Biological fate, functional properties, and design strategies for oral delivery systems for cinnamaldehyde
Xiaolan
Weng
a,
Chi-Tang
Ho
 b and
Muwen
Lu
b and
Muwen
Lu
 *a
*a
aGuangdong Provincial Key Laboratory of Nutraceuticals and Functional Foods, College of Food Science, South China Agricultural University, Guangzhou 510642, China. E-mail: muwen90@scau.edu.cn; Tel: +18664527647
bDepartment of Food Science, Rutgers University, New Brunswick, NJ 08901, USA
First published on 9th May 2024
Abstract
Cinnamaldehyde (CA) is the main bioactive component extracted from the internal bark of cinnamon trees with many health benefits. In this paper, the bioavailability and biological activities of cinnamaldehyde, and the underlying molecular mechanism are reviewed and discussed, including antioxidant, cardioprotective, anti-inflammatory, anti-obesity, anticancer, and antibacterial properties. Common delivery systems that could improve the stability and bioavailability of CA are also summarized and evaluated, such as micelles, microcapsules, liposomes, nanoparticles, and nanoemulsions. This work provides a comprehensive understanding of the beneficial functions and delivery strategies of CA, which is useful for the future application of CA in the functional food industry.
1. Introduction
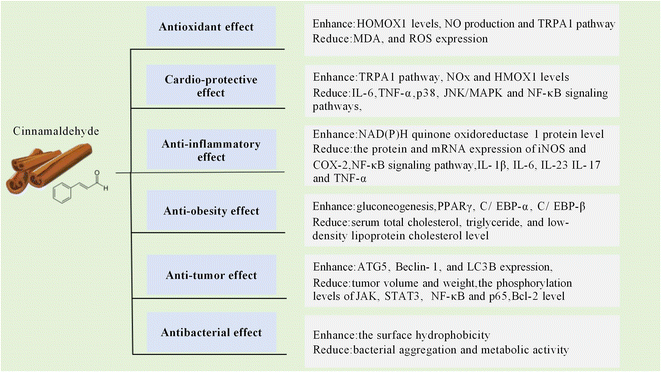
Cinnamon is a popular spice around the world, which contains a variety of vitamins, minerals, and phytochemicals with many health benefits.1 Cinnamaldehyde (CA) is the major bioactive constituent extracted from the inner bark of various tree species within the Cinnamomum genus with concentrations as high as 90.5% in Cinnamomum verum and 85.3% in Cinnamomum cassia.2 The biosynthetic pathway leading to CA synthesis begins in the leaves and bark tissues of the plant.3 The photosynthetic process facilitates the generation of the precursor molecule phenylalanine, which subsequently undergoes a complicated biosynthetic pathway, leading to the formation of cinnamic acid.4 The cinnamic acid is then transported to the bark tissue and reduced to CA through a catalytic reaction.5CA has been reported to exhibit diverse pharmacological properties, including antioxidant,6 cardioprotective,7,8 anti-inflammatory,9,10 anti-obesity,11,12 antitumor,13,14 and antibacterial activities15–17 (as shown in Fig. 1). However, due to the low water solubility and susceptibility to oxygen, light and high temperature, the stability and bioavailability of CA are negatively affected.18 Consequently, much effort has been made in recent decades to develop delivery systems for CA to enhance its biological efficacy. In this review, the bioavailability, pharmacological properties and underlying molecular mechanisms of CA are reviewed and discussed. Furthermore, various delivery systems for improving the bioavailability of CA are also summarized.
2. Bioavailability of cinnamaldehyde
A variety of studies had been conducted to evaluate the oral bioavailability of CA in the aspect of absorption, tissue distribution, metabolism, and excretion.19–21 Zhu et al. reported that after oral administration, CA was partially metabolized into cinnamic acid in the stomach and small intestine and was almost completely converted into cinnamic acid in the livers of rats.22 Sapienza et al. studied the primary metabolic pathway of CA in rats and reported that CA was firstly oxidized into benzoic acid by β-oxidation, which then reacted with glycine to form hippuric acid, and was excreted in urine. The major urinary metabolite was hippuric acid.1,23Yong et al. studied the pharmacokinetics of CA after oral administration using male SD rats and found that the area under the blood concentration-time curve was 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 648.38 ± 482.32 (mg min) L−1.24 The peak concentration of 301.61 ± 67.91 mg L−1 was reached at 20 minutes after oral administration. Ji et al. reported that the half-life for CA reached 8.7 ± 0.7 h after oral administration in rats.25 It was found that CA in rat tissues was mainly distributed in the gastrointestinal tract, livers, and kidneys in male Fischer 344 rats after the oral administration of 14C-labelled CA.20,23 According to Zhao et al., CA could not be detected after 24 hours of oral administration, indicating that CA was completely metabolized within one day.26
648.38 ± 482.32 (mg min) L−1.24 The peak concentration of 301.61 ± 67.91 mg L−1 was reached at 20 minutes after oral administration. Ji et al. reported that the half-life for CA reached 8.7 ± 0.7 h after oral administration in rats.25 It was found that CA in rat tissues was mainly distributed in the gastrointestinal tract, livers, and kidneys in male Fischer 344 rats after the oral administration of 14C-labelled CA.20,23 According to Zhao et al., CA could not be detected after 24 hours of oral administration, indicating that CA was completely metabolized within one day.26
3. Biological efficacy of cinnamaldehyde
3.1 Antioxidant effect
The antioxidant properties of CA had been examined in numerous studies, which are summarized in Table 1. Tanaka et al. reported that CA had an effective antioxidant effect in the ultraviolet radiation B-induced keratinocytes by enhancing the expression of heme oxygenase 1 (HMOX1) (an enzyme responsible for oxidative degradation of the heme group).27 Uchi et al. discovered that CA effectively inhibited the nuclear translocation of aryl hydrocarbon receptor (AHR) induced by benzo(α)pyrene in human immortalized keratinocyte (HaCaT) cells, thereby inhibiting the overproduction of reactive oxygen species (ROS).28 CA had also been found to significantly inhibit ROS production in peripheral blood mononuclear cells of patients with rheumatoid arthritis, mouse skin tissue, and young mouse hepatocytes,27,29,30 suggesting great potential in the treatment of oxidative stress-induced diseases. Mitamura et al. claimed that CA suppressed the expression of periostin (a multifunctional matricellular protein in inflammatory microenvironments) through transforming growth factor (TGF)-β1 and the interleukin (IL)-13 signaling pathway by activating nuclear factor erythroid-derived 2-like 2 (Nrf2).31 Another study using rats fed a high-fat diet (HFD) revealed that CA significantly reduced the HFD-induced oxidative stress by restoring changes in the concentration of nitric oxide metabolites (NOx) in the serum and cerebellum.32 Therefore, CA has great potential to be used as a promising dietary supplement to prevent oxidation.| In vitro/in vivo model | Observations | Ref. |
|---|---|---|
| Ultraviolet radiation b-induced keratinocytes | Enhance induction of HMOX1 expression | 27 |
| Mouse skin tissue | Downregulate the expression of MDA | 27 |
| Decrease lipid peroxidation | ||
| Decrease ROS | ||
| HaCaT cells | Inhibit nuclear translocation of AHR | 28 |
| Decrease ROS | ||
| Rheumatoid arthritis patient peripheral blood mononuclear cells | Reduce ROS | 30 |
| Young mouse hepatocytes | Reduce ROS | 29 |
| Dermal fibroblasts | Decrease POSTN | 31 |
| Rats fed a high-fat diet | Decrease MDA in the serum and brain | 32 |
| Restore the concentration of NOx | ||
| Downregulate lipid peroxidation levels | ||
| Diminish weight gain | ||
| Albino Wistar rats | Inhibit the activity of superoxide dismutase (SOD) | 91 |
| Decrease the expression of CAT | ||
| Fat-sucrose diet/streptozotocin (STZ) rat model | Enhance reduced glutathione (GSH) and ascorbate of placental content | 92 |
| Suppress MDA and total non-enzymatic antioxidant power | ||
| Reduce flavonoids and total ascorbate | ||
| Monosex Nile tilapia fingerlings (Oreochromis niloticus) | Decrease muscle MDA | 93 |
| Activate the activity of glutathione reductase | ||
| Cynoglossus semilaevis | Reduce the accumulation of MDA | 94 |
| Increase the activities of antioxidant enzymes including superoxidase dismutase, CAT, total antioxidant capacity and glutathione peroxidase | ||
| Myocardial ischemia/reperfusion injury rat model | Increase the levels of GSH and SOD | 95 |
| Inhibit the activity of MDA |
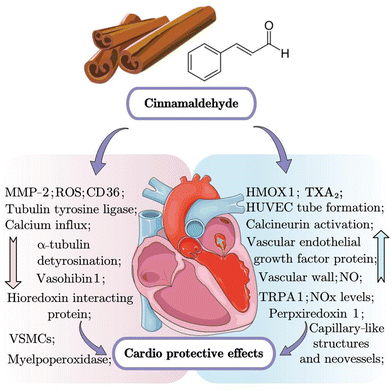
3.2 Cardioprotective effect
Researchers had reported that CA could protect against cardiovascular disorders in various cell and animal models.33–35 Li et al. found that CA inhibited the proliferation, migration and inflammation of the oxidized low-density lipoprotein-induced vascular smooth muscle cells (VSMC), and foam cell formation by reducing the overexpression of MMP-2 and lectin-like oxidized low density lipoprotein receptor-1, upregulating the expression of HMOX1.33 According to Zheng et al., CA ameliorated abnormal myocardial morphology including edema, unclear striations, and some areas of necrosis and apoptosis in myocardial ischemia/hypoxia model through activation of the phosphatidylinositol 3-kinase/protein kinase B (PI3K/AKT) signaling pathway.36 Many studies had reported the vasodilatory effect of CA by inhibiting calcium influx and release.35,37,38 According to a study using an adult male white rabbit model of subarachnoid hemorrhage, CA increased the cross-sectional areas of the basilar artery and decreased the arterial wall thickness via multiple pathways, such as transient receptor potential ankyrin-1 (TRPA1) agonism, resulting in the release of calcitonin gene-related peptides, indicating the neuroprotective and cerebral vasospasm-preventing properties of CA.38 Therefore, CA exerts effective cardioprotective properties by activation of the TRPA1 channel and regulating the expression levels of HMOX1 and MMP-2 via the PI3K/AKT signaling pathway (as summarized in Fig. 2 and Table 2).| In vitro/in vivo model | Observations | Ref. |
|---|---|---|
| Fructose-exposed H9c2 cells | Restore fructose-induced changes in TGF-β, p-Smad2/3 and Smad4 protein levels; | 96 |
| Reduce nicotinamide adenine dinucleotide phosphate oxidase and xanthine oxidase activities; | ||
| Attenuate ox-ldl-induced ROS overproduction, hioredoxin-interacting protein overexpression and CD36 upregulation; | ||
| Neonatal rat cardiac myocytes and adult mouse cardiac myocytes | Inhibit the expression of vasohibin 1, small vasohibin binding protein and tubulin tyrosine ligase and α-tubulin detyrosination; | 97 |
| enhance calcineurin activation caused by phenylephrine; | ||
| reduce store-operated Ca2+ entry and stromal interaction molecule-1/orai1 translocation; | ||
| protect T-tubule structure, calcium handling, and sarcomere contractility; | ||
| Human umbilical vein endothelial cells (HUVEC) | Activate the phosphatidylinositol 3-kinase and mitogen-activated protein kinase pathways; | 98 |
| enhance the secretion of vascular endothelial growth factor and HUVEC tube formation; | ||
| reduce HUVEC tube damage | ||
| The mouse Matrigel plug assay | Form more capillary-like structures and neovessels, and thicken the vascular wall; | |
| increase vascular endothelial growth factor protein around the damaged tissues | ||
| The adult male white rabbit model of subarachnoid hemorrhage | Decrease the level of TXA2, an important regulator of vascular tension; | 38 |
| activate TRPA1, resulting in the release of the calcitonin gene-related peptide | ||
| VSMC | Suppress the migration of VSMC induced by human oxidized low-density lipoprotein; | 33 |
| upregulate the expression of HMOX1; | ||
| block the cell cycle of VSMC in the S phase; | ||
| suppress the overexpression of MMP-2, p38, JNK/MAPK and NF-κB signaling pathways | ||
| Thoracic aortas of male Sprague-Dawley rats | Activate the TRPA1 pathway; | 37 |
| release the calcitonin gene-related peptide; | ||
| inhibit calcium influx and release | ||
| Rabbits on a high cholesterol diet | Reduce atherosclerotic lipid deposition, myeloperoxidase activity and high-cholesterol diet aortic intima/media ratio; | 99 |
| increase NOx levels; | ||
| decrease expression of cholesteryl ester transfer protein and myeloperoxidase | ||
| Thoracic aorta of male Wistar rats | Reduce the exaggerated contraction of vascular and the formation of advanced glycation end products (AGEs); | 8 |
| stimulate nitric oxide(NO) production; | ||
| Myocardial ischemia/reperfusion injury rat model | Reverse the decrease of left ventricular ejection fraction, left ventricular fractional shortening and stroke volume; | 95 |
| Reverse the increase of left ventricular internal diameter at end-systole and left ventricular internal diameter at end-diastole; | ||
| inhibit the elevations of cardiac troponin I, creatine kinase-MB, lactate dehydrogenase, and aspartate aminotransferase; | ||
| suppress the increase of mRNA and protein expression levels of gasdermin (GSDMD); | ||
| decrease the expression levels of IL-6, necrosis factor-alpha (TNF-α), nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3), pro-caspase-1, caspase-1, and apoptosis-associated speck-like protein containing a carboxy-terminal CARD (ASC) | ||
| Zucker diabetic fatty rats | Inhibit platelet derived growth factor-induced VSMC proliferation; | 100 |
| enhance the level of glutamate-cysteine ligase catalytic subunit and peroxiredoxin 1 | ||
| Mice subjected to subcutaneous administration of phenylephrine | Inhibit the elevation of hypertrophic genes, including Nppa, Nppb and Mhy7; | 101 |
| prevent the increase of the interventricular septal thickness at both systole and diastole, as well as the increase of the phosphorylation of calcium/calmodulin-dependent protein kinase II and extracellular signal-related kinase |
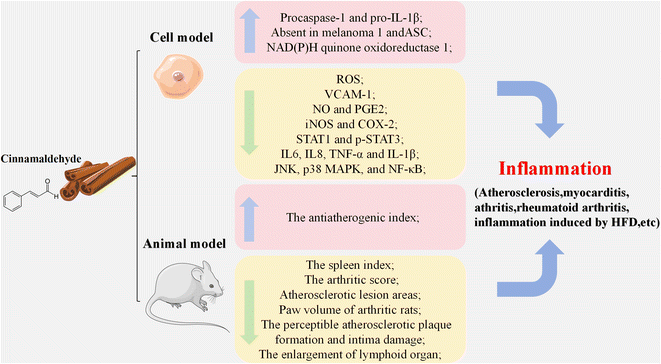
3.3 Anti-inflammatory effect
Multiple studies had indicated the potential application of CA as an anti-inflammatory agent as shown in Fig. 3 and Table 3.39–42 According to Kim et al., CA effectively suppressed U937 monocytic cells adhesion to human umbilical vein endothelial cells by reducing the expression of vascular cell adhesion protein 1 (VCAM-1).42 Zhu et al. discovered that CA improved the immune function in helminth-infected rodents by suppressing the expression of toll-like receptor (TLR) and regulating T-cell populations in mesenteric lymph nodes.9trans-Cinnamaldehyde (TCA) had been found to reduce the proliferation of pro-inflammatory cytokines in C2C12 mouse skeletal muscle cells treated with lipopolysaccharide (LPS) by suppressing the expression levels of TLR-4 and inhibiting the NF-κB signaling pathway, as well as downregulating the expression of nitric oxide (NO) and prostaglandin E2 (PGE2), inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2).39,43 According to Li et al., CA could alleviate cartilage destruction in the C28/I2 human chondrocyte cell line via decreasing the mRNA expression levels of MMP-1, MMP-3 and MMP-13 as well as reducing the levels of p-p38 and p-JNK, demonstrating an anti-inflammatory effect in vitro and a protective effect on chondrocytes in vivo.10 Mateen et al. discovered that CA exerted an anti-inflammatory effect in peripheral blood mononuclear cells (PBMCs) from patients with rheumatoid arthritis by effectively inhibiting the secretion of pro-inflammatory cytokines TNF-α and IL-6 and regulating the equilibrium of cytokines secreted by Th1 and Th2 cells.30 Another study stated that CA alleviated collagen-induced swollen paw volume in rats with arthritis by inhibiting the phosphorylation of janus kinase 2 (JAK2) and decreasing the expression of p-signal transducer and activator of transcription 1 (STAT1) and p-STAT3.44 Therefore, CA has great potential to be used as an anti-inflammatory agent by suppressing the pro-inflammatory mediators and cytokines, such as TNF-α, IL-6, IL-8 IL-10, IL-17 and IL-1β.| In vitro/in vivo model | Observations | Ref. |
|---|---|---|
| U937 | Reduce the expression of VCAM-1; | 42 |
| C2C12 mouse skeletal muscle cells | Inhibit the expression of common pro-inflammatory mediators such as NO and PGE2 by suppressing the protein and mRNA expression of iNOS and COX-2; | 39 |
| suppress the expression levels of TLR-4; | ||
| inhibit the NF-κB signaling pathway | ||
| C28/I2 cells | Alleviate cartilage destruction; | 10 |
| Reduce the mRNA expression levels of MMP-1, MMP-3, MMP-13, ADAMTS-4 and ADAMTS-5 | ||
| PBMC | Inhibit the secretion of pro-inflammatory cytokines | 30 |
| Regulate the equilibrium of cytokines secreted by Th1 and Th2 cells | ||
| MH7A | Inhibit the phosphorylation of JAK2; | 44 |
| decrease the expression of STAT1 and p-STAT3; | ||
| alleviate the collagen-induced arthritis by decreasing in paw swelling and significant reduction in histologic changes; | ||
| reduce the serum levels of pro-inflammatory cytokines IL-1β and IL-6 | ||
| BV-2 microglial cells | Inhibit the activation of extracellular-regulated kinase, JNK, p38 MAPK, and NF-κB; | 102 |
| decrease microglia-mediated neuroblastoma cell death | ||
| RAW 264.7 murine macrophage cells | Inhibit the expression of Porphyromonas gingivalis (Pg) supernatant-induced IL-6, TNF-α and IL-1β; | 103 |
| attenuate the elevation of ROS; | ||
| inhibit the activation of the NF-κB signaling pathway | ||
| Human periodontal ligament cells | Suppress the expression of Pg supernatant-induced IL-6, IL-8, TNF-α and IL-1β; | |
| inhibit the elevation of monocyte chemoattractant protein 1, intercellular adhesion molecule 1 and VCAM-1 induced by the Pg supernatant; | ||
| attenuate the elevation of ROS; | ||
| THP-1 cells | Inhibit the release of TNF-a and IL-1β; | 104 |
| suppress the phosphorylation of JNK, p38, and NF-κB; | ||
| increase the expression of absent in melanoma 1, ASC, procaspase-1 and pro-IL-1β | ||
| Human keratinocytes | Activate the Nrf2 pathway; | 105 |
| increase HMOX1; | ||
| enhance the protein level of NAD(P)H quinone oxidoreductase 1 | ||
| Female C57BL/6 mice | Downregulate inflammation-related pathways in both healthy and helminth-infected rodents; | 9 |
| alter immune-related genes and NRF2-related xenobiotic-metabolizing pathways | ||
| Raw 264.7 murine macrophage cells | Suppress the protein and mRNA expression of iNOS; | 43 |
| decrease the mRNA expression level and secretion of IL-1β, IL-6 and TNF-α | ||
| Endotoxin-induced mice | Reverse endotoxin-induced body weight loss and the enlargement of lymphoid organ; | 106 |
| decrease endotoxin-induced levels of peripheral nitrate/nitrite; | ||
| downregulate IL-1β, IL-18, TNF-α, interferon-γ, and high-mobility group box 1 protein; | ||
| inhibit the activation of NF-κB and caspase-1; | ||
| inhibit the expression of caspase-recruitment domain and NLRP3; | ||
| enhance the expression of TLR4, myeloid differentiation protein 2, and myeloid differentiation primary response gene 88 | ||
| Male Wistar rats fed with HFD | Reduce the anti-atherogenic index | 107 |
| Overactive bladder-related murine model | Inhibit overexpression of macrophage migration inhibitory factor and TLR4 | 108 |
| Complete Freund's adjuvant-induced arthritis in mice | Downregulate the expressions of TNF-α, NF-κB and COX-2 | 109 |
| Reduce the levels of IL-1β, IL-6, IL-23 and IL-17 | ||
| ApoE−/− mice | diminish atherosclerotic lesion areas; | 40 |
| reduce the perceptible atherosclerotic plaque formation and intima damage; | ||
| Female Wistar rats | Decrease the spleen index; | 41 |
| decrease the arthritic score; | ||
| reduce paw volume of arthritic rats |
3.4 Anti-obesity effect
CA had been reported to have an anti-obesity effect by reducing body weight and fat accumulation, as well as preventing obesity-related metabolism disorders, as summarized in Table 4.45–49 Neto et al. claimed that CA treatment reduced the size of white adipose tissue (WAT) adipocytes by decreasing the expression of peroxisome proliferator-activated receptor gamma (PPARγ).46 In another rat model of early obesity, CA was found to decrease the synthesis of lipid in the liver possibly through attenuating the increments of the mRNA expressions of sterol regulatory element-binding protein (SREBP1c) and acetyl-CoA carboxylase 1 (ACC1).47 Hoi et al. found that CA facilitated the release of serotonin via the TRPA1 pathway in differentiated Caco-2 cells, thereby alleviating the reduction of fatty acid uptake.50 CA treatment could also lead to a reduction in triglyceride accumulation (an indicator of fat content) in 3T3-L1 cells via the activation of TRPA1 channels.51 Abdelmageed et al. stated that CA improved lipid metabolism disorders by attenuating the levels of triacylglycerols, total cholesterol and very-low-density lipoprotein cholesterol.49 Therefore, CA treatment could be an effective approach for treating obesity and related metabolic disorders.| In vitro/in vivo model | Observations | Ref. |
|---|---|---|
| Caco-2 cells | Alleviate the reduction of fatty acid uptake; | 50 |
| induce serotonin release; | ||
| 3T3-L1 cells | Activate of TRPA1 channels; | 51 and 110 |
| reduce in triglyceride accumulation; | ||
| decrease the content of microRNA (miR)320; | ||
| increase the content of miR26-b; | ||
| downregulate the expression levels of key lipogenic transcription factors PPARγ, C/EBP-α, C/EBP-β, and lipogenic markers FAS gene and protein levels | ||
| Primary human omental preadipocytes | Enhance insulin-induced activation of Akt2 and glucose uptake; | 111 |
| Alpha mouse liver 12 cells | increase the expression of methyltransferase 3; | 11 |
| upregulate the levels of capric acid, gamma-linolenic acid, arachidonic acid, and docosapentaenoic acid; | ||
| increase CYP4F40 expression; | ||
| alleviate steatosis | ||
| Male C57BL/6J rat model fed an HFD | Decrease HFD-induced body weight gain; | 45 |
| diminish plasma free fatty acid and leptin levels; | ||
| inhibit the elevation of serum total cholesterol, triglyceride, and low-density lipoprotein cholesterol level; | ||
| enhance the expression of the peroxisome PPARγ, PR domain-containing 16 and PPARγ coactivator 1α proteins | ||
| HepG2 cells | Decrease the activity of G6Pase; | 12 |
| inhibit glucose metabolism of α-enolase; | ||
| enhance gluconeogenesis; | ||
| increase the phosphorylation level of AMP-activated protein kinase | ||
| STZ-induced T2D rat model | Decrease serum alanine aminotransferase and aspartate aminotransferase activity, serum AGEs and its receptors in the aorta; | 49 |
| decrease hepatic MDA; | ||
| increase hepatic and aortic glutathione and SOD; | ||
| inhibit steatosis and inflammation in the liver tissue | ||
| Male rat model of early obesity | Reduce Srebf1c and Acaca expression; | 46 and 47 |
| diminish WAT and brown adipose tissue adipocyte; | ||
| reduce expression of lipogenesis-related genes like PPARγ and Dgat2; | ||
| increase BAT thermogenesis markers such as PPARα, Fgf21 and Ucp1; | ||
| reduce lipogenesis marker expression such as PPARγ and lipoprotein lipase | ||
| Male Wistar rats fed an HFD | Decrease the NOx level of HFD rats; | 48 |
| reduce NO-induced insulin secretion; | ||
| inhibit iNOS activity of HFD rats | ||
| Male db/db mice | Decrease the level of GHbA1c; | 12 |
| decrease glycerophosphocholine levels; | ||
| diminish long-chain fatty acids, such as myristoleic acid and oleic acid; | ||
| upregulate 1-stearoyl-2-oleoyl-sn-glycerol 3-phosphocholine |
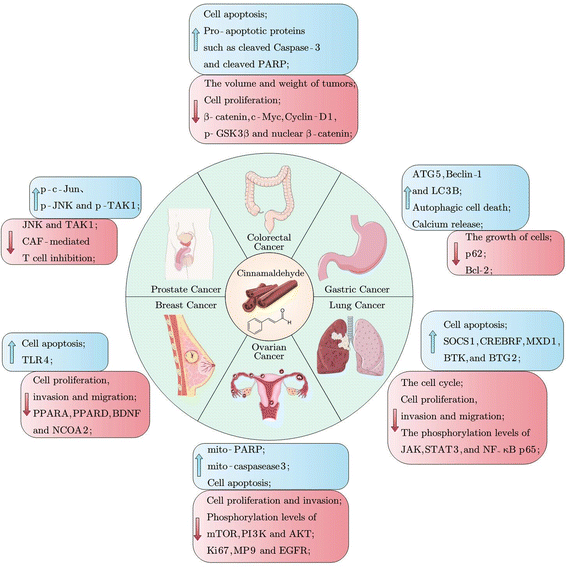
3.5 Anti-cancer effect
Cancer is one of the leading causes of death worldwide, and its prevention could be aided by the supplementation of various dietary phytochemicals.52 CA exerted anti-cancer effects in various cancer cells through promoting cell apoptosis and inhibiting cell proliferation, migration, and invasion, as shown in Fig. 4 and Table 5.53–55 Chen et al. discovered that CA treatment suppressed cell proliferation in A549, NCIH1650, SK-MES-1, and NCI-H226 cells by elevation of suppressors of cytokine signaling 1, BTG anti-proliferation factor 2 and Bruton tyrosine kinase via the JAK/STAT signaling pathway.55 CA suppressed the cell viability of the MDA-MB-231 human breast cancer cell line by reducing the elevated expression of proliferation-related proteins mammalian target of rapamycin, PI3K, and proliferating cell nuclear antigen (PCNA), indicating the potential of CA against breast cancer.52 Mei et al. discovered that CA treatment attenuated the inhibition of T cells mediated by cancer-associated fibroblasts (CAFs) by altering the expression levels of TLR4 pathway-related proteins such as phospho-c-Jun N-terminal kinase (p-JNK), JNK, p-TAK1, TAK1, and p-c-Jun, thus activating the TLR4 pathway.56 Kim et al. reported that CA mediated autophagy in NCI-N87 and MKN-74 cells by downregulating p62 expression and upregulating ATG5, Beclin-1, and LC3-II expression, thereby inhibiting the growth of gastric cancer cells.57 In a visfatin-induced proliferation xenograft animal model, tumor volume and weight decreased in mice treated with CA via downregulation of the expression of PCNA.52 Therefore, CA has great potential in the prevention and treatment of cancers, including breast cancer, gastric cancer, lung cancer, prostate cancer, colorectal cancer, bladder cancer, and ovarian cancer.58–60| Cancer model | Cell model | Effect and mechanism | Ref. |
|---|---|---|---|
| Breast cancer | MDA-MB-231 cells | Suppress cell proliferation, invasion, and migration; | 52 and 53 |
| induce apoptosis; | |||
| downregulate the levels of key proteins such as PPARA, PPARD, BDNF and NCOA2; | |||
| Upregulate the expression of TLR4; | |||
| Visfatin-induced proliferation xenograft animal model | decrease tumor volume and weight were in mice; | ||
| diminish the expression of PCNA protein, intracellular and extracellular nicotinamide phosphoribosyl transferase protein | |||
| Gastric cancer | NCI-N87 cells | Induce autophagic cell death and accelerate calcium release; | 57 |
| upregulate ATG5, Beclin-1, LC3B, GRP78, p-PERK, p-eIF2α and CHOP; | |||
| MKN-74 cells | Increase caspase 3 and 9 cleavage; | ||
| downregulae Bcl-2 and p62 expression; | |||
| Lung cancer | A549 cells | Decrease the relative cell activity; | 54 and 55 |
| arrest cell cycle; | |||
| increase the number of apoptotic cells; | |||
| NSCLC cells | Induce cell apoptosis and suppress cell proliferation; | 55 | |
| inhibit cell invasion and migration; | |||
| enhance the expression levels of cytokine signaling 1, CREB3 regulatory factor, MAX dimerization protein 1, BTG anti-proliferation factor 2, Bruton tyrosine kinase, lncRNAsLINC01504, LUCAT1, LINC01484, THUMPD3-AS1, and LINC01783; | |||
| decrease the phosphorylation levels of JAK, STAT3, NF-κB and p65 | |||
| Subcutaneous tumor implantation model | Decrease the volume and weight of tumors; | ||
| Prostate cancer | CAFs | Alter the expression levels of TLR4 pathway-related proteins such as phospho-c-Jun N-terminal kinase (p-JNK), JNK, p-TAK1, TAK1, and p-c-Jun; | 56 |
| relieve CAF-mediated T cell inhibition; | |||
| Colorectal cancer | Xenograft mouse model | Decrease tumor volume and weight were in mice; | 59 |
| downregulate Bcl-2; | |||
| upregulate Bax, pro-apoptotic proteins such as cleaved caspase-3 and cleaved PARP | |||
| Human CRC cell lines HCT116 and SW480 | Induce cell apoptosis and inhibit proliferation; | ||
| upregulate pro-apoptotic proteins such as cleaved caspase-3 and cleaved PARP; | |||
| downregulate β-catenin, c-Myc, Cyclin-D1, p-GSK3β and nuclear β-catenin; | |||
| Ovarian cancer | A2780 and SKOV3 cells | Inhibit proliferation and invasion, and phosphorylation levels of mTOR, PI3K, and AKT; | 60 |
| increase the expression of mito-PARP and mito-caspase-3; | |||
| induce cell apoptosis | |||
| Subcutaneous xenograft model of the A2780 cells in nude mice | Decrease tumor weight in mice; | ||
| suppress the expression levels of Ki67, MP9 and EGFR |
3.6 Antibacterial effect
Many studies had shown that CA exerted antibacterial activity by inhibiting the growth of infectious microorganisms.61–65 He et al. reported that CA inhibited the growth of Streptococcus mutans by decreasing bacterial aggregation and metabolic activity, and increasing surface hydrophobicity.65 CA had also been reported to inhibit the proliferation of Escherichia coli (E. coli.) in the type 2 human epithelial cell model by reducing the aggregation and adhesion of E. coli.64 Similarly, CA exerted an anti-adhesive effect on Salmonella in human colon carcinoma cells via suppressing the type I fimbriae.66 Yu et al. discovered that the intervention of CA decreased the structural integrity of cells by reducing the concentration of extracellular polymer proteins in Campylobacter, thereby inhibiting its biofilm formation.62 Usai et al. discovered that CA had a synergistic effect with antibiotics against a wide range of Gram-negative and Gram-positive bacteria, particularly drug-resistant strains and superbugs, as determined by measuring the fractional inhibitory concentration index.61 Therefore, CA showed antibacterial activity by inhibiting the growth of microorganisms, destabilizing bacterial biofilms, reducing the aggregation and adhesion of bacterial cells, and functioning as a synergistic agent with antibiotics.4. Novel delivery systems for cinnamaldehyde
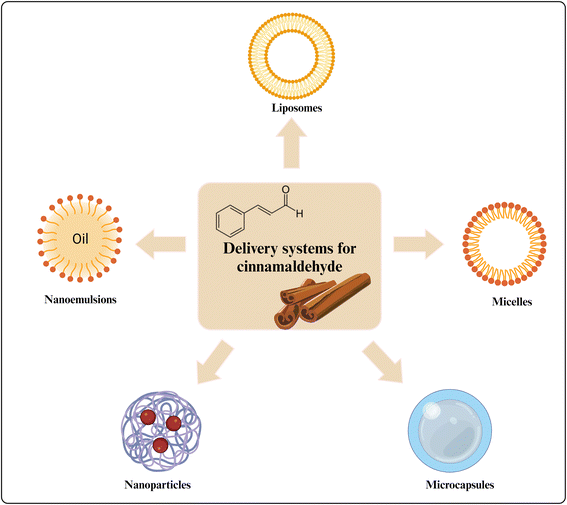
Due to the poor water solubility and sensitivity to oxygen, light, and high temperature, the biological efficacy of CA was greatly limited.67 To enhance its bioavailability, various delivery systems for CA had been designed, including micelles, microcapsules, liposomes, nanoparticles and nanoemulsions, as depicted in Fig. 5.68–70 In this section, the novel delivery systems for CA were summarized and discussed.4.1 Micelles
Micelles had been developed as nanocarriers for hydrophobic nutraceuticals, consisting of spherical, self-assembled amphiphilic block copolymers with diameters between 10 and 100 nanometers.71 Cartaya et al. encapsulated CA into poly(lactic-co-glycolic acid)-poly(siloxane) pluronic micelles using a direct dissolution method to prepare antioxidant response-activated nanospheres, providing complete drug integrity protection and selective targeting of cells, effectively inhibiting the proliferation and migration of vascular smooth muscle cells.72 CA-loaded micelles significantly increased the content of GSH compared to free unformulated CA. Raffai et al. reported that CA-loaded micelles were synthesized by the Michael addition reaction.73 Those CA-loaded micelles possessed vasodilator properties, shown to relieve coronary vasospasm and inhibit the Ca2+ influx in porcine coronary arteries. Deng et al. developed a pH-sensitive charge-conversion CA polymeric prodrug micelle using a reversible addition–fragmentation chain transfer polymerization method.74 The CA-loaded micelles had been proven to have a reduced particle size of 227 nm and enhanced anti-tumor effects on 143B osteosarcoma cells compared with free CA, indicating that micelles had great potential to reduce particle sizes and enhance the bioavailability of CA.4.2 Microcapsules
Microcapsules have been widely used to protect bioactive compounds from oxidation and volatilization, effectively improving the drug stability.21,75,76 Li et al. prepared a three-matrix system of methyl cellulose/carboxymethyl chitosan/sodium alginate using a spray drying method to encapsulate CA with a 50% encapsulation rate and a 4% loading capacity.77 Physicochemical and biological studies showed that CA-loaded microcapsules exhibited enhanced resistance to oxidation, hydrolysis, and thermal decomposition, with prolonged storage and improved bioavailability. Xiao et al. claimed that the encapsulation of CA using β-cyclodextrin significantly elevated the plasma CA concentration while reducing the levels of its metabolites in male C57/BL6 mice.21 Notable distinctions in richness and diversity were observed in the fecal microbiota compared to the CA group, indicating that the CA microcapsules were effective in enhancing CA absorption with a pronounced modulatory effect on gut microbiota. Chen et al. prepared CA-loaded microcapsules using tannic acid with an encapsulation efficiency of 92.53 ± 5.20%.75 Biological studies showed that the microcapsules effectively improved the antifungal activity of CA against Aspergillus brasiliensis. Wong et al. stated that the sodium alginate–chitosan at a mass ratio of 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 had an encapsulation rate of 95.25% and a loading capacity of 81.42% for CA.78 Physiochemical characterization had proved that the sodium alginate and chitosan-wrapped CA had a prolonged release and improved absorption.
1 had an encapsulation rate of 95.25% and a loading capacity of 81.42% for CA.78 Physiochemical characterization had proved that the sodium alginate and chitosan-wrapped CA had a prolonged release and improved absorption.
4.3 Liposomes
Liposomes were spherical vesicles composed of dispersed amphipathic lipids, featuring a hydrophilic head and a hydrophobic tail within an aqueous phase, which were considered ideal delivery systems for nutraceuticals due to their high drug loading rate and low biological toxicity.79 Xue et al. developed inulin-modified liposomes using the thin-film hydration method, achieving a particle size of 72.52 ± 0.71 nm and an encapsulation efficiency of 70.71 ± 0.53%.79 The inulin-modified liposomes inhibited particle aggregation, resulting in the prevention of CA leakage as well as increased storage stability and antioxidant activity. Sang et al. prepared a polymyxin B-modified liposomal system loaded with CA (P-CA-Lipo) using the thin film evaporation method, with a particle size of 150 nm, an encapsulation efficiency of 31.93 ± 0.6%, and a drug loading of 14.99 ± 0.28%.80 Compared to unformulated samples, the CA release from P-CA-Lipo was more stable and durable, exhibiting higher water solubility and stability, and improved antibacterial activity through enhanced bacterial targeting and penetration. Makwana et al. found that polydiacetylene N-hydroxysuccinimide liposomes loaded with CA could fuse with the bacterial cell membranes and release CA directly into the cells, thereby enhancing the antibacterial effect.814.4 Nanoparticles
Nanoparticles were tiny materials with particle sizes ranging from 1 to 100 nanometers, which had been widely used as nanocarriers in drug delivery.82–84 Compared with large molecules, nanoparticles were more easily absorbed by cells, thereby enhancing the pharmacological and therapeutic properties of drugs. Liu et al. developed CA-loaded nanoparticles (NPs-C) using a thermally induced gelation method, with a particle size of approximately 185 nm, an encapsulation efficiency of 76.57%, and a loading capacity of 19.02%.84 Compared with free CA, the NPs-C were more stable in harsh environments (extreme pH, high ionic strength, and elevated temperatures), with extended storage time and sustained antimicrobial activity. Li et al. discovered that solid-lipid nanoparticles containing CA could reduce the toxic metabolite malonaldehyde in strawberry cells, enhance the activity of antioxidant enzymes, and prevent cell damage.83 Physicochemical and biological studies had demonstrated that nanoparticles effectively enhanced the sustained release and mitigated the toxic effects of high CA concentrations on strawberry cells. Gursu et al. found that CA-loaded poly(DL-lactide-co-glycolide) (PLGA) nanoparticles had an enhanced stability, with a particle size of 130.4 nm, an encapsulation efficiency of 93%, and zeta potential values ranging from −3.54 to −3.86 mV.82 CA-PLGA nanoparticles demonstrated stronger antifungal and antibiofilm properties, achieving higher sterilization effects with significantly lower amounts of CA. Therefore, nanoparticles could enhance the stability and retention for CA, with sustained release and improved bio-efficacy.4.5 Nanoemulsions
Nanoemulsions were emulsions with a droplet size ranging from 10 to 1000 nm, which could effectively enhance the solubility of bioactive compounds due to high surface area and dispersibility.18 Hojati et al. prepared the CA-loaded nanoemulsion with a zeta potential of −0.60 mV and a droplet size of 146.1 nm.85 The CA-loaded nanodroplets demonstrated a reduced minimum inhibitory concentration and minimum bactericidal concentrations against S. aureus, E. coli, and C. perfringens, suggesting increased antimicrobial activity. Liu et al. used Tween 80 to encapsulate CA within a water-in-oil emulsifier, fabricating a CA nanoemulsion with an average particle size of 94.37 ± 2.12 nm and a polydispersity index (PDI) of 0.227 ± 0.008.86 The CA nanoemulsion reduced the volatility of CA and provided sustained release, effectively reducing loss in meat exudate and extending the shelf life of frozen pork. Otoni et al. prepared a CA-loaded oil-in-water nanoemulsion with particle sizes ranging from 41.32 to 271.95 nm and PDI ranging from 0.221 to 0.301.87 Microbiological assessment showed that the permeability of CA through microbial cells was enhanced due to the increased surface area of the nanoemulsion, thereby effectively enhancing the antibacterial activity.5. Summary
As a natural aromatic aldehyde, CA has wide applications in the essence and aroma industry, as well as the pharmaceutical industry because of its diverse biological activities, including antioxidant, cardioprotective, anti-inflammatory, anti-obesity, and anti-tumor. After oral administration, CA could be rapidly absorbed in the stomach and proximal small intestine, which was metabolized, and finally excreted in the urine. Studies have investigated the safety of CA.88–90 The toxicity study using animal models indicated that the acute oral lethal dose (LD50) value of CA was 3.4 g kg−1 in white rats, white mongrel mice, and guinea pigs, indicating that CA exerted a low level of toxicity.1,90 Common delivery technologies to enhance the stability and bioavailability of CA were also summarized, including micelles, microcapsules, liposomes, nanoparticles, and nanoemulsions, which exhibited good load capacity and encapsulation capability. Currently, these delivery systems face limitations in human applications since an in vitro model could not completely mimic the physiological conditions of human organs. Therefore, future studies are needed to apply more in vivo models to evaluate the bioefficacy of the abovementioned delivery systems, which could better explore the specific mechanisms underlying the enhanced bioavailability of CA. This study could improve our understanding of the biological functions and molecular mechanisms of CA, providing the scientific reference for the future application of CA in the functional food industry.Abbreviations
| ADAMTS | A disintegrin and metalloproteinase with thrombospondin motifs |
| AGEs | Advanced glycation end products |
| AHR | Aryl hydrocarbon receptor |
| AKT | Protein kinase B |
| ASC | Apoptosis-associated speck-like protein containing a carboxy-terminal CARD |
| BTG2 | BTG anti-proliferation factor 2 |
| BTK | Bruton tyrosine kinase |
| C/EBP | CCAAT/enhancer binding protein |
| CA | Cinnamaldehyde |
| CAFs | Cancer-related fibroblasts |
| CAT | Catalase |
| COX-2 | Cyclooxygenase-2 |
| FAS | Fatty acid synthase |
| GPx-1 | Glutathione peroxidase-1 |
| GSDMD | Gasdermin |
| GSH | Reduced glutathione |
| HaCaT | Human immortalized keratinocytes |
| HFD | High-fat diet |
| HMOX1 | Heme oxygenase 1 |
| HUVEC | Human umbilical vein endothelial cells |
| IL | Interleukin |
| iNOS | inducible NO synthase |
| JAK2 | Janus kinase 2 |
| JNK | C-Jun-amino terminal kinase |
| MAPK | Mitogen-activated protein kinases |
| MDA | Malondialdehyde |
| miR | MicroRNA |
| MMP | Matrix metalloproteinases |
| NF-κB | Nuclear factor kappa-B |
| NLRP3 | Nucleotide-binding oligomerization domain-like receptor family pyrin domain-containing 3 |
| NO | Nitric oxide |
| NOx | Nitric oxide metabolites |
| NPs-C | CA-loaded nanoparticles |
| Nrf2 | Nuclear factor erythroid-derived 2-like 2 |
| PBMC | Peripheral blood mononuclear cell |
| P-CA-Lipo | Polymyxin B-modified liposomal system loaded with CA |
| PCNA | Proliferating cell nuclear antigen |
| PDI | Polydispersity index |
| Pg | Porphyromonas gingivalis |
| PGE2 | Prostaglandin E2 |
| PI3K | Phosphatidylinositol 3-kinase |
| p-JNK | Phospho-c-Jun N-terminal kinase |
| PLGA | Poly(DL-lactide-co-glycolide) |
| PPAR | Proliferator-activated receptor |
| SOCS1 | Cytokine signaling 1 |
| SOD | Superoxide dismutase |
| STAT | Transcription |
| STZ | Streptozotocin |
| TCA | trans-Cinnamaldehyde |
| TGF | Transforming growth factor |
| TLR | Toll-like receptor |
| TNF-α | Tumor necrosis factor-α |
| TRPA1 | Transient receptor potential ankyrin-1 |
| VCAM-1 | Vascular cell adhesion protein 1 |
| VSMC | Vascular smooth muscle cell |
| WAT | White adipose tissue |
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was financially supported by the Program for Guangdong Introducing Innovative and Entrepreneurial Teams (Grant No. 2019ZT08N291), the Guangdong Basic and Applied Basic Research Foundation (Grant No. 2021A1515012124), and the Science and Technology Program of Guangzhou, China (Grant No. 2023A04J0760). The creation of Fig. 5 was supported by BioRender.com.References
- S. Shreaz, W. A. Wani, J. M. Behbehani, V. Raja, M. Irshad, M. Karched, I. Ali, W. A. Siddiqi and L. T. Hun, Cinnamaldehyde and Its Derivatives, a Novel Class of Antifungal Agents, Fitoterapia, 2016, 112, 116–131 CrossRef CAS PubMed.
- A. A. Doyle and J. C. Stephens, A Review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents, Fitoterapia, 2019, 139, 104405 CrossRef CAS PubMed.
- U. M. Senanayake, R. B. H. Wills and T. H. Lee, Biosynthesis of Eugenol and Cinnamic Aldehyde in Cinnamomum Zeylanicum, Phytochemistry, 1977, 16, 2032–2033 CrossRef CAS.
- J.-Q. Kong, Phenylalanine Ammonia-Lyase, a Key Component Used for Phenylpropanoids Production by Metabolic Engineering, RSC Adv., 2015, 5, 62587–62603 RSC.
- J. Koukol and E. E. Conn, The Metabolism of Aromatic Compounds in Higher Plants, J. Biol. Chem., 1961, 236, 2692–2698 CrossRef CAS PubMed.
- V. Suryanti, F. R. Wibowo, S. Khotijah and N. Andalucki, Antioxidant Activities of Cinnamaldehyde Derivatives, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 333, 012077 CrossRef.
- M. A. Nystoriak, P. J. Kilfoil, P. K. Lorkiewicz, B. Ramesh, P. J. Kuehl, J. McDonald, A. Bhatnagar and D. J. Conklin, Comparative Effects of Parent and Heated Cinnamaldehyde on the Function of Human iPSC-Derived Cardiac Myocytes, Toxicol. in Vitro, 2019, 61, 104648 CrossRef CAS PubMed.
- M. M. Tarkhan, K. S. Balamsh and H. M. El-Bassossy, Cinnamaldehyde Protects from Methylglyoxal–induced Vascular Damage: Effect on Nitric Oxide and Advanced Glycation End Products, J. Food Biochem., 2019, 43(7), e12907 CrossRef PubMed.
- L. Zhu, A. I. S. Andersen-Civil, L. J. Myhill, S. M. Thamsborg, W. Kot, L. Krych, D. S. Nielsen, A. Blanchard and A. R. Williams, The Phytonutrient Cinnamaldehyde Limits Intestinal Inflammation and Enteric Parasite Infection, J. Nutr. Biochem., 2022, 100, 108887 CrossRef CAS PubMed.
- G. Li and Y. Song, Cinnamaldehyde Induces the Expression of MicroRNA-1285-5p and MicroRNA-140-5p in Chondrocytes to Ameliorate the Apoptosis and Inflammatory Response, Cartilage, 2023, 14, 375–385 CrossRef CAS PubMed.
- R. Xu, X. Xiao, S. Zhang, J. Pan, Y. Tang, W. Zhou, G. Ji and Y. Dang, The Methyltransferase METTL3-Mediated Fatty Acid Metabolism Revealed the Mechanism of Cinnamaldehyde on Alleviating Steatosis, Biomed. Pharmacother., 2022, 153, 113367 CrossRef CAS PubMed.
- J. Gao, M. Zhang, R. Niu, X. Gu, E. Hao, X. Hou, J. Deng and G. Bai, The Combination of Cinnamaldehyde and Kaempferol Ameliorates Glucose and Lipid Metabolism Disorders by Enhancing Lipid Metabolism via AMPK Activation, J. Funct. Foods, 2021, 83, 104556 CrossRef CAS.
- A. Nile, J. Shin, J. Shin, G. S. Park, S. Lee, J.-H. Lee, K.-W. Lee, B. G. Kim, S. G. Han, R. K. Saini and J.-W. Oh, Cinnamaldehyde-Rich Cinnamon Extract Induces Cell Death in Colon Cancer Cell Lines HCT 116 and HT-29, Int. J. Mol. Sci., 2023, 24, 8191 CrossRef CAS PubMed.
- Y.-T. Kuo, C.-H. Liu, S. H. Wong, Y.-C. Pan and L.-T. Lin, Small Molecules Baicalein and Cinnamaldehyde Are Potentiators of Measles Virus-Induced Breast Cancer Oncolysis, Phytomedicine, 2021, 89, 153611 CrossRef CAS PubMed.
- M. Albano, B. P. Crulhas, F. C. B. Alves, A. F. M. Pereira, B. F. M. T. Andrade, L. N. Barbosa, A. Furlanetto, L. P. D. S. Lyra, V. L. M. Rall and A. F. Júnior, Antibacterial and Anti-Biofilm Activities of Cinnamaldehyde against S. Epidermidis, Microb. Pathog., 2019, 126, 231–238 CrossRef CAS PubMed.
- G.-F. Du, X.-F. Yin, D.-H. Yang, Q.-Y. He and X. Sun, Proteomic Investigation of the Antibacterial Mechanism of Trans -Cinnamaldehyde against Escherichia Coli, J. Proteome Res., 2021, 20, 2319–2328 CrossRef CAS PubMed.
- M. Y. Wani, A. Ahmad, F. M. Aqlan and A. S. Al-Bogami, Modulation of Key Antioxidant Enzymes and Cell Cycle Arrest as a Possible Antifungal Mode of Action of Cinnamaldehyde Based Azole Derivative, Bioorg. Med. Chem. Lett., 2022, 73, 128922 CrossRef CAS PubMed.
- B. Muhoza, B. Qi, J. D. Harindintwali, M. Y. F. Koko, S. Zhang and Y. Li, Encapsulation of Cinnamaldehyde: An Insight on Delivery Systems and Food Applications, Crit. Rev. Food Sci. Nutr., 2023, 63, 2521–2543 CrossRef CAS PubMed.
- S. Banerjee and S. Banerjee, Anticancer Potential and Molecular Mechanisms of Cinnamaldehyde and Its Congeners Present in the Cinnamon Plant, Physiologia, 2023, 3, 173–207 CrossRef.
- A. Niknejad, S. M. Razavi, Y. Hosseini, Z. N. Arab, A. H. Abdolghaffari and S. Momtaz, Cinnamon Modulates Toll-Like Receptors: A New Therapeutic Approach for Diabetes, Rev. Bras. Farmacogn., 2024, 34, 223–235 CrossRef CAS.
- Y. Xiao, F. Zhang, H. Xu, C. Yang, X. Song, Y. Zhou, X. Zhou, X. Liu and J. Miao, Cinnamaldehyde Microcapsules Enhance Bioavailability and Regulate Intestinal Flora in Mice, Food Chem.: X, 2022, 15, 100441 CAS.
- R. Zhu, H. Liu, C. Liu, L. Wang, R. Ma, B. Chen, L. Li, J. Niu, M. Fu, D. Zhang and S. Gao, Cinnamaldehyde in Diabetes: A Review of Pharmacology, Pharmacokinetics and Safety, Pharmacol. Res., 2017, 122, 78–89 CrossRef CAS PubMed.
- P. P. Sapienza, G. J. Ikeda, P. I. Warr, S. L. Plummer, R. E. Dailey and C. S. Lin, Tissue Distribution and Excretion of 14C-Labelled Cinnamic Aldehyde Following Single and Multiple Oral Administration in Male Fischer 344 Rats, Food Chem. Toxicol., 1993, 31, 253–261 CrossRef CAS PubMed.
- Z. Yong, W. Xingqi, H. Jie, H. Rongfeng and C. Xiaoqin, Formulation, Production, in Vitro Release and in Vivo Pharmacokinetics of Cinnamaldehyde Sub-Micron Emulsions, Pharm. Dev. Technol., 2020, 25, 676–685 CrossRef PubMed.
- B. Ji, Y. Zhao, Q. Zhang, P. Wang, J. Guan, R. Rong and Z. Yu, imultaneous Determination of Cinnamaldehyde, Cinnamic Acid, and 2-Methoxy Cinnamic Acid in Rat Whole Blood after Oral Administration of Volatile Oil of Cinnamoni Ramulus by UHPLC-MS/MS: An Application for a Pharmacokinetic Study, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2015, 1001, 107–113 CrossRef CAS PubMed.
- H. Zhao, Q. Yang, Y. Xie, J. Sun, H. Tu, W. Cao and S. Wang, Simultaneous Determination of Cinnamaldehyde and Its Metabolite in Rat Tissues by Gas Chromatography–Mass Spectrometry, Biomed. Chromatogr., 2015, 29, 182–187 CrossRef CAS PubMed.
- Y. Tanaka, H. Uchi and M. Furue, Antioxidant Cinnamaldehyde Attenuates UVB-Induced Photoaging, J. Dermatol. Sci., 2019, 96, 151–158 CrossRef CAS PubMed.
- H. Uchi, M. Yasumatsu, S. Morino-Koga, C. Mitoma and M. Furue, Inhibition of Aryl Hydrocarbon Receptor Signaling and Induction of NRF2-Mediated Antioxidant Activity by Cinnamaldehyde in Human Keratinocytes, J. Dermatol. Sci., 2017, 85, 36–43 CrossRef CAS PubMed.
- L. Yin, S. Hussain, T. Tang, Y. Gou, C. He, X. Liang, Z. Yin, G. Shu, Y. Zou, H. Fu, X. Song, H. Tang, F. Xu and P. Ouyang, Protective Effects of Cinnamaldehyde on the Oxidative Stress, Inflammatory Response, and Apoptosis in the Hepatocytes of Salmonella Gallinarum-Challenged Young Chicks, Oxid. Med. Cell. Longevity, 2022, 2022, 1–21 Search PubMed.
- S. Mateen, M. T. Rehman, S. Shahzad, S. S. Naeem, A. F. Faizy, A. Q. Khan, M. S. Khan, F. M. Husain and S. Moin, Anti-Oxidant and Anti-Inflammatory Effects of Cinnamaldehyde and Eugenol on Mononuclear Cells of Rheumatoid Arthritis Patients, Eur. J. Pharmacol., 2019, 852, 14–24 CrossRef CAS PubMed.
- Y. Mitamura, M. Murai, C. Mitoma and M. Furue, NRF2 Activation Inhibits Both TGF-β1- and IL-13-Mediated Periostin Expression in Fibroblasts: Benefit of Cinnamaldehyde for Antifibrotic Treatment, Oxid. Med. Cell. Longevity, 2018, 2018, 1–10 CrossRef PubMed.
- Z. Ataie, H. Mehrani, A. Ghasemi and K. Farrokhfall, Cinnamaldehyde Has Beneficial Effects against Oxidative Stress and Nitric Oxide Metabolites in the Brain of Aged Rats Fed with Long-Term, High-Fat Diet, J. Funct. Foods, 2019, 52, 545–551 CrossRef CAS.
- W. Li, W. Zhi, J. Zhao, Q. Yao, F. Liu and X. Niu, Cinnamaldehyde Protects VSMCs against Ox-LDL-Induced Proliferation and Migration through S Arrest and Inhibition of P38, JNK/MAPKs and NF-κB, Vasc. Pharmacol., 2018, 108, 57–66 CrossRef CAS PubMed.
- L. Lu, Y. Xiong, J. Zhou, G. Wang, B. Mi and G. Liu, The Therapeutic Roles of Cinnamaldehyde against Cardiovascular Diseases, Oxid. Med. Cell. Longevity, 2022, 2022, 1–23 Search PubMed.
- G. Das, S. Gonçalves, J. Basilio Heredia, A. Romano, L. A. Jiménez-Ortega, E. P. Gutiérrez-Grijalva, H. S. Shin and J. K. Patra, Cardiovascular Protective Effect of Cinnamon and Its Major Bioactive Constituents: An Update, J. Funct. Foods, 2022, 97, 105045 CrossRef CAS.
- B. Zheng, J. Qi, Y. Yang, L. Li, Y. Liu, X. Han, W. Qu and L. Chu, Mechanisms of Cinnamic Aldehyde against Myocardial Ischemia/Hypoxia Injury in Vivo and in Vitro: Involvement of Regulating PI3K/AKT Signaling Pathway, Biomed. Pharmacother., 2022, 147, 112674 CrossRef CAS PubMed.
- K. Bian, H.-X. Shi, Ferid and Y.-L. Xue, Vasodilatory Effects of Cinnamaldehyde and Its Mechanism of Action in the Rat Aorta, Vasc. Health Risk Manage., 2011, 7, 273–280 CrossRef PubMed.
- B. Gürer, H. Kertmen, P. Kuru Bektaşoğlu, Ö. Ç. Öztürk, H. Bozkurt, A. Karakoç, A. T. Arıkök and E. Çelikoğlu, The Effects of Cinnamaldehyde on Early Brain Injury and Cerebral Vasospasm Following Experimental Subarachnoid Hemorrhage in Rabbits, Metab. Brain Dis., 2019, 34, 1737–1746 CrossRef PubMed.
- C. Park, H. Lee, S. Hong, I. M. N. Molagoda, J.-W. Jeong, C.-Y. Jin, G.-Y. Kim, S. H. Choi, S. H. Hong and Y. H. Choi, Inhibition of Lipopolysaccharide-Induced Inflammatory and Oxidative Responses by Trans-Cinnamaldehyde in C2C12 Myoblasts, Int. J. Med. Sci., 2021, 18, 2480–2492 CrossRef CAS PubMed.
- W. Li, W. Zhi, J. Zhao, W. Li, L. Zang, F. Liu and X. Niu, Cinnamaldehyde Attenuates Atherosclerosis via Targeting the IκB/NF-κB Signaling Pathway in High Fat Diet-Induced ApoE−/− Mice, Food Funct., 2019, 10, 4001–4009 RSC.
- S. Mateen, S. Shahzad, S. Ahmad, S. S. Naeem, S. Khalid, K. Akhtar, W. Rizvi and S. Moin, Cinnamaldehyde and Eugenol Attenuates Collagen Induced Arthritis via Reduction of Free Radicals and Pro-Inflammatory Cytokines, Phytomedicine, 2019, 53, 70–78 CrossRef CAS PubMed.
- N. Kim, N. Trinh, S. Ahn and S. Kim, Cinnamaldehyde Protects against Oxidative Stress and Inhibits the TNF-α-induced Inflammatory Response in Human Umbilical Vein Endothelial Cells, Int. J. Mol. Med., 2020, 46(1), 449–457 CAS.
- M. E. Kim, J. Y. Na and J. S. Lee, Anti-Inflammatory Effects of Trans-Cinnamaldehyde on Lipopolysaccharide-Stimulated Macrophage Activation via MAPKs Pathway Regulation, Immunopharmacol. Immunotoxicol., 2018, 40, 219–224 CrossRef CAS PubMed.
- W.-X. Cheng, S. Zhong, X.-B. Meng, N.-Y. Zheng, P. Zhang, Y. Wang, L. Qin and X.-L. Wang, Cinnamaldehyde Inhibits Inflammation of Human Synoviocyte Cells Through Regulation of Jak/Stat Pathway and Ameliorates Collagen-Induced Arthritis in Rats, J. Pharmacol. Exp. Ther., 2020, 373, 302–310 CrossRef CAS PubMed.
- J. Zuo, D. Zhao, N. Yu, X. Fang, Q. Mu, Y. Ma, F. Mo, R. Wu, R. Ma, L. Wang, R. Zhu, H. Liu, D. Zhang and S. Gao, Cinnamaldehyde Ameliorates Diet-Induced Obesity in Mice by Inducing Browning of White Adipose Tissue, Cell. Physiol. Biochem., 2017, 42, 1514–1525 CrossRef CAS PubMed.
- J. G. O. Neto, S. K. Boechat, J. S. Romão, L. R. B. Kuhnert, C. C. Pazos-Moura and K. J. Oliveira, Cinnamaldehyde Treatment during Adolescence Improves White and Brown Adipose Tissue Metabolism in a Male Rat Model of Early Obesity, Food Funct., 2022, 13, 3405–3418 RSC.
- J. G. O. Neto, S. K. Boechat, J. S. Romão, C. C. Pazos-Moura and K. J. Oliveira, Treatment with Cinnamaldehyde Reduces the Visceral Adiposity and Regulates Lipid Metabolism, Autophagy and Endoplasmic Reticulum Stress in the Liver of a Rat Model of Early Obesity, J. Nutr. Biochem., 2020, 77, 108321 CrossRef CAS PubMed.
- Z. Ataie, M. Dastjerdi, K. Farrokhfall and Z. Ghiravani, The Effect of Cinnamaldehyde on iNOS Activity and NO-Induced Islet Insulin Secretion in High-Fat-Diet Rats, Evidence-Based Complementary Altern. Med., 2021, 2021, 1–8 CrossRef PubMed.
- M. E. Abdelmageed, G. S. Shehatou, R. A. Abdelsalam, G. M. Suddek and H. A. Salem, Cinnamaldehyde Ameliorates STZ-Induced Rat Diabetes through Modulation of IRS1/PI3K/AKT2 Pathway and AGEs/RAGE Interaction, Naunyn-Schmiedeberg's Arch. Pharmacol., 2019, 392, 243–258 CrossRef CAS PubMed.
- J. K. Hoi, B. Lieder, M. Pignitter, J. Hans, J. P. Ley, J. Lietard, K. Hoelz, M. Somoza and V. Somoza, Identification of Cinnamaldehyde as Most Effective Fatty Acid Uptake Reducing Cinnamon-Derived Compound in Differentiated Caco-2 Cells Compared to Its Structural Analogues Cinnamyl Alcohol, Cinnamic Acid, and Cinnamyl Isobutyrate, J. Agric. Food Chem., 2019, 67, 11638–11649 CrossRef CAS PubMed.
- J. K. Hoi, B. Lieder, B. Liebisch, C. Czech, J. Hans, J. P. Ley and V. Somoza, TRPA1 Agonist Cinnamaldehyde Decreases Adipogenesis in 3T3-L1 Cells More Potently than the Non-Agonist Structural Analog Cinnamyl Isobutyrate, ACS Omega, 2020, 5, 33305–33313 CrossRef CAS PubMed.
- Y.-F. Chiang, H.-Y. Chen, K.-C. Huang, P.-H. Lin and S.-M. Hsia, Dietary Antioxidant Trans-Cinnamaldehyde Reduced Visfatin-Induced Breast Cancer Progression: In Vivo and In Vitro Study, Antioxidants, 2019, 8, 625 CrossRef CAS PubMed.
- Y. Liu, T. An, D. Wan, B. Yu, Y. Fan and X. Pei, Targets and Mechanism Used by Cinnamaldehyde, the Main Active Ingredient in Cinnamon, in the Treatment of Breast Cancer, Front. Pharmacol., 2020, 11, 582719 CrossRef CAS PubMed.
- J. Park and S. H. Baek, Combination Therapy with Cinnamaldehyde and Hyperthermia Induces Apoptosis of A549 Non-Small Cell Lung Carcinoma Cells via Regulation of Reactive Oxygen Species and Mitogen-Activated Protein Kinase Family, Int. J. Mol. Sci., 2020, 21, 6229 CrossRef CAS PubMed.
- R. Chen, J. Wu, C. Lu, T. Yan, Y. Qian, H. Shen, Y. Zhao, J. Wang, P. Kong and X. Zhang, Systematic Transcriptome Analysis Reveals the Inhibitory Function of Cinnamaldehyde in Non-Small Cell Lung Cancer, Front. Pharmacol., 2021, 11, 611060 CrossRef PubMed.
- J. Mei, J. Ma, Y. Xu, Y. Wang, M. Hu, F. Ma, Z. Qin, R. Xue and N. Tao, Cinnamaldehyde Treatment of Prostate Cancer-Associated Fibroblasts Prevents Their Inhibitory Effect on T Cells Through Toll-Like Receptor 4, Drug Des., Dev. Ther., 2020, 14, 3363–3372 CrossRef CAS PubMed.
- T. W. Kim, Cinnamaldehyde Induces Autophagy-Mediated Cell Death through ER Stress and Epigenetic Modification in Gastric Cancer Cells, Acta Pharmacol. Sin., 2022, 43, 712–723 CrossRef CAS PubMed.
- Z. Aminzadeh, N. Ziamajidi, R. Abbasalipourkabir, S. Daei, S. Helbi and A. Moridnia, Antitumor Activities of Aqueous Cinnamon Extract on 5637 Cell Line of Bladder Cancer through Glycolytic Pathway, Int. J. Inflammation, 2022, 2022, 1–9 CrossRef PubMed.
- C. Wu, Y. Zhuang, J. Zhou, S. Liu, R. Wang and P. Shu, Cinnamaldehyde Enhances Apoptotic Effect of Oxaliplatin and Reverses Epithelial-Mesenchymal Transition and Stemnness in Hypoxic Colorectal Cancer Cells, Exp. Cell Res., 2019, 383, 111500 CrossRef CAS PubMed.
- Y. Wang, Y. Li, L. Wang, B. Chen, M. Zhu, C. Ma, C. Mu, A. Tao, S. Li, L. Luo, P. Ma, S. Ji and T. Lan, Cinnamaldehyde Suppressed EGF-Induced EMT Process and Inhibits Ovarian Cancer Progression Through PI3K/AKT Pathway, Front. Pharmacol., 2022, 13, 779608 CrossRef CAS PubMed.
- F. Usai and A. Di Sotto, Trans-Cinnamaldehyde as a Novel Candidate to Overcome Bacterial Resistance: An Overview of In Vitro Studies, Antibiotics, 2023, 12, 254 CrossRef CAS PubMed.
- H. H. Yu, Y. J. Song, H. Yu, N. Lee and H. Paik, Investigating the Antimicrobial and Antibiofilm Effects of Cinnamaldehyde against Campylobacter Spp. Using Cell Surface Characteristics, J. Food Sci., 2020, 85, 157–164 CrossRef CAS PubMed.
- L. Sun, G. Rogiers and C. W. Michiels, The Natural Antimicrobial Trans-Cinnamaldehyde Interferes with UDP-N-Acetylglucosamine Biosynthesis and Cell Wall Homeostasis in Listeria Monocytogenes, Foods, 2021, 10(7), 1666 CrossRef CAS PubMed.
- W. A. Pereira, C. D. S. Pereira, R. G. Assunção, I. S. C. Da Silva, F. S. Rego, L. S. R. Alves, J. S. Santos, F. J. R. Nogueira, A. Zagmignan, T. T. Thomsen, A. Løbner-Olesen, K. A. Krogfelt, L. C. N. Da Silva and A. G. Abreu, New Insights into the Antimicrobial Action of Cinnamaldehyde towards Escherichia Coli and Its Effects on Intestinal Colonization of Mice, Biomolecules, 2021, 11, 302 CrossRef CAS PubMed.
- Z. He, Z. Huang, W. Jiang and W. Zhou, Antimicrobial Activity of Cinnamaldehyde on Streptococcus Mutans Biofilms, Front. Microbiol., 2019, 10, 2241 CrossRef PubMed.
- L. Yin, Y. Dai, H. Chen, X. He, P. Ouyang, X. Huang, X. Sun, Y. Ai, S. Lai, L. Zhu and Z. Xu, Cinnamaldehyde Resist Salmonella Typhimurium Adhesion by Inhibiting Type I Fimbriae, Molecules, 2022, 27, 7753 CrossRef CAS PubMed.
- R. H. Olmedo, C. M. Asensio and N. R. Grosso, Thermal Stability and Antioxidant Activity of Essential Oils from Aromatic Plants Farmed in Argentina, Ind. Crops Prod., 2015, 69, 21–28 CrossRef CAS.
- A. Sedaghat Doost, M. Nikbakht Nasrabadi, V. Kassozi, H. Nakisozi and P. Van Der Meeren, Recent Advances in Food Colloidal Delivery Systems for Essential Oils and Their Main Components, Trends Food Sci. Technol., 2020, 99, 474–486 CrossRef CAS.
- B. Muhoza, S. Xia, X. Wang, X. Zhang, Y. Li and S. Zhang, Microencapsulation of Essential Oils by Complex Coacervation Method: Preparation, Thermal Stability, Release Properties and Applications, Crit. Rev. Food Sci. Nutr., 2022, 62, 1363–1382 CrossRef CAS PubMed.
- R. Delshadi, A. Bahrami, A. G. Tafti, F. J. Barba and L. L. Williams, Micro and Nano-Encapsulation of Vegetable and Essential Oils to Develop Functional Food Products with Improved Nutritional Profiles, Trends Food Sci. Technol., 2020, 104, 72–83 CrossRef CAS.
- N. Majumder, N. G. Das and S. K. Das, Polymeric Micelles for Anticancer Drug Delivery, Ther. Delivery, 2020, 11, 613–635 CrossRef CAS PubMed.
- A. E. Cartaya, H. Lutz, S. Maiocchi, M. Nalesnik and E. M. Bahnson, Delivery of Cinnamic Aldehyde Antioxidant Response Activating nanoParticles (ARAPas) for Vascular Applications, Antioxidants, 2021, 10, 709 CrossRef CAS PubMed.
- G. Raffai, B. Kim, S. Park, G. Khang, D. Lee and P. M. Vanhoutte, Cinnamaldehyde and Cinnamaldehyde-Containing Micelles Induce Relaxation of Isolated Porcine Coronary Arteries: Role of Nitric Oxide and Calcium, Int. J. Nanomed., 2014, 2557 CrossRef PubMed.
- J. Deng, S. Liu, G. Li, Y. Zheng, W. Zhang, J. Lin, F. Yu, J. Weng, P. Liu and H. Zeng, pH-Sensitive Charge-Conversion Cinnamaldehyde Polymeric Prodrug Micelles for Effective Targeted Chemotherapy of Osteosarcoma in Vitro, Front. Chem., 2023, 11, 1190596 CrossRef CAS PubMed.
- W. Chen, S. Xia and C. Xiao, Complex Coacervation Microcapsules by Tannic Acid Crosslinking Prolong the Antifungal Activity of Cinnamaldehyde against, Aspergillus Brasiliensis, Food Biosci., 2022, 47, 101686 CrossRef CAS.
- R. Mu, H. Zhang, Z. Zhang, X. Li, J. Ji, X. Wang, Y. Gu and X. Qin, Trans-Cinnamaldehyde Loaded Chitosan Based Nanocapsules Display Antibacterial and Antibiofilm Effects against Cavity-Causing Streptococcus Mutans, J. Oral Microbiol., 2023, 15, 2243067 CrossRef PubMed.
- M. Li, C. Li, Y. Zhou, H. Tian, Q. Deng, H. Liu, L. Zhu and X. Yin, Optimization of Cinnamaldehyde Microcapsule Wall Materials by Experimental and Quantitative Methods, J. Appl. Polym. Sci., 2021, 138, 49667 CrossRef CAS.
- S. T. S. Wong, A. Kamari, A. M. Jaafar, M. Z. Hussein, H. Othman, H. Abdullah, N. Yusof and N. Hashim, Longer Mosquito Control Using a Sodium Alginate–Chitosan Nanocarrier for Cinnamaldehyde in Larvicide Formulations, Environ. Chem. Lett., 2020, 18, 1345–1351 CrossRef CAS.
- M. Xue, J. Wang and M. Huang, Inulin-Modified Liposomes as a Novel Delivery System for Cinnamaldehyde, Foods, 2022, 11, 1467 CrossRef CAS PubMed.
- N. Sang, L. Jiang, Z. Wang, Y. Zhu, G. Lin, R. Li and J. Zhang, Bacteria-Targeting Liposomes for Enhanced Delivery of Cinnamaldehyde and Infection Management, Int. J. Pharm., 2022, 612, 121356 CrossRef CAS PubMed.
- S. Makwana, R. Choudhary, N. Dogra, P. Kohli and J. Haddock, Nanoencapsulation and Immobilization of Cinnamaldehyde for Developing Antimicrobial Food Packaging Material, LWT–Food Sci. Technol., 2014, 57, 470–476 CrossRef CAS.
- B. Y. Gursu, I. Dag and G. Dikmen, Antifungal and Antibiofilm Efficacy of Cinnamaldehyde-Loaded Poly(DL-Lactide-Co-Glycolide) (PLGA) Nanoparticles against Candida Albicans, Int. Microbiol., 2022, 25, 245–258 CrossRef CAS PubMed.
- S. Li, J. Chen, Y. Liu, Q. Zheng, W. Tan, X. Feng, K. Feng and W. Hu, Application of Cinnamaldehyde Solid Lipid Nanoparticles in Strawberry Preservation, Horticulturae, 2023, 9, 607 CrossRef.
- Q. Liu, H. Cui, B. Muhoza, E. Duhoranimana, S. Xia, K. Hayat, S. Hussain, M. U. Tahir and X. Zhang, Fabrication of Low Environment-Sensitive Nanoparticles for Cinnamaldehyde Encapsulation by Heat-Induced Gelation Method, Food Hydrocolloids, 2020, 105, 105789 CrossRef.
- N. Hojati, S. Amiri and M. Radi, Effect of Cinnamaldehyde Nanoemulsion on the Microbiological Property of Sausage, J. Food Meas. Charact., 2022, 16, 2478–2485 CrossRef.
- F. Liu, C. Yu, S. Guo, B.-S. Chiou, M. Jia, F. Xu, M. Chen and F. Zhong, Extending Shelf Life of Chilled Pork Meat by Cinnamaldehyde Nano Emulsion at Non-Contact Mode, Food Packag. Shelf Life, 2023, 37, 101067 CrossRef CAS.
- C. G. Otoni, M. R. D. Moura, F. A. Aouada, G. P. Camilloto, R. S. Cruz, M. V. Lorevice, N. D. F. F. Soares and L. H. C. Mattoso, Antimicrobial and Physical-Mechanical Properties of Pectin/Papaya Puree/Cinnamaldehyde Nanoemulsion Edible Composite Films, Antimicrobial and Physical-Mechanical Properties of Pectin/Papaya Puree/Cinnamaldehyde Nanoemulsion Edible Composite Films, Food Hydrocolloids, 2014, 41, 188–194 CrossRef CAS.
- D. Bickers, P. Calow, H. Greim, J. M. Hanifin, A. E. Rogers, J. H. Saurat, I. G. Sipes, R. L. Smith and H. Tagami, A toxicologic and dermatologic assessment of cinnamyl alcohol, cinnamaldehyde and cinnamic acid when used as fragrance ingredients, Food Chem. Toxicol., 2005, 43, 799–836 CrossRef CAS PubMed.
- J. Michiels, J. Missotten, N. Dierick, D. Fremaut, P. Maene and S. De Smet, In vitro degradation and in vivo passage kinetics of carvacrol, thymol, eugenol and trans-cinnamaldehyde along the gastrointestinal tract of piglets, J. Sci. Food Agric., 2008, 88, 2371–2381 CrossRef CAS.
- M. Honma, M. Yamada, M. Yasui, K. Horibata, K. Sugiyama and K. Masumura, In vivo and in vitro mutagenicity of perillaldehyde and cinnamaldehyde, Genes Environ., 2021, 43, 30 CrossRef CAS PubMed.
- U. K. Sharma, R. Kumar, R. Ganguly, A. Gupta, A. K. Sharmaand and A. K. Pandey, Cinnamaldehyde, An Active Component of Cinnamon Provides Protection against Food Colour Induced Oxidative Stress and Hepatotoxicity in Albino Wistar Rats, Vegetos, 2018, 31, 123 CrossRef.
- A. Hosni, S. A. El-twab, M. Abdul-Hamid, E. Prinsen, H. AbdElgawad, A. Abdel-Moneim and G. T. S. Beemster, Cinnamaldehyde Mitigates Placental Vascular Dysfunction of Gestational Diabetes and Protects from the Associated Fetal Hypoxia by Modulating Placental Angiogenesis, Metabolic Activity and Oxidative Stress, Pharmacol. Res., 2021, 165, 105426 CrossRef CAS PubMed.
- S. A. Amer, A. E. Metwally and S. A. A. Ahmed, The Influence of Dietary Supplementation of Cinnamaldehyde and Thymol on the Growth Performance, Immunity and Antioxidant Status of Monosex Nile Tilapia Fingerlings (Oreochromis Niloticus), Egypt. J. Aquat. Res., 2018, 44, 251–256 CrossRef.
- Y. Wang, Q. Wang, K. Xing, P. Jiang and J. Wang, Dietary Cinnamaldehyde and Bacillus Subtilis Improve Growth Performance, Digestive Enzyme Activity, and Antioxidant Capability and Shape Intestinal Microbiota in Tongue Sole, Cynoglossus Semilaevis, Aquaculture, 2021, 531, 735798 CrossRef CAS.
- F. Luan, Z. Lei, X. Peng, L. Chen, L. Peng, Y. Liu, Z. Rao, R. Yang and N. Zeng, Cardioprotective Effect of Cinnamaldehyde Pretreatment on Ischemia/Reperfusion Injury via Inhibiting NLRP3 Inflammasome Activation and Gasdermin D Mediated Cardiomyocyte Pyroptosis, Chem.-Biol. Interact., 2022, 368, 110245 CrossRef CAS PubMed.
- L.-L. Kang, D.-M. Zhang, C.-H. Ma, J.-H. Zhang, K.-K. Jia, J.-H. Liu, R. Wang and L.-D. Kong, Cinnamaldehyde and Allopurinol Reduce Fructose-Induced Cardiac Inflammation and Fibrosis by Attenuating CD36-Mediated TLR4/6-IRAK4/1 Signaling to Suppress NLRP3 Inflammasome Activation, Sci. Rep., 2016, 6, 27460 CrossRef CAS PubMed.
- J. Tian, X.-L. Shan, S.-N. Wang, H.-H. Chen, P. Zhao, D.-D. Qian, M. Xu, W. Guo, C. Zhang and R. Lu, Trans-Cinnamaldehyde Suppresses Microtubule Detyrosination and Alleviates Cardiac Hypertrophy, Eur. J. Pharmacol., 2022, 914, 174687 CrossRef CAS PubMed.
- X. Yuan, L. Han, P. Fu, H. Zeng, C. Lv, W. Chang, R. S. Runyon, M. Ishii, L. Han, K. Liu, T. Fan, W. Zhang and R. Liu, Cinnamaldehyde Accelerates Wound Healing by Promoting Angiogenesis via Up-Regulation of PI3K and MAPK Signaling Pathways, Lab. Invest., 2018, 98, 783–798 CrossRef CAS PubMed.
- O. A. A. Nour, G. S. G. Shehatou, M. A. Rahim, M. S. El-Awady and G. M. Suddek, Cinnamaldehyde Exerts Vasculoprotective Effects in Hypercholestrolemic Rabbits, Naunyn-Schmiedeberg's Arch. Pharmacol., 2018, 391, 1203–1219 CrossRef CAS PubMed.
- N. E. Buglak, W. Jiang and E. S. M. Bahnson, Cinnamic Aldehyde Inhibits Vascular Smooth Muscle Cell Proliferation and Neointimal Hyperplasia in Zucker Diabetic Fatty Rats, Redox Biol., 2018, 19, 166–178 CrossRef CAS PubMed.
- D. Qian, J. Tian, S. Wang, X. Shan, P. Zhao, H. Chen, M. Xu, W. Guo, C. Zhang and R. Lu, Trans-cinnamaldehyde protects against phenylephrine-induced cardiomyocyte hypertrophy through the CaMKII/ERK pathway, BMC Complementary Med. Ther., 2022, 22, 115 CrossRef CAS PubMed.
- M. Hajinejad, M. Ghaddaripouri, M. Dabzadeh, F. Forouzanfar and S. Sahab-Negah, Natural Cinnamaldehyde and Its Derivatives Ameliorate Neuroinflammatory Pathways in Neurodegenerative Diseases, BioMed Res. Int., 2020, 2020, 1–9 CrossRef PubMed.
- Y. Ou, M. Yan, G. Gao, W. Wang, Q. Lu and J. Chen, Cinnamaldehyde Protects against Ligature-Induced Periodontitis through the Inhibition of Microbial Accumulation and Inflammatory Responses of Host Immune Cells, Food Funct., 2022, 13, 8091–8106 RSC.
- J. Chung, S. Kim, H. A. Lee, M. H. Park, S. Kim, Y. R. Song and H. S. Na, Trans-Cinnamic Aldehyde Inhibits Aggregatibacter Actinomycetemcomitans -Induced Inflammation in THP-1-Derived Macrophages via Autophagy Activation, J. Periodontol., 2018, 89, 1262–1271 CrossRef CAS PubMed.
- R. Vallion, K. Hardonnière, A. Bouredji, M.-H. Damiens, C. Deloménie, M. Pallardy, P.-J. Ferret and S. Kerdine-Römer, The Inflammatory Response in Human Keratinocytes Exposed to Cinnamaldehyde Is Regulated by Nrf2, Antioxidants, 2022, 11, 575 CrossRef CAS PubMed.
- S.-C. Lee, S.-Y. Wang, C.-C. Li and C.-T. Liu, Anti-Inflammatory Effect of Cinnamaldehyde and Linalool from the Leaf Essential Oil of Cinnamomum Osmophloeum Kanehira in Endotoxin-Induced Mice, J. Food Drug Anal., 2018, 26, 211–220 CrossRef CAS PubMed.
- B. S. Ismail, B. Mahmoud, E. S. Abdel-Reheim, H. A. Soliman, T. M. Ali, B. H. Elesawy and M. Y. Zaky, Cinnamaldehyde Mitigates Atherosclerosis Induced by High-Fat Diet via Modulation of Hyperlipidemia, Oxidative Stress, and Inflammation, Oxid. Med. Cell. Longevity, 2022, 2022, 1–15 Search PubMed.
- L.-L. Chen, M.-H. Lee, C.-L. Chang, K.-T. Liou, S.-H. Liu, C.-M. Chern, H.-I. Chen, Y.-C. Shen and Y.-H. Wang, Suppression of Inflammatory and Fibrotic Signals by Cinnamon (Cinnamomum Cassia) and Cinnamaldehyde in Cyclophosphamide-Induced Overactive Bladder in Mice, Evidence-Based Complementary Altern. Med., 2021, 2021, 1–13 Search PubMed.
- G. S. El-Tanbouly and R. S. Abdelrahman, Novel Anti-Arthritic Mechanisms of Trans-Cinnamaldehyde against Complete Freund's Adjuvant-Induced Arthritis in Mice: Involvement of NF-κB/TNF-α and IL-6/IL-23/IL-17 Pathways in the Immuno-Inflammatory Responses, Inflammopharmacology, 2022, 30, 1769–1780 CrossRef CAS PubMed.
- Y. Naghiaee, M. Vakili, M. Mohammadi, A. Mohiti and J. Mohiti-Ardakani, Comparing the Effect of Cinnamaldehyde and Metformin on Expression of MiR320 and MiR26-b in Insulin Resistant 3T3L1 Adipocytes, Phytomed. Plus, 2021, 1, 100122 CrossRef.
- Y. Urasaki and T. T. Le, A Composition of Phytonutrients for Glycemic and Weight Management, Nutrients, 2022, 14, 3784 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |