Open Access Article
Open Access ArticleSelf-assembly and salt-induced thermoresponsive properties of amphiphilic PEG/cation random terpolymers in water†
Rikuto
Kanno
,
Makoto
Ouchi
 and
Takaya
Terashima
and
Takaya
Terashima
 *
*
Department of Polymer Chemistry, Graduate School of Engineering, Kyoto University, Katsura, Nishikyo-ku, Kyoto 615-8510, Japan. E-mail: terashima.takaya.2e@kyoto-u.ac.jp
First published on 24th January 2023
Abstract
Herein, we report the self-assembly and salt-induced thermoresponsive properties of amphiphilic random terpolymers consisting of hydrophilic poly(ethylene glycol) (PEG) and quaternary ammonium cations, and hydrophobic dodecyl groups in water. The random terpolymers self-assembled into size-controlled multichain micelles in pure water or in water containing NaCl. The micelle size increased upon increasing the content of the cationic groups in the total hydrophilic monomer units (∼50 mol%) and turned larger in the presence of NaCl than that in pure water. More uniquely, the random terpolymer micelles showed lower critical solution temperature-type solubility in water containing salts such as NaCl, while the solutions of the polymer micelles in pure water were transparent even upon heating to over 90 °C. The cloud point (Cp) temperature of the aqueous polymer micelle solution was controlled by the concentration of NaCl or the composition of the terpolymers. The critical concentration of NaCl for thermoresponsive solubility depended on the PEG/cation composition of the terpolymers. For example, a PEG/cation/dodecyl (1/1/2) random terpolymer micelle exhibited thermoresponse in water containing more than 0.5 M NaCl; the Cp of the aqueous solution decreased from 86 °C to 59 °C upon increasing the concentration of NaCl from 0.5 M to 2.0 M. The Cp of their terpolymers increased upon increasing the content of quaternary ammonium cations.
Introduction
Thermoresponsive polymers are one class of stimuli-responsive polymers1–9 in which the morphology, self-assembly, and solubility can be controlled by temperature as a reversible and non-destructive response. Such thermoresponsive polymers are widely employed as functional materials for various applications including carriers for drug delivery systems,6 injectable gelators,7 and water purification systems.8 Lower critical solution temperature (LCST)-type thermoresponsive polymers can switch the solubility in water or organic solvents upon heating: the homogeneous polymer solution is turbid or phase-separated upon heating above the cloud point (Cp) temperature and reversibly becomes homogeneous upon cooling below the Cp. Various LCST-type thermoresponsive polymers10–27 have been designed and developed for targeted thermoresponse, functions, and applications, e.g., poly(N-isopropylacrylamide) (PNIPAM),10–14 Pluronics,15–17 and poly(ethylene glycol) (PEG) or poly(ethylene oxide) (PEO)-based compounds and (co)polymers.18–23 Polymers with LCST-type solubility in water mostly consist of both hydrophilic units and hydrophobic units. The thermoresponsive solubility is caused by the dehydration of polymer chains in water by changing the affinity or interaction of water molecules with the hydrophilic units. Thus, the Cp temperatures of thermoresponsive polymers in water depend on not only the hydrophobic/hydrophilic balance of polymer chains (monomer species and/or copolymer composition) and the local hydrophobicity around hydrophilic units but also the solvent conditions such as solvent quality (mixed solvent systems), externally added salts, and pH, in addition to the total concentration and three-dimensional architectures14 of polymers.Among them, the copolymerization of thermoresponsive (or hydrophilic) monomers and hydrophobic monomers is a promising strategy to control the Cp temperature of polymer aqueous solutions and the phase transition temperatures of hydrogels. We have designed amphiphilic random copolymers bearing hydrophilic PEG chains and hydrophobic alkyl pendants and created thermoresponsive micelles and gels in water.28–33 The copolymers induce chain folding via the association of hydrophobic alkyl groups in water to form single- or multi-chain micelles. The size and aggregation number can be controlled by the pendant structures, composition, and chain length. The micelles consist of thermoresponsive PEG shells and hydrophobic cores and thereby show LCST-type solubility in water. The Cp of their polymer micelles can also be tuned in a wide range of temperature between 30 °C and 85 °C by varying the hydrophilic/hydrophobic balance of the copolymers: copolymer composition,29,30 the length of hydrophobic alkyl groups or hydrophilic PEG chains,30,31 the backbone structures (acrylate vs. methacrylate),32 and random block copolymerization with a long PEG chain.33 Owing to such fine controllability of the Cp, stepwise thermoresponsive systems are established by mixing two polymer micelles with different Cp temperatures into one solution.30
Another strategy to control the thermoresponsive behavior involves the addition of salts into aqueous solutions of thermoresponsive polymers,11,34–44i.e., tuning the solvent conditions. The Cp temperatures of PNIPAM and PEG/PEO-based compounds in water often change in the presence of salts. Typically, the Cp for the aqueous solutions of PNIPAM containing salts is known to decrease in the order of salts originating from Hofmeister Series because the affinity between water molecules and the amide groups changes in the presence of salts.11 In addition to such non-ionic polymers, ionic polymers such as carboxylated poly(allylamine)s also show LCST-type solubility in aqueous solutions containing salts.34 We also found that the fused micelles of cationic random copolymers and PEG-bearing random copolymers did not show thermoresponsive solubility in pure water, in contrast to PEG copolymer micelles; however, in the presence of large amounts of NaNO3, PEG-bearing copolymers were separated from fused micelles to exhibit thermoresponsive solubility.45
Given these unique results and findings, we anticipated that if thermoresponsive PEG chains, quaternary ammonium cations, and hydrophobic alkyl groups are introduced into amphiphilic polymers as thermoresponsive, hydrophilic, and hydrophobic pendants, the resulting random terpolymers may not only form size-controlled micelles but also show thermoresponsive solubility in water in the presence of salts, because of the following features: (1) the Cp temperatures of PEG/PEO-based compounds in water often decrease by adding salts, since the dehydration of the ethylene oxide units is promoted by salts.35–37 (2) Electrostatic repulsion between cationic polymer chains in water would also be suppressed via shielding effects if appropriate amounts of salts are added.46 (3) The local affinity of their PEG and cationic units to water could be modulated by salts. Considering these possibilities, the thermoresponsive properties of the terpolymer micelles could be controlled by the molecular structure (e.g., composition) and salt concentration and/or species added into aqueous solutions, leading to diverse possibilities in designing thermoresponsive polymer materials typically toward bio-applications.
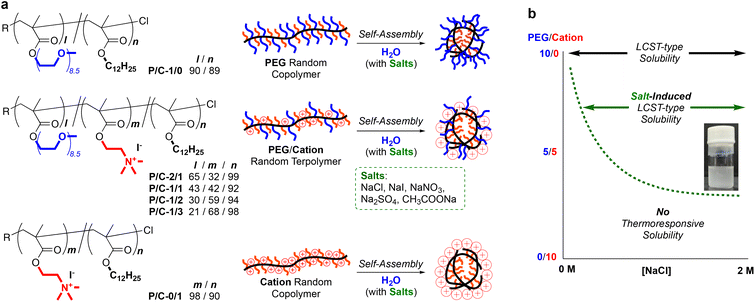
Herein, we report the self-assembly and salt-induced thermoresponsive properties of amphiphilic random terpolymers bearing PEG chains, quaternary ammonium cations, and hydrophobic dodecyl groups in water (Fig. 1a). The random terpolymers induced self-assembly to form size-controlled multichain micelles in pure water or in water containing NaCl. The terpolymer micelles exhibited LCST-type thermoresponsive solubility in water in the presence of salts. Importantly, the critical concentration of NaCl required for the thermoresponsive solubility depended on the composition (PEG/cation ratio) of the terpolymers (Fig. 1b). The cloud point temperatures also varied by the concentration of salts in water and the composition of the terpolymers. This work provides a new piece of knowledge in creating thermoresponsive polymer materials and related stimuli-responsive functional materials.
Results and discussion
Design and synthesis of PEG/cation random terpolymers
We designed amphiphilic random ter(co)polymers bearing hydrophilic PEG chains and/or quaternary ammonium cations and hydrophobic dodecyl groups. The composition of the hydrophobic dodecyl group was set to 50 mol% in their total monomer units. In the hydrophilic units (50 mol%), the molar ratio of the PEG chains (P) and cationic groups (C) was varied: P/C = 1/0, 2/1, 1/1, 1/2, 1/3, and 0/1. For the random ter(co)polymers coded as P/C-1/0, -2/1, -1/1, -1/2, -1/3, and -0/1, the total degree of polymerization (DP) was controlled to 180–190. PEG/cation random terpolymers (P/C-2/1, -1/1, -1/2, and -1/3) were synthesized by living radical copolymerization of poly(ethylene glycol) methyl ether methacrylate (PEGMA), 2-(dimethylamino)ethyl methacrylate (DMAEMA), and dodecyl methacrylate (DMA) with a ruthenium catalyst [Ru(Cp*)Cl(PPh3)2/4-dimethylamino-1-butanol] and a chloride initiator (ethyl-2-chloro-2-phenylacetate: ECPA), followed by the quaternization of the terpolymers with methyl iodide (Scheme S1†). As control samples, we produced PEGMA/DMA or DMAEMA/DMA copolymers (P/C-1/0 or P/C-0/1) via living radical copolymerization with a ruthenium catalyst [Ru(Ind)Cl(PPh3)2/n-Bu3N] and ECPA. The copolymerization behavior was monitored by 1H NMR measurements of the polymerization solutions sampled at predetermined periods. PEGMA, DMAEMA, and DMA were consumed at the same rate, independent of the feed ratio of their monomers (Fig. S1†). This indicates that the reactivity ratio of the three monomers is almost one and the three monomers are randomly introduced into the resulting terpolymers. All the monomers were smoothly copolymerized up to over 70% monomer conversion to give well-controlled random ter(co)polymers with a targeted composition, DP, and narrow molecular weight distribution (Mn = 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900–47
900–47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400, Mw/Mn = 1.13–1.27, by size exclusion chromatography (SEC), Table 1 and Fig. S1†). The number of PEGMA, DMAEMA, and DMA units in the ter(co)polymers (l/m/n) was estimated from the area ratio of their pendant units to the initiator fragment by 1H NMR (Fig. S2–S4†).
400, Mw/Mn = 1.13–1.27, by size exclusion chromatography (SEC), Table 1 and Fig. S1†). The number of PEGMA, DMAEMA, and DMA units in the ter(co)polymers (l/m/n) was estimated from the area ratio of their pendant units to the initiator fragment by 1H NMR (Fig. S2–S4†).
| Polymera | PEG/cation/dodecylb (mol%) | l/m/nb (NMR) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (SEC) c (SEC) |
M
w/Mn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (SEC) c (SEC) |
M
n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d (NMR) d (NMR) |
|---|---|---|---|---|---|
| a P/C-2/1, P/C-1/1, P/C-1/2, and P/C-1/3 were synthesized by living radical copolymerization of poly(ethylene glycol) methyl ether methacrylate (PEGMA), 2-(dimethylamino)ethyl methacrylate (DMAEMA), and dodecyl methacrylate (DMA), followed by the quaternization of the terpolymers with methyl iodide. P/C-1/0 was prepared by living radical copolymerization of poly(ethylene glycol) methyl ether methacrylate (PEGMA) and DMA. P/C-0/1 was synthesized by living radical copolymerization of 2-(dimethylamino)ethyl methacrylate (DMAEMA) and dodecyl methacrylate (DMA), followed by the quaternization of the copolymer with methyl iodide. b The contents of PEG, cation, and dodecyl units (mol%) and the degree of polymerization of PEGMA (l), DMAEMA (m), and DMA (n) of the ter(co)polymers determined by 1H NMR. c Number-average molecular weight (Mn) and molecular weight distribution (Mw/Mn) of P/C-1/0 determined by SEC in DMF (10 mM LiBr) or the DMAEMA-based precursors of P/C-2/1, P/C-1/1, P/C-1/2, P/C-1/3, and P/C-0/1 determined by SEC in THF (20 mM Et3N) with PMMA standard calibration. d The number-average molecular weight (Mn) of the ter(co)polymers determined by 1H NMR. Mn values of P/C-2/1, P/C-1/1, P/C-1/2, P/C-1/3, and P/C-0/1 were calculated, considering that the ter(co)polymers bear n of quaternary ammonium iodide [–CH2N(CH3)3I] units. | |||||
| P/C-1/0 | 50/0/50 | 90/—/89 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.27 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
| P/C-2/1 | 33/16/51 | 65/32/99 | 42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.13 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
| P/C-1/1 | 24/24/52 | 43/42/92 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.15 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
| P/C-1/2 | 16/33/51 | 30/59/94 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.13 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| P/C-1/3 | 12/36/52 | 21/68/98 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.13 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
| P/C-0/1 | 0/52/48 | -/98/90 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.27 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
The PEGMA/DMAEMA/DMA terpolymers and the DMAEMA/DMA copolymer were mixed with methyl iodide in THF/acetonitrile (1/1, v/v) at 0 °C for 24 h, and the resulting products were analyzed by 1H NMR. In all the products, the methyl and methylene proton signals originating from the DMAEMA pendants [–CH2N(CH3)2 at 2.5 and 2.2 ppm], observed for their DMAEMA-based precursors, completely disappeared, and the proton signals derived from quaternized groups [-CH2N(CH3)3I] in turn appeared at around 3.2 ppm. Thus, the quaternization of their DMAEMA-based ter(co)polymers quantitatively proceeded to give P/C-2/1, -1/1, -1/2, -1/3, and -0/1. The number-average molecular weight of P/C-1/0, -2/1, -1/1, -1/2, -1/3, and -0/1 was estimated to be 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600–65
600–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 by 1H NMR.
900 by 1H NMR.
Similarly, PEG/cation (1/1) random terpolymers with different DPs (DP = 76, 107, and 135), as well as P/C-1/1 (DP = 177), were prepared by ruthenium-catalyzed living radical copolymerization of PEGMA, DMAEMA, and DMA, followed by the quaternization of their precursors with methyl iodide. Those terpolymers were designed to evaluate the effects of DP on the self-assembly behavior and thermoresponsive solubility in water (Table S1 and Fig. S1†).
Micellization of PEG/cation random terpolymers and copolymers in water
The self-assembly of PEG/cation random ter(co)polymers (P/C-1/0, -2/1, -1/1, -1/2, -1/3 and -0/1) into micelles in water was evaluated by SEC coupled with multi-angle laser light scattering (MALLS) in water containing 100 mM NaNO3 as an eluent. NaNO3 is added into the eluent to reduce the ionic interaction between the polymer micelles and the SEC column for the efficient separation and analysis of the cationic polymer micelles by SEC. Their polymer micelles were prepared as follows: The bulk polymers were dissolved in pure water or in water containing 2 M NaCl to evaluate the effects of NaCl on the micelles. The aqueous solutions were sonicated for 10 minutes at room temperature. The solutions of the cationic ter(co)polymers were placed in a water bath kept at 50 or 40 °C. After 24 h, the solutions were gradually cooled at room temperature and filtered with a membrane filter (pore size = 0.45 μm) before measurements. The aqueous solutions of the polymer micelles (1 mg mL−1, 0.1 mL) were injected into a SEC-MALLS system to determine the absolute weight-average molecular weight (Mw,H2O) and aggregation number (Nagg) of their micelles (Fig. 2 and Table 2). The apparent size of their micelles was evaluated by SEC on poly(ethylene oxide) (PEO) standard calibration.| Samplesa | Solventsa |
M
w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (calcd) b (calcd) |
M
w,H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) c (MALLS) c (MALLS) |
N
agg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d d |
R
h![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e (nm) e (nm) |
Cp![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) f (°C) f (°C) |
|---|---|---|---|---|---|---|
a Micelles of P/C-1/0, P/C-2/1, P/C-1/1, P/C-1/2, P/C-1/3, and P/C-0/1 prepared in pure water or water containing 2 M NaCl were used for SEC-MALLS and cloud point measurements. The micelle solutions prepared in water containing 100 mM NaNO3 or 2 M NaCl were employed for DLS measurements.
b Weight-average molecular weight of the polymer chains calculated with Mn (NMR) and Mw/Mn (SEC): Mw (calcd) = Mn (NMR) × Mw/Mn (SEC). The Mn (NMR) used for P/C-2/1, P/C-1/1, P/C-1/2, P/C-1/3, and P/C-0/1 micelles was re-calculated, assuming that all the counter anions for the micelles were changed to NO3− originating from NaNO3 included in the eluent of SEC-MALLS.
c Absolute weight-average molecular weight of the polymer micelles (Mw,H2O) was determined by the SEC-MALLS system in water containing 100 mM NaNO3. The aqueous micelle solutions ([polymer] = 1 mg mL−1, 0.1 mL) were injected to SEC-MALLS in water containing 100 mM NaNO3 as an eluent.
d Aggregation number (Nagg) of P/C-2/1, P/C-1/1, P/C-1/2, P/C-1/3, and P/C-0/1 micelles was estimated as follows: Nagg = Mw,H2O (MALLS)/Mw (calcd). Nagg of P/C-1/0 micelles: Nagg = Mw,H2O (MALLS)/Mw,DMF (MALLS), Mw,DMF (MALLS) = 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.
e Hydrodynamic radius (Rh) of the polymer micelles was determined by DLS in H2O containing 100 mM NaNO3 or 2 M NaCl at 25 °C: [polymer] = 1 mg mL−1.
f Cloud point (Cp) temperature was determined by monitoring the transmittance of the aqueous solutions (pure H2O or 2 M NaClaq) of their polymer micelles ([polymer] = 4 mg mL−1) at 670 nm upon heating from 25 °C to 90 °C. Cp was defined as a temperature, at which the transmittance became 90%. 100.
e Hydrodynamic radius (Rh) of the polymer micelles was determined by DLS in H2O containing 100 mM NaNO3 or 2 M NaCl at 25 °C: [polymer] = 1 mg mL−1.
f Cloud point (Cp) temperature was determined by monitoring the transmittance of the aqueous solutions (pure H2O or 2 M NaClaq) of their polymer micelles ([polymer] = 4 mg mL−1) at 670 nm upon heating from 25 °C to 90 °C. Cp was defined as a temperature, at which the transmittance became 90%.
|
||||||
| P/C-1/0 Micelle | H2O or 100 mM NaNO3aq | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
204![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.5 | 6.0 | 60 |
| P/C-1/0 Micelle | 2 M NaClaq | 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
222![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.8 | 8.1 | 38 |
| P/C-2/1 Micelle | H2O or 100 mM NaNO3aq | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
285![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.0 | 7.6 | — |
| P/C-2/1 Micelle | 2 M NaClaq | 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
487![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
6.8 | 8.3 | 49 |
| P/C-1/1 Micelle | H2O or 100 mM NaNO3aq | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
348![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
5.6 | 9.1 | — |
| P/C-1/1 Micelle | 2 M NaClaq | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
577![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
9.3 | 9.5 | 59 |
| P/C-1/2 Micelle | H2O or 100 mM NaNO3aq | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
426![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
7.2 | 9.5 | — |
| P/C-1/2 Micelle | 2 M NaClaq | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
745![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
13 | 9.8 | 77 |
| P/C-1/3 Micelle | H2O or 100 mM NaNO3aq | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
522![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
9.0 | 9.7 | — |
| P/C-1/3 Micelle | 2 M NaClaq | 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 670 670![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
29 | 17 | — |
| P/C-0/1 Micelle | H2O or 100 mM NaNO3aq | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
228![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
3.9 | 6.9 | — |
| P/C-0/1 Micelle | 2 M NaClaq | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
289![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
4.9 | 7.8 | — |
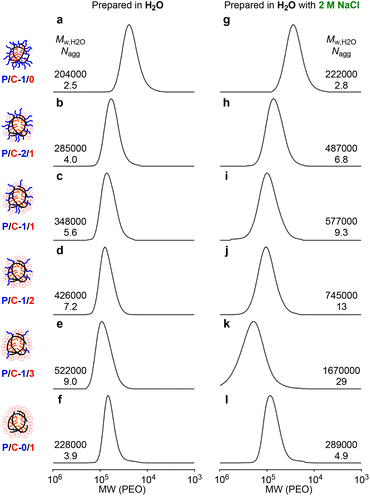
All the polymer micelles prepared in pure water or in water containing 2M NaCl showed unimodal SEC curves (Fig. 2, Mw/Mn < 1.4). The molecular weight of P/C-1/0, -2/1, -1/1, -1/2, and -1/3 micelles in pure water by PEO calibration increased upon increasing the content of cation units, while the Mn of their polymer chains by 1H NMR was relatively close (56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–65
000–65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, Table 1). The Mw,H2O of their polymer micelles was determined to be 204
800, Table 1). The Mw,H2O of their polymer micelles was determined to be 204![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–522
000–522![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 by SEC-MALLS. The Mw,H2O was larger than the weight-average molecular weight of the polymer chains calculated from Mn by 1H NMR and Mw/Mn by SEC [Mw (calcd) = Mn (NMR) × Mw/Mn, P/C-2/1, -1/1, -1/2, -1/3 and -0/1] or Mw,DMF determined by SEC-MALLS in DMF (P/C-1/0). The Nagg values of their polymer micelles [Nagg = Mw,H2O/Mw (calcd) or Mw,H2O/Mw,DMF] were calculated to be 2.5, 4.0, 5.6, 7.2, 9.0, and 3.9, respectively (Table 2), demonstrating that all the polymers formed multichain micelles in pure water. Their Nagg values for P/C-2/1, -1/1, -1/2, -1/3 and -0/1 were calculated on the assumption that all the counter anions were replaced to NO3- originating from the salt included in the eluent for SEC-MALLS.
000 by SEC-MALLS. The Mw,H2O was larger than the weight-average molecular weight of the polymer chains calculated from Mn by 1H NMR and Mw/Mn by SEC [Mw (calcd) = Mn (NMR) × Mw/Mn, P/C-2/1, -1/1, -1/2, -1/3 and -0/1] or Mw,DMF determined by SEC-MALLS in DMF (P/C-1/0). The Nagg values of their polymer micelles [Nagg = Mw,H2O/Mw (calcd) or Mw,H2O/Mw,DMF] were calculated to be 2.5, 4.0, 5.6, 7.2, 9.0, and 3.9, respectively (Table 2), demonstrating that all the polymers formed multichain micelles in pure water. Their Nagg values for P/C-2/1, -1/1, -1/2, -1/3 and -0/1 were calculated on the assumption that all the counter anions were replaced to NO3- originating from the salt included in the eluent for SEC-MALLS.
The Mw,H2O of the polymer micelles prepared in 2 M NaClaq was also determined by SEC-MALLS (Fig. 2g–l). The Mw,H2O and Nagg of P/C-2/1, -1/1, -1/2, -1/3 and -0/1 micelles formed in 2 M NaClaq were larger than those in pure water though P/C-1/0 micelles prepared in pure water or in 2 M NaClaq had close Mw,H2O and Nagg values (Fig. 2a and g). We have recently revealed that a cationic random copolymer bearing dodecyl groups, close to P/C-0/1, forms a larger micelle in 1 M NaNO3 than that in pure water by identical SEC-MALLS measurements.45 Importantly, the cationic polymer micelles can maintain their sizes formed in aqueous solutions containing salts even though the salt concentration of the eluent for SEC analysis is different from that for micelle preparation. This is because cationic polymer micelles, as well as PEG copolymer micelles, hardly induce dynamic chain exchange in diluted SEC conditions. The formation of the larger micelles of P/C-2/1, -1/1, -1/2, and -1/3 in 2 M NaClaq is also confirmed by SEC-MALLS. This fact supports that the random terpolymer micelles also maintain their sizes during SEC analyses though the dynamic properties of their terpolymer micelles have not been clarified yet.
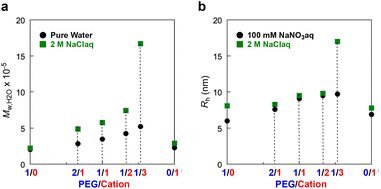
The formation of large micelles of their cationic random (co)terpolymers in the presence of NaCl would be related to the following reasons: (1) hydrophobic effect in water is enhanced by the addition of sodium chloride. (2) Sodium chloride in their micelle solutions suppresses the electrostatic repulsion of cationic groups and that between cationic polymers, since the Debye screening length of polyelectrolytes shortens in accordance with ionic strength in the solutions. Except for the cationic copolymer P/C-0/1, there was a clear relationship between the polymer composition and the micellar size: The more cation pendants a polymer has, the larger micelle (Mw,H2O by SEC-MALLS) it forms in both pure water and 2M NaClaq (Fig. 3a). The P/C-0/1 micelle has a relatively small size in not only 2 M NaClaq but also pure water. This is probably due to several factors including intermolecular electrostatic repulsion of the cationic groups and/or its small content of DMA (48 mol%) compared with the other ter(co)polymers (50–52 mol%). The latter reason is proposed by our previous finding that the size of PEG-bearing random copolymer micelles dramatically changed by a small difference in the composition.28–30
P/C-1/0, -2/1, -1/1, -1/2, -1/3, and -0/1 micelles in water containing 100 mM NaNO3 or 2 M NaCl were analyzed by dynamic light scattering (DLS) (Table 2, Fig. 3b and Fig. S5†). The hydrodynamic radius (Rh) of their micelles also increased with increasing Mw,H2O. The P/C-1/0 micelle had a relatively large Rh in water with 2 M NaCl, even though its Mw,H2O value was not changed much. It has previously been reported that sodium cations interact with oxygen atoms on PEGMA side chains, rendering the PEG units a little bit cationic.47,48 Given that, the larger hydrodynamic radius of the P/C-1/0 micelle in 2 M NaCl water may be due to the interaction of the sodium cation with the PEG shell; the PEG shells more expand to decrease electrostatic repulsion. Another possibility involves a morphological change in the PEGMA/DMA micelle caused by the interaction between PEG units and sodium cations, as reported for the self-assembly of PSt-b-PEO block copolymers.49
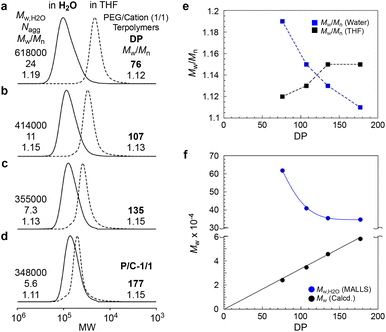
To investigate the effect of DP on micellization, the aqueous solutions of PEG/cation (1/1) random terpolymers with different DPs (76, 107, and 135) were analyzed by SEC-MALLS, compared with P/C-1/1 (DP = 177). All the terpolymers formed multichain micelles in water (Nagg = 5.6–24, Fig. 4a–d, Table S1†). The apparent molecular weight distribution (Mw/Mn by PEO standard calibration) and Mw,H2O (by MALLS) of the micelles depended on the DP of the terpolymers. The Mw,H2O of the micelles gradually decreased from 618![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 355
000 to 355![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 upon increasing the DP from 76 to 135 and then became almost constant (∼350
000 upon increasing the DP from 76 to 135 and then became almost constant (∼350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) at a DP of 177 (Fig. 4f). The Mw/Mn of the micelles gradually became narrow from 1.19 to 1.11 upon increasing the DP (Fig. 4e).
000) at a DP of 177 (Fig. 4f). The Mw/Mn of the micelles gradually became narrow from 1.19 to 1.11 upon increasing the DP (Fig. 4e).
In general, the molecular weight of PEGMA-based random copolymer micelles (Mw,H2O by MALLS) is determined by the composition and independent of DP, if the DP is smaller than the threshold DP that specifically leads to unimer micelles.29,30 Thus, the DP dependence of Mw,H2O on PEG/cation random terpolymer micelles implies that the composition distribution included in the terpolymers remarkably affects the molecular weight and size distribution of multichain micelles. Though the monomer reactivity ratio of PEGMA, DMAEMA, and DMA is close to 1, the random terpolymers with a relatively small DP would have a composition distribution broader than those with a large DP (>135), as reported by related PEG random copolymers.50 Typically, in a PEG/cation (1/1) terpolymer with a DP of 76, more hydrophobic terpolymer chains consisting of a larger DMA content form larger micelles, and more cationic terpolymer chains also form larger micelles owing to the repulsion of the ionic groups. Therefore, we revealed that controlling the DP of PEG/cation random terpolymers to about 200 was important to produce size-controlled micelles with a narrow molecular weight distribution in water.
Thermoresponsive properties of PEG/cation polymer micelles in water
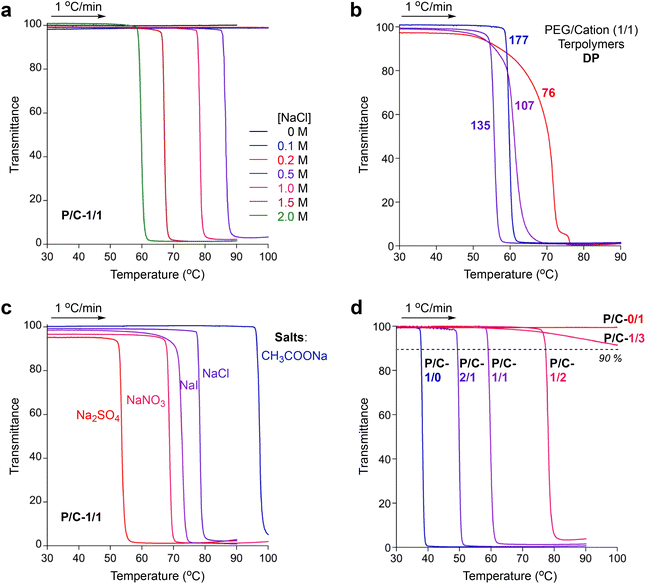
PEG-based copolymers are known to show lower critical solution temperature (LCST)-type thermoresponsive solubility in water; the aqueous solutions turn turbid at a certain cloud point (Cp) temperature upon heating. The Cp is often affected by the salts included in aqueous solutions due to the promotion of dehydration of PEG units.35–37 Additionally, repulsive force between ionic polymers and groups is also suppressed by salts in water due to the shielding effects of ionic groups.46 Thus, random terpolymer micelles containing both thermoresponsive PEG chains and cationic groups may show unique thermoresponsive solubility dependent on salts in water.We first investigated the thermoresponsive solubility of a P/C-1/1 micelle in water containing NaCl at various concentrations (0–2.0 M, Fig. 5a). The transmittance of the aqueous solutions was monitored at λ = 670 nm upon heating or cooling at a rate of 1 °C min−1. The Cp was determined as a temperature, at which the transmittance of the solution reached 90% in the heating process. The micelle solutions in pure water or in water containing NaCl below 0.2 M were transparent even at 90 or 100 °C and did not show thermoresponsive solubility, whereas the micelle solutions containing NaCl above 0.5 M turned turbid upon heating at certain temperatures. Such salt-mediated LCST-type solubility of the P/C-1/1 micelle is close to that of carboxylated polyallylamines in the presence of divalent alkaline earth metal ions.34 The Cp of a P/C-1/1 micelle decreased from 86 °C to 59 °C upon increasing the concentration of NaCl from 0.5 M to 2.0 M (Fig. 5a). At any concentration of NaCl above 0.5 M, the thermoresponse of the P/C-1/1 micelle was sharp and reversible (Fig. S6†).
PEG/cation (1/1) random terpolymers with different DPs (76–135), as well as P/C-1/1 (DP = 177), also showed thermoresponsive solubility in water containing 2 M NaCl upon heating. The thermoresponse of the terpolymers turned sharp upon increasing the DP (Fig. 5b). This is because the composition distribution of the terpolymers turns narrow upon increasing the DP, as described above. Thus, the design of a PEG/cation random terpolymer with a large DP (P/C-1/1) was also important to achieve a sharp thermoresponse in water containing 2 M NaCl.
We then examined the effects of the added salt species on the thermoresponsive behaviors of P/C-1/1. We measured the Cp of the aqueous solutions of P/C-1/1 including 1 M of NaCl, NaNO3, NaI, Na2SO4, and CH3COONa (Fig. 5c and Fig. S11†). The polymer formed small micelles in the presence of all the salts (Rh = 9.9–15 nm by DLS, Fig. S12†). The Cp dramatically changed from 97 °C to 53 °C depending on the salt species in this order: Cp = 97 °C (CH3COONa), 79 °C (NaCl), 70 °C (NaI), 68 °C (NaNO3), and 53 °C (Na2SO4). Although the order of Cp temperatures was not related to Hofmeister Series, sodium salts derived from strong acid and strong base (NaI, NaCl, NaNO3, and Na2SO4) tend to effectively decrease the Cp, in contrast to CH3COONa obtained from a weak acetic acid and a strong base. Na2SO4 led to the lowest Cp due to twice the amount (mole) of a sodium cation against the other salts. These results suggest that the Cp of the terpolymer depends on the concentration of a sodium cation freely separated from their salts.
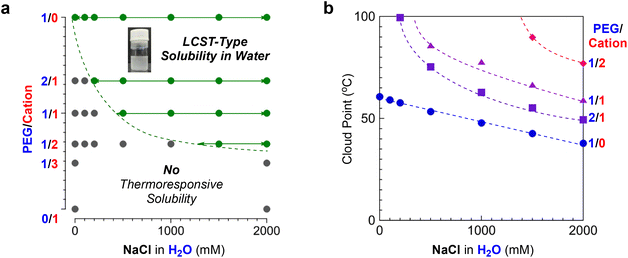
To evaluate the effects of polymer composition on Cp values, we further investigated the thermoresponsive solubility of P/C-1/0, -2/1, -1/2, -1/3 and -0/1 micelles in water. In pure water, the PEG/cation random terpolymers (P/C-2/1, -1/2, and -1/3), as well as P/C-1/1, did not show thermoresponsive solubility, though P/C-1/0 exhibited the Cp at 60 °C (Table 2). In contrast, the terpolymers showed thermoresponsive solubility or aggregation in 2 M NaCl aqueous solutions. The Cp increased from 38 °C to 77 °C as the contents of cation units in polymer chains increased in this order: P/C-2/1, 1/1, and 1/2 (Fig. 5d). P/C-1/3 containing a large amount of cation units did not clearly exhibit the Cp upon heating in 2 M NaClaq, whereas the transmittance of the solution slightly decreased above 70 °C due to the formation of larger aggregates as described later. P/C-0/1 not containing PEG units kept 100% transmittance without a thermoresponse both in pure water and in water containing 2 M NaCl. These results indicate that LCST-type solubility or aggregation of PEG/cation random terpolymers containing 50 mol% dodecyl units in 2 M NaClaq depends on the content of PEGMA units: the critical PEG/cation ratio (minimum content of PEGMA units) for the thermoresponsive solubility is found to be between 1/2 and 1/3.
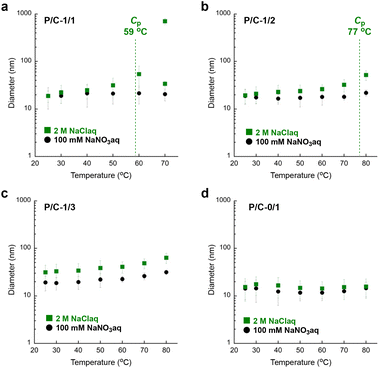
We further examined the thermoresponsive aggregation of P/C-2/1, -1/1, -1/2, -1/3 and -0/1 micelles in water containing 100 mM NaNO3 or 2 M NaCl by temperature-variation DLS measurements (Fig. 6 and Fig. S13–S18†). As shown in Fig. 6a, the diameter of a P/C-1/1 micelle in 2 M NaClaq gradually increased from 19 nm to 54 nm upon heating from 30 °C to 60 °C. Above 60 °C (>Cp), the P/C-1/1 solution showed a multimodal size distribution including quite large aggregates with over several hundred nanometers. In contrast, the micelle diameter was constant at ∼20 nm in the temperature range between 30 °C and 80 °C in water containing a small amount of NaNO3 (100 mM). In 2 M NaClaq, P/C-2/1 also showed a multimodal size distribution containing quite large aggregates above the Cp, whereas P/C-1/2 and P/C-1/3 micelles exhibited a gradual and subtle increase of the diameter (Fig. 6b, c and Fig. S18†). This means that the P/C-1/3 micelle induces thermoresponsive aggregation in 2 M NaClaq although remarkable LCST-type solubility was not observed in the diluted concentration (1–4 mg mL−1). In contrast, the diameter of a cationic P/C-0/1 micelle was almost constant, independent of temperature and the presence/absence of NaCl (Fig. 6d).
As shown in Fig. 5a, the P/C-1/1 micelle has its critical salt concentration ([NaCl] = 0.5 M) that is required to induce thermoresponsive solubility. Given the outcome, we systematically investigated the critical NaCl concentration for the thermoresponsive solubility of other PEG/cation random ter(co)polymer micelles (P/C-1/0, -2/1, and -1/2) in water (Fig. S7–S9†). As shown in Fig. 7a, the critical salt concentration gradually increased upon increasing the content of cationic groups in the hydrophilic monomer units. In all the cases, the Cp decreased upon increasing the content of PEG units or the concentration of NaCl (Fig. 7b). Given the results, the salt-mediated thermoresponsive solubility of their PEG/cation random terpolymer micelles is affected by the following two factors: (1) the competition between the dehydration from PEG chains and the strong hydration of quaternary ammonium cation pendants, and (2) the electrostatic repulsion between cation pendants. If the concentration of NaCl is under the threshold value, the hydration of cation moieties can compensate for the dehydration of PEG chains by heating, so the PEG/cation terpolymer micelle solution does not exhibit cloud points. In such a situation, the shielding effect by salts is insufficient and thus the formation of quite large aggregates, leading to thermoresponsive solubility, is suppressed by the electrostatic repulsion between cation pendants. On the other hand, in the presence of a large amount of NaCl above the threshold point, the terpolymer micelle solution recovers thermoresponsive solubility like PEG copolymer micelle solutions, because the salt promotes the dehydration from PEG chains and the electrostatic repulsion is effectively shielded.
Conclusions
In summary, we investigated the self-assembly and thermoresponsive properties of amphiphilic random terpolymers bearing hydrophilic PEG chains and quaternary ammonium cations, and hydrophobic dodecyl groups in pure water or in water containing salts. The random terpolymers formed size-controlled multichain micelles in both pure water and water containing NaCl; the micelle size depended on the ratio of PEG and cation units and the absence or presence of NaCl. More uniquely, the terpolymers exhibited LCST-type thermoresponsive solubility in water in the presence of salts such as NaCl. The cloud point temperature was controlled by the ratio of PEG and cation units, salt concentration, and salt species. The salt-mediated thermoresponsive polymer micelles developed herein would open new strategies to design stimuli-responsive polymer materials and self-assemblies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Japan Society for the Promotion of Science KAKENHI Grants (JP19K22218, JP20H02787, JP20H05219, JP22H04539), the Ogasawara Foundation for the Promotion of Science & Engineering, the Noguchi Institute, and the Iketani Science and Technology Foundation.References
- D. Neugebauer, Polym. Int., 2007, 56, 1469–1498 CrossRef CAS.
- J. F. Lutz, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3459–3470 CrossRef CAS.
- R. Liu, M. Fraylich and B. R. Saunders, Colloid Polym. Sci., 2009, 287, 627–643 CrossRef CAS.
- D. Roy, W. L. A. Brooks and B. S. Sumerlin, Chem. Soc. Rev., 2013, 42, 7214–7243 RSC.
- R. Hoogenboom and H. Schlaad, Polym. Chem., 2017, 8, 24–40 RSC.
- S. Mura, J. Nicolas and P. Couvreur, Nat. Mater., 2013, 12, 991–1003 CrossRef CAS PubMed.
- M. R. Matanović, J. Kristl and P. A. Grabnar, Int. J. Pharm., 2014, 472, 262–275 CrossRef PubMed.
- K. Dutta and S. De, J. Mater. Chem. A, 2017, 5, 22095–22112 RSC.
- M. A. C. Stuart, W. T. S. Huck, J. Genzer, M. Müller, C. Ober, M. Stamm, G. B. Sukhorukov, I. Szleifer, V. V. Tsukruk, M. Urban, F. Winnik, S. Zauscher, I. Luzinov and S. Minko, Nat. Mater., 2010, 9, 101–113 CrossRef PubMed.
- T. Kawaguchi, Y. Kojima, M. Osa and T. Yoshizaki, Polym. J., 2008, 40, 455–459 CrossRef CAS.
- Y. Zhang, S. Furyk, D. E. Bergbreiter and P. S. Cremer, J. Am. Chem. Soc., 2005, 127, 14505–14510 CrossRef CAS PubMed.
- M. Heskins and J. E. Guillet, J. Macromol. Sci., Part A, 1968, 2, 1441–1455 CAS.
- A. Halperin, M. Kröger and F. M. Winnik, Angew. Chem., Int. Ed., 2015, 54, 15342–15367 CrossRef CAS PubMed.
- S. Ida, Y. Toyama, S. Takeshima and S. Kanaoka, Polym. J., 2020, 52, 359–363 CrossRef CAS.
- D. Cohn, G. Lando, A. Sosnik, S. Garty and A. Levi, Biomaterials, 2006, 27, 1718–1727 CrossRef CAS PubMed.
- H. Abdeltawab, D. Svirskis, B. J. Boyd, A. Hill and M. Sharma, Int. J. Pharm., 2021, 604, 120748 CrossRef CAS PubMed.
- S. A. Pillai, V. Kumar, K. Kuperkar, D. Ray, V. K. Aswal and S. Kumar, Colloids Interface Sci. Commun., 2021, 42, 100414 CrossRef CAS.
- A. Hoth and J.-F. Lutz, Macromolecules, 2006, 39, 893–896 CrossRef.
- J. F. Lutz, K. Weichenhan, Ö. Akdemir and A. Hoth, Macromolecules, 2007, 40, 2503–2508 CrossRef CAS.
- I. Akar, R. Keogh, L. D. Blackman, J. C. Foster, R. T. Mathers and R. K. O'Reilly, ACS Macro Lett., 2020, 9, 1149–1154 CrossRef CAS PubMed.
- Y. Liu, Y. Lei and Y. Chen, Macromolecules, 2022, 55, 5149–5163 CrossRef CAS.
- Y. Koda, T. Terashima and M. Sawamoto, ACS Macro Lett., 2015, 4, 1366–1369 CrossRef CAS PubMed.
- M. A. Ward and T. K. Georgiou, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 775–783 CrossRef CAS.
- T. Manouras, E. Koufakis, S. H. Anastasiadis and M. Vamvakaki, Soft Matter, 2017, 13, 3777–3782 RSC.
- Q. Zhang, J. D. Hong and R. Hoogenboom, Polym. Chem., 2013, 4, 4322–4325 RSC.
- T. Yoshida, S. Kanaoka, H. Watanabe and S. Aoshima, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 2712–2722 CrossRef CAS.
- P. Dinda, M. Anas, P. Banerjee and T. K. Mandal, Macromolecules, 2022, 55, 4011–4024 CrossRef CAS.
- M. Shibata, T. Terashima and T. Koga, Macromolecules, 2021, 54, 5241–5248 CrossRef CAS.
- Y. Hirai, T. Terashima, M. Takenaka and M. Sawamoto, Macromolecules, 2016, 49, 5084–5091 CrossRef CAS.
- S. Imai, Y. Hirai, C. Nagao, M. Sawamoto and T. Terashima, Macromolecules, 2018, 51, 398–409 CrossRef CAS.
- M. Shibata, M. Matsumoto, Y. Hirai, M. Takenaka, M. Sawamoto and T. Terashima, Macromolecules, 2018, 51, 3738–3745 CrossRef CAS.
- G. Hattori, Y. Hirai, M. Sawamoto and T. Terashima, Polym. Chem., 2017, 8, 7248–7259 RSC.
- M. Hibino, K. Tanaka, M. Ouchi and T. Terashima, Macromolecules, 2022, 55, 178–189 CrossRef CAS.
- J. Emoto, Y. Kitayama and A. Harada, Macromolecules, 2022, 55, 7204–7211 CrossRef CAS.
- P. Bahadur, K. Pandya, M. Almgren, P. Li and P. Stilbs, Colloid Polym. Sci., 1993, 271, 657–667 CrossRef CAS.
- M. Desai, N. J. Jain, R. Sharma and P. Bahadur, J. Surfactants Deterg., 2000, 3, 193–199 CrossRef CAS.
- K. Patel, P. Bahadur, C. Guo, J. H. Ma, H. Z. Liu, Y. Yamashita, A. Khanal and K. Nakashima, Eur. Polym. J., 2007, 43, 1699–1708 CrossRef CAS.
- V. Baddam, V. Aseyev, S. Hietala, E. Karjalainen and H. Tenhu, Macromolecules, 2018, 51, 9681–9691 CrossRef CAS.
- V. Baddam, L. Välinen, L. Kuckling and H. Tenhu, Polym. Chem., 2022, 13, 3790–3799 RSC.
- E. Karjalainen, V. Aseyev and H. Tenhu, Macromolecules, 2014, 47, 7581–7587 CrossRef CAS.
- E. Karjalainen, N. Suvarli and H. Tenhu, Polym. Chem., 2020, 11, 5870–5883 RSC.
- N. Isobe and S. Shimizu, Phys. Chem. Chem. Phys., 2020, 22, 15999–16006 RSC.
- L. Liberman, P. W. Schmidt, M. L. Coughlin, A. M. Ya'Akobi, I. Davidovich, J. Edmund, S. P. Ertem, S. Morozova, Y. Talmon, F. S. Bates and T. P. Lodge, Macromolecules, 2021, 54, 2090–2100 CrossRef CAS.
- Y. Xu, C. Wang, K. C. Tam and L. Li, Langmuir, 2004, 20, 646–652 CrossRef CAS PubMed.
- R. Kanno, K. Tanaka, T. Ikami, M. Ouchi and T. Terashima, Macromolecules, 2022, 55, 5213–5221 CrossRef CAS.
- M. Muthukumar, Macromolecules, 2017, 50, 9528–9560 CrossRef CAS PubMed.
- J. Xia, P. L. Dubin and Y. Kim, J. Phys. Chem., 1992, 96, 6805–6811 CrossRef CAS.
- I. Tomatsu, A. Hashizume, S. Yusa and Y. Morishima, Macromolecules, 2005, 38, 7837–7844 CrossRef CAS.
- L. Zhang, K. Yu and A. Eisenberg, Science, 1996, 272, 1777–1779 CrossRef CAS PubMed.
- M. Shibata, T. Terashima and T. Koga, Eur. Polym. J., 2022, 168, 111091 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, SEC, 1H NMR, DLS, and thermoresponsive solubility. See DOI: https://doi.org/10.1039/d3py00013c |
| This journal is © The Royal Society of Chemistry 2023 |