Open Access Article
Open Access ArticleNext generation microfluidics: fulfilling the promise of lab-on-a-chip technologies
Umut A.
Gurkan
 *a,
David K.
Wood
*a,
David K.
Wood
 *b,
Dorn
Carranza
c,
Luke H.
Herbertson
*b,
Dorn
Carranza
c,
Luke H.
Herbertson
 c,
Scott L.
Diamond
d,
E.
Du
c,
Scott L.
Diamond
d,
E.
Du
 e,
Suvajyoti
Guha
e,
Suvajyoti
Guha
 c,
Jorge
Di Paola
c,
Jorge
Di Paola
 f,
Patrick C.
Hines
f,
Patrick C.
Hines
 gh,
Ian
Papautsky
gh,
Ian
Papautsky
 i,
Sergey S.
Shevkoplyas
i,
Sergey S.
Shevkoplyas
 j,
Nathan J.
Sniadecki
j,
Nathan J.
Sniadecki
 k,
Vamsee K.
Pamula
k,
Vamsee K.
Pamula
 l,
Prithu
Sundd
m,
Asif
Rizwan
n,
Pankaj
Qasba
n and
Wilbur A.
Lam
l,
Prithu
Sundd
m,
Asif
Rizwan
n,
Pankaj
Qasba
n and
Wilbur A.
Lam
 *o
*o
aCase Western Reserve University, USA. E-mail: umut@case.edu
bUniversity of Minnesota, USA. E-mail: dkwood@umn.edu
cUS Food and Drug Administration, USA
dUniversity of Pennsylvania, USA
eFlorida Atlantic University, USA
fWashington University in St. Louis, USA
gWayne State University School of Medicine, USA
hFunctional Fluidics, Inc., USA
iUniversity of Illinois Chicago, USA
jUniversity of Houston, USA
kUniversity of Washington, USA
lBaebies, Inc., USA
mVERSITI Blood Research Institute and Medical College of Wisconsin, USA
nNational Heart, Lung, and Blood Institute, USA
oEmory University School of Medicine, USA. E-mail: wilbur.lam@emory.edu
First published on 5th March 2024
Abstract
Microfluidic lab-on-a-chip technologies enable the analysis and manipulation of small fluid volumes and particles at small scales and the control of fluid flow and transport processes at the microscale, leading to the development of new methods to address a broad range of scientific and medical challenges. Microfluidic and lab-on-a-chip technologies have made a noteworthy impact in basic, preclinical, and clinical research, especially in hematology and vascular biology due to the inherent ability of microfluidics to mimic physiologic flow conditions in blood vessels and capillaries. With the potential to significantly impact translational research and clinical diagnostics, technical issues and incentive mismatches have stymied microfluidics from fulfilling this promise. We describe how accessibility, usability, and manufacturability of microfluidic technologies should be improved and how a shift in mindset and incentives within the field is also needed to address these issues. In this report, we discuss the state of the microfluidic field regarding current limitations and propose future directions and new approaches for the field to advance microfluidic technologies closer to translation and clinical use. While our report focuses on using blood as the prototypical biofluid sample, the proposed ideas and research directions can be extrapolated to other areas of hematology, oncology, biology, and medicine.
Ever since the emergence of the microfluidics (MF) field almost 40 years ago, MF researchers predicted that these technologies would bring about “lab-on-chip” devices that would revolutionize clinical diagnostics and translational research. By leveraging technologies and processes the computer chip industry developed decades earlier, MF devices can significantly reduce reagent usage, chemical reaction times, and cost on a single “chip,” which, in turn, could enable novel point-of-care (POC) and point-of-need solutions for clinical diagnostics. While there have been several successful technologies that use microscale sample volumes or microfluidic components (e.g., blood gas and chemistry analyzers, blood glucose and hemoglobin A1C tests),1 especially recently during the COVID-19 pandemic in the form of lateral flow assays,2 by and large, the MF field has not yet fulfilled its potential in the research or clinical areas. One of the major limitations of the new MF technologies is the cost barrier to designing, prototyping, and manufacturing these devices at scale for widespread use. Cost structure, value proposition, performance characteristics, and use case scenarios of MF point-of-care technologies should be carefully considered with respect to centralized clinical laboratory tests.1 POC technologies create a complex economic scenario since the unit cost per test may be greater compared to the unit cost per test with high throughput laboratory automation. However, the POC testing approach potentially offers substantial healthcare savings by enabling rapid delivery of results to the patient and reduction in laboratory facility infrastructure and operational costs.3 Therefore, it is important to perform a health economics and cost-effectiveness analysis for a new MF point-of-care technology by considering the potential cost savings and effectiveness. For example, a costly but more effective POC test may be adopted and paid for by the existing diagnostic-related group (DRG) or health-related group (HRG) payment structures within the established healthcare systems.3 Our thesis is that a combination of technical and, perhaps even more importantly, value and incentive challenges have ultimately limited the utility of these technologies thus far. On the one hand, technical issues include a lack of standardization in design and fabrication methods and the materials used for fabrication. Less discussed in the literature but even more important are a lack of communication and collaboration between researchers, technology developers, manufacturers, regulators, users, and payors, and a potential misalignment of incentives among the various stakeholder groups.
How can we use microfluidics to enable the next generation of applications?
Medical applications of MF include manipulating and analyzing small-volume biological samples, such as saliva or blood, to screen, diagnose diseases, monitor health and treatments.1,4 Biotechnology applications include the high-throughput screening of chemical, biological, and genetic materials in new drug and biomolecule discovery.5–9 Environmental applications include testing and analyzing small water, air, and soil samples for toxins and contaminants.10,11 However, for medical and biotechnology applications, non-technical and technical challenges prevent MF technologies from making a much-needed broader impact.Misalignment in incentives
MF technology developers are typically researchers in engineering and scientific disciplines that do not necessarily “speak” the same language as the biomedical community who need the technology. MF technology developers also rarely communicate with end users (i.e., biomedical researchers and clinicians) before designing or developing a new technology.12 This lack of communication from the beginning leads to a lack of understanding of the end user's perspective, which, in turn, leads to a misunderstanding of the problem and the specific unmet needs of the end user. Therefore, most MF technologies are relegated to become proof-of-concept methods in articles published in technology-focused journals, with a strong likelihood of not crossing paths with others outside the field. In other words, in most cases, what the MF field offers is not tailored to answer the specific needs or wants of the biomedical user community.Indeed, most MF developers typically focus on an exciting new design, technology, or solution without a clear and informed understanding of the specific problem and the user's needs. The academic motives of most technology developers are primarily and typically publishing in top journals, which are needed for promotion and tenure. However, these academic success metrics often do not align with clinical translational requirements, typically comprising non-academic activities such as customer interviews, designing for simplicity and ease of use, reproducibility, scalable manufacturability, and understanding the regulatory and reimbursement processes. On the other hand, most biomedical researchers and MF end users see MF technologies as promising tools that can generate new and unique data. As such, biomedical researchers, as ‘customers’ of MF technologies, do not care about how sophisticated or smart the MF design is; they care about whether the solution reliably and reproducibly solves a significant problem that they may have. Therefore, MF platforms should meet these expectations by offering the utmost biological and physiological relevance without compromising simplicity, accessibility, usability, throughput, manufacturability, and robustness.
Technological challenges
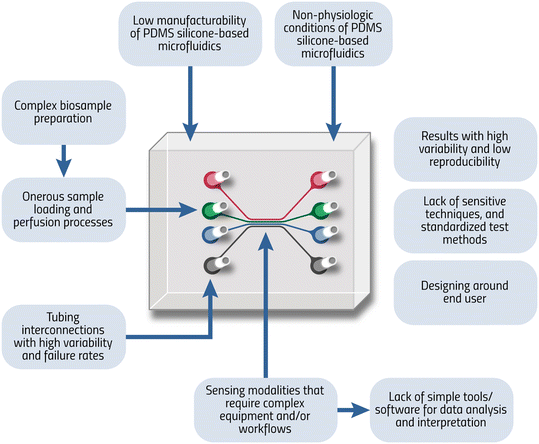
To increase adoption, the field should improve MF devices technologically and should focus on three key areas (Fig. 1): (1) improved recapitulation of in vivo conditions;13–16 (2) standardization of design and fabrication processes;9,17–21 and (3) improving usability and reducing the dependence on technical expertise, peripheral equipment and laboratory infrastructure.18,19MF systems can be designed to better recapitulate in vivo conditions by using physiologically relevant materials, such as biomolecules, extracellular matrix proteins, or hydrogels.22 MF technologies can be designed to incorporate biological cues, such as signaling molecules and cell surface proteins. 3D design and mimicking of physiological flow properties, shear rates, and dynamic control of temperature, pH, and oxygen levels could help better represent physiology.23 Incorporating living cells into MF system design and organ-on-a-chip approaches could also help better mimic in vivo conditions and disease pathophysiology.
In addition, standardization of design, fabrication, and usability can be achieved by developing guidelines, best practices, and recommendations for materials, dimensions, and system integration.17,19 A common MF design software could be developed with standard material types, designs, connectors, and fabrication considerations. Standardized fabrication protocols, including material selection, fabrication methods, leakage tests,24 and quality control processes could be developed. In addition, an open-source, public MF design repository can be created to store and share MF device designs and fabrication methods. Workshops and committees can be formed to adopt guidelines and industry standards for MF devices.18 Different industry standards could be developed for different MF modalities (e.g., continuous flow, discrete flow, digital) and applications (e.g., medical, biotechnology). For example, workshop and committee reports focused on material and design standardization may lead to publications, or production of standards reviewed and published by the American Society for Testing Materials (ASTM) or the International Organization for Standardization (ISO).
