Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Milk fat globule membrane and its polar lipids: reviewing preclinical and clinical trials on cognition
Álvaro Luque-Uría a,
María V. Calvo
a,
María V. Calvo a,
Francesco Visioli
a,
Francesco Visioli bc and
Javier Fontecha
bc and
Javier Fontecha *a
*a
aFood Lipid Biomarkers and Health Group, Institute of Food Science Research (CIAL, CSIC-UAM), Madrid 28049, Spain. E-mail: j.fontecha@csic.es; alvaro.l.u@csic.es; mv.calvo@csic.es
bDepartment of Molecular Medicine, University of Padova, 35121 Padova, Italy. E-mail: francesco.visioli@unipd.it
cIMDEA-Food, Madrid 28049, Spain
First published on 27th May 2024
Abstract
In most parts of the world, life expectancy is increasing thanks to improved healthcare, public health policies, nutrition, and treatment. This increase in lifespan is often not accompanied by an increase in health span, which severely affects people as they age. One notable consequence of this is the increasing prevalence of neurodegenerative diseases such as mild cognitive impairment, dementia, and Alzheimer's disease. Therefore, dietary and pharmaceutical measures must be taken to reduce the burden of such pathologies. Among the different types of nutrients found in the diet, lipids and especially polar lipids are very important for cognition due to their abundance in the brain. Amid the most studied sources of polar lipids, milk fat globule membrane (MFGM) stands out as it is abundant in industrial by-products such as buttermilk. In this narrative review, we discuss the latest, i.e. less than five years old, scientific evidence on the use of MFGM and its polar lipids in cognitive neurodevelopment in early life and their potential effect in preventing neurodegeneration in old age. We conclude that MFGM is an interesting, abundant and exploitable source of relatively inexpensive bioactive molecules that could be properly formulated and utilized in the areas of neurodevelopment and cognitive decline. Sufficiently large randomized controlled trials are required before health-related statements can be made. However, research in this area is progressing rapidly and the evidence gathered points to biological, health-promoting effects.
1 Introduction
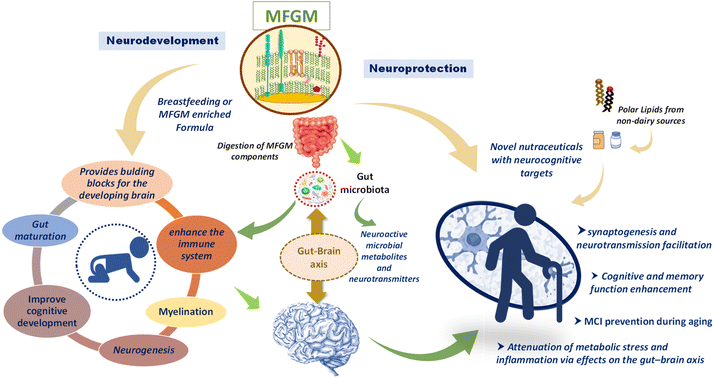
Lifespan is increasing in most of the world, thanks to improved health care, public health policies, nutrition, and treatments.1 This fact is often not accompanied by an increase in health span, greatly affecting aged individuals.2 As the elderly represent a larger portion of the population in many developed countries, some age-related health diseases are becoming more ubiquitous. One notable example are neurodegenerative illnesses such as mild cognitive impairment (MCI), which can be defined as a “cognitive decline greater than that expected for an individual's age and education level, but does not interfere notably with activities of daily life”.3 MCI can lead to more serious ailments such as dementia and Alzheimer's disease (AD).4 Therefore, rapid diagnosis and effective treatments are required to prevent further complications. Unfortunately, dementia and AD are still poorly etiologically understood and clinically treated. However, several studies do suggest that there are preventive and therapeutic ways to delay the onset of cognitive impairments or even alleviate them.5–8In this respect, milk fat globule membrane (MFGM) stands out as a potential nutraceutical, due to its high polar lipids content. MFGM is produced by the mammary epithelial cells and is structurally composed of a triple layer of phospholipids and cholesterol with incorporated proteins and glycoproteins. Even though the constituent/bioactive properties of MFGM and its presumed mode(s) of action are yet to be fully elucidated, most published research points to lipids, namely polar lipids as the most prominent components of MFGM. Because of their abundance in industrial by-products such as buttermilk or cheese whey, among others, the exploitation of MFGM by the supplement industry is gaining traction and some promising results have been published in the literature.9
It must be underscored that, in addition to providing energy, lipids are very important for cognition owing to their abundance in the brain10 and because they are precursors of chemical messengers.8,11 In particular, whereas non-polar lipids such as triacylglycerols and cholesterol esters mainly act as energy reservoirs, polar lipids have more biologically-relevant properties.11,12 Indeed, they are indispensable components of cell membranes, but they are also associated with brain functionality and development.11,13,14 The total lipid concentration in the brain accounts for more than 50% of its dry weight for infants and this percentage significantly increases as we grow older.15 Of note, the majority of lipids found in brain tissues are long-chain polyunsaturated fatty acids (LCPUFA) and phospholipids.16
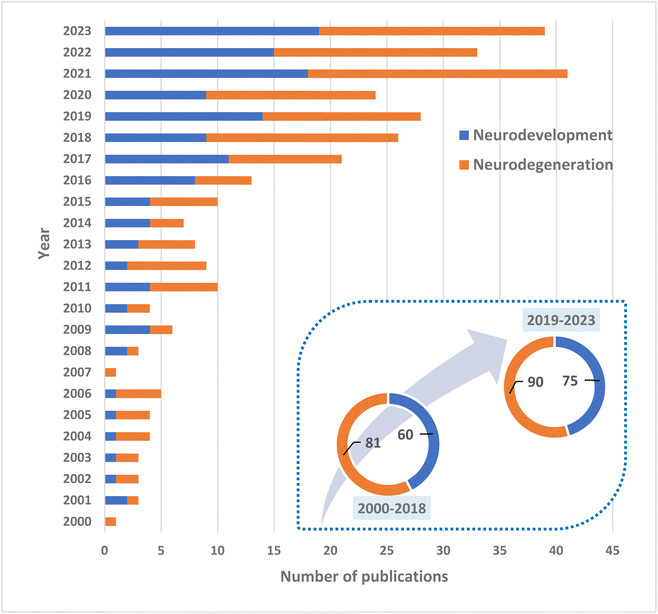
Therefore, much investigation is being devoted to polar lipids (whose proportion in MFGM is rather high) as potential pharma-nutritional agents that might promote the neurodevelopment in infants and lessen the burden of cognitive impairment during aging17,18 (Fig. 1).
In this regard, an important field of study that is being developing over the last few years concerns infant formulas composition as they affect the development and metabolism of children.19–22 Furthermore, the rationale that supplementing MCI patients with MFGM would provide bioactive lipids to lessen the burden of such disease, has been progressively gaining force.
In this narrative review we discuss the most recent scientific evidence on the use of MFGM and its polar lipids in the field of cognitive neurodevelopment in the early stages of life, as well as its possible action in the prevention of neurodegeneration during aging.
2 Methods
We searched Google Scholar, Science Direct and PubMed, from the year 2019 onwards to provide an update of previous reviews published on this topic.9,14,19–21 Only articles written in English were included in this review. The terms ‘polar lipids’, ‘phospholipids’, ‘sphingolipids’, ‘cognition’, ‘infant’, ‘MFGM’, ‘milk fat globule membrane’ ‘neurodegeneration’, ‘neurodevelopment’, ‘preclinical’, ‘clinical’, ‘trial’ were used alone or in combination.3 Results
3.1 MFGM as an available source of polar lipids
As mentioned, polar lipids can be conveniently rubricked as phospholipids and sphingolipids, and are often referred to as complex lipids.22,23 Phospholipids are composed of glycerol, fatty acids, and a polar head made of a phosphate group and typically an organic base linked to it. They are ubiquitous in all types of biological membranes, and they present an amphipathic behavior: hydrophilic, polar heads (phosphate group and organic bases), and hydrophobic tails (fatty acids).19 Phospholipids are crucial for many biological functions, and they play major roles in membrane permeability, fluidity, and deterioration, as well as in the interactions among other proteins.24 Among the most common phospholipids we can find phosphatidyl ethanolamine (PE), phosphatidyl serine (PS), phosphatidyl inositol (PI), and phosphatidyl choline (PC). Regarding sphingolipids, the most abundant is sphingomyelin (SM), which consists of a molecule of sphingosine linked to a fatty acid through an amide bond, also linked to a phosphocholine group.25 Sphingomyelin is a sphingolipid unique to animal cells and, similarly to phospholipids, it is present in most membranes. It is closely related to the uptake of iron inside the cell, and it is also believed that it is responsible for the distribution of cholesterol inside the cell.19,26Phospholipids and sphingolipids can be biosynthesized.27,28 However, they are also present in a wide variety of foods, of both animal and vegetal origin.29 Table 1 reports the most common food products where polar lipids can be found.
| Origin | Food | % Polar lipids (g per 100 g fat) | % (g per 100 g total polar lipids) | ||||
|---|---|---|---|---|---|---|---|
| % PE | % PC | % PS | % PI | % SM | |||
| Data obtained from references.19,29–33 | |||||||
| Vegetal | Soy beans | 11.1 | 26.2 | 44 | nd | 14 | nd |
| Vegetal | Peanuts | 1.27 | 8.1 | 43.5 | nd | 24.2 | nd |
| Animal | Egg yolk | 28–33 | 10–20 | 65–75 | nd | 0.5–2.0 | 2.0–5.0 |
| Animal | Beef | 14–18 | 20–30 | 58–65 | 2.0–4.0 | 5.0–7.0 | 5.0–7.0 |
| Animal | Chicken liver | 43–47 | 30–34 | 42–48 | 5.0–7.0 | nd | 10–12 |
| Animal | Pork liver | 73.5 | 21.3 | 58.2 | 1.3 | 7.2 | 4.5 |
| Animal | Beef brain | 76 | 35.8 | 24.1 | 16.0 | 4.5 | 17.4 |
| Animal | Squid | 64–67 | 8.0–12 | 70–75 | 6.0–8.0 | nd | 7.0–11 |
| Animal | Herring (dark muscle) | 33 | 26.5 | 53.6 | 13.9 | nd | 4.5 |
| Animal | Mackerel (dark muscle) | 38 | 38.4 | 22.7 | 9.8 | nd | 29.1 |
| Animal | Milk | 0.7–2.3 | 20–42 | 20–40 | 2–14 | 0.6–12 | 16–35 |
| Animal | Buttermilk | 4.5–35.3 | 17.0–44.8 | 17.3–46.0 | 8.0–18.0 | 2.4–17.3 | 12.1–21.5 |
| Animal | Cheese whey | 5.3–24 | 27.4–41.1 | 19.0–32.2 | 3.9–9.3 | 2.8–3.7 | 9.9–16.4 |
| Animal | Cream | 0.3–5.6 | 17.7–45.6 | 14.6–34 | 6.7–14.8 | 6.8–15.4 | 11.9–28.6 |
| Animal | Butter serum | 47–48.4 | 27–31 | 24.9–27 | 6.9–10 | 9.0–11.2 | 23.8–28.9 |
Cogent to this review, phospholipids are important constituents of larger structures such as milk fat globules (MFG). These MFG are made of a triglyceride core surrounded by the MFGM, which acts as a protective layer to prevent coalescing and lipolysis of triacylglycerols in the presence of enzymes or other molecules.9 The MFGM consist of a lipidic tri-layer made up of about 40% in weight of polar lipids, rendering a very rich source of phospholipids.34,35 MFGM can be found both in human and ruminant milks, with similar compositions and structures.36 The unique features of the MFGM (Fig. 2) and its possible role in neurodevelopment have been subject to numerous preclinical and clinical studies. In the following sections, the most recent preclinical and clinical studies that used dietary polar lipids for neurodevelopment or to alleviate neurodegeneration are reviewed.
3.2 Preclinical studies
As mentioned above, the use of MFGM as potential supplements is gaining great traction.9,37,38 One field of pharma-nutritional applications of MFGM is infant development, where an adequate nutrition is indispensable for a proper neurodevelopment of newborns and young infants.39 Table 2 summarizes the most recent preclinical studies on this subject, subdivided into rodents (Table 2A) and pigs (Table 2B). Over the last few years, a decent amount of publications was dedicated to preclinical studies with rodents, but only a few of them focused on pigs. A notable example of the effects of MFGM in neurodevelopment was provided by Brink et al.,40 who tested how MFGM affect the neurodevelopment of rat pups as compared with other supplements, i.e. a bovine polar lipid concentrate, different concentrations of sialic acid, and a non-fat milk control drink. The authors reported that T-maze scores of rats treated with MFGM were higher than those of pups supplemented with the other substances, although these effects were no longer significant long-term. One study was conducted by Fil et al.,41 in which pigs underwent magnetic resonance imaging and behavioral tests after pigs followed a diet supplemented with polar lipids or just a control diet. The results showed that a minimal difference was observed in terms of exploratory behavior but similar results were obtained when recognition memory was tested.| Objective | Methodology | Supplementation | Duration | Dose | Conclusions | Ref. |
|---|---|---|---|---|---|---|
| MS: maternal supplementation; IS: infant supplementation; bw: body weight; AIN: American institute of nutrition; PD: postnatal day; PW: postnatal week. | ||||||
| (A) Rodents | ||||||
| Examination of neurodevelopment and long-term programming effects on offspring after MFGM supplementation to obese dams | Rats (n = 24) were divided in control and high-fat diet group, which were supplemented with control or MFGM (n = 6 for each) | (MS) Lab-made polar lipid-enriched MFGM from raw milk MFGM mixtures supplied by Fonterra. | Pregnancy and lactation | Control diet was 10% energy from fat and high-fat diet was 60%. MFGM administered was 400 mg per kg bw during pregnancy and lactation | MFGM diet proved to be an efficient tool for offspring neurodevelopment against a high-fat diet induced cognitive impairment | 44 |
| Investigate whether MFGM supplementation in obese rats promotes neurodevelopment in offspring | Control rats (n = 12) or obese rats (n = 12) were supplemented with MFGM (n = 6) or continued a control diet (n = 6) | (MS) Commercial MFGM (Arla) | Pregnancy and lactation | Diet consisted of 60% calories from fat and MFGM supplementation was of 400 mg per kg bw for control and obese rats | Results proved that neurodevelopment was partly promoted with MFGM supplementation by modulating gut microbiota in offspring | 45 |
| Investigate the effect on neuromuscular system of dietary MFGM supplementation | Growing rats (n = 64) were supplemented with one of four different diets, including a control diet, and three bovine-derived MFGM supplements | (IS) Commercial MFGM (Fonterra) | 60 days (PD 10 to PD 70) | MFGM supplements: complex milk lipid mixture (0.64 mg g−1 day−1), beta serum concentrate (5.05 mg g−1 day−1) and polar-lipid-enriched complex milk lipid without MFGM protein (1.78 mg g−1 day−1) | Dietary supplementation of all bovine-derived MFGM supplements enriched neuromuscular development, shifting adult muscle phenotype | 46 |
| (IS) Determine whether MFGM enhances cognition in rats compared to other individual components | Growth restricted rat pups were supplemented with one of the five different treatments tested (n = 51 per group) | (IS) Commercial MFGM and phospholipid concentrate (Lacprodan MFGM-10, Lacprodan PL-20, Arla) | 20 days (PD 2 to PD 21) | MFGM, phospholipid concentrate and a non-fat milk control each at 100 mg per kg (bw), sialic acid 200 mg per kg bw and sialic acid 2 mg per kg bw | MFGM group exhibited enhanced results in a T-maze test, but no differences were observed in the Morris Walter Maze or brain serologies | 40 |
| (IS) Evaluation of the effects of a dietary intervention with MFGM and a prebiotic blend on maternal separated rats | Dietary supplementation to rats with a control or three different diets (n = 12 per group) was conducted | (IS) Commercial MFGM from whey protein concentrate (Mead Johnson Nutrition) | 13 weeks (from PD 21) | Diets supplemented with galacto-oligosaccharide 20.86 g kg−1 + polydextrose 6.44 g kg−1, MFGM 15.9 g kg−1 or a combination of both | MFGM and prebiotic blend mixture impacted microbiota and improved effects of early-life stress (visceral hypersensitivity) in maternal-separated rats | 47 |
| (IS) Investigation of the effects of a diet mimicking breastmilk on early-life stress and after prolonged Western-style diet | Control (n = 50) or early life stress (n = 50) mice were fed with standard or MFGM-enriched (n = 25 for each group) infant milk formula followed by Western-style diet | (IS) Commercial bovine MFGM phospholipids (Fonterra) Danone Nutricia Research | Infant formula for 26 days (PD 16 to PD 42). Western style diet for 188 days (until PD 230) | Diets followed AIN standards. Bovine-MFGM was added in the enriched milk formula | Protective effects of infant milk formula and harmful effects of early-life stress disappear after extended Western-style diet | 48 |
| (IS) Evaluate the effects of a diet rich in coffee polyphenols and MFGM in mice | Mice were fed with a control, high coffee polyphenol or MFGM-enriched diet, or a combination of the latter two (n = 12 per group) | (IS) Commercial MFGM from butter serum (LECICO GmbH) | 1 month or 8 months (from 2 months of age) | Polyphenol diet contained 2% coffee polyphenols and MFGM diet 1% MFGM, base feed followed the AIN standards | Both diets proved to increase survival rates and improve retention of long-term memory. A preventive effect on brain ageing was observed for both experiments | 49 |
| (IS) Assess the effect of MFGM supplementation on maternal-separated rats across lifespan | Maternal-separated or non-separated rats were fed a control diet or a diet rich in MFGM | (IS) Commercial MFGM from whey protein concentrate (Mead Johnson Nutrition) | 100 days (PD 1 to PD 100) | Control and MFGM-enriched diets contained 5.3 g kg−1 DHA/ARA and MFGM diet contained 15.9 g kg−1 whey protein concentrate MFGM | Visceral hypersensitivity was ameliorated with MFGM diet but little to no improvements were observed in spatial memory or neural or glial networks | 50 |
| (IS) Explore the early postnatal temporal profiling of brain lipidome as well as the effect of a polar lipid chronic supplementation in rats | Pups were distributed in a control group (n = 16) fed with a standard diet supplemented with oil or a target group (n = 16) supplemented with polar lipids | (IS) Polar lipids extracted from whey protein concentrate | 43 days (PD 7 to PD 50) | Polar lipid extract obtained from α-lactalbumin-enriched whey protein concentrate and consisted of 3.3 mg per 10 μL per g bw | Polar lipid dietary intake modulated the brain lipid composition during early stages for rat pups and could support early brain development | 51 |
| Determination of behavioral improvements in mice after MFGM intervention | Mice followed a control (n = 10) or MFGM-enriched (n = 10) diet | (IS) Commercial MFGM (Hilmar Ingredients) | 18 weeks (from 8–9 weeks old) | MFGM administered as saline solution at 400 mg per kg bw by intragastric gavage and control group was supplemented a sterile saline solution | Mice supplemented with MFGM presented more neurons and better spatial memory than control group | 52 |
| Analyze bioavailability of bovine MFGM after oral administration in rodents | Mice and rats were administered a control (mice: n = 18; rats n = 12) or a bovine-MFGM-enriched (mice n = 18; rats n = 12) diet (mice 8 weeks, rats 10 weeks) | (IS) Commercial bovine MFGM (Lacprodan MFGM-10, Arla) | 8 weeks (PW 4 to PW 12) | Diets were isocaloric and followed AIN standards, enriched diet contained 2.3% (w/w) bovine-MFGM and whey protein | Plasma phospholipid profiles varied, increasing sphingomyelin levels. Similar results were obtained in the prefrontal cortex | 53 |
| (B) Pigs | ||||||
| Evaluation of longitudinal micro and macrostructure development on pigs after following an early-life polar lipid supplementation | Pigs followed a polar lipid supplemented diet (n = 14) and a control diet (n = 14) | Commercial bovine milk-based milk replacer treatments formulated with whey protein (TestDiet) | 4 weeks (PD 2 to PW 4) | Control diet did not contain polar lipids and polar-lipid diet contained 475 ± 59 mg SM, 564 ± 143 mg PE, 432 ± 47 mg PC 190 ± 37 mf PI per 100 g. Both diets were isocaloric (4.26 kcal kg−1) | Early-life polar lipid supplementation had limited effect on neurodevelopment, object recognition and exploratory behavior | 41 |
| Investigate whether dairy-derived emulsifiers increased lipid absorption and neurodevelopment in newborn piglets | Piglets followed a soy-lecithin (n = 25) or whey protein diet enriched in extracellular vesicles (n = 24) or phospholipids (n = 25) | Commercial whey protein (Arla) | 19 days (PD 1 to PD 19) | Piglets were fed every 2–3 h varying amounts of parenteral (120–0 mL kg−1 day−1) and enteral (32–180 mL kg−1 day−1) nutrition | No differences were observed in hippocampal lipid composition or short-term memory, although plasma lipid profile was altered | 54 |
| Investigate the different effects of a bovine MFGM supplementation on the diet of young pigs | Pigs (n = 18 per group) followed a control or two different MFGM-enriched diets | Commercial whey protein (Lacprodan MFGM-10, Arla) | 30 days (PD 2 to PD 31) | Whey protein concentrate added to the MFGM supplemented diets at 2.5 and 5 g L−1 | Serum lipoprotein levels increased in those pigs following the diet, but no significant differences were found elsewhere | 55 |
| Comparison of neurodevelopment between normal born and intrauterine growth restricted piglets fed with a formula containing vegetable oils or bovine milk fat products | Normal born piglets (n = 18) and intrauterine growth restricted piglets (n = 18) were fed two different formulas supplemented with vegetable oils or bovine milk fat (n = 9 for each group) | Commercial bovine whey protein (Arla) | 3 weeks (PD 3 to PD 24–25) | Vegetable formula (90 g L−1 vegetable oils) or milk formula (119 g L−1 fresh bovine cream) were fed to piglets every 3 h in doses between 160 to 280 mL kg−1 day−1 | Minor effects were observed between the diet and the weight and no improvement on brain structure and functional impairments for intrauterine growth restricted piglets | 56 |
These results are in concordance with another study carried out in aged rats, showing an enhanced spatial working memory in MFGM-supplemented animals, even though no significant differences were observed in the hippocampus and frontal cortex of the tested animals.42 Another work reported that chronic supplementation with MFGM improved measures of spatial long-term memory accuracy and cognitive flexibility, but failed to improve anxiety, general mobility or exploratory behavior43 (Table 3).
| Objective | Methodology | Supplementation | Duration | Dose | Conclusions | Ref. |
|---|---|---|---|---|---|---|
| Evaluate cognitive and biochemical effects of MFGM concentrate supplementation in aged rats | Aged rats (n = 30) followed a control diet or were supplemented with an MFGM cookie piece | Lab-made MFGM concentrate from buttermilk | 4 months (from 15 months to 19 months of age) | MFGM cookie piece corresponded to a 0.5 g per animal per day supplement | MFGM supplementation could restore bioactive lipids in brain and prevent or slow cognitive impairment | 42 |
| Assess the effects of chronic MFGM diet on motor skills, anxiety and long-term memory in adult rats | Early-life rats were supplemented with a control diet (n = 14) or a polar lipid extract supplement (n = 14) | Lab-made polar lipid extract from whey protein concentrate | 65 days (PD 7 to PD 72) | Polar lipid extract (PLE) was made of 14.6 g SM, 14.5 g PC 1.1 g PS, 5.5 g PE and 1.1 g PI per 100 g, rats were fed different amounts, considering the animal's bw | Long-term spatial memory accuracy and cognitive flexibility were enhanced, but it failed to improve recognition memory, anxiety, motor coordination or locomotion | 43 |
| Investigation of cognition effects on aged rats after a diet supplemented with polar lipids from krill oil and buttermilk | Aged rats followed one of four different diets: control with refined olive oil (n = 8), and supplemented diets in polar lipids from buttermilk (n = 11), krill oil (n = 10) or a combination of both (n = 12) for 3 months | Lab-made polar lipid-enriched lollipops from buttermilk or krill oil | 3 months (from 18 months of age) | Animals followed a low polar lipid standard diet and the polar lipid-rich supplements (phospho- and sphingolipids) were added in the form of jelly lollipops | Emotional memory (contextual fear conditioning) was lower in aged rats supplemented with concentrates, but no other behavioral parameter was significantly altered | 57 |
| Determine the role of a diet rich in whey protein powder with MFGM in Alzheimer's disease in mice | Wild type and triple transgenic Alzheimer's disease (AD) mice (n = 30) fed with protein powder and MFGM | Commercial whey protein powder with MFGM (ByHealth Co. Ltd) | 3 months (from 4 months of age) | Whey protein powder with MFGM was provided to the target group at 3.4 g kg−1 day−1 wild type and AD control groups followed the same diet without whey protein powder | Pathology was alleviated by inhibiting neuroinflammation in the brains of AD mice by targeting the peroxisome proliferator-activated receptor | 58 |
| Examine the impact of a diet enriched in MFGM on fat storage, neuroinflammatory processes and myelination in obese mice | Mice were exposed to a control diet (n = 43), high fat diet (n = 23) or a high fat diet enriched in MFGM (n = 27) for 24 weeks to induce obesity | Commercial whey protein lipid concentrates high in MFGM | 24 weeks (from PW 12) | High fat diet was 45% kcal of fat, enriched diet in MFGM substituted 3% of the previous diet with whey protein concentrate | Supplementation benefitted hippocampal-dependent spatial memory, functional connectivity and anti-inflammatory processes in obese mice | 59 |
3.3 Clinical studies
In addition to the aforementioned preclinical studies, some research focused on clinical tests, using infant, adolescent, and adult volunteers. One of the most important aspects to consider when carrying out clinical studies is the safety and tolerability of the products administered to the volunteers. In this sense, Jiang et al.60 assessed whether an MFGM supplementation in infant formula was well tolerated by infants ranging from 0 to 12 months of age. The results proved that there was a similar number of incidences and of a similar caliber as the group that was consuming the standard formula. The same research group showed in another publication that the group consuming the supplemented infant formula reduced the gap existing between breastfed infants and formula fed infants in terms of cognitive performance on Bayley Scales of Infant and Toddler Development III (Bayley-III) test.61 Moreover, it was proven that the levels of serum gangliosides increased for the MFGM group. Other recent clinical studies assessing neurodevelopment in infants are reported in Table 4.| Objective | Methodology | Supplementation | Duration | Dose | Conclusions | Ref. |
|---|---|---|---|---|---|---|
| Evaluation of the safety and tolerability of including MFGM in infant formulas, as well as neurodevelopment in healthy infants | A formula supplemented with MFGM or a standard formula was given to infants (n = 212), tolerance and safety events were recorded. Also, Bayley-III test was conducted at 6 and 12 months | Commercial MFGM and formulas (Fonterra) | 1 year (infants 0 to 12 months of age) | Difference in total phospholipid content between MFGM-enriched and control formula was 32.1 mg in the initial formula and 39 mg in the follow-on formula | Enriched formula was well tolerated by infants from 0 to 12 months of age. Cognitive tests reduced the gap between the MFGM group and a breastfed group | 60 and 61 |
| Evaluation of neurodevelopment, general health and growth of infants receiving a formula supplemented with MFGM and lactoferrin | Cognitive and medical results were obtained after infants (n = 291) received the treatment and 6 months later | Commercial bovine whey protein (Lacprodan MFGM-10, Arla) | 1 year (infants started at 10–14 days of age) | Enriched formula contained 5 g L−1 bovine MFGM from whey protein concentrate and lactoferrin, control was a cow milk-based formula | Infants taking the supplemented diet showed accelerated neurodevelopment after the year of treatment and less medical complications | 62 |
| Analyze the neurodevelopment of children at 5.5 years of age after consuming an enriched MFGM and lactoferrin diet during one year at 12 months of age | Infants who followed a previous diet were invited to a cognitive and behavioral follow-up study (n = 116) | Commercial bovine whey protein (Lacprodan MFGM-10, Arla) | 1 year (until 12 months of age) | Enriched formula contained 5 g L−1 bovine MFGM from whey protein concentrate and lactoferrin, control was a cow milk-based formula | Results proved that infants following the MFGM and lactoferrin diet improved intelligence and executive function at 5.5 years of age | 63 |
| Assess long-term neurodevelopment, growth and plasma cholesterol of children of 6–6.5 years of age that followed a MFGM-enriched low-energy and low-protein diet at 6 months of age. | Anthropometric tests and plasma lipid analyses were carried out at 6 years of age and behavioral tests at 6.5 years of age as a follow-up study (n = 240) | Commercial bovine whey protein (Lacprodan MFGM-10, Arla) | 4 months (less than 2 months old) | Experimental formula contained 4% MFGM and lower energy and proteins (60 kcal per 100 mL and 1.20 mg per 100 mL) standard formula was 66 kcal per 100 mL and 1.27 mg per 100 mL | No significant differences were observed among children following supplemented diet on neurodevelopment, growth or plasma cholesterol | 64 |
| Examination of the benefits of MFGM consumption in early infancy on physical growth and brain development | Infants of 4 months of age of less (n = 340) received a diet enriched with MFGM or a standard formula, and the analyzed data was compared to breastfed infants (n = 200) | Commercial bovine whey protein (Lacprodan MFGM-10, Arla) | 1 year (less than 4 months old) | Both formulas contained 17 mg DHA, 25 mg ARA and 1.9 g protein per 100 kcal, but enriched fraction of MFGM contained 5 g L−1 whey protein concentrate | Results proved that during the first 2 years of age infants taking the supplemented diet followed normal growth i | 69 and 70 |
| Analysis of long-term effects of MFGM-, LC-PUFA- and symbiotic-supplemented infant formula on children aged 6 compared to breastfed infants | Infants from 0–2 months old followed a diet of MFGM-enriched infant formula (n = 39) or a standard formula (n = 37) for up to 18 months, cognitive test were performed at 6 years of age including breastfed infants (n = 32) | Infant formulas were provided by Laboratorios Ordesa, SL | 18 months (less than 2 months old) | MFGM enriched formula contained 10% w/w total protein as MFGM. LC-PUFAs included DHA and ARA, symbiotics, gangliosides, nucleotides and sialic acid were added | Greater neurodevelopment observed for children who followed the supplemented diet | 71 and 72 |
| Establishment of a relationship between major human milk nutrients and temperament and cognition on infants up to 18 months old | Human breast milk was obtained from the participant mothers and cognition and behavioral tests were conducted to infants (n = 54) | Breast milk | 18 months (less than 6 months old) | Support of behavioral and cognitive traits was found in phospholipids, long chain polyunsaturated fatty acids and choline | 73 | |
| Study the relationship between the consumption of sphingomyelin during early life with myelination and cognitive development | Infants (n = 88) fed with three different infant nutrition products; product A (n = 39), product B (n = 28), product C (n = 21) were considered for the study. Mullen scales of early learning were utilized to assess cognitive development and brain MRI for myelin content | Commercial infant formulas | 3 months (first 3 months of age) | Infants had to be fed at least 80% of the times with these products during the first 3 months of age to be included in the study | Results indicated that cognitive development was influenced by the amount of sphingomyelin in infant formulas, as verbal development in infants following a diet higher in sphingomyelin showed a higher rate of change | 74 |
Two other works presented concordant results when the effects on infants following a diet supplemented with MFGM and lactoferrin were tested. In the first study, infants following the aforementioned diet during one year showed an enhanced neurodevelopment compared to control groups, and these results were validated once a follow-up study was conducted at 5.5 years of age.62,63 Nevertheless, another study assessed long-term results on neurodevelopment of infants that started a low-energy and low-protein formula supplemented with MFGM for up to six months since they were born. After six years, the results showed no significant neurodevelopment, growth, or plasma cholesterol improvement for those infants compared to the control group.64
Lastly, Table 5 gathers the most recent publications regarding the effects of MFGM and polar lipid supplementation on neurodegeneration in aged individuals. For example, the effects on psychological health of a diet supplemented with MFGM on healthy adults were studied.65 In this trial, the volunteers were separated in three different groups, two of them receiving different MFGM amounts with the diet and a placebo group. Volunteers followed the diet for 12 weeks and several questionnaires about psychological health and sleep quality were administered at the beginning and at weeks 6 and 12. The results showed that those individuals taking MFGM presented significantly lower stress scores than the placebo group both after six and 12 weeks. There are a few studies that are worth mentioning although they were not included in the tables due to the absence of treatment with MFGM or polar lipids, for example, Chakraborty et al. (2021) carried out an evaluation of the prevalence of mild cognitive impairment on type 2 diabetes mellitus patients by conducting cognitive tests and blood lipidomics to diabetic patients.66 They found out that triacylglycerol as well as phosphatidyl choline levels decreased in patients suffering mild cognitive impairment whereas ceramide levels increased, Costa et al. (2019) carried out a quantification of phospholipids as potential biomarkers of Alzheimer's disease (AD) and determined phospholipase A2 activity in blood of mild cognitive impairment and AD patients.67 They extracted and consequently analyzed blood from healthy individuals (n = 25) as well as mild cognitive impaired (n = 20) and AD patients (n = 34), finding out that lipid metabolites in plasma could be indicators of the transition between healthy and diseased brains. Lastly, Muñoz-Garach et al. (2021) assessed the relationship between the intake of milk and dairy products and cognitive decline of high cardiovascular risk individuals.68 The intake of milk and dairy products consumed was estimated by nutritional questionnaires and cognitive tests were conducted to all individuals (n = 6426). Interestingly, they concluded that a greater intake of milk and dairy products could be linked to a greater cognitive decline; however, whole-fat milk was associated with less cognitive impairment.
| Objective | Methodology | Supplementation | Duration | Dose | Conclusions | Ref. |
|---|---|---|---|---|---|---|
| Assess the effect of a diet enriched with MFGM on psychological health for a group of healthy adults | Participants (n = 122) were consuming different MFGM amounts or placebo and took questionnaires at baseline and after 6 and 12 weeks | Commercial MFGM from buttermilk (Fonterra) | 12 weeks | Participants were given 1 or 2 sachets daily containing 600 mg MFGM or a placebo containing maltodextrin. | MFGM groups showed lower stress scores than placebo group, suggesting that MFGM consumption could lead to reducing anxiety or improve general psychological health | 65 |
| Test whether a MFGM and protein -enriched snack could improve the physical performance in aged woman | Women of 70 years of age or more were supplemented (n = 51) with a snack rich in MFGM and protein or not (n = 50) for 12 weeks followed by some physical tests | Lab-made products from lactose-free buttermilk concentrate (Valio Ltd.) | 12 weeks | Intervention group was given either a chocolate milkshake with 3.6 mg MFGM or a protein powder with 3.9 mg MFGM | Balance tests improved significantly in the intervention group compared to the control group, no other significant changes were observed | 75 |
| Evaluate the effects of an MFGM-enriched milk on healthy or mildly cognitively impaired subjects | Subjects of 65 years of age or more (n = 44) were administered with MFGM-enriched milk for 14 weeks. Cognitive tests and lipidomic analyses were performed. | Lab-made functional milk drinks from buttermilk | 14 weeks | Participants of control group were given 200 mL day−1 of skim milk (0.01 mg of PL) and target group 200 mL day−1 MFGM milk (0.38 mg of PL) | Individuals participating proved an improvement in the episodic memory after the 14 weeks, significantly improved among females. | 76 |
4 Discussion
Research on the role of polar lipids and, especially, MFGM in neurodevelopment and neurodegeneration has intensified in recent years (Fig. 1). Much of this research is concentrating on diet supplementation of MFGM as a potential nutraceutical, as reported by many animal and human investigations. Preclinical and clinical data are promising yet still scant to draw firm conclusions. Undoubtedly, polar lipids access the brain and are incorporated into cerebral phospholipids.77,78In order to determine and unravel the possible mechanisms of action of the lipid-based interventions reviewed here, some studies have been carried out over the last few years to better understand the behavior of polar lipids and the different interactions of the MFGM with cells. For example, one recent study described the interaction between fluorescently labelled MFGM obtained from human breast milk with Caco-2 cells, i.e. basic components of the intestinal epithelial barrier. In that study, MFGM was labelled with two different fluorochromes that induce fluorescence resonance energy transfer. By using inhibitors of endocytosis, it was proven that the interaction led to a fusion of the MFGM and the Caco-2 cell membranes.79 This study adds to the potential interactions of polar lipids and infant development at the earliest stage of life, because milk MFGM were not fully digested, approximating the situation in which infants with a very immature digestive system would obtain nutrients from their mothers.
In the field of neuroscience, the administration of milk polar lipids to aged rats was able to modulate the miRNA expression80 and to improve hippocampal insulin resistance and synaptic signaling.81 A recent paper by Zhou et al.52 reported that the provision of MFGM increased the number of neurons in the dentate gyrus of the hippocampus and modulated the expression of proteins that are known to promote synapse formation and signaling pathways that are related to cognitive processes.
Considering the different works presented in this review, it can be observed that the majority of publications are focused on pre-clinical trials, and very few works have tackled clinical trials on neurodegeneration with aged population. One common trend that can be appreciated in the results shown on the tables is that for those studies carried out for a short period of time (i.e. a few weeks), little to no positive outcome has been noticed, whereas longer studies of several months tend to provide better results. Therefore, it can be concluded that more long-term studies are required in order to provide a better understanding of the role of polar lipids in the wide field of cognition. It is undeniable that different commercial or lab-made products will not present the exact lipidic composition. That is why another remarkable point to consider is the type of supplementation that was employed in the investigations. It can be observed that there are several studies, both pre-clinical and clinical, that use the same products with different outcomes. For example, the most commonly used commercial products come from Arla and Fonterra. However, the success of the results observed did not follow an exact correlation with the type of supplementation but rather on the duration of the process. As previously mentioned, those investigations carried out for an extended period of time, i.e. a year, presented more promising results, regardless of the type of product consumed. Despite the small differences of the products used, an extended period of time is definitely required, especially for humans.
Among the many bioactive lipidic components of MFGM, particular attention is being paid to PS and PC, in addition to sphingomyelin and gangliosides. While the former have been mostly studied in peripheral nervous system diseases, the latter are being used as supplements because of their purported effects in e.g. memory and brain development.37,82,83 Moreover, it is believed that sphingomyelin plays a crucial role in maintaining the membrane structure of MFGM.37,84
The amount of PS in MFGM ranges from 2% to 7%85–87 and this phospholipid is highly concentrated in the brain, between 12 and 20%.88,89 PS plays important roles in e.g. mitochondrial integrity, neurotransmitter release, and memory formation.90 PS has been studied in trials of dementia and MCI, which largely reported the ameliorative effects of PS on memory and learning.31,57,91–96
Another important PL component of MFGM is PC, which makes up approximately a third of the total amount of PL found85 and is crucial for the synthesis of acetylcholine and, downstream, for memory formation and mood.97 Choline and/or its metabolites are also indispensable to the structural integrity and signaling function of cell membranes,97,98 and it is the major source of methyl groups in the diet.98 Pertinent to this review, choline intake during gestation and the early postnatal period has been shown to enhance cognitive performance in childhood, adulthood and into old age in multiple animal models and in some human studies.99 Even though excessive dietary choline intake might lead to the microbiota-mediated formation of trimethylamine N-oxide,100 its role in memory formation and dementia prevention is being actively investigated.101 It must be underscored that it is currently difficult to disentangle the neuroprotective actions of choline from those of choline-containing phospholipids such as PC, namely PC species that contain high proportions of DHA. Yet, most author concur that the available evidence suggests that an adequate and concomitant dietary supply of choline and DHA is necessary to maintain plasma and cerebral PC-DHA concentrations, which might contribute to slow the onset of cognitive decline with aging, and potentially to delay or ameliorate the pathogenetic process in AD.100
5 Conclusions
In conclusion, MFGM are an interesting, abundant, and exploitable source of relatively inexpensive bioactive molecules that could be properly formulated and employed in the neurodevelopment and cognitive decline fields.Large, adequately sized randomized controlled trials and, as discussed before, of an extended duration, are certainly needed before making health claims. However, research in this area is rapidly accelerating and accumulated evidence is supportive of biological, salubrious effects. A balanced interaction between academy and industry will augment an effective way to produce and study nutraceuticals and functional foods with high yet palatable concentrations of MFGM that could be employed at the two spectra of lifespan, with interesting and useful pharma-nutritional outcomes.
Author contributions
ALU: conceptualization, investigation, methodology, writing – original draft, writing – review & editing. MVC and FV: conceptualization, investigation, methodology, supervision, writing – review & editing. JF: conceptualization, investigation, methodology, supervision, writing – review & editing, funding acquisition.Conflicts of interest
The authors declare no conflict of interest associated with this work.Acknowledgements
This study was supported by the Project PID2020-114821RB-I00 funded by MCIN/AEI/10.13039/501100011033 and, in part, by PRIN 2022NZNZH8.References
- A. Y. Chang, V. F. Skirbekk, S. Tyrovolas, N. J. Kassebaum and J. L. Dieleman, Measuring Population Ageing: An Analysis of the Global Burden of Disease Study 2017, Lancet Public Health, 2019, 4(3), e159–e167, DOI:10.1016/S2468-2667(19)30019-2.
- GBD 2015 Mortality and Causes of Death Collaborators, Global, Regional, and National Life Expectancy, All-Cause Mortality, and Cause-Specific Mortality for 249 Causes of Death, 1980–2015: A Systematic Analysis for the Global Burden of Disease Study 2015, Lancet, 2016, 388(10053), 1459–1544, DOI:10.1016/S0140-6736(16)31012-1.
- S. Gauthier, B. Reisberg, M. Zaudig, R. C. Petersen, K. Ritchie, K. Broich, S. Belleville, H. Brodaty, D. Bennett, H. Chertkow, J. L. Cummings, M. de Leon, H. Feldman, M. Ganguli, H. Hampel, P. Scheltens, M. C. Tierney, P. Whitehouse and B. Winblad, Mild Cognitive Impairment, Lancet, 2006, 367(9518), 1262–1270, DOI:10.1016/S0140-6736(06)68542-5.
- R. C. Petersen and S. Negash, Mild Cognitive Impairment: An Overview, CNS Spectr., 2008, 13(1), 45–53, DOI:10.1017/S1092852900016151.
- K. Laver, S. Dyer, C. Whitehead, L. Clemson and M. Crotty, Interventions to Delay Functional Decline in People with Dementia: A Systematic Review of Systematic Reviews, BMJ Open, 2016, 6(4), e010767, DOI:10.1136/bmjopen-2015-010767.
- A. N. McLaren, M. A. LaMantia and C. M. Callahan, Systematic Review of Non-Pharmacologic Interventions to Delay Functional Decline in Community-Dwelling Patients with Dementia, Aging Mental Health, 2013, 17(6), 655–666, DOI:10.1080/13607863.2013.781121.
- C. B. Hall, R. B. Lipton, M. Sliwinski, M. J. Katz, C. A. Derby and J. Verghese, Cognitive Activities Delay Onset of Memory Decline in Persons Who Develop Dementia, Neurology, 2009, 73(5), 356–361, DOI:10.1212/WNL.0b013e3181b04ae3.
- N. V. Bhagavan and C.-E. Ha, Lipids I: Fatty Acids and Eicosanoids, in Essentials of Medical Biochemistry, Elsevier, 2015, pp. 269–297. DOI:10.1016/B978-0-12-416687-5.00016-6.
- J. Fontecha, L. Brink, S. Wu, Y. Pouliot, F. Visioli and R. Jiménez-Flores, Sources, Production, and Clinical Treatments of Milk Fat Globule Membrane for Infant Nutrition and Well-Being, Nutrients, 2020, 12(6), 1607, DOI:10.3390/nu12061607.
- R. P. Bazinet and S. Layé, Polyunsaturated Fatty Acids and Their Metabolites in Brain Function and Disease, Nat. Rev. Neurosci., 2014, 15(12), 771–785, DOI:10.1038/nrn3820.
- A. Pérez-Gálvez, M. Jarén-Galán, J. Garrido-Fernández, M. V. Calvo, F. Visioli and J. Fontecha, Activities, Bioavailability, and Metabolism of Lipids from Structural Membranes and Oils: Promising Research on Mild Cognitive Impairment, Pharmacol. Res., 2018, 134, 299–304, DOI:10.1016/j.phrs.2018.07.013.
- D. Richard, P. Bausero, C. Schneider and F. Visioli, Polyunsaturated Fatty Acids and Cardiovascular Disease, Cell. Mol. Life Sci., 2009, 66(20), 3277–3288, DOI:10.1007/s00018-009-0085-4.
- N. V. Bhagavan and C.-E. Ha, Lipids II: Phospholipids, Glycosphingolipids, and Cholesterol, in Essentials of Medical Biochemistry, Elsevier, 2015, pp. 299–320. DOI:10.1016/B978-0-12-416687-5.00017-8.
- L. Zheng, M. Fleith, F. Giuffrida, B. V. O'Neill and N. Schneider, Dietary Polar Lipids and Cognitive Development: A Narrative Review, Adv. Nutr., 2019, 10(6), 1163–1176, DOI:10.1093/advances/nmz051.
- J. S. O'Brien and E. L. Sampson, Lipid Composition of the Normal Human Brain: Gray Matter, White Matter, and Myelin*, J. Lipid Res., 1965, 6(4), 537–544, DOI:10.1016/S0022-2275(20)39619-X.
- E. E. Birch, Y. S. Castañeda, D. H. Wheaton, D. G. Birch, R. D. Uauy and D. R. Hoffman, Visual Maturation of Term Infants Fed Long-Chain Polyunsaturated Fatty Acid–Supplemented or Control Formula for 12 Mo2, Am. J. Clin. Nutr., 2005, 81(4), 871–879, DOI:10.1093/ajcn/81.4.871.
- F. Visioli and E. Burgos-Ramos, Selected Micronutrients in Cognitive Decline Prevention and Therapy, Mol. Neurobiol., 2016, 53(6), 4083–4093, DOI:10.1007/s12035-015-9349-1.
- A. Loughman, H. M. Staudacher, T. Rocks, A. Ruusunen, W. Marx, A. O’Neil and F. N. Jacka, Diet and Mental Health, Mod. Trends Psychiatry, 2021, 32, 100–112, DOI:10.1159/000510422.
- P. Castro-Gómez, A. Garcia-Serrano, F. Visioli and J. Fontecha, Relevance of Dietary Glycerophospholipids and Sphingolipids to Human Health, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2015, 101, 41–51, DOI:10.1016/j.plefa.2015.07.004.
- L. R. Brink and B. Lönnerdal, Milk Fat Globule Membrane: The Role of Its Various Components in Infant Health and Development, J. Nutr. Biochem., 2020, 85, 108465, DOI:10.1016/j.jnutbio.2020.108465.
- G. S. Raza, K.-H. Herzig and J. Leppäluoto, Invited Review: Milk Fat Globule Membrane—A Possible Panacea for Neurodevelopment, Infections, Cardiometabolic Diseases, and Frailty, J. Dairy Sci., 2021, 104(7), 7345–7363, DOI:10.3168/jds.2020-19649.
- H. F. González and S. Visentin, Nutrients and Neurodevelopment: Lipids, Arch. Argent. Pediatr., 2016, 114(5), 472–476, DOI:10.5546/aap.2016.eng.472.
- D. J. Hanahan and G. A. Thompson, Complex Lipids, Annu. Rev. Biochem., 1963, 32(1), 215–240, DOI:10.1146/annurev.bi.32.070163.001243.
- B. Szachowicz-Petelska, I. Dobrzyńska, M. Skrodzka, B. Darewicz, Z. A. Figaszewski and J. Kudelski, Phospholipid Composition and Electric Charge in Healthy and Cancerous Parts of Human Kidneys, J. Membr. Biol., 2013, 246(5), 421–425, DOI:10.1007/s00232-013-9554-7.
- B. M. Quinville, N. M. Deschenes, A. E. Ryckman and J. S. Walia, A Comprehensive Review: Sphingolipid Metabolism and Implications of Disruption in Sphingolipid Homeostasis, Int. J. Mol. Sci., 2021, 22(11), 5793, DOI:10.3390/ijms22115793.
- M. Aureli, N. Loberto, V. Chigorno, A. Prinetti and S. Sonnino, Remodeling of Sphingolipids by Plasma Membrane Associated Enzymes, Neurochem. Res., 2011, 36(9), 1636–1644, DOI:10.1007/s11064-010-0360-7.
- A. A. Farooqui, L. A. Horrocks and T. Farooqui, Glycerophospholipids in Brain: Their Metabolism, Incorporation into Membranes, Functions, and Involvement in Neurological Disorders, Chem. Phys. Lipids, 2000, 106(1), 1–29, DOI:10.1016/S0009-3084(00)00128-6.
- A. H. Merrill Jr, Sphingolipid and Glycosphingolipid Metabolic Pathways in the Era of Sphingolipidomics, Chem. Rev., 2011, 111(10), 6387–6422, DOI:10.1021/cr2002917.
- J. S. Cohn, A. Kamili, E. Wat, R. W. S. Chung and S. Tandy, Dietary Phospholipids and Intestinal Cholesterol Absorption, Nutrients, 2010, 2(2), 116–127, DOI:10.3390/nu2020116.
- J. L. Weihrauch and Y.-S. Son, Phospholipid Content of Foods, J. Am. Oil Chem. Soc., 1983, 60(12), 1971–1978, DOI:10.1007/BF02669968.
- M. Schverer, S. M. O'Mahony, K. J. O'Riordan, F. Donoso, B. L. Roy, C. Stanton, T. G. Dinan, H. Schellekens and J. F. Cryan, Dietary Phospholipids: Role in Cognitive Processes across the Lifespan, Neurosci. Biobehav. Rev., 2020, 111, 183–193, DOI:10.1016/j.neubiorev.2020.01.012.
- L. Anto, S. W. Warykas, M. Torres-Gonzalez and C. N. Blesso, Milk Polar Lipids: Underappreciated Lipids with Emerging Health Benefits, Nutrients, 2020, 12(4), 1001, DOI:10.3390/nu12041001.
- R. Lordan, A. Tsoupras and I. Zabetakis, Phospholipids of Animal and Marine Origin: Structure, Function, and Anti-Inflammatory Properties, Molecules, 2017, 22(11), 1964, DOI:10.3390/molecules22111964.
- B. Y. Fong, C. S. Norris and A. K. H. MacGibbon, Protein and Lipid Composition of Bovine Milk-Fat-Globule Membrane, Int. Dairy J., 2007, 17(4), 275–288, DOI:10.1016/j.idairyj.2006.05.004.
- I. H. Mather and T. W. Keenan, Origin and Secretion of Milk Lipids, J. Mammary Gland Biol. Neoplasia, 1998, 3(3), 259–273, DOI:10.1023/a:1018711410270.
- H. J. J. Mohamed, E. K. H. Lee, K. C. K. Woo, R. Sarvananthan, Y. Y. Lee and Z. A. Mohd Hussin, Brain–Immune–Gut Benefits with Early Life Supplementation of Milk Fat Globule Membrane, JGH Open, 2022, 6(7), 454–461, DOI:10.1002/jgh3.12775.
- D. Yao, C. S. Ranadheera, C. Shen, W. Wei and L.-Z. Cheong, Milk Fat Globule Membrane: Composition, Production and Its Potential as Encapsulant for Bioactives and Probiotics, Crit. Rev. Food Sci. Nutr., 2023, 1–16, DOI:10.1080/10408398.2023.2249992.
- C. Wang, X. Qiao, Z. Gao, L. Jiang and Z. Mu, Advancement on Milk Fat Globule Membrane: Separation, Identification, and Functional Properties, Front. Nutr., 2021, 8, 807284, DOI:10.3389/fnut.2021.807284.
- K. Cohen Kadosh, L. Muhardi, P. Parikh, M. Basso, H. J. J. Mohamed, T. Prawitasari, F. Samuel, G. Ma and J. M. W. Geurts, Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children—An Update and Novel Insights, Nutrients, 2021, 13(1), 199, DOI:10.3390/nu13010199.
- L. R. Brink, J. P. Gueniot and B. Lönnerdal, Effects of Milk Fat Globule Membrane and Its Various Components on Neurologic Development in a Postnatal Growth Restriction Rat Model, J. Nutr. Biochem., 2019, 69, 163–171, DOI:10.1016/j.jnutbio.2019.03.013.
- J. E. Fil, S. Joung, J. Hauser, A. Rytz, C. A. Hayes and R. N. Dilger, Influence of Dietary Polar Lipid Supplementation on Memory and Longitudinal Brain Development, Nutrients, 2021, 13(8), 2486, DOI:10.3390/nu13082486.
- S. Baliyan, M. V. Calvo, D. Piquera, O. Montero, F. Visioli, C. Venero and J. Fontecha, Milk Fat Globule Membrane Concentrate as a Nutritional Supplement Prevents Age-Related Cognitive Decline in Old Rats: A Lipidomic Study of Synaptosomes, Food Res. Int., 2023, 163, 112163, DOI:10.1016/j.foodres.2022.112163.
- S. Sultan, J. Hauser, M. Oliveira, A. Rytz, N. Preitner and N. Schneider, Effects of Post-Natal Dietary Milk Fat Globule Membrane Polar Lipid Supplementation on Motor Skills, Anxiety, and Long-Term Memory in Adulthood, Front. Nutr., 2021, 8, 737731, DOI:10.3389/fnut.2021.737731.
- Q. Yuan, H. Gong, M. Du and X. Mao, Supplementation of Milk Polar Lipids to Obese Dams Improves Neurodevelopment and Cognitive Function in Male Offspring, FASEB J., 2021, 35(4), e21454, DOI:10.1096/fj.202001974RRR.
- Q. Yuan, H. Gong, M. Du, T. Li and X. Mao, Milk Fat Globule Membrane Supplementation to Obese Rats during Pregnancy and Lactation Promotes Neurodevelopment in Offspring via Modulating Gut Microbiota, Front. Nutr., 2022, 9, 945052, DOI:10.3389/fnut.2022.945052.
- J. F. Markworth, B. Durainayagam, V. C. Figueiredo, K. Liu, J. Guan, A. K. H. MacGibbon, B. Y. Fong, A. C. Fanning, A. Rowan, P. McJarrow and D. Cameron-Smith, Dietary Supplementation with Bovine-Derived Milk Fat Globule Membrane Lipids Promotes Neuromuscular Development in Growing Rats, Nutr. Metab., 2017, 14(1), 9, DOI:10.1186/s12986-017-0161-y.
- S. M. O’Mahony, K. A. McVey Neufeld, R. V. Waworuntu, M. M. Pusceddu, S. Manurung, K. Murphy, C. Strain, M. C. Laguna, V. L. Peterson, C. Stanton, B. M. Berg, T. G. Dinan and J. F. Cryan, The Enduring Effects of Early-Life Stress on the Microbiota–Gut–Brain Axis Are Buffered by Dietary Supplementation with Milk Fat Globule Membrane and a Prebiotic Blend, Eur. J. Neurosci., 2020, 51(4), 1042–1058, DOI:10.1111/ejn.14514.
- M. R. Abbink, L. Schipper, E. F. G. Naninck, C. M. H. de Vos, R. Meier, E. M. van der Beek, P. J. Lucassen and A. Korosi, The Effects of Early Life Stress, Postnatal Diet Modulation, and Long-Term Western-Style Diet on Later-Life Metabolic and Cognitive Outcomes, Nutrients, 2020, 12(2), 570, DOI:10.3390/nu12020570.
- K. Unno, K. Taguchi, T. Hase, S. Meguro and Y. Nakamura, Coffee Polyphenol, Chlorogenic Acid, Suppresses Brain Aging and Its Effects Are Enhanced by Milk Fat Globule Membrane Components, Int. J. Mol. Sci., 2022, 23(10), 5832, DOI:10.3390/ijms23105832.
- J. M. Collins, V. Caputi, S. Manurung, G. Gross, P. Fitzgerald, A. V. Golubeva, J. Popov, C. Deady, T. G. Dinan, J. F. Cryan and S. M. O'Mahony, Supplementation with Milk Fat Globule Membrane from Early Life Reduces Maternal Separation-Induced Visceral Pain Independent of Enteric Nervous System or Intestinal Permeability Changes in the Rat, Neuropharmacology, 2022, 210, 109026, DOI:10.1016/j.neuropharm.2022.109026.
- M. Oliveira, K. Koshibu, A. Rytz, F. Giuffrida, S. Sultan, A. Patin, M. Gaudin, A. Tomezyk, P. Steiner and N. Schneider, Early Life to Adult Brain Lipidome Dynamic: A Temporospatial Study Investigating Dietary Polar Lipid Supplementation Efficacy, Front. Nutr., 2022, 9, 898655, DOI:10.3389/fnut.2022.898655.
- Y. Zhou, X. Zou, R. Feng, X. Zhan, H. Hong, Y. Luo and Y. Tan, Improvement of Spatial Memory and Cognitive Function in Mice via the Intervention of Milk Fat Globule Membrane, Nutrients, 2023, 15(3), 534, DOI:10.3390/nu15030534.
- R. Davies, J. A. van Diepen, L. R. Brink, S. Bijlsma, K.-A. M. Neufeld, J. F. Cryan, S. M. O'Mahony, I. Bobeldijk and G. Gross, Lipidome Analysis in Brain and Peripheral Plasma Following Milk Fat Globule Membrane Supplementation in Rodents, Mol. Nutr. Food Res., 2022, 66(22), 2200177, DOI:10.1002/mnfr.202200177.
- N. L. Henriksen, K. Aasmul-Olsen, R. Venkatasubramanian, M. K. E. Nygaard, R. R. Sprenger, A. B. Heckmann, M. S. Ostenfeld, C. S. Ejsing, S. F. Eskildsen, A. Müllertz, P. T. Sangild, S. B. Bering and T. Thymann, Dairy-Derived Emulsifiers in Infant Formula Show Marginal Effects on the Plasma Lipid Profile and Brain Structure in Preterm Piglets Relative to Soy Lecithin, Nutrients, 2021, 13(3), 718, DOI:10.3390/nu13030718.
- J. E. Fil, S. A. Fleming, M. Chichlowski, G. Gross, B. M. Berg and R. N. Dilger, Evaluation of Dietary Bovine Milk Fat Globule Membrane Supplementation on Growth, Serum Cholesterol and Lipoproteins, and Neurodevelopment in the Young Pig, Front. Pediatr., 2019, 7, 417, DOI:10.3389/fped.2019.00417.
- N. L. Henriksen, K. S. Asmussen, X. Pan, P.-P. Jiang, Y. Mori, L. I. Christiansen, R. R. Sprenger, C. S. Ejsing, S. Pankratova and T. Thymann, Brain Lipidomics and Neurodevelopmental Outcomes in Intrauterine Growth Restricted Piglets Fed Dairy or Vegetable Fat Diets, Sci. Rep., 2022, 12(1), 3303, DOI:10.1038/s41598-022-07133-3.
- A. García-Serrano, J. Tomé-Carneiro, M. C. Crespo, M. V. Calvo, I. Pereda-Pérez, S. Baliyan, E. Burgos-Ramos, O. Montero, A. Dávalos, C. Venero, F. Visioli and J. Fontecha, Concentrates of Buttermilk and Krill Oil Improve Cognition in Aged Rats, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2020, 155, 102077, DOI:10.1016/j.plefa.2020.102077.
- Y. Li, Z.-H. Zhang, S.-L. Huang, Z.-B. Yue, X.-S. Yin, Z.-Q. Feng, X.-G. Zhang and G.-L. Song, Whey Protein Powder with Milk Fat Globule Membrane Attenuates Alzheimer's Disease Pathology in 3×Tg-AD Mice by Modulating Neuroinflammation through the Peroxisome Proliferator-Activated Receptor γ Signaling Pathway, J. Dairy Sci., 2023, 106(8), 5253–5265, DOI:10.3168/jds.2023-23254.
- I. A. C. Arnoldussen, M. C. Morrison, M. Wiesmann, J. A. van Diepen, N. Worms, M. Voskuilen, V. Verweij, B. Geenen, N. P. Gualdo, L. van der Logt, G. Gross, R. Kleemann and A. J. Kiliaan, Milk Fat Globule Membrane Attenuates High Fat Diet-Induced Neuropathological Changes in Obese Ldlr−/−.Leiden Mice, Int. J. Obes., 2022, 46(2), 342–349, DOI:10.1038/s41366-021-00998-w.
- B. Jiang, Y. Xia, L. Zhou, X. Liang, X. Chen, M. Chen, X. Li, S. Lin, N. Zhang, L. Zheng, M. Tao, P. Petocz, S. Gallier, A. Rowan and B. Wang, Safety and Tolerance Assessment of Milk Fat Globule Membrane-Enriched Infant Formulas in Healthy Term Chinese Infants: A Randomised Multicenter Controlled Trial, BMC Pediatr., 2022, 22(1), 465, DOI:10.1186/s12887-022-03507-8.
- Y. Xia, B. Jiang, L. Zhou, J. Ma, L. Yang, F. Wang, H. Liu, N. Zhang, X. Li, P. Petocz and B. Wang, Neurodevelopmental Outcomes of Healthy Chinese Term Infants Fed Infant Formula Enriched in Bovine Milk Fat Globule Membrane for 12 Months - a Randomized Controlled Trial, Asia Pac. J. Clin. Nutr., 2021, 30(3), 401–414, DOI:10.3316/informit.165063824812712.
- F. Li, S. S. Wu, C. L. Berseth, C. L. Harris, J. D. Richards, J. L. Wampler, W. Zhuang, G. Cleghorn, C. D. Rudolph, B. Liu, D. J. Shaddy and J. Colombo, Improved Neurodevelopmental Outcomes Associated with Bovine Milk Fat Globule Membrane and Lactoferrin in Infant Formula: A Randomized, Controlled Trial, J. Pediatr., 2019, 215, 24–31.e8, DOI:10.1016/j.jpeds.2019.08.030.
- J. Colombo, C. L. Harris, J. L. Wampler, W. Zhuang, D. J. Shaddy, B. Y. Liu and S. S. Wu, Improved Neurodevelopmental Outcomes at 5.5 Years of Age in Children Who Received Bovine Milk Fat Globule Membrane and Lactoferrin in Infant Formula Through 12 Months: A Randomized Controlled Trial, J. Pediatr., 2023, 261, 113483, DOI:10.1016/j.jpeds.2023.113483.
- N. Timby, M. Adamsson, E. Domellöf, T. Grip, O. Hernell, B. Lönnerdal and M. Domellöf, Neurodevelopment and Growth until 6.5 Years of Infants Who Consumed a Low-Energy, Low-Protein Formula Supplemented with Bovine Milk Fat Globule Membranes: A Randomized Controlled Trial, Am. J. Clin. Nutr., 2021, 113(3), 586–592, DOI:10.1093/ajcn/nqaa354.
- N. Davies, C. Frampton, M. Fuad and R. Slykerman, The Effect of Supplementation with Milk Fat Globule Membranes on Psychological Health: A Randomized Clinical Trial in Healthy Adults with Moderate Stress, J. Funct. Foods, 2023, 105, 105585, DOI:10.1016/j.jff.2023.105585.
- A. Chakraborty, S. Hegde, S. K. Praharaj, K. Prabhu, C. Patole, A. K. Shetty, S. S. Mayya, R. V. Acharya, H. M. Hande, M. M. Prabhu and D. Upadhya, Age Related Prevalence of Mild Cognitive Impairment in Type 2 Diabetes Mellitus Patients in the Indian Population and Association of Serum Lipids With Cognitive Dysfunction, Front. Endocrinol., 2021, 12, 798652, DOI:10.3389/fendo.2021.798652.
- A. C. Costa, H. P. G. Joaquim, O. Forlenza, L. L. Talib and W. F. Gattaz, Plasma Lipids Metabolism in Mild Cognitive Impairment and Alzheimer's Disease, World J. Biol. Psychiatry, 2019, 20(3), 190–196, DOI:10.1080/15622975.2017.1369566.
- A. Muñoz-Garach, I. Cornejo-Pareja, M.Á Martínez-González, M. Bulló, D. Corella, O. Castañer, D. Romaguera, J. Vioque, Á.M Alonso-Gómez, J. Wärnberg, J. A. Martínez, L. Serra-Majem, R. Estruch, M. R. Bernal-López, J. Lapetra, X. Pintó, J. A. Tur, J. López-Miranda, A. Bueno-Cavanillas, M. Delgado-Rodríguez, P. Matía-Martín, L. Daimiel, V. M. Sánchez, J. Vidal, L. Prieto, E. Ros, F. Fernández-Aranda, L. Camacho-Barcia, C. Ortega-Azorin, M. Soria, M. Fiol, L. Compañ-Gabucio, L. Goicolea-Güemez, J. Pérez-López, N. Goñi, J. Pérez-Cabrera, E. Sacanella, J. C. Fernández-García, L. Miró-Moriano, M. Gimenez-Gracia, C. Razquin, I. Paz-Graniel, P. Guillem, M. D. Zomeño, M. Moñino, A. Oncina-Canovas, I. Salaverria-Lete, E. Toledo, J. Salas-Salvadó, H. Schröder, F. J. Tinahones and P.-P. Investigators, Milk and Dairy Products Intake Is Related to Cognitive Impairment at Baseline in Predimed Plus Trial, Mol. Nutr. Food Res., 2021, 65(7), 2000728, DOI:10.1002/mnfr.202000728.
- R. Toro-Campos, C. Algarín, P. Peirano, M. Peña, T. Murguia-Peniche, S. S. Wu and R. Uauy, Effect of Feeding Mode on Infant Growth and Cognitive Function: Study Protocol of the Chilean Infant Nutrition Randomized Controlled Trial (ChiNuT), BMC Pediatr., 2020, 20(1), 225, DOI:10.1186/s12887-020-02087-9.
- A. M. Jaramillo-Ospina, R. Toro-Campos, T. Murguía-Peniche, J. L. Wampler, S. S. Wu, C. L. Berseth and R. Uauy, Added Bovine Milk Fat Globule Membrane in Formula: Growth, Body Composition, and Safety through Age 2: An RCT, Nutrition, 2022, 97, 111599, DOI:10.1016/j.nut.2022.111599.
- A. Nieto-Ruiz, J. A. García-Santos, J. Verdejo-Román, E. Diéguez, N. Sepúlveda-Valbuena, F. Herrmann, T. Cerdó, R. De-Castellar, J. Jiménez, M. G. Bermúdez, M. Pérez-García, M. T. Miranda, M. C. López-Sabater, A. Catena and C. Campoy, Infant Formula Supplemented With Milk Fat Globule Membrane, Long-Chain Polyunsaturated Fatty Acids, and Synbiotics Is Associated With Neurocognitive Function and Brain Structure of Healthy Children Aged 6 Years: The COGNIS Study, Front. Nutr., 2022, 9, 820224, DOI:10.3389/fnut.2022.820224.
- A. Nieto-Ruiz, E. Diéguez, N. Sepúlveda-Valbuena, F. Herrmann, T. Cerdó, F. López-Torrecillas, R. De-Castellar, J. Jiménez, M. Pérez-García, M. T. Miranda, A. Catena, J. A. García-Santos, M. G. Bermúdez and C. Campoy, The Effects of an Infant Formula Enriched with Milk Fat Globule Membrane, Long-Chain Polyunsaturated Fatty Acids and Synbiotics on Child Behavior up to 2.5 Years Old: The COGNIS Study, Nutrients, 2020, 12(12), 3825, DOI:10.3390/nu12123825.
- T. Li, T. M. Samuel, Z. Zhu, B. Howell, S. Cho, K. Baluyot, H. Hazlett, J. T. Elison, D. Wu, J. Hauser, N. Sprenger, H. Zhu and W. Lin, Joint Analyses of Human Milk Fatty Acids, Phospholipids, and Choline in Association with Cognition and Temperament Traits during the First 6 Months of Life, Front. Nutr., 2022, 9, 919769, DOI:10.3389/fnut.2022.919769.
- N. Schneider, J. Hauser, M. Oliveira, E. Cazaubon, S. C. Mottaz, B. V. O'Neill, P. Steiner and S. C. L. Deoni, Sphingomyelin in Brain and Cognitive Development: Preliminary Data, eNeuro, 2019, 64, ENEURO.0421-18.2019, DOI:10.1523/ENEURO.0421-18.2019.
- S. K. Jyväkorpi, R. T. Niskanen, M. Markkanen, K. Salminen, T. Sibakov, K.-M. Lehtonen, S. Kunvik, K. H. Pitkala, A. M. Turpeinen and M. H. Suominen, Effect of Milk Fat Globule Membrane- and Protein-Containing Snack Product on Physical Performance of Older Women—A Randomized Controlled Trial, Nutrients, 2023, 15(13), 2922, DOI:10.3390/nu15132922.
- M. V. Calvo, V. Loria-Kohen, C. Díaz-Mardomingo, S. García-Herranz, S. Baliyan, J. Tomé-Carneiro, G. Colmenarejo, F. Visioli, C. Venero and J. Fontecha, Milk Fat Globule Membrane-Enriched Milk Improves Episodic Memory: A Randomized, Parallel, Double-Blind, Placebo-Controlled Trial in Older Adults, J. Funct. Foods, 2023, 111, 105849, DOI:10.1016/j.jff.2023.105849.
- B. J. Klievik, A. D. Tyrrell, C. T. Chen and R. P. Bazinet, Measuring Brain Docosahexaenoic Acid Turnover as a Marker of Metabolic Consumption, Pharmacol. Ther., 2023, 248, 108437, DOI:10.1016/j.pharmthera.2023.108437.
- R. P. Bazinet, A. H. Metherel, C. T. Chen, S. R. Shaikh, A. Nadjar, C. Joffre and S. Layé, Brain Eicosapentaenoic Acid Metabolism as a Lead for Novel Therapeutics in Major Depression, Brain, Behav., Immun., 2020, 85, 21–28, DOI:10.1016/j.bbi.2019.07.001.
- V. Martínez-Sánchez, M. V. Calvo, I. Viera, J. Girón-Calle, J. Fontecha and A. Pérez-Gálvez, Mechanisms for the Interaction of the Milk Fat Globule Membrane with the Plasma Membrane of Gut Epithelial Cells, Food Res. Int., 2023, 173, 113330, DOI:10.1016/j.foodres.2023.113330.
- M. C. Crespo, J. Tomé-Carneiro, D. Gómez-Coronado, E. Burgos-Ramos, A. García-Serrano, R. Martín-Hernández, S. Baliyan, J. Fontecha, C. Venero, A. Dávalos and F. Visioli, Modulation of miRNA Expression in Aged Rat Hippocampus by Buttermilk and Krill Oil, Sci. Rep., 2018, 8(1), 3993, DOI:10.1038/s41598-018-22148-5.
- J. Tomé-Carneiro, N. Fernández-Alonso, C. Tomás-Zapico, F. Visioli, E. Iglesias-Gutierrez and A. Dávalos, Breast Milk microRNAs Harsh Journey towards Potential Effects in Infant Development and Maturation. Lipid Encapsulation Can Help, Pharmacol. Res., 2018, 132, 21–32, DOI:10.1016/j.phrs.2018.04.003.
- A. Harris, A. Roseborough, R. Mor, K. K.-C. Yeung and S. N. Whitehead, Ganglioside Detection from Formalin-Fixed Human Brain Tissue Utilizing MALDI Imaging Mass Spectrometry, J. Am. Soc. Mass Spectrom., 2020, 31(3), 479–487, DOI:10.1021/jasms.9b00110.
- I. B. Sukhov, M. F. Lebedeva, I. O. Zakharova, K. V. Derkach, L. V. Bayunova, I. I. Zorina, N. F. Avrova and A. O. Shpakov, Intranasal Administration of Insulin and Gangliosides Improves Spatial Memory in Rats with Neonatal Type 2 Diabetes Mellitus, Bull. Exp. Biol. Med., 2020, 168(3), 317–320, DOI:10.1007/s10517-020-04699-8.
- M. Murata, N. Matsumori, M. Kinoshita and E. London, Molecular Substructure of the Liquid-Ordered Phase Formed by Sphingomyelin and Cholesterol: Sphingomyelin Clusters Forming Nano-Subdomains Are a Characteristic Feature, Biophys. Rev., 2022, 14(3), 655–678, DOI:10.1007/s12551-022-00967-1.
- S. Danthine, C. Blecker, M. Paquot, N. Innocente and C. Deroanne, Évolution Des Connaissances Sur La Membrane Du Globule Gras Du Lait: Synthèse Bibliographique, Le Lait, 2000, 80(2), 209–222, DOI:10.1051/lait:2000120.
- Z. Haddadian, G. T. Eyres, P. Bremer and D. W. Everett, Polar Lipid Composition of the Milk Fat Globule Membrane in Buttermilk Made Using Various Cream Churning Conditions or Isolated from Commercial Samples, Int. Dairy J., 2018, 81, 138–142, DOI:10.1016/j.idairyj.2018.01.012.
- M. V. Calvo, M. C. Martín-Hernández, A. García-Serrano, M. P. Castro-Gómez, L. Alonso-Miravalles, R. García-Martín, J. Megino-Tello, L. Alonso and J. Fontecha, Comprehensive Characterization of Neutral and Polar Lipids of Buttermilk from Different Sources and Its Milk Fat Globule Membrane Isolates, J. Food Compos. Anal., 2020, 86, 103386, DOI:10.1016/j.jfca.2019.103386.
- H.-Y. Kim, B. X. Huang and A. A. Spector, Phosphatidylserine in the Brain: Metabolism and Function, Prog. Lipid Res., 2014, 56, 1–18, DOI:10.1016/j.plipres.2014.06.002.
- L. Svennerholm, Distribution and Fatty Acid Composition of Phosphoglycerides in Normal Human Brain, J. Lipid Res., 1968, 9(5), 570–579, DOI:10.1016/S0022-2275(20)42702-6.
- A. B. Scholey, D. A. Camfield, M. E. Hughes, W. K. Woods, C. K. Stough, D. J. White, S. V. Gondalia and P. D. Frederiksen, A Randomized Controlled Trial Investigating the Neurocognitive Effects of Lacprodan® PL-20, a Phospholipid-Rich Milk Protein Concentrate, in Elderly Participants with Age-Associated Memory Impairment: The Phospholipid Intervention for Cognitive Ageing Reversal (PLICAR): Study Protocol for a Randomized Controlled Trial, Trials, 2013, 14(1), 404, DOI:10.1186/1745-6215-14-404.
- L. Amaducci, Phosphatidylserine in the Treatment of Alzheimer's Disease: Results of a Multicenter Study, Psychopharmacol. Bull., 1988, 24(1), 130–134 CAS.
- R. R. Engel, W. Satzger, W. Günther, N. Kathmann, D. Bove, S. Gerke, U. Münch and H. Hippius, Double-Blind Cross-over Study of Phosphatidylserine vs. Placebo in Patients with Early Dementia of the Alzheimer Type, Eur. Neuropsychopharmacol., 1992, 2(2), 149–155, DOI:10.1016/0924-977x(92)90025-4.
- T. Crook, W. Petrie, C. Wells and D. C. Massari, Effects of Phosphatidylserine in Alzheimer's Disease, Psychopharmacol. Bull., 1992, 28(1), 61–66 CAS.
- P. J. Delwaide, A. M. Gyselynck-Mambourg, A. Hurlet and M. Ylieff, Double-Blind Randomized Controlled Study of Phosphatidylserine in Senile Demented Patients, Acta Neurol. Scand., 1986, 73(2), 136–140, DOI:10.1111/j.1600-0404.1986.tb03254.x.
- T. Cenacchi, T. Bertoldin, C. Farina, M. G. Fiori and G. Crepaldi, Cognitive Decline in the Elderly: A Double-Blind, Placebo-Controlled Multicenter Study on Efficacy of Phosphatidylserine Administration, Aging, 1993, 5(2), 123–133, DOI:10.1007/BF03324139.
- M. J. Glade and K. Smith, Phosphatidylserine and the Human Brain, Nutrition, 2015, 31(6), 781–786, DOI:10.1016/j.nut.2014.10.014.
- L. E. Louck, K. C. Cara, K. Klatt, T. C. Wallace and M. Chung, The Relationship of Circulating Choline and Choline-Related Metabolite Levels with Health Outcomes: A Scoping Review of Genome-Wide Association Studies and Mendelian Randomization Studies, Adv. Nutr., 2024, 15(2), 100164, DOI:10.1016/j.advnut.2023.100164.
- S. Zeisel, Choline, Other Methyl-Donors and Epigenetics, Nutrients, 2017, 9(5), 445, DOI:10.3390/nu9050445.
- J. K. Blusztajn, B. E. Slack and T. Mellott, Neuroprotective Actions of Dietary Choline, Nutrients, 2017, 9(8), 815, DOI:10.3390/nu9080815.
- F. Scarmozzino, A. Poli and F. Visioli, Microbiota and Cardiovascular Disease Risk: A Scoping Review, Pharmacol. Res., 2020, 159, 104952, DOI:10.1016/j.phrs.2020.104952.
- Q. Tian, B. A. Mitchell, A. E. Corkum, R. Moaddel and L. Ferrucci, Metabolites Associated with Memory and Gait: A Systematic Review, Metabolites, 2022, 12(4), 356, DOI:10.3390/metabo12040356.
| This journal is © The Royal Society of Chemistry 2024 |