Structure design mechanisms and inflammatory disease applications of nanozymes
Yi
Lu
a,
Cheng
Cao
 a,
Xinni
Pan
b,
Yanlei
Liu
a,
Xinni
Pan
b,
Yanlei
Liu
 *a and
Daxiang
Cui
*a and
Daxiang
Cui
 *ac
*ac
aInstitute of Nano Biomedicine and Engineering, Shanghai Engineering Research Centre for Intelligent Diagnosis and Treatment Instrument, Department of Instrument Science and Engineering, School of Electronic Information and Electrical Engineering, Shanghai Jiao Tong University, 800 Dongchuan Road, Shanghai 200240, People's Republic of China. E-mail: liuyanlei@sjtu.edu.cn
bShanghai Sixth People's Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, Shanghai, China
cNational Engineering Center for Nanotechnology, Shanghai 200240, People's Republic of China. E-mail: dxcui@sjtu.edu.cn
First published on 3rd November 2022
Abstract
Nanozymes are artificial enzymes with high catalytic activity, low cost, and good biocompatibility, and have received ever-increasing attention in recent years. Various inorganic and organic nanoparticles have been found to exhibit enzyme-like activities and are used as nanozymes for diverse biomedical applications ranging from tumor imaging and therapeutics to detection. However, their further clinical applications are hindered by the potential toxicity and long-term retention of nanomaterials in vivo. Clarifying the catalytic mechanism of nanozymes and identifying the key factors responsible for their behavior can guide the design of nanozyme structure, enlighten the ways to improve their enzyme-like activities, and minimize the dosage of nanozymes, leading to reduced toxicity to the human body for a real biomedical application prospect. In particular, inflammation occurring in numerous diseases is closely related to reactive oxygen species, and the active oxygen scavenging ability of nanozymes potentially exerts excellent therapeutic effects on inflammatory diseases. In this review, we systematically summarize the structure–activity relationship of nanozymes, including regulation strategies for size and morphology, surface structure, and composition. Based on the structure–activity mechanisms, a series of chemically designed nanozymes developed to target various inflammatory diseases are briefly summarized.
1 Introduction
A nanozyme is a kind of organic or inorganic nanomaterial with intrinsic enzyme-like properties, with a diameter ranging from several nanometers to hundreds of nanometers. Nanozymes have become a hot spot in the field of enzyme catalysis as a potential surrogate and competitor for natural enzymes. Typically, nanozymes show enzyme-like catalytic activity similar to natural enzymes (such as peroxidase, catalase, oxidase, etc.) under specific physical conditions (mild temperature and physiological pH). The nanozyme is different from the artificial enzyme, which imitates the natural enzyme deliberately. The nanozyme has active centers or electronic transport structures similar to the natural enzyme, which means intrinsic catalytic activities. Usually, a nanozyme can perform several different enzyme-like properties with better stability and superior catalytic performance than natural enzymes. In addition, most nanozymes have low cost, stability, simple synthesis methods, and ease of mass production compared with natural enzymes. Therefore, in recent years, nanozymes have been widely used by scientists in the fields of biomedical sensing, therapeutics, and environmental protection. The research on nanozymes mainly focuses on finding new materials with enzyme-like activity, regulating their enzyme-like catalytic activity, and applying these nanozymes to new fields. The best-known characteristic of the nanozyme is that it has many different types of enzyme activities, such as Peroxidase (POD)-like activity, Catalase (CAT)-like activity, Superoxide dismutase (SOD)-like activity, Oxidase (OXD)-like activity, Glutathione peroxidase (GPx)-like activities and so on, which can regulate toxic reactive oxygen and nitrogen species (RONS) in the human body into nontoxic substances. Peroxidase (POD)-like activity can act on peroxide (such as H2O2) to generate transient reactive intermediates like free radicals, which quickly react with another substrate. POD-like nanozymes act like natural Horseradish peroxidase (HRP), and their kinetics follow the same Michaelis–Menten kinetics equation. Catalase (CAT)-like activity can decompose H2O2 into molecular H2O and O2 as natural catalases do. Oxidase (OXD)-like activity can catalyze the colorimetric oxidation reaction of TMB in the presence of O2. Superoxide dismutase (SOD)-like activity can catalyze the dismutation reaction of superoxide (O2˙−) to produce O2 and H2O2.Since Gao et al. proposed in 2007 that Fe3O4 has POD-like enzyme activity,1 researchers have found intrinsic enzyme-like activity in various materials. To date, the reported nanozymes can be roughly divided into three categories as follows: (1) metal-based nanozymes like platinum (Pt),2 palladium (Pd),3 and iridium (Ir),4etc.; (2) metal oxide-based nanozymes like Mn3O4,5 CeO2,6 Fe3O4,7etc.; and (3) organic framework materials like MOF,8 COF,9etc. These nanozymes can interact with various toxic RONS, including H2O2 and O2˙−, to turn them into non-toxic substances like H2O, O2, etc., highlighting their potential as a potent RONS scavenger. The excellent properties of nanozymes qualify them to regulate the redox balance in the body and sequentially treat RONS-related diseases. However, the potential side effects of long-term toxicity have always been an obstacle to the clinical application of nanozymes. It is a recognized and feasible aim to further improve the catalytic activity of nanozymes in order to reduce the dose of nanozymes during treatment. Through the continuous optimization of synthetic methods and the deepening of theoretical calculations, scientists can continuously improve the catalytic ability of nanozymes. They could optimize the electronic structure of materials and lower the Gibbs free energy of enzyme-induced reactions by rational design. By enhancing the catalytic efficiency of materials and reducing the dosage, the potential toxicity to organs in vivo would be minimized, and nanozymes could be better applied in the biological field.
Both reactive oxygen species (ROS) and reactive nitrogen species (RNS) at low and moderate concentrations are signaling molecules involved in mitogenic response or defense against infectious agents. However, excessive production of RONS or their inefficient scavenging leads to oxidative and nitrosative stress, respectively. This condition is potentially dangerous, as it may alter the inflammatory response and lead to lipid and protein modifications, DNA damage, apoptosis, or cancerogenic cell transformation.10,11 Researchers could apply nanozymes to treat oxidative stress-induced diseases by scavenging RONS and alleviating oxidative stress in the human body, as RONS and subsequent inflammatory activation are associated with the etiology of neurodegenerative diseases like Alzheimer's and Parkinson's disease,12,13 inflammatory-related diseases like inflammatory bowel disease (IBD),14 acute liver injury (ALI),15 and ischemia-reperfusion (IS).16 In addition to neurodegenerative diseases and inflammatory diseases, researchers also use nanozymes for tumor treatment. Nanozymes could alleviate the hypoxic and acidic microenvironment inside the tumor to improve the efficacy of tumor photothermal therapy, photodynamic therapy, or radiotherapy.16–18 Also, nanozymes can help reverse the drug resistance of the tumor.20
Inflammation plays a crucial role in many diseases. Some have become the focus of increasing attention due to their high prevalence and increased risk of death and disability. There are many pathogenic factors for inflammation, including environmental chemicals, physical injuries such as scratches, pathogens, radiation, psychosocial stress, etc. Therefore, understanding of the pathogenesis and etiology of various types of inflammation is still in the research stage. Due to the lack of knowledge about diverse inflammation etiology, there is still a shortage of efficient early diagnosis and specific pharmaceuticals for some inflammation conditions. At the same time, people always underestimate the incidence of inflammatory diseases, and therapies are delayed because of insufficient awareness; consequently, the best treatment window for drugs may be lost, resulting in death or disability.19,20 Taking IBD, ALI, acute kidney injury (AKI), and cerebral ischemic stroke (CIS) as examples, these inflammatory diseases have high mortality and disability rates.21–23 In addition, they also face the problems of a lack of therapeutic drugs or a short treatment window, resulting in severe consequences like death or disability.24 At the same time, the medical cost is exceptionally high, so it is urgent to develop new therapeutic methods for these inflammatory diseases.
Research shows that ROS can induce inflammatory responses, leading ultimately to dysfunction and tissue injury.25,26 Therefore, it is known that alleviating oxidative stress as the target to treat inflammatory diseases has good feasibility. Nanozymes, as efficient antioxidants, can quickly scavenge RONS to protect cells, neurons, and tissues from ROS-induced oxygen stress and inflammation. Additionally, in the study of different inflammatory disease models, it was found that some nanozymes can partly suppress inflammatory-related cellular events through the prevention of influencing multiple signaling pathways, e.g., NF-κB, inhibiting the concentration of the pro-inflammatory cytokine (e.g., tumor necrosis factor (TNF), interleukin (IL), growth factor (GF), etc.), reducing the recruitment of related immune cells (e.g., macrophages, neutrophils, etc.) and inhibiting their activation, to achieve further anti-inflammation effects.27–30 Hence, utilizing nanozymes as a ‘drug’ for these inflammatory diseases is feasible and has great potential for clinical translation.
In conclusion, in this review, we will discuss the regulation of enzyme activity and the application of nanozymes in inflammatory diseases (Scheme 1). We systematically summarize the research on key factors that affect the catalytic activity of Pt, Mn, and Ce-based nanozymes, including modification, modulating the size and morphology of nanozymes and changing surface valence state, oxygen vacancy concentration, and composition. We aim to systematically understand the enzymatic features, kinetics, and mechanisms of nanozymes to find an effective method for the rational design and manufacture of high-performance nanozymes. Also, the application of nanozymes in IBD, ALI, AKI, and CIS is discussed to demonstrate the promising application value of nanozymes in inflammatory diseases. Moreover, we discuss methods for improving the utilization efficiency of nanozymes and reducing their potential toxicity in the human body. The application of nanozymes in other fields or other inflammatory diseases will not be repeated here. Readers are referred to numerous critical reviews and references for more information.
2 Modulation of catalytic properties of metal-based nanozymes
So far, more than one thousand nanozymes have been reported, their composition involving various elements. Among them, iron oxide nanozymes, gold-based nanozymes, carbon-based nanozymes, etc., have comprehensively summarized enzymatic features and novel applications due to early research. Readers are referred to plenty of reviews and references for more information.31–33 Herein, we briefly summarize newly developed Pt-based and Mn-based nanozymes, as well as Ceria nanozymes which have a broad prospect in the field of inflammation. We systematically summarize the research on improving the catalytic properties and biocompatibility of these three kinds of nanozymes by adjusting the size, morphology, valence, oxygen vacancy concentration, and composition of the nanozymes to improve their catalytic activity and find the relationship between structure and activity. We can thus see the key factors that affect the activity of nanozymes and gain inspiration for their improvement or design, and help nanozymes be better applied in the biomedical field in the future.2.1 Platinum-based nanozymes
As a noble metal, Platinum (Pt) is well known for its excellent corrosion resistance and catalytic activity. It is reported that Pt-based nanozymes exhibit multiple antioxidant enzyme-mimicking activities, including SOD-like, CAT-like, OXD-like, and POD-like activities, all exhibiting superior catalytic performances. Because of the various enzyme-like activities and excellent catalytic performance, Pt-based nanozymes have been used in in vitro detection,34,35 wound healing,36 tumor treatment,37–40 and other fields.Pt-based nanozymes exhibit superior and stable enzyme-like activities proved through theoretical calculations. Gao et al., using density functional theory (DFT) calculations, investigated the performance and mechanism of SOD-like, CAT-like, OXD-like, and POD-like activities on Pt (111), Au (111), and Ag (111) surfaces (Fig. 1(a)).41,42 It was proved that Pt (111) surface has the lowest adsorption energies (Eads) and activation energy (Eact) of its CAT, POD, OXD, and SOD-like reactions (Fig. 1(b) and (c)). Thus, Pt (111) has the most superior catalytic activity among these three surfaces, both in thermodynamic and kinetic aspects.
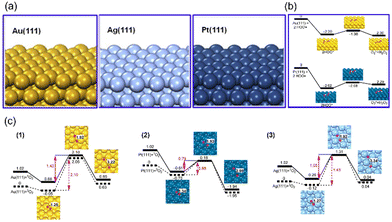 | ||
| Fig. 1 (a) The structure of Au (111), Ag (111), and Pt (111) surfaces. Reproduced from ref. 42 with permission from Elsevier, copyright 2015. (b) Rearrangement of two ˙OH on facets Au (111) and Pt (111). (c) 2D potential energy surfaces for O2 adsorption and dissociation on facets (1) Au (111), (2) Pt (111), (3) Ag (111). (b) and (c) have been reproduced from ref. 41 with permission from the American Chemical Society, copyright 2015. | ||
In order to further improve the catalytic activity of Pt-based nanozymes, scientists have researched changing the particle size and micromorphology, modifying the surface, and adjusting material surface valence or composition. From Table 1, we can see that the Pt nanozyme with a particle size of 5 nm shows better enzyme activity under the same pH and temperature conditions than that with a particle size of 20 nm2. Similarly, Shirahata et al. compared Pt nanoparticles (Pt nps) with a diameter of 1 nm, 2 nm, 3 nm, and 3–5 nm.43 They found that the catalytic activity of 1 nm Pt nps was the best. In addition, using DNA, BSA, and other proteins as templates, Pt nanozymes with particle size less than 5 nm also showed outstanding catalytic activity.44–46 The change of size can quickly improve the specific surface area of the nanoparticles so as to obtain better catalytic activity. It is worth noting that when comparing the effects of different particle sizes on the catalytic activity of Pt nanozymes, we should not only ensure the consistency of test conditions (such as the same temperature and pH range), but the synthetic scheme of materials should also be unified as far as possible.47 Different material synthesis methods, such as using BSA or other proteins as templates, will result in different catalytic activity performance compared with naked Pt nanoparticles, which may be caused by the difference between positively charged and negatively charged surfaces. Furthermore, the catalytic activity can also be enhanced by utilizing the excessively high specific surface free energy of single-atom nanozymes. Chen et al. directly transformed the Pt nanoparticles into single atoms.48 These Pt single atoms showed remarkable enhancement in POD-like activity and kinetics with a well-defined electronic and geometric structure. Besides changing the particle size, designing the morphology is also an effective method to improve the enzyme catalytic activity. For example, Pt nanozymes with high-index facets are always more active because of the high density of atomic steps, ledges, and kinks on high-index planes, which usually serve as active sites for breaking chemical bonds.49 Compared with {111}, {110}, {100} surfaces, Pt nanoparticles with high-index planes such as {730}, {311}, {210}, {310} generally have better catalytic activity. Tian et al. first synthesized tetrahexahedral platinum nanocrystals (THH Pt NCs) bounded by 24 facets of high-index planes ∼{730} and vicinal planes such as {210} and {310} (Fig. 2(a)–(e)).49 The characterization proved that they have high oxidation activity. Similarly, Yang et al. synthesized Pt nanorods enclosed by high-index {311} facets and found that they have enhanced catalytic performance for methanol selective oxidation.51 Gao et al. prepared high-index {730} faceted Pt concave nanocubes.50 Different from the idea of Tian, Gao et al. exposed the high-index crystal surface by preparing concave surfaces with negative curvature, which is not favored by thermodynamics owing to its higher energy (Fig. 2(f)–(h)). These high-index planes significantly improved the POD-like catalytic activity for an ultrasensitive colorimetric immunoassay of the human prostate-specific antigen (PSA).
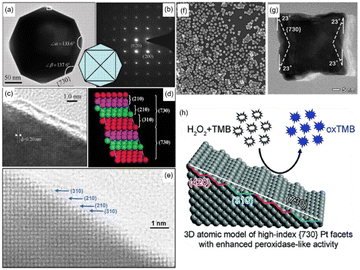 | ||
| Fig. 2 (a) Transmission electron microscope (TEM) image of the THH Pt NCs recorded along [001] direction. (b) Corresponding SAED pattern with square symmetry, showing the single-crystal structure of the THH Pt NCs. (c) High-resolution TEM image recorded from the boxed area marked in (a). (d) Atomic model of Pt (730) with a high density of stepped surface atoms. The (730) surface is made of (210) and (310) subfacets. (e) High-resolution TEM (HRTEM) image recorded from another THH Pt NC to reveal surface atomic steps in the areas made of (210) and (310) subfacets. (a)–(e) have been adapted from ref. 49 with permission from The American Association for the Advancement of Science, copyright 2007. (f) TEM image of synthesized Pt concave nanocubes with special concave morphology. (g) HRTEM {730} faceted Pt concave nanocube with measurements of the projection angles along the [001] zone axis. (h) Schematic illustration of the concave morphology. (f)–(h) have been adapted from ref. 50 with permission from The Royal Society of Chemistry, copyright 2017. | ||
| Pt-nanozymes | Size (diameter) nm | Substrate | K m (mM) | V max (10−8 M s−1) | Surface ligands | pH | Temperature (°C) | Mimicking activity | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Pt | 2.5 | H2O2 | 205.6 | 6.51 | Citrate | NA | NA | POD | 54 |
| TMB | 0.1206 | 9.79 | |||||||
| Pt | 5 | H2O2 | 47.2 | 17 | Citrate | 4.6 | RT | POD | 2 |
| TMB | 11.4 | 35 | |||||||
| Pt | 20 | H2O2 | 123.6 | 5.1 | Citrate | 4.6 | RT | POD | 2 |
| TMB | 1.3 | 14 | |||||||
| Ft-Pt | 15.22 | H2O2 | 187.25 | 3.2 × 104 | Apoferritin | 4.0 | 37 | POD | 52 |
| TMB | 0.22 | 55.8 | |||||||
| H2O2 | 420.60 | 8.4 × 104 | 7.4 | 37 | CAT | ||||
| Hep-Pt | 1.71 | H2O2 | 165 | 11.98 | Heparin | 4 | 25 | POD | 46 |
| TMB | 0.016 | 7.18 | |||||||
| DNA-Pt | 2.9 | H2O2 | 48 | 56.8 | DNA | 4.0 | 25 | POD | 45 |
| TMB | 0.056 | 58.2 | |||||||
| BSA-Pt | 2 | H2O2 | 41.8 | 16.7 | BSA | 4.0 | 25 | POD | 44 |
| TMB | 0.119 | 21.0 | |||||||
| PVP-Pt | 3 | H2O2 | 79 | 172 | Polyvinyl pyrrolidone (PVP) | 7.4 | RT | POD | 55 |
| TMB | 2.3 | 281 | |||||||
| H2O2 | 160 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 250 |
7.4 | RT | CAT | ||||
| CM-Pt | 3.8 | H2O2 | 63.86 | 29.0 | β-Casein | 4.0 | 20 | POD | 56 |
| TMB | 0.052 | 16.4 | |||||||
| HIF-Pt-CNCs | 43.9 | H2O2 | NA | 12.9 | PVP | 4.0 | 37 | POD | 50 |
| TMB | NA | 15.2 | |||||||
| FePt | 28 | TMB | 0.03 | 1.42 | Polyoxyethylene cholesteryl ether | 4.0 | 37 | Oxidase | 57 |
| Au2Pt | 42 | H2O2 | 5.045 | 14.11 | Polyethylene glycol (PEG) | 4.5 | 20 | POD | 17 |
| TMB | 0.05378 | 18.41 | |||||||
| H2O2 | 7.7066 | 90.18 | 7 | 20 | CAT | ||||
| AgPt | 44.3 | H2O2 | 76.05 | 3.57 | BSA | 4 | 37 | POD | 58 |
| OPD | 0.129 | 2.49 | |||||||
| H2O2 | 62.98 | 610 | 11 | RT | CAT | ||||
| PVP-PtCuNCs | 47.2 | H2O2 | 75.55 | 35.88 | PVP | 4.0 | 20 | POD | 59 |
| TMB | 0.033 | 26.78 | |||||||
| H2O2 | 9.94 | 0.2616 | 7.4 | 25 | CAT | ||||
| CDs (carbon dots)@PtNPs | 32 | H2O2 | 0.87 | 10.2 | Polyethyleneimine (PEI) | 4.0 | RT | POD | 36 |
| TMB | 0.079 | 17.6 | |||||||
| Au@Pt | Pt nanodots 3 nm | H2O2 | 0.0095 | 10.2 | Poly(styrenesulfate) | 4.5 | 30 | POD | 60 |
| TMB | 0.006 | 2.62 | 4.5 | 30 | Oxidase | ||||
| HCS@Pt | ∼220 | H2O2 | 0.04853 | 21.7871 | PEG | 4.5 | 25 | POD | 39 |
| TMB | 1.779 | 51.1538 | |||||||
| TMB | 0.3520 | 0.8243 | Oxidase | ||||||
| PtNPs@MnO2 | ∼250 | TMB | 0.015 | 15.6 | NA | 4 | RT | Oxidase | 61 |
| PtFe@Fe3O4 | NA | H2O2 | 53.55 | 10.78 | PEG | 4.5 | NA | POD | 37 |
| TMB | 0.213 | 5.477 | |||||||
| HRP | H2O2 | 0.34 | 9.48 | 4.0 | 35 | POD | 62 | ||
| H2O2 | 3.7 | 8.71 | 3.5 | 40 | POD | 31 | |||
| TMB | 0.434 | 10.0 | 3.5 | 40 | POD | 31 | |||
| Catalase | H2O2 | 54.30 | 1620 | 8 | CAT | 31 |
As is known to all, many catalytic reactions take place on the nanozyme surface. Additional surface coating or surface modification is also an idea to improve the enzyme activity of materials. Using small molecules, ions, and polymers to modify the nanozyme surfaces, mainly by physisorption, can expose the active sites and change the surface valence state of the material to improve the enzyme catalytic activity of the material. For Pt nanozymes, another critical role of surface modification is to avoid the aggregation of Pt nanoparticles sequentially to enhance their biocompatibility.
DNA,34,45 Apoferritin,52 and Bovine serum albumin (BSA),44,53etc. are commonly used for surface modification. They are usually used as synthetic templates to control the particle size and improve the stability and biocompatibility of materials. However, it is worth mentioning that, sometimes, due to the existence of templates, the reduction process of Pt ions will be hindered, resulting in a decline in catalytic activity. Fu et al. used two different kinds of DNA as templates to synthesize Pt nanozymes.45 The physicochemical properties, including diameters and charge states, are strongly associated with the DNA templates and the molar ratios of [Pt precursor]/[DNA]. DNA template can stabilize Pt nanoparticles and avoid aggregation, but to a certain extent it hinders the reduction of Pt2+. This causes a certain amount of Pt2+ ions to be left on the surface, which hinders further improving its affinity with the H2O2 substrate. In comparison, with the increase of the proportion of Pt0 on the surface, the affinity with H2O2 increases. Fan et al. used apoferritin to synthesize 1–2 nm Pt nanoparticles (Pt-Ft NPs) with CAT and POD-like enzyme activities.52 The use of apoferritin can also avoid the aggregation of Pt nanoparticles and improve their stability. The Pt-Ft NPs were composed of 74% Pt0 and 26% Pt2+. However, the hydrodynamic diameters of Pt-Ft NPs are about 15 nm, which perhaps accounts for the relatively low enzyme-like activity of Pt-FT NPs. Li et al. selected BSA as the template to synthesize Pt-based peroxidase nanomimetics with an average diameter of 2 nm, which possessed highly POD-like activity.44
In addition, Gu et al. synthesized Heparin-reduced Pt nanoclusters with enhanced POD-like activities but without oxidase-like characteristics.46 The surface-remaining heparin enhances its affinity with TMB substrates, contributing to outstanding POD-like activity.
Other metals which have enzyme-like properties, such as Au and Fe, were also used to synthesize Pt-based bimetallic nanomaterials. As a reasonable strategy, the synthesis of bimetallic nanomaterials with cheap metals can not only lessen the utilization of Pt but also enhance the catalytic performance. However, not all bimetallic nanozymes can perform higher catalytic activities. By forming bimetallic nanozymes, the electronic structure inside the material will change, thus changing their ability to facilitate electron transfer. The research shows that when Pt is alloyed with Ag or Au, the increase of Ag/Pt or Au/Pt ratio results in a sharp decrease in the POD and OXD-like activity.63,64 The research of Yin's group indicates that appropriate alloying of Pt with Ru can enhance both pro- and anti-oxidant capabilities.65 Controlling the atomic ratio of Ru to less than 25% can significantly improve the POD-like and OXD-like activity. Li et al. synthesized hollow PtCo nanozymes with greatly improved catalytic properties.66 And with the help of Co elements, the hollow PtCo nanozyme exhibit improved catalytic ability. Zhang et al. synthesized mesoporous Pd@Pt nanoparticles that have greatly enhanced the enzyme activity.67 In addition to the change of electronic structure, the mesoporous structure formed on the surface of Pt also provided a super large specific surface area for the material.
In conclusion, scientists can further improve the enzyme catalytic activity of Pt nanozymes by reducing the particle size, exposing high-index planes, modifying functional groups, and doping with other metal elements. Pt nanozymes possess stable POD, CAT, SOD, and OXD-like activities under a wide range of pH values and temperatures. In this way, Pt-based nanozymes show great potential in various applications. However, at this stage, the research on regulating their catalytic activity mostly depends on the simple characterization of enzyme-like reactions and the rough calculation of Michaelis–Menten kinetics parameters of POD-like activity or OXD-like activity. However, the simple characterization and kinetic analysis of enzyme-like activity are strongly affected by the chemical environment of the enzyme reaction test or the environment of material synthesis. So, it is virtually impossible to make a horizontal comparison between different regulation methods and synthesis methods. In the future, the change in the electronic structure of nanozymes or Gibbs free energy of enzyme-like reaction will become a more important research direction to judge whether this regulation method works or not. In this way, the structure–activity relationships of Pt nanozymes will be particularly elucidated.
2.2 Manganese-based nanozymes
A manganese (Mn)-based nanozyme is a nanozyme with the Mn atom as the active center, and usually means manganese oxide and its salts, such as MnO2, Mn3O4, Mn3(PO4)2, MnFe2O4, etc. Most of them possess multiple enzyme-like activities. Taking advantage of their excellent enzyme-like properties, Mn-based nanozymes are widely used in the treatment of inflammatory diseases,68–70 neurodegenerative diseases,71 and cancer,72,73 and in the field of in vitro detection.74–77 Besides, Mn-based nanozymes are used as contrast agents in MRI because Mn2+ is paramagnetic in nature.78 Here, we will briefly introduce the optimization of enzyme-like activities of Mn-based nanozymes from three aspects: MnO2, Mn3O4, and other Mn-based nanozymes.MnO2 shows great structural flexibility with several crystal types, such as α-MnO2, β-MnO2, and γ-MnO2. The coordination number of Mn and the specific surface area of these three crystal types are different. Three tunnel-structure polymorphs of MnO2 open up various possibilities for regulating its catalytic performance.82 In recent years, in order to improve the enzyme-like activities of MnO2 nanozymes, scientists have mainly focused on improving the specific surface area and shortening the electron transmission distance of materials by changing the morphology, adjusting the oxygen vacancy concentration, and doping with metals, as well as increasing or exposing active sites.
To evaluate the relationship between different morphology and catalytic activities, Han et al. synthesized one-dimensional (1D) MnO2 nanowires using genetically engineered filamentous phages as biotemplates.79 When comparing bulk MnO2 with phage-templated tetraglutamate-fused phage (M13-E4)@MnO2, the small size effect, the large surface area, and the homogeneous distribution of nanocrystals could improve the POD-like activity of MnO2 nanomaterials. In addition, the authors eliminated the possibility that the higher POD-like activity resulted from M13 itself or free manganese ions. Furthermore, through regulation of the synthesis temperature, the authors further verified that the specific surface area and uniformity of nanocrystals on phase are the sources of higher enzyme-like activity. Wan et al. synthesized MnO2 nanospheres, nanosheets, nanowires, nanocomplexes, and nanosticks to investigate the effect of morphology on enzyme catalytic activities83 (Fig. 3(a)–(e)). The results showed that the POD-like activity of nanospheres and nanowires was relatively good. However, the stability of the enzyme catalytic activity of the nanospheres was poor, and would decrease by nearly 50% after 24 hours. Consequently, the study further verified the superiority of 1D nanowires in POD-like enzyme catalytic activity. 1D nanowires can improve the electron transfer efficiency and show surface effect and size effect to improve the enzyme catalytic activity. Moreover, Jiao et al. proposed a hierarchical manganese dioxide nanoflowers (MnO2 NFs) structure, a typical δ-MnO2 crystalline structure.85 This rational, open structure enlarged the specific surface area and shortened the transport path length of electrons and cations. Hence, the material exhibited good OXD-like activity.
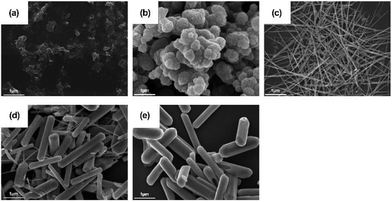 | ||
| Fig. 3 SEM images of (a) MnO2 nanosheets, (b) MnO2 nanospheres, (c) MnO2 nanowires, (d) MnO2 nanocomplexes, and (e) MnO2 nanosticks. Scale bar 1 μm. (a)–(e) have been reproduced from ref. 83 with permission from Elsevier, copyright 2011. | ||
Li et al. synthesized Co–γ-MnO2 and Ce–γ-MnO2 by doping, and it was found that doping with Ce makes the nanoparticle show mixed crystals consisting of γ-MnO2 and α-MnO2.86 The mixed crystal structure of MnO2 exhibits increased specific surface area with more surface oxygen vacancies and surface defects. These properties ultimately led to a higher CAT-like activity. Liu et al. used doping with Cu or Zn to improve the oxygen vacancy concentration on the surface of MnO2.84 This also changed the layer stacking, surface area, and electronic structure. As a result, CAT-like activity can be improved by a higher oxygen vacancy concentration. However, there was no distinct correlation between SOD-like activity and the oxygen vacancy concentration (Fig. 4). The data show that chloride and phosphate ligands on the material surface play a vital role in SOD-like activity. However, according to Li et al., the excessive increase of oxygen vacancy on the surface of MnO2 may hinder the internal electron transport of the material,87 and to some extent, it led to a decrease in the catalytic activity of the material. However, this study did not analyze the enzyme-like activity of the material.
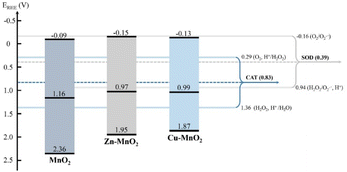 | ||
| Fig. 4 The band structure diagram and the redox potentials of different redox couples of MnO2, Zn–MnO2, Cu–MnO2 nanozymes in the CAT and SOD catalytic reactions. Adapted from ref. 84 with permission from Elsevier, copyright 2020. | ||
In conclusion, the benefit of these different crystalline structures of MnO2 is the morphology regulation which can further improve its enzyme catalytic activity. At the same time, increasing the concentration of oxygen vacancies on the surface of MnO2 is also an effective method. However, it should be noted that an oxygen vacancy concentration that is too high may hinder electron transport and finally reduce enzyme-like activity. Moreover, there are rare studies on the influence of different crystal planes on enzyme catalytic activity. In the future, we expect more research on how to improve MnO2 enzyme-like activity so as to explore further the key points affecting enzyme-like activities.
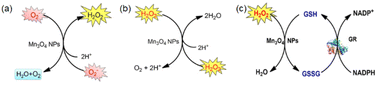 | ||
| Fig. 5 (a) Scheme showing SOD-like activity of Mn3O4 NPs. (b) Scheme showing CAT-like activity of Mn3O4 NPs. (c) Scheme showing GPx-like activity of Mn3O4 NPs. (a)–(c) have been adapted from ref. 89 with permission from John Wiley and Sons, copyright 2018. | ||
Generally speaking, the enzyme-like activity is closely related to the particle size, specific surface area, morphology, and surface valence of the material. For metal oxide, increasing the oxygen vacancy concentration on the surface is also a means to improve the enzyme activity of the material.
Singh et al. synthesized five different shapes of Mn3O4.89 Among them, Mn3O4 nanoflowers exhibited remarkably high activity in all three antioxidant enzyme-like activities: CAT-like, GPx-like, and SOD-like activities. Although the flower-like morphology leads to a large particle size, it also provides a very high specific surface area. Meanwhile, Mn3O4 nanoflowers also have larger pores that act as active site pockets similar to naturally occurring enzymes, providing the most suitable environment for enzyme-like activity. Also, the redox cycling between Mn2+ and Mn3+ in the material plays a critical role during the catalytic activity. Singh et al. explored the effect of the Mn3+/Mn2+ ratio by preparing oxidized-MnF and reduced-MnF.93 They found that increasing the Mn3+/Mn2+ ratio can further improve the CAT-like and GPx-like enzyme-like activities. And both oxidized-MnF and reduced-MnF show more excellent SOD-like enzyme activities, indicating that both oxidation states play crucial roles in SOD mimetic activity. Yao et al. also proved the importance of Mn atoms with different valence states on the nanozyme surface by comparing Mn3O4 with CeO2.94 Furthermore, Adhikari et al. proved that the Mn2+ is the catalytic center of GPx-like activities using DFT calculation.71 They proved the mechanism of the GPx-like reaction on the Mn2+ catalytic center and computed the Gibbs free energy (ΔG = −6.3 kcal mol−1). Thus, the adsorbed H2O2 will spontaneously split on the Mn2+ center, forming a peroxide species (Fig. 6(a) and (b)).
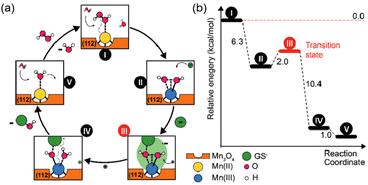 | ||
| Fig. 6 (a) Possible mechanism of GPx-like action of C–Mn3O4 NPs. (b) Energy profile for the reaction scheme performed by density functional theory. (a) and (b) have been adapted from ref. 71 with permission from John Wiley and Sons, copyright 2020. | ||
Similar to MnO2, adjusting the oxygen vacancy concentration on the surface of Mn3O4 is also a way to improve its enzyme activity.95 Lu et al. increased the oxygen vacancy concentration in Mn3O4 by adjusting oxygen partial pressure in the sintering process. Mn3O4 with higher oxygen vacancy concentration exhibited excellent OXD-like activity. In addition, the introduction of oxygen vacancy changed the structure of the material surface, and the molar ratio of Mn2+/Mn3+ on the Mn3O4 was greatly improved. The surface electronic structure, surface valence state, and oxygen vacancy played essential roles in enhancing the OXD-like activity.92
Overall, Mn3O4 exhibits exponential multi-enzyme-like properties that mainly come from the Mn2+ and Mn3+ couples on the surface. In addition, those oxygen vacancies on the surface may also act as the active center. However, there is still a lack of research on the relationship between oxygen vacancies on the surface and the enzyme-like activities of CAT-, SOD- and GPx-like activities. It is expected that scientists can use theoretical calculation and other methods to put forward more possibilities for the improvement of Mn3O4 intrinsic enzyme-like activities in the future.
Several studies on the regulation of the enzyme catalytic activity of MnFe2O4 enzymes have mainly focused on a specific enzyme-like activity. The combination of Mn ions and Fe ions gives this nanoparticle multiple potential applications, such as triggering the Fenton reaction and photothermal therapy for cancer.
In addition, single-atom Mn also displays CAT-like, OXD-like, and POD-like activities. The coordination of single-atom manganese to nitrogen atoms in hollow zeolitic imidazolate frameworks can realize tumor treatment.103
To sum up, single-atom Mn, manganese oxides, and manganese salts display various enzyme-like activities. In the future, the research direction for Mn-based nanozymes will focus more on evaluating the balance between its various enzyme activities and discovering the critical point of its enzyme-like activities. With the help of theoretical calculation, we can further study the relationship between structure and enzyme-like activities. At the same time, because Mn oxides and salts have been applied in the electrochemical catalysis and photocatalysis fields, using catalytic activity improvement schemes similar to the adjustment of enzyme activity will also be a research direction.
2.3 Cerium oxide-based nanozymes
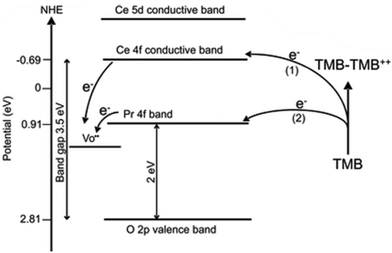
Ce is a well-known rare earth element with a unique 4f15d16s2 external electronic shell. The peculiar 4f orbitals of equal energy endow cerium with unique physical and chemical properties. Ceria (CeO2) is a nanocrystalline material derived from cerium, among the most studied metal oxides. Excellent catalytic performance distinguishes CeO2 for multipurpose industrial application, especially in catalysis-related fields.104 In addition, the applications of ceria in the biomedical field have been receiving increasing attention. It has played an essential role in the treatment of ROS-related diseases such as radiation protection,105 bone tissue repair and regeneration,106,107 angiogenesis, tumor, and inflammation,108–110 for its prospective oxidation resistance, antibacterial property, low toxicity, and good biocompatibility. Ceria has exhibited a variety of enzyme-like activities such as POD-like,111 OXD-like,112 CAT-like,113 and SOD-like activities.113 In addition, ceria also shows a unique phosphate-like activity.114 Because of its excellent application prospects, the structure–activity relationship of ceria nanozymes has been studied more deeply. Ceria nanomorphology and size are important factors affecting its catalytic abilities. Deshpande et al. synthesized nanocerium oxide particles with diameters of 3 nm, 6 nm and 30 nm, and proved that with the decrease of the particle diameter, the concentration of Ce3+ ions on the particle surface will increase due to the formation of oxygen vacancies, which made them show outstanding ROS scavenging ability.115 Similarly, Pulido-Reyes et al. synthesized nanoceria with spherical shape and diameters of approximately 5, 7, and 18 nm.116 They found that when the size was reduced to 5 nm, CeO2 nanozymes had the highest surface content of Ce3+ sites and the best SOD-like activities. Furthermore, Baldim et al. examined the SOD and CAT mimetic catalytic activities of nanoceria with sizes of 4, 5, 7.8, 23 and 28 nm.113 The results showed that the SOD-like activity was enhanced for smaller particles due to the largest Ce3+ fraction. But for CAT-like activity, particles sized 7.8 nm exhibited the best catalytic activity. Through further analysis, the authors speculate that the adsorption of H2O2 molecules at the particle surface modulates the efficacy of the decomposition process and should also be taken into account. This indicates that the nanoceria CAT-like activity depends mainly on particle size, Ce3+ fraction and H2O2 adsorption properties. These two reports listed above suggested that the surface density of Ce3+ active sites played a significant role in the SOD catalytic activity. Ahn et al. further proved that ceria with smallest particle size possessed the best OXD-like activities.117In addition to the impact of size on the enzyme-like activities of nanoceria, the morphology of the ceria nanostructure can also exert influence on the properties. Tian et al. synthesized five different ceria nanostructures: CeO2 nanoparticles, CeO2 cubes, CeO2 octahedrons, non-porous nanorods and porous nanorods. Among these, porous nanorods of ceria demonstrated a robust POD-like activity. And they revealed that a higher surface Ce3+ fraction, larger surface area and higher concentration of oxygen vacancies led to improved catalytic activity of ceria. Furthermore, Yang et al. synthesized ceria nanorods and nanocubes with similar Ce3+/Ce4+ ratio and oxygen vacancy content.118 They demonstrated that different exposed facets may also influence the enzyme-mimetic activities. They found that CeO2 nanocubes with exposed {100} facets exhibit a higher POD-like activity than CeO2 nanorods with exposed {110} facets. And for POD-like activities, CeO2 nanocubes with exposed {100} facets exhibit a higher POD but lower SOD-like activity than those of the nanorods with exposed {110} facets.
Naganuma et al. utilized a nitrogen gas purge method to control the crystal shape of ceria nanoparticles.119 By comparing the CAT mimetic and SOD mimetic activities of nanopolyhedra, nanocubes and nanorods, they found that nanopolyhedra with dominant {111} planes exhibit the highest CAT-like activities. This may be explained by the large percentage of Ce4+ ions and the highest oxygen vacancy content. The nanoceria with a higher concentration of Ce3+ ions on the {100} planes further enhanced the SOD mimetic activity. Moreover, the phosphatase mimetic activity of ceria nanoparticles is also affected by different shapes. Trenque et al. elaborated nano-octahedra, nanocubes or nanorods by hydrothermal synthesis to describe the reaction mechanism of phosphatase mimetic activity.121 They found that both specific surface area and different facets play important roles in the phosphatase-like activity of nanoceria. Among them, nano-octahedral with {111} facets exhibit the best phosphatase mimetic activity. Also, these authors elucidated that the coordination between the phosphoryl oxygen and a metallic atom remains the most likely adsorption mechanism for paraoxon. Fisher et al. investigated the relationship between morphology and decomposition of H2O2.122 They found that the ceria nanorods exhibited the best catalytic activity because of the highest oxygen vacancy concentration and the highest percentages of Ce3+ in the surface. In addition, {100} facets were concluded to be more active than {111} facets for decomposition of H2O2. Furthermore, Xu et al. proposed that a broom-like porous hierarchical structure CeO2 has a higher concentration of oxygen vacancies and shorter electron transmission distance that result in enhancement of catalytic activity.123
The optimization of the catalyst's structural composition is essential to regulate its catalytic performance. Scientists usually use doping with new metals or synthesis of a bimetallic alloy to change the structure of materials. By introducing metal with catalytic activities, such as Pt,6 Mn,72 Lanthanum,124 or Zirconium,125 the structure of CeO2 is changed and the concentration of its active sites is adjusted. Hao et al. synthesized Praseodymium (Pr)-doped mesoporous CeO2 to possess numerous oxygen vacancies and ultimately increase catalytic activities.126 Pr and Ce are adjacent to each other on the periodic table. In that case, the addition of Pr greatly reduces the bandgap of ceria and thus improves its photocatalytic properties. Similarly, Jiang et al. also modified ceria nanocubes with Pr, and they found with increasing concentration of Pr the ceria nanocubes exhibited an enhanced oxidase-like activity.120 They found that the Pr incorporated into the ceria nanocubes produced a lower energy level, thus the electron transfer was much easier for Pr-modified CeO2 NC samples, leading to their higher oxidase activity (Fig. 7). They also found a relationship between morphology and the Pr concentration.
 | ||
| Fig. 7 Electron transfer between TMB and CeO2 nanocube (path 1) or 10% Pr–CeO2 nanocube during TMB oxidation. Adapted from ref. 120 with permission from the American Chemical Society, copyright 2017. | ||
Other than Pr, which is adjacent to Ce on the periodic table of elements, scientists have used other lanthanide series metal elements such as Lanthanum (La)124 and Ytterbium (Yb)127 as well. Besides, utilizing metals with high catalytic activities (e.g., Pt, Pd) can further improve ceria nanoparticle enzyme mimetic activities by changing the electronic structure and components. Yan et al. developed single-atom Pt/CeO2 with enhanced CAT-like, SOD-like, POD-like and GPx-like activities.6 Single-atom Pt induced the lattice expansion and enhanced the Ce3+/Ce4+ ratio on the surface, and it brought more oxygen vacancies. Furthermore, the Pt single atoms with oxygen vacancies from the active lattice oxygen on the uppermost CeO2 can provide a catalytically active site and thus further improve the multi-enzyme activities (Fig. 8(a) and (b)).
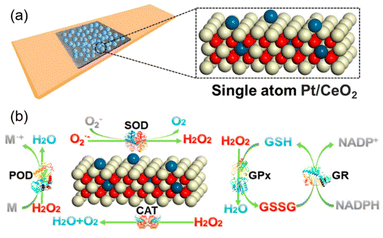 | ||
| Fig. 8 (a) Design of single-atom Pt/CeO2 bandage. (b) Enzyme-mimetic properties of single-atom Pt/CeO2. (a) and (b) have been adapted from ref. 6 with permission from the American Chemical Society, copyright 2019. | ||
All these factors significantly increased the endogenous catalytic activity of Pt/CeO2.
Li et al. proposed a new hybrid of Pd nanoparticles dispersed on CeO2 nanotubes (Pd NPs/CeO2 NTs).128 Pd NPs/CeO2 NTs hybrid exhibited a synergy effect to enhance POD-like activity. Specifically, Pd causes the change of electronic states on the surface of CeO2 and thus contributes to a shorter distance between O and Pd. Consequently, the oxygen atom is readily reduced, and at the same time, Ce4+ is easily transferred to the low oxidation of Ce3+, thus increasing the Ce3+/Ce4+ ratio. Zhu et al. reported the enzymatic properties of Ti-doped CeO2 nanozymes.129 They found that the OXD-like activity slightly increased while the SOD-like activity declined on doping with Ti. The different performance of these two kinds of catalytic activities may contribute to the fundamentally different chemical mechanisms of these two enzymatic activities; the SOD-like activity appears to be sensitive to shape, while the oxidase activity is not. Guo et al. reported an effective strategy to modulate the POD-like activity of nanoceria by doping with the first row of transition metals (i.e., Mn, Fe, Co, Ni, and Cu) with the same doping ratio.130 From their experiments, the POD-like activities of these nanoparticles followed the order: Mn1Ce10 > Co1Ce10 > Fe1Ce10 > Cu1Ce10 > CeO2 > Ni1Ce10 (Fig. 9). Despite the decreased Ce3+ fraction with Mn doping, both the surface oxygen (Oβ) and the synergistic effect between Ce and Mn played key roles in the POD-mimicking activity of the Mn-doped nanoceria.
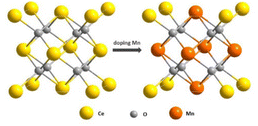 | ||
| Fig. 9 Schematic illustration of engineering nanoceria by transition metal doping for enhanced peroxidase mimics. Adapted from ref. 130 with permission from John Wiley and Sons, copyright 2019. | ||
Depositing other metal oxides on the surface of cerium oxide may also change surface structure to produce highly catalytic antioxidants. Han et al. deposited manganese ions on the surface of cerium oxide nanocrystals to form strained layers of manganese oxide islands, increasing the number of oxygen vacancies131 (Fig. 10(a)). In addition, the CeO2 core and Mn3O4 islands experience a tensile strain due to a large mismatch between the lattice parameters (CeO2 2.71 Å and Mn3O4 2.36 Å) of these two metal oxides (Fig. 10(b)). All these factors induced a significant increase in both CAT-like and SOD-like activities. It could be easily seen that the higher concentration of oxygen vacancies often correlated with exposed Ce3+ catalytic sites and increased intrinsic catalytic activity.
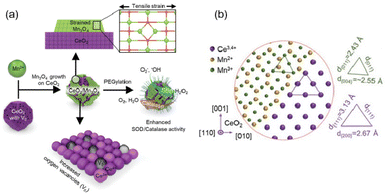 | ||
| Fig. 10 (a) Schematic illustration of highly catalytic CeO2/Mn3O4 nanocrystals. (b) Schematic atomic arrangement of heterostructured CeO2/Mn3O4 nanocrystals. (a) and (b) have been adapted from ref. 131 with permission from John Wiley and Sons, copyright 2020. | ||
Therefore, scientists further studied the relationship between the Ce3+/Ce4+ ratio and oxygen vacancy concentration to further elucidate the impact of Ce3+/Ce4+ ratio and oxygen vacancies on enzyme-like activities. The crystal structure of cerium oxide shows that most of the cerium atoms are present in the +4 state in the crystalline lattice. However, scientists found that the reduction of ceria nanoparticle size leads to an increase in oxygen vacancy concentration and Ce3+ content. Fisher et al. found that the concentration of Ce3+ increases with the rise of oxygen vacancy content.122 They classified oxygen vacancies into Schottky-type and Frenkel-type oxygen vacancy defects (Fig. 11). For Frenkel-type oxygen vacancy defects, the oxygen anions are displaced from their lattice positions to interstitial sites. These dislocations consequently create vacancies at the original lattice sites. This may also account for the phenomenon that both the highest Ce3+ concentration and oxygen vacancy content are found on the surface of ceria nanorods with the best catalytic performance among ceria nanorods, ceria nanocubes, and ceria nano-octahedra. According to the characterization by XPS and Raman data, most oxygen vacancies on the surface of nanorods are Schottky-type oxygen vacancies, and Frenkel-type vacancies also exist.
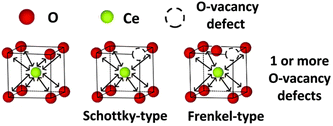 | ||
| Fig. 11 Schematic diagram of Schottky-type defect and Frenkel-type defect. Adapted from ref. 122 with permission from the Royal Society of Chemistry, copyright 2019. | ||
The enzyme-like catalytic activity of nanozymes is also closely related to their surface valence state and oxygen vacancies. Heckert et al. found that a decrease in the Ce3+/Ce4+ ratio correlates directly with a loss of SOD mimetic activity.132 Pirmohamed et al. also confirmed the relationship between SOD mimetic activity and Ce3+/Ce4+ ratio, but they found that the CAT-like activity appears to be inversely related to the steady-state cerium oxidation state of the nanoparticle, which shows lower CAT-like activity with the increase of Ce3+/Ce4+ ratio.133 Similarly, Naganuma et al. proved that with higher Ce3+ content, the ceria nanoparticles would exhibit enhanced SOD-like activity, and nanoparticles with higher Ce4+ content would improve the CAT-like activity.119 The different trends of CAT-like and SOD-like activities may be related to the SOD-mimic and CAT-mimic reactions.
SOD-mimic reaction:
| O2˙− + Ce3+ + 2H+ → H2O2 + Ce4+ |
Catalase-mimic reactions:
| H2O2 + 2Ce4+ → O2 + 2Ce3+ + 2H+ |
Scientists also examined the relationship between Ce3+ content and phosphate-like activity. Dowding et al. found that ceria nanoparticles with an increased level of Ce4+ exhibit better phosphate-like activity.134 Furthermore, decreasing oxygen vacancies may also play a role in increasing the phosphate activity of the nanoparticles because oxygen vacancies can strongly coordinate with the –OH group and decrease the availability of –OH on the surface. However, Kuchma et al. reached the opposite conclusion.135 They used simulations from first principles and concluded that Ce(III) sites are the more active site in the hydrolysis reaction. They found that the activation energy for dephosphorylation is lower for the Ce(III)–Ce(III) center than for either the Ce(IV)–Ce(III) center or the Ce(IV)–Ce(IV) center, which is in agreement with the finding that dephosphorylation is inhibited when cerium is in the +4 oxidation state.
In conclusion, changing the particle size, morphology, structure, surface valence, and oxygen vacancy concentration can efficiently improve the activity of nanoceria enzymes. The enhancement of enzyme activity can be realized by increasing Ce3+ concentration and oxygen vacancy concentration, exposing specific facets, and changing the surface structure of the material. By finding the structure–activity roles, scientists could get inspiration to design nanozymes from the beginning to achieve highly efficient ceria nanozymes. Moreover, this may ultimately provide a variety of solutions for the further application of nanoceria in the fields of catalysis and biomedicine.
3 Nanozymes for inflammatory diseases
Oxidative stress arises from the imbalance of RONS inside the human body, and can cause damage to liposomes, proteins, and DNA, and significant destruction of cell structure and function, supporting protease activity through the inactivation of antiproteases and by modulating the formation of inflammatory mediators and adhesion molecules. Many of the pathophysiological effects of RONS may occur simultaneously or sequentially. Therefore, imbalanced RONS in the body will lead to a series of diseases, such as inflammation, neurodegenerative diseases, and cancer.136,137 In addition, oxidative stress can cause negative effects such as aging and slow wound healing.138,139 Nanozymes usually exhibit many kinds of enzyme-like activities that can efficiently alleviate oxidative stress in organisms. By scavenging reactive oxygen species, they can regulate redox balance to realize disease treatment. Scientists have investigated their potential in treating a variety of inflammatory diseases. Here, we mainly focus on the treatment with nanozymes of inflammatory diseases such as IBD, ALI, AKI, and CSI.3.1 Inflammatory bowel diseases
Inflammatory bowel disease (IBD) is a non-specific, chronic and recurrent gastrointestinal inflammatory disease with unclear etiology and pathogenesis. IBD includes two chronic idiopathic inflammatory diseases: Ulcerative Colitis (UC) and Crohn's disease (CD). The incidence and prevalence of IBD are increasing worldwide.140Because of its recurrence and unpredictable disease process, IBD is not curable. The treatment goals are to minimize symptoms, improve quality of life, and minimize the progression and complications of the disease. There are some clinical treatments for IBD, such as glucocorticoids,141 oral Janus kinase (JAK) inhibitor,142 and some low molecular weight drugs. Usually, the treatment is expensive and has potentially negative consequences, which lays a heavy burden on patients and their families. Therefore, it is urgent to develop new and effective drugs.
ROS has a long-standing implication in both the etiology and the progression of UC143 (Fig. 12). Both previous and recent studies showed oxidative stress in patients with untreated CD, and a positive correlation was found between free radical formation and pro-inflammatory cytokine content.144 At the same time, the production of reactive oxygen species and mitochondrial function were reversed during disease remission, showing the important role played by mitochondria and oxidative stress in CD development.145 Therefore, eradicating ROS may have great potential for ameliorating the pro-inflammatory microenvironment and promoting mucosal healing in IBD so as to treat UC or CD.30 Nanozymes, as a kind of nanomaterial with multiple enzyme-like activities, are characterized by their ability to eliminate reactive oxygen species and relieve oxidative stress (Table 2).
| Application | Nanozyme | Catalytic activity | In vivo models | Method of administration | Dosage | Ref. |
|---|---|---|---|---|---|---|
| Inflammatory bowel disease | PPB | CAT, POD, SOD | Dextran sulfate sodium (DSS)-induced colitis mouse model | Intravenously injected on days 1, 3, 5, and 7 | 10 mg kg−1 | 29 |
| Ce NP-PEG | CAT, SOD | DSS-induced colitis mouse model | Intravenously injected on days 3, 5, and 7 | 1 mg kg−1 | 30 | |
| CeO2@MMT | CAT, SOD | DSS-induced colitis mouse model | Orally administrated on days 5, 7, and 9 | 55 mg kg−1 | 146 | |
| Mn3O4 | CAT, SOD | DSS-induced colitis mouse model | Intraperitoneally injected on days 7, 8 and 9 | 0.10 mg kg−1 | 148 | |
| 2,4,6-Trinitro benzene sulfonic acid (TNBS) induced CD model | Intraperitoneally injected on days 9, 10 and 11 | |||||
| Manganese Prussian blue nanozymes (MPBZ) | CAT, POD, SOD | DSS-induced colitis mouse model | Orally administrated on days 1, 3, 5, 7 | 0.3 mg ml−1, 3 ml | 149 | |
| Fe/N-doped hollow carbon nanospheres | CAT, POD, SOD, OXD | DSS-induced colitis mouse model | Orally administrated on days 1, 3, 5, 7 | 0.5 mg ml−1, 0.12 ml | 150 | |
| NiCo2O4@PVP | CAT, POD, SOD, OXD | DSS-induced colitis mouse model | Orally administrated on the days 1, 3, 5, and 7 | 8 mg kg−1 | 151 | |
| Ulcerative colitis | CCZM | SOD | DSS-induced colitis mouse model | Intravenously injected on days 2, 4, 6 | 2 mg kg−1 | 147 |
| Colitis-associated colon cancer | Rh-PEG NDs | CAT, SOD | DSS-induced colitis mouse model | Intravenously injected on day 1 | 10 mg ml−1 0.1 ml | 14 |
| Scavenge ˙NO, ONOO− | CT-26 tumor-bearing mouse model |
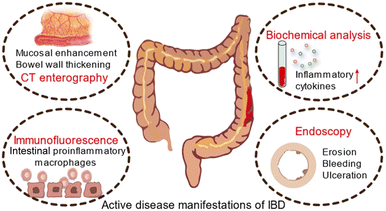 | ||
| Fig. 12 Schematic diagram of active disease manifestations of IBD. Adapted from ref. 30 with permission from Springer Nature, copyright 2022. | ||
Therefore, it is possible to use nanozymes in the treatment of IBD. Zhao et al. constructed poly(vinylpyrrolidone)-modified Prussian blue (PPB) to improve material stability and biosafety.29 The prepared PPB had POD-like, CAT-like, and SOD-like enzyme activities that could scavenge ROS and inhibit proinflammatory cytokines, significantly reducing colitis in mice without distinct side effects. In addition, the researchers found that PPB is a potent NF-κB inhibitor, suppressing the NF-κB activation pathway, resulting in a dramatic decrease of inflammatory cytokines, including TNF-α and IL-6. Zeng et al. synthesized biocompatible drug-free ceria nanoparticles (CeNP-PEG) with CAT-like and SOD-like activities, and ˙OH scavenging abilities.30 CeNP-PEG ameliorated the proinflammatory microenvironment via persistently scavenging ROS, down-regulating the levels of several proinflammatory cytokines, and restraining the proinflammatory profile of macrophages and Th1/Th17 response. The mechanism may be related to the inhibition of the co-activation of NF-κB and JAK2/STAT3 pathways.
In addition, over-produced RNS (e.g., ˙NO and ONOO−) can also induce cell damage, DNA fragmentation, and lipid oxidation, and have a long-standing implication in both the etiology and progression of UC and CD.144 Miao et al. reported that polyethylene glycol (PEG)-coated (PEGylated) ultrasmall rhodium nanodots (Rh-PEG NDS) can scavenge RONS and have photothermal activities for anti-inflammatory and antitumor theranostics in colon diseases.14 Benefiting from multi-enzyme activities, the regeneration of thick colonic epithelial in mice was observed, proving that the material has the potential to restore the disrupted intestinal barrier (Fig. 13(a)). According to these studies, it can be seen that alleviating RONS from inflammatory sites is an effective method to treat IBD, which suggests great potential in clinical application.
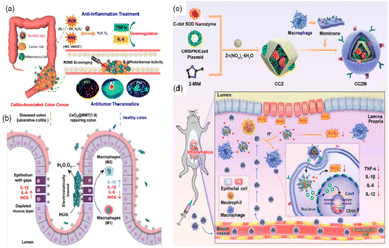 | ||
| Fig. 13 (a) Schematic illustration of Rh nanozymes with intrinsic RONS scavenging and photothermal activities for simultaneous anti-inflammation and antitumor theranostics in colon diseases. Adapted from ref. 14 with permission from the American Chemical Society, copyright 2020. (b) Therapeutic effects of CeO2@MMT on IBD. Adapted from ref. 146 with permission from John Wiley and Sons, copyright 2020. (c) Synthesis of C-dot nanozyme@CD98 CRISPR/Cas9 plasmid@ZIF-8 (named as CCZ). CCZM were finally constructed by cloaking with macrophage membrane onto CCZ. (d) Applications of CCZM in UC treatment. (c) and (d) have been adapted from ref. 147 with permission from the American Chemical Society, copyright 2022. | ||
The significant disadvantages of nanozymes are insufficient targeting and lacking the ability to bind to substrates specifically, which may cause side effects or affect the effectiveness of the treatment. Therefore, it is necessary to rationally design the nanozyme to improve targeting, bind specific substrates, and offer a potential treatment option for patients with IBD. Zhao et al. coupled multi-enzyme-mimicking CeO2 nanoparticles with montmorillonite (MMT).146 The negatively charged MMT can be orally administered and specifically adsorbed onto the positively charged inflamed colon tissue via electrostatic interactions for targeted delivery. MMT is well tolerated by the digestive tract and has been used clinically. It can alleviate diarrhea through physical binding, cover mucosa, and remove toxins. In addition, the CeO2 nanoparticles exhibit SOD-like and CAT-like enzyme activities and ˙OH scavenging ability. Assembled by in situ growth of CeO2 on MMT, the obtained CeO2@MMT can specifically target inflamed colon, scavenge excessive ROS, downregulate the level of inflammatory factors, and relieve symptoms (Fig. 13(b)).
In addition to using positive and negative charges to target inflammatory sites, Ma et al. used macrophage membrane to improve the targeting ability of their biomimetic system.147 They designed C-dot nanozyme@CD98 CRISPR/Cas9 plasmid@ZIF-8@Macrophage membrane (CCZM) (Fig. 13(c)). The upregulation of transmembrane glycoprotein CD98 plays an important role in the initiation and progression of UC. The authors used the CRISPR/Cas9 system to down-regulate the expression of CD98 so as to reduce the severity of inflammation values of the inflammation conditions induced the disaggregation of ZIF-8, leading to the targeted release of C-dots and plasmid. Ultimately, the macrophage membrane was modified on the outer layer of ZIF-8 to improve the circulation time, target inflammatory sites, and provide simultaneous multi-drug delivery ability to the material. As expected, this biomimetic system exhibited pH-responsiveness, immune escape, and inflammation-targeting capability simultaneously.
Based on this, the authors designed a biomimetic pH-responsive metal–organic framework (MOF) carrier to deliver carbon nanodot (C-dots) nanozymes and clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) (CRISPR/Cas9) system for site-specific treatment of UC (Fig. 13(d)). In this system, the C-dots nanozyme showed excellent SOD enzyme activity, and could scavenge ROS effectively. The low pH values of the inflammation conditions induced the disaggregation of ZIF-8, leading to the targeted release of C-dots and plasmid. Ultimately, the macrophage membrane was modified on the outer layer of ZIF-8 to improve the circulation time, target inflammatory sites, and provide simultaneous multi-drug delivery ability to the material. As expected, this biomimetic system exhibited pH-responsiveness, immune escape, and inflammation-targeting capability simultaneously.
3.2 Acute liver injury
Acute liver failure (ALF) is a short, intense clinical syndrome with a variety of causes resulting in a rapid loss in hepatocyte function.152 Prior to the availability of liver transplantation, mortality of ALF was extremely high, often exceeding 90%.152 There are various etiologies of ALF, including Acetaminophen (APAP) overdose, hepatitis, drug-induced liver injury (DILI), and hepatic ischemia-reperfusion (HIRI). Despite there being several different etiologies of ALF, ROS has long been implicated in the pathophysiology of various acute liver injuries (ALIs).153 In this way, we can translate these findings to the clinic and develop therapeutic agents like antioxidant drugs. Nanozymes, well known for their antioxidant performance, may quickly scavenge those ROS in the liver so as to protect cells and the liver from oxidant stress. Here, we will introduce the application of nanozymes in ALI from the aspects of APAP-induced ALI, DILI, HIRI, and so on. According to the statistics, in the United States and European countries APAP abuse accounts for most ALI cases,19,152,154 and the cases of APAP-induced ALI show an increasing trend year by year.155 In the last few decades, intense investigation of the mechanism of hepatotoxicity has established the role played by oxygen free radicals in the etiopathogenesis of APAP-induced ALI.153 The increased generation of free radicals leads to the dysfunction of mitochondria, lipid peroxidation, and centrilobular necrosis. N-Acetylcysteine (NAC) is the only clinically available antidote but its therapeutic time window is rather narrow.156 In this way, nanozymes, as an ideal antioxidant drug, have great potential in the treatment of APAP-induced ALI.Utilizing the SOD-like, CAT-like, and GPx-like enzyme activities of Cu5.4O, Liu et al. used ultrasmall Cu5.4O nanoparticles (Cu5.4O USNPs) for the treatment of APAP-induced ALI.157 By controlling the hydrodynamic size at about 4.5 nm, the Cu5.4O USNPs exhibited cytoprotective effects at an extremely low dosage and realized significant treatments (Fig. 14(a)). Meanwhile, according to in vivo experiments, approximately 70% of Cu5.4O USNPs were excreted within 48 h post-injection. Thus, it is proved that the ultrasmall size of Cu5.4O USNPs enables rapid renal clearance, low long-term toxicity, and high biocompatibility. At the same time, Li et al. reported ceria nanozymes (CeNZs) with SOD-like, CAT-like activities and ˙OH scavenging ability for the treatment of APAP-induced ALI.158 By using their CAT-like enzyme activities, CeNZs can generate abundant oxygen to alleviate hypoxia and specifically suppress the activation of CD86+ macrophages to relieve inflammation and promote hepatocyte regeneration. Both the protective effect of CeNZs and treatment at the late stages have been proved via in vivo experiments, which demonstrated the therapeutic ability of CeNZs (Fig. 14(b)).
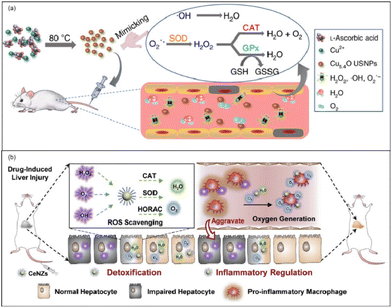 | ||
| Fig. 14 (a) Schematic preparation of Cu5.4O USNPs with multi-enzyme like activities. Adapted from ref. 157 with permission from Springer Nature, copyright 2020. (b) Schematic illustration of the ROS scavenging and oxygen generation abilities of CeNZs to treat drug-induced liver injury based on their multi-enzymatic activities. Adapted from ref. 158 with permission from Elsevier, copyright 2020. | ||
In addition to APAP-induced ALI, drug-induced liver injury caused by anthracycline (e.g., daunorubicin (DNR), and doxorubicin, which are commonly used as antitumor agents) is gradually increasing. Bai et al. demonstrated artificial Prussian blue nanozymes (PBZs) with CAT-like, SOD-like, and POD-like enzyme activities to prevent anthracycline-induced liver injury.159 PBZs could efficiently eliminate ROS, reduce deoxyribonucleic acid damage, and decrease the levels of inflammatory cytokines and chemokines. Also, PBZs could upregulate antioxidative genes by activating the Nrf2 pathway to reduce oxidative stress and protect against anthracycline-induced liver injury.
Intraperitoneal injection of lipopolysaccharide (LPS) in mice is a common way to establish acute liver damage. Lipopolysaccharide/D-Galactosamine (LPS/D-Gal)-induced acute liver injury is characterized by significant inflammatory responses and is widely applied in inflammation research.160 Li et al. utilized Er3+-doped CeO2−x nanoprobes with high CAT-like, SOD-like, and GPx-like enzyme activities for LPS-induced ALI.161 By comparing the protein expression levels of TNF-α and IL-1β in the liver, the function of anti-inflammation was proved. Furthermore, Zhao et al. explored cellular vesicles as templates and carriers to elevate the therapeutic performance and biocompatibility of Ce nanozymes (Ce-ReVs).15 By using nano-sized red cell vesicles (ReVs) as templates they synthesized ultrasmall Ce nanocrystals with ultrahigh Ce(III) content to enhance antioxidant performances. Also, by using red cell vesicles the Ce-ReVs can quickly accumulate at the inflamed sites. Moreover, these authors hybridized ReVs with mesenchymal stem cell-derived exosomes to realize the additional repair function of highly damaged tissues. And during in vivo research, they found strong evidence of disrupted vascular structure and increased blood flow in colitis, inspiring the use of these pathophysiological features to treat ALI. Also, the approach of using cellular vesicles and exosomes for additional repair is worth learning.
Hepatic ischemia-reperfusion injury (HIRI) is an acute liver injury that often happens during liver transplantation, hepatic resection, or trauma.162,163 Using some antioxidant agents, it was proved that the administration of exogenous antioxidants, particularly in the early stages of reperfusion, can significantly decrease the severity of HIRI.163 In this way, using nanozyme as an antioxidant could be a feasible way to treat HIRI.
Ni et al. used the CeO2 nanoparticles (NPs) with CAT-like and SOD-like activity and the ability to scavenge ˙OH to regulate oxidative stress in the liver.164 It was proved that ceria NPs can scavenge ROS, restore SOD levels, and alleviate lipid peroxidation/DNA damage. At the same time, the ceria NPs successfully inhibited the activation of Kupffer cells and monocyte/macrophages, minimized the recruitment and infiltration of neutrophils, and suppressed subsequent inflammatory responses (Fig. 15(a)). Researchers exploited the ‘disadvantage’ of the mononuclear phagocyte system (MPS, e.g., liver, spleen) which often sequesters most of the injected nanoparticles. In this way, most ceria NPs will be preferentially taken up by the liver to directly prevent HIRI. Similarly, Katsumi et al. developed Pt nanoparticles (Pt-NPs) to scavenge superoxide anion, hydrogen peroxide, and hydroxyl radicals27 to prevent oxidative stress, and partially suppress inflammatory-related cellular events through prevention of NF-κB pathway activation in the liver after ischemia/reperfusion. Interestingly, by comparing Pt NPs of different diameters (30 nm and 106 nm), researchers found that although the Pt NPs with larger diameters had higher accumulation in the liver, Pt NPs with smaller diameters had better therapeutic effects because of their better enzymatic activity.
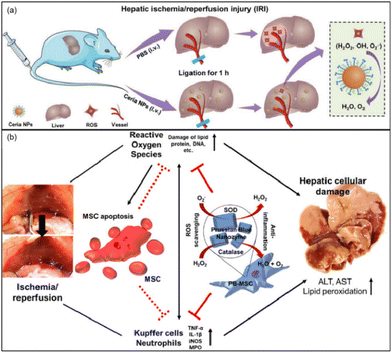 | ||
| Fig. 15 (a) Schematic illustration of preventing IRI by ceria NPs. Adapted from ref. 164 with permission from John Wiley and Sons, copyright 2019. (b) Prussian blue (PB) nanoparticles impregnated into MSC for hepatic ischemia-reperfusion injury alleviation. Adapted from ref. 28 with permission from the American Chemical Society, copyright 2021. | ||
In order to achieve better therapeutic efficiency, Sahu et al. incorporated Prussian blue (PB) into mesenchymal stem cells (MSCs) for HIRI alleviation.28 MSC-based therapy holds great potential to heal injured tissues/organs through paracrine factor secretion and immunomodulation. However, the transplanted cells exhibited low viability due to the elevated oxidative stress levels. Nevertheless, PB nanoparticles as a biocompatible nanozyme exhibiting SOD-like, POD-like and CAT-like activities could be an ideal ROS-scavenging nanozyme to protect MSCs. The analysis of cytokines and serum in tissues showed that PB not only improved the survival rate of MSCs under high oxidative stress, but also remarkably promoted the paracrine effect and anti-inflammatory properties of MSCs (Fig. 15(b)). PB and MSC combined could support each other and be an effective and viable strategy in the treatment of HIRI.
In conclusion, nanozymes have shown great potential for therapeutic effects on DILI, HIRI, and other acute liver injury models (Table 3). Nanozymes can protect the liver from oxidative stress, by scavenging the reactive oxygen species in inflammatory sites, downregulating the production of the inflammatory cytokines, and inhibiting the activation of related signal pathways. Since nanoparticles can accumulate in the mononuclear phagocyte system (e.g., liver, spleen) after entering the body,164 the targeted delivery of nanoparticles to disease sites can be easily realized.
| Application | Nanozyme | Catalytic activity | In vivo models | Method of administration | Dosage | Ref. |
|---|---|---|---|---|---|---|
| DILI | Cu5.4O USNPs | GPx, SOD, CAT | APAP-treated mice | Intravenously injected | 2 μg kg−1 | 157 |
| CeNZs | SOD, CAT | APAP-treated mice | Intravenously injected | 1.5 mg kg−1 | 158 | |
| PBZs | SOD, CAT, POD | Anthracycline-treated mice | Intravenously injected | 5 mg kg−1; 10 mg kg−1; 20 mg kg−1 | 159 | |
| ALF | Er3+-doped CeO2−x | SOD, CAT, GPx | LPS-treated mice | Intravenously injected | 2 mg kg−1 | 161 |
| Ce-ReVs | Scavenge ˙OH and O2˙− | D-GalN/LPS-treated mice | Intravenously injected | 5 × 1013 particles per kg | 15 | |
| Liver fibrosis | CeO2 | SOD | CCl4-treated rats | Intravenously injected | 0.1 mg kg−1 | 165 |
| MoS2 | SOD, CAT, POD, GPx | CCl4-treated mice | Intravenously injected | 0.5 mg kg−1 | 166 | |
| HIRI | CeO2 NPs | SOD, CAT | Hepatic IRI | Intravenously injected | 0.6 mg kg−1 | 164 |
| Pt-NPs | SOD, CAT | Hepatic IRI | Intravenously injected | 50 μg kg−1 | 27 | |
| MSC-PB | SOD, CAT, POD | Hepatic IRI | Intravenously injected through hepatic vein | 1 × 105 cells per mice | 28 |
Therefore, the treatment of acute liver injury with nanozymes has good clinical translation potential and provides new aspects to understanding the pathology of ALI.
3.3 Acute kidney injury
Acute kidney injury (AKI) is a syndrome generally characterized by abrupt deterioration in kidney function.167 According to the statistics, AKI occurs in approximately 10–15% of patients admitted to hospital, and the incidence in intensive care patients is more than 50%.168 Annually, AKI leads to about 1.7 million deaths worldwide.169 The incidence of AKI is increasing and is associated with considerable morbidity and mortality.167,170 Although the pathophysiology of AKI varies due to complex etiologies, oxidative stress has been proved as the predominant pathogenesis for AKI.169 The overproduction of ROS and RNS may lead to cellular apoptosis, necrosis, altered gene expression, progression of tissue damage, promotion of fibrosis, and abnormal kidney function.171 Nanozymes as ideal antioxidants have great potential to protect the kidneys from oxidative stress. Significantly, for the treatment of AKI, researchers pay much attention to the hydrodynamic diameter of nanozymes. A hydrodynamic diameter of ∼5–6 nm is suggested to minimize nanozyme toxicity. This hydrodynamic diameter is associated with higher renal filtration and urinary excretion.172 Because of the small particle size, nanozymes can pass through the glomerular capillary wall in the kidneys, resulting in higher biodistribution and clearance of nanomaterials intravenously injected into the body.Most nanoparticles with larger size will be nonspecifically taken up by mononuclear phagocyte systems (e.g., liver and spleen). Therefore, the hydrodynamic diameter of nanozymes is important in the treatment of AKI. Here, we will discuss the application of nanozymes in AKI caused by sepsis, drugs with nephrotoxicity, and rhabdomyolysis.
Sepsis is a systemic inflammatory response syndrome that results from the host's deleterious response to infection. Patients with AKI associated with sepsis have a significantly increased mortality relative to those with AKI of other etiology.173 The pathogenesis of acute renal injury caused by sepsis has not been fully elucidated. Inflammation, oxidative stress, and renal tubular epithelial cell injury are all potential mechanisms.173
Yu et al. designed a ROS-responsive nano-drug delivery system for sepsis-induced AKI.174 They utilized ceria nanoparticles with multi-enzyme activities modified with triphenylphosphine (TPP), followed by coating with ROS-responsive organic polymer (mPEG-TK-PLGA) and loaded atorvastatin (Atv/PVP-TCeria NPs). The mPEG-TK-PLGA coating can improve biocompatibility and mono-dispersity, and the thioketal bond in mPEG-TK-PLGA can be readily cleaved by ROS, to responsively release ceria nanoparticles and atorvastatin (Fig. 16). Atorvastatin has an ameliorative effect on AKI, and is loaded in the nano-drug delivery system to reduce the dosage of ceria nanoparticles and increase the therapeutic effect. Furthermore, TPP was used to help ceria nanoparticles target mitochondria, improving the efficiency of eliminating excessive ROS, which demonstrated great potential in treating sepsis-induced AKI according to the in vivo experiments. Besides sepsis-induced AKI, drugs with nephrotoxicity may account for 25% of AKI cases.175 In particular, chemotherapy-associated nephrotoxicity is increasingly recognized as a significant side effect of chemotherapy. About 17.5% of cancer patients develop AKI,176 and among them, cisplatin-induced AKI is widely studied due to this drug's long history as an ideal chemotherapeutic agent since 1978. Studies have demonstrated that the mechanism of AKI induced by cisplatin is related to its direct nephrotoxicity and oxidative stress.177 Through the generation of ROS, cisplatin can damage the structure and functional constituents of renal cells.175,178
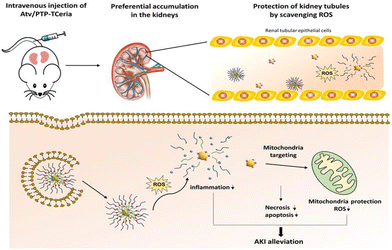 | ||
| Fig. 16 Atv/PTP-TCeria NPs could passively target the kidneys and release drug responding to high-level ROS and would target mitochondria to scavenge excessive ROS for ameliorating AKI. Adapted from ref. 174 with permission from the American Chemical Society, copyright 2021. | ||
Zhang et al. synthesized ultrasmall Prussian blue nanozymes (PB NZs) with 4.5 nm in diameter as theranostic agents for magnetic resonance (MR)/photoacoustic (PA) dual modal imaging guided AKI treatment.179 PB NZs exhibited CAT-like, POD-like and SOD-like activities, which can effectively eliminate RONS (including ˙NO and ONOO−) in the kidneys (Fig. 17(a)). According to their excellent enrichment in the kidneys, multi-enzyme mimetic abilities, and rapid blood elimination and kidney excretion, ultrasmall PB NZs exhibited superior AKI treatment efficacy in cisplatin-induced AKI. Also, the treatment of rhabdomyolysis-induced AKI (RM-AKI) has been proved. Similarly, Rosenkrans et al. designed selenium-doped carbon quantum dots (SeCQDs) to prevent cisplatin-induced AKI.180 They exhibited prominent renal accumulation and showed great potential as a treatment option for AKI (Fig. 17(b)).
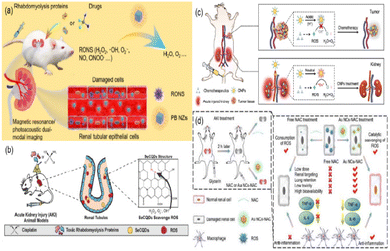 | ||
| Fig. 17 (a) Schematic illustration of Prussian blue nanozymes (PB NZs) as theranostic agent clearance for magnetic resonance/photoacoustic dual-modal imaging-guided drug-induced AKI or RM-AKI treatment through the effective removal of various reactive oxygen/nitrogen species (RONS). Adapted from ref. 179 with permission from Springer Nature, copyright 2021. (b) Scheme showing that the specific renal accumulation of SeCQDs allows prevention and treatment of cisplatin-induced AKI of different origins. Adapted from ref. 180 with permission from the authors, copyright 2020. (c) Schematic illustration of catalytic activity-tunable CNPs for AKI prevention during chemotherapy. Adapted from ref. 109 with permission from Springer Nature, copyright 2021. (d) Schematic illustration of anti-inflammatory activities of ultrasmall Au NCs-NAC for RM-AKI alleviation as compared with free NAC. Adapted from ref. 184 with permission from the authors, copyright 2021. | ||
Nanozymes as ideal antioxidants can scavenge ROS to treat AKI and protect kidney structures. However, nanozymes may indiscriminately reduce oxidative stress in tumor tissues, and are argued to spur the growth and metastasis of tumors, hindering their potential application for preventing chemotherapy-induced AKI in cancer patients. To solve this problem, Weng et al. exploit the pathophysiological differences between kidney and tumor, especially the pH discrepancy, to reconcile AKI prevention and potent chemotherapy.109 They designed a catalytic tunable ceria nanoparticle (CNP). Utilizing the acidic tumor environment, the CNPs become inert facing excessive H+ (Fig. 17(c)).
These H+ may disrupt the re-exposure of active-catalytic sites, and thus do not interfere with ROS generated for cancer therapy. And CNPs exhibit CAT-like and SOD-like activities for scavenging ROS in the neutral microenvironment of the kidneys. Furthermore, CNPs can activate the Nrf2/Keap1 signaling pathway for efficient ROS elimination. In this way, CNPs possess multi-enzyme like activities to protect the kidneys in chemotherapeutic agents-induced AKI but without compromising anti-tumor potency under an acidic tumor microenvironment. Moreover, the protective effect of CNPs is also observed in ischemia-reperfusion (IR)-induced AKI.
Acute kidney injury as a complication of rhabdomyolysis is quite common, with a reported incidence ranging from 13% to approximately 50%.181 It has been proved that ROS play a key role in RM-AKI. Thereby nanozymes with antioxidant properties can attenuate the oxidative stress, militating against subsequent renal damage.182
Ni et al. designed molybdenum-based polyoxometalate (POM) nanoclusters with preferential renal uptake as novel nano-antioxidants for kidney protection.183 These POM nanoclusters possessed the capability to scavenge H2O2, O2˙− and ˙OH. As smart, self-adaptive nano-theranostics, POM with ultra-small size showed high rates of accumulation in the kidneys over a long period (>24 h). In this way, POM nanoclusters are promising for the treatment of both RM-AKI and cisplatin-induced AKI. Although long-term retention in the body can completely remove excess ROS, studies in recent years have found that longer retention of nanoparticles may cause potential toxicity to other organs. From this, Zhang et al. reported that ultrasmall PVP-coated iridium nanoparticles (Ir NPs-PVP) with POD-like, SOD-like, and CAT-like activities scavenge excessive RONS in the kidneys.4 Meanwhile, because of an ultrasmall size, Ir NPs-PVP demonstrated preferential renal uptake following intravenous administration and could be rapidly excreted through the urine. These findings proved that ultrasmall Ir NPs-PVP have good compatibility and low toxicity, and have enormous promise for treating cisplatin-induced AKI and RM-AKI. Zhang et al. also designed ultrasmall gold nanoclusters capped with NAC (Au-NAC) with SOD-like, CAT-like, and POD-like activities for RM-AKI treatment184 (Fig. 17(d)). The Au-NAC displayed preferential renal enrichment (<2 h) in AKI mice.
In addition to RM-AKI, cisplatin-induced AKI, and AKI caused by sepsis, some scholars have focused on intra-abdominal infection-induced AKI. Manne et al. used the SOD-like and CAT-like activities of CeO2 to attenuate the damage caused by peritonitis to renal glomeruli and tubules.185 Also, it can reduce the level of superoxide in the kidneys and inflammation, and diminish renal apoptosis.
To sum up, nanozymes can efficaciously protect the kidneys from oxidative stress, whether in sepsis-induced AKI, cisplatin-induced AKI, RM-AKI, or intra-abdominal infection-induced AKI (Table 4). For the treatment of acute renal injury, the hydrodynamic parameters size of the nanozyme should below the kidney filtration threshold, so as to pass through the glomerulus and be excreted within a certain time scale.172 In the future, researchers will put more effort into improving the renal delivery efficiency and at the same time achieving rapid renal clearance based on high treatment efficiency. In this way, the accumulation of nanoparticles in the body and toxic side effects will both be minimized. Utilizing nanozymes as antioxidants for treating AKI triggered by various insults can improve treatment efficiency, and has great potential for clinical translation.
| Application | Nanozyme | Diameter (nm) | Catalytic activity | In vivo models | Method of administration | Dosage | Ref. |
|---|---|---|---|---|---|---|---|
| Sepsis-induced AKI | Atv/PTP-TCeria NP | 25.5 | SOD, CAT | LPS-induced AKI model | Intravenous injection | 1 mg kg−1 | 174 |
| Chemotherapy-induced AKI | Ceria NPs | 9.7 | SOD, CAT | Cisplatin-induced AKI model | Intravenous injection | 0.5 mg kg−1; 1.5 mg kg−1 | 109 |
| Ischemia-reperfusion (IR)-induced AKI model | 180 | ||||||
| 1.5 mg kg−1 | |||||||
| SeCQD | 40 | Scavenge H2O2, ˙OH and O2˙− | 50% glycerol treated mice | Intravenous injection | 1 μg in 200 μl PBS | 186 | |
| Cisplatin-induced AKI model | 50 μg in 200 μl PBS | ||||||
| RM-AKI | Ceria NPs | 3–4 | SOD, CAT, POD | 50% glycerol-treated mice | Intravenous injection | 2 mg in 150 μl PBS | 179 |
| PB | 4.5 | SOD, CAT, POD | 50% glycerol-treated mice | Intravenous injection | 100 μg in 100 μl PBS 100 μg in 100 μl PBS | 183 | |
| Cisplatin-induced AKI model | |||||||
| POM | 3 | Scavenge H2O2, ˙OH and O2˙− | 50% glycerol-treated mice | Intravenous injection | 1 mg in 100 μl PBS | 4 | |
| Cisplatin-induced AKI model | |||||||
| Ir-PVP | 1.5 | SOD, CAT, POD | 50% glycerol-treated mice | Intravenous injection | 500 μg | 184 | |
| Cisplatin-induced AKI model | |||||||
| Au-NAC | 3–4 | SOD, CAT, POD | 50% glycerol-treated mice | Intravenous injection | 200 μg in 100 μl PBS | 187 | |
| RuO2 | 2 | SOD, CAT, GPx | 50% glycerol-treated mice | Intravenous injection | 5 mg kg−1 | 188 | |
| Intra-abdominal infection-induced AKI | CeO2 NPs | 10–40 | SOD, CAT | Cecal inoculums-treated rats | Intravenous injection | 0.5 mg kg−1 | 174 |
3.4 Cerebral ischemic stroke reperfusion
Stroke is an acute cerebrovascular disease caused by the sudden rupture of cerebral blood vessels or the transient or permanent reduction of the blood flow in brain caused by the blockage of a cerebral artery. Stroke is the second leading cause of death, causing 9% of deaths worldwide.21,189 The incidence and mortality rate of stroke remain elevated for several years.190Stroke can be simply classified into two categories, hemorrhagic and ischemic, with ischemic stroke accounting for up to 85% of all cases.191 The only treatment for ischemic stroke approved in the USA acts to clear the thrombus that impedes blood flow, either by mechanical means or with an intravenous tissue-type plasminogen activator (tPA).190 However, using mechanical means is traumatic, while the effect of tPA is limited and with a short treatment window. Besides, the reperfusion caused by thrombolysis will lead to the production of oxygen boost-mediated free radicals and lead to severe consequences.192,193
In addition, although there are antioxidant enzymes in the brain that can remove RONS spontaneously, ischemic stroke prevents their expression.194 This makes it impossible to completely remove excessive RONS and avoid tissue loss in the infarct rim with differential consequences for stroke recovery.195 Alleviating oxidative stress during a stroke can effectively break the vicious circle between excess RONS and brain injury. Nanozymes have excellent potential for ischemic stroke treatment and improving prognosis, especially in addressing injury caused by reperfusion. Nevertheless, it is worth noting that a significant issue in the clinical translation of nanozymes in treating ischemia-reperfusion injury is whether the nanozyme can cross the blood–brain barrier (BBB) or not.
Scientists have mainly focused on feasibility studies in the early stage of using nanoparticles to treat cerebral ischemia-reperfusion injury. As early as 2009, Reddy et al. first encapsulated natural SOD in biodegradable nanoparticles (SOD-NPs) to reduce ischemia-reperfusion injury.196 Reddy et al. found that SOD-NPs could cross the BBB to the brain parenchyma, possibly because of the increased permeability of the BBB during reperfusion. The success of using natural enzymes to treat ischemia-reperfusion injury provided the reference for using nanoparticles with enzyme-like activities as an ideal antioxidant to treat cerebral ischemia-reperfusion.
In 2011, Takamiya et al. produced a Platinum nanoparticle (nPt) with SOD-like and CAT-like activities to treat ischemic stroke.197 Treatment with nPt greatly reduced infarct volume and improved motor function. Treatment with nPt nanoparticles inhibited the activation of MMP and alleviated the significant reduction of collagen IV through the scavenging of superoxide anion. In this way, nPt treatment achieved significant neurobehavioral and neuroprotective effects. Similarly, in 2012, Kim et al. synthesized ultrasmall ceria nanoparticles (<4 nm) that exhibited SOD-like and CAT-like activities.198 They demonstrated that the intravenously injected ceria nanoparticles could not permeate normal brain tissue but were able to penetrate into the ischemic brain tissue. This was likely possible because ischemia leads to extensive breakdown of the blood–brain barrier, which facilitates the passage of ceria nanoparticles.
The studies listed above indicate the promise of nanozymes for prevention of cerebral ischemia-reperfusion injury. In recent years, scientists have explored special injection methods to help nanoparticles effectively cross the BBB to reach the ventricle.
For example, Liu et al. used preinjection into lateral ventricles to circumvent the BBB and achieve more precise control of local nanozyme delivery.199 Direct injection into lateral ventricles can also minimize the adverse effects in normal tissues involved in systemic therapy and circumvent occluded vessels that may prevent delivery to the ischemic region. Using a rat model of ischemic stroke, the authors showed that preinjection of PEG-MeNPs can significantly decrease the infarct area of ischemic brain with negligible side effects. Yan et al. reported a dietary strategy to promote stoke healing using iron oxide (Fe3O4) nanoparticles with CAT-like, POD-like, and SOD-like activities.200 They found that after intracerebroventricular injection, Fe3O4 nanozymes accumulated in the ventricle and could not be detected in the blood vessels, compared with the oral administration of Fe3O4 nanozymes. And when orally administered, Fe3O4 nanozymes were widely distributed in the cerebral vascular walls in mouse brain within 24 h post-administration. These points may account for the failure of injected Fe3O4 nanozymes to protect the brain from neuronal damage. Therefore, these results indicate that orally administrated Fe3O4 nanozymes play a vital role in neuroprotection and maintaining the balance of the antioxidant reduction system to overcome the destruction of BBB permeability and nerve injury.
However, it is nearly impossible to directly inject nanoparticles into cerebral ventricles in clinical practice, and it has a high risk. Therefore, how to use modification and other means to lift the efficiency of nanozymes penetrating the BBB has become an important issue for the clinical translation of nanozymes.
To help nanozymes cross the BBB, Huang et al. used Human serum albumin (HSA) to modify Mn3O4 nanozyme.68 HSA–Mn3O4 exhibited SOD-like, CAT-like and GSH-Px-like activities. In addition, HSA–Mn3O4 can release manganese ions into the body's blood circulation, promoting SOD2 expression. Therefore, HSA–Mn3O4 can reduce neuronal damage, improve neurological function and ultimately constrain ischemic stroke reperfusion-induced nervous system injury (Fig. 18(a)).
 | ||
| Fig. 18 (a) Schematic illustration of the HSA–Mn3O4 synthetic route and ROS scavenging capacity in reperfusion-induced injury in ischemic stroke.68 Adapted from ref. 68 with permission from the American Chemical Society, copyright 2022. (b) Schematic diagram of MNET salvaging in an acute ischemic stroke via combining free radical scavenging and natural oxygen sponge effect.201 Adapted from ref. 201 with permission from the American Chemical Society, copyright 2020. (c) Illustration of MPBzyme@NCM therapy for ischemic damage and long-term neurological functional recovery. Adapted from ref. 203 with permission from the American Chemical Society, copyright 2021. | ||
Shi et al. relied on the stealth effect of natural erythrocytes and the BBB-crossing ability of T7 peptide to realize efficient enrichment of nanoparticles in the infarct area.201 They reported that an engineered nanosponge (Mn3O4@nanoerythrocyte-T7 (MNET)) exerted a distinct therapeutic effect in two stages of an ischemic stroke, combining regulating hypoxia after ischemic stroke and oxygen-boost after thrombolysis. This oxygen-sponge effect is attributed to Hemoglobin (Hb), a nature oxygen transporter, which has reversible oxygen-transport capacity: releasing oxygen in a hypoxia environment and storing oxygen in an oxygen-enriched environment. Benefiting from the stealth effect of natural erythrocytes, the BBB-crossing ability of the T7 peptides, oxygen-sponge effect of Hb, and high antioxidant activity of Mn3O4, MNET exerted an obvious targeting ability and played a major role in cerebral infarction. Before thrombolysis, MNET can rescue neurocytes via rapid free radical scavenging and timely oxygen supply; after thrombolysis, it suppresses the oxygen-boost via oxygen storage, and scavenges free radicals to avoid reperfusion injury. This nanosponge system provides a broad clinical application of nanozymes in ischemic stroke (Fig. 18(b)).
In addition to using specific modifications to improve the BBB penetration rate, specific physiological changes after stroke may also provide inspiration for designing a nanozyme platform. For example, overreactive astrocytes and activated microglia will release a burst of highly toxic and proinflammatory factors, including RONS, which further exacerbate ischemic brain damage. Furthermore, following ischemia/reperfusion injury, a large influx and activation of glial cells causes an intense inflammatory response and glial scar formation, ultimately disturbing the self-repair ability of brain tissues. Liu et al. developed a Co-doped Fe3O4 nanozyme that is capable of scavenging excess RONS in reperfusion injury to provide neuroprotection against ischemic stroke.202 The Co-doped Fe3O4 exhibited uptake by neurons, astrocytes, microglia, and endothelial cells, and it was found to be accumulated in the rim of the penumbra, providing neuroprotection 72 h post-stroke in transient middle cerebral artery occlusion (MCAO). Similar results were also confirmed in the photothrombotic stroke model. Specifically, Co-doped Fe3O4 nanozymes reduced neurological deficits and the infarct volume, decreased stroke-induced astrogliosis, attenuated microglial abundance, and increased neuronal survival in these stroke models.
In addition, some scholars have taken advantage of neutrophil-like cell membrane. Feng et al. synthesized a neutrophil-like cell-membrane-coated mesoporous Prussian blue nanozyme (MPBzyme@NCM) with SOD-like and CAT-like activities.203 It can realize noninvasive active-targeting therapy based on the innate connection between inflamed brain microvascular endothelial cells and neutrophils after stroke. After being intravenously administered, MPBzyme@NCM is delivered into the damaged brain and taken up by microglia to exhibit long-term in vivo therapeutic efficacy, including polarization of microglia toward M2, reduced recruitment of neutrophils, decreased apoptosis of neurons, and proliferation of neural stem cells, neuronal precursors, and neurons (Fig. 18(c)).
In conclusion, nanozymes can effectively alleviate oxidative stress, reduce the level of proinflammatory cytokines, inhibit activation of astrogliosis and microglia in the brain to achieve the neuroprotective effect, reduce the infarct area and ameliorate neurological deficits during ischemic stroke reperfusion injury. The feasibility of alleviating oxidative stress as a target for the treatment of stroke was proved through in vivo and in vitro experiments. The BBB plays a vital role in maintaining the stability of brain tissue, preventing harmful substances from entering the brain through the blood, and this cannot be circumvented. Crossing the BBB is always an obstacle for nanoparticles to reach the clinic. By modifying HSA, T7 peptide, or neutrophil cell membrane, scientists have realized the targeted delivery of nanozymes across the blood–brain barrier and verified their effectiveness in mouse models. These studies provide a direction for the future investigations of nanozyme treatments, to help cross the BBB post-ischemia, including exploring BBB-penetrable modifications or utilizing specific physiological changes like the recruitment of immune cells. They also provide inspiration to realize nanozyme therapy for other brain diseases, such as Alzheimer's and Parkinson's disease.
However, because of the significant physiological difference between animal models and humans, especially the thickness and nature of the BBB, whether nanozymes can cross the human blood–brain barrier is still unclear. In the future, research hotspots for the clinic translation of nanozymes will be in achieving targeted nanozyme delivery through simple modification or a simple drug delivery method, crossing the blood–brain barrier, improving nanozyme utilization, and reducing the potential toxicity of nanozyme interaction with biological systems (Table 5).
| Application | Nanozyme | Catalytic activity | In vivo models | Method of administration | Dosage | Ref. |
|---|---|---|---|---|---|---|
| Ischemia-reperfusion | SOD-nanoparticles | SOD | Focal cerebral ischemia-reperfusion injury model | Intracarotid arterial administration | 35 mg kg−1 | 196 |
| Cerebral ischemia | Cerium oxide nanoparticles | Scavenge H2O2, ˙OH, O2˙−, ˙NO, ONOO− | Mouse hippocampal brain slice model of ischemia | 204 | ||
| Cerebral ischemic stroke | Platinum nanoparticle | SOD, CAT | Transient middle cerebral artery occlusion (tMCAO) model | Intravenous injection | 4.0 μmol kg−1 | 205 |
| Ceria nanoparticles | SOD, CAT | Focal ischemia-reperfusion model | Intravenous injection | 0.5 mg kg−1; 0.7 mg kg−1 | 198 | |
| Hollow Prussian blue | POD, CAT, SOD | Middle cerebral artery occlusion (MCAO) model | Intracerebroventricular injection | 206 | ||
| PEG-melanin nanoparticles | SOD | Middle cerebral artery occlusion (MCAO) model | Intraventricular injection | 10 mg ml−1 | 199 | |
| PEG–Fe3O4 | POD, CAT, SOD | Middle cerebral artery occlusion (MCAO) model | Oral administration | 50 mg kg−1 | 200 | |
| Intracerebroventricular injection | 1.5 mg kg−1 | |||||
| 3 mg kg−1 | ||||||
| HSA–Mn3O4 | SOD, CAT, GSH-Px | Middle cerebral artery occlusion (MCAO) model | Intravenous injection | 0.4 mg kg−1 | 68 | |
| Co–Fe3O4 | CAT | Middle cerebral artery occlusion (MCAO) model | Intravenous injection | 2 mg kg−1 | 202 | |
| Photothrombotic stroke model | ||||||
| MPBzyme@NCM | SOD, CAT | Transient middle cerebral artery occlusion (tMCAO) model | Intravenous injection | 20 mg kg−1 | 203 | |
| MNET | Scavenge ˙OH and O2˙− | Middle cerebral artery occlusion (MCAO) model | Intravenous injection | 4 μg ml−1 | 201 |
4. Summary and outlook
In this review, we have systematically summarized recent progress in regulating the enzyme-like activities of Pt-based nanozymes, Mn-based nanozymes, and Ceria nanozymes. Furthermore, we discussed the applications of nanozymes in inflammatory bowel diseases, acute liver injury, acute kidney injury, and cerebral ischemic stroke reperfusion.Most studies have shown that multi-enzyme-like activities are influenced by five main factors: morphology, size, specific facets, chemical composition, and surface modification. The latest research on the structure–activity relationship of nanozymes can be summarized as follows.
(1) Size and morphology regulation of nanozymes
In most studies, nanozymes with smaller sizes and fine-tuned morphology (e.g., nanorods, porous nanosphere) may lead to larger specific surface areas and increased oxygen vacancy content. Nevertheless, this does not exactly obey the size effect once their surface active sites are influenced by other factors, such as electron transmission distance or ligand effects. In addition, active site adjustment can also be achieved by regulating size or morphology. Porous hierarchical structures like nanoflower or broom-like morphology can increase the concentration of oxygen vacancies and shorten electron transmission distance to enhance catalytic activity.
(2) Surface structure regulation of nanozymes
Surface structure regulation means specifically exposing specific facets of high-index facets to enhance the catalytic performance of nanozymes. Specific facets are those facets that exhibit relatively higher enzyme-like activities. The higher enzyme-like activities are usually attributed to a more considerable amount of dangling bonds or different atomic arrangements, which sometimes may also contribute to the exposure of catalytically active atoms. For example, ceria nanocubes with exposed {100} facets exhibit a higher POD-like activity but lower SOD-like activity than those of the nanorods with exposed {110} facets. However, both Mn-based nanozymes and Pt-nanozymes lack deep research on the relationship between specific facets and enzyme-like activities. Furthermore, high-index facets are thermodynamically more unstable and active than lower energy surfaces. They may have a high density of atomic steps, ledges, and kinks, which usually serve as active sites for breaking chemical bonds. In a word, the nanozymes’ catalytic activity is closely related to their surface structure.
(3) Regulation of the composition of nanozymes
Optimization of the catalyst's composition is essential to improve its catalytic performance. There are several ways to regulate the composition of nanozymes, including directly depositing other metal ions onto the surface of nanozymes, simply synthesizing bimetallic alloys with a co-reduction process, doping with other metal atoms, and so on. By changing the composition of nanozymes, the content of oxygen vacancies and exposed catalytically active atoms will increase. Also, doping with other atoms will change the electronic structure of nanozymes. Sometimes, there are significant discrepancies in lattice parameters between core and shell which may induce the surface structure to experience a tensile strain, to expand the lattice and ultimately increase the catalytic capability. Otherwise, growing nanomaterials with different catalytic activities can effectively broaden the utilization of nanozymes.
(4) Surface modification of nanozymes
Since the catalytic reactions mainly occur on the surface of nanozymes, surface modification can not only enhance the biological targeting effects but also improve their catalytic activities. Generally speaking, scientists use DNA, BSA, and other proteins as templates to control the size of nanozymes and change the surface state, so as to enhance the catalytic activities. Moreover, the protein corona formation can also affect the catalytic activity of nanozymes by affecting their surface properties. Besides, some scientists have modified pH-responsive carriers or specific peptides on the surface of nanozymes to improve targeted binding on specific substrates and the stability of nanozymes in the human body.
Overall, the number of active sites may be the most essential part in the design of nanozymes. The catalytic activity and mechanisms of the nanozymes mainly depend on the steric configuration and numbers of active centers rather than the size, structure, and composition. However, the relationship between catalytic activities and the architecture of nanozymes needs further elucidation to facilitate the design of nanozymes and ultimately promote the enzyme-like activities of nanozymes. Furthermore, more precise characterization methods of SOD-like and CAT-like activities need to be explored to allow us to judge the efficiency of different enzyme activity regulation methods. Thus, the fundamental understanding of the relationship between the catalytic activities and the structure of nanozymes is essential in designing nanozymes.
The tunable catalytic properties of nanozymes provide an opportunity to design biosafe agents for medical applications. With the enhancement of the catalytic ability of nanozymes, the potential toxicity to organs in vivo could be minimized. In this review, we have gathered recent research on the development of inflammatory disease treatment based on nanozymes in inflammatory bowel diseases, acute liver injury, acute kidney injury, and cerebral ischemic stroke reperfusion. The overproduction of ROS and RNS and inflammation are the fundamental problems that limit the recovery and treatment of injuries, resulting in cellular apoptosis, necrosis, altered gene expression, or even progression of tissue damage. Thus, it is crucial to eliminate excess RONS for inflammatory disease therapy. The intrinsic catalytic activities of nanozymes can efficiently scavenge RONS, reduce the level of proinflammatory cytokines, and inhibit the activation of immune cells to relieve inflammation. Numerous studies, as we concluded, have demonstrated the feasibility and responsiveness of nanozymes in inflammatory treatment applications.
Although nanozymes have many advantages in the area of inflammation-related diseases, they will still face many challenges in the future. Firstly, compared with natural enzymes, nanozymes generally have various enzyme-like activities, but their catalytic activity is still insufficient when compared with natural enzymes. Therefore, the synthesis of nanozymes with high catalytic performance will be a hot topic in the future. At the same time, in order to increase the biocompatibility of nanozymes additional coatings have been designed, which may hinder the performance of enzyme-like activities to some extent. To date, some studies have demonstrated that the biological coating may promote enzyme-like activities, but the mechanism still needs further elucidation. Furthermore, a standard for comparing enzyme-like activities of different nanozymes needs to be established. As the characterization and kinetic analysis of enzyme-like activity is strongly affected by the chemical environment of the enzyme reaction test or the environment of material synthesis, it is virtually impossible to make a horizontal comparison between different nanozymes. Also, the relationship between the enzyme-like activities and the internal electronic structure needs to be elaborated, which will also inspire scientists to design nanozymes from the very beginning and promote the biomedical application of nanozymes. In addition to the improvement of enzyme-like activities, the morphology of the nanozymes also needs to be regulated. In order to improve the enzyme-like activities, scientists have used some bamboo-like or flower-like morphology to increase the number of active sites, but the homogeneity of these kinds of nanozymes is generally poor and may impede their biological application. Meanwhile, low selectivity and targeting ability have been obstacles to their further applications in biomedical fields. In addition, the ability to cross barriers (e.g., blood–brain-barrier or glomerular capillary wall in the kidney, etc.) is also an obstacle for nanozymes in clinical applications. To address the dilemma, rationally designed nanozymes are significant for achieving targeted delivery. Scientists have designed pH-responsive nanozymes or taken advantage of a specific peptide with stealth ability, or clinically used drugs to improve the targeting ability of nanozymes and reduce their potential toxicity. Furthermore, scientists have modified cell membranes or peptides on the surface of the nanozyme to improve biocompatibility and help nanozymes to cross barriers. Although great progress in the biocompatibility and specificity of nanozymes has been achieved in the last ten years, the selectivity and specificity of nanozymes should be further improved and optimized. More detailed biological data, such as the long-term toxicity, pharmacodynamics, immunogenicity, and catalytic activity of nanozymes in vivo, are still needed to narrow the gap between basic research and clinical application.
All in all, nanozymes with reusability, storage stability, low cost, and mass production have great potential in the treatment of inflammatory diseases. However, the above-mentioned issues need to be addressed for future clinical translation. Herein, we have summarized the influencing factors of nanozyme activity and their application in inflammation-related diseases. Based on the laws of structure–activity interaction, and strengths in clinical usage, we propose a feasible scheme for their further application in inflammation-related diseases from the perspective of material design. In the future, more emphasis should be put on enhancing the specific catalytic ability, minimizing treatment dosage, overcoming in vivo toxicity and improving the targeting capability of nanozymes for therapeutics.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We gratefully acknowledge support from the National Natural Science Foundation of China (grant numbers 81803094).References
- L. Z. Gao, J. Zhuang, L. Nie, J. B. Zhang, Y. Zhang, N. Gu, T. H. Wang, J. Feng, D. L. Yang, S. Perrett and X. Yan, Nat. Nanotechnol., 2007, 2, 577–583 CrossRef CAS PubMed.
- M. Moglianetti, E. De Luca, P. A. Deborah, R. Marotta, T. Catelani, B. Sartori, H. Amenitsch, S. F. Retta and P. P. Pompa, Nanoscale, 2016, 8, 3739–3752 RSC.
- S. Lei, J. Zhang, N. T. Blum, M. Li, D. Y. Zhang, W. Yin, F. Zhao, J. Lin and P. Huang, Nat. Commun., 2022, 13, 1298 CrossRef CAS PubMed.
- D.-Y. Zhang, M. R. Younis, H. Liu, S. Lei, Y. Wan, J. Qu, J. Lin and P. Huang, Biomaterials, 2021, 271, 120706 CrossRef CAS PubMed.
- N. Singh, M. A. Savanur, S. Srivastava, P. D'Silva and G. Mugesh, Angew. Chem., Int. Ed., 2017, 56, 14267–14271 CrossRef CAS PubMed.
- R. Yan, S. Sun, J. Yang, W. Long, J. Wang, X. Mu, Q. Li, W. Hao, S. Zhang, H. Liu, Y. Gao, L. Ouyang, J. Chen, S. Liu, X. D. Zhang and D. Ming, ACS Nano, 2019, 13, 11552–11560 CrossRef CAS.
- M. Huo, L. Wang, Y. Chen and J. Shi, Nat. Commun., 2017, 8, 357 CrossRef PubMed.
- C. Liu, J. Xing, O. U. Akakuru, L. Luo, S. Sun, R. Zou, Z. Yu, Q. Fang and A. Wu, Nano Lett., 2019, 19, 5674–5682 CrossRef CAS.
- L. Zhang, G. P. Yang, S. J. Xiao, Q. G. Tan, Q. Q. Zheng, R. P. Liang and J. D. Qiu, Small, 2021, 17, e2102944 CrossRef.
- M. Valko, D. Leibfritz, J. Moncol, M. T. D. Cronin, M. Mazur and J. Telser, Int. J. Biochem. Cell Biol., 2007, 39, 44–84 CrossRef CAS.
- M. Valko, H. Morris, M. Mazur, P. Rapta and R. F. Bilton, Biochim. Biophys. Acta, 2001, 1527, 161–166 CrossRef CAS.
- H. J. Kwon, D. Kim, K. Seo, Y. G. Kim, S. I. Han, T. Kang, M. Soh and T. Hyeon, Angew. Chem., Int. Ed., 2018, 57, 9408–9412 CrossRef CAS PubMed.
- H. J. Kwon, M. Y. Cha, D. Kim, D. K. Kim, M. Soh, K. Shin, T. Hyeon and I. Mook-Jung, ACS Nano, 2016, 10, 2860–2870 CrossRef CAS.
- Z. Miao, S. Jiang, M. Ding, S. Sun, Y. Ma, M. R. Younis, G. He, J. Wang, J. Lin, Z. Cao, P. Huang and Z. Zha, Nano Lett., 2020, 20, 3079–3089 CrossRef CAS PubMed.
- J. Zhao, Y. Wang, W. Wang, Y. Tian, Z. Gan, Y. Wang, H. He, W. Chen, X. Zhang, Y. Wu, R. Jia, M. Shi, W. Wei and G. Ma, Nano Today, 2021, 40, 101282 CrossRef CAS.
- Y. Hao, Y. Chen, X. He, Y. Yu, R. Han, Y. Li, C. Yang, D. Hu and Z. Qian, Adv. Sci., 2020, 7, 2001853 CrossRef CAS.
- M. Wang, M. Chang, Q. Chen, D. Wang, C. Li, Z. Hou, J. Lin, D. Jin and B. Xing, Biomaterials, 2020, 252, 120093 CrossRef CAS.
- Y. Zhang, F. Wang, C. Liu, Z. Wang, L. Kang, Y. Huang, K. Dong, J. Ren and X. Qu, ACS Nano, 2018, 12, 651–661 CrossRef CAS.
- W. Bernal, Semin. Liver Dis., 2003, 23, 227–237 CrossRef PubMed.
- J. M. Roth, Proc. (Bayl. Univ. Med. Cent.), 2011, 24, 257–259 Search PubMed.
- G. B. D. Mortality and C. Causes of Death, Lancet, 2016, 388, 1459–1544 CrossRef PubMed.
- Y. Qiu, W. Ren, Y. Liu, W. E. Chen, X. H. Pan and J. J. Ren, EClinicalMedicine, 2020, 27, 100544 CrossRef.
- R. Bellomo, J. A. Kellum and C. Ronco, Lancet, 2012, 380, 756–766 CrossRef.
- J. K. Triantafillidis, E. Merikas and F. Georgopoulos, Drug Des., Dev. Ther., 2011, 5, 185–210 CrossRef CAS.
- L. Baud and R. Ardaillou, Am. J. Physiol., 1986, 251, F765–F776 CAS.
- R. H. Bhogal, R. Sutaria and S. C. Afford, Liver Transplant, 2011, 17, 95–95 CrossRef PubMed.
- H. Katsumi, K. Fukui, K. Sato, S. Maruyama, S. Yamashita, E. Mizumoto, K. Kusamori, M. Oyama, M. Sano, T. Sakane and A. Yamamoto, Metallomics, 2014, 6, 1050–1056 CrossRef CAS.
- A. Sahu, J. Jeon, M. S. Lee, H. S. Yang and G. Tae, ACS Appl. Mater. Interfaces, 2021, 13, 25649–25662 CrossRef CAS PubMed.
- J. Zhao, X. Cai, W. Gao, L. Zhang, D. Zou, Y. Zheng, Z. Li and H. Chen, ACS Appl. Mater. Interfaces, 2018, 10, 26108–26117 CrossRef CAS.
- F. Zeng, Y. Shi, C. Wu, J. Liang, Q. Zhong, K. Briley, B. Xu, Y. Huang, M. Long, C. Wang, J. Chen, Y. Tang, X. Li, M. Jiang, L. Wang, Q. Xu, L. Yang, P. Chen, S. Duan, J. Xie, C. Li and Y. Wu, J Nanobiotechnol., 2022, 20, 107 CrossRef CAS.
- L. Gao, K. Fan and X. Yan, Theranostics, 2017, 7, 3207–3227 CrossRef CAS.
- H. Wei and E. Wang, Chem. Soc. Rev., 2013, 42, 6060–6093 RSC.
- H. Zhao, R. Zhang, X. Yan and K. Fan, J. Mater. Chem. B, 2021, 9, 6939–6957 RSC.
- Y. Sun, J. Wang, W. Li, J. Zhang, Y. Zhang and Y. Fu, Biosens. Bioelectron., 2015, 74, 1038–1046 CrossRef CAS.
- X. Lin, Y. Liu, Z. Tao, J. Gao, J. Deng, J. Yin and S. Wang, Biosens. Bioelectron., 2017, 94, 471–477 CrossRef CAS.
- M. Liang, Y. Wang, K. Ma, S. Yu, Y. Chen, Z. Deng, Y. Liu and F. Wang, Small, 2020, 16, e2002348 CrossRef PubMed.
- S. Li, L. Shang, B. Xu, S. Wang, K. Gu, Q. Wu, Y. Sun, Q. Zhang, H. Yang, F. Zhang, L. Gu, T. Zhang and H. Liu, Angew. Chem., Int. Ed., 2019, 58, 12624–12631 CrossRef CAS.
- S. Liang, X. R. Deng, Y. Chang, C. Q. Sun, S. Shao, Z. X. Xie, X. Xiao, P. A. Ma, H. Y. Zhang, Z. Y. Cheng and J. Lin, Nano Lett., 2019, 19, 4134–4145 CrossRef CAS.
- Z. Xu, P. Sun, J. Zhang, X. Lu, L. Fan, J. Xi, J. Han and R. Guo, Chem. Eng. J., 2020, 399, 125797 CrossRef CAS.
- Y. Li, K. H. Yun, H. Lee, S. H. Goh, Y. G. Suh and Y. Choi, Biomaterials, 2019, 197, 12–19 CrossRef CAS PubMed.
- X. Shen, W. Liu, X. Gao, Z. Lu, X. Wu and X. Gao, J. Am. Chem. Soc., 2015, 137, 15882–15891 CrossRef CAS.
- J. Li, W. Liu, X. Wu and X. Gao, Biomaterials, 2015, 48, 37–44 CrossRef CAS PubMed.
- T. Hamasaki, T. Kashiwagi, T. Imada, N. Nakamichi, S. Aramaki, K. Toh, S. Morisawa, H. Shimakoshi, Y. Hisaeda and S. Shirahata, Langmuir, 2008, 24, 7354–7364 CrossRef CAS.
- W. Li, C. Bin, H. X. Zhang, Y. H. Sun, J. Wang, J. L. Zhang and Y. Fu, Biosens. Bioelectron., 2015, 66, 251–258 CrossRef CAS.
- Y. Fu, X. Zhao, J. Zhang and W. Li, J. Phys. Chem. C, 2014, 118, 18116–18125 CrossRef CAS.
- H. Gu, Q. Huang, J. Zhang, W. Li and Y. Fu, Colloids Surf., A, 2020, 606, 125455 CrossRef CAS.
- Z. R. Wang, R. F. Zhang, X. Y. Yan and K. L. Fan, Mater. Today, 2020, 41, 81–119 CrossRef CAS.
- Y. Chen, P. Wang, H. Hao, J. Hong, H. Li, S. Ji, A. Li, R. Gao, J. Dong, X. Han, M. Liang, D. Wang and Y. Li, J. Am. Chem. Soc., 2021, 143, 18643–18651 CrossRef CAS.
- N. Tian, Z. Y. Zhou, S. G. Sun, Y. Ding and Z. L. Wang, Science, 2007, 316, 732–735 CrossRef CAS PubMed.
- Z. Gao, S. Lv, M. Xu and D. Tang, Analyst, 2017, 142, 911–917 RSC.
- L. Yang, X. Song, M. Qi, L. Xia and M. Jin, J. Mater. Chem. A, 2013, 1, 7316–7320 RSC.
- J. Fan, J. J. Yin, B. Ning, X. Wu, Y. Hu, M. Ferrari, G. J. Anderson, J. Wei, Y. Zhao and G. Nie, Biomaterials, 2011, 32, 1611–1618 CrossRef CAS.
- E. De Luca, D. Pedone, M. Moglianetti, D. Pulcini, A. Perrelli, S. F. Retta and P. P. Pompa, ACS Omega, 2018, 3, 15389–15398 CrossRef CAS PubMed.
- G. W. Wu, S. B. He, H. P. Peng, H. H. Deng, A. L. Liu, X. H. Lin, X. H. Xia and W. Chen, Anal. Chem., 2014, 86, 10955–10960 CrossRef CAS PubMed.
- D.-Y. Zhang, H. Liu, M. R. Younis, S. Lei, C. Yang, J. Lin, J. Qu and P. Huang, Chem. Eng. J., 2021, 409, 127371 CrossRef CAS.
- Y. Liu, Y. Zheng, D. Ding and R. Guo, Langmuir, 2017, 33, 13811–13820 CrossRef CAS PubMed.
- K. Shah, S. Bhagat, D. Varade and S. Singh, Colloids Surf., A, 2018, 553, 50–57 CrossRef CAS.
- M. Gharib, A. Kornowski, H. Noei, W. J. Parak and I. Chakraborty, ACS Mater. Lett., 2019, 1, 310–319 CrossRef CAS.
- Y. Liu, Y. Qing, L. Jing, W. Zou and R. Guo, Langmuir, 2021, 37, 7364–7372 CrossRef CAS.
- W. He, Y. Liu, J. Yuan, J. J. Yin, X. Wu, X. Hu, K. Zhang, J. Liu, C. Chen, Y. Ji and Y. Guo, Biomaterials, 2011, 32, 1139–1147 CrossRef CAS PubMed.
- J. Liu, L. Meng, Z. Fei, P. J. Dyson and L. Zhang, Biosens. Bioelectron., 2018, 121, 159–165 CrossRef CAS PubMed.
- X. Liu, Q. Wang, H. Zhao, L. Zhang, Y. Su and Y. Lv, Analyst, 2012, 137, 4552–4558 RSC.
- W. W. He, X. N. Han, H. M. Jia, J. H. Cai, Y. L. Zhou and Z. Zheng, Sci. Rep., 2017, 7, 40103 CrossRef CAS PubMed.
- X. N. Hu, A. Saran, S. Hou, T. Wen, Y. L. Ji, W. Q. Liu, H. Zhang, W. W. He, J. J. Yin and X. C. Wu, RSC Adv., 2013, 3, 6095–6105 RSC.
- C. Liu, Y. Yan, X. Zhang, Y. Mao, X. Ren, C. Hu, W. He and J. J. Yin, Nanoscale, 2020, 12, 3068–3075 RSC.
- S. Li, W. Sun, Y. Luo, Y. Gao, X. Jiang, C. Yuan, L. Han, K. Cao, Y. Gong and C. Xie, J. Mater. Chem. B, 2021, 9, 4643–4653 RSC.
- J. Zhang, Y. Zhong, C. Zhang, J. Zhang and Z. Zhuang, J. Phys. Chem. Lett., 2022, 13, 2137–2143 CrossRef CAS PubMed.
- G. Huang, J. Zang, L. He, H. Zhu, J. Huang, Z. Yuan, T. Chen and A. Xu, ACS Nano, 2022, 16, 431–452 CrossRef CAS PubMed.
- J. Shen, A. Chen, Z. Cai, Z. Chen, R. Cao, Z. Liu, Y. Li and J. Hao, Bioact. Mater., 2022, 12, 153–168 CrossRef CAS PubMed.
- S. Kumar, I. M. Adjei, S. B. Brown, O. Liseth and B. Sharma, Biomaterials, 2019, 224, 119467 CrossRef CAS PubMed.
- A. Adhikari, S. Mondal, M. Das, P. Biswas, U. Pal, S. Darbar, S. S. Bhattacharya, D. Pal, T. Saha-Dasgupta, A. K. Das, A. K. Mallick and S. K. Pal, Adv. Healthc. Mater., 2021, 10, e2001736 CrossRef PubMed.
- F. Abbas, T. Jan, J. Iqbal, M. S. Haider Naqvi and I. Ahmad, Mater. Chem. Phys., 2016, 173, 146–151 CrossRef CAS.
- M. Wang, H. Li, B. Huang, S. Chen, R. Cui, Z. J. Sun, M. Zhang and T. Sun, Adv. Healthc. Mater., 2021, 10, e2100090 CrossRef PubMed.
- A. Lee, W. Kang and J. S. Choi, Nanomaterials, 2021, 11, 3046 CrossRef CAS PubMed.
- X. Wang, F. Wen, L. He, J. Su, P. Jiang and D. He, Anal. Chim. Acta, 2022, 1198, 339564 CrossRef CAS PubMed.
- L. Han, H. Zhang, D. Chen and F. Li, Adv. Funct. Mater., 2018, 28, 180018 Search PubMed.
- J. Wu, W. Lv, Q. Yang, H. Li and F. Li, Biosens. Bioelectron., 2021, 171, 112707 CrossRef CAS PubMed.
- D. Zhu, F. Liu, L. Ma, D. Liu and Z. Wang, Int. J. Mol. Sci., 2013, 14, 10591–10607 CrossRef PubMed.
- L. Han, J. Shi and A. Liu, Sens. Actuators, B, 2017, 252, 919–926 CrossRef CAS.
- P. Prasad, C. R. Gordijo, A. Z. Abbasi, A. Maeda, A. Ip, A. M. Rauth, R. S. DaCosta and X. Y. Wu, ACS Nano, 2014, 8, 3202–3212 CrossRef CAS PubMed.
- Y. Huang, Z. Liu, C. Liu, E. Ju, Y. Zhang, J. Ren and X. Qu, Angew. Chem., Int. Ed., 2016, 55, 6646–6650 CrossRef CAS PubMed.
- J. Jia, P. Zhang and L. Chen, Appl. Catal., B, 2016, 189, 210–218 CrossRef CAS.
- Y. Wan, P. Qi, D. Zhang, J. Wu and Y. Wang, Biosens. Bioelectron., 2012, 33, 69–74 CrossRef CAS PubMed.
- S. Liu, K. Li, D. Shao, Q. Shen, S. Huang, H. Ji, Y. Xie and X. Zheng, Appl. Surf. Sci., 2020, 534, 147649 CrossRef CAS.
- L. Jiao, L. Zhang, W. Du, H. Li, D. Yang and C. Zhu, Nanoscale, 2018, 10, 21893–21897 RSC.
- X. Li, J. Ma, L. Yang, G. He, C. Zhang, R. Zhang and H. He, Environ. Sci. Technol., 2018, 52, 12685–12696 CrossRef CAS PubMed.
- L. Li, X. Feng, Y. Nie, S. Chen, F. Shi, K. Xiong, W. Ding, X. Qi, J. Hu, Z. Wei, L.-J. Wan and M. Xia, ACS Catal., 2015, 5, 4825–4832 CrossRef CAS.
- S. M. Antao, L. A. Cruickshank and K. S. Hazrah, Minerals, 2019, 9, 343 CrossRef CAS.
- N. Singh, M. Geethika, S. M. Eswarappa and G. Mugesh, Chemistry, 2018, 24, 8393–8403 CrossRef CAS PubMed.
- S. K. Apte, S. D. Naik, R. S. Sonawane, B. B. Kale, N. Pavaskar, A. B. Mandale and B. K. Das, Mater. Res. Bull., 2006, 41, 647–654 CrossRef CAS.
- X. Jiang, P. Gray, M. Patel, J. Zheng and J. J. Yin, J. Mater. Chem. B, 2020, 8, 1191–1201 RSC.
- W. Lu, J. Chen, L. Kong, F. Zhu, Z. Feng and J. Zhan, Sens. Actuators, B, 2021, 333, 129560 CrossRef CAS.
- N. Singh, M. A. Savanur, S. Srivastava, P. D'Silva and G. Mugesh, Nanoscale, 2019, 11, 3855–3863 RSC.
- J. Yao, Y. Cheng, M. Zhou, S. Zhao, S. Lin, X. Wang, J. Wu, S. Li and H. Wei, Chem. Sci., 2018, 9, 2927–2933 RSC.
- J. Xi, C. Zhu, Y. Wang, Q. Zhang and L. Fan, RSC Adv., 2019, 9, 16509–16514 RSC.
- M.-Q. Wang, C. Ye, S.-J. Bao and M.-W. Xu, Microchim. Acta, 2017, 184, 1177–1184 CrossRef CAS.
- N. K. Dega, A. B. Ganganboina, H. L. Tran, E. P. Kuncoro and R. A. Doong, Talanta, 2022, 237, 122957 CrossRef CAS PubMed.
- R. Ragg, A. M. Schilmann, K. Korschelt, C. Wieseotte, M. Kluenker, M. Viel, L. Volker, S. Preiss, J. Herzberger, H. Frey, K. Heinze, P. Blumler, M. N. Tahir, F. Natalio and W. Tremel, J. Mater. Chem. B, 2016, 4, 7423–7428 RSC.
- S. Carregal-Romero, A. B. Miguel-Coello, L. Martinez-Parra, Y. Marti-Mateo, P. Hernansanz-Agustin, Y. Fernandez-Afonso, S. Plaza-Garcia, L. Gutierrez, M. D. M. Munoz-Hernandez, J. Carrillo-Romero, M. Pinol-Cancer, P. Lecante, Z. Blasco-Iturri, L. Fadon, A. C. Almansa-Garcia, M. Moller, D. Otaegui, J. A. Enriquez, H. Groult and J. Ruiz-Cabello, Small, 2022, 18, e2106570 CrossRef PubMed.
- V. Verma, M. Kaur and S. Sharma, Bull. Mater. Sci., 2019, 42, 120 CrossRef.
- V. Figueroa-Espí, A. Alvarez-Paneque, M. Torrens, A. J. Otero-González and E. Reguera, Colloids Surf., A, 2011, 387, 118–124 CrossRef.
- Y. Peng, Z. Wang, W. Liu, H. Zhang, W. Zuo, H. Tang, F. Chen and B. Wang, Dalton Trans., 2015, 44, 12871–12877 RSC.
- Y. Zhu, W. Wang, J. Cheng, Y. Qu, Y. Dai, M. Liu, J. Yu, C. Wang, H. Wang, S. Wang, C. Zhao, Y. Wu and Y. Liu, Angew. Chem., Int. Ed., 2021, 60, 9480–9488 CrossRef CAS PubMed.
- T. Montini, M. Melchionna, M. Monai and P. Fornasiero, Chem. Rev., 2016, 116, 5987–6041 CrossRef CAS PubMed.
- R. Zhao, H. Liu, Y. Li, M. Guo and X. D. Zhang, Bioconjugate Chem., 2021, 32, 411–429 CrossRef CAS PubMed.
- H. Li, P. Xia, S. Pan, Z. Qi, C. Fu, Z. Yu, W. Kong, Y. Chang, K. Wang, D. Wu and X. Yang, Int. J. Nanomed., 2020, 15, 7199–7214 CrossRef CAS PubMed.
- J. M. Li, F. Kang, X. S. Gong, Y. Bai, J. J. Dai, C. R. Zhao, C. Dou, Z. Cao, M. M. Liang, R. Dong, H. Jiang, X. C. Yang and S. W. Dong, FASEB J., 2019, 33, 6378–6389 CrossRef CAS PubMed.
- P. Rozhin, M. Melchionna, P. Fornasiero and S. Marchesan, Nanomaterials, 2021, 11, 2259 CrossRef CAS PubMed.
- Q. Weng, H. Sun, C. Fang, F. Xia, H. Liao, J. Lee, J. Wang, A. Xie, J. Ren, X. Guo, F. Li, B. Yang and D. Ling, Nat. Commun., 2021, 12, 1436 CrossRef CAS PubMed.
- E. O. Gubernatorova, X. Liu, A. Othman, W. T. Muraoka, E. P. Koroleva, S. Andreescu and A. V. Tumanov, Adv. Healthc. Mater., 2017, 6, 1700176 CrossRef PubMed.
- X. Wei, X. Li, Y. Feng and S. Yang, RSC Adv., 2018, 8, 11764–11770 RSC.
- H. Cheng, S. Lin, F. Muhammad, Y.-W. Lin and H. Wei, ACS Sens., 2016, 1, 1336–1343 CrossRef CAS.
- V. Baldim, F. Bedioui, N. Mignet, I. Margaill and J. F. Berret, Nanoscale, 2018, 10, 6971–6980 RSC.
- P. Janoš, J. Ederer, M. Došek, J. Štojdl, J. Henych, J. Tolasz, M. Kormunda and K. Mazanec, Environ. Sci.: Nano, 2019, 6, 3684–3698 RSC.
- S. Deshpande, S. Patil, S. V. N. T. Kuchibhatla and S. Seal, Appl. Phys. Lett., 2005, 87, 133113 CrossRef.
- G. Pulido-Reyes, I. Rodea-Palomares, S. Das, T. S. Sakthivel, F. Leganes, R. Rosal, S. Seal and F. Fernandez-Pinas, Sci. Rep., 2015, 5, 15613 CrossRef CAS PubMed.
- H. T. Ahn, H.-R. An, Y. C. Hong, S. C. Lee, T. N. Le, X. A. Le, H. S. Kwak, Y.-S. Lee, Y. Jeong, J.-I. Park, H. Kim, M. Lee, S. J. Yoo, S.-G. Lee, K. Choi, Y.-B. Lee, M. I. Kim and H. U. Lee, Sens. Actuators, B, 2020, 320, 128404 CrossRef CAS.
- Y. Yang, Z. Mao, W. Huang, L. Liu, J. Li, J. Li and Q. Wu, Sci. Rep., 2016, 6, 35344 CrossRef CAS PubMed.
- T. Naganuma, Nano Res., 2016, 10, 199–217 CrossRef.
- L. Jiang, S. Fernandez-Garcia, M. Tinoco, Z. Yan, Q. Xue, G. Blanco, J. J. Calvino, A. B. Hungria and X. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 18595–18608 CrossRef CAS PubMed.
- I. Trenque, G. C. Magnano, M. A. Bolzinger, L. Roiban, F. Chaput, I. Pitault, S. Briancon, T. Devers, K. Masenelli-Varlot, M. Bugnet and D. Amans, Phys. Chem. Chem. Phys., 2019, 21, 5455–5465 RSC.
- T. J. Fisher, Y. Zhou, T. S. Wu, M. Wang, Y. L. Soo and C. L. Cheung, Nanoscale, 2019, 11, 4552–4561 RSC.
- B. Xu, Q. Zhang, S. Yuan, M. Zhang and T. Ohno, Chem. Eng. J., 2015, 260, 126–132 CrossRef CAS.
- K. Korschelt, R. Schwidetzky, F. Pfitzner, J. Strugatchi, C. Schilling, M. von der Au, K. Kirchhoff, M. Panthofer, I. Lieberwirth, M. N. Tahir, C. Hess, B. Meermann and W. Tremel, Nanoscale, 2018, 10, 13074–13082 RSC.
- H. Issa Hamoud, B. Azambre and G. Finqueneisel, J. Chem. Technol. Biotechnol., 2016, 91, 2462–2473 CrossRef CAS.
- S. Hao, J. Hou, P. Aprea and F. Pepe, Appl. Catal., B, 2014, 160–161, 566–573 CrossRef CAS.
- M. A. Małecka, J. J. Delgado, L. Kępiński, J. J. Calvino, S. Bernal, G. Blanco and X. Chen, Catal. Today, 2012, 187, 56–64 CrossRef.
- X. Li, Z. Pu, H. Zhou, W. Zhang, X. Niu, Y. He, X. Xu, F. Qiu, J. Pan and L. Ni, J. Mater. Sci., 2018, 53, 13912–13923 CrossRef CAS.
- A. Zhu, K. Sun and H. R. Petty, Inorg. Chem. Commun., 2012, 15, 235–237 CrossRef CAS PubMed.
- W. Guo, M. Zhang, Z. Lou, M. Zhou, P. Wang and H. Wei, ChemCatChem, 2019, 11, 737–743 CrossRef CAS.
- S. I. Han, S. W. Lee, M. G. Cho, J. M. Yoo, M. H. Oh, B. Jeong, D. Kim, O. K. Park, J. Kim, E. Namkoong, J. Jo, N. Lee, C. Lim, M. Soh, Y. E. Sung, J. Yoo, K. Park and T. Hyeon, Adv. Mater., 2020, 32, e2001566 CrossRef PubMed.
- E. G. Heckert, A. S. Karakoti, S. Seal and W. T. Self, Biomaterials, 2008, 29, 2705–2709 CrossRef CAS PubMed.
- T. Pirmohamed, J. M. Dowding, S. Singh, B. Wasserman, E. Heckert, A. S. Karakoti, J. E. King, S. Seal and W. T. Self, Chem. Commun., 2010, 46, 2736–2738 RSC.
- J. M. Dowding, S. Das, A. Kumar, T. Dosani, R. McCormack, A. Gupta, T. X. T. Sayle, D. C. Sayle, L. von Kalm, S. Seal and W. T. Self, ACS Nano, 2013, 7, 4855–4868 CrossRef CAS PubMed.
- M. H. Kuchma, C. B. Komanski, J. Colon, A. Teblum, A. E. Masunov, B. Alvarado, S. Babu, S. Seal, J. Summy and C. H. Baker, Nanomedicine, 2010, 6, 738–744 CrossRef CAS PubMed.
- H. Jaeschke, J. Gastroenterol. Hepatol., 2000, 15, 718–724 CrossRef CAS PubMed.
- H. Wang, Z. Cui, X. Wang, S. Sun, D. Zhang and C. Fu, BioMed Res. Int., 2021, 2021, 9980127 Search PubMed.
- B. C. Dickinson and C. J. Chang, Nat. Chem. Biol., 2011, 7, 504–511 CrossRef CAS PubMed.
- R. Tian, J. Xu, Q. Luo, C. Hou and J. Liu, Front. Chem., 2020, 8, 831 CrossRef CAS PubMed.
- N. A. Molodecky, I. S. Soon, D. M. Rabi, W. A. Ghali, M. Ferris, G. Chernoff, E. I. Benchimol, R. Panaccione, S. Ghosh, H. W. Barkema and G. G. Kaplan, Gastroenterology, 2012, 142, 46–54 CrossRef PubMed.
- F. Leonard, S. Srinivasan, X. W. Liu, E. M. Collnot, M. Ferrari, C. M. Lehr and B. Godin, Eur. J. Pharm. Biopharm., 2020, 151, 61–72 CrossRef CAS PubMed.
- S. C. Davies, I. M. Hussein, T. M. Nguyen, C. E. Parker, R. Khanna and V. Jairath, Cochrane Database Syst. Rev., 2020, CD012381, DOI:10.1002/14651858.CD012381.pub2.
- D. N. Seril, J. Liao, G. Y. Yang and C. S. Yang, Carcinogenesis, 2003, 24, 353–362 CrossRef CAS PubMed.
- A. Piechota-Polanczyk and J. Fichna, Naunyn-Schmiedebergs Arch. Pharmacol., 2014, 387, 605–620 CrossRef CAS PubMed.
- B. Beltran, P. Nos, F. Dasi, M. Iborra, G. Bastida, M. Martinez, J. E. O'Connor, G. Saez, I. Moret and J. Ponce, Inflamm. Bowel Dis., 2010, 16, 76–86 CrossRef PubMed.
- S. Zhao, Y. Li, Q. Liu, S. Li, Y. Cheng, C. Cheng, Z. Sun, Y. Du, C. J. Butch and H. Wei, Adv. Funct. Mater., 2020, 30, 2004692 CrossRef CAS.
- Y. Ma, W. Gao, Y. Zhang, M. Yang, X. Yan, Y. Zhang, G. Li, C. Liu, C. Xu and M. Zhang, ACS Appl. Mater. Interfaces, 2022, 14, 6358–6369 CrossRef CAS PubMed.
- Y. Cheng, C. Cheng, J. Yao, Y. Yu, Y. Liu, H. Zhang, L. Miao and H. Wei, Adv. Ther., 2021, 4, 2100081 CrossRef CAS.
- J. Zhao, W. Gao, X. Cai, J. Xu, D. Zou, Z. Li, B. Hu and Y. Zheng, Theranostics, 2019, 9, 2843–2855 CrossRef CAS PubMed.
- L. Fan, P. Sun, Y. Huang, Z. Xu, X. Lu, J. Xi, J. Han and R. Guo, ACS Appl. Bio Mater., 2020, 3, 1147–1157 CrossRef CAS PubMed.
- N. Zhao, F. E. Yang, C. Y. Zhao, S. W. Lv, J. Wang, J. M. Liu and S. Wang, Adv. Healthc. Mater., 2021, 10, e2101618 CrossRef PubMed.
- W. M. Lee, Semin. Respir. Crit. Care Med., 2012, 33, 36–45 CrossRef PubMed.
- A. Ramachandran and H. Jaeschke, Curr. Opin. Toxicol., 2018, 7, 17–21 CrossRef PubMed.
- A. Canbay, C. Jochum, L. P. Bechmann, S. Festag, R. K. Gieseler, Z. Yuksel, P. Lutkes, F. H. Saner, A. Paul and G. Gerken, Z. Gastroenterol., 2009, 47, 807–813 CrossRef CAS PubMed.
- P. Nourjah, S. R. Ahmad, C. Karwoski and M. Willy, Pharmacoepidemiol Drug Saf, 2006, 15, 398–405 CrossRef PubMed.
- C. I. Ghanem, M. J. Perez, J. E. Manautou and A. D. Mottino, Pharmacol. Res., 2016, 109, 119–131 CrossRef CAS PubMed.
- T. Liu, B. Xiao, F. Xiang, J. Tan, Z. Chen, X. Zhang, C. Wu, Z. Mao, G. Luo, X. Chen and J. Deng, Nat. Commun., 2020, 11, 2788 CrossRef CAS PubMed.
- F. Li, Y. Qiu, F. Xia, H. Sun, H. Liao, A. Xie, J. Lee, P. Lin, M. Wei, Y. Shao, B. Yang, Q. Weng and D. Ling, Nano Today, 2020, 35, 100925 CrossRef CAS.
- H. Bai, F. Kong, K. Feng, X. Zhang, H. Dong, D. Liu, M. Ma, F. Liu, N. Gu and Y. Zhang, ACS Appl. Mater. Interfaces, 2021, 13, 42382–42395 CrossRef CAS PubMed.
- L. Li, C. L. Duan, Y. Zhao, X. F. Zhang, H. Y. Yin, T. X. Wang, C. X. Huang, S. H. Liu, S. Y. Yang and X. J. Li, Int. Immunopharmacol., 2017, 51, 99–106 CrossRef CAS PubMed.
- Y. Li, Y. Li, Y. Bai, L. Lin and Y. Sun, J. Mater. Chem. B, 2020, 8, 8634–8643 RSC.
- G. Szabo and J. Petrasek, Nat. Rev. Gastroenterol. Hepatol., 2015, 12, 387–400 CrossRef CAS PubMed.
- E. E. Montalvo-Jave, T. Escalante-Tattersfield, J. A. Ortega-Salgado, E. Pina and D. A. Geller, J. Surg. Res., 2008, 147, 153–159 CrossRef CAS PubMed.
- D. Ni, H. Wei, W. Chen, Q. Bao, Z. T. Rosenkrans, T. E. Barnhart, C. A. Ferreira, Y. Wang, H. Yao, T. Sun, D. Jiang, S. Li, T. Cao, Z. Liu, J. W. Engle, P. Hu, X. Lan and W. Cai, Adv. Mater., 2019, 31, e1902956 CrossRef PubMed.
- D. Oro, T. Yudina, G. Fernandez-Varo, E. Casals, V. Reichenbach, G. Casals, B. Gonzalez de la Presa, S. Sandalinas, S. Carvajal, V. Puntes and W. Jimenez, J. Hepatol., 2016, 64, 691–698 CrossRef CAS PubMed.
- X. Zhang, S. Zhang, Z. Yang, Z. Wang, X. Tian and R. Zhou, Nanoscale, 2021, 13, 12613–12622 RSC.
- O. Rewa and S. M. Bagshaw, Nat. Rev. Nephrol., 2014, 10, 193–207 CrossRef CAS PubMed.
- C. Ronco, R. Bellomo and J. A. Kellum, Lancet, 2019, 394, 1949–1964 CrossRef CAS PubMed.
- L. Wang, Y. Zhang, Y. Li, J. Chen and W. Lin, Nano Res., 2020, 14, 920–933 CrossRef.
- R. Murugan and J. A. Kellum, Nat. Rev. Nephrol., 2011, 7, 209–217 CrossRef CAS PubMed.
- B. B. Ratliff, W. Abdulmahdi, R. Pawar and M. S. Wolin, Antioxid. Redox Signal., 2016, 25, 119–146 CrossRef CAS PubMed.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165–1170 CrossRef CAS PubMed.
- J. T. Poston and J. L. Koyner, Br. Med. J., 2019, 364, k4891 CrossRef PubMed.
- H. Yu, F. Jin, D. Liu, G. Shu, X. Wang, J. Qi, M. Sun, P. Yang, S. Jiang, X. Ying and Y. Du, Theranostics, 2020, 10, 2342–2357 CrossRef CAS PubMed.
- J. A. Kellum and J. R. Prowle, Nat. Rev. Nephrol., 2018, 14, 217–230 CrossRef PubMed.
- L. De Chiara, G. Lugli, G. Villa, V. Raglianti, F. Husain-Syed, F. Ravaglia, P. Romagnani and E. Lazzeri, Int. J. Mol. Sci., 2022, 23, 2638 CrossRef CAS PubMed.
- N. A. G. dos Santos, M. A. C. Rodrigues, N. M. Martins and A. C. dos Santos, Arch. Toxicol., 2012, 86, 1233–1250 CrossRef PubMed.
- J. Y. C. Soo, J. Jansen, R. Masereeuw and M. H. Little, Nat. Rev. Nephrol., 2018, 14, 378–393 CrossRef CAS PubMed.
- D. Y. Zhang, H. Liu, K. S. Zhu, T. He, M. R. Younis, C. Yang, S. Lei, J. Wu, J. Lin, J. Qu and P. Huang, J. Nanobiotechnol., 2021, 19, 266 CrossRef CAS PubMed.
- Z. T. Rosenkrans, T. Sun, D. Jiang, W. Chen, T. E. Barnhart, Z. Zhang, C. A. Ferreira, X. Wang, J. W. Engle, P. Huang and W. Cai, Adv. Sci., 2020, 7, 2000420 CrossRef CAS PubMed.
- X. Bosch, E. Poch and J. M. Grau, N. Engl. J. Med., 2009, 361, 62–72 CrossRef CAS PubMed.
- D. Singh, R. Kaur, V. Chander and K. Chopra, J. Med. Food, 2006, 9, 443–450 CrossRef CAS PubMed.
- D. Ni, D. Jiang, C. J. Kutyreff, J. Lai, Y. Yan, T. E. Barnhart, B. Yu, H. J. Im, L. Kang, S. Y. Cho, Z. Liu, P. Huang, J. W. Engle and W. Cai, Nat. Commun., 2018, 9, 5421 CrossRef CAS PubMed.
- D.-Y. Zhang, T. Tu, M. R. Younis, K. S. Zhu, H. Liu, S. Lei, J. Qu, J. Lin and P. Huang, Theranostics, 2021, 11, 9904–9917 CrossRef CAS PubMed.
- N. Manne, R. Arvapalli, V. A. Graffeo, V. V. K. Bandarupalli, T. Shokuhfar, S. Patel, K. M. Rice, G. K. Ginjupalli and E. R. Blough, Cell. Physiol. Biochem., 2017, 42, 1837–1846 CrossRef CAS PubMed.
- D. Y. Zhang, H. Liu, C. Li, M. R. Younis, S. Lei, C. Yang, J. Lin, Z. Li and P. Huang, ACS Appl. Mater. Interfaces, 2020, 12, 56830–56838 CrossRef CAS PubMed.
- Z. Liu, L. Xie, K. Qiu, X. Liao, T. W. Rees, Z. Zhao, L. Ji and H. Chao, ACS Appl. Mater. Interfaces, 2020, 12, 31205–31216 CrossRef CAS PubMed.
- N. D. Manne, R. Arvapalli, N. Nepal, T. Shokuhfar, K. M. Rice, S. Asano and E. R. Blough, J. Nanobiotechnol., 2015, 13, 75 CrossRef PubMed.
- R. Rodrigo, R. Fernandez-Gajardo, R. Gutierrez, J. M. Matamala, R. Carrasco, A. Miranda-Merchak and W. Feuerhake, CNS Neurol. Disord. Drug Targets, 2013, 12, 698–714 CrossRef CAS PubMed.
- M. J. Poellmann, J. Bu and S. Hong, Nanomedicine, 2018, 13, 2327–2340 CrossRef CAS PubMed.
- D. Mozaffarian, E. J. Benjamin, A. S. Go, D. K. Arnett, M. J. Blaha and M. B. Turner, Circulation, 2016, 133, E38–E360 Search PubMed.
- G. A. Donnan, H. Ma, S. Schwarz, D. Georgiadis and W. Hacke, Adv. Neurol., 2003, 92, 347–359 Search PubMed.
- M. Fisher, Adv. Neurol., 2003, 92, 401–408 Search PubMed.
- M. Spranger, S. Krempien, S. Schwab, S. Donneberg and W. Hacke, Stroke, 1997, 28, 2425–2428 CrossRef CAS PubMed.
- M. Furlan, G. Marchal, F. Viader, J. M. Derlon and J. C. Baron, Ann. Neurol., 1996, 40, 216–226 CrossRef CAS PubMed.
- M. K. Reddy and V. Labhasetwar, FASEB J., 2009, 23, 1384–1395 CrossRef CAS PubMed.
- M. Takamiya, Y. Miyamoto, T. Yamashita, K. Deguchi, Y. Ohta and K. Abe, Neuroscience, 2012, 221, 47–55 CrossRef CAS PubMed.
- C. K. Kim, T. Kim, I. Y. Choi, M. Soh, D. Kim, Y. J. Kim, H. Jang, H. S. Yang, J. Y. Kim, H. K. Park, S. P. Park, S. Park, T. Yu, B. W. Yoon, S. H. Lee and T. Hyeon, Angew. Chem., Int. Ed., 2012, 51, 11039–11043 CrossRef CAS PubMed.
- Y. Liu, K. Ai, X. Ji, D. Askhatova, R. Du, L. Lu and J. Shi, J. Am. Chem. Soc., 2017, 139, 856–862 CrossRef CAS PubMed.
- B. C. Yan, J. Cao, J. Liu, Y. Gu, Z. Xu, D. Li and L. Gao, ACS Biomater. Sci. Eng., 2021, 7, 299–310 CrossRef CAS PubMed.
- J. Shi, W. Yu, L. Xu, N. Yin, W. Liu, K. Zhang, J. Liu and Z. Zhang, Nano Lett., 2020, 20, 780–789 CrossRef CAS PubMed.
- Y. Liu, X. Wang, X. Li, S. Qiao, G. Huang, D. M. Hermann, T. R. Doeppner, M. Zeng, W. Liu, G. Xu, L. Ren, Y. Zhang, W. Liu, E. Casals, W. Li and Y. C. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 46213–46224 CrossRef CAS PubMed.
- L. Feng, C. Dou, Y. Xia, B. Li, M. Zhao, P. Yu, Y. Zheng, A. M. El-Toni, N. F. Atta, A. Galal, Y. Cheng, X. Cai, Y. Wang and F. Zhang, ACS Nano, 2021, 15, 2263–2280 CrossRef CAS PubMed.
- A. Y. Estevez, S. Pritchard, K. Harper, J. W. Aston, A. Lynch, J. J. Lucky, J. S. Ludington, P. Chatani, W. P. Mosenthal, J. C. Leiter, S. Andreescu and J. S. Erlichman, Free Radicals Biol. Med., 2011, 51, 1155–1163 CrossRef CAS PubMed.
- M. Takamiya, Y. Miyamoto, T. Yamashita, K. Deguchi, Y. Ohta, Y. Ikeda, T. Matsuura and K. Abe, J. Neurosci. Res., 2011, 89, 1125–1133 CrossRef CAS PubMed.
- K. Zhang, M. Tu, W. Gao, X. Cai, F. Song, Z. Chen, Q. Zhang, J. Wang, C. Jin, J. Shi, X. Yang, Y. Zhu, W. Gu, B. Hu, Y. Zheng, H. Zhang and M. Tian, Nano Lett., 2019, 19, 2812–2823 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |