Bioactive compounds and benefits of by-products of Amazon babassu oil production: potential for dietary supplement, biomedical and food applications
Rayssa Cruz
Lima
 abcde,
Anna Paula Azevedo de
Carvalho
abcde,
Anna Paula Azevedo de
Carvalho
 *abcde,
Antonio Eugenio Castro Cardoso de
Almeida
a and
Carlos Adam
Conte-Junior
*abcde,
Antonio Eugenio Castro Cardoso de
Almeida
a and
Carlos Adam
Conte-Junior
 abcde
abcde
aGraduate Program in Sanitary Surveillance (PPGVS), National Institute of Health Quality Control, Oswaldo Cruz Foundation (FIOCRUZ), Rio de Janeiro, RJ 21040900, Brazil. E-mail: annacarvalho@iq.ufrj.br
bDepartment of Biochemistry, Chemistry Institute, Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941909, Brazil
cResearch Support Group on Nanomaterials, Polymers, and Interaction with Biosystems (BioNano), Chemistry Institute, Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941909, Brazil
dCenter for Food Analysis (NAL), Technological Development Support Laboratory (LADETEC), Federal University of Rio de Janeiro, Rio de Janeiro, RJ 21941598, Brazil
eAnalytical and Molecular Laboratorial Center (CLAn), Chemistry Institute, Federal University of Rio de Janeiro, Cidade Universitária, Rio de Janeiro, RJ 21941909, Brazil
First published on 15th May 2024
Abstract
Babassu coconut (Attalea speciosa syn. Orbignya phalerata) contains an oil-rich nut and is primarily found in South America's Amazon region. Future market researchers predict an increase in the babassu oil market from USD 227.7 million in 2022 to USD 347.0 million by 2032, and the yield of babassu oil from babassu-processed waste could reach 90%. Of these, mesocarp flour is an underrated by-product used only for animal feed purposes by local producers. This comprehensive review focuses on advances in knowledge and understanding of phytochemicals from babassu oil by-products considering the mechanisms of action – covering antioxidant, antimicrobial, antiparasitic, anti-inflammatory, antithrombotic, immunomodulatory, and anticancer effects. Babassu coconut fruit contains free fatty acids, (poly)phenols, phytosterols, and triterpenes. Pytochemicals, antiparasitic and antibacterial activities of babassu mesocarp flour were shown, but fungi and viruses can get more attention. Beyond its antioxidant capacity, babassu mesocarp flour showed potential as a dietary food supplement. Aqueous suspensions of mesocarp flour with a higher preference for cancer cells than normal cells and an antithrombotic effect were also identified, probably related to the antioxidant capacity of its secondary metabolites. Mesocarp flour, a starch-rich fraction, is promising for application as biodegradable packaging to improve the oxidative stability of foods. Finally, low-added value fractions can be considered bio-waste/co-products, and their phytochemicals may attract interest for applications in medicine and nutrition. Toxicological concerns, trends, and gaps are discussed for the future of foods and related sciences.
Introduction
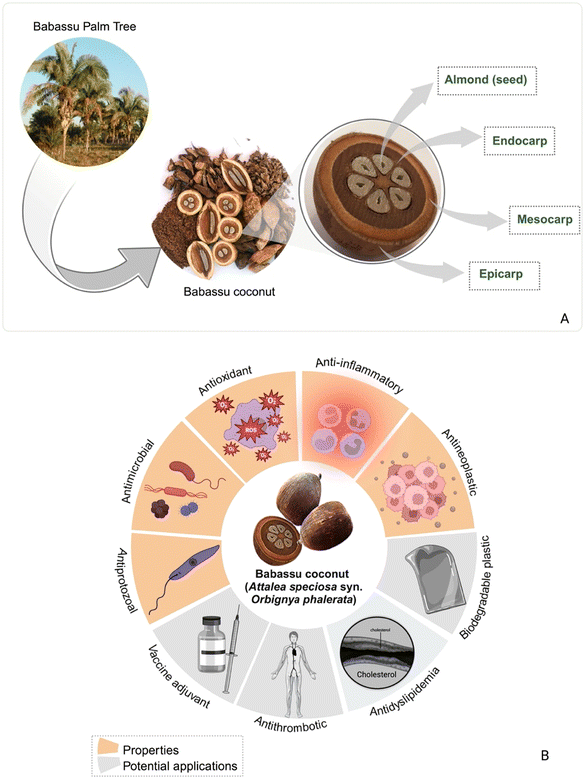
The babassu palm tree belongs to the Arecaceae, Attalea genus,1,2 and is mainly found in South America's Amazon region. Martius first described the babassu palm in 1826 as Attalea speciosa and Orbignya phalerata in Bolivia in 1844.3 Later, babaçu palms in Brazil were examined by May and colleagues in 1985, described as Orbignya spp.4 and more profound knowledge was provided by Anderson and colleagues in 1991.5 These are the prominent scientific names found in the literature for the babassu palm. A more recent publication analyzed nomenclature and strongly recommended that Attalea speciosa Mart ex. Spreng be adopted.6 However, as Attalea speciosa is the accepted name in plant nomenclature indexes, and Orbignya phalerata was widely adopted by the scientific community, and currently used, in this study, we adopted these two prominent names in the binomial nomenclature system synonymizing A. speciosa under O. phalerata.Babassu (or “babaçu”) coconut contains an oil-rich nut yielding babassu oil,7 a light-yellow vegetable oil that is extracted from babassu palm seeds. Babassu oil is extracted from seed kernels and is often used in food, cosmetics, and skin products.8–10 Future market insight researchers predicted an increase in the babassu oil market from USD 227.7 million in 2022 to USD 347.0 million by 2032.11 The babassu fruit has four parts, from the outermost to the inner (Fig. 1A): epicarp, mesocarp, endocarp, and almond (seed);12 among these, the seed nut is the high-added-value fraction, which is used to manufacture the oil, but represents just 7% of the total weight of the fruit.13 The epicarp, mesocarp, and endocarp represent 11%, 23%, and 59%, respectively.2 All seedless parts are a by-product of oil production or agri-food waste. Thus, in the processing of babassu coconut to yield oil from the seed kernel, the residue volume can make up 90% of the processed babassu fruit and 35% of the residual seed material (bagasse, peel).
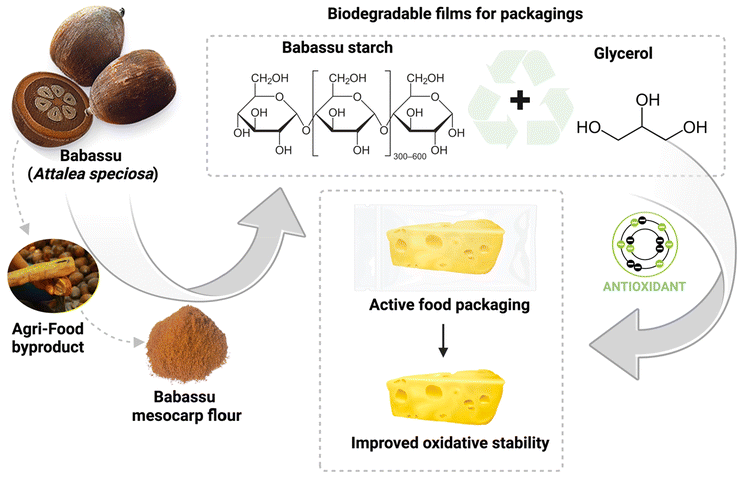
Beyond its economic value, some previously published studies have shown that it is a product with several uses for local producers, including food, construction, cosmetic, ritual, and domestic,14 once babassu oil and nut residues have been used for feed, skincare, and fuel purposes. A synopsis previously published based on the local population's knowledge reported the medicinal effects of babassu consumption (as it ameliorates the effects of abdominal pain, constipation, rheumatism, inflammation of the uterus and ovaries, arthritis, and menstrual pains and it is gastroprotective).15 Since then, researchers’ attention has increasingly focused on new products and technologies based on babassu derivatives for food science and technology, biomedical and pharmaceutical applications, aiming at delivery systems for nutraceutical foods and novel therapy approaches.16–18 In the last two decades, there have been reports in the literature on the significant health benefits and biological properties of babassu derivatives and nut residues for relevant applications in food additives and packaging and in the medical and pharmaceutical19 fields: antiinflammation,17,20,21 immunomodulation,18,21–24 antioxidant,13,25–29 antimicrobial,2,16,21,25,30–32 anticancer,23,33,34 antiprotozoal,22 and antithrombotic properties,35 and potential food supplements against dyslipidemia have all been described.36 Furthermore, babassu mesocarp showed potential to treat leishmaniasis24 and other infectious diseases caused by intracellular pathogens.18,24 Nut residues were proposed for use in materials, food science and technology, fuels, medicine, animal feed, cosmetics, etc. (Fig. 1B).37 For example, babassu mesocarp is a starch-rich fraction that stands out as a bioactive and biodegradable material for active packaging applications to extend the shelf life of foods.13,25–29 The mesocarp could be a nutritious alternative to wheat flour in baking,38 and an alternative adjuvant for developing novel vaccines and platforms against infectious diseases.18,24 In materials engineering, babassu oil by-products (endocarp, mesocarp, epicarp, and activated carbon) were predicted to be promising adsorptive materials with potential as adsorptive species for inorganic and organic molecules.39 The epicarp and endocarp have the potential for applications in handicrafts and as organic manure.40 Babassu nut shells were also highly feasible for producing bioenergy and charcoal.41
In the last few years, our research group has been dedicated to understanding the health benefits of nutrients and bioactive compounds found in native species of Brazilian flora, with a particular interest in edible parts, agro-industrial by-products, and food waste.42 We have discussed several opportunities, from plant extracts to dietary supplements and novel technologies in the clinical and preclinical investigation stage with high potential for human nutrition and in the prevention/treatment of chronic or infectious diseases.43 Therefore, we recently showed a green protocol to obtain babassu mesocarp flour extract rich in phytochemicals (i.e., phenolic and flavonoids) with significant antioxidant/antimicrobial effects and high feasibility to extend shelf lives and inhibit the oxidation of foods.44 Additional investigations were recently published to show the capacity of babassu mesocarp flour as a food additive, specifically as an innovative alternative to reduce sodium in cheese, once it showed an antimicrobial effect and extended the shelf life of a salt-reduced fresh cheese.45 In addition, we combined nanobiotechnology concepts with babassu oil to produce photoprotective nanoemulsions containing babassu (O. phalerata Mart.) lipophilic extract as a promising formulation for developing photoprotective products in sunscreen.9
Despite the high potential of babassu seed oil (high value-added fraction) as an anti-inflammatory and antioxidant agent for pharmaceutical applications previously discussed, to the best of our knowledge, there has been little focus on its bioactive compounds and their effects on human health and diseases, considering the main mechanisms of action. Moreover, the literature has not focused on its co-products and waste (low value-added fractions) to use nanobiotechnology approaches to improve the therapeutic efficacy of its vegetable oil. This review often associates the mechanism with bioactive compounds such as phytochemicals or their primary metabolites such as essential fatty acids screened in the epicarp, endocarp, mesocarp, and nut oil. Specifically, for each technology, potential applications in medicine and food technology, the babassu derivative and its high-added value compounds, underlying mechanisms, and novel opportunities are further discussed in detail.
Literature search methods
This review article contains results of searches for all biological activities already found for different fractions of babassu coconut (epicarp, mesocarp, endocarp, and nut) evaluated as drug delivery systems and dietary supplements. We searched for articles in the Science Direct, Scopus, PubMed, Web of Science, and Embase databases with keywords and synonyms for babassu (babassu OR babaçu OR “Attalea speciosa” OR “Orbignya phalerata”) AND biological/health benefits (antioxidant OR antimicrobial OR anti-inflammatory). However, we also accepted and included in our review research papers evaluating other biological activities/health benefits associated with different fractions of babassu coconut, considered co-products or waste after babassu oil extraction.Primary and secondary metabolites in babassu (A. speciosa syn. O. phalerata) coconut
Several authors studied the babassu palm tree's leaves and different fruit parts (i.e., mesocarp, epicarp, nut/seeds). They presented antioxidant, antimicrobial (antibacterial, antifungal, and antiseptic), immunomodulatory, anti-inflammatory, antinociceptive, and wound healing capacity in the preclinical stage of an investigation. The main phytochemicals are often chemically characterized and discussed in the papers overviewed, considering oil seeds and mesocarp flour extracts as sources (Fig. 2). Oil seed covers significant quantities of polyunsaturated fatty acids (PUFA)46,47 but also minor amounts of (poly)phenols, tri-, diglycerides, terpenes, and phytosterols.48Table 1 shows some babassu derivatives rich in phytochemicals reviewed in this work based on the preparation method and there is an overview of the primary class of phytochemicals screened in studies. This table is quite revealing in several ways. First, babassu derivative products studied are rich in PUFA (omega 3, 6, and 9) that are of nutritional value to the human body and growth. An exciting mixture of ω3 and ω6 was identified in palm tree leaf extracts (ω3/ω6 ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5)32 recommended by regulatory agencies as being crucial for health promotion and to reduce the risk of cardiovascular and other chronic diseases.49,50 Moreover, researchers identified apigenin as the main flavonoid in the dichloromethane fraction (DF) of babassu leaf ethanolic extract by nuclear magnetic resonance (NMR) and this was confirmed by other chromatographic analysis. This result is interesting because once the authors investigated the babassu leaf DF and apigenin using nociception models (acetic acid-induced abdominal writhing, formalin, and hot plate) they observed strong evidence of the antinociceptive activity of O. speciosa Mart. (babassu) leaves. Moreover, the authors also studied the possible mechanisms involved. They concluded that the in vivo antinociceptive effect of babassu leaves due to apigenin might be associated, in part, with opioid and cholinergic systems.
