 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An algae-derived partially renewable epoxy resin formulation for glass fibre-reinforced sustainable polymer composites†
Dimitrios
Apostolidis‡
,
William E.
Dyer‡
,
Clemens A.
Dransfeld
and
Baris
Kumru
*
Aerospace Structures & Materials Department, Faculty of Aerospace Engineering, Delft University of Technology, 2629 HS Delft, The Netherlands. E-mail: b.kumru@tudelft.nl
First published on 2nd January 2024
Abstract
Utilization of sustainable feedstocks to fabricate renewable thermosetting epoxy resins has been of great interest recently; however, their translation into composite structures and benchmark comparisons are poorly understood. Phloroglucinol is a phenolic molecule obtained from brown algae, and its epoxidized form is a high viscosity, high reactivity monomer. In this study, the potential of epoxidized phloroglucinol as a laminating resin was examined in comparison with a bisphenol A diglycidyl ether (BADGE) epoxy monomer employing the Epikure 04908 linear amine hardener system. Utilization of a reactive diluent for PHTE resin was necessary for room temperature laminating applications to reduce viscosity, and the thermomechanical properties of PHTE-based resins and composites are superior to those of BADGE systems.
Polymer composites use polymer matrices and various fillers across different lengthscales (nano to macro) to construct advanced materials with upgraded functions; i.e. conductivity, photoactivity, and mechanical reinforcement.1–4 Fibre-reinforced polymer composites (FRPCs) shaped the modern aviation, automotive and wind turbine industries by presenting lightweight, durable and mechanically superior structures.5 Thermosetting resins are the dominant class of matrices used for reinforcing fibres, such as carbon and glass, using manufacturing engineering to form defect-free structures.6 Currently, the composite market is dominated by the use of diglycidyl ether of bisphenol A (DGEBA) as an epoxy monomer, which is generated through the reaction of bisphenol A with epichlorohydrin (epichlorohydrin can be made 100% biobased on an industrial scale) in the presence of a base.7 A hardener is selected among multifunctional amines or anhydrides based on the target application and provides an exothermic ring-opening reaction with multifunctional epoxy monomers to form a thermoset.8,9 Additives such as reactive diluents (low viscosity epoxy compounds) and accelerators (imidazoles) are found in delicate industrial resin recipes to adjust processability and curing cycles.10
Bisphenol A (BPA) is a substance of concern as it possesses major toxicity as an endocrine disruptor.11 Additionally, its synthesis from fossil feedstocks (phenol and acetone in the presence of a strong acid catalyst) fails to satisfy the green chemistry principle ‘utilization of renewable feedstocks’.12 Hence, polyphenolic compounds from renewable resources spark interest in replacing BPA and other petroleum-derived chemicals for epoxy resin formation.13 Despite the utilization of renewable compounds such as citric acid, cardanol and linseed oil to form epoxy resins even on a commercial scale, their aliphatic structures fail to satisfy when thermomechanical performance criteria are vital.14–16 Meanwhile, many renewable starting compounds such as resveratrol are studied for epoxy resin formation.17 However, the majority of research utilizes petroleum-derived analogues of such molecules while implying the potential of generation of such molecules from renewable resources, which in reality is strictly hindered by scalability.18 Additionally, many articles report successful resin formation starting from renewable epoxy compounds; however, their translation into fibre-reinforced composites is rarely defined in the literature.19–21 Phloroglucinol is a phenolic compound with the chemical formula C6H3(OH)3, which can be extracted from brown algae in high yields.22 Other bio-synthetic pathways using genetically modified plants and bacteria are also being explored.23,24 Resin formulations using epoxidized phloroglucinol (phloroglucinol trisepoxy, PHTE) in the presence of anhydride hardeners have been reported by Mija and colleagues, whereas they further pursued carbon fibre-reinforced polymer composite formation using a PHTE-based resin with ester bonds exhibiting recyclability (the matrix dissolved in ethylene glycol solution at 170 °C after 2.5 h).25,26
Epikote-Epikure 04908 is a low-viscosity laminating system with high industrial production volume and diverse applicability, which employs DGEBA as an epoxy constituent. It has a long pot life at room temperature enabling ease of impregnation, and post-curing at 80 °C is recommended to achieve full conversion. In this project, the potential of renewable PHTE in a laminating system will be studied by replacing PHTE with BADGE and utilizing the Epikure 04908 hardener. PHTE will be screened as a renewable starting product for epoxy resin formation due to its sustainable origin and trifunctionality as well as aromaticity promising good mechanical performance.8,27 Glass fibres were used as reinforcing fabrics for composite manufacturing. The resin and composite were analyzed in detail and compared against the commercial reference material Epikote-Epikure 04098, a bisphenol A diglycidyl ether (BADGE) based system with an aliphatic amine hardener (Scheme 1).
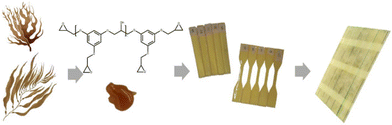 | ||
| Scheme 1 Chemical formula of PHTE and the so-formed resin and glass fibre reinforced composite images. | ||
First of all, structural analyses via1H-NMR and 13C-NMR were performed on the as-received PHTE sample to identify the oligomerization level and epoxy equivalent weight. Based on clear peak allocations and integration as given in Fig. 1a, we estimate an EEW of 112 g mol−1 (the supplier suggests 8.3 meq g−1 which is around 120 g mol−1 by titration). We confirmed the oligomeric mixture content of PHTE by the ratio of He peaks (ring-opened epoxy hydrogens) and 13C-NMR confirmed the solvent-free pure chemical structure (Fig. S1 and 2†).
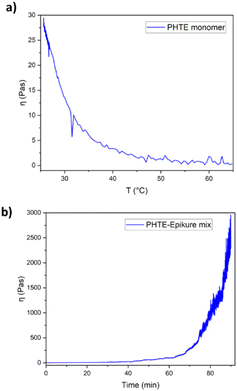 | ||
| Fig. 1 (a) Viscosity profile of PHTE with increased temperature and (b) viscosity of the PHTE–Epikure mix at RT. | ||
The FT-IR spectra of Epikure 04098 and PHTE are presented in Fig. S3†, depicting characteristic signals arising from functional groups (i.e. epoxy, amino, and the benzene ring), that are useful to monitor during curing reactions. Based on the epoxy equivalent weight (EEW) of 120 g mol−1 of PHTE28 and the amine equivalent weight (AEW) of 50 g mol−1 for the hardener,29 a mixture ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was chosen for the generation of the resin system. Experimental details are presented in the ESI.† The viscosity of pure PHTE was measured as 30 Pas at RT, which is highly viscous (Fig. 1a). Even at higher temperatures at around 60 °C, viscosity still presents a high value, which presents challenges for vacuum infusion and hand layup. Hence, it was decided to reduce the viscosity of the mixture using the 1,2-epoxy-3-phenoxypropane monofunctional reactive diluent since the addition of the Epikure 04098 hardener was not sufficient for reducing the viscosity (6 Pas was measured). A 10 wt% reactive diluent was used, and the viscosity of this system was 2 Pas shortly after mixing, an acceptable viscosity for a comparable study (Fig. 1b) but still much higher than that of the Epikote-Epikure 04098-reactive diluent, which is 0.1 Pas shortly after mixing. Additionally, the viscosity of PHTE–Epikure increases to 30 Pas in 43 minutes showing a relatively fast curing time. Cure kinetics is related to monomer activation energy and the hardener structure; therefore the activation energy of PHTE resin in the presence of the Epikure hardener was studied through different heating rates and fitting a Kissinger model. The calculated Ea of the curing reaction was 37.2 kJ mol−1 (Table S1 and Fig. S4a and b†), which is on the low end for epoxy-amine reactions.30,31 This high reactivity and viscosity led to difficulty in composite manufacturing as will be discussed later.
1 was chosen for the generation of the resin system. Experimental details are presented in the ESI.† The viscosity of pure PHTE was measured as 30 Pas at RT, which is highly viscous (Fig. 1a). Even at higher temperatures at around 60 °C, viscosity still presents a high value, which presents challenges for vacuum infusion and hand layup. Hence, it was decided to reduce the viscosity of the mixture using the 1,2-epoxy-3-phenoxypropane monofunctional reactive diluent since the addition of the Epikure 04098 hardener was not sufficient for reducing the viscosity (6 Pas was measured). A 10 wt% reactive diluent was used, and the viscosity of this system was 2 Pas shortly after mixing, an acceptable viscosity for a comparable study (Fig. 1b) but still much higher than that of the Epikote-Epikure 04098-reactive diluent, which is 0.1 Pas shortly after mixing. Additionally, the viscosity of PHTE–Epikure increases to 30 Pas in 43 minutes showing a relatively fast curing time. Cure kinetics is related to monomer activation energy and the hardener structure; therefore the activation energy of PHTE resin in the presence of the Epikure hardener was studied through different heating rates and fitting a Kissinger model. The calculated Ea of the curing reaction was 37.2 kJ mol−1 (Table S1 and Fig. S4a and b†), which is on the low end for epoxy-amine reactions.30,31 This high reactivity and viscosity led to difficulty in composite manufacturing as will be discussed later.
Rheology analysis revealed a gel point time of 197.5 minutes for the system, taken as the intersection of G′ and G′′. This theoretically gave a large processing window for pre-preg systems (Fig. S5†). However, the pot life was revealed to be around 40 minutes before large increases in viscosity were observed (Fig. 1b). When working with a bulk material, the working time was much lower due to a lower surface area to volume ratio leading to more trapped heat from the exothermic reaction and therefore faster reaction times – a critical mass of 40 g was determined and the material became solid in roughly 5 minutes using our given apparatus. The pot life contrasts sharply with the given Epikote-Epikure reference system pot life of 300 minutes. Such high reactivity together with a low critical mixing mass lowers the potential applicability of the PHTE–Epikure system in composite formation compared to Epikote-Epikure.
Thermogravimetric analysis (TGA) measurements show high thermal stability for the cured resin system up to 320 °C, after which about 50% of the resin degrades within 40 °C, as can be observed in the TGA in Fig. S6a.† After 460 °C, secondary degradation takes place with almost no mass left at 800 °C, indicating a low char yield (Fig. S6a†). A very similar thermal degradation profile was observed for the Epikote-Epikure resin system, in which the degradation starts at 20 °C less than the PHTE–Epikure resin (Fig. S6b†). The glass transition of the resin is observed at 123 °C by differential scanning calorimetry measurements (Fig. 2a). DMA performed on pure resin samples cured at room temperature reveals an initial storage modulus (E′) of 2000 MPa and a loss modulus (E′′) of 80 MPa at room temperature (Fig. S7a†). An initial Tg of 60 °C (a peak of tan δ) is observed in the 1st heating cycle, which increased to 125 °C following post-curing at 140 °C for 1 h (Fig. 2b). Hence, based on DMA data, the necessity of post-curing to fabricate a fully crosslinked resin was derived; therefore, post-curing was applied to PHTE resin formulations throughout this study. DMA showed a Tg of 60 °C for the Epikote-Epikure 04908-reactive diluent post-cured resin, which is shockingly low (Fig. S7b†). One can conclude that a PHTE-based formulation offers better thermomechanical performance compared to the BADGE-based system. Mid-range IR spectroscopy (550–4000 cm−1) revealed an absorption band at 907 cm−1 corresponding to the C–O stretching of the oxirane ring. This peak is highly pronounced in the PHTE monomer (Fig. S3†). The RT cured sample shows a large decrease in this peak, indicating that the curing reaction is proceeding; however, post-curing at 140 °C eliminates the oxirane C–O absorption band. This suggests that the curing reaction is not fully complete at room temperature, in line with the DMA data (Fig. S7a†).
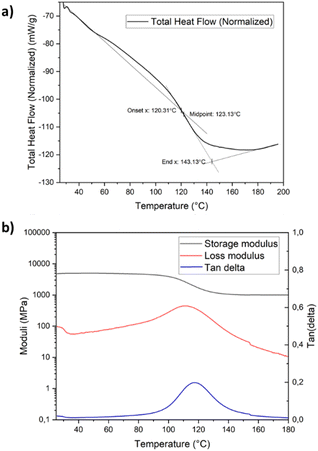 | ||
| Fig. 2 (a) Differential scanning calorimetry diagram of the cured PHTE resin and (b) dynamic mechanical analysis of the cured PHTE resin. | ||
Samples for mechanical testing of the resin were manufactured following the standards ISO178 and ASTM-D638 (Tables S2 & S3†). The cured PHTE resin showed a flexural strength of 80 MPa and a flexural modulus of 3600 MPa, determined according to ISO178 (Fig. S8 & Table S4†). The standard deviation from the values for the different specimens was calculated to be 15 MPa and 507 MPa, respectively, as shown in the stress–strain curve in Fig. 3a. For the tensile tests, only three out of five tensile samples returned usable results, as two samples were damaged at the grip locations due to the applied clamp forces (Fig. S9†). Out of those three samples, a tensile strength and tensile modulus of 56 MPa and 4000 MPa were obtained, with a total strain of 3.6% determined by ASTM-D638 (Fig. 3b & Table S5†). The standard deviation was calculated to be 5 MPa and 273 MPa, respectively, as shown in the stress–strain curve in Fig. 3b. The PHTE resin shows an increase in both tensile and flexural strength in comparison with the Epikote-Epikure 04908 system (Fig. S10†). These results show that PHTE produces a stiffer system compared to the BADGE reference. Additionally, higher water absorption behaviour was observed for the PHTE resin compared to Epikote.
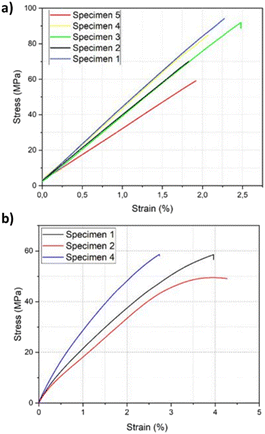 | ||
| Fig. 3 Stress–strain curves of PHTE: (a) flexural testing of the resin and (b) tensile testing of the PHTE resin. | ||
The PHTE–Epikure 04908-reactive diluent resin was found to have an average density of 1.2 g cm−3 at 23 °C following ASTM-D792 (Table S6†). The Epikote-Epikure 04908 resin has a density of 1.12 g cm−3 at room temperature; hence, the density of the PHTE–Epikure 04908-reactive diluent resin is higher compared to the BADGE system, which might be attributed to a higher crosslinking degree. This can be explained by the branched PHTE structure leading to a more tightly-packed polymer system compared to the oligomeric BADGE system. Gel content was found to be 99% following ASTM D2765-16 (Table S7†). The mass of the sample increased by 0.76% after being submerged in water for 24 h at 25 °C (Table S8†). To further determine the hydrophilicity of the resin, the water contact angle of different samples was measured (Fig. S11†). Out of five samples, an average contact angle of 55.2 was measured, indicating slight hydrophilicity of the resin system (Table S9†).
The chemical and mechanical properties of the PHTE-derived resin are summarized in Table 1 in comparison with the Epikote-Epikure 04908 resin system. It can be concluded that the PHTE resin has comparable properties to the BADGE-based resin, and increases in flexural properties and Tg are noted. In the next step, composite formulation is attempted.
| Properties | Unit | Epikote-Epikure resin | PHTE–Epikure resin |
|---|---|---|---|
| a T g determined by the peak of tan δ in DMA measurements. | |||
| Density | g cm−3 | 1.12 | 1.2 |
| Tensile strength | MPa | 55 ± 3 | 57 ± 5 |
| Tensile strain | % | 3.75 | 3.6 |
| Tensile modulus | MPa | 3770 ± 700 | 3950 ± 270 |
| Flexural strength | MPa | 54 ± 2.5 | 80 ± 15 |
| Flexural modulus | MPa | 3560 ± 290 | 3600 ± 500 |
| Water absorption | % | 0.16 | 0.76 |
T
g![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a a |
°C | 60 | 125 |
A composite was manufactured through wet hand layup, using the HEXFORCE 7581 glass fiber satin weave, weighing 303 g m−2, with [0,90,0,90]s layup. A 300 × 160 mm laminate was manufactured to obtain 5 tensile samples of 250 mm length and 25 mm width. The resin was degassed for 20 minutes before saturating the reinforcement using a brush and applying the vacuum bag. The laminate was left to cure at room temperature for 24 h followed by post-curing at 140 °C for 1 h (Fig. S12, details are given in the ESI†). From the laminate, samples for DMA testing, tensile testing, laser microscopy, SEM, swelling tests and resin burn-off tests were obtained. A laminate using Epikote-Epikure 04908 with 10 wt% diluent was manufactured in the same way to compare its properties with the PHTE-based laminate.
The initial fibre volume fraction, matrix volume fraction and void content of the PHTE-based laminate were determined, following ASTM D3171-99 and ASTM D2734-94, to be equal to 42.21%, 51.88% and 5.91% respectively (Table S10†). The high void content is attributed to the reactive nature of PHTE and manufacturing defects. An increase in viscosity after 40 minutes led to impregnation difficulties. The void content was verified via optical microscopy images (Fig. S13†). Microscopy analysis revealed a void content of 4.71% (Table S10†). It is observed that some of the voids are as big as 1000 μm, which could easily lead to the initiation of cracks within the composite. The adhesion of the resin to the fibers was not investigated; however, scanning electron microscopy (SEM) images were taken to visually determine how the resin adhered to the fibers. It was observed that in few cases, voids were present around individual fibers and fiber batches (Fig. S14†). The gel content test showed a percentage decrease of 0.97% after the samples were submerged in toluene for 72 h (Table S7†). The water absorption test of the composite exhibited a 0.98% mass increase after being submerged in water for 24 h at 25 °C, and the average water contact angle of five samples was determined to be 56.14°.
The mechanical properties of the composites were investigated following ASTM-D3039-08. Five samples for tensile testing of the resin were manufactured following the guidelines of the standard (Table S11 and Fig. S15†). In the case of specimen 5 of PHTE, slipping was observed during the test, even though tabs were present on the specimen. This is also seen in the sudden drop of stress present in the stress–strain curve. For that reason, the results of this specimen were not considered for the determination of the tensile strength. The PHTE-based composite showed a tensile strength of 292 ± 57 MPa and a tensile modulus of 26 ± 1.7 GPa, respectively, compared to the tensile strength of 247 ± 29 MPa and the tensile modulus of 21 ± 1.7 GPa of the Epikote-Epikure 04908 manufactured laminate (Table S12†). The stress–strain curves of the two composites shown in Fig. 4 further support these findings. Hence, PHTE-based composites offer improved thermomechanical properties.
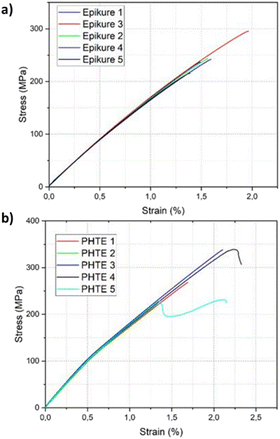 | ||
| Fig. 4 Stress–strain curves obtained from the tensile test of (a) the Epikote-Epikure 04908-based composite and (b) the PHTE–Epikure 04908-based composite. | ||
Conclusions
In this manuscript, replacing petroleum-derived DGEBA with renewable PHTE in a laminating epoxy resin system is examined. PHTE is a high-viscosity oligomer mixture as evidenced by NMR and a 10 wt% reactive diluent was used in both PHTE and DGEBA recipes. PHTE is a highly reactive resin with a low activation energy as evidenced by curing studies, and a low critical mixing mass (30 g) was observed. Due to the oligomeric structure with altered molar masses and reactivities, post-curing at 140 °C was necessary to achieve full conversion for the PHTE-based recipe, whereas DGEBA-based Epikote-Epikure 04908 required post-curing at 80 °C. Thermomechanical analyses elucidated the superior properties of the PHTE resin by means of glass transition (60 vs. 125 °C), flexural and tensile modulus as well as stiffness. A higher degree of water absorption (0.16 vs. 0.76% in 24 hours), however, was monitored. Difficulties arise when composite manufacturing is intended, mainly due to the high reactivity of the PHTE resin system. Glass fibre-reinforced composites using hand layup were generated and once again PHTE shed light on its potential use as a laminating resin system by offering improved composite properties. Overall, by replacing DGEBA with PHTE in the Epikote-Epikure recipe, a 60% biobased carbon-containing high performance resin system can be engineered.Renewable-sourced PHTE is a suitable candidate to replace DGEBA in laminating systems, yet few important considerations must be taken into account. First, the mixed oligomeric structure of commercially available PHTE causes disparities in curing cycles and enthalpy calculations, hence distilling ‘purer’ products would offer higher quality thermosetting resins. Additionally, in this study, a 10 wt% diluent was employed for both DGEBA and PHTE systems, where in reality, Epikote-Epikure 04908 is employed without a diluent. Even in this case, the properties of the PHTE resin with a diluent are comparable to those of Epikote-Epikure 04908 (the properties of commercial Epikote-Epikure 04908 are given in ESI Table S13†). Manufacturing large-area complex composites at room temperature is challenging for the PHTE resin, which requires high amounts of reactive diluents, yet low activation energies limit mixing masses. In the future, PHTE resin can be formulated for high-temperature activating systems (such as DICY and DDS) for high-performance applications. PHTE can be a great fit for fast-curing resin systems. Alternatively, the high reactivity of PHTE can be harnessed for frontal polymerization studies.
Author contributions
D. A. and W. E. D. have contributed equally by performing synthesis, composite manufacturing and analysis. C. A. D. has advised the project from a resin and composite engineering perspective. B. K. is involved in project development, supervision, writing and submission.Conflicts of interest
The authors underline that there are no conflicts to declare.Acknowledgements
The authors greatly acknowledge the Delft University of Technology – Faculty of Aerospace Engineering and Department of Aerospace Structures & Materials for continuous support. Thanks to Dr Roy Awater for assistance with physical analysis training, Dave Ruijtenbeek for mechanical analysis advice, Victor Horbowiec for composite manufacturing advice, and Huub Urselmann for generous help in composite manufacturing.References
- H. Khatoon, S. Iqbal and S. Ahmad, in Thermoset Composites: Preparation, Properties And Applications, ed. A. Khan, S. A. Bhawani, A. M. Asiri and I. Khan, 2018, vol. 38, pp. 189–216 Search PubMed.
- Y. Yang, T. Chen, D. Q. Pan, J. Gao, C. T. Zhu, F. Y. Lin, C. H. Zhou, Q. D. Tai, S. Xiao, Y. B. Yuan, Q. L. Dai, Y. B. Han, H. P. Xie and X. Y. Guo, Nano Energy, 2020, 67, 104246 CrossRef CAS.
- O. Faruk, A. K. Bledzki, H. P. Fink and M. Sain, Prog. Polym. Sci., 2012, 37, 1552–1596 CrossRef CAS.
- C. Esen and B. Kumru, Ind. Eng. Chem. Res., 2022, 61, 10616–10630 CrossRef CAS.
- W. E. Dyer and B. Kumru, Macromol. Chem. Phys., 2023, 2300186 CrossRef CAS.
- D. K. Rajak, D. D. Pagar, P. L. Menezes and E. Linul, Polymers, 2019, 11, 1667 CrossRef CAS PubMed.
- H. Sukanto, W. W. Raharjo, D. Ariawan, J. Triyono and M. Kaavesina, Open Eng., 2021, 11, 797–814 CrossRef CAS.
- J.-P. Pascault and R. J. J. Williams, in Epoxy Polymers, 2010, pp. 1–12 Search PubMed.
- F. Rothenhäusler, M. Kettenbach and H. Ruckdaeschel, Macromol. Mater. Eng., 2023, 308, 2300122 CrossRef.
- F. Rothenhäusler and H. Ruckdaeschel, Polymers, 2022, 14, 4331 CrossRef.
- A. Usman, S. Ikhlas and M. Ahmad, Toxicol. Lett., 2019, 312, 222–227 CrossRef CAS.
- A. Langsdorf, M. Volkmar, D. Holtmann and R. Ulber, Bioresour. Bioprocess., 2021, 8, 19 CrossRef.
- E. A. Baroncini, S. K. Yadav, G. R. Palmese and J. F. Stanzione, J. Appl. Polym. Sci., 2016, 133, 44103 CrossRef.
- A. Kadam, M. Pawar, O. Yemul, V. Thamke and K. Kodam, Polymer, 2015, 72, 82–92 CrossRef CAS.
- F. Jaillet, E. Darroman, A. Ratsimihety, R. Auvergne, B. Boutevin and S. Caillol, Eur. J. Lipid Sci. Technol., 2013, 116, 63–73 CrossRef.
- A. Nicolau, R. M. Mariath, E. A. Martini, D. D. Martini and D. Samios, Mater. Sci. Eng., C, 2010, 30, 951–962 CrossRef CAS.
- Y. Tian, M. Ke, X. Wang, G. Wu, J. Zhang and J. Cheng, Eur. Polym. J., 2021, 147, 110282 CrossRef CAS.
- R. Gerardy, R. Morodo, J. Estager, P. Luis, D. P. Debecker and J. C. M. Monbaliu, Top. Curr. Chem., 2019, 377, 111–145 CrossRef PubMed.
- N. Wang, X. W. Feng, J. K. Pei, Q. K. Cui, Y. J. Li, H. Y. Liu and X. X. Zhang, ACS Sustainable Chem. Eng., 2022, 10, 3604–3613 CrossRef CAS.
- C. H. Chen, S. H. Tung, R. J. Jeng, M. M. Abu-Omar and C. H. Lin, Green Chem., 2019, 21, 4475–4488 RSC.
- J. Y. Dai, Y. Y. Peng, N. Teng, Y. Liu, C. C. Liu, X. B. Shen, S. Mahmud, J. Zhu and X. Q. Liu, ACS Sustainable Chem. Eng., 2018, 6, 7589–7599 CrossRef CAS.
- Y. J. Li, X. T. Fu, D. L. Duan, X. Y. Liu, J. C. Xu and X. Gao, Mar. Drugs, 2017, 15, 49 CrossRef PubMed.
- M. Liu, Y. M. Ding, H. L. Chen, Z. Zhao, H. Z. Liu, M. Xian and G. Zhao, BMC Microbiol., 2017, 17, 10 CrossRef PubMed.
- A. Meyer, I. Saaem, A. Silverman, V. A. Varaljay, R. Mickol, S. Blum, A. V. Tobias, N. D. Schwalm, W. Mojadedi, E. Onderko, C. Bristol, S. T. Liu, K. Pratt, A. Casini, R. Eluere, F. Moser, C. Drake, M. Gupta, N. Kelley-Loughnane, J. P. Lucks, K. L. Akingbade, M. P. Lux, S. Glaven, W. Crookes-Goodson, M. C. Jewett, D. B. Gordon and C. A. Voigt, ACS Synth. Biol., 2019, 8, 2746–2755 CrossRef CAS.
- A. Mija, E. Louisy, S. Lachegur, V. Khodyrieva, P. Martinaux, S. Olivero and V. Michelet, Green Chem., 2021, 23, 9855–9859 RSC.
- R. Dinu, U. Lafont, O. Damiano and A. Mija, Front. Mater., 2023, 10, 1242507 CrossRef.
- I. P. Singh, J. Sidana, P. Bansal and W. J. Foley, Expert Opin. Ther. Pat., 2009, 19, 847–866 CrossRef CAS PubMed.
- Specific Polymers, 2023, PHTE technical data sheet.
- Hexion/Bakelite, 2023, Epikote-Epikure 04908 technical data sheet.
- S. F. Lau, ACS Symp. Ser., 1992, 496, 170–181 CrossRef CAS.
- P. I. Karkanas and I. K. Partridge, J. Appl. Polym. Sci., 2000, 77, 1419–1431 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3lp00174a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |
