Open Access Article
Open Access ArticleDirect mechanocatalysis by resonant acoustic mixing (RAM)†
Cameron B.
Lennox
 ab,
Tristan H.
Borchers
ab,
Lori
Gonnet
ab,
Tristan H.
Borchers
ab,
Lori
Gonnet
 ab,
Christopher J.
Barrett
ab,
Christopher J.
Barrett
 b,
Stefan G.
Koenig
b,
Stefan G.
Koenig
 *c,
Karthik
Nagapudi
*c,
Karthik
Nagapudi
 *c and
Tomislav
Friščić
*c and
Tomislav
Friščić
 *ab
*ab
aSchool of Chemistry, University of Birmingham, Birmingham, B15 2TT, UK. E-mail: t.friscic@bham.ac.uk
bDepartment of Chemistry, McGill University, 801 Sherbrooke St. W., Montreal, Quebec H3H 0B8, Canada
cSmall Molecule Pharmaceutical Sciences, Genentech Inc., One DNA Way, South San Francisco, CA 94080, USA
First published on 18th May 2023
Abstract
We demonstrate the use of a metal surface to directly catalyse copper-catalysed alkyne–azide click-coupling (CuAAC) reactions under the conditions of Resonant Acoustic Mixing (RAM) – a recently introduced and scalable mechanochemical methodology that uniquely eliminates the need for bulk solvent, as well as milling media. By using a simple copper coil as a catalyst, this work shows that direct mechanocatalysis can occur in an impact-free environment, relying solely on high-speed mixing of reagents against a metal surface, without the need for specially designed milling containers and media. By introducing an experimental setup that enables real-time Raman spectroscopy monitoring of RAM processes, we demonstrate 0th-order reaction kinetics for several selected CuAAC reactions, supporting surface-based catalysis. The herein presented RAM-based direct mechanocatalysis methodology is simple, enables the effective one-pot, two-step synthesis of triazoles via a combination of benzyl azide formation and CuAAC reactions on a wide scope of reagents, provides control over reaction stoichiometry that is herein shown to be superior to that seen in solution or by using more conventional CuCl catalyst, and is applied for simple gram-scale synthesis of the anticonvulsant drug Rufinamide.
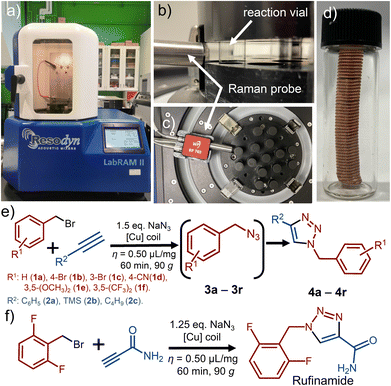
Mechanochemistry is a versatile synthetic approach which1–4 avoids the need for bulk solvents, making it intrinsically more green and environmentally-friendly,5,6 and can offer access to reactions and products otherwise difficult to achieve.7,8 While mechanosynthesis traditionally relies on impact and mixing resulting from the use of balls or screws, Resonant Acoustic Mixing (RAM, Fig. 1a–c)9,10 has recently emerged as a strategy to conduct mechanochemical reactions without such milling or crushing media. Instead, RAM achieves reactivity by shaking materials at a low acoustic frequency (e.g., 60 Hz), with energy input modulated through changes in the vertical acceleration of the reaction vessel (0–100 g, where g = 9.81 m s−2). By avoiding the need for milling media, RAM enables simplification of reaction design, avoids product contamination resulting from chipping and abrasion, and facilitates scale-up. So far, RAM has been shown to enable a simple, efficient, and rapid approach to metal–organic frameworks, cocrystals, mechano-redox catalysis, as well as several metal-catalyzed transformations.11
An exciting emergent opportunity in ball-milling mechanochemistry is direct mechanocatalysis, where components of the milling assembly itself (e.g., balls and/or vessel walls) acts as the metal catalyst.12–17 This methodology, pioneered by the Mack and Borchardt groups, has so far been applied to several copper-, palladium-, silver- or nickel-based transformations.8b,12,15,18–20 The key advantages of direct mechanocatalysis are straightforward introduction, removal, and reusability of metal catalyst, as well as simplicity of product recovery, but the methodology is challenged by the need to manufacture specialised ball-milling equipment.
As direct mechanocatalysis involves dynamic contact between the reaction mixture and a metal, we speculated it should be compatible with RAM, potentially offering an even simpler, more efficient synthesis by bringing into contact the reaction mixture and a metal surface, without the need to design specialised equipment or generate impact by using milling media.
Here, we provide a proof-of-principle demonstration of direct mechanocatalysis by RAM, focusing on the copper-catalyzed alkyne–azide click-coupling (CuAAC) as a model reaction. RAM enabled a surprisingly uncomplicated approach to direct mechanocatalysis, replacing custom-made milling equipment with simple copper wire. This straightforward reaction platform allowed fast (≤60 minutes) direct mechanocatalysis, without copper-based milling media or vessels ubiquitous in previous examples of copper-based direct mechanocatalysis. While the herein developed process is applicable to a range of solid or liquid benzyl bromide and alkyne reactants, we also show that it permits selective mono- or bis-derivatization, as well as desymmetrization of a symmetrical dialkyne reactant, and is readily scalable to at least one gram, as demonstrated in the RAM synthesis of the anticonvulsant API Rufinamide.
As the model reaction we used the one-pot, two-step reaction of benzyl bromide (1a) and phenylacetylene (2a) in the presence of 1.5 equivalents (50% excess) of NaN3. The reaction is expected to proceed via the intermediate benzyl azide (3a) to form the click coupling product 4a (Fig. 1e). As the catalyst, we used a piece of copper wire of 0.9 mm diameter (#20-gauge), wound into a coil of 45 mm × 10 mm dimensions. The coil was wedged between the vial cap and the bottom of the vial, preventing it from moving during mixing. The reaction was conducted by RAM in the presence of a liquid additive, with the ratio of the volume of liquid additive to the weight of reaction mixture η = 0.50 μL mg−1. As the liquid additive, we explored dimethylsulfoxide (DMSO), water, methanol (MeOH), and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by volume mixture of water and DMSO, among which DMSO alone was found to be the most effective. While the initial attempt of reaction gave 4a in conversion below 4 ± 3% after 60 minutes (Table 1, Entry 2), subsequent attempts using the same copper coil gave 4a quantitatively within 60 minutes (Fig. 2a, Table 1, Entry 3, also ESI†).
1 by volume mixture of water and DMSO, among which DMSO alone was found to be the most effective. While the initial attempt of reaction gave 4a in conversion below 4 ± 3% after 60 minutes (Table 1, Entry 2), subsequent attempts using the same copper coil gave 4a quantitatively within 60 minutes (Fig. 2a, Table 1, Entry 3, also ESI†).
| Entry | Copper source | Copper treatment | Conversionb (%) |
|---|---|---|---|
| a Reactions conducted by RAM of equimolar (2 mmol) amounts of 1a and 2a with 1.5 equivalents of NaN3, and DMSO (η = 0.50 μL mg−1) at 90 g for 60 minutes. b Based on triplicate experiments and 1H NMR analysis following dissolution of the entire reaction mixture in CDCl3. c RAM of equimolar amounts of 1a and 2a with 1.5 equivalents of NaN3, 3 mol% (relative to 1a) copper salt, with DMSO (η = 0.50 μL mg−1) at 90 g for 60 minutes. d 300 mg of solid with DMSO (η = 0.50 μL mg−1). e Coil soaked for 24 h in 2 mL alkyne 2a in a sealed vial at room temperature. f RAM of 300 mg SiO2, 256 μL 2a, and DMSO (η = 0.50 μL mg−1) at 90 g for 60 minutes, followed by washing with EtOAc. g Reaction performed at a three times larger scale: 6 mmol of 1a and 2a each and 9 mmol of NaN3, with DMSO (η = 0.50 μL mg−1) at 90 g for 60 minutes. | |||
| 1 | — | n/a | 0 |
| 2 | Cu coil | n/a | 4 ± 3 |
| 3 | Cu coil | 1a, 2a, NaN3a | >95 |
| 4 | CuClc | n/a | 31 ± 4 |
| 5 | Cu(OAc)2·H2Oc | n/a | 26 ± 6 |
| 6 | Cu coil | Celite | 6 ± 1 |
| 7 | Cu coil | SiO2d | 36 ± 2 |
| 8 | Cu coil | 2a | 14 ± 6 |
| 9 | Cu coil | SiO2d followed by 2ae | >95 |
| 10 | Cu coil | 2a and SiO2f | >95 |
| 11 | Cu coil | 125 °C for 24 h | 34 ± 2 |
| 12 | Cu coil | CH3COOHe followed by 125 °C for 24 h | 68 ± 1 |
| 13 | Cu coil | 1a, 2a, NaN3a,g | >95g |
Similar behaviour was observed when the reaction was conducted using 20 minute cycles: improvement in reactivity was seen only after the third cycle (Fig. 2a, also ESI†). Overall, these observations indicate an induction period of ca. 60 minutes, after which the copper coil becomes a highly effective catalyst.12 For comparison, the analogous one-pot reaction using either CuCl or copper(II) acetate monohydrate as the catalyst (3 mol% relative to 1a) gave no more than 30% conversion after 60 minutes of RAM (Table 1, Entries 4 and 5, and ESI†). Using either MeOH or water as a liquid additive led to maximum conversions of ca. 80% and 30% at η-value of 0.50 μL mg−1, respectively, while the mixture of water and DMSO led to conversions below 10% in all cases (see ESI†).
Using a piece of uncoiled copper wire (45 mm length, diameter 0.9 mm, #20-gauge) gave significantly lower conversion to 4a (24 ± 4%), indicating the importance of metal surface. We also explored the potential effect of atmosphere on this direct RAM mechanocatalysis procedure, revealing only 13 ± 4% conversion under an argon atmosphere after 60 minutes, while conducting the same reaction in an O2 atmosphere led to complete conversion to 4a. No product arising from a Glaser-type homocoupling was ever observed. X-ray photoelectron spectroscopy (XPS) on a copper coil before and immediately after a 60 minute RAM reaction revealed the appearance Cu(I) after RAM, which upon exposure to air further oxidized to Cu(II). We conclude that the process of activating the copper coil is related to the formation of Cu(I) species on the surface, which requires the presence of oxygen (see ESI†).
Next, we investigated the possibility to deliberately activate the copper coil through different treatments (Table 1, Entries 6–12). Considering that cleaning the metal surface might play a role, we investigated RAM in the presence of abrasive Celite, which did not improve reactivity in the first cycle. Using SiO2 as the additive, however, led to 36 ± 2% conversion. We also explored the effect of soaking a fresh copper coil in 2a for 24 h before use, with little improvement on reactivity. However, soaking in 2a in a coil that had previously been treated with SiO2 led to quantitative conversion. Indeed, quantitative conversion to 4a was achieved by RAM within 60 minutes, by using a coil that has been primed by RAM in the presence of SiO2 and 2a for 60 minutes at 90 g. This priming procedure resulted in a Cu(I)-containing surface, as observed by XPS, suggesting that the induction period involves a surface-cleaning process and oxidation to an activated, likely copper(I)-acetylide, layer. Importantly, if the copper coil is stored in an inert Ar atmosphere immediately after activation, it remains catalytically active for at least two weeks. However, if stored in air, the coil loses catalytic activity within 24 hours and needs to be re-activated. This also indicates that coil activation involves the formation of surface Cu(I) species.
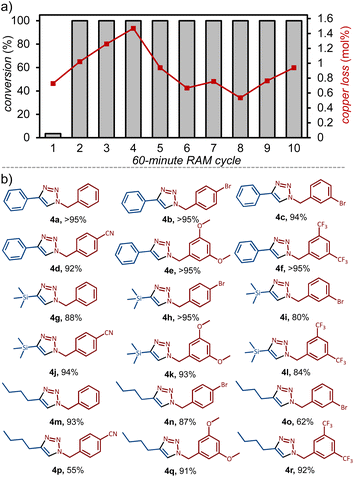
To investigate whether coil activation and reaction conversion might be related to leaching of copper from the coil, the conversion to 4a and any changes to the weight of the coil were measured over a set of 10 sequential 60 minutes reaction cycles. The analysis was performed for four separate copper coils, amounting to a total of 40 experiments, which revealed an average copper loss of 0.9 mol ± 0.3% (relative to 2a) in each reaction cycle (Fig. 2a, also ESI†). For each of the four coils, we also verified the amount of copper leached in the third cycle by inductively coupled plasma mass spectrometry (ICP-MS), revealing a loss of 0.81 mol%, consistent with the value obtained by weighing. No correlation was observed between the amount of copper leached and reaction conversion after each reaction cycle – after the first cycle, all reactions exhibited quantitative conversion (Fig. 2a). As using 3 mol% of CuCl as the catalyst led to only ca. 30% conversion to 4a, we conclude that a significant amount of catalysis must be occurring on the metal coil surface (Table 1, Entries 3–5, also ESI†).21
Moreover, we have conducted the catalytic synthesis of 4a using a three-fold increase of starting materials (Table 1, Entry 13). Under these conditions, the amount of copper lost from the coil averaged to 0.95 ± 0.3 mg across four experiments, i.e. the amount of copper lost from the coil was now only 0.25 ± 0.08 mol% with respect to the alkyne reactant, while the conversion remained at quantitative level. These observations are consistent with the entire copper coil being exposed to the sample throughout the RAM process, and also support the view that the majority, if not all, of catalysis is taking place on the metal surface and not via copper lost by abrasion.
With reaction conditions for direct mechanocatalysis by RAM in hand, we extended the method to a range of liquid and solid alkyl bromides 1a–1f and alkynes: phenylacetylene (2a), trimethylsilylacetylene (2b), and 1-hexyne (2c). With few exceptions, the expected cycloaddition products 4a–4r (Fig. 2b) were obtained after workup in near-quantitative conversions and >90% isolated yields, following RAM of an equimolar (2 mmol) amount of alkyl bromide and alkyne, with 1.5 equivalents NaN3 in the presence of a copper coil and DMSO liquid additive (η = 0.50 μL mg−1). The workup consisted of adding 2 mL of ethyl acetate (EtOAc) to the reaction vial, followed by RAM for an additional 5 minutes, and filtration to remove excess NaN3 and the byproduct NaBr. The cycloaddition product was then precipitated by adding 3 mL of a water:MeOH mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v), followed by another 5 minutes of RAM and filtration to yield white or yellow solids. For 4g–4l, work-up involved washing with a mixture of concentrated aqueous solution of NH4Cl and EtOAc (1
2 v/v), followed by another 5 minutes of RAM and filtration to yield white or yellow solids. For 4g–4l, work-up involved washing with a mixture of concentrated aqueous solution of NH4Cl and EtOAc (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v), before evaporation in vacuo to yield a yellow solid or oil.‡
2 v/v), before evaporation in vacuo to yield a yellow solid or oil.‡
To verify that the reaction was indeed taking place by RAM rather than during subsequent analysis, the synthesis of 4a was interrupted after 20 minutes, and immediately accessed by 1H-NMR in CDCl3 which revealed ca. 25% conversion, and then again 24 hours later, revealing no change in conversion. The products were characterised by 1H-, 13C-NMR, FTIR spectroscopy, and HR-MS, and 4e was additionally characterized by single crystal X-ray diffraction, confirming that [2 + 3] cycloaddition took place (see ESI†. Investigation of selected samples by scanning electron microscopy (SEM) revealed that crude materials from RAM synthesis were composed of well-developed micrometre-sized crystals (see ESI†).
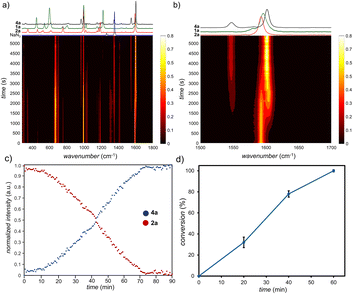
Noting that the RAM platform should be highly amenable to in situ monitoring,22 we devised a setup for real-time reaction monitoring using Raman spectroscopy, previously not described in the context of RAM mechanochemistry (see ESI†). Specifically, real-time reaction monitoring was done using a RamanRxn1™ analyser by Kaiser Optical Systems Inc., equipped with a power tuneable 1–400 mW 785 nm Raman probe. Spectra were recorded with an integration time of 5–10 seconds and 3–5 accumulations to optimise the signal-to-noise ratio. All spectra were dark and intensity corrected using the Holograms® software package before being processed with MATLAB. In a typical monitoring experiment, a 1-dram volume glass vial was placed in the custom-made sample holder containing an entrance slit (Fig. 1b, also ESI†). The 785 nm Raman probe was then placed approximately 10 mm from the vial, with the focus on the vial centre (see ESI†). Using this monitoring setup enabled us to observe linear kinetics for the formation of 4a (Fig. 3a–c).
Accuracy of the method was validated by ex situ monitoring of the reaction progress by 1H NMR in separate experiments, which also showed a linear profile (Fig. 3d). A linear kinetic profile was also observed for the coupling of 2a with the solid reactant 3,5-dimethoxybenzyl bromide (1e) to give the triazole 4e (see ESI†). Overall, the experimentally observed linear kinetic profiles indicate 0th-order reaction kinetics, consistent with surface catalysis.
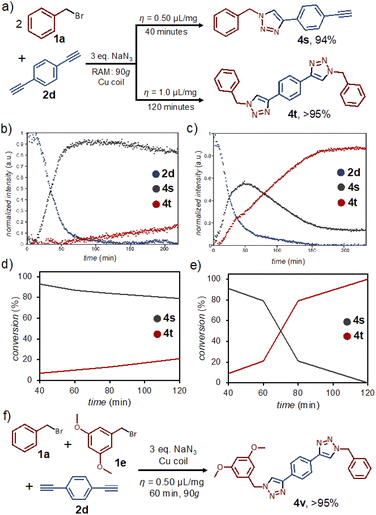
Mechanochemical reactions by ball milling have previously been shown to permit excellent control of reaction stoichiometry, enabling selective functionalization of substrates bearing multiple reaction sites.23 In order to investigate if such selectivity is accessible in RAM, we explored the reactivity of the symmetrical dialkyne p-diethynylbenzene (2d) (Fig. 4a). Real-time Raman spectroscopy monitoring revealed that the reaction selectivity is strongly affected by η, even independent of reaction stoichiometry. Specifically, following the reaction of 2d and 1a in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 respective stoichiometric ratio in the presence of DMSO (η = 0.50 μL mg−1) revealed almost complete conversion into the non-symmetrical 4s within 60 minutes. After 4 hours, only a small amount of the symmetrical 4t was observed. At η = 1.0 μL mg−1, however, the reaction rapidly proceeds further and 4s only appears as a reaction intermediate, quantitatively yielding 4t after 2 hours (Fig. 4b and c).14 The ability to selectively obtain either 4s or 4t by RAM contrasts with reactivity observed in solution: performing the same reaction under dilute conditions (10 mol% CuCl, 25 mL DMSO) produced after 24 hours only 4t, in 28% conversion.
2 respective stoichiometric ratio in the presence of DMSO (η = 0.50 μL mg−1) revealed almost complete conversion into the non-symmetrical 4s within 60 minutes. After 4 hours, only a small amount of the symmetrical 4t was observed. At η = 1.0 μL mg−1, however, the reaction rapidly proceeds further and 4s only appears as a reaction intermediate, quantitatively yielding 4t after 2 hours (Fig. 4b and c).14 The ability to selectively obtain either 4s or 4t by RAM contrasts with reactivity observed in solution: performing the same reaction under dilute conditions (10 mol% CuCl, 25 mL DMSO) produced after 24 hours only 4t, in 28% conversion.
The observations made by in situ Raman monitoring were also confirmed by 1H NMR analysis of the entire reaction mixture, which revealed selective formation of 4s at η = 0.50 μL mg−1 within 40 minutes, and switching of the selectivity to 4t at η = 1.0 μL mg−1 within 2 hours (Fig. 4d, e). The formation of 4s and 4t was verified by 1H- and 13C-NMR, FTIR spectroscopy and HR-MS (see ESI†). The reaction kinetics based on 1H-NMR analysis of reaction mixtures in separate experiments was similar, but not identical to that based on Raman monitoring, most likely because the latter is limited to only a small fraction of the sample surface. The selectivity for the non-symmetrical 4s at lower η-values might be related to poorer mixing in the presence of NaBr byproduct. This view was supported by two separate experiments. First, the introduction of one equivalent of NaBr to a mixture of 1a and 2a was found to prevent the normally high-yielding formation of 4a within 60 minutes. Second, complete conversion to the di-substituted 4t is readily achieved within 60 minutes of RAM if previously isolated 4s is used as the reactant in combination with one equivalent of 1a.
A similar stepwise process was also observed for the solid reactant 1e, as established by 1H-NMR analysis. The reaction of the dialkyne 2d with 1e was much faster, however, leading to complete conversion to the disubstituted product 4u at η = 0.50 μL mg−1 in 60 minutes (see ESI†).
The ability to achieve desymmetrisation of the dialkyne 2d, and the notably higher reactivity observed for 1e compared to 1a under RAM conditions, led us to explore the one-pot synthesis of a non-symmetrical target bis(triazole) 4v (Fig. 4f) directly from equimolar amounts of 1a, 1e, and the dialkyne 2d. After 60 minutes of RAM with a copper coil, 3 equivalents of NaN3 and DMSO liquid additive (η = 0.50 μL mg−1), the reaction selectively and quantitatively gave targeted 4v, as confirmed by 1H- and 13C-NMR, 1H–1H COSY-NMR, FTIR spectroscopy and HR-MS (see ESI†). The selectivity of this process is remarkable, as the analogous solution reaction using CuCl as catalyst (10 mol%, 25 mL DMSO) gave a mixture of 4s (25%), 4t (18%), 4u (21%), 4v (19%), along with the mono-triazole product (16%) arising from a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 reaction of 1a and 1e after 24 hours. Moreover, the high selectivity appears to be associated to using the copper coil as the catalyst, as the RAM reaction using CuCl catalyst (10 mol%) also led to a mixture of products 4s (62%), 4t (2%), 4u (23%) and 4v (5%) after 60 minutes. We speculate that selectivity of direct mechanocatalysis for 4v might be related to adsorption of the reactant 2d on the coil surface, and continue to investigate this effect.
1 reaction of 1a and 1e after 24 hours. Moreover, the high selectivity appears to be associated to using the copper coil as the catalyst, as the RAM reaction using CuCl catalyst (10 mol%) also led to a mixture of products 4s (62%), 4t (2%), 4u (23%) and 4v (5%) after 60 minutes. We speculate that selectivity of direct mechanocatalysis for 4v might be related to adsorption of the reactant 2d on the coil surface, and continue to investigate this effect.
Finally, to evaluate the applicability of RAM direct mechanocatalysis for the synthesis of a functional molecule, we targeted Rufinamide, an anti-epileptic triazole-containing API. The RAM reaction of equimolar amounts of 2,5-difluorobenzyl bromide and propiolamide with DMSO (η = 0.50 μL mg−1), 1.5 equivalents of NaN3 and a copper coil, gave Rufinamide in 88% isolated yield after 60 minutes at 90 g, as confirmed by 1H- and 13C-NMR, FTIR spectroscopy and HR-MS (see ESI†). The reaction was readily scalable at least 5-fold, to provide 1.2 grams of Rufinamide within 1 hour. This creates an alternative synthetic route to the high temperatures, long reaction times, and multi-step processes currently used in solution.24
Conclusions
In summary, we have provided the first proof-of-principle for direct mechanocatalysis by resonant acoustic mixing. This work shows that RAM enables using a simple copper coil as the catalyst for rapid, simple, and high-yielding direct-mechanocatalytic copper-catalyzed alkyne–azide coupling on a wide range of substrates, enabling also the simple and gram-scale synthesis of the API Rufinamide. Direct mechanocatalysis by RAM also enabled stoichiometric selectivity greatly superior to that seen when using a more conventional CuCl catalyst, either in solution or under ball-milling conditions, enabling the high-yielding, rapid and one-pot synthesis of non-symmetrical products from an initially symmetrical dialkyne reactant. Herein introduced technique for real-time Raman spectroscopy monitoring, validated by stepwise 1H NMR spectroscopy analysis, reveals that reactions follow a 0th-order kinetic profile consistent with surface catalysis. Overall, this work shows that direct mechanocatalysis can occur in a mild, impact-free environment, relying solely on high-speed mixing of reagents against a copper surface. This offers a basis for the further simplification and development of highly selective mechanochemical surface-catalysed synthesis using the so far poorly explored but readily scalable RAM platform. More broadly, reactions involving raw metals either as catalysts or reactants are emerging as a particularly interesting area of mechanochemistry, and the herein presented work opens an exciting new entry to such developments, based on the RAM technology.25Data availability
Details of experimental procedures, and selected NMR, FTIR spectroscopy, HR-MS, ICP-MS, SEM, XPS, and single crystal X-ray diffraction data are provided in the ESI.†Author contributions
All authors have contributed to the writing of this manuscript. Development of mechanochemical synthesis procedure and characterization was performed by CBL and LG, in situ Raman spectroscopy monitoring was done by CBL and THB. The research was organized and coordinated by SGK, KN, and TF.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the support of the NSERC Discovery Grant (RGPIN-2017-06467); NSERC John C. Polanyi Award (JCP 562908-2022); Tier-1 Canada Research Chair Program (TF); NSERC CGS-D Scholarship (CBL); Leverhulme International Professorship (TF) and the University of Birmingham. GenenTech, Inc. is acknowledged for support.Notes and references
- (a) J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC; (b) J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC; (c) W. Pickhardt, S. Grätz and L. Borchardt, Chem.–Eur. J., 2020, 26, 12903–12911 CrossRef CAS PubMed; (d) O. Maurin, P. Verdié, G. Subra, F. Lamaty, J. Martinez and T.-X. Métro, Beilstein J. Org. Chem., 2017, 13, 2087–2093 CrossRef CAS PubMed; (e) T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed.
- (a) D. Tan and F. García, Chem. Soc. Rev., 2019, 48, 2274–2292 RSC; (b) N. R. Rightmire and T. P. Hanusa, Dalton Trans., 2016, 45, 2352–2362 RSC.
- (a) C. Bolm and J. G. Hernández, Angew. Chem., Int. Ed., 2019, 58, 3285–3299 CrossRef CAS PubMed; (b) P. A. Julien, C. Mottillo and T. Friščić, Green Chem., 2017, 19, 2729–2747 RSC.
- (a) P. Baláž, M. Achamovičová, M. Baláž, P. Billik, Z. Cherkezova-Zheleva, J. M. Criado, F. Delogu, E. Dutková, E. Gaffet, F. J. Gotor, R. Kumar, I. Mitov, T. Rojac, M. Senna, A. Streletskii and K. Wieczorek-Ciurowa, Chem. Soc. Rev., 2013, 42, 7571–7637 RSC; (b) A. A. L. Michalchuk, E. V. Boldyreva, A. M. Belenguer, F. Emmerling and V. V. Boldyrev, Front. Chem., 2021, 9, 685789 CrossRef CAS PubMed.
- (a) S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC; (b) E. Boldyreva, Chem. Soc. Rev., 2013, 42, 7719–7738 RSC; (c) A. Bose and P. Mal, Beilstein J. Org. Chem., 2019, 15, 881–900 CrossRef CAS PubMed; (d) R. T. O'Neill and R. Boulatov, Nat. Rev. Chem., 2021, 5, 148–167 CrossRef PubMed; (e) D. E. Crawford, C. K. G. Miskimmin, A. B. Albadarin, G. Walker and S. L. James, Green Chem., 2017, 19, 1507–1518 RSC.
- (a) D. Tan, L. Loots and T. Friščić, Chem. Commun., 2016, 52, 7760–7781 RSC; (b) E. Colacino, A. Porcheddu, C. Charnay and F. Delogu, React. Chem. Eng., 2019, 4, 1179 RSC.
- (a) J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007–4019 CrossRef PubMed; (b) K. Kubota, R. Takahashi and H. Ito, Chem. Sci., 2019, 10, 5837–5842 RSC.
- (a) R. F. Koby, T. P. Hanusa and N. D. Schley, J. Am. Chem. Soc., 2018, 140, 15934–15942 CrossRef CAS PubMed; (b) R. A. Haley, A. R. Zellner, J. A. Krause, H. Guan and J. Mack, ACS Sustainable Chem. Eng., 2016, 4, 2464–2469 CrossRef CAS; (c) F. Cuccu, L. DeLuca, F. Delogu, E. Colacino, N. Solin, R. Mocci and A. Porcheddu, ChemSusChem, 2022, 15, e202200362 CrossRef CAS PubMed.
- (a) J. G. Osorio, E. Hernández, R. J. Romañach and F. J. Muzzio, Powder Technol., 2016, 297, 349–356 CrossRef CAS; (b) A. Vandenberg and K. Wille, Constr. Build. Mater., 2018, 164, 716–730 CrossRef; (c) D. H. Leung, D. J. Lamberto, L. Liu, E. Kwong, T. Nelson, T. Rhodes and A. Bak, Int. J. Pharm., 2014, 473, 10–19 CrossRef CAS PubMed.
- (a) C. J. Wright, P. J. Wilkinson, S. E. Gaulter, D. Fossey, A. O. Burn and P. P. Gill, Propellants, Explos., Pyrotech., 2021, 46, 1–15 CrossRef; (b) M. Solares-Briones, G. Coyote-Dotor, J. C. Páez-Franco, M. R. Zermeño-Ortega, C. M. de le O Contreras, D. Canseco-González, A. Avila-Sorrosa, D. Morales-Morales and J. M. Germán-Acacio, Pharmaceutics, 2021, 13, 790 CrossRef CAS PubMed.
- (a) L. Gonnet, C. B. Lennox, J.-L. Do, I. Malvestiti, S. G. Koenig, K. Nagapudi and T. Friščić, Angew. Chem., Int. Ed., 2022, 61, e202115030 CrossRef CAS PubMed; (b) L. Gonnet, T. H. Borchers, C. B. Lennox, J. Vainauskas, Y. Teoh, H. M. Titi, C. J. Barrett, S. G. Koenig, K. Nagapudi and T. Friščić, Faraday Discuss., 2023, 241, 128–149 RSC; (c) H. M. Titi, J.-L. Do, A. J. Howarth, K. Nagapudi and T. Friščić, Chem. Sci., 2020, 11, 7578–7584 RSC; (d) K. Nagapudi, E. Y. Umanzor and C. Masui, Int. J. Pharm., 2017, 521, 337–345 CrossRef CAS PubMed; (e) F. Effaty, L. Gonnet, S. G. Koenig, K. Nagapudi, X. Ottenwaelder and T. Friščić, Chem. Commun., 2023, 59, 1010–1013 RSC.
- A similar induction period was seen for direct mechanocatalysis by ball-milling: (a) W. Su, J. Yu, Z. Li and Z. Jiang, J. Org. Chem., 2011, 76, 9144–9150 CrossRef CAS PubMed; (b) L. Chen, D. Leslie, M. G. Coleman and J. Mack, Chem. Sci., 2018, 9, 4650–4661 RSC; (c) T. L. Cook, J. A. Walker and J. Mack, Green Chem., 2013, 15, 617 RSC; (d) C. G. Vogt, S. Grätz, S. Lukin, I. Halasz, M. Etter, J. D. Evans and L. Borchardt, Angew. Chem., Int. Ed., 2019, 58, 18942–18947 CrossRef CAS PubMed.
- Y. Zhao, S. V. Rocha and T. M. Swager, J. Am. Chem. Soc., 2016, 138, 13834–13837 CrossRef CAS PubMed.
- Appearance of a mono-substituted intermediate in the mechanochemical click reaction of a di-alkyne was previously observed for the ball-milling reaction of 2d with decyl azide, see: R. Thorwirth, A. Stolle, B. Ondruschka, A. Wild and U. S. Schubert, Chem. Commun., 2011, 47, 4370–4372 RSC.
- (a) D. A. Fulmer, W. C. Shearouse, S. T. Medonza and J. Mack, Green Chem., 2009, 11, 1821–1825 RSC; (b) S. Hwang, S. Grätz and L. Borchardt, Chem. Commun., 2022, 58, 1661–1671 RSC; (c) R. A. Haley, J. Mack and H. Guan, Inorg. Chem. Front., 2017, 4, 52–55 RSC; (d) M. Wolgemuth, M. Mayer, M. Rappen, F. Schmidt, R. Saure, S. Grätz and L. Borchardt, Angew. Chem., Int. Ed., 2022, 61, e202212694 Search PubMed.
- S. Jung and H. J. Yoon, Angew. Chem., Int. Ed., 2021, 59, 4883–4887 CrossRef PubMed.
- (a) W. Pickhardt, S. Grätz and L. Borchardt, Chem.–Eur. J., 2020, 26, 12903–12911 CrossRef CAS PubMed; (b) L. Chen, M. O. Bovee, B. E. Lemma, K. S. M. Keithley, S. L. Pilson, M. G. Coleman and J. Mack, Angew. Chem., Int. Ed., 2015, 54, 11084–11087 CrossRef CAS PubMed.
- (a) W. Su, J. Yu, Z. Li and Z. Jiang, J. Org. Chem., 2011, 76, 9144–9150 CrossRef CAS PubMed; (b) C. G. Vogt, S. Grätz, S. Lukin, I. Halasz, M. Etter, J. D. Evans and L. Borchardt, Angew. Chem., Int. Ed., 2019, 58, 18942–18947 CrossRef CAS PubMed.
- (a) Y. Sawama, N. Yasukawa, K. Ban, R. Goto, M. Niikawa, Y. Monguchi, M. Itoh and H. Sakiji, Org. Lett., 2018, 20, 2892–2896 CrossRef CAS PubMed; (b) Y. Sawama, M. Niikawa and H. Sajiki, J. Synth. Org. Chem., Jpn., 2019, 77, 1070–1077 CrossRef CAS.
- C. G. Vogt, M. Oltermann, W. Pickhardt and S. Grätz, Adv. Energy Sustainability Res., 2021, 2, 2100011 CrossRef CAS.
- The Borchardt group has recently shown that catalysis in the Suzuki-Miyaura reactions conducted using a palladium-based milling assembly occurs exclusively on the ball, rather than abraded material, see: W. Pickhardt, C. Beaković, M. Mayer, M. Wohlgemuth, F. J. L. Kraus, M. Etter, S. Grätz and L. Borchardte, Angew. Chem., Int. Ed., 2022, 61, e202205003 CrossRef CAS PubMed.
- (a) A. A. L. Michalchuk, K. S. Hope, S. R. Kennedy, M. V. Blanco, E. V. Boldyreva and C. R. Pulham, Chem. Commun., 2018, 54, 4033–4036 RSC; (b) A. G. Buzanich, C. T. Cakir, M. Radtke, M. B. Haider, F. Emmerling, P. F. M. de Oliveira and A. A. L. Michalchuk, J. Chem. Phys., 2022, 157, 214202 CrossRef PubMed; (c) G. I. Lampronti, A. A. L. Michalchuk, P. P. Mazzeo, A. M. Belenguer, J. K. M. Sanders, A. Bacchi and F. Emmerling, Nat. Commun., 2021, 12, 6134 CrossRef CAS PubMed.
- (a) V. Štrukil, D. Margetić, M. D. Igrc, M. Eckert-Maksić and T. Friščić, Chem. Commun., 2012, 48, 9705–9707 RSC; (b) M. Ferguson, N. Giri, X. Huang, D. Apperley and S. L. James, Green Chem., 2014, 16, 1374–1382 RSC; (c) J. L. Howard, Y. Sagatov, L. Repusseau, C. Schotten and D. L. Browne, Green Chem., 2017, 19, 2798–2802 RSC; (d) T. Seo, K. Kubota and H. Ito, J. Am. Chem. Soc., 2020, 142, 9884–9889 CrossRef CAS PubMed; (e) R. Schmidt, A. Stolle and B. Ondruschka, Green Chem., 2012, 14, 1673 RSC.
- (a) R. Portmann and A. G. Novartis, Basel, Switzerland. U.S. Patent 6,156,907, 2000; (b) P. Bodhuri, S. P. Green, A. Karadeolian, and E. G. Cammisa, Apotex Technologies Inc., WO2014121383A1, 2014.
- For recent ball-milling examples, see: (a) A. C. Jones, M. T. J. Williams, L. C. Morrill and D. L. Browne, ACS Catal., 2022, 12, 13681–13689 CrossRef CAS PubMed; (b) Q. Cao, J. L. Howard, E. Wheatley and D. L. Browne, Angew. Chem., Int. Ed., 2018, 130, 11509–11513 CrossRef; (c) R. R. A. Bolt, L. C. Morrill, E. Richards and D. L. Browne, Angew. Chem., Int. Ed., 2021, 60, 23128–23133 CrossRef PubMed; (d) W. I. Nicholson, J. L. Howard, G. Magri, A. C. Serastram, A. Khan, R. R. A. Bolt, L. C. Morrill, E. Richards and D. L. Browne, Angew. Chem., Int. Ed., 2021, 60, 23128–23133 CrossRef CAS PubMed; (e) P. Gao, J. Jiang, S. Maeda, K. Kubota and H. Ito, Angew. Chem., Int. Ed., 2022, 41, e202207118 Search PubMed; (f) R. Takahashi, A. Hu, P. Gao, Y. Pang, T. Seo, J. Jiang, S. Maeda, H. Takaya, K. Kubota and H. Ito, Nat. Commun., 2021, 12, 6691 CrossRef CAS PubMed; (g) V. S. Pfennig, R. C. Viellella, J. Nikodemus and C. Bolm, Angew. Chem., Int. Ed., 2022, 61, e202116514 CrossRef CAS PubMed; (h) I. R. Speight and T. P. Hanusa, Molecules, 2020, 25, 570 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2223762. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3sc01591b |
| ‡ Workup was developed to minimise overall solvent consumption, with aqueous MeOH serving to hydrolyze any remaining benzyl bromide or benzyl azide reactant. |
| This journal is © The Royal Society of Chemistry 2023 |