5)32 recommended by regulatory agencies as being crucial for health promotion and to reduce the risk of cardiovascular and other chronic diseases.49,50 Moreover, researchers identified apigenin as the main flavonoid in the dichloromethane fraction (DF) of babassu leaf ethanolic extract by nuclear magnetic resonance (NMR) and this was confirmed by other chromatographic analysis. This result is interesting because once the authors investigated the babassu leaf DF and apigenin using nociception models (acetic acid-induced abdominal writhing, formalin, and hot plate) they observed strong evidence of the antinociceptive activity of O. speciosa Mart. (babassu) leaves. Moreover, the authors also studied the possible mechanisms involved. They concluded that the in vivo antinociceptive effect of babassu leaves due to apigenin might be associated, in part, with opioid and cholinergic systems.
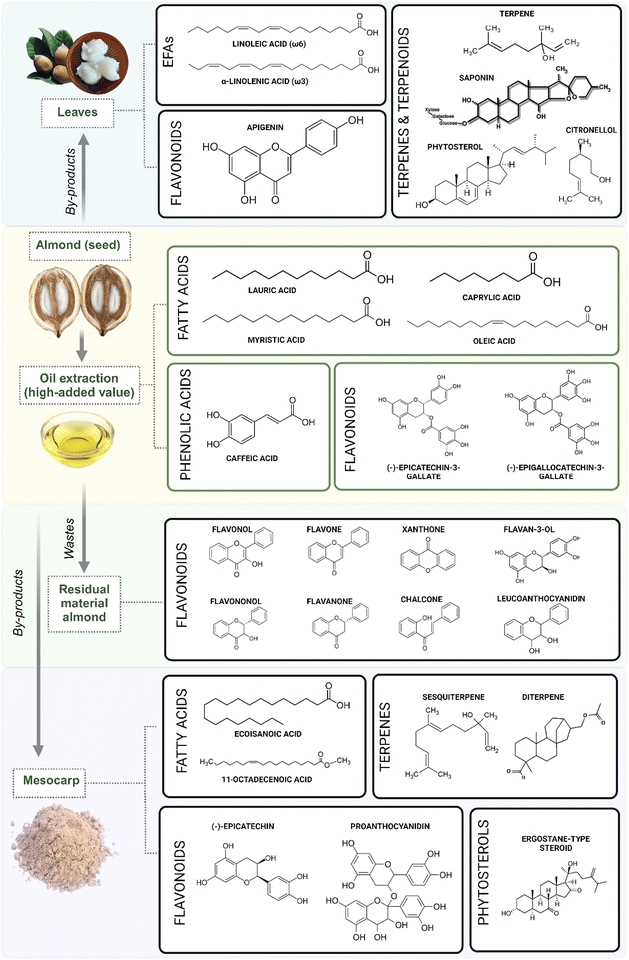 | ||
| Fig. 2 Primary metabolites and phytochemicals found in babassu fruit and by-products generated during babassu oil extraction from seed oil. | ||
| Babassu by-product | Extraction method (solvent) | Primary metabolites/phytochemicals | Potential health benefits | Ref. | ||
|---|---|---|---|---|---|---|
| Identification/quantification method | Chemical compounds | Results | ||||
| AlCl3: aluminium chloride; —: not reported; 1H and 13C NMR: proton (1H) and carbon-13 (13C) nuclear magnetic resonance; GC/MS: gas chromatography/mass spectrometry; HPLC: high-performance liquid chromatography; TLC: thin-layer chromatography; GAE: gallic acid equivalent; CE: catechin equivalent; QE: quercetin equivalent; MW: molecular weight; UAE: ultrasound-assisted extraction. | ||||||
| Leaves | Solvent extraction (ethanol), dichloromethane fraction | TLC | Complex mixtures of terpenes, steroids, and possibly other flavonoids | Isolation and identification of apigenin | Antinociceptive effects | Pinheiro et al. 201251 |
| HPLC | Apigenin | Retention time 11.51 min, 98% purity | ||||
| 1H and 13C NMR | 5,7,4-Trihydroxyflavone (apigenin) | |||||
| Leaves | Soxhlet (ethanol) | GC-MS | Linolenic acid (ω3) | A = 20.65 | Antimicrobial potential | Oliveira et al., 201632 |
| Linoleic acid (ω6) | A = 4.62 | |||||
| Palmitic acid | A = 3.27 | |||||
| Capric acid | A = 2.26 | |||||
| Stearic acid | A = 1.93 | |||||
| Citronellol | A = 7.65 | |||||
| Mesocarp | Solvent extraction (ethanol) | Spectrophotometry (Folin–Ciocalteu) | (Poly)phenols | 56% (55% phenolic acids and 1% flavonoids) | Antimicrobial, antiseptic, immunomodulation | Barroqueiro et al., 201621 |
| Mesocarp | Maceration (hydro alcoholic), ethyl acetate fraction | Spectrophotometry (Folin–Ciocalteu) | Total (poly)phenols | 646.50 mg GAE/g | Antioxidant activity | Romero Hernández et al. 201752 |
| Spectrophotometry (vanillin-HCl) | Proanthocyanidins | 453.70 mg CE/g | ||||
| HPLC, GS/MS | (−)-Epicatechin oligomers | MW: 290 to 1154 g mol−1 | ||||
| Mesocarp | Successive maceration (methanol); chromatographic fractionation | GC/MS, 1H and 13C NMR | Arachidic acids | 38.67% | — | de Farias et al. 201948 |
| 11-Octadecenoic acids | 21.73% | |||||
| GC/MS | Phytosterols | 32.0% | ||||
| Sesquiterpene (nerolidol) | 24.9% | |||||
| Diterpene (17-acetoxy-19-kauranal;) | 15.2% | |||||
| Maceration (methanol); liquid–liquid partition | 1H and 13C NMR | Phytosterol–ergostane-type steroids | ||||
| Residual almond material after oil extraction | Soxhlet (methanol) | Qualitative tests–phytochemical screening | Phenols, leucoanthocyanidins, flavones, flavonols, flavononols, flavonones, xanthones, chalcones, aurones, catechins | Antioxidant | Nobre et al., 201853 | |
| Mesocarp | UAE (ethanol) (varying mesocarp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratio w/v from 1 ethanol ratio w/v from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to 1 4 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25) 25) |
Spectrophotometry (Folin–Ciocalteu) | Total (poly)phenols | Up to 5124.86 mg GAE per 100 g | Antioxidant and antimicrobial | Lima et al., 202344 |
| Spectrophotometry (AlCl3 complexation) | Total flavonoids | Up to 493.53 mg QE per 100 g | ||||
Babassu mesocarp as a dietary antioxidant for health promotion
‘Oxidative stress’ is a term associated with an imbalance between oxidative products and antioxidant agents,54 and this results in a loss of homeostasis, contributing to health emergencies, such as chronic kidney diseases,55 atherosclerosis,56 and neurodegenerative disorders (i.e., Parkinson's disease, Alzheimer's disease, amyotrophic lateral sclerosis, multiple sclerosis).57 Moreover, food (i.e., meat, fish, milk, cheese) is also susceptible to oxidation due to components such as lipids and proteins. So, oxidation of these compounds could increase the consumption of malefic compounds known as “cholesterol oxidation products” (COPs), which are associated with the emergence of diseases such as those mentioned in the previous sentence. Internal and external factors can induce oxidation, and reactive species from oxygen and nitrogen molecules can cause biochemical reactions. Reactive oxygen species (ROS) and reactive nitrogen species (RNS) can interact with macromolecules and produce oxidized molecules. Thus, ingesting antioxidants from food, for example, could reduce oxidative molecules in food and the body.13,58 Perhaps other fields of antioxidant applications can take advantage of phenolic compounds inside different babassu fruit waste fractions (mesocarp, epicarp, and endocarp) to improve the oxidative stability of foods.13 A hydroalcoholic extract of babassu (HEB) showed a total phenolic compound (TPC) content of 414.40 mg GAE per g. However, that extract when subjected to liquid–liquid fractionation using ethyl acetate (EAF) and a hydroalcoholic fraction (HAF) showed higher TPC amounts of 646.50 and 508.90 mg GAE per g, respectively. Nevertheless, the results observed in the antioxidant analyses (FRAP, DPPH, and ABTS) did not change for the different samples. In FRAP, the authors obtained values for the HEB, EAF, and HAF of 11.46, 15.41, and 4.89 (mmol Fe2+/g), respectively. For the DPPH assay, HEB, EAF, and HAF showed IC50 values of 4.00, 3.38, and 3.82 (μg mL−1), respectively. And for ABTS analyses, the results were reported as IC50 activity, with values of 2.74, 2.04, and 2.98 (μg mL−1) for HEB, EAF, and HAF, respectively.59 Very recently, ultrasound-assisted ethanolic extraction of mesocarp flour by applying different mesocarp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratios (1
ethanol ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, and 1
10, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 w/v) showed high antioxidant capacity with 3327.43, 3167.82, and 4037.56 FRAP (μmol TEAC/100 g), respectively. Moreover, in the DPPH assay, mesocarp
25 w/v) showed high antioxidant capacity with 3327.43, 3167.82, and 4037.56 FRAP (μmol TEAC/100 g), respectively. Moreover, in the DPPH assay, mesocarp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol ratios of 1
ethanol ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 and 1
4 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25 w/v showed similar EC50 values (37.23 and 36.78, respectively). This antioxidant capacity is probably associated with high phenolic contents and flavonoids recovered by the greener ultrasound-assisted extraction protocol, as observed by phytochemical screening.44
25 w/v showed similar EC50 values (37.23 and 36.78, respectively). This antioxidant capacity is probably associated with high phenolic contents and flavonoids recovered by the greener ultrasound-assisted extraction protocol, as observed by phytochemical screening.44
Antimicrobial potential of babassu coconut mesocarp
According to the World Health Organization (WHO), antimicrobial resistance has emerged as a global challenge in health promotion and therapy for infectious diseases.60 Thus, several nanobiotechnology scientists are researching novel antimicrobial alternatives. Mesocarp and seed oil from babassu are potential candidates for novel tools to manage infectious diseases caused by Gram-positive pathogens due to their antimicrobial activity. The starch-rich mesocarp fraction has attracted attention from researchers (Table 2) due to polysaccharide chains (linear amylose formed by D-glucose α-1–4 bonds, branched amylopectin formed by α-1–4 bonds, and α-1–6 D-glucose bonds)61 due to interesting properties like biocompatibility, biodegradability, and non-toxicity.2 A pioneering study showed that the aqueous extract of babassu mesocarp from the lyophilized hydroalcoholic (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) extract showed antimicrobial activity against 7 strains of S. aureus, including 2 strains considered MRSA (methicillin-resistant S. aureus) isolated hospital strain: wound discharge and nasal exudate.62 Barroqueiro et al. (2016) demonstrated that the mesocarp ethanol extract showed antimicrobial activity only against Gram-positive bacteria (Enterococcus faecalis, Staphylococcus aureus, and MRSA). The authors chemically screened mesocarp with 56% total (poly)phenols (including 55% phenolic acid and 1% flavonoids).21 These compounds were described to have antimicrobial activity through the mechanism of cell wall damage by inhibiting the enzymatic system essential to synthesizing the cell wall.63 Moreover, the authors also reported in vivo effects against lethal sepsis.21 Later, a drug delivery system based on babassu mesocarp/carboxymethylcellulose films loaded with tannic acid showed an antimicrobial effect against S. aureus. Thus, the authors suggest that the babassu delivery system helps carry antibiotics to treat MRSA.25 According to Dong et al. (2018), tannic acid acts against the S. aureus peptidoglycan of the cell wall, promoting cell membrane construction.64 In contrast, the green synthesis of spherically shaped silver nanoparticles (AgNPs) used babassu starch as a reduction/stabilizer agent to provide anatomizing antimicrobial effects against both S. aureus (Gram-positive) and Escherichia coli (Gram-negative). A greater antimicrobial effect was observed against E. coli.2 Recently, Lima et al. (2023) first identified the antimicrobial activity of babassu mesocarp ethanolic extract against other Gram-negative bacteria (Salmonella enteritidis) beyond E. coli using different solid–liquid ratios in the extraction protocol.44 The authors reported concentrations of 40, 100, and 250 mg mL−1 and an inhibition halo range from 9.70 to 11.83 mm for E. coli and 10.45 to 12.48 mm for S. enteritidis. This result is probably because of a thinner cell wall, which favors the interaction between AgNPs and the lipopolysaccharides of the bacterial wall.2 This interaction promotes cell death by membrane damage, affecting bacterial respiration, leading to pores in the membrane and nutrient release.65
1) extract showed antimicrobial activity against 7 strains of S. aureus, including 2 strains considered MRSA (methicillin-resistant S. aureus) isolated hospital strain: wound discharge and nasal exudate.62 Barroqueiro et al. (2016) demonstrated that the mesocarp ethanol extract showed antimicrobial activity only against Gram-positive bacteria (Enterococcus faecalis, Staphylococcus aureus, and MRSA). The authors chemically screened mesocarp with 56% total (poly)phenols (including 55% phenolic acid and 1% flavonoids).21 These compounds were described to have antimicrobial activity through the mechanism of cell wall damage by inhibiting the enzymatic system essential to synthesizing the cell wall.63 Moreover, the authors also reported in vivo effects against lethal sepsis.21 Later, a drug delivery system based on babassu mesocarp/carboxymethylcellulose films loaded with tannic acid showed an antimicrobial effect against S. aureus. Thus, the authors suggest that the babassu delivery system helps carry antibiotics to treat MRSA.25 According to Dong et al. (2018), tannic acid acts against the S. aureus peptidoglycan of the cell wall, promoting cell membrane construction.64 In contrast, the green synthesis of spherically shaped silver nanoparticles (AgNPs) used babassu starch as a reduction/stabilizer agent to provide anatomizing antimicrobial effects against both S. aureus (Gram-positive) and Escherichia coli (Gram-negative). A greater antimicrobial effect was observed against E. coli.2 Recently, Lima et al. (2023) first identified the antimicrobial activity of babassu mesocarp ethanolic extract against other Gram-negative bacteria (Salmonella enteritidis) beyond E. coli using different solid–liquid ratios in the extraction protocol.44 The authors reported concentrations of 40, 100, and 250 mg mL−1 and an inhibition halo range from 9.70 to 11.83 mm for E. coli and 10.45 to 12.48 mm for S. enteritidis. This result is probably because of a thinner cell wall, which favors the interaction between AgNPs and the lipopolysaccharides of the bacterial wall.2 This interaction promotes cell death by membrane damage, affecting bacterial respiration, leading to pores in the membrane and nutrient release.65
| Babassu part | Babassu derived product | Method | Pathogen | Dosage | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Microbiocidal effect | ||||||
| Almond (seed) | Pure oil | Acridine orange method | Diarrheagenic E. coli (EPEC) (serotype 0111: H- AL-, eae+, eaf+, bfp+) | 100 ng mL−1 | Cell viability: 94.3 ± 3.1% | Honório-França et al., 201418 |
| Phagocytosis index: 63.5 ± 9.4% | ||||||
| Bactericidal index: 45.9 ± 5.4% | ||||||
| Almond (seed) | O/W microemulsion (babassu oil dispersed in aqueous phase) | 100 ng mL−1 | Cell viability: 98.0 ± 0.8% | |||
| Phagocytosis index: 69.1 ± 12.3% | ||||||
| Bactericidal index: 31.8 ± 5.2% | ||||||
| Babassu part | Babassu product/technology | Method | Pathogen | Dosage | Outcome | Ref. |
|---|---|---|---|---|---|---|
| Antifungal effect | ||||||
| Leaves | Ethanolic extract | Disk diffusion (Kirby–Bauer) | C. albicans; | 25, 50, and 100 mg mL−1 | No effect | Oliveira et al., 201632 |
| C. parapsilosis | ||||||
| Babassu part | Babassu product/technology | Method | Pathogen | Dosage | Outcome (MIC/MBC/IZ) | Ref. |
|---|---|---|---|---|---|---|
| O/W: oil-in-water; MIC: minimum inhibitory concentration; MBC: minimum bactericidal concentration; IZ: inhibition zone; MRSA: methicillin-resistant S. aureus; PLA: polylactic acid polymer AgNP: silver nanoparticles; CMC: carboxymethylcellulose; BHI: brain heart infusion; MCFA: medium-chain fatty acid; ES + SC: prepared using electrospinning (ES) and solvent casting technique (SC).a Antibacterial activity of pristine tannic acid (5 mg mL−1) was 10.0 ± 1.00 mm and 10.7 ± 1.53 mm against S. aureus and E. coli, respectively.b S. aureus was resistant to standard antibiotics (5 μg ciprofloxacin IZ: ≤32 mm for E. coli and IZ: ≥28 mm for P. aeruginosa; 10 μg gentamicin IZ: ≤18 mm for E. coli and IZ: ≥21 mm for P. aeruginosa). | ||||||
| Antibacterial effect | ||||||
| Mesocarp | Aqueous extract from lyophilized hydroalcoholic extract (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Disk diffusion | S. aureus ATCC 6538 – AM 103 | 30 mg mL−1 | IZ: 18 mm | Caetano et al. 200262 |
| S. aureus ATCC 9144 – AM 108 | IZ: 18 mm | |||||
| S. aureus AM 189 | IZ: 17 mm | |||||
| S. aureus AM 211 | IZ: 16 mm | |||||
| S. aureus AM 221 | IZ: 14 mm | |||||
| S. aureus AM 349 | IZ: 16 mm | |||||
| S. aureus AM 355 | IZ: 16 mm | |||||
| Leaves | Ethanolic extract | Disk diffusion | S. aureus, E. coli, E. faecalis, P. aeruginosa | 25, 50, and 100 mg mL−1 | No effect | Oliveira et al., 201632 |
| Mesocarp flour | Mesocarp extract powder | Disk diffusion | E. coli | 250 mg mL−1 | IZ: 0 mm | Barroqueiro et al., 201621 |
| 500 mg mL−1 | IZ: 0 mm | |||||
| P. aeruginosa | 250 mg mL−1 | IZ: 0 mm | ||||
| 500 mg mL−1 | IZ: 0 mm | |||||
| E. faecalis | 250 mg mL−1 | IZ: 12.4 ± 0.2 mm | ||||
| 500 mg mL−1 | IZ: 14.4 ± 0.4 mm | |||||
| S. aureus | 250 mg mL−1 | IZ: 15.0 ± 0.3 mm | ||||
| 500 mg mL−1 | IZ: 18.5 ± 0.9 mm | |||||
| MRSA | 250 mg mL−1 | IZ: 15.3 ± 0.3 mm | ||||
| 500 mg mL−1 | IZ: 17.4 ± 0.3 mm | |||||
| Almond (seeds) | Oil | Microdilution method | S. aureus ATCC 12692 | 8–512 μg mL−1 | MIC: 512 μg mL−1 | Nobre et al., 201853 |
| S. aureus Sa 358 | MIC: 256 μg mL−1 | |||||
| P. aeruginosa ATCC 15442 | MIC: 512 μg mL−1 | |||||
| E. coli ATCC 25922 | MIC: 64 μg mL−1 | |||||
| E. coli Ec 27 | MIC: 32 μg mL−1 | |||||
| Mesocarp | (+glycerol 5 wt%) films loaded with tannic acid | Disk diffusion | S. aureus | 5 mg mL−1 of tannic acida | IZ: 7.0 ± 1.00 mm | de Sousa Leal et al., 201825 |
| E. coli | IZ: 6.0 mm | |||||
| Mesocarp | (+glycerol 2 wt% + CMC) films loaded with tannic acid | S. aureus | 5 mg mL−1 of tannic acida | IZ: 6.0 mm | de Sousa Leal et al., 201825 | |
| E. coli | IZ: 6.0 mm | |||||
| Mesocarp flour | Mesocarp extract powder | Broth dilution (BHI) | E. facealis ATCC 29212 | 0.9 to 500 mg mL−1 | MIC: 7.8 mg mL−1 | Barroqueiro et al., 201621 |
| S. aureus ATCC 25922 | MIC: 31.2 mg mL−1 | |||||
| MRSA | MIC: 31.2 mg mL−1 | |||||
| E. coli ATCC 25922 | No effect | |||||
| P. aeruginosa ATCC 27853 | No effect | |||||
| Babassu oil | MCFA hydrolyzed oil (purchased from Sweet Natural Botanicas-USA) | Broth microdilution | C. perfringens UGent 56 | 0.14–4.5 mg ml−1 | MIC: 0.56 mg ml−1 | Hovorková et al., 201830 |
| C. perfringens CIP 105178 | MIC: 0.56 mg ml−1 | |||||
| E. cecorum CCM 3659 | MIC: 4.5 mg ml−1 | |||||
| E. cecorum CCM 4285 | MIC: 2.25 mg ml−1 | |||||
| L. monocytogenes | MIC: >4.5 mg ml−1 | |||||
| S. aureus | MIC: 1.13 mg ml−1 | |||||
| S. aureus (MRSA) | MIC: 31.2 mg mL−1 | |||||
| Almond (seeds) | Oil | Broth dilution (BHI) | S. aureus SA–ATCC 6538 | 1024 to 1.0 μg mL−1 | MIC: 812.75 μg mL−1 | Machado et al., 201931 |
| K. pneumoniae KP-ATCC 10031 | MIC: 406.37 μg mL−1 | |||||
| S. aureus SA–10 | MIC: ≥1024 μg mL−1 | |||||
| B. cereus BC-ATCC 33018 | MIC: ≥1024 μg mL−1 | |||||
| E. coli EC-ATCC 10536 | MIC: ≥1024 μg mL−1 | |||||
| P. aeruginosa PA-ATCC 9027 | MIC: ≥1024 μg mL−1 | |||||
| S. flexneri EC-ATCC 12022 | MIC: ≥1024 μg mL−1 | |||||
| P. vulgaris PV-ATCC 13315 | MIC: ≥1024 μg mL−1 | |||||
| Mesocarp starch | +AgNP | Microdilution | E. coli ATCC 25922 | 3.37 to 27 μg Ag mL−1 | MIC: 6.75 μg mL−1 | Araruna et al., 20202 |
| MBC: 6.75 μg mL−1 | ||||||
| S. aureus ATCC 29213 | MIC: 13.5 μg mL−1 | |||||
| MBC: >27 μg mL−1 | ||||||
| Commercial Babassu oil | PLA–babassu oil membranes (by ES + SC) | Disk diffusion | E. coli ATCC 23784 | 1% (v/v) of babassu oil was added to PLA membranes | No effect | Fernandes et al., 202116 |
| S. aureus ATCC 11632b | No effect | |||||
| P. aeruginosa ATCC 9027b | IZ: 0.40 mm | |||||
| Mesocarp | Ethanolic extract | Disk diffusion | E. coli ATCC 25922 | 250 mg mL−1 | IZ: 9.70 ± 1.00 mm | Lima et al., 202344 |
| 100 mg mL−1 | IZ: 9.59 ± 0.60 mm | |||||
| 40 mg mL−1 | IZ: 11.83 ± 1.42 mm | |||||
| Mesocarp | Ethanolic extract | Disk diffusion | S. enteritidis ATCC 13076 | 250 mg mL−1 | IZ: 12.48 ± 0.43 mm | Lima et al., 202344 |
| 100 mg mL−1 | IZ: 10.45 ± 0.54 mm | |||||
| 40 mg mL−1 | IZ: 11.56 ± 0.76 mm | |||||
Antimicrobial and wound healing potential of babassu coconut oil
Nut oil is the high-added-value fraction of babassu widely studied in terms of antimicrobial activity (Table 2). Due to its antimicrobial and anti-inflammatory activities, researchers applied it in a wound healing-drug delivery system based on polylactide (PLA) membranes (produced by electrospinning and solvent casting techniques).16 PLA/babassu oil membranes inhibited Pseudomonas aeruginosa growth16 but did not inhibit E. coli and S. aureus growth. Babassu nut oil is rich in medium-chain saturated fatty acids, mainly lauric acid.20 Reports in the literature generally recognize that free fatty acids can pass across the cell membrane,66 promoting intracellular acidification and growth inhibition.67 However, the fatty acid could change the plasma membrane, changing membrane permeability and disrupting the electron transport chain.30 Other associated mechanisms involve the influence of enzyme activity, nutrient take up, and the generation of toxic peroxidation and autoxidation products.68Babassu added as an oily phase O/W microemulsion increased the bactericidal index, phagocyte activity, and consequently, phagocytosis of enteropathogenic (diarrheagenic) E. coli (EPEC). Phagocytes produce free radicals and promote the antimicrobial effect, so the authors highlighted the potential of babassu nut oil as a potential candidate for vaccine adjuvants in immunotherapy in future investigations.18 Later, Machado et al. (2019) reported that fixed oil extracted from babassu nuts presented antimicrobial activity against both Gram-positive (S. aureus) and Gram-negative (Klebsiella pneumoniae) bacteria; this was mainly attributed to the free fatty acid profile.31 Likewise, the hydrolyzed oil containing medium-chain fatty acids also inhibited the activity of several Gram-positive bacteria (i.e., S. aureus, Enterococcus decorum, and Listeria monocytogenes), mainly Clostridium perfringens with an outstanding MIC value of 0.56 mg mL−1.30 The oil comprised fatty acids, primarily lauric acid,30 previously associated with antimicrobial activity.31,68–70 The detergent property of fatty acids acts against the amphipathic structure of the cell membrane, solubilizing its components (i.e., lipids and proteins), altering the membrane structure and hence, affecting processes that are essential to bacteria like metabolic process, the electron transport chain, and oxidative phosphorylation.31 It also damages the membrane through complex nutrient absorption enzyme inactivation, causes toxic peroxidation generation,68,69 and affects bacterial efflux pumps due to the hydrophobic compounds. On the other hand, the ethanolic extract from leaves of the A. speciosa palm tree prepared by a Soxhlet protocol32 did not present antimicrobial activity against S. aureus, E. faecalis, E. coli, and P. aeruginosa by disk diffusion (Table 2).32 However, gas chromatography (GC/MS) analysis revealed high levels of citronellol – a widely known antimicrobial metabolite – and higher contents of essential fatty acids (omega 3, omega 6, palmitic, and capric acid) of importance in human nutrition, indicating the biotechnology potential of A. speciosa palm trees. The extracts did not show antifungal activity.32 Nobre et al. (2018) observed the antimicrobial activity of the fixed oil of babassu by MIC analysis. The authors reported a MIC range from 32 to 512 μg mL−1 against bacteria such as S. aureus, P. aeruginosa, and E. coli. and the same study observed the association of this fixed oil with an antibiotic from the aminoglycoside class, which showed a significant reduction in MIC values, conferring a synergistic action with the antibiotic.53
Anti-inflammatory and immunomodulatory capacity
Beyond the potential of babassu coconut fractions as food ingredients, as previously discussed in this review, their immunomodulatory capacity makes them a novel substance for the pharmaceutical and chemical industries.Table 3 shows a brief overview of the anti-inflammatory activity from babassu derivatives (mainly nut oil) reported in the literature, showing their potential in folk medicine to treat skin wounds.20 The bioactive polysaccharide glucan in babassu aqueous extract showed a significant capacity to inhibit the increased vascular permeability induced by acetic acid.71 An ethanolic extract made with babassu almond was evaluated for benign prostatic hyperplasia (BPH) treatment. However, the chemical composition (rich in oil) was not soluble in culture media (aqueous). So, a B8 viscogel was used to intercalate the extract of Orbignya speciosa kernels, resulting in the development of a nanoparticulate system (NanoOSE), which was a stock solution containing 30 mg mL−1, and this was diluted to obtain a 300 μg mL−1 solution. The cell culture was treated with NanoOSE, which effectively induced morphological changes in the cell structure, diminished cell proliferation, and triggered necrosis/apoptosis in both BPH cells and tissues.72 A significant reepithelization effect of babassu mesocarp aqueous extract (BMAE) on the healing process of skin surgical wounds of rats was recognized in the experimental group compared with the control group by microscopic analysis.73 Later, the same research group found a pleurodesis action, revealing BMAE as a potential therapeutic agent for patients with spontaneous pneumothorax or malignant pleural effusion. In vivo, interventions with BMAE were highly irritating to the rats’ pleura and lung parenchyma. Numerous adhesions and inflammation were observed macroscopically without significant changes evidenced microscopically.73 When any injury or stress occurs to the membrane cell, there is a stimulus to repair the problem; hence, inflammation occurs.74 The mechanism includes activation via the toll-like receptor (TLR) with inflammatory gene activation75 and increased synthesis and secretion of mediators like cytokines, tumor necrosis factor-α (TNF-α), and interleukin.76 The fatty acid-rich babassu oil contains natural phenols like phytosterols and tocopherols (Table 1). Natural (poly)phenols have been found to interfere with protein kinase C (PKC) signaling pathways, which are essential mediators of immune cells.77 Therefore, in an in vivo model of induced inflammatory reaction, Barbosa et al. (2012) reported higher anti-inflammatory activity after oral administration of unrefined babassu oil (0.18 mL per dose) compared to mineral oil in the control group.78
| Babassu derivative | Product/technology used | In vivo model | Inflammation model | Administration mode | Dosage | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; IL-8: interleukin 8; O/W: oil-in-water; PMA: phorbol 12-myristate 13-acetate; AA: arachidonic acid; —: not reported/not identified. | |||||||
| Mesocarp | Aqueous extract | Male BALB/c mice | Acetic acid-induced vascular permeability | Orally | 100 mg kg−1 | Inhibited the increase in vascular permeability | Silva and Parente 200171 |
| Mesocarp flour | Aqueous extract | Adult Wistar rats | Skin surgical wounds – punch | Topical anesthetics | 25 mg mL−1 | Reepithelization effect on the healing process | Amorim et al. 200673 |
| Mesocarp flour | Aqueous extract | Male Wistar rats | Pleurodesis model | Intrapleural injection | 25 mg mL−1 for 50 mg kg−1 | Irritation to the pleura and pulmonary parenchyma without microscopic pleura adhesion | Amorim et al. 200673 |
| Mesocarp flour | Mesocarp extract | Swiss mice | Sepsis induction | Subcutaneous | 125 mg kg−1 | Inhibition: TNF-α, IL-6, and IL-8 production | Barroqueiro et al., 201621 |
| 250 mg kg−1 | Inhibition: peritoneal cell migration; TNF-α and IL-6 production | ||||||
| Seed oil | 100% (pure oil) | Male Swiss and BALB/c mice | PMA-induced ear edema | Topical | 10 μL per ear | 58% (p < 0.001) edema inhibition | Reis et al., 201717 |
| Seed oil | 100% (pure oil) | AA-induced ear edema | Topical | 10 μL per ear | 78.5% (p < 0.001) edema inhibition | ||
| Seed oil | 100% (pure oil) | Male Swiss and BALB/c mice | PMA-induced ear edema | Oral | 100 mg kg−1 | 23.5% (p < 0.001) edema inhibition | Reis et al., 201717 |
| 300 mg kg−1 | 39.7% (p < 0.001) edema inhibition | ||||||
| 1000 mg kg−1 | 51.9% (p < 0.001) edema inhibition | ||||||
| Seed oil | 12.2% oil diluted in acetone | Male Swiss and BALB/c mice | PMA-induced ear edema | Topical | — | 17.5% (not significant) edema inhibition | Reis et al., 201717 |
| Seed oil | 12.2% oil in O/W microemulsion | PMA-induced ear edema | Topical | — | 66.2% (p < 0.001) edema inhibition | ||
| Seed oil | Unrefined oil | Male golden hamsters | Ischemia/reperfusion-induced (hamster cheek pouch) | Oral | 0.02, 0.06, and 0.08 mL per dose | Lower: ischemia-induced micro-vascular leaks during reperfusion, histamine-induced microvascular permeability, leucocyte adhesion, and cytokine levels. | Barbosa et al., 201278 |
| Seed oil | Fixed oil | Male Swiss mice | Ear edema induced by multiple applications of croton oil | Topical | 10 μL per ear | Reduced ear thickness, epidermal hyperplasia, and myeloperoxidase activity | Santos et al., 202020 |
Likewise, in vivo interventions with babassu mesocarp extract (125 and 250 mg kg−1) showed anti-inflammatory activity in mice with induced sepsis (the inflammatory process was triggered due to puncture injuries on the cecum of mice) due to no pro-inflammatory cytokine production (e.g. TNF-α, interleukin 1 (IL-1) and 6 (IL-6)).21 Subcutaneous administration of 125 mg kg−1 mesocarp extract provided 40% survival after 24 hours, maintaining the survival rate on the 10th day. In contrast, 250 mg kg−1 administration showed 60% survival after 24 hours, decreasing to 40% after 36 hours, and maintaining the survival rate on the 10th day.21 We provided a schematic representation of the in vivo immunomodulatory effect after subcutaneous administration of babassu mesocarp extract, providing a new way to inhibit the inflammatory chain (Fig. 3). TNF-α is the first pro-inflammatory cytokine, promoting increased leukocyte recruitment to the inflammatory regions. Monocytes produce IL-6 and endothelial cells, fibroblasts, and other cells like TNF-α are also responsible for inducing inflammatory activity. Phorbol 12-myristate 13-acetate (PMA) can induce the activation of an inflammatory mechanism through PKC, activation of nuclear factor kappa B (NF-κβ), and the production of TNF-α, cyclooxygenase-2 (COX2), and prostaglandin E2 (PGE2). Furthermore, mast cell infiltration with mediator liberation increases permeability and neutrophil influx.79,80 Topical administration of pure lauric acid (10 μL per ear) inhibited PMA-induced ear edema by 90.3% (p < 0.001), while dexamethasone inhibited ear edema by 79.7% (p < 0.001).17 Interestingly, pure babassu oil (10 μL per ear) inhibited ear edema by 54.1% (p < 0.001) and demonstrated an anti-inflammatory ability,17 probably associated with the presence of lauric acid as its main component (data from Table 3 can be compared with data in Table 1, which shows the chemical profiles). Such a compound can interfere with arachidonic acid metabolism, inhibiting phospholipase A2 (PLA2), COX2, and lipoxygenase (LOX), and hence, PMA-induced inflammation. Interestingly, the authors also argued that babassu oil could have a possible barrier effect on ear surfaces. Thus, babassu oil was also systematically (100–1000 mg kg−1 oral dosages) tested against PMA-induced ear edema and showed up to 51.9% (p < 0.001) edema inhibition at a dose of 1000 mg kg−1; the nonsteroidal anti-inflammatory drug indomethacin (10 mg kg−1) inhibited ear edema by 62.0% (p < 0.001). Furthermore, the topical anti-inflammatory potential was also confirmed against another inflammation model: babassu oil (10 μL per ear) reduced arachidonic acid-induced ear edema by 78.5% (p < 0.001). In contrast, the non-selective COX-1/2 inhibitor indomethacin (0.5 mg per ear) reduced ear edema by 61.5% (p < 0.001).17
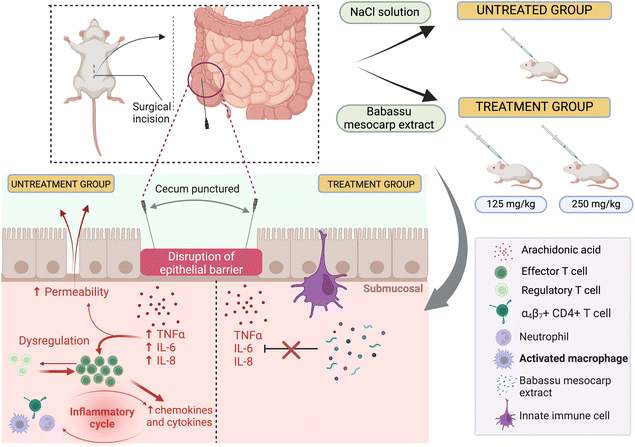 | ||
| Fig. 3 Immunomodulatory, anti-inflammatory mechanism of babassu mesocarp ethanolic extract on a treated group and inflammatory process in an untreated group through an in vivo assay. | ||
Later, the potential of using pure babassu oil in folk medicine for skin wound healing was suggested by the in vivo anti-inflammatory effect of nut oil in a chronic ear edema model, as proved by reduced ear thickness, epidermal hyperplasia, and myeloperoxidase activity after the topical administration of 10 μL babassu oil per ear.20
Healing potential of babassu mesocarp
The healing process is a multifaceted biological event encompassing inflammation, chemotaxis, cellular proliferation, differentiation, and remodeling.81 An aqueous extract made with 25 mg mL−1 babassu mesocarp was evaluated. The authors observed that mice with colon anastomosis treated with 50 mg per kg per weight doses could be healed.82 In another study, an aqueous extract of mesocarp at the same concentration (25 mg mL−1) applied intra-peritoneally via a single dose of 50 mg kg−1 was observed to target stomach healing by aiding the bringing together of the edges of the gastrorrhaphy in animals that had been sacrificed on the 7th day of the postoperative period.83 At the same concentration (25 mg mL−1), the aqueous extract of babassu mesocarp was applied inside the peritoneal cavity of the rats in a single dose of 50 mg kg−1 to observe the healing process of linea alba. The authors observed a potential healing effect based on the animals treated with the extract, which had better results for the tensiometric parameter and more excellent resistance.84 Under the same conditions, an aqueous extract of babassu mesocarp induced better healing of surgical injuries of the bladder.85 An apparent circular incision of 2 cm in diameter with a metallic puncture was made in mice. It was possible to observe the healing effect of the aqueous extract (similar concentration and dose to those previously described), and it was possible to observe that the aqueous extract had an essential effect on healing at the microscopic level, on mononuclear variables and collagen fibers.86 The healing of colonic anastomosis in rats was evaluated by administration aqueous mesocarp extract at 50 mg per kg per body weight. The results showed superior healing of the cecum when compared to the control group.87 With the same objective, an aqueous extract made with babassu powder at a concentration of 25 mg mL−1in vivo was administered orally by daily gavage at a dose of 50 mg kg−1. This study agrees with other works already observed in the literature, reporting the effectiveness of babassu extract in the surgical healing process, with excellent parameters observed in the healing process for cecorrhaphy in rats.81 An effect was observed in peptic ulcer treatment: rats were treated orally with an aqueous extract made with 2 g kg−1 babassu mesocarp, and results demonstrated the potential therapeutic effect in the treatment of gastric ulcer, with behavior like that of the control group treated with omeprazole.88Babassu almond oil in emulsions
Nanobiotechnology concepts using babassu oil in a microemulsion system for topical delivery were related to striking results of topical and systemic anti-inflammatory effects against ear edema induced by PMA. Babassu oil showed short-term stability in oil-in-water (O/W) nanoemulsions by emulsification phase inversion and was considered a promising dispersive system for pharmaceutical and cosmetic applications.89 Later, Reis et al. (2017) evaluated the in vivo anti-inflammatory activity of a babassu nanocarrier based on an oil-in-water (O/W) microemulsion using babassu as an oily phase. A classical microemulsion system formulation (9% water as the aqueous phase, 12.2% babassu as the oil phase, and 48.8% surfactant system) inhibited ear edema by 66.2% (p < 0.001), showing an enhanced activity promoted by the microemulsification of babassu oil, since babassu oil at 12.2% (final concentration in the O/W microemulsion) did not have a significant effect (please see Table 3). Microemulsification of babassu oil seems to enhance the skin permeation of active anti-inflammatory compounds (e.g., lauric acid) found in the oil, reaching the same percentage of edema inhibition as pure babassu oil with a lower oil concentration, producing a remarkable outcome.Babassu mesocarp in drug delivery systems towards parasitic and infectious diseases
Drug-releasing systems have attracted significant attention from the pharmaceutical industry for drug administration due to several therapeutic benefits such as efficiency, principal selectivity, long-term efficacy, and minimized side effects.90 Thus, drug release from natural products and biocompatible biopolymers has been studied, targeting novel alternatives to treat neglected infectious diseases of high mortality, such as leishmaniasis.22,25 Leishmaniasis is an endemic disease with a substantial impact on human health and high prevalence in 90 countries and tropical areas.91 What is quite interesting is that standard anti-leishmanial drugs present limitations such as severe toxicity and drug resistance.92 As a drug alternative treatment against leishmaniasis, da Silva et al. (2018) showed that an aqueous extract of mesocarp (BMAE) encapsulated by poly(lactic-co-glycolic acid) (PLGA) was effective against promastigote forms of Leishmania amazonensis.22 At the same time, other research groups reported the starch-rich mesocarp of babassu (Orbignya sp.) and carboxymethylcellulose (CMC) films as a tannic acid releasing matrix with in vitro cytotoxicity on sarcoma-180 (91.86 ± 9.97%) and promastigote forms of Leishmania major (100%).25 Moreover, the babassu mesocarp–CMC films showed controlled drug release (release of 71.01% of TA from the matrix after 24 h) and good antioxidant activity by DPPH (∼79–82%) and ABTS (82–89%) assays.22Other potential health benefits of babassu low-added-value fractions
Table 4 displays other potential health benefits of babassu derivatives due to their antiprotozoal, anticancer, antithrombotic, and anti-dyslipidemia effects reported in the literature in the preclinical stage of an investigation.| Babassu fraction | Drug delivery system | Organism | Dosage | Outcome | Ref. |
|---|---|---|---|---|---|
| Antiprotozoal effects | |||||
| Mesocarp | MMP-loaded PLGA microparticles | Promastigote forms of Leishmania amazonensis | 300 μL, 10 mg mL−1 | IC50 = 12 pg mL−1 (P < 0.05) | Silva et al., 201822 |
| Mesocarp | Babassu mesocarp–CMC films loaded with TA | Promastigote forms of Leishmania major | 6.25 to 800 800 μg mL−1; SI: 0.94 | Release of 71.01% of TA of the matrix after 24 h; Antioxidant activity; IC50 = 100% | de Sousa Leal et al., 201825 |
| Babassu fraction | Extract | Model | Intervention/dose | Outcome | Ref. |
|---|---|---|---|---|---|
| In vivo anticancer potential | |||||
| Epicarp/mesocarp | Crude ethanol extract (by maceration) | Against leukaemic cell lines (HL-60, K562, K562-Leucena 1); | Dose-dependent | Antineoplastic potential: higher cytotoxicity against tumoral than non-tumoral cells; stimulation of PFK activity observed on HL-60 cells | Rennó et al., 200834 |
| MCF-7 tumor cells, mouse fibroblast cell line 3T3-L1, and fresh human lymphocytes | |||||
| Mesocarp flour | Aqueous extract | In vitro and in vivo assay: C3H/HePas mice peritoneal cellular | 10 and 20 mg kg−1 | Macrophage activation (in vitro and in vivo), cytotoxic activity, and induction of inflammatory mediators | Nascimento et al., 200633 |
| Mesocarp flour | Aqueous extract | In vivo: male Swiss mice with inoculation of MCF-7 tumor cells | 100 μL of aqueous extract at 20 mg mL−1 | Induction of antitumor immune response; increased marker expression for granulocytes (Ly6G-LY6C), increased antigen presenting cells | Almeida et al. 201423 |
| Babassu fraction | Extract | Model | Intervention | Outcome | Ref. |
|---|---|---|---|---|---|
| MMP: aqueous extract of babassu mesocarp; PLGA: poly(lactic-co-glycolic acid); IC50: in vitro – 50% inhibitory concentration; PFK: 6-phosphofructokinase; TA: tannic acid as a standard drug; CMC: carboxymethylcellulose; SI: selectivity index; TNF-α: tumor necrosis factor-α; MFC-7: human breast cancer cell line. | |||||
| Antithrombotic effect | |||||
| Mesocarp flour | Aqueous extract | In vivo assay – C57Bl/6 mice | 500 mg per kg per day | Slowed coagulation process; NO production; thrombosis inhibition | Azevedo et al., 200735 |
| Babassu as a food supplement – treatment strategy for dyslipidemia | |||||
| Mesocarp flour | Aqueous extract | In vivo – Swiss mice | Oral supplementation/5 mg kg−1 | Reduction in cholesterol levels | Soares et al., 202193 |
| Mesocarp flour | Aqueous extract | In vivo – Swiss mice | Oral supplementation + resistance training/5 mg kg−1 | Reduction in TNF-α | Soares et al., 202193 |
| Body weight | |||||
| Retroperitoneal and interstitial fat deposits | |||||
| Triglyceride levels | |||||
| Total number of lymphocytes | |||||
| Antinociceptive effects | |||||
| Palm tree leaves | Ethanolic extract | In vivo – Swiss mice (hot plate test) | Oral/10 mg kg−1 | 48.7% writhing inhibition | Pinheiro et al. 201294 |
| Dichloromethane fraction | 66.8% writhing inhibition | ||||
Babassu mesocarp as a dietary supplement against dyslipidemia
After the COVID-19 pandemic affected the whole world, authorities have warned of other problems that have arisen, like obesity.95 The WHO highlights that the “obesity epidemic” is increasing in Europe, with more than 60% overweight or obese adults and 7.9% overweight or obese children under five.96 High body fat levels are an essential risk factor for cardiovascular, endocrine, and metabolic disorders like type 2 diabetes.97 Recently, babassu mesocarp was proposed as a food supplement during training resistance and as a medicine to treat dyslipidemia.93 An in vivo assay showed that babassu mesocarp aqueous extract had an immunomodulatory effect (increasing helper T lymphocyte count TCD4+ and CD69+) on lymphocyte, macrophage, and cytokine production; in addition, it had the capacity to control cholesterol and triglyceride levels, reducing TNF-α, body weight, retroperitoneal, and interstitial fat deposits.93Anticancer potential of babassu by-products and waste
Developing novel chemotherapy strategies with minor side effects for cancer treatment is one of the significant global challenges concerning health. In this review, we also identified preclinical interventions with extracts of babassu by-products (mesocarp) and wastes (epicarp) with antitumor effects showing a higher propensity for tumoral cells than normal cells.34 The aqueous extract from the babassu mesocarp showed anti-tumoral activity due to increased macrophage activity. This can be caused by the ability to induce the production of inflammatory metabolites that promote increased cellular influx to the peritoneal cavity, MHC class II expression, and the spreading ability, and result in the production of NO, TNF, and H2O2.33 The ethanolic extract of babassu epicarp and mesocarp showed a dose-dependent antineoplastic action against leukemic cell lines (HL-60, K562, K562-Leucena 1), human breast cancer cell line MCF-7, mouse fibroblast cell line 3T3-L1 and fresh human lymphocytes.34 Cytotoxicity activity occurs through inhibiting a key glycolytic enzyme, 6-phosphofructo1-kinase (PFK). At the same time, the modification of cells, like a reduction in the size of cells, formation of rounded cells, and nuclear condensation are morphological singns of apoptosis.98,99The potential of BAE as an immunological adjuvant was also confirmed by reports of its antitumor immunity. Animals stimulated with MCF-7 tumor cells pretreated with BAE (97% cell viability) showed increased helper and cytotoxic T cells. It also showed an increase in phagocytic cells with PAMPs (pathogen-associated molecular patterns) on the cell surface, and some PAMPs can recognize carbohydrates such as mannose. This result may explain why animals treated with babassu extract showed better granulocytic cell activity, as the composition of the extract, rich in carbohydrates, induced this response, suggesting a pro-inflammatory effect.23
Babassu mesocarp to avoid thrombosis events
Thrombosis, a venous disease that occurs when blood clots block blood vessels, is a serious but preventable disease that can cause disability and death.100 An in vivo antithrombotic effect of babassu (O. phalerata) mesocarp flour suspended in 1 L of distilled water was reported by Azevedo et al. (2007); this suggests it is a prophylactic agent to avoid thrombosis events. An antithrombotic effect with a slow coagulation process was observed as a positive effect on coagulation factor levels, nitric oxide (NO) production by macrophages, and 88.9% reduction in the necrosis of the tail of carrageenin-induced thrombosis in mice orally treated (500 mg per kg per day) with babassu mesocarp.35 NO was reported to be an essential agent against the thrombotic effect,33,101 associated with its action on blood vessel relaxation, platelet adhesion, endothelial regeneration, and inhibition of leukocyte chemotaxis.35 The antithrombotic effect of babassu mesocarp might be related to the antioxidant capacity of its bioactive compounds and nutrients. Some studies reported the antithrombosis role of food nutrition and dietary supplements102via inhibition of platelet activation mechanisms103 or by its relationship between oxidative stress and thrombosis, once several nutrients and foods bioactive compounds are antioxidants.103,104Babassu mesocarp as potential active food packaging material
It is important to comment on the viable usability of raw mesocarp flour instead of starch isolated from the mesocarp to make bioactive films. Although higher antioxidant and total phenolic compound (TPC) contents were observed using the raw babassu mesocarp flour105 and its isolated starch to produce films, the packaging's mechanical and functional properties (resistance, WVP, transparency, water resistance, and hydrophobicity) were compromised due to the higher protein and fiber contents.13 However, suppose the application interest is for use as a food ingredient. In that case, direct use of the agro-industrial residue, babassu mesocarp flour, offers the best nutritional value and bioactive properties.Antioxidant biodegradable films
Due to their prominent flexibility, softness, strength, transparency, water and moisture resistance, and barrier properties, conventional synthetic plastic films are widely used in everyday life, supermarkets, mulch films, and the packaging industries.106 Currently, the use of synthetic plastics has triggered a worldwide alert, as accelerated use and incorrect disposal promote negative impacts on the environment and animal and human life.106–108 Thus, the scientific community is looking for greener alternatives, such as biodegradable plastics.109–111 Babassu mesocarp is a by-product of babassu oil extraction, mainly composed of cellulose and polysaccharides, and thus, a potential green and natural raw material alternative for the production of food-derived biopolymers and biodegradable plastics. The babassu mesocarp is a starch-rich fraction (∼71%) that can be combined with a plasticizer agent to overcome the brittle nature of starch to produce biodegradable films.112 Furthermore, babassu mesocarp's phenolic compounds and antioxidant properties29 make it an exciting alternative to synthetic plastics for active plastic coating/packaging applications that come into direct contact with food and attractive for food packaging and biomedical applications (Fig. 4).When an aqueous extract of O. phalerata mesocarp was made by suspending mesocarp powder in demineralized water (at a concentration of 20%), the authors did not observe a strong in vitro antioxidant effect by thiobarbituric acid-reactive substances (TBARS), nitric oxide (NO), and hydroxyl radical scavenging assays.27 The authors observed a positive reaction for (poly)phenols in phytochemical screening, suggesting that the compounds extracted may interact with OH radicals, reactive oxygen species (ROS), instead of with NO, a reactive nitrogen species.27
On the other hand, starch was successfully isolated from babassu mesocarp with swelling power and antioxidant activity using steeping in water (WS), in alkaline (KS),29 and an acid (AS) medium.13 However, although alkaline steeping provided purer starch (99%) in a higher yield (85%), a more significant loss of total phenolic compounds (TPC) (8.2 mg of GAE per 100 g) was caused compared to water steeping (63.7 mg of GAE per 100 g).13,29 This behavior can be understood by the possibility of an alkaline medium generating phenoxides from phenolic compounds related to chromophores that can be oxidized by air, producing a darker material, as confirmed by the authors during color evaluation.29 Thus, the authors verified that the acid medium isolated starch with higher antioxidant activity (70%) due to the considerable content of TPC.13 Nonetheless, films prepared with babassu mesocarp starch by steeping in water showed high potential as packaging materials for oxidation-sensitive foods. The WS method yielded purer starch films with a significant content of amylose and, hence, higher mechanical and functional properties when the authors plasticized it with glycerol (i.e., stronger, more rigid, less water-vapor permeable (WVP), more crystalline, with a denser and ordered homogeneous structure).13 Later, this same group evaluated the effect of varied plasticizer types to obtain films based on babassu starch isolated by WS, AS, and KS methods with lower hydrophilicity and good mechanical properties. Each extraction method produces starch with a particular chemical structure and composition and hence affects the plasticizer–starch interaction in the film.28 The authors showed that sorbitol was the most suitable for AS and KS starch films. In contrast, glycerol was the most suitable plasticizer for WS starch, producing films with mechanical resistance.28
Safety and toxicological concerns
This review has discussed how researchers focus on the bioactive compounds of babassu and its by-products due to their valuable applications in the health care, cosmetics for skin care, and food sectors. Considering the increasing attention being paid to research on babassu waste using nanotechnology concepts (i.e., nanoemulsions, nanocomposites, and hybrids with organic and inorganic materials), the analysis of chemical migration (i.e., nanoparticles, plasticizers, phytochemicals) from food packaging is needed.Moreover, tannins, saponins, and flavonoids are the most common phytochemicals in babassu mesocarp, and this review shows their valuable therapeutic activities and potential for food packaging applications. Although other plant extracts of these compounds have been reported to have low toxicity, evaluating acute toxicity by oral administration of single/multiple doses of babassu by-product extracts is fundamental to advancing toxicological research and risk assessment. It is crucial to mention other materials science strategies that could also contribute to advancing the safety of babassu mesocarp and its phytochemicals in drug delivery or novel chemotherapy approaches to treat cancer. Recently, films based on babassu mesocarp were reported as strong candidates for drug release once they served as a release matrix for tannic acid with in vitro toxicity against leishmania parasites and tumor cell strains without high toxicity on normal cells.34 The in vivo low toxicity of babassu mesocarp extract was proved by oral administration of a single high dose (up to 5000 mg kg−1) of babassu mesocarp extract to mice once no alterations were identified at the tissue level or body and organ weight (i.e., glucose, triacyl glyceride, cholesterol, and creatinine).113 However, some changes in biochemistry levels (i.e., increase in alkaline phosphatase and reduction in urea) with a long-lasting effect were observed, which the authors considered to be an accidental finding and unrelated to treatment.113 In 2017, researchers revisiting Amazonian plants commented that babassu seed oil for dermatologic and cosmetic applications was increasing rapidly in applications such as skin rehydration and soothing; anti-inflammatory, antiaging, and antiparasitic effects; hair care; burn and wound healing; and the amelioration of rosacea and psoriasis. However, such usage did not consider possible harmful effects and is limited in empirical knowledge.8
Conclusions and outlook
The nut of babassu coconut (the high added-value product of babassu coconut) presents benefits attributed to its bioactive compounds, which may be influenced by how the raw material is obtained, extraction methods, and processing. As it is a natural product, the processes for obtaining beneficial metabolites must be detailed and standardized for reproducible results. Considering babassu by-products, their low-added fractions are rich in bioactive compounds, such as primary (essential fatty acids, polysaccharides) and secondary metabolites (i.e., (poly)phenols) with antioxidant capacity, antiparasitic properties, and microbiocidal and antimicrobial activity. Thus, recent preclinical investigations showed the potential of using babassu by-products and waste in cancer therapy, for their immunomodulation and anti-inflammatory properties, as drug delivery agents to avoid thrombotic events, to treat hypercholesterolemia, dyslipidemia, and infectious and parasitic diseases, as biodegradable materials in food packaging, and as food additive substituents (i.e., salt replacer in cheese) with antimicrobial effects.Babassu mesocarp starch could be an alternative for active and biodegradable plastic packaging materials. Thus, bioplastic made with babassu appears to be an alternative to protect foods while improving their oxidation stability.
Other studies showed the potential of babassu mesocarp flour as a dietary supplement for dyslipidemia and trends in other metabolic disorders. Likewise, the high capacity of babassu oil to inhibit pro-inflammatory cytokines was improved using concepts of nanobiotechnology (i.e., micro/nano-emulsification), looking to boost the anti-inflammatory behavior.
Technology based on babassu derivatives (i.e., mesocarp and seed oil) also appears in this review as a potential tool to improve the human immune system through its application as a vaccine adjuvant. Moreover, the macrophage production ability of babassu mesocarp was also discussed and related to its in vivo anti-tumoral activity. We identified gaps and future opportunities for mesocarp and other low-added fractions of babassu coconut (i.e., endocarp and epicarp) that can be conveniently studied, proposing their application as novel substances for the pharmaceutical and chemical industry. For example, the epicarp and endocarp were discussed as (i) fiber-reinforcement filler for composite materials, (ii), valuable raw materials for lignocellulosic-feedstock biorefinery, and (iii) an immunomodulatory biomaterial for immunomodulation strategies in tissue repair. On the other hand, nanoemulsification of babassu components can be conveniently evaluated in delivery systems for food science and technology, like food additives and functional foods.
Finally, as demonstrated by in vivo investigations, babassu showed an antithrombotic effect, which might be related to the antioxidant capacity of its bioactive compounds and nutrients. However, the possible consumption of babassu oil or its by-products within a balanced diet may be considered before hypothesizing possible health effects. Until now, advances have been shown only in the preclinical stage of investigations since papers considered in this review related to health effects obtained results from in vitro or animal models, and no human studies have been reported so far.
Author contributions
Rayssa Cruz Lima: formal analysis, investigation, writing – original manuscript preparation. Anna Paula Azevedo de Carvalho: conceptualization, methodology, supervision, project administration, writing – original manuscript preparation, funding acquisition. Antonio Eugenio Castro Cardoso de Almeida: supervision, writing – reviewing and editing. Carlos Adam Conte-Junior: supervision, writing – reviewing and editing. All authors read and approved the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) [grant numbers E-26/201.402/2023, E-26/210.385/2022, E-26/200.621/2022, E-26/200.891/2021]; the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant numbers 313119/2020-1, 152936/2022-0]; and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) [grant number 88887.696241/2022-00] for financial support.References
- J. T. de Medeiros-Costa, Estágio Atual da Taxonomia dos Gêneros e Espécies da Unidade Attalea (Palmae) no Brasil, EMBRAPA-UEPAE de Teresina, Teresina, Documentos 4, 1984, https://ainfo.cnptia.embrapa.br/digital/bitstream/item/95435/1/DOC040001.pdf, (Accessed April 4, 2024).
- F. B. Araruna, F. O. S. Araruna, L. P. L. A. Pereira, M. C. A. Brito, P. V. Quelemes, A. R. de Araújo-Nobre, T. M. de Oliveira, D. A. da Silva, J. R. S. A. Leite, D. F. Coutinho, M. O. R. Borges and A. C. R. Borges, Green Syntheses of Silver Nanoparticles Using Babassu Mesocarp Starch (Attalea Speciosa Mart. Ex Spreng.) and Their Antimicrobial Applications, Environ. Nanotechnol., Monit. Manage., 2020, 13, 100281 Search PubMed
.
-
S. F. Glassman, Preliminary taxonomic studies in the palm genus Attalea H.B.K, Field Museum of Natural History, Chicago, Ill, 1977, vol. 38, no.5 Search PubMed
.
- P. H. May, A. B. Anderson, J. M. F. Frazão and M. J. Balick, Babassu Palm in the Agroforestry Systems in Brazil's Mid-North Region, Agrofor. Syst., 1985, 3, 275–295 CrossRef
.
-
A. Anderson, P. H. May and M. Balick, The Subsidy from Nature - Palm Forests, Peasantry, and the Development of Amazon Frontier, Columbia University Press, New York, 1991 Search PubMed
.
- M. M. Cavallari and M. M. Toledo, What Is the Name of the Babassu? A Note on the Confusing Use of Scientific Names for This Important Palm Tree, Rodriguésia, 2016, 67, 533–538 CrossRef
.
- D. V. Johnson, NON-WOOD FOREST PRODUCTS 10/Rev. 1, Tropical palms ––2010 revision, Rome, Food and Agriculture Organization of the United Nations, 2010.
- B. Burlando and L. Cornara, Revisiting Amazonian Plants for Skin Care and Disease, Cosmetics, 2017, 4, 25 CrossRef
.
- I. R. S. Vieira, A. C. L. N. Silva, N. R. Castro, C. D. S. C. Pinto, Z. M. F. de Freitas, E. Ricci-Júnior, E. P. dos Santos, A. L. Camara, M. C. P. Costa and C. A. Conte-Junior, Development and Characterization of Photoprotective Nanoemulsions Containing Babassu (Orbignya Phalerata Mart.) Lipophilic Extract, Braz. J. Pharm. Sci., 2023, 59, e23011, DOI:10.1590/s2175-97902023e23011
.
- M. S. do Rosário, M. I. R. Gauto, A. C. L. N. Silva, J. S. Sales, F. D. S. Pereira, E. P. dos Santos, E. R. Júnior and M. C. P. Costa, Estudo de estabilidade de emulsão cosmética com potencial de creme hidratante para o tratamento da xerose cutânea utilizando o óleo de babaçu (Orbignya phalerata martius)/Study of stability of cosmetic emulsion with potential of hydrating cream for the treatment of cutaneous xerosis using babassu oil (Orbignya phalerata martius), Braz. J. Dev., 2021, 7, 29552–29570 CrossRef
.
- Future Market Insights, Babassu Oil Market Outlook, https://www.futuremarketinsights.com/reports/babassu-oil-market, (Accessed April 4, 2024).
- M. P. Soler, A. D. A. Vitali and E. F. Muto, Tecnologia de Quebra Do Coco Babaçu (Orbignya Speciosa),
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/char_0063_0332.gif) .
. ![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[c with combining low line]](https://www.rsc.org/images/entities/char_0063_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[o with combining low line]](https://www.rsc.org/images/entities/char_006f_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/char_006c_0332.gif) .
. ![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[l with combining low line]](https://www.rsc.org/images/entities/char_006c_0332.gif)
![[i with combining low line]](https://www.rsc.org/images/entities/char_0069_0332.gif)
![[m with combining low line]](https://www.rsc.org/images/entities/char_006d_0332.gif)
![[e with combining low line]](https://www.rsc.org/images/entities/char_0065_0332.gif)
![[n with combining low line]](https://www.rsc.org/images/entities/char_006e_0332.gif)
![[t with combining low line]](https://www.rsc.org/images/entities/char_0074_0332.gif) ., 2007, 27, 717–722 Search PubMed
., 2007, 27, 717–722 Search PubMed .
- B. C. Maniglia, L. Tessaro, A. A. Lucas and D. R. Tapia-Blácido, Bioactive Films Based on Babassu Mesocarp Flour and Starch, Food Hydrocolloids, 2017, 70, 383–391 CrossRef CAS
.
- S. E. González-Pérez, M. Coelho-Ferreira, P. de Robert and C. L. L. Garcés, Conhecimento e Usos Do Babaçu (Attalea Speciosa Mart. e Attalea Eichleri (Drude) A. J. Hend.) Entre Os Mebêngôkre-Kayapó Da Terra Indígena Las Casas, Estado Do Pará, Brasil, Acta Bot. Bras., 2012, 26, 295–308 CrossRef
.
- M. De Fátima Agra, P. F. De Freitas and J. M. Barbosa-Filho, Synopsis of the Plants Known as Medicinal and Poisonous in Northeast of Brazil, Rev. Bras. Farmacogn., 2007, 17, 114–140 CrossRef
.
- D. M. Fernandes, W. S. Barbosa, W. S. P. Rangel, I. M. M. Valle, A. Paula, D. S. Matos, F. G. Melgaço, M. L. Dias, E. Ricci-Júnior, L. C. P. da Silva, L. C. L. de Abreu and M. S. d. S. B. Monteiro, Polymeric Membrane Based on Polyactic Acid and Babassu Oil for Wound Healing, Mater. Today Commun., 2021, 26, 102173 CrossRef CAS
.
- M. Y. F. A. Reis, S. M. dos Santos, D. R. Silva, M. V. Silva, M. T. S. Correia, D. M. A. F. Navarro, G. K. N. Santos, F. Hallwass, O. Bianchi, A. G. Silva, J. V. Melo, A. B. Mattos, R. M. Ximenes, G. Machado and K. L. A. Saraiva, Anti-Inflammatory Activity of Babassu Oil and Development of a Microemulsion System for Topical Delivery, Evidence-Based Complementary Altern. Med., 2017, 2017, 1–14 CrossRef PubMed
.
- A. Honorio-França, R. Pessoa, E. França, E. Ribeiro, P. Lanes, N. Chaud and L. Moraes, Microemulsion of Babassu Oil as a Natural Product to Improve Human Immune System Function, Drug Des., Dev. Ther., 2014, 215, 21–31 CrossRef PubMed
.
- L. C. Paixão, E. R. C. Borba, I. C. Sousa, A. K. D. Barros Filho, D. F. Coutinho, L. M. Costa-Junior, M. S. S. Cartagenes, M. O. D. R. Borges, R. M. Ribeiro, I. C. Abreu, A. C. R. Borges and F. S. Monteiro, Aplicações Farmacêutica Bioprodutos Do Babaçu, Rev. Cent. Cienc. Saude, 2021, 21, 35–44 Search PubMed
.
- J. A. A. Santos, J. W. da Silva, S. M. dos Santos, M. D. F. Rodrigues, C. J. A. Silva, M. V. da Silva, M. T. S. Correia, J. F. C. Albuquerque, C. M. L. Melo, T. G. Silva, R. D. Martins, F. C. A. Aguiar-Júnior and R. M. Ximenes, In Vitro and In Vivo Wound Healing and Anti-Inflammatory Activities of Babassu Oil (Attalea Speciosa Mart. Ex Spreng., Arecaceae), Evidence-Based Complementary Altern. Med., 2020, 2020, 1–10 CrossRef PubMed
.
- E. S. B. Barroqueiro, D. S. Prado, P. S. Barcellos, T. A. Silva, W. S. Pereira, L. A. Silva, M. C. G. Maciel, R. B. Barroqueiro, F. R. F. Nascimento, A. G. Gonçalves and R. N. M. Guerra, Immunomodulatory and Antimicrobial Activity of Babassu Mesocarp Improves the Survival in Lethal Sepsis, Evidence-Based Complementary Altern. Med., 2016, 2016, 6–13 Search PubMed
.
- M. C. P. da Silva, J. M. Brito, A. D. S. Ferreira, A. A. M. Vale, A. P. A. dos Santos, L. A. Silva, P. V. S. Pereira, F. R. F. Nascimento, R. Nicolete and R. N. M. Guerra, Antileishmanial and Immunomodulatory Effect of Babassu-Loaded PLGA Microparticles: A Useful Drug Target to Leishmania Amazonensis Infection, Evidence-Based Complementary Altern. Med., 2018, 2018, 1–14 Search PubMed
.
- C. S. C. De Almeida, J. M. F. Godinho-Júnior, T. S. F. Cunha, F. R. F. do Nascimento and A. P. A. dos Santos, Antitumor Immune Response by Mcf7 Tumor Cells Pretreated with Babassu Mesocarp, Cad. Pesqui., 2014, 21, 30–39 CrossRef
.
- R. N. Guerra, V. M. Silva, L. S. Aragão-França, P. R. Oliveira, R. Feitosa, F. R. Nascimento and L. C. Pontes-de-Carvalho, Babassu Aqueous Extract (BAE) as an Adjuvant for T Helper (Th)1-Dependent Immune Responses in Mice of a Th2 Immune Response-Prone Strain, BMC Immunol., 2011, 12, 13 CrossRef CAS
.
- A. S. Leal, R. de Araújo, G. R. Souza, G. L. N. Lopes, S. T. Pereira, M. Muálem, M. M. M. Alves, H. M. Barreto, A. L. M. Carvalho, P. M. P. Ferreira, D. Silva, F. A. A. Carvalho, J. A. D. Lopes and L. C. C. Nunes, In Vitro Bioactivity and Cytotoxicity of Films Based on Mesocarp of Orbignya Sp. and Carboxymethylcellulose as a Tannic Acid Release Matrix, Carbohydr. Polym., 2018, 201, 113–121 CrossRef
.
- D. S. Santos, L. D. de Moura, M. A. Radicchi, R. B. Azevedo, M. C. P. Costa, P. E. N. de Souza, A. L. Camara, J. R. da Silva, L. A. Muehlmann and J. P. F. Longo, Nanoemulsion Improves Babassu Palm Oil (Orbignya Phalerata) Antioxidant Properties, Braz. Arch. Biol. Technol., 2021, 64, e21190387, DOI:10.1590/1678-4324-2021190387
.
- A. P. D. S. Silva, G. S. Cerqueira, L. C. C. Nunes and R. M. de Freitas, Effects of an Aqueous Extract of Orbignya Phalerata Mart on Locomotor Activity and Motor Coordination in Mice and as Antioxidant in Vitro, Pharmazie, 2012, 67, 260–263 Search PubMed
.
- B. C. Maniglia, L. Tessaro, A. P. Ramos and D. R. Tapia-Blácido, Which Plasticizer Is Suitable for Films Based on Babassu Starch Isolated by Different Methods?, Food Hydrocolloids, 2019, 89, 143–152 CrossRef CAS
.
- B. C. Maniglia and D. R. Tapia-Blácido, Isolation and Characterization of Starch from Babassu Mesocarp, Food Hydrocolloids, 2016, 55, 47–55 CrossRef CAS
.
- P. Hovorková, K. Laloučková and E. Skřivanová, Determination of in Vitro Antibacterial Activity of Plant Oils Containing Medium-Chain Fatty Acids against Gram-Positive Pathogenic and Gut Commensal Bacteria, Czech J. Anim. Sci., 2018, 63, 119–125 CrossRef
.
- J. Machado, M. do Socorro Costa, S. Tintino, F. Rodrigues, C. Nobre, H. Coutinho, J. da Costa, I. de Menezes and E. de Sousa, Antibiotic Activity Potentiation and Physicochemical Characterization of the Fixed Orbignya Speciosa Almond Oil against MDR Staphylococcus Aureus and Other Bacteria, Antibiotics, 2019, 8, 28 CrossRef CAS PubMed
.
- A. I. T. De Oliveira, T. S. Mahmoud, G. N. L. D. Nascimento, J. F. M. Da Silva, R. S. Pimenta and P. B. De Morais, Chemical Composition and Antimicrobial Potential of Palm Leaf Extracts from Babaçu (Attalea Speciosa), Buriti (Mauritia Flexuosa), and Macaúba (Acrocomia Aculeata), Sci. World J., 2016, 2016, 1–6 Search PubMed
.
- F. R. F. Nascimento, E. S. B. Barroqueiro, A. P. S. Azevedo, A. S. Lopes, S. C. P. Ferreira, L. A. Silva, M. C. G. Maciel, D. Rodriguez and R. N. M. Guerra, Macrophage Activation Induced by Orbignya Phalerata Mart., J. Ethnopharmacol., 2006, 103, 53–58 CrossRef PubMed
.
- M. N. Rennó, G. M. Barbosa, P. Zancan, V. F. Veiga, C. S. Alviano, M. Sola-Penna, F. S. Menezes and C. Holandino, Crude Ethanol Extract from Babassu (Orbignya Speciosa): Cytotoxicity on Tumoral and Non-Tumoral Cell Lines, An. Acad. Bras. Cienc., 2008, 80, 467–476 CrossRef PubMed
.
- A. P. S. Azevedo, J. C. Farias, G. C. Costa, S. C. P. Ferreira, W. C. Aragão-Filho, P. R. A. Sousa, M. T. Pinheiro, M. C. G. Maciel, L. A. Silva, A. S. Lopes, E. S. B. Barroqueiro, M. O. R. Borges, R. N. M. Guerra and F. R. F. Nascimento, Anti-Thrombotic Effect of Chronic Oral Treatment with Orbignya Phalerata Mart., J. Ethnopharmacol., 2007, 111, 155–159 CrossRef PubMed
.
- M. C. R. Soares, M. C. P. Silva, F. A. S. Almeida-Junior, J. R. Nascimento, F. R. F. Nascimento and R. N. M. Guerra, Effect of Babassu Mesocarp As a Food Supplement During Resistance Training, J. Med. Food, 2021, 24(4), 411–421 CrossRef CAS PubMed
.
- L. S. de Araujo, G. B. S. da Silva, F. E. Torresan, D. D. C. Victoria, L. E. Vicente, E. L. Bolfe and C. V. Manzatto, https://ainfo.cnptia.embrapa.br/digital/bitstream/item/159940/1/Serie-Documentos-108-Luciana.pdf, 2016, 1–29.
- M. Cardoso Vieira, R. Pischke Garske, P. de Souza Rocha, L. da Fontoura Xavier Costa, A. R. Nunes Paiva and R. C. S. Thys, Babassu Mesocarp Flour: A Nutritive Brazilian By-Product for Gluten-Free Muffins, J. Culin. Sci. Technol., 2023, 21, 517–532 CrossRef
.
- J. S. da Silva, M. L. dos Santos, E. C. da Silva Filho, M. D. G. F. D. M. Carvalho and L. C. C. Nunes, Subprodutos Do Babaçu (Orbignya Sp) Como Novos Materiais Adsortivos: Uma Revisão, Matéria, 2019, 24(3), e-12415, DOI:10.1590/s1517-707620190003.0730
.
- E. Melo, F. Michels, D. Arakaki, N. Lima, D. Gonçalves, L. Cavalheiro, L. Oliveira, A. Caires, P. Hiane and V. Nascimento, First Study on the Oxidative Stability and Elemental Analysis of Babassu (Attalea Speciosa) Edible Oil Produced in Brazil Using a Domestic Extraction Machine, Molecules, 2019, 24, 4235 CrossRef CAS PubMed
.
- T. de Paula Protásio, P. Fernando Trugilho, A. A. da Silva César, A. Napoli, I. C. N. Alves de Melo and M. Gomes da Silva, Babassu Nut Residues: Potential for Bioenergy Use in the North and Northeast of Brazil, SpringerPlus, 2014, 3, 124 CrossRef PubMed
.
- R. S. Lima, A. P. A. Carvalho and C. A. Conte-Junior, Health from Brazilian Amazon Food Wastes: Bioactive Compounds, Antioxidants, Antimicrobials, and Potentials against Cancer and Oral Diseases, Crit. Rev. Food Sci. Nutr., 2022, 63(33), 12453–12475, DOI:10.1080/10408398.2022.2101983
.
- A. P. A. de Carvalho and C. A. Conte-Junior, Health Benefits of Phytochemicals from Brazilian Native Foods and Plants: Antioxidant, Antimicrobial, Anti-Cancer, and Risk Factors of Metabolic/Endocrine Disorders Control, Trends Food Sci. Technol., 2021, 111, 534–548 CrossRef
.
- R. C. Lima, A. P. A. de Carvalho, B. D. da Silva, L. Torres Neto, M. R. D. S. de Figueiredo, P. H. T. Chaves, A. E. C. C. de Almeida and C. A. Conte-Junior, Green Ultrasound-Assisted Extraction of Bioactive Compounds of Babassu (Attalea Speciosa) Mesocarp: Effects of Solid-Liquid Ratio Extraction, Antioxidant Capacity, and Antimicrobial Activity, Appl. Food Res., 2023, 3, 100331 CrossRef CAS
.
- R. C. Lima, A. P. A. de Carvalho, C. A. Lelis, D. J. Faria, B. D. da Silva, M. R. da Silva de Figueiredo, P. H. T. Chaves, A. E. C. C. de Almeida and C. A. Conte-Junior, An innovative alternative to reduce sodium in cheese: Babassu coconut byproduct improving quality and shelf-life of reduced-sodium Minas fresh cheese, Innovative Food Sci. Emerging Technol., 2024, 92, 103601 CrossRef CAS
.
- J. L. Serra, A. M. D. C. Rodrigues, R. A. de Freitas, A. J. D. A. Meirelles, S. H. Darnet and L. H. M. da Silva, Alternative Sources of Oils and Fats from Amazonian Plants: Fatty Acids, Methyl Tocols, Total Carotenoids and Chemical Composition, Food Res. Int., 2019, 116, 12–19 CrossRef CAS PubMed
.
- R. A. Ferrari and M. P. Soler, Obtention and Characterization of Coconut Babassu Derivatives, Sci. Agric., 2015, 72, 291–296 CrossRef
.
- G. B. de Farias, J. L. da Silva Rodrigues, M. N. de Sousa Ribeiro, L. O. Magalhães, A. G. Ferreira and M. da Paz Lima, Chemical Constituent Analysis of the Babassu (Orbignya Phalerata Mart.) Mesocarp, Univ. Sci., 2019, 24, 323–335 CrossRef CAS
.
- WHO and FAO Joint Consultation, Fats and Oils in Human Nutrition, Nutr. Rev., 1995, 53(7), 202–205, DOI:10.1111/j.1753.4887.1995.tb01552.x
.
- A. P. Simopoulos, The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic Diseases, Exp. Biol. Med., 2008, 233, 674–688 CrossRef CAS PubMed
.
- M. M. G. Pinheiro, F. Boylan and P. D. Fernandes, Antinociceptive Effect of the Orbignya Speciosa Mart. (Babassu) Leaves: Evidence for the Involvement of Apigenin, Life Sci., 2012, 91, 293–300 CrossRef CAS PubMed
.
- V. C. Silva, J. R. Barboza, R. P. Dutra, V. C. da Silva, J. R. Barboza, R. P. Dutra, M. C. A. Batista, K. S. Veras, J. W. Godinho, J. W. C. de Mesquita, L. S. S. de Mesquita, F. M. M. do Amaral and M. N. S. Ribeiro, Identification of Phenolic Compounds by LC/MS-MS and Antioxidant and Anti Tyrosinase Activities of the Attalea Speciosa Mart. Ex Spreng. Mesocarp, J. Chem. Pharm. Res., 2017, 9, 268–276 Search PubMed
.
- C. B. Nobre, E. O. Sousa, C. J. Camilo, J. F. Machado, J. M. F. L. Silva, J. R. Filho, H. D. M. Coutinho and J. G. M. Costa, he Fruits of “Babaçu” (Orbignia Speciose) and “Buriti” (Mauritia Flexuosa), Food Chem. Toxicol., 2018, 121, 423–429 CrossRef CAS PubMed
.
- H. Sies, Oxidative Stress: Concept and Some Practical Aspects, Antioxidants, 2020, 9, 1–6 CrossRef PubMed
.
- K. Daenen, A. Andries, D. Mekahli, A. Van Schepdael, F. Jouret and B. Bammens, Oxidative Stress in Chronic Kidney Disease, Pediatr. Nephrol., 2019, 34, 975–991 CrossRef PubMed
.
- A. J. Kattoor, N. V. K. Pothineni, D. Palagiri and J. L. Mehta, Oxidative Stress in Atherosclerosis, Curr. Atheroscler. Rep., 2017, 19, 42, DOI:10.1007/s11883-017-0678-6
.
- M. T. Islam, Oxidative Stress and Mitochondrial Dysfunction-Linked Neurodegenerative Disorders, Neurol. Res., 2017, 39, 73–82 CrossRef CAS PubMed
.
- S. A. Petropoulos, F. Di Gioia, N. Polyzos and N. Tzortzakis, Natural Antioxidants, Health Effects and Bioactive Properties of Wild Allium Species, Curr. Pharm. Des., 2020, 26, 1816–1837 CrossRef CAS PubMed
.
- V. C. Da Silva, J. R. Barboza, R. P. Dutra, M. Cristina, A. Batista, K. S. Veras, J. W. Godinho, W. C. De Mesquita, L. Santos, S. De Mesquita, M. M. do Amaral, M. Nilce and S. Ribeiro, Identification of Phenolic Compounds by LC/MS-MS and Antioxidant and Anti Tyrosinase Activities of the Attalea Speciosa Mart. Ex Spreng. Mesocarp, J. Chem. Pharm. Res., 2017, 2017, 268–276 Search PubMed
.
- W. H. Organization, Antimicrobial resistance: global report on surveillance, World Health Organization, 2014, https://www.who.int/publications/i/item/9789241564748, (Accessed April 4, 2024).
- B. R. Ramadoss, M. P. Gangola, S. Agasimani, S. Jaiswal, T. Venkatesan, G. R. Sundaram and R. N. Chibbar, Starch Granule Size and Amylopectin Chain Length Influence Starch in Vitro Enzymatic Digestibility in Selected Rice Mutants with Similar Amylose Concentration, J. Food Sci. Technol., 2019, 56, 391–400 CrossRef CAS PubMed
.
- N. Caetano, A. Saraiva, R. Pereira, D. Carvalho, M. C. B. Pimentel and M. B. S. Maia, Determinação de Atividade Antimicrobiana de Extratos de Plantas de Uso Popular Como Antiflamatório, Rev. Bras. Farmacogn., 2002, 12, 132–135 CrossRef
.
- A. Matsuda, A. Jacob, R. Wu, M. Aziz, W.-L. Yang, T. Matsutani, H. Suzuki, K. Furukawa, E. Uchida and P. Wang, Novel Therapeutic Targets for Sepsis: Regulation of Exaggerated Inflammatory Responses, J. Nippon Med. Sch., 2012, 79, 4–18 CrossRef CAS PubMed
.
- G. Dong, H. Liu, X. Yu, X. Zhang, H. Lu, T. Zhou and J. Cao, Antimicrobial and Anti-Biofilm Activity of Tannic Acid against, Staphylococcus Aureus, Nat. Prod. Res., 2018, 32, 2225–2228 CrossRef CAS PubMed
.
- O. M. Bondarenko, M. Sihtmäe, J. Kuzmičiova, L. Ragelienė, A. Kahru and R. Daugelavičius, Plasma Membrane Is the Target of Rapid Antibacterial Action of Silver Nanoparticles in Escherichia Coli and Pseudomonas Aeruginosa, Int. J. Nanomed., 2018, 13, 6779–6790 CrossRef CAS PubMed
.
- R. W. Schwenk, G. P. Holloway, J. J. F. P. Luiken, A. Bonen and J. F. C. Glatz, Fatty Acid Transport across the Cell Membrane: Regulation by Fatty Acid Transporters, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2010, 82, 149–154 CrossRef CAS PubMed
.
- C. Q. Sun, C. J. O'Connor, S. J. Turner, G. D. Lewis, R. A. Stanley and A. M. Roberton, The Effect of PH on the Inhibition of Bacterial Growth by Physiological Concentrations of Butyric Acid: Implications for Neonates Fed on Suckled Milk, Chem.-Biol. Interact., 1998, 113, 117–131 CrossRef CAS PubMed
.
- A. P. Desbois and V. J. Smith, Antibacterial Free Fatty Acids: Activities, Mechanisms of Action and Biotechnological Potential, Appl. Microbiol. Biotechnol., 2010, 85, 1629–1642 CrossRef CAS PubMed
.
- J.-Y. Lee, Y.-S. Kim and D.-H. Shin, Antimicrobial Synergistic Effect of Linolenic Acid and Monoglyceride against Bacillus Cereus and Staphylococcus Aureus, J. Agric. Food Chem., 2002, 50, 2193–2199 CrossRef CAS PubMed
.
- B. C. L. Chan, X. Q. Han, S. L. Lui, C. W. Wong, T. B. Y. Wang, D. W. S. Cheung, S. W. Cheng, M. Ip, S. Q. B. Han, X.-S. Yang, C. Jolivalt, C. B. S. Lau, P. C. Leung and K. P. Fung, J. Pharm. Pharmacol., 2014, 67, 107–116 CrossRef PubMed
.
- B. P. da Silva and J. P. Parente, An Anti-Inflammatory and Immunomodulatory Polysaccharide from Orbignya Phalerata, Fitoterapia, 2001, 72, 887–893 CrossRef CAS PubMed
.
- P. A. V. R. De Souza, A. Palumbo, L. M. Alves, V. P. De Souza, L. M. Cabral, P. D. Fernandes, C. M. Takiya, F. S. Menezes and L. E. Nasciutti, Effects of a Nanocomposite Containing Orbignya Speciosa Lipophilic Extract on Benign Prostatic Hyperplasia, J. Ethnopharmacol., 2011, 135, 135–146 CrossRef PubMed
.
- E. Amorim, J. E. F. Matias, J. C. U. Coelho, A. C. L. Campos, H. J. Stahlke Jr, J. R. R. Timi, L. C. D. A. Rocha, A. T. R. Moreira, D. Z. Rispoli and L. M. Ferreira, Efeito do Uso Tópico do Extrato Aquoso de Orbignya Phalerata (Babaçu) na Cicatrização de Feridas Cutâneas: Estudo Controlado em Ratos, Acta Cir. Bras., 2006, 21, 67–76 CrossRef PubMed
.
- R. Etienne, F. P. D. Viegas and C. Viegas Jr, Pathophysiological Aspects of Inflammation and Drug Design: An Updated Overview, Rev. Virtual Quim., 2021, 13, 167–191 CrossRef CAS
.
- P. C. Calder, N. Ahluwalia, R. Albers, N. Bosco, R. Bourdet-Sicard, D. Haller, S. T. Holgate, L. S. Jönsson, M. E. Latulippe, A. Marcos, J. Moreines, C. M'Rini, M. Müller, G. Pawelec, R. J. J. van Neerven, B. Watzl and J. Zhao, Consideration of Biomarkers to Be Used for Evaluation of Inflammation in Human Nutritional Studies, Br. J. Nutr., 2013, 109, S1–S34 CrossRef PubMed
.
- M. Rogero and P. Calder, Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids, Nutrients, 2018, 10, 432 CrossRef PubMed
.
- A. Azzi, E. Aratri, D. Boscoboinik, S. Clément, N. K. Özer, R. Ricciarelli and S. Spycher, Molecular Basis of A–tocopherol Control of Smooth Muscle Cell Proliferation, BioFactors, 1998, 7, 3–14 CrossRef CAS PubMed
.
- M. D. C. L. Barbosa, E. Bouskela, F. Z. Cyrino, A. P. S. Azevedo, M. C. P. Costa, M. D. G. C. de Souza, D. S. Santos, F. L. Barbosa, L. F. A. Guerra and M. D. D. S. Nascimento, Effects of Babassu Nut Oil on Ischemia/Reperfusion-Induced Leukocyte Adhesion and Macromolecular Leakage in the Microcirculation: Observation in the Hamster Cheek Pouch, Lipids Health Dis., 2012, 11, 158 CrossRef CAS PubMed
.
- Y. H. Shin, S.-H. Yoon, E.-Y. Choe, S.-H. Cho, C.-H. Woo, J.-Y. Rho and J.-H. Kim, Effects of Babassu Nut Oil on Ischemia/Reperfusion-Induced Leukocyte Adhesion and Macromolecular Leakage in the Microcirculation: Observation in the Hamster Cheek Pouch, Exp. Mol. Med., 2007, 39, 97–105 CrossRef CAS PubMed
.
- R. Medeiros, M. F. Otuki, M. C. W. Avellar and J. B. Calixto, Mechanisms Underlying the Inhibitory Actions of the Pentacyclic Triterpene α-Amyrin in the Mouse Skin Inflammation Induced by Phorbol Ester 12-O-Tetradecanoylphorbol-13-Acetate, Eur. J. Pharmacol., 2007, 559, 227–235 CrossRef CAS PubMed
.
- C. L. Scheibe, J. M. Ribas-Filho, N. G. Czeczko, O. Malafaia, L. E. D. Barboza, F. M. Ribas, E. Wendler, O. Torres, F. C. Lovato and J. G. S. Scapini, Schinus Terebinthifolius Raddi (Aroeira) and Orbignya Phalerata Mart. (Babassu) Effect in Cecorrahphy Healing in Rats, Acta Cir. Bras., 2016, 31, 402–410 CrossRef PubMed
.
- R. N. Baldez, O. Malafaia, N. G. Czeczko, N. L. P. Martins, L. M. Ferreira, C. A. P. M. Ribas, G. Salles, R. P. Del Claro, L. D. O. M. Dos Santos, L. G. Neto and L. R. R. De Araújo, Healing of Colonic Anastomosis with the Use of Extract Aqueous of Orbignya Phalerata (Babassu) in Rats, Acta Cir. Bras., 2006, 21, 29–36 CrossRef PubMed
.
- C. P. Batista, O. J. M. Torres, J. E. F. Matias, A. T. R. Moreira, D. Colman, J. H. F. de Lima, M. M. Macri, R. J. Rauen Jr, L. M. Ferreira and A. C. T. de Freitas, Efeito Do Extrato Aquoso de Orbignya Phalerata (Babaçu) Na Cicatrização Do Estômago Em Ratos: Estudo Morfológico e Tensiométrico, Acta Cir. Bras., 2006, 21, 26–32 CrossRef PubMed
.
- S. B. De Brito Filho, J. E. F. Matias, H. J. Stahlke, O. J. M. Torres, J. R. R. Timi, S. B. Tenório, E. M. Tâmbara, Â.G Carstens, R. V. Campos and M. Myamoto, Análise Da Cicatrização Na Linha Alba Com Uso de Extrato Aquoso de Orbignya Phalerata (Babaçu). Estudo Controlado Em Ratos, Acta Cir. Bras., 2006, 21, 76–88 CrossRef
.
- E. C. Ferreira, J. E. F. Matias, A. C. L. Campos, R. Tâmbara-Filho, L. C. A. Rocha, J. R. R. Timi, H. N. Sado, D. G. Sakamoto, A. R. D. Tolazzi and M. P. Soares Filho, Análise Da Cicatrização Da Bexiga Com o Uso Do Extrato Aquoso Da Orbignya Phalerata (Babaçu): Estudo Controlado Em Ratos, Acta Cir. Bras., 2006, 21, 33–39 CrossRef PubMed
.
- N. L. P. Martins, O. Malafaia, J. M. Ribas-Filho, M. Heibel, R. N. Baldez, P. R. L. de Vasconcelos, H. Moreira, M. Mazza, P. A. N. Nassif and T. Z. Wallbach, Análise Comparativa Da Cicatrização Da Pele Com o Uso Intraperitoneal de Extrato Aquoso de Orbignya Phalerata (Babaçu), Acta Cir. Bras., 2006, 21, 66–75 CrossRef PubMed
.
- C. E. S. Silva, O. J. dos Santos, J. M. Ribas-Filho, F. I. Tabushi, M. H. Kume, L. B. Jukonis and I. F. Cella, Effect of Carapa Guianensis Aublet (Andiroba) and Orbignya Phalerata (Babassu) in Colonic Healing in Rats, Rev. Col. Bras. Cir., 2015, 42, 399–406 CrossRef PubMed
.
- O. J. M. Torres, O. J. dos Santos, R. S. de Moura, H. O. Serra, V. P. Ramos, S. P. D. C. Melo and C. M. B. Loureiro, Activity of Orbignya Phalerata and Euterpe Edules in the Prevention and Treatment of Peptic Ulcer in Rats, Arq. Bras. Cir. Dig., 2018, 31, 3–6 Search PubMed
.
- V. C. Gumiero and P. A. da Rocha Filho, Babassu Nanoemulsions Have Physical and Chemical Stability, J. Dispers. Sci. Technol., 2012, 33, 1569–1573 CrossRef CAS
.
- J. F. Coelho, P. C. Ferreira, P. Alves, R. Cordeiro, A. C. Fonseca, J. R. Góis and M. H. Gil, Drug Delivery Systems: Advanced Technologies Potentially Applicable in Personalized Treatments, EPMA J., 2010, 1, 164–209 CrossRef PubMed
.
- World Health Organization, Leishmaniasis, https://apps.who.int/neglected_diseases/ntddata/leishmaniasis/leishmaniasis.html, (Accessed August 4, 2023).
- J. Brindha, M. M. Balamurali and K. Chanda, An Overview on the Therapeutics of Neglected Infectious Diseases—Leishmaniasis and Chagas Diseases, Front. Chem., 2021, 12, 9, 622286 Search PubMed
.
- M. C. R. Soares, M. C. P. Silva, F. A. S. Almeida-Junior, J. R. Nascimento, F. R. F. Nascimento and R. N. M. Guerra, Effect of Babassu Mesocarp As a Food Supplement During Resistance Training, J. Med. Food, 2021, 24(4), 411–421 CrossRef CAS PubMed
.
- M. M. G. Pinheiro, F. Boylan and P. D. Fernandes, Antinociceptive Effect of the Orbignya Speciosa Mart. (Babassu) Leaves: Evidence for the Involvement of Apigenin, Life Sci., 2012, 91, 293–300 CrossRef CAS PubMed
.
- C. Boutari and C. S. Mantzoros, A 2022 Update on the Epidemiology of Obesity and a Call to Action: As Its Twin COVID-19 Pandemic Appears to Be Receding, the Obesity and Dysmetabolism Pandemic Continues to Rage On, Metabolism, 2022, 133, 155217 CrossRef CAS PubMed
.
- R. O. for Europe, World Health Organization, WHO European Regional Obesity Report 2022, World Health Organization. Regional Office for Europe, 2022, https://www.who.int/europe/publications/i/item/9789289057738, (Accessed April 4, 2024).
- T. M. Powell-Wiley, P. Poirier, L. E. Burke, J.-P. Després, P. Gordon-Larsen, C. J. Lavie, S. A. Lear, C. E. Ndumele, I. J. Neeland, P. Sanders and M.-P. St-Onge, Obesity and Cardiovascular Disease: A Scientific Statement From the American Heart Association, Circulation, 2021, 143, e984–e1010, DOI:10.1161/CIR.0000000000000973
.
- D. D. Meira, M. M. Marinho-Carvalho, C. A. Teixeira, V. F. Veiga, A. T. Da Poian, C. Holandino, M. S. de Freitas and M. Sola-Penna, Clotrimazole Decreases Human Breast Cancer Cells Viability through Alterations in Cytoskeleton-Associated Glycolytic Enzymes, Mol. Genet. Metab., 2005, 84, 354–362 CrossRef CAS PubMed
.
- I. Vermes, C. Haanen and C. Reutelingsperger, Flow Cytometry of Apoptotic Cell Death, J. Immunol. Methods, 2000, 243, 167–190 CrossRef CAS PubMed
.
- Centers for Disease Control and Prevention (CDC), Venous Thromboembolism (Blood Clots). What is Venous Thromboembolism? https://www.cdc.gov/ncbddd/dvt/facts.html, (Accessed April 4, 2024).
- J. E. Barbato and E. Tzeng, Nitric Oxide and Arterial Disease, J. Vasc. Surg., 2004, 40, 187–193 CrossRef PubMed
.
- H. Wang, C. Wang and M. Guo, Impact of Polymerized Whey Protein/Pectin Thickening (PP) System on Physical Properties and Volatile Compounds of Goat Milk Kefir Mild and Kefir, J. Food Sci., 2021, 86(3), 1014–1021 CrossRef CAS PubMed
.
- F. Violi, D. Pastori, P. Pignatelli and R. Carnevale, Nutrition, Thrombosis, and Cardiovascular Disease, Circ. Res., 2020, 126, 1415–1442 CrossRef CAS PubMed
.
- A. F. Aldairi, R. A. Alyamani, A. Al-Hazmi, I. F. Halawani, A. A. Alsubaihi, S. Idris, N. A. Fallatah, A. Gassas, A. A. Almalki, A. Qasem, M. M. Ghaith, H. Almasmoum, A. A. Alghamdi, M. Allahyani, R. A. Almaimani, H. Banni and M. Alkhanabashi, Antioxidant and Antithrombotic Effects of Green Mussels (Perna Canaliculus) in Rats, J. Food Biochem., 2021, 45(9), e13865 CrossRef CAS PubMed
.
- A. I. Lopes, J. Santos-Jr, D. C. Da Silva, L. J. S. Da Silva, A. K. Barros, H. A. Villa-Vélez and A. A. Santana, Characterization of Pectin Biofilms with the Addition of Babassu Mesocarp and Whey Protein Concentrate, Am. J. Mater. Sci., 2017, 2017, 64–70 Search PubMed
.
- The Future of Plastic, Nat. Commun., 2018, 9(2157), 1–3, DOI:10.1038/s41467-018-04565-2
https://www.nature.com/articles/s41467-018-04565-2#citeas .
- C. J. Rhodes, Plastic Pollution and Potential Solutions, Sci. Prog., 2018, 101, 207–260 CrossRef PubMed
.
- World Economic Forum, The New Plastics Economy—Rethinking the future of plastics, 2016, https://www.weforum.org/publications/the-new-plastics-economy-rethinking-the-future-of-plastics/, (Accessed April 4, 2024).
- A. P. A. de Carvalho and C. A. Conte-Junior, Health Benefits of Phytochemicals from Brazilian Native Foods and Plants: Antioxidant, Antimicrobial, Anti-Cancer, and Risk Factors of Metabolic/Endocrine Disorders Control, Trends Food Sci. Technol., 2021, 111, 534–548 CrossRef
.
- A. P. A. de Carvalho and C. A. Conte-Junior, Food-Derived Biopolymer Kefiran Composites, Nanocomposites and Nanofibers: Emerging Alternatives to Food Packaging and Potentials in Nanomedicine, Trends Food Sci. Technol., 2021, 116, 370–386 CrossRef
.
- I. R. S. Vieira, A. P. A. de Carvalho and C. A. Conte-Junior, Recent Advances in Biobased and Biodegradable Polymer Nanocomposites, Nanoparticles, and Natural Antioxidants for Antibacterial and Antioxidant Food Packaging Applications, Compr. Rev. Food Sci. Food Saf., 2022, 21, 3673–3716 CrossRef CAS PubMed
.
- D. C. Da Silva, I. A. Lopes, L. J. S. Da Silva, M. F. Lima, A. K. D. Barros-Filho, H. A. Villa-Vélez and A. A. Santana, Physical Properties of Films Based on Pectin and Babassu Coconut Mesocarp, Int. J. Biol. Macromol., 2019, 130, 419–428 CrossRef CAS PubMed
.
- E. S. B. Barroqueiro, F. S. B. Barroqueiro, M. T. Pinheiro, M. C. G. Maciel, P. S. Barcellos, L. A. Silva, A. S. Lopes, F. R. F. Nascimento and R. N. M. Guerra, Evaluation of Acute Toxicity of Babassu Mesocarp in Mice, Rev. Bras. Farmacogn., 2011, 21, 710–714 CrossRef
.
| This journal is © The Royal Society of Chemistry 2024 |