 Open Access Article
Open Access ArticleDevelopment of electrical conductivity-based photosensitive switching devices using metal complexes with Schiff base ligands†
Shibashis
Halder

Department of Chemistry, T. N. B. College, Bhagalpur, Bihar 812007, India. E-mail: haldershibashis@gmail.com
First published on 28th September 2023
Abstract
Investigations of the electrical conductivity as well as the charge transportation properties of various types of metal complexes are observed to be an emerging trend in contemporary research. Scientific analyses on the implementation of metal complexes in the fabrication of electrical conductivity based photosensitive switching devices are being carried out by different research groups. But, until now only a few research articles have been reported which are related precisely to this research area. Given the current energy crisis faced by civilization, the development of energy efficient sun light-dependent photosensitive switching devices is a real requirement. Given that the processes of synthesis of Schiff base molecules are uncomplicated as well as cost efficient, the preparation of Schiff base-containing metal complexes is also simple and less expensive. Consequently, large scale production of Schiff base-containing metal complexes along with their industrial execution in the development of photo-switching devices is considered to be highly significant. From this perspective, the recent advancements in the area of research where Schiff base-containing metal complexes have been applied in the fabrication of electrical conductivity-based photosensitive switching devices have been discussed in this review article. It is noteworthy that researchers in the fields of chemistry, physics and material science should benefit from this overview.
Introduction
In recent years, a growing trend has been observed towards the investigation of the electrical conductivity as well as the charge transportation properties of different kinds of metal complexes.1–7 The application of metal–organic hybrid materials in the making of electronic devices is catching the attention of today's researchers.8–17The nature of the combination between the metal centres and the organic moieties within the structural architecture of a metal complex as well as the extent of resonance are considered as pivotal factors for determining the conducting properties of the material.18–20 For a long time, Schiff-base molecules have played a significant role in developing stable complexes due to their strong tendency for complexation with metal ions.21–31 Recently, numerous metal complexes with conjugated Schiff base ligands have been reported to exhibit fascinating electrical conducting properties.32–36
An electrical conductivity-based photosensitive switching device may be identified as a device which can exhibit I–V characteristics that display enhanced magnitude of conductivity in the presence of light compared to that in the dark.
The simplistic route of synthesis for Schiff base compounds correspondingly makes the development of metal complexes, containing Schiff base molecules as ligands, time efficient as well as less expensive. In addition to this, slight modification by incorporation of different substituent groups within the structural architecture of Schiff base molecules results in the modulation of various significant characteristics of the metal complexes developed with those Schiff base molecules. Due to these reasons, Schiff base containing-metal complexes can be considered as an ideal choice for the fabrication of electrical conductivity based photosensitive switching devices.37–39
Since the large scale production of Schiff base containing metal complexes is reasonably easy, their industrial implementation in making photo-switching devices is easily achievable and therefore highly demanded in contemporary research.
Herein, a detailed overview was presented regarding the reported metal complexes (containing Schiff base molecules as ligands), which were implemented in the development of electrical conductivity-based photosensitive switching devices.
Discussion
For the fabrication of an electrical conductivity based photosensitive switching device, the material should be layered as a thin film on the surface of ITO (indium tin oxide) covered glass. To clean the glass substrate coated with indium tin oxide, initially either acetone or isopropanol is utilized, subsequently washing with water; finally an ultrasonication process is implemented. Thereafter, to dry it, generally a vacuum chamber is used. To prepare the material's thin film on the surface of the ITO coated glass, initially preparation of a finely dispersed solution of the required material is carried out in a proper solvent with the help of the ultrasonication method. After obtaining the well dispersed solution of the sample, the development of the thin layer is carried out by depositing the sample on the surface of the glass coated with ITO following the spin coating technique. To modulate the layer's thickness, the rate as well as the time of spinning has to be controlled. Prior to aluminium-deposition as the front contact electrode, the already placed thin layer of the sample has to be appropriately desiccated using a vacuum oven at nearly 100 °C. The deposition of the Al-electrode can be carried out over the thin film with the help of the vacuum coating technique at a pressure of nearly 10−6 Torr. For the characterization of the electrical properties of the material, the current(I)–voltage(V) characteristics of the designed device are monitored by applying any kind of source meter utilizing the two-probe method.40,41 The I–V features of the designed device may be monitored in the dark as well as under illuminated conditions. The construction of an electrical conductivity-based photosensitive switching device is depicted in Fig. 1.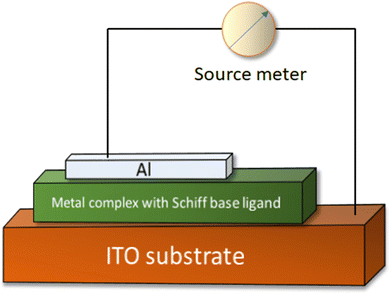 | ||
| Fig. 1 Schematic depiction of an electrical conductivity-based photosensitive switching device fabricated with a metal complex with a Schiff base ligand. | ||
Any material's electrical property can be measured as its efficacy in charge transportation. Transportation of electrical charge has a direct connection with the carrier mobility (i.e. m) as well as with the charge carrier concentration (i.e. n). The structural architectures as well as the electronic properties of the systems play pivotal roles in controlling these variables. Fundamentally, the charge transport processes within solid materials can be established via two mechanistic pathways: the hopping charge transport mechanism and band-like (or ballistic) transport mechanism.42–44 The hopping charge transport mechanism suggests the transfer of charge carriers between non-bonded discrete sites. This charge transportation pathway is mainly attributed to the conducting properties of organic semiconductors as well as disordered materials. Contrary to this, the pathway of band-like transportation suggests strong interactions in between suitable sites (for the development of uninterrupted energy bands) that exist within delocalized charge carriers. This charge transportation pathway can be ascribed to the conducting properties of the inorganic as well as hybrid materials.45–48
Fascinatingly, it has been observed that the conductivity of the hopping type is always triggered due to thermal energy, i.e.; with increase in temperature the conductivity of the material will increase. This observation can be exemplified with the equation as follows,
σ = σ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−(T0/T)1/d] exp[−(T0/T)1/d] |
In addition to this, the conducting properties of semiconductor materials are also found to display Arrhenius dependency,49–52
σ = σ0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp[−(Ea/kT)] exp[−(Ea/kT)] |
Researches in the field where Schiff-base containing metal complexes were reported to exhibit photo-sensitive conducting properties first emerged almost a decade ago. Now, detailed discussions on these photo-sensitive conducting complexes are being presented. Table 1 contains the structure of these complexes along with their conductivity values. Table S1, ESI† contains a list of abbreviations.
