiogenesis and osteogenesis. (B) The photothermal effect of the TiO2@UCN/Qr/LA coating. (C) In vivo therapy of osteosarcoma reflected by reduced tumor size. (D) In vitro anti-biofilm efficiency against S. aureus. Reproduced with permission.41 Copyright 2022, Elsevier. | ||
PDA is a promising photothermal material due to its good biocompatibility, high photothermal conversion efficiency, and more importantly, its active groups (–OH, –NH2) could provide plenty of covalent binding sites for grafting functional molecules.155 Therefore, PDA can be solely applied as a photosensitive agent for photothermal modification of titanium implants,56,62 or serves as a grafting medium for other functional molecules while providing photothermal activity simultaneously.23,46,58,65 Zeng et al.156 designed a novel biocompatible coating using PDA as a binder to immobilize IR820 and Daptomycin (DAP), to functionalize titanium implants with antibiotic/photodynamic/photothermal triple therapeutic properties. In this coating, PDA not only served as a medium for binding IR820 and DAP, but also possessed photothermal and pro-osteogenic properties. This study suggested the PDA-IR820-DAP coating reached an anti-bacterial efficiency of over 97% in vitro and in vivo, ascribed to the release of DAP and the combination of hyperthermia generated by PDA and singlet oxygen (1O2) produced by IR820 under 808 nm NIR light irradiation (Fig. 4). Yuan et al.61 proposed an interfacial NIR light-activable functionalization strategy for titanium surface by integrating mesoporous PDA (MPDA) nanoparticles, which were loaded with carbonic oxide (CO) nanogenerators (Fe3(CO)12) (CO@MPDA). The hyperthermia generated by MPDA under NIR light irradiation triggered the on-demand release of CO gas, which in turn potentiated mild PTT, thus promoting bacteria elimination. Moreover, the on-demand delivery of CO could alleviate the infection-induced inflammatory response by driving the polarization of macrophages toward anti-inflammation M2-phenotype and inhibiting the secretion of pro-inflammatory cytokines, thereby constructing a pro-regenerative microenvironment. This study also suggested that the anti-inflammation and M2-type macrophage polarization effects of NIR light-triggered CO release was achieved by participating in the activation of HO-1 antioxidant system. The eliminated residual bacteria, alleviated accompanying inflammation, and promoted bone healing of this phototherapeutic strategy were verified in an induced implant-associated infection model. Similarly, MPDA has been employed as the vehicle in previous studies for loading multiple functional molecules (such as s-nitrosoglutathione as a NO donor, ICG as a photosensitizer for PDT, and luteolin as a quorum sensing inhibitor), displaying multiple therapeutic functions along with photothermal activity.48,49,54,66
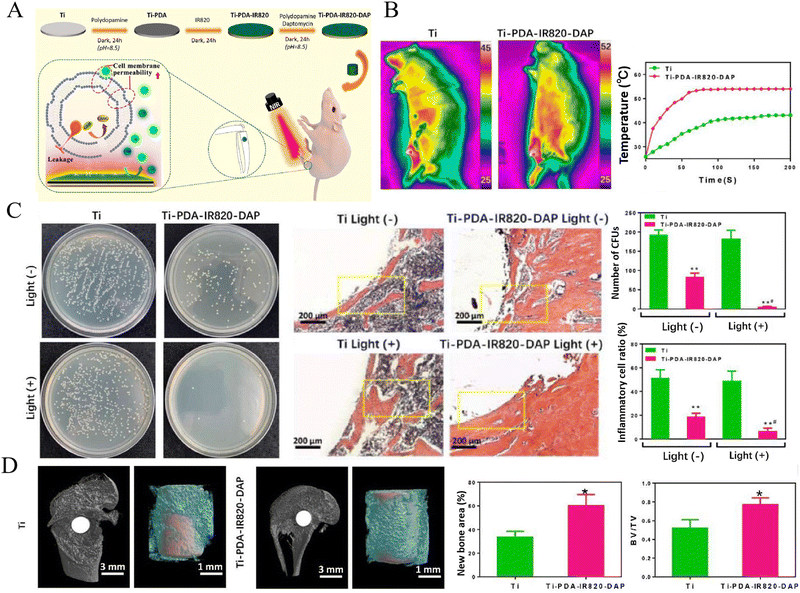 | ||
| Fig. 4 A novel NIR light-triggered antibiotic/photodynamic/photothermal coating for eliminating S. aureus biofilm. (A) Schematic of the Ti-PDA-IR820-DAP coating and its anti-biofilm mechanisms under NIR light irradiation. (B) The real-time temperature changes of the Ti-PDA-IR820-DAP coating in vivo. (C) In vivo antibacterial efficiency and H&E staining of inflammatory cells infiltrated into peri-implant tissue (** Compared with the Ti group, p < 0.01; # Compared with the Ti-PDA-IR820-DAP light (−) group, p < 0.05). D In vivo bone regeneration and osseointegration reflected by micro-CT (* Compared with the Ti group, p < 0.05). Reproduced with permission156 Copyright 2020, Elsevier. | ||
Red phosphorous (RP) has been used as a promising photothermal reagent since it was for the first time reported by Tan et al.23 In their studies, RP film was deposited on titanium implants by chemical vapor deposition (CVD), serving as an antibacterial coating with the synergic PDT of IR780.52 Subsequently, the authors presented another RP-based NIR light-triggered implant design, in which a hydrogel containing NO release donor (RSNO) was formed on the RP film deposited on titanium surfaces.47 Under NIR light irradiation, peroxynitrite (˙ONOO−) was generated by the released NO to eradicate the Methicillin-resistant S. aureus (MRSA) biofilm with the synergy of RP induced hyperthermia and ˙O2−. In addition, RP could be conducted to form P–N heterojunction with calcium titanate (CTO), which enhanced the photocatalytic and photothermal properties of titanium implants under 808 nm NIR light irradiation, as reported by Mao et al.69 The in vivo antibacterial efficacy against MRSA of Ti-CTO/RP implants reached 99.42% ± 0.22%, while maintaining superior biocompatibility and osteoconductivity.
Though various photocatalytic and photothermal molecules have been reported to effectively improve the PDT and PTT effects of titanium implants, the potential toxicity of these materials remained worrying. Many studies addressed this problem by immobilizing biocompatible peptides (such as RGD) on titanium implants,35,46,52,58,61 but the long-term stability and safety still need further validation. Additionally, photostimulation-responsive implants in most of the published studies were constructed to achieve PDT or PTT for bacterial infection or bone tumor, the excessive ROS and hyperthermia may have severe side effects on healthy tissues and cells, which is a dilemma for this strategy.
2.2. Magnetic field (MF) -responsive strategies
MF is another highly promising noninvasive external trigger to design SSR biomaterials, ascribed to its high tissue penetration capacity, mild side effects and high controllability. First of all, external static magnetic field (SMF) can directly exert biological effects on cells and have been proposed to enhance the osseointegration of implants by a number of studies.157,158 For example, Leesungbok et al.159 evaluated the effect of SMF on the osseointegration of SLA implants and found that a neodymium magnet could promote early bone formation. The biological effect of MF has also been utilized to functionalize titanium implants. Li et al.70,71 reported Fe-doped hydroxyapatite or TiO2 coatings on titanium surfaces fabricated by microarc oxidation (MAO), to improve the in vitro and in vivo osteogenic performance. The promoted osteogenesis of osteoblasts was presumed to be associated with MF-induced ion channel on/off switching, cytoskeleton remodeling, cell membrane potential changing and membrane bending, and etc. MF-responsive biomaterial system is usually based on magnetic nanoparticles (MNPs), principally Fe3O4 NPs, which has been extensively studied as a therapeutic reagent for tumor therapy utilizing their magnetic hyperthermia characteristics. Initially, MNPs were used to design drug delivery and on-demand release system on TNT-modified titanium surfaces reported by Aw et al.72 In this study, the authors designed a MF-triggered drug delivery titanium surface by sequentially loading DOPA-Fe3O4 NPs and amphiphilic polymer micelles as drug carriers into TNTs. Under SMF, MNPs at the bottom of TNTs were attracted and then mobilized micelles, enabling them to diffuse out of TNTs. In another study, TNTs loaded with Fe3O4 NPs were coated by chitosan, which further delayed drug release.160Recently, the mobilization characteristic of MNPs under MF was employed to improve the pro-osteogenic activity of titanium surfaces, which could provide mechanical stimulus for osteoblastic cells and thus affect cell behaviors. Zhuang et al.73 designed a MF-responsive coating by electrochemically depositing Fe3O4/mineralized collagen (MC) on titanium surfaces, which could generate mechanical stimulus on osteoblasts by Fe3O4-induced coating deformation when exposing to SMF. This study suggested that the osteogenic differentiation of osteoblasts was enhanced on this MF-responsive coating, which could originate from magnetically actuated mechanotransduction signaling pathways. Subsequently, the authors further studied the mode and intensity of the MF-triggered mechanical stimulus acting on MSCs, and demonstrated that the periodic mechanical stimulus (12 h per day) displayed significantly enhanced osteogenic differentiation of MSCs compared with static mode.74 In another study, the Fe3O4/PDA coating was fabricated on 3D-printed porous titanium scaffolds by a one-step method, and the synergistic effect of MNPs and SMF on promoting osteogenesis of BMSCs was verified. The pro-osteogenic mechanism of MF-actuated and MNPs-induced mechanical stimulus might be the complicated crosstalk among TGFβ, BMP, and integrin-mediated mechanotransduction signaling pathways.75
Apart from Fe3O4 nanoparticles, a new type of superparamagnetic Fe-doped hydroxyapatite (named as HYH-Fe) was fabricated via a two-step doping method, along with the doping of lanthanide (Ln) ions (Yb and Ho) which endowed hydroxyapatite (HA) particles with in vivo fluorescence and micro-CT tracking property (Fig. 5).24 In this study, HYH-Fe was coated on titanium surfaces with a dismountable NdFeB magnetic rod embedded in the implant. The three-month in vivo experiments revealed synergistic pro-osteogenic effects of SMF and superparamagnetic HYH-Fe particles as reflected by improved new bone formation and enhanced push-out/shear strength, and effective long-term in vivo tracking properties of decomposed Yb/Ho-doped HA. The synergistic effects on promoting bone formation could attributed to the mechanical stimulation exerted by HYH-Fe particles under SMF, which have been demonstrated important and beneficial for maintaining the mass and normal functioning of natural bone, as well as the regeneration and reconstruction of bone defects.161
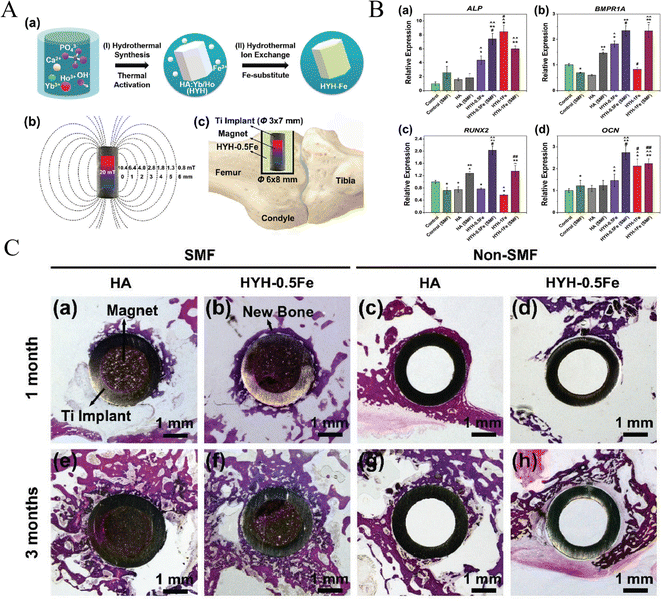 | ||
| Fig. 5 A new superparamagnetic Fe-doped hydroxyapatite (HYH-Fe) on titanium surface promoted osteogenesis/osteointegration surrounding implants under SMF. (A) Schematic of the two-step doping process for fabricating HYH-Fe coating (a), the generation of SMF (b) and the in vivo surgical procedure (c). (B) Osteogenic genes expression of osteoblasts (* VS Control, ^ VS HA, # VS HYH-0.5Fe, ∼ VS HYH-1Fe for p < 0.05; ** VS Control (SMF), ^^ VS HA (SMF), ## VS HYH-0.5Fe (SMF) for p < 0.05). (C) In vivo bone regeneration and osteointegration. Reproduced with permission.24 Copyright 2019, Wiley-VCH. | ||
The MF-responsive strategy is promising to functionalize titanium implants, but still needs further developments. One major obstacle should be of great concern is the biosafety of incorporated magnetic materials (such as Fe3O4 NPs), which could be dose-/time-dependent toxic in vivo. Thus, the adsorption, metabolism and interaction of magnetic materials with cells and tissues need systematically studied. In addition, the application mode and parameters of MF for improving the biological performance of implants are expected to be optimized.
2.3. Electrical stimulation (ES)-responsive strategies
There have been a number of studies focusing on utilizing electrical field to control stem cell fate.162 ES can affect cellular activities and have been demonstrated to maintain BMSCs stemness, promote cell proliferation and differentiation, consequently accelerating tissue regeneration.87,163 Recently, Pettersen et al. reviewed the literature that attempted to enhance the osseointegration of titanium implants by using them as electrodes.133 Although there were nonuniform experiment protocols and inconsistent ES parameters applied in different reports, most studies implied beneficial effects of ES on promoting osseointegration. This review addressed that osteoblasts and bone tissues were sensitive to electric field magnitude, the duration and frequency of stimulation, and the protocol used to deliver ES. It was also revealed that further researches on the optimization of ES parameters and reporting standards should be done before the clinical translation of this strategy.133Many efforts have been made to improve the electroconductivity of titanium implants.77,78 For example, Mazare et al.76 reported a kind of ‘black’ TiO2 nanotube arrays with enhanced electrical conductivity, which was produced by annealing in Ar/H2 (10% H2) at 550 °C. The ‘black’ TiO2 nanotube arrays did not alter the adhesion and focal contact formation of MSCs, while with the engagement of electric field by using the implant as an electrode, faster cell growth and elevated intracellular calcium levels of MSCs were found. In another study, for antibacterial purposes, carbon-doped TNTs (TNT-C) were fabricated to render the implant with capacitive characteristic after charging by an external electrical current.79 This capacitance-based platform could deform the morphology of bacteria and increase their intracellular ROS levels, consequently killing bacteria while maintaining osteoblast growth.
In addition to utilizing the titanium substrate itself, various electroactive or electroconductive materials, particularly conductive polymers, have been applied to design ES-responsive titanium implants.81,83 Among these materials, polypyrrole (PPy) were most frequently reported, ascribed to its electrical conductivity and redox activity under ES. Nanostructured PPy incorporated with anionic drugs, such as penicillin/streptomycin (P/S) or dexamethasone (Dex), were coated on titanium surfaces, serving as ES-triggered on-demand drug delivery platform.84,85 Xie et al. fabricated ES-responsive polydopamine-polypyrrole microcapsules (PDA-PPy-MCs) which possessed the cell affinity of PDA, electrical conductivity and redox characteristics of PPy, and the microstructure of MCs, and electrochemically deposited them on 3D-printed porous titanium scaffolds. The applied external ES could trigger on-demand release of Dex loaded within PDA-PPy-MCs, and on the other hand, directly modulate cellular behaviors synergistically with the biological effects of PDA and the porous microstructure of MCs.82 In particular, conductive polymers can scavenge ROS by providing electrons, but the lack of cell affinity and osteoinductivity hindered their application in tissue engineering. To cope with this issue, an electroactive, cell affinitive, persistent ROS-scavenging, and osteoinductive PPy-PDA-HA film was in situ coated on porous titanium scaffolds via an layer-by-layer pulse electrodeposition (LBL-PED) method.80 In this design, PDA NPs were entangled with and doped into PPy to enhance its cell affinity and ROS-scavenging efficiency, which was verified in this study. Additionally, the osteogenic differentiation of BMSCs was also promoted by HA's osteoinductivity and PPy-mediated ES synergistically, as reflected by a high-throughput stimulation experiment.
Naturally existed endogenous electric field plays an important role in tissue development and regeneration. At bone defect sites, endogenous electronegative potentials appear immediately after injury and directly affect cell proliferation and differentiation, thereby mediating bone repair.164 Inspired by this innate feature of bone defects, a built-in electric field was designed to improve the biological healing of implants.87 This study used epitaxial growth technique to organize the crystal panel of BiFeO3 (BFO) at the atomic scale, forming an BFO nanofilm on SrTiO3 (STO) implant surfaces. A built-in electric field was established between the electropositive BFO nanofilms (after polarization) and electronegative bone defect walls, which was proved to facilitate implant osseointegration in the rat femur. Custom-designed PCR array test revealed the sequentially initiated calicium signaling, cell adhesion and spreading, and PI3K-AKT signaling in MSCs might contribute to the enhanced in vivo bone formation.87 Another study utilizing ES to alter the surface potential of implants was reported by Shi et al.,86 which endowed titanium implants with anti-biofilm and pro-osteogenic activity. In this study, the authors designed a self-powered triboelectric nanogenerator (TENG) based on vertical contact separation between the polytetrafluoroethylene (PTFE) film, which was etched with nanorods, and the TNT surface. The TENG could harvest and convert mechanical energy produced by daily body motions to ES, and thus building a negatively charged implant surface, which displayed effective antibacterial and pro-osteogenic activity without adverse side effects (Fig. 6).86
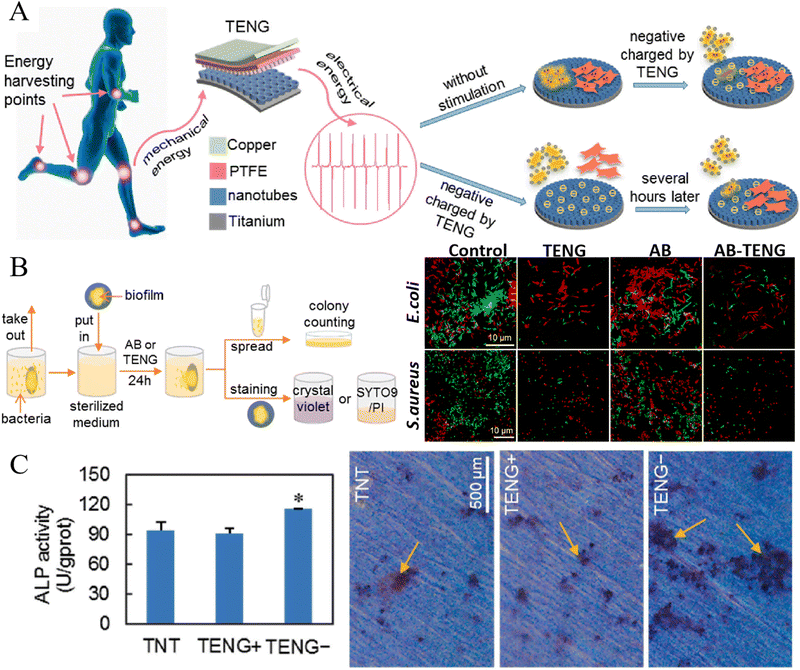 | ||
| Fig. 6 Electrical stimulation produced by a self-powered triboelectric nanogenerator (TENG) improved the anti-biofilm and osteogenic activity of titanium surface. (A) Schematic of action and mechanism of TENG in the construction of antimicrobial and osteogenesis promoting surfaces. (B) Experimental processes of antimicrobial assays (left) and fluorescent LIVE/DEAD staining of bacteria (right) (AB: using antibiotics as a control group). (C) Osteogenic differentiation of osteoblasts reflected by ALP activity (left) and alizarin red staining (right) (*p < 0.05). Reproduced with permission.86 Copyright 2020, Elsevier. | ||
ES-responsive implants fabricated with electroconductive materials actually serve as electrodes, which deliver ES to cells from external electrical devices, making it difficult to implement in vitro and in vivo evaluations. The important role of endogenous ES in tissue development and regeneration has been revealed, and inspired by this phenomenon, some studies designed built-in devices to provide noninvasive wireless electrical signals to regulate cell functions and promote tissue regeneration.86,87 Moreover, the piezoelectric effect of bone has also been adopted as an intriguing and promising approach to design new SSR systems for bone tissue engineering. A number of piezoelectric materials have been developed to construct bone grafts, which could generate electrical signals under mechanical triggers to direct cell activities.165,166 Since most published studies on piezoelectric implants utilized ultrasound as the external stimuli, relevant contents will be discussed in the next section.
2.4. Ultrasound (US)-responsive strategies
US has been extensively utilized in biomedical fields due to its non-ionizing nature and deep tissue penetration ability. In addition to its application in medical imaging, ultrasonic therapy has attracted increasingly significant attention in recent years. Sonodynamic therapy (SDT) can generate ROS under the cavitation effect triggered by US, and has displayed promising prospects for treating deep-sited tumors or infection.167,168 Dental alveolar bone is an important and special part of the skeletal system, which contains teeth and periodontium. Titanium implants are widely used to restore lost teeth and plenty of studies focusing on the surface modification of titanium have been conducted to improve the osseointegration of dental implants.169–171 As for SSR strategies applied to dental implants, Pourhajibagher et al.91 reported an ex vivo study on the application of SDT, simultaneously with PDT, to inhibit polymicrobial periopathogenic biofilms on titanium dental implant surfaces. In this study, indocyanine green (ICG) was employed as the photostimulation-sonosensitizer, which displayed synergistic anti-biofilm effect under NIR light and US stimulation. However, the PDT and SDT activity in the study were not verified via detecting ROS production, and ulterior in vitro and in vivo studies were absent. Considering the special biological characteristics of alveolar bone and peripheral soft tissues (gingiva and alveolar mucosa), as well as distinct pathogens in oral environment, the development of SSR dental implants are supposed to be differentiated and further efforts are desiderated.Besides incorporating additional sonosensitizers, TiO2 can be employed to render titanium implants with SDT activity, which can generate ROS via electron–hole separation triggered by US.88 Su et al.26 fabricated an oxygen-deficient sulfur (S)-dopped TiO2 (Ti–S–TiO2−x) layer by heating titanium implant in an argon and sulfur atmosphere, which could generate ˙OH and 1O2 under US stimulation and produce hyperthermia under NIR light irradiation. The in vitro and in vivo antibacterial experiments revealed a high antibacterial efficiency against S. aureus and enhanced osseointegration under the simultaneous treatments of US and NIR light. More importantly, the S-doped titanium implant displayed unchanged structures and properties after immersion in water for 6 months, suggesting long-term stable antibacterial performance under US stimulation.
Unfortunately, like PDT and chemodynamic therapy (CDT), the SDT efficiency is also limited by the hypoxia environment of deep tissues, especially in infectious microenvironment.172,173 Guan et al.96 reported a potential solution to this predicament by proposing a sonothermal therapy. In their work, red phosphorous (RP) was deposited on titanium surface by chemical vapor deposition, forming a Ti–RP interface. This heterojunction could convert the US-induced mechanical energy into heat by activating the motion of interfacial electrons, thus producing hyperthermia on implant surfaces. The surface was further coated with mesoporous silicon dioxide nanoparticles (MSNs) which could release NO responding to hyperthermia, to realize enhanced antibacterial effect on deep-sited MRSA infection.96 Moreover, US has been reported to construct non-invasive and external stimuli-triggered local drug delivery platforms based on TNT-modified titanium surfaces, though still at the preliminary stage. Initially, ultrasonic waves were directly used to provoke drug-loaded nano-carriers in TNTs, and the parameters of US stimulation (pulse length, time, amplitude and power intensity) were optimized.174 In another study, TNTs were treated with 1H,1H,2H,2H- perfluorooctyl-triethoxysilane (POTS) to obtain superhydrophobic surfaces, and US was employed to remove trapped air layers on this superhydrophobic surface, thus triggering the drug release from TNTs.89
Since the piezoelectricity of natural bone was elaborated by scholars, many studies have been exploring piezoelectric materials to promote the regeneration of bone defects.93,94 Recently, the US-induced mechanical stimulation has been utilized to activate piezoelectric coating as reported by Chen et al.90 In this study, a piezoelectric BaTiO3 coating was hydrothermally formed on the surface of MAO-treated Ti6Al4V scaffolds, which was polarized by the periodic US-induced mechanical stimulation, consequently generating an induced current of 10–17.5 μA on implant surfaces. In vitro experiments unveiled that this piezodynamic therapy promoted the mitochondria activation of BMSCs at the initial stage, and intervened cell cycle which weakened the apoptotic damage. Significantly, the synergistic effects of US and piezoelectricity of the scaffold upregulated the expression of osteogenic genes, indicating a promising method to repair bone defects.90 The in vivo enhanced osteogenesis of US-induced piezoelectric effect was demonstrated in another study, in which improved osseointegration on BaTiO3/Ti surface was observed 6 and 12 weeks after implantation in large segmental bone defects in rabbits’ radius.92 More recently, an US-driven piezoelectric surface for immunoregulation to rescue titanium implant infection was reported.95 The implant was coated in situ by piezoelectric BaTiO3 (BTO-Ti) and subsequently deposited with AuNPs (piezoTi) as cocatalysts for efficient piezocatalysis. Under US irradiation, the formed piezotronic implant promoted ROS generation and stimulated macrophages’ potent phagocytosis and antibacterial activity. With the synergistic effects of US-triggered piezodynamic therapy and immunoregulation, a high antibacterial efficiency against S. aureus and enhanced osteointegration were achieved in an osteomyelitis model (Fig. 7).
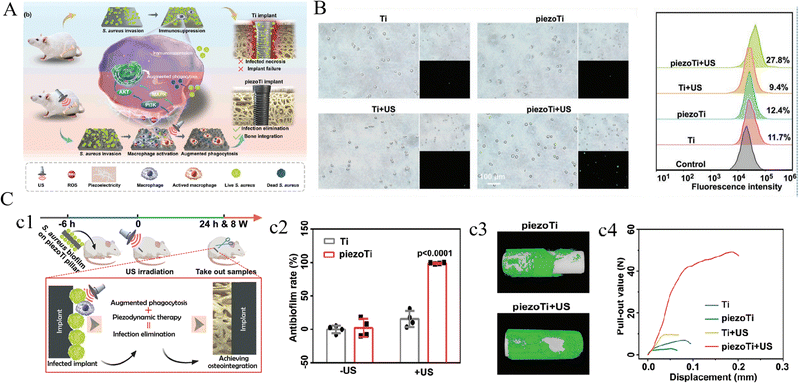 | ||
| Fig. 7 An US-driven piezodynamic surface for immunoregulation to rescue titanium implant infection. (A) Schematic illustrating the synergy of piezodynamic and immunotherapy enhanced the antibacterial performance of piezoTi implants under US irradiation. (B) Phagocytosis of US-driven macrophages against fluorescence-labeled S. aureus detected by fluorescent imaging (left) and flow cytometry (right). (C) Scheme and timeline of the animal experiments (c1), in vivo antibacterial efficiencies (c2) and osseointegration of implants reflected by micro-CT (c3) and push-out test (c4). Reproduced with permission.95 Copyright 2023, Wiley-VCH. | ||
Though US possesses the merits of deep tissue penetration ability and noninvasiveness, the biomedical application of US-triggered sonodynamic/sonothermal therapy meets a similar dilemma to PDT/PTT, i.e., excessive ROS or hyperthermia is detrimental to cells and tissues, which could cause severe side effects. The US-driven piezoelectric strategy is promising to construct US-responsive titanium implants, since BTO coatings could be fabricated in situ on titanium surface. This piezoelectric coating can achieve US-to-ES signal convert and couple the electrophysiological property of bone defects. But relevant studies are insufficient, further efforts are expected to optimize the therapy protocols.
3. Internal stimuli-responsive strategies
The occurrence and progress of pathologies are always accompanied with changes of physical and/or biochemical characteristics in the local microenvironment. These changes can be detected as internal triggers by some materials and stimulate their structural transformation, ultimately exerting biological effects on peripheral cells and tissues. The internal and external stimuli-responsive strategies have intrinsic differences: the responsiveness of external stimuli-responsive strategies is passively activated by manipulated external triggers, which can be controlled and optimized to achieve favorable biological effects; while internal stimuli-responsive strategies can proactively detect clues in microenvironment and response to them automatically, which is ‘smarter’ but also uncontrollable. In this section, we will introduce recent progress about internal stimuli-responsive implants.3.1. pH-responsive strategies
Physiological microenvironment of healthy body is mildly alkaline, but it would turn into mildly acidic in some pathological conditions, such as chronic inflammation, bacterial infection and tumor.175,176 This phenomenon caused by pathological progress has been exploited by many researchers to design pH-responsive biomaterials with therapeutic and/or regenerative functions.177,178 Accordingly, various pH-sensitive materials have been developed to construct titanium implants, including natural biomolecules (such as chitosan and silk fibroin),97–103 synthetic polymers (such as PMAA),25 inorganic molecules (such as metal–organic frameworks (MOFs) and ZnO)106–108,179 and pH-sensitive bonds/linkers (such as Schiff base bonds, metal ions coordination bonds and boronic ester bonds).108–113,180Chitosan (Chi) is a natural cationic polysaccharide and has been widely used to fabricate biomaterials for bone regeneration, ascribed to its biocompatible and osteoinductive nature.181 Specifically, protonated amino groups and enhanced dissolution of Chi in acidic microenvironment can result in accelerating drug release loaded in Chi.182 Therefore, many researches have been reported to fabricate Chi-based pH-responsive drug release systems on implant surfaces through various methods, including electrophoretic deposition and layer-by-layer (LBL) self-assembly.98–101 For example, Ning et al.102 designed a pH-responsive Chi coating on titanium surface via electrophoretic deposition, which contained mesoporous silica nanoparticles (MSNs) loaded with copper peptide glycyl-L-histidyl-L-lysine-Cu2+ (GHK-Cu). The pH-responsive release of Cu2+ was validated, and in vitro experiments in this study suggested pretty cytocompatibility, outstanding antibacterial properties against E. coli and S. aureus.
Another pH-sensitive natural biomolecule is silk fibroin (SF), which has been reported to build coatings loaded with antibacterial reagents on implant surfaces. Zhou et al.100 fabricated an AgNPs/gentamicin (Gen)-loaded SF coating on titanium surfaces with a Chi barrier layer, to render implants with self-defensive antibacterial and pro-osteogenic properties. The pH-responsive release of drugs and antibacterial effect were verified by in vitro assays. Afterward, the pH-responsive activity of SF were further studied by constructing differently structured SF coatings, which revealed that α-structured SF displayed better pH-dependent and long-lasting drug release and antibacterial activity, as well as better biocompatibility and pro-osteogenic performance.103 This study also elucidated that the responsive mechanism of SF could be associated with the transformation of molecular conformation from α-helices to β-sheets responding to decreased pH, which resulted in weakened binding force of drugs and compactness of the coating. More recently, antimicrobial peptides (AMPs) with osteogenic fragments were synthesized and grafted on the AgNP-loaded SF coatings (AgNPs@AP/SF).97 The infection-triggered pH-responsive drug release and antibacterial activity, as well as enhanced osseointegration, were validated in vitro and in vivo (Fig. 8).
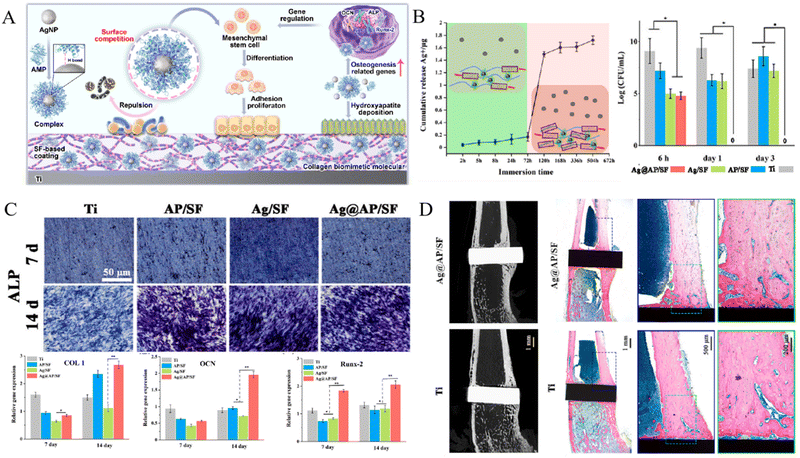 | ||
| Fig. 8 An infection-triggered AgNPs@AP/SF coating for antibacterial therapy and promoting osseointegration. (A) Scheme illustrating antibacterial and pro-osteogenic mechanisms of SF-based functional coating. (B) pH-controlled release of Ag+ from Ag@AP/SF coating (left) and killing effect against bacteria (right). (C) Osteogenic differentiation of BMSCs reflected by ALP activity (upper) and osteogenic genes expression (lower). (D) In vivo osseointegration of implants. (*p < 0.05, **p < 0.01) Reproduced with permission.97 Copyright 2023, Elsevier. | ||
Some synthetic polymers have been reported to construct pH-responsive drug release platforms on implant surfaces. Chen et al.25 turned the TNT-modified titanium surface into a Pandora's box by loading AMPs (HHC36 peptides) into nanotubes and then decorating pH-responsive poly (methacrylic acid) (PMAA) polymers at the orifice as a molecular gate (Ti-NTs-P-A). Under physiological conditions PMMA polymers swelled to seal TNTs, while they collapsed to release cargoes if pH decreased. In this study, the pH-dependent release of AMPs and anti-bacterial effect against multiple microorganisms of the Pandora's box were verified in vitro and in vivo. And this system also displayed satisfying biocompatibilities and pro-osteogenic activity. In another study, Zhang et al.104 utilized positively-charged carboxyl groups to shield cationic quaternary ammonium salts (QAs) from toxicity and constructed a pH-sensitive antibacterial copolymer coating on titanium surfaces via an LBL method. When bacterial infection occurred, the acidic environment could deionize carboxyl groups which led to invalidation of the shielding effect of positive charges, and as a result, the bactericidal activity of cationic QAs was emancipated.
In addition, MOFs have also been conducted to design pH-responsive titanium implants. Chen et al.106 reported an osteoporotic microenvironment-responsive nanocatalytic biofunctional MOF coating, which was constructed in situ by a hydrothermal method on titanium surfaces. The acidic osteoporotic microenvironment would promote the protonation of phosphate in the p-xylylenebisphosphonate (PXBP)-based bio-MOF and accelerate the release of incorporated Ce/Sr ions, which could serve as antioxidant enzymes (catalase (CAT) and superoxide dismutase (SOD)) to scavenge ROS in the osteoporotic microenvironment. The amended mitochondrial function of MSCs, enhanced osteogenesis and inhibited osteoclastogenesis under osteoporotic conditions, as well as the boosted in vivo osseointegration in ovariectomy (OVX) rats, were demonstrated in this study (Fig. 9). The application of pH-responsive MOFs for tackling bacterial infection was also presented in another study, in which osteogenic growth peptide (OGP)-loaded cobalt-metal organic frameworks (ZIF-67) were synthesized on TNTs.107 The decomposition of pH-sensitive ZIF-67 in the bacterial infection microenvironment caused the release of therapeutic Co2+ and induced a mildly alkaline microenvironment, contributing to the strong antibacterial effect against E. coli, S. aureus, S. mutans, and MRSA. Simultaneously, the release of OGP and alkaline microenvironment, as well as the consequent anti-inflammation effect, promoted the osteogenesis of MSCs and osseointegration of implants in vivo.
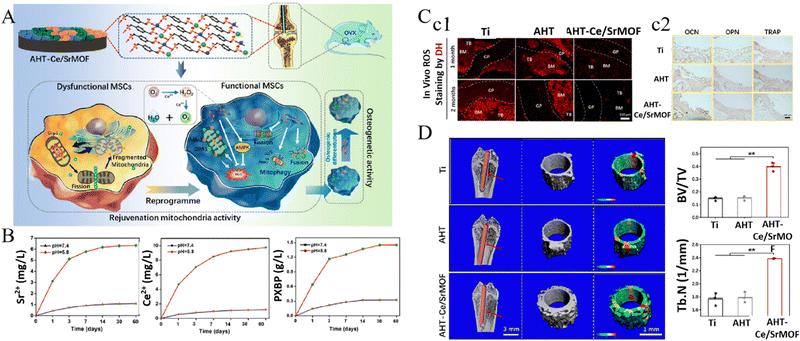 | ||
| Fig. 9 Acidic osteoporotic microenvironment-responsive nanocatalytic bio-MOF coating on titanium implants promoted bone regeneration through MSC reprogramming. (A) Schematic illustrating the fabrication of the bio-MOF coating and the mechanism of reprogramming MSCs in osteoporosis to enhance osteogenesis. (B) pH-responsive release of Sr2+, Ce2+ and PXBP. (C) In vivo scavenging of ROS (c1) and osteogenic/osteoclastic proteins expression (c2). (D) Bone regeneration around implants (*p < 0.05 and **p < 0.01). Reproduced with permission.106 Copyright 2022, American Chemical Society. | ||
Another pH-responsive strategy was to incorporate pH-sensitive bonds/linkers, such as coordination bonds formed with Cu2+, Fe3+, Zn2+ or Ag+, boronic ester bonds, and acetal linker, which disrupted in acidic environment, resulting in release of metal ions and linked drugs.109–113,180 For example, Yang et al.108 prepared pH-triggered drug delivery nanoparticles grafted on titanium surfaces for the reconstruction of large bony defects after osteosarcoma resection. ZnO nanoparticles were modified with 3-carboxyphenylboronic acid (PBA), which further linked naringin (a natural polymethoxylated flavonoid) via boronic ester bonds. The bacterial infection and osteosarcoma-induced acidic condition triggered the disruption of boronic ester and decomposition of ZnO nanoparticles, resulting in the release of Zn2+ and naringin. The functionalized titanium surface could inhibit bacteria growth, induce osteosarcoma cell apoptosis, and promote osteogenesis of osteoblasts, suggesting promising application prospects for the reconstruction of osteosarcoma-related bone defects.108
3.2. ROS-responsive strategies
ROS plays an essential role in physiological bone remodeling, but disturbed redox balance of cells by aging, trauma, inflammation and many other pathological progress results in excessive production of ROS.183 Relevant ROS molecules include superoxide anion (O2−), hydrogen peroxide (H2O2) and hydroxyl radical (˙OH) which can be generated during cell metabolism or produced when exposing to pathogens, chemicals or ultraviolet radiations, etc. Increasing evidence indicates that excessive ROS-induced oxidative stress (OS) plays an adverse role in bone homeostasis, thus ROS has become a promising therapeutic target for the treatment of bone metabolic disorders and improvement of bone regeneration in OS microenvironment.184,185A certain level of ROS is necessary for maintaining the physiological functions of cells, and it is reported that ultralow levels of ROS could hinder host defense reaction.186 Recently, ROS-responsive materials have been proposed which were used as imaging agents and intelligent on-demand drug-delivery vehicles in response to high-level ROS.140,141 One of the responsive mechanisms can be summarized as changes of physical properties of materials (especially solubility) in response to ROS. For example, the solubility of poly(propylene sulfide) (PPS), thioether-containing polymers, tellurium-/selenium-containing polymers was enhanced in ROS-rich microenvironment due to the hydrophobic-to-hydrophilic transition under oxidation, resulting in accelerated release of encapsulated compounds.187 Another responsive mechanism lies in cleavage of chemical bonds in, for example, poly(thioketal) (TK), poly(proline), phenylboronic acid and ester-containing polymers, which leads to polymer degradation and compound release.
ROS-responsive strategy has been applied to design titanium implants, though studies are limited. It is known that the ROS level in osteoporotic microenvironment is significantly higher than that in healthy tissues, which further promotes osteoclastic activity and hinders bone defect restoration.188 Chen et al.114 designed a biomimetic multilayered coating incorporated with α-melanocyte-stimulating hormone (α-MSH) by an LBL technique on titanium implant, in which ROS-responsive linkers formed by the boronic acid groups of 4-nitrophenyl 4-(4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolan-2) benzyl carbonate (NBC) and the polyhydroxy groups of dextran amine. The coating could be broken down in ROS-rich osteoporotic microenvironment and release α-MSH to promote in situ osteogenesis of MSCs and inhibit osteoclast, consequently improving bone remolding in osteoporotic rats. In another study, the authors fabricated a ROS-responsive titanium-hydroxyapatite implant to recruit MSCs in osteoporotic microenvironment.28 In this coating design, Apt 19S (a DNA aptamer) was used to identify MSCs owing to its high affinity with alkaline phosphatase protein on MSCs’ membrane and guide them to migrate to peri-implant areas. The ROS-responsive boronic ester bonds formed between the catechol of Apt 19S and the boronic acid group of NBC, which were linked onto HA coatings through phosphorylated osteogenic growth peptides (p-OGP). This study validated the improved in vitro cell migration and in vivo recruitment of MSCs, as well as promoted osteogenesis in osteoporotic environment (Fig. 10).28
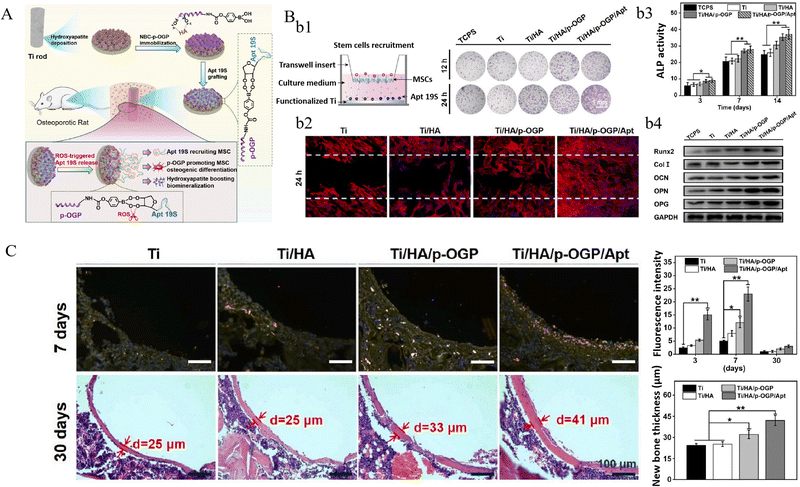 | ||
| Fig. 10 An ROS-responsive hydroxyapatite coating on titanium implant for MSC recruitment and bone formation in osteoporosis microenvironment. (A) Schematic of the hydroxyapatite coating and its ROS-responsive release of Apt 19S for MSC recruitment and promoting osteogenesis. (B) The transwell (b1) and scratching (b2) assays to evaluate MSC migration, ALP activity (b3) and osteogenic proteins expression (b4) of MSCs on different surfaces. (C) Immunofluorescence of CD29/CD90 labeled MSCs at the bio-implant interface (upper), and the new bone thickness around the implants (lower). (*p < 0.05 and **p < 0.01). Reproduced with permission.28 Copyright 2022, Elsevier. | ||
The biological effects and relative molecular mechanisms of ROS have been unveiled in the past years, especially its negative roles in many pathologies. Considerable numbers of works focusing on scavenge ROS through versatile methods have emerged, representatives of which includes enzyme-like nanomaterial-catalytic strategies and loading antioxidative pharmaceutical molecules. For example, CaCO3-Quercetin-chromium nanoparticles were synthesized and packaged in an gelatin/chitosan hydrogel coating on titanium surfaces (Ti/Gel/CaCO3-Qu-Cr), to ameliorate the ROS level and amend energy metabolism of osteoporotic MSCs.116 The hydrogel crosslinked via 2,2′-[propane-2,2-diylbis (thio)] diacetic acid could be cleaved by high-level ROS, while in turn, the consequently released quercetin in CaCO3-Qu-Cr nanoparticles could scavenge ROS. With the promotional effect of Qu-Cr combination on glucose metabolism, enhanced osteoporotic osseointegration was achieved on Ti/Gel/CaCO3-Qu-Cr implants. However, as stated above, a certain level of ROS is necessary for maintaining physiological functions of cells, and uncontrolled elimination of ROS might bring side effects with it. To avoid the potential risk, a negative feedback mechanism based on ROS-responsive strategies is advocated: scavengers would be activated in response to excessive ROS, otherwise, they turn to inactive while the amount of ROS decreased to a physiological level.
3.3. Enzyme-responsive strategies
Enzymes are natural biomacromolecules in organisms and participate in catalyzing various molecular reactions in cells and tissues, which are essential for maintaining physiological microenvironment. The occurrence and progress of many diseases may be accompanied with disordered enzyme expression or activity, and inspired by this phenomenon, some enzyme-responsive biomaterials have been designed and developed in recent years.189 For constructing enzyme-responsive titanium implants, this strategy has been mainly used to design on-demand drug release systems in response to bacterial infectious microenvironment.Pathogenic bacteria, particularly S. aureus, secrete a high level of hyaluronidase (HAase) at infectious sites, which could invade into healthy tissues and cause deep-sited bacterial infection.190 Some studies conjugated antibiotics or antimicrobial peptides, such as vancomycin, cecropin B, lauric acid and etc., with sodium hyaluronic (SH) or hyaluronic acid (HA), and then coated them on drug-containing TNTs through LBL methods.118–120 For example, Yu et al.117 fabricated HA-gentamicin (Gen) conjugates and chitosan multilayers which were coated on deferoxamine (DFO)-loaded TNTs (TNT/DFO/HA-Gen). When exposing to bacterial infectious microenvironment, HA was cleaved by the over-expressed hyaluronidase and resulted in decomposition of coatings and release of Gen and DFO, which exhibited in vitro antibacterial effects and pro-regenerative properties. In another study, ciprofloxacin (CIP)-loaded MPDA on titanium surface was covered by a layer of sodium hyaluronate-catechol (HAc) coating, and with the synergy of hyaluronidase-triggered CIP release and NIR light-triggered photothermal effect, an effective bacteriostatic efficacy was achieved without sacrificing osteogenesis (Fig. 11).66
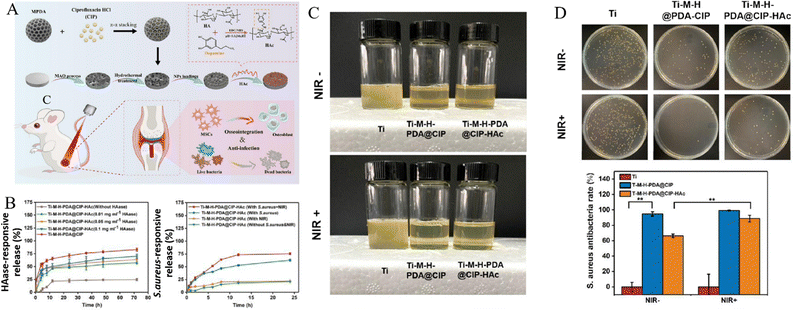 | ||
| Fig. 11 Sodium hyaluronate-catechol (HAc) coating on titanium surfaces achieved HAase-triggered antibiotics release and effective bacteriostatic effect without sacrificing osteogenesis. (A) Scheme of fabricating HAc-coated titanium implants which anchored antibiotics (CIP)-loaded MPDA (PDA@CIP). (B) Time-dependent CIP release curves responded to HAase or S. aureus. (C) Intuitive enrichment culture of S. aureus on implants retrieved from SD rats’ femurs. (D) In vivo antibacterial efficiency of implants (**p < 0.01). Reproduced with permission.66 Copyright 2023, Elsevier. | ||
Matrix metalloproteases (MMPs) are a series of zinc-dependent proteases responsible for degradation of extracellular matrix components during tissue regeneration and remodeling.191 In recent years, the MMP-responsive strategy have been applied for drug delivery and regenerative medicine.192,193 For example. MMP-9 is involved in osteoclast-mediated bone remolding and also secreted by immune cells such as neutrophils, macrophages, and B-lymphocytes. Fischer et al.121 synthesized a dynamic coating on titanium by covalently co-immobilizing an antimicrobial peptide (GL13K) and an MMP-9 cleavable peptide (MMP9-CP). In this study, the authors verified MMP9-dependent release of GL13K when cultured with osteoclasts, and proposed potential applications of the MMP9-responsive multifunctional titanium surface design by conjugating with pro-osteogenic, immunomodulatory or other functional motifs. Moreover, glutamyl endonuclease (V8 enzyme) is an exogenous product of S. aureus which is related to the virulence of S. aureus infection. Ding et al.122 developed an enzyme-responsive nanoplatform on titanium surfaces by assembling poly-L-glutamic acid (PG), the amide linkages of which can be degraded by glutamyl endonuclease. The multilayered PG films on Ag-containing mesoporous silica nanoparticles (MSNs) particles could be destroyed by the over-expressed V8 enzymes in bacterial infectious microenvironment, resulting in on-demand release of Ag+. The antibacterial effects and promoted osseointegration in bacterial infection environment were verified in this study.
A large number of studies have demonstrated the feasibility of internal-responsive strategies to functionalize titanium, especially pH-responsive implants. Other internal clues which can be exploited to design responsive systems remains to be developed. Recently, Zhou et al.194 reported a thermoresponsive antimicrobial titanium surface to enable dynamic adaptation to different stages after implantation. In this study, thermoresponsive polymers could respond to the increased temperature caused by bacterial infection and collapse to expose AMPs, otherwise the surface remained pro-osteogenic phenotype. Internal-responsive strategies detect biochemical changes in niches and spontaneously respond to them to exert therapeutic effects. This automatic responsive logic demands highly precise responsiveness of materials to ensure on-demand sufficient therapy, and long-term stability of materials in complex microenvironment to prevent undesired therapy, which needs further validation.
4. Multiple stimuli-responsive strategies
Bone tissue regeneration and reconstruction is a long-term dynamic process, while with the occurrence and development of pathological factors, the complexity increase by a large margin. Combination therapy, i.e., combined application of multiple therapies, is often conducted to cope with complicated clinical scenarios. Similarly, many studies reported multiple stimuli-responsive strategies that could simultaneously respond to multiple external or internal triggers and exert multiple therapeutic or pro-regenerative effects.195 For instance, as mentioned above, Su et al.26 presented a rapid photo-sonotherapy for the treatment of implant-related bacterial infection by sulfur (S)-doping of TiO2. More recently, another photothermal and sonodynamic implant design was reported, in which two-dimensional (2D) photo-sonosensitive black phosphorus nanosheets (BPNSs) were attached to implants via PDA coating (Ti/PDA/BP).30 The surface could induce mild hyperthermia under 808 nm NIR light irradiation and produce ˙OH stimulated by US. In this study, the Ti/PDA/BP coating displayed a high antibacterial efficiency against S. aureus with combined 10 min NIR light and 20 min US treatments, and remarkably promoted osteogenesis and osseointegration (Fig. 12).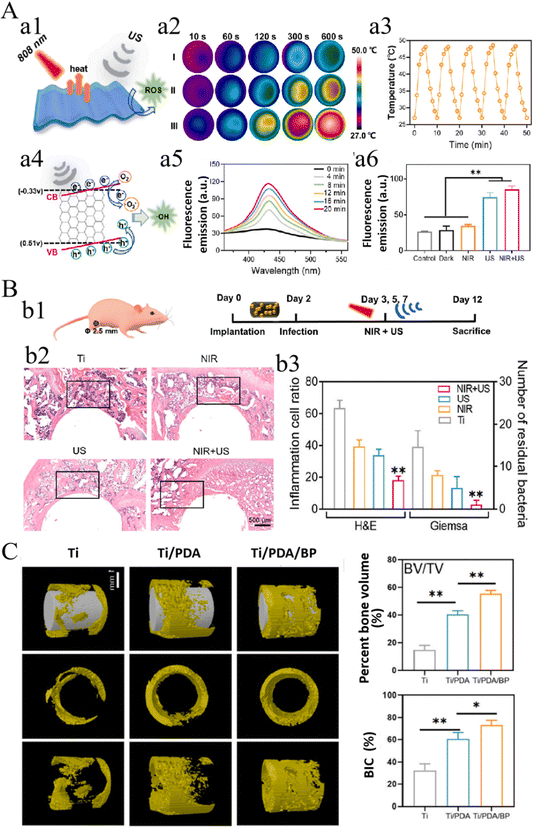 | ||
| Fig. 12 Combined photothermal and sonodynamic Ti/PDA/BP coating for efficient bacterial inhibition and bone-implant integration. (A) Schematic illustrating of the SDT/PTT of Ti/PDA/BP coating (a1), NIR light-triggered hyperthermia (a2-a3, I: Ti, II: Ti/PDA, III: Ti/PDA/BP) and US-triggered production of ˙OH (a4) detected by fluorescence intensity (a5) and DCFH-DA (a6). (B) Schematic diagram of the antibacterial animal experiment (b1), H&E staining of peri-implant tissues (b2) and quantification of residual bacteria and inflammation cells (b3). (C) In vivo bone regeneration and osseointegration of implants. (*p < 0.05, **p < 0.01). Reproduced with permission.30 Copyright 2023, Elsevier. | ||
In addition to adopting multiple external stimuli, attempts combining external signals with internal triggers to construct multiple stimuli-responsive implants have also been reported. Teng et al.45 reported a NIR light-actuated and pH-responsive implant platform by incorporating iodine immobilized metal–organic framework (ZIF-8) onto titanium surfaces for the antibacterial therapy on orthopedic implants. ZIF-8 stands out of regular MOF materials because of its favorable photocatalytic property under NIR light irradiation, and responsiveness to decreased pH which is a significant change of the bacterial infection microenvironment. In this study, the NIR light and pH-responsive release of iodine was evaluated, and the synergistic antibacterial effect of responsively released iodine and NIR light-actuated production of ROS were verified in vitro and in vivo. In addition, topographical cues on implants derived from the surface treatment procedures (MAO and hydrothermal treatment) promoted the osteogenesis of BMSCs and improved new bone formation in vivo. Besides NIR light and pH-responsiveness, ZIF-8 was reported recently to augment microwave-induced hyperthermia therapy.196 In this study, ZIF-8 nanoparticles were integrated on 3D-printed titanium scaffolds as drug carriers to eradiate osteosarcoma and promote osteogenesis via the synergy of microwave thermo-chemotherapy and immunotherapy. Another study combining pH and NIR light-responsive strategies was reported earlier, in which polydopamine-ferrocene (PDA-Fc) was used to functionalize TiO2 nanorods for implant-related bacterial infection treatment.65 The redox-active PDA-Fc could be activated in the acidic microenvironment to produce ROS, especially ˙OH, which showed over 95 and 92% antibacterial efficacy against MRSA and E. coli. Moreover, with the synergy of the hydrothermal effect of PDA under NIR light irradiation, the antibacterial capability was further improved to inhibit biofilm formation.
Multiple stimuli-responsive strategies are expected to functionalize implants through the synergy of different modalities in order to tackle complicated clinical problems. It is a misunderstanding to construct multiple stimuli-responsive platforms by simply involving various responsive materials, which would increase the cost and complexity of manufacturing processes. For the combination therapy under complicated clinical conditions, designing facile multiple stimuli-responsive implants with synergistic effects for pathological microenvironment reverse and bone regeneration is advocated. Recently, a dual pH/ROS-responsive coating simultaneously with photothermal property was constructed on titanium surface.197 This coating was fabricated by tannic acids and gallium ions, forming metal-phenolic networks, which displayed antioxidative and antibacterial effects, and ultimately promoted the osseointegration of implants. This study provided a feasible approach for developing multiple stimuli-responsive coating on titanium, but the incorporating of mental ions still remained worrying.
5. Conclusions
With this work, recent progress in the design and construction of smart stimuli-responsive titanium implants and their applications for tackling pathological microenvironment and promoting osteogenesis was summarized (Table 2). Generally, frequently-used responsive strategies have been applied to SSR implants, and endowed implants with diverse functional properties, including antibacterial infection, eliminating residual tumors, promoting osteogenesis and regulating osteoimmunology. Regarding external stimuli, photostimulation (especially NIR light) were predominantly employed to design SSR implants. In addition to incorporating various photosensitive molecules (PDA, RP, IR780 and etc.), chemical modification or elemental doping of nano-TiO2 was conducted to improve the photo-responsiveness of titanium. Photo-responsive implants could either directly exert photocatalytic/photothermal/photoelectric therapeutic effects, or enable spatiotemporally controlled decoupling of bioactive compounds under irradiation. Since externally applied magnetic fields and electrical stimulations themselves can directly regulate cells and tissues, some studies used these stimuli to promote the osteogenic or antibacterial performance of implants. Otherwise, corresponding MF or ES-responsive implants were also presented by involving magnetic nanoparticles or electroactive/electroconductive materials, which generated mechanical or electrical clues to direct cells’ functions, or enabled on-demand drug release. Similar to photostimulation, ultrasound can actuate sonosensitizers on implant surfaces to generate ROS or hyperthermia, thus directly exerting antibacterial effects. Particularly, US-induced mechanical clues can be employed to stimulate piezoelectricity, thus some studies fabricated US-responsive piezoelectric coatings on implants to promote osteogenesis. As for internal stimuli, versatile pH/ROS/enzyme-responsive materials were additively incorporated on implant surfaces to embed or graft bioactive or therapeutic molecules for diverse scenarios, among which pH-responsive strategies were most predominantly reported.However, there are still several concerns of different nature for these responsive strategies. For example, although studies on photostimulation-triggered implant functionalization have been extensively reported and achieved satisfying therapeutic results in experiments, the poor tissue penetration capacity of light hindered their application in deep-sited bone tissues, especially for large-sized segmental bone defects. Besides, the overproduction of ROS and hyperthermia of NIR light-actuated PDT and PTT could exert sever hazardous effects on adjacent tissues, which is a dilemma of these strategies. ES-responsive implants fabricated with electroconductive materials actually serve as electrodes and deliver ES from external electrical devices in an invasive approach, making it difficult to be implemented for in vivo applications. MF and US possessed the merit of excellent tissue penetration capacity and are feasible to remotely actuate the responsiveness of SSR implants in a non-invasive approach, to stimulate bioactive molecule delivery or other functions. But the therapeutic efficiency of external stimuli-responsive strategies and optimized stimulation pattern still need further investigated in vivo. Other than external stimuli-responsive strategies, internal ones can proactively detect clues in the microenvironment and response to them automatically, which is ‘smarter’ but also more difficult to control. These internal stimuli exist inherently in a healthy microenvironment, and it is crucial to manipulate the precision of responsive materials to detect subtle changes of these stimuli when pathological progress occurs, in case disturbing physiological homeostasis.
Although rapid and significant progress has been achieved, smart stimuli-responsive titanium implants are still in its preliminary stage and further developments are desired before their clinical applications. Herein, several challenges and potential future directions are proposed.
(I) Many established SSR strategies were based on incorporating other responsive materials, including synthesized polymers or small molecules, which decomposed in response to external or internal stimuli to exert functional effects. Therefore, the in vivo biosafety of these materials and their decomposition products should be further investigated. In addition, since bone tissue regeneration, particularly segmental bone defects, needs weeks or even months, not mention that bone remodeling lasts a lifetime, SSR implants' long-term stability and responsiveness are supposed to be verified, especially through the in vivo studies.
(II) The responsive effects of SSR materials are generally dependent on the pattern of triggering stimuli they received. Furthermore, compared with other clinical scenarios, the applications of external stimuli to bony tissues are quite different due to their special physical and mechanical properties. Hence, the excitation parameters of external stimuli, such as the intensity, duration, frequency and etc., need to be explored and optimized before their clinical applications. However, internal stimuli are particularly more complicated to control, since they may vary among different patients and even at different stages of disease progression.
(III) Most of reported SSR implants were constructed through complex procedures which involved multiple reagents, for the purpose of: (1) successfully incorporating responsive materials or improving their stability; (2) alleviating toxicity or strengthening therapeutic effects; (3) achieving multiple functions and etc. The complex fabricating procedures increased concerns of biosafety, batch-to-batch reproducibility and cost-effectiveness. Developing novel materials and simplified fabricating methods are encouraging.
(IV) Bacterial infection is one of the principle causes for implant failures, thus most of reported SSR implants were committed to preventing or treating bacterial infection. However, in recent years an increasing number of studies have been revealed that aseptic inflammation occurs around implants could be responsible for considerable implant failures. Besides, the basic pathological microenvironment of many bone-related metabolic diseases, such as osteoporosis, are characterized by chronic inflammation, which hampers bone tissue regeneration and remodeling. SSR implants’ potential application directions can be extended further, for example, to promote bone tissue regeneration in pathological microenvironment by regulating local inflammatory reactions.
Author contributions
Jinkai Zhang and Yu Zhuang: writing – original draft, visualization. Ruilong Sheng, Helena Tomás, João Rodrigues and Guangyin Yuan: writing-review and editing. Xudong Wang and Kaili Lin: conceptualization and supervision. João Rodrigues, Xudong Wang and Kaili Lin: funding acquisition. All authors discussed and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (32271379, 82072396, 82302667), Program of Shanghai Academic/Technology Research Leader (20XD1433100), Science and Technology Commission of Shanghai Municipality (22YF1421300, 21490711700, 21DZ2294600), the Interdisciplinary Program of Shanghai Jiao Tong University (YG2021ZD12), and CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5-037). R. S., H.T. and J. R. are grateful to FCT-Fundação para a Ciência e a Tecnologia through the CQM Base Fund - UIDB/00674/2020, and Programmatic Fund - UIDP/00674/2020, ARDITI-Agência Regional para o Desenvolvimento da Investigação Tecnologia e Inovação through funds from Região Autónoma da Madeira-Governo Regional. R. S. also thanks FCT for the FCT-individual employment grant 2021.00453. CEECIND.Notes and references
- S. Kurtz, K. Ong, E. Lau, F. Mowat and M. Halpern, J. Bone Jt. Surg., Am. Vol., 2007, 89A, 780–785 CrossRef.
- J. R. Perez, D. Kouroupis, D. J. Li, T. M. Best, L. Kaplan and D. Correa, Front. Bioeng. Biotechnol., 2018, 6, 00105 CrossRef PubMed.
- R. A. Namanloo, M. Ommani, K. Abbasi, M. Alam, A. Badkoobeh, M. Rahbar, H. K. Arasteh, E. Hajmohammadi, R. S. Soufdoost and S. A. Mosaddad, Adv. Mater. Sci. Eng., 2022, 2022, 2489399 CrossRef.
- G. Battafarano, M. Rossi, V. De Martino, F. Marampon, L. Borro, A. Secinaro and A. Del Fattore, Int. J. Mol. Sci., 2021, 22, 1128 CrossRef CAS PubMed.
- Q. Ding, J. Cui, H. Shen, C. He, X. Wang, S. G. F. Shen and K. Lin, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2021, 13, e1669 CAS.
- T. Takizawa, N. Nakayama, H. Haniu, K. Aoki, M. Okamoto, H. Nomura, M. Tanaka, A. Sobajima, K. Yoshida, T. Kamanaka, K. Ajima, A. Oishi, C. Kuroda, H. Ishida, S. Okano, S. Kobayashi, H. Kato and N. Saito, Adv. Mater., 2018, 30, 1703608 CrossRef PubMed.
- D. D. Bosshardt, V. Chappuis and D. Buser, Periodontology, 2017, 73, 22–40 CrossRef PubMed.
- H. Wang, C. Yuan, K. Lin, R. Zhu and S. Zhang, Front. Bioeng. Biotechnol., 2021, 9 Search PubMed.
- H. Wang, J. Liu, C. Wang, S. G. Shen, X. Wang and K. Lin, J. Mater. Sci. Technol., 2021, 63, 18–26 CrossRef CAS.
- H. Wang, C. Lin, X. Zhang, K. Lin, X. Wang and S. G. Shen, ACS Appl. Mater. Interfaces, 2019, 11, 7615–7625 CrossRef CAS PubMed.
- Y. Sun, Y. Li, Y. Zhang, T. Wang, K. Lin and J. Liu, Mater. Sci. Eng., C, 2021, 131, 112482 CrossRef CAS PubMed.
- L. Liu, X. Wang, Y. Zhou, M. Cai, K. Lin, B. Fang and L. Xia, J. Mater. Chem. B, 2020, 8, 2754–2767 RSC.
- T. Geng, Y. Wang, K. Lin, C. Zhang, J. Wang, Y. Liu, C. Yuan and P. Wang, Front. Bioeng. Biotechnol., 2022, 10, 1011482 CrossRef PubMed.
- J. Cui, L. Xia, K. Lin and X. Wang, J. Mater. Chem. B, 2021, 9, 9505–9513 RSC.
- J. Zhang, W. Zhou, H. Wang, K. Lin and F. Chen, J. Mater. Sci. Technol., 2019, 35, 336–343 CrossRef CAS.
- J. Zhang, J. Liu, C. Wang, F. Chen, X. Wang and K. Lin, Bioact. Mater., 2020, 5, 9–16 CrossRef PubMed.
- J. Zhang, C. Zhao, R. Sheng, K. Lin, X. Wang and S. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 30571–30581 CrossRef CAS PubMed.
- F. Lini, P. P. Poli, M. Beretta, I. Cortinovis and C. Maiorana, Int. J. Oral Maxillofac. Implants, 2019, 34, 999–1006 CrossRef PubMed.
- B. Abar, N. Kwon, N. B. Allen, T. Lau, L. G. Johnson, K. Gall and S. B. Adams, Foot Ankle Int., 2022, 30, 750–761 CrossRef.
- J. D. Oliver, J. Banuelos, A. Abu-Ghname, K. S. Vyas and B. Sharaf, Ann. Plast. Surg., 2019, 82, S289–S294 CrossRef CAS.
- D. Aggarwal, V. Kumar and S. Sharma, J. Controlled Release, 2022, 344, 113–133 CrossRef CAS PubMed.
- Y. Zeng, J. Hoque and S. Varghese, Acta Biomater., 2019, 93, 152–168 CrossRef CAS.
- L. Tan, J. Li, X. Liu, Z. Cui, X. Yang, S. Zhu, Z. Li, X. Yuan, Y. Zheng, K. W. K. Yeung, H. Pan, X. Wang and S. Wu, Adv. Mater., 2018, 30, 1801808 CrossRef PubMed.
- X. Li, Q. Zou, Y. Man and W. Li, Small, 2019, 15, 1901617 CrossRef.
- J. Chen, X. Shi, Y. Zhu, Y. Chen, M. Gao, H. Gao, L. Liu, L. Wang, C. Mao and Y. Wang, Theranostics, 2020, 10, 109–122 CrossRef CAS.
- K. Su, L. Tan, X. Liu, Z. Cui, Y. Zheng, B. Li, Y. Han, Z. Li, S. Zhu, Y. Liang, X. Feng, X. Wang and S. Wu, ACS Nano, 2020, 14, 2077–2089 CrossRef CAS PubMed.
- Y. Zhang, K. Hu, X. Xing, J. Zhang, M. R. Zhang, X. Ma, R. Shi and L. Zhang, Macromol. Biosci., 2021, 21, e2000194 CrossRef PubMed.
- M. Chen, Y. Sun, Y. Hou, Z. Luo, M. Li, Y. Wei, M. Chen, L. Tan, K. Cai and Y. Hu, Bioact. Mater., 2022, 18, 56–71 CAS.
- M. Chen, M. Li, X. Ren, F. Zhou, Y. Li, L. Tan, Z. Luo, K. Cai and Y. Hu, ACS Nano, 2023, 17, 15942–15961 CrossRef CAS PubMed.
- J. Zeng, C. Gu, X. Geng, K. Lin, Y. Xie and X. Chen, Biomaterials, 2023, 297, 122122 CrossRef CAS PubMed.
- M. Kaur and K. Singh, Mater. Sci. Eng., C, 2019, 102, 844–862 CrossRef CAS PubMed.
- V. A. Ponomarev, E. A. Orlov, N. A. Malikov, Y. V. Tarasov, A. N. Sheveyko, E. S. Permyakova, K. A. Kuptsov, I. A. Dyatlov, S. G. Ignatov, A. S. Ilnitskaya, N. A. Gloushankova, B. Subramanian and D. V. Shtansky, Appl. Surf. Sci., 2020, 516, 146068 CrossRef CAS.
- K. Suzuki, T. Yokoi, M. Iwatsu, M. Furuya, K. Yokota, T. Mokudai, H. Kanetaka and M. Kawashita, J. Australas. Ceram. Soc., 2021, 9, 1448–1456 CrossRef.
- M. M. M. Abir, Y. Otsuka, K. Ohnuma and Y. Miyashita, J. Mech. Behav. Biomed. Mater., 2022, 125, 104888 CrossRef CAS PubMed.
- G. Zhang, X. Zhang, Y. Yang, R. Chi, J. Shi, R. Hang, X. Huang, X. Yao, P. K. Chu and X. Zhang, Biomater. Sci., 2020, 8, 391–404 RSC.
- S. Oh, K.-S. Moon, J.-H. Moon, J.-M. Bae and S. Jin, J. Nanomater., 2013, 2013, 1–7 Search PubMed.
- J. Xu, X. Zhou, Z. Gao, Y.-Y. Song and P. Schmuki, Angew. Chem., Int. Ed., 2016, 55, 593–597 CrossRef CAS.
- G. Zhang, Y. Yang, J. Shi, X. Yao, W. Chen, X. Wei, X. Zhang and P. K. Chu, Biomaterials, 2021, 269, 120634 CrossRef CAS PubMed.
- Z. Wu, Q. Tian, J. Wang, Y. Feng, L. Li, C. Xu, J. Lv and Z. Lv, Colloids Surf., B, 2022, 211, 112296 CrossRef CAS PubMed.
- X. Zhang, G. Zhang, M. Chai, X. Yao, W. Chen and P. K. Chu, Bioact. Mater., 2021, 6, 12–25 CAS.
- G. Zhang, Z. Wu, Y. Yang, J. Shi, J. Lv, Y. Fang, Z. Shen, Z. Lv, P. Li, X. Yao, W. Chen, X. Wei, P. K. Chu and X. Zhang, Chem. Eng. J., 2022, 428, 131155 CrossRef CAS.
- W. Zhang, J. Gu, K. Li, J. Zhao, H. Ma, C. Wu, C. Zhang, Y. Xie, F. Yang and X. Zheng, Mater. Sci. Eng., C, 2019, 102, 458–470 CrossRef CAS PubMed.
- K. Suzuki, M. Iwatsu, T. Mokudai, M. Furuya, K. Yokota, H. Kanetaka, M. Shimabukuro, T. Yokoi and M. Kawashita, Molecules, 2023, 28, 650 CrossRef CAS PubMed.
- Y. Xue, L. Zhang, F. Liu, Y. Zhao, J. Zhou, Y. Hou, H. Bao, L. Kong, F. Ma and Y. Han, Adv. Healthcare Mater., 2022, 11, e2200998 CrossRef PubMed.
- W. Teng, Z. Zhang, Y. Wang, Y. Ye, E. Yinwang, A. Liu, X. Zhou, J. Xu, C. Zhou, H. Sun, F. Wang, L. Zhang, C. Cheng, P. Lin, Y. Wu, Z. Gou, X. Yu and Z. Ye, Small, 2021, 17, 2102315 CrossRef CAS PubMed.
- G. Zhang, X. Zhang, Y. Yang, H. Zhang, J. Shi, X. Yao and X. Zhang, Adv. Mater. Interfaces, 2020, 7, 1901706 CrossRef CAS.
- Y. Li, X. Liu, B. Li, Y. Zheng, Y. Han, D.-F. Chen, K. W. K. Yeung, Z. Cui, Y. Liang, Z. Li, S. Zhu, X. Wang and S. Wu, ACS Nano, 2020, 14, 8157–8170 CrossRef CAS PubMed.
- K. Xu, Z. Yuan, Y. Ding, Y. He, K. Li, C. Lin, B. Tao, Y. Yang, X. Li, P. Liu and K. Cai, Appl. Mater. Today, 2021, 24, 101155 CrossRef.
- Z. Yuan, B. Tao, Y. He, C. Mu, G. Liu, J. Zhang, Q. Liao, P. Liu and K. Cai, Biomaterials, 2019, 223, 119479 CrossRef CAS PubMed.
- M. Li, L. Li, K. Su, X. Liu, T. Zhang, Y. Liang, D. Jing, X. Yang, D. Zheng, Z. Cui, Z. Li, S. Zhu, K. W. K. Yeung, Y. Zheng, X. Wang and S. Wu, Adv. Sci., 2019, 6, 1900599 CrossRef PubMed.
- X. Wang, Y. Li, H. H. Y. Tong, P. Yuan, K.-L. Wong and Y. Yang, J. Lumin., 2020, 222, 117108 CrossRef CAS.
- B. Huang, L. Tan, X. Liu, J. Li and S. Wu, Bioact. Mater., 2019, 4, 17–21 Search PubMed.
- Z. Zhang, Y. Wang, W. Teng, X. Zhou, Y. Ye, H. Zhou, H. Sun, F. Wang, A. Liu, P. Lin, W. Cui, X. Yu, Y. Wu and Z. Ye, Biomaterials, 2021, 274, 120853 CrossRef CAS PubMed.
- J. Hu, Y. Ding, B. Tao, Z. Yuan, Y. Yang, K. Xu, X. Li, P. Liu and K. Cai, Bioact. Mater., 2022, 18, 228–241 CAS.
- T. Yang, D. Wang and X. Liu, Colloids Surf., B, 2019, 173, 833–841 CrossRef CAS PubMed.
- X. Ren, R. Gao, H. C. van der Mei, Y. Ren, B. W. Peterson and H. J. Busscher, ACS Appl. Mater. Interfaces, 2020, 12, 34610–34619 CrossRef CAS PubMed.
- S. Zhao, Y. Xu, W. Xu, Z. Weng, F. Cao, X. Wan, T. Cui, Y. Yu, L. Liao and X. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 30044–30051 CrossRef CAS.
- Z. Yuan, B. Tao, Y. He, J. Liu, C. Lin, X. Shen, Y. Yu, C. Mu, P. Liu and K. Cai, Biomaterials, 2019, 217, 119290 CrossRef CAS PubMed.
- D. Ding, Q. Zeng, F. He and Z. Chen, Materials, 2021, 14, 4031 CrossRef CAS PubMed.
- X. Wang, L. Tan, X. Liu, Z. Cui, X. Yang, K. W. K. Yeung, P. K. Chu and S. Wu, Biomater. Sci., 2018, 6, 2460–2471 RSC.
- Z. Yuan, J. Wu, Z. Fu, S. Meng, L. Dai and K. Cai, Adv. Funct. Mater., 2022, 32, 2200374 CrossRef CAS.
- Y. Ding, Z. Yuan, J.-W. Hu, K. Xu, H. Wang, P. Liu and K.-Y. Cai, Rare Met., 2022, 41, 673–688 CrossRef CAS.
- X. Zhang, J. Qiu, J. Tan, D. Zhang, L. Wu, Y. Qiao, G. Wang, J. Wu, K. W. K. Yeung and X. Liu, Carbon, 2022, 192, 209–218 CrossRef CAS.
- X. Han, G. Zhang, M. Chai and X. Zhang, Biomed. Mater., 2021, 16, 025018 CrossRef CAS.
- J. Song, H. Liu, M. Lei, H. Tan, Z. Chen, A. Antoshin, G. F. Payne, X. Qu and C. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 8915–8928 CrossRef CAS.
- D. Li, D. Wang, Y. He, B. Tao, X. Liu, Y. Yang, L. Tan, Y. Zhang, J. Hu, W. Yang, Y. Tang and K. Cai, J. Mater. Sci. Technol., 2023, 143, 93–106 CrossRef CAS.
- X. Long, L. Duan, W. Weng, K. Cheng, D. Wang and H. Ouyang, Colloids Surf., B, 2021, 207, 111996 CrossRef CAS PubMed.
- X. Long, Y. Yi, X. Wang, X. Duan, Y. Sun, C. Wu, W. Weng, B. Xu, K. Cheng, H. Wang and J. Lin, ACS Biomater. Sci. Eng., 2020, 6, 2020–2028 CrossRef CAS PubMed.
- C. Mao, W. Zhu, Y. Xiang, Y. Zhu, J. Shen, X. Liu, S. Wu, K. M. C. Cheung and K. W. K. Yeung, Adv. Sci., 2021, 8, 2002211 CrossRef CAS.
- K. Li, F. Dai, T. Yan, Y. Xue, L. Zhang and Y. Han, ACS Biomater. Sci. Eng., 2019, 5, 2208–2221 CrossRef CAS PubMed.
- K. Li, T. Yan, Y. Xue, L. Guo, L. Zhang and Y. Han, J. Mater. Chem. B, 2018, 6, 5756–5767 RSC.
- M. S. Aw, J. Addai-Mensah and D. Losic, J. Mater. Chem., 2012, 22, 6561 RSC.
- J. Zhuang, S. Lin, L. Dong, K. Cheng and W. Weng, Acta Biomater., 2018, 71, 49–60 CrossRef CAS PubMed.
- S. Lin, J. Li, L. Dong, K. Cheng, J. Lin and W. Weng, ACS Biomater. Sci. Eng., 2019, 5, 6446–6453 CrossRef CAS PubMed.
- Z. Huang, Y. He, X. Chang, J. Liu, L. Yu, Y. Wu, Y. Li, J. Tian, L. Kang, D. Wu, H. Wang, Z. Wu and G. Qiu, Adv. Healthcare Mater., 2020, 9, 2000318 CrossRef CAS PubMed.
- A. Mazare, J. Park, S. Simons, S. Mohajernia, I. Hwang, J. E. Yoo, H. Schneider, M. J. Fischer and P. Schmuki, Acta Biomater., 2019, 97, 681–688 CrossRef CAS PubMed.
- J. Park, A. Mazare, H. Schneider, K. von der Mark, M. J. Fischer and P. Schmuki, Tissue Eng., Part C, 2016, 22, 809–821 CrossRef CAS PubMed.
- K. Gulati, S. Maher, S. Chandrasekaran, D. M. Findlay and D. Losic, J. Mater. Chem. B, 2016, 4, 371–375 RSC.
- G. Wang, H. Feng, L. Hu, W. Jin, Q. Hao, A. Gao, X. Peng, W. Li, K.-Y. Wong, H. Wang, Z. Li and P. K. Chu, Nat. Commun., 2018, 9, 2055 CrossRef PubMed.
- T. Zhou, L. Yan, C. Xie, P. Li, L. Jiang, J. Fang, C. Zhao, F. Ren, K. Wang, Y. Wang, H. Zhang, T. Guo and X. Lu, Small, 2019, 15, 1805440 CrossRef PubMed.
- B. Zhu, Y. Li, F. Huang, Z. Chen, J. Xie, C. Ding and J. Li, Biomater. Sci., 2019, 7, 4730–4737 RSC.
- C. Xie, P. Li, L. Han, Z. Wang, T. Zhou, W. Deng, K. Wang and X. Lu, NPG Asia Mater., 2017, 9, e358 CrossRef CAS.
- D. Chen, M. Gao, Y. Fu, X. Xu and Z. Hao, Electrochim. Acta, 2015, 182, 841–846 CrossRef CAS.
- S. Sirivisoot, R. Pareta and T. J. Webster, Nanotechnology, 2011, 22, 085101 CrossRef PubMed.
- S. Sirivisoot, R. A. Pareta and T. J. Webster, J. Biomed. Mater. Res., Part A, 2011, 99A, 586–597 CrossRef CAS PubMed.
- R. Shi, J. Zhang, J. Tian, C. Zhao, Z. Li, Y. Zhang, Y. Li, C. Wu, W. Tian and Z. Li, Nano Energy, 2020, 77, 105201 CrossRef CAS.
- Y. Liu, X. Zhang, C. Cao, Y. Zhang, J. Wei, Y. J. Li, W. Liang, Z. Hu, J. Zhang, Y. Wei and X. Deng, Adv. Funct. Mater., 2017, 27, 173771 Search PubMed.
- F. Li, Q. Pan, Y. Ling, J. Guo, Y. Huo, C. Xu, M. Xiong, M. Yuan, Z. Cheng, M. Liu and J. Lin, Chem. Eng. J., 2023, 460 CAS.
- J. Zhou, M. A. Frank, Y. Yang, A. R. Boccaccini and S. Virtanen, Mater. Sci. Eng., C, 2018, 82, 277–283 CrossRef CAS PubMed.
- J. Chen, S. Li, Y. Jiao, J. Li, Y. Li, Y.-L. Hao and Y. Zuo, ACS Appl. Mater. Interfaces, 2021, 13, 49542–49555 CrossRef CAS PubMed.
- M. Pourhajibagher, A. R. Rokn, H. R. Barikani and A. Bahador, Photodiagn. Photodyn. Ther., 2020, 31, 101834 CrossRef CAS PubMed.
- B. Fan, Z. Guo, X. Li, S. Li, P. Gao, X. Xiao, J. Wu, C. Shen, Y. Jiao and W. Hou, Bioact. Mater., 2020, 5, 1087–1101 Search PubMed.
- K. Cai, Y. Jiao, Q. Quan, Y. Hao, J. Liu and L. Wu, Bioact. Mater., 2021, 6, 4073–4082 CAS.
- W. Liu, D. Yang, X. Wei, S. Guo, N. Wang, Z. Tang, Y. Lu, S. Shen, L. Shi, X. Li and Z. Guo, J. Biomater. Appl., 2020, 35, 544–552 CrossRef CAS.
- K. Li, W. Xu, Y. Chen, X. Liu, L. Shen, J. Feng, W. Zhao, W. Wang, J. Wu, B. Ma, S. Ge, H. Liu and J. Li, Adv. Funct. Mater., 2023, 33, 2214522 CrossRef CAS.
- W. Guan, L. Tan, X. Liu, Z. Cui, Y. Zheng, K. W. K. Yeung, D. Zheng, Y. Liang, Z. Li, S. Zhu, X. Wang and S. Wu, Adv. Mater., 2021, 33, 2006047 CrossRef CAS.
- W. Zhou, T. Bai, L. Wang, Y. Cheng, D. Xia, S. Yu and Y. Zheng, Bioact. Mater., 2023, 20, 64–80 CAS.
- S. Amrani, A. Atwal and F. Variola, RSC Adv., 2015, 5, 93666–93675 RSC.
- W. Song, L. Zhao, K. Fang, B. Chang and Y. Zhang, J. Mater. Chem. B, 2015, 3, 8567–8576 RSC.
- W. Zhou, Y. Li, J. Yan, P. Xiong, Q. Li, Y. Cheng and Y. Zheng, Sci. Rep., 2018, 8, 13432 CrossRef PubMed.
- B. Tao, Y. Deng, L. Song, W. Ma, Y. Qian, C. Lin, Z. Yuan, L. Lu, M. Chen, X. Yang and K. Cai, Colloids Surf., B, 2019, 177, 242–252 CrossRef CAS.
- C. Ning, J. Jiajia, L. Meng, Q. Hongfei, W. Xianglong and L. Tingli, Mater. Sci. Eng., C, 2019, 104, 109746 CrossRef CAS PubMed.
- Z. Wenhao, T. Zhang, J. Yan, Q. Li, P. Xiong, Y. Li, Y. Cheng and Y. Zheng, Acta Biomater., 2020, 116, 223–245 CrossRef PubMed.
- J. Bai, X. Zuo, X. Feng, Y. Sun, Q. Ge, X. Wang and C. Gao, ACS Appl. Mater. Interfaces, 2019, 11, 36939–36948 CrossRef CAS.
- F. Zhang, Q. Hu, Y. Wei, W. Meng, R. Wang, J. Liu, Y. Nie, R. Luo, Y. Wang and B. Shen, Chem. Eng. J., 2022, 435, 134802 CrossRef CAS.
- M. Chen, D. Wang, M. Li, Y. He, T. He, M. Chen, Y. Hu, Z. Luo and K. Cai, ACS Nano, 2022, 16, 15397–15412 CrossRef CAS PubMed.
- B. Tao, C. Lin, Y. He, Z. Yuan, M. Chen, K. Xu, K. Li, A. Guo, K. Cai and L. Chen, Chem. Eng. J., 2021, 423, 130176 CrossRef CAS.
- Y. Yang, B. Tao, Y. Gong, R. Chen, W. Yang, C. Lin, M. Chen, L. Qin, Y. Jia and K. Cai, J. Biomed. Mater. Res., Part A, 2020, 108, 2190–2205 CrossRef CAS PubMed.
- Y. Liu, C. Xie, F. Zhang and X. Xiao, J. Nanomater., 2019, 2019, 1–7 Search PubMed.
- Y. Dong, H. Ye, Y. Liu, L. Xu, Z. Wu, X. Hu, J. Ma, J. L. Pathak, J. Liu and G. Wu, Colloids Surf., B, 2017, 158, 127–136 CrossRef CAS.
- T. Wang, X. Liu, Y. Zhu, Z. D. Cui, X. J. Yang, H. Pan, K. W. K. Yeung and S. Wu, ACS Biomater. Sci. Eng., 2017, 3, 816–825 CrossRef CAS.
- Y. Lee, J. Huang, Z. Bing, K. Yuan, J. Yang, M. Cai, S. Zhou, B. Yang, W. Teng, W. Li and Y. Wang, J. Mater. Sci.: Mater. Med., 2022, 33, 63 CrossRef CAS PubMed.
- L. Zhang, Y. Yang, Y. H. Xiong, Y. Q. Zhao, Z. Xiu, H. M. Ren, K. Zhang, S. Duan, Y. Chen and F. J. Xu, Bioact. Mater., 2023, 25, 1–12 CAS.
- M. Chen, Y. Hu, Y. Hou, Y. Sun, M. Chen, M. Li, L. Tan, Z. Luo and K. Cai, Appl. Mater. Today, 2021, 23, 101059 CrossRef.
- X. Li, K. Xu, Y. He, B. Tao, K. Li, C. Lin, J. Hu, J. Wu, Y. Wu, S. Liu, L. Peng, H. Wang and K. Cai, Biomaterials, 2022, 287, 121683 CrossRef CAS PubMed.
- M. Chen, M. Li, Y. Wei, C. Xue, M. Chen, Y. Fei, L. Tan, Z. Luo, K. Cai and Y. Hu, Biomaterials, 2022, 291, 121878 CrossRef CAS PubMed.
- Y. Yu, Q. Ran, X. Shen, H. Zheng and K. Cai, Colloids Surf., B, 2020, 185, 110592 CrossRef CAS.
- X. Shen, F. Zhang, K. Li, C. Qin, P. Ma, L. Dai and K. Cai, Mater. Des., 2016, 92, 1007–1017 CrossRef CAS.
- L. Sutrisno, Y. Hu, X. Shen, M. Li, Z. Luo, L. Dai, S. Wang, J. L. Zhong and K. Cai, Mater. Sci. Eng., C, 2018, 89, 95–105 CrossRef CAS.
- Z. Yuan, S. Huang, S. Lan, H. Xiong, B. Tao, Y. Ding, Y. Liu, P. Liu and K. Cai, J. Mater. Chem. B, 2018, 6, 8090–8104 RSC.
- N. G. Fischer, X. Chen, K. Astleford-Hopper, J. He, A. F. Mullikin, K. C. Mansky and C. Aparicio, Mater. Sci. Eng., C, 2021, 125, 112108 CrossRef CAS PubMed.
- Y. Ding, Y. Hao, Z. Yuan, B. Tao, M. Chen, C. Lin, P. Liu and K. Cai, Biomater. Sci., 2020, 8, 1840–1854 RSC.
- S. Amirthalingam, A. K. Rajendran, Y. G. Moon and N. S. Hwang, Mater. Horiz., 2023, 10, 3325–3350 RSC.
- M. Zhang, W. Hu, C. Cai, Y. Wu, J. Li and S. Dong, Mater. Today Bio, 2022, 14, 100223 CrossRef CAS PubMed.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- A. Gelmi and C. E. Schutt, Adv. Healthcare Mater., 2021, 10, 2001125 CrossRef CAS PubMed.
- P. Abdollahiyan, B. Baradaran, M. de la Guardia, F. Oroojalian and A. Mokhtarzadeh, J. Controlled Release, 2020, 328, 514–531 CrossRef CAS PubMed.
- L. Kuang, J. Huang, Y. Liu, X. Li, Y. Yuan and C. Liu, Adv. Funct. Mater., 2021, 31, 2105383 CrossRef CAS.
- Y. Zhang, C. Fang, S. Zhang, R. E. Campbell and M. J. Serpe, ACS Appl. Mater. Interfaces, 2021, 13, 7051–7059 CrossRef CAS PubMed.
- S. Li, C. Wei and Y. Lv, Curr. Stem Cell Res. Ther., 2020, 15, 428–440 CrossRef CAS PubMed.
- Y. Zhang, J. Li and P. Habibovic, Bioact. Mater., 2022, 15, 372–381 CAS.
- C. R. Luculescu, A. M. Acasandrei, C. C. Mustaciosu, M. Zamfirescu, M. Dinescu, B. S. Calin, A. Popescu, D. Chioibasu, D. Cristian and I. A. Paun, Appl. Surf. Sci., 2018, 433, 166–176 CrossRef CAS.
- E. Pettersen, J. Anderson and M. Ortiz-Catalan, J. Neuroeng. Rehabil., 2022, 19, 31 CrossRef PubMed.
- T. Levingstone, B. Ali, C. Kearney and N. Dunne, J. Biomed. Mater. Res., Part B, 2021, 109, 1622–1633 CrossRef CAS PubMed.
- R. Das, E. J. Curry, T. T. Le, G. Awale, Y. Liu, S. Li, J. Contreras, C. Bednarz, J. Millender, X. Xin, D. Rowe, S. Emadi, K. W. H. Lo and T. D. Nguyen, Nano Energy, 2020, 76, 105028 CrossRef CAS PubMed.
- Z. Geng, X. Wang, Y. Yu, L. Ji, J. Wang and C. Liu, Chem. Eng. J., 2021, 420, 127674 CrossRef CAS.
- C. Dou, J. Li, J. He, F. Luo, T. Yu, Q. Dai, Y. Chen, J. Xu, X. Yang and S. Dong, Bioact. Mater., 2021, 6, 4697–4706 CAS.
- Z. Bao, Z. Gu, J. Xu, M. Zhao, G. Liu and J. Wu, Chem. Eng. J., 2020, 396, 125353 CrossRef CAS.
- H. Zhang, H. Xiong, W. Ahmed, Y. Yao, S. Wang, C. Fan and C. Gao, Chem. Eng. J., 2021, 409, 128147 CrossRef CAS.
- C. Shen, M. Gao, H. Chen, Y. Zhan, Q. Lan, Z. Li, W. Xiong, Z. Qin, L. Zheng and J. Zhao, J. Nanobiotechnol., 2021, 19, 395 CrossRef CAS PubMed.
- X. Ren, H. Liu, X. Wu, W. Weng, X. Wang and J. Su, Front. Bioeng. Biotechnol., 2022, 9, 820468 CrossRef PubMed.
- C. Yang, X. Gao, M. R. Younis, N. T. Blum, S. Lei, D. Zhang, Y. Luo, P. Huang and J. Lin, Chem. Eng. J., 2021, 408, 127959 CrossRef CAS.
- J. Safari and Z. Zarnegar, J. Saudi Chem. Soc., 2014, 18, 85–99 CrossRef CAS.
- H. Wei, J. Cui, K. Lin, J. Xie and X. Wang, Bone Res., 2022, 10, 17 CrossRef CAS PubMed.
- A. L. Linsebigler, G. Q. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735–758 CrossRef CAS.
- W. Ren, Y. Yan, L. Zeng, Z. Shi, A. Gong, P. Schaaf, D. Wang, J. Zhao, B. Zou, H. Yu, G. Chen, E. M. B. Brown and A. Wu, Adv. Healthcare Mater., 2015, 4, 1526–1536 CrossRef CAS PubMed.
- G. Ou, Z. Li, D. Li, L. Cheng, Z. Liu and H. Wu, Nano Res., 2016, 9, 1236–1243 CrossRef CAS.
- T. Ueno, T. Ikeda, N. Tsukimura, M. Ishijima, H. Minamikawa, Y. Sugita, M. Yamada, N. Wakabayashi and T. Ogawa, Biomaterials, 2016, 108, 177–186 CrossRef CAS PubMed.
- F. Iwasa, N. Hori, T. Ueno, H. Minamikawa, M. Yamada and T. Ogawa, Biomaterials, 2010, 31, 2717–2727 CrossRef CAS PubMed.
- S. G. Ullattil, S. B. Narendranath, S. C. Pillai and P. Periyat, Chem. Eng. J., 2018, 343, 708–736 CrossRef CAS.
- H. Wu, L. Xie, M. He, R. Zhang, Y. Tian, S. Liu, T. Gong, F. Huo, T. Yang, Q. Zhang, S. Guo and W. Tian, Acta Biomater., 2019, 97, 597–607 CrossRef CAS PubMed.
- W. Xu, M. Qi, X. Li, X. Liu, L. Wang, W. Yu, M. Liu, A. Lan, Y. Zhou and Y. Song, J. Electroanal. Chem., 2019, 842, 66–73 CrossRef CAS.
- H. S. Jung, P. Verwilst, A. Sharma, J. Shin, J. L. Sessler and J. S. Kim, Chem. Soc. Rev., 2018, 47, 2280–2297 RSC.
- J. Chen, C. Ning, Z. Zhou, P. Yu, Y. Zhu, G. Tan and C. Mao, Prog. Mater. Sci., 2019, 99, 1–26 CrossRef CAS PubMed.
- D. Wu, X. Duan, Q. Guan, J. Liu, X. Yang, F. Zhang, P. Huang, J. Shen, X. Shuai and Z. Cao, Adv. Funct. Mater., 2019, 29, 1900095 CrossRef.
- J. Zeng, Y. Wang, Z. Sun, H. Chang, M. Cao, J. Zhao, K. Lin and Y. Xie, Chem. Eng. J., 2020, 394, 125017 CrossRef CAS.
- Y. He, L. Yu, J. Liu, Y. Li, Y. Wu, Z. Huang, D. Wu, H. Wang, Z. Wu and G. Qiu, FASEB J., 2019, 33, 6069–6081 CrossRef CAS PubMed.
- C. M. M. Nunes, C. L. Ferreira, D. V. Bernardo, C. C. R. Lopes, L. Collino, V. C. da Silva Lima, D. de Camargo Reis Mello, L. M. R. de Vasconcellos and M. A. N. Jardini, Clin. Oral Investig., 2021, 25, 2925–2937 CrossRef PubMed.
- R. Leesungbok, S.-J. Ahn, S.-W. Lee, G.-H. Park, J.-S. Kang and J.-J. Choi, J. Oral Implantol., 2013, 39, 248–255 CrossRef.
- K. Gulati, L. Johnson, R. Karunagaran, D. Findlay and D. Losic, Biomacromolecules, 2016, 17, 1261–1271 CrossRef CAS PubMed.
- O. A. Tertuliano and J. R. Greer, Nat. Mater., 2016, 15, 1195 CrossRef CAS PubMed.
- G. Thrivikraman, S. K. Boda and B. Basu, Biomaterials, 2018, 150, 60–86 CrossRef CAS PubMed.
- B. Yu, Z. Qiao, J. Cui, M. Lian, Y. Han, X. Zhang, W. Wang, X. Yu, H. Yu, X. Wang and K. Lin, Biomaterials, 2021, 276, 120997 CrossRef CAS PubMed.
- M. Zhao, B. Song, J. Pu, T. Wada, B. Reid, G. Tai, F. Wang, A. Guo, P. Walczysko, Y. Gu, T. Sasaki, A. Suzuki, J. V. Forrester, H. R. Bourne, P. N. Devreotes, C. D. McCaig and J. M. Penninger, Nature, 2006, 442, 457–460 CrossRef CAS PubMed.
- W. Liu, X. Li, Y. Jiao, C. Wu, S. Guo, X. Xiao, X. Wei, J. Wu, P. Gao, N. Wang, Y. Lu, Z. Tang, Q. Zhao, J. Zhang, Y. Tang, L. Shi and Z. Guo, ACS Appl. Mater. Interfaces, 2020, 12, 51885–51903 CrossRef CAS PubMed.
- H. Wu, H. Dong, Z. Tang, Y. Chen, Y. Liu, M. Wang, X. Wei, N. Wang, S. Bao, D. Yu, Z. Wu, Z. Yang, X. Li, Z. Guo and L. Shi, Biomaterials, 2023, 293, 121990 CrossRef CAS PubMed.
- B. Geng, X. Yang, P. Li, W. Shi, D. Pan and L. Shen, ACS Appl. Mater. Interfaces, 2021, 13, 45325–45334 CrossRef CAS PubMed.
- Y. Yu, L. Tan, Z. Li, X. Liu, Y. Zheng, X. Feng, Y. Liang, Z. Cui, S. Zhu and S. Wu, ACS Nano, 2021, 15, 10628–10639 CrossRef CAS PubMed.
- Y. C. Shin, J.-H. Bae, J. H. Lee, I. S. Raja, M. S. Kang, B. Kim, S. W. Hong, J.-B. Huh and D.-W. Han, Biomater. Res., 2022, 26, 11 CrossRef CAS PubMed.
- S. Wang, X. Zhao, Y. Hsu, Y. He, F. Wang, F. Yang, F. Yan, D. Xia and Y. Liu, Acta Biomater., 2023, 169, 19–44 CrossRef CAS PubMed.
- S. Maekawa, Y.-D. Cho, F. Kauffmann, Y. Yao, J. V. Sugai, X. Zhong, C. Schmiedeler, N. Kinra, A. Moy, L. Larsson, J. Lahann and W. V. Giannobile, Adv. Mater. Interfaces, 2022, 9, 2200531 CrossRef CAS PubMed.
- H. S. Jung, J. Han, H. Shi, S. Koo, H. Singh, H.-J. Kim, J. L. Sessler, J. Y. Lee, J.-H. Kim and J. S. Kim, J. Am. Chem. Soc., 2017, 139, 7595–7602 CrossRef CAS PubMed.
- L. Shi, F. Hu, Y. Duan, W. Wu, J. Dong, X. Meng, X. Zhu and B. Liu, ACS Nano, 2020, 14, 2183–2190 CrossRef CAS PubMed.
- M. S. Aw and D. Losic, Int. J. Pharm., 2013, 443, 154–162 CrossRef CAS PubMed.
- B. A. Webb, M. Chimenti, M. P. Jacobson and D. L. Barber, Nat. Rev. Cancer, 2011, 11, 671–677 CrossRef CAS PubMed.
- X. Chen, A. Jaiswal, Z. Costliow, P. Herbst, E. A. Creasey, N. Oshiro-Rapley, M. J. Daly, K. L. Carey, D. B. Graham and R. J. Xavier, Nat. Immunol., 2022, 23, 1063 CrossRef CAS PubMed.
- J. Shinn, N. Kwon, S. A. Lee and Y. Lee, Int. J. Pharm. Invest., 2022, 52, 427–441 CrossRef CAS PubMed.
- H. Ding, P. Tan, S. Fu, X. Tian, H. Zhang, X. Ma, Z. Gu and K. Luo, J. Controlled Release, 2022, 348, 206–238 CrossRef CAS PubMed.
- Y. Xiang, X. Liu, C. Mao, X. Liu, Z. Cui, X. Yang, K. W. K. Yeung, Y. Zheng and S. Wu, Mater. Sci. Eng., C, 2018, 85, 214–224 CrossRef CAS PubMed.
- T. Zhang, C. Xie, Y. Liu, F. Zhang and X. Xiao, Mater. Lett., 2018, 215, 95–98 CrossRef CAS.
- M. Rinaudo, Prog. Polym. Sci., 2006, 31, 603–632 CrossRef CAS.
- H. Du, M. Liu, X. Yang and G. Zhai, Drug Discovery Today, 2015, 20, 1004–1011 CrossRef CAS PubMed.
- B. D'Autreaux and M. B. Toledano, Nat. Rev. Mol. Cell Biol., 2007, 8, 813–824 CrossRef PubMed.
- J. Li, C. Deng, W. Liang, F. Kang, Y. Bai, B. Ma, C. Wu and S. Dong, Bioact. Mater., 2021, 6, 3839–3850 CAS.
- H. Byun, G. N. Jang, J. Lee, M.-H. Hong, H. Shin and H. Shin, Biofabrication, 2021, 13, 034101 CrossRef CAS PubMed.
- P.-A. Mouthuy, S. J. B. Snelling, S. G. Dakin, L. Milkovic, A. C. Gasparovic, A. J. Carr and N. Zarkovic, Biomaterials, 2016, 109, 55–68 CrossRef CAS PubMed.
- Y. Yao, H. Zhang, Z. Wang, J. Ding, S. Wang, B. Huang, S. Ke and C. Gao, J. Mater. Chem. B, 2019, 7, 5019–5037 RSC.
- S. C. Manolagas, Endocr. Rev., 2010, 31, 266–300 CrossRef CAS PubMed.
- M. Shahriari, M. Zahiri, K. Abnous, S. M. Taghdisi, M. Ramezani and M. Alibolandi, J. Controlled Release, 2019, 308, 172–189 CrossRef CAS PubMed.
- C. B. Ibberson, C. L. Jones, S. Singh, M. C. Wise, M. E. Hart, D. V. Zurawski and A. R. Horswill, Infect. Immun., 2014, 82, 4253–4264 CrossRef PubMed.
- M. D. Sternlicht and Z. Werb, Annu. Rev. Cell Dev. Biol., 2001, 17, 463–516 CrossRef CAS PubMed.
- Q. Yao, L. Kou, Y. Tu and L. Zhu, Trends Pharmacol. Sci., 2018, 39, 766–781 CrossRef CAS PubMed.
- W. Zhou, Z. Duan, J. Zhao, R. Fu, C. Zhu and D. Fan, Bioact. Mater., 2022, 17, 1–17 CAS.
- H. Zhou, S. Ye, M. Xu, L. Hao, J. Chen, Z. Fang, K. Guo, Y. Chen and L. Wang, Biomaterials, 2023, 301 Search PubMed.
- S. Dong, Y. Chen, L. Yu, K. Lin and X. Wang, Adv. Funct. Mater., 2020, 30, 1907071 CrossRef CAS.
- L. Ma, J. Zhou, Q. Wu, G. Luo, M. Zhao, G. Zhong, Y. Zheng, X. Meng, S. Cheng and Y. Zhang, Biomaterials, 2023, 301, 122236 CrossRef CAS PubMed.
- K. Xu, C. Mu, C. Zhang, S. Deng, S. Lin, L. Zheng, W. Chen and Q. Zhang, Biomaterials, 2023, 301, 122268 CrossRef CAS PubMed.
Footnote |
| † The authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |