Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unveiling the future of steel corrosion inhibition: a revolutionary sustainable odyssey with a special emphasis on N+-containing ionic liquids through cutting-edge innovations
Sanjukta
Zamindar
 ab,
Sukdeb
Mandal
ab,
Sukdeb
Mandal
 ab,
Manilal
Murmu
ab,
Manilal
Murmu
 c and
Priyabrata
Banerjee
c and
Priyabrata
Banerjee
 *ab
*ab
aElectric Mobility and Tribology Research Group, CSIR-Central Mechanical Engineering Research Institute, Mahatma Gandhi Avenue, Durgapur 713209, West Bengal, India. E-mail: pr_banerjee@cmeri.res.in
bAcademy of Scientific and Innovative Research (AcSIR), Ghaziabad 201002, Uttar Pradesh, India
cDepartment of Nuclear and Quantum Engineering, Korea Advanced Institute of Science and Technology, Daejeon 34141, South Korea
First published on 13th May 2024
Abstract
The advancement of sustainable and green technologies for the mitigation of corrosion, driven by the escalating awareness of ecological considerations and stringent environmental regulations, is of paramount importance. Over the past decade, significant attention has been directed towards inhibiting metallic corrosion using ionic liquids (ILs) owing to their intriguing characteristic properties and their capacity to be adsorbed, followed by the formation of an effective barrier film onto metallic surfaces. This review comprehensively explores the application of ILs with a special emphasis on N+-containing ILs as “next-generation corrosion inhibitors” for metal substrates, specifically steel surfaces, across various aggressive environments, highlighting the trend towards “hype vs. hope”. Additionally, this review elucidates the adsorption mechanism and intriguing factors that trigger the adsorption process of ILs by employing state-of-the-art experimental as well as computational approaches to gain a comprehensive understanding of their corrosion inhibition potential.
1. Introduction
Corrosion is an inevitable process that involves the irreversible degradation of metals or alloys through chemical or electrochemical reactions with their surroundings. Corrosion causes severe destruction of metallic resources, resulting in health hazards and/or loss of flora and fauna, including mankind, along with huge economic impacts across the world.1–5 Its pervasive impact affects the economic growth trajectories of both developed and developing nations. According to a study conducted by the National Association of Corrosion Engineers (NACE), the United States experiences an economic loss that is roughly equivalent to 3.1% of the country's Gross Domestic Product (GDP) due to corrosion.6,7Metal and their alloys find widespread industrial usage, such as manufacturing, architecture, transportation, and oil refineries, but their ensuing corrosion due to the adverse environments has become an alarming issue.8,9 Steel, being an affordable and robust material, is one of the most widely utilised go-to building materials globally and is used in several infrastructures, buildings, vehicles, machinery, and other applications. Among the other construction materials, it offers the best strength-to-weight ratio and is fully recyclable. Large construction projects, mainly those constructed of steel, require a long life span of infrastructural materials. During this time, metallic structures are exposed to several adverse environments and many other harsh conditions that result in the deterioration of the metallic structures. Accordingly, the longevity of the metallic bodies is highly compromised. Therefore, to enhance longevity and protect such type of vital assets in terms of economic and environmental aspects, corrosion protection is highly essential. The intrinsic ability of a metal to chemically react with its surroundings is the primary factor in determining the magnitude of the ensuing loss. Elemental composition, temperature, mechanical variables, electrical potential difference, pH, and environmental circumstances are some of the factors that affect the rate of metal corrosion. Corrosion may be minimized using appropriate techniques, such as the application of potential corrosion inhibitors and protective coatings.10–21
In recent years, several corrosion mitigation strategies have been developed. The application of synthetic inhibitors has become the most promising approach owing to their low cost and ease of utilization in several industries. One of the most commonly used methods to combat aqueous corrosion of various metals and alloys is the application of organic compounds as inhibitors. The majority of effective inhibitors consist of an organic scaffold containing heteroatoms (nitrogen, oxygen, phosphorous, and sulphur) and unsaturation (e.g., double or triple bonds) that function as active centres for donor–acceptor (D–A) type interaction with the metal atom.3,22–26
The molecular structure of organic molecules plays a crucial role in their ability to act as effective corrosion inhibitors. These organic molecules contain unsaturated conjugated bonds, aromatic and/or heterocyclic moieties, and polar functional groups, such as nitro (–NO2), amine (–NH2), hydroxyl (–OH), carboxyl (–COOH), ester (–COOR), amide (–CONH2), etc. These functional groups enable the organic molecules to adhere to the metal substrate and form a protective layer on its surface, thereby preventing corrosion. The unsaturated conjugated bonds and aromatic and/or heterocyclic moieties further enhance the efficacy of organic molecules as corrosion inhibitors. The functional groups act as highly proficient D–A centers that strongly interact with metal atoms, effectively facilitating the adsorption of the inhibitor molecules onto the exposed metal exterior. Inhibitor molecules adsorbed on metal surfaces can hinder corrosion processes, including anodic, cathodic, or both, at the metal–electrolyte interface.6,27–32
Nevertheless, traditional organic inhibitors face limitations due to tedious synthetic processes, low thermal stability, reduced chemical stability, and a gradual loss of protective efficacy over time.33–35 Therefore, recognizing the imperative for environmentally friendly and sustainable corrosion prevention technologies has become paramount. This shift is fuelled by heightened awareness of ecological considerations and adherence to stringent environmental regulations. Over the past decade, significant attention has been directed towards inhibiting metallic corrosion using ILs owing to their intriguing characteristics, such as their low vapor pressure, non-inflammable nature, higher thermos-chemical stability, and their capability to get adsorbed onto metallic surfaces.36 ILs are molten salts basically comprising anions and cations that have an organic molecular composition with intriguing physical–chemical characteristics.37,38
Furthermore, by combining the proper cation–anion pair, the physicochemical characteristics of ILs can be tailored to accomplish specific functions. Several “tailor-made” functional ILs can be developed for target-specific materials and functional applications due to their tunable characteristics. Consequently, ILs play a unique role in the formulation of novel functional materials because of their various scientific and technological applications, most remarkably in the protection of steel from corrosion damage. The beauty within the molecular structure of the IL helps in its quick film formation ability on the metallic surface. It has been observed that N+-containing ILs have grabbed superior attention because of their facile synthesis, high yield, and better shielding nature towards corrosion.39,40 Moreover, the inadequate solubility of typical organic and polymeric chemical substances in polar electrolytic media confines the application of these substances as corrosion inhibitors. This constraint is easily overcome by employing ILs because they exhibit high solubility in polar aqueous environments.41–43
There are several recent reports in the literature that deal with the comparative insight regarding the adsorption of ILs on metal surfaces to retard the rate of corrosion inhibitory actions.44–46 This review thoroughly explores how N+-based ILs can be used as “green” corrosion inhibitors to effectively reduce the corrosion rate of mild steel in various electrolytic environments. The mechanisms and adsorption behaviours of the most important five types of ILs based on ammonium, pyridinium, piperidinium, pyrrolidinium, and imidazolium cations are discussed. Additionally, the consideration of structural features, including chain length, heteroatom incorporation, ring structure, and π-electron moieties, influencing their ability to adhere to the mild steel surface and their subsequent protection ability in various electrolytic media are highlighted. To advance our understanding of their real-world corrosion inhibition potential, both modern experimental techniques and cutting-edge computational approaches have been comprehensively discussed. These dual investigative approaches aim to provide a nuanced and interconnected understanding of adsorption and corrosion inhibition, which ultimately contribute to the design and adaptation of more effective and easy-going corrosion prevention strategies for different environmental contexts based on green ILs.
2. Evolution of ILs in corrosion control
The evolution of ILs in corrosion inhibition represents a transformative journey in materials science, revolutionizing the way of protecting metals from corrosion.47 Subsequent years have seen an increase in the design and synthesis of tailored ILs, marking notable advancements in the contemporary field. In the early stages of this evolution, around the turn of the century, researchers began to recognize the potential of ILs as effective corrosion inhibitors, stemming from their unique physicochemical properties. Researchers have been driven in the field of corrosion mitigation with ILs by diverse combinations of cations paired with anions, all aimed at optimizing their inhibitive performance. The focus then shifted towards understanding the intricate mechanisms underlying corrosion inhibition, including adsorption and film formation.This deeper understanding has led to the design of ILs for enhanced efficacy in diverse corrosive environments. The evolution also witnessed an expansion beyond the laboratory, with ILs finding practical applications in specific industries, such as oil and gas, where corrosion poses significant challenges.
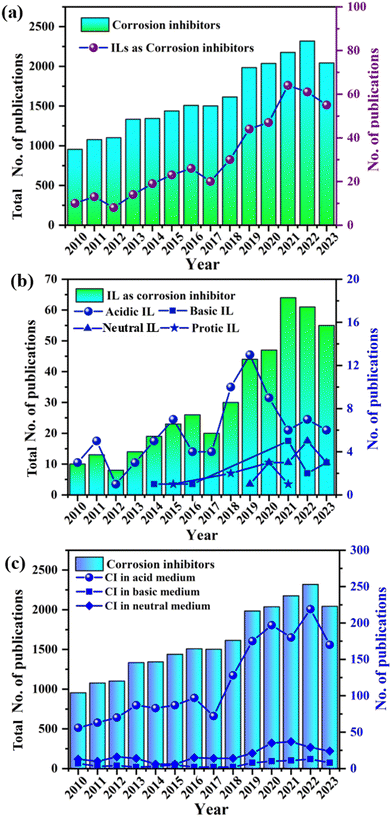
Continuous advancements in the contemporary field have yielded environmentally benign IL-based formulations that offer robust corrosion protection and minimize environmental impacts. The year-wise progression, as illustrated in Fig. 1, reflects a dynamic journey marked by relentless innovation and a deeper understanding of how ILs can serve as game changers to combat corrosion. The future has immense potential for further refinement and expansion of this technology, promising even more effective and sustainable solutions for corrosion inhibition across various industries. Currently, the primary obstacle facing the application of ILs as corrosion inhibitors is their cost. To overcome the cost barrier of ILs towards corrosion inhibition application, a techno-commercial study is required. Another effective and environmentally viable approach to overcome the cost barrier of ILs is the synthesis of deep eutectic solvents (DES) from the ILs. It has been reported that the production cost of DES is around 80% less than that of ILs. Commonly available DES is mainly choline-based ILs in association with urea, ethylene glycol, and diethanolamine. In the future, several other N-based ILs can be used for the production of DES for effective corrosion inhibition.
Another challenge of ILs in corrosion inhibition applications is their toxicity in some cases. ILs may be toxic in solute form for animals, including humans, in some cases. Therefore, it is highly necessary to formulate eco-friendly ILs for corrosion inhibition application.48–50
3. Classification of ILs
The year 1914 marked a significant turning point in the field of chemistry with the introduction of ethylammonium nitrate, [EtNH3][NO3], the first IL to be recognized in scientific literature. This colourless to slightly yellowish liquid, with a melting point of 12 °C, indicated a new era in the field of science and engineering.47,51,52 Nearly three decades later, in 1940, Frank Hurley and Tom Weir made another groundbreaking discovery through the development of room-temperature stable ILs. While conducting experiments with organic salts, they observed that a warm powder of alkyl pyridinium chloride, when combined with aluminium chloride, transformed into a clear, colourless liquid.47 This remarkable observation marked the birth of a new class of materials. Despite their immense potential, ILs have remained largely overlooked for several decades, confined to the field of chemical curiosities. However, the tide began to turn in the 1970s when scientists rediscovered these intriguing liquids and their unique properties.53–55Categorizing ILs into well-defined groups is a complex endeavour due to the various factors that influence their properties. These factors include the structural diversity of both cations and anions, which form the building blocks of ILs. The positively charged ions in ILs exhibit a wide structural variety, including imidazolium, pyridinium, and piperidinium-based cations.56 Each cation type imparts distinct properties to the IL, such as melting point, viscosity, and solubility. However, the negatively charged ions also contribute significantly to the overall properties of ILs. Common anions include chloride (Cl−), bromide (Br−), and nitrate (NO3−).56 The choice of anion can influence the IL's polarity, thermal stability, and miscibility with other solvents. ILs also have mobile ions responsible for their ionic conductivities. Moreover, these compounds have been initially categorized into several classes depending on their multi-functional properties since their invention. For instance, based on physical characteristics such as solubility, water-miscibility, viscosity, conductive nature, and the basicity or acidity of the constituent ionic species, ILs have been categorized into different groups.57 These classes are neutral IL, acidic IL, basic IL, IL with amphoteric anions, functionalized IL, protic IL, chiral IL, supported IL, bio-IL, poly IL, and energetic IL. A categorization system was established to classify IL compounds into three generations: first-generation, second-generation, and third-generation. This classification is based on the time frame when these compounds were developed and introduced. The categorization system helps identify each generation of IL compound's characteristics and properties and their potential applications in various fields.58 First-generation ILs contain bulky cations in their molecular structure, and they are not so stable in the presence of air and water. The evolution of second-generation ILs with the development of water/air stable IL was reported by Wilkes and Zawrotko in 1992 based on 1-ethyl-3-methylimidazolium cation and suitable anions, such as OAc− and NO3−.59 At the very beginning of the 21st century, the third-generation ‘task-specific’ IL was developed to overcome the drawback of the earlier developed ILs.60–62 Additionally, another two categories of ILs, cationic IL and anionic IL, have also been found in the literature.63 However, based on the well-established distinction between proton-donating and nonproton-donating functionality, the two most prevalent IL families are protic IL and aprotic IL.64,65 However, this classification is also not so rigorous. Generally, ILs comprise two components, e.g., the cationic and anionic parts, similar to the two glasses of a spectacle. The cationic and anionic fragments that frequently comprise ILs are presented in Fig. 2.
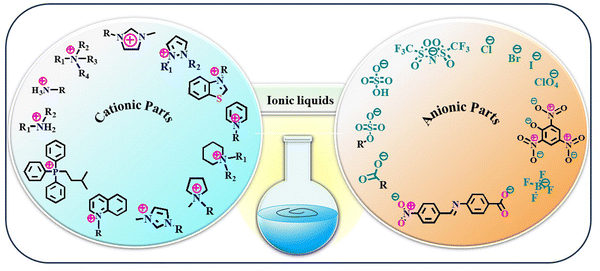 | ||
| Fig. 2 Structural representation of some common cationic and anionic counterparts that combine to form ILs. | ||
Accordingly, the prolonged classifications of ILs can be made based on constituent ions within its molecular scaffold. The anionic fragment may be an organic or inorganic anion such as Cl−, Br−, iodide (I−), nitrate (NO3−) perchlorate (ClO4−), carboxylate (HCOO−) bistriflimide ((CF3SO2)2N−), tetrafluoroborate (BF4−), and sulphate (SO42−). The cationic component may be a derivative of ammonium, imidazolium, pyrazolium, pyrrolidinium, phosphonium, thiazolium, piperidinium, pyridinium, etc., with variable substituents such as alkyl groups or aromatic rings.
4. Traits and implementations of ILs
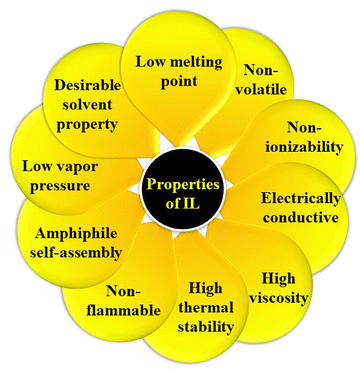
However, ILs are a fascinating class of compounds that have captivated the scientific community with their remarkable properties. Unlike traditional salts, such as sodium chloride (NaCl) or potassium chloride (KCl), which exist as crystalline solids at room temperature, ILs are liquids comprising constituent ions. This difference arises from the asymmetrical and bulky nature of the constituent ions in ILs, which weaken ionic interactions and lowers the melting point significantly.55,66–68Fig. 3 represents a pictorial depiction of several intriguing properties of ILs.One of the most intriguing aspects of ILs is their tunability. By carefully designing the combination of cations and anions, scientists can tailor the physical characteristics of ILs, including their viscosity, density, thermal stability, melting point, and boiling point. This allows for the creation of ILs with specific properties that are ideal for various applications.36,37,69–72
Furthermore, ILs possess a diverse range of other remarkable properties, such as
(i) Low vapour pressure: ILs are essentially non-volatile, making them environmentally friendly and safe to handle.73
(ii) Non-flammability: unlike many organic solvents, ILs are highly resistant to combustion.
(iii) Extreme stability: ILs are remarkably stable over a wide range of pH and temperature conditions, making them suitable for harsh environments.74
(iv) Long-term stability: ILs do not decompose readily, which enhances long-term sustainability.
(v) Amphiphile self-assembly: ILs can be used to create self-assembled structures with unique properties that can be exploited in various applications, such as drug delivery and nanotechnology.
(vi) High dissolving power: ILs are excellent solvents for various organic and inorganic compounds, including gases, such as H2, CO, and CO2.75
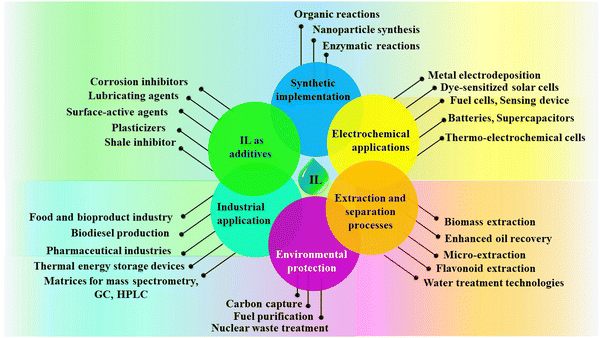
These impressive properties have opened up a vast range of potential applications for ILs in various sectors, as follows.
(i) Green solvents: ILs are explored as replacements for traditional solvents in various chemical processes, offering a more sustainable and environmentally friendly alternative.76
(ii) Energy storage: ILs are investigated for use in batteries, fuel cells, and other energy storage devices due to their high ionic conductivity and stability.77
(iii) Pharmaceuticals: ILs are used to develop new drugs and drug delivery systems, taking advantage of their unique properties.38
(iv) Catalysis: ILs can be used as catalysts in various chemical reactions, offering improved efficiency and selectivity.78
(v) Separation technologies: ILs are used to develop new and improved methods for separating and purifying various chemicals and materials.79
(vi) Lubricants: ILs are investigated as lubricants for high-temperature and high-pressure applications due to their exceptional thermal stability and low volatility.80
The various applications of ILs are pictorially depicted in Fig. 4. Research on ILs is growing rapidly, and new applications are being discovered all the time. With their unique properties and vast potential, ILs are poised to revolutionize various industries and contribute significantly to a more sustainable future. Because only about 300 ILs are currently commercialized, one can imagine how many opportunities in this field remain untapped and why this field of chemistry is so alluring.72 Despite the notable advancements, the cost still stands as an obstacle and restricts most applications to specialized, small-scale implementations.81
5. ILs as a revolutionary choice over organic corrosion inhibitors
ILs offer several advantages over other organic corrosion inhibitors, making them a preferred choice for various applications. Here are some key explanations for preferring ILs:(i) High thermal stability: ILs exhibit superior thermal stability, often withstanding temperatures exceeding 200 °C. This makes them suitable for high-temperature corrosion inhibition applications where conventional inhibitors may degrade or decompose.82
(ii) Low vapour pressure: ILs generally have very low vapour pressures, minimizing evaporation and inhalation hazards. This is particularly important in confined spaces or when working with volatile organic compounds (VOCs).83
(iii) Tunable properties: by selecting appropriate combinations of cations and anions, the characteristics of ILs can be tuned. This allows for fine-tuning of properties, such as viscosity, miscibility, and solubility, enabling better surface protection applications.84,85
(iv) High solubilities: ILs often exhibit high solubilities in a wide range of solvents, including water, organic solvents, and supercritical fluids. This versatility makes them applicable as corrosion inhibitors in various environments and on different substrates.
(v) Adsorption and film-forming propensity: ILs have a strong affinity for metal surfaces, allowing them to form a protective barrier that hinders corrosion reactions. This adsorption ability is enhanced by the presence of specific cations and anions.86 ILs can form thin, stable films on metal surfaces, providing long-lasting protection against corrosion.86,87
(vi) Synergistic effect: ILs can be combined with other corrosion inhibitors to induce synergistic effects, resulting in enhanced protective ability.
(vii) Reusability: ILs can often be recovered and reused, making them a more cost-effective solution than disposable organic inhibitors.
(viii) Versatility: ILs can be used as corrosion inhibitors for a wide range of metals, including carbon steel, stainless steel, copper, aluminium, and magnesium. An extensive variety of metals, including carbon steel, stainless steel, copper, aluminium, and magnesium, can be protected from corrosion damage by the application of ILs.
(ix) Eco-friendliness: many ILs are considered more environmentally friendly than traditional organic inhibitors due to their lower toxicity and biodegradability.88
6. A comprehensive analysis of the anti-corrosive performance of N+-based ILs for steel substrates
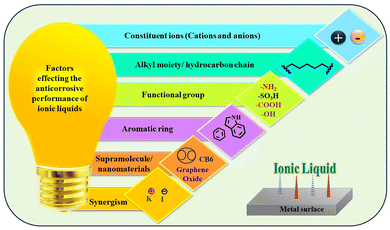
The advent of ILs has introduced a new era of corrosion inhibition, offering a radiant shield against the relentless degradation of iron metals and alloys. Pioneering experimental techniques have illuminated the intricate mechanisms underlying the anti-corrosive prowess of ILs, paving the way for a transformative era in safeguarding metallic structures.As demonstrated in Fig. 5, several imperative factors significantly affect the adsorption process of ILs onto metal surfaces: the nature of the constituent ions, the presence of specific functional groups, such as –COOH, –SO3H, –NH2, and –OH, and the number and length of alkyl chains.
Aromatic moieties and the presence of external ions can further enhance this process, resulting in synergistic effects.
The cationic part of the IL plays a key role in anchoring the IL film to the metal surface, forming strong bonds through chemisorption. However, non-bonding electrons of heteroatoms and the π-electron cloud of aromatic moieties facilitate physisorption, further strengthening the adhesion of the film on the metal. Existing alkyl groups within the molecule with their hydrophobic nature act as a barrier against the corrosive ingredients present in the exposed environments. As the length and number of carbon chains increase, the hydrophobicity of the film becomes more pronounced, providing a robust barrier property.
Understanding the intricate interplay of these factors is crucial for designing and developing ILs with optimal corrosion inhibition properties. Recent advancements in research have shed light on the exciting potential of N+-containing ILs in this regard. These ILs, with their unique structural features, offer enhanced adsorption capabilities and superior film-forming properties, making them even more effective corrosion inhibitors for iron alloys, specifically steel surfaces.
In this discussion, five types of N+-based ILs (e.g., ammonium, pyridinium, piperidinium, pyrrolidinium, and imidazolium cation containing ILs) have emerged as potential contenders in the field of steel protection from corrosion in various aggressive mediums. This comprehensive exploration is illuminated by advanced analytical techniques that provide a comprehensive understanding of their efficacy in corrosion inhibition.
(i) Ammonium-based ILs (AmILs): these types of ILs are characterized by the presence of an ammonium-based cation and an appropriate anion. The cationic part of AmILs usually consists of 1°,2°,3° or 4° ammonium cation, and the anionic parts can be varied as Cl−, sulfonate (–SO3H), and HCOO−. AmILs are known for their excellent film-forming ability, which acts as a physical barrier, hindering access to corrosive agents penetrating the metal surface.
(ii) Piperidinium-based ILs (PiILs): these ILs combine the advantages of both pyridinium and ammonium-based ILs. The piperidine ring structure offers a stronger adsorption ability than ammonium while maintaining the film-forming capacity.
(iii) Pyridinium-based ILs (PyILs): the heterocyclic structure of the pyridinium cation provides a strong adsorption ability because of the presence of a lone pair of electrons on the N-atom. This leads to a strong chemisorption of PiILs with the metal surface, enhancing the efficacy of the ILs as a corrosion inhibitors.
(iv) Pyrolidinium-based ILs (PyrILs): similar to piperidinium ILs, these ILs also possess a five-membered nitrogen-containing ring. However, the presence of an additional carbon atom in the ring leads to increased hydrophobicity, further enhancing the effectiveness of the protective film.
(v) Imidazolium-based ILs (ImILs): these are the most extensively studied N+-based ILs for corrosion inhibition. The presence of two nitrogen atoms in the imidazole ring allows for multiple adsorption sites, leading to a robust and long-lasting interaction with the metal surface. Additionally, the aromatic character of the imidazolium cation contributes to its excellent thermal stability, making it suitable for high-temperature applications.
6.1. AmILs as anticorrosive agents
AmILs have emerged as promising green alternatives in the field of corrosion mitigation strategies. These ILs, composed of ammonium cations paired with various anions, exhibit unique properties that make them effective anticorrosive agents. The remarkable ability of AmILs to form a stable barrier film on metal exteriors significantly impedes corrosion processes.89–91 The ionic nature of these compounds facilitates their interaction with metal surfaces, forming a robust barrier that hinders the penetration of corrosive agents. Additionally, the tunable nature of AmILs allows for the customization of their chemical structure to enhance specific anticorrosive properties, making them adaptable to diverse industrial applications.92 Their low volatility, thermal stability, and negligible vapour pressure contribute to their suitability for corrosion protection in various environments. As researchers continue to explore and optimize the use of AmILs, their application as anticorrosive agents holds great promise for advancing corrosion control strategies while aligning with the increasing demand for sustainable and eco-friendly solutions.93,94In several sub-classes of ILs, the AmILs have demonstrated exceptional anti-corrosive activity. The chemical constituents of cations are mostly responsible for their anticorrosive effectiveness. Several studies have revealed that the inhibitory mechanism of AmILs is predominately based on the physisorption process of ILs onto metallic substrates. Additionally, AmILs have several advantages over other ILs, including surfactant nature, reduced toxicity compared to heterocyclic group-based ILs, cost-effectiveness, and less tedious synthesis procedures, that provide high-purity reaction products. Another important feature of AmILs is their ‘tensioactive property’, i.e., the property by which a chemical species containing polar and non-polar moieties can change the value of surface tension.90
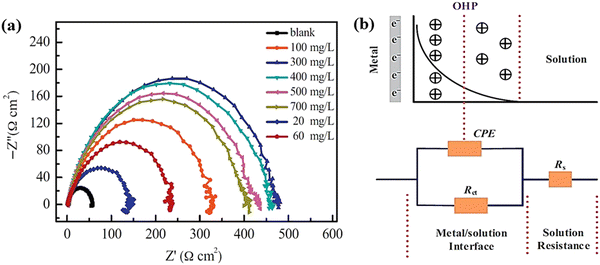 | ||
| Fig. 6 (a) Nyquist plot for the carbon steel working electrode in the presence of 0.1 M H2SO4 solution without and with different concentrations of BKC, (b) potential distribution diagram at the interface between the metal and the electrolytic solution (above) and analogous circuit used to fit the Nyquist plot (bellow) [reprinted with permission from ref. 95, ©2015 Elsevier]. | ||
 | ||
| Fig. 7 After evaluating API-X52 steel as a working electrode in the presence of 1 M HCl solution, the inhibition efficiency of (a) TMA and (b) TTA as functions of the immersion duration and temperature [reprinted with permission from ref. 90, ©2018 Elsevier]. | ||
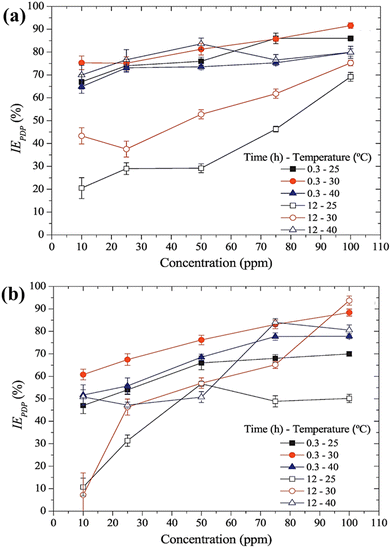
Another two AmILs, namely glycine propyl ester lauryl sulphate (GlyC3LS) and glutamic acid propyl ester lauryl sulphate (GluC3LS), were synthesized, and their protective capability was thoroughly investigated by Zehra et al.98 In this study, mild steel was used as the target metal, and 1 M HCl was used as the electrolytic solution for the corrosion inhibition studies. The adherence of both the studied AmILs on the metal surface occurs through both physisorption and chemisorption processes; subsequently, the IE reaches 99.81% (in electrochemical impedance spectroscopy i.e., EIS or potentiodynamic polarization i.e., PDP). Because of the variation in the cationic parts of the two ILs, GluC3LS acted as a better inhibitor than GlyC3LS, as supported by electrochemical and theoretical analyses. The additional hydrocarbon chain attached to the –COOH functional group within GluC3LS facilitates strong adsorption on the test sample.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The presence of the supramolecular part enhanced the water solubility of the IL. In that reported work, EIS, PDP, cyclic voltammetry (CV), and electrochemical noise measurement (EN) were executed for electrochemical investigations of corrosion inhibition studies. Consequently, it was revealed that the anticorrosive efficiency reached up to 97.97% with the application of a 100 mg L−1/0.032 mM concentration of IL. From the CV experiment, it was found that the oxidation curve diminished with the addition of CB6-based [3]rotaxane within 1 M NaOH and 1 M NaCl solution. The reduction reaction on the metal electrode occurs before 0.3 V in the alkali medium without an inhibitor. The outcomes indicate retardation in the reduction reaction, which is a diminished rate in the hydrogen evolution reaction. To evaluate the change in electrochemical noise, an EN experiment was performed for the carbon steel sample without and with various concentrations (25, 50, 75 and 100 mg L−1) of CB6-based [3]rotaxane in a 1 M NaOH + 1 M NaCl solution mixture for a duration of 900 minutes, as shown in Fig. 8.
1). The presence of the supramolecular part enhanced the water solubility of the IL. In that reported work, EIS, PDP, cyclic voltammetry (CV), and electrochemical noise measurement (EN) were executed for electrochemical investigations of corrosion inhibition studies. Consequently, it was revealed that the anticorrosive efficiency reached up to 97.97% with the application of a 100 mg L−1/0.032 mM concentration of IL. From the CV experiment, it was found that the oxidation curve diminished with the addition of CB6-based [3]rotaxane within 1 M NaOH and 1 M NaCl solution. The reduction reaction on the metal electrode occurs before 0.3 V in the alkali medium without an inhibitor. The outcomes indicate retardation in the reduction reaction, which is a diminished rate in the hydrogen evolution reaction. To evaluate the change in electrochemical noise, an EN experiment was performed for the carbon steel sample without and with various concentrations (25, 50, 75 and 100 mg L−1) of CB6-based [3]rotaxane in a 1 M NaOH + 1 M NaCl solution mixture for a duration of 900 minutes, as shown in Fig. 8.
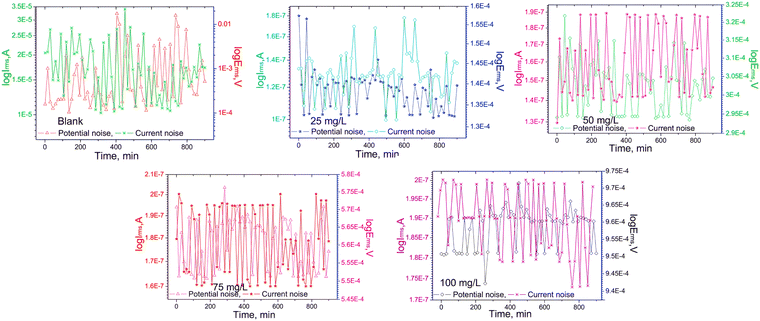 | ||
| Fig. 8 Electrochemical noise measurement of carbon steel in a solution of 1 M NaOH + 1 M NaCl, both with and without the CB6-based [3]rotaxane inhibitor. [Reprinted with permission from ref. 97, ©2022 Elsevier.] | ||
The experimental results clearly indicate that the potential and current noise for inhibitor-free steel samples were significantly in the higher (more positive) potential and current regions. This fact can be attributed to the presence of free OH− and Cl− ions because of their less noise resistance. This outcome indicates a higher rate of corrosion. However, the electrochemical noise is reduced to the lower (more negative) region of potential and current in the presence of IL. This phenomenon leads to a decrease in the corrosion current and potential value with the addition of IL. ![[R with combining macron]](https://www.rsc.org/images/entities/i_char_0052_0304.gif) rms values, which denote the degree of inhibition efficacy, were calculated from the EN experiments. The low value of
rms values, which denote the degree of inhibition efficacy, were calculated from the EN experiments. The low value of ![[R with combining macron]](https://www.rsc.org/images/entities/i_char_0052_0304.gif) rms (136.3 Ω) for the IL-free system indicates less inhibition, which means more corrosion. This phenomenon also leads to a decrease in the corrosion current and potential value with the addition of IL. The
rms (136.3 Ω) for the IL-free system indicates less inhibition, which means more corrosion. This phenomenon also leads to a decrease in the corrosion current and potential value with the addition of IL. The ![[R with combining macron]](https://www.rsc.org/images/entities/i_char_0052_0304.gif) rms value increased significantly with the addition of the IL in the aggressive electrolyte solution, indicating better corrosion resistivity. The 100 mg L−1 of the supramolecular IL in the electrolyte mixture showed 97.33% IE, as obtained from EN experiments. The amount of charge transfer from the anodic region to the cathodic region on the working electrode surface was significantly low in the presence of IL.
rms value increased significantly with the addition of the IL in the aggressive electrolyte solution, indicating better corrosion resistivity. The 100 mg L−1 of the supramolecular IL in the electrolyte mixture showed 97.33% IE, as obtained from EN experiments. The amount of charge transfer from the anodic region to the cathodic region on the working electrode surface was significantly low in the presence of IL.
The role of this supramolecular IL in protecting low carbon steel in the presence of NaOH and NaCl mixture is illustrated in Fig. 9. The corrosion process of metals studied by 1 M NaOH and NaCl electrolytic solutions is a complex phenomenon. The OH− and Cl− ions are adsorbed primarily on the Fe surface.
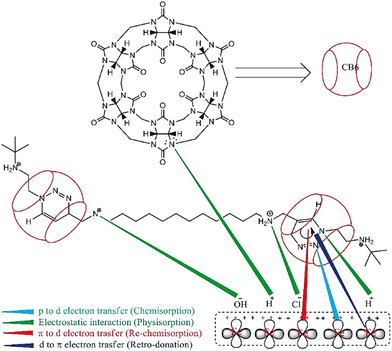 | ||
| Fig. 9 Schematic illustration of the protective mechanism of CB6-based [3]rotaxane to retard the rate of corrosion of low-carbon steel in 1 M NaOH + 1 M NaCl [reprinted with permission from ref. 97, ©2022 Elsevier]. | ||
When IL is added to the solution, the adsorbed OH− and Cl− ions are neutralized by the protonated N atoms of the IL. This phenomenon is considered a physisorption process via physical interaction or electrostatic interaction. However, the p-orbital electrons of N and π orbital electrons are transferred to the vacant d-orbital of Fe. These is termed chemisorption. Again, d-orbital electrons from Fe are transferred to the vacant π-orbital of CB6-based [3] rotaxane, i.e., retro-donation occurs.
The corrosion inhibition performances of some AmIL-based inhibitors are tabulated in Table 1.
| Sl. no. | Ionic liquid (Structure and IUPAC name) | Metal surface/medium | Adsorption mechanism | Isotherm model | Highlights | Ref. |
|---|---|---|---|---|---|---|
| 1 |
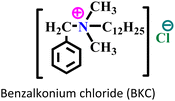
|
Carbon steel/0.1 M H2SO4 | Both physisorption and chemisorption | Langmuir | (i) IE reaches 88.34% (EIS) and 83.52% (PDP)@300 mg L−1 | 95 |
| (ii) The presence of Cl− ions increased the adsorption capability of the AmIL on the carbon-steel surface through bridging | ||||||
| (iii) The presence of a long alkyl chain facilitates extra surface coverage | ||||||
| 2 |
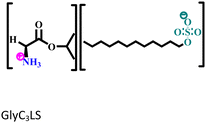
|
Mild steel/1 M HCl | Both physisorption and chemisorption | Langmuir | (i) IE of these biodegradable amino acid-based ILs: 98.64% for GlyC3LS and 99.81% for GluC3LS@100 ppm at 60 °C | 98 |
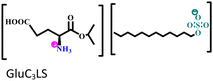
|
(ii) Corrosion inhibition analysis was performed in a wide temperature range (30, 40, 50 and 60 °C) | |||||
| (iii) GluC3LS shows better corrosion inhibition efficiency than GlyC3LS because of the combined effect of the hydrocarbon chain and –COOH functional group | ||||||
| 3 |
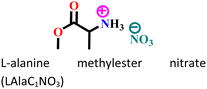
|
Mild steel/1 M HCl | Physisorption and chemisorption | Langmuir | (i) Corrosion inhibition analysis was performed in a wide temperature range (30, 40, 50 and 60 °C) | 42 |
| (ii) IE: 84% @0.003 M | ||||||
| (iii) The presence of heteroatoms facilitates adsorption | ||||||
| 4 |
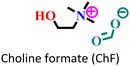
|
Mild steel/1 M HCl | Mixed adsorption | Langmuir | (i) Corrosion inhibition analysis was performed in a wide temperature range | 88 |
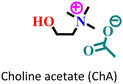
|
(ii) IE of these ILs: 96.9% for ChF and 99.5% for ChA@2 × 10−3 M at 50 °C | |||||
| (iii) The presence of an alyl group facilitates better adsorption in ChA | ||||||
| 5 |
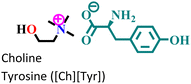
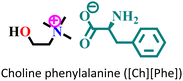
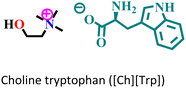
|
Mild steel/1 M HCl | Physisorption and predominant chemisorption | Langmuir | (i) IE: [Ch][Phe] > [Ch][Trp] > [Ch][Tyr] | 99 |
|
(ii) The aromatic nature of the –Ph ring increases the IE, while the decreased aromaticity reduces the IE in the following ILs | |||||
|
||||||
| 6 |
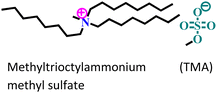
|
API-X52 steel/1 M HCl | Physisorption | Langmuir | (i) IE: TMA > TTA | 90 |
|
(ii) Increasing numbers of –Me groups facilitate steric crowding in the polar head group in the case of TTA. Accordingly, the adsorption tendency of TTA is diminished compared to that of TMA |
6.2. PiIL as an anticorrosive agent
PiILs are the category of ILs in which a nitrogen-containing six-membered heterocyclic ring is present along with a positive charge centred on nitrogen. PiILs are emerging as a groundbreaking class of corrosion inhibitors, exhibiting exceptional efficacy and environmental compatibility. These innovative molecules, characterized by their piperidinium cations paired with various anions, offer a unique approach to combat corrosion, which is a ubiquitous and costly problem across diverse industries. Their potent anticorrosive action stems from their remarkable film-forming capability.45 PiILs are readily adsorbed onto the metal surface, leading to a robust, adherent layer that serves as an impenetrable barrier for corrosive agents, such as oxygen, chloride ions, and acidic solutions.100 This effectively shields the underlying metal from corrosive attack, drastically decreasing the corrosion process and extending the operational lifespan of valuable assets. Unlike traditional corrosion inhibitors, which often rely on volatile and potentially hazardous components, PiILs are engineered for sustainability. Their low volatility and negligible vapour pressure significantly reduce their environmental impact, minimizing the risk of air and water contamination. Furthermore, their inherent biodegradability provides an added advantage of environmental responsibility, aligning with the increasing demand for eco-friendly solutions. Beyond their environmental benefits, PiILs offer exceptional versatility and tunability. The chemical structure of these ILs can be readily tailored by modifying the cation or anion, enabling researchers to fine-tune specific properties, such as adsorption strength, thermal stability, and solubility. This customizable nature of PiILs allows for the development of tailor-made corrosion inhibitors for diverse applications, ranging from protecting pipelines and storage tanks in the oil and gas industry to safeguarding aircraft components and medical implants. The impressive performance and environmental advantages of PiILs have ignited widespread interest in corrosion research. Their emergence marks a significant step towards a sustainable future where valuable assets are safeguarded effectively and responsibly, ushering in a new era of corrosion control strategies.101 PiILs play a crucial role in the field of IL-based corrosion mitigation strategies.6.3. PyIL as an anticorrosive agent
A positively charged six-membered ring comprising 5 carbon atoms and 1 nitrogen atom renders the structure of the heterocyclic pyridinium cation consisting of six delocalized π-electrons within the conjugated cyclic structure. In the domain of the IL-based corrosion inhibition strategy, PyILs play a pivotal role.35,102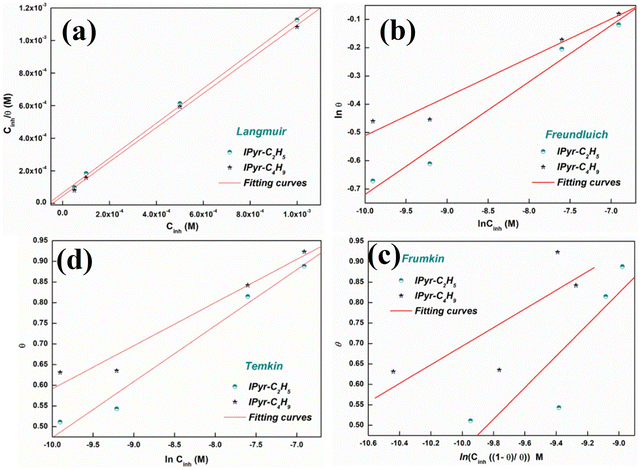 | ||
| Fig. 10 (a) Langmuir, (b) Freundluich, (c) Frumkin and (d) Temkin isotherm models for understanding the adsorption mechanism of IPyr-C2H5 and IPyr-C4H9 on mild steel surface in the presence of 1 M HCl at 298 K. [Reprinted with permission from ref. 103, ©2020 Elsevier.] | ||
The Langmuir adsorption isotherm model was the undisputed choice for describing the PyIL adsorption mechanism, which is also supported by the obtained high linear regression coefficients (R2 > 0.999) value. The strong adsorption of the PyILs is depicted by the high value of equilibrium constant (155.57 × 102 M−1 for IPyr-C4H9 and 218.93 × 102 M−1 for IPyr-C2H5). These findings suggest that the film formation of PyILs on the metal surface involves both physical and chemical adsorption mechanisms.
The sequential order of inhibitory performance is IPyr-C2H5 < IPyr-C4H9, which is because of their large specific surface area. The longer carbon chain of IPyr-C4H9 facilitates its adsorption on iron surfaces more predominately than IPyr-C2H5. On the contrary, Hajjaji et al. shed light on the corrosion inhibition effectiveness of two ILs, namely, (E)-4-(2-(4-fluorobenzylidene)hydrazinecarbonyl)-1-propylpyridin-1-ium iodide (denoted as Ipyr-C3H7) and (E)-4-(2-(4-fluorobenzylidene) hydrazinecarbonyl)-1-pentylpyridin-1-ium iodide (denoted as Ipyr-C5H11).104 The electrochemical results revealed that the synthesized PyILs act as mixed-type inhibitors with a predominant anodic type in nature. Electrochemical investigations, such as EIS and PDP, were performed to analyse the protective capabilities of the PyILs against mild steel corrosion in HCl electrolytes. From the EIS experiments, the obtained increased polarization resistance indicates that the corrosion inhibitors are adsorbed on the metal surface in the metal–electrolyte interface by dislodging previously adsorbed water molecules. The decreased corrosion current density with the addition of ILs obtained from the PDP study reveals the strong adsorption of ILs to the metal surface, specifically the anodic sites. The negatively charged I− ions are adsorbed on the positively charged metal surface through Coulombic interaction, resulting in a bridge type of linkage formation. This may also be referred to as the occurrence of physisorption. The presence of the aromatic ring, azomethine linkage, and –NH– moiety within the IL scaffold facilitates the sharing of electron clouds with the vacant orbitals of metal atoms, thus resulting in chemisorption. The value of the standard adsorption-free energy ranges from −20 kJ mol−1 to −40 kJ mol−1, which signifies the mixed type (i.e., chemical and physical) of the adsorption nature of the ILs. It makes sense that at elevated temperatures, the long chain of Ipyr-C5H11 ILs cannot sufficiently cover the metal surface. With an increase in carbon chain length, the corrosion inhibition efficacy of the current derivatives reaches a maximum when there are four carbons. Consequently, following the elongation of more than four carbons, the inhibitory efficacy gradually starts to decrease.
Similarly, the corrosion suppressing ability of cetylpyridinium picrate (CPP) IL on a mild steel working electrode in the presence of 1 M HCl is another approach of PyIL aspects.15 Gravimetric analysis revealed that the effectiveness of the CPP ILs increased with concentration and test solution temperature, culminating at 99.04% at 70 °C when the concentration was very low or close to 8 × 10−7 M, which was further supported by EIS and PDP analyses. XPS and SEM surface morphology investigations validated the development of a hydrophobic organic–inorganic hybrid protective film. This was the main factor in the strong adsorption of the IL onto the metal surface. The strong binding energy (443.63 kcal mol−1) obtained in the MD simulation study is predominantly attributed to the electrostatic interaction between the IL and the pre-adhered chloride ions onto the metal surface. The D–A interaction between the heteroatoms and aromatic moieties within the ILs and the metal surface helps in the strong adsorption of the ILs, and a self-assembled layer is formed on the metal plane. In addition, the longer cetyl chain within the CPP IL triggered a hydrophobic nature. Consequently, the electrolyte and metal surface were restricted from making contact. The hydrophobic nature induced by the cetyl chain was ascertained from a contact angle (CA) study. At 30 °C, the CA values of polished mild steel and mild steel after immersion in an acidic solution were found to be 53° and 46°, respectively. The addition of CPP IL into the corrosive solution increased the CA up to 92°, which reflects the hydrophobic nature of the steel surface.
The surface was then desiccated in a nitrogen environment. The SKP system (SKP5050, KP Technology) was used to obtain surface Volta potential maps of the metal samples through SKP measurement. The SKP is a precise and non-destructive method that provides surface information about electrodes. This technique is renowned as a non-contact process and can analyze potential distribution, measure electrochemical properties, and detect localized corrosion topographies of metals. SKP analysis is conducted in various environments, such as vacuum, open-air, and even humid environments. During the experimentation, a metallic probe, connected to the instrument, scans across the surface of the metallic sample and calculates the Volta potential (i.e., contact potential difference between the vibrating tip and the metal specimen) by evaluating the alternating current. Fig. 11(a–d) depicts the potential distribution diagrams of the studied samples obtained by SKP before and after the weight loss analysis.
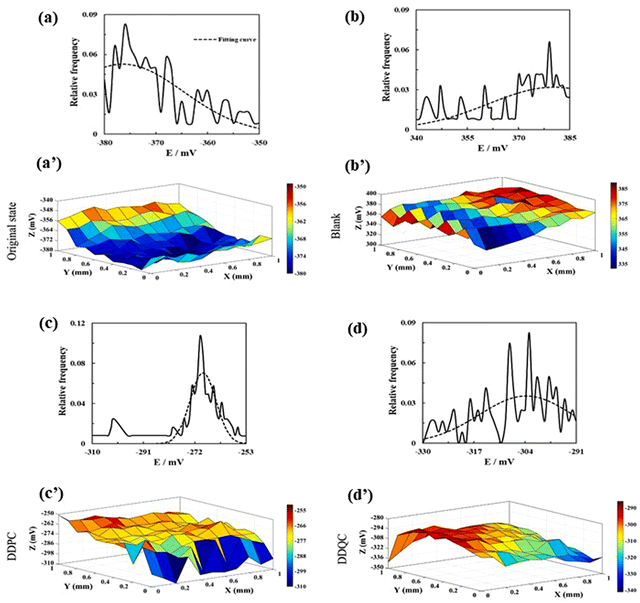 | ||
| Fig. 11 Relative frequency histogram of SKP diagrams. SKP potential distribution diagrams for carbon steel (a) and (a′) in the original state, (b) and (b′) after being immersed in a sour brine solution, (c) and (c′) with DDPC, and (d) and (d′) with DDQC. [Reprinted with permission from ref. 105, ©2023 Elsevier.] | ||
The potential distribution of the specimen prior to immersion was discovered to be less than 1, indicating its original state. In the case of the blank sample, the potential distribution shifted towards a positive value, indicating severe corrosion of its surface. When corrosion inhibitors viz., DDPC and DDQC, were present, it shifted towards negative values. This phenomenon suggests the adsorption of the PyILs onto the metal exterior, thereby suppressing the corrosion process and effective protection of the metal surface.
Furthermore, when comparing the potential distribution diagram of the inhibitors, it was observed that DDQC had a lesser value than DDPC, indicating that DDQC had a more significant effect. This observation is consistent with the results obtained from previous electrochemical studies, such as PDP and EIS, and gravimetric analysis, such as weight loss experiments. Fig. 11(a′–d′) illustrates the relative frequency histogram of the SKP potential distribution for each sample, and the position of these distributions was determined by fitting a Gaussian function. From the fitting analysis, the potential distribution of the polished sample with electrolyte, DDPC, and DDQC was found to be −376 mV, 380 mV, −269 mV, and −304 mV, respectively. The corrosion inhibition performances of some PyIL-based inhibitors are summarized in Table 2.
| Sl. no. | Ionic liquid (Structure/IUPAC name) | Metal surface and medium | Adsorption mechanism | Isotherm model | Highlights | Ref. |
|---|---|---|---|---|---|---|
| 1 |
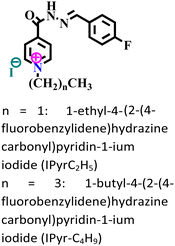
|
Mild steel/1 M HCl | Physisorption and chemisorption | Langmuir | (i) IE: 88.8% for IPyrC2H5 and 92.3% for IPyr-C4H9@10−3 M and 298 K | 103 |
| (ii) The larger chain length of the alkyl group is responsible for the better adsorption of IPyr-C4H9 | ||||||
| 2 |
|
X65 carbon steel/brine solution | Chemisorption | Langmuir | (i) IE: 97.4% for DDPC and 98.3% for DDQC@100 mg L−1 and 20 °C in dynamic EIS analysis | 105 |
|
||||||
| (ii) The presence of an aromatic ring increased the protective nature of DDQC | ||||||
| 3 |
|
Mild steel/1 M HCl | Physisorption and chemisorption | Langmuir | (i) IE: 92.34% @8 × 10−7 M and 30 °C | 15 |
| (ii) Interestingly, the IE increased with an increase in the concentration and temperature because of the formation of the hybrid organic–inorganic protective layer. 99.04% IE was achieved @8 × 10−7 M and 70 °C | ||||||
| 4 |
|
Mild steel/15% HCl | Physisorption and chemisorption | Dubinin Radushkevich | (i) IE: 90.3% for BPC and 79.8% for BPH@100 mg L−1 and 308 K | 35 |
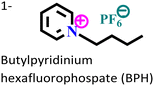
|
||||||
| (ii) The large size of the anion decreases the electrostatic attraction between the IL and the charged metal surface in the case of BPH | ||||||
| 5 |
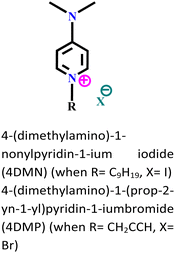
|
Carbon steel/3.5 wt% NaCl | Both physisorption and chemisorption | — | (i) IE: 93.8% for 4DMN and 91.9% for 4DMP@5 × 10−3 mol L−1 and 303 K. | 106 |
| (ii) The presence of iodide ions synergistically increased the electrostatic interaction of 4DMN | ||||||
| 6 |
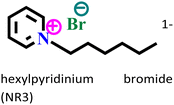
|
Carbon steel/1 M HCl | Physisorption as well as chemisorption | Langmuir | (i) IE: 81.3% @3 × 10−3 mol L−1 and 294 K | 107 |
| (ii) Acts as a mixed-type inhibitor |
6.4. PyrIL as an anticorrosive agent
The PyrILs are composed of pyrrolidinium cations and various anions. The PyrILs demonstrate excellent adhesion to metal exteriors and form a durable protective film that inhibits corrosion processes.108,109 The unique molecular structure of PyrILs contributes to their high thermal stability, low volatility, and strong affinity for metal substrates. This combination of attributes enables them to impede the penetration of corrosive agents effectively, thereby safeguarding metal surfaces from degradation.110–112 Because PyrILs come from non-renewable resources, it has been shown that their biodegradability and biocompatibility are inadequate.33 In this context, PyrILs are considered preferable contenders in the domain of corrosion mitigation. L-Proline nitrate IL (denoted as [Pro][NO3]) was synthesized, and its effectiveness as a green corrosion PyrIL was thoroughly investigated.336.5. ImIL as an anticorrosive agent
The ImILs represent a significant breakthrough in the field of corrosion protection. The term ImILs refers to the ILs, where the imidazole ring contains a positive charge. The imidazole ring was integrated into IL for the first time in 1984, and since then, it has become one of the centrepieces of interest because of its outstanding propensity to yield cationic molten salts (i.e., ILs).113 These novel compounds, constructed with an imidazolium cation and diverse anions, exhibit exceptional film-forming abilities. This enables them to create a robust and durable barrier on metal surfaces, effectively shielding them from the detrimental effects of corrosion-inducing agents, such as oxygen, chloride ions, and acidic solutions.88,114–116 Imidazolium salts constitute the most promising subclass of the ILs because their melting point, solubility, conductivity, and viscosity can be tuned by changing the substituents at the nitrogen atoms and counter ions. The imidazole scaffold is a five-membered hetero-aromatic ring containing three carbon atoms and two nonadjacent nitrogen atoms. ILs solely have a high degree of water solubility due to their strong polarity. Recent studies have revealed a considerable augmentation in the application of ImILs as green and protective agents of metal surfaces because of their low toxicity, biodegradability, economic viability and water solubility. The incorporation of the long-chain alkyl group enhances anticorrosive activity by boosting the hydrophobicity of ImILs. It has been conceptualized that the non-polar alkyl chain (tail part) points towards the bulk electrolyte, while the electron-rich polar hydrophilic imidazolium moiety (head part) adheres to the metallic surface. In the current scenario, ImILs have exquisite applications in the field of corrosion inhibition.43,117,118 For instance, Guo et al. reported an ImIL, namely, 1-dodecyl-3-methyl imidazolium hydrogen sulfate (DMIMHS), as a green corrosion inhibitor for the protection of carbon steel during oil and gas corrosion.119 CO2-saturated brine solution (NaCl) was selected as the experimental corrosive medium, and the electrochemical investigations were carried out at 313 K temperature. In the EIS study, it was observed that the capacitive arc diameter increased significantly with the addition of the DMIMHS inhibitor to the electrolytic solution, indicating the protective nature of DMIMHS. The increase in the phase angle peak suggests that frequency dispersion occurs on the electrode surface. It is noteworthy that the curves obtained in the Nyquist plot were not perfectly semicircles, signifying that the metal–electrolyte interface does not possess an ideal capacitive nature. The lack of an ideal capacitive nature of the electrode occurs because of the surface roughness, chemical inhomogeneity, and protective layer formation on the electrode. This occurrence is called the frequency dispersion effect. In the Nyquist plot, the radius of the semicircle curve increases, and the time constant value shifts to a smaller frequency zone with the immersion time of the working electrode in the corrosive medium. This outcome reflects the formation of the barrier films of the corrosion product on the working electrode surface. The SEM images of the top surface views may sometimes not provide accurate information regarding the surface of the metal. It was found from the cross-section morphologies of the samples immersed for 48 hours in CO2-saturated NaCl brine without DMIMHS and for 120 hours with DMIMHS that a layer of corrosion product was formed in the metal-inhibitor interface after the immersion of the targeted metal. With the increase in immersion time, i.e., after immersion of 120 hours, the nature of the corrosion product layer did not change, but the thickness of that film increased significantly.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N). This suggests that the imidazolium counterpart may exist on the steel exterior. The N 1s signals, as depicted in Fig. 12(f), display a signal at around 400.1 eV, which corresponds to the amine group (–NR3).
N). This suggests that the imidazolium counterpart may exist on the steel exterior. The N 1s signals, as depicted in Fig. 12(f), display a signal at around 400.1 eV, which corresponds to the amine group (–NR3).
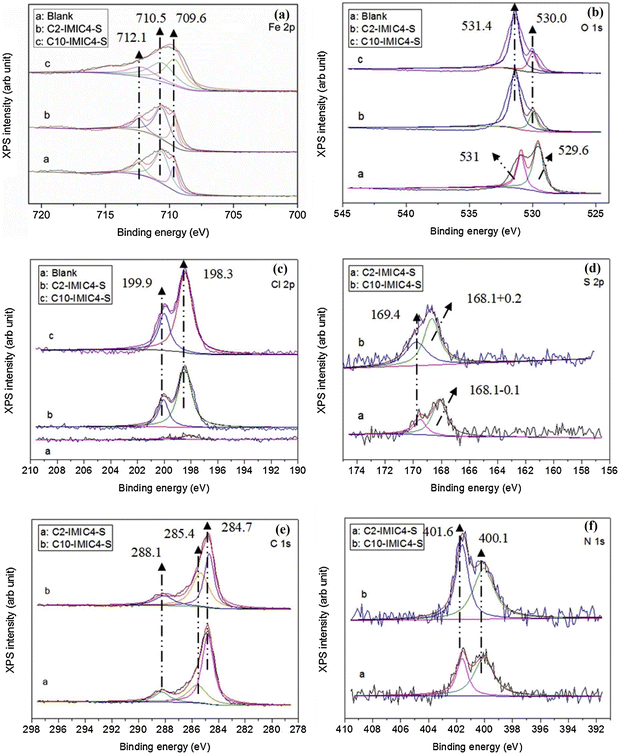 | ||
| Fig. 12 XPS spectra for (a) Fe 2p, (b) O 1s, (c) Cl 2p, (d) S 2p, (e) C 1S, and (f) N 1s elements on the steel surface after 24 hours of immersion with and without 15 mM IL in 0.5 HCl solution [reprinted with permission from ref. 62, ©2019 Elsevier]. | ||
However, the signal at 401.6 eV is attributed to the quaternary nitrogen (NR4+) that indicates a coordinated nitrogen atom and a C–N–Fe bonding. By analyzing the peaks in the C 1s and N 1s spectra together, it can be inferred that the bonding of C and N species with the iron surface occurs in distinct ways, suggesting that the ImILs, along with the anion, can adhere to the steel surface as a whole. Hence, chemical adsorption is supported by XPS analysis.
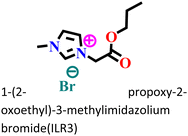
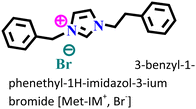
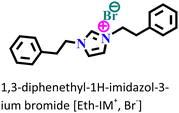
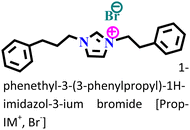
It was found that the ImIL with a larger alkyl end triggered a more hydrophobic nature. In addition, the adsorptive nature of ImILs on the steel exterior results in the displacement of water molecules, which helps reduce the attack of hostile species. Consequently, a drop in double-layer capacitance was observed. Similarly, Haldhar et al. synthesized and characterized three ImILs, namely, 1-(2-methoxy-2-oxoe thyl)-3-methylimidazolium bromide (ILR1), 1-(2-ethoxy-2-oxoethyl)-3-methylimidazolium bromide (ILR2), and 1-(2-propoxy-2-oxoethyl)-3-methylimidazolium bromide (ILR3) with different chain lengths.123 From the electrochemical and morphological analyses, the IE order was achieved as ILR1 < ILR2 < ILR3 for the protection of A1010 steel in 1 M HCl media. Even in the AFM study, the surface roughness of the mild steel immersed in 1 M HCl was found to be very high, while surface roughness started diminishing with the addition of ImILs. Notably, the surface roughness was significantly lower in the presence of ILR3 than in the presence of ILR1 and ILR2. The extended chain length of ILR3 was ascribed to its superior adsorption and strong corrosion inhibition capabilities compared to ILR2 and ILR1. Recently, Hajjaji et al. synthesized three new eco-friendly ImILs, namely, 3-benzyl-1-phenethyl-1H-imidazol-3-ium bromide [Met-IM+, Br−], 1,3-diphenethyl-1H-imidazol-3-ium bromide [Eth-IM+, Br−] and 1-phenethyl-3-(3-phenylpropyl)-1H-imidazol-3-ium bromide [Prop-IM+, Br−].124 The anticorrosive effectiveness of these as-synthesized ILs in the presence of 37% HCl medium has been investigated thoroughly experimentally and theoretically. Electrochemical experimentations (EIS and PDP) were carried out in a 37% HCl medium in the absence and presence of various dosages of the ILs at various temperatures. The single semicircles obtained in the Nyquist plot indicated a charge transfer transition in the metal–electrolyte interface, i.e., controlled corrosion was taking place. The depressed nature of the semicircles was obtained due to the surface roughness and the surface inhomogeneity. The data obtained from EIS were nicely fitted to the Randles equivalent circuit model. It was also observed that the Rct values of the electrolyte solvent increased with the gradual addition of ILs. As the Rct values are directly proportional to the adsorption capability of the compound, greater adsorption was attributed to [Prop-IMp, Br−] because of its highest Rct value. The order of IE of the ILs was [Prop-IM+, Br−] (97.3%) > [Eth-IM+, Br−] (95.7%) > [Met-IM+, Br−] (95.5%). These findings also indicate the stronger adsorption efficiency of [Prop-IM+, Br−] than the other two ILs. The adsorption nature was understood from the Arrhenius plot, i.e., ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) icorrvs. 1000/T and ln (icorr/T) vs. 1000/T plots. From these plots, the activation energy (Ea), activation entropy (ΔHa) and activation enthalpy (ΔSa) were successfully calculated from the slopes and intercepts. The high value of Ea indicates the physisorption of the ILs. The endothermic nature of the metal dissolution reaction process was established by the high positive values of ΔHa.
icorrvs. 1000/T and ln (icorr/T) vs. 1000/T plots. From these plots, the activation energy (Ea), activation entropy (ΔHa) and activation enthalpy (ΔSa) were successfully calculated from the slopes and intercepts. The high value of Ea indicates the physisorption of the ILs. The endothermic nature of the metal dissolution reaction process was established by the high positive values of ΔHa.
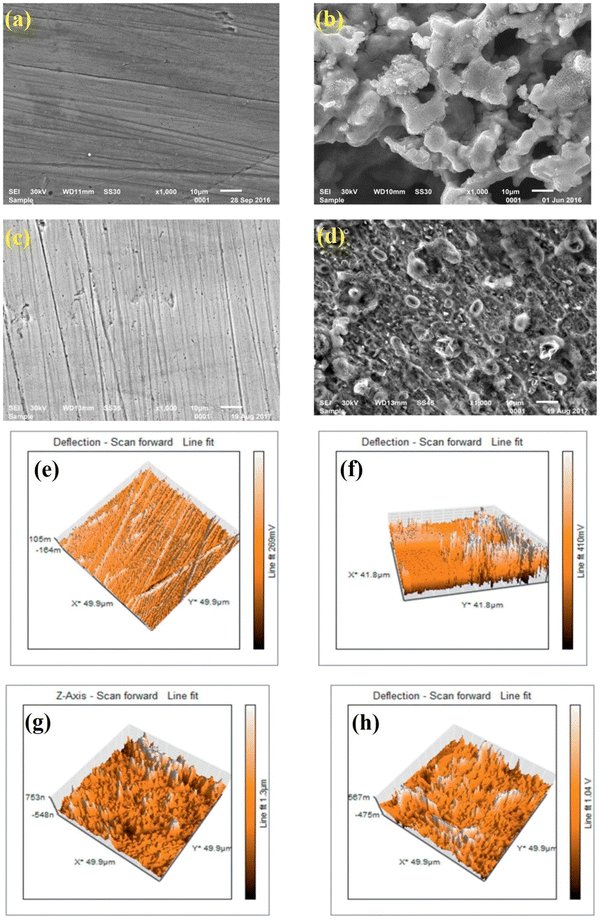 | ||
| Fig. 13 SEM (a–d) and AFM (e–h) images of (a and e) the polished mild steel, (b and f) after immersion within 0.5 M H2SO4 without IL, (c and g) with 10−2 M [FBMIm]Br, and (d and h) with 10−5 M [FBMIm]Br. [Reprinted with permission from ref. 126, ©2019 Elsevier.] | ||
At 298 K, the morphological images of the pre- and post-corroded surfaces depict the surface protection of ImIL at a higher concentration (10−2 M) than that of a lower concentration (10−5 M). The surface roughness was also significantly low in the case of 10−2 M [FBMIm]Br/H2SO4 solution. The increase in corrosion retardation is based on two main factors: first, the adsorption of [FBMIm]Br on the metal surface and second, the synergistic effect induced by bromide ions. The negatively charged ions (SO42−) of corrosive media compete with Br− ions in adsorption on metals. Thus, the formation of an insoluble cationic barrier of ImILs is intensely expedited.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of IL
1 ratio of IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KI showed 96.2% IE, wherein the same concentration of the ImIL exhibited 94.8% IE. The theoretical findings supported the experimental outcomes.
KI showed 96.2% IE, wherein the same concentration of the ImIL exhibited 94.8% IE. The theoretical findings supported the experimental outcomes.
Similarly, other ILs, such as (E)-4-(4-nitrobenzylideneamino)benzoate (SNBB), 3,3′-diethylthiadicarbocyanine iodide (DI), and Thioflavin T (TT), are also preferable contenders to combat the adverse effects of corrosion.128,129
| Sl. no. | Ionic liquid (Structure/IUPAC name) | Metal surface and medium | Adsorption mechanism | Isotherm model | Highlights | Ref. |
|---|---|---|---|---|---|---|
| 1 |
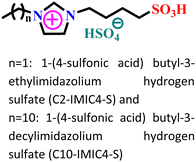
|
Carbon steel/0.5 M HCl | Physisorption, chemisorption and mixed | Langmuir | (i) IE: 80.8% for C2-IMIC4-S and 97.9% C10-IMIC4-S@15 mM and 25 °C | 62 |
| (ii) The larger chain length of C10-IMIC4-S is responsible for its better adsorption on metals | ||||||
| 2 |
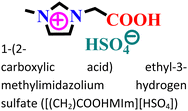
|
Mild steel/0.5 M HCl | Both physisorption and chemisorption | Langmuir | (i) IE: 75.8% for [(CH2)COOHMIm][HSO4], 89.5% for [(CH2)2COOHMIm][HSO4] and 93.0% for [(CH2)3COOHMIm][HSO4]@10 mM and 298 K | 120 |
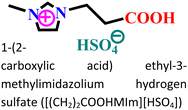
|
||||||
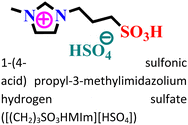
|
||||||
| (ii) The functional groups and the additional alkyl group within the cationic part increased the inhibitory process of the [(CH2)3SO3HMIm][HSO4] IL | ||||||
| 3 |
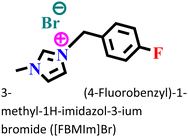
|
Mild steel-/0.5 M H2SO4 | Physical and chemical adsorption | Langmuir | (i) IE: 98.90% @0.01 M and 298 K | 126 |
| (ii) The synergistic effect of bromide (Br−) results in a better protective nature of this IL | ||||||
| 4 |
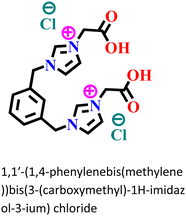
|
Carbon steel/0.5 M HCl | Predominant chemisorption | Langmuir | (i) IE: 94.8% for 1 mM IL and 96.2% for IL![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KI of 1 KI of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
127 |
| (ii) IL and KI inhibited the corrosion process through strong synergism | ||||||
| 5 |
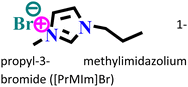
|
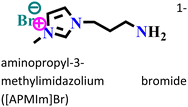
Mild steel/0.5 M H2SO4 | Physicochemical adsorption | Langmuir | (i) IE: 60.4% for [PrMIm]Br, 86.4% [APMIm]Br and 91.0% for [BMImB]Br2@10 mM and 303 K | 125 |
|
||||||
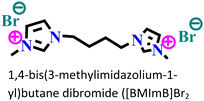
|
(ii) An increase in corrosion IE was triggered by an increase in the chain length within the cationic part | |||||
| 6 |
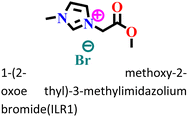
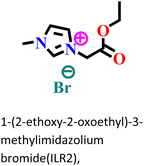
|
A1010 steel/1 M HCl | Physisorption and chemisorption | Langmuir | (i) IE: 84.50% for ILR1, 92.95% for ILR2 and 95.77 for ILR3@1 mM and 298 K | 123 |
|
||||||
| (ii) Increased IE resulted in increased surface coverage with elongation of the aliphatic chain length | ||||||
|
||||||
| 7 |
|
Mild steel/37% HCl | Predominantly chemisorption | Langmuir | (i) IE: 95.5% for [Prop-IM+, Br−], 95.7% for [Eth-IM+, Br−] and 97.3% for [Met-IM+, Br−]@10−3 M | 124 |
|
||||||
| (ii) An increase in the carbon number within the aliphatic moiety facilitated better adsorption of the IL, which was further associated with the +I effects of the alkyl groups | ||||||
|
||||||
| 8 |
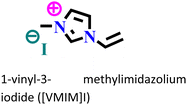
|
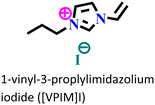
X70 steel/0.5 M H2SO4 | Physisorption and chemisorption | Langmuir | (i) IE: 96% for [VMIM]I, 97% for [VPIM]I and 99.4% [VBIM]I@5 mM and 298 K | 122 |
| (ii) An increase in IE was associated with an increase in alkyl chain length | ||||||
|
||||||
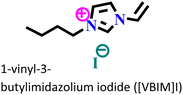
|
||||||
| 9 |
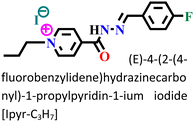
|
Mild steel/1 M HCl | Both physisorption and chemisorption | Langmuir | (i) IE: 88.6% for Ipyr-C3H7, 87.8% for Ipyr-C5H11@10−3 M and 298 K | 104 |
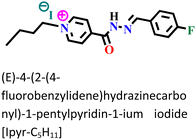
|
||||||
| (ii) These ILs showed better protective ability with varying temperature ranges and different immersion times | ||||||
| (iii) An increase in IE was observed for the IL with the alkyl group of four C, but after more increase in the alkyl group, IE decreased because of agglomeration |
7. Cutting-edge computational approaches to assess the performance of the ILs in corrosion inhibition
Cutting-edge computational approaches are revolutionizing the field of corrosion inhibition. As computational power continues to increase and algorithms become more sophisticated, we can expect even more accurate and reliable predictions of the performance of ILs. This leads to the development of more effective and sustainable solutions for protecting metallic substances. Accordingly, the intriguing factors influencing the corrosion suppression performance of the ILs are discussed in light of computational modeling analysis. Undoubtedly, a thorough understanding of the inhibition process requires electronic-level insights between the interacting inhibitor molecules and metal substrates. Consequently, different computational approaches, such as density functional theory (DFT), density functional tight binding (DFTB), molecular dynamic (MD) and Monte Carlo (MC) simulations, for the congregation of results coming from different experimental outcomes can be an additional authenticate tool to strengthen the inferences. With these consequences, several researchers and scientists worldwide have also performed proper DFT studies, which strengthen the observations that come from experimental observations. However, before going for modelling, it is always an imperative criterion to consider a system wherein the electrons reside, and its quantitative approach is very crucial. In this context, both DFT and DFTB calculations are important. Over the last few decades, research on corrosion inhibition mechanisms has effectively utilized first-principles calculations based on the fundamental equations of quantum theory, which explain the behaviour of electrons, atoms, and molecules. DFT has been utilized to understand electronic or quantum chemical parameters, while DFTB can do so in a more directive manner.131,132Several regulating factors, such as chain lengths, functional groups, and aromaticity, affecting corrosion inhibition effectiveness have been well explained through several efficient computational aspects.131,133,134 In addition, the complicacy of the corrosion inhibition mechanism has driven the adaptation of the quantitative structure activity relationship (QSAR) model. QSAR analysis increasingly influences the electronic nature of the molecules described by wave functions. Quantum descriptors incorporating both electronic and molecular information are preferred for the development of the QSAR model. These descriptors provide predictions regarding the theoretical IE of the studied system.
7.1. Insight through the first principle calculation
DFT provides a powerful framework for simulating the interactions between ILs and metal surfaces at the atomic level. DFT analysis allows for the evaluation of the adsorption energy (Eads) of ILs on metal surfaces. This information is crucial for understanding the strength of the bond between the IL and the metal, which directly impacts the effectiveness of corrosion inhibition. By analyzing the charge density distribution and the interaction between the orbitals of the IL and the metal, researchers can gain insights into the mechanism of corrosion inhibition. By computationally screening large numbers of ILs, researchers can identify promising candidates for further experimental evaluation.106,135Computational studies based on DFT are commonly employed to illustrate the donor–acceptor nature of the two interacting species.136–139 Various important electronic parameters, such as the highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbitals (LUMO), and associated energies (EHOMO and ELUMO), their energy difference (ΔE), electronegativity, electron affinity, and the fraction of electrons transferred between the inhibitor and metal, are determined from DFT calculations. The electronic distribution in the frontier molecular orbitals (FMOs i.e., HOMO and LUMO) is admirably an important aspect in understanding the electron-donating or accepting sites. Generally, the donor property of the inhibitor increases with an increase in the HOMO value. In a recent work based on cetylpyridinium picrate (CPP) IL, it is observed that the electron cloud is mainly located on the anionic part of the protonated and neutral IL, as depicted in Fig. 14(a) and (b).15 This suggests that the anionic part is mainly responsible for the electron donation or acceptance properties. It is worth mentioning that the electronic orbitals strongly participate in the adsorption process. In the case of 1-octyl-3-methylimidazolium L-prolinate ([Omim]Lpro) ImIL, the cationic part is mainly responsible for chemisorption because of its lower ΔE value.140
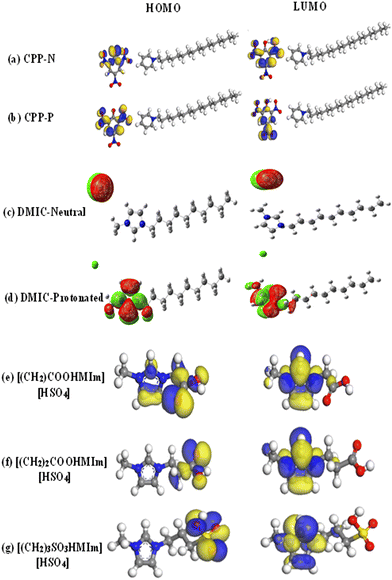 | ||
| Fig. 14 Pictorial representation of the HOMO and LUMO orbitals of (a) CPP-N, (b) CPP-P, (c) DMIC-Neutral, (d) DMIC-Protonated, (e) [(CH2)COOHMIm] [HSO4], (f) [(CH2)2COOHMIm] [HSO4] and (g) [(CH2)3SO3HMIm] [HSO4] (red colour: oxygen, grey colour: carbon, blue colour: nitrogen, white colour: hydrogen, yellow colour: sulphur). [Reprinted with permission from ref. 15, ©2023 Elsevier, ref. 39, ©2021 Elsevier, and ref. 115, ©2020 Elsevier.] | ||
From Fig. 14(c) and (d), it is evident that the distribution of HOMO in both neutral and protonated forms of 1-decyl-3-methylimidazolium chloride (DMIC) is centred on the chloride ion. The LUMO is concentrated on the cationic portions of the imidazoline rings of the neutral and protonated forms, that is the anionic counterpart takes part in electron donation during the adsorption process. Additionally, the cationic imidazolium part is involved in the electron acceptance process. Higher EHOMO, lower ELUMO and lower ΔE values in the case of neutral form than the protonated form of the ImIL suggest better adherence of the neutral form on the P110 steel when immersed in 15% HCl medium. To understand the effect of functional group, the HOMO–LUMO plots of [(CH2)COOHMIm][HSO4], [(CH2)2COOHMIm][HSO4] and [(CH2)3SO3HMIm][HSO4] play a vital role (vide, Fig. 14(e), (f) and (g)). Particularly, the partial imidazole ring and –COOH group contain the majority of the HOMO of the cation of [(CH2)COOHMIm][HSO4]. However, HOMO is mostly found in the –COOH group for [(CH2)2COOHMIm][HSO4]. In contrast, the –SO3H group is the central point of the HOMO of [(CH2)3SO3HMIm][HSO4]. Therefore, functional groups play a principal role in adsorption through electron donation.
Additionally, in several recent studies, the identification of local reactive sites within the inhibitor has been aided by the analysis of Fukui indices (FIs).14,17,42 Herein, fK+ and fK−, are designated as the corresponding Fukui functions that induce the respective sites to react via nucleophilic and electrophilic reaction pathways, respectively. In a recent study, FI analysis was executed to unveil the location of the reactive atoms within the L-alanine methyl ester nitrate (LAlaC1NO3) AILs.42 It was observed that the nucleophilic attacks were more facilitated on the anionic parts of the IL as the fK+ values were exceptionally high on those sites.
7.2. Insight through DFTB
Although the DFT approach is the most popular and widely used ab initio technique, this first principal calculation is unable to provide atomistic details regarding the inhibition mechanism of large inhibitors. Accordingly, theoretical approaches to address the adsorption issue of large-sized inhibitors must be found to gain a deeper understanding of the mechanistic roadmap of the inhibition process. Researchers have realized that studying the adsorption phenomena of organic macromolecules on metal surfaces may be possible using this quasi-empirical DFTB method. DFTB consolidates the tight binding technique with the higher accuracy and efficiency of the ab initio DFT approach.141In this regard, an investigation of the adsorption of 1-butylpyridinium cations onto the Fe(110) plane was carried out using the DFTB study.35 It was observed that the electron-rich benzene moiety of the corrosion inhibitors 1-butylpyridinium chloride (BPC) and 1-butylpyridinium hexafluorophospate (BPH) allowed their cationic components to be adsorbed horizontally on the Fe(110) plane vide, as depicted in Fig. 15(a) and (b). The 1-butylpyridinium moiety of the BPC and BPH ILs has a considerable adsorption capability, as indicated by the extremely negative magnitude of Eads. Fig. 15(c) depicts the plotted charge density differences triggered by the adsorption of 1-butylpyridinium cation on the Fe(110) surface. Fig. 15(c) demonstrates that when the 1-butylpyridinium moiety comes closer to the iron surface atoms, the adsorption of the 1-butylpyridinium moiety occurs. The contact and electron sharing between the 1-butylpyridinium moiety and the iron surface atom resulted in the blue area displaying electron-rich sites and the red region displaying electron-deficient sites.
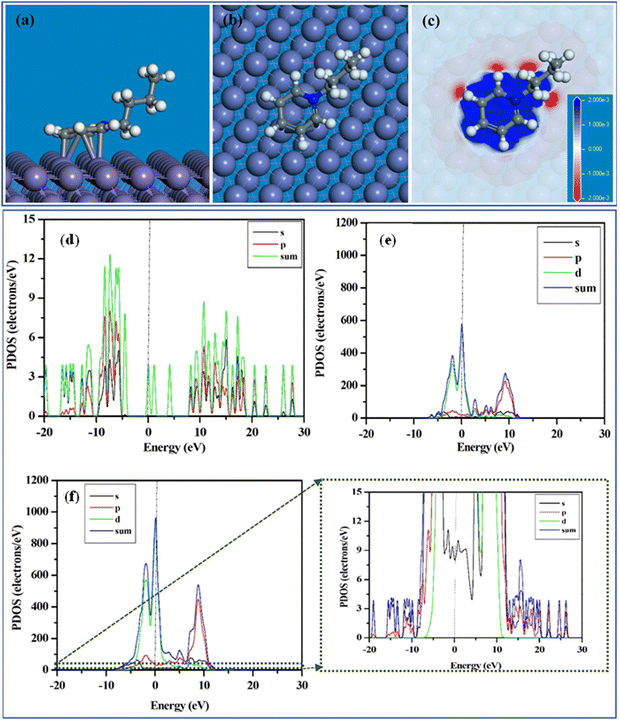 | ||
| Fig. 15 (a) Side view, (b) top view, (c) charge density difference plot of the equilibrium adsorption configuration of 1-butylpyridinium cation on Fe(110) surface; partial density of states (PDOS) of (d) 1-butylpyridinium cation, (e) iron surface and (c) 1-butylpyridinium cation after adsorption on Fe(110) surface. [Reprinted with permission from ref. 35, ©2023 Elsevier.] | ||
Accordingly, it could be concluded that the 1-butylpyridinium moiety appears to promote the adsorption of the ILs. To shed further light on the nature of the interactions, the projected density of states (PDOS) to the ILs and the Fe atoms that bond to the respective cationic moiety were plotted. Fig. 15(d) and (e) illustrate the PDOS plot of the 1-butylpyridinium moiety and iron surface before adsorption. Fig. 15(f) illustrates the PDOS plot that emerged owing to the interactions of the 1-butylpyridinium moiety on the Fe(110) surface. Notably, the vertical dotted line indicates that the Fermi energy level was set to zero. The inhibitor peaks are sharper prior to adsorption, but these peaks start broadening with adsorption, indicating robust adsorption between the cationic part of the PyIL and the Fe surface. Similarly, compared to the PDOS peaks for Fe before adsorption, the Fe peaks after adsorption are not as sharp before adsorption. Accordingly, from the PDOS analysis, it can be inferred that the presence of an aromatic moiety enhanced the adherence tendency of the ILs onto the targeted metal exterior.
7.3. Insight through the light of MD simulation
In the current scenario, MD simulation has attracted superior attention in the scientific community owing to its simpler, more affordable technique and capability to produce molecular insights.142 In other words, the key objective of MD simulation is to gather molecular-level information regarding the structure and dynamics of a classical main-body system under equilibrium, as governed by the classical Newtonian mechanism.143 MD simulation can assist in determining the macroscopic parameters of a system, such as the coordination number, radial distribution function (RDF), average potential energy, diffusion, and density of systems containing a significant number of molecules.142,144 An analysis of MD simulations can reveal the details regarding the orientation of molecules on metal surfaces, as well as the interaction energy (Eint) and binding energy (Ebind). MD simulation is considerably utilised in the vicinity of corrosion inhibition by ILs. If the total energy of the simulated system, the energy of the IL and the energy of metal with the simulated corrosive electrolytic solution are ET, EIL and EM+E respectively, then the Eint and Ebind are as follows (videeqn (1) and (2)):15,145–149| Eint = ET − (EM+E + EIL) | (1) |
| Ebind = −Eint | (2) |
The higher the negative value of Eint, the better the interaction between the IL and the metal plane. From the orientation of the ILs, it is clear which structural part of the IL is actually taking part in adsorption and how. Accordingly, the effect of the regulating factors can be explained satisfactorily. Recent works based on MD simulation have been discussed, vide infra. Recently, in 2021, Singh et al. reported the anticorrosive behaviour of three commercially available PiILs, namely, IL-1, IL-2, and IL-3, for retarding the corrosion rate of Q235 steel in the presence of 1 N HCl.100 In the MD simulation study, the three ILs with different chain lengths were separately allowed to get adsorbed on the Fe(110) plane in the presence of 150 H2O, 15 H3O+ and 15 Cl− ions. The equilibrium adsorption configurations revealed the flat horizontal orientation of the ILs, videFig. 16.
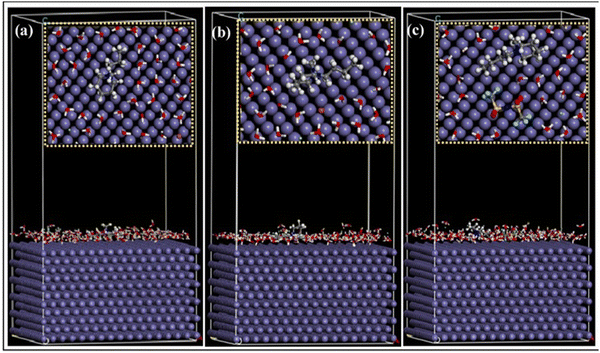 | ||
| Fig. 16 Side view of the optimized adsorption configuration of (a) IL-1; (b) IL-2 and (c) IL-3 on the Fe(110) plane in a simulated acid medium (i.e., in the presence of 150 H2O, 15H3O+ and 15Cl−) obtained from MD simulation (inset: top view). [Reprinted with permission from ref. 100, ©2021 Elsevier.] | ||
The Eint values for IL-1, IL-2, and IL-3 were found to be −660.56 kJ mol−1, −710.46 kJ mol−1, and −906.56 kJ mol−1, respectively. This outcome implies that the ILs interact in the sequence IL-2 > IL-1 > IL-3, validating the experimental outcomes. The effect of alkyl chain length was lucidly elaborated through the MD simulation. From the Eint values, it was observed that IL-2 and IL-3 have a higher interaction capability with the metal, which may be attributed to the presence of longer alkyl chains. However, IL-3 possesses a better interacting capability than that of IL-2. This fact can be explained by the presence of the larger anionic moiety within IL-3, which facilitates better surface coverage.
To achieve the effect of the aromatic moiety on the anticorrosive nature of AmILs, an MD simulation study accompanied by an RDF analysis was performed.97 In the MD simulation study, the three AmILs were allowed to get adsorbed on the Fe(110) surface in the presence of H2O, H3O+ and Cl− ions. As per the equilibrium adsorption model, when the ILs bind to the Fe(110) crystal surface, it does not allow for the presence of any H2O, H3O+ and Cl− ions between the ILs and the metal exterior. This indicates that the ILs replace any pre-adsorbed solvent or other ionic species in the electrolyte medium and form a robust shielding layer in the metal electrolyte interface. Similarly, the adsorption patterns of the [Ch]+ cations of ILs are almost identical on the Fe(110) surface. Therefore, the discrepancy in corrosion mitigation effectiveness can be ascribed primarily to the alterations in anions. The anionic parts of the AmILs, including the benzene ring portion, amino group, and indole moiety, orient themselves in a parallel fashion on the Fe surface, while the carboxyl group exerts itself outward. The carboxyl group aids in enhancing the surface coverage and facilitates strong bonding with the underlying surface, leading to more stable adsorption. Based on the results of the MD simulations, it can be concluded that the pre-occupied solvent or other corrosive species can be replaced by corrosion inhibitors. The effective estimation of bond length can be achieved by applying RDF, denoted by g(r).99,150–153 It is possible to extract insights into the interaction between the simulated metal surface and the AmILs by examining the peaks. Chemisorption of the ILs was evidenced by the appearance of the first peak within the range of 1–3.5 Å, while physisorption was confirmed by the appearance of a peak greater than 3.5 Å. These outcomes indicated that chemisorption is the predominant factor during the adsorption of the AmILs. The corrosion inhibition is schematically presented in Fig. 17.99
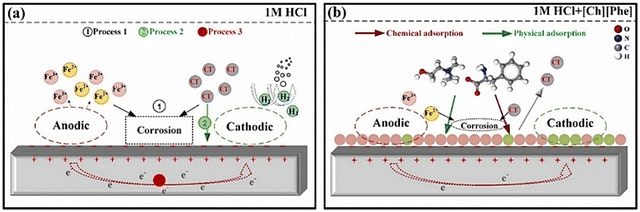 | ||
| Fig. 17 Schematic representation of the (a) corrosion mechanism of mild steel in the presence of 1 M HCl and (b) corrosion inhibition mechanism of mild steel in the presence of [Ch][Phe] in 1 M HCl solution. [Reprinted with permission from ref. 99, ©2023 Elsevier.] | ||
The metal surface can be significantly safeguarded against corrosion by acidic solutions when choline amino acid AmIL corrosion inhibitors are adsorbed on it in a mixed manner, i.e., physisorption and chemisorption. It is evident from process-1 in Fig. 17(a) that there is a diminishing tendency of electrochemical reactions (cathodic and anodic), resulting in a drop in electron transfer within the metal surface, vide process 3. Moreover, the adsorption of the AmILs leads to the displacement of Cl− ions pre-adsorbed on the mild steel exterior, that is desorption of Cl− takes place. As depicted in Fig. 17(b), it is clearly visible that the adsorbed Cl− anions attract cations of the AmILs through strong electrostatic interaction. Consequently, the physisorption of AmILs takes place, and the hydrogen generation reaction in cathodic sites is reduced. However, heteroatoms, such as N and O, and unsaturated bonds participate in donor–acceptor interactions with the molecular orbitals of the metal, which facilitates chemisorption of the ILs. Both the theoretical analysis and experimental findings yield a similar order of IE: [Ch][Phe] > [Ch][Trp] > [Ch][Tyr].104 RDF analysis is employed to identify the type of interaction between the adsorbate and the adsorbent, whether it is physisorption, chemisorption, or both. Fig. 18 illustrates that the first peak values for each bond length of Ipyr-C3H7 and Ipyr-C5H11 adsorption on the Fe(110) surface at a simulated temperature of 298 K suggest chemisorption interaction for Fe–N9 (2.77 Å), Fe–N10 (2.86 Å), and Fe–N17 (2.79 Å) bond lengths.
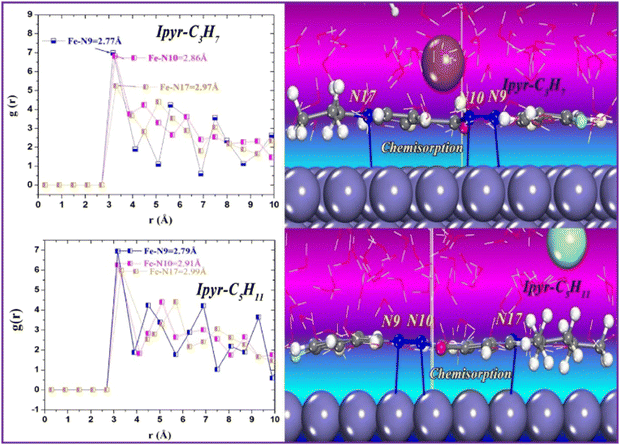 | ||
| Fig. 18 RDF analysis of adsorbed Ipyr-C3H7 and Ipyr-C5H11 on Fe(110) surface in a simulated acid medium at 298 K temperature obtained from MD simulation. [Reprinted with permission from ref. 104, ©2021 Elsevier.] | ||
Organic corrosion inhibitor molecules typically work through adsorption on a specified metal surface. The presence of an aromatic ring, azomethine linkage, and –NH– moiety present within the IL scaffold facilitates the chemisorption of the IL on the targeted metal substrate. These aforementioned factors allow the electron cloud to be shared with the empty orbitals of the metal.
Cao et al. synthesized two task-specific Brønsted acid ILs (BAILs), namely, 1-(4-sulfonic acid) butyl-3-ethyl imidazolium hydrogen sulfate and 1-(4-sulfonic acid) butyl-3-decyl imidazolium hydrogen sulfate, designated as C2-IMIC4-S and C10-IMIC4-S,62 respectively. Molecular orbital theory and MD simulation studies were conducted to corroborate the experimental observations. The equilibrium configuration after the adsorption of the cations of C2-IMIC4-S and C10-IMIC4-S on the Fe(001) surface in vacuum and the aqueous phase was considered. It can be observed that the cations of both ILs are projected in a parallel fashion, which is advantageous for achieving high coverage and effective protection against corrosion. The orientation of the imidazole ring of both the C2-IMIC4-S and C10-IMIC4-S molecules is parallel to the simulated metal surface, promoting high surface coverage and efficient IE. The Eads of the cations on the Fe(001) surface in the presence of solution followed the order of C10-IMIC4-S (−1110.74 kJ mol−1) < C2-IMIC4-S (−237.30 kJ mol−1). A more negative Eint or a more positive Ebind value indicates a higher stability of the adsorption of IL cations on the Fe(001) surface, which could lead to superior inhibition performance.
Similarly, the effects of alkyl chain length were explored for three ImILs, namely, 3-benzyl-1-phenethyl-1H-imidazol-3-ium bromide [Met-IM+, Br−], 1,3-diphenethyl-1H-imidazol-3-ium bromide [Eth-IM+, Br−] and 1-phenethyl-3-(3-phenylpropyl)-1H-imidazol-3-ium bromide [Prop-IM+, Br−] through MD simulation.124 The ILs were horizontally oriented onto the Fe(110) surface. The computed adsorption energies were found to be −197.151, −187.249 and −182.243 kcal mol−1 for [Prop-IM+, Br−], [Eth-IM+, Br−], [Met-IM+, Br−], respectively. The greater the Eads, the greater the binding of the species to the surface. Accordingly, the MD simulation study also supported the idea that [Prop-IM+, Br−] has a better adsorptive capability. Henceforth, it acted as a better corrosion inhibitor among the three ILs. In this case, the strong adsorption involved may be attributed to either physisorption, chemisorption or both. The positively charged species is responsible for physisorption because it is electrostatically attached to the Cl− ions on the metal surface. As reported, the ILs form coordinate bonds via the sharing of electrons from the filled orbitals of heteroatoms to the vacant d-orbitals of Fe metal, which facilitates chemisorption. Additionally, the π-electrons situated in the aromatic rings also take part in the donor–acceptor-like interaction, resulting in back donation, also known as retro-donation. The large surface area of the ILs helps cover the surface of the underlying metal; thus, the metal is protected from an aggressive environment. In another study, the ImIL, namely, DMIMHS, was allowed to get adsorbed on Fe(110) and FeCO3(104) at the molecular level to understand the adsorption sites along with the adsorption mechanism of the ImIL on a metal substrate.119 The top and side views of the adsorption of DMIMHS are presented on Fe(110) and FeCO3(104) surfaces, as depicted in the first two columns of Fig. 19(a), (b) and (c). The equilibrium adsorption of nine DMIMHS ILs on the Fe(110) and FeCO3(104) surfaces is depicted in the middle two columns of Fig. 19(a), (b) and (c). It was found that the DMIMHS ILs are adsorbed horizontally on the Fe(110) surface, but in the case of the adsorption on the FeCO3(104) plane, the anionic part of DMIMHS, that is HSO4−, is adsorbed on the surface, and the aromatic imidazole moiety exerts itself in the outwards of the surface. The calculated binding energy values for the adsorption of DMIMHS were found to be 280.01 kcal mol−1 and 35.21 kcal mol−1 for the Fe(110) and FeCO3(104) surfaces, respectively. These results suggest a better interaction of IL, leading to efficient adsorption and better protection of the iron surface. Based on the experimental and theoretical findings, a probable interaction mechanism is proposed for the protective mechanism of the IL on the targeted surfaces, videFig. 20.
 | ||
| Fig. 19 Optimized adsorption configuration of the adsorption of one DMIMHS on Fe(110) (first column) and FeCO3(104) (middle two columns): (a) overall view, (b) top view and (c) side view; optimized adsorption configuration of nine DMIMHS on (d) Fe(110) and (e) FeCO3(104). [Reprinted with permission from ref. 119, ©2022 Elsevier.] | ||
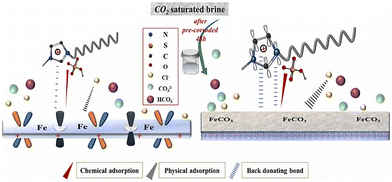 | ||
| Fig. 20 Protective mechanism of DMIMHS towards the corrosion inhibition of Fe and FeCO3 surface. [Reprinted with permission from ref. 119, ©2022 Elsevier.] | ||
From DFT analysis, it was confirmed that the HOMO orbital electron density is mainly situated in the anionic part, that is HSO4−, while the electron density of LUMO is mainly concentrated in the imidazole rings. In the presence of the brine solution, DMIMHS IL is dissociated into two ionic parts: the cationic part is the imidazolium moiety and the anionic part is the HSO4−. Accordingly, in the presence of NaCl medium, the HSO4− parts are involved in electron donation to the vacant d orbitals of Fe, resulting in chemisorption. On the contrary, the imidazolium moiety takes part in electron acceptance from the filled d orbitals of Fe, resulting in retro-donation. DMIMHS is adsorbed onto the Fe and FeCO3 surfaces via mixed adsorption, thus leading to the creation of protective layers on substrates.
In the light of theoretical evaluations, FMOs analysis, electrostatic potential analysis, reactivity indicators analysis and Fukui analysis were performed. The equilibrium adsorption configuration of CB6-based [3]rotaxane on the Fe(110) surface was successfully obtained through an MD simulation study.97 From the side and top views, it can be observed that the IL is adsorbed onto the metal surface in a parallel fashion, as presented in Fig. 21(a). The IL can form a dense protective barrier onto the metal surface to prevent the penetration of corrosive particles, as observed in the density field distribution study, as presented in Fig. 21(b). Additionally, the parallel orientation of the IL after adsorption on the metal surface was confirmed by relative concentration distribution analysis, as presented in Fig. 21(c). The z-axis of the Fe(001) plane possesses a greater value of relative concentration. The relative concentration of water molecules was found to increase in the absence of IL molecules, whereas it decreased in the presence of IL molecules. This signifies that the presence of IL molecules hinders the approach of water molecules towards the underlying metal body, as depicted in Fig. 21(d).
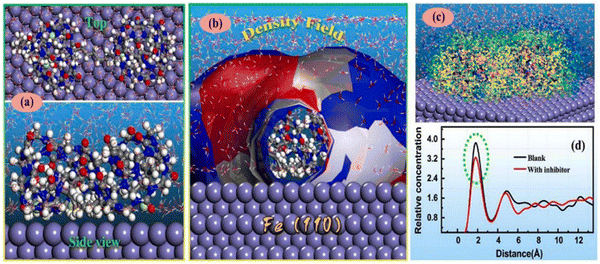 | ||
| Fig. 21 (a) Optimized adsorption configuration of CB6-based [3]rotaxane on the Fe(110) surface, (b) density field distribution, (c) concentration distribution analysis, and (d) concentration distribution of water. [Reprinted with permission from ref. 97, ©2022 Elsevier.] | ||
Again, the diffusion behaviour of a molecule can be reliably measured by its diffusion coefficient (D).95 The diffusion coefficient indicates how fast a corrosive species can migrate in inhibitor films. Higher inhibition effectiveness results originate from lower D values, i.e., a higher degree of corrosion prevention by the inhibitor film. The Einstein diffusion equation is typically used to obtain D from the mean square displacement (MSD). The MSD diagram through the inhibitor membranes displayed a subtle and minimal increase throughout the first simulation process. However, when the simulation period was prolonged, the MSD diagram showed a steady rise in the vacuum. The present D-values of H2O computed in vacuum and membranes are 2.2 × 10−10 and 2.0 × 10−11 m2 s−1, respectively for thirty BKC cations and thirty chloride anions containing inhibitor film. Accordingly, the inhibitor membranes could efficiently restrict the migration of corrosive particles. For a better understanding, the free volume present within the inhibitor film was analyzed. The free volume within the inhibitor film indicates the available cavity for the migration of corrosive species. On the contrary, a small free volume indicates a better protective nature of the inhibitor. The equilibrium configuration and the free volume distribution with and without chloride ions are displayed in Fig. 22.
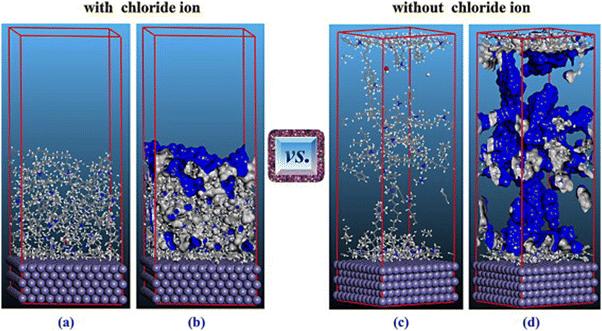 | ||
| Fig. 22 (a) and (c) Equilibrium configuration and (b) and (d) free volume distribution of the inhibitor film of BKC cations with and without chloride ions.[Reprinted with permission from ref. 95, ©2015 Elsevier.] | ||
The fraction free volumes of the inhibitor film were 52% and 65% in the presence and absence of chloride ions, respectively. From these observations, it can be concluded that the presence of chloride ions has a huge impact on the protective nature of the inhibitors.6,45
7.4. Insight through MC simulation
Similar to the MD simulation, the MC simulation has become a popular computational simulation approach to comprehend the as-desired observables. MC simulations are simpler than MD simulations because there is no need to consider forces.135,154–156 The descriptors derived from MC simulation are as follows: total energy (ET) represents the summation of the energies of the inhibitor and the metal surface; Eads represents the energy released following the adsorption of the adsorbate molecules (herein, IL inhibitors); rigid energy (Erigid) represents the energy released following the adsorption of one mole of energetically non-relaxed adsorbate; deformation energy (Edef) represents the energy released following the relaxation of one mole of adsorbate on the adsorbent surface; and the energy of the adsorbate following the removal of one adsorbate component denoted by dEads/dNi.157,158 By increasing the Eads value, the interaction between the ILs and the metal is regarded to be stronger, that is better surface protection is achieved. Typically, the lowest energy adsorption sites are obtained using MC simulation techniques to determine the favoured adsorption sites on the metal surface. In several studies, MC simulation has been accepted as an efficient tool in determining the influence of the triggering effects on the adherence of the ILs on the metal surface.42 Recently, an MC simulation study was performed to investigate the orientation of two PILs, namely, DDPC and DDQC, after adsorption on the metallic surface.105Fig. 23(a) and (c) depict the actual arrangement of the ILs. Herein, adsorption energies were obtained as −119.27 kcal mol−1 and −194.43 kcal mol−1 for DDPC and DDQC, respectively, that is DDQC served as a better surface protective agent and was further validated by theoretical analysis.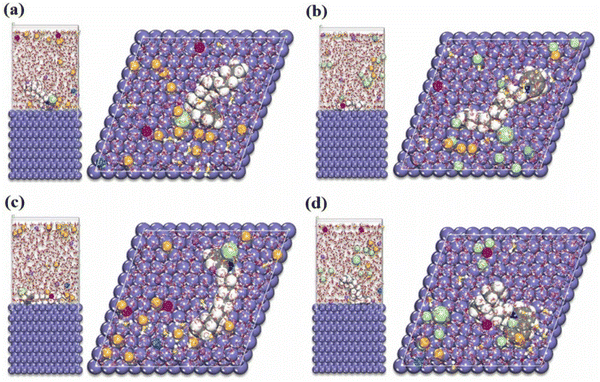 | ||
| Fig. 23 Adsorption configurations obtained from (a) MC and (b) MD simulation for DDPC and (c) MC and (d) MD simulation for DDQC. [Reprinted with permission from ref. 105, ©2023 Elsevier.] | ||
During the MC simulation, inhibitors were subjected to orient themselves in a horizontal fashion, in which pyridine rings orient themselves in a planar way in the “decorative direction”. In this orientation, the heteroatoms, herein nitrogen, and electron-rich moieties come closer to the metal surface to make strong adsorption feasible.
7.5. Insight through QSAR analysis
QSAR analysis has now become an important tool to elucidate the relationship between the structural and physicochemical properties of ILs with corrosion inhibition efficiencies. To correlate the quantum chemical descriptors with the experimental findings, both linear and non-linear equations are used, as proposed by Lukovits.159–162 These models include regression coefficients, various quantum chemical parameters and the experimental concentrations of the inhibitors. The linear and non-linear equations are expressed as mentioned in eqn (3) and (4), respectively:| IEtheory = AXiCi + B | (3) |
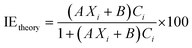 | (4) |
Seven linear equations and eight non-linear equations were developed by Murulana et al.163 It has been observed that at least two quantum chemical parameters are absolutely necessary to achieve a strong agreement with the experimentally obtained results. Yesudass et al. investigated the correlation between the electronic parameters and subsequent corrosion inhibition efficiencies of five IMILs, namely, 1-ethyl-3-methylimidazolium ethylsulfate [EMIM]+ [EtSO4]−, 1-ethyl-3-methylimidazolium acetate [EMIM]+ [Ac]−, 1-butyl-3-methylimidazolium thiocyanate [BMIM]+ [SCN]−, 1-butyl-3-methylimidazolium acetate [BMIM]+ [Ac]− and 1-butyl-3-methylimidazolium dicyanamide [BMIM]+ [DCA]− to suppress the mild steel corrosion rate in HCl.164 The QSAR analysis signifies that the IE of the ImILs is heavily influenced by the descriptors, including molecular weight, dipole moment and fraction of electron transferred from ImIL to the metal surface. The increased IE of [EMIM]+ [EtSO4]− compared to [EMIM]+ [Ac]− is due to the presence of more electronegative heteroatoms, S than O. Besides, the presence of an extended alkyl chain within the [BMIM]+ compared to [EMIM]+ results in better IE in [BMIM]+ series. In another study by Chen and co-workers, thirty-six ImILs with various anions, including halides, such as Cl−, Br−, and I− along with N(CN)2−, BF4−, PF6− and varying alkyl chain lengths with 0, 1, 2, 3, 4, 5 and 6 number of carbons were studied to analyse the nature of adsorption on iron surface.165 It was observed that the Eads values of the ImILs were significantly influenced by the alkyl chain length of the cations, as demonstrated by applying the dispersion-corrected DFT (DFT-D3) method.
Additionally, DFT showed higher accuracy in predicting the adsorption process similar to MD simulation. It was observed that the Eads value increased with the enhancement of the radius of the halides (i.e., Cl− < Br−< I−) for the first three ImILs. For the remaining three ImILs, BF4− and PF6− are adsorbed through fluorine atoms and N(CN)2− is adsorbed through the N atom. To correlate the molecular descriptors with the DFT-calculated adsorption energies, the QSAR model was introduced. In this work, the training set and test set ratio was 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, and three machine learning algorithms (e.g., multiple linear regression (MLR), random forest regression (RFR), and support vector regression (SVR)) were compared. Among the three algorithms, SVR achieved higher accuracy (coefficient of determination (R2) ∼ 0.94) and lower error (Root Mean Square Error (RMSE) ∼ 10.2%). Accordingly, it was found that the QSAR model is suitable for predicting the adsorption energies of the ImILs. In a comprehensive theoretical analysis by Quadri et al., traditional linear regression and non-linear multilayer perceptron neural network (MLPNN) were employed to establish the correlation between six selected descriptors and the experimental IE of the set of thirty ILs.166 The linear model, with the sum of square error (SSE) 1156.53, demonstrated a moderate ability to describe the relationship between the selected sets of the DFT parameters obtained using the B3LYP functional and 6-31G+d,p basis set. However, a significantly improved model was achieved using standardized variables and a less biased MLPNN model. The non-linear model produced superior performance metrics, including a Mean Square Error (MSE) of 29.9242, RMSE of 5.4703, Median Absolute Deviation (MAD) of 4.9628 and Mean Absolute Percentage Error (MAPE) of 5.7809. Furthermore, the constructed models were used to theoretically evaluate the preventive performance of five new ILs to protect steel surfaces against HCl environments. In a recent study, the influence of alkyl chain lengths on the imidazolium cations and the size of the counter ions of six ImILs, namely, 1-ethyl-3-methylimidazolium chloride [EMIm Cl], 1-butyl-3-methylimidazolium chloride [BMIm Cl], 1-butyl-3-methylimidazolium hexafluorophosphate [BMIm PF6], 1-butyl-3-methylimidazolium tetrafluoroborate [BMIm BF4], 1-butyl-3-methylimidazolium bromide [BMIm Br], and 1-hexyl-3-methylimidazolium chloride [HMIm Cl], and their mixtures with an anionic surfactant, sodium dodecyl sulfate (SDS) on the IE of mild steel exposed to HCl was investigated through experimental, ab initio analysis and QSAR model.151 The study revealed that an increase in the length of the alkyl chains or the size of the counter ions significantly increases the protective efficiency against corrosion. In this work, one linear and non-linear equations were developed to successfully correlate the quantum chemical parameters and experimentally obtain inhibition efficiencies. The high R2 values (0.937 and 0.987), low SSE (0.003 and 0.005) and RMSE (0.0128 and 0.0059) for both the linear and non-linear equations indicate a strong relation between the dipole moment and the inhibition efficiencies. From the equations, it can be inferred that a high dipole moment contributes to a greater IE. A recent report unveiled the Group Contribution Method (GCM) accompanied by the QSAR model to carry out a more insightful investigation of seventy-four ILs as efficient corrosion inhibitors for the protection of carbon steel under a static HCl environment. Seventy-four ILs were categorized into twenty-nine functional groups of cations and seventeen functional groups of anions to acquire 760 data points. The model achieved good performance after the forth iteration by achieving the training sets (R2 of 0.639 and average absolute average deviation (AARD) of 14.9%), a validation set (R2 of 0.319 and AARD of 14.4%), and an overall data point assessment (R2 of 0.324 and AARD of 14.3%).
20, and three machine learning algorithms (e.g., multiple linear regression (MLR), random forest regression (RFR), and support vector regression (SVR)) were compared. Among the three algorithms, SVR achieved higher accuracy (coefficient of determination (R2) ∼ 0.94) and lower error (Root Mean Square Error (RMSE) ∼ 10.2%). Accordingly, it was found that the QSAR model is suitable for predicting the adsorption energies of the ImILs. In a comprehensive theoretical analysis by Quadri et al., traditional linear regression and non-linear multilayer perceptron neural network (MLPNN) were employed to establish the correlation between six selected descriptors and the experimental IE of the set of thirty ILs.166 The linear model, with the sum of square error (SSE) 1156.53, demonstrated a moderate ability to describe the relationship between the selected sets of the DFT parameters obtained using the B3LYP functional and 6-31G+d,p basis set. However, a significantly improved model was achieved using standardized variables and a less biased MLPNN model. The non-linear model produced superior performance metrics, including a Mean Square Error (MSE) of 29.9242, RMSE of 5.4703, Median Absolute Deviation (MAD) of 4.9628 and Mean Absolute Percentage Error (MAPE) of 5.7809. Furthermore, the constructed models were used to theoretically evaluate the preventive performance of five new ILs to protect steel surfaces against HCl environments. In a recent study, the influence of alkyl chain lengths on the imidazolium cations and the size of the counter ions of six ImILs, namely, 1-ethyl-3-methylimidazolium chloride [EMIm Cl], 1-butyl-3-methylimidazolium chloride [BMIm Cl], 1-butyl-3-methylimidazolium hexafluorophosphate [BMIm PF6], 1-butyl-3-methylimidazolium tetrafluoroborate [BMIm BF4], 1-butyl-3-methylimidazolium bromide [BMIm Br], and 1-hexyl-3-methylimidazolium chloride [HMIm Cl], and their mixtures with an anionic surfactant, sodium dodecyl sulfate (SDS) on the IE of mild steel exposed to HCl was investigated through experimental, ab initio analysis and QSAR model.151 The study revealed that an increase in the length of the alkyl chains or the size of the counter ions significantly increases the protective efficiency against corrosion. In this work, one linear and non-linear equations were developed to successfully correlate the quantum chemical parameters and experimentally obtain inhibition efficiencies. The high R2 values (0.937 and 0.987), low SSE (0.003 and 0.005) and RMSE (0.0128 and 0.0059) for both the linear and non-linear equations indicate a strong relation between the dipole moment and the inhibition efficiencies. From the equations, it can be inferred that a high dipole moment contributes to a greater IE. A recent report unveiled the Group Contribution Method (GCM) accompanied by the QSAR model to carry out a more insightful investigation of seventy-four ILs as efficient corrosion inhibitors for the protection of carbon steel under a static HCl environment. Seventy-four ILs were categorized into twenty-nine functional groups of cations and seventeen functional groups of anions to acquire 760 data points. The model achieved good performance after the forth iteration by achieving the training sets (R2 of 0.639 and average absolute average deviation (AARD) of 14.9%), a validation set (R2 of 0.319 and AARD of 14.4%), and an overall data point assessment (R2 of 0.324 and AARD of 14.3%).
8. Mechanism of adsorption on the iron surface
Similar to their traditional organic counterparts, ILs exert their effectiveness by obstructing both the anodic and cathodic sites on the metal surface. In simpler terms, these ILs impede or decelerate the rate of oxidation in the anode and reduction in the cathode (i.e., redox reactions), introducing a deliberate slowdown to the chemical processes involved.167–170According to Likhanova et al., the redox reactions of a divalent metal in an acidic environment, i.e., in presence of H2SO4 (SO42− ion) and HCl (Cl− ion), both with and without ILs, may be expressed as follows, as presented in eqn (5)–(8):171
(i) Anodic reactions in the absence of IL
| M + nH2O ↔ M(H2O)n(ads) | (5) |
| M(H2O)n(ads) + SO42−/2Cl− ↔ M[(H2O)nSO42−/2Cl−]ads | (6) |
| M[(H2O)n SO42−/2Cl−]ads ↔ M[(H2O)nSO4/2Cl−]ads + 2e− | (7) |
| M[(H2O)n SO4/2Cl]ads + 2e− ↔ M2+ + OH− + H+ + SO42−/2Cl− | (8) |
The SO42− and Cl− anions accelerate the anodic oxidation reactions. Alternatively, the introduction of IL hinders the anodic oxidation process due to the adsorption of the cations (IL+) of these ILs. The quick oxidation of the metallic constituent results in electron gathering at the anodic site. Consequently, the cationic constituents of the ILs exhibit a preference for adsorption at the anodic site, thereby retarding the oxidation reaction. The underlying reaction mechanism is expressed using eqn (9):
(ii) Anodic reactions in the presence of IL
| M[(H2O)nSO42−/2Cl−]ads + IL+↔ M[(H2O)nSO4/2Cl IL]ads− | (9) |
The adsorption of cations at the anode is accelerated by counter ions, such as sulphate and chloride ions.
(iii) Cathodic reactions in the absence of IL
When corrosion inhibiting substances are not present in aqueous electrolytes, cathodic reactions occur, as shown in eqn (10)–(12):
| M + H3O+ ↔ M(H3O)ads+ | (10) |
| M(H3O)ads+ ↔ M(H3O)ads + e− | (11) |
| M(H3O)ads + e− + H+ ↔ M + H2O + H2 | (12) |
(iv) Cathodic reactions in the presence of IL, as denoted in eqn (13) and (14):
| M + IL+ ↔ M(IL)ads+ | (13) |
| M(IL)ads+ + e− ↔ M(IL)ads | (14) |
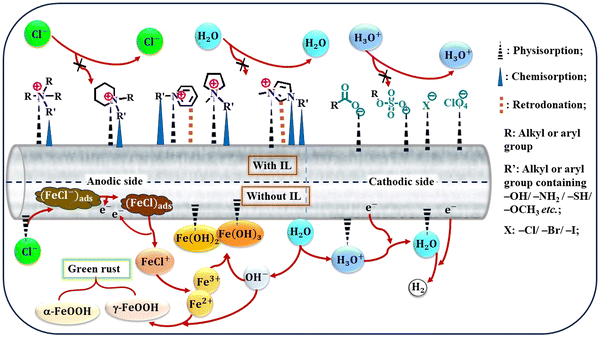 | ||
| Fig. 24 Mechanism of corrosion within HCl environment and mechanism of corrosion retardation process in the presence of IL. | ||
Furthermore, chemisorption plays a crucial role in anchoring ILs to the metal surface. Specific functional groups present in the ILs, such as the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N moiety in ImILs, act as active adsorption centres. These groups can form strong chemical bonds with the iron atoms through donor–acceptor interactions, involving the lone pairs of electrons on the N-atoms and the unoccupied orbitals on the Fe. The extent of chemisorption is influenced by factors such as the type of functional groups, the structure of the ILs, and the surface properties of the iron. This combined action of electrostatic and chemisorption mechanisms leads to the development of a stable shield on the iron surface. This film effectively blocks the active sites of corrosion, which hinders the migration of corrosive species to the metal surface, thereby preventing the occurrence of the redox process involved in corrosion. The adsorption phenomena are influenced by various factors such as the concentration of the ILs, the temperature, and the presence of other species in the electrolyte. Understanding the specific adsorption mechanism of each IL-iron system is crucial for optimizing the design and application of these promising corrosion inhibitors.
C–N moiety in ImILs, act as active adsorption centres. These groups can form strong chemical bonds with the iron atoms through donor–acceptor interactions, involving the lone pairs of electrons on the N-atoms and the unoccupied orbitals on the Fe. The extent of chemisorption is influenced by factors such as the type of functional groups, the structure of the ILs, and the surface properties of the iron. This combined action of electrostatic and chemisorption mechanisms leads to the development of a stable shield on the iron surface. This film effectively blocks the active sites of corrosion, which hinders the migration of corrosive species to the metal surface, thereby preventing the occurrence of the redox process involved in corrosion. The adsorption phenomena are influenced by various factors such as the concentration of the ILs, the temperature, and the presence of other species in the electrolyte. Understanding the specific adsorption mechanism of each IL-iron system is crucial for optimizing the design and application of these promising corrosion inhibitors.
9. Conclusion and future perspectives
In conclusion, the quest for green and sustainable corrosion prevention technologies has led to a significant focus on the application of ILs as next-generation corrosion inhibitors. Over the past decade, the escalating awareness of ecological considerations and the imposition of stringent environmental regulations have underscored the paramount importance of advancing corrosion mitigation methods. In response to these requirements, ILs have emerged as promising contenders, endowed with unique properties, such as low volatility, non-flammability, and high thermal and chemical stability. Their exceptional ability to get adsorbed onto various metallic surfaces adds versatility to their protective function. This comprehensive review has thoroughly explored the diverse applications of N+-containing ILs as efficient corrosion inhibitors for the protection of steel surfaces in diverse corrosive mediums. This exploration extends across various electrolytic environments, providing insights into the adaptability and efficacy of ILs in different corrosion scenarios.Moreover, the review delves into the intricate mechanisms and adsorption behaviours of ILs on metallic surfaces. A concerted effort is made to employ state-of-the-art experimental and computational approaches to ensure a nuanced and comprehensive understanding of the corrosion inhibition potential of ILs. This amalgamation of methodologies advances our knowledge and positions ILs as transformative agents in the field of sustainable corrosion prevention.
As the global community increasingly recognizes the importance of eco-friendly practices and stringent adherence to environmental standards, the role of ILs in corrosion science becomes more pronounced. Their promise as agents of change in sustainable materials protection resonates as a beacon for future endeavours in corrosion research and underscores the ongoing evolution towards greener technologies in corrosion prevention.
Future avenues for exploration
In a nutshell, this review has portrayed a vivid picture of the tremendous potential of ILs as game changers in the field of corrosion inhibition. By leveraging their unique properties, tunability, and environmental compatibility, ILs are poised to revolutionize this field, ushering in a new era of sustainable and effective corrosion protection. By pursuing further research focused on addressing their current limitations and exploring their full potential, we can pave the way for the widespread adoption of these remarkable materials, thereby contributing to a more sustainable future for our planet and its resources.
Author contributions
Sanjukta Zamindar: conceptualization, methodology, software, visualization, writing – original draft preparation. Sukdeb Mandal: visualization, writing – reviewing and editing. Manilal Murmu: investigation, validation, writing – reviewing and editing. Priyabrata Banerjee: supervision, validation, writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The PB is very thankful to the Science and Engineering Research Board, Department of Science and Technology, Government of India (project no. GAP-240712, reference no. 01DU20003A). SZ acknowledges the Department of Science and Technology (DST), Government of India, for her fellowship (IF200407). SM acknowledges the University Grants Commission (UGC), Government of India, for his fellowship [212/CSIR-UGC NET DEC.2017].References
- A. G. Nurioglu, A. C. C. Esteves and G. de With, J. Mater. Chem. B, 2015, 3, 6547–6570 RSC.
- U. Z. Husna, K. A. Elraies, J. A. B. M. Shuhili and A. A. Elryes, J. Pet. Explor. Prod. Technol., 2022, 12, 1075–1094 CrossRef CAS.
- C. Verma, D. K. Verma, E. E. Ebenso and M. A. Quraishi, Heteroat. Chem., 2018, 29, e21437 CrossRef.
- M. J. Nine, M. A. Cole, D. N. H. Tran and D. Losic, J. Mater. Chem. A, 2015, 3, 12580–12602 RSC.
- P. Banerjee, M. Murmu, S. Zamindar and N. C. Murmu, Carbon Allotropes, De Gruyter, 2022, pp. 175–200 Search PubMed.
- S. Gurjar, S. K. Sharma, A. Sharma and S. Ratnani, Appl. Surf. Sci. Adv., 2021, 6, 100170 CrossRef.
- A. R. Prasad, A. Kunyankandy and A. Joseph, Corrosion Inhibitors in the Oil and Gas Industry, Wiley, 2020, pp. 135–150 Search PubMed.
- B. D. B. Tiu and R. C. Advincula, React. Funct. Polym., 2015, 95, 25–45 CrossRef CAS.
- N. Tiwari, R. K. Mitra and M. Yadav, Surf. Interfaces, 2021, 22, 100770 CrossRef CAS.
- M. Murmu, S. K. Saha, N. C. Murmu and P. Banerjee, J. Mol. Liq., 2019, 278, 521–535 CrossRef CAS.
- B. Kulyk, M. A. Freitas, N. F. Santos, F. Mohseni, A. F. Carvalho, K. Yasakau, A. J. S. Fernandes, A. Bernardes, B. Figueiredo, R. Silva, J. Tedim and F. M. Costa, Crit. Rev. Solid State Mater. Sci., 2021, 1–48 Search PubMed.
- R. Ding, W. Li, X. Wang, T. Gui, B. Li, P. Han, H. Tian, A. Liu, X. Wang, X. Liu, X. Gao, W. Wang and L. Song, J. Alloys Compd., 2018, 764, 1039–1055 CrossRef CAS.
- A. Dehghani, G. Bahlakeh and B. Ramezanzadeh, Chem. Eng. J., 2020, 400, 125860 CrossRef CAS.
- A. Singh, K. R. Ansari, P. Bedi, T. Pramanik, I. H. Ali, Y. Lin, P. Banerjee and S. Zamindar, J. Phys. Chem. Solids, 2023, 172, 111064 CrossRef CAS.
- M. Mobin Huda, S. Zamindar and P. Banerjee, J. Mol. Liq., 2023, 385, 122403 CrossRef.
- J. Haque, V. Srivastava, M. A. Quraishi, D. Singh Chauhan, H. Lgaz and I.-M. Chung, Corros. Sci., 2020, 172, 108665 CrossRef CAS.
- S. K. Saha, M. Murmu, N. C. Murmu and P. Banerjee, J. Mol. Struct., 2021, 1245, 131098 CrossRef CAS.
- M. O. Valappil, F. Forouzandeh, X. Li, S. Luong, M. Atwa and V. I. Birss, Electrochim. Acta, 2022, 422, 140444 CrossRef CAS.
- M. Ozhukil Valappil, F. Forouzandeh, M. Atwa, S. Luong and V. I. Birss, ECS Meet. Abstr., 2021, MA2021–02, 541 CrossRef.
- F. Forouzandeh, X. Li, D. W. Banham, F. Feng, S. Ye and V. Birss, J. Electrochem. Soc., 2018, 165, F3230–F3240 CrossRef CAS.
- R. P. Wong, J. E. Wong and V. I. Birss, Can. J. Chem., 2004, 82, 1536–1544 CrossRef CAS.
- J. Wang, L. An, J. Wang, J. Gu, J. Sun and X. Wang, Adv. Colloid Interface Sci., 2023, 321, 103031 CrossRef CAS PubMed.
- J. Saranya, M. Sowmiya, P. Sounthari, K. Parameswari, S. Chitra and K. Senthilkumar, J. Mol. Liq., 2016, 216, 42–52 CrossRef CAS.
- N. N. Hau and D. Q. Huong, J. Mol. Struct., 2023, 1277, 134884 CrossRef CAS.
- S. Vikneshvaran and S. Velmathi, ChemistrySelect, 2019, 4, 387–392 CrossRef CAS.
- N. K. Gupta, C. Verma, R. Salghi, H. Lgaz, A. K. Mukherjee and M. A. Quraishi, New J. Chem., 2017, 41, 13114–13129 RSC.
- H. Assad and A. Kumar, J. Mol. Liq., 2021, 344, 117755 CrossRef CAS.
- C. Verma, L. O. Olasunkanmi, E. E. Ebenso and M. A. Quraishi, J. Mol. Liq., 2018, 251, 100–118 CrossRef CAS.
- R. Aslam, G. Serdaroglu, S. Zehra, D. Kumar Verma, J. Aslam, L. Guo, C. Verma, E. E. Ebenso and M. A. Quraishi, J. Mol. Liq., 2022, 348, 118373 CrossRef CAS.
- D. S. Chauhan, C. Verma and M. A. Quraishi, J. Mol. Struct., 2021, 1227, 129374 CrossRef CAS.
- X. Ren, S. Xu, X. Gu, B. Tan, J. Hao, L. Feng, W. Ren, F. Gao, S. Zhang, Y. Xiao and L. Huang, J. Colloid Interface Sci., 2021, 585, 614–626 CrossRef CAS PubMed.
- D. Sukul, A. Pal, S. K. Saha, S. Satpati, U. Adhikari and P. Banerjee, Phys. Chem. Chem. Phys., 2018, 20, 6562–6574 RSC.
- R. Aslam, M. Mobin Huda, M. Shoeb, M. Murmu and P. Banerjee, J. Ind. Eng. Chem., 2021, 100, 333–350 CrossRef CAS.
- M. Mobin Huda, M. Shoeb, R. Aslam and P. Banerjee, J. Mol. Struct., 2022, 1262, 133027 CrossRef.
- A. Singh, K. R. Ansari, I. H. Ali, Y. Lin, M. Murmu and P. Banerjee, J. Mol. Liq., 2023, 376, 121408 CrossRef CAS.
- C. Verma, E. E. Ebenso and M. A. Quraishi, J. Mol. Liq., 2017, 233, 403–414 CrossRef CAS.
- K. Goossens, K. Lava, C. W. Bielawski and K. Binnemans, Chem. Rev., 2016, 116, 4643–4807 CrossRef CAS PubMed.
- S. N. Pedro, C. S. R. Freire, A. J. D. Silvestre and M. G. Freire, Int. J. Mol. Sci., 2020, 21, 8298 CrossRef CAS PubMed.
- C. Verma, L. O. Olasunkanmi, I. Bahadur, H. Lgaz, M. A. Quraishi, J. Haque, E.-S. M. Sherif and E. E. Ebenso, J. Mol. Liq., 2019, 273, 1–15 CrossRef CAS.
- T. Gu, Z. Chen, X. Jiang, L. Zhou, Y. Liao, M. Duan, H. Wang and Q. Pu, Corros. Sci., 2015, 90, 118–132 CrossRef CAS.
- C. Verma, E. E. Ebenso, M. A. Quraishi and C. M. Hussain, Mater. Adv., 2021, 2, 3806–3850 RSC.
- R. Aslam, M. Mobin Huda, M. Murmu, P. Banerjee and J. Aslam, J. Mol. Liq., 2021, 334, 116469 CrossRef CAS.
- A. Singh, K. R. Ansari, M. A. Quraishi and P. Banerjee, J. Mol. Liq., 2021, 323, 114608 CrossRef CAS.
- E. Berdimurodov, A. Kholikov, K. Akbarov, L. Guo, S. Kaya, K. P. Katin, D. Kumar Verma, M. Rbaa, O. Dagdag and R. Haldhar, J. Electroanal. Chem., 2021, 901, 115794 CrossRef CAS.
- L. Souza, E. Pereira, L. Matlakhova, V. A. F. Nicolin, S. N. Monteiro and A. R. G. de Azevedo, J. Mater. Res. Technol., 2023, 22, 2186–2205 CrossRef CAS.
- Y. L. Kobzar and K. Fatyeyeva, Chem. Eng. J., 2021, 425, 131480 CrossRef CAS.
- S. K. Singh and A. W. Savoy, J. Mol. Liq., 2020, 297, 112038 CrossRef CAS.
- K. J. Kulacki and G. A. Lamberti, Green Chem., 2008, 10, 104–110 RSC.
- M. Kumar, N. Trivedi, C. R. K. Reddy and B. Jha, Chem. Res. Toxicol., 2011, 24, 1882–1890 Search PubMed.
- L. Ma, Q. Lin, Y. Song, B. Zhao and M. Fan, Open Life Sci., 2020, 15, 466–475 Search PubMed.
- S. Sugden and H. Wilkins, J. Chem. Soc., 1929, 1291–1298 RSC.
- Z. Lei, B. Chen, Y.-M. Koo and D. R. MacFarlane, Chem. Rev., 2017, 117, 6633–6635 CrossRef PubMed.
- Q. Zhang and J. M. Shreeve, Chem. Rev., 2014, 114, 10527–10574 CrossRef CAS PubMed.
- C. Reichardt, J. Org. Chem., 2022, 87, 1616–1629 CrossRef CAS PubMed.
- R. Hayes, G. G. Warr and R. Atkin, Chem. Rev., 2015, 115, 6357–6426 CrossRef CAS PubMed.
- F. Javed, F. Ullah, M. R. Zakaria and H. M. Akil, J. Mol. Liq., 2018, 271, 403–420 CrossRef CAS.
- A. R. Hajipour and F. Rafiee, Org. Prep. Proced. Int., 2015, 47, 249–308 CrossRef CAS.
- A. P. M. Tavares, O. Rodríguez and E. A. Macedo, Ionic Liquids—New Aspects for the Future, ed. J.-I. Kadokawa, InTech, Rijeka, Croatia, 2013, ch. 20, p. 537. Search PubMed.
- J. S. Wilkes and M. J. Zaworotko, J. Chem. Soc., Chem. Commun., 1992, 965 RSC.
- M. D. Morton and C. K. Hamer, Sep. Purif. Technol., 2018, 196, 3–9 CrossRef CAS.
- S. Zafari, A. A. Sarabi and B. Movassagh, Corros. Eng. Sci. Technol., 2020, 55, 589–601 CrossRef CAS.
- S. Cao, D. Liu, H. Ding, J. Wang, H. Lu and J. Gui, Corros. Sci., 2019, 153, 301–313 CrossRef CAS.
- J. S. Sandhu, Green Chem. Lett. Rev., 2011, 4, 289–310 CrossRef.
- T. L. Greaves and C. J. Drummond, Chem. Rev., 2008, 108, 206–237 CrossRef CAS PubMed.
- C. A. Angell, N. Byrne and J.-P. Belieres, Acc. Chem. Res., 2007, 40, 1228–1236 CrossRef CAS PubMed.
- A. Kuchenbuch and R. Giernoth, ChemistryOpen, 2015, 4, 677–681 CrossRef CAS PubMed.
- P. Sun and D. W. Armstrong, Anal. Chim. Acta, 2010, 661, 1–16 CrossRef CAS PubMed.
- S. Jeong, S. Li, G. B. Appetecchi and S. Passerini, Energy Storage Mater., 2019, 18, 1–9 CrossRef.
- M. Deetlefs, K. R. Seddon and M. Shara, Phys. Chem. Chem. Phys., 2006, 8, 642–649 RSC.
- S. Dewilde, W. Dehaen and K. Binnemans, Green Chem., 2016, 18, 1639–1652 RSC.
- T. L. Greaves and C. J. Drummond, Chem. Soc. Rev., 2008, 37, 1709 RSC.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- J. Flieger and M. Flieger, Int. J. Mol. Sci., 2020, 21, 6267 CrossRef CAS PubMed.
- H.-B. Wu, B. Zhang, S.-H. Liu and C.-C. Chen, J. Loss Prev. Process Ind., 2020, 66, 104196 CrossRef CAS.
- T. Nakashima and N. Kimizuka, Polym. J., 2012, 44, 665–671 CrossRef CAS.
- B. Nanda, M. S. Sailu, P. M. Priyaranjan, R. K. P. Ranjan and B. M. Nanda, Mater. Today Proc., 2021, 47(5), 1234–1240 CrossRef CAS.
- Himani, A. Pratap Singh Raman, M. Babu Singh, P. Jain, P. Chaudhary, I. Bahadur, K. Lal, V. Kumar and P. Singh, J. Mol. Liq., 2022, 364, 119989 CrossRef CAS.
- K. Sood, Y. Saini and K. K. Thakur, Mater. Today Proc., 2023, 81, 739–744 CrossRef CAS.
- P. Izák, F. D. Bobbink, M. Hulla, M. Klepic, K. Friess, Š. Hovorka and P. J. Dyson, ChemPlusChem, 2018, 83, 7–18 CrossRef PubMed.
- M.-D. Bermúdez, A.-E. Jiménez, J. Sanes and F.-J. Carrión, Molecules, 2009, 14, 2888–2908 CrossRef PubMed.
- L. Chen, M. Sharifzadeh, N. Mac Dowell, T. Welton, N. Shah and J. P. Hallett, Green Chem., 2014, 16, 3098–3106 RSC.
- Z. Zhou, H. Matsumoto and K. Tatsumi, Chem. – Eur. J., 2005, 11, 752–766 CrossRef CAS PubMed.
- M. Yoshizawa, W. Xu and C. A. Angell, J. Am. Chem. Soc., 2003, 125, 15411–15419 CrossRef CAS PubMed.
- F. Philippi and T. Welton, Phys. Chem. Chem. Phys., 2021, 23, 6993–7021 RSC.
- J.-M. Lee, Chem. Eng. J., 2011, 172, 1066–1071 CrossRef CAS.
- H. Asfour, G. Y. Elewady, E. G. Zaki and A. E.-A. S. Fouda, ACS Omega, 2023, 8, 41077–41099 CrossRef CAS PubMed.
- S. Herrmann, M. Kostrzewa, A. Wierschem and C. Streb, Angew. Chem., Int. Ed., 2014, 53, 13596–13599 CrossRef CAS PubMed.
- M. Mobin, R. Aslam, R. Salim and S. Kaya, J. Colloid Interface Sci., 2022, 620, 293–312 CrossRef CAS PubMed.
- S. Gao, Y. Huang, Y. Xiong, X. Guo, J. Zhang and L. Wang, J. Mol. Liq., 2023, 386, 122467 CrossRef CAS.
- P. Arellanes-Lozada, O. Olivares-Xometl, N. V. Likhanova, I. V. Lijanova, J. R. Vargas-García and R. E. Hernández-Ramírez, J. Mol. Liq., 2018, 265, 151–163 CrossRef CAS.
- E. Udabe, M. Forsyth, A. Somers and D. Mecerreyes, Mater. Adv., 2020, 1, 584–589 RSC.
- H. M. Abd El-Lateef, A. H. Tantawy and A. A. Abdelhamid, J. Surfactants Deterg., 2017, 20, 735–753 CrossRef CAS.
- O. Olivares-Xometl, E. Álvarez-Álvarez, N. V. Likhanova, I. V. Lijanova, R. E. Hernández-Ramírez, P. Arellanes-Lozada and J. L. Varela-Caselis, J. Adhes. Sci. Technol., 2018, 32, 1092–1113 CrossRef CAS.
- P. Arellanes-Lozada, O. Olivares-Xometl, N. V. Likhanova, E. M. Arce-Estrada, I. V. Lijanova, L. Lartundo-Rojas and M. de Consuelo Mendoza-Herrera, Int. J. Electrochem. Sci., 2016, 11, 7785–7800 CrossRef CAS.
- L. Guo, S. Zhu and S. Zhang, J. Ind. Eng. Chem., 2015, 24, 174–180 CrossRef CAS.
- A. Dhawan, B. A. Laurent, J. Moloney and C. M. Silvernail, Molecules having one hydrophobic group and two identical hydrophilic ionic groups and compositions thereof, Australia Pat., AU2021204283B2, September 22, 2022 Search PubMed.
- E. Berdimurodov, A. Kholikov, K. Akbarov, L. Guo, S. Kaya, K. P. Katin, D. K. Verma, M. Rbaa and O. Dagdag, Colloids Surf., A, 2022, 633, 127837 CrossRef CAS.
- S. Zehra, M. Mobin, R. Aslam, H. Lgaz and I.-M. Chung, J. Mol. Struct., 2021, 1240, 130505 CrossRef CAS.
- E. Li, Y. Li, S. Liu and P. Yao, Colloids Surf., A, 2023, 657, 130541 CrossRef CAS.
- A. Singh, K. R. Ansari, P. Banerjee, M. Murmu, M. A. Quraishi and Y. Lin, Colloids Surf., A, 2021, 623, 126708 CrossRef CAS.
- B. Anandkumar, R. P. George and J. Philip, Anal. Chim. Acta, 2020, 1126, 38–51 CrossRef CAS PubMed.
- S. M. Ali, K. M. Emran and M. Messali, Prog. Org. Coat., 2019, 130, 226–234 CrossRef CAS.
- F. El-Hajjaji, E. Ech-chihbi, N. Rezki, F. Benhiba, M. Taleb, D. S. Chauhan and M. A. Quraishi, J. Mol. Liq., 2020, 314, 113737 CrossRef CAS.
- F. E. L. Hajjaji, R. Salim, M. Taleb, F. Benhiba, N. Rezki, D. S. Chauhan and M. A. Quraishi, Surf. Interfaces, 2021, 22, 100881 CrossRef CAS.
- D. Iravani, N. Esmaeili, A. Berisha, E. Akbarinezhad and M. H. Aliabadi, Colloids Surf., A, 2023, 656, 130544 CrossRef CAS.
- M. Messali, S. M. Almutairi, A. Ait Mansour, R. Salghi and H. Lgaz, J. Mater. Res. Technol., 2023, 27, 8292–8307 CrossRef CAS.
- S. Ben Aoun, RSC Adv., 2017, 7, 36688–36696 RSC.
- E. K. Ardakani, E. Kowsari, A. Ehsani and S. Ramakrishna, Microchem. J., 2021, 165, 106049 CrossRef CAS.
- A. M. El-Shamy, K. Zakaria, M. A. Abbas and S. Zein El Abedin, J. Mol. Liq., 2015, 211, 363–369 CrossRef CAS.
- M. Zunita and Y. J. Kevin, Results Eng., 2022, 15, 100562 CrossRef CAS.
- A. H. E. Moustafa, H. H. Abdel-Rahman, M. Hagar, M. R. Aouad, N. Rezki and S. A. A. Bishr, Sci. Rep., 2023, 13, 19197 CrossRef CAS PubMed.
- O. Al-Rashed and A. Abdel Nazeer, Materials, 2022, 15, 2326 CrossRef CAS PubMed.
- M. D. Green and T. E. Long, Polym. Rev., 2009, 49, 291–314 CrossRef CAS.
- S. Velusamy, S. Sakthivel, L. Neelakantan and J. S. Sangwai, J. Earth Sci., 2017, 28, 949–961 CrossRef CAS.
- M. T. Zaky, M. I. Nessim and M. A. Deyab, J. Mol. Liq., 2019, 290, 111230 CrossRef CAS.
- C. Liu, P. Du, F. Nan, H. Zhao and L. Wang, Surf. Topogr.: Metrol. Prop., 2018, 6, 024004 CrossRef CAS.
- E. Kowsari, M. Payami, R. Amini, B. Ramezanzadeh and M. Javanbakht, Appl. Surf. Sci., 2014, 289, 478–486 CrossRef CAS.
- I. Lozano, E. Mazario, C. O. Olivares-Xometl, N. V. Likhanova and P. Herrasti, Mater. Chem. Phys., 2014, 147, 191–197 CrossRef CAS.
- Y. Guo, J. Xue, J. Zhang, Q. Chen, L. Fan, C. Tang, K. Ren, A. Fu and Q. Bi, Colloids Surf., A, 2022, 651, 129135 CrossRef CAS.
- S. Cao, D. Liu, H. Ding, H. Lu and J. Gui, J. Colloid Interface Sci., 2020, 579, 315–329 CrossRef CAS PubMed.
- E. E. El-Katori, M. I. Nessim, M. A. Deyab and K. Shalabi, J. Mol. Liq., 2021, 337, 116467 CrossRef CAS.
- L. Feng, S. Zhang, Y. Qiang, S. Xu, B. Tan and S. Chen, Mater. Chem. Phys., 2018, 215, 229–241 CrossRef CAS.
- R. Haldhar, C. Jayprakash Raorane, V. K. Mishra, T. Periyasamy, A. Berisha and S.-C. Kim, J. Mol. Liq., 2023, 372, 121168 CrossRef CAS.
- F. El Hajjaji, E. Ech-chihbi, R. Salim, A. Titi, M. Messali, B. El Ibrahimi, S. Kaya and M. Taleb, Mater. Sci. Eng. B, 2023, 289, 116232 CrossRef CAS.
- X. Zeng, X. Zheng, L. Guo, Q. Xu, H. Huang and B. Tan, J. Mol. Liq., 2021, 324, 115063 CrossRef CAS.
- Bhaskaran, P. D. Pancharatna, S. Lata and G. Singh, J. Mol. Liq., 2019, 278, 467–476 CrossRef CAS.
- S. Cao, D. Liu, H. Ding, J. Wang, H. Lu and J. Gui, J. Mol. Liq., 2019, 275, 729–740 CrossRef CAS.
- M. Talebian, K. Raeissi, M. Atapour, B. M. Fernández-Pérez, Z. Salarvand, S. Meghdadi, M. Amirnasr and R. M. Souto, Appl. Surf. Sci., 2018, 447, 852–865 CrossRef CAS.
- L. Feng, S. Zhang, Y. Lu, B. Tan, S. Chen and L. Guo, Appl. Surf. Sci., 2019, 483, 901–911 CrossRef CAS.
- O. A. Al-Rashed and A. A. Nazeer, J. Mol. Liq., 2019, 288, 111015 CrossRef CAS.
- E. E. Ebenso, C. Verma, L. O. Olasunkanmi, E. D. Akpan, D. K. Verma, H. Lgaz, L. Guo, S. Kaya and M. A. Quraishi, Phys. Chem. Chem. Phys., 2021, 23, 19987–20027 RSC.
- A. A. Bahraq, I. B. Obot, M. A. Al-Osta and M. Ibrahim, Constr. Build. Mater., 2024, 412, 134808 CrossRef CAS.
- C. Verma, M. A. Quraishi and K. Y. Rhee, Chem. Eng. J., 2022, 430, 132645 CrossRef CAS.
- S. Donkor, Z. Song, L. Jiang and H. Chu, J. Mol. Liq., 2022, 359, 119260 CrossRef CAS.
- C. Verma, H. Lgaz, D. K. Verma, E. E. Ebenso, I. Bahadur and M. A. Quraishi, J. Mol. Liq., 2018, 260, 99–120 CrossRef CAS.
- S. K. Saha, P. Ghosh, A. Hens, N. C. Murmu and P. Banerjee, Phys. E, 2015, 66, 332–341 CrossRef CAS.
- S. Sengupta, M. Murmu, S. Mandal, H. Hirani and P. Banerjee, Colloids Surf., A, 2021, 617, 126314 CrossRef CAS.
- S. Mandal, S. Zamindar, S. Sarkar, M. Murmu, L. Guo, S. Kaya, H. Hirani and P. Banerjee, J. Adhes. Sci. Technol., 2022, 1–17 Search PubMed.
- S. Mandal, S. Bej and P. Banerjee, J. Mol. Liq., 2023, 381, 121789 CrossRef CAS.
- X. Zheng, S. Zhang, M. Gong and W. Li, Ind. Eng. Chem. Res., 2014, 53, 16349–16358 CrossRef CAS.
- L. Guo, C. Qi, X. Zheng, R. Zhang, X. Shen and S. Kaya, RSC Adv., 2017, 7, 29042–29050 RSC.
- S. K. Saha, A. Dutta, P. Ghosh, D. Sukul and P. Banerjee, Phys. Chem. Chem. Phys., 2016, 18, 17898–17911 RSC.
- J. Li, Handbook of Materials Modeling, Ohio State University, 2005, pp. 565–588 Search PubMed.
- S. K. Saha, A. Dutta, P. Ghosh, D. Sukul and P. Banerjee, Phys. Chem. Chem. Phys., 2015, 17, 5679–5690 RSC.
- S. Kaya, B. Tüzün, C. Kaya and I. B. Obot, J. Taiwan Inst. Chem. Eng., 2016, 58, 528–535 CrossRef CAS.
- N. I. N. Haris, S. Sobri, Y. A. Yusof and N. K. Kassim, Metals, 2020, 11, 46 CrossRef.
- S. Kaya, P. Banerjee, S. K. Saha, B. Tüzün and C. Kaya, RSC Adv., 2016, 6, 74550–74559 RSC.
- M. Murmu, S. Sengupta, R. Pal, S. Mandal, N. C. Murmu and P. Banerjee, RSC Adv., 2020, 10, 33401–33416 RSC.
- C. Verma, L. O. Olasunkanmi, I. B. Obot, E. E. Ebenso and M. A. Quraishi, RSC Adv., 2016, 6, 53933–53948 RSC.
- R. Hsissou, F. Benhiba, O. Dagdag, M. El Bouchti, K. Nouneh, M. Assouag, S. Briche, A. Zarrouk and A. Elharfi, J. Colloid Interface Sci., 2020, 574, 43–60 CrossRef CAS PubMed.
- A. Yousefi, S. Javadian, N. Dalir, J. Kakemam and J. Akbari, RSC Adv., 2015, 5, 11697–11713 RSC.
- R. Hsissou, F. Benhiba, M. El Aboubi, S. Abbout, Z. Benzekri, Z. Safi, M. Rafik, H. Bahaj, M. Kaba, M. Galai, N. Wazzan, S. Briche, S. Boukhris, A. Zarrouk, M. EbnTouhami and M. Rafik, Chem. Phys. Lett., 2022, 806, 139995 CrossRef CAS.
- A. Singh, K. R. Ansari, Y. Lin, M. A. Quraishi, H. Lgaz and I.-M. Chung, J. Taiwan Inst. Chem. Eng., 2019, 95, 341–356 CrossRef CAS.
- L. H. Madkour, S. Kaya and I. B. Obot, J. Mol. Liq., 2018, 260, 351–374 CrossRef CAS.
- K. F. Khaled and A. El-Maghraby, Arab. J. Chem., 2014, 7, 319–326 CrossRef CAS.
- A. Kasprzhitskii and G. Lazorenko, J. Mol. Liq., 2021, 331, 115782 CrossRef CAS.
- K. Cherrak, M. E. Belghiti, A. Berrissoul, M. El Massaoudi, M. El Faydy, M. Taleb, S. Radi, A. Zarrouk and A. Dafali, Surf. Interfaces, 2020, 20, 100578 CrossRef CAS.
- H. El Aadad, M. Galai, M. Ouakki, A. Elgendy, M. E. Touhami and A. Chahine, Surf. Interfaces, 2021, 24, 101084 CrossRef.
- I. Lukovits, I. Bakó, A. Shaban and E. Kálmán, Electrochim. Acta, 1998, 43, 131–136 CrossRef CAS.
- I. Lukovits, A. Shaban and E. Kálmán, Russ. J. Electrochem., 2003, 39, 177–181 CrossRef CAS.
- G. Tian and K. Yuan, J. Mol. Model., 2021, 27, 195 CrossRef CAS PubMed.
- E. E. Ebenso, M. M. Kabanda, L. C. Murulana, A. K. Singh and S. K. Shukla, Ind. Eng. Chem. Res., 2012, 51, 12940–12958 CrossRef CAS.
- L. C. Murulana, A. K. Singh, S. K. Shukla, M. M. Kabanda and E. E. Ebenso, Ind. Eng. Chem. Res., 2012, 51, 13282–13299 CrossRef CAS.
- S. Yesudass, L. O. Olasunkanmi, I. Bahadur, M. M. Kabanda, I. B. Obot and E. E. Ebenso, J. Taiwan Inst. Chem. Eng., 2016, 64, 252–268 CrossRef CAS.
- M.-F. Chen, Y. Chen, Z. Jia Lim and M. Wah Wong, J. Mol. Liq., 2022, 367, 120489 CrossRef CAS.
- T. W. Quadri, L. O. Olasunkanmi, O. E. Fayemi, E. D. Akpan, H.-S. Lee, H. Lgaz, C. Verma, L. Guo, S. Kaya and E. E. Ebenso, Comput. Mater. Sci., 2022, 214, 111753 CrossRef CAS.
- C. Verma, S. H. Alrefaee, M. A. Quraishi, E. E. Ebenso and C. M. Hussain, J. Mol. Liq., 2021, 321, 114484 CrossRef CAS.
- M. A. Deyab, M. T. Zaky and M. I. Nessim, J. Mol. Liq., 2017, 229, 396–404 CrossRef CAS.
- M. F. Shehata, A. M. El-Shamy, K. M. Zohdy, E.-S. M. Sherif and S. Zein El Abedin, Appl. Sci., 2020, 10, 1444 CrossRef CAS.
- W. Xiang, C. Zhao, C. Zhang, X. Wang, X. Li, S. Liu, C. Sun, Q. Yu, B. Yu, M. Cai and L. Shi, Langmuir, 2024, 40(1), 389–402 CrossRef CAS PubMed.
- N. V. Likhanova, M. A. Domínguez-Aguilar, O. Olivares-Xometl, N. Nava-Entzana, E. Arce and H. Dorantes, Corros. Sci., 2010, 52, 2088–2097 CrossRef CAS.
- N. V. Likhanova, N. López-Prados, D. Guzmán-Lucero, O. Olivares-Xometl, I. V. Lijanova, P. Arellanes-Lozada and J. Arriola-Morales, J. Adhes. Sci. Technol., 2022, 36, 845–874 CrossRef CAS.
- O. Olivares-Xometl, I. V. Lijanova, N. V. Likhanova, P. Arellanes-Lozada, H. Hernández-Cocoletzi and J. Arriola-Morales, J. Mol. Liq., 2020, 318, 114075 CrossRef CAS.
- P. Arellanes-Lozada, V. Díaz-Jiménez, H. Hernández-Cocoletzi, N. Nava, O. Olivares-Xometl and N. V. Likhanova, Corros. Sci., 2020, 175, 108888 CrossRef CAS.
- S. Gurjar, S. K. Sharma, A. Sharma and S. Ratnani, Electrochem. Sci. Adv., 2022, 2, e2100110 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |