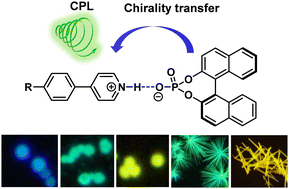
Well-defined luminescent micro/nanostructures are important building blocks for miniaturized photonic devices. In this work, a simple method is employed to prepare organic microspheres and crystalline microrods with circularly polarized luminescence (CPL) properties. The in situ protonation and co-assembly of a series of pyridine-functionalized chromophores and a chiral binaphthol phosphoric acid give rise to blue-to-yellow emissive amorphous microspheres and green- and yellow-emissive crystalline microrods with good fluorescence quantum yields (9.6–64.6%). The luminescence dissymmetry factors (glum) of microrods (∼10−3) are found to be one order of magnitude larger than those of microspheres. These microstructures and related materials are further characterized by fluorescence microscopy, scanning electron microscopy, single-crystal and powder X-ray diffraction, and Fourier transform-infrared spectroscopy analyses. The well-defined morphologies and promising luminescence properties of these microstructures make them potentially useful for chiral photonic applications.