Graphene family in cancer therapy: recent progress in cancer gene/drug delivery applications
Negin
Borzooee Moghadam
,
Manizheh
Avatefi
,
Mahnaz
Karimi
and
Matin
Mahmoudifard
 *
*
Department of Industrial and Environmental Biotechnology, National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran. E-mail: matinmahmodifard@yahoo.com; m_mahmodifard@nigeb.ac.ir
First published on 14th February 2023
Abstract
In the past few years, the development in the construction and architecture of graphene based nanocomplexes has dramatically accelerated the use of nano-graphene for therapeutic and diagnostic purposes, fostering a new area of nano-cancer therapy. To be specific, nano-graphene is increasingly used in cancer therapy, where diagnosis and treatment are coupled to deal with the clinical difficulties and challenges of this lethal disease. As a distinct family of nanomaterials, graphene derivatives exhibit outstanding structural, mechanical, electrical, optical, and thermal capabilities. Concurrently, they can transport a wide variety of synthetic agents, including medicines and biomolecules, such as nucleic acid sequences (DNA and RNA). Herewith, we first provide an overview of the most effective functionalizing agents for graphene derivatives and afterward discuss the significant improvements in the gene and drug delivery composites based on graphene.
1. Introduction
Cancer is a leading cause of mortality for people all over the globe. New cancer diagnoses in 2020 were 19 million individuals, while cancer deaths were estimated to be 10 million people. By 2035, the number of cancer patients is expected to have doubled.1 Cancer is a term referred to a disease caused by genetic or environmental factors that result in critical-gene mutations. Proliferation, invasion, and metastasis are the core characteristics of this illness and the main reasons for its challenging therapy. New, powerful, and beneficial technologies for rapid and effective cancer detection and treatment are urgently required due to the increasing rise in cancer suffering. Diagnostic procedures based on imaging, tissue, blood, genetics, and immunology have become popular in recent years for the early detection of cancer.2Many cancer treatment approaches, including surgery, chemotherapy, hormone therapy, radiation therapy, and immune therapy, have been discovered and are now being employed to cure the disease.3 Surgery is the most effective technique for removing solid tumors, and it is often used in conjunction with chemotherapy, which is considered to be one of the most important cancer treatment options.4
Chemotherapy is beneficial in treating malignancies such as acute myelogenous leukemia, acute lymphoblastic leukemia, lung cancer, ovarian cancer, etc. However, there have been several reported adverse effects. Several side effects arise owing to the non-specific action of the medications, severe systemic toxicity, and cancer cell resistance. Hair loss, nausea, vomiting, and anemia are just a few examples. Cooperative treatments, such as chemotherapy–radiotherapy, surgery–chemotherapy, and so on, have been discovered to be even more successful for cancer therapy.4,5
These approaches don’t all lead to a complete cure due to various reasons, including tumor metastasis, tumor cell drug resistance, fast removal of drugs from the body (short half-life of drugs), non-cancer-cell-specific lethality, low biocompatibility, possible association with cancer stem cells, etc. Henceforth, designing modern approaches with fewer side effects and high specificity is promptly required for cancer therapy.6
Nanotechnology and other cutting-edge technologies have made it possible to overcome the obstacles mentioned. Nanotechnology is often regarded as the manufacturing technology of the twenty-first century because it permits the creation of novel materials by atomic and molecular level manipulation of existing substances.
Nanoparticles are microscopic particles sized 1–100 nm with unique physiochemical properties. Because they are so much smaller than cells, they can interface with the cell membrane and be taken within the cell, making them very useful in biomedicine.7 However, these attributes can change depending on the nanoparticles’ structure, size, and shape. Many characteristics, such as the kind of material and the number of dimensions (1D,8–10 2D, and 3D), are used to classify nanomaterials into distinct categories. Nanomaterials based on carbon, in particular, have gained attention for their unique characteristics.
Carbon-based nanomaterials, such as the graphene family, are the pioneers in biomedicine and cancer nano-based therapeutics. These nano-sized materials are optimal for biology and medicine usage because they possess high conductivity and stability with specific optical traits.11
Graphene derivatives such as graphene oxide (GO), nano graphene oxide (NGO), reduced graphene oxide (rGO), graphene nano-ribbons (GNR), graphene quantum dots (GQD), etc. are extensively used in drug delivery. They are basically made from graphene sheets that go through chemical reactions.12
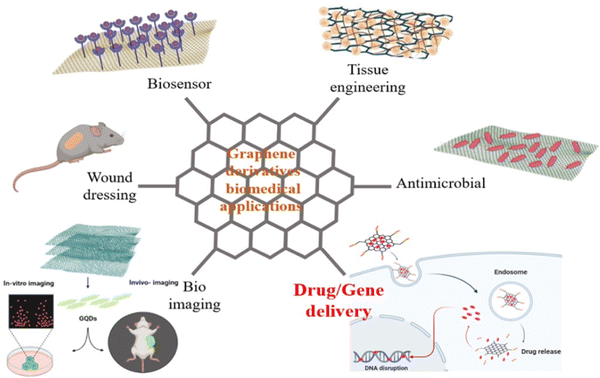
Today, graphene derivatives are tackling the most serious shortcomings of present diagnostic13 and therapeutic techniques14 such as tissue engineering,15,16 bioimaging,17,18 gene/drug delivery,19 biosensing,17,20–22 wound dressing,23 and anti-bacterial substances,24 and thus promoting them. Graphene derivatives have wonderful physio-chemical and electrical properties such as ease of functionalization, the ability to couple with various molecules such as drugs and nucleic acid sequences, high surface area, biocompatibility, low toxicity, electrospun mediated synthesis of various materials, and direct-targeted delivery.25 The most important biomedical applications of graphene derivatives are illustrated in Fig. 1.
Moreover, graphene family nanomaterials can absorb a wide range of external light such as ultraviolet (UV) and infrared light, and this excellent feature endows them with superior optical characteristics. For instance, light energy may be used to induce hyperthermia in the graphene family. Graphene-based nanomaterials, on the other hand, can contain a range of photosensitizers and create reactive oxygen species (ROS) under laser irradiation, allowing for effective cancer eradication by photodynamic treatment (PDT). Furthermore, cancer treatment may benefit significantly from the exceptional immunological properties of the graphene family. Small graphene particles have been shown to activate immune cells, stimulate the production of cytokines, and control the immunological response, according to previous studies.26
Thus, nano-graphene-based cancer therapies such as chemotherapy (chemo), photodynamic therapy(PDT), molecular therapy, immune therapy, photothermal therapy (PTT), and combined therapies like co-drug–gene delivery, chemo-PTT, chemo-PTT-PDT, etc., are now widely used in the treatment of cancer patients.
Graphene and its derivatives have been shown in recent investigations to be capable of smart and controlled delivery of various kinds of molecules, meaning they can carry loads of drugs, nucleic acids, proteins, etc., and deliver them to a particular destination (e.g. tissue or cancer cells). Besides, they can be engineered to release their cargo at a more gradual pace. Another outstanding property of the graphene family is that they can be easily tracked due to their remarkable optical features.4,27
In a nutshell, graphene derivatives can securely carry and deliver numerous biomolecules into the target cell/tissue without causing any damage to the body. In addition, they may release their payload in reaction to a trigger (light, heat, ultrasound, etc.), low pH (acidic tumor environment), or other specific circumstances. It is also feasible to transport medications, genes, RNAs, and many chemicals and biological components concurrently. These capabilities have made them particularly well-suited for biological and medical applications, including cancer treatment. This review aims to present a comprehensive overview of current developments in cancer treatment employing gene/drug delivery systems based on graphene derivatives.4,27
1.1 Graphene derivatives
Recently, graphene's application in biomedicine, especially in DNA sequencing, biosensor construction, and cell proliferation, has been demonstrated. Additionally, its water insolubility properties have slighted its application in biomedicine. The preliminary investigations on graphene's potential in drug delivery were done in 2008 by Liu et al.28 In this project, poly(ethylene glycol) (PEG)-GO was fabricated, and afterward, the water-insoluble SN38 drug was non-covalently attached. Thenceforth, a great deal of work has been done on graphene derivatives to create more efficient nanocarriers for anticancer purposes.
Moreover, GO is known as a highly advantageous material in various kinds of cancer therapies such as immunotherapy,32 biosensing,33 extracellular matrix (ECM) therapy,34 and gene/drug delivery.35 The widespread use of GO in cancer treatment strategies demonstrates its distinct structural properties, for instance, its high biocompatibility, good water solvability, high surface area, and easy functionalization.
Studies indicate that tumor cells are more vulnerable to extreme temperatures than normal cells.36 Thus, due to rGO's brilliant potential for light to heat conversion under NIR light and its photoacoustic properties, it has good photothermal properties that have increased its application in cancer therapy.37 Besides, it has a vast surface area with various functional groups, making it a lot more biocompatible and a good candidate for drug and gene delivery.
When employed for gene delivery, functionalized rGO has been tested and deemed successful in the endosomal escape after entering the cell.38 rGO serves as the general foundation in the construction of many nanocomposites that are employed in cancer therapy methods such as chemotherapy (drug delivery),39 phototherapy,40 and combined therapies,36 as well as a biosensor.41
NGO is renowned for its high surface area, which allows for the connection of a large number of drug molecules and functional groups. NGO can be employed as a stable and biocompatible nanocarrier for drug delivery on the condition that hydrophilic polymers are added as functionalizing agents to increase the biocompatibility and stability of NGO in physiological solutions.42
In the past few years, oxidized graphene nanoribbons (O-GNRs) have been used for gene delivery because of their unique properties, such as a large amount of loading capacity for nucleic acid sequences with no limitation in size, without getting functionalized.47 To further improve the GNR's efficiency and obtain a higher transfection rate, GNRs can be grafted with functional groups such as nitrogen (amination) and oxygen (reduction), or coated with PEG, PEI, and chitosan biopolymers. Thereby, these surface and edge changes make them more biocompatible and hydrophilic.44
GQDs are qualified to sense different molecules such as cancer cell-specific biomolecules accessible on the cancer cell membrane or the ones released in its environment. Besides, they can also sense pH alterations.50 GQDs solely are confirmed to have anticancer effects. GQDs can pass through the cell membrane and even the nuclear membrane, connect to the cell's DNA structure through π–π and electrostatic bonds, and cause irreversible damage resulting in apoptosis.51 For example, Qi et al.51 designed a GQD-based nanocarrier for selective targeting and elimination of cancer cells through DNA interaction. First, they added amine to the GQDs; afterward, they used nucleus-targeting TAT peptides (TAT-NGs) as a means of selective nucleus targeting. Then folic acid (FA)-PEG was loaded for selective targeting of cancer cells. The resulting nanocomplex, FA-PEG-GQD-TAT, successfully passed the biocompatibility, cancer cell targeting, and cellular internalization tests. It passed through the membrane, interacted with the cell's DNA, and induced apoptosis. Finally, the undeniable application of this nanocomplex in cancer therapy was determined.
Moreover, GQDs are highly applicable in visualization therapies owing to their marvelous optical properties, photostability, and biocompatibility.49 Even though GQD-based nanocomposites have recently been used to deliver anticancer drugs such as imatinib for leukemia treatment,52 they may also be incorporated into cancer treatment through other procedures such as ROS production in sonodynamic cancer therapies.53 Drugs should be connected on the surface of GQDs, which enhances the risk of drug release in non-targeted tissues resulting in systemic toxicity. To address this issue, scientists have proposed multifunctional composites based on GQDs that are commonly paired with visualization therapy.54
1.2 Graphene-based material functionalization for cancer therapy
Biomedical materials should have certain qualities such as stability and solvability in water and physiological solutions, amongst other considerations. One of graphene's fundamental properties is water resistance and forming aggregates in water. Moreover, graphene is well known for its distinctive feature of being easily functionalized, and consequently, graphene's undesirable properties can be modified via functionalization. Chemical processes that result in interactions between graphene and other functional groups through covalent and noncovalent bonds improve the performance of graphene derivatives in biomedical applications.12,55,56Various functional groups have been utilized to design efficient graphene based drug/gene delivery systems. Functional biomolecules such as peptides, polymers, magnetic particles, etc., are qualified to not only increase the nanosystem's efficiency by increasing its biocompatibility and half-life and reducing systemic toxicity, but also increase its targeting specificity, all of which can benefit cancer therapy through designing more efficient carriers.55,56 In the following section, different kinds of functional groups that can be used for graphene derivatives’ functionality are briefly introduced.
1.2.2.1 Polyethylenimine. Polyethylenimine (PEI) is a polymer with positive ions that cannot be found in nature. PEI is applied non-covalently on the graphene's surface to elevate the gene delivery efficiency. Owing to the high concentrations of nitrogen atoms (positively charged) on its surface, PEI can strongly bind to nucleic acids (negatively charged). PEI can also ease the nanocarriers’ cellular uptake through endocytosis. After internalization, PEI-positive ions facilitate the nano-carrier separation from the endosomes via the “proton sponge effect.” The outcomes display that PEI addition offers the graphene family members with extra stability and solubility hence performing as a superior carrier.57,58
1.2.2.2 Polyamidoamine dendrimer. The polyamidoamine dendrimer (PAMAM) is a biodegradable polymer synthesized in different structures that vary in characteristics owing to the construction procedure. PAMAM endows the nanocarrier with the advantage of more gene-loading capacity over other polymers due to allowing a higher number of surface amine groups, and furthermore facilitates DNA linkage to the nanocarrier and maintains its efficient transportation.59 A fourth-generation PAMAM dendrimer displayed an optimal and adaptive structural design to accept small interfering RNA (siRNA) with effective binding and releasing capabilities in an energetically advantageous manner among different dendrimer generations. Simultaneous and effective delivery alongside subsequent release through the proton sponge effect protects siRNA against destruction by enzymes. Furthermore, the combination of graphene based materials with PAMAM has been demonstrated to offer promises in cancer therapy.60 For example GO-PEG-PAMAM has been utilized in a study conducted by Wang et al.61 as an efficient antimicroRNA-21 delivery agent that successfully suppressed cancer migration and invasion.
1.2.3.1 Polyethylene glycol. The most important characteristics a nano-carrier should possess are hydrophilicity and the ability to stay in blood circulation long enough to deliver/release its cargo. One crucial material that has met our expectations is polyethylene glycol (PEG). PEG is a chemical compound with hydrophilic and lipophilic properties. This biocompatible polymer can decrease noxiousness, enhance liquid diffusion, and undergo no changes in physiological solutions when coated on the nanocarrier's surface. Consequently, PEG can prolong the blood circulation time by blocking any interaction with proteins. As a result, it decreases antigenicity and immunogenicity.62,63 Notably, PEG surface density is a crucial factor that determines the effectiveness of the coating.64
1.2.3.2 Chitosan. Chitosan (CS) is a non-synthetic, linear cationic polysaccharide with a variety of applications that may provide the graphene family with solubility, antibacterial activity, reduced toxicity, biocompatibility, and low allergenicity. This bioactive polymer65,66 has the potential to influence the adsorption and desorption of drug molecules on the graphene family's surface and maintain their stability.67,68 Besides, divalent cations, like chitosan, can facilitate DNA linkage on the graphene oxide surface. All these features indicate that chitosan can improve the efficiency of the gene/drug delivery systems based on graphene for curing cancer.69
1.2.3.3 Polydopamine. Polydopamine (PDA) is a mussel-inspired material developed from mussel adhesive proteins that have a significant wet adhesion capacity. PDA is the main component of melanin that can be found in abundance in nature. It is known as a universal adhesion molecule due to its excellent characteristics such as perfect biocompatibility, non-toxicity, hydrophilicity, and high dispersibility in various solvents because of plenty of functional groups such as amino, carboxyl, phenol, and imine groups. Specifically, catechol and primary amine groups endow PDA with excellent adhesion, metal coordination, and antioxidant capacity.70
Many sorts of interactions with the PDA surface make it an ideal platform for the attachment and release of small-molecule medicines and RNA/DNA therapeutics. A handful of studies indicate that PDA-coated surfaces can contain a higher quantity of RNA molecules and deliver them to the target cells.71
PDA decreases the side effects of using non-biocompatible materials when constructing a nanocarrier. Besides, studies have reported that PDA has no toxic effects on normal body cells such as endothelial cells, fibroblasts, and neuron cells or cancer cells. Biodegradability is another significant property of this polymer which is very favorable in biomedical applications, especially cancer therapy.72,73
1.2.3.4 Poly(D,L-lactic-co-glycolic acid). PLGA (poly(D,L-lactic-co-glycolic acid)) is a green and biocompatible polymer produced through fermentation of sugars and is potentially biodegradable, making it a favorable material for biomedical usage. PLGA has been widely utilized in delivery systems carrying drugs, nucleic acid sequences (DNA, RNA, etc.), and proteins with prolonged release which is entirely dependent on the cargo size, weight, and type of interaction with PLGA. PLGA is also capable of encapsulating the cargo and protecting it from degradation caused by enzymes.74 Alongside, PLGA can protect the whole nanocomplex from the O2 and H2O molecules in the physiological environments to maintain the carrier's function due to its hydrophobic structure. Moreover, it endows the carriers with other features such as the capability of targeted drug delivery to specific organs like the liver and brain.75 These wonderful features are increasing PLGA's use in cancer treatment over time.
1.2.3.5 Poly-L-arginine. Poly-D-arginine (P-L-Arg) is a macro ion of particular interest because of its unique characteristics and biocompatibility with the environment and living organisms. It is a natural cationic polymer consisting of L-arginine amino acid, active in physiological environments. P-L-Arg has been utilized in a handful of cancer therapy studies for delivering genetic sequences or drug/vaccine molecules because of its excellent properties. It has also been used in a handful of studies proving its anti-bacterial and biosensor functionalities.76
1.2.4.1 Gold nanoparticles (AuNPs). AuNPs have a wide range of applications in biomedicine due to their unique properties. They can be easily constructed in various sizes and configurations that results in different properties. Additionally, their biological inertness is a vital characteristic for biomedical uses due to AuNP's special surface properties.77,78 Similar to rGO, Au nanoparticles take advantage of photoacoustic properties and convert NIR light into heat that can be applied in designing multi-functional nanocarriers based on graphene with PTT capability for cancer treatment.79,80
1.2.4.2 Magnetic particles (IO, SPION, Fe2O3, Fe3O4). Iron oxide nanoparticles (IONPs) are widely employed as contrast agents in magnetic resonance imaging (MRI). Moreover, they may be coupled with graphene-based nanomaterials for biomedical and therapeutic usages.36
Magnetic particles, especially Fe3O4, have increased drug delivery efficiency in nanocomposites due to their exceptional properties. A problem to address about magnetic particles is that they are easily agglomerated in physiological solutions, which has a direct effect on the drug delivery dimensions and its scale down.81 Therefore, by adding graphene to these nanostructures, nanocomposites with synergistic capabilities can be created. Superparamagnetic characteristics, for example, have been seen in graphene-SPION nanocomposites created by coating graphene nanosheets with SPIONs. Because of this, the hybrid nanocomposites made of graphene and other graphene-based materials have the potential to be used in a wide range of applications such as drug/gene delivery and bioimaging.82
Cell targeting peptides (CTPs) and cell penetrating peptides (CPPs) can also be used for more specific cell targeting and easier internalization. CPPs are a family of varied amino acid sequences capable of entering cells through their membrane. A wide range of bioactive cargos, including proteins, nucleic acid sequences, and drugs, may be delivered into cancer cells with high specificity and efficiency via CPPs and thus reduce the systemic toxicity caused by non-specific drug delivery systems.83 However, because of their cytotoxicity and tendency to become entrapped in endosomes, CPPs have poor performance when used alone, and thus forming a multifunctional complex to compensate for each unit's defects is required.84
For example, N-formylmethionyl-leucyl-phenylalanine (fMLP), which is a chemotactic peptide,85 was introduced into the graphene family to facilitate the delivery of anticancer drugs for the elimination of cancer cells.86,87 R8, MPG-2H1, and Oligo-arginines are other types of CPPs that assist nanocarriers’ cell entrance and endosomal escape.88 When these peptides are used in the structure of a nanocarrier, they can guide their cargo right through the cell membrane and block the cell's proteolytic system to ensure protein-based cargo safety.89
Another peptide sequence with gene delivery applications is cRGDfV (cyclo(Arg-Gly-Asp-DPhe-Val)). The cRGDfV peptide inhibits angiogenesis and has synergistic activity with VEGF-siRNA in angiogenesis suppression. Some peptides are capable of targeting and entering cells of a specific organ besides passing through the cell membrane.90 For example, PV7 (PKKKRKV) is a peptide for targeting the nucleus through nuclear-localized signals,91 and MitP is a mitochondrion-targeting peptide.92
Other organic materials such as dextran (DEX) have also been employed for GO functionalization. DEX is a hydrophilic glucose-derived homopolysaccharide with high biodegradability. DEX can be introduced into different nanoparticles such as the graphene family to enhance their colloidal stability even more than when they are coated with PEG. Another advantage of DEX is more reactive sites which can provide more possible interactions with other functional groups. DEX-coated nanoparticles, on the other hand, are more likely to evade the immune system because they are less likely to bind to proteins.95
Here we are going to discuss functionalized graphene family members’ applications as nanoagents in cancer gene therapy.
2. Gene delivery
Gene delivery is the result of humankind's ambition to eradicate illnesses that has brought forth gene therapy. Gene therapy is any modification, addition, deletion, replacement, repair, or regulation of genetic sequences in particular cells to treat diseases. These alterations are done in three major ways: one, gene silencing which is done by delivering DNA or RNAs (miRNA, siRNA, shRNA, mRNA,…) into a malfunctioning target cell, aiming to deactivate one specific gene function;96,97 two, gene replacement that is done by delivering plasmids (pDNA), and three, using gene nucleases for altering mutations in a specific gene. A variety of nucleases, including zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and Cas-associated nucleases in the CRISPR-Cas (clustered regulatory interspaced short tandem repeats (CRISPR)-associated protein) system, are used to modify genetic mutations.98Besides cancer, various genetic diseases such as sickle cell anemia, hemophilia, and cystic fibrosis can also be treated by gene therapy techniques.43
Gene therapy is the best possible procedure for cancer therapy that mostly depends on the stability of the carrier. The carrier should provide a safe and guaranteed therapeutic agent transfer to the desired cells (nucleus, cytoplasm, and other organelles).99 Viral and non-viral vectors can be used as a gene carrier. Non-viral gene vectors have advantages over viral vectors, such as avoiding immune response, low toxicity, low cost of production, and low mutation rates because they cannot be integrated into the genome.100 On the other hand, the non-viral vectors have low transfection and gene expression efficiency.57 Today, graphene-based nanomaterials have gained much attention as a non-viral vector and are widely used in cancer therapy (Table 1). Scientists have extensively investigated the nano gene transfer agents for cancer treatment in the last decade. Plain nucleotide sequences are, however, unable to penetrate the cell membrane, and they have a short half-life (10 min for DNA and 1 min for RNA). Besides, they are immediately eliminated from the blood by nucleases. So, carriers with prolonged life in the body are extensively needed for gene delivery operations.43,101 Studies indicate that graphene-based nanomaterials can be used as efficient nanocarriers to transport various therapeutic anticancer factors, including nucleic acid sequences (DNA, RNA, etc.).102 Graphene family members can act as reliable nanocarriers for cancer gene therapy because they are biologically compatible and exhibit a high affinity for nucleic acid sequences such as DNA and RNAs through hydrophobic interactions, π-stacking, and van der Waals forces.30
| Nanocarrier | Nucleic acid sequence type | Cell line | Highlights | Ref. |
|---|---|---|---|---|
| PEG-NGO | GFP and EGFR SspDNA | A549 | – Successful transfection of cancer cells. | 103 |
| – GFP and EGFR gene expressions were suppressed. | ||||
| GO-PEG-FA-PyNH2 | hTERT siRNA | HeLa | – Successful siRNA delivery | 63 |
| GO-PEI | CXCR4-siRNA | MDA-MB-231 | – Efficient delivery agent. | 104 |
| – Reduced tumor invasiveness. | ||||
| – Anti-metastatic potential. | ||||
| GO-PAMAM-PEG (GDP) | EPAC1 siRNA | MDA-MB-231 HUVEC | – Good stability in physiological solutions. | 60 |
| – Low cytotoxicity. | ||||
| – Excellent cellular penetrance. | ||||
| – Significant transfection efficacy. | ||||
| – The siRNA was delivered and released in a pH-dependent manner. | ||||
| – The therapy successfully suppressed the EPAC1 target protein. | ||||
| – Cancer progression and metastasis were hindered successfully. | ||||
| PEG-GO-PEI-FA | Anti-PLK1 (PLK1-homo-581) siRNA | SKOV3 | – High uptake efficiency. | 105 |
| – Mild cytotoxicity. | ||||
| – Blockage of cancer cell growth. | ||||
| – Anti-cancer properties for cancer cells with high FA expression. | ||||
| GO–chitosan | Bcl2 siRNA | Saos-2 and MG-63 cells/peppas model | – pH-dependent siRNA release. | 106 |
| – Expression of inflammation-related critical genes (IL-6, TGF-β, TNF-α) was negligible in both RAW 264.7 and bone marrow-derived macrophage treatment. | ||||
| GO | – Mimic miRNA-124, miRNA-137, | U87, U118, U87, U251, T98 | – Nanocomplexes were transferred through electroporation. | 107 |
| rGO | – antisense miRNA-21, –miRNA-221, miRNA-222. | – GO-antisense miRNA-21 is – GO-antisense miRNA-21 is the best nanocomplex used to reduce miRNA-21 expression and thus upregulate the expression of its target genes, resulting in elevated apoptotic cell death in glioblastoma cancer cells. | ||
| GO-PEI | 4 miRNA; miRNA-194-5p, miRNA-125b-5p, miRNA-122-5p, and let-7c-5p | Intrahepatic cholangiocarcinoma (ICC) samples | – Efficient transfection, slightly more than lipofectamine 2000. | 53 |
| – The selected miRNA expression increased once more, and consequently, their target gene expression was repressed. | ||||
| – The conformation of tumor cells and features of cancer stem cells such as colony formation, round shape of the tumor, tumor weight, and drug resistance were all decreased. | ||||
| – Excellent potential for cancer therapy. | ||||
| GO-PEI | Anti-miRNA-214 | OSCC tumor, Cal27, and SCC9 cell lines. | – Therapy method: injection into tumor mass. | 108 |
| – Successful dysfunction of miRNA-214. | ||||
| – Successful suppression of migration, invasion, and growth of cancer cells. | ||||
| – Increased apoptosis. | ||||
| GQD-PEI | mRNA | Huh-7 | – Effective condensation of mRNA molecules and safe transportation to cancer cells. | 109 |
| – Low transfection efficiency (25%). | ||||
| GO-PEI-PEG | cas9/sgRNA | AGS | – The first study to transfer large functional complexes weighing ∼180 kDa. | 110 |
| – Effective suppression of EGFP protein expression. | ||||
| GOCL | Cy3-pDNA | HeLa, HEK-239 | – Good cell internalization. | 111 |
| – High biocompatibility. | ||||
| – High transfection efficiency (90%). | ||||
| C-dot-PEG-pDNA-TNF-α-CS-CGO | TNF-α pDNA | HeLa | – Anti-angiogenesis. | 69 |
| – High transfection efficacy. | ||||
| GOAS-pEGFP-p53 | GFP, Tp53 dsDNA | BT-20 | – Apoptosis induction. | 41 |
| – Increased transfection rate (90%). | ||||
| GO-PLL-SDGR | Anti-VEGF siRNA | HUVEC, HeLa | – High transfer rate and solubility in aqueous solutions. | 112 |
| –>40% expression reduction. | ||||
| – Hindered tumor growth rate (51%). | ||||
| GO-R8/anti-HER2 (GRH)-survivin-siRNA | Survivin siRNA | MCF-7 | – Successful suppression of survivin at the mRNA level (42%) and the protein level (50%). | 113 |
| – No noticeable toxicity. | ||||
| GRcR/VEGF-siRNA | VEGF-siRNA | HeLa | – Successful HeLa cell internalization. | 90 |
| – Effective in silencing VEGF (48%). | ||||
| – Tumor growth and angiogenesis were suppressed. | ||||
| rGON-PLPEG-R8 | Cell death siRNA | MCF-7 | – High penetrance rate (82%). | 86 |
| – Induced cell death (50%). | ||||
| GPND-HEPG2 siRNA | VEGFa siRNA | HEPG2 | – VEGFa was suppressed at both mRNA and protein levels. | 114 |
| – Tumor cell growth was inhibited. | ||||
| DNA/RNA co-delivery | ||||
| GO-PEG-R8-CPP | Anti-c-Myc siRNA and EGFP pDNA | MCF-7 MDA-MB 231 | – High cytocompatibility. | 58 |
| – Reduction of c-Myc and EGFP expression. | ||||
| GQD-PEG-PLA | Mir-21 and survivin gene probe | HeLa | – Providing the capability of monitoring gene delivery. | 115 |
| – Fluorescence is monitored when target mirs are identified. | ||||
There are various kinds of receptors on each cell's surface. When normal cells become cancerous, they increase the construction of specific receptors that aid their viability and are crucial for their persistent proliferation. Scientists may now use these overexpressed receptors to target cancer cells specifically. Cancer cell receptors may be of any kind depending on the organ or tissue from which they originated.116
For instance, the overexpression of folic acid (FA) receptors has been observed in ovarian, breast, colon, and lung cancer.117
Another receptor is EGFR (epidermal growth factor receptor) receptors which are overexpressed in many tumor sites including lung,103 colon,101 glioblastoma,118 colorectal cancer (CRC),119 and nasopharyngeal carcinoma.120 Another famous cancer cell receptor is CD44 with the hyaluronic acid (HA) ligand that is amplified on many cancer cells such as CT26 (colorectal cancer), B16-F10 (melanoma), and 4T1 (breast cancer).121 Other cancer cell receptors that have been used in graphene-based gene/drug delivery are as follows:
– Formyl peptide receptor (FPR) in HeLa (cervical cancer) cells.85
– Glycyrrhetinic acid (GA) which is well known as a liver-targeting ligand and has proven beneficial in nanomaterial functionalization for hepatocellular carcinoma (HEPG2) cell therapy.114
– Estrogen receptors found in abundance in cervical and breast cancer cells (MCF7, MDA-MB-231); their specific regulator is tamoxifen citrate (TC).122
– Vitamin receptors such as biotin, vitamin B12, and riboflavin are mainly targeting and selective ligands for their overexpressed receptors on cancer cells.116
Above all, either the easy functionalization or the graphene family's high biocompatibility, low toxicity, and unique optical, physicochemical, and photo-thermal properties have made them the prominent transporter and increased their application as a nanocarrier for gene therapy.57,123
In the following, different kinds of nucleic acid sequences that can be used for gene therapy are introduced briefly, and then we further investigate the graphene family-based nanocarriers used for carrying various nucleic acid sequences and genetic materials to cancer cells with the aim of cancer gene therapy.
A schematic of a graphene-based gene/drug delivery system can be seen in Fig. 2.
 | ||
| Fig. 2 A schematic representation of the targeted gene/drug delivery procedure utilizing graphene derivatives as nanocarriers. | ||
In the following paragraphs, various kinds of nucleic acid sequences used for gene therapy applications are elaborated.
2.1 Nucleic acid sequences
RNA and DNA (single- or double-stranded) may all be probed using PNAs. Thus, PNAs were mainly used in developing gene therapy drugs by binding tightly to DNA or mRNA molecules and stopping the transcription or translation process in the target cell.57
In a project, Baek et al.103 developed an efficient single-stranded PNA delivery platform with fluorescence properties based on PEG-NGO for low toxic lung cancer (A549) cell gene therapy. Single-stranded PNAs against green fluorescent protein (GFP) and epidermal growth factor receptor (EGFR) genes were loaded on PEG-NGO as a biocompatible nanocarrier and then successfully transferred to the A549 cancer cells. PNAs were released in the low pH environment of endosomes and easily escaped them. Thus, GFP and EGFR gene expressions were suppressed.
Many studies have used MBs as early cancer diagnosis agents,127 which may help cancer therapy by determining specific gene expression in real-time.128 Graphene derivatives are one of the potential transfer agents for genetic materials. In a pioneering study conducted by Lu et al.,129,130 a PEG-grafted NGO was used to design a nanocarrier for transferring oligonucleotides, such as a MB, to HeLa cells (cervix cancer) for targeting the mRNA transcripts of the survivin gene that is known to be associated with the pathway of cancer. As mentioned before, PEG is used to block DNase1 and other enzymatic activity on the MB as well as reduce cytotoxicity even at high concentrations of NGO (100 mg L−1). The MB was loaded on the surface of PEG-NGO, transferred, and separated successfully from its carrier at the destination, to recognize its target by binding to it. Thus, it was proven that this nanocarrier can protect oligonucleotides from enzyme attacks and deliver them safely into the target cancer cells and bond to their target mRNA.
When trying to attach double-stranded DNAs to the graphene-based nanocarriers for targeted delivery, scientists found that double-stranded DNA absorbance on GO surfaces is not as easy as linking an ssDNA through π–π stacking interaction, and additional functionalization is required.
Therefore, scientists decided to use RNA molecules as gene therapy agents and transfer them to cancer cells, but due to the fact that RNA molecules are small, single-stranded, cannot penetrate cells, and are easily degraded by the RNase enzymes in the body environment, many chose graphene-based nanocarriers as reliable transporting agents for cancer gene therapy by RNAs (Fig. 3).
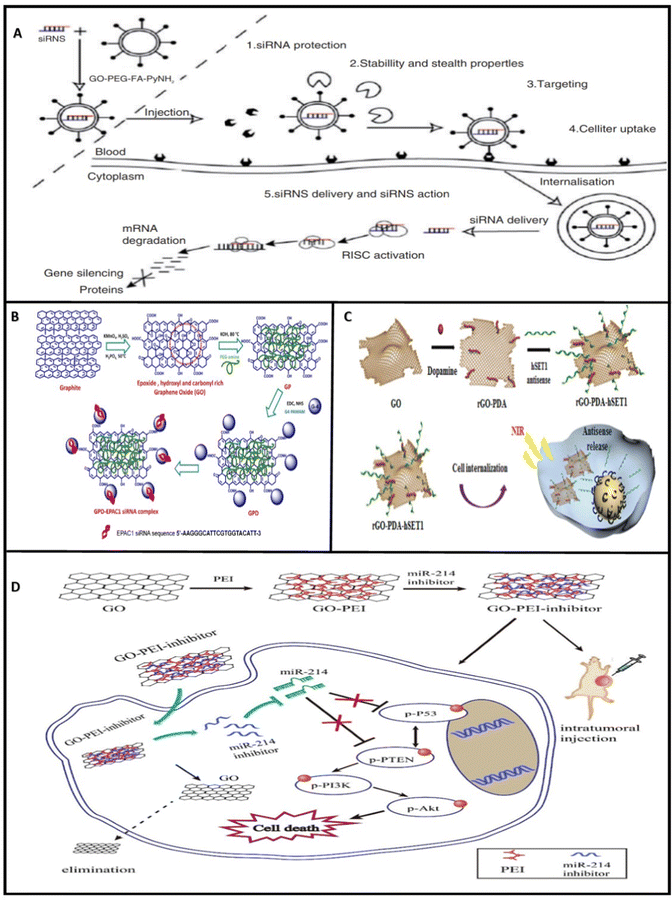 | ||
| Fig. 3 Schematic illustration of (A) graphene oxide nanoparticles with folate decoration (GO-PEG-FA-PyNH2) targeting cancer cells and cell internalization.135 (B) GDP-EPAC siRNA complex construction.60 (C) rGO-PDA nanocarrier transferring the hSET1 antisense to cancer cells.136 (D) microRNA-214 delivery by GO-PEI and the subsequent regulation of downstream genes.108 Reprinted with permission from ref. 60, 108, 135 and 136. | ||
2.2.3.1 RNA interference. RNA interference (RNAi) is a process in which RNA molecules are used to regulate gene expression at different levels such as translation, mRNA, or post-transcription levels. In this regard, Wan et al.135 synthesized a GO-based nanocarrier to transfect RNAi targeting HIF-1α mRNA and protein in patu8988 (pancreatic cancer) cells (Fig. 3). PEG, FA, and pyrene methylamine hydrochloride were linked to GO successfully. After successful transfection, the patu8988 cells no more increased in size or number. Alongside, invasion and metastasis were hindered in pancreatic cancer cells. Moreover, HIF-1α suppression by the nanocarrier increased apoptosis as a response to Glut-1 (glucose transporter-1) and F-FDG (F-fluorodeoxyglucose) repression at the mRNA level. Thus, the outcomes displayed that the tumor expansion was hindered.
In order to mitigate the RNAi impact in gene therapy applications, short hairpin RNA (shRNA) and siRNA are often used. Various medical conditions are well-suited to siRNA therapy because of its temporary impact and ease of production. It is possible to achieve high potency and long-term effects with low copy numbers by optimizing shRNA designs for processing by the body's own machinery. Below we further explore the two ncRNA's applications in graphene based nanocarriers for cancer treatment.137
2.2.3.2 Small interfering RNA. It is possible to transport siRNAs, or small interfering ribonucleic acids, directly into cancer cells, where they may be used to silence mRNAs with specific sequences and limit protein turnover.
Yang et al.63 designed a nano transporter by functionalizing GO for pre-planned transfer of hTERT (human telomerase reverse transcriptase) siRNA. First, GO was decorated with PEG and FA (folic acid). PEG improved solubility and biocompatibility, and reduced toxicity. At the same time, FA was chosen for its tumor-targeting properties. Afterward, the hTERT siRNA was linked to GO-PEG-FA via a π–π stacking mechanism mediated by PyNH2. GO-PEG-FA-PyNH2 was ideally disseminated in the blood and successfully delivered the siRNA into the HeLa cells. Consequently, the target gene expression was favorably regulated at the mRNA level, and it was validated that the nanocarrier is capable of siRNA delivery to cancer cells.
In another project, Huang et al.104 used PEI grafted GO to transfer anti-CXCR4 (C-X-C chemokine receptor type 4) siRNA to MDA-MB-231 invasive breast cancer cells with aggressive features. According to CXCR4 gene expression in relation to reducing metastasis, the anti-CXCR4 siRNA was chosen to be transferred into an invasive breast cancer cell line to evaluate its effectiveness against metastasis. The outcomes indicate that this nano transporter acted efficiently as a delivery agent and had anti-metastatic potential.
A separate investigation conducted by Yadav et al.60 illustrates the construction of a PEG conjugated GO-PAMAM nanocomposite for anti-EPAC1(gene) siRNA delivery to MDA-MB-231 and HUVEC cells. Notably, EPAC1 overexpression can result in metastasis in breast cancer cells. The nanocomplex exhibited good stability in physiological solutions, low cytotoxicity, excellent cellular penetrance, and significant transfection efficacy. Besides, the siRNA was delivered and released in a pH-dependent manner and successfully suppressed the target protein. Breast cancer progression and metastasis were highly hindered due to this therapy.
Further, Wang et al.105 developed a nanocomposite of GO for delivering Anti-PLK1 (PLK1-homo-581) siRNA to ovarian cancer cells. GO was grafted with PEG, PEI, and FA in this nanocomplex to enhance siRNA transfection efficiency. The outcome of using this ∼261 nm-sized complex for SKOV3 (ovarian cancer) cell gene therapy was high uptake efficacy, mild cytotoxicity, and a blockage of cancer cell growth. It can be concluded that PEG-GO-PEI-FA successfully killed ovarian cancer cells and has the potential to act as a siRNA delivery system for any FA-positive cancer treatment.
In a study by Saravanabhavan et al.106 an effective nano delivery agent based on GO was constructed to transfer siRNA into Saos-2 and MG-63 cells/Peppas model, as an osteosarcoma drug delivery agent. GO-chitosan was linked to bcl2 targeting siRNA, and the siRNA was uncoupled in the acidic pH of the tumor environment leisurely. The expression of critical genes contributing to the inflammation process, IL-6, TGF-β, and TNF-α, was monitored before and after treatment in both RAW 264.7 and bone marrow-derived macrophages, which was negligible. Also, the nano transporter showed good biocompatibility and efficacy. Despite the presence of ROS as a result of a stressful scenario, slight changes in the inflammatory cytokines were seen due to the use of this gene delivery nanocarrier.
Although siRNAs possess many identical and excellent properties, and thus, have been used widely, they are easily destructed by nucleases and aren’t able to pass through cell membranes because of their high charge. Another molecule compensating for these defects is shRNA which acts like siRNAs but much better.118
2.2.3.3 shRNA. shRNAs are short hairpin ribonucleic acids that act similar to but not identical to siRNAs in regulating gene expression. They have been used in constructing multifunctional gene therapy nanocarriers based on graphene, which will be discussed in the gene/drug co-delivery section.138,139
2.2.3.4 microRNA. Another group of noncoding RNAs with a high regulatory function are microRNAs (miRNAs) which repress gene expression post-transcriptionally. Thus, using microRNAs as a cargo of gene therapy complexes may be an efficient way to cure diseases.140 Kutwin et al.107 used two members of the graphene family, GO and rGO, for microRNA delivery to glioblastoma cell lines (U87, U118, U87, U251, and T98).
The microRNAs were chosen from previously studied141 anti-tumor (miRNA-124 and miRNA-137) and tumor-inducing (miRNA-221, miRNA-222, and miRNA-21) microRNAs in glioblastoma. miRNA-124 and miRNA-137 have been confirmed to cause sensitivity to chemotherapy and radiation as well as reduce cell growth and proliferation. Meanwhile, it has been demonstrated that miRNA-221 and miRNA-222 are capable of elevating tumorigenesis and invasion. Alongside, studies indicate that in more than 70% of glioblastoma patients, miRNA-21 is attested to be overexpressed, even more than any other microRNA in glioblastoma. Subsequently, its down regulation can initiate apoptosis.
To this end, Kutwin et al. decided to design graphene-based complexes and transport mimic miRNA-124 and miRNA-137, and antisense miRNA-21, -miRNA-221, and miRNA-222 to various cell lines of glioblastoma through electroporation, intending to cure it. Antisense miRNAs are ssRNA sequences that conjugate with their target mRNA and therefore reduce its translation into protein, while mimic mirs act the opposite.141,142 Above all, they concluded that GO-antisense miRNA-21 is the best nanocomplex used to reduce miRNA-21 expression and thus upregulate the expression of its target genes, resulting in elevated apoptotic cell death in glioblastoma cancer cells.
In another study, Yang et al.143 designed a novel nano transporter based on GO-PEI for delivering multiple miRNAs to intrahepatic cholangiocarcinoma (ICC) samples for the first time. Based on TCGA data analysis, four of the most significantly downregulated microRNAs (miRNA-194-5p, miRNA-125b-5p, miRNA-122-5p, and let-7c-5p) were chosen for transfection. The results indicated that the GO-PEI-4 miRNA was transferred efficiently, a cut above lipofectamine 2000. On the other hand, the expression of the selected miRNAs increased once more, and consequently, their target gene expression was repressed. On top of that, the conformation of tumor cells and features of cancer stem cells such as colony formation, round shape of the tumor, tumor weight, and drug resistance were all decreased. These satisfying results indicate that this nanocarrier has excellent potential for cancer therapy.
In a study by Ou et al.108 GO-PEI was most recently utilized as a transfer agent for delivering anti-miRNA-214 to squamous cell carcinoma tumor cells (OSCC). Anti-miRNAs are chemically modified oligonucleotides altered through chemical reactions with the potential for targeting and high affinity for attaching to miRNAs. Besides, anti-miRNAs can withstand nuclease attacks.144 MiRNA-214 acts as an oncomir (microRNAs that are associated with the process of causing cancer) in OSCC. The results display the efficient transfection of the GO-PEI-anti miRNA complex by its injection into the tumor mass. As a result of miRNA-214 dysfunctioning, invasion, migration, and tumor growth were suppressed, and apoptosis was increased in the OSCC tumor cells (Cal27 and SCC9).
Another investigation led by Liu et al.109 was performed for the first time to shed light on gene delivery applications of GQDs for cancer therapy. They developed functionalized GQDs (GQD-PEI) for mRNA delivery to Huh-7 (hepatocarcinoma) cells. The functionalized GQDs effectively condensed mRNA molecules and transported them safely into the cancer cells but with low efficiency (25%).
2.2 DNA and RNA co-delivery
Various nucleic acid sequences can be delivered to cancer cells to increase the efficiency of tumor suppression through the knockdown of cancer survival genes/mRNAs. For instance, siRNA and DNA molecules can be transported together at once into the cancer cells. In a study designed by Imani et al.58 a GO-based nano-platform was developed by introducing PEG-diamine, R8, and CPP to GO for breast cancer cell gene therapy. Anti-c-Myc siRNA and EGFP pDNA were efficiently co-delivered by this nano transporter to MCF-7 and MDA-MB 231 cell lines. The cellular uptake was increased by 85% via simultaneous R8 and PEG-diamine usage, which also increased the cytocompatibility of the nanocarrier. The results indicate c-Myc protein knockdown and EGFP expression, which determines the efficient delivery of the nanocarrier and the functionality of the nucleic acid sequences.Many attempts have been made to design multirole nanocarriers for efficient gene therapy of cancer. For example, Dong et al.115 developed a gene carrier based on GQDs grafted with PEG and PLA. Afterward, multiple mir-21-specific and surviving gene probes were grafted. Gene probes generate fluorescence signals when the target mirs are identified, and this is found to be a way to observe the target genes’ regulation. Besides, GQDs, due to their photoluminescence properties, can be monitored when uptaken by the HeLa cells. Overall, It can be concluded that multifunctional GQDs can be utilized for developing upgraded nanocarriers with various applications (Fig. 4).
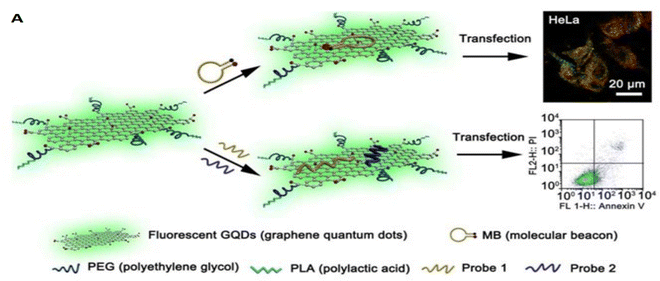 | ||
| Fig. 4 Schematic illustration of (A) DNA/RNA co delivery by GQDs for cancer treatment.115 Reprinted with permission from ref. 115. | ||
3. Small molecular drug delivery
Drug delivery is concerned with the various formulation, construction, and storage methodologies used to transport pharmaceutical substances throughout the body to their intended target areas to treat an illness or create a secondary effect. The primary obstacles in drug delivery processes are loading the correct quantity of medication, conveying it quickly, safely, and in active form to or into the target cell/tissue/organ, and controlling the drug's release.145The advancement of nanotechnology in biomedical domains, particularly cancer treatment via the invention of drug delivery platforms, has now gained considerable interest. Using nanocarriers to transport drugs is one of the most successful cancer treatment strategies. It may lessen the side effects and raise the efficacy of the therapy by overcoming difficulties such as drug resistance, quick clearance of medications from the blood circulation, and general toxicity to the body.6,100
One significant impediment to developing drug delivery systems is the hydrophobic nature of drugs. Graphene-based nanocarriers are renowned for being easily functionalized, and through this procedure, a hydrophilic system can be designed. Two different methods have been used to address this issue. First, functionalizing the nanocarrier with hydrophilic agents (polymers, peptides, etc.) that most studies approve. The second method is injecting the nanographene oxide into the hydrophobic drug crystals without affecting the structure or physical characteristics of the drug crystals by employing the distributed nGOs as nucleation sites for crystallization. In this method, nano sized GOs dispersed in the solution provide nucleating sites for crystallization and, meanwhile, are inserted into the drug crystals without changing their physical properties.146
Other perfect features of the graphene family that have aided drug delivery for designing efficient nanocarriers are their high loading capacity besides low toxicity and direct-targeted delivery, and controlled release of drugs. Therefore, the graphene family has opened up new paths for targeted cancer therapy where conventional chemotherapy cannot meet all requirements.
Scientists have long been concerned about the toxicity of the designed drug delivery nanocarriers, especially whether the loaded drug triggers the immune system or harms other organs on its way to the target tumor. Most recently, Farhanfar et al.147 have assessed the inflammatory response and impacts of an innocuous stable anticancer drug termed ginsenoside Rh2 on the immune systems of breast cancer mouse models (balb/c). Their outcomes demonstrated an insignificant increase in white blood cells and inflammatory reactions, which determines the high efficacy of the designed nanoplatform, graphene-arginine-ginsenoside Rh2 (G-Arg-Rh2).
3.1. Drugs used in cancer chemotherapy
Various chemotherapeutic drugs have been used in graphene-based systems for killing cancer cells through initiating the apoptosis pathway148 and reducing proliferation,149 cell viability or metastasis in cancer cells, such as 5-fluorouracil (5-FU),150 doxorubicin (DOX),151 paclitaxel (PTX),117 cisplatin (CisPt),152 protocatechuic acid (PCA),153 zolendronic acid (ZOL)154 and mitoxantrone (MitX).155 In the following sections, the recent drug delivery platforms based on graphene derivatives for cancer therapy are introduced.Functionalized GQDs have been employed by Li 2022 et al.157 as a nanocarrier for colon cancer therapy. GQDs were grafted with PEG and PEI and furnished with DOX and GFP plasmid and formed a star-shaped nanocarrier. GQDs-polymer-DOX conjugates (GECD) successfully entered the cell membrane and demonstrated pH-responsive drug release, and successfully suppressed cancer cell proliferation. The in vivo studies confirmed the carrier's high anti-cancer capability when tested in a mouse xenograft model. Above all, it was demonstrated that the GECD nano-star could be used as a potential nanocarrier for future cancer therapy applications.
Iron oxide grafted graphene nanocarriers (GIOPMPC) have been developed by Perumal and coworkers for thyroid cancer cell drug delivery. DOX was chosen as the anticancer drug and loaded onto GIOPMPC. GIOPMPC alone has been shown to have negligible toxicity; however, significant toxicity was observed via effects on apoptosis, cell proliferation, and DNA damage when loaded with DOX.158
Besides focused drug delivery and long-lasting circulation time, steady drug release (consistent medication release) is of paramount importance for a nanocarrier to act efficiently. Farahani et al.159 designed a graphene-based nanocarrier stabilized with BSA, decorated with chitosan, and grafted with DOX. By maintaining drug release in acidic pH, this nanocarrier was suitable for reducing SKBR-3 breast cancer cell proliferation.
For breast cancer treatment, Ghamkari and coworkers160 developed a special polymer nanostructure for the oral delivery of DOX to breast cancer patients. The nanocomplex GO/(PHEMA-gPLA)-b-PEG-b-(PHEMA-gPLA) was scaled at 51 nm and created through chemical reactions involving (PHEMA-gPLA):poly(2-hydroxyethyl methacrylate)-g-poly(lactide), GO, and PEG, followed by loading of DOX. High biocompatibility, good cell internalization, ROS production, and pH-responsive medication release were the highlights of this nanocarrier to serve as a potentially efficient nanocarrier for oral drug administration.
The conventional protein targeting agents are unable to specifically target the desired cancer cell due to their affinity for other body cells with the same or similar protein pattern. To address this problem and boost the specificity of the carrier, Han et al.161 constructed a dual-targeting pH-responsive GO-based nanocarrier for cancer therapeutics delivery with the assistance of molecularly imprinted polymers (MIPs) technology. Apart from providing high specificity for targeting CEA-expressing cells, the MIP-tech-constructed polymers displayed increased resistance to enzyme attacks, chemical interactions, and rough environmental conditions. Boronic acid grafted magnetic graphene oxide was then conjugated to dopamine, a functional monomer. The resulting nanostructure demonstrated both magnetic properties and high biocompatibility. Due to the remarkable specificity of these nanocarriers for CEA, the tumor cells’ viability was lowered, and toxicity was increased. Accordingly, tumor cells may now be targeted without any need for protein–ligand modification.
Pooresmalili and coworkers162 participated in the development of a magnetic (Fe3O4)-GO (MG) nanocarrier for anticancer drug delivery that was furnished with copolymer brushes (PB) of N-isopropyl acrylamide (NIPAM) and acrylate cyclodextrin (Ac CD). First, vinylic groups, which serve as the base molecules for the growth of acrylic groups, were added through the MG remodeling process using triethoxyvinylsilane (TEVS), followed by loading DOX. The designed biocompatible nanocarrier was found to have a high anticancer capability, reducing MCF-7 and MCF-10A breast cancer cell growth due to specific cell internalization and stimuli-responsive (pH–heat) drug release.
On the surface of many cancer cells, such as HeLa, A549, MCF-7, 4T1, etc.,163 biotin receptors are increased in number. Vinothini and colleagues116 developed a Carrageenan graphene-based nanocarrier for biotin-mediated delivery of DOX to cervical cancer cells. Carrageenan is a linear polysaccharide with an algal source that confers the nano platform with high biocompatibility and negative charge, which may aid in the dispersion of GO-based nanocarriers. This nanocarrier, GO-κ-Car-biotin, possessed 94% DOX entrapment and pH-sensitive drug release and was shown to penetrate HeLa cancer cells over normal epithelial cells preferentially.
Zhang et al.164 loaded proapoptotic peptides (KLA) alongside DOX on GO via disulfide and π–π bonds to develop a dual-sensitivity, pH-responsive drug delivery system. Following that, DOX@GO-SS-KLA was coated with bovine serum albumin (BSA) to enhance the carrier's stability in physiological conditions (DOX@GO-SS-KLA/BSA). The research findings indicate that this carrier was successful in MCF-7 cancer cell internalization and death.
Martin et al.85 used fMLP to increase GO's qualification in targeting and delivering DOX to HeLa (cervical cancer) cells through the formyl peptide receptor (FPR). GOfMLP acted efficiently in triggering cancer cells’ rapid entry and elimination via apoptosis. Amazingly, this nanocomplex possesses a self-degradation ability. It was well suited to influence neutrophil degranulation that results in its degradation. Given that the FPR is present on various cancer cell membranes, the capability of targeting multiple tumor types at once is indeed a solid and positive point for this carrier.
GO is easily recognized and eliminated from the blood by the immune cells (macrophages). Coating GO with red blood membrane vesicles is another technique to boost either its stability in physiological solutions and blood or its hemolysis capacity. Xie 2021 et al.165 introduced the red blood cell (RBC) membrane on the surface of GO to provide a more stable targeting drug carrier for cancer treatment. Finally, DOX was loaded on RBC-GO. The resultant nanocarrier demonstrated perfect stability, biocompatibility, and a pH-responsive drug delivery profile. RBC-GO-DOX was proven to be extremely cytotoxic in high densities for MCF-7 cells. Therefore, when nanocomplexes are uptaken, the cancer cells are eliminated, and the tumor size shrinks.
Additionally, GO can be engaged in electrospun GO construction for a variety of applications, including cancer-related treatments. In a project by Samadi et al.,166 a nanoplatform inclusive of an electrospun chitosan/PLA/GO/TiO2/DOX nanofibrous structure was innovated for controlled release of DOX for cancer therapy. Although nanofibrous scaffolds are mainly used in tissue engineering, they have shown promise in cancer therapy due to their ideal properties. In the initial steps of fabricating a nanofibrous structure, a polymer should be dissolved in an organic solvent. Here, the graphene oxide/TiO2/doxorubicin (GO/TiO2/DOX) nanoplatform got into solution with the chitosan/poly(lactic acid) (PLA) dissolvent. Following that, using a high-force electric device (electrospinning), nano-scale fibers are generated, which results in a porous configuration with a large surface area. Owing to its high (98%) drug loading capacity and pH-responsive controlled diffusion, the nanofibrous scaffold was capable of reducing in vitro systemic toxicity and selective killing of lung cancer cells (A549 cell line) in vitro.
One peptide named HN-1 (TSPLNIHNGQKL) has been substantiated to target OSCC cells specifically, and therefore Li et al.167 developed the idea of constructing an NGO-PEG-based nanocarrier for DOX transfer to CAL-27 and SCC-25 oral squamous cell carcinoma cells. DOX@NGO-PEG-HN-1 demonstrated good cancer cell targeting, internalization, and high toxicity with pH-dependent drug release.
In another study, GQD-based nanospheres were developed and tested for drug delivery-based cancer therapy. Pooresmaeil et al.168 constructed a gelatin-coated magnetic GQD nanocomplex, abbreviated (Fe3O4/GQDs@GM), for transporting DOX to breast cancer cells (MDA-MB 231) to trigger apoptosis. The increased drug loading capacity of the composing nano platform (30%) compared to the gelatin microsphere (GM) alone (29%) and its pH-dependent drug release mechanism, as well as its superior biocompatibility and biodegradability, all contribute to this nanocarrier's efficiency as a drug delivery agent.
Magnetic particles are easily agglomerated in physiological solutions. To circumvent the limitations, Karimi et al.81 suggested adding a green protective shell of maltose disaccharide to envelope the magnetic particles. In this method, magnetic carbons (C@Fe3O4) are first coated with maltose disaccharide molecules and then with a third-generation triazine dendrimer (Fe3O4@C@TD-G3). Finally, Fe3O4@C@TD-G3 interacts with GQDs and generates Fe3O4@C@TDGQDs microspheres. This nanocarrier was utilized for DOX delivery to the A549 cell line. pH-sensitive drug release, no toxicity, and low cost are the key advantages of this drug delivery nanocarrier.
Drug leakage has always been a fundamental issue in designing drug delivery nanocarriers. To address this issue, Xu et al.169 suggested polymer-shelling the nanoparticles and drugs. So they developed and compared molecularly imprinted polymers (GMIPs) and non-imprinted polymers (GNIPs). The nanopolymer nanocarrier imprinted with DOX (drug), GQDs (photothermal agent), and 1-vinyl-3-dodecyl imidazolium bromide (antimicrobe) demonstrated lower drug leakage and ‘burst effect’. NIR light has been used as a drug-release trigger. This nanocomposite is proven to be effective for tracking drug delivery, providing its safety and hindering leakage through its transport to the specific target site with the help of NIR light.
N-GQD nanocarriers for DOX delivery to HeLa and MCF-7 cancer cells have been developed in a project designed by Frieler et al.156 which are capable of delivering and fluorescence tracking of doxorubicin, resulting in an IC50 reduction of over 1.5 and allowing for the use of up to 10 times lower doses of the drug for the same therapeutic effect. They employed nitrogen-doped GQDs for two main reasons: one, enhanced biocompatibility, and two, multicolor visible/near-infrared fluorescence imaging.
As mentioned above, DOX has been delivered as a chemotherapeutic agent to cancer cells employing graphene derivatives. The construction and delivery procedures of some of these carriers are summarized in Fig. 5.
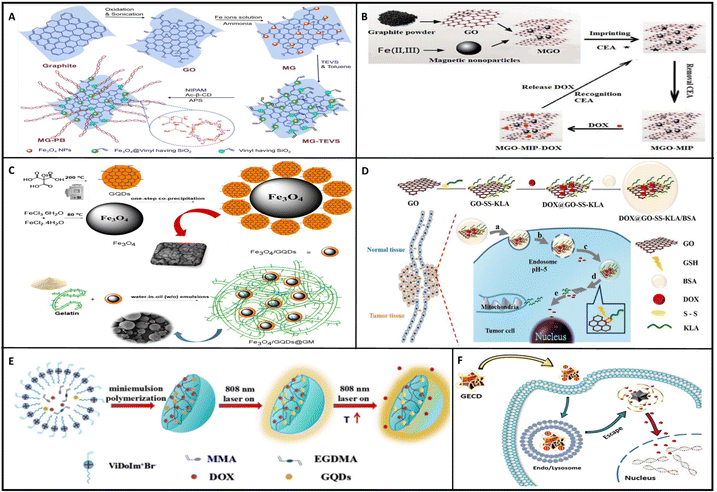 | ||
| Fig. 5 Cancer chemotherapy utilizing graphene-based nanocarriers; DOX delivery to cancer cells. (A) Preparation procedure of the MG-PB nanocarrier for DOX delivery to breast cancer cells.162 (B) DOX delivery to cancer cells with graphene-based nanocarriers furnished with MIP-tech-constructed polymers.169 (C) Gelatin-coated magnetic GQD nanocarrier for DOX delivery to breast cancer cells.168 (D) Pro-apoptotic peptide-loaded GO for DOX delivery.164 (E) Polymer-shelling GQD nanoparticles and drugs for reducing drug leakage and systemic toxicity.170 (F) GECD nanostar (GQD and polymers) drug delivery procedure.157 All figures are reprinted with permission from ref. 157, 162, 164 and 168–170. | ||
Not long ago, Makharza et al.173 designed a super-paramagnetic (MF = 7 tesla, MS = 15 emu g−1) NGO-derived nanocarrier, γ-Fe2O3@NGO, for targeted delivery of CisPt to glioma cancer cells through a magnetic guide. CisPt was loaded after magnetic γ-Fe2O3 nanoparticles were grafted onto NGO sheets for easing focused delivery. Due to the nanoplatform's high preference for CisPt, the release rate of the drug was prolonged (80% per 250 h). CisPt exhibited minimal toxicity in the absence of the nanocarrier. One obvious advantage of using γ-Fe2O3 as a magnetic agent in this nanocarrier besides its magnetic properties is its high stability and biocompatibility. Above all, we can conclude that this magnetic nanoparticle is capable of efficient and focused delivery of CisPt to U87 cells.
Sahne et al.179 used a layer-by-layer technique to graft GO nanoparticles with a monolayer of polymers named carboxymethylcellulose (CMC) and poly N-vinylpyrrolidone (PVP) for chemotherapeutics delivery to cancer cells. With the aim of enabling these 60 nm-sized nanoparticles for targeting cancer cells through their folic acid receptors, GO was first grafted with PEG and afterward coated with folic acid antibodies before CUR loading. The CMC membrane has a vast room (94%) for shelling CUR. CMC/PVP GO NPs effectively inhibited Saos2 and MCF7 cell growth in vitro (76% and 81%). In vivo tests revealed a 76% tumor suppression rate, elevated cell death (apoptosis and angiogenesis), and reduced cell growth with no apparent toxicity.
As a means of enhancing GQD's capacity to transport more medicines, Ghanbari et al.180 constructed a drug-loaded tryptophan-conjugated graphene quantum dot (Trp-GQD) nanocomposite, which on the one hand elevates the drug loading capability (23%) through its critical properties such as higher biocompatibility, solubility, and antioxidant and anti-inflammatory properties, and on the other hand, increases adsorption and emission in the UV area due to its cyclic structure. As a result, a pH-dependent, nontoxic, trackable nanocarrier with increased CUR delivery capability to MCF-7 cells was developed.
Razaghi et al.181 developed a pH-responsive drug delivery system based on fluorinated graphene oxide (FGO), loaded with the linoleic acid-CUR conjugate. Studies on the MCF-7 cell line revealed high toxicity (60%) as a result of in vitro drug delivery tests of this nanocarrier. In vivo studies on tumor-bearing BALB/c mice also resulted in an inhibition of tumor growth with no significant side effects. Above all, this nanocarrier had acceptable anti-tumor activity and could act as a potential candidate for elevating MRI contrast.
Most recently, Paknia182 and coworkers developed and characterized a nanocarrier both in the lab and using bioinformatics. In this project, a multi-functionalized GO was constructed by using magnetic nanoparticles (Fe3O4) and a hyperbranched polyglycerol (HPG) polymer for CUR delivery to cancer cells. HPG endowed the nanocarrier with elevated biocompatibility and the MNPs were placed between the branches just before the CUR was introduced. The therapeutic and anatomical potential of CUR was determined via a bioinformatics server and the results showed that the drug loading capacity was impressive (∼198%) and its release was pH-dependent. Besides, it was shown that after treatment with GO-HPG-MNPs-(CUR), apoptosis and toxicity were increased in cancer cells but MCF-7 cells displayed less sensitivity and more resistance in comparison to SH-SY5Y cells which may be due to its special therapeutic effects on the nervous system, predicted by bioinformatics studies. In conclusion, GO-HPG-MNPs-(CUR) seems to show the fundamental properties of an efficient nanocarrier for cancer therapy purposes.
In another investigation, an anticancer nanoplatform based on rGO-5-FU embedded alginate beads was introduced as an efficient carrier by Boddu et al.186 owing to the high loading capacity of rGO for drugs and the high biocompatibility of hydrogels. Apart from its high loading capacity, rGO possesses other advantages like better thermal stability and efficiency of the beads. The beads demonstrate pH-dependent drug release and considerable anticancer functions against MCF-7 cells. It is good to mention that the crosslinking agents used for connecting drugs and rGO may have affected the drug's release rate, such as Mg2+, which also displayed a remarkable swelling degree.
According to the 2019 National Clinical Cancer Network (NCCN), paclitaxel (PTX) is recommended as a front-line treatment (category 1) for gastric cancer patients since PTX could efficiently inhibit spindle apparatus function and thus suppress tumor cells’ proliferation.187
Vinothini and coworkers117 investigated a modified graphene oxide-methyl acrylate (GO-g-MA) nanocarrier for targeted anti-cancer drug delivery to breast cancer (MDA-MB-231) cells. MA is a biologically compatible synthetic polymer with many biomedical applications. In this investigation, GO-g-MA is grafted with folic acid, a targeting ligand for breast cancer cells. Paclitaxel (PTX) was assembled through π–π stacking and hydrophobic interactions on the surface of the GO-g-MA/FA carrier. This nanocarrier demonstrates 39% toxicity in vitro. The in vivo results indicate that this nanosystem was not only capable of maintaining the mitochondria's function, in spite of chemotherapy, but also restoring mammary cells’ mitochondrial membrane integrity and citric acid cycle enzymes at normal levels, which were disrupted during breast carcinogenesis.
A drug delivery system for Siha (human cervical adenocarcinoma) cell therapy based on CLB grafted rGO-FA coated with gelatin has been developed and named CLB-FADDO by Singh and coworkers.188 FA was employed to trigger and extend cell death through apoptosis in human cervical adenocarcinoma cells. Alongside, gelatin was employed to increase graphene sheet's stability and biocompatibility in physiological and aqueous environments via covering the nanocarrier and acting as a reducing agent. This biodegradable nanocarrier demonstrated high drug loading efficiency, pH-dependent release, targeted delivery, and reduced toxicity compared to the free drug.
For instance, Matiyani et al.192 have constructed a polymer grafted GO (PVP-GO) with magnetic properties to deliver QSR to MDA-MB-231 cancer cells. Polyvinyl pyrrolidone (PVP) is a hydrophilic polymer used for the functionalization of GO to make it more biocompatible, and then magnetic Fe3O4 nanoparticles can be introduced to provide external magnetic control over the nanoparticle concentration. Afterward, QSR is loaded with a loading capacity of 1.69 mg mg−1 and can be released pH-dependently. This smart nanocarrier was highly toxic for cancer cells but highly biocompatible when tested on normal cells (HEK 293T cells) which increased its chance of being used as a cancer therapy agent in the future.
Abdollahi et al.92 collaborated in the development of biocompatible, magnetic nanoparticles adequate for the targeted delivery of medicines to cancer cells. MTX alone demonstrated less cytotoxicity when tested on HeLa and MCF-7 cell lines than when linked to the prepared nanocarrier, GOMNP/PEGA. MTX loaded on a PEGA (polyethylene glycol bis amine) grafted graphene oxide/iron oxide nanocarrier demonstrated high blood compatibility.
Above all, many different drugs have been employed on graphene derivatives for cancer-drug delivery. These nanocarriers were designed, constructed, and used as illustrated in Fig. 6.
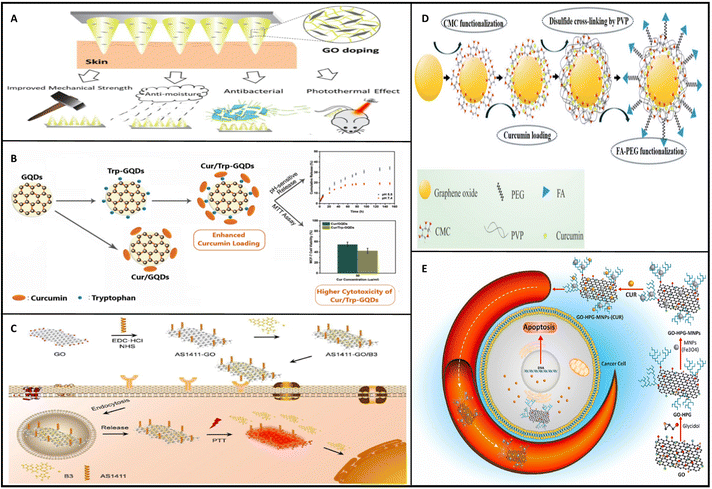 | ||
| Fig. 6 Graphene derivatives used for drug delivery to cancer cells. (A) GO doped polymeric microneedles enhanced the properties for drug delivery and cancer therapy applications.174 (B) GQD nanocomplexes furnished with Trp and CUR for cancer therapy applications.180 (C) B3 loaded AS1411 grafted GQD construction and application in cancer chemotherapy.190 (D) construction procedure of CMC and PVP grafted GO nanoparticles for targeted and safe-CUR delivery for cancer therapy applications.179 (E) GO-HPG-MNPs-(CUR) development procedure and mechanism of action.182 Reprinted with permission from ref. 174, 179, 180, 182 and 190. | ||
3.2 Dual drug delivery platforms
Although chemotherapy is the most assuring cancer treatment method, cancer cells invent their unique ways of fighting against chemotherapy. Studies indicate that 90% of cancer treatment failures are due to drug resistance, so scientists have proposed dual drug delivery platforms based on graphene derivatives for more effective cancer therapy (Table 2).| Mono drug delivery platforms | ||||
|---|---|---|---|---|
| Nanocomposite | Drug | Cell line/in vivo | Highlights | Ref. |
| G-Arg- | Rh2 | Breast cancer Balb/c mouse model. | – Increase in inflammatory responses of WBC. | 147 |
| – High efficient delivery. | ||||
| Graphene-BSA-chitosan | DOX | SKBR-3 | – Reduce breast cancer cell proliferation. | 202 |
| – Acidic pH drug release pattern. | ||||
| Iron oxide grafted graphene nanocarriers (GIOPMPC) | DOX | Thyroid cancer cell | – GIOPMPC had negligible toxicity. | 158 |
| – Apoptosis, cell proliferation, and DNA damage were increased when loaded with DOX. | ||||
| GO/(PHEMA-gPLA)-b-PEG-b-(PHEMA-gPLA) | DOX | In vivo | – Efficient oral drug delivery agent. | 160 |
| – High biocompatibility. | ||||
| – Good cell internalization. | ||||
| – ROS production. | ||||
| – pH responsive medication release. | ||||
| MGO-MIP- | DOX | HepG2 | – Demonstrated high selectivity recognition for CEA without interference. | 99 |
| L02 | – High selectivity for targeting and killing cancer cells compared to normal cells. | |||
| – pH-dependent drug release. | ||||
| – High biocompatibility. | ||||
| MG-PB | DOX | MCF-7 | – Heat and pH responsive drug release. | 162 |
| MCF-10A | – Nanocarrier was solely nontoxic, while it exhibited high toxicity when loaded with DOX. | |||
| – Successful cell uptake. | ||||
| GO-κ-Car-biotin | DOX | HeLa | – High biocompatibility. | 116 |
| – pH-responsive drug delivery. | ||||
| – 94% drug entrapment. | ||||
| – Selective targeting of cancer cells. | ||||
| GO-SS-KLA | DOX | MCF-7 | – Dual sensitive drug delivery system (pro-apoptotic peptic KLA and DOX) | 164 |
| – BSA coating increased biocompatibility. | ||||
| – Successful cell internalization and killing. | ||||
| – pH responsive drug release. | ||||
| GOfMLP | DOX | HeLa | – Targeted delivery to HeLa cells through the formyl peptide receptor (FPR). | 85 |
| – Multiple tumor targeting possibilities due to the introduction of FPR on various cancer cells. | ||||
| – Rapid cancer cell entry. | ||||
| – Inducing apoptosis. | ||||
| – Self-degradation ability through influencing neutrophil degranulation. | ||||
| RBC-GO | DOX | MCF-7 | – RBC membrane introduction increased the stability and biocompatibility of the carrier in the blood. | 165 |
| – pH-responsive drug delivery. | ||||
| – Toxic for cancer cells, tumor size shrinks, and cancer cells are eliminated. | ||||
| Chitosan/PLA/GO/TiO2/ | DOX | – High drug loading capacity (98%) due to the porous configuration and large surface area. | 166 | |
| – Reduced toxicity | ||||
| NGO-PEG-HN-1 | DOX | CAL-27 | – Specific cancer cell targeting. | 167 |
| SCC-25 | – Good cell internalization. | |||
| – High toxicity when loaded with the drug. | ||||
| – pH-dependent drug release. | ||||
| Fe3O4/GQDs@GM | DOX | MDA-MB 231 | – Increased drug loading capacity (30%) compared to gelatin microsphere (GM) solely. | 168 |
| – pH-triggered drug release. | ||||
| – Superior biocompatibility and biodegradability. | ||||
| Fe3O4@C@TDGQDs | DOX | A549 | – pH-sensitive drug release. | 81 |
| – No toxicity. | ||||
| – Low cost of preparation. | ||||
| GMIP (GQD, MMA, EGDMA, ViDoIm+Br−) | DOX | — | – Reduced drug leakage. | 169 |
| – NIR triggered drug release. | ||||
| – Potential carrier to be used for cancer therapy. | ||||
| N-GQD | DOX | HeLa | – Ten times reduced drug usage compared to using the drug alone | 156 |
| MCF-7 | – IC50 = 1.5 | |||
| – Enhanced biocompatibility. | ||||
| – The capability of two multicolor visible/near-infrared fluorescence imaging. | ||||
| γ-Fe2O3@NGO | CisPt | U87 | – Magnetic guide. | 173 |
| – Targeted delivery. | ||||
| – Prolonged drug release rate (80% per 250 h). | ||||
| – High anticancer property. – Negligible toxicity of nanocarrier (not loaded with anticancer drug). | ||||
| GO fortifying polymeric micro-needles | HA15 | Melanoma bearing mouse model | – Increased mechanical strength. | 174 |
| – Antibacterial and anti-moisture properties. | ||||
| – Photothermal effect/on-demand drug release pattern. | ||||
| – Dissolvable. | ||||
| Fe3O4-GO (MGO) | Temozolomide | C6 | – No toxicity (40–120 μg mL−1). | 175 |
| – High loading capacity. | ||||
| – pH-dependent drug release. | ||||
| – In vivo results: suppression of cancer cells. | ||||
| FA-GNR | TC | MCF-7 | – Low drug leakage and elevated drug cargo delivery. | 176 |
| MDA-MB-231 | – Targeted delivery. | |||
| – In vivo toxicity is unknown. | ||||
| FA-OGNR | RXF | MCF-7 | – Loading efficiency (37%). | 177 |
| MDA-MB-231 | – Entrapment efficiency (56%). | |||
| – Time-, dose-, and pH-dependent drug release behaviors. | ||||
| CMC/PVP GO-FA NPs | CUR | MCF-7 | – 60 nm sized nanoparticles. | 179 |
| Saos-2 | – Specific targeting of FA receptor positive cancer cells. | |||
| – 94% CUR was shelled. | ||||
| – In vivo tests revealed a 76% tumor suppression rate, elevated cell death (apoptosis and angiogenesis), and reduced cell growth with no apparent toxicity. | ||||
| Trp-GQDs | CUR | MCF-7 | – Tryptophan elevated drug loading capacity (23%). | 180 |
| – Higher biocompatibility and solubility. | ||||
| – Antioxidant and anti-inflammatory properties. | ||||
| – Trp increased adsorption and emission in the UV light area. | ||||
| – pH-dependent, nontoxic, and traceable nanocarrier. | ||||
| FGO-linoleic acid- | CUR | MCF-7 | – pH-responsive drug release. | 181 |
| Balb/c mice | – Inhibition of tumor growth. | |||
| – No systemic toxicity. | ||||
| – A potential candidate for MRI contrast. | ||||
| GO-HPG-MNPs | CUR | SH-SY5Y | – Increased biocompatibility due to employing HPG. | 182 |
| MCF-7 | – Impressive drug loading capacity (∼198%). | |||
| – pH-dependent drug release. | ||||
| – MCF-7 cells displayed less sensitivity and more resistance in comparison to SH-SY5Y cells, which may be due to their special therapeutic effects on the nervous system (bioinformatics studies). | ||||
| HA-GO | Met | TNBC cells | – Cell migration and EMT were decreased by affecting the miR-10b/PTEN pathway, pFAK/integrin1, and E-cadherin expression. | 184 |
| – Decreased stemness by targeting stemness markers such as Nanog, oct4, and sox2. | ||||
| – No side effects on other organs were observed. | ||||
| GO/NHs | FU | MCF-7 | – Negligible toxicity. | 185 |
| – Increased apoptosis. | ||||
| – Increased expression of apoptotic proteins such as P53, PARP, cleaved PARP, Bcl-2, and Bax. | ||||
| rGO alginate beads | 5-FU | MCF-7 | – Mg2+, a crosslinking agent, may have affected the drug release rate and high swelling degree. | 186 |
| – pH-dependent drug release. | ||||
| – Considerable anticancer function. | ||||
| GO-g-MA/FA | PTX | MDA-MB-231 | – Biocompatible. | 117 |
| In vitro | – 39% toxicity in vitro. | |||
| – In vivo tests: though drug delivery was perfectly done, mitochondria were not damaged. | ||||
| – Mitochondrial integrity and citric acid cycle enzymes were back to normal after therapy. | ||||
| FADDO | CLB | Siha | – FADDO (CLB grafted rGO-FA coated with gelatin) | 188 |
| – Induced cell death in cervical cancer cells (apoptosis). | ||||
| – Gelatin increased stability and biocompatibility. | ||||
| – High drug loading capability. | ||||
| – pH-dependent release. | ||||
| – Reduced systemic toxicity compared to free drugs. | ||||
| CS-GN-CP-PEG2-VC | Cyclophosphamide | Molecular dynamics simulations | – High drug loading capacity. | 189 |
| – Efficient drug delivery and drug release at 35 °C. | ||||
| GO–PVP–Fe3O4 | QSR | MDA-MB-231 | – Increased biocompatibility. | 192 |
| – pH-controlled drug release. | ||||
| – High toxicity for cancer cells. | ||||
| GOMNP/PEGA | MTX | HeLa | – Higher toxicity when loaded on the nanocarrier. | 92 |
| MCF-7 | – High blood compatibility. | |||
| GO-PEG | Erlotinib | NPC | – Successful delivery of erlotinib to NPC cells. | 198 |
| – Destroyed cancer cells. | ||||
| – Reduced tumor progression. | ||||
| GO–CH–Ma-UL | Ulvan lacuta | Glioblastoma | – pH-controlled drug release. | 199 |
| – Biocompatible and nontoxic to RBC and normal cells while toxic to cancer cells. | ||||
| Ag-rGONCs | ChR | MDA-MB-468 | – Enhanced thermal stability. | 201 |
| Au-rGONCs | MDA-MB-231 | – ROS production resulted in increased efficiency. | ||
| – The toxicity of ChR solely, compared to the designed nanocarrier, was negligible. | ||||
| – Minor toxicity was observed when tested on normal fibroblast cells. | ||||
| GOMNP-MitP | MiTX | HeLa | – Magnetic field triggering drug release. | 197 |
| MCF-7 | – Direct targeting of cancer cells’ mitochondria. | |||
| – Disturbs ATP production by decreasing mitochondrial membrane potential. | ||||
| – Caused apoptosis. | ||||
| GO-DEX-Apt | CUR | 4T1 | – Efficient entrance to nucleolin-overexpressed cells. | 95 |
| MCF-7 | – High toxicity for cancer cells. | |||
| FA-CMCS/AGO | DOX | L929 | – High drug loading capacity (95%). | 203 |
| HeLa | ||||
| MCF7 | ||||
| GO-PEG | Cur | — | – Immune system escape. | 204 |
| – Efficient cancer therapy. | ||||
| GO/Fe3O4 | TMZ | C6 | – High potential drug delivery system. | 175 |
| GO | Ag NPs | HT-29 | – Green formulated. | 93 |
| HCT 116 | – Suppressed 50% of cancer cells. | |||
| HCT-8 | – Highest anticancer potential against HT-29. | |||
| HRT-18 | ||||
| Ramos.2G6.4C10 | ||||
| OVA–PMMA–GO | DOX | CACO-2 (gastric cancer) | – Enhanced permeability. | 205 |
| – Successful drug loading and controlled pH-dependent release. | ||||
| – 62% cancer cell death after treatment. | ||||
| rGO-Fe3O4-GL-PF | Quercetin | A549 | – Enhanced physiological stability and dispersibility. | 82 |
| MRC-5 | – GL increases the cancer elimination potential of the nanocarrier. | |||
| – drug loading efficiency: 11 wt%. | ||||
| GO-ZnFe2O4 | DOX | HeLa | – Higher toxicity. | 206 |
| – RO production. | ||||
| – Nuclear and mitochondrial damage. | ||||
| – Apoptosis induction. | ||||
| – Noninvasive MR imaging. | ||||
| GO/IO/Au | Quercetin | MCF-7 | – Highly biocompatible. | 207 |
| HEK-293 | – High magnetic properties. | |||
| – Potent drug carrier. | ||||
| – Effective drug delivery. | ||||
| GO | DOX | HCT-116 | – Induced apoptosis and autophagy. | 208 |
| – Significant anticancer effects. | ||||
| Dual drug delivery platforms | ||||
| GO-PCH-g-HPG | DOX | MCF-7 | – Biocompatible. | 209 |
| CUR | – pH-sensitive drug release. | |||
| – Efficient cell internalization. | ||||
| p-GO | DOX | CAL-27 and MCF- | – Boost apoptosis | 210 |
| CisPt | 7 | – Minimum systemic toxicity | ||
| Cs-rGO | 5-FU | HT-29 | – Successful inhibition of cancer cell growth. | 39 |
| CUR | – Minor toxicity | |||
| GO-PVP | GEF | PA-1 ovarian cancer cells | – possesses a greater release profile than a single drug delivery system. | 211 |
| QSR | – Increased toxicity. | |||
| rGO-g-PSEMA/Fe3O4 | DOX | MCF-7 | – Apoptosis induction (75%). | 212 |
| CisPt | – Easy cell internalization due to small size (<70 nm). | |||
| GO | CUR | AGS | – Simultaneous use of CUR and DOX to reduce side effects and elevate efficiency. | 213 |
| DOX | PC3 | – pH sensitive drug release. | ||
| A2780 | – High loading efficiency and drug release (80% for DOX and 13% for CUR). | |||
| GQD-PEG-PEI | DOX | Tested on the mouse xenograft model. | – A star-shaped nanocarrier. | 157 |
| GFP plasmid | – Successful cell entrance. | |||
| – pH-dependent drug release. | ||||
| – Successfully suppressed cancer cell proliferation. | ||||
| FA-GO-PEG- | PCA | HEPG2 | – Size: 9–40 nm | 214 |
| CA | HT-29 | – Non-toxic to normal cells. | ||
| – Highly toxic to cancer cells with high expression of FA receptors | ||||
| GO-PCH-g-HPG | CUR | MCF-7 | – Manageable drug release (92h) at pH = 7.4 | 215 |
| PTX | – Cancer cells were successfully eliminated. | |||
| DS-CNP- | DOX | HER2+ breast cancer cells | – Antitumor properties. | 216 |
| Herceptin | – pH-dependent drug release. | |||
| – Degradable and biocompatible. | ||||
| – Nontoxic carrier. | ||||
| APT-CGO | DOX | AGS | – Targeted drug delivery using AS1411 as a targeting agent. | 213 |
| CUR | – CUR influences many signaling pathways like inflammation, proliferation, apoptotic cell death, and angiogenesis, thus altering their gene expressions. | |||
| – Suppressed NF-κB, CDK2, and AKT at the gene level while enhancing RB1 at the protein level. | ||||
| KGO | CPT | MDA-MB-231 | – Increased aqueous solubility. | 217 |
| GEF | – Drug release profile (38%). | |||
| – 82% of cancer cells were eliminated. | ||||
| MiRGD-GQD | CUR | HUVEC | – Multifunctional theranostic nanocarriers, with high potential for targeting, internalization, and drug delivery to tumor cells. | 218 |
| DOX | 4T1-induced breast cancer BALB/c mouse | |||
Bullo et al.214 proposed multiple drug delivery with the assistance of GO nanocarriers for enhanced treatment of drug-resistant tumors. They constructed a potential FA-GO-PEG-PCA-chlorogenic acid (CA) nanocarrier for dual-drug delivery to HEPG2 (liver cancer) and HT-29 (colon cancer) cells. This nanocarrier was sized at 9–40 nm and was discovered to be non-toxic to normal cells but highly toxic to liver cancer cells, owing to FA ligands.
In a study on breast cancer, Asgari et al.215 developed a GO-based nanocarrier wrapped with pullulan nanofibers through an electrospinning technique. First, poly(epichlorohydrin) (PCH) molecules were loaded onto the edge-hydroxyl groups of GO. Afterward, to form a nanocarrier covered with oxygen groups, the PCH hydroxyl groups were coated with hyperbranched polyglycerol (HPG). Finally, two anti-breast cancer drugs, PTX and CUR, were grafted onto the GO-PCH-g-HPG nanocarrier and encircled with pullulan nanofibers through an electrospinning process. The drugs were released manageably over time within 92 hours in the physiological pH (7.4) condition and killed the MCF-7 cancer cells.
To improve HER2-positive breast cancer treatment, Ko and colleagues216 participated in the construction of new dual stimuli-responsive degradable carbon-based nanoparticles (DS-CNPs), a GO-dependent nanocarrier grafted with PEG for co-delivery of DOX and herceptin. HER2 (human epidermal growth factor receptor 2) is a receptor available on some breast cancer cells (HER2+).216 Moreover, these receptors have been widely used to target HER2+ cells and deliver drugs such as herceptin (monoclonal antibody) to decrease the proliferation of breast cancer cells. Thus, the HER2 linked on the outer surface of cancer cells eases the cellular uptake of DS-CNP-DOX. Above all, they successfully designed an anti-tumor, pH-dependent, degradable, nontoxic carrier for drug/gene delivery in breast cancer cells both in vivo and in vitro.
Yaghoubi and coworkers178 invented a remarkable drug delivery composite AS1411-carboxylated graphene oxide (APT-CGO) grafted with CUR and DOX for delivering chemical and nature-derived drugs to AGS cells. Using AS1411 as an aptamer, nanocarriers may be transferred to AGS cancer cells with more precision, increasing the drug's potency. CUR influences many signaling pathways like inflammation, proliferation, apoptotic cell death, and angiogenesis, thus altering their gene expressions. It was, therefore, necessary to examine the differential expression of RB1, NF-κB, CDK2, and AKT genes and RB1 and CDK2 proteins. The results indicate successful delivery of drugs, and when it came to gene and protein expression, it suppressed NF-κB, CDK2, and AKT at the gene level while enhancing RB1 at the protein level.
Tiwari and coworkers217 employed potassium-contained GO (KGO) grafted with camptothecin (CPT) and gefitinib (GEF) as chemotherapeutic agents to develop a novel fluorescent dual drug-loaded nanocarrier for more efficient cancer therapy. The results indicate that K-GO was highly hydrophilic, which increases its aqueous solubility, and its release profile for anti-cancer drugs was 38% which was able to eliminate 82% of MDA-MB-231 cancer cells after treatment.
Most recently, Ghafary et al.219 designed a nanocarrier by employing a MiRGD peptide loaded with CUR or DOX as chemotherapeutic agents and GQDs as tracking agents for targeted drug delivery to cancer cells by targeting integrin receptors located on their outer surface. The results of in vitro (HUVEC cells) and in vivo (4T1-induced breast cancer BALB/c mouse) treatments showed that these multifunctional theranostic nanocarriers have high potential for targeting, internalization, and drug delivery to tumor cells.
In conclusion, a handful of studies have demonstrated that combined chemotherapy results in better cancer suppression through increasing cytotoxicity. Since these discoveries, it is now possible to create more effective chemotherapeutic regimens by delivering anticancer medications in combination with graphene-derivative nanocarriers (Fig. 7).
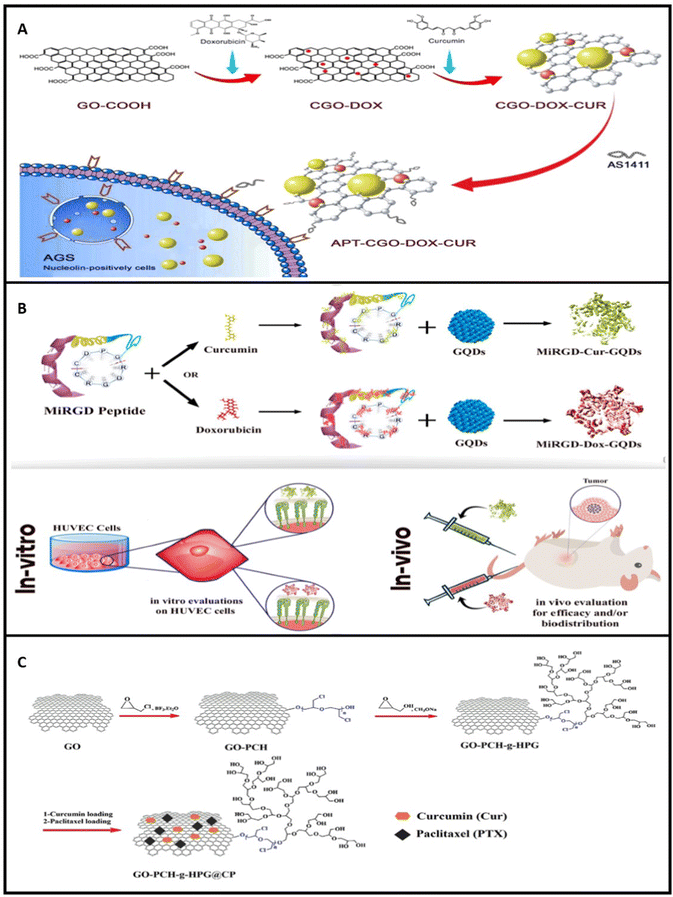 | ||
| Fig. 7 The schematic illustration of cancer dual-drug delivery agents based on graphene derivatives. Reprinted with permission from ref. 213, 218, 215. (A) APT-CGO-DOX-CUR drug-codelivery nanosystem development for cancer therapy.213 (B) Nanocomposite prepared from GQDs, loaded with the miRGD peptide and CUR/DOX for cancer drug delivery purposes.218 (C) GO grafted with pullulan nanofibers and HPG, and loaded with PTX and CUR chemotherapeutics for cancer treatment objectives.215 | ||
In the following (Table 2) we further explore the graphene family based nanocarriers utilized to deliver various drugs to cancer cells with the aim of cancer drug delivery.
4. Combined therapies
Simultaneous delivery of different biomolecules with biomedical properties such as photothermal, photodynamic, or even bioimaging properties can be a step forward to more effective cancer treatment. Graphene derivatives are a perfect choice for combined therapies due to their remarkable properties, and the combination of these favorable properties with the high gene/drug delivery capability of graphene derivatives has assisted cancer therapy scientists in recent years (Table 3 and Fig. 8).| Nanocarrier | Cargo | In vivo/in vitro | Highlights | Ref. | |
|---|---|---|---|---|---|
| Gene/drug delivery | GPF | DOX-VEGF siRNA | Both | – VEGF was downregulated at both mRNA (46%) and protein (52%) levels | 220 |
| – Anti-tumor effects were observed. | |||||
| – In vivo trials displayed significant VEGF suppression and tumor inhibition (66%). | |||||
| – No remarkable toxicity. | |||||
| CPN@GO-CET | CPT11, shRNA | In vivo | – Injected inside the tumor. | 118 | |
| – Mitochondria-specific targeting. | |||||
| – pH-dependent drug release. | |||||
| – Increased apoptosis rate. | |||||
| – Reduced cancer cell migration. | |||||
| GPPF/ | CQ, cell death control siRNA labelled with FITC | MCF-7 | – Stable structure. | 221 | |
| – pH-dependent drug release (95% at lysosomal pH). | |||||
| – Efficient intracellular gene delivery. | |||||
| Tf-HPAA-GO | Docetaxel (DOC) and MMP-9 shRNA | Both | – High cytotoxicity towards cancer cells. | 222 | |
| – Good delivery efficiency of the developed complex in vivo and in vitro. | |||||
| GO-HAP | HSV-TK | Cancer cells | – Induces DNA damage and apoptosis. | 223 | |
| – Suppresses cell proliferation by successful transfer of pDNA. | |||||
| Chemo/PTT | GO@Au-His@a-ZnO | Apt, DOX | A549 | – High loading capacity | 224 |
| – Stability | |||||
| – Negligible toxicity | |||||
| – High biocompatibility. | |||||
| – Photothermal conversion efficiency | |||||
| – Targeted delivery. | |||||
| – pH and NIR triggered drug release | |||||
| GCA-PPP (graphene-calcium alginate-PLGA-PEG-PLGA) | 5-FU | In situ treatment | – Graphene microsphere based drug delivery. | 225 | |
| – Manageable step-shaped drug release diagram. | |||||
| – Antitumor activity only under NIR light irradiation. | |||||
| – The hydrogel containing the nanocomplexes will be injected into the desired area. | |||||
| – Photothermal stability. | |||||
| GO-AS1411 | B3 | A549 | – Specific targeting of nucleoin-overexpressed cancer cells. | 190 | |
| – Photothermal and pH-sensitive drug release. | |||||
| – Killing a major population of cancer cells when using NIR light combined with nanocarrier drug delivery. | |||||
| MGO-PEG | CET, DOX | CT-26, in vivo | – Negligible toxicity | 119 | |
| – Considerable tumor size reduction. | |||||
| GO | IR820-LA, DOX | Both | – Fluorescence imaging potential. | 226 | |
| – Active cancer treatment guidance. | |||||
| MGO-FA | TCA, DOX | Both | – pH and NIR-dependent drug release. | 29 | |
| – High tumor suppression (85%). | |||||
| GO-ADH | HA-MTX | Both | – Biocompatible. | 227 | |
| – Innocuous to blood cells. | |||||
| – Stable. | |||||
| – Nontoxic. | |||||
| – Capable of targeting tumor cells in different stages of development. | |||||
| TFGP | DOX | LO2, SMMC-7721 | – Reduced toxicity. | 228 | |
| – Dual-targeting properties. | |||||
| – Constant drug release. | |||||
| AUNRs/GO@PDA | DOX | MCF-7 | – High toxicity for cancer cells. | 229 | |
| – pH-dependent and NIR-responsive drug release behavior. | |||||
| – High drug loading capacity (86%). | |||||
| CMC-rGO/CHO-PEG | DOX | L-929 | – Good distribution. | 230 | |
| – Hydrophilic nature. | |||||
| – pH-dependent drug release. | |||||
| RGD-GO-PEG | DOX | Hep-G2 | – Good suppression of cancer cells (78%). | 231 | |
| – Provided cancer treatment using redox response. | |||||
| GS/LB | DOX | C6 | – NIR-dependent drug release. | 232 | |
| – Efficient cancer cell elimination. | |||||
| – Highly stable and biocompatible. | |||||
| HAp@GO | DOX | MG-63 | – Improved PTT and efficient cancer treatment. | 233 | |
| – pH-dependent and NIR- | |||||
| controlled drug release. | |||||
| – High drug loading capacity. | |||||
| rGO@msilica | DOX | A549, sw620 | – Effective cancer cell elimination under NIR light exposure. | 234 | |
| – pH sensitive and controllable drug release. | |||||
| Gene/PTT | PDA-rGO | — | 136 | ||
| Chemo/PTT | FGO-ADH-HA-Fe3O4 | DOX | A549 | – The fluorescence “switch off” process was used to track DOX loading. | 235 |
| – A549 cancer cells with a high amount of HA receptors were specifically targeted and killed by this nanocarrier. | |||||
| APT-GO-CO-PGA (A-G-C-P) | DOX | HeLa | – Drug release was controlled with pH and NIR light. | 236 | |
| – Toxic to cancer cells under NIR light. | |||||
| GO-CO-γPGA | MiTX | MDA-MB-231 | – MiTX encapsulation efficiency and release rate in acidic pH were 73% and 56% in 120 h. | 237 | |
| – Increased apoptosis. | |||||
| – The nanocarrier was loaded with breast cancer cells’ exosomes to use their targeting ability for breast cancer cells and targeted drug delivery. | |||||
| MGO@GEL@PAC | PAC | MCF-7 | – High biocompatibility. | 238 | |
| – Drug release rate was enhanced at lower pH values. | |||||
| – It was able to eliminate cancer cells specifically. | |||||
| S-MTN@IG (mesoporous silica with GO) | Imatinib | HCT-116 | – Able to reach the tumor environment and reduce cancer cell proliferation in the presence of NIR light and kill the cancer cells through imatinib release. | 239 | |
| HT-29 | |||||
| MG–NH2–PEG | DOX | MCF-7 | – Negligible toxicity (survival rate > 85%), but the drug-loaded platform could kill the cancer cells with the help of photothermal and magnetic localization methods. | 202 | |
| – More than 80% cell internalization. | |||||
| GOF-BSA/ | DOX | HeLa | – High cell toxicity under NIR light. | 170 | |
| – High stability and pH-responsive drug release (54% DOX release at 42 °C). | |||||
| rGO/DA/AU NPs/ | DOX | — | – 0.852 mg/mg DOX loading capacity and 67% drug release in acidic pH. | 240 | |
| – PTT properties which endowed it with the potential of being used as a cancer therapeutic agent. | |||||
| Silica-CTAB-(carbanosilica) | DOX | 4T1 | – Enables image-guided tumor eradication by chemo-phototherapy. | 241 | |
| L929/ | – 31% drug loading capability. | ||||
| In vivo | – Under NIR light, these nanocomplexes are qualified to cause a 68% reduction in tumor mass and 89% of 4T1 cancer cells were killed. | ||||
| GO-PEG-FA | DOX | MCF7 | – Small size. | 242 | |
| MDA-MB-231 | – NIR-dependent drug delivery. | ||||
| – Localized hyperthermia. | |||||
| – selectively killed breast cancer cells. | |||||
| – IC50 up to 12 times lower in non-cancerous cells. | |||||
| – Used plasma etching as a low cost method to functionalize GO. | |||||
| Chemodynamic/PTT | rGO@ | MnO2 | HeLa | – GSH molecules present in the tumor cells convert MnO2 to Mn+. | 42 |
| – HO− is produced via the Fenton reaction by the help of Mn+ molecules under NIR light. | |||||
| – PTT accelerates these reactions by producing high temperature. | |||||
| – This nanocarrier acted as a promising candidate for elimination of cancer cells. | |||||
| Chemo/immune/PTT | rGO/SB | MiTX | 4T1 mouse mammary tumor model. | – SB-431542 (SB), employed as an immune-triggering agent. | 243 |
| – The nanocarrier could perfectly destroy the primary tumors and the distance metastasis in 70% of high metastatic and poor immunogenic mouse models when exposed to NIR light. | |||||
| – The mice not only experienced longevity but also devised a tumor type specific immunity to combat reactivated tumor cells. | |||||
| Chemo/PDT | PEG-GO-FA/ICG | TH287 (MTH1 inhibitor) | SaOS-2 | – Effective transportation of TH287. | 244 |
| MNNG/ | – Proliferation and migration in cancer cells were suppressed. | ||||
| HOS | – ER-stress induced apoptosis and autophagy were increased. | ||||
| MG63 | |||||
| U2OS | |||||
| MrGO-AA-g-4-HC | CPT | MCF-7 | – 4-hydroxy coumarin endows the nanocarrier with the capability of ROS production when exposed to UV light. | 245 | |
| – High toxicity against cancer cells. | |||||
| PEG–GO–FA/ICG– | Rg3 | In vivo/in vitro (osteosarcoma derived cancer stem cells) | – Successful inhibition of cancer stem cells. | 246 | |
| – NIR light increased treatment efficiency and reduced tumor progression. | |||||
| Gene/PTT | NGO-PEG-PEI | Plk1 siRNA | HeLa | – NIR light increased the transfer rate by making the cell membrane permeable by generating heat. | 247 |
| – Increased intracellular trafficking. | |||||
| HDAc1, K-RAS targeting siRNAs | MIA PaCa-2/in vivo | – Biocompatible and noncytotoxic. | 248 | ||
| – High anti-tumor effect when exposed to NIR light. | |||||
| – 80% of the tumor had shrunk after treatment. | |||||
| – This nanocarrier was efficacious in suppressing tumor cell proliferation, blocking the cell cycle, and triggering apoptosis. | |||||
| rGADA (rGO@AuNSDODAB/DOPE-FA) | Krasl | Pancreatic cancer cells/in vivo | – Liver metastasis with pancreatic origin was suppressed after treatment. | 249 | |
| – Outstanding photothermal property and astonishing photoacoustic and photothermal imaging functioning. | |||||
| GO-PEI-P-I-Arg- | miR-101 | MCF7 | – Combination with PTT increased the therapy's efficiency by elevating apoptosis. | 250 | |
| MDA-MB-231 | – Reduced side effects and rapid treatment. | ||||
| – miRNA-101 successfully suppressed stathmin1 expression in cancer cells. | |||||
| Chemo/PDT/PTT | NCGO@DOX-FA | DOX | HeLa | – pH and heat triggered drug release. | 251 |
| NCGO@MeB-FA | MCF-7 | – High drug loading capacity, vast surface area, | |||
| – Photostability and targeted delivery (FA receptors) | |||||
| GQDs@DOX/PB | DOX | HeLa | – MeB acts as a photosensitizer and produces ROS under NIR light. | 75 | |
| MeB@DOX/PB | MCF-7 | – Single oxygen production. | |||
| ACNGHox | AQ4N | In vivo/in vitro (L02) | – NGO/Ce6 endowed the nanocomplex with PTT/PDT properties. | 252 | |
| – Hypoxia-activated chemotherapy occurs. | |||||
| – CD44 is a targeting agent. | |||||
| GO-PEG-PSA | PTX | HGC-27 | – Blocks p-glycoprotein pump and, as a result, resistance to PTX by triggering ROS production through NIR light exposure. | 187 | |
| – As a result of high ROS and damage to mitochondria, ATP production was reduced, and consequently, the PGP pump was deactivated. | |||||
| – Increased cell death. | |||||
| Chemo/fluorescence | GO@PEG/AU/Apt | DOX | HT-29 and MCF-7 (MUC+) | – The fluorescence light can follow a turn-off/on procedure with the help of the MUC aptamer. | 253 |
| – MUC1 was employed in their developed nanocarrier and successfully delivered DOX to breast, colon (MUC+), and hepatic (MUC−) cancer cells. | |||||
| – More toxicity was monitored in MUC+ cell lines. | |||||
| LDH@SGQD-VP16 | VP16 | HGC-27 | – VP16 was employed as both a therapeutic and a visualization agent. | 54 | |
| – pH-dependent drug release. | |||||
| – VP16 endowed the nanocarrier with the ability only to target the cancer cells, induce apoptosis, and reduce cancer cell proliferation. | |||||
| GQDs@GE11 | CDDP | CNE-2 | – Enhanced cancer cell elimination by employing two drugs simultaneously. | 120 | |
| DOX | – The targeting agent, GE11 peptide, was used for specific targeting of EGFR receptors on cancer cells. | ||||
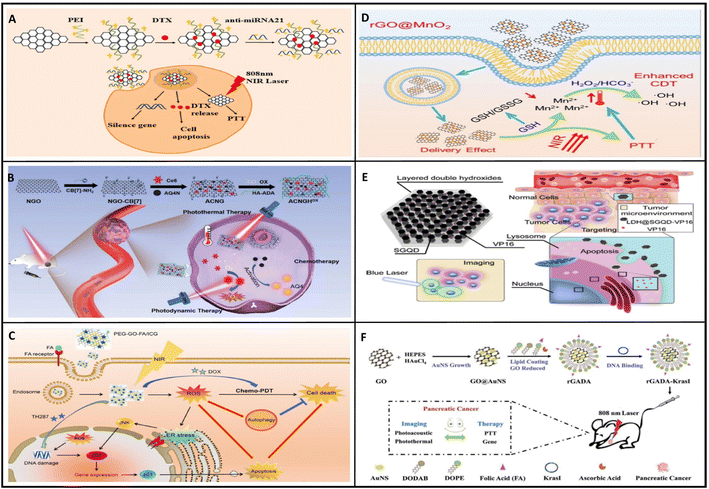 | ||
| Fig. 8 Summary of various combined therapies based on graphene derivatives used for cancer treatment. Reprinted with permission from ref. 42, 54, 244, 249, 252 and 254. (A) GO functionalized with PEI was furnished with DTX and anti-miRNA-21 for combining gene/drug delivery and PTT in triple-negative breast cancer.254 (B) Schematic overview of ACNGHOX preparation with NGO nanoparticles for PTT/PDT and hypoxia activated chemotherapy of cancer cells.252 (C) PEG, FA, and ICG were attached to GO for targeted chemo-PDT of osteosarcoma cancer cells.244 (D) rGO functionalized with MnO2 NPs for chemodynamic-PTT of cancer cells triggered by NIR light, heat and ROS production.42 (E) SGQDs were grafted with VP16 and LDHs as visualization and chemotherapeutic agents for pH-sensitive drug delivery to cancer cells.54 (F) preparative process of the rGADA-KrasL nanosystem based on GO and AU nanoparticles with gene-PTT potential and dual-modal imaging properties.249 | ||
4.1 Gene/drug co-delivery
Graphene derivatives are capable of delivering medications at lower dosages than conventional chemotherapy, alongside DNA/RNAs, directed unharmed into the target tissue/cells with fewer adverse effects.255 Besides, combined therapies are usually employed to treat particular cancer types which display resistance to monotherapies such as chemotherapy. One crucial advantage of combined therapy is that the cancer cells are targeted from different aspects, enhancing the effectiveness of the attack and reducing the chance of therapy resistance.For example, in a study, Izadi and coworkers121 utilized carboxylated graphene oxide (CGO) linked to trimethyl chitosan (TMC) and HA for drug/gene therapy in CD44+ cancer cells such as CT26, B16-F10 (melanoma), and 4T1. To stop tumor expansion and migration, they loaded HIF-1α siRNA and dinaciclib on the CGO-TMC-HA nanoplatforms for the first time. They realized that tumor growth, migration, and angiogenesis are blocked due to CDK (cyclin-dependent kinase) and HIF-1α genes’ effective suppression. Among the significant HIF-1 target molecules and prominent cancer hallmarks are cyclins and CDKs, which together play a crucial role in cell division, proliferation, and promotion throughout the cell cycle. The hif-1 gene upregulates CDK gene expression, and thus promotes the cell cycle.
In a study by Gu et al.,256 a co-delivery nanocarrier system based on GO-PAMAM for DOX and MMP-9 shRNA plasmid dual delivery to breast cancer cells was introduced. GO-PAMAM, with a surface rich in amines, can supply extra delivery capacity. The designed transporter is further stable and biocompatible, which enhances the efficiency of the treatment.
Besides the co-delivery of genes and drugs, tracing nanocarriers can also effectively help targeted delivery. GQDs have recently been used for traceable drug delivery. In this regard, Lo et al.256 designed a GQD-based nano transferor for cancer chemotherapy with low toxicity, known as GIGED. GFP and DOX were loaded on the PEI-grafted GQD. This complex is prepared to target colon tumor cells through particular antibodies. Besides, EGFRs are vital keys for the nanocarriers’ easy entrance to HCT116 (colon cancer) cells. In vivo trials displayed that DOX release is pH-dependent, and the designed complex efficiently suppressed tumor growth.
Liu and coworkers257 designed a novel NGO-based nanoplatform for dual transfer of anti-mir-21 and CisPt to A549 cancer cells. Anti-miR-21 targets mir-21 and anti-apoptotic Bcl-2 protein, and CisPt is one of the well-known anticancer drugs. As a result of GO-anti-mir-21-CisPt efficient transfer at once, increased cytotoxicity and apoptosis in cancer cells were observed. In a recent study done by Yang et al.,258 GO was utilized as a nanocarrier for transferring antimir-21 and DOX to MDA-MB-231 cells. This nanocarrier delivered DOX and cDNA-21 efficiently into the cancer cells. As a result, mir-21 was silenced, and DOX killed the cancer cells even in low doses.
Several graphene based transporters can be used for MRI detection of the exact tumor location. For example, Yang et al.259 developed an NGO-based nanocomplex bonded to gadolinium for dual delivery of the epirubicin (EPI) drug and Let-7g miRNA to U87 cells. As a tumor suppressor, the Let-7 microRNA family of nine members inhibits the Ras oncogene family's expression.260 The results demonstrate that Gd-NGO has high transfer efficacy and effectively hampers cancer cell proliferation.
Most recently, Chen et al.254 have developed a triple functionalized nanocarrier based on GO for gene/drug/PTT therapy of triple-negative breast cancer. GO was initially furnished with PEI to increase the stability and drug loading capacity, docetaxel (DTX) as the chemotherapeutic agent, and anti-miRNA-21 as the gene therapy agent. The overall in vitro results are encouraging due to reduced proliferation and metastasis of triple-negative breast cancer cells after treatment.
It can be concluded that graphene has successfully fulfilled almost all of the critical conditions for a carrier to be a successful gene/drug co-delivery agent in recent years, owing to graphene's great functionalization capability, which overcomes the constraints of unfunctionalized graphene.
GO is also used in “self-killing” gene/drug dual therapy. Cheang and coworkers223 participated in the construction of a GO-Hap (hydroxyapatite) based gene therapy system for delivering herpes simplex virus thymidine kinase gene (HSV-TK) to cancer cells. HSV-TK/GCV (Cymevene) is a well-known composite that can cause DNA damage and induce apoptosis when co-delivered with ganciclovir, an antiviral drug that hinders DNA synthesis. The results demonstrate that GO–HAp/p-HRE/ERE-Sur-TK/GCV can efficiently transfer pDNA, block cell multiplication, and induce apoptosis in cancer cells. Besides, it is good to note that the measured cytotoxic effects of this nanocomposite on normal breast cells are at the minimum level.
4.2 Chemo/photothermal therapy (chemo/PTT)
According to the results of the investigations, scientists have found that tumor cells could be suppressed in the 41–43 °C heat of NIR light. The vitality of normal cells may, however, be adversely affected by heating (photothermal treatment). Researchers have developed graphene-based nanocomplexes for PTT applications to address this problem. These nanocomplexes have been extensively applicable in reducing the mortality of normal cells while enhancing the heat impact exclusively on cancer cells. Graphene-based nanocarriers are becoming more popular because they can be used as platforms to combine chemotherapy with PTT to increase cancer therapies’ efficiency and simultaneously reduce side effects, owing to their superior photothermal and drug transport capabilities.261,262Previously, drug release from nanocarriers has been mainly dependent on pH. However, most recently, Zhuang et al.225 suggested a graphene microsphere-based drug delivery platform for a 5-FU release procedure that occurs as required. This platform is made up of graphene and calcium alginate, which are mixed to produce a microsphere which is afterward coupled with 5-FU and a triblock polymer to make a heat-sensitive hydrogel abbreviated as PLGA-PEG-PLGA or PPP. This nanocarrier was first injected into the desired area in a hydrogel structure, and the heat produced by NIR exposure resulted in drug release. This biocompatible platform exhibited photothermal stability and an exceptional heating plateau with a step-shaped drug release diagram. The anti-tumor activity of the platform only occurred under NIR light radiation, which we can regulate. This nano-based cancer therapy system seems to be a promising method for tumors with various shapes.
Wang et al.235 developed the idea of using fluorinated/magnetic graphene for cancer cell chemo-photothermal dual-therapy. First, HA and subsequently Fe3O4 were grafted onto fluorinated graphene. Finally, the fluorescence “switch off” process was used to track DOX loading. A549 cancer cells with a high amount of HA receptors were specifically targeted and killed by this nanocarrier.
Gao and coworkers236 designed a nanoplatform for the chemo-photothermal therapy of cervical cancer. First, in order to increase dispersion and solubility, GO was grafted with chitosan and -polyglutamic acid (-PGA), named GO–CO–PGA (G–C–P). GCP was then linked to a nucleolin (C23) targeting nucleic acid aptamer NH2-AS1411 (APT), resulting in the formation of APT–GO–CO-PGA (A–G–C–P). The cervical cancer cell surface is overexpressed with C23 allowing for targeted therapy. Finally, DOX was loaded, and AGCPD was prepared. Drug release was controlled with pH and NIR light. Unlike the carrier (AGCP) alone, the nanoplatform (AGCPD) was found to be toxic, and much more so after NIR irradiation. Both in vitro tests performed on HeLa cells and in vivo tests on nude mice approved the nanoplatform's high biocompatibility and cellular uptake. When applying the nanoplatform under NIR light, increased antitumor effects were observed with no tissue damage. That was in contrast to the results from using free DOX.
But in another most recent project guided by Chen and coworkers,237 GO–CO which was modified with γ-polyglutamic acid, GO–CO-γPGA, was loaded with breast cancer cells’ exosomes to use their targeting ability for breast cancer cells and deliver mitoxantrone as a chemotherapeutic agent to MDA-MB-231. The mitoxantrone encapsulation efficiency and release rate in acidic pH were 73% and 56% in 120 h, respectively, which resulted in elevated apoptosis induction. These features have endowed this nanocomplex with the capability of advanced treatment of breast cancer due to local drug concentration.
In another example of GO-assembly chemo-PTT simultaneous cancer therapy, Isiklan et al.238 structured a magnetic graphene oxide (MGO) grafted with gelatin to deliver paclitaxel (PAC) to MCF-7 cancer cells in a high biocompatible nanocarrier. With the assistance of NIR light, the drug release rate of this nanocomplex MGO@GEL@PAC was enhanced in lower pH conditions, and it was able to eliminate cancer cells specifically.
GO is not always the engineered core in a chemo-PTT nanocomplex. In a study by Gautam et al.,239 silica-based mesoporous titania (SMTN) is furnished with GO (G) and imatinib (I) (drug) and pegylated, respectively. GO is responsible for ROS production and the photothermal effect of this nanocarrier. The resulted carrier, named SMTN@IG-P, displayed enhanced drug loading and release capacity, a NIR-sensitive drug release property, and high toxicity toward cancer cells (HCT-116 and HT-29). This carrier was able to reach the tumor environment and reduce cancer cell proliferation in the presence of NIR light and kill the cancer cells through imatinib release. This graphene-decorated nanocarrier generally demonstrates adequate drug delivery and PTT characteristics for cancer treatment.
Farani et al.202 introduced a combined therapy platform for simultaneous chemo-PTT therapy with magnetic guidance assembled on the GO core. Following GO amination, it was endowed with magnetic properties using Fe3O4 nanoparticles, and subsequently, it was grafted with PEG. Finally, DOX was loaded on the magnetic carrier as an anticancer drug for the MCF-7 cell line. Cellular internalization of the nanoplatform was more than 80%. The carrier (MG-NH2-PEG) revealed negligible toxicity (survival rate >85%), but the drug-loaded platform could kill the cancer cells with the help of photothermal and magnetic localization methods.
In another study, Xu and coworkers170 developed a novel nanocarrier with PTT properties by constructing a graphene organic framework (GOF) grafted with BSA and DOX. GOF-BSA/DOX could produce high temperatures when exposed to NIR light and displayed high cell toxicity. Moreover, this nanocarrier was highly stable and displayed pH-responsive drug release which was increased when exposed to NIR light (54% DOX release at 42 °C and pH = 5). These results indicate that these porous GOF based nanocarriers have the potential to be employed in future combined therapies for cancer elimination.
Most recently, Mirza-Aghayan et al.240 developed a novel rGO nanocomplex functionalized with dopamine (DA) and Au NPs for intelligent DOX delivery. This nanocarrier displayed 0.852 mg mg−1 DOX loading capacity and 67% drug release in acidic pH besides good PTT properties, which has endowed it with the potential of being used as a cancer therapeutic agent.
Another graphene derivative recently employed as a drug delivery agent with PTT properties is GQDs. The usage of GQDs as a delivery agent was not common due to their ability to cause high systemic toxicity; Prasad et al.241 proposed a solution for the interaction of the drugs with other tissues and the systemic toxicity of the GQD based drug delivery systems. They constructed a GQD-fixed mesoporous silica nanocarrier in which porous silica acts as a shell for the GQD and medicines. Localized cancer therapy has been made possible through these NIR-sensitive GQD-based nanocomposites (chemo-photothermal therapy). NIR can improve the penetration and retention of nanocomposites inside solid tumors. Besides their 31% drug loading capability, under NIR light, these nanocomplexes are qualified to cause a 68% reduction in tumor mass. This nanocomplex enables image-guided tumor eradication by chemo-phototherapy. The in vitro assay was done using fibroblastic L929 normal mouse adipose tissue and 4T1 cancer cells. The nanocarrier was found to be highly biocompatible with normal cells, and the nanocomplex successfully killed 89% of the 4T1 cancer cells with the assistance of NIR light.
4.3 Chemodynamic-PTT
Cancer therapy efficiency can be improved by combining chemodynamic therapy and photothermal therapy, which nowadays has been proposed as a successful technique. This type of combined therapy has been recently developed by employing graphene-based materials due to their undeniable photothermal properties. In a study, Ma et al.42 designed a nanoplatform based on rGO grafted manganese dioxide nanoparticles (MnO2 NPs) for chemodynamic (CDT)-PTT therapy of HeLa cells. It is preferable to employ MnO2 NPs rather than Mn2+ ions because of their low toxicity and long-lasting properties. When the carrier manages to enter the cell, GSH molecules are oxidized by MnO2, and Mn2+ is produced. H2O2 is converted to HO−via the Fenton reaction, which is accelerated by the PTT-generated high temperature. As a result, this nanocarrier is a promising candidate for the elimination of cancer cells.In another project, Mauro and coworkers242 developed a nanocarrier, GO-PEG grafted with FA and DOX, for chemo/PTT of breast cancer cells. GO is capable of converting NIR light to heat and thus killing cancer cells. The active targeting agent for breast cancer cells, FA, was coupled to the end of PEG chains. The results indicate that the generated heat increased intracellular DOX delivery and MCF-7 and MDA-MB-231 cell death due to hyperthermia. This nanocarrier exhibited acceptable photothermal efficiency and drug delivery properties.
4.4 Chemo/immune/PTT
Another combined therapy method for healing high-stage cancer was developed by Zhou et al.243 through designing a nanocarrier based on reduced graphene oxide, which was capable of simultaneous chemotherapy, photothermal therapy, and immunotherapy and may be employed as an anticancer vaccine. The nanocarrier is comprised of a chemotherapy agent, mitoxantrone (MitX), and a transforming growth factor beta (TGF-β) inhibitor, SB-431542 (SB), employed as an immune-triggering agent, which are all grafted on rGO with PTT properties. This nanocarrier, rGO/MTX/SB, was tested on a high metastatic and poor immunogenic 4T1 mouse mammary tumor model, and it was exposed to NIR light. The NIR light could demolish the primary tumors and hinder distance metastasis, and 70% of the models not only experienced longevity but also developed a tumor-type-specific immunity to combat reactivated tumor cells. This could be the beginning of a tumor-specific vaccination strategy, which was determined by the infiltration of CD8+ and regulatory T cells in tumors.4.5 Chemo/PDT
Another noninvasive method for cancer therapy is PDT (photodynamic therapy). PDT is a process in which reactive oxygen species (ROS) are generated through the energy transfer from a particular wavelength of light to surrounding oxygen molecules, mediated by a photosensitizer such as methylene blue (MeB), 4-hydroxy coumarin (4-HC),245 and indocyanine green (ICG). ROS and single oxygen molecules can kill cells by damaging them by oxidizing their vital macromolecules. PDT is fast, repeatable, and on top of that, very low invasive, which can ease cancer therapy.75,244PDT effectiveness may be improved by increasing cellular sensitivity to ROS by blocking the DNA oxidative damage repair enzyme MTH1. Thus, Huang and colleagues244 used a GO-based nanocarrier to deliver TH287 (MTH1 inhibitor) and DOX to cancer cells alongside performing PDT (chemo-PDT). Their developed nanocarrier based on GO is grafted with PEG, FA, photosensitizer indocyanine green (ICG), TH287, and DOX. As a result of the effective transport of DOX and TH287 with the PEG-GO-FA/ICG carrier, proliferation and migration were suppressed, and endoplasmic reticulum (ER)-stress-induced apoptosis and autophagy were enhanced in MNNG/HOS, MG63, U2OS, and SaOS-2 (osteosarcoma cancer) cells.
In another work, Vinothini and colleagues245 created a magnetic nanocomposite from rGO grafted with a chemo drug, CPT, and a photosensitizer agent, 4-hydroxy coumarin (4-HC), linked with the help of allylamine (AA). MrGO-AA-g-4-HC loaded with CPT exhibits pH-dependent behavior in drug release and displays high toxicity against the MCF-7 cancerous cell line. When the nanocarrier is exposed to UV light, the nanocarrier's ability to suppress cancer cells increases due to increased ROS production by 4-HC. This nanocomplex disclosed exceptional cell apoptosis and death that have made this dual therapy a potential method for cancer healing.
LU and coworkers246 employed ginsenoside Rg3, a ginseng derivative with antitumor properties, to treat osteosarcoma, a high metastatic and drug-resistant bone cancer. To reach this objective, GO was grafted with a photosensitizer (PS), indocyanine green (ICG), PEG, and FA. Afterward, Rg3 was introduced, and the resulting nanocarrier was used simultaneously with PDT on osteosarcoma cells both in vivo and in vitro. The osteosarcoma-derived cancer stem cells were successfully inhibited in vitro, and NIR light boosted that effect. The in vivo results indicate that NIR light was shown to be effective by enhancing the inhibitory effect of PEG-GO-FA/ICG-Rg3 on tumor progression. According to the results, we can conclude that an efficient cancer combined-therapy method has been developed.
Graphene's unique properties have made PDT and chemotherapeutic medicines delivered through graphene-based nanocarriers more effective and faster in the treatment of cancer than either of these approaches alone.
4.6 Gene/PTT
As mentioned above, graphene-based materials like GO and rGO can carry nucleotide sequences (DNA, RNA) on their surface and deliver them specifically, due to their unique surface chemistry and high surface area. They can also respond to light, especially absorbing NIR light and converting it to heat. Thus, graphene is a promising material for photothermal and gene therapy applications because of its unique properties.100One of the prior studies on light-controllable gene transportation was guided by Feng et al.247 in 2013. A dual polymer (PEI and PEG) functionalized GO-based nanocarrier NGO-PEG-PEI was synthesized with the capability of transferring plasmid Polo-like kinase 1 (Plk1)-siRNA to HeLa cells with high efficiency with the help of NIR light. They used NIR light to increase the transfer rate of nano transporters such that the mild elevation in heat can increase the penetrability of the plasma membrane. In addition, the accelerated intracellular trafficking of nanocarriers with the help of photothermal therapy was pioneered by these researchers.
To obtain a superior transfection rate, PEG-FA grafted GO was linked to PAH9 (poly-allylamine hydrochloride) for the pancreatic cancer gene/thermal therapy by Yin and coworkers.248 Then, HDAc1 and K-Ras targeting siRNAs were loaded and delivered to the MIA PaCa-2 cells efficiently alongside NIR light emission. The results indicated that this nanocarrier was efficacious in suppressing tumor cell proliferation, blockage of the cell cycle, and triggering apoptosis. Regarding the cytotoxic effects of the nanocarrier, it is good to note that GO was biologically safe; It had shown no recognizable side effects before getting metabolized and was shortly emitted from the body. Collectively, the results confirmed the high anti-tumor effect of the nanocomplex/NIR. Also, the in vivo growth of the tumor was inhibited up to 80% after treatment.
Graphene-based gene therapy may also benefit from adding lipid bilayers to the payload. Lipid bilayers increase biocompatibility and stability, and protect genes from cellular enzyme breakdown, allowing for gene therapy to be successfully implemented. According to this fact, Jia et al.249 synthesized an AuNS grafted rGO covered with a cationic lipid bilayer of DODAB/DOPE bonded to FA named rGO@AuNSDODAB/DOPE-FA (rGADA) for co-gene/photothermal therapy. A mutated K-Ras gene plasmid (Krasl) was efficiently transported by rGADA into pancreatic cancer cells, both in vivo and in vitro. It was shown that liver metastasis with pancreatic origin was suppressed due to the treatment. Collectively, the results confirmed the outstanding photothermal property and astonishing photoacoustic and photothermal imaging functioning/behavior of this nanocomplex.
In another investigation on GO-PEG application as a nanocarrier, Assali et al.250 demonstrated that GO-PEI has been exploited as a dual gene/photothermal therapy agent for cancer therapy. GO-PEI was linked to a nucleic acid polymer (P-L-Arg) as an actor for guiding the carrier to the tumor cell and easing its entrance. GO-PEI-P-I-Arg exhibited higher infrared absorption, higher loading capacity, better cellular entrance, and easier endosomal escape. Besides, loading mir-101 on this complex, which acts as a tumor suppressor miRNA, facilitates the stathmin1 suppression in MCF7 and MDA-MB-231 cells. As a result of using GO-PEI-P-I-Arg-miR-101 in combination with laser exposure, a significant rise in apoptosis was observed.
According to recent studies, it can be concluded that gene therapy and photothermal treatment have been used as an efficient healing procedure that reduces healing duration and negative side effects in patients.100
Babavalian and coworkers136 employed polydopamine grafted rGO to fabricate an innovative nanocarrier for gene/PTT of solid tumors. PDA, a nature-derived biocompatible polymer, was used to graft rGO before it was furnished with histone methyltransferase complex subunit SET1 (hSET1) antisense, a NIR absorption agent and a suppressor for cancer cell proliferation. The resulting nanocarrier, rGO-PDA-hSET1, displayed higher photothermal properties that not only induced apoptosis but also increased hSET1 antisense release. Moreover, rGO-PDA demonstrated no toxicity, high biocompatibility, and good bonding capability with oligonucleotides, increasing its potential to be further used as a gene delivery/PTT agent for enhanced elimination of solid tumors.
4.7 Chemo/PDT/PTT
Most recently, graphene-derived nanocarriers have been designed and constructed with triple capabilities to enhance the efficiency of cancer treatment methods more than ever. Liang et al.251 invented a GO-based nanocarrier sensitive to pH and heat, used for cancer healing with synergistic triple capabilities: PTT-PDT-chemotherapy. GO is grafted with two drugs: DOX and MeB, which form NCGO@DOX-FA and NCGO@MeB-FA complexes. Among the unique properties of this nanocarrier are its high carrying capability for drugs, a vast surface zone, photostability, and targeted delivery. The FA receptors guide the complexes perfectly through the cancer cells, and the drug is released as a result of the acidic pH or heat.Liang et al.251 synthesized another shelled GQD nanocomposite by encrusting GQDs–DOX or MeB–DOX into the center of bovine serum albumin (BSA) grafted PLGA core–shell NPs (GQDs@DOX/PB and MeB@DOX/PB NPs). MeB acts as a photosensitizer for PDT and produces ROS under a specific wavelength of light to kill cancer cells.
Besides pH-dependent DOX delivery, this nanocarrier can efficiently eliminate HeLa and MCF-7 cancer cell lines when exposed to NIR light (photothermal therapy) and with single oxygen production (photodynamic therapy).
A further investigation was designed by Ding et al.252 resulting in the construction of an NGO-based cancer-targeting nanoparticle as a potential drug delivery agent through noncovalent functionalization via cucurbit [7] uril (CB[7]). Accordingly, CB [7] was loaded on NGO, and the resultant NGO-CB [7] was grafted with a photosensitizer (chlorin e6) and a hypoxia-responsive prodrug (AQ4N, banoxantrone dihydrochloride). Following that, a CB[7] guest (OX, oxaliplatin) and a CD44 targeting molecule that elevates biocompatibility, ADA-hyaluronic acid (ADA-HA), were loaded. Owing to the presence of NGO/Ce6, this nanoplatform may operate as a PTT-PDT agent alongside a dual-chemotherapy agent due to OX and AQ4N for the treatment of L02 (human fetal hepatocyte line) and B16 (murine melanoma) cells. This drug delivery platform provides a promising multi-modality cancer therapy system, both in vivo and in vitro.
As previously stated, medication resistance may pose significant complications over the course of cancer treatment. Drug resistance in gastric cancer (GC), for example, is caused by P-glycoprotein (P-gp) activity pumping out PTX. Thus, GUO et al.187 proposed that the deactivation of this pump may simplify GC treatment. They constructed a triple-purpose, pH-sensitive drug delivery nanocarrier composed of GO-PEG shelled with oxidized sodium alginate (OSA), and then grafted it with PTX (PTX@GO-PEG-OSA). It is well-established that most cellular pumps need adenosine triphosphate (ATP) to function; as a result, a deficiency of ATP is synonymous with an absence of pumping. Accordingly, elevated NIR irradiation increased heat and ROS production in cancer cells and consequently damaged the enzymes in mitochondria, so the ATP generation was lowered, and hence P-gp activity was suppressed. P-gp inhibition halts multidrug resistance in cancer cells, restoring chemotherapeutic susceptibility to PTX-resistant GC cells (HGC-27/PTX), and thus cell death occurs.
Above all, we may conclude that PDT/PTT coupled with chemotherapy may effectively thermally ablate cancer cells targeted by graphene's inherent NIR absorption capabilities. This graphene-composed vehicle seems promising in cancer therapy.
4.8 Chemo/fluorescence theranostics
Besides all its marvelous features as a transporter, the graphene family possesses fluorescence properties that aid in its bioimaging applications as shown in our previous work19 where we have studied HA-GQDs’ application as a drug delivery and cancer cell imaging agent. Nanocarriers based on graphene derivatives can be monitored when used as drug delivery agents, in vivo and in vitro. Non-invasive imaging, which utilizes fluorescence microscopy and flow cytometry, helps us keep track of the nanocarriers and even the amount of drug release.253In an investigation designed by Esmaeili et al.,253 it was demonstrated that the fluorescence light produced by GO could follow a turn “on/off” procedure. In this study, they employed an aptamer (MUC1) in their developed nanocarrier that not only delivered DOX to breast, colon (MUC+), and hepatic (MUC−) cancer cells but also served as a key for GO fluorescence. As predicted, the cellular toxicity of this nanocarrier was more significant in MUC+ (HT-29 and MCF-7) cancer cells.
In another project conducted by Wu et al.,54 first, GQDs were doped with sulfur (SGQD). Following that, a layered double hydroxides (LDHs) and etoposide (VP16) were loaded as visualization and chemotherapeutic agents. The VP16 carrying rate is reported at 28% in the LDH@SGQD-VP16 nanocomplex. Besides, it features a pH-dependent mode of drug release which facilitates medication release in the tumor environment. The mentioned nanocomplex is endowed with VP16-enhanced curing properties, including the ability to protect normal cells from drug damage, increase apoptosis, and inhibit tumor cell proliferation (tested in vitro). To sum up, this nanocomplex was 2.7 times more effective in targeting and killing HGC-27 tumor cells than VP16 alone, because of properties like PH-dependant drug release and induced apoptosis.
By using cancer cell-targeting peptides, the tumor targeting approach becomes more efficient. In a project, Yu et al.120 developed a tri-functional GQD-based nanocomposite for treating nasopharyngeal carcinoma and used an anti-EGFR peptide named GE11 to target EGFRs on CNE-2 (nasopharyngeal carcinoma cell line) (GQDs@GE11). Two chemotherapeutic drugs (cisplatin (CDDP) and DOX) were loaded on the GQDs@GE11 surface for better cancer cell elimination. When carrying and releasing DOX, differences in the emission and excitation spectrum of GQDs were detected, which can be used for sensitive detection of drug release in single cells. The carriers were capable of transmitting 67 mg g−1 of DOX and 50 mg g−1 of CDDP. The overall results indicate that this nanocarrier possesses good tumor targeting and cancer cell inhibition features when used to treat nasopharyngeal cancer.
To increase GQDs’ fluorescence stability Sheng et al.263 proposed chitosan-wrapped GQDs grafted with CYT (anticancer drug). The GQDs-CYT were shelled in chitosan gels to form a composite with pH-dependent drug release. Moreover, the fluorescence stability of GQDs in the presence of chitosan gel may be due to inhibited agglomeration caused by chitosan gel.
In conclusion, GQDs’ fluorescence property is another positive point for their usage in bioimaging and combined therapies for cancer treatment.
5. Toxicology aspect
The employment of graphene based biomaterials in in vivo biomedicine and cancer treatment has always been a matter of contention. Although the toxicity of graphene derivatives for bacteria and cancer cells is a desired quality, due to concerns over their potential toxicity, and the lack of knowledge regarding their metabolism, and long term effects on various body cell types, tissues, and organs, their employment in biomedicine may be severely restricted.264The toxicity of graphene based materials is mainly based on their chemical response to the environment which is fundamentally dependent on their preparation process, and to be specific, on the additive materials and the choice of precursors, in the synthesis process. Furthermore, toxicological and biocompatibility considerations for graphene derivatives include their surface chemistry, size, dose, production technique, and degradation residues that can affect human health directly or indirectly.265–268
Determining and researching these properties is crucial when discussing the use of these nanomaterials in biomedicine, since even a short period of adjacency with body cells and tissues can lead to inflammation, irritation, toxicity, and teratogenicity.269,270 The fact that graphene derivatives can elicit systemic effects should not be neglected, since several processes will be implicated, including absorption, distribution in different organs, and excretion. Even more important, in in vivo therapies, the duration of exposure to nanomaterials is far greater which can lead to genotoxic, epigenetic, and carcinogenic effects or even blood hemolysis, thrombosis, and coagulation.265,266
Recent investigations on the toxicity and physiological role of graphene nanoparticles have shown a wide range of conclusions with a focus on how small changes in their structure may change their properties. Some studies have stated that low concentrations of GO can enter the blood circulation and damage many organs such as the liver, brain,271 kidneys,272 and lungs.273 It is also shown that GO can enter maternal milk, cross the blood–brain and placental barrier and even harm the fetus in many ways. However, others indicate the improbability of absorbance of GO derivatives and GQDs that are functionalized with PEG, into blood, and their rapid excretion through faeces.
Both in vivo and in vitro experiments are crucial for determining the safety of these nanomaterials. In the following sections, a brief discussion is devoted to in vitro and in vivo toxicity of graphene derivatives.
5.1 In vitro toxicity
Before running in vivo tests, every biological carrier should be tested in vitro for its clinical efficacy to be evaluated; that being the case, usually different organelle functions and oxidative stress response would be evaluated. Various factors can determine the graphene-derived nanomaterials’ cytotoxicity namely their size, dosage, time of exposure, and concentration, which accordingly can affect their internalization process. Graphene-based nanomaterials are hazardous in a variety of cell lines, with effects ranging from organelle death to alterations in the cells’ ability to operate normally. Damage to the plasma membrane and mitochondria has been seen not only in PC12 and HepG2 cell lines but also in normal cells exposed to these nanomaterials.274 A further study found that when rat macrophages were exposed to graphene, reactive oxygen species (ROS) production increased.274,275 The translation of microRNAs has also been shown to be influenced by them in a few cell lines.276 Aside from cancer, geno-toxicity is a less prevalent but nonetheless important side effect of graphene exposure.277For instance, GQDs have been reported to cause oxidative stress which affects cell DNA (in vitro).278 Testing the nanomaterials in living organisms, or in vivo, will follow in vitro experiments to ensure their safety before they may be used in biomedical treatments.
5.2 In vivo toxicity
For imitating the human body, animal models such as primates and mice are used as the human body mimicry models for large-scale in vivo screening of nanomaterials’ function and their effects on organelles and cytoplasmic membrane integration. In one study, the neurodevelopmental toxicity of GO was tested on zebrafish, due to its correspondence with the human genome.279 This research results demonstrated that even low dosages of GO, 10 μg mL−1, could cause side effects like decreased hatching rate and increased behavioral hypoactivity which is a probable result of elevated oxidative stress. Graphene-based nanomaterials can also cause cellular membrane disruption. For example, the sharp edges of GO can damage sperm cell membranes through physical contact.280 In another study conducted by Wen et al.281 the long-term effects of GO distribution were observed for half a year in vivo. The outcomes displayed that GO was responsible for lung and spleen chronic inflammation. Also, cell membrane damage was noticed as the cause of brachychronic liver injuries. Furthermore, the dosage and the duration of treatment with these nanomaterials can also affect the side effects associated with pulmonary parameters such as lung injury, resulting from Nabil and coworkers’ project.282Another drawback of graphene derivatives is their strong protein adsorption capacity which is mainly due to their vast surface area. Because of the potential for adverse repercussions, including the nanoparticle's inability to carry out its intended therapeutic role (such as drug delivery), linkage with proteins is a concern when discussing the biosafety and toxicity of these materials. However, graphene derivatives’ binding capacity is not necessarily seen as a drawback, as they have been used as a promising agent in protein purification. Additional toxicity reduction and expanded drug delivery potential can be achieved by pre-functionalizing graphene derivatives with a specific protein.283
Another consequence of protein adsorption on graphene derivatives, which causes the nanoparticle to grow in size, is the blocking of capillaries. Also, alteration in the protein structure after linking to graphene-based nanoparticles’ surface is another potential source of unexpected consequences. A further major concern that must be addressed when injecting these nanoparticles into the bloodstream is the potential for hemolysis to occur. It has been shown via research that the hematotoxicity of GO decreases with increasing particle size and that even when the particles coalesce, they are less likely to cause hemolysis.284,285 Regarding the biodistribution of these nanomaterials, research has shown that larger GO derivatives (1–5 μm) are stored in the lungs, whilst smaller ones (110–500 nm) are stored in the liver. But it has been shown that GO levels below 50 mg L−1 pose no danger to cells.286
As specified by the European Food Safety Authority (EFSA),287 the modified 90 day toxicity test is the minimum criterion for in vivo toxicity assessment of an ingested nanomaterial. However, to the best of our knowledge, the scientific literature is devoid of research with exposure durations of 90 days or more following Organization for Economic Co-operation and Development (OECD) protocols.264 Due to their short exposure duration, the published studies are insufficient for assessing the potential sub-chronic toxicity of this substance. Nonetheless, these investigations might shed light on appropriate dosages and target organs. The gastrointestinal system was the primary focus of the investigations conducted in this field, while a few papers have expanded their scope to include studies on the liver,288 kidneys,289 gut microbiota, and reproduction system.270 The results were inconclusive due to many reasons such as the usage of varying dosages and substances. However, the mechanisms used in causing toxicity were found to be apoptosis, oxidative damage, and inflammation that could result in increased gut permeability, decreased number of intestinal crypts, shorter villi, or histopathological abnormalities. In terms of reproductive and developmental toxicity, oral consumption of GO in mice during gestational days 7–16 resulted in the lowered weight of dam and living fetuses, an increase in fetal mortality rates, and delayed skeletal development, and these effects were shown to be dose-dependent.270 Graphene derivatives may also be orally consumed in other ways due to their probable presence in all levels of the food chain. We must keep in mind that the discharge of graphene-based nanomaterials into the water and soil near industrial sites generating graphene derivatives, in particular GO, can be harmful to the ecosystem and its organisms. Thus, GO being consumed by human beings and various other organisms is inevitable because of its water solubility leading to its existence in all levels of the food chain, which can have detrimental effects on the environment265,266 such as reducing the soil bacteria in number and decreasing their viability and activity.267
Other smaller-sized graphene derivatives, such as GQDs, demonstrated high organ uptake with a minimum of 25% in the small intestine and a minimum of 90% in kidneys in which in vivo mutagenicity and A:T to G:C alterations and frame shift mutations have also been noted.290 Controversial results were also expressed that insisted on no toxic effects of graphene-based nanoparticles.264
5.3 Gap between research and practical applications
Today, a variety of strategies are available for minimizing graphene nanoparticles’ negative effects such as surface modification and functionalization, investigating the molecular processes of toxicity, using less toxic graphene derivatives, or finding new sources and green pathways for synthesizing more biocompatible nanoparticles. Using glucose and deionized water as a precursor, GQDs were synthesized by de Menezes and co-workers through a low-price efficient procedure, in which the constructed GQDs were reported to be generally safe but at the same time dose-based mutagenic.290 However, graphene-based nanomaterials should be evaluated regarding their cytotoxicity and biocompatibility because even a small change in the temperature of the synthesis process or dosage can make a huge difference in their properties.267,286Finally, although some research suggests that graphene-based nanomaterials are safe for biological uses, the findings of more recent toxicity studies have cast doubt on this. More research following international norms is needed to investigate the safety of these remarkable nanoparticles for medical employment, as in vivo results may not be accurate due to conflicts in material utilization or duration of investigations.
6. Conclusion and future remarks
Every day, new cancer therapy methods are being developed due to the ever-increasing rate of cancer death and, consequently, demand for cancer therapeutics. Recently, scientists have come up with new approaches to cancer treatment, using graphene derivatives as therapeutic carriers. Graphene derivatives have considerable potential as gene/drug delivery platforms for cancer therapy and can address the concerns around defects of viral vectors such as carcinogenicity and immunogenicity due to graphene's unique characteristics such as vast surface area, high stability, optical and photoluminescence properties, and easy and low-cost functionalization. On top of all the impressive qualities of graphene-based nanomaterials, there are challenges associated with their use in biomedicine that must be overcome.Critical problems that need to be resolved are graphene derivatives’ toxicology concerns and the immune system's response to the presence of these nanocarriers in the body fluids or tissues. These symptoms may include inflammatory responses in the lungs and kidneys, decreasing heart rate, embryonic development problems, affecting gut and colon morphology and microbiota diversity, and so on. These are some of the obstacles in the way of promoting the in vitro tests to in vivo remediation. It is evident that in vitro experiments can’t mimic a natural body environment, and these tests are vital for the progression of the nanotherapeutics investigation in animals and human bodies for further improvements. To assess the long-term impact of nanoparticles on tissue and organ function, in vivo tests that adhere to OECD requirements and last 90 days or longer are essential. Therefore, more precise information on their toxicology, the potential for application in biomedical treatments, and means of reducing toxicity might be made available.
To achieve this goal, the functionalization of graphene-based nanomaterials by polymers such as PEI, PEG, chitosan, polydopamine, etc., peptides, and dendrimers like PAMAM can be suggested. Moreover, multi-functionalization and employing green precursors and green procedures for graphene derivatives’ synthesis can be among the most favorable solutions because they can endow them with new properties, such as high biocompatibility, low systemic toxicity, and the ability to escape the immune system. Moreover, dual delivery of therapeutics such as gene and drug co-delivery, dual drug delivery, and dual gene delivery are primary methods of increasing the efficiency of therapy while producing synergistic effects in methods such as gene/drug delivery combined with PTT, PDT, bioimaging, etc. which can boost the therapeutic effects of these nanocarriers. Moreover, other than targeted delivery, the transfer process must be done in the shortest time possible without the cargo getting damaged, leaking, or causing systemic toxicity, which can be soothed even more with external guidance such as a magnet, laser, etc.
Another possible solution to increase in vivo applications is the targeted delivery of cancer therapeutics by introducing suitable targeting peptides/protein/molecules which can guide the nanocarriers directly to their destination. For these means, analyzing cancer proteomics can be a good help in finding specific surface biomarkers overexpressed in each cancer type and thus developing anticancer nanocarriers.
In this review, we generally focused on recent advances in graphene-based gene/drug delivery systems that are expected to facilitate the development of innovative and efficient cancer therapy systems that can overcome current issues. Even though there has been a tremendous amount of research, just a few graphene-based medications have been used in clinical trials due to systemic toxicity and uncertainty of long-term outcomes. Hence, there is an immediate need to implement novel green methods for synthesizing green-graphene derivatives that are both more biocompatible and less hazardous. In addition, providing standard protocols and specific standard materials for graphene-based nanomaterial synthesis are also other suggestions to make these nanomaterials safer for biomedical usage, as even small changes in time, precursors, functionalizing molecules, and temperature can result in physiochemical changes in the synthesized nanoparticles.
Despite what has been mentioned, this scenario can be improved by devoting efforts to studying the tumor microenvironment, signaling pathways, and the immune system's role in carcinogenesis and cancer therapy.
Abbreviation
| 2D | Two dimensional |
| 4-HC | 4-Hydroxy coumarin |
| 5-FU | 5-Fluorouracil |
| ADH | Adipicdihydrazide |
| ALL | Acute lymphocytic leukemia |
| AML | Acute myeloid leukemia |
| ATP | Adenosine triphosphate |
| Au-NPs | Gold nanoparticles |
| AUNRs | Gold nanorods |
| BPEI | Branched polyethyleneimine |
| CA | Chlorogenic acid |
| Chemo | Chemotherapy |
| CIS | Cisplatin |
| CMC | Carboxymethylcellulose |
| CML | Chronic myelogenous leukemia |
| CP | Cisplatin |
| CPG | Cytosine-phosphate-guanine |
| CPN | Chitosan-g-poly(N-isopropylacrylamide) |
| CPPs | Cell penetrating peptides |
| cRGDfV | Cyclo(Arg-Gly-Asp-DPhe-Val) |
| CRISPR-Cas | Clustered regulatory interspaced short tandem repeats (CRISPR)-associated protein |
| CS | Chitosan |
| CTPs | Cell targeting peptides |
| CUR | Curcumin |
| CXCR4 | C-X-C chemokine receptor type 4 |
| CY3 | Cyanine dye |
| DA | Dopamine |
| DEX | Dextran |
| DNA | Deoxyribonucleic acid |
| DOX | Doxorubicin |
| dsDNA | Double stranded DNA |
| ECM | Extracellular matrix |
| EGFR | Epidermal growth factor receptor |
| FA | Folic acid |
| F-FDG | F-fluorodeoxyglucose |
| FGO | Fluorinated graphene oxide |
| fMLP | N-Formylmethionyl-leucyl-phenylalanine |
| G0 | Gap phase |
| GFP | Green fluorescent protein |
| Glut-1 | Glucose transporter-1 |
| GM | Gelatin microsphere |
| GNR | Graphene nanoribbon |
| GO | Graphene oxide |
| GPF | GO-poly-L-lysine hydrobromide/folic acid |
| GPND | GA-PEG-NGO-Dendrimer |
| GPPF | FA, R8, and PEG-diamine multifunctionalized GO |
| GQD | Graphene quantum dot |
| GS/LB | Mesoporous silica-coated rGO/lipid bilayer |
| H2O | Water |
| HAP | Nanoscale hydroxyapatite |
| HPAA | Hyperbranched poly(amido amine) |
| HPG | Hyperbranched polyglycerol |
| hTERT | Human telomerase reverse transcriptase |
| IC50 | Half-maximal inhibitory concentration |
| ICG | Indocyanine green |
| IL | Interleukin |
| IONPs | Iron oxide nanoparticles |
| lncRNAs | Long-noncoding RNAs |
| LSPR | Localized surface plasmon resonance |
| MA | Methyl acrylate |
| MB | Molecular beacon |
| MeB | Methylene blue |
| MGO | Magnetic graphene oxide |
| miRNA, mir | Micro RNA |
| MitP | Mitochondrion targeting peptide |
| MitX | Mitoxantrone |
| MRI | Magnetic resonance imaging |
| mRNA | Messenger RNA |
| MTX | Methotrexate |
| ncRNAs | Noncoding RNAs |
| NGO | Nano graphene oxide |
| NIR | Near infrared |
| nm | Nanometer |
| NPC | Nasopharyngeal carcinoma |
| NPs | Nanoparticles |
| NRs | Nanorods |
| O2 | Oxygen |
| O-GNRs | Oxidized graphene nanoribbons |
| PAH9 | 9-Hydroxy coumarin |
| PAMAM | Polyamidoamine dendrimer |
| PB | Polymeric brush |
| PEGA | Polyethylene glycol bis amine |
| PC3 | Prostate cancer cell line |
| PCA | Protocatechuic acid |
| PCH | Poly(epichlorohydrin) |
| PDA | Polydopamine |
| pDNA | Plasmid DNA |
| PDT | Photodynamic treatment |
| PEG | Poly(ethylene glycol) |
| PEI | Polyethylenimine |
| pH | Potential of hydrogen |
| PHEMA | Polyhydroxyethyl methacrylate |
| PL | Phospholipid |
| P-l-Arg | Poly-L-arginine |
| PLGA | Poly(D,L-lactic-co-glycolic acid) |
| PLL | Poly-L-lysine |
| PNA | Peptide nucleic acid |
| PTT | Photothermal therapy |
| PTX | Paclitaxel |
| PV7 | PKKKRKV |
| PVP | Poly N-vinylpyrrolidone |
| QSR | Quercetin |
| R8 | Octaargenine |
| RBC | Red blood cell |
| RGD | Arginine-glycine-aspartic acid |
| rGO | Reduced graphene oxide |
| RNA | Ribonucleic acid |
| RNAi | Interfering RNA |
| ROS | Reactive oxygen species |
| RXF | Raloxifene hydrochloride |
| shRNA | Small hairpin RNA |
| siRNA | Small interfering RNA |
| SN38 | 7-Ethyl-10-hydroxycamptothecin |
| SPIONs | Superparamagnetic iron oxide nanoparticles |
| ssDNA | Single-stranded DNA |
| STAT3 | Signal transducer and activator of transcription 3 |
| TALENs | Transcription activator-like effector nucleases |
| TAT | Transactivator of transcription |
| TAT-NGs | Nucleus targeting TAT peptides |
| TFGP | Tf/FA-GO-PF68 |
| TGF-β | Transforming growth factor beta |
| TLR9 | Toll-like receptor 9 |
| TMC | Trimethyl chitosan |
| TNF-α | Tumor necrosis factor alpha |
| TOP2 | Topoisomerase-II |
| WBC | White blood cell |
| ZFNs | Zinc-finger nucleases |
| ZOL | Zoledronic acid |
Data availability
Not applicable for this study.Author contributions
Negin Borzoee Moghadam wrote the first draft of the paper. Matin Mahmoudifard has a role in supervision and writing – review & Editing paper. All other authors contributed to drafting the first version of the manuscript. All authors participated in writing modified versions and read and approved the final manuscript.Animal research
Not applicable for this study.Consent to participate
Not applicable for this study.Consent to publish
Not applicable for this study.Conflicts of interest
There is no conflict of interest to declare.Acknowledgements
This study was made possible by a grant from the National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran.References
- H. Sung,
et al., Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA: Cancer J. Clin., 2021, 71(3), 209–249 Search PubMed
.
- R. A. Weinberg, How cancer arises, Sci. Am., 1996, 275(3), 62–70 CrossRef CAS PubMed
.
- A. Sudhakar, History of cancer, ancient and modern treatment methods, J. Cancer Sci. Ther., 2009, 1(2), 1 Search PubMed
.
- Z. Cheng,
et al., Nanomaterials for cancer therapy: Current progress and perspectives, J. Hematol. Oncol., 2021, 14(1), 1–27 CrossRef PubMed
.
- F. Alemi,
et al., Graphene oxide and reduced graphene oxide: Efficient cargo platforms for cancer theranostics, J. Drug Delivery Sci. Technol., 2020, 60, 101974 CrossRef CAS
.
- B. S. Dash,
et al., Functionalized reduced graphene oxide as a versatile tool for cancer therapy, Int. J. Mol. Sci., 2021, 22(6), 2989 CrossRef CAS PubMed
.
- C. Kher and S. Kumar, The Application of Nanotechnology and Nanomaterials in Cancer Diagnosis and Treatment: A Review, Cureus, 2022, 14(9) Search PubMed
.
- E. Ekrami, M. Khodabandeh Shahraky, M. Mahmoudifard, M. S. Mirtaleb and P. Shariati,
et al., Biomedical applications of electrospun nanofibers in industrial world: a review, Int. J. Polym. Mater. Polym. Biomater., 2022, 72(7), 561–575 CrossRef
.
- M. Mahmoudifard, A. M. Shoushtari and M. Shanehsaz, Quantum dot/polyvinyl alcohol composite nanofibers membrane as highly sensitive fluorescence quenching-based sensors, Fibers Polym., 2014, 15(9), 1797–1803 CrossRef CAS
.
- S. Zamanlui,
et al., Enhanced chondrogenic differentiation of human bone marrow mesenchymal stem cells on PCL/PLGA electrospun with different alignments and compositions, Int. J. Polym. Mater. Polym. Biomater., 2018, 67(1), 50–60 CrossRef CAS
.
- A. Aghebati-Maleki,
et al., Nanoparticles and cancer therapy: Perspectives for application of nanoparticles in the treatment of cancers, J. Cell. Physiol., 2020, 235(3), 1962–1972 CrossRef CAS PubMed
.
- D. Sahu,
et al., Graphene, graphene-derivatives and composites: fundamentals, synthesis approaches to applications, J. Compos. Sci., 2021, 5(7), 181 CrossRef CAS
.
- E. Ekrami,
et al., Potential Diagnostic Systems for Coronavirus Detection: a Critical Review, Biol. Proced. Online, 2020, 22, 21 CrossRef CAS PubMed
.
- F. Barati, M. Avatefi, N. Borzooee Moghadam, S. Asghari, E. Ekrami and M. Mahmoudifard, A review of graphene quantum dots and their potential biomedical applications, J. Biomater. Appl., 2022, 37(7), 1137–1158, DOI:10.1177/08853282221125311
.
- M. Mahmoudifard,
et al., The different fate of satellite cells on conductive composite electrospun nanofibers with graphene and graphene oxide nanosheets, Biomed. Mater., 2016, 11(2), 025006 CrossRef PubMed
.
- S. Hosseinzadeh,
et al., The nanofibrous PAN-PANi scaffold as an efficient substrate for skeletal muscle differentiation using satellite cells, Bioprocess Biosyst. Eng., 2016, 39(7), 1163–1172 CrossRef CAS PubMed
.
- F. Barati, A. Arpanaei and M. Mahmoudifard, Highly efficient detection of cancer-derived exosomes using modified core–shell electrospun nanofibers as a capture substrate and antibody immobilized-graphene quantum dots as a signaling agent, Anal. Methods, 2020, 12(28), 3670–3681 RSC
.
- A. M. Barati F, N. B. Moghadam, S. Asghari, E. Ekrami and M. Mahmoudifard, A review of graphene quantum dots and their potential biomedical applications., J. Biomater. Appl., 2022, 37(7), 1137–1158 CrossRef PubMed
.
- N. Vahedi, F. Tabandeh and M. Mahmoudifard, Hyaluronic acid- Graphene Quantum Dot Nanocomposite: Potential Target Drug Delivery and Cancer Cell Imaging, Biotechnol. Appl. Biochem., 2021, 69(3), 1068–1079 CrossRef PubMed
.
- M. M. Sahar Asghari, The detection of the captured CTCs on the core shell nanofibrous membrane using hyaluronic acid-functionalized graphene quantum dots, J. Biomed. Mater. Res., Part B, 2023, 1–12 Search PubMed
.
- S. Asghari,
et al., Nanostructure Materials: Efficient Strategies for Circulating Tumor Cells Capture, Release, and Detection, Biotechnol. Bioprocess Eng., 2021, 26(4), 529–545 CrossRef CAS
.
- S. Asghari,
et al., The role of the nanofibers in lateral flow assays enhancement: a critical review, Int. J. Polym. Mater. Polym. Biomater., 2022, 1–14, DOI:10.1080/00914037.2022.2090360
.
- S. R. Ur Rehman,
et al., Graphene Oxide
Loaded Hydrogel for Enhanced Wound Healing in Diabetic Patients, Annu. Int. Conf. IEEE Eng. Med. Biol. Soc., 2019, 2019, 3943–3946 Search PubMed
.
- I. H. Ali,
et al., Antimicrobial and Wound-Healing Activities of Graphene-Reinforced Electrospun Chitosan/Gelatin Nanofibrous Nanocomposite Scaffolds, ACS Omega, 2022, 7(2), 1838–1850, DOI:10.1021/acsomega.1c05095
.
- A. Shafiee, S. Iravani and R. S. Varma, Graphene and graphene oxide with anticancer applications: Challenges and future perspectives, MedComm, 2022, 3(1), e–118, DOI:10.1002/mco2.118
.
- L. Tang,
et al., Versatile carbon nanoplatforms for cancer treatment and diagnosis: strategies, applications and future perspectives, Theranostics, 2022, 12(5), 2290 CrossRef CAS PubMed
.
- H. Sachdeva,
et al., Graphene-based nanomaterials for cancer therapy, Mater. Today: Proc., 2021, 43, 2954–2957 CAS
.
- M. Hoseini-Ghahfarokhi,
et al., Applications of graphene and graphene oxide in smart drug/gene delivery: is the world still flat?, Int. J. Nanomed., 2020, 15, 9469 CrossRef CAS PubMed
.
- T. Gong,
et al., Triformyl cholic acid and folic acid functionalized magnetic graphene oxide nanocomposites: Multiple-targeted dual-modal synergistic chemotherapy/photothermal therapy for liver cancer, J. Inorg. Biochem., 2021, 223, 111558 CrossRef CAS PubMed
.
- V. P. Jain,
et al., Advanced functionalized nanographene oxide as a biomedical agent for drug delivery and anti-cancerous therapy: a review, Eur. Polym. J., 2021, 142, 110124 CrossRef CAS
.
- H. Hashemzadeh and H. Raissi, Understanding loading, diffusion and releasing of doxorubicin and paclitaxel dual delivery in graphene and graphene oxide carriers as highly efficient drug delivery systems, Appl. Surf. Sci., 2020, 500, 144220 CrossRef CAS
.
- M. A. Yunus,
et al., Stimulation of innate and adaptive immune cells with graphene oxide and reduced graphene oxide affect cancer progression, Arch. Immunol. Ther. Exp., 2021, 69(1), 1–16 CrossRef PubMed
.
- H. Y. Kim,
et al., In vivo visualization of endogenous miR-21 using hyaluronic acid-coated graphene oxide for targeted cancer therapy, Biomaterials, 2017, 121, 144–154 CrossRef PubMed
.
- M. Sosnowska,
et al., Graphene oxide nanofilm and chicken embryo extract decrease the invasiveness of HepG2 liver cancer cells. Cancer, Nanotechnology, 2021, 12(1), 1–33 Search PubMed
.
- A. Rhazouani,
et al., Synthesis and toxicity of graphene oxide nanoparticles: A literature review of in vitro and in vivo studies, BioMed Res. Int., 2021, 2021, 5518999 Search PubMed
.
- K. Moloudi,
et al., Iron oxide/gold nanoparticles-decorated reduced graphene oxide nanohybrid as the thermo-radiotherapy agent, IET Nanobiotechnol., 2020, 14(5), 428–432 CrossRef PubMed
.
- G. Wei,
et al., Photothermal and photodynamic therapy reagents based on rGO-C 6 H 4-COOH, RSC Adv., 2016, 6(5), 3748–3755 RSC
.
- H. Kim and W. J. Kim, Photothermally controlled gene delivery by reduced graphene oxide–polyethylenimine nanocomposite, Small, 2014, 10(1), 117–126 CrossRef CAS PubMed
.
- S. Dhanavel,
et al., 5-Fluorouracil and curcumin co-encapsulated chitosan/reduced graphene oxide nanocomposites against human colon cancer cell lines, Polym. Bull., 2020, 77(1), 213–233 CrossRef CAS
.
- C.-H. Lin, Y.-C. Chen and P.-I. Huang, Preparation of multifunctional dopamine-coated zerovalent iron/reduced graphene oxide for targeted phototheragnosis in breast cancer, Nanomaterials, 2020, 10(10), 1957 CrossRef CAS PubMed
.
- V. Mirzaie,
et al., Nano-Graphene Oxide-supported APTES-Spermine, as Gene Delivery System, for Transfection of pEGFP-p53 into Breast Cancer Cell Lines, Drug Des., Dev. Ther., 2020, Volume 14, 3087–3097 CrossRef PubMed
.
- B. Ma, Y. Nishina and A. Bianco, A glutathione responsive nanoplatform made of reduced graphene oxide and MnO2 nanoparticles for photothermal and chemodynamic combined therapy, Carbon, 2021, 178, 783–791 CrossRef CAS
.
- A. P. Johnson, H. Gangadharappa and K. Pramod, Graphene nanoribbons: A promising nanomaterial for biomedical applications, J. Controlled Release, 2020, 325, 141–162 CrossRef CAS PubMed
.
- O. V. Zakharova,
et al., Graphene nanoribbons: Prospects of application in biomedicine and toxicity, Nanomaterials, 2021, 11(9), 2425 CrossRef CAS PubMed
.
- H.-C. C. Foreman,
et al., Gene delivery to mammalian cells using a graphene nanoribbon platform, J. Mater. Chem. B, 2017, 5(12), 2347–2354 RSC
.
- S. M. Mousavi,
et al., Graphene nano-ribbon based high potential and efficiency for DNA, cancer therapy and drug delivery applications, Drug Metab. Rev., 2019, 51(1), 91–104 CrossRef CAS PubMed
.
- S. M. Chowdhury,
et al., Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme, Nanomedicine, 2015, 11(1), 109–118 CrossRef CAS PubMed
.
- V. Kansara, S. Tiwari and M. Patel, Graphene quantum dots: A review on the effect of synthesis parameters and theranostic applications, Colloids Surf., B, 2022, 217, 112605 CrossRef CAS PubMed
.
- T. A. Tabish,
et al., Graphene quantum dot–based electrochemical biosensing for early cancer detection. Current Opinion in, Electrochemistry, 2021, 30, 100786 CAS
.
- D. Iannazzo, C. Celesti and C. Espro, Recent advances on graphene quantum dots as multifunctional nanoplatforms for cancer treatment, Biotechnol. J., 2021, 16(2), 1900422 CrossRef CAS PubMed
.
- L. Qi,
et al., Biocompatible nucleus-targeted graphene quantum dots for selective killing of cancer cells via DNA damage, Commun. Biol., 2021, 4(1), 1–12 CrossRef PubMed
.
- D. M. Felix,
et al., Graphene quantum dots decorated with imatinib for leukemia treatment, J. Drug Delivery Sci. Technol., 2021, 61, 102117 CrossRef CAS PubMed
.
- H. Yang,
et al., Functionalized graphene oxide as a nanocarrier for multiple suppressive miRNAs to inhibit human intrahepatic cholangiocarcinoma, Nano Sel., 2021, 2(7), 1372–1384 CrossRef CAS
.
- B. Wu,
et al., Trifunctional Graphene Quantum Dot@ LDH Integrated Nanoprobes for Visualization Therapy of Gastric Cancer. Advanced Healthcare, Materials, 2021, 10(16), 2100512 CAS
.
- M. O. Ansari,
et al., Graphene and graphene-based materials in biomedical applications, Curr. Med. Chem., 2019, 26(38), 6834–6850 CrossRef CAS PubMed
.
- J. Sturala,
et al., Chemistry of graphene derivatives: Synthesis, applications, and perspectives, Chem. – Eur. J., 2018, 24(23), 5992–6006 CrossRef CAS PubMed
.
- K. Ghosal and K. Sarkar, Biomedical Applications of Graphene Nanomaterials and Beyond, ACS Biomater. Sci. Eng., 2018, 4(8), 2653–2703 CrossRef CAS PubMed
.
- R. Imani, F. Mohabatpour and F. Mostafavi, Graphene-based nano-carrier modifications for gene delivery applications, Carbon, 2018, 140, 569–591 CrossRef CAS
.
- K. Sarkar, G. Madras and K. Chatterjee, Dendron conjugation to graphene oxide using click chemistry for efficient gene delivery, RSC Adv., 2015, 5(62), 50196–50211 RSC
.
- N. Yadav,
et al., Stable dispersions of covalently tethered polymer improved graphene oxide nanoconjugates as an effective vector for siRNA delivery, ACS Appl. Mater. Interfaces, 2018, 10(17), 14577–14593 CrossRef CAS PubMed
.
- F. Wang,
et al., Imaging Dendrimer-Grafted Graphene Oxide Mediated Anti-miR-21 Delivery With an Activatable Luciferase Reporter, ACS Appl. Mater. Interfaces, 2016, 8(14), 9014–9021 CrossRef CAS PubMed
.
- L. Zhang,
et al., PEGylated reduced graphene oxide as a superior ssRNA delivery system, J. Mater. Chem. B, 2013, 1(6), 749–755 RSC
.
- X. Yang,
et al., The preparation of functionalized graphene oxide for targeted intracellular delivery of siRNA, J. Mater. Chem., 2012, 22(14), 6649–6654 RSC
.
- J. S. Suk,
et al., PEGylation as a strategy for improving nanoparticle-based drug and gene delivery, Adv. Drug Delivery Rev., 2016, 99, 28–51 CrossRef CAS PubMed
.
- R. C. Cheung,
et al., Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications, Mar. Drugs, 2015, 13(8), 5156–5186 CrossRef CAS PubMed
.
- A. Muxika,
et al., Chitosan as a bioactive polymer: Processing, properties and applications, Int. J. Biol. Macromol., 2017, 105(Pt 2), 1358–1368 CrossRef CAS PubMed
.
- J.-W. Shen,
et al., Molecular dynamics study on the adsorption and release of doxorubicin by chitosan-decorated graphene, Carbohydr. Polym., 2020, 248, 116809 CrossRef CAS PubMed
.
- Z. Jafari,
et al., Synthesis and application of chitosan/tripolyphosphate/graphene oxide hydrogel as a new drug delivery system for Sumatriptan Succinate, J. Mol. Liq., 2020, 315, 113835 CrossRef CAS
.
- J. A. Jaleel,
et al., Carbon dot festooned and surface passivated graphene-reinforced chitosan construct for tumor-targeted delivery of TNF-α gene, Int. J. Biol. Macromol., 2019, 127, 628–636 CrossRef PubMed
.
- Y. Fu,
et al., Polydopamine antibacterial materials, Mater. Horiz., 2021, 8(6), 1618–1633 RSC
.
- J. H. Ryu, P. B. Messersmith and H. Lee, Polydopamine Surface Chemistry: A Decade of Discovery, ACS Appl. Mater. Interfaces, 2018, 10(9), 7523–7540 CrossRef CAS PubMed
.
- M. E. Lynge,
et al., Polydopamine—a nature-inspired polymer coating for biomedical science, Nanoscale, 2011, 3(12), 4916–4928 RSC
.
- X. Liu,
et al., Polyamidoamine dendrimer and oleic acid-functionalized graphene as biocompatible and efficient gene delivery vectors, ACS Appl. Mater. Interfaces, 2014, 6(11), 8173–8183 CrossRef CAS PubMed
.
- E. M. Elmowafy, M. Tiboni and M. E. Soliman, Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles., J. Pharm. Invest., 2019, 49(4), 347–380 CrossRef CAS
.
- J. Liang,
et al., Versatile Nanoplatform Loaded with Doxorubicin and Graphene Quantum Dots/Methylene Blue for Drug Delivery and Chemophotothermal/Photodynamic Synergetic Cancer Therapy, ACS Appl. Bio Mater., 2020, 3(10), 7122–7132 CrossRef CAS PubMed
.
- M. Morga,
et al., Poly-L-Arginine Molecule Properties in Simple Electrolytes: Molecular Dynamic Modeling and Experiments, Int. J. Environ. Res. Public Health, 2022, 19(6), 3588 CrossRef CAS PubMed
.
- J.-P. Sun,
et al., Photoacoustic response optimization of gold nanorods in the near-infrared region, Results Phys., 2022, 34, 105209 CrossRef
.
- Y.-C. Lin,
et al., Multimodal bioimaging using nanodiamond and gold hybrid nanoparticles, Sci. Rep., 2022, 12(1), 5331 CrossRef CAS PubMed
.
- F. Yousefimehr,
et al., Facile fabricating of rGO and Au/rGO nanocomposites using Brassica oleracea var. gongylodes biomass for non-invasive approach in cancer therapy, Sci. Rep., 2021, 11(1), 11900 CrossRef CAS PubMed
.
- G. Wei,
et al., Photothermal and photodynamic therapy reagents based on rGO–C6H4–COOH, RSC Adv., 2016, 6(5), 3748–3755 RSC
.
- S. Karimi and H. Namazi, Simple preparation of maltose-functionalized dendrimer/graphene quantum dots as a pH-sensitive biocompatible carrier for targeted delivery of doxorubicin, Int. J. Biol. Macromol., 2020, 156, 648–659 CrossRef CAS PubMed
.
-
X. J. Lee, et al., Functionalization of Graphene for Nanodelivery of Drugs, in Synthesis, Technology and Applications of Carbon Nanomaterials, ed. S. A. Rashid, R. N. I. Raja Othman, and M. Z. Hussein, 2019, Elsevier, ch. 7, pp. 157–176 Search PubMed
.
- A. Shoari,
et al., Delivery of Various Cargos into Cancer Cells and Tissues via Cell-Penetrating Peptides: A Review of the Last Decade, Pharmaceutics, 2021, 13(9) DOI:10.3390/pharmaceutics13091391
.
- L. Gui,
et al., Cell-Penetrating Peptides and Polymers for Improved Drug Delivery, ChemNanoMat, 2020, 6(8), 1138–1148 CrossRef CAS
.
- C. Martín,
et al., A Biodegradable Multifunctional Graphene Oxide Platform for Targeted Cancer Therapy, Adv. Funct. Mater., 2019, 29(39), 1901761 CrossRef
.
- R. Imani,
et al., Dual-functionalized graphene oxide for enhanced siRNA delivery to breast cancer cells, Colloids Surf., B, 2016, 147, 315–325 CrossRef CAS PubMed
.
- L. Ren,
et al., Functionalized graphene oxide for anti-VEGF siRNA delivery: preparation, characterization and evaluation in vitro and in vivo, RSC Adv., 2017, 7(33), 20553–20566 RSC
.
- S. M. Ghafary,
et al., Simultaneous Gene Delivery and Tracking through Preparation of Photo-Luminescent Nanoparticles Based on Graphene Quantum Dots and Chimeric Peptides, Sci. Rep., 2017, 7(1), 9552 CrossRef PubMed
.
- A. Kloss,
et al., The cell-penetrating peptide octa-arginine is a potent inhibitor of proteasome activities, Eur. J. Pharm. Biopharm., 2009, 72(1), 219–225 CrossRef CAS PubMed
.
- J. Li,
et al., Preparation and Characterization of Functionalized Graphene Oxide Carrier for siRNA Delivery, Int. J. Mol. Sci., 2018, 19(10), 3202 CrossRef PubMed
.
- T. Ren,
et al., Engineered polyethylenimine/graphene oxide nanocomposite for nuclear localized gene delivery, Polym. Chem., 2012, 3(9), 2561–2569 RSC
.
- Z. Abdollahi,
et al., PEGAylated graphene oxide/superparamagnetic nanocomposite as a high-efficiency loading nanocarrier for controlled delivery of methotrexate, J. Biotechnol., 2019, 298, 88–97 CrossRef CAS PubMed
.
- Y. Lu,
et al., Synthesis of a reusable composite of graphene and silver nanoparticles for catalytic reduction of 4-nitrophenol and performance as anti-colorectal carcinoma, J. Mater. Res. Technol., 2021, 12, 1832–1843 CrossRef CAS
.
-
M. Ay, et al., Quercetin, in Nutraceuticals, 2021, Elsevier, pp. 749–755 Search PubMed
.
- M. Alibolandi,
et al., Fabrication of aptamer decorated dextran coated nano-graphene oxide for targeted drug delivery, Carbohydr. Polym., 2017, 155, 218–229 CrossRef CAS PubMed
.
- R. Goswami,
et al., Gene Therapy Leaves a
Vicious Cycle, Front. Oncol., 2019, 9(297) DOI:10.3389/fonc.2019.00297
.
- S. Patil,
et al., The Development of Functional Non-Viral Vectors for Gene Delivery, Int. J. Mol. Sci., 2019, 20(21), 5491 CrossRef CAS PubMed
.
- N. Sayed,
et al., Gene therapy: Comprehensive overview and therapeutic applications, Life Sci., 2022, 294, 120375 CrossRef CAS PubMed
.
- X. M. Han,
et al., Functionalization and optimization-strategy of graphene oxide-based nanomaterials for gene and drug delivery, Am. J. Transl. Res., 2020, 12(5), 1515–1534 CAS
.
- L. Liu,
et al., Recent progress of graphene oxide-based multifunctional nanomaterials for cancer treatment, Cancer Nanotechnol., 2021, 12(1), 18 CrossRef CAS
.
- P.-Y. Lo,
et al., GFP Plasmid and Chemoreagent Conjugated with Graphene Quantum Dots as a Novel Gene Delivery Platform for Colon Cancer Inhibition In Vitro and In Vivo, ACS Appl. Bio Mater., 2020, 3(9), 5948–5956 CrossRef CAS PubMed
.
- H. Sharma and S. Mondal, Functionalized Graphene Oxide for Chemotherapeutic Drug Delivery and Cancer Treatment: A Promising Material in Nanomedicine, Int. J. Mol. Sci., 2020, 21(17), 6280 CrossRef CAS PubMed
.
- A. Baek,
et al., Polyethylene Glycol-Engrafted Graphene Oxide as Biocompatible Materials for Peptide Nucleic Acid Delivery into Cells, Bioconjugate Chem., 2018, 29(2), 528–537 CrossRef CAS PubMed
.
- Y. P. Huang,
et al., Suppression of Breast Cancer Cell Migration by Small Interfering RNA Delivered by Polyethylenimine-Functionalized Graphene Oxide, Nanoscale Res. Lett., 2016, 11(1), 247 CrossRef PubMed
.
- Y. Wang,
et al., Functionalized Folate-Modified Graphene Oxide/PEI siRNA Nanocomplexes for Targeted Ovarian Cancer Gene Therapy, Nanoscale Res. Lett., 2020, 15(1), 57 CrossRef CAS PubMed
.
- S. S. Saravanabhavan,
et al., Graphene oxide functionalized with chitosan based nanoparticles as a carrier of siRNA in regulating Bcl-2 expression on Saos-2 & MG-63 cancer cells and its inflammatory response on bone marrow derived cells from mice, Mater. Sci. Eng., C, 2019, 99, 1459–1468 CrossRef CAS PubMed
.
- M. Kutwin,
et al., MicroRNA Delivery by Graphene-Based Complexes into Glioblastoma Cells, Molecules, 2021, 26(19), 5804 CrossRef CAS PubMed
.
- L. Ou,
et al., Efficient miRNA Inhibitor Delivery with Graphene Oxide-Polyethylenimine to Inhibit Oral Squamous Cell Carcinoma, Int. J. Nanomed., 2020, 15, 1569–1583 CrossRef CAS PubMed
.
- Y. Liu,
et al., A Novel Graphene Quantum Dot-Based mRNA Delivery Platform, ChemistryOpen, 2021, 10(7), 666–671 CrossRef CAS PubMed
.
- H. Yue,
et al., Graphene oxide-mediated Cas9/sgRNA delivery for efficient genome editing, Nanoscale, 2018, 10(3), 1063–1071 RSC
.
- R. Di Santo,
et al., Microfluidic-generated lipid-graphene oxide nanoparticles for gene delivery, Appl. Phys. Lett., 2019, 114, 233701 CrossRef
.
- L. Ren,
et al., Functionalized graphene oxide for anti-VEGF siRNA delivery: preparation, characterization and evaluation in vitro and in vivo, RSC Adv., 2017, 7, 20553–20566 RSC
.
- X. Wang,
et al., Anti-HER2 functionalized graphene oxide as survivin-siRNA delivery carrier inhibits breast carcinoma growth in vitro and in vivo, Drug Des., Dev. Ther., 2018, 12, 2841–2855 CrossRef CAS PubMed
.
- Y. Qu,
et al., Glycyrrhetinic acid-modified graphene oxide mediated siRNA delivery for enhanced liver-cancer targeting therapy, Eur. J. Pharm. Sci., 2019, 139, 105036 CrossRef CAS PubMed
.
- H. Dong,
et al., Multifunctional Poly(L-lactide)-Polyethylene Glycol-Grafted Graphene Quantum Dots for Intracellular MicroRNA Imaging and Combined Specific-Gene-Targeting Agents Delivery for Improved Therapeutics, ACS Appl. Mater. Interfaces, 2015, 7(20), 11015–11023 CrossRef CAS PubMed
.
- K. Vinothini,
et al., Development of biotin molecule targeted cancer cell drug delivery of doxorubicin loaded κ-carrageenan grafted graphene oxide nanocarrier, Mater. Sci. Eng., C, 2019, 100, 676–687 CrossRef CAS PubMed
.
- K. Vinothini,
et al., Folate receptor targeted delivery of paclitaxel to breast cancer cells via folic acid conjugated graphene oxide grafted methyl acrylate nanocarrier, Biomed. Pharmacother., 2019, 110, 906–917 CrossRef CAS PubMed
.
- Y. J. Lu,
et al., Injectable Thermo-Sensitive Chitosan Hydrogel Containing CPT-11-Loaded EGFR-Targeted Graphene Oxide and SLP2 shRNA for Localized Drug/Gene Delivery in Glioblastoma Therapy, Int. J. Mol. Sci., 2020, 21(19), 7111 CrossRef CAS PubMed
.
- Y. J. Lu,
et al., Magnetic Graphene Oxide for Dual Targeted Delivery of Doxorubicin and Photothermal Therapy, Nanomaterials, 2018, 8(4), 193 CrossRef PubMed
.
- C. Yu,
et al., Graphene quantum dots-based targeted nanoprobes detecting drug delivery, imaging, and enhanced chemotherapy of nasopharyngeal carcinoma, Bioeng. Transl. Med., 2022, 7(2), e10270 CAS
.
- S. Izadi,
et al., Codelivery of HIF-1α siRNA and Dinaciclib by Carboxylated Graphene Oxide-Trimethyl Chitosan-Hyaluronate Nanoparticles Significantly Suppresses Cancer Cell Progression, Pharm. Res., 2020, 37(10), 196 CrossRef CAS PubMed
.
- G. Cui,
et al., Graphene-based nanomaterials for breast cancer treatment: promising therapeutic strategies, J. Nanobiotechnol., 2021, 19(1), 211 CrossRef CAS PubMed
.
- E. Keles,
et al., Recent progress in nanomaterials for gene delivery applications, Biomater. Sci., 2016, 4(9), 1291–1309 RSC
.
- C.-H. Lu, C.-L. Zhu, J.-J. Li, J.-J. Liu, X. Chen and H.-H. Yang, Using graphene to protect DNA from cleavage during cellular delivery, Chem. Commun., 2010, 46(18), 3116–3118 RSC
.
- X. Wang,
et al., A persistent luminescence resonance energy transfer-based molecular beacon probe for the highly sensitive detection of microRNA in biological samples, Biosens. Bioelectron., 2022, 198, 113849 CrossRef CAS PubMed
.
- B. M. Wile,
et al., Molecular beacon-enabled purification of living cells by targeting cell type–specific mRNAs, Nat. Protoc., 2014, 9(10), 2411–2424 CrossRef CAS PubMed
.
- S. Zou,
et al., Intercalating methylene blue in molecular beacon for sensitive detection of salivary TNF-α towards early diagnosis of oral cancer, Sens. Diagn., 2022, 1(4), 731–738 RSC
.
- X. H. Peng,
et al., Real-time detection of gene expression in cancer cells using molecular beacon imaging: new strategies for cancer research, Cancer Res., 2005, 65(5), 1909–1917 CrossRef CAS PubMed
.
- K. Liu,
et al., Green and facile synthesis of highly biocompatible graphene nanosheets and its application for cellular imaging and drug delivery, J. Mater. Chem., 2011, 21(32), 12034–12040 RSC
.
- C.-H. Lu, Using graphene to protect DNA from cleavage during cellular delivery, Chem. Commun., 2010, 46(18), 3116–3118, 10.1039/b926893f
.
-
Mitochondrial and Chloroplast Plasmids. Extrachromosomal Elements in Lower Eukaryotes, ed. R. B. Wickner, A. Hinnebusch, A. M. Lambowitz, I. C. Gunsalus, A. Hollaender, Springer, Boston, MA, US, 1987, pp. 81–146 Search PubMed
.
-
K. U. Esser K, C. Lang-Hinrichs, P. Lemke, H. D. Osiewacz, U. Stahl and P. Tudzynski, Plasmids of Eukaryotes: fundamentals and Applications, Springer-Verlag, Berlin, 1986 Search PubMed
.
- J. P. Falese, A. Donlic and A. E. Hargrove, Targeting RNA with small molecules: from fundamental principles towards the clinic, Chem. Soc. Rev., 2021, 50(4), 2224–2243 RSC
.
- G. J. Goodall and V. O. Wickramasinghe, RNA in cancer, Nat. Rev. Cancer, 2021, 21(1), 22–36 CrossRef CAS PubMed
.
- R. Wan,
et al., The target therapeutic effect of functionalized graphene oxide nanoparticles graphene oxide–polyethylene glycol–folic acid-1-pyrenemethylamine hydrochloride-mediated RNA interference of HIF-1α gene in human pancreatic cancer cells, J. Biomater. Appl., 2019, 34(2), 155–177 CrossRef CAS PubMed
.
- A. Babavalian,
et al., Reduced polydopamine coated graphene for delivery of Hset1 antisense as A photothermal and gene therapy of breast cancer, J. Drug Delivery Sci. Technol., 2022, 73, 103462 CrossRef CAS
.
- D. D. Rao,
et al., siRNA vs. shRNA: Similarities and differences, Adv. Drug Delivery Rev., 2009, 61(9), 746–759 CrossRef CAS PubMed
.
- T. R. Brummelkamp, R. Bernards and R. Agami, A system for stable expression of short interfering RNAs in mammalian cells, Science, 2002, 296(5567), 550–553 CrossRef CAS PubMed
.
- P. J. Paddison,
et al., Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells, Genes Dev., 2002, 16(8), 948–958 CrossRef CAS PubMed
.
- P. J. Dexheimer and L. Cochella, MicroRNAs: From Mechanism to Organism, Front. Cell Dev. Biol., 2020, 8, 409 CrossRef PubMed
.
- A. Buruiană,
et al., The Roles of miRNA in Glioblastoma Tumor Cell Communication: Diplomatic and Aggressive Negotiations, Int. J. Mol. Sci., 2020, 21(6), 1950 CrossRef PubMed
.
-
D. L. N. Cox and M. Michael, in Lehninger principles of biochemistry, ed. W. H. Freeman, New York, 5th edn, 2008, p. 1045 Search PubMed
.
- H. Yang,
et al., Functionalized graphene oxide as a nanocarrier for multiple suppressive miRNAs to inhibit human intrahepatic cholangiocarcinoma, Nano Sel., 2021, 2(7), 1372–1384 CrossRef CAS
.
- D. J. Hogan,
et
al., Anti-miRs competitively inhibit microRNAs in Argonaute complexes, PLoS One, 2014, 9(7), e100951 CrossRef PubMed
.
- H. Park, A. Otte and K. Park, Evolution of drug delivery systems: From 1950 to 2020 and beyond, J. Controlled Release, 2022, 342, 53–65 CrossRef CAS PubMed
.
- M. S. Islam,
et al., Direct incorporation of nano graphene oxide (nGO) into hydrophobic drug crystals for enhanced aqueous dissolution, Colloids Surf., B, 2020, 189, 110827 CrossRef CAS PubMed
.
- S. D. Farhangfar,
et al., Evaluating the blood toxicity of functionalized graphene-arginine with anticancer drug ginsenoside Rh2 in balb/c mouse model with breast cancer, Iran. J. Pediatr. Hematol. Oncol., 2022, 12(1), 10–16 Search PubMed
.
- G. Niu,
et al., Melatonin and doxorubicin co-delivered via a functionalized graphene-dendrimeric system enhances apoptosis of osteosarcoma cells, Mater. Sci. Eng., C, 2021, 119, 111554 CrossRef CAS PubMed
.
- S. Bera,
et al., Inhibition of microtubule assembly and cytotoxic effect of graphene oxide on human colorectal carcinoma cell HCT116, Arch. Biochem. Biophys., 2021, 708, 108940 CrossRef CAS PubMed
.
- D. Saminathan,
et al., 5-Fluorouracil and curcumin co-encapsulated chitosan/reduced graphene oxide nanocomposites against human colon cancer cell lines, Polym. Bull., 2020, 77 Search PubMed
.
- M. Xie,
et al., Layer-by-layer modification of magnetic graphene oxide by chitosan and sodium alginate with enhanced dispersibility for targeted drug delivery and photothermal therapy, Colloids Surf., B, 2019, 176, 462–470 CrossRef CAS PubMed
.
- N. F. Rosli,
et al., Graphene Oxide Nanoplatelets Potentiate Anticancer Effect of Cisplatin in Human Lung Cancer Cells, Langmuir, 2019, 35(8), 3176–3182 CrossRef CAS PubMed
.
- K. Buskaran,
et al., Anticancer Molecular Mechanism of Protocatechuic Acid Loaded on Folate Coated Functionalized Graphene Oxide Nanocomposite Delivery System in Human Hepatocellular Carcinoma, Materials, 2021,(4), 14 Search PubMed
.
- G. Boran,
et al., Synergistic effect of graphene oxide and zoledronic acid for osteoporosis and cancer treatment, Sci. Rep., 2020, 10(1), 1–12 CrossRef PubMed
.
- S. Suryaprakash,
et al., Graphene oxide cellular patches for mesenchymal stem cell-based cancer therapy, Carbon, 2018, 129, 863–868 CrossRef CAS
.
- M. Frieler,
et al., Effects of doxorubicin delivery by nitrogen-doped graphene quantum dots on cancer cell growth: experimental study and mathematical modeling, Nanomaterials, 2021, 11(1), 140 CrossRef CAS PubMed
.
- G.-Y. Lee,
et al., Integration of PEG and PEI with graphene quantum dots to fabricate pH-responsive nanostars for colon cancer suppression in vitro and in vivo, FlatChem, 2022, 31, 100320 CrossRef CAS
.
- S. Perumal,
et al., Noncovalent functionalized graphene nanocarriers from graphite for treating thyroid cancer cells, ACS Biomater. Sci. Eng., 2021, 7(6), 2317–2328 CrossRef CAS PubMed
.
- S. Gooneh-Farahani,
et al., A pH-sensitive nanocarrier based on BSA-stabilized graphene-chitosan nanocomposite for sustained and prolonged release of anticancer agents, Sci. Rep., 2021, 11(1), 1–14 CrossRef PubMed
.
- A. Ghamkhari,
et al., Development of a graphene oxide-poly lactide nanocomposite as a smart drug delivery system, Int. J. Biol. Macromol., 2021, 169, 521–531 CrossRef CAS PubMed
.
- S. Han,
et al., Drug-loaded dual targeting graphene oxide-based molecularly imprinted composite and recognition of carcino-embryonic antigen, RSC Adv., 2020, 10(19), 10980–10988 RSC
.
- M. Pooresmaeil and H. Namazi, Fabrication of a smart and biocompatible brush copolymer decorated on magnetic graphene oxide hybrid nanostructure for drug delivery application, Eur. Polym. J., 2021, 142, 110126 CrossRef CAS
.
- J.-F. Shi,
et al., Synthesis and anticancer activity of Boc-Gly-Pro dipeptide-annonaceous acetogenin prodrugs targeting fibroblast activation protein or other hydrolytic enzymes, Med. Chem. Res., 2022, 31(4), 605–616 CrossRef CAS
.
- J. Zhang,
et al., Dual-sensitive graphene oxide loaded with proapoptotic peptides and anticancer drugs for cancer synergetic therapy, Langmuir, 2019, 35(18), 6120–6128 CrossRef CAS PubMed
.
- M. Xie,
et al., The camouflage of graphene oxide by red blood cell membrane with high dispersibility for cancer chemotherapy, J. Colloid Interface Sci., 2021, 591, 290–299 CrossRef CAS PubMed
.
- S. Samadi,
et al., Fabrication of chitosan/poly(lactic acid)/graphene oxide/TiO(2) composite nanofibrous scaffolds for sustained delivery of doxorubicin and treatment of lung cancer, Int. J. Biol. Macromol., 2018, 110, 416–424 CrossRef CAS PubMed
.
- R. Li,
et al., Graphene oxide loaded with tumor-targeted peptide and anti-cancer drugs for cancer target therapy, Sci. Rep., 2021, 11(1), 1–10 CrossRef CAS PubMed
.
- M. Pooresmaeil and H. Namazi, pH-sensitive ternary Fe3O4/GQDs@ G hybrid microspheres; Synthesis, characterization and drug delivery application, J. Alloys Compd., 2020, 846, 156419 CrossRef CAS
.
- Y. Xu,
et al., A novel controllable molecularly imprinted drug delivery system based on the photothermal effect of graphene oxide quantum dots, J. Mater. Sci., 2019, 54(12), 9124–9139 CrossRef CAS
.
- C. Xu,
et al., Nanoarchitectured Graphene Organic Framework for Drug Delivery and Chemo-photothermal Synergistic Therapy, J. Biomater. Appl., 2022, 8853282221108482 Search PubMed
.
- A. Moammeri,
et al., pH-Responsive, Adorned Nanoniosomes for Codelivery of Cisplatin and Epirubicin: Synergistic Treatment of Breast Cancer, ACS Appl. Bio Mater., 2022, 5(2), 675–690 CrossRef CAS PubMed
.
- A. Vasanthakumar,
et al., Design of Bio-Graphene-Based Multifunctional Nanocomposites Exhibits Intracellular Drug Delivery in Cervical Cancer Treatment, ACS Appl. Bio Mater., 2022, 5(6), 2956–2964 CrossRef CAS PubMed
.
- S. A. Makharza,
et al., Magnetic Graphene Oxide Nanocarrier for Targeted Delivery of Cisplatin: A Perspective for Glioblastoma Treatment, Pharmaceuticals, 2019,(2), 12 Search PubMed
.
- Y. Chen,
et al., Multifunctional Graphene-Oxide-Reinforced Dissolvable Polymeric Microneedles for Transdermal Drug Delivery, ACS Appl. Mater. Interfaces, 2020, 12(1), 352–360 CrossRef CAS PubMed
.
- L. H. Wang,
et al., A composite of graphene oxide and iron oxide nanoparticles for targeted drug delivery of temozolomide, Pharmazie, 2020, 75(7), 313–317 CAS
.
- A. S. Abu Lila,
et al., Tamoxifen-loaded functionalized graphene nanoribbons for breast cancer therapy, J. Drug Delivery Sci. Technol., 2021, 63, 102499 CrossRef CAS
.
- A. S. Abu Lila,
et al., Folic acid-conjugated raloxifene-loaded graphene-based nanocarrier: Fabrication, characterization and antitumor screening, Colloids Surf., A, 2021, 625, 126971 CrossRef CAS
.
- F. Yaghoubi,
et al., Multiresponsive carboxylated graphene oxide-grafted aptamer as a multifunctional nanocarrier for targeted delivery of chemotherapeutics and bioactive compounds in cancer therapy, Nanotechnol. Rev., 2021, 10(1), 1838–1852 CrossRef CAS
.
- F. Sahne, M. Mohammadi and G. D. Najafpour, Single-Layer Assembly of Multifunctional Carboxymethylcellulose on Graphene Oxide Nanoparticles for Improving in Vivo Curcumin Delivery into Tumor Cells, ACS Biomater. Sci. Eng., 2019, 5(5), 2595–2609 CrossRef CAS PubMed
.
- N. Ghanbari,
et al., Tryptophan-functionalized graphene quantum dots with enhanced curcumin loading capacity and pH-sensitive release, J. Drug Delivery Sci. Technol., 2021, 61, 102137 CrossRef CAS
.
- M. Razaghi,
et al., Highly fluorinated graphene oxide nanosheets for anticancer linoleic-curcumin conjugate delivery and T2-Weighted magnetic resonance imaging: In vitro and in vivo studies, J. Drug Delivery Sci. Technol., 2020, 60, 101967 CrossRef CAS
.
- F. Paknia,
et al., The convergence of in silico approach and nanomedicine for efficient cancer treatment; in vitro investigations on curcumin loaded multifunctional graphene oxide nanocomposite structure, J. Drug Delivery Sci. Technol., 2022, 71, 103302 CrossRef CAS
.
- P. Saraei,
et al., The beneficial effects of metformin on cancer prevention and therapy: a comprehensive review of recent advances, Cancer Manage. Res., 2019, 11, 3295–3313 CrossRef CAS PubMed
.
- A. Basu,
et al., Hyaluronic acid engrafted metformin loaded graphene oxide nanoparticle as CD44 targeted anti-cancer therapy for triple negative breast cancer, Biochim. Biophys. Acta, Gen. Subj., 2021, 1865(3), 129841 CrossRef CAS PubMed
.
- M. Ashjaran,
et al., Stimuli-responsive polyvinylpyrrolidone-NIPPAm-lysine graphene oxide nano-hybrid as an anticancer drug delivery on MCF7 cell line, Artif. Cells, Nanomed., Biotechnol., 2019, 47(1), 443–454 CrossRef CAS PubMed
.
- A. Boddu,
et al., Encapsulation of 5-Fluorouracil Treated Reduced Graphene Oxide in Sodium Alginate Matrix for Controlled and pH-Responsive Drug Delivery, ChemistrySelect, 2021, 6(25), 6533–6540 CrossRef CAS
.
- W. Guo,
et al., Graphene oxide (GO)-based nanosheets with combined chemo/photothermal/photodynamic therapy to overcome gastric cancer (GC) paclitaxel resistance by reducing mitochondria-derived adenosine-triphosphate (ATP), J. Nanobiotechnol., 2021, 19(1), 1–19 CrossRef PubMed
.
- G. Singh,
et al., Fabrication of chlorambucil loaded graphene-oxide nanocarrier and its application for improved antitumor activity, Biomed. Pharmacother., 2020, 129, 110443 CrossRef CAS PubMed
.
- Z. Shariatinia and A. Mazloom-Jalali, Molecular dynamics simulations on chitosan/graphene nanocomposites as anticancer drug delivery using systems, Chin. J. Phys., 2020, 66, 362–382 CrossRef CAS
.
- P. Du,
et al., Tumor microenvironment and NIR laser dual-responsive release of berberine 9-O-pyrazole alkyl derivative loaded in graphene oxide nanosheets for chemo-photothermal synergetic cancer therapy, J. Mater. Chem. B, 2020, 8(18), 4046–4055 RSC
.
- M. Ghanbari-Movahed,
et al., Recent advances in improved anticancer efficacies of camptothecin nano-formulations: A systematic review, Biomedicines, 2021, 9(5), 480 CrossRef CAS PubMed
.
- M. Matiyani,
et al., Polymer grafted magnetic graphene oxide as a potential nanocarrier for pH-responsive delivery of sparingly soluble quercetin against breast cancer cells, RSC Adv., 2022, 12(5), 2574–2588 RSC
.
- J. Bourhis,
et al., Avelumab and cetuximab as a therapeutic combination: An overview of scientific rationale and current clinical trials in cancer, Cancer Treat. Rev., 2021, 97, 102172 CrossRef CAS PubMed
.
- J. Song,
et al., The Multiple Roles of Glucose-6-Phosphate Dehydrogenase in Tumorigenesis and Cancer Chemoresistance, Life, 2022, 12(2), 271 CrossRef CAS PubMed
.
- A. S. Doghish,
et al., Graphene oxide and its nanocomposites with EDTA or chitosan induce apoptosis in MCF-7 human breast cancer, RSC Adv., 2021, 11(46), 29052–29064 RSC
.
- S. S. Abolmaali, A. M. Tamaddon and R. Dinarvand, A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis, Cancer Chemother. Pharmacol., 2013, 71(5), 1115–1130 CrossRef CAS PubMed
.
- H. Zhu,
et al., Mitochondrion targeting peptide-modified magnetic graphene oxide delivering mitoxantrone for impairment of tumor mitochondrial functions, Chin. Chem. Lett., 2021, 32(3), 1220–1223 CrossRef CAS
.
- M.-Y. Lan,
et al., Polyethylene glycol-coated graphene oxide loaded with erlotinib as an effective therapeutic agent for treating nasopharyngeal cancer cells, Int. J. Nanomed., 2020, 15, 7569 CrossRef CAS PubMed
.
- S. Kesavan,
et al., Ulvan loaded graphene oxide nanoparticle fabricated with chitosan and d-mannose for targeted anticancer drug delivery, J. Drug Delivery Sci. Technol., 2021, 65, 102760 CrossRef CAS
.
- P. Seetharaman,
et al., Isolation and characterization of anticancer flavone chrysin (5,7-dihydroxy flavone)-producing endophytic fungi from Passiflora incarnata L. leaves, Ann. Microbiol., 2017, 67(4), 321–331 CrossRef CAS
.
- S. Gnanasekar,
et al., Chrysin-anchored silver and gold nanoparticle-reduced graphene oxide composites for breast cancer therapy, ACS Appl. Nano Mater., 2020, 3(5), 4574–4585 CrossRef CAS
.
- M. Ramezani Farani,
et al., PEGylation of graphene/iron oxide nanocomposite: assessment of release of doxorubicin, magnetically targeted drug delivery and photothermal therapy, Appl. Nanosci., 2020, 10(4), 1205–1217 CrossRef CAS
.
- T. Anirudhan, V. C. Sekhar and V. Athira, Graphene oxide based functionalized chitosan polyelectrolyte nanocomposite for targeted and pH responsive drug delivery, Int. J. Biol. Macromol., 2020, 150, 468–479 CrossRef CAS PubMed
.
- J. Charmi,
et al., Polyethylene glycol (PEG) decorated graphene oxide nanosheets for controlled release curcumin delivery, Heliyon, 2019, 5(4), e01466 CrossRef CAS PubMed
.
- S. Prabakaran,
et al., Polymethyl methacrylate–ovalbumin @ graphene oxide drug carrier system for high anti-proliferative cancer drug delivery, Appl. Nanosci., 2019, 9(7), 1487–1500 CrossRef CAS
.
- R. Ali,
et al., Graphene oxide/zinc ferrite nanocomposite loaded with doxorubicin as a potential theranostic mediu in cancer therapy and magnetic resonance imaging, Ceram. Int., 2022, 48(8), 10741–10750 CrossRef CAS
.
- A. S. Saqezi,
et al., Synthesis of Graphene Oxide/Iron Oxide/Au Nanocomposite for Quercetin Delivery, J. Inorg. Organomet. Polym. Mater., 2022, 32(5), 1541–1550 CrossRef CAS
.
- S. R. Banoon and A. Ghasemian, The characters of graphene oxide nanoparticles and doxorubicin against HCT-116 colorectal cancer cells in vitro, J. Gastrointest. Cancer, 2022, 53(2), 410–414 CrossRef CAS PubMed
.
- A. Pourjavadi, S. Asgari and S. H. Hosseini, Graphene oxide functionalized with oxygen-rich polymers as a pH-sensitive carrier for co-delivery of hydrophobic and hydrophilic drugs, J. Drug Delivery Sci. Technol., 2020, 56, 101542 CrossRef CAS
.
- X. Pei,
et al., PEGylated nano-graphene oxide as a nanocarrier for delivering mixed anticancer drugs to improve anticancer activity, Sci. Rep., 2020, 10(1), 1–15 CrossRef PubMed
.
- H. Tiwari,
et al., Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells, Colloids Surf., B, 2019, 178, 452–459 CrossRef CAS PubMed
.
- S. Astani,
et al., Co-delivery of cisplatin and doxorubicin by carboxylic acid functionalized poly(hydroxyethyl methacrylate)/reduced graphene nanocomposite for combination chemotherapy of breast cancer cells, J. Biomater. Sci., Polym. Ed., 2021, 32(5), 657–677 CrossRef CAS PubMed
.
- F. Yaghoubi,
et al., A functionalized graphene oxide with improved cytocompatibility for stimuli-responsive co-delivery of curcumin and doxorubicin in cancer treatment, Sci. Rep., 2022, 12(1), 1–18 CrossRef PubMed
.
- S. Bullo,
et al., Dual drugs anticancer nanoformulation using graphene oxide-PEG as nanocarrier for protocatechuic acid and chlorogenic acid, Pharm. Res., 2019, 36(6), 1–11 CAS
.
- S. Asgari,
et al., Encapsulation of drug-loaded graphene oxide-based nanocarrier into electrospun pullulan nanofibers for potential local chemotherapy of breast cancer, Macromol. Chem. Phys., 2021, 222(15), 2100096 CrossRef CAS
.
- N. R. Ko,
et al., Dual pH-and GSH-responsive degradable PEGylated graphene quantum dot-based nanoparticles for enhanced HER2-positive breast cancer therapy, Nanomaterials, 2020, 10(1), 91 CrossRef CAS PubMed
.
- H. Tiwari,
et al., Dual Drug Loaded Potassium-Contained Graphene Oxide as a Nanocarrier in Cocktailed Drug Delivery for the Treatment of Human Breast Cancer, Curr. Drug Delivery, 2022 DOI:10.2174/1567201819666220524152558
.
- S. M. Ghafary,
et al., Design and preparation of a theranostic peptideticle for targeted cancer therapy: Peptide-based codelivery of doxorubicin/curcumin and graphene quantum dots, Nanomedicine, 2022, 42, 102544 CrossRef PubMed
.
- S. Moasses Ghafary,
et al., Design and preparation of a theranostic peptideticle for targeted cancer therapy: Peptide-based codelivery of doxorubicin/curcumin and graphene quantum dots, Nanomedicine, 2022, 42, 102544 CrossRef CAS PubMed
.
- Q. Sun,
et al., Doxorubicin and anti-VEGF siRNA co-delivery via nano-graphene oxide for enhanced cancer therapy in vitro and in vivo, Int. J. Nanomed., 2018, 13, 3713 CrossRef CAS PubMed
.
- R. Imani,
et al., Microencapsulated Multifunctionalized Graphene Oxide Equipped with Chloroquine for Efficient and Sustained siRNA Delivery, BioMed Res. Int., 2022, 2022, 5866361 Search PubMed
.
- T. Liu,
et al., Transferrin-targeting redox hyperbranched poly(amido amine)-functionalized graphene oxide for sensitized chemotherapy combined with gene therapy to nasopharyngeal carcinoma, Drug Delivery, 2019, 26(1), 744–755 CrossRef CAS PubMed
.
- T.-Y. Cheang,
et al., Graphene oxide–hydroxyapatite nanocomposites effectively deliver HSV-TK suicide gene to inhibit human breast cancer growth, J. Biomater. Appl., 2018, 33(2), 216–226 CrossRef CAS PubMed
.
- M. Zhang,
et al., Multifunctional nanocomposites for targeted, photothermal, and chemotherapy, Chem. Mater., 2018, 31(6), 1847–1859 CrossRef
.
- Y. Zhuang,
et al., Constructing an on-demand drug release system composed of thermosensitive PPP hydrogel and drug-laden alginate/graphene microspheres to treat tumorous defect, J. Mater. Sci., 2022, 57, 4754–47770 CrossRef CAS
.
- C. Huang,
et al., Tailored graphene oxide-doxorubicin nanovehicles via near-infrared dye-lactobionic acid conjugates for chemo-photothermal therapy, J. Colloid Interface Sci., 2019, 545, 172–183 CrossRef CAS PubMed
.
- H. Zhang,
et al., Multifunctional nanosystem based on graphene oxide for synergistic multistage tumor-targeting and combined chemo-photothermal therapy, Mol. Pharmaceutics, 2019, 16(5), 1982–1998 CrossRef CAS PubMed
.
- T. Lu,
et al., Preparation and anti-cancer activity of transferrin/folic acid double-targeted graphene oxide drug delivery system, J. Biomater. Appl., 2020, 35(1), 15–27 CrossRef CAS PubMed
.
- Z. Qi,
et al., Gold nanorods/graphene oxide nanosheets immobilized by polydopamine for efficient remotely triggered drug delivery, J. Mater. Sci., 2020, 55(29), 14530–14543 CrossRef CAS
.
- W. Liu,
et al., Reduced graphene oxide (rGO) hybridized hydrogel as a near-infrared (NIR)/pH dual-responsive platform for combined chemo-photothermal therapy, J. Colloid Interface Sci., 2019, 536, 160–170 CrossRef CAS PubMed
.
- M. Wei,
et al., Reductive response and RGD targeting nano-graphene oxide drug delivery system, J. Drug Delivery Sci. Technol., 2019, 53, 101202 CrossRef CAS
.
- X. Cui,
et al., Biomimetic light-activatable graphene-based nanoarchitecture for synergistic chemophotothermal therapy, Chem. Eng. J., 2021, 420, 127710 CrossRef CAS
.
- R. Sang,
et al., HAp@ GO drug delivery vehicle with dual-stimuli-triggered drug release property and efficient synergistic therapy function against cancer, J. Biomed. Mater. Res., Part A, 2019, 107(10), 2296–2309 CrossRef CAS PubMed
.
- X. Liu,
et al., Reduced Graphene Oxide/Mesoporous Silica Nanocarriers for pH-Triggered Drug Release and Photothermal Therapy, ACS Appl. Bio Mater., 2020, 3(5), 2577–2587 CrossRef CAS PubMed
.
- D. Wang,
et al., Fluorescence Turn-off Magnetic Fluorinated Graphene Composite with High NIR Absorption for Targeted Drug Delivery, ChemNanoMat, 2021, 7(1), 71–77 CrossRef CAS
.
- R. Gao,
et al., AS1411-targeted graphene oxide nano-drug delivery system for chemo-photothermal therapy of cervical cancer, Res. Sq., 2022 DOI:10.21203/rs.3.rs-1256751/v1
.
- Q. Chen,
et al., Construction of an exosome-functionalized graphene oxide based composite bionic smart drug delivery system and its anticancer activity, Nanotechnology, 2022, 33(17), 175101 CrossRef PubMed
.
- N. Işıklan, N. A. Hussien and M. Türk, Synthesis and drug delivery performance of gelatin-decorated magnetic graphene oxide nanoplatform, Colloids Surf., A, 2021, 616, 126256 CrossRef
.
- M. Gautam,
et al., Stealth Polymer-Coated Graphene Oxide Decorated Mesoporous Titania Nanoplatforms for In Vivo Chemo-Photodynamic Cancer Therapy, Pharm. Res., 2020, 37(8), 162 CrossRef CAS PubMed
.
- M. Mirza-Aghayan,
et al., Synthesis and characterization of a novel multi-functionalized reduced graphene oxide as a pH-sensitive drug delivery material and a photothermal candidate, Appl. Surf. Sci., 2022, 583, 152568 CrossRef CAS
.
- R. Prasad,
et al., Ultrahigh Penetration and Retention of Graphene Quantum Dot Mesoporous Silica Nanohybrids for Image Guided Tumor Regression, ACS Appl. Bio Mater., 2021, 4(2), 1693–1703 CrossRef CAS PubMed
.
- N. Mauro,
et al., Folic acid-functionalized graphene oxide nanosheets via plasma etching as a platform to combine NIR anticancer phototherapy and targeted drug delivery, Mater. Sci. Eng., C, 2020, 107, 110201 CrossRef CAS PubMed
.
- F. Zhou,
et al., Photo-activated chemo-immunotherapy for metastatic cancer using a synergistic graphene nanosystem, Biomaterials, 2021, 265, 120421 CrossRef CAS
.
- X. Huang,
et al., Delivery of MutT homolog 1 inhibitor by functionalized graphene oxide nanoparticles for enhanced chemo-photodynamic therapy triggers cell death in osteosarcoma, Acta Biomater., 2020, 109, 229–243 CrossRef CAS PubMed
.
- K. Vinothini,
et al., A magnetic nanoparticle functionalized reduced graphene oxide-based drug carrier system for a chemo-photodynamic cancer therapy, New J. Chem., 2020, 44(14), 5265–5277 RSC
.
- S. L. Lu,
et al., Graphene Oxide Nanoparticle-Loaded Ginsenoside Rg3 Improves Photodynamic Therapy in Inhibiting Malignant Progression and Stemness of Osteosarcoma, Front. Mol. Biosci., 2021, 8, 663089 CrossRef PubMed
.
- L. Feng,
et al., Polyethylene glycol and polyethylenimine dual-functionalized nano-graphene oxide for photothermally enhanced gene delivery, Small, 2013, 9(11), 1989–1997 CrossRef CAS PubMed
.
- F. Yin,
et al., SiRNA Delivery with PEGylated Graphene Oxide Nanosheets for Combined Photothermal and Genetherapy for Pancreatic Cancer, Theranostics, 2017, 7(5), 1133–1148 CrossRef CAS PubMed
.
- X. Jia,
et al., Functionalized Graphene@Gold Nanostar/Lipid for Pancreatic Cancer Gene and Photothermal Synergistic Therapy under Photoacoustic/Photothermal Imaging Dual-Modal Guidance, Small, 2020, 16(39), e2003707 CrossRef PubMed
.
- A. Assali,
et al., Cationic graphene oxide nanoplatform mediates miR-101 delivery to promote apoptosis by regulating autophagy and stress, Int. J. Nanomed., 2018, 13, 5865 CrossRef CAS PubMed
.
- J. Liang,
et al., Ph and thermal dual-responsive graphene oxide nanocomplexes for targeted drug delivery and photothermal-chemo/photodynamic synergetic therapy, ACS Appl. Bio Mater., 2019, 2(12), 5859–5871 CrossRef CAS PubMed
.
- Y.-F. Ding,
et al., Supramolecular nanomedicine derived from cucurbit [7] uril-conjugated nano-graphene oxide for multi-modality cancer therapy, Biomater. Sci., 2021, 9(10), 3804–3813 RSC
.
- Y. Esmaeili,
et al., Hierarchical multifunctional graphene oxide cancer nanotheranostics agent for synchronous switchable fluorescence imaging and chemical therapy, Microchim. Acta, 2020, 187(10), 1–15 CrossRef PubMed
.
- W. Chen,
et al., Polyethylenimine modified graphene oxide for effective chemo-gene-photothermal triples therapy of triple-negative breast cancer and inhibits metastasis, J. Drug Delivery Sci. Technol., 2022, 74, 103521 CrossRef CAS
.
- M. Nejabat, F. Charbgoo and M. Ramezani, Graphene as multifunctional delivery platform in cancer therapy, J. Biomed. Mater. Res., Part A, 2017, 105(8), 2355–2367 CrossRef CAS PubMed
.
- Y. Gu,
et al., A polyamidoamne dendrimer functionalized graphene oxide for DOX and MMP-9 shRNA plasmid co-delivery, Mater. Sci. Eng., C, 2017, 70(Pt 1), 572–585 CrossRef CAS PubMed
.
- P. Liu,
et al., Platinated graphene oxide: A nanoplatform for efficient gene-chemo combination cancer therapy, Eur. J. Pharm. Sci., 2018, 121, 319–329 CrossRef CAS PubMed
.
- Z. Yang,
et al., Simultaneous Delivery of antimiR-21 and Doxorubicin by Graphene Oxide for Reducing Toxicity in Cancer Therapy, ACS Omega, 2020, 5(24), 14437–14443 CrossRef CAS PubMed
.
- H.-W. Yang,
et al., Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging, Biomaterials, 2014, 35, 6534–6542 CrossRef CAS PubMed
.
- M. Fana,
et al., PAMAM Dendrimer Nanomolecules Utilized as Drug Delivery Systems for Potential Treatment of Glioblastoma: A Systematic Review, Int. J. Nanomed., 2020, 15, 2789–2808 CrossRef CAS PubMed
.
- W. Zhang,
et al., Photothermal/pH Dual-Responsive Drug Delivery System of Amino-Terminated HBP-Modified rGO and the Chemo-Photothermal Therapy on Tumor Cells, Nanoscale Res. Lett., 2018, 13(1), 379 CrossRef PubMed
.
- J. Yan,
et al., Let-7i miRNA and platinum loaded nano-graphene oxide platform for detection/reversion of drug resistance and synergetic chemical-photothermal inhibition of cancer cell, Chin. Chem. Lett., 2022, 33(2), 767–772 CrossRef CAS
.
- Y. Sheng,
et al., pH-sensitive drug delivery based on chitosan wrapped graphene quantum dots with enhanced fluorescent stability, Mater. Sci. Eng., C, 2020, 112, 110888 CrossRef CAS PubMed
.
- O. Cebadero-Dominguez,
et al., Hazard characterization of graphene nanomaterials in the frame of their food risk assessment: A review, Food Chem. Toxicol., 2022, 164, 113014 CrossRef CAS PubMed
.
- J. Li,
et al., Promising Graphene-Based Nanomaterials and Their Biomedical Applications and Potential Risks: A Comprehensive Review, ACS Biomater. Sci. Eng., 2021, 7(12), 5363–5396 CrossRef CAS PubMed
.
- M. M. Ghazimoradi,
et al., Epigenetic effects of graphene oxide and its derivatives: A mini-review, Mutat. Res., Genet. Toxicol. Environ. Mutagen., 2022, 878, 503483 CrossRef CAS PubMed
.
- A. N. Ghulam,
et al., Graphene Oxide (GO) Materials—Applications and Toxicity on Living Organisms and Environment, J. Funct. Biomater., 2022, 13(2), 77 CrossRef CAS PubMed
.
-
M. T. Rohit Srivastava, K. Mukesh Kumar and B. Rohan, Next Generation Graphene Nanomaterials for Cancer Theranostic Applications, Springer, Singapore, 2021, vol. X, p. 132 Search PubMed
.
- Y. Liu,
et al., Bio-transformation of Graphene Oxide in Lung Fluids Significantly Enhances Its Photothermal Efficacy, Nanotheranostics, 2018, 2(3), 222–232 CrossRef PubMed
.
- X. Liu,
et al., Altered gut microbiome accompanying with placenta barrier dysfunction programs pregnant complications in mice caused by graphene oxide, Ecotoxicol. Environ. Saf., 2021, 207, 111143 CrossRef CAS PubMed
.
- H. R. H. Mohamed,
et al., Estimation of genomic instability and mutation induction by graphene oxide nanoparticles in mice liver and brain tissues, Environ. Sci. Pollut. Res. Int., 2020, 27(1), 264–278 CrossRef CAS PubMed
.
- A. K. Patlolla,
et al., Toxicity Evaluation of Graphene Oxide in Kidneys of Sprague-Dawley Rats, Int. J. Environ. Res. Public Health, 2016, 13(4), 380 CrossRef PubMed
.
- A. Wang,
et al., Role of surface charge and oxidative stress in cytotoxicity and genotoxicity of graphene oxide towards human lung fibroblast cells, J. Appl. Toxicol., 2013, 33(10), 1156–1164 CrossRef CAS PubMed
.
- C. Xie,
et al., Elucidating the origin of the surface functionalization - dependent bacterial toxicity of graphene nanomaterials: Oxidative damage, physical disruption, and cell autolysis, Sci. Total Environ., 2020, 747, 141546 CrossRef CAS PubMed
.
- Y. Li,
et al., The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways, Biomaterials, 2012, 33(2), 402–411 CrossRef CAS PubMed
.
- S. Yadav,
et al., An Update on Graphene Oxide: Applications and Toxicity, ACS Omega, 2022, 7(40), 35387–35445 CrossRef CAS PubMed
.
- S. Syama and P. V. Mohanan, Safety and biocompatibility of graphene: A new generation nanomaterial for biomedical application, Int. J. Biol. Macromol., 2016, 86, 546–555 CrossRef CAS PubMed
.
- R. E. Li,
et al., Graphene quantum dots potently block copper-mediated oxidative DNA damage: implications for cancer intervention, React. Oxygen Species, 2018, 6(18), 406 Search PubMed
.
- X. Yang,
et al., Developmental neurotoxicity and immunotoxicity induced by graphene oxide in zebrafish embryos, Environ. Toxicol., 2019, 34(4), 415–423 CAS
.
- N. K. Nirmal, K. K. Awasthi and P. J. John, Effects of Nano-Graphene Oxide on Testis, Epididymis and Fertility of Wistar Rats, Basic Clin. Pharmacol. Toxicol., 2017, 121(3), 202–210 CrossRef CAS PubMed
.
- K.-P. Wen,
et al., Accumulation and toxicity of intravenously-injected functionalized graphene oxide in mice, J. Appl. Toxicol., 2015, 35(10), 1211–1218 CrossRef CAS PubMed
.
- N. A. El-Yamany,
et al., Graphene oxide nanosheets induced genotoxicity and pulmonary injury in mice, Exp. Toxicol. Pathol., 2017, 69(6), 383–392 CrossRef CAS PubMed
.
- G. Duan,
et al., Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane, Nanoscale, 2015, 7(37), 15214–15224 RSC
.
- K.-H. Liao,
et al., Cytotoxicity of Graphene Oxide and Graphene in Human Erythrocytes and Skin Fibroblasts, ACS Appl. Mater. Interfaces, 2011, 3(7), 2607–2615 CrossRef CAS PubMed
.
- A. Deb, N. G. Andrews and V. Raghavan, Honokiol–camptothecin loaded graphene oxide nanoparticle towards combinatorial anti-cancer drug delivery, IET Nanobiotechnol., 2020, 14(9), 796–802 CrossRef PubMed
.
- V. Rossa,
et al., Nanocomposites based on the graphene family for food packaging: historical perspective, preparation methods, and properties, RSC Adv., 2022, 12(22), 14084–14111 RSC
.
- EFSA Scientific Committee, S. More, V. Bampidis, D. Benford, C. Bragard, T. Halldorsson, A. Hernández-Jerez, S. Hougaard Bennekou, K. Koutsoumanis, C. Lambré, K. Machera, H. Naegeli, S. Nielsen, J. Schlatter, D. Schrenk, V. Silano, D. Turck, M. Younes, J. Castenmiller, Q. Chaudhry, F. Cubadda, R. Franz, D. Gott, J. Mast, A. Mortensen, A. G. Oomen, S. Weigel, E. Barthelemy, A. Rincon, J. Tarazona and R. Schoonjans, Guidance on risk assessment of nanomaterials to be applied in the food and feed chain: human and animal health, EFSA J., 2021, 19(8), e06768 Search PubMed
.
- H. Li,
et al., Ameliorative effect of graphene nanosheets against arsenic-induced toxicity in mice by oral exposure, Environ. Sci. Pollut. Res. Int., 2021, 28(17), 21577–21588 CrossRef CAS PubMed
.
- A. K. Patlolla,
et al., Toxicity Evaluation of Graphene Oxide in Kidneys of Sprague-Dawley Rats, Int. J. Environ. Res. Public Health, 2016, 13(4), 380 CrossRef PubMed
.
- F. D. de Menezes,
et al., Graphene quantum dots unraveling: Green synthesis, characterization, radiolabeling with 99mTc, in vivo behavior and mutagenicity, Mater. Sci. Eng., C, 2019, 102, 405–414 CrossRef CAS PubMed
.
| This journal is © The Royal Society of Chemistry 2023 |