| S. no. | Structure | Conductivity (under dark) (S m−1) | Conductivity (in light) (S m−1) | Ref. |
|---|---|---|---|---|
| 1 |
|
1.01 × 10−6 | 2.16 × 10−6 | 53 |
| 2 |
|
4.53 × 10−5 | 1.93 × 10−4 | 54 |
| 3 |
|
2.90 × 10−4 | 7.16 × 10−4 | 55 |
| 4 |
|
8.26 × 10−2 | 22.07 × 10−2 | 56 |
| 5 |
|
2.02 × 10−2 | 8.55 × 10−2 | 57 |
| 6 |
|
3.63 × 10−3 | 4.13 × 10−3 | 58 |
| 7 |
|
2.13 × 10−3 | 3.19 × 10−3 | 59 |
| 8 |
|
3.99 × 10−3 | 7.13 × 10−3 | 59 |
| 9 |
|
1.26 × 10−6 | 6.72 × 10−5 | 60 |
| 10 |
|
1.07 × 10−7 | 2.44 × 10−7 | 60 |
| 11 |
|
1.78 × 10−7 | 6.15 × 10−7 | 60 |
| 12 |
|
7.47 × 10−4 | 23.12 × 10−4 | 61 |
| 13 |
|
2.94 × 10−6 | 6.12 × 10−6 | 62 |
| 14 |
|
2.92 × 10−7 | 3.66 × 10−7 | 62 |
| 15 |
|
0.88 × 10−8 | 2.38 × 10−8 | 63 |
| 16 |
|
8.24 × 10−5 | 14.03 × 10−5 | 64 |
| 17 |
|
7.0 × 10−5 | 3.5 × 10−4 | 65 |
| 18 |
|
2.0 × 10−5 | 4.9 × 10−4 | 65 |
| 19 |
|
6.49 × 10−4 | 21.29 × 10−4 | 66 |
| 20 |
|
13.1 × 10−6 | 11.2 × 10−5 | 67 |
| 21 |
|
5.4 × 10−6 | 13.5 × 10−5 | 67 |
| 22 |
|
0.87 × 10−3 | 2.45 × 10−3 | 68 |
| 23 |
|
3.89 × 10−4 | 8.25 × 10−4 | 69 |
| 24 |
|
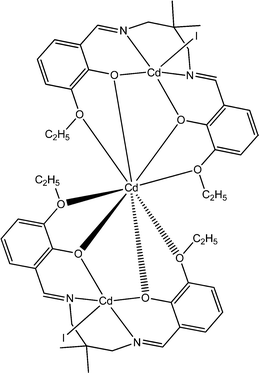
1.81 × 10−4 | 4.72 × 10−4 | 69 |
| 25 |
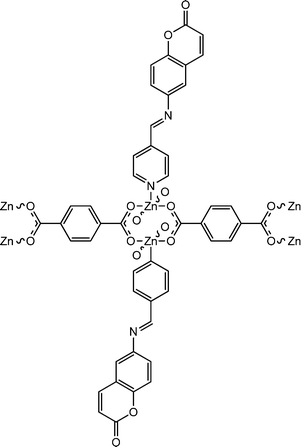
|
8.07![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × × ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−3 10−3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
9.26![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) × × ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10−3 10−3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
70 |
| 26 |
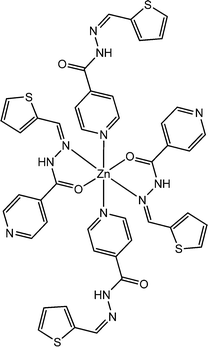
|
2.53 × 10−9 | 5.97 × 10−5 | 71 |
| 27 |
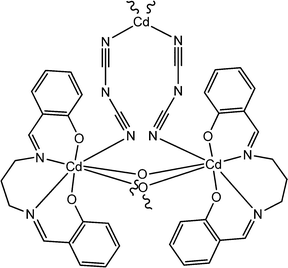
|
3.69 × 10−4 | 10.68 × 10−4 | 72 |
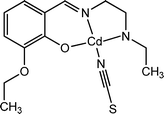
Roy et al. reported the synthesis and characterization of a new thiocyanate bridged two dimensional metal organic framework, [CdL1(μ-1,3-SCN)2]n (1), [where HL1 represents 2-(2-(ethylamino)ethyliminomethyl)-6-ethoxyphenol] (Fig. 2(a)).53 The band gap determined by the authors suggested the possibility of the material to possess some conducting properties. To fabricate an electrical conductivity-based photosensitive switching device the material was reported to be sandwiched as a thin film in between an ITO coated glass surface and metallic aluminium. The conductivity of this device was reported as 2.16 × 10−8 S cm−1 and 1.01 × 10−8 S cm−1 in the presence of light and in the dark, respectively (Fig. 2(b)). The authors reported that the repetitive measurements of the conductivity of the device were performed at a fixed bias voltage of 10 V.
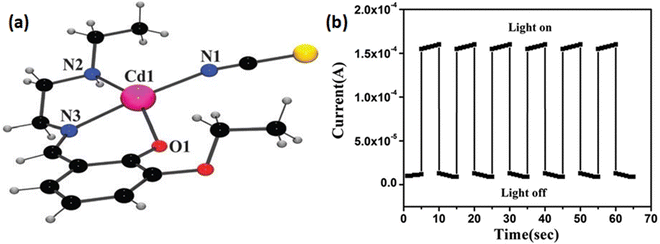 | ||
| Fig. 2 (a) A perspective view of the asymmetric unit of 1; (b) conductivity of ITO/1/Al configuration at light-on and light-off conditions. Reproduced with permission from ref. 53. Copyright 2015 Royal Society of Chemistry. | ||
Halder et al. reported a thiocyanate bridged Cd(II) MOF, [Cd(3-bpd)(SCN)2]n (2) (3-bpd represents 1,4-bis(3-pyridyl)-2,3-diaza-1,3-butadiene) (Fig. 3(a)).54 This metal organic framework was reported to be well-characterized using X-ray diffraction analysis, elemental analysis, thermogravimetric analysis and various spectroscopic techniques. X-ray diffraction study revealed the existence of a 2D ripple like polymeric network. To study the conducting characteristics of the MOF a device was reported to be fabricated by sandwiching the material in between ITO coated glass and metallic Al. The conducting properties of the metal organic framework were reported to be monitored in dark conditions as well as under the illumination of light also. The authors reported that the conductivity of the MOF enhanced in the presence of light compared to that in the dark. It was reported that on enhancement of the incident radiation intensity, the photosensitivity of the fabricated device further improved. The conductivity of the device was reported as 4.53 × 10−7 S cm−1 and 1.93 × 10−6 S cm−1 in dark conditions and under the illumination of light at a constant bias voltage of 1.0 V, respectively (Fig. 3(b)). According to the authors, this assembly can be successfully implemented as a conductivity based photo-switching device.
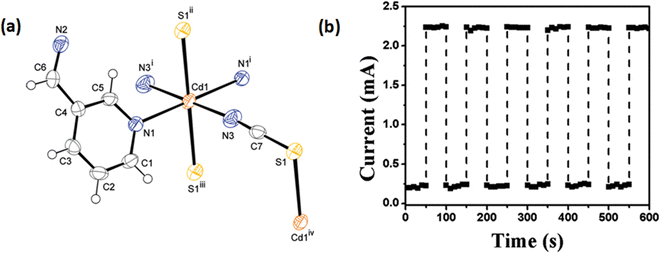 | ||
| Fig. 3 (a) A thermal ellipsoid plot of an asymmetric unit of 2 drawn with 50% ellipsoidal probability; (b) conductivity of the device fabricated with 2 at light-on and light-off conditions (bias voltage = 1.0 V and intensity of incident light = 80 mW cm−2). Reproduced with permission from ref. 54. Copyright 2015 Royal Society of Chemistry. | ||
Halder et al. again reported a newly synthesized thiocyanate bridged 2D cadmium-based MOF, [Cd(4-bpd)(SCN)2]n (3) (where 4-bpd represents 1,4-bis(4-pyridyl)-2,3-diaza-1,3-butadiene).55 X-ray diffraction analysis of the single crystal of the complex displayed a captivating 2D ladder-like molecular design. A specific device fabrication was reported to be carried out by the authors where the MOF was sandwiched between ITO covered glass and aluminium. This specially designed device was observed to display some fascinating properties related to electrical conductivity. The constructed device's I–V features were recorded under light illuminated conditions and also in the dark. The current–voltage curves obtained for this device exhibited a non-linear pattern. The values of the electrical conductance of the configured device were presented by the authors as 2.90 × 10−4 S m−1 and 7.16 × 10−4 S m−1, in the dark and in light illuminated conditions, respectively. The conductivity was reported to be measured by applying a bias voltage sequentially within a range of ±5 V. The authors investigated various parameters to rationalise that the configured device can be proficient to work as an electrical conductivity-based photosensitive switching device. These results were justified well with the support from density functional theoretical analyses. In accordance with the theoretical interpretations, it was reported that under the irradiation of light there occurs a significant decrease in the magnitude of the energy necessary for the allowable electronic transition (valence band to conduction band). This may be attributed to the reason that under the illumination of light, slight fluctuations occur in several bond lengths of the 4-bpd moiety. As said by the authors, this may be the reason for the enhancement in electrical conductivity.
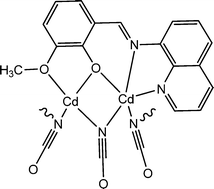
Ghorai and co-workers reported a newly synthesized Cd-based 1D coordination polymer (4). This coordination polymer comprises a Schiff base ligand based on a quinoline moiety together with a cyanate moiety.56 A device was configured by sandwiching this coordination polymer in between ITO layered glass and metallic aluminium. This configured device was described to display fascinating electrical characteristics. The device's I–V curves exhibited rectifying non-linear nature. In addition to this, it was reported that the electrical conductivity of the fabricated device increases under the illumination of light compared to that in the dark. The conductivity values were reported as 8.26 × 10−2 S m−1 and 22.07 × 10−2 S m−1 under dark condition and in the presence of light, respectively. The conductivity measurement was performed by applying a bias voltage of 2 V. The authors carried out theoretical analyses to support these experimental findings. The direct band gap obtained from DFT analysis was reported as 1.836 eV, which is in good agreement with the value of the band gap obtained experimentally (2.16 eV). The enhancement of conductivity of the material in the presence of light was also nicely explained by theoretical analysis.
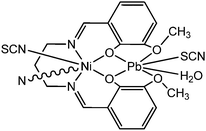
Roy and co-workers reported a new lead(II)/nickel(II) coordination polymer [(NCS)Pb(H2O)-L2Ni(NCS)]n (5) (where H2L2 represents N,N′-bis(3-methoxysalicylidene)propane-1,3-diamine).57 The characterization of this coordination polymer was performed by numerous spectroscopic methods, which include X-ray single crystal diffraction analyses. The value of the optical band-gap of the material was calculated as 3.18 eV with the help of the Tauc plot. This finding signifies semiconducting behaviour of the material. This coordination polymer was sandwiched between the ITO coated glass surface and Al to generate a device to study its electrical properties. It was reported by the authors that the conductivity of this device enhanced under the irradiation of light compared to that under dark conditions. The values of electrical conductivity of the configured device were reported as 2.02 × 10−4 S cm−1 in the dark and 8.55 × 10−4 S cm−1 in the presence of light.
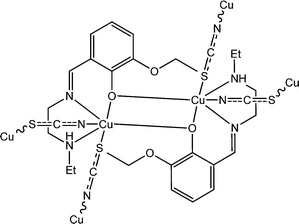
Khan et al. reported a newly synthesized Cu-based coordination polymer, [Cu2L32(μ-1,3-SCN)2]n (6) (HL3 represents 2-(((2-(ethylamino)ethylimino)methyl)-6-ethoxyphenol).58 The Schiff-base ligand was reported to be tetradentate in nature with N2O2 donor sites. Single crystal X-ray diffractrometric analyses reveal the presence of thiocyanate bridging (end-to-end) within the structural architecture of the complex. To study the photo-sensitive sensing properties of this coordination polymer, a device was designed where the material was layered as a thin film in between metallic Al and the glass surface covered with indium tin oxide. This configured device exhibited the conductivity of 3.63 × 10−5 S cm−1 under dark conditions whereas the conductivity was reported to be increased to 4.13 × 10−5 S cm−1 on irradiation of light. The photosensitivity of the device was reported as 2.833. The conductivity measurements were reported to be carried out at a bias voltage of 2 V.
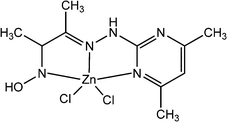
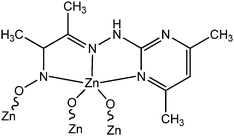
Konar et al. reported two newly synthesized Zn(II) based complexes, [Zn(Pymox)Cl2] (7) and [Zn6(Pymox)6(μ2-O)3] (8) (“Pymox” represents 3-[(4,6-dimethyl-pyrimidine-2-yl)-hydrazono]-butan-2-one oxime).59 The values of the optical band-gap obtained from Tauc plot were reported as 3.43 eV and 2.36 eV for [Zn(Pymox)Cl2] and [Zn6(Pymox)6(μ2-O)3], respectively. The X-ray single crystal diffraction analyses confirmed the mononuclear and hexanuclear nature of [Zn(Pymox)Cl2] and [Zn6(Pymox)6(μ2-O)3], respectively. To evaluate the conducting properties of these complexes, both of them were layered as a thin film in between an indium tin oxide covered glass surface and metallic aluminium. It was reported that both the devices exhibited enhancement in conductivity under light irradiation compared to that in the dark. For [Zn(Pymox)Cl2], the conductivity were measured as 2.13 × 10−3 S m−1 and 3.19 × 10−3 S m−1 in the dark and in the presence of light, respectively within a successive bias voltage of ± 2 V. Whereas for [Zn6(Pymox)6(μ2-O)3] the conductivity was reported as 3.99 × 10−3 S m−1 in the dark and 7.13 × 10−3 S m−1 under the illumination of light.
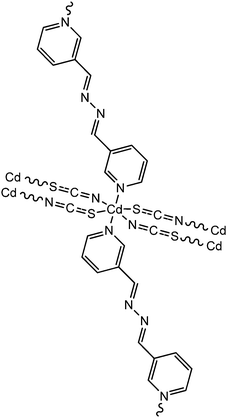
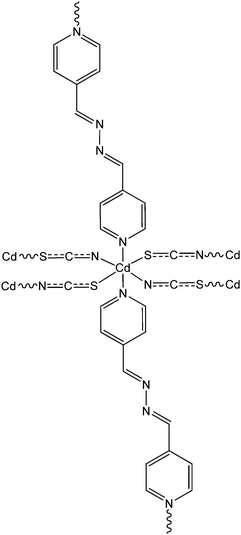
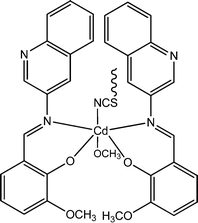
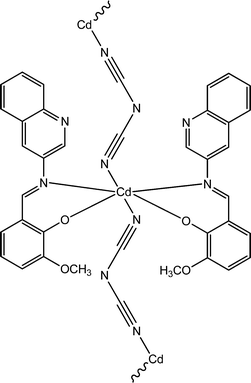
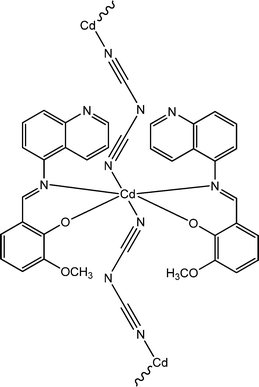
Recently, three newly synthesized Cd-based coordination polymers (9, 10 and 11) were reported by Ghorai and co-workers.60 These coordination polymers consisted of Schiff-base ligands containing quinoline moieties. As revealed by an X-ray single crystal diffraction study, pseudohalides were involved pivotally to generate the polymeric structure of these complexes. Devices were configured to analyse the electrical properties by developing thin films of these complexes between indium tin oxide coated glass and aluminium. The I–V curves obtained from these devices showed rectifying non-linear character. The electrical conductivities of all these devices were found to get enhanced under the illumination of light than in the dark. The values of the conductivity in dark conditions were reported as 1.26 × 10−6 S m−1, 1.78 × 10−7 S m−1 and 1.07 × 10−7 S m−1 for the devices configured with 9, 10 and 11, respectively. Whereas, after irradiation under light the conductivity values were measured as 6.72 × 10−5 S m−1, 6.15 × 10−7 S m−1 and 2.44 × 10−7 S m−1 for the devices fabricated with 9, 10 and 11, respectively. The conductivity of these devices was reported to be measured by applying a bias voltage successively within a range of ±2 V. Among these three complexes, 9 exhibited the highest photosensitivity whereas the lowest photosensitivity was recorded for 11. As reported by the authors, the transportation of electrons among the neighbouring Cd-centres can be attributed to the delocalized π-electrons of the organic ligands as well as those of the pseudohalide moieties.
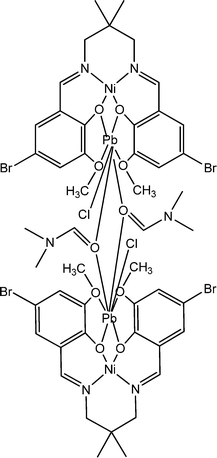
Interesting electrical properties of a newly synthesized nickel(II)/lead(II) tetranuclear complex (12) were reported by Roy and co-workers.61 Characterization by X-ray single crystal diffraction study disclosed the occurrence of two [(NiO2)Pb] centres linked through two dimethylformamide moieties. Tauc's plot helped to evaluate the optical band-gap, which was found to be 3.08 eV. This finding recommended the authors regarding the probable semiconducting characteristics of the material. To analyse the electrical properties, a device was configured with this material in such a way that it was layered as a thin film between indium tin oxide covered glass and Al. The configured device exhibited conductivity values of 23.12 × 10−4 S m−1 under the irradiation of light and 7.47 × 10−4 S m−1 in the dark. The conductivity measurements were reported to be carried out by implementing a bias voltage successively within a range of ±2 V. In this case, the current–voltage curve was found to be non-linear.
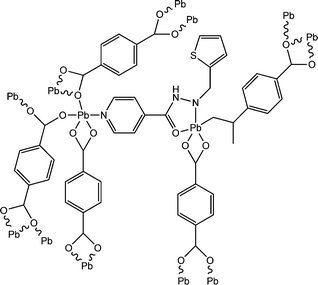
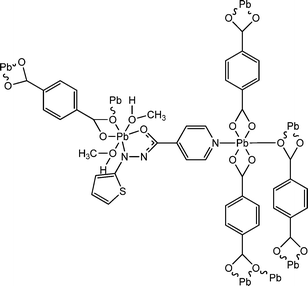
Two newly synthesized coordination polymers, [Pb2(bdc)1.5(aiz)]n (13) and [Pb2(bdc)1.5(aiz)-(MeOH)2]n (14), were reported by Dey and co-workers (where, “H2bdc” represents 1,4-benzene dicarboxylic acid and “aiz” represents (E)-N′-(thiophen-2-ylmethylene)isonicotinohydrazide).62 X-ray single crystal diffractometric study suggested the polymeric structural architecture of these coordination polymers. Calculation of the optical band-gaps for both of the complexes was reported to be carried out with the help of UV-visible spectroscopic analyses. Theoretical studies were performed by the authors to justify these experimental findings. The optical band gaps obtained from Tauc's plot were reported as 3.46 eV for 13 and 3.01 eV for 14. The values of the theoretical band gaps obtained from DFT computation are 2.84 eV for 13 and 2.52 eV for 14. The obtained values of the band-gaps provide indications regarding the probable semiconducting properties of both the coordination polymers. To properly investigate the electrical properties, specific kinds of devices were designed by incorporating the complexes as a thin layer between metallic aluminium and a glass surface covered with ITO. The current–voltage plots for both the devices displayed enhancement in electricity under the illumination of light compared to that under dark conditions. The device configured with [Pb2(bdc)1.5(aiz)]n exhibited enhanced value of conductivity compared to the device configured with [Pb2(bdc)1.5(aiz)-(MeOH)2]n. The values of electrical conductance for the device fabricated with [Pb2(bdc)1.5(aiz)]n were measured as 6.12 × 10−6 S m−1 and 2.94 × 10−6 S m−1 under the illumination of light and in the dark, respectively. While the obtained values of the electrical conductivity for the device developed with [Pb2(bdc)1.5(aiz)-(MeOH)2]n were found to be 3.66 × 10−7 S m−1 and 2.92 × 10−7 S m−1 under the illumination of light and in dark conditions, respectively.
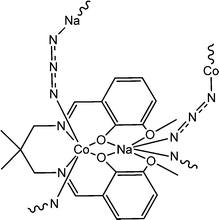
Interesting electrical properties of a newly synthesized sodium–cobalt complex (15) (Fig. 4(a)) were reported by Ghosh and co-workers.63 X-ray single crystal diffraction analyses revealed the existence of end to end azide bridging within the structural architecture. The experimentally obtained band gap specified the probable semiconducting characteristics of the hybrid metal complex. To study the conducting properties, a device was configured by layering the complex as a thin film between aluminium and a glass surface covered with indium tin oxide. Fascinatingly it was observed that the conductivity of the fabricated device got enhanced under the irradiation of light than in dark conditions. As reported, the configured device exhibited conductivities of 0.88 × 10−8 S m−1 in dark conditions and 2.38 × 10−8 S m−1 under the illumination of light (Fig. 4(b)). The conductivity measurements were performed by implementing a bias voltage of ±2 V. The authors reported the value of photosensitivity as 1.45. To rationalise these experimental findings, the authors carried out theoretical analyses. Density functional theory calculation suggested the theoretical band gap as 1.80 eV and justified its enhanced conductivity in the presence of light.
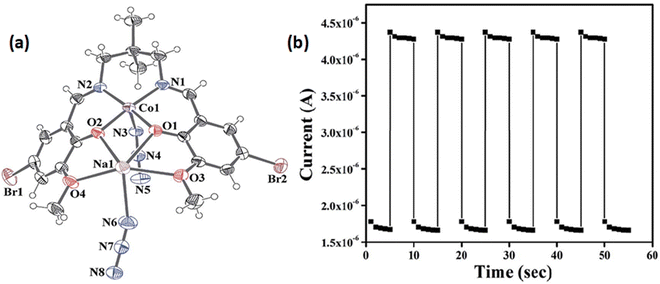 | ||
| Fig. 4 (a) A thermal ellipsoid plot of a hetero-binuclear unit of 15 drawn with 40% ellipsoidal probability; (b) conductivity of the ITO/15/Al configuration under light-on and light-off conditions. Reproduced with permission from ref. 63. Copyright 2019 Royal Society of Chemistry. | ||
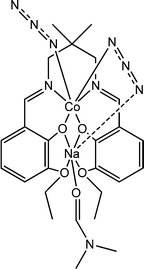
Interesting conducting characteristics of a newly synthesized Schiff base containing a hetero-dinuclear sodium(I)/cobalt(III) complex (16) was reported by Roy et al.64 X-ray single crystal diffraction analyses revealed the presence of captivating C–H⋯π(N3) contacts within the structural architecture. The band-gap was found to be 2.85 eV. Encouraged by this finding, the authors designed a specific kind of device to study the conducting properties of the material by layering it as a thin film between metallic Al and an ITO covered glass surface. The I–V curve obtained for this device showed non-linearity. It was reported that under irradiation of light the conductivity of the configured device enhanced compare to that under dark conditions. The measured value of conductivity of this device was mentioned as 14.03 × 10−5 S m−1 under the illumination of light and 8.24 × 10−5 S m−1 in the dark. The outstanding photosensitivity displayed by the device fabricated with this dinuclear complex can be attributed to its greater conductivity under the illumination of light. The double donor–acceptor characteristics of the dinuclear complex may be attributed to the above discussed observations. The electron rich portions within the complex are the aromatic ring together with the imine bonds; while the electron deficient portions are the metal centres accompanied by the azide moiety. As stated by the authors, on light illumination, a transfer of charge takes place from the electron enriched portion to the electron deficient portion within the molecule. The consequence of this is the enhancement of the electrical conductivity under the irradiation of light.
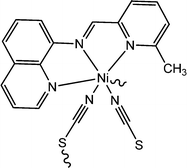
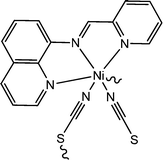
Captivating electrical properties of two nickel-containing 1D coordination polymers were reported by Ghorai and co-workers.65 Both the coordination polymers were reported to consist of Schiff base compounds as ligands. These coordination polymers are designated here as [Ni(L4)(NCS)2]n (17) and [Ni(L5)(NCS)2]n (18) (where L4 represents (E)-N-(pyridin-2-ylmethylene)quinolin-8-amine and L5 represents (E)-N-((6-methylpyridin-2-yl)methylene)quinolin-8-amine). As revealed by the analyses done with X-ray single crystal diffractometry, both the above mentioned coordination polymers were found to be isostructural. They are also observed to consist of thiocyanato moieties as zigzag bridges. As reported by the authors, there also exist feeble inter-chain interactions amongst sulphur atoms. To investigate the conducting properties, devices were fabricated by layering both the coordination polymers as a thin film in between aluminium and a glass surface covered with indium tin oxide. It was observed that both the devices exhibit enhancement in conductivity under the irradiation of light compared to that in the dark. The values of the conductivity obtained for the device configured with [Ni(L4)(NCS)2]n were reported as 7.0 × 10−5 S m−1 in dark conditions and 3.5 × 10−4 S m−1 under the irradiation of light. Similarly, the conductivities for the device designed with [Ni(L5)(NCS)2]n were recorded as 2.0 × 10−5 S m−1 in the dark and 4.9 × 10−4 S m−1 in the presence of light. For both of the devices, the obtained I–V plots exhibited rectifying non-linear nature. The capacity of donation of π-electrons of the Schiff-base moieties existing within the Ni(II) complexes may be the reason behind the enhancement of conductivity in the case of both the configured devices under the illumination of light. These fascinating conducting properties may be tuned simply by inserting or altering different substituent groups within the Schiff-base moieties. As stated by the authors, the device configured with [Ni(L5)(NCS)2]n exhibited increased conductivity under the illumination of light, which may be attributed to the existence of an additional electron-donating methyl-substituent in L5 (which is missing in the case of L4).
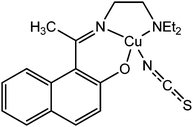
A newly synthesized thiocyanate-containing copper complex, [CuL6(NCS)] (19), was reported by Khan et al. (where HL6 represents 1-(((2-(diethylamino)ethylimino)ethyl)naphthalene-2-ol).66 This complex was found to contain a Schiff base ligand having a naphthalene moiety. As reported by the authors the calculated optical band gap of the material is 2.92 eV. This finding provided an indication about the semiconducting characteristics of the material. To investigate the electrical property of the complex a device was configured by layering the complex as a thin film in between metallic aluminium and ITO covered glass surface. The I–V plots of this configured device were found to display non-linear nature. It was reported that under dark conditions, the conductivity of the device gets reduced compare to that under the irradiation of light. The values of conductivity of the device fabricated with [CuL6(NCS)] were recorded as 21.29 × 10−4 S m−1 under the irradiation of light and 6.49 × 10−4 S m−1 in the dark. The conductivity measurements were carried out by implementing a bias voltage of ±2 V. To justify the experimental findings the authors carried out theoretical analyses. The theoretical band gap was obtained by DFT calculation, which matched well with the experimentally obtained band gap suggesting the semiconducting nature of the material.
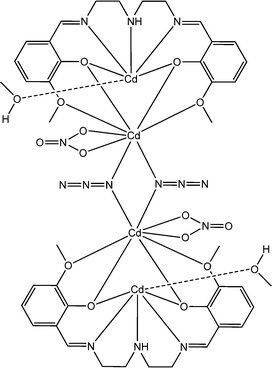
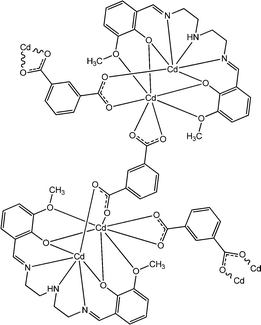
Recently, interesting conducting properties of two novel cadmium-based complexes, [Cd4L72(NO3)2(μ1,1-N3)2(CH3OH)2] (20) and {[Cd2L7(IPA)]2·(CH3OH)}n (21), were reported by Ghosh and co-workers (where H2L7 represents N,N′-bis(3-methoxysalicylidene)-diethylenetriamine and IPA represents isophthalic acid).67 Both of these complexes contain Schiff base ligands as constituents. As revealed by X-ray single crystal diffraction analyses, [Cd4L72(NO3)2(μ1,1-N3)2(CH3OH)2] displays a tetranuclear structural architecture whereas {[Cd2L7(IPA)]2·(CH3OH)}n is 1D polymeric in nature. As stated by the authors, both of these two complexes were utilized in the fabrication of a specific type of device where the complexes were layered as a thin film in between aluminium and an ITO covered glass surface. Both of these devices were reported to show enhanced conductivity under the irradiation of light compared to that in the dark. According to the authors, these characteristics of the materials make them potential candidates to be used for the fabrication of electrical conductivity-based photo-switching devices. The conductivity of the device configured with [Cd4L72(NO3)2(μ1,1-N3)2(CH3OH)2] was measured as 13.1 × 10−8 S cm−1 in the dark and 11.2 × 10−7 S cm−1 under the irradiation of light. Similarly, for the device configured with {[Cd2L7(IPA)]2·(CH3OH)}n the conductivity was recorded as 5.4 × 10−8 S cm−1 under dark conditions and 13.5 × 10−7 S cm−1 in the presence of light.
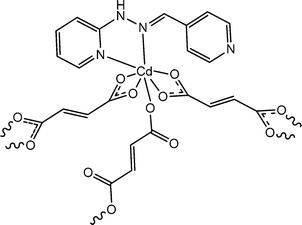
Electrical properties of a newly prepared 2D cadmium-based coordination polymer, {[Cd4(ppmh)4(fum)4(H2O)4]·3H2O}n (22), were discussed by Chandra et al. (where “H2fum” represents fumaric acid and “ppmh” represents N-pyridin-2-yl-N′-pyridin-4-ylmethylene-hydrazine).68 As disclosed by the characterization through X-ray single crystal diffraction analyses, within the structural architecture there exist fumarato bridging amongst contiguous cadmium centres using bidentate coordination. The optical band-gap of the material was measured as 3.29 eV. This value indicated the probable semiconducting characteristics of the coordination polymer. Computational analyses carried out by the authors also justified this finding. To investigate the electrical properties of the material, a device was reported to be designed in such a way that the coordination polymer is placed as a thin film in between metallic aluminium and a glass surface covered with ITO. The I–V curves obtained from this device showed non-linear character. It was reported that the conductivity of the device got reduced in the dark compared to that in light. The values of conductivity were found to be 2.45 × 10−3 S m−1 under the irradiation of light and 0.87 × 10−3 S m−1 in the dark. The conductivity measurements were performed on the implementation of a bias voltage of ±3 V.
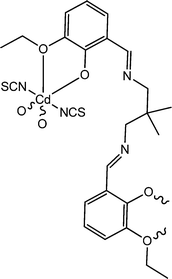
Recently, interesting conducting properties of two newly synthesized cadmium-based complexes were reported by Roy et al. Both of these complexes contain Schiff base ligands as a constituent unit. Among these two, one was trinuclear in nature (23) and the other one (24) was a coordination polymer.69 The structural features of these complexes were revealed by characterization through X-ray single crystal diffraction analyses. For the trinuclear complex, the band-gap was calculated as 3.43 eV and in the case of the coordination polymer it was found to be 3.21 eV. These findings gave indications about the semiconducting characteristics of the materials. To study the electrical properties of the complexes, specific kinds of devices were configured where the materials were layered as a thin film in between aluminium and a glass surface covered with indium tin oxide. The current–voltage plots of the devices displayed rectifying non-linear character. The values of conductance of the device configured with the trinuclear complex (23) were measured as 1.81 × 10−4 S m−1 in dark conditions and 4.72 × 10−4 S m−1 under the illumination of light. The values of conductance of the device configured with the coordination polymer (24) were recorded as 8.25 × 10−4 S m−1 under the illumination of light and 3.89 × 10−4 S m−1 in dark conditions. The conductivity measurements were carried out by implementing a bias voltage successively within a range of ±2 V. As stated by the authors, in the cases of both these materials the charge transportation largely occurs via space, which can be explained by the hopping transport method. For the justification of the experimental findings the authors carried out theoretical calculations. DFT analysis helped to obtain a theoretical band gap, which was in good agreement with the experimental findings.
Paul et al. reported interesting electrical properties of a newly synthesized two dimensional Zn(II)-based coordination polymer, [Zn2(BDC)4(QPR)2(H2O)]n (25) (where, H2BDC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) represents
represents![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,4-benzenedicarboxylic acid and QPR
1,4-benzenedicarboxylic acid and QPR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) represents
represents![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7-[(pyridin-4-ylmethylene)-amino]-chromen-2-one).70 According to the authors, this coordination polymer was apparently the firstly reported coumarinyl-pyridyl molecular architecture containing a terephthalate moiety as a linker. With the help of the Tauc plot, the authors measured the optical band-gap and it was reported as 2.91
7-[(pyridin-4-ylmethylene)-amino]-chromen-2-one).70 According to the authors, this coordination polymer was apparently the firstly reported coumarinyl-pyridyl molecular architecture containing a terephthalate moiety as a linker. With the help of the Tauc plot, the authors measured the optical band-gap and it was reported as 2.91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) eV. This result indicated the semiconducting properties of the material. To investigate the electrical properties of the coordination polymer a device was designed by layering the material as a thin film in between metallic aluminium and a glass surface covered with ITO. The conductivity of the configured device was measured as 8.07 × 10−3 S m−1 in the dark and 9.26 × 10−3 S m−1 in the presence of light. This observation makes the coordination polymer a capable candidate to be used in the fabrication of electrical conductivity-based photo-switching devices.
eV. This result indicated the semiconducting properties of the material. To investigate the electrical properties of the coordination polymer a device was designed by layering the material as a thin film in between metallic aluminium and a glass surface covered with ITO. The conductivity of the configured device was measured as 8.07 × 10−3 S m−1 in the dark and 9.26 × 10−3 S m−1 in the presence of light. This observation makes the coordination polymer a capable candidate to be used in the fabrication of electrical conductivity-based photo-switching devices.
Recently, Debsharma et al. reported a newly synthesized two dimensional zinc-based coordination polymer, containing a tridentate Schiff base ligand. The coordination polymer was designated as {[Zn(SIZ)2]·DMF}n (26) (where HSIZ represents (E)-N′-(thiophen-2-ylmethylene)isonicotinohydrazide and DMF represents N,N-dimethylformamide).71 X-ray single crystal diffraction analyses revealed the presence of π⋯π interactions, C–H⋯π interactions and H-bonding, which build up the fascinating structural architecture of the complex. From the Tauc plot, the direct and indirect optical band-gaps of the polymeric material were evaluated as 2.91 eV and 2.80 eV, respectively. To analyse the conducting properties of the material, it was layered as a thin film in between Al and a glass surface covered with indium tin oxide. This configured device exhibited conductivity of 2.53 × 10−9 S m−1 in dark conditions whereas the conductivity was reported to be enhanced to 5.97 × 10−5 S m−1 upon irradiation of light. The conductivity measurements were carried out by implementing a bias voltage of ±1 V. These observations justified the probable application of this coordination polymer in making electrical conductivity-based photo-switching devices.
Recently, Majumdar and co-workers have reported interesting electrical properties of a newly synthesized cadmium-based coordination polymer (27) containing Salen-type ligands.72 The optical band gap was calculated as 3.54 eV, which indicated its semiconducting nature. To investigate the electrical properties of this material, a device was configured by layering this coordination polymer as a thin film between ITO covered glass surface and metallic aluminium. This configured device was found to exhibit enhanced conductivity under illumination of light compared to that in the dark. The values of conductivity were reported as 3.69 × 10−4 S m−1 in the dark and 10.68 × 10−4 S m−1 under the illumination light.
It has been observed that the coordination interaction of organic ligands with different kinds of metal ions as well as metal clusters, occasionally, results in the enhancement of the charge carrier mobility, which consequently increases the electrical conductivity of the material. Nonetheless, this cannot be considered as a general phenomenon; actually the mechanistic route related with the electronic movement plays a pivotal role in determining the conductivity of the material. As discussed previously, secondary interaction together with structural features are considered as important aspects for electrical conductivity. In many instances, it has been observed that under light irradiation tiny changes in bond angles or in dihedral angles occur within the structural architecture of the molecules, which affects the charge transport capabilities of the materials. These eventually result in an enhancement in conductivity. For polyheteronuclear complexes, the transportation of charge can be influenced by the nature of the binding of the metal centres as well as the inhomogeneities present within the structure; nonetheless, rigorous research should be done to get into the details behind the mechanism of charge transportation for this type of complex.
Although this is not a general observation, but usually it can be observed that the devices fabricated with either one dimensional or two dimensional coordination polymers are found to exhibit greater conductivity in both illuminated conditions and in the dark. More detailed and rigorous research in this field would definitely help in determining the specific pattern of the effect of dimensionality of materials on the conductivity of the configured devices.
Conclusion
This article basically presents a summary related to the recent advancement in the area of research where Schiff base-containing metal complexes have been applied in the fabrication of electrical conductivity-based photosensitive switching devices. To date only a handful of research articles have been published related to this specific research area. In this era of energy crisis, the development of energy efficient sun light-dependent photo-switching devices is a genuine requirement. Therefore, the fabrication of electrical conductivity-based photo-switching devices using less expensive as well as easily synthesizable Schiff base-containing metal complexes can be considered as exceedingly significant as well as a requirement for contemporary research. In consideration of the need of civilization, crucial discussion on the development of different kinds of photo-switching device is really relevant. Since the synthesis process of Schiff base molecules is straightforward and cost efficient, the preparation of Schiff base-containing metal complexes can also be considered as simple and less expensive. For this reason, the large scale production of Schiff base-containing metal complexes and their industrial implementation in the fabrication of photo-switching devices are highly appreciable. It is noteworthy that this overview should benefit researchers in the fields of chemistry, physics and material science.Author contributions
Shibashis Halder: conceptualization, analysis, formal analysis, visualization, investigation, resource collection, writing – original draft manuscript, writing – review and editing, and supervision.Conflicts of interest
The author declares no conflict of interest.References
- M. Mladenović and N. Vukmirovic, Adv. Funct. Mater., 2015, 25, 1915–1932 CrossRef.
- J. Terao, A. Wadahama, A. Matono, T. Tada, S. Watanabe, S. Seki, T. Fujihara and Y. Tsuji, Nat. Commun., 2013, 4, 1691–1699 CrossRef PubMed.
- S. Stafström, Chem. Soc. Rev., 2010, 39, 2484–2499 RSC.
- W. Wu, Y. Liu and D. Zhu, Chem. Soc. Rev., 2010, 39, 1489–1502 RSC.
- J. Cornil, D. Beljonne, J.-P. Calbert and J.-L. Brédas, Adv. Mater., 2001, 13, 1053–1067 CrossRef CAS.
- B. Dutta and S. Halder, ChemistrySelect, 2023, 8, e202301586 CrossRef CAS.
- H. Wang, Q.-L. Zhu, R. Zou and Q. Xu, Chem, 2017, 2, 52–80 CAS.
- P. J. Low, Dalton Trans., 2005, 2821–2824 RSC.
- A. A. Talin, A. Centrone, A. C. Ford, M. E. Foster, V. Staliva, P. Haney, R. A. Kenney, V. Szalai, F. E. Gabaly, H. P. Yoon, F. Leonard and M. D. Allendorf, Science, 2013, 343, 66–69 CrossRef PubMed.
- A. Nath, K. S. Asha and S. Mandal, Chem. – Eur. J., 2021, 27, 11482–11538 CrossRef CAS PubMed.
- I. Stassen, N. Burtch, A. Talin, P. Falcaro, M. Allendorf and R. Ameloot, Chem. Soc. Rev., 2017, 46, 3185–3241 RSC.
- J. Wu, J. Chen, C. Wang, Y. Zhou, K. Ba, H. Xu, W. Bao, X. Xu, A. Carlsson, S. Lazar, A. Meingast, Z. Sun and H. Deng, Adv. Sci., 2020, 7, 1903003 CrossRef CAS PubMed.
- M. D. Allendorf, A. Schwartzberg, V. Stavila and A. A. Talin, Chem. – Eur. J., 2011, 17, 11372–11388 CrossRef CAS PubMed.
- L.-T. Zhang, Y. Zhou and S.-T. Han, Angew. Chem., Int. Ed., 2021, 133, 15320–15340 CrossRef.
- J. Nicks, K. Sasitharan, R. R. R. Prasad, D. J. Ashworth and J. A. Foster, Adv. Funct. Mater., 2021, 31, 2103723 CrossRef CAS.
- W. Zhao, J. Peng, W. Wang, S. Liu, Q. Zhao and W. Huang, Coord. Chem. Rev., 2018, 377, 44–63 CrossRef CAS.
- W.-J. Li, J. Liu, Z.-H. Sun, T.-F. Liu, J. Lu, S.-Y. Gao, C. He, R. Cao and J.-H. Luo, Nat. Commun., 2016, 7, 11830 CrossRef CAS PubMed.
- X. Wang, Y. Wang, M. A. Silver, D. Gui, Z. Bai, Y. Wang, W. Liu, L. Chen, J. Diwu, Z. Chai and S. Wang, Chem. Commun., 2018, 54, 4429–4432 RSC.
- M. Sadakiyo, H. Kasai, K. Kato, M. Takata and M. Yamauchi, J. Am. Chem. Soc., 2014, 136, 1702–1705 CrossRef CAS PubMed.
- L. Zhai, J.-W. Yu, J. Zhang, W.-W. Zhang, L. Wang and X.-M. Ren, Dalton Trans., 2019, 48, 12088–12095 RSC.
- H. Keypour, M. Shayesteh, A. Sharifi-Rad, S. Salehzadeh, H. Khavasi and L. Valencia, J. Organomet. Chem., 2008, 693, 3179–3187 CrossRef CAS.
- M. J. MacLachlan, M. K. Park and L. K. Thompson, Inorg. Chem., 1996, 35, 5492–5499 CrossRef CAS PubMed.
- S. Yamada, Coord. Chem. Rev., 1999, 190–192, 537–555 CrossRef CAS.
- X. Liu and J.-R. Hamon, Coord. Chem. Rev., 2019, 389, 94–118 CrossRef CAS.
- E. L. Gavey and M. Pilkington, Coord. Chem. Rev., 2015, 296, 125–152 CrossRef CAS.
- M. R. Maurya, Coord. Chem. Rev., 2019, 383, 43–81 CrossRef CAS.
- A. K. Gupta, A. Dhir and C. P. Pradeep, Inorg. Chem., 2016, 55, 7492–7500 CrossRef CAS PubMed.
- E. T. Aljohani, M. R. Shehata and A. M. Abu-Dief, Appl. Organomet. Chem., 2021, 35, e6169 CrossRef CAS.
- A. M. Abu-Dief, R. Díaz-Torres, E. C. Sañudo, L. H. Abdel-Rahman and N. Aliaga-Alcalde, Polyhedron, 2013, 64, 203–208 CrossRef CAS.
- L. H. Abdel-Rahman, A. M. Abu-Dief, F. M. Atlam, A. A. H. Abdel-Mawgoud, A. A. Alothman, A. M. Alsalme and A. Nafady, J. Coord. Chem., 2020, 73, 3150–3173 CrossRef CAS.
- A. A. Al-Shamry, M. M. Khalaf, H. M. A. El-Lateef, T. A. Yousef, G. G. Mohamed, K. M. K. El-Deen, M. Gouda and A. M. Abu-Dief, Materials, 2023, 16, 83 CrossRef CAS PubMed.
- C. E. Carraher Jr, J. Chem. Educ., 1981, 58, 921–934 CrossRef.
- S. Djebbar-Sid, O. Benali-Baitich and J. P. Deloume, Polyhedron, 1997, 16, 2175–2182 CrossRef CAS.
- Y.-B. Dong, L. Wang, J.-P. Ma, X.-X. Zhao, D.-Z. Shen and R.-Q. Huang, Cryst. Growth Des., 2006, 6, 2475–2485 CrossRef CAS.
- J. Zhang, L. Xu and W.-Y. Wong, Coord. Chem. Rev., 2018, 355, 180–198 CrossRef CAS.
- X. Li, Y. Jiao and S. Li, Eur. Polym. J., 1991, 27, 1345–1351 CrossRef CAS.
- S. Mahato, A. Mondal, M. Das, M. Joshi, P. P. Ray, A. Roy Choudhury, C. M. Reddy and B. Biswas, Dalton Trans., 2022, 51, 1561–1570 RSC.
- S. Karadeniz, D. E. Yilid, H. H. Gullu, D. A. Kose, A. A. Hussaini and M. Yildirim, J. Mater. Sci.: Mater. Electron., 2022, 33, 18039–18053 CrossRef CAS.
- Q. Li, J. Meng and Z. Li, J. Mater. Chem. A, 2022, 10, 8107–8128 RSC.
- F. Ahmed, B. Dutta and M. H. Mir, Dalton Trans., 2021, 50, 29–38 RSC.
- S. Naaz, P. Das, A. Frontera, B. Dutta, S. Khan, P. P. Ray and M. H. Mir, Cryst. Growth Des., 2022, 22, 5189–5197 CrossRef CAS.
- L. Sun, M. G. Campbell and M. Dincă, Angew. Chem., Int. Ed., 2016, 55, 3566–3579 CrossRef CAS PubMed.
- T. C. Narayan, T. Miyakai, S. Seki and M. Dincă, J. Am. Chem. Soc., 2012, 134, 12932–12935 CrossRef CAS PubMed.
- S. S. Park, E. R. Hontz, L. Sun, C. H. Hendon, A. Walsh, T. V. Voorhis and M. Dincă, J. Am. Chem. Soc., 2015, 137, 1774–1777 CrossRef CAS PubMed.
- F. Ahmed, S. Halder, B. Dutta, S. Islam, C. Sen, S. Kundu, C. Sinha, P. P. Ray and M. H. Mir, New J. Chem., 2017, 41, 11317–11323 RSC.
- B. Dutta, A. Dey, C. Sinha, P. P. Ray and M. H. Mir, Dalton Trans., 2019, 48, 11259–11267 RSC.
- T. Panda and R. Banerjee, Proc. Natl. Acad. Sci., India, Sect. A, 2014, 84, 331–336 CrossRef CAS.
- K. Naskar, S. Sil, N. Sahu, B. Dutta, A. M. Z. Slawin, P. P. Ray and C. Sinha, Cryst. Growth Des., 2019, 19, 2632–2641 CrossRef CAS.
- E. M. M. Ibrahim, L. H. Abdel-Rahman, A. M. Abu-Dief, A. Elshafaie, S. K. Hamdan and A. M. Ahmed, Mater. Res. Bull., 2018, 107, 492–497 CrossRef CAS.
- A. Elshafaie, L. H. Abdel-Rahman, A. M. Abu-Dief, S. K. Hamdan, A. M. Ahmed and E. M. M. Ibrahim, NANO, 2018, 13, 1850074 CrossRef CAS.
- L. H. A. Rahman, A. M. Abu-Dief, R. M. El-Khatib, S. M. Abdel-Fatah, A. M. Adam and E. M. M. Ibrahim, Appl. Organomet. Chem., 2018, 32, e4174 CrossRef.
- E. M. M. Ibrahim, L. H. Abdel-Rahman, A. M. Abu-Dief, A. Elshafaie, S. Kamel Hamdan and A. M. Ahmed, Phys. Scr., 2018, 93, 055801 CrossRef.
- S. Roy, A. Dey, P. P. Ray, J. Ortega-Castro, A. Frontera and S. Chattopadhyay, Chem. Commun., 2015, 51, 12974–12976 RSC.
- S. Halder, A. Layek, K. Ghosh, C. Rizzoli, P. P. Ray and P. Roy, Dalton Trans., 2015, 44, 16149–16155 RSC.
- S. Halder, A. Dey, A. Bhattacharjee, J. Ortega-Castro, A. Frontera, P. P. Ray and P. Roy, Dalton Trans., 2017, 46, 11239–11249 RSC.
- P. Ghorai, A. Dey, P. Brandão, J. Ortega-Castro, A. Bauza, A. Frontera, P. P. Ray and A. Saha, Dalton Trans., 2017, 46, 13531–13543 RSC.
- S. Roy, S. Halder, M. G. B. Drew, P. P. Ray and S. Chattopadhyay, ACS Omega, 2018, 3, 12788–12796 CrossRef CAS PubMed.
- S. Khan, S. Halder, P. P. Ray, S. Herrero, R. González-Prieto, M. G. B. Drew and S. Chattopadhyay, Cryst. Growth Des., 2018, 18, 651–659 CrossRef CAS.
- S. Konar, A. Dey, S. Ray Choudhury, K. Das, S. Chatterjee, P. P. Ray, J. Ortega-Castro, A. Frontera and S. Mukhopadhyay, J. Phys. Chem. C, 2018, 122, 8724–8734 CrossRef CAS.
- P. Ghorai, A. Dey, A. Hazra, B. Dutta, P. Brandão, P. P. Ray, P. Banerjee and A. Saha, Cryst. Growth Des., 2019, 19, 6431–6447 CrossRef CAS.
- S. Roy, A. Dey, M. G. B. Drew, P. P. Ray and S. Chattopadhyay, New J. Chem., 2019, 43, 5020–5031 RSC.
- S. Dey, S. Sil, B. Dutta, K. Naskar, S. Maity, P. P. Ray and C. Sinha, ACS Omega, 2019, 4, 19959–19968 CrossRef CAS PubMed.
- K. Ghosh, S. Sil, P. P. Ray, J. Ortega-Castro, A. Frontera and S. Chattopadhyay, RSC Adv., 2019, 9, 34710–34719 RSC.
- S. Roy, S. Halder, A. Dey, K. Harms, P. P. Ray and S. Chattopadhyay, New J. Chem., 2020, 44, 1285–1293 RSC.
- P. Ghorai, A. Dey, P. Brandão, S. Benmansour, C. J. Gómez García, P. P. Ray and A. Sahaw, Inorg. Chem., 2020, 59, 8749–8761 CrossRef CAS PubMed.
- S. Khan, S. Halder, A. Dey, B. Dutta, P. P. Ray and S. Chattopadhyay, New J. Chem., 2020, 44, 11622–11630 RSC.
- T. K. Ghosh, S. Jana, S. Jana and A. Ghosh, New J. Chem., 2020, 44, 14733–14743 RSC.
- A. Chandra, D. Das, J. O. Castro, K. Naskar, S. Jana, A. Frontera, P. P. Ray and C. Sinha, Inorg. Chim. Acta, 2021, 518, 120253 CrossRef CAS.
- S. Roy, A. Dey, R. M. Gomila, J. Ortega-Castro, A. Frontera, P. P. Ray and S. Chattopadhyay, Dalton Trans., 2022, 51, 5721–5734 RSC.
- S. Paul, B. Dutta, P. Das, S. Halder, M. Shit, P. P. Ray, K. Jana and C. Sinha, Appl. Organomet. Chem., 2023, 37, e7160 CrossRef CAS.
- K. Debsharma, S. Dey, D. Das, S. Halder, J. Ortega-Castro, S. Sarkar, B. Dutta, S. Maity, K. Jana, A. Frontera, P. P. Ray and C. Sinha, CrystEngComm, 2023, 25, 162–172 RSC.
- D. Majumdar, S. Roy, A. Dey and D. Sutradhar, J. Mol. Struct., 2023, 1294, 136438 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ma00557g |
| This journal is © The Royal Society of Chemistry 2023 |