Moreover, miniaturization with integrated sensors and actuators can reduce the dependence of MF technologies on complex peripheral equipment and research laboratory infrastructure to achieve ‘on-chip’ fluid handling, measurement, and control.25 Designing microfluidic technologies for POC applications with low-cost fabrication and ease-of-use as primary design criteria could also reduce the need for infrastructure and expertise, such as high-resolution microscopy.26 Microfluidic designs should incorporate strategies to improve robustness, reducing the influences from environmental variations in temperature, humidity, pressure, vibration, and light sources during transportation and use. Automation, integration, and online sensing, monitoring, and control strategies could be incorporated, as needed, to reduce the need for manual interventions. Integrated data capture and analysis methods, including computer vision and artificial intelligence algorithms, could enable data analysis on-device or in the cloud. However, these technological advances should not be utilized at the expense of decreasing usability or overly increasing the complexity of the system, which may prevent user adoption.
A common challenge for continuous flow MF systems is the choice of materials, such as poly (dimethyl siloxane) (PDMS) which has known limitations (Fig. 1).27–30 PDMS has remained a workhorse material for MF research because it is a convenient material for developing new designs, prototyping, training students, helping understand how cells move in this space, and learning the problems and limitations of this technology (e.g., channel expansion, leakages, clotting issues). Thus, PDMS is appropriate for hypothesis-driven research but not ideal for commercial or clinical applications, since it is difficult to scale for manufacturing. In addition, the infrastructure needs for photolithography-based PDMS elastomer-based fabrication can be a barrier as it requires a micro/nano-fabrication facility and equipment. Alternatively, injection molding-based manufacturing of MF systems is scalable and could reduce the cost of MF device fabrication once optimized and when fabrication is done at a high scale. However, injection mold-based manufacturing initial cost can be very high due to master-mold design31 and this approach works best for feature sizes on the order of 100 micrometers or more, while some manufacturers are working towards making even smaller sized features. Recently, micro injection molding has shown promise in the replication of microfeatures. More research on mold materials, tooling technologies, molding, and demolding processes is still needed to improve its repeatability and resolution to a few micrometers. 3D printing is an emerging promising technology for MF system prototyping and preliminary studies, as this technology is more accessible, lower cost, and widely available. 3D printing ink materials and printable polymers can be formulated to better mimic biology.32 3D printing technology is not currently suitable for low-cost, high-volume manufacturing of single-use cartridges or consumables. Due to the high cost associated with setting up the initial manufacturing infrastructure for MF device fabrication (e.g., creating custom injection molds),31 it may be challenging for MF developers and start-up companies to update designs quickly or change manufacturing methods or vendors easily once manufacturing starts. Therefore, before starting large-scale manufacturing, it may be reasonable to use cost-effective prototyping methods (e.g., hot embossing, elastomer casting, and 3D printing) to produce smaller quantities of products for analytical validation, clinical validation, usability, and feasibility studies.26
How can we increase the adoption of microfluidics in biomedical and clinical research laboratories?
MF and lab-on-a-chip technologies have made an especially noteworthy impact in hematology and vascular biology due to MF technologies' inherent ability to mimic physiologic flow conditions in blood vessels and capillaries.20,33–43 For example, MF technology allowed researchers to integrate sensors and measure the physical forces of blood and biochemical behaviors in hemostasis and platelet research.39 However, MF technology has had a more modest impact in other fields, such as cancer.44Building on existing successful examples and showcasing the benefits and results, we can improve accessibility and broaden the adoption of MF technologies in biomedical research by standardization, education and training, funding and grants, access to resources, and interdisciplinary collaborations. Improving usability with standardization of design and fabrication ensures that devices are easily adopted and utilized in biomedical research laboratories. The accessibility of MFs could be improved by human-centered designs that reduce dependence on complex fabrication methods, laboratory infrastructure, and complex workflows with the end user in mind. Dissemination and training programs could raise awareness of the MF capabilities and encourage biomedical researchers to adopt these technologies. Users who would prefer to purchase MF for their research could benefit from a national MF design and fabrication resource center or an MF translational research institute from which biomedical researchers could order research-grade devices that are not yet commercially available. These centers or institutes could host a database of established standard designs and MF developers would be interested in depositing their device designs to increase dissemination and visibility. A centralized translational research center of excellence model with relevant expertise would let academic MF researchers to focus on innovation, novelty, and breaking new grounds, rather than confining their thinking and research strategies by manufacturability, regulatory processes, reimbursement potential, or translation steps. Makerspaces at universities could increase the accessibility of MF system design and prototyping among undergraduate and graduate students.45
Challenges remain in increasing the acceptability of MF as an in vitro model of disease pathophysiology due to both technical and, interestingly, cultural issues. For example, MF technologies can be extremely sensitive to intra-patient and inter-patient variability. As such, while MF researchers may believe that they are providing insight into the heterogeneity of the disease, the biologist users may attribute the heterogeneity solely to the variability of the MF devices themselves. These challenges can be addressed by conducting comprehensive clinical validation studies to demonstrate MF technology's ability to mimic the relevant biological and physiological underpinnings of healthy and diseased states sufficiently and accurately.46,47 Improved communication between technology developers and biomedical researchers is needed to clearly define the advantages and limitations of MF systems in recapitulating disease pathophysiology. Whether it is simply improving the understanding of the translational researcher's needs at the incipience of the project or adopting a mindset and willingness to achieve longer-term goals in close collaboration with the end users themselves, the culture of MF technology developers needs to move beyond just publishing technology-based papers in the short term. In addition, investing in developing more biologically and physiologically relevant MF technologies that can better mimic the complexity of biological systems and disease states is needed, but it should be coupled with open communication with biomedical experts about what constitutes a “good” model and what validation data are required as well as ensuring that the system maintains accessibility, simplicity, and usability for the specific type of researcher in mind.
How can we translate MF technologies from the bench to the clinic?
Several key barriers to translating MF technologies from the laboratory to the clinic remain. Technical challenges include, as discussed above, limitations around usability, a lack of standardization, and difficulty scaling up from the laboratory to a commercial or clinical setting. Regulatory and reimbursement challenges also exist.15,48 MF technologies may be subjected to different regulatory processes. MF technologies are not currently represented in clinical diagnostic tests covered by existing billing codes, so new MF diagnostics may require unique billing codes (Current Procedural Terminology, CPT or Proprietary Laboratory Analyses, PLA) that may not be covered by insurance or reimbursement codes limiting their accessibility for clinical use. Even when there is a perfect match between a clinical problem for which MF is the only solution, the reimbursement may be based on other existing, lower cost technologies that may not be economically viable for the MF product. For example, a metabolic panel test in a preemie can be performed using a fraction of blood volume on an MF device and may cost 2× more than standard tests; however, since there is no commensurate higher reimbursement for a MF device, though it is clinically advantageous, it may not be economically feasible in this scenario. Overall, regulatory and reimbursement barriers limit the accessibility of MF for clinical use. There is limited understanding and acceptance of MF technologies among healthcare providers and patients. Another challenge is limited access to necessary resources, specialized equipment, or trained personnel in clinical settings. MF technologies can generate large amounts of measurements and data, and management, storage, and data analysis can be a barrier to translation. There is a clear gap in bridging MF research from the bench to the clinic. Multiple partners from the academy, clinic, corporate, regulatory, and federal agencies should be engaged at a larger scale to move MF into the clinical space and to lower barriers. MF researchers should consider collaborating with multidisciplinary teams (clinicians, engineers, human factors and usability experts, manufacturers, and software developers). MF centers of excellence could train biomedical engineers, guide clinically relevant MF research, and inform applications at the POC with the clinical end user's needs in mind. Organizing joint workshops and interlaboratory studies to address these issues may also be beneficial.MF device submissions to the United States Food and Drug Administration (FDA) have substantially increased in recent years19 and are projected to increase even more in the coming years. The reliability, failure modes,24 and performance monitoring of MF devices are areas of concern for the FDA. Common testing methods, interconnections, and inter-compatibility standards are needed, such as modularity, assembly, and flow control in MF devices.19 The Office of Science and Engineering Laboratories (OSEL) at the FDA aims to accelerate patient access to innovative, safe, and effective medical devices through regulatory science. OSEL develops innovative regulatory science tools, science-based approaches, or methodologies to help assess the safety or effectiveness of a medical device or emerging technology.49 Regulatory Science Tools provide a peer-reviewed resource for medical device companies to use where standards and qualified Medical Device Development Tools (MDDTs) do not yet exist. These tools are published in the FDA's Catalog of Regulatory Science Tools to Help Assess New Medical Devices.50 The current MF regulatory research program at OSEL includes forecasting and technology trends in MF and developing preclinical testing protocols for device evaluation, materials and fabrication, and failure modes.24 Another area is using MF to evaluate therapeutic products and better understand the in vivo environment. MF-based organ-on-chip technologies can also recapitulate aspects such as efficacy and adverse effects in vitro. To expedite translation, innovation, and commercialization, it is important to talk to experts at the right time and ask the right questions at different stages of research. As such, MF technology developers should be knowledgeable about the regulatory processes from the early stages of development. While academic researchers are incentivized to publish, these technologies will not be adopted if robustness, repeatability, and reproducibility are not demonstrated. Therefore, learning about the regulatory requirements early in the process can shape their research in ways that can lead to downstream translation and user adoption. This could be achieved by inviting regulatory experts to technical conferences or by organizing workshops on regulatory science for MF researchers. As discussed earlier, translational MF research and development could be performed at centers or institutes of excellence with appropriate technical, manufacturing, regulatory, and reimbursement expertise.
We need to accelerate translation by reducing the cost and shortening the time to market to achieve equitable widespread access. In the journey from basic science to translation, forming partnerships with manufacturers, businesses, the government, users, clinicians, and scientists is important. The process starts in the academic laboratory. For example, it takes an average of $34 million in funds and 6 years to commercialize a POC diagnostic device in the US.1 Though, most venture capitalist investors hope to see market potential or returns in a much shorter timeframe than 6 years. To enable execution, we should strive to minimize the need for funds raised, minimize time to market, and work in a need-centric, problem-oriented, informed, disciplined, bottom-up fashion. The following steps can help shorten the translational timeline: (1) explore the market and understand reimbursement and customer needs before prototyping; (2) study the regulatory aspects, engage regulatory experts early, and consult the World Health Organization list of diagnostics for global health applications; (3) design for low-cost and large-volume manufacturing with the user in mind; and (4) start clinical validation studies early, minimize preclinical study duration, and maximize clinical trial duration.
Adapting MF technologies for resource-limited settings is highly challenging and an active area of research. While many healthcare decisions are driven by diagnostic testing, there is a push to reduce overall healthcare costs around the world and therefore developing MF technologies that are economical for low-resource settings is bound to be economically beneficial for everyone. For example, complex equipment and labor-intensive microscopy-based sensing can potentially be eliminated by coupling electronics sensors with MF devices for in vitro blood testing.51–53 Coupling the electrical field with fluidics could enable POC testing and may improve the throughput and specificity of the impedance flow cytometry.54 Researchers can also design low-cost MF devices with minimal or no supporting equipment and accessories in resource-limited settings.26 Opportunities exist for MF applications in rapid diagnostic tools that can be used on-site, such as drawing blood from a finger stick. The community should strive to design user-friendly and field-ready devices and form partnerships with industry and the government. Most researchers are focused on innovation and development but not on scale-up and performance or large-scale collaborations, and more research is needed in this area. For example, the ability to predict sickle cell pain crises is an unmet need and opportunity for the MF field. Smart, low-cost MF applications have been developed with successful proof-of-concept prototypes (e.g., smartphone microscopy, microflow cytometry, flow assay, mobile ELISA).
There is a need to support the use case of a new MF technology in a clinical environment, show the economic benefit of adoption, and have a reimbursement strategy that ensures payer engagement. Clinical adoption remains challenging, and most providers prefer to continue using a test they are familiar with. There is significant potential and opportunities for MF-based diagnostics in the Clinical Lab Improvement Amendment (CLIA) and Lab-Developed-Test (LDT) environment and for MF-based diagnostics in clinical trials.46,47 Adopting a strategy to make the test available as a clinical send-out through the CLIA/LDT environment is possible. More research support is needed to accelerate MF translation to the clinic to create MF technology-based standards for clinical studies, a uniform calibration across platforms, standardized shipping conditions for biological samples, blood and biological sample acquisition methods for micro-volume samples, and better reimbursement for novel functional blood testing (MF-based technology).55
Next-next generation MF systems should improve the capabilities of current analytical systems, integrate MF into existing devices to enhance capabilities, and create interoperability standards between different formats of MFs to allow continuous, discrete, and digital multifunctional capabilities. The field should prioritize pre-analytical and post-analytical systems to fully exploit MF advances. For example, low-sample volume collection devices (<100 μL) are not readily available, which is an issue for pediatric and neonatal patients. Because some MF devices have mobile POC capabilities, they can collect and aggregate information to obtain actionable data for emerging epidemics. An ideal next generation MF diagnostic device would be instrument-free, room temperature-stable, inexpensive to allow equitable global access, and not a single purpose, but a platform that can perform multiple assays required to diagnose and manage a certain disease.
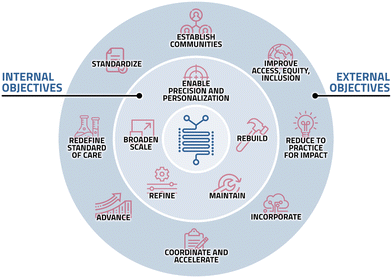
In summary, the following areas of opportunity were identified to help rebuild, refine, broaden, and translate the MF field: first, the MF field should look inward to strengthen the scientific fundamentals and advance the frontiers of the field (Fig. 2), which requires several steps:
• Develop: more sensitive test methods that can detect common failure modes associated with MF technology; guidelines with tips and tricks to aid innovators and accelerate innovation.
• Rebuild: standardize the design and fabrication to emphasize scale-up, improve usability, and reduce dependence on peripheral equipment and research laboratory infrastructure with the end user in mind.
• Innovate: with scale-up and end user in mind.
• Refine: reimagine materials, designs, and dynamic control systems to better recapitulate biology, physiology, and in vivo conditions without sacrificing usability.
• Broaden scale: improve the capability to scale up (>liters) and, conversely, the ability to scale down (<μL) sample volumes.
• Enable precision and personalization: develop and validate MF biomarker assays and multiplex, high-throughput, high-data content biomarkers for precision and personalized medicine.
• Maintain: a pipeline of MF investigators and translational researchers via research, education, and training.
Second, the MF field should look outward to other stakeholders (e.g., funding agencies, regulatory groups, clinical and scientific consortia, private sector) to accelerate the translation and transform medicine, which requires collective action, specifically:
• Establish: communities of all stakeholders, multidisciplinary teams, and end-user needs and education/collaboration to improve adoption.
• Coordinate and accelerate: regulatory tools to streamline device clearance.
• Standardize: establish large cooperative research groups and work with partners to establish and disseminate much-needed industry standards.
• Reduce to practice for impact: translate published yet still immature MF phenomena for use-inspired biomedical solutions for robust integration into existing clinical assays.
• Advance: updating, re-engineering/re-purposing, or replacing current, antiquated diagnostics in various use cases with novel MF solutions.
• Incorporate into cutting-edge technologies: incorporation of MF into precision medicine, omics platforms, AI/ML/Big Data systems, and smartphone-based/digital health platforms.
• Improve access, equity, and inclusion: leverage MF to ensure equitable access to diagnostics in the US and globally and meet unmet clinical needs.
• Redefine the standard of care: incorporate MF into clinical trials as clinical endpoints (diagnostics, biomarkers).
Disclaimer
The views expressed in this article are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute, the National Institutes of Health, the U.S. Food and Drug Administration, or the U.S. Department of Health and Human Services.Author contributions
U. A. G., D. K. W., and W. L. drafted the manuscript. D. C., L. H., S. L. D., E. D., S. G., J. D., P. H., I. P., S. S. S., N. J. S., V. K. P., P. S., A. R., P. Q. reviewed, edited, revised, and/or provided feedback and suggestions on the manuscript draft. All authors have participated in discussions, reviewed, and approved the final version of the manuscript.Conflicts of interest
U. A. G. and Case Western Reserve University have financial interests in Hemex Health Inc., BioChip Labs Inc., and Xatek Inc. U. A. G. has financial interests in DxNow Inc. Financial interests include licensed intellectual property, stock ownership, research funding, employment, and consulting. The competing interests of Case Western Reserve University employees are overseen and managed by the Conflict of Interests Committee according to a Conflict-of-Interest Management Plan. S. L. D. and the University of Pennsylvania have a financial interest in FloBio LLC. V. K. P. is the founder and has stock ownership in Baebies. N. J. S. has a financial interest in Stasys Medical Corporation and Curi Bio. P. C. H. and Wayne State University have financial interests in Functional Fluidics, Inc., Financial interests include licensed intellectual property, stock ownership, research funding, and employment. The competing interests of Wayne State University employees are overseen and managed by the Conflict of Interests Committee according to a Conflict-of-Interest Management Plan.Acknowledgements
To help develop the next generation microfluidics research, National Heart, Lung, and Blood Institute (NHLBI) sought input from the MF research community and convened a two-day virtual workshop entitled, “Next Generation Microfluidics” on November 4-5, 2021. This workshop aimed to bring together MF experts in the basic sciences, in preclinical research, in clinical and translational research- and regulatory sciences to chart a roadmap for the future of MF field and lab-on-a-chip technology research. The panel was asked to evaluate the field's current, near, and future directions and the hurdles faced to enable the next generation of MF applications in biology and medicine. The authors acknowledge and thank the National Heart, Lung, and Blood Institute for organizing the Next Generation Microfluidics Workshop, where these ideas and concepts were presented and discussed. The authors thank the Case Western Reserve University Strategic Partnerships and Research Collaborative (SPARC) for their critical review (Dr. Juliann Reineke) and for the graphical design of the illustrations (Emily Hromi). Authors from the FDA would like to acknowledge Dr. Rucha Natu for the valuable feedback she provided for the FDA presentation.References
- C. D. Chin, V. Linder and S. K. Sia, Lab Chip, 2012, 12, 2118–2134 RSC.
- E. A. Tarim, M. Anil Inevi, I. Ozkan, S. Kecili, E. Bilgi, M. S. Baslar, E. Ozcivici, C. Oksel Karakus and H. C. Tekin, Biomed. Microdevices, 2023, 25, 10 CrossRef CAS PubMed.
- A. St John and C. P. Price, Clin. Biochem. Rev., 2013, 34, 61–74 Search PubMed.
- P. Pattanayak, S. K. Singh, M. Gulati, S. Vishwas, B. Kapoor, D. K. Chellappan, K. Anand, G. Gupta, N. K. Jha, P. K. Gupta, P. Prasher, K. Dua, H. Dureja, D. Kumar and V. Kumar, Microfluid. Nanofluid., 2021, 25, 99 CrossRef PubMed.
- S. M. Bjork and H. N. Joensson, Curr. Opin. Biotechnol., 2019, 55, 95–102 CrossRef CAS PubMed.
- Y. Liu and X. Jiang, Lab Chip, 2017, 17, 3960–3978 RSC.
- J. Bahnemann and A. Grunberger, Adv. Biochem. Eng./Biotechnol., 2022, 179, 1–16 CrossRef CAS PubMed.
- K. Illath, S. Kar, P. Gupta, A. Shinde, S. Wankhar, F. G. Tseng, K. T. Lim, M. Nagai and T. S. Santra, Biomaterials, 2022, 280, 121247 CrossRef CAS PubMed.
- P. S. Dittrich and A. Manz, Nat. Rev. Drug Discovery, 2006, 5, 210–218 CrossRef CAS PubMed.
- J. C. Jokerst, J. M. Emory and C. S. Henry, Analyst, 2012, 137, 24–34 RSC.
- X. Zhu, K. Wang, H. Yan, C. Liu, X. Zhu and B. Chen, Environ. Sci. Technol., 2022, 56, 711–731 CrossRef CAS PubMed.
- N. Convery and N. Gadegaard, Micro Nano Eng., 2019, 2, 76–91 CrossRef.
- S. N. Bhatia and D. E. Ingber, Nat. Biotechnol., 2014, 32, 760–772 CrossRef CAS PubMed.
- W. Sun, Z. Luo, J. Lee, H. J. Kim, K. Lee, P. Tebon, Y. Feng, M. R. Dokmeci, S. Sengupta and A. Khademhosseini, Adv. Healthcare Mater., 2019, 8, e1801363 CrossRef PubMed.
- H. Wang, P. C. Brown, E. C. Y. Chow, L. Ewart, S. S. Ferguson, S. Fitzpatrick, B. S. Freedman, G. L. Guo, W. Hedrich, S. Heyward, J. Hickman, N. Isoherranen, A. P. Li, Q. Liu, S. M. Mumenthaler, J. Polli, W. R. Proctor, A. Ribeiro, J. Y. Wang, R. L. Wange and S. M. Huang, Clin. Transl. Sci., 2021, 14, 1659–1680 CrossRef PubMed.
- S. Browne, E. L. Gill, P. Schultheiss, I. Goswami and K. E. Healy, Stem Cell Rep., 2021, 16, 2058–2075 CrossRef CAS PubMed.
- D. R. Reyes and H. van Heeren, J. Res. Natl. Inst. Stand. Technol., 2019, 124, 1–22 CrossRef PubMed.
- C. M. Klapperich, Expert Rev. Med. Devices, 2009, 6, 211–213 CrossRef PubMed.
- D. R. Reyes, H. van Heeren, S. Guha, L. Herbertson, A. P. Tzannis, J. Ducree, H. Bissig and H. Becker, Lab Chip, 2021, 21, 9–21 RSC.
- M. Azul, E. F. Vital, W. A. Lam, D. K. Wood and J. D. Beckman, Transl. Res., 2022, 246, 1–14 CrossRef CAS PubMed.
- J. Zhou and I. Papautsky, Microsyst. Nanoeng., 2020, 6, 113 CrossRef CAS PubMed.
- Y. Alapan, K. Icoz and U. A. Gurkan, Biotechnol. Adv., 2015, 33, 1727–1743 CrossRef CAS PubMed.
- J. White, M. Lancelot, S. Sarnaik and P. Hines, Clin. Hemorheol. Microcirc., 2015, 60, 201–213 CAS.
- V. Silverio, S. Guha, A. Keiser, R. Natu, D. R. Reyes, H. van Heeren, N. Verplanck and L. H. Herbertson, Front. Bioeng. Biotechnol., 2022, 10, 01–17 Search PubMed.
- S. Tasoglu, U. A. Gurkan, S. Wang and U. Demirci, Chem. Soc. Rev., 2013, 42, 5788–5808 RSC.
- K. Qua, S. M. Swiatkowski, U. A. Gurkan and C. M. Pelfrey, J. Clin. Transl. Sci., 2021, 5, e207 CrossRef PubMed.
- K. J. Regehr, M. Domenech, J. T. Koepsel, K. C. Carver, S. J. Ellison-Zelski, W. L. Murphy, L. A. Schuler, E. T. Alarid and D. J. Beebe, Lab Chip, 2009, 9, 2132–2139 RSC.
- E. Berthier, E. W. Young and D. Beebe, Lab Chip, 2012, 12, 1224–1237 RSC.
- N. Bhattacharjee, A. Urrios, S. Kang and A. Folch, Lab Chip, 2016, 16, 1720–1742 RSC.
- S. B. Campbell, Q. Wu, J. Yazbeck, C. Liu, S. Okhovatian and M. Radisic, ACS Biomater. Sci. Eng., 2021, 7, 2880–2899 CrossRef CAS PubMed.
- H. Becker, Lab Chip, 2009, 9, 2759–2762 RSC.
- A. V. Nielsen, M. J. Beauchamp, G. P. Nordin and A. T. Woolley, Annu. Rev. Anal. Chem., 2020, 13, 45–65 CrossRef PubMed.
- G. V. Grigorev, A. V. Lebedev, X. Wang, X. Qian, G. V. Maksimov and L. Lin, Biosensors, 2023, 13(1), 117 CrossRef CAS PubMed.
- G. Simitian, M. Virumbrales-Muñoz, C. Sánchez-de-Diego, D. J. Beebe and D. Kosoff, Lab Chip, 2022, 22, 3618–3636 RSC.
- M. Labib and S. O. Kelley, Mol. Oncol., 2021, 15, 1622–1646 CrossRef CAS PubMed.
- A. Aich, Y. Lamarre, D. P. Sacomani, S. Kashima, D. T. Covas and L. G. de la Torre, Front. Mol. Biosci., 2020, 7, 558982 CrossRef CAS PubMed.
- S. M. Hastings, M. T. Griffin and D. N. Ku, Platelets, 2017, 28, 427–433 CrossRef CAS PubMed.
- K. Haase and R. D. Kamm, Regener. Med., 2017, 12, 285–302 CrossRef CAS PubMed.
- T. V. Colace, G. W. Tormoen, O. J. McCarty and S. L. Diamond, Annu. Rev. Biomed. Eng., 2013, 15, 283–303 CrossRef CAS PubMed.
- M. Toner and D. Irimia, Annu. Rev. Biomed. Eng., 2005, 7, 77–103 CrossRef CAS PubMed.
- R. An and U. A. Gurkan, Curr. Opin. Hematol., 2022, 29, 327–334 CrossRef CAS PubMed.
- S. L. Diamond and J. M. Rossi, Lab Chip, 2021, 21, 3667–3674 RSC.
- M. A. Jimenez, E. Tutuncuoglu, S. Barge, E. M. Novelli and P. Sundd, Haematologica, 2015, 100, e390–e393 CrossRef CAS PubMed.
- Z. Zhang and S. Nagrath, Biomed. Microdevices, 2013, 15, 595–609 CrossRef CAS PubMed.
- D. I. Walsh III, D. S. Kong, S. K. Murthy and P. A. Carr, Trends Biotechnol., 2017, 35, 383–392 CrossRef PubMed.
- D. D. Pittman, P. C. Hines, D. Beidler, D. Rybin, A. L. Frelinger, A. D. Michelson, K. Liu, X. Gao, J. White, A. U. Zaidi, R. J. Charnigo and M. U. Callaghan, Blood, 2021, 137, 2010–2020 CrossRef CAS PubMed.
- P. C. Hines, M. U. Callaghan, A. U. Zaidi, X. Gao, K. Liu, J. White and M. Tarasev, Br. J. Haematol., 2021, 194, 1074–1082 CrossRef CAS PubMed.
- E. M. Cox, A. V. Edmund, E. Kratz, S. H. Lockwood and A. Shankar, Clin. Transl. Sci., 2020, 13, 451–461 CrossRef PubMed.
- FDA, Office of Science and Engineering Laboratories, https://www.fda.gov/about-fda/cdrh-offices/office-science-and-engineering-laboratories, 2024 Search PubMed.
- FDA, Catalog of Regulatory Science Tools to Help Assess New Medical Devices, https://cdrh-rst.fda.gov/, 2024 Search PubMed.
- D. Maji, M. De La Fuente, E. Kucukal, U. D. S. Sekhon, A. H. Schmaier, A. Sen Gupta, U. A. Gurkan, M. T. Nieman, E. X. Stavrou, P. Mohseni and M. A. Suster, J. Thromb. Haemostasis, 2018, 16, 2050–2056 CrossRef CAS PubMed.
- Y. Man, D. Maji, R. An, S. P. Ahuja, J. A. Little, M. A. Suster, P. Mohseni and U. A. Gurkan, Lab Chip, 2021, 21, 1036–1048 RSC.
- E. Du, S. Ha, M. Diez-Silva, M. Dao, S. Suresh and A. P. Chandrakasan, Lab Chip, 2013, 13, 3903–3909 RSC.
- D. Dieujuste, Y. Qiang and E. Du, Biotechnol. Bioeng., 2021, 118, 4041–4051 CrossRef CAS PubMed.
- FDA, FDA Proposes Rule Aimed at Helping to Ensure Safety and Effectiveness of Laboratory Developed Tests, https://www.fda.gov/news-events/press-announcements/fda-proposes-rule-aimed-helping-ensure-safety-and-effectiveness-laboratory-developed-tests Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |