Single junction binary and ternary polymer solar cells-based D–A structured copolymer with low lying HOMO energy level and two nonfullerene acceptors†
Mukhamed L.
Keshtov
*a,
Dmitry Y.
Godovsky
a,
Ilya E.
Ostapov
a,
Vladimir G.
Alekseev
b,
Hemraj
Dahiya
 c,
Rahul
Singhal
d,
Fang-Chung
Chen
c,
Rahul
Singhal
d,
Fang-Chung
Chen
 ef and
Ganesh D.
Sharma
ef and
Ganesh D.
Sharma
 *c
*c
aA. N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences, Vavilova St., 28, 119991 Moscow, Russian Federation. E-mail: keshtov@ineos.ac.ru
bDepartment of Inorganic and Analytical Chemistry Tver State University, Sadovyi per. 35, Tver, 170002, Russian Federation
cDepartment of Physics and Electronics Communication Engineering, The LNM Institute for Information Technology, Jamdoli, Jaipur, Rajasthan 302031, India. E-mail: gdsharma@lnmiit.ac.in; gdsharma273@gmail.com
dDepartment of Physics, MNIT, Jaipur, Rajasthan, India
eDepartment of Photonics, National Yang Ming Chiao Tung University, Hsinchu, Taiwan
fCenter for Emergent Functional Matter Science, National Yang Ming Chiao Tung University, Hsinchu, Taiwan
First published on 21st September 2022
Abstract
A donor–acceptor (D–A) conjugated copolymer denoted as P(DTB-BDD) consisting of benzodithiophenedione (BDD) as a strong acceptor and dithienobenzene (DTB) as a weak donor was prepared, and its optical and electrochemical properties were analyzed. P(DTB-BDD) exhibited a deeper highest occupied molecular orbital and an optical bandgap of 1.74 eV. Polymer solar cells (PSCs) were fabricated through blending P(DTB-BDD) with two non-fullerene acceptors, i.e., narrow bandgap Y6 and medium bandgap DBTBT-IC. The power conversion efficiencies (PCEs) of the PSCs using the bulk heterojunction active layer based on P(DTB-BDD):DBTBT-IC and P(DTB-BDD):Y6 were 13.16% and 12.62%, respectively. The larger open circuit voltage of the DBTBT-IC based PSCs as compared with that of the Y6 counterpart was due to the up-shifted LUMO energy level of DBTBT-IC, and the high short circuit current for the Y6 based PSCs may be associated with the extended absorption profile of Y6. Taking advantage of the high open circuit voltage and short circuit current of the devices based on DBTBT-IC and Y6, the ternary PSCs were prepared by optimizing the weight ratios between two acceptors and maintaining a constant amount of P(DTB-BDD); the resulting ternary PSCs based on P(DTB-BDD)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBTBT-IC
DBTBT-IC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6 (1.0
Y6 (1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0) showed an improved PCE of 16.32%, which is greater than those for the binary PSCs. The enrichment in the PCE of the ternary device may be concomitant with the effective exploitation of excitons via energy transfer from DBTBT-IC to Y6 and increased the D/A interfacial area for more effective charge transfer. The open circuit voltage of the PSC based on ternary lies in between that for the Y6 and DBTBT-IC-based PSCs, demonstrating the formation of an alloy for the two acceptors.
1.0) showed an improved PCE of 16.32%, which is greater than those for the binary PSCs. The enrichment in the PCE of the ternary device may be concomitant with the effective exploitation of excitons via energy transfer from DBTBT-IC to Y6 and increased the D/A interfacial area for more effective charge transfer. The open circuit voltage of the PSC based on ternary lies in between that for the Y6 and DBTBT-IC-based PSCs, demonstrating the formation of an alloy for the two acceptors.
Design, System, ApplicationIn this manuscript, we have designed and synthesized a wide bandgap D–A conjugated polymer and used it as a donor, along with two non-fullerene acceptors (medium bandgap and small bandgap). After the optimization of active layer engineering, the ternary polymer solar cells attained a power conversion efficiency of 16.32%. Our results demonstrated that this donor polymer might be suitable for high efficiency polymer solar cells for commercial applications. |
1. Introduction
Polymer solar cells (PSCs), which feature large area production and compatibility with flexible substrates at low cost, eco-friendly processing, and semitransparency, have attracted considerable attention in the last few years.1–6 In PSCs, a bulk heterojunction (BHJ) active layer consisting of a polymer or small molecule as a donor material (D) and an acceptor material (A) is generally used, and it affords sufficient D/A interfaces to dissociate the photogenerated excitons into free charges, which are subsequently collected by the electrodes, generating photocurrent.7,8 With the uninterrupted invention in designing donor and acceptor materials, engineering the device architecture, optimization of BHJ active layers, and the development of the non-fullerene acceptors (NFAs), particularly ITIC series9,10 and Y-series NFAs,11–14 the power conversion efficiencies (PCEs) more than 18% have been attained.15–19 NFAs have more degree of freedom, allowing higher electron affinity tunability and absorbing incident visible NIR radiation more strongly and are more stable and easier to synthesize as compared to fullerene counterparts. The recent progress in low bandgap NFAs has unlocked a new opportunity for designing new wide-bandgap polymer donors for PSCs.20–22 The PCE values of BHJ-PSCs are determined by multiplying the open circuit voltage (VOC) with short circuit current (JSC), and fill factor (FF), and then, the whole product is to be divided by the incident power. The light-absorbing efficiency of the BHJ active layer is directly linked to the JSC of PSC. The energy difference between the HOMO level of the donor and the LUMO level of the acceptor is directly linked with the value of VOC. To improve the above-mentioned photovoltaic parameters, new efficient wide-bandgap polymer donors with the deep-lying HOMO level and extraordinary hole-mobility but with complementary absorption spectra to the narrow bandgap acceptor are to be designed.The concept of the D–A tactic has been effectively employed to develop efficient organic semiconducting materials. The optical bandgap and the HOMO and LUMO levels of D–A organic semiconducting materials could be easily adjusted via the intermolecular charge transfer (ICT) by selecting the appropriate combination of D and A units23–26 Moreover, the D–A copolymers also exhibit high hole mobility due to the intermolecular D–A interactions. In 2010, Zhou et al. introduced a concept of a strong acceptor–weak donor approach for synthesizing ideal copolymers for PSCs.27 The weak donor helps to maintain the deep HOMO level and the strong acceptor assists in reducing the bandgap via the ICT process. Many D–A copolymers have been designed by different research groups and reported PCE in the range of 16–17% for OSCs using them as donors along with NFSMAs as acceptors.28–32
In D–A wide bandgap copolymers, benzothiadiazole (BT),33 isoindigo (IID),34 and diketopyrrolopyrrole (DPP)35 are used as strong acceptor units and benzodithiophenedione (BDD) as a relatively weak acceptor. The BDD acceptor unit has an enormous planar arrangement and PSC-based polymers centered on BDD attained high PCE.36–40 The combination of the weak donor and strong acceptor is used to develop the D–A polymer, which exhibits a low-lying HOMO level and thus helps to obtain a high VOC of the photovoltaic devices.27,41 The typical approach for designing a weak donor might be realized by reducing the electron donating ability of the thiophene unit, which can be realized by mixing it with a comparatively electron-deficient unit. This approach has been effectively adopted to achieve the deep HOMO level of polymers.42
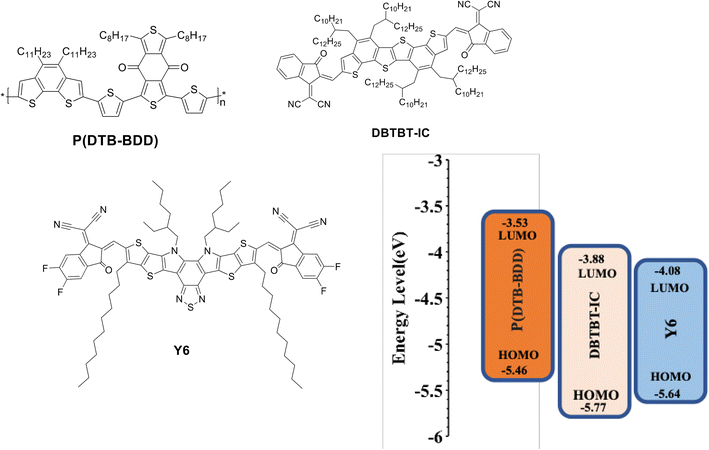
Herein, taking advantage of the above concept, we have synthesized a D–A copolymer denoted as P(DTB-BDD) comprising BDD as a strong acceptor and dithienobenzene (DTB) as a weak donor. P(DTB-BDD) attained a strong absorption in the wavelength range from 300 nm to 700 nm with optical bandgap and HOMO levels of about 1.74 eV and −5.46 eV, respectively. As the absorption profile is complementary with the well-known narrow bandgap A–DA′D–A non-fullerene acceptor Y6, we have fabricated PSCs using P(DTB-BDD) as the donor and Y6 as the acceptor; the optimized PSCs based on P(DTB-BDD):Y6 exhibited an overall PCE of 12.62% (VOC = 0.83 V, JSC = 23.76 mA cm−2, and FF = 0.64). Although the JSC of the PSC is quite high, the low value of PCE is due to the low value of VOC and FF as compared to other Y6 based PSCs. In order to enhance the efficiency of the PSCs, a medium bandgap acceptor DBTBT-IC43 with a high lying LUMO energy level (−3.88 eV) as a second acceptor is used to fabricate the ternary PSCs. Our group has also fabricated the binary PSCs and the improved P(DTB-BDD):DBTBT-IC-based PSCs showed overall PCE of 13.16% (VOC = 1.05 V, JSC = 19.06 mA cm−2, and FF = 0.68). We have incorporated DBTBT-IC as a guest acceptor in the host binary P(DTB-BDD):Y6 active layer to take advantage of the high VOC and FF for PSCs based on P(DTB-BDD):DBTBT-IC layer, and the optimized ternary PSCs achieve PCE of 16.32%, is greater than the binary counterparts. The increased PCE for ternary PSCs is associated with the improved interfacial area of D/A in the ternary BHJ layer for more effective exciton dissociation and also with efficient utilization of excitons through the transfer of energy from DBTBT-IC to Y6. The VOC of ternary PSCs lies in between that for Y6 and DBTBT-IC-based PSCs, demonstrating the development of an alloy between these two acceptors.
2. Results and discussion
2.1. Synthesis of P(DTB-BDD)
In Scheme 1, the synthetic process for preparing P(DTB-BDD) is shown, and their characterization is given in the ESI.† The monomers (4,5-diundecylbenzo[1,2-b:6,5-b′]dithiophene-2,7-diyl)bis(trimethylstannane) (M1)44 and 1,3-bis(5-bromothiophen-2-yl)-5,7-dioctylbenzo[1,2-c:4,5-c′]dithiophene-4,8-dione-2 (M2)45 were synthesized as reported in the literature. The polymerization based on Stille cross-coupling between M1 and M2 was used to prepare the target copolymer P(DTB-BDD). The catalyst was palladium (Pd(Ph3P)4). P(DTB-BDD) was extracted as black solid powders with 86% yields and refined further using Soxhlet extraction to eliminate the catalyst and oligomers.Elemental analysis and 1H NMR spectroscopy were used to examine the composition and structure of P(DTB-BDD) (Fig. S1, ESI†). P(DTB-BDD) was soluble in a wide range of organic solvents. For determining the polydispersity index (PDI) and molecular weight of P(DTB-BDD), gel permeation chromatography (GPC) was used at 60 °C with 1, 2, 4-trichlorobenzene and polystyrene as the eluent and standard, respectively. P(DTB-BDD) has a number average molecular weight (Mn) of 32.3 kDa and a PDI of 1.96, respectively.
The thermal property of P(DTB-BDD) was examined using thermo-gravimetric (TGA) analysis (Fig. S3, ESI†). P(DTB-BDD) has a thermal decomposition temperature Td of 395 °C (5% weight loss) and thus possesses high thermal stability.
2.2. Optical characterization
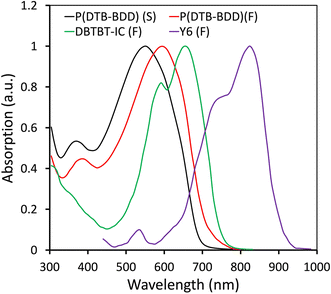
The absorption spectra of P(DTB-BDD) are shown in Fig. 1, where a dilute chloroform solution was used and the film was cast on a quartz substrate, and related data are summarized in Table 1. It is seen that P(DTB-BDD) exhibited wide absorption spectra covering the 300–700 nm wavelength region (Fig. 1). P(DTB-BDD) exhibited two main absorption bands in the dilute chloroform solution. The band that lies in the wavelength range of 300–450 nm (absorption maxima at 366 nm) is associated with the π–π* transition in the polymer backbone, while the absorption band seen in the 500–700 nm region with absorption maxima at 548 nm may be accredited to intramolecular charge transfer (ICT) among DTB and BDD. In contrast to that in solution, the absorption maxima of the P(DTB-BDD) thin film shifted to the red region of the solar spectrum, which is usual in conjugated D–A organic semiconducting materials. The redshifted ICT absorption band is due to a number of intermolecular interactions and their effects on the energy states and therefore absorption features.46 The optical bandgap of P(DTB-BDD) estimated from the absorption edge was 1.74 eV. The absorption profiles of DBTBT-IC and Y6 are also shown in Fig. 1, which are complementary to P(DTB-BDD).The HOMO and LUMO levels of P(DTB-BDD) were determined using electrochemical cyclic voltammetry (CV) (Fig. S4†), and the related data are shown in Table 1. To determine the HOMO/LUMO levels using the onset of the oxidation (Eoxonset) and reduction (Eredonset) potentials, the following expressions were taken into consideration:
| EHOMO = −q(Eoxonset + 4.43) eV and ELUMO = −q(Eredonset + 4.43) eV. |
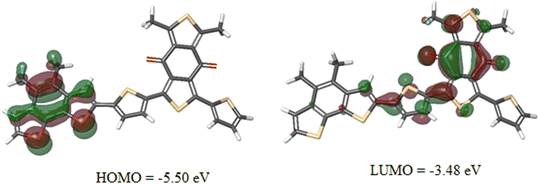
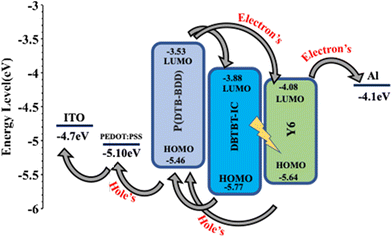
The values of HOMO/LUMO were found to be −5.46/−3.53 eV. The deep-lying HOMO of the copolymer donor is beneficial for both the steadiness of the PSC in ambient conditions and achieving high VOC.
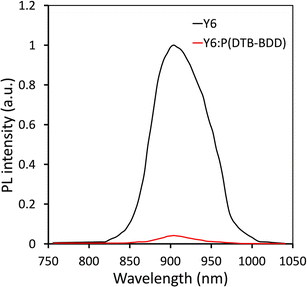
The photoluminescence (PL) spectra of neat Y6 film and its blend is studied with P(DTB-BDD) since the HOMO offset P(DTB-BDD)/Y6 is about 0.18, which is not as much of the threshold value required for hole transfer from Y6 to P(DTB-BDD) (Fig. 4). We found that when Y6 is blended with P(DTB-BDD), the intensity of its PL peaks is dramatically reduced, suggesting the efficient hole transfer from Y6 to P(DTB-BDD), with even the HOMO offset below 0.3 eV and even zero, as seen in most non-fullerene-based PSCs.47–49 As the HOMO offset between DBTBT-IC and P(DTB-BDD) is roughly 0.31 eV, hole transfer from DBTBT-IC to P(DTB-BDD) is possible.
Using the conventional PSCs, we investigated the photovoltaic functioning of P(DTB-BDD) as a donor in combination with non-fullerene acceptors (DBTBT-IC and Y6) (detailed device fabrication is given in ESI†). Initially, we adjusted the performance by assessment of the donor–acceptor weight ratio and discovered that P(DTB-BDD)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6 (1
Y6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) and P(DTB-BDD)
1.2) and P(DTB-BDD)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DBTBT-IC (1
DBTBT-IC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2) had the best photovoltaic performance (Tables S1 and S2†). To further enhance the photovoltaic performance of the PSCs using a solvent vapor annealing (SVA) treatment, the active layer was exposed to SVA for 40 s in a THF atmosphere. Fig. 5a represents the J–V plots of the binary PSCs under illumination, and Table 2 lists the photovoltaic performance statistics.
1.2) had the best photovoltaic performance (Tables S1 and S2†). To further enhance the photovoltaic performance of the PSCs using a solvent vapor annealing (SVA) treatment, the active layer was exposed to SVA for 40 s in a THF atmosphere. Fig. 5a represents the J–V plots of the binary PSCs under illumination, and Table 2 lists the photovoltaic performance statistics.
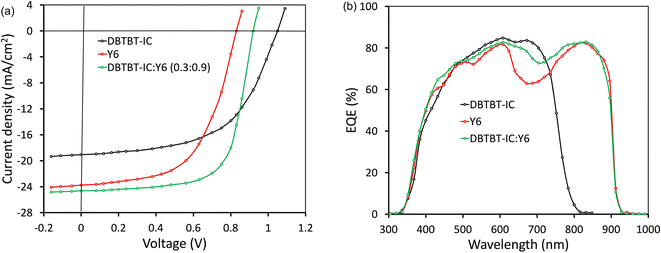 | ||
| Fig. 5 (a) J–V characteristics (AM1.5G, 100 mW cm−2) under illumination and (b) EQE response of binary and ternary PSCs. | ||
The optimized PSCs based P(DTB-BDD):DBTBT-IC and P(DTB-BDD):Y6 active layers attained PCE values of 13.61% and 12.62%, respectively. DBTBT-IC-based PSCs showed higher VOC as compared to Y6 counterparts that are correlated to the upshifted LUMO level of DBTBT-IC (−3.88 eV) relative to Y6 (−4.08 eV) since the VOC is straightforwardly proportional to the HOMO–LUMO energy offset of the donor and acceptor. Since the JSC is connected to the photon harvesting efficiency of the BHJ active layer, the trend in the JSC values agrees with the absorption profile of the acceptors utilized in the BHJ active layer. The external quantum efficiency (EQE) response of the PSCs was also measured to obtain further information about the changes in JSC values (Fig. 5b). The EQE spectra of P(DTB-BDD):Y6 are wider and extend up to 910 nm, whereas P(DTB-BDD):DBTBT-IC is confined to 810 nm, which accords with the absorption profile of acceptors. The JSC values computed from the assimilation of EQE spectra for P(DTB-BDD):DBTBT-IC and P(DTB-BDD):Y6 are 18.81 and 23.48 mA cm−2, respectively, which are in accordance with the J–V characteristics data (Table 2).
The ternary active layer technique via mixing three organic semiconducting materials in common solvents,50–57 which uses the simplicity of BHJ as in binary counterparts, is commonly employed to boost the PCE of PSCs. In this method, the complementary absorption spectra of either two donors and one acceptor or two acceptors and one donor were mixed together to enhance the light-harvesting efficiency and well-matched energy levels to increase the D/A interfacial area for exciton dissociation or energy transfer between two acceptors or two donors. The ternary photoactive layer-based PSCs have achieved a PCE of about 19%.58,59 In our binary BHJ PSCs, the JSC for Y6 is higher as compared to DBTBT-IC, whereas the FF and VOC for the DBTBT-IC device were superior to that of the Y6 counterpart. We have incorporated DBTBT-IC as the third component in the host P(DTB-BDD):Y6 to form the ternary BHJ active layer. To fabricate ternary PSCs, weight ratios between the two acceptors were altered while keeping the amount of P(DTB-BDD) and overall concentration of the ternary mixture constant. Table S3† lists the photovoltaic properties for ternary PSCs with various weight ratios (ESI†). The optimized ternary film was then treated with SVA, as is done with binary active layers. Fig. 5a shows the J–V under optimum ternary PSC illumination, with the accompanying photovoltaic data reported in Table 2. The JSC of the ternary PSCs (24.64 mA cm−2) is higher than the binary counterparts, which is also corroborated by the EQE response of ternary PSCs (Fig. 5b). Fig. 5b shows that the EQE values for ternary PSC are greater than those for Y6-based PSC in the 610–800 nm range, where DBTBT-IC absorption is higher than that of Y6, implying that more excitons are created due to DBTBT-IC absorption in the ternary active layer, enhancing the JSC. According to the EQE spectra, the JSC value is around 24.43 mA cm−2, which is consistent with J–V characteristics (Table 2).
The ternary PSC's VOC value is around 0.92 V, which is higher than the Y6-based PSC but lower than that of DBTBT-IC. We used cyclic voltammetry to determine the HOMO/LUMO values of mixed DBTBT-IC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6 (0.3
Y6 (0.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.9), which are around −5.71 eV/−3.95 eV. The increase in the VOC of the ternary PSCs may be due to the LUMO level of the blend acceptor being pushed upwards, forming an alloy between two acceptors and behaving as a mixed acceptor.60
0.9), which are around −5.71 eV/−3.95 eV. The increase in the VOC of the ternary PSCs may be due to the LUMO level of the blend acceptor being pushed upwards, forming an alloy between two acceptors and behaving as a mixed acceptor.60
We have investigated the energy transfer between two acceptors via thin film photoluminescence (PL) measurements (Fig. 6a and b). The DBTBT-IC generated a significant PL in the 700–850 nm range, with a peak at 764 nm (excited at 648 nm), which was almost quenched in the DBTBT-IC:Y6 blend, but the PL strength corresponding to Y6 was increased in the blend compared to pristine Y6 (Fig. 6a). Furthermore, as represented in Fig. 6b, the PL emission band of DBTBT-IC overlaps considerably with the absorption profile of Y6. As demonstrated in Fig. 7, these measurements demonstrate efficient energy transmission from DBTBT-IC to Y6.61,62 Furthermore, we built acceptor-only devices to learn more about the possibilities of charge transfer between two acceptors. We found that the JSC value of the DBTBT-IC:Y6 device is similar to that of the pristine acceptor device, showing that charge transfer is not occurring between the two acceptors. Fig. 7 depicts the energy and charge transmission in the ternary device. The excitons are dissociated at the P(DTB-BDD)/Y6 and P(DTB-BDD)/DBTBT-IC D/A interfaces after light absorption by the active layer, and the electrons from the P(DTB-BDD) are transported to both acceptors and finally collected by the cathode, whereas the holes from both Y6 and DBTBT-IC are transferred to P(DTB-BDD) and finally collected by the anode.
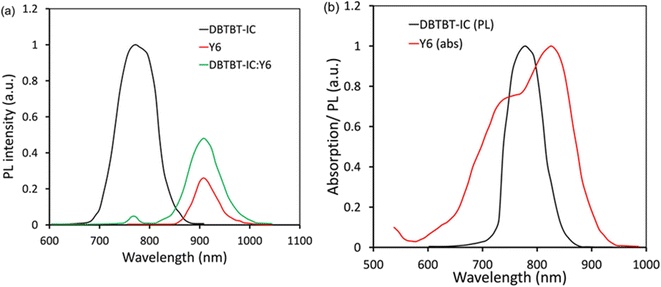 | ||
| Fig. 6 (a) PL response of pristine DBTBT-IC, Y6, and DBTBT-IC:Y6 excited at 648 nm, and (b) PL and absorption response of DBTBT-IC and Y6. | ||
Charge transport plays a deciding role in efficient PSCs. Thus, we have estimated the hole (μh) and electron (μe) mobility in the active layers using the single carrier devices and fitting the dark J–V characteristics with the space charge limited current (SCLC) model (Fig. 8a and b).63 The values of μh and μe are compiled in Table 3. It can be seen that the μh/μe ratio for the P(DTB-BDD):DBTBT-IC and P(DTB-BDD):Y6 are about 1.54 and 1.86. This value for P(DTB-BDD):DBTBT-IC is lower than that for P(DTB-BDD):Y6, specifying that the charge transport is further balanced in the former device relative to the latter. The trend in the ratio μh/μe is well in agreement with the FF values of PSCs (Table 2). It can be seen from the table that both the μh and μe have increased for ternary devices and the degree of enhancement is more for μe and compared to μh leading the μh/μe is about 1.16, demonstrating further balanced charge transportation in the ternary devices and reliable with the increased value of FF.
 | ||
| Fig. 8 Dark J–V plots for (a) hole only and (b) electron only devices and their fitting with SCLC model for binary and ternary devices. | ||
| Acceptor | μ h (cm2 V−1 s−1) | μ e (cm2 V−1 s−1) | μ h/μe | G max (1028 m−3 s−1) | P diss | P coll |
|---|---|---|---|---|---|---|
| DBTBT-IC | 3.78 × 10−4 | 2.45 × 10−4 | 1.54 | 1.36 | 0.962 | 0.814 |
| Y6 | 3.68 × 10−4 | 1.98 × 10−4 | 1.86 | 1.53 | 0.943 | 0.798 |
DBTBT-IC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6 (0.2 Y6 (0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0) 1.0) |
4.03 × 10−4 | 3.48 × 10−4 | 1.16 | 1.62 | 0.982 | 0.854 |
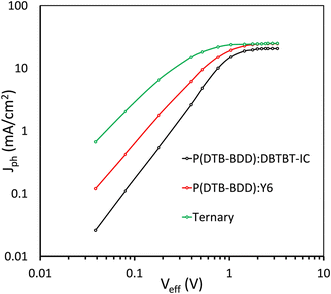
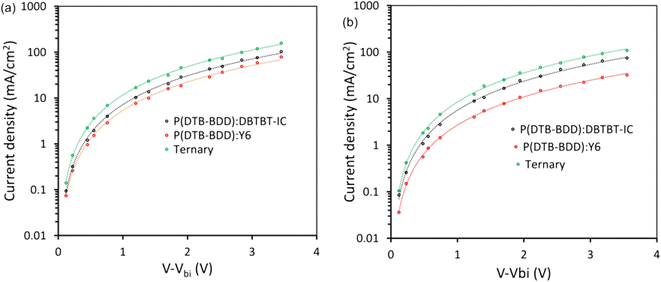
We examined the photocurrent density (Jph)-effective voltage (Veff) results to learn more about the maximal exciton generation rate (Gmax), exciton generation probability (Pdiss), and charge collection probability (Pcoll) in these devices64 (Fig. 9). After an initial linear increase in Jph as Veff increased, the Jph achieved a saturation value Jsat that was independent of Veff, showing that the majority of photogeneration excitons are efficiently separated into charge carriers. The Gmax can be estimated as Gmax = Jsat/qL, where q is the elementary charge and L is the active layer thickness. In Table 3, the Gmax values for these devices are compiled. The values of Gmax for P(DTB-BDD):DBTBT-IC, P(DTB-BDD):Y6 and P(DTB-BDD):DBTBT-IC:Y6 are 1.36 × 1028, 1.53 × 1028, and 1.62 × 1028 m−3 s−1, respectively, and is the highest for P(DTB-BDD):DBTBT-IC:Y6 and are consistent with the EQE response as well as JSC of the PSCs. The values of Pdiss and Pcoll calculated from the Jph/Jsat at the short circuit and maximum power point conditions, respectively, are given in Table 3. The values of Pdiss/Pcoll are higher for P(DTB-BDD):DBTBT-IC as compared to P(DTB-BDD):Y6 and which are in line with the values of FF, as the FF is directly related to the charge collection probability. The values of Pdiss/Pcoll are further increased for the ternary PSCs, demonstrating improved exciton dissociation owing to the increased interfacial area and charge collection due to the well-adjusted charge transport in the ternary film.
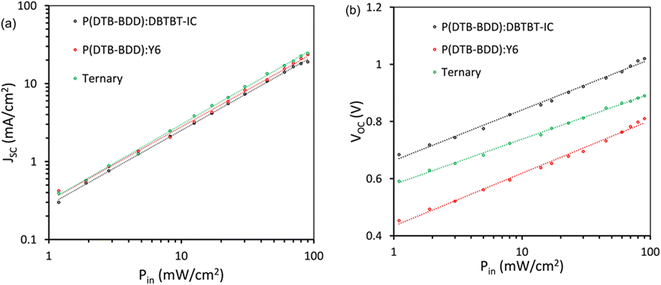
In the study of charge recombination kinetics in our PSCs, we looked at the relationship between JSC/VOC and illumination intensity (Pin)65 (Fig. 10a and b). The power law JSC ∝ (Pin)α, follows the dependency of JSC with Pin (Fig. 10a), where α is the exponent factor and provides information about the extent of recombination (bimolecular). Optimized P(DTB-BDD):DBTBT-IC, P(DTB-BDD):Y6, and P(DTB-BDD):DBTBT-IC:Y6 based PSCs have α values of 0.958, 0.943, and 0.981, respectively. The ternary device's value of α is higher than its binary counterparts, directing that bimolecular recombination is reduced for ternary BHJ films with higher JSC and FF values.
The formula, VOC (nkT/q)ln(Pin), describes VOC's dependency on Pin (Fig. 10b), where the absolute temperature is represented by T, Boltzmann's constant by k, and electronic charge by q. When the number n is approximately 2, under the open circuit condition, the monomolecular or trap-assisted recombination of charges is the primary recombination process, whereas when the value of n is near unity, the bimolecular recombination is dominant. The ternary device's value of n is around 1.13, which is lower than the binary counterpart's values of 1.26 and 1.34 for P(DTB-BDD):DBTBT-IC and P(DTB-BDD):Y6, respectively, indicating the suppression of trap-assisted recombination in the ternary device and elevate the value of FF.
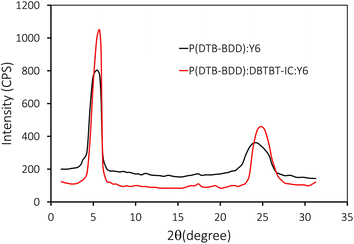
The X-ray diffraction (XRD) curves of pristine P(DTB-BDD), optimized P(DTB:BDD):Y6, and P(DTB-BDD):DBTBT-IC:Y6 films were recorded to get information about the molecular ordering and crystallinity of binary P(DTB-BDD):Y6 and P(DTB-BDD):DBTBT-IC:Y6 films. Fig. S7† depicts the XRD pattern of pure P(DTB-BDD). The thin film of P(DTB-BDD) revealed two different diffraction heights at 2θ = 5.06° and 24.64° respectively, corresponding to lamellar (100) with d-spacing of 1.714 nm and stacking diffraction (010) with a stacking distance of 0.354 nm. Fig. 11 shows the XRD patterns of binary P(DTB-BDD):Y6 and ternary P(DTB-BDD):DBTBT-IC:Y6. Both binary and ternary films displayed two unique diffraction peaks, namely (100)/(010) peaks at 2θ −5.42°/24.34° and 5.74°/24.78° for binary and ternary films, respectively.
The ternary film has a higher intensity of both diffraction peaks than its binary counterparts. For binary/ternary films, the d-spacing for lamellar/stacking is about 1.582/0.358 nm and 1.48/0.351 nm, respectively. The binary/ternary film's crystal coherence length (CCL) in lamellar and π–π stacking directions is about 5.87/2.63 nm and 7.08/3.65 nm, respectively. The ternary film's lamellar d-spacing and stacking distances were found to be lower than their binary equivalents. The CCL values for the ternary film are superior to their binary counterparts, which is beneficial for phase separation in the active layer and improves the charge transport. The charge transport is aided by the small stacking distance and large CCL value near the exciton diffusion length, resulting in a greater FF for ternary PSC compared to its binary ones.66
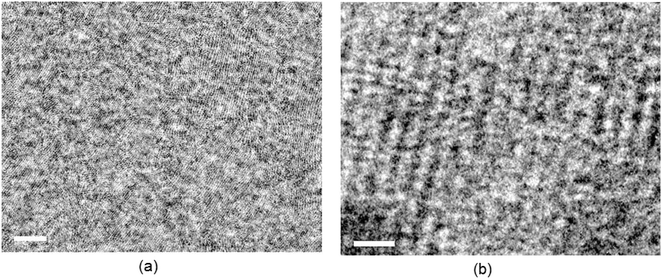
The morphology of the ternary films is also helpful in overcoming the morphology limitations of the binary BHJ active layer, and transmission electron microscopy (TEM) pictures were used to analyze the phase separation and morphology in the photoactive layers (Fig. 12). The photoactive layer's domain size should not be either tiny or too large for an efficient PSC. The charge transport is hampered by a small domain size, whereas the exciton dissociation is restricted by a large domain size. As seen in these images, the ternary films attained better phase separation than that of the binary counterpart, which is advantageous for efficient exciton dissociation and charge transport, causing a high FF and overall greater PCE for the ternary PSC as compared with binary ones.67,68
3. Conclusion
We have created a D–A copolymer P(DTB-BDD) with a weak DTB (donor) unit and a relatively weak acceptor BDD and investigated its optical and electrochemical properties. With a deep HOMO level of −5.46 eV, P(DTB-BDD) showed strong absorption spectra in the wavelength region of 300–700 nm. Using the middle bandgap acceptor DBTBT-IC and the narrow bandgap acceptor Y6, the photovoltaic performance of P(DTB-BDD) as the donor was examined. The PCE of the BHJ-PSCs based on P(DTB-BDD):DBTBT-IC and P(DTB-BDD):Y6 was 13.16% and 12.62%, respectively. The VOC and FF values for P(DTB-BDD):DBTBT-IC are greater than those of the P(DTB-BDD):Y6 device, but the JSC value for P(DTB-BDD):Y6 is larger compared to that for P(DTB-BDD):DBTBT-IC. Using the benefits of DBTBT-IC and Y6, we generated ternary PSCs by mixing a small quantity of DBTBT-IC into the P(DTB-BDD):Y6, attained a PCE of 16.32%, higher than binary equivalents. The higher exciton generation rate is due to the broader absorption pattern from 300 to 900 nm, efficient exciton utilization through energy transmission from DBTBT-IC to Y6, and a reduction in both bimolecular recombination and trap-assisted recombination. The increase in the VOC of the ternary PSCs also indicates the formation of an alloy between the two acceptors.Conflicts of interest
There are no competing interests to be declared by the authors.Acknowledgements
The present study was supported by RFBR (No. 21-53-53037). We gratefully acknowledge the financial support from the Ministry of Science and Higher Education of the Russian Federation using the equipment of the Center for molecular composition studies of INEOS RAS for NMR, DSC studies and elemental analysis. GDS and his research group are grateful to the Department of Science and Technology for financial support through INDO-Russia and BRICS projects.References
- S. Lee, D. Jeong, C. Kim, C. Lee, H. Kang, H. Y. Woo and B. J. Kim, Eco-friendly polymer solar cells: Advances in green solvent processing and material design, ACS Nano, 2020, 14, 14493–14527 CrossRef PubMed.
- D. Wang, H. Liu, Y. Li, G. Zhou, L. Zhan, H. Zhu, X. Lu, H. Chen and C. Z. Li, High performance and eco-friendly semitransparent organic solar cells for greenhouse applications, Joule, 2021, 5, 945–957 CrossRef CAS.
- L. Hong, H. Yao, Y. Cui, Z. Ge and J. Hou, Recent advances in high efficiency organic solar cells fabricated by eco-compatible solvents at relatively large area scale, APL Mater., 2020, 8, 120901 CrossRef CAS.
- L. X. Chen, Organic solar cells: Recent progress and challenges, ACS Energy Lett., 2019, 4, 2537–2539 CrossRef CAS.
- M. Riede, D. Spoltore and K. Leo, Organic solar cells- The path to commercial success, Adv. Energy Mater., 2021, 11, 2002653 CrossRef CAS.
- C. Y. Liao, T. T. Hsiao, K. W. Tsai, N. W. Teng, W. L. Li, J. L. Wu, J. C. Kao, C. C. Lee, C. M. Yang, H. S. Tan, K. H. Chung and Y. M. Chang, Photoactive materials for highly efficient and all solution organic photovoltaic modules: Study on the efficiency, stability and synthetic complexity, Sol. RRL, 2021, 5, 2000749 CrossRef CAS.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Polymer photovoltaic cells: enhanced efficiencies via a network of internal donor-acceptor heterojunctions, Science, 1995, 270, 1789–1791 CrossRef CAS.
- J. Nelson, Polymer: fullerene bulk heterojunction solar cells, Mater. Today, 2011, 14, 462–470 CrossRef CAS.
- Y. Lin, J. Wang, Z. G. Zhang, H. Bai, Y. Li, D. Zhu and X. Zhan, An electron acceptor challenging fullerene for efficient polymer solar cells, Adv. Mater., 2015, 27, 1170–1174 CrossRef CAS.
- F. Zhao, S. Dai, Y. Wu, Q. Zhang, J. Wang, L. Jiang, Q. Ling, Z. Wei, W. Ma, W. You, C. Wang and X. Zhan, Single junction binary blend nonfullerene polymer solar cells with 12.1% efficiency, Adv. Mater., 2017, 29, 1700144 CrossRef.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H. L. Yip, T. K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Single junction organic solar cells with over 15% efficiency using fused ring acceptor with electron deficient core, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- Y. Yang, The original design principles of the Y-series non-fullerene acceptors from Y1 to Y6, ACS Nano, 2021, 15, 18679–18682 CrossRef CAS.
- Q. Wei, J. Yuan, Y. Yi, C. Zhang and Y. Zou, Y6 and its derivatives: molecular design and physical mechanism, Natl. Sci. Rev., 2021, 8, nwab121.2021 Search PubMed.
- A. Armin, W. Li, O. J. Sanberg, Z. Ziao, L. Ding, J. Nelson, D. Neher, K. Vandewal, S. Shoaee, T. Wang, H. Ade, T. Heumuller, C. Brabec and P. Mersdith, A history and perspective of nonfullerene acceptors for organic solar cells, Adv. Energy Mater., 2021, 11, 2003570 CrossRef CAS.
- S. Chen, L. Feng, T. Jia, J. Jing, Z. Hu, K. Zhang and F. Huang, High performance polymer solar cells with efficiency over 18% enabled by asymmetric side chain engineering of nonfullerene acceptors, Sci. China: Chem., 2021, 64, 1192–1199 CrossRef CAS.
- C. Li, J. Zhou, J. Song, J. Xu, H. Zhang, X. Zhang, J. Guo, L. Zhu, D. Wei, G. Han, J. Min, Y. Zhang, Z. Xie, Y. Yi, H. Yan, F. Gao, F. Liu and Y. Sun, Nonfullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells, Nat. Energy, 2021, 6, 605–613 CrossRef CAS.
- J. Qin, L. Zhang, C. Zuo, Z. Xiao, Y. Yuan, S. Yang, F. Hao, M. Cheng, K. Sun and Q. Bao, A chlorinated copolymer donor demonstrates a 18.13% power conversion efficiency, J. Semicond., 2021, 42, 010501 CrossRef CAS.
- K. Jin, Z. Xiao and L. Ding, 18.69% PCE for organic solar cells, J. Semicond., 2021, 42, 060502 CrossRef.
- H. Meng, C. Liao, M. Deng, X. Xu, L. Yu and Q. Peng, 18.77% efficiency organic solar cells promoted by aqueous solution processed cobalt (II) acetate hole transport layer, Angew. Chem., Int. Ed., 2021, 133, 22728–22735 CrossRef.
- B. Zheng, L. Huo and Y. Li, Benzodithiophenedione based polymers: Recent advances in organic photovoltaics, NPG Asia Mater., 2020, 12, 3 CrossRef CAS.
- X. Xu, G. Zhang, Y. Li and Q. Peng, The recent progress of wide bandgap donor polymers towards non-fullerene organic solar cells, Chin. Chem. Lett., 2019, 30, 809–825 CrossRef CAS.
- K. He, P. Kumar, Y. Yuan and Y. Li, Wide bandgap polymer donors for high efficiency non-fullerene acceptor based organic solar cells, Mater. Adv., 2021, 2, 115–145 RSC.
- Y. Ma. Z. Kang and Q. Zheng, J. Mater. Chem. A, 2017, 5, 1860–1872 RSC.
- L. Sun, X. Xu, S. Song, Y. Zhang, C. Miao, X. Liu, G. Xing and S. Zhang, Macromol. Rapid Commun., 2019, 40, 1900074 CrossRef PubMed.
- W. Li, K. H. Hendriks, M. M. Wienk and R. A. J. Janssen, Acc. Chem. Res., 2016, 49, 78–85 CrossRef CAS PubMed.
- K. Mullen and W. Pisula, Donor-acceptor polymers, J. Am. Chem. Soc., 2015, 137, 9503–9505 CrossRef PubMed.
- H. Zhou, L. Yang, S. Stoneking and W. You, A weak donor -strong acceptor strategy to design ideal polymers for organic solar cells, ACS Appl. Mater. Interfaces, 2010, 2, 1377–1383 CrossRef CAS.
- X. Yuan, Y. Zhao, Y. Zhang, D. Xie, W. Deng, J. Li, H. Wu, C. Duan, F. Huang and Y. Cao, Achieving 16% efficiency for polythiophene organic solar cells with a cyano-substituted polythiophene, Adv. Funct. Mater., 2022, 32, 2201142 CrossRef CAS.
- X. Yuan, Y. Zhao, D. Xie, L. Pan, X. Liu, C. Duan, F. Huang and Y. Cao, Polythiophenes for organic solar cells with efficiency surpassing 17%, Joule, 2022, 6, 647–661 CrossRef CAS.
- B. Yin, Z. Chen, S. Pang, X. Yuan, Z. Liu, C. Duan, F. Huang and Y. Cao, The renaissance of oligothiophene based donor -acceptor polymers in organic solar cells, Adv. Energy Mater., 2022, 6, 2104050 CrossRef.
- X. Yuan, Y. Zhao, T. Zhan, J. Oh, J. Zhou, J. Li, X. Wang, Z. Wang, S. Pang, P. Cai, C. Yang, Z. He, Z. Xie, C. Duan, F. Huang and Y. Cao, A donor polymer based on 3-cyanothiophene with superior batch-to batch reproducibility for high efficiency organic solar cells, Energy Environ. Sci., 2021, 14, 5530–5540 RSC.
- S. Pang, Z. Wang, X. Yuan, L. Pan, W. Deng, H. Tang, H. Wu, S. Chen, C. Duan, F. Huang and Y. Cao, A facile synthesized polymer featuring B-N covalent bond and small singlet-triplet gap for high performance organic solar cells, Angew. Chem., 2021, 60, 8813–8817 CrossRef CAS.
- X. Gong, G. Li, C. Li, J. Zhang and Z. Bo, Benzothiazole based conjugated polymers for high performance polymer solar cells, J. Mater. Chem. A, 2015, 3, 20195–20200 RSC.
- R. Stalder, J. Mei and J. R. Reynolds, Isoindigo based donor-acceptor conjugated polymers, Macromolecules, 2010, 43, 8348–8352 CrossRef CAS.
- W. Li, K. H. Hendriks, M. M. Wienk and R. A. J. Janssen, Diketopyrrolopyrrole polymers for organic solar cells, Acc. Chem. Res., 2016, 49, 78–85 CrossRef CAS.
- B. Zheng, L. Huo and Y. Li, Benzodithiophene based polymers: Recent advances in organic photovoltaics, NPG Asia Mater., 2020, 12, 3 CrossRef CAS.
- W. Zhao, D. Qian, S. Zhang, S. Li, Q. Inganas, F. Gao and J. Hou, Fullerene free polymer solar cells with over 11% efficiency and excellent thermal stability, Adv. Mater., 2016, 28, 4734–4739 CrossRef CAS.
- Y. Cui, H. Yao, J. Zhang, T. Zhang, Y. Wang, L. Hong, K. Xian, B. Xu, S. Zhang, J. Peng, Z. Wei, F. Gao and J. Hou, Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open circuit voltages, Nat. Commun., 2019, 10, 2515 CrossRef PubMed.
- Z. C. Wen, H. Yin and X. T. Hao, Recent progress of PM6:Y6 based high efficiency organic solar cells, Surf. Interfaces, 2021, 23, 100921 CrossRef CAS.
- H. Bin, T. P. A. Van der Pol, J. Li, B. T. Van Gorkom, M. M. Wienk and R. A. J. Janssen, Efficient organic solar cells with small energy loss based on wide bandgap trialkylsilyl-substiuted donor polymer and a nonfullerene acceptor, Chem. Eng. J., 2022, 435, 134878 CrossRef CAS.
- Y. J. Kim, H. N. Kim, M. C. Hwang, Y. H. Kim and C. E. Park, A weak donor/strong acceptor alternating copolymer for efficient bulk heterojunction solar cells, Synth. Met., 2014, 198, 93–100 CrossRef CAS.
- Z. Shi, W. H. Ka, X. Wang, C. Vijila, F. Wang, G. Li, W. W. Tjiu, J. Li and J. Xu, Low bandgap weak donor-strong acceptor conjugated polymer for organic solar cells, RSC Adv., 2015, 5, 98876–98879 RSC.
- M. L. Keshtov, I. O. Konstantinov, S. A. Kuklin, A. R. Khokhlov, I. E. Ostapov, A. S. Peregudov, M. I. Buzin, C. Dou, H. Dahiya and G. D. Sharma, Energy Technol., 2021, 9, 2001100 CrossRef CAS.
- M. L. Keshtov, I. O. Konstantinov, I. E. Ostapov, A. R. Khokhlov, V. G. Alekseev, Z. H. Xie, H. Dahiya and G. D. Sharma, Macromol. Chem. Phys., 2021, 222, 2100053 CrossRef CAS.
- C. B. Nielsen and T. Bjornholm, New regiosymmetrical dioxopyrrolo- and dihydropyrrolo-functionalized Polythiophenes, Org. Lett., 2004, 6, 3381–3384 CrossRef CAS PubMed.
- S. Z. Hassan, H. J. Cheon, C. Choi, S. Yoon, M. Kang, J. Cho, Y. H. Jang, S. K. Kwon, D. S. Chung and Y. H. Kim, Molecular engineering of a donor-acceptor polymer to realize single band absorption toward a red-selective thin-film organic photodiode, ACS Appl. Mater. Interfaces, 2019, 11, 28106–28114 CrossRef CAS.
- C. Sun, S. Qin, R. Wang, S. Chen, F. Pan, B. Qiu, Z. Shang, L. Meng, C. Zhang, M. Xiao, C. Yang and Y. Li, High efficiency polymer solar cells with efficient hole transfer at zero highest occupied molecular orbital offset between methylated polymer donor and brominated acceptor, J. Am. Chem. Soc., 2020, 142, 1465–1474 CrossRef CAS PubMed.
- S. Chen, Y. Wang, L. Zhang, J. Zhao, Y. Chen, D. Zhu, H. Yao, G. Zhang, W. Ma, R. H. Friend, P. C. Y. Chow, F. Gao and H. Yan, Efficient non-fullerene organic solar cells with small driving forces for both hole and electron transfer, Adv. Mater., 2018, 45, 1804215 CrossRef PubMed.
- C. Li, Q. Yue, H. Wu, B. Li, H. Fan and X. Zhu, Small bandgap non-fullerene acceptor enables efficient PTB7-Th solar cells with near 0 eV HOMO offset, J. Energy Chem., 2021, 52, 60–66 CrossRef CAS.
- C. Zhao, J. Wang, X. Zhao, Z. Du, R. Yang and J. Tang, Recent advances, challenges and prospects in ternary organic solar cells, Nanoscale, 2021, 13, 2181–2208 RSC.
- W. Xu, X. Ma, J. H. Son, S. Y. Jeong, L. Niu, C. Xu, S. Zhang, Z. Zhou, J. Gao, H. Y. Woo, J. Zhang and F. Zhang, Smart ternary strategy in promoting the performance of polymer solar cells based on bulk heterojunction or layer-by layer structure, Small, 2022, 18, 2104215 CrossRef CAS.
- Z. Fan, Y. Wang, S. Xu, S. Hou, C. Zhuang and B. Wang, Versatile third components in organic ternary solar cells, Sol. Energy, 2022, 231, 732–757 CrossRef CAS.
- X. Xu, Y. Li and Q. Peng, Ternary blend organic solar cells: understanding the morphology from recent progress, Adv. Mater. DOI:10.1002/adma.202107476.
- T. Wang, X. Wang, R. Yang and C. Li, Recent advances in ternary organic solar cells based on Forster resonance energy transfer, Sol. RRL, 2021, 5, 2100496 CrossRef.
- Y. C. Chen, C. Y. Hsu, R. Y. Y. Lin, K. C. Ho and J. T. Lin, Materials for the active layer of organic photovoltaics: Ternary solar cell approach, ChemSusChem, 2013, 6, 20–35 CrossRef CAS PubMed.
- W. Zhang, J. Huang, X. Lv, M. Zhang, W. Liu, T. Xu, J. Ning, A. Hexig, F. Liu, A. Xu and C. Zhan, Chlorinated phthalimide polymer donor as ultra-wide bandgap and deep HOMO guest for achieving highly efficient polymer solar cells, Chin. Chem. Lett., 2022 DOI:10.1016/j.cclet.2022.04.034.
- W. Xu, Y. Chang, X. Zhu, Z. Wei, X. Zhang, X. Sun, K. Lu and Z. Wei, Organic solar cells based on small molecule donor and polymer acceptor, Chin. Chem. Lett., 2022, 33, 123–132 CrossRef CAS.
- P. Bi, S. Zhang, Z. Chen, Y. Xu, Y. Cui, T. Zhang, J. Ren, J. Qin, L. Hong, X. Hao and J. Hou, Reduced non-radiative charge recombination enables organic photovoltaic cell approaching 19% efficiency, Joule, 2021, 5, 2408–2419 CrossRef CAS.
- Y. Cui, Y. Xu, H. Yao, P. Bi, J. Zhang, Y. Zu, T. Zhang, J. Qin, J. Ren, Z. Chen, C. He, X. Hao, Z. Wei and J. Hou, Single junction organic photovoltaic cell with 19% efficiency, Adv. Mater., 2021, 33, 2102420 CrossRef CAS.
- P. P. Khlyabich, M. Sezen-Edmonds, J. B. Howard, B. C. Thompson and Y. L. Loo, Formation of organic alloys in ternary-blend solar cells with two acceptors having energy offsets exceeding 0.4 eV, ACS Energy Lett., 2017, 9, 2149–2156 CrossRef.
- J. S. Huang, T. Goh, X. Li, M. Y. Sfeir, E. A. Bielinski, S. Tomasulo, M. L. Lee, N. Hazari and A. D. Taylor, Polymer bulk heterojunction solar cells employing forster resonance energy transfer, Nat. Photonics, 2013, 7, 479–485 CrossRef CAS.
- R. Lv, D. Chen, X. Liao, L. Chen and Y. Chen, A terminally tertrafluorinated non-fullerene acceptor for well performing alloy ternary solar cells, Adv. Funct. Mater., 2019, 29, 1805872 CrossRef.
- J. Dacuna and A. Salleo, “Modelling space charge limited currents in organic semiconductors” Extracting trap density and mobility, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 195209 CrossRef.
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Device physics of polymer: fullerene bulk heterojunction solar cells, Adv. Mater., 2007, 19, 1551–1566 CrossRef CAS.
- I. Riedel, J. Parisi, V. Dyakonov, L. Lusten, D. Vanderzande and J. C. Hummelen, Effect of temperature and illumination on the electrical characteristics of polymer fullerene bulk heterojunction solar cells, Adv. Funct. Mater., 2004, 14, 38–44 CrossRef CAS.
- L. Zhang, F. Yang, X. Meng, S. Yang, L. Ke, C. Zhou, H. Yan, X. Hu, S. Zhang, W. Ma and Y. Yuan, Regulating crystallization to maintain balanced carrier mobility via ternary strategy in blade coated flexible organic solar cells, Org. Electron., 2021, 89, 106027 CrossRef CAS.
- Y. Xie, F. Yang, Y. Li, M. A. Uddin, P. Bi, B. Fan, Y. Cai, X. Hao, H. Y. Woo, W. Li, F. Liu and Y. Sun, Morphology control enables efficient ternary organic solar cells, Adv. Mater., 2018, 30, 1803045 CrossRef.
- K. Jiang, G. Zhang, G. Yang, J. Zhang, Z. Li, T. Ma, H. Hu, W. Ma, H. Ade and H. Yan, Multiple cases of efficient Nonfullerene ternary organic solar cells enabled by an effective morphology control method, Adv. Energy Mater., 2018, 8, 1701370 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2me00166g |
| This journal is © The Royal Society of Chemistry 2023 |