 Open Access Article
Open Access ArticleAnti-corrosion applications of 2D transition metal based layered materials
Yuqin
Tian
a,
Qiaoxin
Yang
a,
Wei
Li
a,
Yuan
Gong
 a,
Qiuping
Zhao
a,
Chunlei
Li
*a and
Xinxin
Sheng
a,
Qiuping
Zhao
a,
Chunlei
Li
*a and
Xinxin
Sheng
 *bc
*bc
aCollege of Petrochemical Technology, Lanzhou University of Technology, Lanzhou 730050, P. R. China. E-mail: licl@lut.edu.cn; 20220045@lut.edu.cn; 232081700023@lut.edu.cn; 222085602026@lut.edu.cn; yuangong@lut.edu.cn; 1134743502@qq.com
bDepartment of Polymeric Materials and Engineering, School of Materials and Energy, Guangdong University of Technology, Guangzhou, 510006, P. R. China. E-mail: xinxin.sheng@gdut.edu.cn; cexxsheng@gmail.com
cGuangdong Provincial Key Laboratory of Functional Soft Condensed Matter, Guangdong University of Technology, Guangzhou 510006, P. R. China
First published on 7th February 2024
Abstract
Metal corrosion causes great economic losses, wastes resources, pollutes the environment and damages personal safety. With the birth of the two-dimensional (2D) material graphene, there has been an upsurge in research on two-dimensional materials. Because of their excellent physical and chemical properties, two-dimensional materials have great application potential and prospects in the field of metal corrosion protection. Two-dimensional transition metal layered materials including layered double hydroxides (LDHs), transition metal carbonitrides (MXenes) and transition metal dichalcogenides (TMDs) have also received extensive attention. This article briefly introduces the above three two-dimensional transition metal layered materials. The current application status of LDHs, TMDs and MXenes in the field of metal anti-corrosion is focused on. Some studies that can be carried out in the future with the two-dimensional transition metal layered materials in corrosion prevention are proposed.
1. Introduction
In 2004, Andre K. Geim and Konstantin Novoselov obtained graphene by a simple exfoliation method.1 Two-dimensional nanomaterials have attracted extensive research attention since then.2,3 Among them, two-dimensional transition metal layered materials including layered double hydroxides (LDHs),4–7 transition metal dichalcogenides (TMDs)8–11 and transition metal carbonitrides (MXenes)12–16 have also received extensive attention. Two-dimensional transition metal layered materials have a large specific surface area,17 excellent physical and chemical properties,18–21 and have been widely used in a lot of fields, including energy storage and conversion,22–25 catalysis,26–29 biomedicine,30,31 metal corrosion and prevention,32,33 flame retardants,34–36etc. This article focuses on two-dimensional transition metal layered materials and their applications in corrosion prevention.1.1. Layered double hydroxides (LDHs)
LDHs, commonly known as hydrotalcites, are typical two-dimensional layered materials composed of two or more metal elements.37,38 In 1942, Feitknacht et al. first prepared LDHs through the co-precipitation method by reacting magnesium chloride, aluminum chloride, etc. with hydroxide. With extensive research, the structure of LDHs has been clarified. The synthesis method has been continuously proposed, and the application field of LDHs is also broadened. LDHs consist of a positively charged brucite-like layer and an interlayered region containing negatively charged anions or solvation molecules.39 The general structural formula of LDHs is [M1−x2+Mx3+(OH)2]x+(An−)x/n·yH2O, where M2+ and M3+ represent divalent and trivalent metal cations in the laminate, An− refers to the interlayer anion, and y is the amount of crystal water.40–44 The structure of LDHs is shown in Fig. 1.45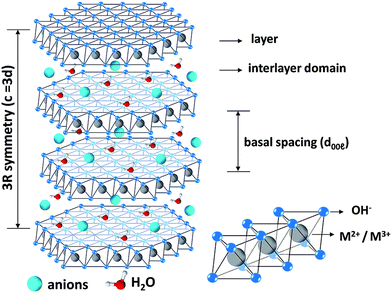 | ||
| Fig. 1 The structure of LDHs. Reproduced with permission from ref. 45. Copyright 2021 Royal Society of Chemistry. | ||
The anions between the layers can maintain the charge balance, making LDHs, as a whole, electrically neutral. LDHs are characteristic of the exchange of interlayer anions with external counterparts, which is ascribed to the weak or trivial crosslinking between the host layers.46 The host laminates of LDHs are orderly combined with interlayer anions through electrostatic interactions, van der Waals forces, and hydrogen bonds. The types of metal elements in the laminates and the interlayer anions can be adjusted to realize the controllable synthesis of LDHs. In addition, the interlayer anions of LDHs are exchangeable, which allows the synthesis of a variety of functional anion-intercalated LDHs. LDHs also have thermal stability and structural memory effects. LDHs have been widely used in biomedicine, catalysis, sensors, optoelectronic devices, thin films, corrosion resistance and other fields.47–49
The preparation methods of LDHs include the co-precipitation method, the roasting reduction method, the ion exchange method, the hydrothermal method and the sol–gel method and so on. The co-precipitation method is to mix the metal salt solution constituting the laminate with the alkali solution under certain conditions to nucleate, and then crystallize at a certain temperature to form LDHs, which is the most commonly used method for preparing LDHs.50–52 However, the preparation of LDHs by the co-precipitation method has problems such as poor crystal form and uneven particle size. The hydrothermal method uses insoluble oxides and hydroxides of metal ions and alkaline solutions as raw materials to prepare LDHs under high temperature and pressure conditions.53 Compared with the co-precipitation method, LDHs prepared by the hydrothermal method have better crystal form and a uniform particle size distribution. Based on the exchangeability of LDH interlayer anions, the ion exchange method replaces anions and inserts the required anions, thereby endowing LDHs with new properties.54,55 Based on the structural memory of LDHs, the metal oxide after LDH roasting is placed in an aqueous solution of the anion to be intercalated at a certain temperature to rebuild the layered structure of LDHs. This method can prepare LDHs with different anions but the same structure as the initial structure.56,57
1.2. Transition metal carbonitrides (MXenes)
In 2011, Yury Gogotsi et al. reported a two-dimensional material obtained by removing Al in Ti3AlC2 with hydrofluoric acid and named it MXene.58 The precursor of MXenes is a class of ternary layered compounds (MAX) with a unified chemical formula Mn+1AXn, where M is a transition metal, A is III or IV groups elements, X is C or N atoms, and n = 1, 2, 3, etc.59,60 In MAX, M atomic layers and A atomic layers are alternately arranged to form a close-packed hexagonal layered structure, and X fills the octahedral gaps (as shown in Fig. 2).61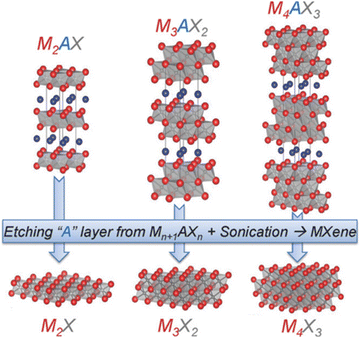 | ||
| Fig. 2 Structures of MAX and the corresponding structures of MXenes. Reproduced with permission from ref. 61. Copyright 2014 Wiley. | ||
In MAX, there is a strong covalent bond or ionic bond between M–X, and a weak metal bond between M–A. So MXene materials can be obtained by etching the atomic layer of A.62 MXenes have hydrophilicity,63 great biocompatibility and electrochemical performance, and high conductivity.64,65 Due to the unique physical and chemical properties of MXenes, they have received extensive attention and research studies in the fields of sensors, biomedicine, catalysis, energy storage and conversion, and multifunctional polymer composites.66–71 The current methods for preparing MXenes mainly include etching and bottom-up methods. HF is the first reported etchant for the successful preparation of MXenes.72 However, due to the strong corrosiveness and high toxicity of HF, with the deepening of MXene research, the process of preparing MXenes has gradually developed in a greener and more environmentally friendly direction. The currently reported etchants include solutions of LiF and HCl, molten fluoride salts, molten transition metal halides, high-temperature alkali solutions, etc.73–75 In addition, electrochemical etching can also be used to prepare MXenes, which provides a new idea for the preparation of MXenes.76 The bottom-up approach is to prepare MXenes by chemical vapor deposition, which can obtain MXenes with large areas, high quality and fewer defects.
1.3. Transition metal dichalcogenides (TMDs)
TMDs have a graphene-like two-dimensional layered structure, and the general formula can be written as MX2, where M represents a transition metal element (such as Mo, W, Ti, etc.), and X refers to chalcogen elements (S, Se and Te).77,78 The structure of a single-layer TMD generally presents an X–M–X sandwich structure. The TMD layers are connected by weak van der Waals forces, and there are strong chemical bonds in each layer plane. In 1963, Robert Frindt obtained an ultrathin MoS2 layer by cutting from the surface of a large MoS2 crystal.79 TMDs have now been widely used in optoelectronic devices, catalysis, quantum information, energy storage, corrosion resistance and other fields.80–83The top-down preparation methods of 2D TMDs mainly include mechanical exfoliation, liquid-phase ultrasonic exfoliation, and intercalation-based exfoliation.84 The method of mechanical exfoliation is easy to operate, but the size and number of layers prepared are uncontrollable. In addition, this method cannot prepare TMDs on a large scale. Liquid exfoliation refers to the direct peeling of TMDs by destroying the van der Waals interaction between TMD layers using ultrasonic waves in a solvent. Intercalation-based exfoliation is used to prepare TMDs by intercalating ions or molecules to increase the interlayer distance and weaken the interlayer interaction. Bottom-up preparation methods include the hydrothermal method and the chemical vapor deposition (CVD) method.
2. Corrosion
2.1. Corrosion mechanism and corrosion damage
Metal materials can easily interact with the surrounding environment to cause metal corrosion, resulting in the loss and degradation of metal materials.85 The huge harm caused by metal corrosion involves various aspects such as public health safety, human health, economic loss, waste of resources, etc. Metals are purified from metal ores by the metal smelting technology.86 From a thermodynamic point of view, the ore exists in a thermodynamically stable state. In the process of extracting pure metal, energy is forced into the ore, so the metal is in a thermodynamically unstable state. Metal corrosion is a process that releases energy and causes the metal to return to the lowest energy stable state. During this process, metal materials would lose their properties. The most common type of corrosion is electrochemical corrosion. The metal comes into contact with the electrolyte solution to form a corroded primary battery, resulting in the destruction of the metal materials and the inability to do any useful work for the outside world.87 Electrochemical corrosion includes three processes, the anodic process, cathodic process, and current loop. Among them, the anode process is that a metal substrate dissolves when it comes into contact with the corrosive medium (M → Mn+ + ne−), and then enters the media in the form of metal ions. The cathode process is that the electrons flowing from the anode are absorbed by oxygen and hydrogen ions, etc. (O2 + 2H2O + 4e− → 4OH−; 2H+ + 2e− → H2↑). There is a current flowing in the middle, forming a current loop.88 As long as any one of these three processes is stopped, corrosion would be prevented. Therefore, metal corrosion prevention measures are aimed at blocking these three processes.2.2. Corrosion prevention measures
Metal corrosion prevention measures include changing the inherent structure of the metal,89 improving the corrosion environment,90 electrochemical protection including cathodic protection and anodic protection,91,92 and metal surface coatings. Forming a covering layer on the metal surface, including metal plating, chemical conversion coatings, polymer coatings, etc., can isolate the contact between the metal materials and corrosive media to greatly prevent the damage of corrosive media.93–95The incorporation of 2D nanomaterials in metal corrosion prevention has been extensively studied. Due to the layered structure, large specific surface area, excellent impermeability, and mechanical properties of two-dimensional materials, they can play a labyrinth effect in coatings. This article mainly focuses on the application of 2D transition metal layered materials in metal corrosion prevention.96
3. Applications of 2D transition metal layered materials in corrosion prevention
3.1. Applications of LDHs in corrosion prevention
Due to the adjustable types of metal ions and anions in LDHs, LDHs can be controllably prepared. In addition, LDHs have excellent ion exchange capacity due to their special 2D structure, which also provides the possibility to insert corrosion inhibitors in the LDH layers.97,98 LDHs can be doped into polymer coatings to improve the performance of polymer coatings, and can also be directly applied to the surface of metal substrates in the form of conversion coatings to protect metals.Bouali et al. proposed the mechanism of ZnAl layered double hydroxide conversion coating on the surface of the AA2024-T3 aluminum alloy.101 Their research work proposed that the LDH conversion coating was controlled by diffusion reactions, which are divided into three stages. In the first stage, the original oxide layer rapidly transformed into the AlOOH intermediate layer. As the pH increased, the AlOOH intermediate layer was partially dissolved, and Al(OH)4− reacted with Zn(OH)+ to form LDH flakes. The LDH flakes generated continued to grow to form LDH conversion coatings in the third stage and protect the surface of the aluminum alloy substrate from corrosion. Zhao et al. prepared LDH coatings containing Ce ions on the surface of the AZ31 magnesium alloy. In addition, their team also successfully prepared 8-hydroxyquinoline (8HQ) intercalated Mg–Al LDH coatings on the surface of the AZ31 magnesium alloy.102 The anti-corrosion mechanism of the LDH conversion coating in the magnesium alloy is shown in Fig. 3. LDHs have anion exchange capacity; Cl− in the corrosive media can be exchanged with 8HQ, which would greatly reduce the damage of the corrosive media to the metal substrate. Secondly, 8HQ can also chelate with Mg2+ to form insoluble precipitate Mg(HQ)2, which can seal the defects of LDH conversion coatings to further reduce the occurrence of corrosion reactions. The effective synergistic effect of corrosion inhibitors and LDHs provides a new idea for the corrosion resistance of magnesium alloys.
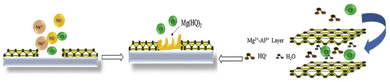 | ||
| Fig. 3 Mechanism of corrosion inhibition of the 8HQ intercalated Mg–Al LDH coating on AZ31. Reproduced with permission from ref. 102. Copyright 2020 Elsevier. | ||
Due to the defects of the simply prepared LDH conversion coatings and the poor performance of the coatings, they cannot provide more long-term stable protection for the metal substrates. Therefore, research studies on LDH conversion coatings have expanded to multifunctional composite coatings, such as endowing the coatings with self-healing, self-cleaning, and superhydrophobic functions to provide longer-term stable and effective protection for the LDH coatings.103,104 Cao et al. prepared a layered ZnAl-LDH-La LDH coating with superhydrophobic function on the surface of an aluminum substrate.105 The ZnAl-LDH coating was first prepared by a hydrothermal method and then soaked in sodium laurate solution to endow the coating with superhydrophobic properties. The prepared coating has long-term stable corrosion resistance performance. Zhang et al. prepared aspartic acid-intercalated Li–Al LDH conversion coatings on 6N01 aluminum alloy. The conversion coatings are dense and uniform, and have self-healing properties.106 Mohammadi et al. used a one-step hydrothermal method to prepare a Zn–Al LDH conversion coating intercalated with benzimidazole on the surface of the AA2024-T3 metal substrate.107 The modification of the corrosion inhibitor benzimidazole molecule effectively improved the corrosion resistance of LDH conversion coatings. Wang et al. used the hydrothermal method to grow LDH coatings in situ by plasma electrolytic oxidation (PEO) (PEO–LDHs) and then modified the PEO–LDH composite coating with sodium dodecyl sulfate (SDS) using an anion exchange reaction (PEO–LDHs–SDS). The corrosion resistance mechanism of the PEO–LDHs–SDS composite coating is shown in Fig. 4. Firstly, LDHs can fill the defects in the PEO coating and capture Cl− through anion exchange. Secondly, SDS can endow the coatings with superhydrophobic properties.108
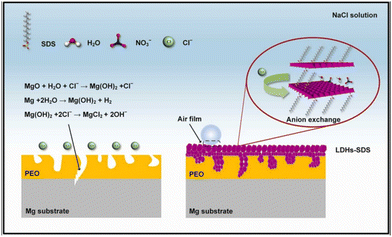 | ||
| Fig. 4 The corrosion resistance mechanism of the PEO–LDHs–SDS composite coating. Reproduced with permission from ref. 108. Copyright 2022 Elsevier. | ||
Jian et al. in situ generated sodium pyrithione modified LDH coatings on the surface of an aluminum alloy, which can improve the corrosion resistance and antifouling performance of aluminum alloy 7075 at the same time.109 Li et al. deposited NiP coating on the surface of Mg–Li alloy, then grew MgAl LDHs in situ on an NiP coating, and finally modified the NiP/MgAl LDH double-layer coating with stearic acid (@SA) to achieve the superhydrophobic function of the composite coatings.110 The preparation process of the NiP/MgAl LDHs@SA coating is shown in Fig. 5. On the one hand, the LDHs generated in situ can fill the defects of NiP coating, and on the other hand, LDHs can capture the corrosion ions. Furthermore, superhydrophobic modification would further inhibit the occurrence of corrosion. The construction of the double coatings provides new ideas for metal corrosion prevention methods. The above works confirm that multifunctional composite coatings can protect metal substrates from damage for a longer period of time.
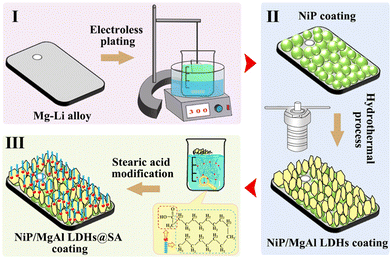 | ||
| Fig. 5 The preparation process of the NiP/MgAl LDHs@SA coating. Reproduced with permission from ref. 110. Copyright 2023 Elsevier. | ||
In addition, ionic liquids, octadecyl-trimethoxy-silane, salicylic acid, etc. have all been reported for the modification of LDHs to enhance the corrosion resistance of LDH coatings.111–113 Other two-dimensional materials such as graphene oxide, transition metal carbonitrides and metal–organic frameworks can also form composite coatings with LDHs to achieve the purpose of synergistically protecting metal substrates from corrosion.114–116 LDH coatings will be more widely used in future.
LDHs can act as fillers in polymer coatings due to their 2D lamellar structure and anion exchange capacity. On the one hand, LDHs can fill the defects of polymer coatings and block the penetration of corrosive media. On the other hand, they have the ability to capture Cl− to slow down the occurrence of corrosion reactions. Zhang et al. designed and constructed a trifunctional filler to improve the corrosion resistance of epoxy coatings (EP). Basalt scales (Bt) were firstly modified with polydopamine (PDA) (Bt@PDA), and then molybdate intercalated LDH (LM) was grown in situ on the surface of Bt@PDA (Bt@PDA@LM).121 The protection mechanism for Bt@PDA@LM/EP coating is shown in Fig. 6. In addition to the blocking effect on corrosive media and the trapping effect on Cl−, the molybdate corrosion inhibitor released by LDH due to anion exchange further inhibited the occurrence of corrosion.
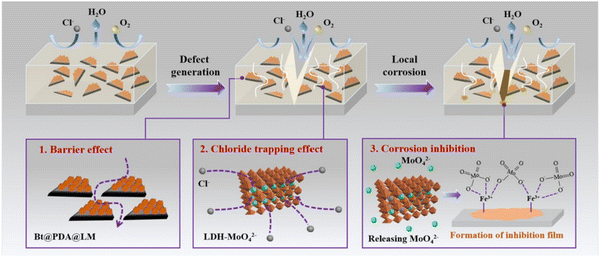 | ||
| Fig. 6 The protection mechanism of the Bt@PDA@LM/EP coating. Reproduced with permission from ref. 121. Copyright 2022 Elsevier. | ||
The work of Wang et al. reported that sodium tripolyphosphate intercalated LDHs as fillers also effectively improved the corrosion resistance of the waterborne epoxy coating.122 The tripolyphosphate ions released by anion exchange have the effect of chelating iron ions to passivate the metal surface, thereby preventing the metal substrate from being damaged by corrosive media. Xu et al. prepared zeolitic imidazolate framework-8 (ZIF-8) in situ on the surface of Zn–Al LDHs and then loaded the corrosion inhibitor 1H-benzotriazole (BTA) PVB/(LDH/ZIF-8@BTA).123 LDH/ZIF-8@BTA were filled in the polyvinyl butyral (PVB) coatings to effectively enhance the passive and active anti-corrosion performance of the composite coatings for metal substrates. LDH-conductive polymer composites can also achieve synergistic anti-corrosion effects. Chetan B. Pawar et al. applied amino silane to surface treat Zn–Al LDHs and Mg–Al LDHs synthesized by the precipitation method, and then grafted conductive polymers polyaniline (PANI), polyorthoanisidine (POA), and PANI–POA copolymer onto the surface of LDHs.124 The addition of the synthesized LDH-conductive polymer composites to epoxy-polyamide based coatings can enhance the pencil hardness, scratch resistance, and corrosion resistance of coatings. LDHs after functional modification can better play a corrosion-resistant role in polymer coatings. Therefore, LDHs have great application prospects in conversion coatings or as nanofillers for polymer coatings.125
3.2. Applications of MXenes in corrosion prevention
There have been a lot of research works reporting the application of MXenes in corrosion prevention. Due to the large specific surface area, excellent mechanical properties and many other superior properties, MXenes can exert the labyrinth effect in polymer coatings to block the penetration of corrosive media. However, there are also some problems in applying MXenes directly into polymer coatings.126 MXenes are prone to agglomerate in polymer coatings, resulting in poor dispersion.127 MXenes are also easily oxidized and have poor stability.128 Moreover, the direct addition of MXenes may cause galvanic corrosion due to the conductivity of MXenes.129 Therefore, before MXenes are added to the polymer coatings, they need to be modified to achieve great dispersion to better protect the metal substrates. MXenes can be modified through covalent or non-covalent interactions.The construction of self-healing coatings enables the coatings to actively protect against corrosion when damaged. Sun et al. combined mesoporous silica nanoparticles loaded tannic acid corrosion inhibitor onto the MXene surface as nanofillers for epoxy coatings.130 In addition to the passive shielding effect on corrosive media, when the coatings were damaged, the corrosion inhibitor tannic acid would be released and react with iron ions to form a self-healing film to achieve active protection of the metal substrate.131 The strategy adopted by Yan et al. was to encapsulate benzotriazole as the corrosion inhibitor and self-healing agent in a 2-methylimidazole zinc salt (ZIF-8) nanocontainer (ZB), and then load the ZB on the amino-functionalized Ti3C2Tx surface (f-Ti3C2Tx-ZB).132 The purpose of amino functionalization of Ti3C2Tx was to improve the dispersion and compatibility of Ti3C2Tx in the epoxy resin matrix. The schematic illustration of the synthesis of f-Ti3C2Tx-ZB is shown in Fig. 7.
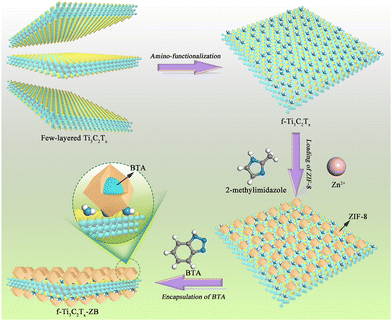 | ||
| Fig. 7 The synthesis process of f-Ti3C2Tx-ZB. Reproduced with permission from ref. 132. Copyright 2021 Elsevier. | ||
The anti-corrosion and anti-wear performance tests indicated that f-Ti3C2Tx-ZB@EP composite coating has the best corrosion resistance and better tribological properties in friction-reducing and wear resistance. When the corrosion reactions occur, it would cause a change in pH, causing the BTA in the ZIF-8 nanocontainers to be released. BTA corrosion inhibitors can form corrosion inhibition films on the surface of the metal substrate to further delay the corrosion rate autonomously. The high wear resistance of the f-Ti3C2Tx-ZB@EP composite coating is mainly due to the lubrication effect of f-Ti3C2Tx and the improvement of plastic deformation resistance. The self-healing function of f-Ti3C2Tx-ZB creates greater possibilities for the practical application of MXenes in anti-corrosion.
MXenes would be gradually oxidized and degraded in air or water, which would also destroy the integrity of the coatings. Therefore, avoiding the oxidation of MXenes is crucial for the application of MXenes in coatings. Related works were carried out based on this. Ning et al. used 1-allyl-3-methylimidazolium bromide ([EMIM]+Br−) to non-covalently modify MXenes (I-Ti3C2Tx) to avoid oxidation or deterioration of MXenes.133 In the antioxidant stability test, after soaking in water for 30 days, Ti3C2Tx dissolved into amorphous TiO2 and disordered carbon structures. On the contrary, I-Ti3C2Tx still retained a 2D lamellar structure similar to fresh Ti3C2Tx, which demonstrated that imidazolium salt can effectively delay the degradation of Ti3C2Tx in an aqueous solution. Excellent oxidation resistance plays a vital role in the application of Ti3C2Tx in coatings. Then, I-Ti3C2Tx was dispersed into the epoxy resin matrix to prepare the composite coatings. The corrosion resistance test results showed that when the added amount of I-Ti3C2Tx was 1.0 wt%, the composite coating maintained a high OCP value during the corrosion immersion process, showing better corrosion resistance. After 240 hours of salt spray test, the surface of the 1.0 wt% I-Ti3C2Tx/EP composite coating remained intact, while the blank EP showed severe corrosion. The Tafel polarization test gave consistent results. 1.0 wt% I-Ti3C2Tx/EP coating has the lowest corrosion rate (1.762 × 10−7 mm per year), and the corrosion rate value was lower than other reported coatings. On the one hand, 1-allyl-3-methylimidazolium bromide effectively delayed the oxidative degradation of MXenes so that MXenes can act as stable barriers. On the other hand, the compatibility between MXenes and the epoxy resin matrix was also enhanced. Wu et al. used cellulose nanofiber to modify Ti3C2Tx to construct Ti3C2Tx@CNF nanohybrids and used them as fillers to enhance the corrosion resistance of waterborne epoxy coatings under the marine alternating hydrostatic pressure (AHP) environment.134 MXene was first etched and then intercalated with dimethyl sulfoxide (DMSO). Then the prepared few-layered delaminated-Ti3C2Tx MXenes were mixed with CNF through strong hydrogen bonding forces. The process of MXenes being modified by CNF is shown in Fig. 8a. The corresponding SEM, TEM and SPM results confirmed the success of CNF modification. As a bioadhesive with rich oxygen-containing functional groups, CNF has the ability to enhance the dispersion and compatibility between Ti3C2Tx and epoxy resin, thereby improving the compactness of the composite coatings and also enhancing the adhesion of the epoxy resin to the metal substrates. The excellent adhesion enables the composite coatings to maintain reliable anti-corrosion performance in the harsher deep-sea environment.
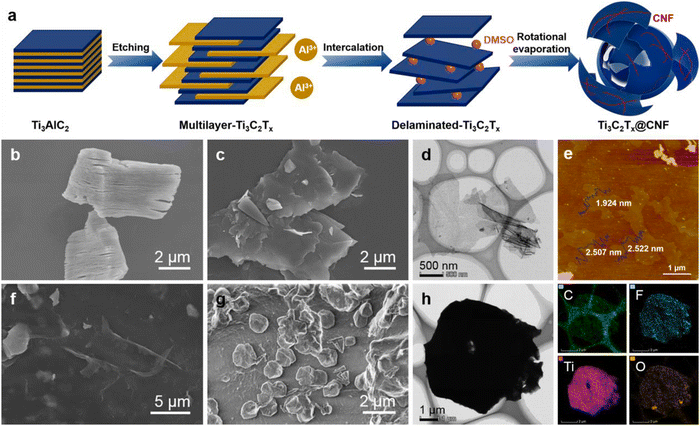 | ||
| Fig. 8 The process of MXenes being modified by CNF (a); SEM images of (b) multilayer-Ti3C2Tx and (c) delaminated- Ti3C2Tx; TEM (d) and SPM (e) images of delaminated-Ti3C2Tx; SEM images of (f) Ti3C2Tx@CNF, and (g) the cross-sectional image of the Ti3C2Tx@CNF film; TEM image of (h) the Ti3C2Tx@CNF topological structure and its elemental mapping. Reproduced with permission from ref. 134. Copyright 2022 Elsevier. | ||
Zhao et al. prepared antioxidant and high-stability MXene sheets through non-covalent functionalization between MXenes and ionic liquid (IL), and used IL@MXene as smart barrier enhancers for WEP coatings.135 IL can quench the reactive oxygen species to passivate Ti3C2Tx. In addition, IL has the ability to disperse and stabilize 2D nanomaterials. Tests on the air stability of MXenes inhibited that IL@MXene still retained the complete crystal structures after aging 30 days, but the unmodified MXene sheets have been highly oxidized and destroyed. The electrochemical test results confirmed that the impedance modulus of the IL@MXene-WEP composite coating was increased by 1–2 orders of magnitude compared with the blank WEP coating. This work also confirmed that when MXenes have excellent antioxidant properties, they can exert better corrosion resistance in the coatings. In our work, poly(tannic acid) was used to modify the MXene, and zinc ions were introduced at the same time.136 Through modification, the MXene@PTA-Zn(II) surface contains abundant hydroxyl groups, which increases the compatibility between the fillers and the epoxy coating, greatly enhancing the corrosion resistance.
Some other works combine MXenes with other nanomaterials such as GO, LDHs, SiO2, etc. to achieve synergistic anti-corrosion effects. Relevant experimental results confirm that this synergistic effect is effective in enhancing corrosion resistance. Wu et al. prepared the MXene/MgAl-LDH composite film modified with yttrium to improve the corrosion resistance of AZ31 Mg alloy.137 Since the few-layer MXene sheets have electronegativity and abundant OH groups, they can effectively adsorb Mg2+ and Al3+ and provide nucleation sites for the in situ growth of LDHs. Therefore, the composite coating layer of MXenes/MgAl-LDH with Y(OH)3 can be synthesized on the AZ31 Mg alloy surface through a one-step hydrothermally chemical conversion method. MXenes can effectively cover the cracks in the LDH coatings to better prevent the penetration of corrosive media. LDHs can further inhibit corrosion through the anion exchange. Furthermore, Y(OH)3 as a corrosion inhibitor can enable active anti-corrosion of the composite coatings. Cai et al. also prepared the composite of MXenes and LDHs and used the composite materials to enhance the corrosion resistance and friction resistance of epoxy resin coatings.138 DFT calculations indicated that the heterostructure of Ti3C2Tx MXene and MgAl-LDH has strong binding stability, and Ti3C2Tx MXene@MgAl-LDH still has great dispersion and compatibility with epoxy resin after standing for 160 days. Shen et al. grafted MXenes on the surface of GO nanosheets through nucleophilic substitution and used the composite materials to improve the corrosion resistance of epoxy zinc-rich coatings.139 The high conductivity Ti3C2 in GO-Ti3C2 can enhance the electrical connection between Zn particles and the steel matrix, providing a new conductive path between the zinc particles and the steel matrix, so that the zinc-rich coatings improve the utilization of zinc particles. In addition, GO can exert an excellent shielding effect on corrosive media. OCP, EIS and salt spray test results all confirmed that GO-Ti3C2 improved the cathodic protection ability of epoxy zinc-rich coatings, which provides new inspiration for the enhancement of corrosion resistance of epoxy zinc-rich coatings. TiO2 can also be used to modify MXenes. When 0.1% Ti3C2&TiO2 was incorporated into the waterborne polyurethane coating, the corrosion resistance of coatings was significantly improved. Ti3C2 and TiO2 repaired micropores and cracks in coatings while also enhancing the hydrophobicity of the waterborne polyurethane coating.140
We prepared polydopamine (PDA) modified MXene–ZrP composites using a one-pot method and applied the MXene–ZrP@PDA (MZP) heterojunction to enhance the corrosion and wear resistance of waterborne epoxy coatings.141 The corrosion test results indicated that the corrosion rate of the MZP/WEP composite coating was reduced by an order of magnitude compared with the blank WEP coating. The two-dimensional nanosheet materials can extend the diffusion path of the corrosion media. In addition, combining ZrP and MXene effectively improves the aggregation effect of MXenes. The surface modification of PDA improves the interfacial compatibility of MZP in epoxy resin. Furthermore, PDA can capture Fe3+ and form Fe3+–PDA complexes to further delay the occurrence of corrosion. MZP composite materials also effectively improve the wear resistance of the MZP/WEP coating. Its average coefficient of friction (COF) was reduced by 82.06% compared with the blank WEP coating. We also grew structurally stable alkoxysilane modified SiO2in situ on the MXene's surface to prepare a 0D/2D nanohybrid (FSiO2-MX).142 The insulating FSiO2 would reduce the current effect of MXenes, thus inhibiting the occurrence of galvanic corrosion. In addition, the amino groups on the surface of SiO2 react with the epoxy resin to further promote resin curing and reduce defects in the WEP coating.
Some other green corrosion inhibitors can also be applied to modify MXenes and achieve better corrosion prevention. L-Cysteine is widely used as a green corrosion inhibitor. Li et al. applied L-cysteine as a surface modifier for MXenes (fMX) to enhance the dispersion performance of MXenes in the epoxy matrix (shown in Fig. 9).143
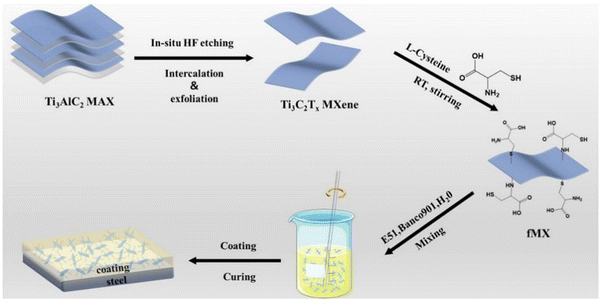 | ||
| Fig. 9 The preparation process of MXene (Ti3C2Tx), fMX and the composite coating. Reproduced with permission from ref. 143. Copyright 2021 Elsevier. | ||
The electrochemical test results indicated that the corrosion rate of the fMX composite coating was reduced by 3 orders of magnitude compared with the blank waterborne epoxy coating. fMX nanosheets have great dispersion in the epoxy matrix. In addition, there is a strong interfacial interaction between fMX and epoxy groups, which delayed the diffusion of corrosive media and improved the corrosion resistance of the waterborne epoxy coating. Chitosan is the product of removing some of the acetyl groups from the natural polysaccharide chitin and is an environmentally friendly green corrosion inhibitor. Our strategy was to apply phosphorylated chitosan to functionally modify MXenes and use the modified MXene as a filler for waterborne epoxy coatings, which can effectively inhibit the galvanic corrosion of MXenes.144 The combination of chitosan and MXenes can achieve a good synergistic effect. The agglomeration tendency of MXenes was eliminated, and chitosan can also play a corrosion inhibition role. Moreover, the adhesion between the coatings and the substrate was also increased, which further enhanced the corrosion resistance of the WEP coatings. Silk fibroin can also be used to modify MXenes, the protein fibers on the surface of SF-MXene nanosheets were beneficial to the interface with the resin improving the mechanical properties of the coating. SF-Ti3C2Tx/epoxy composite coatings exhibit excellent protection and delayed failure capabilities in harsh deep-sea environments.145 Sheng et al. reported that MXene is also a great phosphating accelerator that can effectively improve the corrosion resistance of the phosphate coating.146,147 In addition, MXenes have also shown promise for preparing functional polymers with superior flame retardancy and smoke suppression ability.
3.3. Applications of TMDs in corrosion prevention
Daniel S. Schulman et al. discovered that single-layer transition metal dichalcogenides have high resistance to electro-oxidation and corrosion.148 This is due to the excellent chemical stability of monomolecular delamination in oxidative corrosion environments, which indicates that TMD materials, especially disulfides, have great prospects and potential economic impact in anti-corrosion applications. Among TMDs, layered molybdenum disulfide (MoS2) has attracted increasing attention in the field of anti-corrosion due to its special hydrophobicity, ultra-thin atomic layer structure, and large specific surface area. Although exposed MoS2 is easily corroded and oxidized, when it is added as the nanofiller to the polymer coatings, the corrosion resistance of the prepared nanocomposite coatings can be increased. Ding et al. prepared layered TMD nanosheets of MoS2 and WS2 through liquid exfoliation technology, and incorporated these two nanosheets into the epoxy matrix.149 The electrochemical test results fully confirmed that the low conductivity of TMDs can effectively cut off the electronic path of microgalvanic corrosion and inhibit the occurrence of corrosion. There have been some reports that using other substances to modify MoS2 to prepare composite polymer coatings can effectively extend the life of the polymer coatings. Xia et al. used PDA to modify MoS2 and PDA@MoS2 to improve the corrosion resistance of epoxy resin.150 Compared with the blank EP coating, the corrosion resistance of the composite MoS2@PDA/EP coating was greatly enhanced. In addition, the adhesion strength of the epoxy coating to the metal matrix has also been enhanced by about 3 MPa. Zhao et al. used sodium dodecyl benzene sulfonate to modify MoS2 and fabricated a 2D MoS2/SDBS composite coating on carbon steel.151 MoS2/SDBS can effectively fill the voids of an epoxy coating to increase corrosion resistance. Gao et al. successfully prepared MoS2/acrylic composite coatings on the surface of galvanized sheets.152 Tannic acid and hydroxyethylidene diphosphonic acid were also added. The addition of MoS2 effectively reduced the corrosion rate and corrosion current density of acrylic coatings. Jing et al. applied gamma-(2,3-epoxypropoxy) propyltrimethoxy silane (KH560) to modify MoS2.153 The silane coupling agent can act as an intermediate bridge to increase the compatibility and interfacial force between the nanofiller and coatings. When 0.8 wt% KH560-MoS2 was incorporated, the corrosion resistance of epoxy coatings was significantly enhanced. Similarly, other two-dimensional sheet materials combined with TMDs can also achieve synergistic anti-corrosion effects. H-BN was added to MoS2 and deposited on the surface of mild steel. Compared with pure MoS2 coating, the corrosion resistance of the MoS2-hBN coating was enhanced and the corrosion rate was significantly reduced.154 Chen et al. uniformly loaded MoS2 on the surface of graphene oxide, and then modified MoS2-RGO with KH560 to improve the dispersion stability of MoS2-RGO in the polymer coatings.155 In this work, MoS2 and RGO achieved a synergistic anti-corrosion effect. Their excellent shielding effect greatly increased the penetration resistance of coatings.Although TMDs can effectively extend the thermal stability, toughness and mechanical properties of the coatings, the physical barrier alone cannot protect the coatings for a long time, and once the coatings are damaged, the damage is irreversible.
Milad Motamedi et al. proposed a strategy of encapsulating corrosion inhibitors Pr and tannate into ZIF8 anchored on the surface of MoS2 to achieve smart anti-corrosion of the coatings.156 EP/Pr-TA-ZIF8@MS composite coatings can control the release of corrosion inhibitors through changes in pH to achieve anti-corrosion. The self-healing protection mechanism of the EP/Pr-TA-ZIF8@MS nanocoating is shown in Fig. 10.
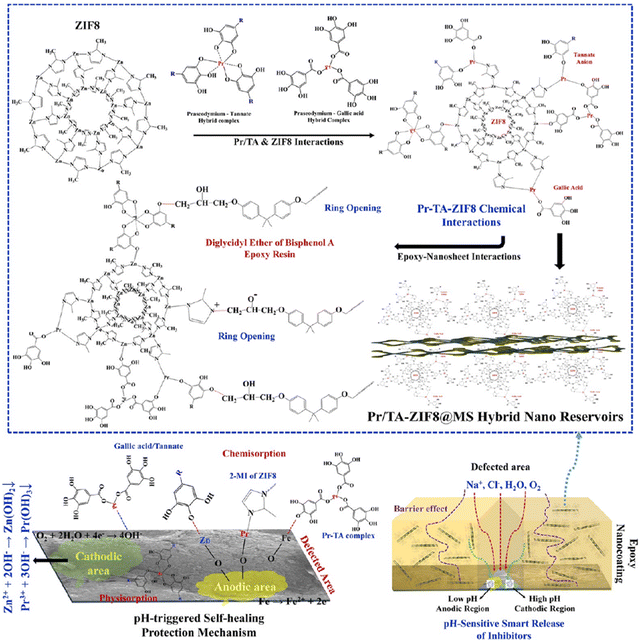 | ||
| Fig. 10 The self-healing protection mechanism of the EP/Pr-TA-ZIF8@MS nanocoating. Reproduced with permission from ref. 156. Copyright 2022 American Chemical Society. | ||
Firstly, the nanosheet materials dispersed in the polymer coatings can enhance the curvature of the diffusion paths of oxygen and water in the epoxy coatings, allowing the EP/Pr-TA-ZIF8@MS composite coatings to have an excellent barrier function. Secondly, as the pH changes, the effective inorganic cations and organic molecules in the nanocontainer would be released to the sensitive active sites of the metal, forming a corrosion inhibition layer adsorbed on the surface of the metal matrix to delay the occurrence of corrosion reactions. Pr3+/Zn2+ and tannate/2-MI in the nano reservoir would be intelligently released to the low pH anode and high pH cathode of the metal matrix at the same time by pH-triggered framework hydrolysis and ion/ligand exchange activities. Hence, adhesion-stabilizing complexes of Pr/Zn-tannate, Pr/Zn-tannate-Fe, and Pr/Zn-MI-Fe adherent/stable complexes are formed at the damaged part of the metal substrate. In addition, the adhesion loss of the EP/Pr-TA-ZIF8@MS nanocomposite coating was reduced by 71.1%, and cathodic debonding was effectively reduced by 60.11%. Compared with the blank EP, the cross-linking density and tensile strength of the EP/Pr-TA-ZIF8@MS composite coating were significantly enhanced. This is an impressive strategy that provides new ideas for the use of nanomaterials in composite coatings and the controlled release of corrosion inhibitors.
4. Summary
4.1. Concluding remarks
This article briefly introduces the structure and properties of two-dimensional transition metal layered materials including LDHs, TMDs and MXenes and their applications in metal corrosion prevention. Due to the two-dimensional lamellar structures and excellent physical and chemical properties, LDHs, MXenes and TMDs have great application prospects in the field of metal anti-corrosion. The conclusions are summarized as follows:1. LDHs can not only act as nanofillers in polymer coatings, but also form chemical conversion coatings to protect metal substrates from corrosion damage.
2. TMDs and MXenes are easily oxidized, corrosion prevention is not obvious when TMDs and MXenes are directly introduced into polymer coatings. Therefore, it is necessary to functionalize two-dimensional transition metal layered materials before introducing them into polymer coatings.
3. Modification can also solve the problem of easy agglomeration of two-dimensional transition metal layered materials in polymer coatings.
4. A lot of research works have confirmed that two-dimensional transition metal layered materials contribute to increasing the corrosion resistance of coatings.
4.2. Outlook
Although existing works have confirmed that two-dimensional transition metal nanomaterials have great permeability resistance and can enhance the corrosion resistance of nanocomposite coatings, there are still some problems that need to be improved. In the future, the application of two-dimensional transition metal nanomaterials in metal corrosion prevention can consider making breakthroughs in the following aspects:1. Two-dimensional transition metal nanomaterials, especially TMDs and MXenes, are easily oxidized, which is extremely detrimental to the performance of the subsequent constructed nanocomposite coatings. Future research should continue to focus on solving this problem to ensure the stability of the two-dimensional transition metal nanomaterials.
2. Single-function coatings can no longer meet the needs of social development. It must be considered that the coatings should exhibit more functions, such as self-healing, superhydrophobic, friction resistance, flame retardant, etc., when two-dimensional transition metal nanomaterials are introduced.
3. The corrosion data of two-dimensional transition metal material nanocomposite coatings in actual application scenarios should be tested and the corrosion data should be monitored in real time.
4. Appropriate models should be established to predict the corrosion process of two-dimensional transition metal material nanocomposite coatings in a more intelligent way.
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the Centrally Guided Local Science and Technology Development Fund Project (Grant No. 22ZY1QA011); the Hongliu Outstanding Young Talent Support Program of Lanzhou University of Technology; and the Lanzhou Talent Innovation and Entrepreneurship Project (Grant No. 2023-RC-46).References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666–669 CrossRef CAS PubMed.
- F. Zhou, Y. Ma, Y. Chen, L. Zhang and X. Sheng, Prog. Org. Coat., 2024, 186, 108007 CrossRef CAS.
- D. Huang, L. Zhang, X. Sheng and Y. Chen, Appl. Therm. Eng., 2023, 232, 121041 CrossRef.
- R. Lv, J. A. Robinson, R. E. Schaak, D. Sun, Y. Sun, T. E. Mallouk and M. Terrones, Acc. Chem. Res., 2015, 48, 56–64 CrossRef CAS PubMed.
- G. Mishra, B. Dash and S. Pandey, Appl. Clay Sci., 2018, 153, 172–186 CrossRef CAS.
- X.-J. Zhao, S.-M. Xu, P. Yin, J.-Y. Guo, W. Zhang, Y. Jie and H. Yan, Chem. Eng. J., 2023, 451, 138500 CrossRef CAS.
- M. A. Nazir, T. Najam, S. Jabeen, M. A. Wattoo, M. S. Bashir, S. S. A. Shah and A. U. Rehman, Inorg. Chem. Commun., 2022, 145, 110008 CrossRef CAS.
- M. Chhowalla, H. S. Shin, G. Eda, L.-J. Li, K. P. Loh and H. Zhang, Nat. Chem., 2013, 5, 263–275 CrossRef PubMed.
- X. Zhang, X.-F. Qiao, W. Shi, J.-B. Wu, D.-S. Jiang and P.-H. Tan, Chem. Soc. Rev., 2015, 44, 2757–2785 RSC.
- Y. Shen, L. Liang, S. Zhang, D. Huang, J. Zhang, S. Xu, C. Liang and W. Xu, Nanoscale, 2018, 10, 1622–1630 RSC.
- E. Lee, Y. S. Yoon and D.-J. Kim, ACS Sens., 2018, 3, 2045–2060 CrossRef CAS PubMed.
- S. Alwarappan, N. Nesakumar, D. Sun, T. Y. Hu and C.-Z. Li, Biosens. Bioelectron., 2022, 205, 113943 CrossRef CAS PubMed.
- X. Guan, Z. Yang, M. Zhou, L. Yang, R. Peymanfar, B. Aslibeiki and G. Ji, Small Struct., 2022, 3, 2200102 CrossRef CAS.
- J. Jiang, S. Bai, J. Zou, S. Liu, J.-P. Hsu, N. Li, G. Zhu, Z. Zhuang, Q. Kang and Y. Zhang, Nano Res., 2022, 15, 6551–6567 CrossRef CAS.
- A. Iqbal, T. Hassan, Z. Gao, F. Shahzad and C. M. Koo, Carbon, 2023, 203, 542–560 CrossRef CAS.
- J. Li, F. Meng, L. Liu, Y. Cui, R. Liu, H. Zheng and F. Wang, Corros. Commun., 2022, 5, 62–72 CrossRef.
- X. Huang, Q. Weng, Y. Chen, L. Zhang and X. Sheng, Surf. Interfaces, 2024, 45, 103911 CrossRef CAS.
- J. Shen, Y. Ma, F. Zhou, X. Sheng and Y. Chen, J. Mater. Sci. Technol., 2024 DOI:10.1016/j.jmst.2024.01.014.
- T. Liu, L. Ma, X. Wang, J. Wang, H. Qian, D. Zhang and X. Li, Corros. Commun., 2021, 1, 18–25 CrossRef.
- Y. Tian, H. Huang, H. Wang, Y. Xie, X. Sheng, L. Zhong and X. Zhang, J. Alloys Compd., 2020, 831, 154906 CrossRef CAS.
- Y. Tian, L. Zhong, X. Sheng and X. Zhang, Adv. Compos. Hybrid Mater., 2022, 5, 1922–1938 CrossRef CAS.
- S. Nahirniak, A. Ray and B. Saruhan, Batteries, 2023, 9, 126 CrossRef CAS.
- Y. Wang, T. Guo, Z. Tian, K. Bibi, Y. Zhang and H. N. Alshareef, Adv. Mater., 2022, 34, 2108560 CrossRef CAS PubMed.
- M. S. Javed, A. Mateen, S. Ali, X. Zhang, I. Hussain, M. Imran, S. S. A. Shah and W. Han, Small, 2022, 18, 2201989 CrossRef CAS.
- K. Li, J. Li, Q. Zhu and B. Xu, Small Methods, 2022, 6, 2101537 CrossRef CAS PubMed.
- Q. Fu, J. Han, X. Wang, P. Xu, T. Yao, J. Zhong, W. Zhong, S. Liu, T. Gao, Z. Zhang, L. Xu and B. Song, Adv. Mater., 2021, 33, 1907818 CrossRef CAS PubMed.
- M. Xu and M. Wei, Adv. Funct. Mater., 2018, 28, 1802943 CrossRef.
- H. Yi, S. Liu, C. Lai, G. Zeng, M. Li, X. Liu, B. Li, X. Huo, L. Qin, L. Li, M. Zhang, Y. Fu, Z. An and L. Chen, Adv. Energy Mater., 2021, 11, 2002863 CrossRef CAS.
- Z. Bi, R. Guo, X. Hu, J. Wang, X. Chen and W. Pan, Nanoscale, 2022, 14, 3367–3386 RSC.
- T. Hu, Z. Gu, G. R. Williams, M. Strimaite, J. Zha, Z. Zhou, X. Zhang, C. Tan and R. Liang, Chem. Soc. Rev., 2022, 51, 6126–6176 RSC.
- A. K. Mia, M. Meyyappan and P. K. Giri, Biosensors, 2023, 13, 169 CrossRef CAS PubMed.
- D. S. Schulman, D. May-Rawding, F. Zhang, D. Buzzell, N. Alem and S. Das, ACS Appl. Mater. Interfaces, 2018, 10, 4285–4294 CrossRef CAS PubMed.
- L. Ma, Y. Qiang and W. Zhao, Chem. Eng. J., 2021, 408, 127367 CrossRef CAS.
- H. Jiang, Y. Xie, R. Zhu, Y. Luo, X. Sheng, D. Xie and Y. Mei, Chem. Eng. J., 2023, 456, 141049 CrossRef CAS.
- Y. Luo, Y. Xie, W. Geng, J. Chu, H. Wu, D. Xie, X. Sheng and Y. Mei, J. Mater. Sci. Technol., 2022, 129, 27–39 CrossRef CAS.
- Y. Cao, M. Weng, M. H. H. Mahmoud, A. Y. Elnaggar, L. Zhang, I. H. El Azab, Y. Chen, M. Huang, J. Huang and X. Sheng, Adv. Compos. Hybrid Mater., 2022, 5, 1253–1267 CrossRef CAS.
- Z. Gu, J. J. Atherton and Z. P. Xu, Chem. Commun., 2015, 51, 3024–3036 RSC.
- J. Yu, Q. Wang, D. O’Hare and L. Sun, Chem. Soc. Rev., 2017, 46, 5950–5974 RSC.
- X. Zhang, Y. Zhang, Y. Lv, Z. Dong, T. Hashimoto and X. Zhou, Corros. Commun., 2022, 6, 67–83 CrossRef.
- V. Zahedi Asl, J. Zhao, Y. Palizdar and M. Junaid Anjum, Corros. Commun., 2022, 5, 73–86 CrossRef.
- J. Li, F. Meng, L. Liu, Y. Cui, R. Liu, H. Zheng and F. Wang, Corros. Commun., 2022, 5, 62–72 CrossRef.
- A. Jagtap, P. G. Wagle, E. Jagtiani and A. P. More, J. Coat. Technol. Res., 2022, 19, 1009–1032 CrossRef CAS.
- A. V. Karim, A. Hassani, P. Eghbali and P. V. Nidheesh, Curr. Opin. Solid State Mater. Sci., 2022, 26, 100965 CrossRef CAS.
- H. N. Tran, D. T. Nguyen, G. T. Le, F. Tomul, E. C. Lima, S. H. Woo, A. K. Sarmah, H. Q. Nguyen, P. T. Nguyen, D. D. Nguyen, T. V. Nguyen, S. Vigneswaran, D.-V. N. Vo and H.-P. Chao, J. Hazard. Mater., 2019, 373, 258–270 CrossRef CAS PubMed.
- R. M. M. Santos, J. Tronto, V. Briois and C. V. Santilli, J. Mater. Chem. A, 2017, 5, 9998–10009 RSC.
- L. Liu, Q. Deng, P. White, S. Dong, I. S. Cole, J. Dong and X.-B. Chen, Corros. Commun., 2022, 8, 40–48 CrossRef.
- M. Zubair, I. Ihsanullah, H. Abdul Aziz, M. Azmier Ahmad and M. A. Al-Harthi, Bioresour. Technol., 2021, 319, 124128 CrossRef CAS PubMed.
- L. Mohapatra and K. Parida, J. Mater. Chem. A, 2016, 4, 10744–10766 RSC.
- V. K. Ameena Shirin, R. Sankar, A. P. Johnson, H. V. Gangadharappa and K. Pramod, J. Controlled Release, 2021, 330, 398–426 CrossRef CAS PubMed.
- W. Feitknecht, Helv. Chim. Acta, 1942, 25, 555–569 CrossRef CAS.
- M. A. Aramendía, Y. Avilés, V. Borau, J. M. Luque, J. M. Marinas, J. R. Ruiz and F. J. Urbano, J. Mater. Chem., 1999, 9, 1603–1607 RSC.
- F. Thevenot, R. Szymanski and P. Chaumette, Clays Clay Miner., 1989, 37, 396–402 CrossRef CAS.
- M. Ogawa and H. Kaiho, Langmuir, 2002, 18, 4240–4242 CrossRef CAS.
- A. V. Radha, P. Vishnu Kamath and C. Shivakumara, Solid State Sci., 2005, 7, 1180–1187 CrossRef CAS.
- M. Meyn, K. Beneke and G. Lagaly, Inorg. Chem., 1993, 32, 1209–1215 CrossRef CAS.
- F. Wong and R. G. Buchheit, Prog. Org. Coat., 2004, 51, 91–102 CrossRef CAS.
- W. Li, J. Lu, J. Chen, G. Li, Y. Jiang, L. Li and B. Huang, J. Chem. Technol. Biotechnol., 2006, 81, 89–93 CrossRef CAS.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- J. Pang, B. Chang, H. Liu and W. Zhou, ACS Energy Lett., 2022, 7, 78–96 CrossRef CAS.
- Z. Othman, H. R. Mackey and K. A. Mahmoud, Chemosphere, 2022, 295, 133849 CrossRef CAS.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- X. Meng, L. Wang, X. Wang, M. Zhen, Z. Hu, S.-Q. Guo and B. Shen, Chemosphere, 2023, 338, 139550 CrossRef CAS PubMed.
- K. Gong, Y. Peng, A. Liu, S. Qi and H. Qiu, Composites, Part A, 2024, 176, 107857 CrossRef CAS.
- X. He, J. Wu, X. Huang, Y. Chen, L. Zhang and X. Sheng, Chem. Eng. Sci., 2024, 283, 119429 CrossRef CAS.
- J. Wu, Y. Chen, L. Zhang and X. Sheng, J. Ind. Eng. Chem., 2024, 129, 424–434 CrossRef CAS.
- Y. Li, S. Huang, S. Peng, H. Jia, J. Pang, B. Ibarlucea, C. Hou, Y. Cao, W. Zhou, H. Liu and G. Cuniberti, Small, 2023, 19, 2206126 CrossRef CAS PubMed.
- X. He, J. Wu, X. Huang, Y. Chen, L. Zhang and X. Sheng, Chem. Eng. Sci., 2024, 283, 119429 CrossRef CAS.
- M. AhadiParsa, A. Dehghani, M. Ramezanzadeh and B. Ramezanzadeh, Adv. Colloid Interface Sci., 2022, 307, 102730 CrossRef CAS PubMed.
- S. Sharma, A. Kumar and E. E. Ebenso, Mater. Lett., 2023, 335, 133789 CrossRef CAS.
- X. Hu, B. Quan, C. Zhu, H. Wen, M. Sheng, S. Liu, X. Li, H. Wu, X. Lu and J. Qu, Adv. Sci., 2023, 10, 2206835 CrossRef CAS PubMed.
- D. Huang, Y. Chen, L. Zhang and X. Sheng, J. Mater. Sci. Technol., 2023, 165, 27–38 CrossRef.
- M. Naguib, M. Kurtoglu, V. Presser, J. Lu, J. Niu, M. Heon, L. Hultman, Y. Gogotsi and M. W. Barsoum, Adv. Mater., 2011, 23, 4248–4253 CrossRef CAS PubMed.
- M. Ghidiu, M. R. Lukatskaya, M.-Q. Zhao, Y. Gogotsi and M. W. Barsoum, Nature, 2014, 516, 78–81 CrossRef CAS.
- P. Urbankowski, B. Anasori, T. Makaryan, D. Er, S. Kota, P. L. Walsh, M. Zhao, V. B. Shenoy, M. W. Barsoum and Y. Gogotsi, Nanoscale, 2016, 8, 11385–11391 RSC.
- T. Li, L. Yao, Q. Liu, J. Gu, R. Luo, J. Li, X. Yan, W. Wang, P. Liu, B. Chen, W. Zhang, W. Abbas, R. Naz and D. Zhang, Angew. Chem., 2018, 130, 6223–6227 CrossRef.
- S. Yang, P. Zhang, F. Wang, A. G. Ricciardulli, M. R. Lohe, P. W. M. Blom and X. Feng, Angew. Chem., Int. Ed., 2018, 57, 15491–15495 CrossRef CAS PubMed.
- X. Yin, C. S. Tang, Y. Zheng, J. Gao, J. Wu, H. Zhang, M. Chhowalla, W. Chen and A. T. S. Wee, Chem. Soc. Rev., 2021, 50, 10087–10115 RSC.
- J. Radhakrishnan, S. Ratna and K. Biswas, Inorg. Chem. Commun., 2022, 145, 109971 CrossRef CAS.
- R. F. Frindt and A. D. Yoffe, Proc. R. Soc. Lond. A, 1963, 273, 69–83 Search PubMed.
- S. Aftab and H. H. Hegazy, Small, 2023, 19, 2205778 CrossRef CAS PubMed.
- A. Alfieri, S. B. Anantharaman, H. Zhang and D. Jariwala, Adv. Mater., 2023, 35, 2109621 CrossRef CAS PubMed.
- S. Ali, S. S. Ahmad Shah, M. Sufyan Javed, T. Najam, A. Parkash, S. Khan, M. A. Bajaber, S. M. M. Eldin, R. A. Tayeb, M. M. Rahman and J. Qi, Chem. Rec., 2023, e202300145 Search PubMed.
- A. Joseph, A. S. Vijayan, C. M. Shebeeb, K. S. Akshay, K. P. John Mathew and V. Sajith, J. Mater. Chem. A, 2023, 11, 3172–3209 RSC.
- R. Yang, Y. Fan, Y. Zhang, L. Mei, R. Zhu, J. Qin, J. Hu, Z. Chen, Y. Hau Ng, D. Voiry, S. Li, Q. Lu, Q. Wang, J. C. Yu and Z. Zeng, Angew. Chem., Int. Ed., 2023, 62, e202218016 CrossRef CAS.
- Y. Shi, B. Yang and P. Liaw, Metals, 2017, 7, 43 CrossRef.
- Y. Tian, Y. Xie, F. Dai, H. Huang, L. Zhong and X. Zhang, Surf. Coat. Technol., 2020, 383, 125227 CrossRef CAS.
- Y. Tian, W. Qiu, Y. Xie, H. Huang, J. Hu, L. Zhong, X. Jiang and X. Zhang, J. Electrochem. Soc., 2020, 167, 101505 CrossRef CAS.
- C. M. Hansson, Metall. Mater. Trans. A, 2011, 42, 2952–2962 CrossRef CAS.
- L. M. Zhang, S. D. Zhang, A. L. Ma, H. X. Hu, Y. G. Zheng, B. J. Yang and J. Q. Wang, Corros. Sci., 2018, 144, 172–183 CrossRef CAS.
- A. Fateh, M. Aliofkhazraei and A. R. Rezvanian, Arabian J. Chem., 2020, 13, 481–544 CrossRef CAS.
- N. K. Prasad, A. S. Pathak, S. Kundu and K. Mondal, Corros. Sci., 2021, 189, 109616 CrossRef CAS.
- T. Vu Dinh, W. W. Sun, Y. Yue, Y. Wu, C. R. Hutchinson and S. Thomas, Corros. Sci., 2018, 145, 67–79 CrossRef CAS.
- S. Kumar, T. Banerjee and D. Patel, Mater. Today: Proc., 2020, 33, 5678–5682 CAS.
- L. A. Hernández-Alvarado, L. S. Hernández, J. Garrido, S. Rivera-Villalobos and M. L. Escudero, Surf. Coat. Technol., 2017, 325, 473–481 CrossRef.
- H. Xu and Y. Zhang, Coatings, 2019, 9, 807 CrossRef CAS.
- Y. Tian, W. Wang, L. Zhong, X. Jiang and X. Zhang, Prog. Org. Coat., 2022, 163, 106655 CrossRef CAS.
- D. Zhang, F. Peng and X. Liu, J. Alloys Compd., 2021, 853, 157010 CrossRef CAS.
- X. Zhang, F. Zhong, X. Li, B. Liu, C. Zhang, B. Buhe, T. Zhang, G. Meng and F. Wang, J. Alloys Compd., 2019, 788, 756–767 CrossRef CAS.
- X. Guo, F. Zhang, D. G. Evans and X. Duan, Chem. Commun., 2010, 46, 5197 RSC.
- L. Guo, W. Wu, Y. Zhou, F. Zhang, R. Zeng and J. Zeng, J. Mater. Sci. Technol., 2018, 34, 1455–1466 CrossRef CAS.
- A. C. Bouali, M. H. Iuzviuk, M. Serdechnova, K. A. Yasakau, D. Drozdenko, A. Lutz, K. Fekete, G. Dovzhenko, D. C. F. Wieland, H. Terryn, M. G. S. Ferreira, I. A. Zobkalo and M. L. Zheludkevich, J. Phys. Chem. C, 2021, 125, 11687–11701 CrossRef CAS.
- M. J. Anjum, J. Zhao, V. Zahedi Asl, G. Yasin, W. Wang, S. Wei, Z. Zhao and W. Qamar Khan, Corros. Sci., 2019, 157, 1–10 CrossRef CAS.
- Y. Zhang, J. Liu, Y. Li, M. Yu, S. Li and B. Xue, J. Coat. Technol. Res., 2015, 12, 595–601 CrossRef CAS.
- K. Lin, X. Luo, X. Pan, C. Zhang and Y. Liu, Appl. Surf. Sci., 2019, 463, 1085–1096 CrossRef CAS.
- Y. Cao, D. Zheng, X. Li, J. Lin, C. Wang, S. Dong and C. Lin, ACS Appl. Mater. Interfaces, 2018, 10, 15150–15162 CrossRef CAS PubMed.
- C. Zhang, X. Luo, X. Pan, L. Liao, X. Wu and Y. Liu, Appl. Surf. Sci., 2017, 394, 275–281 CrossRef CAS.
- I. Mohammadi, T. Shahrabi, M. Mahdavian and M. Izadi, Surf. Coat. Technol., 2021, 409, 126882 CrossRef CAS.
- H. Wang, Y. Song, X. Chen, G. Tong and L. Zhang, Corros. Sci., 2022, 208, 110699 CrossRef CAS.
- W. Jian, Z. Jin, J. Yang, G. Meng, H. Liu and H. Liu, J. Ind. Eng. Chem., 2022, 113, 419–430 CrossRef CAS.
- D. Li, X. Cui, X. Wen, Y. Li, E. Liu, G. Jin and W. Zheng, J. Alloys Compd., 2023, 947, 169666 CrossRef CAS.
- Y. Jiang, S. Gao, Y. Liu, H. Huangfu, X. Guo and J. Zhang, Surf. Coat. Technol., 2022, 440, 128504 CrossRef CAS.
- N. Huang, Y. Wang, Y. Zhang, L. Liu, N. Yuan and J. Ding, Surf. Coat. Technol., 2023, 463, 129539 CrossRef CAS.
- X. Dai, L. Wu, W. Ci, W. Yao, Y. Yuan, Z. Xie, B. Jiang, J. Wang, A. Andrej and F. Pan, Corros. Sci., 2023, 220, 111285 CrossRef CAS.
- Y. Chen, L. Wu, W. Yao, J. Wu, Y. Yuan, Z. Xie, B. Jiang and F. Pan, Colloids Surf., A, 2022, 655, 130339 CrossRef CAS.
- Y. Wu, L. Wu, W. Yao, B. Jiang, J. Wu, Y. Chen, X. Chen, Q. Zhan, G. Zhang and F. Pan, Electrochim. Acta, 2021, 374, 137913 CrossRef CAS.
- Y. Chen, L. Wu, W. Yao, J. Wu, J. Xiang, X. Dai, T. Wu, Y. Yuan, J. Wang, B. Jiang and F. Pan, J. Mater. Sci. Technol., 2022, 130, 12–26 CrossRef CAS.
- N. S. Sangaj and V. C. Malshe, Prog. Org. Coat., 2004, 50, 28–39 CrossRef CAS.
- N. Sharma and S. Sharma, Mater. Today: Proc., 2021, 44, 4498–4502 CAS.
- H. Xu and Y. Zhang, Coatings, 2019, 9, 807 CrossRef CAS.
- C. Liu, S. Qiu, P. Du, H. Zhao and L. Wang, Nanoscale, 2018, 10, 8115–8124 RSC.
- M. Zhang, F. Xu, D. Lin, J. Peng, Y. Zhu and H. Wang, Chem. Eng. J., 2022, 446, 137078 CrossRef.
- N. Wang, H. Gao, J. Zhang, L. Li, X. Fan and X. Diao, Prog. Org. Coat., 2019, 135, 74–81 CrossRef CAS.
- T. Xu, Y. Zhao, J.-H. Zhou and J.-M. Hu, Prog. Org. Coat., 2022, 164, 106695 CrossRef CAS.
- C. B. Pawar, P. D. Desai, H. N. Bagde and A. P. More, Arab. J. Sci. Eng., 2023, 48, 7739–7753 CrossRef CAS.
- C. Ding, J. Wu, Y. Liu, X. Sheng, X. Cheng, X. Xiong and W. Zhao, Molecules, 2023, 28, 5199 CrossRef CAS PubMed.
- H. Jiang, Y. Xie, Y. Jiang, Y. Luo, X. Sheng, Y. Mei and D. Xie, Appl. Surf. Sci., 2024, 649, 159111 CrossRef CAS.
- J. Ding, H. Zhao and H. Yu, Chem. Eng. J., 2022, 430, 132838 CrossRef CAS.
- H. Cao, M. Fang, W. Jia, X. Liu and Q. Xu, Composites, Part B, 2022, 228, 109427 CrossRef CAS.
- A. Sadanandan, S. A. Thomas, M. E. Khan, M. S. Alomar, M. R. Pallavolu and J. Cherusseri, Prog. Org. Coat., 2023, 183, 107757 CrossRef CAS.
- X. Sun, C. Ma, F. Ma, T. Wang, C. Feng and W. Wang, J. Alloys Compd., 2022, 928, 167202 CrossRef CAS.
- H. Jiang, Y. Xie, R. Zhu, Y. Luo, X. Sheng, D. Xie and Y. Mei, Chem. Eng. J., 2023, 456, 141049 CrossRef CAS.
- H. Yan, X. Fan, M. Cai, S. Song and M. Zhu, J. Colloid Interface Sci., 2021, 602, 131–145 CrossRef CAS PubMed.
- Y. Ning, D. Jian, S. Liu, F. Chen, Y. Song, S. Li and B. Liu, Carbon, 2023, 202, 20–30 CrossRef CAS.
- Y. Wu, W. Zhao, S. Liu and L. Wang, Chem. Eng. J., 2022, 438, 135483 CrossRef CAS.
- H. Zhao, J. Ding, M. Zhou and H. Yu, ACS Appl. Nano Mater., 2021, 4, 3075–3086 CrossRef CAS.
- J. Wu, Y. Chen, L. Zhang and X. Sheng, Prog. Org. Coat., 2023, 182, 107706 CrossRef CAS.
- Y. Wu, L. Wu, W. Yao, B. Jiang, J. Wu, Y. Chen, X. Chen, Q. Zhan, G. Zhang and F. Pan, Electrochim. Acta, 2021, 374, 137913 CrossRef CAS.
- M. Cai, X. Fan, H. Yan, Y. Li, S. Song, W. Li, H. Li, Z. Lu and M. Zhu, Chem. Eng. J., 2021, 419, 130050 CrossRef CAS.
- L. Shen, W. Zhao, K. Wang and J. Xu, J. Hazard. Mater., 2021, 417, 126048 CrossRef CAS PubMed.
- F. Zhang, W. Liu, S. Wang, H. Shi, C. Liu, L. Liang and K. Pi, Polymer, 2021, 230, 124086 CrossRef CAS.
- X. He, S. Li, J. Wu, Y. Chen, L. Zhang and X. Sheng, Ind. Eng. Chem. Res., 2022, 61, 12576–12589 CrossRef CAS.
- X. He, J. Wu, S. Li, Y. Chen, L. Zhang and X. Sheng, Prog. Org. Coat., 2022, 171, 107042 CrossRef CAS.
- S. Li, H. Huang, F. Chen, X. He, Y. Ma, L. Zhang, X. Sheng, Y. Chen, E. Shchukina and D. Shchukin, Prog. Org. Coat., 2021, 161, 106478 CrossRef CAS.
- X. He, S. Li, R. Shen, Y. Ma, L. Zhang, X. Sheng, Y. Chen, D. Xie and J. Huang, Adv. Compos. Hybrid Mater., 2022, 5, 1699–1711 CrossRef CAS.
- J. Chen and W. Zhao, Chem. Eng. J., 2021, 423, 130195 CrossRef CAS.
- J. Wu, Y. Chen, L. Zhang and X. Sheng, J. Ind. Eng. Chem., 2024, 129, 424–434 CrossRef CAS.
- X. He, J. Wu, Y. Chen, L. Zhang and X. Sheng, Appl. Surf. Sci., 2022, 603, 154455 CrossRef CAS.
- D. S. Schulman, D. May-Rawding, F. Zhang, D. Buzzell, N. Alem and S. Das, ACS Appl. Mater. Interfaces, 2018, 10, 4285–4294 CrossRef CAS PubMed.
- J. Ding, H. Zhao, X. Zhao, B. Xu and H. Yu, J. Mater. Chem. A, 2019, 7, 13511–13521 RSC.
- Z. Xia, G. Liu, Y. Dong and Y. Zhang, Prog. Org. Coat., 2019, 133, 154–160 CrossRef CAS.
- X. Zhao, B. Zhang, Z. Jin, C. Chen, Q. Zhu and B. Hou, RSC Adv., 2016, 6, 97512–97522 RSC.
- F. Gao, A. Du, R. Ma, C. Lv, H. Yang, Y. Fan, X. Zhao, J. Wu and X. Cao, Colloids Surf., A, 2020, 587, 124318 CrossRef CAS.
- Y. Jing, P. Wang, Q. Yang, Y. He and Y. Bai, Mater. Res. Express, 2019, 6, 086473 CrossRef CAS.
- J. Ding, H. Zhao, X. Zhao, B. Xu and H. Yu, J. Mater. Chem. A, 2019, 7, 13511–13521 RSC.
- C. Chen, Y. He, G. Xiao, Y. Xia, H. Li and Z. He, Appl. Surf. Sci., 2018, 444, 511–521 CrossRef CAS.
- M. Motamedi, S. Mohammadkhah, M. Ramezanzadeh, H. Eivaz Mohammadloo and B. Ramezanzadeh, ACS Appl. Mater. Interfaces, 2022, 14, 31170–31193 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |
