A dual functional molecule for perovskite/P3HT interface to achieve stable perovskite solar cells†
Hyuntae
Choi
a,
Haeryang
Lim
a,
Heesu
Kim
b,
Jeongin
Lim
b,
Minji
Park
b,
Chandra Shakher
Pathak
 b and
Seulki
Song
b and
Seulki
Song
 *b
*b
aDepartment of Chemical Engineering, Pohang University of Science and Technology (POSTECH), 77 Cheongam-Ro, Nam-gu, Pohang, Gyeongbuk, Korea 37673
bDepartment of Chemical Engineering and Applied Chemistry, Chungnam National University (CNU), 99 Daehak-Ro, Yuseong-gu, Daejeon, Republic of Korea 34134. E-mail: sksong@cnu.ac.kr
First published on 11th July 2023
Abstract
After the introduction of spiro-OMeTAD as a hole transport material (HTM), the efficiency of perovskite solar cells (PSCs) has skyrocketed. However, dopants in spiro-OMeTAD make PSCs vulnerable to moisture and heat. Furthermore, the cost of spiro-OMeTAD is quite high, impeding the commercialization of PSCs. Therefore, the demand for HTMs that are dopant-free and cost-effective is increasing. In this regard, P3HT satisfies these criteria, exhibiting low-cost, long term stability, and high hole mobility. Despite these advantages, the PSCs with P3HT showed a lower efficiency compared to those PSCs with spiro-OMeTAD. Herein, we newly synthesized and employed a dual functional molecule, octylammonium azide (OAN3). The ammonium ion could interact with perovskite and passivate the defects in perovskite and the azide moiety could provide crosslinking sites with P3HT, improving both efficiency and long-term stability. As a result, the perovskite solar cells (PSCs) with OAN3 obtained a maximum efficiency of 20.0%. In contrast, bare PSCs achieved a maximum efficiency of 13.8%. In addition, PSCs with OAN3 maintained 90% and 82% of their initial efficiency under RH = 50–60% condition. However, the bare PSCs retained 38% of their initial efficiency under the same conditions.
Introduction
The halide perovskite, well known as the ABX3 structure (A: monovalent cation, B: divalent cation, and X: halides), has presented remarkable applicability to diverse optoelectronic devices such as solar cells, light emitting diodes, sensors, and laser.1–3 Among them, perovskite solar cells (PSCs) showed incredible progress in the past decade, achieving a certified efficiency of 25.7% and are expected to be commercialized.4 Currently, the commercialization of PSCs is a significant issue that cannot be postponed any longer in terms of climate change and eco-friendly energy shortage. However, to be commercialized, several issues remain, such as scalability, toxicity of lead, and instability issue.5 In particular, the instability of PSCs is the major obstacle interrupting the commercialization of PSCs despite their excellent photovoltaic performance.6–9To date, 2,2′,7,7′-tetrakis[N,N-di(4methoxylphenyl)amino]-9,9′-spirobifluorene (spiro-OMeTAD) is widely adopted as a hole transporting material (HTM) in the state-of-the-art PSCs.10–13 However, spiro-OMeTAD requires dopants (i.e., Li-TFSI and tBP) to improve the intrinsic low hole mobility.14,15 These dopants could adversely affect the long-term stability of PSCs by facilitating the degradation of the perovskite material. In particular, Li-TFSI possesses a hygroscopic nature, which promotes the adsorption of moisture and perovskite degradation.16 Moreover, tBP forms a complex with PbI2 and causes the decomposition of the perovskite layer.17 Even worse, tBP gradually evaporates at ambient conditions and could cause severe instability under heat, deteriorating film morphology and performance of PSCs.18 Owing to the aforementioned issues, introducing spiro-OMeTAD as an HTM results in poor long-term stability against moisture and heat.19,20 Recently, to address these issues, novel doping strategies have been proposed to effectively oxidize spiro-OMeTAD without using Li-TFSI, including radical doping and CO2 doping.21–23 Although the stability is quite improved by adopting these methods, the long-term stability is still low. These problems are pointed out as a huge obstacle to realizing the commercialization of PSCs.24
Various strategies have been proposed, such as developing non-hygroscopic dopants,25,26 fabricating bi-layer structure,27,28 and synthesizing dopant-free HTMs, to improve the poor stability resulting from the conventional HTM model.29–33 Among them, developing dopant-free HTM is a fundamental solution in that it is capable of achieving high efficiency and stability and realizing the commercialization of PSCs. However, most dopant-free HTMs require expensive monomers and many synthesis steps, which leads to high costs. In terms of cost-effectiveness and robust long-term stability, poly(3-hexylthiophene) (P3HT) was paid attention as a promising HTM.34 In addition, P3HT exhibited high mobility, which is one of the significant factors in determining the performance of HTM.32 However, when adopting P3HT, the perovskite film has to be contacted with alkyl side chains of P3HT, which leads to a poor interface between perovskite and P3HT.
This phenomenon induces non-radiative recombination and deteriorates the performance of PSCs.34,35 In order to solve this problem, several researchers have introduced molecules at the interface between perovskite and P3HT.36,37 In addition, doping P3HT with different small molecules is also a preferred strategy to improve efficiency.38–40 Recently, different additives that could affect the packing and orientation are also devised: J. Seo and J. H. Noh group introduced alkyl halide-based small molecules to the top of perovskite film and controlled the packing of P3HT.34,41 In addition, Hu group employed the SMe-TATPYr molecule and improved the hole mobility by directly changing the packing of P3HT.42 Recently, J. Gao group has constructed molecular bridges (MDN) to passivate the defects and form a charge transport channel.35 However, these research studies have a limitation in that they have used a secondary bonding between the additive and P3HT, which is not strong enough compared to a covalent bond.
To effectively enhance the stability of PSCs, the perovskite surface, HTM, and interface between perovskite and HTM should all be considered. Defects existing in the perovskite surface must be well controlled, the interface with the HTM must be well formed, and the electrical properties of the HTM itself must also be maintained high. However, the studies controlling perovskite defects, perovskite/HTM interface and properties of HTM have been implemented individually. Research that could control the aforementioned three parts at once could be regarded as a core technology for the commercialization of PSCs.
To achieve this, we designed and synthesized a new dual functional interface modulating material, octylammonium azide (OAN3) (Fig. 1). The ammonium ion has been reported to passivate the defects in perovskites.43,44 In addition, the azide moiety could provide a crosslinking site with P3HT, strengthening the interfacial contact between perovskite and P3HT.45,46 As a result, the P3HT with OAN3 and bare PSCs showed a PCE of 20.0% and 13.8%, respectively, mainly resulting from increased open-circuit voltage (VOC) and fill factor (FF). Moreover, the PSCs treated with OAN3 exhibited improved stability under humid conditions. The OAN3 treated PSCs maintained 90% and 82% of their initial efficiency (with and without crosslinking, respectively) for 500 h under 50–60% relative humidity. In contrast, bare PSCs have retained only 38% of their initial efficiency. We believe that our dual functional molecule strategy could provide a guideline to achieve a stable and efficient perovskite solar cell.
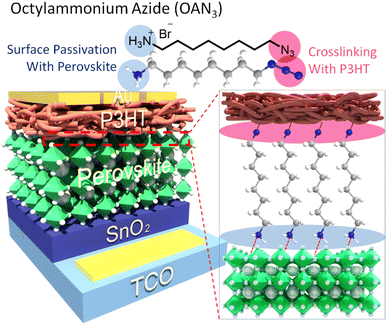 | ||
| Fig. 1 Schematic illustration of perovskite solar cells with crosslinkable molecule, octylammonium azide (OAN3). | ||
Results and discussion
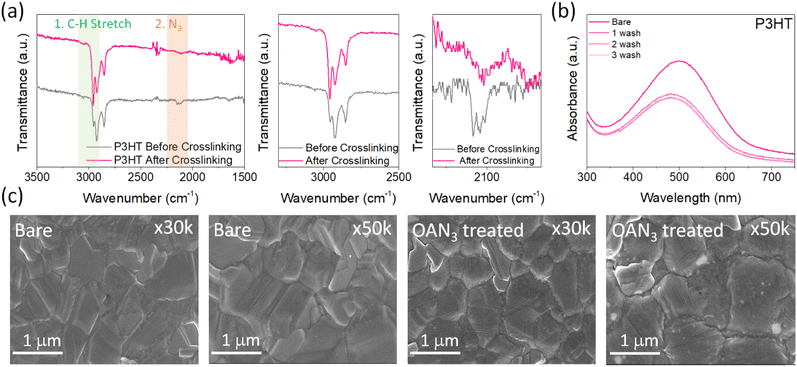
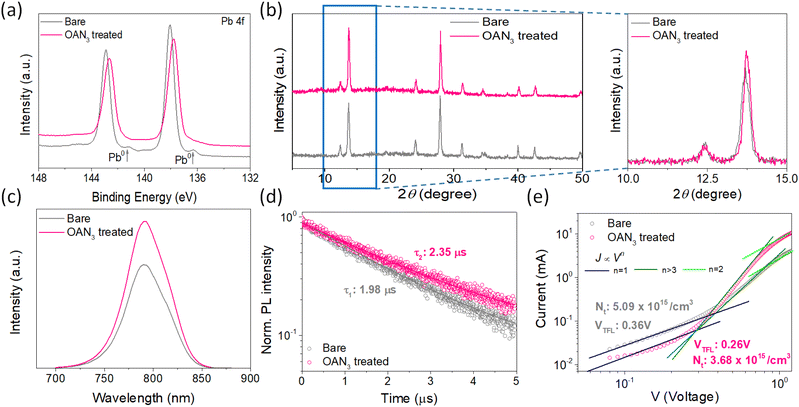
The synthetic scheme and characterization of OAN3 are provided in Scheme S1 and Fig. S1–S3.† Azide moiety is known to generate nitrene after UV light irradiation, inducing insertion reactions with C–H bonds.45,46 The Fourier transform infrared (FT-IR) spectroscopy of OAN3 with P3HT was performed to confirm the crosslinking after UV irradiation. The characteristic peak of azide groups via the asymmetric stretching band (N3) appeared in the 2000–2200 region (Fig. 2a).45,46 After crosslinking, we could check that the corresponding peak is decreased. Furthermore, as the evidence of crosslinking, the C–H stretching peak increased (Fig. 2a). We could also confirm the crosslinking of the interface through UV-Vis spectra. We prepared the film with the configuration of glass/OAN3/P3HT (Fig. 2b). The absorbance of crosslinked P3HT is decreased after the first washing, as the crosslinking only occurred in the interface between OAN3 and P3HT and not in the full P3HT film. When we measured the UV-Vis spectra after irradiation, the absorbance remained almost at its initial state even after three times of washing, indicating the crosslinking proceeded. This phenomenon could also be confirmed by film images of washed P3HT before and after the crosslinking process (Fig. S4†). After confirming the crosslink ability of OAN3, we checked the interaction of OAN3 with perovskite crystals. We used scanning electron microscope (SEM) to figure out the difference in the perovskite film surface after OAN3 treatment (Fig. 2c). The grain size of perovskite film before and after OAN3 are similar, indicating that the morphology of perovskite film is not significantly affected.In addition, we measured X-ray photoelectron spectroscopy (XPS) to figure out the interaction between perovskite and OAN3 (Fig. 3a). As a result, in the bare perovskite, the XPS peaks of Pb 4f appeared at 142.9 and 138.05 eV, respectively. In contrast, the XPS peaks of Pb 4f in the OAN3 treated perovskite appeared at 142.65 and 137.8 eV. This result indicates that the ammonium ions in the OAN3 molecule could interact with Pb atoms. Furthermore, Pb metallic peak (Pb0) is reduced (141.1 and 136.3 eV), which indicates the reduction of non-radiative recombination.47 In addition, the peak shift of I 3d occurred, resulting from the hydrogen bond (N–H⋯I) interaction between N–H in OAN3 and iodide in PbI2 (Fig. S5†).48 From the aforementioned XPS results, OAN3 could interact with both Pb and I ions, respectively. We also measured the 1H NMR for OAN3 and the mixture of OAN3 and PbI2 (Fig. S6†). The peak from ammonium ion (7.77 ppm) in the bare OAN3 shifted toward the up-field (7.69 ppm) after PbI2 addition. This result could support the fact that the interaction between perovskite and OAN3 exists. The positive effect of OAN3 could be found in the X-ray diffraction (XRD) measurement (Fig. 3b). The perovskite film exhibits peaks at 2θ = 13.7°, 28.0°, and 42.7°, corresponding to the (100), (200), and (300) perovskite lattice planes.49
The crystallinity of perovskite crystal is increased after incorporating OAN3. In addition, the lifetime of charge carriers is elongated, as shown in the steady-state and time-resolved photoluminescence (SSPL and TRPL) (Fig. 3c and d). The TRPL measurement was performed with the configuration of glass/perovskite/OAN3. The τ1 value of bare perovskite is 1.98 μs. In the meantime, the τ1 value of perovskite treated with OAN3 is 2.35 μs, indicating the reduction of the non-radiative recombination. In addition, we also measured the TRPL of the samples with the configuration of glass/perovskite/OAN3/P3HT to confirm the charge extraction ability of OAN3 treatment (Fig. S7†). In the TRPL, the τ1 value for OAN3-treated film was examined to be 0.43 ns, which is smaller than that of bare perovskite-base film (0.65 ns).50 This result implies that the OAN3 could facilitate charge transfer from perovskite to P3HT. Also, the absorbance of the perovskite treated with OAN3 is slightly improved compared to bare perovskite (Fig. S8†). Lastly, to quantitatively measure the defect densities, we fabricated hole-only devices with the structure of ITO/PEDOT:PSS/perovskite/spiro-OMeTAD/Au. As a result, the perovskite with OAN3 showed VTFL of 0.26 V and Nt of 3.68 × 1015 cm−3. In contrast, the perovskite without OAN3 showed a larger VTFL value of 0.36 V and Nt of 5.09 × 1015 cm−3, indicating that OAN3 could passivate the perovskite surface effectively. From the aforementioned results, we could confirm that the OAN3 could impart positive effects on perovskite crystals.51,52
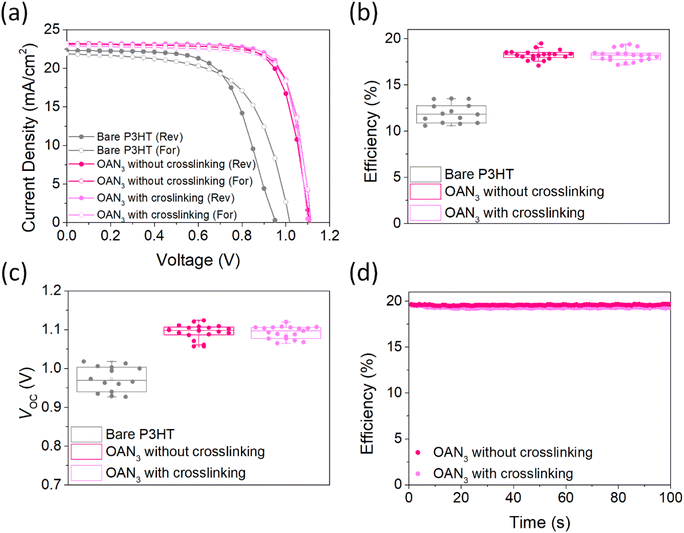
We fabricated PSCs with OAN3 using the following configuration: ITO/SnO2/(FAPbI3)0.95(MAPbBr3)0.05/OAN3/P3HT/Au(Ag) (Fig. 1 and S9†). We first optimized the concentration of P3HT and OAN3 to find out the optimal composition (Fig. S10 and S11†). Finally, we have fabricated PSCs with the optimal composition (Fig. 4a and Table 1). The OAN3 device exhibited a PCE of 20.0% with short-circuit current (JSC) of 23.2 mA cm−2, open-circuit voltage (VOC) of 1.12 V, and fill factor (FF) of 76.9%. The pristine device obtained a PCE of 13.8%, JSC of 22.0 mA cm−2, VOC of 1.02 V, and FF of 62%. Furthermore, the PSCs with OAN3 after crosslinking achieved 19.8 (JSC of 23.1 mA cm−2, VOC of 1.11 V, and FF of 77.1%), which indicates that crosslinking does not deteriorate the efficiency of PSCs. In the photovoltaic histograms of PSCs with OAN3, we have confirmed that the VOC and FF are the major factors in achieving higher efficiency compared to bare PSCs (Fig. 4b, c and S12†). This improvement resulted from a synergistic effect between suppressed trap density, reduced non-radiative recombination, and improved crystallinity of perovskite crystal. In addition, we have measured the external quantum efficiency (EQE) spectra, and the integrated JSC for the OAN3 device (22.8 mA cm−2) well-matched with the value obtained from the J–V curves (Fig. S13†). We further examined the stabilized power output test for the PSCS with OAN3 (Fig. 4d). PSCs with OAN3 achieved a PCE of 19.8% and 19.5% before and after crosslinking, respectively.
| Perovskite | Scan direction | J SC [mA cm−2] | V OC [V] | FF [%] | PCE [%] |
|---|---|---|---|---|---|
| Bare | Reverse | 22.3 | 0.96 | 64.2 | 13.6 |
| Forward | 22.0 | 1.02 | 62.0 | 13.8 | |
| OAN3 without crosslinking | Reverse | 23.2 | 1.12 | 76.9 | 20.0 |
| Forward | 23.2 | 1.11 | 76.1 | 19.7 | |
| OAN3 with crosslinking | Reverse | 23.1 | 1.11 | 77.1 | 19.8 |
| Forward | 23.0 | 1.12 | 76.5 | 19.7 |
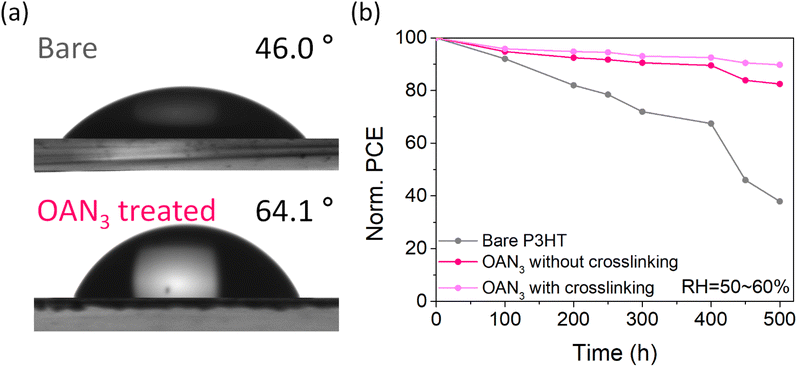
Finally, the long-term stability of the PSCs was measured by keeping the devices under humid conditions. To confirm the moisture-resistivity of perovskites before and after P3HT, we measured the contact angles for the perovskite with and without OAN3 (Fig. 5a). The contact angle for the perovskite film with OAN3 showed a higher angle of 64.1°, compared to that of the bare perovskite film (46.0°). After that, the unencapsulated devices were stored in RH 50–60% under ambient conditions. The OAN3-based devices (with and without crosslinking, respectively) retained 90% and 82% of their initial PCE after 500 h. In contrast, the pristine device only maintained 38% of the initial PCE during the same period (Fig. 5b, S14 and S15†). We consider that crosslinking of P3HT and OAN3 can improve long-term stability by preventing interfacial delamination, which is one of the major factors for PSC degradation.53,54 These results demonstrate that our strategy of using a crosslinkable molecule (OAN3) could achieve highly stable and efficient P3HT-based PSCs.
Conclusions
In this study, we newly designed and synthesized a small molecule, octylammonium azide (OAN3). The ammonium ion is able to passivate the defects in perovskite, and the azide group could provide a crosslinking site with P3HT, strengthening the interfacial contact between perovskite and HTM. As a result, the P3HT with OAN3 device showed a PCE of 20.0%, which is mainly owing to an improved VOC and FF compared to the control device (13.8%). Moreover, the OAN3–P3HT PSCs maintained 90% and 82% of initial efficiency after 500 h under 50–60% relative humidity without encapsulation. In contrast, the bare PSCs retained only 38% of initial efficiency after the same period of time. We believe that OAN3 treatment and crosslinking strategy could endow a high robustness of the PSCs against moisture due to the increased hydrophobicity and compact interfacial contact between perovskite and P3HT. Our dual-functional molecule strategy could provide a guideline to develop novel species of surface modifiers, which could improve the efficiency and stability simultaneously.Author contributions
S. Song designed and organized the project. The experiments were carried out by H. Choi, H. Lim, H. Kim, J. Lim, M. Park, and C. S. Pathak with the guidance of S. Song. The OAN3 molecule was synthesized and characterized by H. Lim. The perovskite solar cells were fabricated by H. Choi, H. Kim, and J. Lim and analysed by H. Choi, M. Park, and C. S. Pathak under comments of S. Song. Lastly, the manuscript was written by H. Choi and H. Lim and advised by S. Song.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Technology Development Program (S3324563) funded by the Ministry of SMEs and Startups (MSS, Korea), by the National Research Foundation of Korea (NRF) grant by the Ministry of Education (NRF-2022R1F1A1074465), and Korea government (MSIT) (2022M3J1A1064317).References
- L. Chouhan, S. Ghimire, C. Subrahmanyam, T. Miyasaka and V. Biju, Chem. Soc. Rev., 2020, 49, 2869–2885 RSC.
- J. Y. Kim, J.-W. Lee, H. S. Jung, H. Shin and N.-G. Park, Chem. Rev., 2020, 120, 7867–7918 CrossRef CAS PubMed.
- P. V. Shinde, A. Patra and C. S. Rout, J. Mater. Chem. C, 2022, 10, 10196–10223 RSC.
- NREL, Best Research-Cell Efficiency Chart, https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf, accessed March, 2023 Search PubMed.
- L. Meng, J. You and Y. Yang, Nat. Commun., 2018, 9, 5265 CrossRef CAS.
- Y. Cheng and L. Ding, Energy Environ. Sci., 2021, 14, 3233–3255 RSC.
- N. Li, X. Niu, Q. Chen and H. Zhou, Chem. Soc. Rev., 2020, 49, 8235–8286 RSC.
- J. Yang, B. D. Siempelkamp, D. Liu and T. L. Kelly, ACS Nano, 2015, 9, 1955–1963 CrossRef CAS PubMed.
- K. Choi, J. Lee, H. Choi, G.-W. Kim, H. I. Kim and T. Park, Energy Environ. Sci., 2020, 13, 5059–5067 RSC.
- H. Min, D. Y. Lee, J. Kim, G. Kim, K. S. Lee, J. Kim, M. J. Paik, Y. K. Kim, K. S. Kim, M. G. Kim, T. J. Shin and S. I. Seok, Nature, 2021, 598, 444–450 CrossRef CAS PubMed.
- T. Zhang, F. Wang, H.-B. Kim, I.-W. Choi, C. Wang, E. Cho, R. Konefal, Y. Puttisong, K. Terado, L. Kobera, M. Chen, M. Yang, S. Bai, B. Yang, J. Suo, S.-C. Yang, X. Liu, F. Fu, H. Yoshida, W. M. Chen, J. Brus, V. Coropceanu, A. Hagfeldt, J.-L. Brédas, M. Fahlman, D. S. Kim, Z. Hu and F. Gao, Science, 2022, 377, 495–501 CrossRef CAS PubMed.
- J. J. Yoo, G. Seo, M. R. Chua, T. G. Park, Y. Lu, F. Rotermund, Y.-K. Kim, C. S. Moon, N. J. Jeon, J.-P. Correa-Baena, V. Bulović, S. S. Shin, M. G. Bawendi and J. Seo, Nature, 2021, 590, 587–593 CrossRef CAS PubMed.
- R. Sun, Q. Tian, M. Li, H. Wang, J. Chang, W. Xu, Z. Li, Y. Pan, F. Wang and T. Qin, Adv. Funct. Mater., 2023, 33, 2210071 CrossRef CAS.
- W. H. Nguyen, C. D. Bailie, E. L. Unger and M. D. McGehee, J. Am. Chem. Soc., 2014, 136, 10996–11001 CrossRef CAS.
- J. Lee, M. M. Byranvand, G. Kang, S. Y. Son, S. Song, G.-W. Kim and T. Park, J. Am. Chem. Soc., 2017, 139, 12175–12181 CrossRef CAS PubMed.
- G.-W. Kim, G. Kang, J. Kim, G.-Y. Lee, H. I. Kim, L. Pyeon, J. Lee and T. Park, Energy Environ. Sci., 2016, 9, 2326–2333 RSC.
- S. Wang, Z. Huang, X. Wang, Y. Li, M. Günther, S. Valenzuela, P. Parikh, A. Cabreros, W. Xiong and Y. S. Meng, J. Am. Chem. Soc., 2018, 140, 16720–16730 CrossRef CAS.
- A. K. Jena, M. Ikegami and T. Miyasaka, ACS Energy Lett., 2017, 2, 1760–1761 CrossRef CAS.
- N. J. Jeon, H. Na, E. H. Jung, T.-Y. Yang, Y. G. Lee, G. Kim, H.-W. Shin, S. I. Seok, J. Lee and J. Seo, Nat. Energy, 2018, 3, 682–689 CrossRef CAS.
- S.-Y. Jeong, H.-S. Kim and N.-G. Park, ACS Appl. Mater. Interfaces, 2022, 14, 34220–34227 CrossRef CAS.
- T. K. Zhang, F. Wang, H. B. Kim, I. W. Choi, C. F. Wang, E. Cho, R. Konefal, Y. Puttisong, K. Terado, L. Kobera, M. Y. Chen, M. Yang, S. Bai, B. W. Yang, J. J. Suo, S. C. Yang, X. J. Liu, F. Fu, H. Yoshida, W. M. M. Chen, J. Brus, V. Coropceanu, A. Hagfeldt, J. L. Bredas, M. Fahlman, D. S. Kim, Z. J. Hu and F. Gao, Science, 2022, 377, 495 CrossRef CAS PubMed.
- W. H. Nguyen, C. D. Bailie, E. L. Unger and M. D. McGehee, J. Am. Chem. Soc., 2014, 136, 10996 CrossRef CAS PubMed.
- J. Kong, Y. Shin, J. A. Röhr, H. Wang, J. Meng, Y. Wu, A. Katzenberg, G. Kim, D. Y. Kim, T. D. Li, E. Chau, F. Antonio, T. Siboonruang, S. Kwon, K. Lee, J. R. Kim, M. A. Modestino, H. Wang and A. D. Taylor, Nature, 2021, 594, 51 CrossRef CAS PubMed.
- F. M. Rombach, S. A. Haque and T. J. Macdonald, Energy Environ. Sci., 2021, 14, 5161–5190 RSC.
- L. Caliò, M. Salado, S. Kazim and S. Ahmad, Joule, 2018, 2, 1800–1815 CrossRef.
- T. Zhang, F. Wang, H.-B. Kim, I.-W. Choi, C. Wang, E. Cho, R. Konefal, Y. Puttisong, K. Terado, L. Kobera, M. Chen, M. Yang, S. Bai, B. Yang, J. Suo, S.-C. Yang, X. Liu, F. Fu, H. Yoshida, W. M. Chen, J. Brus, V. Coropceanu, A. Hagfeldt, J.-L. Brédas, M. Fahlman, D. S. Kim, Z. Hu and F. Gao, Science, 2022, 377, 495–501 CrossRef CAS PubMed.
- X. Ji, T. Zhou, X. Ke, W. Wang, S. Wu, M. Zhang, D. Lu, X. Zhang and Y. Liu, J. Mater. Chem. A, 2020, 8, 5163–5170 RSC.
- G.-W. Kim, G. Kang, K. Choi, H. Choi and T. Park, Adv. Energy Mater., 2018, 8, 1801386 CrossRef.
- G.-W. Kim, J. Lee, G. Kang, T. Kim and T. Park, Adv. Energy Mater., 2018, 8, 1701935 CrossRef.
- W. Wang, J. Zhou and W. Tang, J. Mater. Chem. A, 2022, 10, 1150–1178 RSC.
- G. Qu, L. Dong, Y. Qiao, D. Khan, Q. Chen, P. Xie, X. Yu, X. Liu, Y. Wang, J. Chen, X. Chen and Z.-X. Xu, Adv. Funct. Mater., 2022, 32, 2206585 CrossRef CAS.
- J. Lee, G.-W. Kim, M. Kim, S. A. Park and T. Park, Adv. Energy Mater., 2020, 10, 1902662 CrossRef CAS.
- Q. Fu, X. Tang, H. Liu, R. Wang, T. Liu, Z. Wu, H. Y. Woo, T. Zhou, X. Wan, Y. Chen and Y. Liu, J. Am. Chem. Soc., 2022, 144, 9500–9509 CrossRef CAS.
- E. H. Jung, N. J. Jeon, E. Y. Park, C. S. Moon, T. J. Shin, T.-Y. Yang, J. H. Noh and J. Seo, Nature, 2019, 567, 511–515 CrossRef CAS.
- D. Xu, Z. Gong, Y. Jiang, Y. Feng, Z. Wang, X. Gao, X. Lu, G. Zhou, J.-M. Liu and J. Gao, Nat. Commun., 2022, 13, 7020 CrossRef CAS PubMed.
- S. S. Mali, J. V. Patil, J. A. Steele, S. R. Rondiya, N. Y. Dzade and C. K. Hong, ACS Energy Lett., 2021, 6, 778–788 CrossRef CAS PubMed.
- J. Song, H. Xie, E. L. Lim, Y. Li, T. Kong, Y. Zhang, X. Zhou, C. Duan and D. Bi, Sol. RRL, 2022, 6, 2100880 CrossRef CAS.
- Q. K. Hu, E. Rezaee, Q. S. Dong, H. Q. Shan, Q. Chen, L. D. Wang, B. C. Liu, J. H. Pan and Z. X. Xu, Sol. RRL, 2019, 3, 1800264 CrossRef.
- C. M. Pelicano and H. Yanagi, J. Energy Chem., 2018, 27, 455–462 CrossRef.
- Y. Zhang, M. Elawad, Z. Yu, X. Jiang, J. Lai and L. Sun, RSC Adv., 2016, 6, 108888–108895 RSC.
- M. J. Jeong, K. M. Yeom, S. J. Kim, E. H. Jung and J. H. Noh, Energy Environ. Sci., 2021, 14, 2419–2428 RSC.
- M.-H. Li, J.-Y. Shao, Y. Jiang, F.-Z. Qiu, S. Wang, J. Zhang, G. Han, J. Tang, F. Wang, Z. Wei, Y. Yi, Y.-W. Zhong and J.-S. Hu, Angew. Chem., Int. Ed., 2021, 60, 16388–16393 CrossRef CAS PubMed.
- J. J. Yoo, S. Wieghold, M. C. Sponseller, M. R. Chua, S. N. Bertram, N. T. P. Hartono, J. S. Tresback, E. C. Hansen, J.-P. Correa-Baena, V. Bulović, T. Buonassisi, S. S. Shin and M. G. Bawendi, Energy Environ. Sci., 2019, 12, 2192–2199 RSC.
- Q. Jiang, Y. Zhao, X. Zhang, X. Yang, Y. Chen, Z. Chu, Q. Ye, X. Li, Z. Yin and J. You, Nat. Photonics, 2019, 13, 460–466 CrossRef CAS.
- M. J. Kim, M. Lee, H. Min, S. Kim, J. Yang, H. Kweon, W. Lee, D. H. Kim, J.-H. Choi, D. Y. Ryu, M. S. Kang, B. Kim and J. H. Cho, Nat. Commun., 2020, 11, 1520 CrossRef CAS.
- B. Liu, R.-Q. Png, L.-H. Zhao, L.-L. Chua, R. H. Friend and P. K. H. Ho, Nat. Commun., 2012, 3, 1321 CrossRef PubMed.
- S. Song, E. Y. Park, B. S. Ma, D. J. Kim, H. H. Park, Y. Y. Kim, S. S. Shin, N. J. Jeon, T. S. Kim and J. Seo, Adv. Energy Mater., 2021, 11, 2003382 CrossRef CAS.
- W. Zhao, J. Xu, K. He, Y. Cai, Y. Han, S. Yang, S. Zhan, D. Wang, Z. Liu and S. Liu, Nano-Micro Lett., 2021, 13, 169 CrossRef CAS.
- Y. Zhou, Z. Wang, J. Jin, X. Zhang, J. Zou, F. Yao, Z. Zhu, X. Cui, D. Zhang, Y. Yu, C. Chen, D. Zhao and Q. Cao, Angew. Chem., 2023, 135, e202300759 CrossRef.
- W.-M. Gu, K.-J. Jiang, F. Li, G.-H. Yu, Y. Xu, X.-H. Fan, C.-Y. Gao, L.-M. Yang and Y. Song, Chem. Eng. J., 2022, 444, 136644 CrossRef CAS.
- X. Ding, H. Wang, C. Chen, H. Li, Y. Tian, Q. Li, C. Wu, L. Ding, X. Yang and M. Cheng, Chem. Eng. J., 2021, 410, 128328 CrossRef CAS.
- H. Wang, W. Zhang, B. Wang, Z. Yan, C. Chen, Y. Hua, T. Wu, L. Wang, H. Xu and M. Cheng, Nano Energy, 2023, 111, 108363 CrossRef CAS.
- J. H. Yun, I. Lee, T.-S. Kim, M. J. Ko, J. Y. Kim and H. J. Son, J. Mater. Chem. A, 2015, 3, 22176–22182 RSC.
- R. Ichwani, V. Uzonwanne, A. Huda, R. Koech, O. K. Oyewole and W. O. Soboyejo, ACS Appl. Energy Mater., 2022, 5, 6011–6018 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta01910a |
| This journal is © The Royal Society of Chemistry 2023 |