Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Trends in authentication of edible oils using vibrational spectroscopic techniques
Banu Ozen *,
Cagri Cavdaroglu
*,
Cagri Cavdaroglu and
Figen Tokatli
and
Figen Tokatli
Izmir Institute of Technology, Department of Food Engineering, Urla, Izmir, Turkiye. E-mail: banuozen@iyte.edu.tr; cagricavdaroglu@iyte.edu.tr; figentokatli@iyte.edu.tr
First published on 5th June 2024
Abstract
The authentication of edible oils has become increasingly important for ensuring product quality, safety, and compliance with regulatory standards. Some prevalent authenticity issues found in edible oils include blending expensive oils with cheaper substitutes or lower-grade oils, incorrect labeling regarding the oil's source or type, and falsely stating the oil's origin. Vibrational spectroscopy techniques, such as infrared (IR) and Raman spectroscopy, have emerged as effective tools for rapidly and non-destructively analyzing edible oils. This review paper offers a comprehensive overview of recent advancements in using vibrational spectroscopy for authenticating edible oils. The fundamental principles underlying vibrational spectroscopy are introduced and chemometric approaches that enhance the accuracy and reliability of edible oil authentication are summarized. Recent research trends highlighted in the review include authenticating newly introduced oils, identifying oils based on their specific origins, adopting handheld/portable spectrometers and hyperspectral imaging, and integrating modern data handling techniques into the use of vibrational spectroscopic techniques for edible oil authentication. Overall, this review provides insights into the current state-of-the-art techniques and prospects for utilizing vibrational spectroscopy in the authentication of edible oils, thereby facilitating quality control and consumer protection in the food industry.
1. Introduction
The Food and Agriculture Organization of the United Nations (FAO) defines food authenticity as “irrefutable verification that a food or food ingredient is in its intended original, unaltered form as declared and represented”.1 Consequently, ensuring food authenticity entails verifying the origin, composition, and inherent characteristics of food products. This process serves to provide essential information about the product attributes to consumers, producers, and regulatory authorities. In addition, economically motivated adulteration, a sub-class of authentication, is also defined as “the intentional substitution or addition of a substance in a product for the purpose of increasing the apparent value of the product or reducing the cost of its production, for economic gain”.1 Edible oils, particularly some high-priced oils such as olive oil or cold-pressed oils, very commonly suffer from various authenticity issues, particularly economically motivated adulteration. The adulteration of high-priced oils with cheaper alternatives or low-quality oils, mislabeling of the oil source or the type, and the false claiming of the origin of the oil are some common authenticity problems encountered in edible oils.2 Crises like pandemics, warfare, and adverse climatic conditions such as drought can lead to a reduction in oil production or disrupt the distribution of the product. This, in turn, causes increases in prices and makes the product more susceptible to fraudulent activities. Recent cases shed light on the significance of this issue. Notably, the German Federal Office for Consumer Protection and Food Safety discovered discrepancies in 40% of scrutinized extra virgin olive oil samples.3 Additionally, nearly all olive oils sold online failed to meet the required standards, leading this authority to designate olive oil as one of the top ten most counterfeited foods in Germany.3 Another recent case involved Spanish and Italian police uncovering olive oils mixed with cloudy oils, a byproduct of olive oil production.4 These cases emphasize the importance of addressing counterfeit practices in the edible oil industry.Depending on its nature, a violation of food authenticity can lead to various consequences, including risks to public health, a decline in consumer confidence, economic repercussions for both consumers and producers, and regulatory actions. Consequences related to public health can occur in direct or indirect ways.5 Direct threats involve immediate toxicity or illness resulting from a single exposure.5 Adulterants present in edible oils have the potential to undermine their nutritional integrity and introduce harmful substances, thereby posing potential health risks. For example, substituting extra virgin olive oil with different vegetable oils can diminish essential nutrients and bioactive compounds, compromising the health benefits typically associated with authentic olive oil. Additionally, this practice may introduce allergens, toxins, or contaminants that are absent in authentic oils. An illustrative example is the Spanish toxic oil syndrome, a health crisis in Spain during the early 1980s, linked to the consumption of adulterated rapeseed oil mislabeled as olive oil.6 In contrast, indirect threats arise from the prolonged and repeated consumption of an affected food or ingredient, with the discovery of health impacts occurring after a delay.5 As issues regarding the authenticity of food arise, consumer trust in the food industry and marketplace diminishes, leading individuals to scrutinize the government's ability to protect the public from harm related to food.7 Regarding the economic effects of food authentication, consumers do not fully realize the value of their purchases due to a breach in authenticity. This affects all consumers who seek genuine food products for various reasons such as their health benefits. Food fraud imposes both direct and indirect costs on the industry. Food authenticity breaches can severely damage the reputation of brands associated with the implicated products. In an interconnected global food supply chain, breaches of authenticity can have cross-border implications. They may lead to disruptions in international trade, increased scrutiny of imports and exports, and the need for improved traceability and transparency measures.5 Moreover, a counterfeit food ingredient can also have consequences on multiple product lines within the food manufacturing industry.5 Responding to food fraud, as a compliance measure with new regulations, may require substantial and expensive measures, such as testing, control mechanisms, or requirements for tamper-proof packaging, among other interventions.5 Violations of food authenticity regulations can result in legal consequences for the parties involved. Besides, regulations need to be continuously updated or new regulations are required due to sophisticated fraud practices.
Regulations about the verification of edible oils cover a range of aspects related to quality assurance and traceability. The Codex outlines definitions for numerous edible oils and establishes standards governing their physical properties and compositional parameters.8 The Codex also has multiple provisions and guidelines such as the General Standard for the Labelling of Prepackaged Foods (CXS 1-1985) and the Guidelines for the Use of Nutrition and Health Claims (CXG 23-1997), among others. These guidelines explicitly forbid the false, misleading, or deceptive labeling of foods. In addition to the Codex, specific organizations like the International Olive Council have instituted their own set of standards. Regulations also exist to protect the authenticity of oils concerning their origin, including measures like Protected Designation of Origin (PDO) and Protected Geographical Indication (PGI).9 These regulations are designed to protect the identities of certain products, emphasizing their distinctive features tied to both their geographical origin and traditional expertise used in their production. A ‘geographical indication’ designation can be given to a product that possesses a clear connection to the location of its production. Codex standards and various other international legal instruments offer a crucial framework for formulating, overseeing, and regulating the guidelines established by national governments. Typically, the authenticity of edible oils is assessed by comparing analysis results against these established standards. Some recently marketed oils may lack such standards, making them susceptible to various types of fraudulent activities. This vulnerability is often heightened due to the considerable value attributed to these oils, primarily stemming from their perceived positive health effects. Moreover, regulations defining compositional parameters can be undermined by incorporating different adulterants in precise proportions. This tactic makes it challenging to distinguish the adulterated oil from the authentic one based on a limited analysis of a few components.
Edible oil authentication is commonly conducted through a range of chromatographic methods.10 These methods facilitate the detection of distinct markers and compositional variations, allowing for the verification of the origin and purity of oils. Nevertheless, these methods typically demand more time and may require intricate procedures for analysis, along with a degree of expertise in the technique. Spectroscopic techniques, particularly vibrational spectroscopic methods, offer a comprehensive approach, allowing the representation of a wide range of constituents in the oil through their spectra. Furthermore, these techniques offer rapid and non-invasive analysis, requiring minimal sample preparation. Portable versions of these spectrometers are also becoming more widespread, making them more accessible. The obtained spectra can be interpreted in both qualitative and quantitative manners, and chemometric methods are commonly employed for evaluating the vibrational spectral data. Consequently, these techniques have demonstrated considerable success in authentication, as evidenced by studies in the literature. A total of 164 studies were identified through a Web of Science search covering the five-year period from 2019 to 2023, using the query “(TS = (oil and ((adulteration) or (authentication)) and ((FTIR) or (NIR) or (Raman)))) and AK = ((authentication) or (adulteration)) and Article (document types) and Article (document types)”. After the elimination of studies on non-edible oils, 100 research articles were obtained (Fig. 1). Among these studies, 38% focused on mid-infrared (MIR) spectroscopy, with near-infrared (NIR) and Raman spectroscopy accounting for 27% and 26%, respectively. Additionally, certain studies investigated the combinations of these methods or offered comparative analyses for edible oil authentication. Particularly, 3% of the total articles in this timeframe explored the simultaneous use of Raman and MIR spectroscopy, while NIR and MIR combinations constituted 2%. These numbers highlight the significance of vibrational spectroscopy for the authentication of edible oils, emphasizing the importance of this subject matter.
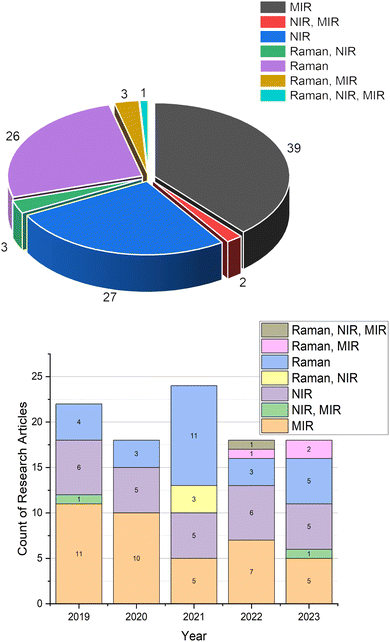 | ||
| Fig. 1 Statistics for the number of papers published about the authentication of edible oils using vibrational spectroscopy according to the Web of Science search. | ||
Numerous literature reviews have explored the application of vibrational spectroscopic techniques in the analysis of edible oils.11–15 Typically, these reviews concentrate mostly on specific spectroscopic methods or provide an overview of authentication methods. This current review, however, provides a summary of recent trends in the field of edible oils over the past five years (2019–2023), with a particular emphasis on the studies related to authentication. Additionally, a basic overview of vibrational spectroscopic techniques and data handling methods is provided before discussing their recent applications.
2. Vibrational spectroscopy
As with any other spectroscopic method, vibrational spectroscopy is also based on the interaction of electromagnetic radiation with the analyzed sample. As a result of this interaction, molecules exhibit vibrations in different forms depending on the bond strength and the atomic masses. Vibrational spectra are typically classified based on the types of molecular vibrations they represent, broadly categorized into two main types: stretching and bending vibrations.16 Stretching vibrations are associated with higher energy and shorter wavelengths, and appear in the higher wavenumber region and they involve changes in bond lengths within molecules and can further be classified into symmetric stretching, involving stretching motions where both atoms on either side of the bond move in the same direction, and asymmetric stretching, involving stretching motions where the atoms on either side of the bond move in opposite directions. Bending vibrations, on the other hand, are related to the changes in bond angles within molecules and can also be subdivided into rocking, involving movements resembling the rocking of a boat where the entire molecule pivots around a particular bond, scissoring, involving motions where atoms move towards or away from each other, causing a change in bond angles, and twisting, involving rotations around bonds resulting in changes in the spatial orientation of atoms.16 Distinctive vibrations associated with each functional group in a molecule are manifested at specific bands within the spectrum. Therefore, vibrational spectroscopy serves as a valuable method for unraveling molecular structures. It offers crucial insights into the intra-molecular forces among atoms within a molecule, the intermolecular forces, and the characteristics of chemical bonds.16 Vibrational spectra can be employed as molecular fingerprints for the characterization and identification of molecules. As an illustration, Fig. 2 shows the spectra obtained with three vibrational spectroscopic methods and assignments of some bands for sesame oil and one of its adulterants, sunflower oil. By analyzing the vibrational modes of molecules, these techniques can identify functional groups and specific chemical bonds. Furthermore, spectra have a direct proportionality to the concentration of the molecules. This characteristic also facilitates quantitative assessment, a task frequently performed using chemometric techniques. Chemometric methods often employ machine learning algorithms, particularly for tasks such as pattern recognition and prediction. Infrared spectroscopy including NIR and MIR spectroscopy and Raman spectroscopy are grouped under vibrational spectroscopic techniques.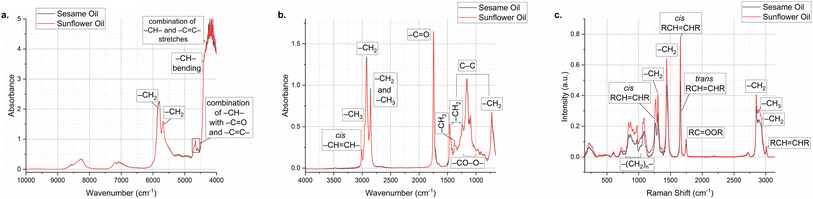 | ||
| Fig. 2 Sesame seed and sunflower oil: (a) near-infrared (NIR) spectra; (b) Fourier-transform infrared (FTIR) spectra; (c) Raman spectra (for peak assignments, see ref. 11–13). | ||
All vibrational spectroscopic techniques share common advantages such as being non-destructive and rapid, requiring minimal sample preparation, and enabling multi-component analysis. The specifics of each technique are detailed in this section.
NIR spectrometers cover the wavelength range of 780 to 2500 nm (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500–4000 cm−1). NIR spectra result from the summation of overtone bands or fundamental absorption bands associated with the transitions of molecular rotations and vibrations. These spectra feature numerous broad and overlapping bands, including those attributed to C–H, N–H, O–H and S–H vibrations.11 Overtone and combination bands limit the detailed structural elucidation of the analyzed sample.11 The NIR absorption bands are generally less intense than their corresponding fundamental MIR absorption bands. However, this serves as an advantage for NIR measurement, as it allows the direct analysis of strongly absorbing and highly light-scattering matrices like suspensions, powders, and pastes.16 Transmission or reflectance modes of NIR spectra can be obtained as a result of the measurements. More recent applications of NIR spectroscopy involve its use in combination with hyperspectral imaging. These two techniques combine spectral and spatial information. During hyperspectral imaging analysis, sensors capture a large number of spectral bands, typically spanning the ultraviolet, visible, and IR regions of the electromagnetic spectrum.17 Unlike traditional imaging, which captures three bands of color (red, green, and blue), hyperspectral imaging captures a much larger number of narrow and adjacent bands across the spectrum.18 This allows for detailed analysis of the spectral characteristics of the imaged scene.18
500–4000 cm−1). NIR spectra result from the summation of overtone bands or fundamental absorption bands associated with the transitions of molecular rotations and vibrations. These spectra feature numerous broad and overlapping bands, including those attributed to C–H, N–H, O–H and S–H vibrations.11 Overtone and combination bands limit the detailed structural elucidation of the analyzed sample.11 The NIR absorption bands are generally less intense than their corresponding fundamental MIR absorption bands. However, this serves as an advantage for NIR measurement, as it allows the direct analysis of strongly absorbing and highly light-scattering matrices like suspensions, powders, and pastes.16 Transmission or reflectance modes of NIR spectra can be obtained as a result of the measurements. More recent applications of NIR spectroscopy involve its use in combination with hyperspectral imaging. These two techniques combine spectral and spatial information. During hyperspectral imaging analysis, sensors capture a large number of spectral bands, typically spanning the ultraviolet, visible, and IR regions of the electromagnetic spectrum.17 Unlike traditional imaging, which captures three bands of color (red, green, and blue), hyperspectral imaging captures a much larger number of narrow and adjacent bands across the spectrum.18 This allows for detailed analysis of the spectral characteristics of the imaged scene.18
The 4000–400 cm−1 (2500–25000 nm) range, on the other hand, corresponds to MIR spectroscopy. MIR spectroscopy provides information regarding the fundamental vibrations of distinct chemical functional groups. Nearly all functional groups, with a focus on C–H, O–H, N–H, or C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups, exhibit absorption in the MIR region.12 The prevalent method of MIR spectroscopy is Fourier-transform infrared (FTIR) spectroscopy. In this approach, the absorption spectrum is acquired using an interference technique. Interferometry involves the superposition of waves to extract information about the properties of the waves or the medium through which they propagate.16 In this technique, an interference pattern is generated, and a mathematical transformation (Fourier-transform) is applied to obtain a spectrum. Fourier-transform spectroscopy offers advantages in terms of simplicity and rapidity in measurements, coupled with a high signal-to-noise ratio.12,16 FTIR spectroscopy commonly employs transmission and attenuated total reflectance (ATR) measurement methods. Reflection, in the form of spectral reflection and diffuse reflection, is also used, particularly for powder-type samples. ATR-FTIR is widely applied for obtaining MIR spectra of edible oils. In ATR mode, IR light strikes the surface of a crystal (typically made from diamond, zinc selenide, or germanium) holding the sample and undergoes internal reflection at the interface between the crystal and sample. During these reflections, a portion of the light is absorbed by the sample. This technique is easy to use and allows for fast and relatively high-sensitivity measurements with a small sample size.
O groups, exhibit absorption in the MIR region.12 The prevalent method of MIR spectroscopy is Fourier-transform infrared (FTIR) spectroscopy. In this approach, the absorption spectrum is acquired using an interference technique. Interferometry involves the superposition of waves to extract information about the properties of the waves or the medium through which they propagate.16 In this technique, an interference pattern is generated, and a mathematical transformation (Fourier-transform) is applied to obtain a spectrum. Fourier-transform spectroscopy offers advantages in terms of simplicity and rapidity in measurements, coupled with a high signal-to-noise ratio.12,16 FTIR spectroscopy commonly employs transmission and attenuated total reflectance (ATR) measurement methods. Reflection, in the form of spectral reflection and diffuse reflection, is also used, particularly for powder-type samples. ATR-FTIR is widely applied for obtaining MIR spectra of edible oils. In ATR mode, IR light strikes the surface of a crystal (typically made from diamond, zinc selenide, or germanium) holding the sample and undergoes internal reflection at the interface between the crystal and sample. During these reflections, a portion of the light is absorbed by the sample. This technique is easy to use and allows for fast and relatively high-sensitivity measurements with a small sample size.
Raman spectroscopy (50–4000 cm−1) also detects vibrational, rotational, and other low-frequency modes in a system. To obtain the spectra, a monochromatic laser light, often in the visible or near-infrared range, is directed onto a sample. Most of the incident photons undergo elastic (Rayleigh) scattering, where they retain the same energy and frequency as the incident light.19 However, a small fraction of the incident photons undergo inelastic scattering, known as Raman scattering. In Raman scattering, the incident photon interacts with the sample molecules, causing a change in the energy state of the molecule.19 The energy difference between the incident and scattered photons corresponds to the vibrational or rotational energy levels of the sample molecules.19 As in MIR spectroscopy, Raman spectroscopy also provides well-resolved spectra, assignable to specific chemical groups; however, spectra are more responsive to molecular structure and symmetry.13 Raman spectroscopy is less susceptible to water as opposed to IR spectroscopy, which has a strong –OH absorption band. One of the limitations of Raman spectroscopy is its constrained sensitivity since only a small proportion of scattered light contributes to Raman scattering.13 However, it was reported that the implementation of advanced Raman technologies, particularly Surface-Enhanced Raman Spectroscopy (SERS), offers a solution to these limitations.13 SERS has the capability to substantially amplify the Raman scattering signal from the analyzed sample while preserving the merits of conventional Raman spectroscopy. This enhancement potential positions SERS as a promising approach for rapid and highly sensitive analysis of trace components in intricate food samples.13 Other forms of Raman spectroscopy such as Fourier transform Raman spectroscopy, spatially offset Raman spectroscopy (SORS), micro-confocal Raman spectroscopy, and resonance Raman spectroscopy are also commonly used for the analysis of food products.13
3. Data analysis
The analysis of the spectral data serves two purposes: classification of observations, which is used to authenticate samples and distinguish them from those that are either not of the same origin or are adulterated and prediction of some target components, such as adulterant concentrations.Spectroscopic instruments generate large amounts of data. Each sample produces a spectrum. The measurements in each spectrum are variables, which are absorption or transmittance values over a wide range of wavelengths (or wavenumbers). The whole data set (spectra) is a matrix or a multivariate data set with n samples and k variables (n ≪ k, in most of the cases). Even the combined spectra from different spectroscopic sources (data fusion) can be helpful to describe the samples in more detail. Spectral data have been effectively evaluated by conventional linear multivariate data analysis. In the recent period covered by this article, other machine learning methods have also been reported in the literature.
Principal Component Analysis (PCA), Soft Independent Modeling of Class Analogy (SIMCA), Partial Least Squares (PLS), and Partial Least Squares Discriminant Analysis (PLS-DA) are projection methods that extract information from highly correlated multivariate data in the form of a smaller number of latent variables, essentially principal components. As part of the analysis, the graphical representation of these new orthogonal variables allows for easy visual inspection of the original samples. Other methods derived from PLS are OPLS (orthogonal projection to latent structure) and OPLS-DA.
PCA is an unsupervised multivariate technique that classifies the observations in a multivariate data set based on the statistical information extracted from the patterns, such as fingerprint regions in the spectra. PCA is usually applied to see natural groupings of observations and to identify possible outliers. SIMCA, on the other hand, is a supervised method as it is composed of independent PCA models of observations from known origins (process, variety, geography, etc.).20,21 The membership of a new sample to the class is determined based on its statistical distance. PLS-DA is also a supervised classification method that requires a data set with different observations belonging to specific classes based on their known characteristics. PLS-DA models are built with this supervision and when new observations are collected, they are assigned to one of these groups with a certain probability.
PLS and OPLS are regression methods used to relate a response matrix Y to an X matrix containing noisy and collinear variables.22,23 In spectroscopic analysis, the X matrix consists of n observations and k variables, which are transmission or absorption values at each wavelength or wavenumber. The Y matrix contains characteristics of the observations such as chemical and physical variables measured with different analytical methods, or the ratio of adulteration. As a result, the target variables in Y are predicted using PLS or OPLS regression models built by maximizing the variation between X and Y.
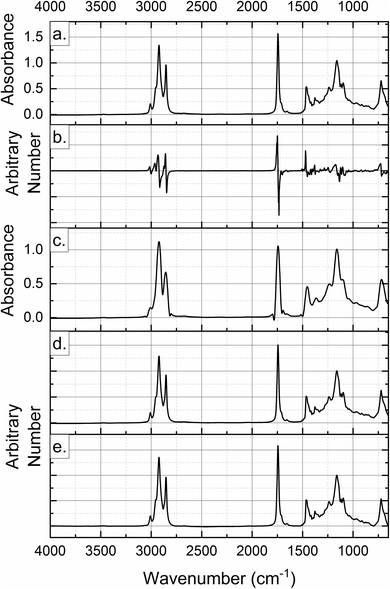
Spectroscopic data contain background noise, some environmental interference and the main information about the chemical structure of materials. Pre-processing of data is required to remove the redundant part of the data and to extract the information that can be used for authentication and prediction. Pre-processing methods are used for noise filtering, data compression and smoothing, and spectral background elimination to remove as much noise and irrelevant information as possible. Some commonly applied techniques are Standard Normal Variate (SNV), first and second derivatives, Multiplicative Scatter Correction (MSC), Orthogonal Signal Correction (OSC), Savitzky–Golay filtering (SG), wavelet transform, and a combination of these to build multivariate models with the best classification and prediction capabilities.24 First and second derivatives enhance features and resolve overlapping peaks in spectral data while eliminating linear baseline drift. SNV normalization removes multiplicative scatter effects by centering and scaling each spectrum individually. MSC corrects scatter effects by transforming each spectrum to match a reference spectrum. SG filtering, another pre-processing method, smooths the data and enhances signal features.24 Some of these pre-processing methods applied to the Raman spectra of sesame oil are shown in Fig. 3. A combination of two or more pre-treatment methods may be more effective in extracting the multivariate information in cases where a single processing technique is unable to enhance the signals. As presented in an example, the fusion application of SNV, MSC, and the second derivative procedures to NIR data for classification purposes resulted in better classification rates than classification using spectroscopic data preprocessed individually by those techniques.25
For the supervised discrimination and prediction models, it is important to check the model performance not only with the calibration data set, but also with an independent validation set to test how good the calibration model is. Correct classification rates for the calibration and validation sets are determined to evaluate the classification success. The statistics used to evaluate the quality of the regression models are R2 values for the calibration, cross-validation and external validation, root mean squared error (RMSE) for the calibration and validation data sets, and residual prediction deviation.
Several spectral data from different sources can also be combined and analyzed with the data fusion approach. In low-level fusion, data from all sources are merged sample-wise into a unified matrix, with each row representing a sample analyzed and each column representing signals or variables measured with different instruments. This combined matrix is utilized to develop a classification or a prediction model. In mid-level fusion, relevant features are first extracted from each data source independently. These extracted features are then concatenated into a single array, which is employed for multivariate classification and prediction tasks. In high-level fusion, distinct classification or prediction models are developed from each data source. The outcomes from each individual model are then amalgamated to derive the final model.26 Parallel Factor Analysis (PARAFAC) is a decomposition technique applied to data containing more than two dimensions, such as time, space, frequency, participants, conditions, and signal characteristics. It is utilized to decompose these arrays and assist in the identification and quantification of independent underlying signals, referred to as ‘components’.27 The model offers multiple applications. It can be directly applied to a three-way array of observations that may have a (possibly incomplete) factorial structure. Alternatively, it can be indirectly applied to the original observations by fitting a set of covariance matrices derived from the observations, with each matrix representing a two-way subset of the data.28 Typically, PARAFAC does not require additional mathematical constraints to achieve a unique decomposition of the data. As a result, it can be utilized in the pre-processing stages to isolate artifacts and in the actual data analysis to extract predominant brain activations or other relevant signal characteristics.29
Spectral data, comprising hundreds or even thousands of absorbance values per observation, can be collected rapidly. These data can be transformed into valuable information through multivariate statistical analysis techniques; however, variable selection can be a crucial step as it improves model predictability and simplicity by eliminating non-informative variables.30 The literature on data analysis and statistics offers numerous proposed methods for variable selection and the genetic algorithm technique is one of those methods.31 Genetic algorithms involve large amounts of input or trials to address optimization problems based on Darwin's theory of evolution and genetic operations. In contrast to traditional optimization methods, such as calculus-based and enumerative approaches, genetic algorithms are more robust and globally applicable, especially in scenarios with limited prior knowledge about the process to be optimized.32 This is due to the probabilistic transition rules of genetic algorithms in contrast to the deterministic rules of calculus-based approaches.33
There has been a growing utilization of machine learning algorithms in chemometric analysis. An example of machine learning algorithms that can be used in the assessment of vibrational spectroscopic data is the random forest algorithm that was introduced by L. Breiman in 2001.34 The technique has achieved significant success as a versatile method for classification and regression. This technique is adaptable to large-scale problems and it can be easily tailored to various specific learning tasks and provide measures of variable importance.35 The method employs a bagging approach to generate multiple distinct training sample sets, each of which is used to construct a decision tree with randomly selected attributes. In a random forest, the development of each decision tree involves the following steps: (I) using the bagging method, random data are selected with replacement from the original training sets to form training sets with differences; (II) a sampling approach is used for feature selection; (III) the decision trees are allowed to grow without pruning. The final outcome is obtained by integrating the results of the decision trees through simple majority voting for the classification problems or mathematical averaging for regression problems.34
Artificial Neural Networks (ANNs), another type of machine learning technique, have demonstrated their effectiveness as a tool for creating dependable models capable of distinguishing between different predefined classes of food matrices.36 In each layer of a neural network, all neurons are defined by identical activation functions that convert input variables into outputs. Essentially, the training process of ANNs involves setting the weights and biases through the training algorithm to approximate a specific function, minimize error, and enhance prediction accuracy. The back propagation-ANN is a simple yet widely used network for spectral data in the authentication and traceability of foods. Factors such as initial weight, learning rate, momentum factor, target error, and epoch time significantly impact the back propagation-ANN's performance. The presence of those adjustable parameters in ANNs can make them susceptible to overfitting.37
Support Vector Machine (SVM) stands as a robust supervised machine learning algorithm, aiming to identify a best-fitting decision boundary that maximally separates objects of distinct classes.38 One of the notable advantages of SVM is that it is based on the theoretical framework of statistical learning theory, adopting the structural risk minimization principle as opposed to the empirical risk minimization principle, which is commonly employed by conventional neural networks. This strategic choice is aimed at mitigating the challenges of overfitting and the curse of dimensionality.39 SVM's adaptability extends to its application in binary classification as well as in regression tasks, showcasing its utility in a diverse range of academic and practical contexts.
4. Applications of vibrational spectroscopic techniques in edible oil authentication
Tables 1–3 (ref. 40–135) provide the summaries of studies conducted in the 2019–2023 period that utilized vibrational spectroscopic techniques for authenticating edible oils. In these studies, researchers investigated different cases of authentication issues ranging from the identification of oils in regard to their designated origin to determining the adulterants in them. The predominant adulterants typically consist of less expensive commercial oils, although cases of utilizing waste cooking oils,136 fried oils,137 deodorized oils,138 and aged oils78 have also been documented. Olive oil authentication accounts for 29.5% of the studies in these tables, making it the most frequently researched oil. Given its high economic and nutritional value, this prominence is not surprising. Additionally, this section identifies the current trends in the field and elaborates on examples illustrating these trends. Current interests include research on authenticating newly introduced oils, identification of oils regarding their designated origin, the adoption of handheld/portable spectrometers and hyperspectral imaging, and the incorporation of contemporary data handling techniques.| Authenticated oil | Statistical method | Content of the study | Reference |
|---|---|---|---|
| Olive oil | PLS, SVM | Quantification of rapeseed oil in olive oil blends using data fusion with NIR and MIR data | 40 |
| Olive oil | PLS | Evaluation of the practicality of a reduced spectral model capable of directly and quantitatively determining the EVOO blends with peanut, sunflower, soybean, sesame, and maize oils | 41 |
| Olive oil | PLS-DA | Comparison of vis-NIR, MIR, and fluorescence spectroscopy techniques for detecting soybean oil adulteration in olive oil | 42 |
| Olive oil | PLS-DA | Development of a robust model to distinguish EVOO and create specific models to differentiate EVOO adulterated with soybean, corn, canola, sunflower, virgin olive, lampante olive, and olive oils | 43 |
| Olive oil | k-NN | Combining temperature-induced spectral variation and two-dimensional correlation to improve accuracy for discrimination of olive oils adulterated with canola, soybean, and corn oils | 44 |
| Olive oil | OPLS-DA | Use of NIR and fluorescence to classify and quantify the adulteration of EVOO with different seed oils and pomace oil | 45 |
| Olive oil | PLS | Use of a smartphone, coupled with image processing to quantify rapeseed oil levels in EVOO | 46 |
| Olive oil | PLS-DA | Comparison of NIR and fluorescence spectroscopy for assessing EVOO quality through geographical origin identification and detection of adulteration with corn, sunflower, and soybean oils | 47 |
| Olive oil | ANN | Application of vis-NIR spectra in the authentication and detection of corn, sunflower, rice, peanut, hazelnut, virgin wheat germ, and virgin cornstarch oil adulterated EVOO | 48 |
| Potato frying oil | SIMCA, PLS | Determination of the type of oil used in potato chip production | 49 |
| Tea seed oil | OC-PLS | Use of data fusion with NIR and fluorescence spectroscopy for the detection of cheap vegetable oils | 50 |
| Vegetable oils | PLS, SVR | Improvement of the prediction accuracy of oil blends containing sesame, soybean, corn, peanut, rapeseed and sunflower oils using different chemometric models | 51 |
| Coriander | LDA, k-NN, PLS | Determination of the authenticity and detection of adulteration with palm olein, canola, and soybean oils | 52 |
| Argan oil | FDA, PLS | Investigation of front-face fluorescence, MIR and NIR spectroscopy for geographical origin differentiation and detection of adulteration with cheaper vegetable oils | 53 |
| Argan oil | PLS | Detection and quantification of argan oil adulteration with cheap vegetable oils | 54 |
| Black cumin oil | SIMCA, PLS | Evaluation of the feasibility of portable FT-NIR, FT-MIR, and Raman spectrometers for the detection of adulteration with canola, corn, soybean, and sunflower oils | 55 |
| Mustard oil | PLS, SVM | Rapid determination of the presence of butter yellow | 56 |
| Chia oil | k-NN, SVM, LDA | Detecting adulteration with rapeseed, sunflower, and corn oils | 57 |
| Sesame and rapeseed oils | SVM, RF | Developing a non-destructive method for the detection of adulteration with soybean oil | 58 |
| Camellia oil | ANN | Enhancing the accuracy of adulteration detection with soybean oil | 59 |
| Camellia oil | DA, PLS | Determining adulteration with corn, rapeseed, and sunflower oils as a real-time monitoring tool | 60 |
| Camellia oil | SVR, RF | Evaluating the potential of UV-vis/NIR spectroscopy for the detection of adulteration with soybean, corn, rapeseed, and peanut oils | 61 |
| Palm oil | LDA, PLS | Determination of the presence of lard | 62 |
| Palm oil | PCA | Detection of the presence of Sudan III and IV dyes | 63 |
| Flaxseed oil | OC-PLS | Assessing adulteration with cottonseed, soybean, rapeseed, maize, and sunflower oils | 64 |
| Peanut oil | PLS | Application of NIR and Raman spectroscopy for the qualitative and quantitative analysis of corn and vegetable oils in peanut oil | 65 |
| Pumpkinseed oil | OPLS-DA, OPLS | Determination of authenticity of retail oils labelled as pumpkin seed oil | 66 |
| Cow and buffalo ghee | PCR, PLS | Adulteration detection with fats and oils, and prediction of adulterant concentration | 67 |
| Oil of Brassica oilseeds | PLS-DS, iPLS | Comparison of NIR and hyperspectral imaging to predict the oil and, fatty acid (erucic) content, and classification of the seed samples | 68 |
| Butter cheese | PLS-DA, PLS | Determination of the substitution with non-dairy fat | 69 |
| Oil mixture capsules | PCA | Rapid screening and detection of adulteration | 70 |
| Authenticated oil | Statistical method | Content of the study | Reference |
|---|---|---|---|
| Olive oil | PLS, SVM | Quantification of rapeseed oil in olive oil blends using data fusion with NIR and MIR data | 40 |
| Olive oil | PLS-DA | Comparison of vis-NIR, MIR, and fluorescence spectroscopic techniques for detecting soybean oil adulteration in olive oil | 42 |
| Olive oil | PLS | Application for the fast identification of adulteration with refined soybean, refined sunflower, refined corn, and refined canola oils | 71 |
| Olive oil | LDA, PLS | Evaluation of data fusion techniques for headspace gas chromatography-ion mobility spectrometry with MIR for geographical identification | 72 |
| Olive oil | PLS-DA | Assessment using UV-vis and MIR spectroscopy for identification and quantification of adulteration with canola and sunflower | 73 |
| Olive oil | GA for pattern recognition | Differentiation using digitally generated data from an IR spectral library for sunflower and corn oil adulterated olive oil | 74 |
| Olive oil | OPLS-DA | Use of MIR and UV-vis spectroscopic data in comparison to pigment profiles for differentiation in regard to the geographical origin and harvest year | 75 |
| Olive oil | PLDS, PLS | Comparison for the authentication and quantification of adulteration with safflower, corn, soybean, canola, sunflower, and sesame oils using GC-MS, hyperspectral imaging, FTIR, Raman and UV-vis spectroscopy | 76 |
| Olive oil | PLS-DA, SIMCA | Development of a spectroscopic methodology for the detection of adulteration with refined canola, hazelnut and safflower oils in ternary mixtures | 77 |
| Olive oil | PLS-DA, OPLS-DA | Evaluating the potentials of UV-vis, FTIR, and fluorescence spectroscopy and data fusion to detect and quantify the adulteration of fresh olive oils with old olive oils | 78 |
| Cod liver and salmon oils | SVM, PLS | Comparison of IR and Raman spectroscopy to detect and quantify palm oil, ester form of omega-3 concentrates, and fish oil as adulterants | 79 |
| Pomegranate seed oil | OPLS-DA, PLS | Comparison of UV-visible, MIR and fluorescence spectroscopic methods for the detection of mixtures containing sunflower oil | 80 |
| Butter oil | PCA | Characterization and quantification of adulteration with soybean oil | 81 |
| Mustard oil | LDA, PCR, PLS | Rapid detection and quantification of adulteration with fried mustard oil | 82 |
| Mustard oil | LDA, PCR, PLS | Determination of adulteration with linseed oil | 83 |
| Eucommia ulmoides seed oil | PCA, PLS, LDA, HCA | Combination and comparison of FTIR and synchronous fluorescence spectroscopy for qualitative and quantitative analysis of adulteration with refined rapeseed, crude rapeseed, peanut oil, corn oil, cotton seed, and sunflower seed oils | 84 |
| Butter | PCA, PLS | Assessment of a fast and rapid technique for determining the adulteration with vegetable oil | 85 |
| Sunflower oil | LR | Determination of sunflower oil purity and sunflower oil concentration in blends with rapeseed and soybean oils | 86 |
| Argan oil | FDA, PLS | Investigation of front-face fluorescence, MIR and NIR spectroscopy for geographical origin differentiation and detection of adulteration with cheaper vegetable oils | 53 |
| Argan oil | PCA, DD-SIMCA, PLS, PCR | Classification of argan oils and detection of adulteration with sunflower and soybean oils | 87 |
| Black cumin seed oil | SIMCA, PLS | Evaluation of the feasibility of portable FT-NIR, FT-MIR, and Raman spectrometers for the detection of adulteration with canola, corn, soybean, and sunflower oils | 55 |
| Black cumin seed oil | PCA, LDA, SIMCA, PLS | Discrimination in binary blends containing refined sunflower oil or soybean oil, and quantification of adulterant levels in binary blends | 88 |
| Camellia oil | PCA, PLS, CVA, SIMCA | Development of a comprehensive classification method for diverse pure edible oils, taking into account oil mixtures with significant variations in species and geographic origin, with a specific application to camellia oil | 89 |
| Camellia oil | PCA, LDA, PLS | Adulteration detection with rapeseed oil | 90 |
| Fish oil | PLS-DA, PLS | The quantitative detection of adulteration with single and multiple terrestrial animal lipids | 91 |
| Fish oil | PCA, PCR | Detection of adulteration of patin fish oil with palm oil | 92 |
| Fish oil | PLS, OPLS, PLS-DA, OPLS-DA | Development of a methodology for detecting pork oil in snakehead fish oil | 93 |
| Nut oil | PCA, PLS, FDA | Development of a chemometric model that identifies adulteration with sunflower and rapeseed oils | 94 |
| Coconut oil | PCA, LDA, PCR, PLS | Development of a methodology for the classification and quantification of adulteration with paraffin oil | 95 |
| Coconut oil | PLS, PCR | Detection of mixing with argemone oil | 96 |
| Coconut oil | PCA, LDA, PCR, PLS | Development of a methodology for the classification of pure coconut oil in refined, bleached and deodorized forms and for the quantification of adulteration with fried coconut oil | 97 |
| Coconut oil | DD-SIMCA | Evaluating authenticity and monitoring adulteration with canola, corn, sunflower and soybean oils | 98 |
| Coconut oil | PCA, LDA, PLS | Detection of adulteration with mustard oil | 99 |
| Grape seed oil | PCA, LDA, SIMCA, PLS | Exploring classification models using full and characteristic spectral regions to provide differentiation from soybean oil and to quantify the level of adulteration | 100 |
| Evening primrose oil | RF, PLS-DA, SIMCA | Comparison of chemometric methods in adulteration detection with soybean, corn, and sunflower oils | 101 |
| Chia and sesame seed oils | OC-PLS, SIMCA | Testing the spectra as input for two different chemometric methods to assess the authenticity of chia and sesame oils by detecting the presence of corn, peanut, soybean and sunflower oils | 102 |
| Perilla oil | OPLS-DA | Discrimination from soybean and corn oils | 103 |
| Linseed oil | Peak comparison | Authentication of a Stradivari 1737 type violin through detection of varnish | 104 |
| Pistachio butter | SIMCA, PLS-DA | Detecting adulteration with peanut, sunflower, and corn oils | 105 |
| Krill oil | SVM, PLS | Investigation of data fusion for adulteration detection with palm oil, omega-3 concentrate, and fish oil as adulterants | 106 |
| Babassu | PCA, LDA, PLS-DA | Assessment of a methodology for adulteration detection with soybean oil | 107 |
| Andiroba, babassu, baru, sweet almond, castor oils | PCA | Assessing data fusion of 1H NMR and 13C NMR for detecting adulteration with commercial oils in high-value oils produced through two different extraction processes | 108 |
| Sunflower oil | PCA, PLS | Development of a spectroscopic methodology for the detection of low levels of safflower as an adulterant | 109 |
| Pumpkin seed, grape seed, black cumin, and sesame seed oils | OPLS-DA, PLS | Comparison of the performances of fluorescence and MIR spectroscopy for adulteration detection with sunflower oil and estimation of several chemical properties including fatty acids, free fatty acid and total phenol contents | 110 |
| Wheat germ oil | PCA, LDA, SIMCA, PLS | Development of classification models for discrimination in binary blends of refined sunflower or soybean oil and quantification of adulteration levels | 111 |
| Authenticated oil | Statistical method | Content of the study | Reference |
|---|---|---|---|
| Olive oil | PLDS, PLS | Comparison for the authentication and quantification of adulteration with safflower, corn, soybean, canola, sunflower, and sesame oils using GC-MS, hyperspectral imaging, FTIR, Raman and UV-vis spectroscopy | 76 |
| Olive oil | PCA, PLS-DA | Establishment of a methodology to authenticate the geographical origin | 112 |
| Olive oil | Intensity ratio | Utilization of the univariate calibration of vegetable oil in EVOO using portable Raman spectroscopy | 113 |
| Olive oil | SIMCA | Identification of the geographical origin of EVOO produced from the arbequina botanical variety by involving receiver operating characteristic curves | 114 |
| Olive oil | GA | Quantification of vegetable oil blends by combining spectroscopic data and metaheuristics-based variable selection | 115 |
| Olive oil | LR | Quantification of rapeseed and corn oil adulteration | 116 |
| Olive oil | MLR | Employment of confocal Raman and fluorescence spectroscopy in the characterization of EVOO along with potential adulterant oils based on their oleic acid and photosensitive substance content | 117 |
| Olive oil | SVR | Identification of adulteration with sunflower oil blended with β-carotene to imitate EVOO | 118 |
| Olive oil | PLS-DA, PLS, MCR-ALS | Discrimination from hazelnut, canola, soybean, and sunflower oils and their mixtures | 119 |
| Olive oil | PCA, PLS, iPLS | Detection of soybean oil and its quantification with a portable system | 120 |
| Olive oil | PLS-DA, DD-SIMCA | Multi-step detection of 4 different adulterants: sunflower, soybean, canola, and hazelnut oils | 121 |
| Olive oil | PLS | Investigation of the potential of the portable system for determining the purity of binary, ternary and quaternary mixtures of corn, rapeseed, and soybean oils | 122 |
| Palm oil | PCA | Evaluating methods for authentication of palm oil and detecting Sudan IV adulteration | 123 |
| Cod liver and salmon oils | SVM, PLS | Comparison of IR and Raman spectroscopy to detect and quantify palm oil, ester form of omega-3 concentrates, and fish oil | 79 |
| Potato frying oil | SIMCA, PLS | Determination of the type of oil used in potato chip production | 49 |
| Soybean oil | PCA | Rapid identification of gutter oil adulteration | 124 |
| Walnut and pumpkin seed oils | PLS | Utilization of a portable system to determine the adulteration with sunflower oil | 125 |
| Black cumin, almond, and walnut oils | PCA, PLS | Determination of adulteration and discrimination between lab-produced and commercially provided cold-pressed oil samples | 126 |
| Black cumin oil | SIMCA, PLS | Evaluation of the feasibility of portable FT-NIR, FT-MIR, and Raman spectrometers for the detection of adulteration with canola, corn, soybean, and sunflower oils | 55 |
| Olive, corn, colza, bean, sunflower, and linseed oils | PCA | Discrimination of edible oils with regard to oil types and oxidation | 127 |
| Chia oil | k-NN, SVM, LDA | Detecting adulteration with rapeseed, sunflower, and corn oils | 57 |
| Avocado, canola, coconut, liquid coconut, corn, grapeseed, olive, peanut, soybean, sunflower, algae, hemp, and safflower oils | PCA, MLR, RF, LR | Classification of oil types and prediction of fatty acid compositions | 128 |
| Krill oil | SVM, PLS | Investigation of data fusion for adulteration detection with palm oil, omega-3 concentrate, and fish oil as adulterants | 106 |
| Sesame oil | OPLS-DA, OC-PLS | Investigation of the potential to discriminate between soybean, corn, rapeseed, and sunflower oils | 129 |
| Corn, canola, rice bran, soy bean, palm olein, and sunflower oils | PLS-DA, SIMCA, PLS | Classification of vegetable oils with regard to the type and prediction of alpha-tocopherol content of the vegetable oils | 130 |
| Peony seed oil | SVM, PLS, PCR, MLR | Defining qualitative and quantitative methods for adulteration with soybean oil | 131 |
| Sunflower oil, coconut oil, groundnut oil, and gingelly oil | Peak intensity | Identification of the palm oil ratio by using the intensities of some representative spectral peaks | 132 |
| Peanut oil | PLS | Application of NIR and Raman spectroscopy for the qualitative and quantitative analysis of corn and vegetable oils in peanut oil | 65 |
| Sunflower, sesame, hemp, walnut, linseed, and oils | Peak intensity | Rapid differentiation of oil types | 133 |
| Milk fat | PCA | Discrimination of milk fat in yogurt from samples blended with corn and sunflower oils | 134 |
| Flaxseed oil | PCA, differential spectrum analysis, derivative spectrum analysis, radarogram analysis | Evaluation of the differences in the spectra of flaxseed, canola, and cottonseed oils and detection of adulteration | 135 |
4.1. Newly introduced oils
One area on which researchers are particularly focused is the investigation of the authentication of unrefined oils. Unrefined oils, particularly those produced through cold pressing, a green extraction technology, have garnered attention. These oils generally have relatively higher levels of bioactive compounds such as polyphenolic compounds, phytosterols and tocopherols contributing to their association with positive health benefits.139 Cold pressed oils are defined as ‘those obtained, without altering the oil, by mechanical procedures only, e.g. expelling or pressing, without the application of heat. They may have been purified by washing with water, settling, filtering and centrifuging only’.8 Despite this definition, specific compositional parameters for each of these oils lack standardized criteria. Moreover, as they are produced in relatively small quantities, these oils are susceptible to adulteration through mixing with more affordable edible oils.There are several studies that aimed to determine the mixing of cold-pressed oils with different adulterants using vibrational spectroscopic techniques in the last 5-year period and they can be found in Tables 1–3. The FTIR spectral data of authentic cold-pressed wheat germ oils and their mixture with sunflower and soybean oils were evaluated with chemometric PCA, Linear Discriminant Analysis (LDA), SIMCA and PLS regression methods.111 The classification thresholds for adulterants were set at levels below 1%. Additionally, the LDA models accurately classified 100% of the studied samples based on their origin.
In a separate research, the study investigated the detection of adulteration in coriander oil, notable for its intriguing fatty acid profile with a 70% composition of petroselinic acid, using data acquired from a portable NIR spectrometer.52 Authentic oils were deliberately mixed with palm olein, canola, and soybean oils at levels ranging from 1% to 90%. Chemometric classification techniques such as PCA, LDA, and k-nearest neighbors (k-NN) were employed. The most effective models were from supervised LDA and k-NN analyses, achieving accuracies surpassing 90%. However, when classifying mixtures containing canola oil with higher petroselinic acid isomer (oleic acid) content, the accuracy of the models was comparatively lower than for other oil blends.
Raman spectroscopy, another vibrational spectroscopic method, was also employed in the discrimination of authentic cold-pressed black cumin, almond and walnut oils from their blends with corn and sunflower oils.126 The use of PLS-DA models resulted in high sensitivity and specificity values, facilitating the effective separation of pure oils from their mixed counterparts.
Another category of oils facing common challenges in authentication is virgin oils. The Codex also outlines a definition for virgin oils, and it is the same definition as cold pressed oils.8 Besides this definition, olive oil can be more specifically categorized as extra virgin and virgin olive oils. While both are recognized as high-quality oils, the key distinction lies in their acidity levels. Extra virgin olive oil has acidity levels below 0.8%, signifying a superior grade, whereas virgin olive oil may have acidity levels of up to 2%, placing it slightly below the stringent standards of extra virgin olive oil.140
Virgin olive oil, a significant component of the Mediterranean-style healthy diet, is particularly susceptible to various authentication issues due to its relatively higher prices, especially those carrying geographical indication status. Vibrational spectroscopic techniques, often coupled with chemometric methods, have been extensively employed in authentication studies of virgin oils. For instance, in a study distinguishing between European and non-European virgin olive oils, Raman spectroscopic data were analyzed using PLS-DA.141 The constructed model correctly classified 72% of EU oils and 84% of non-EU oils from validation set samples not involved in model building. In another study, the LDA of FTIR spectral data demonstrated 100% sensitivity and specificity values in identifying three PDO olive oils from Portugal.142
Another noteworthy virgin oil, virgin coconut oil, receives attention for its unique composition, containing significant amounts of short- and medium-chain fatty acids, including lauric acid with recognized antibacterial and antiviral properties.95,98 The discrimination of paraffin oil-adulterated virgin coconut oil at levels ranging from 1% to 18% was performed using FTIR spectroscopy.95 The data were assessed using LDA with 13 identified wavenumbers, achieving a 100% correct classification rate. Furthermore, PLS regression proved effective in quantifying the adulterant, establishing a 1% threshold with high accuracy. In a separate study, the differentiation of palm olein containing virgin coconut oils at levels ranging from 1% to 70% from authentic samples was accomplished by analyzing FT-NIR spectral data through discriminant analysis.143 The quantification of palm olein in virgin coconut oil was achieved with an accuracy of ±2.20%. However, the application of the same approaches did not allow for differentiation between virgin and refined coconut oils.
4.2. Identification of the designated origin of oils
The distinctive qualities of some oils are often linked to the region of their production. Consequently, oils that hold geographical indication status command higher prices, and mislabeling is common in such cases. False claims about the specified origin of olive oils are recognized as a key concern in the authentication of these oils.144 Numerous studies have investigated the potential of vibrational spectroscopic techniques to distinguish oils based on their geographic origin, with a predominant focus on olive oils.141,145–147 Nevertheless, there is a growing body of research exploring the application of these techniques to other types of oils.It is suggested that camellia oil derived from camellia flowers cultivated in a specific region of China (Hainan) possesses distinctive qualities attributed to the unique natural environment of this area. Consequently, a study aimed to assess the potential of fatty acid composition and NIR spectra for tracing the geographical origin of camellia oil from this particular region.148 The collected data underwent analysis to evaluate the classification capabilities of PLS-DA, random forest (RF), SVM, and convolutional neural network (CNN) algorithms. The findings revealed that the CNN model based on NIR spectra and the CNN model employing data fusion achieved prediction accuracies of 97.92% and 98.75%, respectively.
An additional case of employing vibrational spectroscopic techniques, specifically FTIR spectroscopy, for the geographical authentication of oils is demonstrated for argan oil. Argan oil is derived from the fruits of a tree exclusive to a distinct region in southwestern Morocco. In a study, argan oil samples from five different regions were categorized based on their geographic origin utilizing FTIR spectral data in conjunction with PCA and SIMCA.87 The outcome revealed a general grouping of oil samples according to their geographical origins for all five classes in both the calibration and validation sets. Nonetheless, the proximity observed between certain oils was attributed to the fact that these regions are neighboring and consequently share similar climates.
Vibrational spectroscopy also finds application in distinguishing the geographic origins of peanut oil.149 Raman spectra were obtained from peanut oil samples originating from various geographical locations. The collected data underwent analysis using stepwise linear discriminant analysis (SLDA), k-NN, support vector machine (SVM), and multi-way analysis of variance. The most effective classification model, formed by integrating SLDA with characteristic spectra, swiftly and precisely identified the origin of peanut oil. The outcomes revealed that the correct classification rate of samples, based on complete spectra, exceeded 90%.
4.3. Handheld/portable instruments
Another notable trend in the vibrational spectroscopy field is the use of portable/handheld spectroscopic instruments. In response to the growing requirements of on-site applications, several portable spectrometer products have been created by shrinking the discrete optical elements found in their larger bench-top counterparts.150 These devices have also found applications in the authentication studies of edible oils. The most common of these portable instruments are those working in the NIR region due to the availability of affordable high performance photodetector arrays.150 Building portable instruments for the MIR range poses greater challenges, since interferometers as an important part of FTIR spectrometers are sensitive to temperature and vibrations.151 In contrast, Raman spectroscopy benefits from advancements in telecom optics, making it relatively easy to construct a compact system. The performance of a standard portable spectrometer often falls short of its laboratory equivalent, particularly in aspects such as resolution, range, signal-to-noise ratio, sampling, and versatility, as noted by Crocombe.151 However, portable instruments can be used by non-technical people in the field since they are generally designed to provide easy-to-interpret results. Several reviews are available in the literature about portable instruments and their applications in different food products.152,153 Tailored portable spectroscopic devices can be constructed for specific products, such as verifying the authenticity of a particular oil or measuring essential compositional parameters. The data handling process for these compact vibrational spectroscopy tools aligns with that of conventional instruments, involving the creation of a database and manipulation of data using various machine learning algorithms.As an illustration of the application of portable spectroscopic tools, scientists explored the potential of using a handheld NIR spectrometer for detecting adulteration in copaiba oil, which is a non-timber forest product widely employed in popular medicine, pharmaceuticals, and cosmetics.154 This investigation focused on identifying adulterants, specifically binary and ternary mixtures of copaiba oil with palm oil, olive oil, sunflower oil, coconut oil, and soybean oil, in addition to copaiba oil subjected to thermal treatment. The optimized DD-SIMCA model used in evaluating the data demonstrated 95% sensitivity for authentic samples. The model's specificity is high at 97.3%, indicating its effectiveness in detecting adulterated samples. With an average efficiency of 92.3%, it was indicated that this method could be a promising tool for quality control and authenticity testing.
Another example of portable technology involves assessing the authenticity of extra virgin olive oil using a portable Raman spectrometer, as demonstrated in a study by Barros.113 The study involved collecting Raman spectra in the 1800–800 cm−1 region for both authentic extra virgin olive oil and samples adulterated with soybean oil. Regression models were developed using chemometric tools such as PLS and interval PLS (iPLS). A univariate model based on the ratio of the CH2 band at 1440 cm−1 and CC = at 1655 cm−1 was also created. The iPLS method exhibited the best results, with an R2 prediction of 0.9967 and a RMSEP of 0.0198 wt%, along with detection and quantification limits of 0.97 and 3.24 wt%, respectively.
The presence of examples featuring portable MIR spectrometers in the literature is comparatively limited when compared to NIR and Raman systems. An example is their application in the authentication assessment of extra virgin olive oil, as well as a comparative performance analysis with a portable Raman system.155 In this study, extra virgin olive oils were blended with lower grade olive oils, corn, sunflower, soybean, and canola oils. Spectral data were then subjected to SIMCA for distinguishing between authentic and mixed oils, while PLS was employed for predicting the quality parameters of olive oil such as fatty acid profile, free fatty acids, and total phenolic content. The assessment of MIR spectra demonstrated a perfect sensitivity value of 100% and a very good specificity of 89% in detecting authentic extra virgin olive oil. Both Raman and MIR techniques identified the adulteration of extra virgin olive oil with vegetable oils, with Raman exhibiting limited resolution in detecting virgin olive oil and olive oil mixtures. PLS models displayed outstanding correlation with R2 validation ≥ 0.92 for the reference tests, indicating standard errors of prediction suitable for quality control applications.
4.4. Hyperspectral imaging
Hyperspectral imaging stands as an emerging technology with potential applications in oil authentication studies. This innovative approach combines spectroscopic and imaging techniques within a single system, offering both spectral and spatial information for objects.156 This integration addresses limitations encountered by conventional imaging or spectroscopic methods alone. In hyperspectral imaging, numerous single-band images are captured at specific wavelengths, forming a three-dimensional hypercube of multivariate image data. This hypercube comprises a spectrum for each pixel in the image.156 An example of this type of image can be found in an article by Kamruzzaman et al.156 Frequently, NIR spectroscopy is integrated with hyperspectral imaging to enhance its capabilities.Various sesame oils with distinct qualities, produced through different methods and originating from diverse geographic locations, are available on the market. In a literature study, a hyperspectral imaging system operating in the visible-NIR region (900–1700 nm) was employed to distinguish between these oils.157 The data obtained were subjected to analysis using PCA and PLS-DA. The study achieved success in differentiating between sesame oil made with roasted whole sesame and sesame oil made with roasted whole sesame and sesame powder. However, challenges were encountered in separating sesame oil made with roasted sesame powder from sesame oil made with roasted whole sesame and sesame powder. Additionally, some success was observed in predicting the levels of oleic acid, linoleic acid, and total phenolic content in sesame oils through the application of PLS analysis on the data.
In a comparative study, the performance of NIR-hyperspectral imaging, FTIR, and Raman spectroscopy for authenticating extra virgin olive oil was evaluated.76 Mixtures of olive oil with safflower oil, corn oil, soybean oil, canola oil, sunflower oil, and sesame oil at levels ranging from 1% to 20% were differentiated from authentic olive oils using spectral data treated with PLS-DA and PLS. The hyperspectral imaging-NIR system achieved a classification accuracy rate of 100%, surpassing the accuracies of 99.8% and 96.6% obtained with FTIR and Raman spectroscopic techniques, respectively. Furthermore, in predicting the adulteration ratio, the hyperspectral imaging-NIR data yielded the most robust PLS model compared to the other spectroscopic methods.
4.5. Trends in data analysis
Recent research in the authentication of edible oils has been influenced by the adoption of variable selection algorithms, the data fusion approach and a growing integration of diverse machine learning techniques, including artificial neural networks and particularly non-linear methods like SVM, alongside traditional chemometric tools. These trends are reshaping the approach to handling vibrational spectroscopic data.In the study published by Zhu et al.,115 a method was presented for quickly quantifying the content of high-value pure vegetable oils, such as extra virgin olive oil, in vegetable blend oils on-site, using Raman spectroscopy and variable selection models. Samples of corn oil-EVOO and peanut oil-EVOO blends were prepared for Raman spectra measurement.115 The study then applied PLS regression and variable selection models, including a genetic algorithm combined with a moving window, particle swarm optimization with a moving window, grey wolf optimizer with a moving window, and whale optimization algorithm with a moving window, for a comparative analysis of the Raman spectra. The developed method accurately quantified the extra virgin olive oil content in the vegetable blend oil samples. Stability tests and limit of detection values confirmed the method's stability and sensitivity. Two-tailed paired t-tests showed no significant difference between the results obtained by the developed method and those obtained by gas chromatography-mass spectrometry.
de Santana et al.101 suggested utilizing the random forest algorithm for detecting adulteration in food products.101 The approach was tested on two cases: evening primrose oils, adulterated with soybean, corn, and sunflower oils, detected using ATR-FTIR spectroscopy and ground nutmeg, adulterated with substances such as cumin, commercial monosodium glutamate, soil, roasted coffee husks, and wood sawdust, identified using NIR diffuse reflectance spectroscopy. The random forest method showed better performance than PLS-DA and similar performance to SIMCA for the primrose oil, while for the ground nutmeg, it outperformed both PLS-DA and SIMCA.
A novel method combining NIR and Raman spectroscopy with a backpropagation neural network (BP-ANN) model was proposed by Kuang et al.59 to enhance the accuracy of detecting camellia oil adulteration. PCA was employed to reduce the dimensionality of the Raman spectral data of pure camellia oil, soybean oil, and their blends. A BP-ANN model was used to distinguish between authentic and adulterated camellia oil. This model achieved a prediction linearity value greater than 0.999 and a mean square error less than 1%.59
The study done by Amsaraj et al.56 demonstrates the use of Fourier-transform near-infrared (FT-NIR) spectroscopy combined with multivariate data analysis for detecting and quantifying butter yellow in mustard oil. The real coded genetic algorithm-least squares-SVM model, with 30 selected variables, showed high precision and accuracy in predicting butter yellow concentrations, significantly reducing the number of variables needed for analysis.56
Four primary pattern recognition methods, mapping and display, cluster analysis, feature selection, and classification, were used in the analysis of MIR spectra of ninety-seven edible oil samples such as avocado, peanut, almond, safflower, and hazelnut oils over a three-year period.158 The results of this study indicated the difficulties associated with considering the various sources of variability that can affect the IR spectra of each of the twenty edible oil varieties examined.
Both the number of published articles (Fig. 1) and the findings from these studies indicate that all vibrational spectroscopic methods are highly effective and successful in authentication studies. The literature consistently highlights that three vibrational methods offer detailed molecular structure information, crucial for identifying different oils, and machine learning techniques applied to the spectra are very effective in detecting subtle differences. Additionally, these techniques require minimal sample preparation and are non-destructive, making them particularly suitable for raw material screening of edible oils for quality control and authentication. However, each method has its own advantages and disadvantages. IR spectroscopy provides high sensitivity and specificity, making it excellent for routine analysis and quantification, while Raman spectroscopy is better suited for samples with high water content. One limitation of these techniques is the overlapping signals from various components, which can make it challenging to distinguish between different oils or identify specific adulterants. Additionally, accurate authentication requires extensive calibration and validation using a large number of reference samples. Moreover, variability in the spectral data can arise from differences in instruments and experimental conditions. Therefore, the standardization of methods and equipment is necessary to ensure reliable results.
No single spectroscopic method or data analysis technique stands out as significantly superior to the others in terms of success rates for the authentication studies. Although a more definitive assessment could be made if all three spectroscopic methods were applied simultaneously to the same set of samples and the data were analyzed collectively, the current literature demonstrates that all vibrational techniques and data analysis methods are effective in detecting low levels of oil adulteration. However, as can be seen in Tables 1–3, PLS based classification and regression techniques have been more commonly used. In general, the reported detection thresholds are typically lower than the levels required for economically unjust gains. These thresholds depend largely on the authentic oil and the type of adulterant used. Authenticating oils with very similar chemical structures or distinguishing them based on their geographical origin or similar factors is more challenging and requires a broad range of authentic samples for successful model building.
As this review indicates, research trends across all three vibrational spectroscopic techniques are similar. For instance, data fusion techniques are increasingly used in spectroscopic data evaluation, and combining vibrational spectroscopic methods is highly effective for this purpose. Vibrational spectroscopic techniques have also been used together with other spectroscopic techniques such as fluorescence or NMR spectroscopy. Another emerging trend is the use of portable instruments, with NIR and Raman spectroscopy being more suitable and thus more commonly applied in the literature (Tables 1–3). Additionally, NIR is gaining prominence as an imaging tool within hyperspectral imaging systems. However, all vibrational spectroscopic techniques are equally valuable in investigating the authenticity of newly marketed oils and identifying the geographical origin of oils. Most edible oil authentication studies using vibrational spectroscopy focus on detecting adulteration, which involves adding cheaper, lower-quality oils to economically valuable oils. As previously mentioned, a significant portion of this research concentrates on extra virgin olive oil, one of the high-priced oils. However, recent literature includes a growing number of studies on various newly marketed oils, addressing both adulteration and designation of origin. These studies consistently employ vibrational spectroscopy, often in combination with various machine learning techniques. Additionally, more studies on the designation of the origin of olive oil using vibrational spectroscopy and machine learning methods have been conducted. Recently, other oils, such as argan and camellia, have also begun to benefit from these types of investigations.
5. Conclusions
Research on the authenticity of edible oils indicates the effectiveness of vibrational spectroscopic methods, particularly when coupled with various chemometric techniques that increasingly use machine learning algorithms. These spectroscopic methods offer rapid analysis once chemometric models are established, enable online use, and provide non-destructive analysis with minimal waste generation. However, successful application in edible oil authentication depends on the well-established sampling protocols. The initial step involves constructing a database comprising both authentic and non-authentic samples. The quality of this database is very important, requiring not only a sufficient number of samples but also diversity across representative compositional ranges. Authentication studies often necessitate analyzing samples from diverse geographical areas, potentially involving multiple laboratories. Therefore, increased inter-laboratory studies are crucial to verify the accuracy, reliability, and comparability of the methods, minimizing disparities arising from equipment configurations. Data analysis techniques play a vital role in vibrational spectroscopic analysis, and advancements in these methods can help mitigate the limitations inherent in these techniques. In addition, vibrational spectroscopic techniques can be combined with each other or with other spectroscopic methods to overcome the challenges.Abbreviations
| ANN | Artificial neural network |
| ATR | Attenuated total reflectance |
| CVA | Canonical variate analysis |
| DA | Discriminant analysis |
| DD-SIMCA | Data driven soft independent modelling of class analogy |
| EVOO | Extra virgin olive oil |
| FAO | The food and agriculture organization of the United Nations |
| FDA | Functional data analysis |
| FTIR | Fourier transform infrared |
| GA | Genetic algorithm |
| HCA | Hierarchical clustering analysis |
| iPLS | Interval partial least squares |
| IR | Infrared |
| k-NN | k-Nearest neighbors |
| LDA | Linear discriminant analysis |
| LR | Linear regression |
| MCR-ALS | Multivariate curve resolution alternating least squares |
| MIR | Middle range infrared |
| MLR | Multiple linear regression |
| MSC | Multiplicative scatter correction |
| NIR | Near infrared |
| OC-PLS | One class partial least squares |
| OPLS-DA | Orthogonal partial least squares |
| OSC | Orthogonal signal correction |
| PARAFAC | Parallel factor analysis |
| PCA | Principal component analysis |
| PCR | Principal component regression |
| PDO | Protected designation of origin |
| PGI | Protected geographical indication |
| PLDS | Partial least distance squares |
| PLS | Partial least squares |
| PLS-DA | Partial least squares discriminant analysis |
| RF | Random forest |
| RMSE | Root mean squared error |
| SIMCA | Soft independent modelling of class analogy |
| SNV | Standard normal variate |
| SVM | Support vector machine |
| SVR | Support vector regression |
Data availability
The data from this study are available from the corresponding author upon reasonable request.Author contributions
Banu Ozen: writing – original draft preparation. Cagri Cavdaroglu: writing – original draft preparation. Figen Tokatli: writing – original draft preparation. All authors read and approved the final manuscript.Conflicts of interest
There are no conflicts to declare.References
- Codex Alimentarius Commission, Discussion Paper On Food Integrity And Food Authenticity, 2024, https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1%26url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FMeetings%252FCX-733-24%252FWorking%2BDocuments%252Ffc24_07e.pdf, accessed 15 January.
- O. Uncu and B. Ozen, Trends Food Sci. Technol., 2020, 100, 164–176 CrossRef CAS.
- C. Varallo, Recent fraudulent practices on wine, oil, and packaging, 2024, https://foodlawlatest.com/2024/01/08/recent-fraudulent-practices-on-wine-oil-and-packaging/, accessed 10 January.
- S. Jones, The Guardian, 2023, https://affidiajournal.com/en/irregularities-in-extra-virgin-olive-oil-german-investigation-reveals, accessed 16 January 2024.
- M. T. Roberts, T. Viinikainen and C. Bullon, International and National Regulatory Strategies to Counter Food Fraud, Food & Agriculture Org., 2022 Search PubMed.
- E. Gelpí, M. P. de la Paz, B. Terracini, I. Abaitua, A. G. de la Cámara, E. M. Kilbourne, C. Lahoz, B. Nemery, R. M. Philen, L. Soldevilla and S. Tarkowski, Environ. Health Perspect., 2002, 110, 457–464 Search PubMed.
- H. Kendall, B. Clark, C. Rhymer, S. Kuznesof, J. Hajslova, M. Tomaniova, P. Brereton and L. Frewer, Trends Food Sci. Technol., 2019, 94, 79–90 CrossRef CAS.
- Codex Alimentarius, CXS 210-1999, 2024, https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1%26url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf, accessed 25 January.
- The Council Of The European Union, Council Regulation (Ec) No 510/2006 on the protection of geographical indications and designations of origin for agricultural products and foodstuffs, 2024, https://eur-lex.europa.eu/eli/reg/2006/510/oj/eng, accessed 25 January.
- W. A. Salah and M. Nofal, J. Sci. Food Agric., 2021, 101, 811–819 CrossRef CAS PubMed.
- X. Li, L. Zhang, Y. Zhang, D. Wang, X. Wang, L. Yu, W. Zhang and P. Li, Trends Food Sci. Technol., 2020, 101, 172–181 CrossRef CAS.
- M. A. A. Mousa, Y. Wang, S. A. Antora, A. D. Al-qurashi, O. H. M. Ibrahim, H.-J. He, S. Liu and M. Kamruzzaman, Crit. Rev. Food Sci. Nutr., 2022, 62, 8009–8027 CrossRef CAS PubMed.
- Y. Xu, P. Zhong, A. Jiang, X. Shen, X. Li, Z. Xu, Y. Shen, Y. Sun and H. Lei, TrAC, Trends Anal. Chem., 2020, 131, 116017 CrossRef CAS.
- A. Sudhakar, S. K. Chakraborty, N. K. Mahanti and C. Varghese, Crit. Rev. Food Sci. Nutr., 2023, 63, 873–901 CrossRef CAS PubMed.
- S. A. Ordoudi, L. Strani and M. Cocchi, Molecules, 2023, 28, 337 CrossRef CAS PubMed.
- D. N. Sathyanarayana, Vibrational Spectroscopy: Theory and Applications, New Age International, 2015 Search PubMed.
- L. Feng, B. Wu, S. Zhu, Y. He and C. Zhang, Front. Nutr., 2021, 8, 680357 CrossRef PubMed.
- J. Ma, D.-W. Sun, B. Nicolai, H. Pu, P. Verboven, Q. Wei and Z. Liu, J. Food Eng., 2019, 261, 100–108 CrossRef CAS.
- D. Cozzolino and J. Chapman, Anal. Bioanal. Chem., 2024, 416, 611–620 CrossRef CAS PubMed.
- R. Vitale, M. Cocchi, A. Biancolillo, C. Ruckebusch and F. Marini, Anal. Chim. Acta, 2023, 1270, 341304 CrossRef CAS PubMed.
- P. Oliveri, Anal. Chim. Acta, 2017, 982, 9–19 CrossRef CAS PubMed.
- S. Wold, M. Sjöström and L. Eriksson, Chemom. Intell. Lab., 2001, 58, 109–130 CrossRef CAS.
- M. Bylesjö, M. Rantalainen, O. Cloarec, J. K. Nicholson, E. Holmes and J. Trygg, J. Chemom., 2006, 20, 341–351 CrossRef.
- Å. Rinnan, F. van den Berg and S. B. Engelsen, TrAC, Trends Anal. Chem., 2009, 28, 1201–1222 CrossRef.
- P. Mishra, A. Biancolillo, J. M. Roger, F. Marini and D. N. Rutledge, TrAC, Trends Anal. Chem., 2020, 132, 116045 CrossRef CAS.
- E. Borràs, J. Ferré, R. Boqué, M. Mestres, L. Aceña and O. Busto, Anal. Chim. Acta, 2015, 891, 1–14 CrossRef PubMed.
- K. R. Murphy, C. A. Stedmon, D. Graeber and R. Bro, Anal. Methods, 2013, 5, 6557–6566 RSC.
- R. A. Harshman and M. E. Lundy, Comput. Stat. Data Anal., 1994, 18, 39–72 CrossRef.
- A. M. Hüsser, L. Caron-Desrochers, J. Tremblay, P. Vannasing, E. Martínez-Montes and A. Gallagher, Neurophotonics, 2022, 9, 045004 Search PubMed.
- R. Leardi, M. B. Seasholtz and R. J. Pell, Anal. Chim. Acta, 2002, 461, 189–200 CrossRef CAS.
- T. Mehmood, K. H. Liland, L. Snipen and S. Sæbø, Chemometr. Intell. Lab., 2012, 118, 62–69 CrossRef CAS.
- S. Riahi, M. R. Ganjali, P. Norouzi and F. Jafari, Sens. Actuators, B, 2008, 132, 13–19 CrossRef CAS.
- D. E. Goldberg, Genetic Algorithms in Search, Optimization, and Machine Learning, Addison-Wesley, Reading MA, 1989 Search PubMed.
- L. Breiman, Mach. Learn., 2001, 45, 5–32 CrossRef.
- G. Biau and E. Scornet, Test, 2016, 25, 197–227 CrossRef.
- I. Gonzalez-Fernandez, M. A. Iglesias-Otero, M. Esteki, O. A. Moldes, J. C. Mejuto and J. Simal-Gandara, Crit. Rev. Food Sci. Nutr., 2019, 59, 1913–1926 CrossRef CAS PubMed.
- N. Liang, S. Sun, C. Zhang, Y. He and Z. Qiu, Crit. Rev. Food Sci. Nutr., 2022, 62, 2963–2984 CrossRef PubMed.
- W. Zheng, X. Fu and Y. Ying, Chemom. Intell. Lab., 2014, 139, 42–47 CrossRef CAS.
- D. Wu, Y. He, S. Feng and D.-W. Sun, J. Food Eng., 2008, 84, 124–131 CrossRef CAS.
- Y. Li, Y. M. Xiong and S. G. Min, Vib. Spectrosc., 2019, 101, 20–27 CrossRef CAS.
- H. Jiang and Q. S. Chen, Molecules, 2019, 24, 2134 CrossRef CAS PubMed.
- X. R. Meng, C. L. Yin, L. B. Yuan, Y. Zhang, Y. Ju, K. H. Xin, W. B. Chen, K. D. Lv and L. Q. Hu, Food Chem., 2023, 405, 134828 CrossRef CAS PubMed.
- L. S. Vieira, C. Assis, M. de Queiroz, A. A. Neves and A. F. de Oliveira, Food Chem., 2021, 345, 128866 CrossRef CAS PubMed.
- W. Sohng, Y. Park, D. Jang, K. Cha, Y. M. Jung and H. Chung, Talanta, 2020, 212, 120748 CrossRef CAS PubMed.
- G. Stavrakakis, A. Philippidis and M. Velegrakis, Food Anal. Methods, 2022, 15, 285–293 CrossRef.
- W. R. Song, Z. Y. Song, J. Vincent, H. Wang and Z. Wang, Talanta, 2020, 216, 120920 CrossRef CAS PubMed.
- A. M. Jiménez-Carvelo, V. A. Lozano and A. C. Olivieri, Food Control, 2019, 96, 22–28 CrossRef.
- S. Violino, C. Benincasa, C. Taiti, L. Ortenzi, F. Pallottino, E. Marone, S. Mancuso and C. Costa, Eur. Food Res. Technol., 2021, 247, 1013–1022 CrossRef CAS.
- S. Y. Yao, D. P. Aykas and L. Rodriguez-Saona, Foods, 2020, 10, 42 CrossRef PubMed.
- O. Hu, J. Chen, P. F. Gao, G. F. Li, S. J. Du, H. Y. Fu, Q. Shi and L. Xu, J. Sci. Food Agric., 2019, 99, 2285–2291 CrossRef CAS PubMed.
- X. H. Bian, D. Y. Wu, K. Zhang, P. Liu, H. B. Shi, X. Y. Tan and Z. G. Wang, Biosensors, 2022, 12, 586 CrossRef PubMed.
- K. C. Kaufmann, K. A. Sampaio, J. F. García-Martín and D. F. Barbin, Food Control, 2022, 132, 108536 CrossRef CAS.
- E. Youssra, A. Filali-Maltouf, B. Belkadi, A. Ferradous, R. Karoui and H. Zaroual, Eur. Food Res. Technol., 2023, 249, 1857–1873 CrossRef.
- S. Farres, L. Srata, F. Fethi and A. Kadaoui, Vib. Spectrosc., 2019, 102, 79–84 CrossRef CAS.
- A. Menevseoglu, Processes, 2022, 10, 503 CrossRef.
- R. Amsaraj and S. Mutturi, Infrared Phys. Technol., 2023, 129, 104543 CrossRef CAS.
- M. Mburu, C. Komu, O. Paquet-Durand, B. Hitzmann and V. Zettel, Foods, 2021, 10, 1798 CrossRef CAS PubMed.
- N. Su, S. Z. Weng, L. S. Wang and T. S. Xu, Biosensors, 2021, 11, 492 CrossRef PubMed.
- J. H. Kuang, N. N. Luo, Z. Q. Hao, J. J. Xu, X. D. He and J. L. Shi, J. Food Meas. Char., 2022, 16, 3208–3215 CrossRef.
- Q. W. Du, M. T. Zhu, T. Shi, X. Luo, B. Gan, L. J. Tang and Y. Chen, Food Control, 2021, 121, 107577 CrossRef CAS.
- Q. Liu, Z. L. Gong, D. P. Li, T. Wen, J. W. Guan and W. F. Zheng, Molecules, 2023, 28, 5943 CrossRef CAS PubMed.
- M. N. Hussain, K. N. Basri, S. Arshad, S. Mustafa, M. F. A. Khir and J. Bakar, Food Anal. Methods, 2023, 16, 349–355 CrossRef.
- S. S. Andoh, K. Nyave, B. Asamoah, B. Kanyathare, T. Nuutinen, C. Mingle, K. E. Peiponen and M. Roussey, Food Addit. Contam.: Part A, 2020, 37, 1049–1060 CrossRef CAS PubMed.
- Z. Yuan, L. X. Zhang, D. Wang, J. Jiang, P. D. Harrington, J. Mao, Q. Zhang and P. W. Li, LWT-Food Sci. Technol., 2020, 125, 109247 CrossRef CAS.
- R. C. Castro, D. S. M. Ribeiro, J. L. M. Santos and R. Páscoa, Talanta, 2021, 230, 122373 CrossRef CAS PubMed.
- S. Balbino, D. Vincek, I. Trtanj, D. Egredija, J. Gajdos-Kljusuric, K. Kraljic, M. Obranovic and D. Skevin, Foods, 2022, 11, 835 CrossRef CAS PubMed.
- K. D. Aparnathi, S. Sharma, B. Antony and B. M. Mehta, Indian J. Dairy Sci., 2019, 72, 12–22 Search PubMed.
- M. L. D. Medeiros, J. P. Cruz-Tirado, A. F. Lima, J. M. D. Netto, A. P. B. Ribeiro, D. Bassegio, H. T. Godoy and D. F. Barbin, J. Food Compos. Anal., 2022, 107, 104403 CrossRef.
- M. L. D. Medeiros, A. F. Lima, M. C. Gonçalves, H. T. Godoy and D. F. Barbin, Food Chem., 2023, 425, 136461 CrossRef CAS PubMed.
- L. M. Leme, F. Nakamura, A. A. C. Tanamati, P. Valderrama and P. H. Março, LWT-Food Sci. Technol., 2019, 109, 179–185 CrossRef CAS.
- P. F. Filoda, L. F. Fetter, F. Fornasier, R. D. D. Schneider, G. A. Helfer, B. Tischer, A. Teichmann and A. Ben da Costa, Food Anal. Methods, 2019, 12, 293–304 CrossRef.
- S. Schwolow, N. Gerhardt, S. Rohn and P. Weller, Anal. Bioanal. Chem., 2019, 411, 6005–6019 CrossRef CAS PubMed.
- M. Didham, V. K. Truong, J. Chapman and D. Cozzolino, Food Anal. Methods, 2020, 13, 601–607 CrossRef.
- I. Sota-Uba, C. G. White, K. Booksh and B. K. Lavine, J. Chemom., 2023, 37, e3469 CrossRef CAS.
- O. Uncu, B. Ozen and F. Tokatli, J. Sci. Food Agric., 2020, 100, 2153–2165 CrossRef CAS PubMed.
- D. Malavi, A. Nikkhah, K. Raes and S. Van Haute, Foods, 2023, 12, 429 CrossRef CAS PubMed.
- S. A. Ordoudi, O. Özdikicierler and M. Z. Tsimidou, Food Control, 2022, 142, 109240 CrossRef CAS.
- O. Uncu and B. Ozen, Food Control, 2019, 105, 209–218 CrossRef CAS.
- F. Ahmmed, D. P. Killeen, K. C. Gordon and S. J. Fraser-Miller, Molecules, 2022, 27, 4534 CrossRef CAS PubMed.
- O. Uncu, A. Napiórkowska, T. K. Szajna and B. Ozen, Microchem. J., 2020, 158, 105128 CrossRef CAS.
- A. I. N. Leite, C. G. Pereira, J. Andrade, N. M. Vicentini, M. J. V Bell and V. Anjos, LWT-Food Sci. Technol., 2019, 109, 63–69 CrossRef CAS.
- R. Jamwal, S. Kumari, B. Balan, S. Kelly, A. Cannavan and D. K. Singh, Spectrochim. Acta, Part A, 2021, 244, 118822 CrossRef CAS PubMed.
- R. Jamwal, S. Kumari, S. Kelly, A. Cannavan and D. K. Singh, Vib. Spectrosc., 2021, 113, 103226 CrossRef CAS.
- K. Q. Hu, Z. Y. Huyan, S. T. H. Sherazi and X. Z. Yu, J. Oleo Sci., 2019, 68, 1073–1084 CrossRef CAS PubMed.
- U. Akram, A. Sahar, A. Sameen, N. Muhammad, M. H. Ahmad, M. I. Khan, M. Usman and H. U. U. Rahman, Int. J. Food Prop., 2023, 26, 167–178 CrossRef CAS.
- A. A. Bunaciu, S. Fleschin and H. Y. Aboul-Enein, Chem. Pap., 2022, 76, 5533–5539 CrossRef CAS.
- F. Taous, T. El Ghali, H. Marah, K. Laraki, M. Islam, A. Cannavan and S. Kelly, Food Anal. Methods, 2022, 15, 3032–3044 CrossRef.
- F. N. Arslan, G. Akin, S. N. K. Elmas, I. Yilmaz, H. G. Janssen and A. Kenar, Food Control, 2019, 98, 323–332 CrossRef CAS.
- Q. Ye and X. H. Meng, Food Chem., 2022, 385, 132661 CrossRef CAS PubMed.
- J. X. Han, R. X. Sun, X. Y. Zeng, J. K. Zhang, R. R. Xing, C. D. Sun and Y. Chen, Molecules, 2020, 25, 2036 CrossRef CAS PubMed.
- B. Gao, S. Xu, L. J. Han and X. Liu, Food Chem., 2021, 343, 128420 CrossRef CAS PubMed.
- A. N. Ikhsan, A. Rohman, M. Mustafidah, R. Martien and L. A. Lestari, Indones. J. Pharm., 2023, 34, 174–181 CAS.
- A. Windarsih, A. W. Indrianingsih, W. Apriyana and A. Rohman, Chem. Pap., 2023, 77, 2859–2870 CrossRef CAS.
- M. El Mouftari, I. Essafi, A. Khalidi, F. Kzaiber, G. A. M. Ali, F. Z. Mahjoubi and A. Oussama, Emergent Mater., 2022, 5, 167–174 CrossRef CAS.
- R. J. Amit, S. Kumari, A. S. Dhaulaniya, B. Balan and D. K. Singh, LWT-Food Sci. Technol., 2020, 118, 108754 CrossRef CAS.
- S. Kumari and D. K. Singh, Vib. Spectrosc., 2023, 126, 103525 CrossRef CAS.
- R. J. Amit, S. Kumari, S. Kelly, A. Cannavan and D. K. Singh, LWT-Food Sci. Technol., 2020, 125, 109250 CrossRef CAS.
- M. D. Neves and R. J. Poppi, Talanta, 2020, 219, 121338 CrossRef CAS PubMed.
- R. J. Amit, S. Kumari, A. S. Dhaulaniya, B. Balan, S. Kelly, A. Cannavan and D. K. Singh, Vib. Spectrosc., 2020, 109, 103066 CrossRef CAS.
- G. Akin, S. N. K. Elmas, F. N. Arslan, I. Yilmaz and A. Kenar, LWT-Food Sci. Technol., 2019, 100, 126–137 CrossRef CAS.
- F. B. de Santana, W. B. Neto and R. J. Poppi, Food Chem., 2019, 293, 323–332 CrossRef CAS PubMed.
- S. D. Rodríguez, M. Gagneten, A. E. Farroni, N. M. Percibaldi and M. P. Buera, Food Control, 2019, 105, 78–85 CrossRef.
- S. M. Park, H. Y. Yu, H. S. Chun, B. H. Kim and S. Ahn, J. Oleo Sci., 2019, 68, 389–398 CrossRef CAS PubMed.
- I. Sandu, P. O. Tanasa, I. C. A. Sandu, I. C. Negru, A. V Sandu and V. Vasilache, Appl. Sci., 2019, 10, 306 CrossRef.
- F. Khanban, A. B. Garmarudi, H. Parastar and G. Toth, Infrared Phys. Technol., 2022, 127, 104369 CrossRef CAS.
- F. Ahmmed, K. C. Gordon, D. P. Killeen and S. J. Fraser-Miller, Molecules, 2022, 27, 4534 CrossRef CAS PubMed.
- S. N. G. Pereira, A. B. S. De Lima, T. D. Oliveira, A. S. Batista, J. C. De Jesus, S. P. B. Ferrao and L. S. Santos, LWT-Food Sci. Technol., 2022, 154, 112857 CrossRef CAS.
- T. A. do Nascimento, T. I. B. Lopes, C. E. D. Nazario, S. L. Oliveira and G. B. Alcantara, Food Res. Int., 2021, 144, 110362 CrossRef CAS PubMed.
- I. Tarhan, M. R. Bakir, O. Kalkan and H. Kara, Food Anal. Methods, 2021, 14, 361–371 CrossRef.
- I. Dogruer, H. H. Uyar, O. Uncu and B. Ozen, Food Chem., 2021, 345, 128815 CrossRef CAS PubMed.
- F. N. Arslan and F. Çaglar, Food Anal. Methods, 2019, 12, 355–370 CrossRef.
- A. Fort, I. Ruisánchez and M. P. Callao, Microchem. J., 2021, 169, 106611 CrossRef CAS.
- I. Barros, L. P. Santos, P. R. Filgueiras and W. Romao, Vib. Spectrosc., 2021, 116, 103294 CrossRef CAS.
- I. Ruisánchez, A. M. Jiménez-Carvelo and M. P. Callao, Talanta, 2021, 222, 121564 CrossRef PubMed.
- J. J. Zhu, Y. W. Rong, X. Jiang, H. Qian, X. H. Yu and Q. S. Chen, J. Food Compos. Anal., 2023, 123, 105503 CrossRef CAS.
- T. K. de Lima, M. Musso and D. B. Menezes, Food Chem., 2020, 333, 127454 CrossRef CAS PubMed.
- H. P. Wang and X. Wan, Spectrochim. Acta, Part A, 2021, 248, 119183 CrossRef CAS PubMed.
- P. P. Fang, H. P. Wang and X. Wan, Food Chem., 2022, 397, 133763 CrossRef CAS PubMed.
- S. V Zade and H. Abdollahi, Microchem. J., 2023, 191, 108867 CrossRef.
- I. Barros, L. S. Paixao, M. H. C. Nascimento, V. Lacerda, P. R. Filgueiras and W. Romao, Vib. Spectrosc., 2021, 116, 103299 CrossRef CAS.
- S. V Zade, E. Forooghi, B. Jannat, F. Hashempour-baltork and H. Abdollahi, Chemom. Intell. Lab., 2023, 240, 104903 CrossRef.
- S. Duraipandian, J. C. Petersen and M. Lassen, Appl. Sci., 2019, 9, 2433 CrossRef CAS.
- S. S. Andoh, T. Nuutinen, C. Mingle and M. Roussey, J. Eur. Opt. Soc., 2019, 15, 21 CrossRef.
- M. K. Su, Q. Jiang, J. H. Guo, Y. X. Zhu, S. Cheng, T. Yu, S. S. Du, Y. F. Jiang and H. L. Liu, LWT-Food Sci. Technol., 2021, 143, 111143 CrossRef CAS.
- A. Becze and D. Simedru, Not. Bot. Horti Agrobot. Cluj-Napoca, 2020, 48, 1426–1438 CrossRef CAS.
- H. T. Temiz, S. D. Velioglu, K. G. Guner and H. M. Velioglu, LWT-Food Sci. Technol., 2021, 146, 111479 CrossRef CAS.
- S. S. Du, M. K. Su, Y. F. Jiang, F. F. Yu, Y. Xu, X. F. Lou, T. Yu and H. L. Liu, ACS Sens., 2019, 4, 1798–1805 CrossRef CAS PubMed.
- H. F. Zhao, Y. L. Zhan, Z. Xu, J. J. Nduwamungu, Y. Z. Zhou, R. Powers and C. M. Xu, Food Chem., 2022, 373, 131471 CrossRef CAS PubMed.
- X. Li, D. Wang, F. Ma, L. Yu, J. Mao, W. Zhang, J. Jiang, L. X. Zhang and P. W. Li, Food Chem., 2023, 405, 134884 CrossRef CAS PubMed.
- T. T. M. Htet, J. Cruz, P. Khongkaew, C. Suwanvecho, L. Suntornsuk, N. Nuchtavorn, W. Limwikrant and C. Phechkrajang, J. Food Compos. Anal., 2021, 103, 104119 CrossRef.
- H. P. Wang, Y. J. Xin, H. Z. Ma, P. P. Fang, C. H. Li, X. Wan, Z. P. He, J. J. Jia and Z. C. Ling, Food Chem., 2021, 362, 130041 CrossRef CAS PubMed.
- S. Shenbagamoorthi, S. K. Devi, K. P. Ananth and N. D. Jayram, Spectrosc. Lett., 2023, 56, 512–520 CrossRef.
- C. Berghian-Grosan and D. A. Magdas, J. Mol. Struct., 2021, 1246, 131211 CrossRef CAS.
- N. N. Y. Karacaglar, T. Bulat, I. H. Boyaci and A. Topcu, J. Food Drug Anal., 2019, 27, 101–110 CrossRef PubMed.
- Y. C. Su, Y. P. Li, C. S. Tan, R. Zeng, Y. S. Hua, J. H. Hu and L. H. Wang, J. Food Sci., 2022, 87, 3407–3418 CrossRef CAS PubMed.
- H. Jin, H. Li, Z. Yin, Y. Zhu, A. Lu, D. Zhao and C. Li, Food Chem., 2021, 362, 130191 CrossRef CAS PubMed.
- S. Farres, L. Srata, M. Chikri, S. Addou and F. Fethi, Grasas Aceites, 2023, 74, e520 CrossRef CAS.
- C. Gertz, B. Matthäus and I. Willenberg, Eur. J. Lipid Sci. Technol., 2020, 122, 1900355 CrossRef CAS.
- D. Rabiej-Kozioł, M. Momot-Ruppert, B. Stawicka and A. Szydłowska-Czerniak, Molecules, 2023, 28 CrossRef PubMed.
- International Olive Council, Guide for the Determination of the Characteristics of Oil-Olives, 2024, https://www.internationaloliveoil.org/wp-content/uploads/2019/11/COI-OH-Doc.-1-2011-Eng.pdf, accessed 4 February Search PubMed.
- N. Tena, R. Aparicio, V. Baeten, D. L. García-González and J. A. Fernández-Pierna, Eur. J. Lipid Sci. Technol., 2019, 121, 1900035 CrossRef CAS.
- S. Lamas, D. Ruano, F. Dias, F. Barreiro, J. A. Pereira, A. M. Peres and N. Rodrigues, Chem. Biodivers., 2024, 21, e202301629 CrossRef CAS PubMed.
- H. Jayatunga, H. Somasiri and K. R. R. Mahanama, Int. J. Food Nutr. Sci., 2020, 5, 38–43 Search PubMed.
- E. Casadei, E. Valli, F. Panni, J. Donarski, J. Farrús Gubern, P. Lucci, L. Conte, F. Lacoste, A. Maquet, P. Brereton, A. Bendini and T. Gallina Toschi, Food Control, 2021, 124, 107902 CrossRef.
- R. Kontzedaki, E. Orfanakis, G. Sofra-Karanti, K. Stamataki, A. Philippidis, A. Zoumi and M. Velegrakis, Molecules, 2020, 25 CrossRef CAS PubMed.
- E. Kakouri, P.-K. Revelou, C. Kanakis, D. Daferera, C. S. Pappas and P. A. Tarantilis, Foods, 2021, 10 CrossRef CAS PubMed.
- N. Rodrigues, F. Peres, S. Casal, A. Santamaria-Echart, F. Barreiro, A. M. Peres and J. Alberto Pereira, Food Chem., 2023, 398, 133945 CrossRef CAS PubMed.
- Z. Deng, J. Fu, M. Yang, W. Zhang, Y.-H. Yun and L. Zhang, J. Food Compos. Anal., 2024, 125, 105730 CrossRef CAS.
- P. Zhu, Q. Yang and H. Zhao, J. Integr. Agric., 2022, 21, 2777–2785 CrossRef CAS.
- D. M. Kita, H. Lin, A. Agarwal, K. Richardson, I. Luzinov, T. Gu and J. Hu, IEEE J. Sel. Top. Quantum Electron., 2017, 23, 340–349 Search PubMed.
- R. A. Crocombe, Appl. Spectrosc., 2018, 72, 1701–1751 CrossRef CAS PubMed.
- J. Müller-Maatsch and S. M. van Ruth, Foods, 2021, 10 CrossRef PubMed.
- L. Rodriguez-Saona, D. P. Aykas, K. R. Borba and A. Urtubia, Curr. Opin. Food Sci., 2020, 31, 136–150 CrossRef.
- A. C. de Oliveira Moreira and J. W. B. Braga, Food Anal. Methods, 2021, 14, 865–872 CrossRef.
- D. P. Aykas, A. D. Karaman, B. Keser and L. Rodriguez-Saona, Foods, 2020, 9 Search PubMed.
- M. Kamruzzaman, Y. Makino and S. Oshita, J. Food Eng., 2016, 170, 8–15 CrossRef CAS.
- J.-Y. Choi and K.-D. Moon, J. Food Compos. Anal., 2020, 90, 103505 CrossRef CAS.
- I. Sota-Uba, M. Bamidale, J. Moulton, K. Booksh and B. K. Lavine, Chemom. Intell. Lab., 2021, 210, 104251 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |